Abstract
Background:
Matrix-associated autologous chondrocyte implantation (ACI) is a well-established treatment for cartilage defects. High-level evidence at midterm follow-up is limited, especially for ACI using spheroids (spherical aggregates of ex vivo expanded human autologous chondrocytes and self-synthesized extracellular matrix).
Purpose:
To assess the safety and efficacy of 3-dimensional matrix-associated ACI using spheroids to treat medium to large cartilage defects on different locations in the knee joint (patella, trochlea, and femoral condyle) at 5-year follow-up.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
A total of 75 patients aged 18 to 50 years with medium to large (4-10 cm2), isolated, single cartilage defects, International Cartilage Repair Society grade 3 or 4, were randomized on a single-blind basis to treatment with ACI at 1 of 3 dose levels: 3 to 7, 10 to 30, or 40 to 70 spheroids/cm2 of defect size. Outcomes were assessed via changes from baseline Knee injury and Osteoarthritis Outcome Score (KOOS), International Knee Documentation Committee score, and modified Lysholm assessments at 1- and 5-year follow-up. Structural repair was evaluated using MOCART (magnetic resonance observation of cartilage repair tissue) score. Treatment-related adverse events were assessed up to 5 years for all patients. The overall KOOS at 12 months was assessed for superiority versus baseline in a 1-sample, 2-sided t test.
Results:
A total of 73 patients were treated: 24 in the low-dose group, 25 in the medium-dose group, and 24 in the high-dose group. The overall KOOS improved from 57.0 ± 15.2 at baseline to 73.4 ± 17.3 at 1-year follow-up (P < .0001) and 76.9 ± 19.3 at 5-year follow-up (P < .0001), independent of the applied dose. The different defect locations (patella, trochlea, and weightbearing part of the femoral condyles; P = .2216) and defect sizes (P = .8706) showed comparable clinical improvement. No differences between the various doses were observed. The overall treatment failure rate until 5 years was 4%. Most treatment-related adverse events occurred within the first 12 months after implantation, with the most frequent adverse reactions being joint effusion (n = 71), arthralgia (n = 14), and joint swelling (n = 9).
Conclusion:
ACI using spheroids was safe and effective for defect sizes up to 10 cm2 and showed maintenance of efficacy up to 5 years for all 3 doses that were investigated.
Registration:
NCT01225575 (ClinicalTrials.gov identifier); 2009-016816-20 (EudraCT number).
Keywords: knee, ACI, cartilage, prospective randomized trial, spheroids, patellofemoral, tibiofemoral
Approximately 1.5 million patients per year in Europe are affected by articular cartilage injuries. 24,48 Because hyaline cartilage is not capable of self-regeneration, unaddressed injuries can cause permanent pain and functional limitation of the joint. 26,49 Furthermore, cartilage defects have been shown to cause progressive degenerative changes, leading to osteoarthritis. 11 For other cartilage repair strategies, such as drilling or microfracture, the quality and durability of the repair tissue have been questioned, ## but autologous chondrocyte implantation (ACI) results in more hyaline-like cartilage. Cartilage treated with ACI has been suggested to have biomechanical characteristics similar to the native cartilage and, thus, is probably more durable. 41 Studies 3,7,42 have shown superiority of ACI over other treatment options (like microfracture), especially for larger defects. In recent years, ACI has become an established treatment option for patients with focal cartilage defects of the knee. 17,23,36
The classic ACI technique has evolved over the years, leading to the development of self-adhesive, cell-based cartilage repair procedures based on a 3-dimensional matrix (eg, as spheroids). The mode of action of matrix-associated ACI with chondrocyte spheroids (Spherox; CO.DON AG) is based on the formation of chondrocytes in a fully autologous 3-dimensional matrix, the so-called spheroid. 1,2 These spheroids are implanted into the defect, and the chondrocytes migrate and synthesize extracellular matrix de novo, thereby refilling the defect.
Although cell dosages have been associated with morphological quality of repair tissue in cell-based procedures, 12,33 absence of dosage effect while demonstrating safe and efficient treatment has been shown up to 4 years for the current clinical trial. 4,37 –39 Here, we present the final assessment of the full study duration of 5 years. This study is a valuable addition to the existing literature, as this is the first randomized clinical trial comparing different cell doses for this length of follow-up. In addition, because the current trial included defects between 4 and 10 cm2 in size, a comparison of mid- to large-sized defects was possible. As well, because the trial included defects of the patella, the trochlea, and the weightbearing femoral condyles, we were able to compare the clinical outcomes of the different compartments, which were not reported for ACI using spheroids.
The purpose of the current study was to report the efficacy and safety of 3 different doses of spheroids in medium to large cartilage defects in different locations of the knee at 5 years after treatment. It was hypothesized that ACI with spheroids would show similar results for defects of the femoral condyles, patella, and trochlea over a 5-year follow-up.
Methods
Study Design
Patients were treated with 3 doses of spheroids of human matrix-associated chondrocytes in a multicenter, phase 2 clinical trial (ClinicalTrials.gov registration No. NCT01225575; EudraCT No. 2009-016816-20) and were followed for a 5-year period. Patients were blinded for their dose, but blinding of physicians was impossible because the applied dose can be deduced from the number of spheroids (single-blind design). An independent radiologist assessed all magnetic resonance imaging scans after study intervention without knowledge of the dose applied or the time point when the image was taken. The design of this trial and its conduct were in full compliance with the protocol and met all legal and regulatory requirements and the Declaration of Helsinki.
After approval by local ethics committees and competent authority following registration of the clinical trial, patients with symptomatic full-thickness cartilage defects of the knee were included between November 2010 and September 2012 at 10 German orthopaedic centers.
Included in the study were patients aged 18 to 50 years with cartilage defects that were International Cartilage Repair Society grade 3 or 4 with a size between 4 and 10 cm2. In all cases, final eligibility was assessed during arthroscopy of the affected knee: Only patients with unipolar, focal, symptomatic chondral and osteochondral defects up to a depth of 6 mm with intact adjacent cartilage were included. 36 These defects included those of the patella, trochlea, and weightbearing femoral condyles. In general, other inclusion and exclusion criteria ensured that each patient’s knees were stable and well aligned without any signs of inflammatory joint disease or infection. The exact inclusion and exclusion criteria are described elsewhere 4,37 and are available at ClinicalTrials.gov.
Treatment
Patients were included in the study after providing written informed consent and were scheduled for a first arthroscopy for cartilage biopsy (baseline). During knee arthroscopy, osteochondral cylinders were harvested using a standardized cartilage biopsy tool (Storz) from the intercondylar notch for subsequent cell expansion. After arthroscopy, including biopsy sampling, patients were stratified for randomization into 2 defect-size groups (4-6.99 cm2 and 7-10 cm2). Central randomization was performed within each defect-size group, and randomization was realized via the CRO during surgery because stratification could not be determined before surgery. Patients were randomized to receive 1 of 3 dose levels: 3 to 7 spheroids/cm2 of defect size (low-dose group; n = 24), 10 to 30 spheroids/cm2 (medium-dose group; n = 25), or 40 to 70 spheroids/cm2 (high-dose group; n = 24).
The spheroids were produced as previously described. 1,2 After production in cell culture, the spheroids were implanted in the debrided defect during a second surgery 6 to 8 weeks after baseline.
The ACI procedure and rehabilitation protocol have been described elsewhere. 39 Briefly, ACI was performed using arthroscopy, arthrotomy, or mini-arthrotomy as standard approach in all patients. Cartilage defects were debrided before spheroids were applied and distributed homogeneously within the defect area.
After surgery, all patients followed a standardized rehabilitation protocol appropriate for the surgical treatments, including partial weightbearing and continuous passive motion for 6 weeks and regaining full weightbearing within weeks 7 to 8 or at the latest after 12 weeks. Full range of motion was encouraged from week 7 onward, proprioceptive and muscular training was increased, and cycling or aqua jogging was permitted. Physical therapy was adjusted to individual joint status and symptoms, and return to high-impact sports was recommended after 12 months at the earliest.
Safety Evaluation Criteria and Analysis
Treatment-related adverse reactions were analyzed using SAS 9.4 for Microsoft Windows software (SAS Institute) by tabulation according to their Medical Dictionary for Regulatory Activities (MedDRA) terms. All results presented are based on the safety population (all patients who signed an informed consent form and were successfully randomized [ie, had at least 1 arthroscopy]).
Efficacy Evaluation Criteria and Analysis
Patients were assessed at baseline; 6 weeks; and 3, 6, 12, 18, 24, 36, 48, and 60 months after ACI using the following instruments: the Knee injury and Osteoarthritis Outcome Score 5 (KOOS; including the 5 subscales of Symptoms, Pain, Function in Daily Living [ADL], Function in Sport and Recreation [Sport/Rec], and Knee-Related Quality of Life [QoL]), the International Knee Documentation Committee score (IKDC-2000; including the Current Health Assessment Form, Subjective Knee Evaluation Form, and Knee Examination Form), and the MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) score the modified Lysholm score. All results presented are based on the intention-to-treat (ITT) population.
Statistical Analysis
Statistical analysis was performed using SAS 9.4 for Microsoft Windows. To assess efficacy, we analyzed the ITT population (ie, all patients who were successfully randomized, received a dose of ACI, and completed the KOOS at baseline).
The primary dosage comparison analysis at 12 months after treatment followed a hierarchical scheme. To begin, the overall KOOS at 12 months after treatment within the high-dose group was assessed for superiority versus KOOS at baseline. The null hypothesis was KOOS12mo − KOOSBaseline = 0 in a 1-sample, 2-sided t test. It was to be rejected if P ≤ .05. If superiority was shown for the high-dose group, the analysis was repeated for the medium-dose group; if superiority was shown again, for the low-dose group. Exploratory intergroup comparisons were made for all dose groups.
At all other follow-up time points and for all secondary efficacy variables, a descriptive analysis following the same analysis scheme was performed. Due to the hierarchical testing regimen, no adjustment for multiple testing was performed.
In subgroup analyses, patient populations were compared with respect to defect location (femoral condyle, trochlea, or patella) or defect size (4-6.99 cm2 or 7-10 cm2) but irrespective of treatment dose. Missing values at follow-up time points were imputed via last observation carried forward. The descriptive analysis comprised counts and percentages (frequency tables) for categorical data and the number of observed cases or arithmetic means and standard deviations for continuous data. Paired t tests for the mean difference between subgroups were performed. All statistical analyses were performed by statistical consultancy company StatConsult GmbH.
Results
A total of 163 patients with unilateral knee defects were screened, and a cohort of 75 patients met the eligibility criteria. These patients were included in the clinical trial and underwent biopsy and randomization. For 2 patients, the chondrocytes failed to grow, and thus no implantation was possible. Because these patients had been randomized and had undergone an invasive procedure (first arthroscopy for biopsy), they were included for the safety analysis even though they were not assessed for efficacy (see flow chart in Niemeyer et al 39 ). Characteristics of the full safety cohort (75 patients) are shown in Table 1.
Table 1.
Study Patients: Baseline Characteristics (N = 75) a
| Characteristic | Value |
|---|---|
| Sex | |
| Female | 22 |
| Male | 53 |
| Age, y | 34 ± 9 |
| Body mass index | 25.2 ± 3.1 (19.0-33.2) |
| Smoker | |
| Yes | 18 |
| No | 57 |
| Defect size, cm2 | |
| At arthroscopy | 5.0 ± 1.9 (0.5 b -8.0) |
| At implantation (after debridement) | 5.6 ± 1.6 (2 b -10) |
| Defect size group, cm2 | |
| 4-6.99 | 65 |
| 7-10 | 10 |
| Defect location (primary) | |
| Femur | 28 c |
| Patella | 47 |
a Data are reported as No. of patients or mean ± SD; the range is provided in parentheses where appropriate.
b Value outside the allowed range was recorded for 1 patient; protocol deviation was documented.
c Of 10 trochleae.
Stability of Efficacy
Assessment of the overall KOOS for the ITT population showed a statistically significant improvement of 16.4 points compared with baseline values at 1 year after treatment (primary assessment) (Table 2). The KOOS improved to a mean value of 76.9 points after 5 years, showing that the major increase from baseline occurred during the first year after treatment. 39 The improvement in KOOS was maintained until 5-year follow-up, with a mean change from baseline of 19.9 KOOS points. All results were independent of dose, as published in an earlier study, 39 and therefore subgroup analysis was performed irrespective of dose.
Table 2.
Outcome Measures at Baseline and 1- and 5-Year Follow-up a
| Outcome Measure | Mean ± SD |
|---|---|
| KOOS overall | |
| Baseline | 57.0 ± 15.2 |
| 1-y final follow-up | 73.4 ± 17.3 b |
| 5-y follow-up | 76.9 ± 19.3 b |
| KOOS Symptoms | |
| Baseline | 70.6 ± 16.1 |
| 1-y final follow-up | 83.5 ± 13.4 b |
| 5-y follow-up | 83.7 ± 16.3 b |
| KOOS Pain | |
| Baseline | 63.2 ± 14.6 |
| 1-y final follow-up | 79.6 ± 18.2 b |
| 5-y follow-up | 81.4 ± 19.4 b |
| KOOS ADL | |
| Baseline | 73.6 ± 17.9 |
| 1-y final follow-up | 86.0 ± 15.9 b |
| 5-y follow-up | 87.0 ± 16.9 b |
| KOOS Sport/Rec | |
| Baseline | 45.7 ± 23.0 |
| 1-y final follow-up | 63.3 ± 25.6 b |
| 5-y follow-up | 69.0 ± 27.0 b |
| KOOS QoL | |
| Baseline | 31.9 ± 17.5 |
| 1-y final follow-up | 54.5 ± 23.9 b |
| 5-y follow-up | 63.6 ± 26.4 b |
| IKDC subjective | |
| Baseline | 52.3 ± 14.3 |
| 1-y final follow-up | 67.7 ± 17.6 b |
| 5-y follow-up | 71.6 ± 20.2 b |
| Lysholm | |
| Baseline | 16.9 ± 3.4 |
| 1-y final follow-up | 19.8 ± 3.7 b |
| 5-y follow-up | 20.6 ± 3.9 b |
| MOCART c | |
| 3-mo follow-up c | 62.9 ± 10.3 |
| 1-y final follow-up | 72.4 ± 13.0 |
| 5-y follow-up | 75.1 ± 14.5 |
a All scores range from 0 (worst) to 100 points (best achievable score) except for modified Lysholm ranking (0-24 points). ADL, activities of daily living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; QoL, quality of life; Sport/Rec, sports and recreation.
b Statistically significant improvement from baseline (P < .0001). For score assessments at other time points, refer to Niemeyer et al. 39
c Because MOCART is an assessment tool for repaired cartilage, no baseline assessment was performed.
The analyses of KOOS subscales showed the strongest overall improvements in Sport/Rec (23.3 points) and QoL (31.7 points). Nevertheless, the other subscale scores displayed significant improvement greater than the reported minimal clinically important difference of 8 to 10 points for the KOOS (Pain, 18.2 points; Symptoms, 13.1 points; ADL, 13.4 points) (Table 2).
These results were supported by the secondary outcome measures IKDC-2000 Subjective Knee Evaluation Form and MOCART score (Table 2). On the IKDC, overall score improvement was shown in 36 patients; no change, in 33 patients (of whom 28 patients had already been scored grade A at baseline); and worsening, in 4 patients at 60 months. Thus, only 9 patients did not improve or remained at the optimal function according to this score. This was even 1 patient less compared with the 4-year evaluation. 39
Effect of Defect Size on Efficacy
Patients with a cartilage defect size of 4 to 10 cm2 were included in this clinical trial. Of the 73 patients treated, 10 patients had defect sizes >7 cm2. Even though this subgroup was quite small, clinically meaningful improvement from baseline was found in KOOS and MOCART score for both groups at 1 year after implantation, which was maintained until final follow-up at 5 years (Table 3).
Table 3.
KOOS and MOCART Scores at 1 Year After Implantation According to Defect Size a
| Defect Size | |||
|---|---|---|---|
| 4-6.99 cm2 (n = 63) | 7-10 cm2 (n = 10) | Pb | |
| KOOS overall | |||
| Baseline | 57.4 ± 14.8 | 54.8 ± 18.2 | |
| 1-y follow-up | 73.0 ± 17.1 (P < .0001) | 75.6 ± 19.1 (P = .0244) | .3837 |
| 5-y follow-up | 77.1 ± 19.4 (P < .0001) | 75.7 ± 19.7 (P = .0021) | .8706 |
| MOCART | n = 50 [53] (60) | n = 8 [9] (9) | |
| 3-mo follow-up c | 63.0 ± 10.0 | 62.5 ± 12.8 | |
| 1-y follow-up | 72.0 ± 12.2 | 75.0 ± 17.7 | |
| 5-y follow-up | 74.2 ± 14.6 | 81.1 ± 12.7 | |
a Data are reported as mean ± SD. P values in parentheses are for comparisons from baseline. KOOS, Knee injury and Osteoarthritis Outcome Score; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue.
b For comparisons between compartments.
c Because MOCART is an assessment tool for repaired cartilage, no baseline assessment was performed.
Comparison Between Tibiofemoral and Patellofemoral Compartments
Overall KOOS values observed in patients with trochlear defects (n = 10), defects of the weightbearing part of the femoral condyles (n = 18), and patellar defects (n = 45) were largely similar (Table 4). Defects of the patellofemoral compartment descriptively showed slightly lower values at baseline (54.6 and 56.5 for patella and trochlea, respectively, vs 63.1 for weightbearing femoral condyles) and an overall greater improvement until 5 years after treatment (change from baseline 22.4 and 22.2 vs 12.7 points for patella, trochlea, and femoral condyles, respectively).
Table 4.
KOOS, IKDC, and MOCART Scores in Patients With Defects of the Patellofemoral and Tibiofemoral Compartments a
| Patellofemoral Compartment | Tibiofemoral Compartment | |||
|---|---|---|---|---|
| Outcome Measure | Patella (n = 45) |
Trochlea (n = 10) |
Weightbearing Condyles (n = 18) |
Pb |
| KOOS overall | ||||
| Baseline | 54.6 ± 15.7 | 56.5 ± 14.8 | 63.1 ± 13.3 | |
| 1-y follow-up | 73.2 ± 18.4 (P < .0001) | 74.9 ± 14.5 (P = .0451) | 72.9 ± 16.8 (P = .0202) | .2216 |
| 5-y follow-up | 77.0 ± 19.8 (P < .0001) | 78.7 ± 16.7 (P = .0124) | 75.8 ± 20.4 (P = .0121) | .1459 |
| KOOS Symptoms | ||||
| Baseline | 69.9 ± 17.1 | 67.5 ± 14.2 | 74.2 ± 14.4 | |
| 1-y follow-up | 83.8 ± 13.8 (P < .0001) | 84.6 ± 13.8 (P = .0102) | 83.7 ± 13.8 (P = .0124) | .5258 |
| 5-y follow-up | 83.7 ± 16.4 (P < .0001) | 84.3 ± 16.4 (P = .0219) | 83.3 ± 16.6 (P = .0401) | .5325 |
| KOOS Pain | ||||
| Baseline | 61.2 ± 19.0 | 59.2 ± 18.1 | 70.5 ± 16.5 | |
| 1-y follow-up | 80.2 ± 18.7 (P < .0001) | 78.1 ± 16.2 (P = .0482) | 78.9 ± 18.7 (P = .0685) | .1528 |
| 5-y follow-up | 81.4 ± 19.2 (P < .0001) | 81.9 ± 16.9 (P = .0032) | 80.9 ± 22.0 (P = .0450) | .1237 |
| KOOS ADL | ||||
| Baseline | 71.2 ± 18.9 | 72.5 ± 15.4 | 80.2 ± 15.8 | |
| 1-y follow-up | 85.0 ± 18.0 (P < .0001) | 86.5 ± 11.9 (P = .0748) | 88.2 ± 12.3 (P = .0303) | .4775 |
| 5-y follow-up | 85.7 ± 17.9 (P < .0001) | 87.4 ± 15.5 (P = .0553) | 90.0 ± 15.5 (P = .0261) | .6265 |
| KOOS Sport/Rec | ||||
| Baseline | 43.1 ± 24.6 | 43.5 ± 21.6 | 53.3 ± 18.9 | |
| 1-y follow-up | 63.1 ± 26.8 (P < .0001) | 63.5 ± 23.2 (P = .1305) | 63.6 ± 25.2 (P = .1322) | .5198 |
| 5-y follow-up | 69.4 ± 27.4 (P < .0001) | 73.0 ± 23.6 (P = .0327) | 65.8 ± 28.7 (P = .0917) | .1930 |
| KOOS QoL | ||||
| Baseline | 28.1 ± 17.1 | 40.0 ± 17.0 | 37.2 ± 16.8 | |
| 1-y follow-up | 54.7 ± 25.6 (P < .0001) | 61.9 ± 21.3 (P = .0636) | 50.0 ± 20.7 (P = .0537) | .1252 |
| 5-y follow-up | 64.7 ± 26.1 (P < .0001) | 66.9 ± 28.4 (P = .0307) | 59.0 ± 27.0 (P = .0049) | .0774 |
| IKDC subjective | ||||
| Baseline | 49.9 ± 14.9 | 53.6 ± 13.1 | 57.3 ± 12.8 | |
| 1-y follow-up | 68.1 ± 18.9 (P < .0001) | 65.7 ± 16.3 (P = .1451) | 67.6 ± 15.7 (P = .0078) | .1399 |
| 5-y follow-up | 71.2 ± 20.5 (P < .0001) | 70.3 ± 19.7 (P = .0312) | 73.4 ± 20.5 (P = .0010) | .3718 |
| MOCART | ||||
| 3-mo follow-up c | 63.2 ± 9.9 | 62.1 ± 9.5 | 62.5 ± 12.7 | |
| 1-y follow-up | 71.0 ± 12.6 | 76.3 ± 13.0 | 74.3 ± 14.4 | |
| 5-y follow-up | 71.0 ± 14.5 | 85.0 ± 13.7 | 79.7 ± 10.9 | |
a Data are reported as mean ± SD. P values in parentheses are for comparisons from baseline. All scores range from 0 (worst) to 100 points (best achievable score). For score assessment at other time points, refer to Niemeyer et al. 38,39 ADL, activities of daily living; IKDC, International Knee Documentation Committee; KOOS, Knee Osteoarthritis Outcome Score; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; QoL, quality of life; Sport/Rec, sports and recreation.
b For comparisons between compartments.
c Because MOCART is an assessment tool for repaired cartilage, no assessment at baseline was performed.
The analyses of KOOS subscales revealed trends similar to the overall KOOS results: Again, defects of the patellofemoral compartment descriptively showed slightly greater improvements. In accordance with results for the full population (Table 2), the strongest overall improvements 5 years after implantation were shown for KOOS QoL, Sport/Rec, and Pain scores in patellar and trochlear defects. The KOOS ADL and Symptoms scores showed slightly smaller improvements (Table 4). The IKDC scores showed comparable values between the compartments as well (Table 4).
For the MOCART score, patellar, trochlear, and weightbearing femoral condyle defects showed comparable improvement overall (Table 4). Here, descriptively, the greatest improvement could be shown for trochlear defects, but considering the small sample size, these results should be interpreted with caution.
Overall Safety
A complete overview of adverse events (related to study treatment) observed in the clinical trial (all participants) until the 4-year follow-up has been previously published. 39 Within the fifth year of follow-up, only 1 additional nonserious adverse event was reported (ligament sprain, mild intensity, probably related, resolved). No further treatment failure (defined as the need for reoperation of the initial cartilage defect) was reported, leading to an overall failure rate of 4% (3/75 patients) over the full 5-year follow-up period. Thus, treatment with matrix-associated ACI with spheroids was regarded as safe. The most common adverse events related to study treatment (adverse reactions) were those of the MedDRA System Organ Class “Musculoskeletal and connective-tissue disorders,” especially joint effusion (n = 71), arthralgia (n = 14), and joint swelling (n = 9). Figure 1 shows a detailed analysis of onset and duration of the most frequent adverse event, joint effusion. As shown, up to 65% of the patients were affected by joint effusion, with the most frequent occurrence until 9 weeks after treatment. The occurrence of joint effusion decreased at around 12 weeks after treatment, leading to a rate of <20% at 6 months after treatment. After the first year, joint effusion was reported only occasionally, and no event was reported in the 4- and 5-year follow-up visits.
Figure 1.
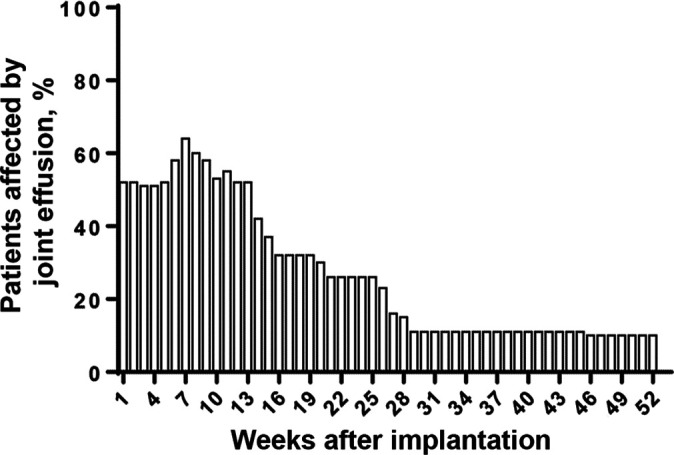
Percentage of patients affected by joint effusion after implantation of Spherox.
The relative frequency of patients affected by adverse reactions was comparable between the tibiofemoral and patellofemoral compartments. The most frequent adverse events for both subgroups were joint effusion (n = 53 vs 18 for patellofemoral compartment vs tibiofemoral compartment, respectively), arthralgia (n = 10 vs 4), and joint swelling (n = 6 vs 3). Most treatment-related adverse events occurred within the first 12 months after ACI treatment. 39
Table 5.
Occurrence of Joint Effusion by Year After Autologous Chondrocyte Implantation With Spheroids a
| 12 mo | 24 mo | 36 mo | 48 mo | 60 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Events | Patients | Events | Patients | Events | Patients | Events | Patients | Events | |
| Cumulative | 57 (76.0) | 64 | 58 (77.3) | 70 | 58 (77.3) | 71 | 58 (77.3) | 71 | 58 (77.3) | 71 |
| Difference from baseline | 57 (76.0) | 64 | 1 (1.3) | 6 | 0 (0.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 |
a Data for patients are expressed as n (%); events are expressed as No. of events. The number of events is larger than the number of patients because patients may have experienced several events.
Discussion
The main study finding was that matrix-associated ACI using spheroids was an effective and safe treatment for cartilage defects of the knee after 5-year follow-up, resulting in significant improvement in KOOS values compared with baseline for patellar (54.6 ± 15.7 vs 77.0 ± 19.8; P < .0001), trochlear (56.5 ± 14.8 vs 78.7 ± 16.7; P = .0124), and femoral condyle defects (63.1 ± 13.3 vs 75.8 ± 20.4; P = .0121).
The 5-year follow-up data confirmed previously published results of the phase 2, prospective, randomized clinical trial and proved the maintenance of stable clinical outcomes. 4,37 –39 Only a few studies have evaluated midterm results of ACI up to 5 years, and only 2 studies have evaluated matrix-associated ACI. 7,46 Brittberg et al 7 found similar results for KOOS subscale scores at 5 years, with the mean defect size (5.1 cm2) being comparable to the value that we found here (5.6 cm2). Brittberg et al furthermore showed statistically significant improvement in KOOS Pain and function subscale scores with ACI versus microfracture. This durability of ACI treatment outcome is of special interest because results published for other treatment options (eg, microfracture) have shown clinical improvement in the short-term but patients experienced deterioration in the mid- to long-term. 30,32,34,47 Defect size and quality of regenerated tissue have been reported to be important factors for deterioration of clinical results in microfracture over time. 18,20,45 In the current study, stable results of large defects could be shown over 5 years, which is further emphasized by the fact that no differences were found between medium (4-6.99 cm2) and large (7-10 cm2) defects (P = .8706). Nevertheless, due to the small sample size, more studies are required to determine a potential upper limit of defect size for ACI.
Repair tissue was evaluated in the current study using the MOCART score. An increase from 1-year to 5-year assessment was seen in the total score (72.4 ± 13.0 vs 75.1 ± 14.5, respectively), and defect filling reached a mean of 18 of 20 points at final follow-up. Surprisingly, numerically higher mean values were shown for larger defects, a finding attributable to the small number of patients and some outliners in the smaller defect group, which inhibited sufficient statistical testing in the subgroups. However, given the overall MOCART score, a high tissue quality may be assumed. This is supported by Grevenstein et al, 21 who found excellent histological results after matrix-associated ACI using spheroids, which probably contributed to the clinical success over time. This was further reflected by the reported treatment failure rate of 4%, which is lower than that reported for other types of ACI as well as other cartilage repair treatment options.
In a 5-year follow-up study, Knutsen et al 27 compared ACI with microfracture and reported a failure rate of 23% for both treatments. However, Knutsen et al evaluated a periosteal-covered ACI, whereas failure rates of matrix-associated ACI have been reported as 2.5% to 10% or 14%. 7,13,46 In a cohort study, 16.7% (14/84) of patients experienced a clinical failure up to 6 years after microfracture, and subchondral bone overgrowth was observed in 62% of patients. 35 In contrast, 5 years after cartilage repair using a cell-free collagen scaffold, 18% of patients (5/28 patients) experienced clinical failure necessitating revision surgery. 43 In a case series of 8 adolescent patients undergoing autologous osteochondral transfer, 37.5% experienced hypertrophy at 28.6 months, 9 whereas Hangody and Fules 22 found a morbidity rate of 3% in 831 patients undergoing autologous osteochondral transfer.
Ebert et al 13 compared the effect of matrix-assisted ACI in patients with tibiofemoral and patellofemoral lesions in a study comprising a sample size considerably greater than the present one (127 tibiofemoral lesions and 67 patellofemoral lesions). The investigators reported significantly better KOOS ADL, QoL, and Sport/Rec scores for the tibiofemoral joint compared with the patellofemoral joint. These differences are in contrast with the present study, as patellofemoral lesions descriptively scored better than tibiofemoral defects. Our results are in accordance with those of other studies showing good clinical outcome of ACI in the patellofemoral compartment. 38,44 These studies used ACI with spheroids that can be applied arthroscopically or in a minimally invasive manner, whereas Ebert et al used arthrotomy to implant matrix-assisted ACI, suggesting that the amount of surgical trauma can contribute to the outcome. Nevertheless, clinical outcome is determined by many factors, and surgical trauma is just one of them. Furthermore, inferior results in patellofemoral lesions have been attributed to different forces that are inherent to inappropriately addressed malalignment. 6 However, Ebert et al found that concomitant patellar realignment had no effect on the functional results for patellofemoral defects.
Ebert et al 13 did not differentiate between patellar and trochlear lesions, which might be important given that Filardo et al 14 reported markedly good outcomes for trochlear lesions and less satisfactory results for patellar lesions. Filardo et al concluded that patellar and trochlear lesions should therefore not be considered together. Those investigators hypothesized that different results may be due to the different compositions of cartilage in the patella and trochlea and that the scaffold used has been shown to produce different results in various weightbearing areas. Therefore, one of the major differences that could further explain the excellent results in patellar defects in the current study might be the matrix. Because no foreign matrix is used in ACI with spheroids, it is a fully autologous product. The matrix is produced during 3-dimensional culture of the chondrocytes by themselves and therefore theoretically might better adapt to the mechanical forces and conditions necessary at the different locations, resulting in better repair tissue.
There is still conflicting evidence on whether the results in the patella and trochlea are different: Gomoll et al 19 showed no differences regarding the defect location in their group with patellofemoral defects, whereas Brittberg et al 7 found a decline in change from baseline in KOOS Sport/Rec score for patients with trochlea defects from 2- to 5-year follow-up (51.3 ± 26.6 vs 38.8 ± 18.9). This effect was even more pronounced in patients with microfracture (KOOS Sport/Rec score, 31.3 ± 33 vs 12.5 ± 29), and a decrease in the KOOS Pain score was also seen in patients with microfracture (25.4 ± 34.1 vs 22.2 ± 33.4). For femoral condyle lesions, these values increased or at least remained stable. Therefore, the patellofemoral compartment of the knee remains a challenge, and a recent expert consensus statement acknowledged some controversies in the optimal therapy for several indications. 10 However, the expert consensus statement underlined the importance of addressing concomitant pathologies to enhance treatment outcomes. This was further emphasized by Ogura et al, 40 who showed high patient satisfaction at 9 years after ACI for bipolar lesions of the patellofemoral compartment, with the best survival rates in patients with concomitant tibial tubercle transfer.
The most common adverse reactions in the current study (related to study treatment) occurred within the first 12 months and consisted of joint effusion, arthralgia, and joint swelling. Occurrence, duration, and type of these adverse reactions can be expected from the type of surgery required for the application of the spheroids. Because the implantation of spheroids may be performed arthroscopically or via minimally invasive surgery, the occurrence of adverse reactions after ACI treatment with spheroids has been described to be similar to that after arthroscopic microfracture. 25
Occurrence of adverse reactions as well as efficiency of treatment showed no correlation to the applied dose at 5-year follow-up, thereby confirming the previous results. 39 Doses ranged from 3-7 spheroids/cm2 to a maximum of 70 spheroids/cm2, which indicates that there is a relatively large effective dose range, and no limits for under- or overdosing could be extracted from these data.
Some limitations have to be mentioned. The subgroups for medium-sized and large defects as well as trochlear defects were quite small, and these analyses were underpowered for sufficient statistical evaluation. Because comparison of defect locations was a secondary outcome, it is possible that these comparisons were underpowered. However, valid implications for linear use might be drawn. In addition, in this phase 2 clinical study, only a few second-look arthroscopies were performed, and this was insufficient for macroscopic and histological analyses of the repair tissue. As a consequence, structural repair was assessed only via magnetic resonance imaging scans and MOCART scoring.
Conclusion
Treatment with ACI using spheroids was safe and effective for defect sizes up to 10 cm2 and showed maintenance of efficacy up to 5 years for all 3 doses that were investigated. ACI with spheroids is a suitable treatment for chondral defects of the patellofemoral as well as the tibiofemoral compartment.
Footnotes
Final revision submitted May 24, 2021; accepted July 14, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: The present study was funded by CO.DON. A.H. has received consulting fees from CO.DON. P.N. has received consulting fees from CO.DON and education support from Aesculap, Arthrex, Geistlich, Moximed, Stryker, and Medi. W.Z. has received consulting fees from CO.DON. S.F. has received consulting fees from Arthrex and Bauerfeind. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Mannheim University Hospital (Central Ethics Committee No. 2009-071F-MA).
References
- 1. Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Miner Res. 2002;17(8):1420–1429. [DOI] [PubMed] [Google Scholar]
- 2. Bartz C, Meixner M, Giesemann P, Roel G, Bulwin GC, Smink JJ. An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. J Transl Med. 2016;14(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. [DOI] [PubMed] [Google Scholar]
- 4. Becher C, Laute V, Fickert S, et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res. 2017;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekkers JE, de Windt TS, Raijmakers NJ, Dhert WJ, Saris DB. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthritis Cartilage. 2009;17(11):1434–1439. [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 7. Brittberg M, Recker D, Ilgenfritz J, Saris DBF; SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343–1351. [DOI] [PubMed] [Google Scholar]
- 8. Brix MO, Stelzeneder D, Chiari C, et al. Treatment of full-thickness chondral defects with Hyalograft C in the knee: long-term results. Am J Sports Med. 2014;42(6):1426–1432. [DOI] [PubMed] [Google Scholar]
- 9. Chadli L, Cottalorda J, Delpont M, Mazeau P, Thouvenin Y, Louahem D. Autologous osteochondral mosaicplasty in osteochondritis dissecans of the patella in adolescents. Int Orthop. 2017;41(1):197–202. [DOI] [PubMed] [Google Scholar]
- 10. Chahla J, Hinckel BB, Yanke AB, et al. An expert consensus statement on the management of large chondral and osteochondral defects in the patellofemoral joint. Orthop J Sports Med. 2020;8(3):2325967120907343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2017;24(3):508–517. [DOI] [PubMed] [Google Scholar]
- 12. Dwivedi G, Chevrier A, Alameh MG, Hoemann CD, Buschmann MD. Quality of cartilage repair from marrow stimulation correlates with cell number, clonogenic, chondrogenic, and matrix production potential of underlying bone marrow stromal cells in a rabbit model. Cartilage. 2021;12(2):237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebert JR, Schneider A, Fallon M, Wood DJ, Janes GC. A comparison of 2-year outcomes in patients undergoing tibiofemoral or patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2017;45(14):3243–3253. [DOI] [PubMed] [Google Scholar]
- 14. Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42(3):626–634. [DOI] [PubMed] [Google Scholar]
- 15. Frisbie DD, Oxford JT, Southwood L, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215–227. [DOI] [PubMed] [Google Scholar]
- 16. Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242–255. [DOI] [PubMed] [Google Scholar]
- 17. Gikas PD, Bayliss L, Bentley G, Briggs TW. An overview of autologous chondrocyte implantation. J Bone Joint Surg Br. 2009;91(8):997–1006. [DOI] [PubMed] [Google Scholar]
- 18. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986–1996. [DOI] [PubMed] [Google Scholar]
- 19. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074–1081. [DOI] [PubMed] [Google Scholar]
- 20. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579–1588. [DOI] [PubMed] [Google Scholar]
- 21. Grevenstein D, Mamilos A, Schmitt VH, et al. Excellent histological results in terms of articular cartilage regeneration after spheroid-based autologous chondrocyte implantation (ACI). Knee Surg Sports Traumatol Arthrosc. 2021;29:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25–32. [DOI] [PubMed] [Google Scholar]
- 23. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. [DOI] [PubMed] [Google Scholar]
- 25. Hoburg A, Niemeyer P, Laute V, et al. Matrix-associated autologous chondrocyte implantation with spheroid technology is superior to arthroscopic microfracture at 36 months regarding activities of daily living and sporting activities after treatment. Cartilage. Published online January 1, 2020. doi:10.1177/1947603519897290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jungmann PM, Gersing AS, Baumann F, et al. Cartilage repair surgery prevents progression of knee degeneration. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):3001–3013. [DOI] [PubMed] [Google Scholar]
- 27. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. [DOI] [PubMed] [Google Scholar]
- 28. Kon E, Filardo G, Berruto M, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39(12):2549–2557. [DOI] [PubMed] [Google Scholar]
- 29. Kon E, Filardo G, Gobbi A, et al. Long-term results after hyaluronan-based MACT for the treatment of cartilage lesions of the patellofemoral joint. Am J Sports Med. 2016;44(3):602–608. [DOI] [PubMed] [Google Scholar]
- 30. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33–41. [DOI] [PubMed] [Google Scholar]
- 31. Kreuz PC, Kalkreuth RH, Niemeyer P, Uhl M, Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. 2019;10(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119–1125. [DOI] [PubMed] [Google Scholar]
- 33. Mesallati T, Buckley CT, Kelly DJ. A comparison of self-assembly and hydrogel encapsulation as a means to engineer functional cartilaginous grafts using culture expanded chondrocytes. Tissue Eng Part C Methods. 2014;20(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 35. Mithoefer K, Venugopal V, Manaqibwala M. Incidence, degree, and clinical effect of subchondral bone overgrowth after microfracture in the knee. Am J Sports Med. 2016;44(8):2057–2063. [DOI] [PubMed] [Google Scholar]
- 36. Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426–435. [DOI] [PubMed] [Google Scholar]
- 37. Niemeyer P, Laute V, John T, et al. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am J Sports Med. 2016;44(8):2005–2014. [DOI] [PubMed] [Google Scholar]
- 38. Niemeyer P, Laute V, Zinser W, et al. Clinical outcome and success rates of ACI for cartilage defects of the patella: a subgroup analysis from a controlled randomized clinical phase II trial (CODIS study). Arch Orthop Trauma Surg. 2020;140(6):717–725. [DOI] [PubMed] [Google Scholar]
- 39. Niemeyer P, Laute V, Zinser W, et al. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1130–1143. [DOI] [PubMed] [Google Scholar]
- 40. Ogura T, Bryant T, Merkely G, Minas T. Autologous chondrocyte implantation for bipolar chondral lesions in the patellofemoral compartment: clinical outcomes at a mean 9 years’ follow-up. Am J Sports Med. 2019;47(4):837–846. [DOI] [PubMed] [Google Scholar]
- 41. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2–12. [DOI] [PubMed] [Google Scholar]
- 42. Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10S–19S. [DOI] [PubMed] [Google Scholar]
- 43. Schuttler KF, Gotschenberg A, Klasan A, et al. Cell-free cartilage repair in large defects of the knee: increased failure rate 5 years after implantation of a collagen type I scaffold. Arch Orthop Trauma Surg. 2019;139(1):99–106. [DOI] [PubMed] [Google Scholar]
- 44. Siebold R, Karidakis G, Fernandez F. Clinical outcome after medial patellofemoral ligament reconstruction and autologous chondrocyte implantation following recurrent patella dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2477–2483. [DOI] [PubMed] [Google Scholar]
- 45. Solheim E, Hegna J, Inderhaug E, Oyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587–1593. [DOI] [PubMed] [Google Scholar]
- 46. Vanlauwe J, Saris DB, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566–2574. [DOI] [PubMed] [Google Scholar]
- 47. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797–804. [DOI] [PubMed] [Google Scholar]
- 48. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. [DOI] [PubMed] [Google Scholar]
- 49. Wluka AE, Ding C, Jones G, Cicuttini FM. The clinical correlates of articular cartilage defects in symptomatic knee osteoarthritis: a prospective study. Rheumatology (Oxford). 2005;44(10):1311–1316. [DOI] [PubMed] [Google Scholar]


