Abstract
Introduction:
Despite their poor tolerance, especially in the elderly, weak opioids (WO) remain commonly prescribed for patients with knee osteoarthritis (KOA). We compared the efficacy and safety of a new wearable transcutaneous electrical nerve stimulation (W-TENS) device with WO for the treatment of moderate-to-severe, nociceptive KOA chronic pain.
Methods:
The study was a non-inferiority, multicentric, prospective, randomized, single-blind, controlled, 2-parallel groups Trial. A total of 110 patients with KOA were included (Kellgren-Lawrence radiographic grade ⩾2; American College of Rheumatology criteria), with chronic moderate-to-severe nociceptive pain (mean 8-day pain intensity (PI) ⩾ 4 on an 11-point numerical rating scale), in failure to non-opioid analgesics, including nonsteroidal anti-inflammatory drugs (NSAIDs). Patients with neuropathic pain were excluded. The co-primary endpoints were mean PI at 3 months (M3) and number of potentially treatment-related adverse events (TRAEs). Secondary outcomes included Western Ontario MAC Master University function subscale (range, 0–68), additional pain and quality of life measures, and responder rates.
Results:
The non-inferiority of W-TENS was demonstrated in both the per protocol (PP) and intent-to-treat (ITT) populations. At M3, PI in PP population was 3.87 (2.12) compared with 4.66 (2.37) [delta: −0.79 (0.44); 95% CI (−1.65, 0.08)] in W-TENS and WO groups, respectively. A planned superiority analysis showed a significant superiority of W-TENS over WO on PI at M3 (p = 0.0124). The number of TRAEs was significantly lower in the W-TENS group (n = 7) than in the WO group (n = 36) (p < 0.001). Other secondary outcomes also favored W-TENS.
Conclusion:
W-TENS was more effective and better tolerated than WO in the treatment of chronic nociceptive KOA pain and offers an interesting non-pharmacological analgesic alternative in the management of KOA.
Trial Registration: ClinicalTrials.gov: NCT03902340
Keywords: clinical trial, knee osteoarthritis, opioids, pain management, TENS
Introduction
Osteoarthritis (OA) is frequent, costly, and a leading cause of disability. It is the most frequent joint disease, 1 one of the most common chronic health conditions in adults 2 and among the most frequent diseases managed in primary health care. 3 It affects around 10% of men and 18% of women aged over 60 years 4 and dramatically increases with age 5 and with the overweight/obesity worldwide epidemic for knee osteoarthritis (KOA). The most prevalent site of osteoarthritis is the knee. 6 Pain is the most frequent symptom of KOA. 1 It is a major cause of disability 7 and is frequently moderate to severe/very severe. Quality of life (QOL) is highly affected by chronic pain8,9 and negatively correlated with its intensity. 10 Approximately 40–60% of people living with KOA pain report inadequate pain relief despite their treatments.11,12
There are discrepancies across the OA treatment guidelines published by the American College of Rheumatology (ACR), 13 Osteoarthritis Research Society International (OARSI), 14 the European League Against Rheumatism (EULAR), and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). 15
The EULAR recommendations for the management of knee OA 16 state that acetaminophen, opioids, and coxibs are recommended based on evidence (1B), while transcutaneous electrical nerve stimulation (TENS), with the same level of evidence, is not recommended. However, the use of acetaminophen as a first-line analgesic is debated because it is conditionally not recommended by OARSI 14 and only conditionally recommended by ACR. 13
Previous guidelines16,17 stated that weak opioids (WO) could be recommended (level 1B). However, OARSI 14 strongly recommends against the use of both oral and transdermal opioids, ESCEO 15 recommends only short-term use of WO such as tramadol, and ACR conditionally recommends the use of tramadol for the management of KOA pain. 13
In all guidelines, non-pharmacological treatments are currently recommended as the first line of therapy in the management of KOA. Among them, TENS is recommended against only by ACR, due to the lack of a high-quality study with an appropriate sample size and control group.
TENS is the therapeutic application of the ‘Gate Control’ theory, which was developed more than 50 years ago. TENS devices are designed to deliver electrical stimulation through adhesive electrodes applied to the skin. TENS reduces pain intensity (PI) by activating a complex neuronal network involving peripheral, spinal, and supraspinal mechanisms. 18 TENS-induced analgesia can be explained by two mechanisms. 19 Conventional TENS, using high-frequency stimulation (80–100 Hz), acts at the segmental level and induces non-painful paresthesia at the site to which it is applied. These pain-free sensations 20 inhibit ascending pain messages in the posterior horn of the spinal cord, at the corresponding metameric level. The analgesic effect persists shortly after stimulation is discontinued. 21 The endomorphinic mode, using low-frequency stimulation (2 Hz), acts at the supraspinal level and induces delayed analgesia (after 15–30 min). The analgesic effect intensifies during stimulation and persists after stimulation is discontinued. 21
Cochrane reviews on TENS reach different conclusions. One highlighted the efficacy of TENS in the management of KOA pain, 22 while another was inconclusive. 23 A recent review was unable to draw any conclusions on the efficacy of TENS. 18 These divergent results may be due to limited data, methodological limitations, and small sample sizes in trials. 18 However, when used as recommended, TENS induces a clinically significant reduction in PI in patients with KOA. 24 As the efficacy of TENS for the management of chronic nociceptive pain is still controversial, 25 we felt it was necessary to conduct a controlled trial on an appropriate patient sample using a high-standard methodology.
The primary objective of our study was to evaluate the efficacy and safety of an innovative wearable transcutaneous electrical nerve stimulation (W-TENS) device compared with WO in KOA patients with chronic moderate-to-severe nociceptive pain.
Methods
Trial design
The study protocol was approved by the Nord-Ouest 1 Institutional Ethics Review Board (France) and prospectively registered as NCT03902340 on ClinicalTrials.gov (The protocol is in supplemental file). Participants provided their written informed consent.
The trial lasted from 19 December 2018 (first patient in) through 5 June 2020 (last patient, last visit).
This was a prospective, multicenter, randomized, single-blinded (for the primary efficacy endpoint), 2-parallel group, non-inferiority, controlled phase III trial comparing W-TENS with WO. The trial comprised two stages: a 3-month controlled period and an optional 3-month follow-up for the W-TENS group.
Patients
Eligible participants were French ambulatory KOA patients ⩾55 years of age, recruited by private-practice or hospital-based rheumatologists, or rehabilitation medicine physicians. All had a Kellgren-Lawrence radiographic grade ⩾2 26 and chronic (⩾3 months) moderate-to-severe nociceptive pain, defined as a PI ⩾4, assessed over the 8 days preceding Day 0 (D0) 27 using an 11-point numerical rating scale (NRS) where 0 = no pain and 10 = maximal pain. All could benefit from a WO prescription after treatment with non-opioid analgesics [acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs)] had failed. The patients were informed about TENS, shown how to use the device, and given the option to try this non-pharmacological analgesic alternative. To be included in the study, patients had to understand how to use W-TENS and WO prescriptions and had to have a smartphone to download the application enabling them to control the intensity of TENS stimulation.
Patients with neuropathic pain, defined as a global DN4 score ⩾4, 28 were excluded.
Randomization and blinding
Randomization was performed using LifeSphere software [Lifesphere EDC (Electronic Data Capture) system, ArisGlobal, USA] and was balanced with permuted blocks of four patients stratified by study centers. The patients were screened and randomized at baseline (D0) and assessed at D0, Month (M) 1, and M3 (study endpoint). Patients in the W-TENS group were also assessed at M6 follow-up. A phone call was planned at Day 15 to evaluate patients’ motivation and identify potential technical and therapeutic issues.
Except for the primary efficacy endpoint, which was collected by phone by a blinded evaluator before each visit, the study was open label.
In the W-TENS group, an advanced, 2-channel, mobile app-enabled, wearable TENS device (actiTENS®, Sublimed SAS, Moirans, France) was used. To ensure the accurate placement of electrodes, the positioning was standardized according to the ‘neurostimulation method for the treatment of moderate to severe chronic nociceptive pain in knee osteoarthritis’ described in United States Patent Application 20200147378 29 as presented in Figure 1. High- and low-frequency stimulations were delivered as follows:
Figure 1.
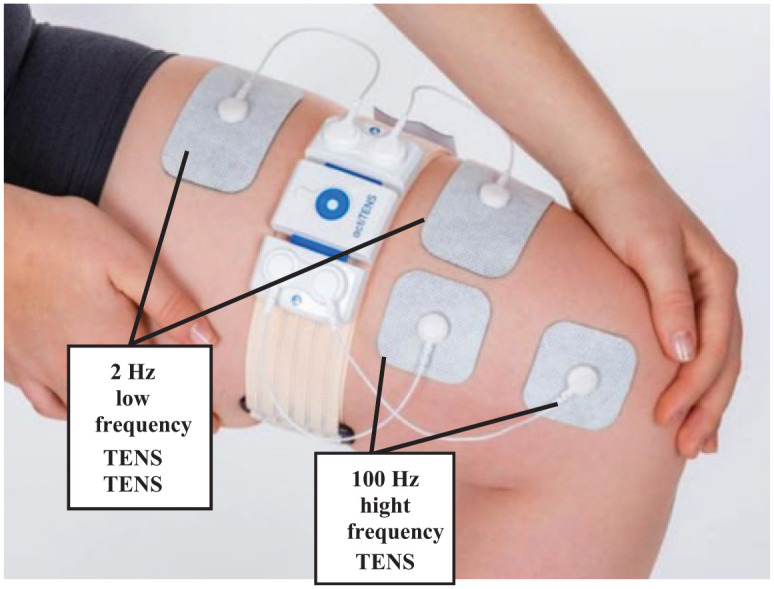
Standardized positioning of W-TENS electrodes. W-TENS is an advanced, mobile app-enabled, wearable TENS delivering electrical stimulation through adhesive electrodes applied to the skin and connected to the box. 50 mm × 50 mm square electrodes are positioned on the path of the infrapatellar nerve, branch of the saphenous nerve and innervating the joint. 50 mm × 90 mm rectangular electrodes are positioned on the quadriceps.
High-frequency (100 Hz) stimulation (i.e. gate control mode) delivered on channel 1 through two square electrodes (50 mm × 50 mm) positioned on the infrapatellar branch of the saphenous nerve innervating the joint.
Low-frequency (2 Hz) stimulation (i.e. endomorphinic mode) delivered on channel 2 through two rectangular electrodes (50 mm × 90 mm) positioned on the quadriceps.
The intensity and duration of stimulation were self-controlled by patients using a mobile application on their smartphone, which was connected to the W-TENS stimulator via Bluetooth. When using high-frequency stimulation, patients had to adjust the intensity so that the stimulation produced a perceptible and not unpleasant tingling feeling. When using low-frequency stimulation, they had to adjust the intensity so that the stimulation produced a not uncomfortable feeling of muscular contraction. 30
In the WO group, investigators were authorized to prescribe the most suitable drug and daily dose (DD), and to switch to another WO and adapt DD when necessary. The five authorized WO were immediate release (IR) or slow release (SR) tramadol; dihydrocodeine and fixed acetaminophen-codeine; acetaminophen-tramadol; and acetaminophen-opium powder combinations with and without caffeine. For treatments delivery in pharmacies, the investigators provided patients with a detailed prescription.
Non-analgesic pharmacological and non-pharmacological treatments remained unchanged. The following rescue analgesics were allowed: acetaminophen prescribed for ⩽5 days/month; an analgesic DD of NSAID, that is, equivalent to 1200 mg/day of ibuprofen for ⩽5 days/month; an anti-inflammatory DD of NSAID in case of flare-up; or arthrocentesis with an intra-articular injection of a corticosteroid, if performed at least 4 weeks before pain assessment.
All other analgesics or anesthetics – for example, treatments for neuropathic pain [antidepressants, antiepileptics (pregabalin, gabapentin), topical treatments (lidocaine or capsaicin patches)], strong opioids and other WO, systemic corticosteroids, viscosupplementation and injectable corticosteroids administered less than 4 weeks before pain assessment – were prohibited.
Baseline and outcome measures
Baseline evaluations included questionnaires on demographics, past medical and surgical history, and all medications taken, knee OA characteristics (including Kellgren-Lawrence grade) and initial scorings of all outcome measures.
Main outcome
The co-primary endpoints were mean PI at M3 – assessed over the preceding 8 days using an 11-point NRS – for efficacy, and the number of potentially treatment-related adverse events (TRAEs) during the 3-month follow-up control period – assessed by investigators as unlikely, possibly, or probably related – for safety.
Secondary outcomes
Secondary efficacy outcomes collected at M1, M3, and M6 (in the W-TENS group only) were (1) patients’ functional status, assessed using the Western Ontario and McMaster University Osteoarthritis Index (WOMAC, Likert-type-format, 0-4) (Global: range 0–96; function subscale: range 0–68); 31 (2) PI; (3) PI differences (PIDx = PIinclusion – PIx); (4) sum of PID (SPID: SPID0-x = Σ [T(x) − T(x − 1)] × PID(x); (5) pain relief (PAR), using a 0–100 mm visual analogue scale (VAS); (6) total pain relief (TOTPAR: TOTPARt0-tx = Σ [T(x) − T(x − 1)] × PAR(x), where x corresponds to M1 or M3 evaluation times; (7) QOL, assessed using EuroQol 5 Dimensions (EQ-5D); 32 and (8) Patient Global Impression of Change (PGIC). 33
The number of patients with a decrease in PI ⩾30% or ⩾50% was calculated at M1 and M3 to provide a responder-to-treatment rate. Since presenting results of therapeutic trials at the patient level using responder rate definitions is now widely recommended by international groups and commonly used in rheumatology trials, post hoc analyses were performed. The proportion of OMERACT-OARSI responders, 34 the proportion of patients with a Minimum Clinically Important Improvement [MCII relative improvement, i.e. ⩾20%, and absolute improvement, i.e. 5 (0–100 scale) or ⩾2 (NRS 0–10)], and the Patients Acceptable Symptom State (PASS, i.e. patients at ⩽40) for pain and WOMAC function were also calculated using normalized 0–100 pain and function scales. 35
In addition, the type and number of all AEs, and the number of patients concerned, were recorded throughout the entire duration of the study.
In the W-TENS group, the number of patients who asked to have the treatment extended for a further 3 months was also recorded.
Statistical analysis
All analyses and generation of individual data listings were performed using statistical software (SAS® version 9.4, SAS Institute Inc., Cary, NC, USA).
The sample size was calculated based on a non-inferiority analysis of the co-primary efficacy endpoint, hypothesizing a clinically relevant difference in means (SD) between the W-TENS and WO groups of over 1.0 (1.3) for PI on the 11-point NRS,36,37 with a non-inferiority margin of 0.825, a statistical power of 85%, a two-tailed alpha level of 5%, and anticipating protocol deviations in 15% of the patients. Based on the calculation, 110 patients (55 per treatment group) had to be recruited to obtain 92 patients in the per protocol (PP) set.
For the primary outcome, the main analysis was performed on the PP population (patients without major protocol deviations), followed by an analysis of the intent-to-treat (ITT) population, that is, all randomized patients in the study who received at least one prescription or attended at least one patient education session.
Non-inferiority analyses (co-primary efficacy endpoint) were performed on the ITT and PP populations. Then, if appropriate – that is, if the absolute value of the 95% confidence interval (CI) of the between-group difference at 3 months was >0 – a superiority analysis of the primary and secondary endpoints was performed on the ITT population. 38
For missing data (MD), the following rules were applied: 39 (1) MD at baseline were not replaced; (2) MD at M1 and M3 were replaced by baseline values (baseline observation carried forward – BOCF); (3) MD at M1 only was replaced by the mean of baseline and M3 values; and (4) MD at M3 were replaced by M1 data (last observation carried forward – LOCF).
Quantitative data were expressed as mean, SD, minimum (Min), median, and maximum (Max) values. Categorical data were expressed as numbers (n) and frequencies (%) and their 95% CI.
The non-inferiority analysis of the co-primary efficacy endpoint (PI at M3) was performed using a one-way analysis of covariance (ANCOVA) with study group (W-TENS/WO) and baseline values as covariates. The non-inferiority of W-TENS was demonstrated if the lower limit of the two-sided 95% CI of the mean between-group difference was above 0.825. In the ITT population, for the co-primary safety endpoint, a chi-square test or Fisher’s exact test was used to compare the proportions of TRAEs between groups.
For the secondary outcomes, the same analyses were performed [i.e. analysis of variance (ANOVA/ANCOVA) with study group and baseline values as covariates, and for qualitative secondary criteria, chi-square or Fisher’s exact tests]. If needed, a time factor and time-by-treatment group interactions were considered in the model.
Results
Patient disposition and demographic characteristics
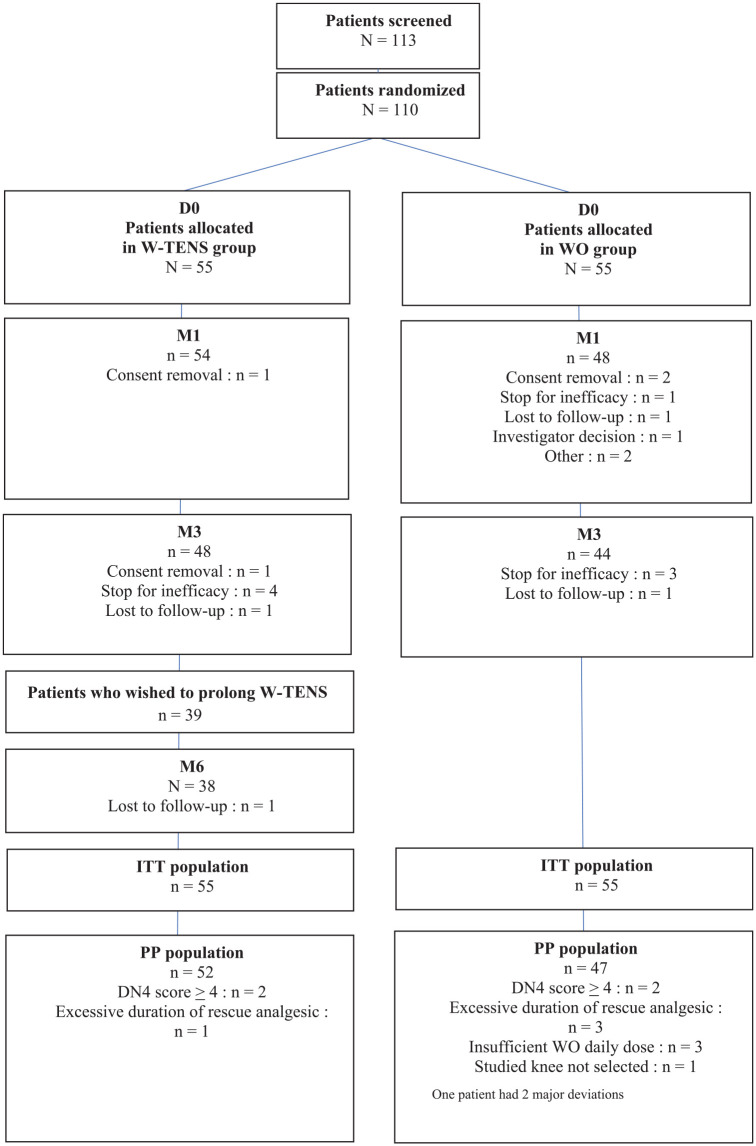
At D0, 113 patients were screened by 15 centers in France (median: 7 patients/center; range: 2–16). Due to screening errors, 3 patients were not randomized, leaving 110 randomized patients in the ITT population (55 per treatment group). As shown in the study flow chart (Figure 2), 11 patients had major deviations (3 in the W-TENS group and 8 in the WO group), leaving 99 patients (52/55 in the W-TENS group and 47/55 in the WO group) in the PP population.
Figure 2.
Consolidated standards of reporting trials (CONSORT) flow diagram showing the patients’ course during the study.
D0, Inclusion day; DN4, Neuropathic Pain Diagnostic Questionnaire–4 Questions; ITT, intent to treat; PP, per protocol; WO, weak opioid; W-TENS, wearable transcutaneous electrical nerve simulation.
Forty-eight patients in the W-TENS group (87.3%) and 44 patients in the WO group (80.0%) completed the 3-month controlled follow-up. The main reasons for discontinuation before the 3-month follow-up visit were lack of efficacy [four patients (7.3%) in the W-TENS group and three in the WO group (5.5%)] and withdrawal of consent [two in the W-TENS group (3.6%) and three in the WO group (5.5%)] (Figure 2).
Demographic and baseline characteristics were well balanced across the groups (Table 1).
Table 1.
Baseline patient characteristics at randomization.
| W-TENS group n = 55 |
WO group n = 55 |
|
|---|---|---|
| Age (years) | ||
| Mean (SD) | 66.9 ± 8.1 | 66.0 ± 7.8 |
| 95% CI ⩾75 years, n (%) |
[64.7–69.1] 8 (14.5) |
[63.9–68.2] 6 (10.9) |
| Min–Max | 55–89 | 55–89 |
| Sex (female) | ||
| N (%) | 37 (67.3) | 34 (61.8) |
| 95% CI | [54.9–79.7] | [49.0–74.7] |
| Weight (kg) (W-TENS group, n = 54) | ||
| Mean (SD) | 77.4 (14.4) | 83.0 (18.3) |
| 95% CI | [73.5–81.3] | [78.1–87.9] |
| Min–Max | 45–106 | 57–147 |
| Height (cm) (W-TENS group, n = 54) | ||
| Mean (SD) | 166.4 (8.5) | 166.9 (10.2) |
| 95% CI | [164.1–168.7] | [164.1–169.6] |
| Min–Max | 150–188 | 150–193 |
| BMI (kg/m2) (W-TENS group, n = 54) | ||
| Mean (SD) | 28.0 (5.0) | 29.8 (5.8) |
| 95% CI | [26.6–29.3] | [28.2–31.4] |
| Min–Max | 19.2–40.9 | 20.4–45.4 |
| Studied knee (right) | ||
| N (%) | 28 (50.9) | 30 (55.6) |
| 95% CI | [37.7–64.1] | [42.3–68.8] |
| DN4 global score (0–10) | ||
| Mean (SD) | 1.0 (1.2) | 1.0 (1.0) |
| 95% CI | [0.65–1.28] | [0.76–1.32] |
| Min–Max | 0.0–5.0 | 0.0–4.0 |
| PI (NRS: 0–10) | ||
| Mean (SD) | 5.9 (1.3) | 5.8 (1.3) |
| 95% CI | [5.5–6.2] | [5.5–6.2] |
| Range | 4.0–9.0 | 4.0–8.0 |
| WOMAC (global score 0–96) (WO group, n = 53) | ||
| Mean (SD) | 47.2 (15.5) | 46.7 (13.5) |
| 95% CI | [43.0–51.4] | [42.9–50.4] |
| Range | 17–74 | 13–80 |
| WOMAC (pain sub-score 0–20) (WO group, n = 53) | ||
| Mean (SD) | 10.9 (2.5) | 10.6 (2.8) |
| 95% CI | [10.2–11.6] | [9.9–11.4] |
| Range | 6–17 | 3–16 |
| WOMAC (function sub-score 0–68) (WO group, n = 54) | ||
| Mean (SD) | 32.0 (12.1) | 31.9 (10.5) |
| 95% CI | [28.7–35.2] | [29.1–34.8] |
| Range | 7–53 | 3–58 |
| WOMAC (stiffness sub-score 0–8) (WO group, n = 54) | ||
| Mean (SD) | 4.3 (2.0) | 4.3 (1.8) |
| 95% CI | [3.8–4.9] | [3.8–4.8] |
| Range | 0–8 | 0–8 |
| EQ-5D (global score 5–12) (WO group, n = 54) | ||
| Mean (SD) | 8.2 (1.4) | 7.7 (1.5) |
| 95% CI | [7.8–8.5] | [7.4–8.1] |
| Range | 6.0–11.0 | 6.0–12.0 |
BMI, body mass index; CI, confidence interval; DN4, Neuropathic Pain Diagnostic Questionnaire–4 questions; EQ-5D, EuroQol 5 Dimensions; NRS, Numerical Rating Scale (0 = non-pain; 10 = Maximal pain as you can imagine); PI, pain intensity; SD, standard deviation; WOMAC, Western Ontario and McMaster University Osteoarthritis Index; WO, weak opioid; W-TENS, wearable transcutaneous electrical nerve stimulation.
Global score: 0–96 (24 items, 0–4), Pain subscore: 0–20 (5 items, 0–4), Function subscore: 0–68 (17 items, 0–4), and Stiffness subscore: 0–8 (2 items, 0–4); EQ-5D = EuroQol 5 dimensions (Global score: 5–12, Mobility: 1–3, Self-care, 1–2, Usual activities: 1–2, Pain/Discomfort: 1–3; Anxiety/Depression: 1–2).
Treatments received in the WO group
The proportions of prescriptions for IR WO were balanced: 25.4% of the prescriptions were for codeine (median DD (MDD): 90 mg), 30.1% for opium powder with or without caffeine (MDD: 35 and 75 mg, respectively), and 22.2% for tramadol with or without acetaminophen (MDD: 112.5 and 100 mg, respectively). SR tramadol accounted for 17.5% of the prescriptions (MDD: 100 mg). WO were mainly prescribed as analgesic monotherapy. Five patients received a combination of SR WO with IR intakes as interdoses. The three patients who were excluded for insufficient DD were on monotherapy. Ten patients (18.5%) had treatment adaptations: five (8.5%) were switched to another WO, six (11.1%) had a DD adjustment, and one had both adaptations.
Rescue analgesics were more frequent in the WO group than in the W-TENS group [12 patients (22.2%) and 4 (7.3%), respectively].
Co-primary efficacy and safety outcomes
The non-inferiority of W-TENS was demonstrated in both the PP and ITT populations (Table 2). In the PP population, PI at M3 was 3.87 (2.12) in the W-TENS group and 4.66 (2.37) in the WO group [delta: −0.79 (0.44); 95% CI (−1.65, 0.08)]. Since the absolute value of the 95% CI of the mean between-group difference in PI [−1.71, −0.12] was >0 in the ITT set, the planned superiority analysis was performed, and demonstrated that W-TENS was significantly superior to WO at M3 (p = 0.0124).
Table 2.
Non-inferiority analyses on PI at M3 (main criterion): ITT and PP populations.
| Group population | Pain intensity at Month
3 p within-group change |
Between-group difference | ||||
|---|---|---|---|---|---|---|
| W-TENS | WO | W-TENS-WO | ||||
| PP Population (n) | 52 | 47 | ||||
| Mean (SD) | 3.87 (2.12) | <0.001 | 4.65 (2.37) | <0.001 | –0.79 (0.44) | Non-inferiority
a
demonstrated 95% CI <0.825 |
| 95% CI | [3.28, 4.46] | [4.03, 5.28] | [–1.65, 0.08] | |||
| ITT Population (n) | 55 | 55 | ||||
| Mean (SD) | 3.84 (2.08) | <0.001 | 4.73 (2.28) | <0.001 | –0.92 (0.40) | Non-inferiority
a
demonstrated 95% CI <0.825 |
| 95% CI | [3.27, 4.40] | [4.18, 5.30] | [–1.71, –0.12] | |||
CI, confidence interval; ITT, intent to treat; PI, pain intensity; PP, per protocol; SD, standard deviation; WO, weak opioid; W-TENS, wearable transcutaneous electrical nerve stimulation.
Least squares means for each study group and study group difference estimate. Corresponding 95% CI.
Non-inferiority margin was 0.825 for pain intensity on numerical rating scale and non-inferiority was demonstrated when 95% CI <0.825.
In the ITT population, the number of potentially TRAEs was significantly lower (p < 0.001) in the W-TENS group (n = 7) than in the WO group (n = 36) during the 3-month controlled follow-up (Table 3). Details of the AEs are provided in Table 4.
Table 3.
Total and potentially treatment-related numbers of adverse events over the 3-month controlled period.
| Treatment groups | p | ||
|---|---|---|---|
| W-TENS | WO | ||
| ITT Population (N) | 55 | 55 | |
| Total number of AE | 24 | 55 | |
| Number of potentially treatment-related AEs: n (%) | 7 (29.2) | 36 (65.5) | <0.0001 |
| 95% CI | [11.0, 47.4] | [52.9, 78.0] | |
AEs, adverse events; CI, confidence interval; n, number; %, percentage; WO, weak opioid; W-TENS, wearable transcutaneous electrical nerve stimulation.
Percentages and corresponding 95% CI.
Table 4.
Number and description of adverse events and serious adverse events collected during the 3-month follow-up period: ITT population.
| W-TENS
group n = 55 |
WO group n = 55 |
p-value | |||
|---|---|---|---|---|---|
| Events n |
Patients n (%) |
Events n |
Patients n (%) |
||
| Adverse events (AEs), n | 24 | 55 | |||
| Potentially treatment-related AEs | 7 | 36 | |||
| Patients with at least one AE | |||||
| n (%) | 7 (12.7) | 16 (29.1) | 0.0348 | ||
| 95% CI | [3.9, 21.5] | [17.1, 41.1] | |||
| Serious AE | 0 (0) | 0 (0) | |||
| AE potentially related to study treatments (by body system) | |||||
| Gastro-intestinal disorders | 0 | 0 | 19 | 12 (21.8) | |
| Abdominal pain | 0 | 0 | 1 | 1 (1.8) | |
| Constipation | 0 | 0 | 7 | 7 (12.7) | |
| Dry mouth | 0 | 0 | 1 | 1 (1.8) | |
| Dyspepsia | 0 | 0 | 1 | 1 (1.8) | |
| Hemorrhoids | 0 | 0 | 1 | 1 (1.8) | |
| Nausea | 0 | 0 | 7 | 6 (10.9) | |
| Vomiting | 0 | 0 | 1 | 1 (1.8) | |
| General disorders and at the application site | 1 | 1 (1.8) | 3 | 3 (5.5) | |
| Erythema at the application site | 1 | 1 (1.8) | 0 | 0 | |
| Fatigue | 0 | 0 | 3 | 3 (5.5) | |
| Clinical examination | 0 | 0 | 1 | 1 (1.8) | |
| Weight gain | 0 | 0 | 1 | 1 (1.8) | |
| Musculoskeletal disorders | 2 | 2 (3.6) | 2 | 2 (3.6) | |
| Arthralgia | 1 | 1 (1.8) | 0 | 0 | |
| Lombalgia | 0 | 0 | 1 | 1 (1.8) | |
| Arthrosis | 0 | 0 | 1 | 1 (1.8) | |
| Plantar fasciitis | 1 | 1 (1.8) | 0 | 0 | |
| Central nervous system disorders | 4 | 4 (7.3) | 8 | 7 (12.7) | |
| Dizziness | 0 | 0 | 3 | 3 (5.5) | |
| Cephalagia | 1 | 1 (1.8) | 1 | 1 (1.8) | |
| Hypoesthesia | 2 | 2 (3.6) | 0 | 0 | |
| Hypotonia | 1 | 1 (1.8) | 0 | 0 | |
| Drowsiness | 0 | 0 | 4 | 4 (7.3) | |
| Skin disorders | 0 | 0 | 3 | 3 (5.5) | |
| Eczema | 0 | 0 | 1 | 1 (1.8) | |
| Pruritus | 0 | 0 | 2 | 2 (3.6) | |
AE, adverse event; CI, confidence interval; % percentage; WO, weak opioids; W-TENS, wearable transcutaneous electrical nerve stimulation.
Number and percentages and corresponding 95% CI.
Numbers of AEs in each body system are in bold.
Secondary outcomes
All secondary outcomes favored the W-TENS group at M1 and M3 (Table 5).
Table 5.
Secondary outcomes at M1 and M3: ITT population.
| Outcomes Mean (SD) |
Month 1 | Month 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| W-TENS group | WO group | Between-group difference | p-value | W-TENS group | WO group | Between-group difference | p-value | |
| WOMAC: Global score | ||||||||
| Mean (SD) | 38.4 (16.8) | 42.9 (15.5) | –4.8 (2.6) | 0.0635 | 33.9 (19.8) | 42.2 (17.2) | –8.5 (3.3) | 0.0106 |
| 95% CI | [33.8, 42.9] | [38.7, 47.7] | [–9.8, 0.3] | [28.6, 39.3] | [37.5, 46.9] | [14.9, –2.0] | ||
| WOMAC: Pain subscore | ||||||||
| Mean (SD) | 9.0 (3.5) | 9.5 (3.5) | –0.6 (0.6) | 0.3047 | 7.9 (4.4) | 9.2 (3.6) | –1.5 (0.8) | 0.0570 |
| 95% CI | [8.0, 9.9] | [8.5, 10.4] | [–1.8, 0.6] | [6.8, 9.1] | [8.2, 10.2] | [–2.9, 0.0] | ||
| WOMAC: Physical function subscore | ||||||||
| Mean (SD) | 25.7 (12.4) | 29.1 (11.6) | –3.5 (1.8) | 0.0631 | 22.7 (14.0) | 28.9 (12.9) | –6.2 (2.3) | 0.0083 |
| 95% CI | [22.3, 29.0] | [25.9, 32.3] | [–7.2, 0.2] | [18.9, 26.5] | [25.4, 32.4] | [–10.8, –1.6] | ||
| WOMAC: Stiffness subscore | ||||||||
| Mean (SD) | 3.7 (2.0) | 4.3 (1.7) | –0.6 (0.3) | 0.0708 | 3.2 (2.3) | 4.0 (1.9) | –0.8 (0.4) | 0.0296 |
| 95% CI | [3.2, 4.3] | [3.9, 4.8] | [–1.2, 0.0] | [2.6, 3.8] | [3.5, 4.5] | [–1.5, –0.1] | ||
| Pain intensity (PI, NRS) | ||||||||
| Mean (SD) | 4.1 (2.1) | 5.3 (1.9) | –1.3 (0.4) | 0.0010 | 3.8 (2.1) | 4.7 (2.3) | –0.9 (0.4) | 0.0174 |
| 95% CI | [3.5, 4.6] | [4.8, 5.8] | [–2.0, –0.5] | [3.3, 4.4] | [4.1, 5.4] | [–1.7, –0.2] | ||
| Pain intensity differences (PID) | ||||||||
| Mean (SD) | 1.8 (2.1) | 0.5 (1.8) | 1.3 (0.4) | 0.0007 | 2.1 (2.3) | 1.1 (2.1) | 0.9 (0.4) | 0.0247 |
| 95% CI | [1.3, 2.4] | [0.0, 1.0] | [0.6, 2.0] | [1.4, 2.7] | [0.5, 1.7] | [0.2, 1.7] | ||
| Patient-responders (PR) rates | ||||||||
| PI reduction ⩾30%: n (%) | ||||||||
| n (%) | 31 (56.4) | 10 (18.2) | < 0.0001 | 29 (52.7) | 19 (34.5) | 0.0545 | ||
| 95% CI | [43.3, 69.5] | [8.0, 28.4] | [39.5, 65.9] | [22.0, 47.1] | ||||
| PI reduction ⩾50%: n (%) | ||||||||
| n (%) | 19 (34.5) | 5 (9.1) | 0.0012 | 21 (38.2) | 13 (23.6) | 0.0988 | ||
| 95% CI | [22.0, 47.1] | [1.5, 16.7] | [25.3, 51.0] | [12.4, 34.9] | ||||
| OMERAC-OARSI | ||||||||
| n (%) | 24 (43.6) | 13 (23.6) | 0.0264 | 30 (54.5) | 18 (32.7) | 0.0211 | ||
| 95% CI | [41.4, 67.7] | [12.4, 34.9] | [41.4, 67.7] | [20.3, 45.1] | ||||
| MCII Pain (absolute) | ||||||||
| n (%) | 31 (56.4) | 14 (25.5) | 0.0010 | 30 (54.5) | 20 (36.4) | 0.0555 | ||
| 95% CI | [43.3, 69.5] | [13.9, 37.0] | [41.4, 67.7] | [23.7, 49.1] | ||||
| MCII Pain (relative) | ||||||||
| n (%) | 34 (61.8) | 14 (25.5) | 0.0001 | 33 (60.0) | 25 (45.5) | 0.1266 | ||
| 95% CI | [49.0, 74.7] | [13.9, 37.0] | [47.1, 72.9] | [32.8, 58.6] | ||||
| MCII WOMAC Function (absolute) | ||||||||
| n (%) | 18 (32.7) | 13 (24.1) | 0.3167 | 21 (38.2) | 13 (24.1) | 0.1119 | ||
| 95% CI | [20.3, 45.1] | [12.7, 35.5] | [25.3, 51.0] | [12.7, 35.5] | ||||
| MCII WOMAC Function (relative) | ||||||||
| n (%) | 28 (50.9) | 17 (31.5) | 0.0394 | 29 (52.7) | 19 (35.2) | 0.0651 | ||
| 95% CI | [37.7, 64.1] | [19.1, 43.9] | [39.5, 65.9] | [22.4, 47.9] | ||||
| PASS (pain) | ||||||||
| n (%) | 29 (52.7) | 14 (25.5) | 0.0034 | 29 (52.7) | 23 (41.8) | 0.2519 | ||
| 95% CI | [39.5, 65.9] | [13.9, 37.0] | [39.5, 65.9] | [28.8, 54.9] | ||||
| PASS (WOMAC Function) | ||||||||
| n (%) | 31 (56.4) | 22 (40.7) | 0.1028 | 36 (65.5) | 22 (40.7) | 0.0097 | ||
| 95% CI | [43.3, 69.5] | [27.6, 53.8] | [52.9, 78.0] | [27.6, 53.8] | ||||
ANOVA, analysis of variance; CI, confidence interval; MCII, minimum clinically important improvement (⩾15 on a 0–100 scale or ⩾2 on a 0–10 NRS for absolute improvement, and ⩾20% for relative improvement on pain and WOMAC function); NRS, Numerical Rating Scale (0 = non-pain; 10 = Maximal pain as you can imagine); OMERAC-OARSI, criteria: improvement in pain or physical function ⩾50% and an absolute change ⩾20 mm; or improvement of ⩾20% with an absolute change ⩾10 mm in at least two of the following three categories: pain, physical function, and patient’s global assessment; PASS, patient acceptable symptom state (⩽40 on a 0–100 scale or ⩽4 on 0–10 NRS for pain and WOMAC Function); PI, pain intensity; PID, pain intensity difference; SD, standard deviation; WO, weak opioid; WOMAC, Western Ontario and McMaster University Osteoarthritis Index, Global score: 0–96 (24 items, 0–4), Pain subscore: 0–20 (5 items, 0–4), Function subscore: 0–68 (17 items, 0–4) and Stiffness subscore: 0–8 (2 items, 0–4); W-TENS, wearable transcutaneous electrical nerve stimulation.
Study group: one-way ANOVA on outcomes (rank data) at M1 and M3, Least squares means, ITT population. Study group: p-value < 0.0001.
Between-study group differences: one-way ANOVA on outcomes (rank data) at M1 and M3. Means (SD) and corresponding 95% confidence interval (CI).
For quantitative data, values are scores (SD) and 95% CI; for responders analyses, results are given as numbers (%) of responders (YES) and 95% CI. Chi-square test or Fisher exact test at M1 and M3.
WOMAC function was significantly lower in the W-TENS group than in the WO group at M3 –22.7 (14.0) and 28.9 (12.9), respectively (p = 0.0083) – but not at M1 (p = 0.0631). The same trend was observed for WOMAC global scores and stiffness subscores, but not for pain subscores, which failed to reach statistical significance at M3.
As shown in Table 5, PI was lower at M1 and M3 in the W-TENS group [4.1 (2.1) and 3.8 (2.1), respectively] than in the WO group [5.3 (1.9) and 4.7 (2.3), respectively], and between-treatment differences were significantly different [Mean (95% CI), −1.3 (−2.0, −0.5), p = 0.0010 and −0.9 (−1.7, −0.2), p = 0.0174] (Table 5). Consequently, PI reductions from baseline, evaluated by PID at M1 and M3, were higher in the W-TENS group [1.8 (2.1) and 2.1 (2.3)] than in the WO group [0.5 (1.8) and 1.1 (2.1), respectively], and between-treatment changes were significantly different [Mean (95% CI), 1.3 (0.6, 2.0) p = 0.0007 and 0.9 (0.2, 1.7), p = 0.0247] (Table 5). Results for SPID, PAR, and TOTPAR are available in Table 6.
Table 6.
Secondary outcomes at M1 and M3: ITT population.
| Outcomes | Month 1 | Month 3 | ||||
|---|---|---|---|---|---|---|
| W-TENS group | WO group | p-value | W-TENS group | WO group | p-value | |
| Sum of pain intensity difference (SPID) | ||||||
| Mean (SD) | 54.5 (7.7) | 16.3 (7.7) | 0.0007 | 176.6 (22.7) | 83.4 (22.7) | 0.0046 |
| 95% CI | [39.2, 69.8] | [0.9, 31.6] | [131.4, 221.6] | [38.3, 128.5] | ||
| Pain relief (PAR, VAS) | ||||||
| Mean (SD) | 55.9 (4.0) | 45.5 (4.2) | 0.0749 | 55.8 (4.0) | 46.6 (4.2) | 0.1154 |
| 95% CI | [48.0, 63.8] | [3.3, 53.8] | [47.9, 63.8] | [38.2, 55.0] | ||
| Total pain relief (TOTPAR) | ||||||
| Mean (SD) | 55.9 (4.0) | 45.6 (4.2) | 0.0749 | 56.2 (3.9) | 46.2 (4.3) | 0.0861 |
| 95% CI | [48.0, 63.8] | [37.3, 53.8] | [48.3, 64.1] | [37.8, 54.6] | ||
| EQ-5D: global score | ||||||
| Mean (SD) | 7.3 (0.1) | 7.8 (0.1) | 0.0171 | 7.0 (0.2) | 7.7 (0.2) | 0.0058 |
| 95% CI | [7.0, 7.6] | [7.5, 8.0] | [6.7, 7.4] | [7.4, 8.0] | ||
| PGIC: degree of change (NRS) | ||||||
| Mean (SD) | 3.6 (1.4) | 4.2 (1.7) | 0.0584 | 3.4 (1.8) | 4.4 (2.3) | 0.0031 |
| 95% CI | [3.2, 4.0] | [3.7, 4.7] | [3.0, 3.9] | [3.7, 5.1] | ||
| PGIC: description of change (NRS) | ||||||
| Mean (SD) | 4.0 (1.7) | 3.3 (1.9) | 0.0392 | 4.4 (1.8) | 3.2 (2.0) | 0.0203 |
| 95% CI | [3.5, 4.5] | [2.7, 3.8] | [3.9, 4.9] | [2.6, 3.] | ||
ANOVA, analysis of variance; CI, confidence interval; EQ-5D, EuroQol 5 dimensions (Global score: 5–12; subscores: Mobility: 1–3, Self-care, 1–2, Usual activities: 1–2, Pain/Discomfort: 1–3; Anxiety/Depression: 1–2); NRS, Numerical Rating Scale; PAR, Pain Relief; PGIC, Patient Global impression of Change. Degree of change (NRS: 0 = much better; 10 = worst) and description of change (NRS: 1 = no change or worst; 7: much better); PID, Pain Intensity Difference; SD, standard deviation; SPID, Sum of Pain Intensity Difference; TOTPAR, Total Pain Relief; VAS, visual analogue scale (0 = no pain relief; 100 = maximal pain relief); WO, weak opioid; W-TENS, wearable transcutaneous electrical nerve stimulation.
Study group: One-way ANOVA on outcomes (rank data) at M1 and M3, Least squares means, ITT population.
Between-study group difference: One-way ANOVA on outcomes (rank data) at M1 and M3. Estimate and corresponding 95% confidence interval (CI).
Study group: p-value <0.0001 except for PID and SPID in WO group: <0.05 at M1 and <0.01 at M3.
The proportions of patients with a decrease in PI ⩾30% and ⩾50% (responder patients – RP) were higher at M1 and M3 in the W-TENS group, but the differences were only significant at M1 [RP30%: 31/55 (56.4%) versus 10/55 (18.2%), p < 0.0001; RP50%: 19/55 (34.5%) versus 5/5 (9.1%), p = 0.0012; Table 5]. OMERACT-OARSI responder rates were significantly higher in the W-TENS group than in the WO group at M1 (43.6% and 23.6%, respectively; p = 0.0264) and at M3 (54.5% versus 32.7%; p = 0.0211). Other responder definitions showed significantly higher percentages in the W-TENS group at M1 [MCII for pain (relative/absolute), MCII for WOMAC function (relative), and PASS for pain] and at M3 (PASS for WOMAC function) (Table 5).
In the W-TENS group, improvement in QOL was significantly greater at M1 and M3. PGIC scores were also significantly higher in the W-TENS group (Table 6).
Thirty-nine patients (70.9% of randomized patients) asked to have the W-TENS treatment extended for a further 3 months, and only one patient discontinued treatment during this non-controlled period (lost to follow-up). For the 38 patients who completed the 3-month extension, the efficacy results obtained at M3 remained stable through M6 [mean PI: 3.32 (0.33) at M6 in the ITT population].
The proportion of patients who reported at least one AE was significantly higher in the WO group than in the W-TENS group [16 (29.1%) and 7 (12.7%), respectively; p = 0.0348]. In the WO group, AEs were systemic and those commonly reported with opioids, that is, dry mouth, constipation, nausea, vomiting, dizziness, drowsiness, and pruritus. In the W-TENS group, AEs were local and related to the use of the device, that is, cutaneous reactions (erythema) (Table 4).
No serious AEs were reported during the controlled period.
No AEs potentially related to W-TENS were reported during the additional 3-month period.
Discussion
Pain is the main symptom in patients with KOA. Its mechanisms include pain sensitization and increased responsiveness of peripheral nociceptors.40,41 To our knowledge, this is the first prospective, randomized, controlled trial comparing a standardized use of TENS (electrode positioning and a predetermined type of stimulation on each channel) with WO analgesics in patients suffering from chronic, moderate-to-severe nociceptive KOA pain.
Our trial showed that, in terms of efficacy, W-TENS not only demonstrated non-inferiority to WO in both the PP and ITT populations, but also superiority in the ITT population. At M1 and M3, the W-TENS group reached the absolute minimal clinically important difference (MCID) for an analgesic [1.8 (2.1) and 2.1 (2.3), respectively], corresponding to a 20 mm reduction in PI (interquartile range: 15–30) on a 0–100 mm VAS – that is, 2 points on a NRS 42 – which equates to ‘much better’. 42 Conversely, in the WO group, a 0.5 (1.8) and a 1.1 (2.1) reduction in PI were observed at M1 and M3, respectively, while a 1-point reduction in PI is required to be considered as a ‘slightly better’ improvement. 42 Consequently, in the W-TENS group compared with the WO group, there were higher proportions of (1) responders to treatment ⩾30% and ⩾50% at M1 [RP30%: 31/55 (56.4%) and 10/55 (18.2%), respectively; p < 0.0001; RP50%: 19/55 (34.5%) and 5/55 (9.1%), respectively; p = 0.0012] and M3 (although not significant); (2) OMERACT-OARSI responders at M1 and M3; and (3) patients achieving MCII relative/absolute for pain, MCII relative for function and PASS for pain at M1, or PASS for function at M3. As relative MCID was estimated as a 32% reduction in PI (interquartile range: 15–41), 43 a 30% reduction in PI reflects a moderately clinically relevant change in chronic pain, while a 50% reduction reflects a more substantial improvement. 44
In this trial, W-TENS had a rapid positive effect, from M1. However, although patients in the W-TENS group continued to improve after M1, between-treatment group differences in responder rates were lower and not significant at M3. The fact that 10 (18.5%) patients had treatment adjustments (switch in WO and daily dose adjustment) could explain these results. Besides, a higher proportion of WO patients received a rescue analgesic (22.2% versus 7.3%).
All secondary outcomes favored the W-TENS group, including the WOMAC index, which is commonly used in KOA trials. 45 In this study, between-treatment differences in WOMAC global scores were statistically significant at M3, mainly due to improvements in ‘function’ and ‘stiffness’ subscores. The WOMAC questionnaire seemed less effective than PI evaluation using a NRS in discriminating between the two analgesic treatments. The reduction in PI in the W-TENS group was associated with a significant improvement in the EQ-5D global score at M1 and M3, confirming the negative correlation between pain severity and QOL. 36
At M3, around 70% of the randomized patients in the W-TENS group asked to prolong the treatment for a further 3 months, and only one patient discontinued before M6. This uncontrolled period confirmed the long-term efficacy and safety of W-TENS.
The clinically and statistically significant efficacy of W-TENS demonstrated in this study is probably due to the specific positioning of the electrodes and the unique design of the actiTENS® product, which encourages and facilitates patient compliance.
Analgesic prescriptions comprised an even balance of the WO recommended for the treatment of KOA pain. 46 In addition, there were few premature drop-outs during the 3-month controlled follow-up, which further substantiates the relevance of our results. Although several new pain treatments are under development, 47 choosing the best treatment for pain in KOA remains a challenge. Controversy surrounding the use of WO was revived after several recent meta-analyses reported that WO might produce symptom relief and even improve function in patients with KOA.48–51 However, opioids are poorly tolerated and associated with an increase in the number of AEs, especially in the elderly, hence a Number-Needed-To-Harm of 5.49,52,53 Besides, their clinical relevance is questionable due to a disputed benefit (mean effect size between 0.3 and 0.7). Previous guidelines16,17 stated that WO could be recommended (level 1B). In recent guidelines endorsed by the American College of Rheumatology/Arthritis Foundation, tramadol is conditionally recommended and non-tramadol opioids are conditionally recommended against. 13 The use of oral opioids is strongly recommended against in the updated OARSI guidelines. 14 Nevertheless, WO are still often used in patients with KOA – when other treatments have failed or are contraindicated – and in patients awaiting a total knee replacement. In our setting, the co-primary safety outcome largely favored W-TENS: the number of patients with at least one potentially TRAE in the W-TENS group (n = 7) was significantly lower than in the WO group (n = 16), which is promising and suggests an interesting balance between the benefits and risks of this treatment.
As symptomatic KOA occurs frequently in elderly patients, and because chronic pain requires long-term treatment, safety is a key issue.54,55 Recently, major concerns have been raised regarding the overuse and misuse of opioids in painful chronic conditions and their consequences on safety.49,56
The AEs observed in the W-TENS group were mainly local cutaneous reactions (erythema, 5.5%) due to the TENS technique, while those observed in the WO group were systemic and well known (dry mouth: 1.8%; constipation: 12.7%; nausea: 10.9%; vomiting: 1.8%; dizziness: 5.5%; drowsiness: 7.3%; and pruritus: 3.6%) and limit their use and effectiveness in clinical practice. 57
The strengths of our study are its high-standard methodology and the sample size, which was calculated in advance to demonstrate the non-inferiority of W-TENS – and its superiority – with a power of 85%.
There are also some limitations to our study: (1) As the intensity and duration of TENS stimulation was self-managed by patients according to their feelings, the time of day, and their habits, the exact daily ‘dose’ administered was not standardized; (2) we did not use a sham TENS device; and (3) the study was not double-blind, and several outcomes were collected in an open fashion. However, it is important to emphasize that the last mean 8-day PI, which was the primary efficacy outcome, was collected by a blind independent assessor at each time point.
Further studies should certainly be performed to assess W-TENS efficacy versus a sham TENS or other treatments and, also, to evaluate the duration of the efficacy in reducing pain intensity in patients with KOA over 12 months and more.
It should be noted that the Food and Drug Administration (FDA) has provided its clearance to market W-TENS as a Class II device under the classification name: Transcutaneous Electrical Nerve Stimulator for Pain Relief. It is ‘available for prescription only and intended to be used as: symptomatic relief and management of chronic, intractable pain … Relief of pain associated with arthritis’. 58
In conclusion, W-TENS achieved significance for both co-primary outcomes and demonstrated a better efficacy and safety profile than WO. Given the age and comorbidities of the KOA population, often prone to polypharmacy and sensitive to adverse reactions, W-TENS could be a relevant non-pharmacological therapeutic alternative to pharmacological analgesics for the management of KOA pain. Its efficacy is certainly further enhanced by standardizing the W-TENS protocol and educating patients on the use of the device.
Overall, our results highlight the good benefit-to-risk ratio of W-TENS in patients with KOA and strongly support the use of W-TENS as a non-pharmacological alternative for the management of KOA.
Supplemental Material
Supplemental material, sj-doc-1-tab-10.1177_1759720X211066233 for Wearable transcutaneous electrical nerve stimulation (actiTENS®) is effective and safe for the treatment of knee osteoarthritis pain: a randomized controlled trial versus weak opioids by Emmanuel Maheu, Sandrine Soriot-Thomas, Eric Noel, Hervé Ganry, Eric Lespessailles and Bernard Cortet in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-doc-2-tab-10.1177_1759720X211066233 for Wearable transcutaneous electrical nerve stimulation (actiTENS®) is effective and safe for the treatment of knee osteoarthritis pain: a randomized controlled trial versus weak opioids by Emmanuel Maheu, Sandrine Soriot-Thomas, Eric Noel, Hervé Ganry, Eric Lespessailles and Bernard Cortet in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We are grateful to the following investigators who contributed to the ArthroTENS study: Profs and Drs P. Fardellone (Amiens), E. Coudeyre (Clermont-Ferrand), Y. Donazzolo (Gieres), A. Amouzougan (Saint-Etienne), L. Grange (Grenoble), T. Conrozier (Belfort), E. Senbel (Marseille), JP. Sanchez (Billère), R. Forestier (Aix-les-Bains), H. Bard (Paris), and E. Gibert (Ivry-sur-Seine).
Footnotes
Author Contributions: Emmanuel Maheu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Sandrine Soriot-Thomas: Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Eric Noel: Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Hervé Ganry: Conceptualization; Data curation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Eric Lespessailles: Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Bernard Cortet: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.M.: punctual interventions, Board member, expertise, speaker at congress for Amgen, Expanscience, Fidia, Mylan, Pierre Fabre, Sublimed, TRB Chemedica; S.S.-T.: Board member, expertise, trainer and speaker at congress for Grünenthal, Sublimed, Kiowa-Kirin Sanofi, Expansciences, Teva, Mylan, Therabel; E.N.: Occasional expertise for Sublimed. H.G.: Expertise for Sublimed, Ludocare, UCB; E.L.: Occasional interventions as an expert or speaker for Amgen, Expanscience, Lilly, MSD, Novartis, Sublimed, Theramex, UCB; B.C.: Occasional interventions as an expert or speaker for Alexion, Amgen, Expanscience, Ferring, Kyowa-Kirin, Lilly, Mylan, MSD, Novartis, Sublimed, Theramex, and UCB.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Sublimed SAS, Moirans, France, manufacturer of actiTENS with the collaboration of Laboratoires Expanscience, Paris La Défense, France.
ORCID iD: Emmanuel Maheu  https://orcid.org/0000-0001-6615-3630
https://orcid.org/0000-0001-6615-3630
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Emmanuel Maheu, Rheumatology Department, St-Antoine Hospital–APHP, 75011 Paris, FrancePrivate Office, 283, Bd Voltaire, Paris, France.
Sandrine Soriot-Thomas, Clinical Research Centre and Orthopaedic and Traumatology Surgery Department, CHU Amiens Picardie, Amiens, France.
Eric Noel, Rheumatologist, Ramsay Santé, Hôpital Privé Jean Mermoz, Centre Orthopédique Santy, Lyon, France.
Hervé Ganry, Hergan Consulting 4U, Amiens, France.
Eric Lespessailles, Rheumatology Department, Regional Hospital of Orleans, University of Orléans, Orléans, France.
Bernard Cortet, Department of Rheumatology, Centre Hospitalier Universitaire de Lille, 59000 Lille, France.
References
- 1. Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage 2020; 28: 242–248. [DOI] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeistra S. Osteoarthritis. Lancet 2019; 393: 1745–1759. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt TW. Approach to osteoarthritis management for the primary care provider. Prim Care 2018; 45: 361–378. [DOI] [PubMed] [Google Scholar]
- 4. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage 2018; 26: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deshpande BR, Katz JN, Solomon DH, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res 2016; 68: 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spitaels D, Mamouris P, Vaes B, et al. Epidemiology of knee osteoarthritis in general practice: a registry-based study. BMJ Open 2020; 10: e031734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrew R, Derry S, Taylor RS, et al. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract 2014; 14: 79–94. [DOI] [PubMed] [Google Scholar]
- 9. Hadi MA, McHugh GA. Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. J Patient Exp 2019; 6: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kowal J, Wilson KG, McWilliams LA, et al. Self-perceived burden in chronic pain: relevance, prevalence, and predictors. Pain 2012; 153: 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conaghan PG, Peloso PM, Everett SV, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology 2015; 54: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson MI, Jones G, Paley CA, et al. The clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain: a protocol for a meta-analysis of randomised controlled trials (RCTs). BMJ Open 2019; 9: e029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Review Arthritis Care Res 2020; 72: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 15. Arden NK, Perry TA, Bannuru RR, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol 2021; 17: 59–66. [DOI] [PubMed] [Google Scholar]
- 16. Jordan KM, Arden N, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis 2003; 62: 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Block JA. Osteoarthritis: OA guidelines: improving care or merely codifying practice. Nat Rev Rheumatol 2014; 10: 324–326. [DOI] [PubMed] [Google Scholar]
- 18. Gibson W, Wand BM, Meads C, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic pain – an overview of Cochrane Reviews. Cochrane Database Syst Rev 2019; 2: CD011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sluka KA, Bjordal JM, Marchand S, et al. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther 2013; 93: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibson W, Wand BMO, Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev 2017; 9: CD011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coutaux A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine 2017; 84: 657–661. [DOI] [PubMed] [Google Scholar]
- 22. Osiri M, Welch V, Brosseau L, et al. Transcutaneous electrical nerve stimulation for the knee osteoarthritis. Cochrane Database Syst Rev 2000; 4: CD002823. [DOI] [PubMed] [Google Scholar]
- 23. Rutjes AW, Nuesch E, Sterchi R, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst Rev 2009; 4: CD002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vance CGT, Dailey DL, Rakel BA, et al. Using TENS for pain control. The state of the evidence. Pain Manag 2014; 4: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 2018; 11: 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthritis. Ann Rheum Dis 1957; 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carr ECJ, Meredith P, Chumbley G, et al. Pain: a quality of care issue during patients’ admission to hospital. J Adv Nurs 2014; 70: 1391–1403. [DOI] [PubMed] [Google Scholar]
- 28. Van den Kerkhof EG, Stitt L, Clark A, et al. Sensitivity of the DN4 in screening for neuropathic pain syndromes. Clin J Pain 2018; 34: 30–36. [DOI] [PubMed] [Google Scholar]
- 29. Soriot-Thomas S, Abraham-Briffod P, Le Bastard F. Neurostimulation method for the treatment of moderate to severe chronic nociceptive pain in knee osteoarthritis. Patent Application Publication. Pub. No: US2020/01477378 A1. 2020, https://www.freepatentsonline.com/20200147378.pdf
- 30. Johnson M. Transcutaneous electrical nerve stimulation (TENS). Research to support clinical practice. Oxford: Oxford University Press, 2014. [Google Scholar]
- 31. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15: 1833–1840. [PubMed] [Google Scholar]
- 32. EuroQol Group (1990). EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 33. Guy W. ECDEU assessment manual for psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM). Rockville, MD: National Institute of Mental Health, 1976, pp. 76–338. [Google Scholar]
- 34. Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 2004; 12: 389–399. [DOI] [PubMed] [Google Scholar]
- 35. Tubach F, Ravaud P, Martin-Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res 2012; 64: 1699–1707. [DOI] [PubMed] [Google Scholar]
- 36. Moore A, Derry S, Eccleston C, et al. Expect analgesic failure; pursue analgesic success. BMJ 2013; 346: f2690. [DOI] [PubMed] [Google Scholar]
- 37. Stauffer ME, Taylor SD, Watson DJ, et al. Definition of nonresponse to analgesic treatment of arthritic pain: an analytical literature review of the smallest detectable difference, the minimal detectable change, and the minimal clinically important difference on the pain visual analog scale. Int J Inflam 2011; 2011: 231926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tripepi G, Chesnaye NC, Dekker FW, et al. Intention to treat and per protocol analysis in clinical trials. Nephrology 2020; 25: 513–517. [DOI] [PubMed] [Google Scholar]
- 39. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on missing data in confirmatory clinical trials. 2010. EMA/CPMP/EWP/1776/99Rev.1, chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.ema.europa.eu%2Fen%2Fdocuments%2Fscientific-guideline%2Fguideline-missing-data-confirmatory-clinical-trials_en.pdf&clen=147855&chunk=true [Google Scholar]
- 40. Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology 2018; 57: 43–50. [DOI] [PubMed] [Google Scholar]
- 41. Lluch E, Torres R, Nijs J, et al. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain 2014; 18: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 42. Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004; 8: 283–291. [DOI] [PubMed] [Google Scholar]
- 43. Olsen MF, Bjerre E, Hansen MD, et al. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 2018; 101: 87–106. [DOI] [PubMed] [Google Scholar]
- 44. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 45. Copsey B, Thompson JY, Vadher K, et al. Problems persist in reporting of methods and results for the WOMAC measure in hip and knee osteoarthritis trials. Qual Life Res 2019; 28: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sarzi-Puttini P, Velluci R, Zuccaro SM, et al. The appropriate treatment of chronic pain. Clin Drug Invest 2012; 32: 21–33. [DOI] [PubMed] [Google Scholar]
- 47. Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol 2020; 16: 673–688. [DOI] [PubMed] [Google Scholar]
- 48. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis: a systematic review and metaanalysis. Rheumatology 2007; 34: 543–555. [PubMed] [Google Scholar]
- 49. Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage 2007; 15: 957–965. [DOI] [PubMed] [Google Scholar]
- 50. da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2014; 17: CD003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith SR, Deshpande BR, Collins JE, et al. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage 2016; 24: 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lo-Ciganic WH, Floden L, Lee JK, et al. Analgesic use and risk of recurrent falls in participants with or at risk of knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017; 25: 1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng C, Dubreuil M, La Rochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA 2019; 12: 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pickering G. Analgesic use in the older person. Curr Opin Support Palliat Care 2012; 6: 207–212. [DOI] [PubMed] [Google Scholar]
- 55. Tracy B, Morrison RS. Pain management in older adults. Clin Ther 2013; 35: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 56. Fuggle N, Curtis E, Shaw, et al. Safety of opioids in osteoarthritis: outcomes of a systematic review and meta-analysis. Drug Aging 2019: 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bugata D, Lorini LF, Fumagalli R, et al. Genetics and opioids: towards more appropriate prescription in cancer pain. Cancers 2020; 12: 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. 510K, https://www.accessdata.fda.gov/cdrh_docs/pdf20/K202159.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tab-10.1177_1759720X211066233 for Wearable transcutaneous electrical nerve stimulation (actiTENS®) is effective and safe for the treatment of knee osteoarthritis pain: a randomized controlled trial versus weak opioids by Emmanuel Maheu, Sandrine Soriot-Thomas, Eric Noel, Hervé Ganry, Eric Lespessailles and Bernard Cortet in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-doc-2-tab-10.1177_1759720X211066233 for Wearable transcutaneous electrical nerve stimulation (actiTENS®) is effective and safe for the treatment of knee osteoarthritis pain: a randomized controlled trial versus weak opioids by Emmanuel Maheu, Sandrine Soriot-Thomas, Eric Noel, Hervé Ganry, Eric Lespessailles and Bernard Cortet in Therapeutic Advances in Musculoskeletal Disease