Abstract
Background: Mentha piperita L. (peppermint) is one of the most widely consumed medicinal herbs that has gained attention from food and pharmaceutical industries due to its distinct aroma and taste. Purpose: Present study was aimed to rationalize the traditional use of peppermint in urolithiasis and to explore its possible underlying mechanism. Research Design: The aqueous methanolic crude extract of Mentha piperita (Mp.Cr) was assessed for phytochemical constituents and antioxidant activity. In vitro crystallization assays were performed to determine the inhibitory effects of Mp.Cr against crystal nucleation, aggregation and growth. In vivo urolithiasis model was developed in rats by the administration of ammonium chloride and ethylene glycol in drinking water. The antiurolithic effects of Mp.Cr were evaluated by analyzing kidney homogenate, biochemical and histological parameters. Results: HPLC analysis showed the presence of epicatechin, quercetin, gallic acid, syringic acid, kaempferol, caffeic acid and coumaric acid. The maximum quantity of quercetin equivalent flavonoid and gallic acid equivalent phenolic content was found to be 63.73 ± .24 mg QE/g and 43.76 ± .6 mg GAE/g of Mp.Cr, respectively. Mp.Cr significantly normalized urinary and serum biochemistry, similar to the standard cystone treatment. Conclusions: The current study validated the preventive and curative potential of Mp.Cr against urolithiasis and justified its traditional use in kidney stone disease.
Keywords: urolithiasis, peppermint, calcium oxalate, ethylene glycol
Introduction
Urolithiasis, the development of urinary stones, has been affecting mankind since prehistoric time, irrespective of racial, cultural and geographical boundaries. It is considered as the third most common disease of the urinary system with an estimated lifelong risk of 1–5% in Asia, 20% in the Middle East and 8-15% in America and European countries. 1 The most commonly reported calculi in humans and animals are oxalate, struvite, cystine, brushite, and urate. However, epidemiological data has shown that 80% of uroliths are composed of calcium oxalate, either alone or mixed with calcium phosphate. 2 Calcium oxalate (CaOx) crystals exist in 2 polymorphic forms; that is, calcium oxalate dihydrate (COD) and calcium oxalate monohydrate (COM). Hyperoxaluria tends to form pointy edged dendritic COM crystals which damage epithelial tissue and adheres strongly to renal epithelium. Preferential formation of dihydrate crystals in urine was suggested as part of the defense against the retention of crystals that spontaneously form, preventing them from attaching to the renal tubular epithelium, thus inhibiting renal inflammation and stone formation. 3
Renal injury is a predisposing factor for the calculogenesis and products of cell damage can act as heterogeneous nidus of CaOx crystal retention. The supersaturated urine acts as a driving force for crystal precipitation and aggregation. The natural stone inhibiting tendency of urine prevents crystal formation. These stone inhibiting agents; such as, citrate and magnesium are masked in individuals with higher risk of developing stones. 4 According to the data obtained from animal studies, urinary crystals and high oxalate concentration induce inflammation in the renal tubular epithelium and prompt to be a major risk factor for urolithiasis. 5 Cell culture studies have shown that crystalluria and hyperoxaluria trigger rennin up-regulation and generate angiotensin-II which activates the synthesis of non-phagocytic NADPH oxidase leading to the increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) causing renal inflammation. 6 ROS also regulate the production of various crystallization modulators that are involved in the cellular inflammatory cascade. ROS-induced renal injury and fibrosis leads to cell death and facilitates crystal formation as in the presence of free radicals; crystal inhibitory molecules become defective thus fail to provide adequate protection resulting in crystal aggregation and growth. 7 Cell death also leads to the new cells formation to repopulate the epithelial tissue. The exposed surface of the newly formed epithelium is favorable for crystal attachment and retention. Crystals retained in the terminal collecting ducts of nephron and produce Randall’ plugs/plagues which will act as crystal nidus when exposed to the pelvic urine. 8
Urolithiasis is mainly a recurrent disease with an estimated relapse rate of 10–23% within 5–10 years and 50–75% within 20 years. The annual incidence of kidney stone disease is rising worldwide which can be linked to changes in climate, lifestyles, and dietary modifications. After every episode, the subsequent relapse rate increases and the recurrence time interval decreases.1,9 Modern therapeutic aids like extracorporeal shock wave lithotripsy (ESWL) and drug treatment have revolutionized the clinical practice but cannot reduce the recurrence of urolithiasis and also have unwanted effects. ESWL may cause renal impairment, hypertension and hemorrhage. The scientific evidence for the effectiveness of pharmaceutical agents (like alkali therapy, allopurinol, thiazide and citrate diuretics) is less convincing against kidney stone disease. Therefore, it is worthwhile to look for alternative therapies such as medicinal plants or phytotherapeutic agents. 10
The use of edible/medicinal plants and their extracts as a source of food and alternative medicine has persisted for centuries in the region of sub-continent. A large number of plants/herbs have been reported against kidney stone disease; 1 however, no pharmacological data is available on the effectiveness of Mentha piperita against urolithiasis till date. Peppermint contains rich source of bioactive compounds 11 and has traditionally been used in the treatment of lithiasis. 12 Mentha piperita has been reported to possess anti-oxidant, anti-microbial, anti-inflammatory, anti-viral, larvicidal, biopesticidal, anticancer, radioprotective effects, genotoxicity and anti-diabetic activities. 13 The present study aims to establish the scientific rationality of antiurolithiatic activity by in vitro CaOx crystallization studies; that is, nucleation, aggregation and oxalate depletion assays and in vivo ethylene glycol–induced urolithiasis model in the male albino rats. Mp.Cr was also assessed for its phytochemical constituents, antioxidant and nephroprotective potential.
Material and Methods
Collection of Plant and Preparation of Crude Extract
Mentha piperita L. (fresh aerial parts), commonly known as peppermint, was collected from province of Punjab, Pakistan, in Aug 2019. Mentha piperita was botanically identified and the specimen was submitted to the herbarium of Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, The Islamia University of Bahawalpur, Pakistan. The voucher number was issued for the specimen; that is, MP-AP-10-20-167 and kept for future reference.
Fresh aerial parts of peppermint were cut into small pieces and washed to remove dust particles. The plant material was soaked in aqueous methanol (30:70) for 3 days and then filtered. This process was repeated twice and the filtrate was concentrated with the help of rotary evaporator (Heidolph, Germany), under reduced pressure. Semi-solid pasty mass of crude extract of Mentha piperita (Mp.Cr; dark greenish brown in color) was then stored in freezer. 14
Phytochemical Analysis, Total Phenolic and Flavonoid Content
Phytochemical screening of Mp.Cr was performed according to the standard test procedures for the qualitative determination of the secondary metabolites; such as alkaloids, glycosides, flavonoids, tannins, saponins, anthraquinones, coumarins and phenolic compounds. 15
Total phenolic content was determined by Folin-Ciocalteu reagent method. About .5 ml of Mp.Cr (1 mg/ml) was mixed with Folin-Ciocalteu reagent (.5 ml, 10%) in a test tube. 5 minutes later Na2CO3 solution (1 ml, 20%) was added to the reaction mixture and was incubated in dark for 10 min. After centrifugation of this reaction mixture, absorbance of supernatant was measured at 750 nm. Flavonoid content of Mp.Cr was determined by AlCl3 colorimetric method. Dilution of Mp.Cr (3.7 ml) was prepared in ethanol and mixed with NaNO2 solution (.5 M; .15 ml) and AlCl3.6H2O solution (.3 M; .15 ml) then incubated for 5 min. NaOH solution (1 M; 1 ml) was added in reaction mixture and absorbance was taken at 506 nm. The total phenolic and flavonoid contents of Mp.Cr were determined from standard curves, constructed from the standard solutions of gallic acid and quercetin, respectively. 16
Determination of Antioxidant Potential
The antioxidant activity of Mp.Cr was determined using methanolic solution of the ‘stable’ free radical 2, 2 diphenyl-1-picrylhydrazyl (DPPH) and compared with ascorbic acid, used as standard. For determination of scavenging effects, different dilutions (5-150 μl/ml) of Mp.Cr and ascorbic acid were prepared. In 3 ml of each dilution, 1 ml of (0.1 mM) DPPH solution was added. Solutions were incubated at room temperature for 30 min and then absorbance was noted at 517 nm. 4 Antioxidant potential was calculated by following equation;
Percent DPPH Radical-Scavenging = [1−(AA−AB)/AA] × 100
(AA = Absorbance of control and AB = Absorbance of sample)
High Performance Liquid Chromatography Analysis
High performance liquid chromatography (HPLC) method was performed for estimation of flavonoids, terpenoids, tannins and phenols. Standard (50 µg/ml) and Mp.Cr (10 mg/ml) solutions were prepared and allowed to stand at 4°C during the experimental procedure. The investigation was performed on Shimadzu LC10-AT VP Liquid Chromatograph equipped with SIL-20A auto sampler (Shimadzu Scientific Instruments, Kyoto, Japan) and SPD-10AV UV VIS Detector. A Shim-Pack CLC-ODS (C-18, 25 cm × 4.6 mm, 5 μm), was used for separation, which was maintained at room temperature. The binary solvent system; ie solvent A (water: acetic acid-94:6, pH = 2.2), and solvent B (acetonitrile) were considered as mobile phase with following gradient elution: 15 minutes for 85%A: 15%B, 15–30 min for 55%A: 45%B and 30–35 min for 0%A: 100%B. The flow rate was 1.0 ml/min and absorbance was noted at wavelength 280 nm.
Evaluation of the in Vitro Crystallization Assay
In vitro crystal nucleation, aggregation and oxalate depletion assays were performed in triplicate manner, according to the method described by Mosquera et al. 17
Nucleation assay
The effects of Mp.Cr on CaOx crystallization were studied by nucleation assay (Patel et al 10 ). The solutions of calcium chloride (.005 M) and sodium oxalate (.0075 M) were prepared in Tris-HCL (0.5 M) and NaCl (.15 M) buffer at pH 6.5. Different dilutions (100–1000 μg/mL) of cystone and Mp.Cr were prepared in distilled water. One milliliter of each dilution (100-1000 μg/mL) of Mp.Cr and cystone was mixed with calcium chloride (3 ml) and then sodium oxalate solution (3 ml) was added in each dilution. These dilutions were incubated in oven at 37°C for half an hour then cooled down to room temperature. Optical density was measured using spectrophotometer (620 nm) and the percent inhibition of crystal nucleation was calculated from following formula:
Percent Inhibition = [1–(OD (Test)/OD (Control))] x 100
Where OD (test) is the optical density of cystone or Mp.Cr and OD (control) is the optical density of the negative control.
Aggregation assay
The effects of Mp.Cr on the aggregation of CaOx crystals were studied. .05 M of calcium chloride and sodium oxalate solutions were prepared separately and then mixed together. This mixture was heated in water bath at 60°C, for an hour and then incubated overnight in oven at 37°C. After drying this mixture, CaOx crystal solution (80 mg/100 mL) was prepared in .05 M Tris-HCl and .5 M NaCl buffer at pH 6.5. One millimeter of each of the dilution of Mp.Cr and cystone was mixed and vortexed with 3 ml of CaOx solution. The mixture was incubated in oven at 37°C for half an hour. Optical density was recorded on spectrophotometer at 620 nm. The percentage inhibition of aggregation was calculated by the formula as described for the nucleation assay.
Oxalate depletion assay
The effects of Mp.Cr on the crystal growth were determined by oxalate depletion assay. Different dilutions of Mp.Cr and cystone were prepared in distilled water. CaOx slurry (3 g/2 ml) was prepared in .05 M sodium acetate buffer at pH 5.7.1 ml of calcium chloride and 1 ml of sodium oxalate (.004 M each) solutions were mixed with 1.5 ml of Tris-HCl (.01 M) and NaCl (.09 M) at pH 7.4. CaOx slurry (30 μl) was added to the reaction mixture and the growth of crystals was observed by the addition of 1 ml of each dilution of the Mp.Cr and cystone to this reaction mixture. The rate of CaOx depletion was determined by measuring the optical density (absorbance) at 214 nm for 10 min. The difference in optical density was calculated and the percentage inhibition of the crystal growth was determined by the formula as described for the nucleation assay.
Diuretic Activity
Diuretic activity of Mp.Cr was studied on rats of either sex, weighing 180 to 220 g. 18 Standard diuretic drug; furosemide (10 mg/kg, i.p.) was used and rest of the groups were given different doses (100, 300 and 500 mg/kg) of Mp.Cr dissolved in water for injection (2 ml/kg, i.p.). Total urine output in 6 hours was calculated and Na+ and K+ levels were also determined.
Animal Model of Urolithiasis
Experiments were performed in accordance with the rules of Pharmacy Animal Ethics Committee (PAEC) of the Islamia University of Bahawalpur, under reference number; PAEC/2020/27. Wistar albino rats (male), weighing 180–250 g, were kept in the polycarbonate cages with sawdust renewed after every 48 h, under controlled temperature of 23 ± 2°C and 12 h light/dark cycle. The animals were divided in different groups, each comprising of 6 animals. Urolithiasis was induced in rats by giving 1% ammonium chloride (AC) (Merck, Germany) for 5 days and .75% ethylene glycol (EG) (Merck, Germany) for 21 days in tap water (Bashir and Gilani, 2011). Normal control and intoxicated groups received distilled water (5 ml/kg, p.o once daily). Treatment groups received different doses of Mp.Cr; ie 100, 300, 500 mg/kg along with lithogenic regimen in prophylactic model and after intoxication for the next 14 days in curative model. Cystone consists of the extracts of Rubia cordifolia, Veronoia Cinerea, Saxifraga ligulata, Cyperus scariosus, Achyranthes aspera, Didymocarpus pedicellata, Onosma bracteatu and the powder form of Hajrul yahood Bhasma and Shilajit (Himalaya, India. and Batch no. 112000673), at the dose of 500 mg/kg, was used as standard antiurolithic drug. 19
Urine analysis
In prophylactic model, at the end of 21st day and in curative model, at the end of 21st and 35th day, animals were placed in metabolic cages for the collection of urine samples. 24 h urine samples were collected for biochemical analysis; such as urine volume, urine pH, and the levels of uric acid, magnesium, calcium, phosphate and total protein levels were determined according to the kit methods, following the kit protocol (HUMAN Diagnostics Worldwide, Germany). Sodium and potassium levels were determined using flame photometer. While fresh 3 h morning urine samples were examined for crystal count using neubauer chamber.
Serum analysis
At the end of 21st day of prophylactic model and at the end of 21st and 35th day of curative model, the rats were anesthetized by using combination of ketamine/xylazine and blood was collected through retro-orbital method on 21st day in curative model, and through cardiac puncture at the end of both the prophylactic and curative models. Serum was separated and analyzed for blood urea nitrogen and creatinine levels by using commercially available kits (Human Diagnostics Worldwide, Germany).
Kidney Homogenate Analysis
Kidney homogenate analysis was performed according to the method followed by Hussain et al, 20 with minor modifications. At the end of prophylactic and curative models, whole kidney was separated and washed. Kidney homogenate was prepared by dissolution of 1 g of kidney tissue in 10 ml of .01 M chilled phosphate buffer saline at pH of 7.4, followed by homogenization. The tissue homogenate was then centrifuged at 10 000 rpm at 4°C for 10–15 min and the supernatant was separated and stored at −20°C for analyzing the levels of MDA, GSH and SOD, following the protocols mentioned of kit method (Elabscience, U.S).
Histological Analysis
At the end of study, animals were anesthetized and kidneys were removed, washed with normal saline and preserved in 10% formalin (Riedel-de Haen, Germany). Tissues were sectioned, stained with hematoxylin and eosin dyes and examined under light microscope (100 X).
Acute Toxicity Assay
Albino rats (180-250 g) of either sex were randomly divided into different groups. Normal control group was treated with distilled water (5 ml/kg; p.o) while other groups received Mp.Cr in increasing doses; that is, .5, 1, 5, 10 g/kg. After the administration of different doses, the animals were observed for various toxic and lethal effects; i.e. convulsions, salivation, sweating, lacrimation, writhing reflex and behavior pattern (grooming, alertness, touch response and pain response) for 24 hours (6 hourly), 48 hours and then daily for 14 days.
Statistical Analysis
The results were calculated through Graph Pad prism 8, using one-way ANOVA and the values were expressed as mean ± SEM.
Results
Phytochemical Analysis, Phenol and Flavonoid Content
Phytochemical screening of Mp.Cr demonstrated the presence of phenols, alkaloids, carbohydrates, flavonoids, glycosides, saponins, tannins and terpenes.
The Folin–Ciocalteu’s assay was used to determine the phenolic contents of Mp.Cr. Mp.Cr exhibited high total phenolic content; ie 63.73 ± .24 mg GAE/g. The total flavonoid content in Mp.Cr was determined based on the formation of flavonoid–aluminum complex. The amount of total flavonoid content was assessed to be; that is, 43.76 ± .6 mg QE/g of Mp.Cr.
Antioxidant Activity
The antioxidant activity of Mp.Cr and ascorbic acid increased in dose-dependent manner and reached the maximum of inhibition; ie 79.39 ± 2.85 and 92.33 ± 3.33%, respectively at the concentration 150 μg/ml. The free radical scavenging activity of Mp.Cr (IC50 = 37.34 ± .21 μg/ml) appeared significantly (P < .05) lower than that of the ascorbic acid (IC50 = 56.8 ± .25 μg/ml).
High Performance Liquid Chromatography Analysis
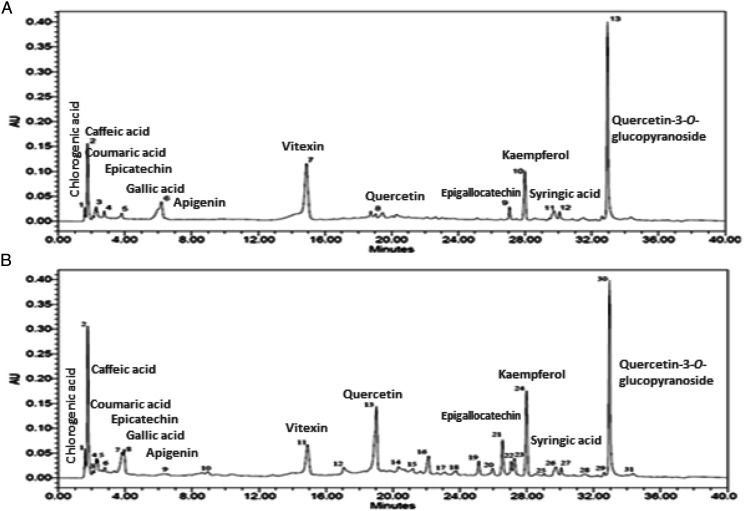
The HPLC analysis revealed the presence of various phytochemical constituents (Figure 1 and Table 1); ie chlorogenic acid (1.63 min), epicatechin (2.58 min), quercetin (19 min), quercetin-3-O-glucopyranoside (33.03 min), gallic acid (3.92), epigallocatechin (27.03 min), syringic acid (30.14 min), kaempferol (27.99 min), caffeic acid (1.85 min) and coumaric acid (2.41 min).
Figure 1.
HPLC profile of (a) chromatogram of Mp.Cr indicating presence of marker phytochemical compounds and (b) chromatogram of standard marker phytochemical compounds.
Table 1.
HPLC Profile of Phytochemical Compounds Detected in the Crude Extract of Mentha piperita (Mp.Cr) and Standard Marker Phytochemical Compounds.
| Sr No | Phytochemical Compounds | Retention Time of Compounds Detected in Mp.Cr (min) | Retention Time of Standard Compounds (min) |
|---|---|---|---|
| 1 | Chlorogenic acid | 1.63 | 1.63 |
| 2 | Caffeic acid | 1.85 | 1.85 |
| 3 | vanillic acid | 2.27 | 2.27 |
| 4 | p-Coumaric acid | 2.41 | 2.41 |
| 5 | Epicatechin | 2.58 | 2.58 |
| 6 | Hydroxycinnamic acid | — | 2.80 |
| 7 | Gallic acid | 3.92 | 3.92 |
| 8 | Quinic acid | — | 3.98 |
| 9 | Apigenin | — | 6.47 |
| 10 | Hispidulin | — | 9.12 |
| 11 | Vitexin | 14.96 | 14.96 |
| 12 | Quercetin-3-O-rutinoside (Rutin) | — | 17.18 |
| 13 | Quercetin | 19.00 | 19.00 |
| 14 | Kaempferol-3-O-rutinoside | — | 20.34 |
| 15 | Hydroxyphenylacetic acid | — | 21.26 |
| 16 | Catechin | — | 22.01 |
| 17 | Isorhamnetin-3-O-glucoside | — | 23.18 |
| 18 | Myricetin | — | 23.83 |
| 19 | Luteolin | — | 25.16 |
| 20 | Apigenin | — | 25.99 |
| 21 | Rhamnetin | — | 26.67 |
| 22 | Epigallocatechin | 27.03 | 27.03 |
| 23 | Sinapic acid | 27.35 | 27.35 |
| 24 | Kaempferol | 27.99 | 27.99 |
| 25 | Butylated hydroxytoluene (BHT) | — | 29.02 |
| 26 | Quercetin-rhamno-di-hexoside | 29.76 | 29.76 |
| 27 | Syringic acid | 30.14 | 30.14 |
| 28 | Hyperoside | — | 31.49 |
| 29 | Naringenin | — | 32.76 |
| 30 | Quercetin-3-O-glucopyranoside | 33.03 | 33.03 |
| 31 | Trans-ferulic acid | — | 34.39 |
Evaluation of the in Vitro Crystallization Assay
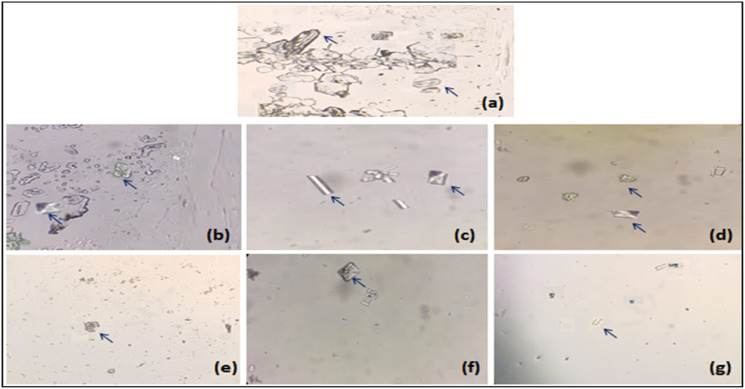
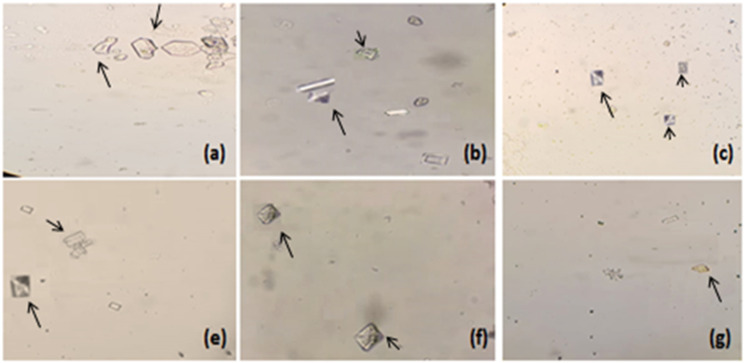
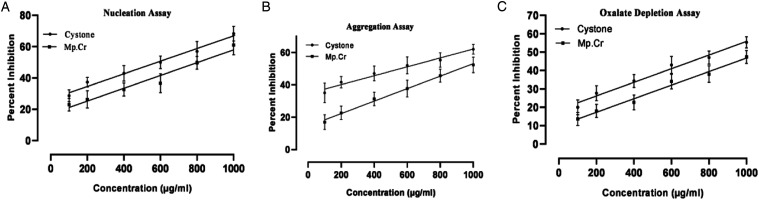
The effects of Mp.Cr on various phases of crystallization were determined by crystal nucleation, aggregation and growth assay. In nucleation assay, rectangular shaped CaOx monohydrate crystals (COM) were observed in control group (Figure 2(a)). Mp.Cr and cystone favored the formation of smooth prism shaped dihydrates crystals (COD) (Figures 2 and 3). The percent inhibition of nucleation, at highest concentration was found to be 61 ± 3.6% as similar to cystone; ie 68.33 ± 2.7% (Figure 4(a)). The percent inhibition in aggregation assay for Mp.Cr was 52.33 ± 2.72%; whereas, cystone showed percent inhibition of 62.00 ± 1.73%, at the concentration of 1000 μg/ml (Figure 4). The percent reduction of crystal growth in oxalate depletion assay was calculated to be 47.33 ± 2.02% (IC50 = 866.76 μg/ml) for 1000 μg as compared to cystone (Figure 2(g)); ie 55.33 ± 1.76% (Figures 2 and 4).
Figure 2.
Microscopic view of in vitro model of crystallization (a) negative control, and cystone; (b) 100 μg/ml, (c) 200 μg/ml, (d) 400 μg/ml, (e) 600 μg/ml, (f) 800 μg/ml and (g) 1000 μg/ml, arrows (→) are showing calcium oxalate crystals (magnification 10 X).
Figure 3.
Microscopic view of in vitro model of crystallization Mp.Cr; (a) 100 μg/ml, (b) 200 μg/ml, (c) 400 μg/ml, (d) 600 μg/ml, (e) 800 μg/ml and (f) 1000 μg/ml, arrows (→) are showing calcium oxalate crystals (magnification 10 X).
Figure 4.
Inhibitory potential of Mp.Cr and cystone against calcium oxalate crystals; (a) nucleation assay, (b) aggregation assay and (c) oxalate depletion assay.
Acute Toxicity Assay
Mp.Cr was found to be safe up to the dose of 10 g/kg as there were no signs of toxicity observed till 14th day.
Diuretic Activity
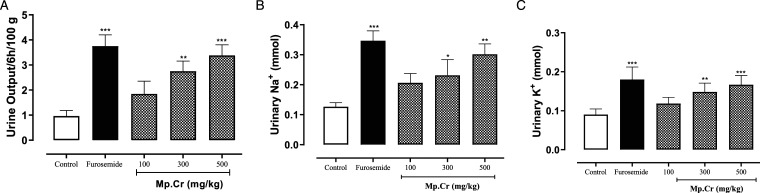
The effects of various doses of Mp.Cr on urine volume, Na+ and K+ excretion is given in Figure 5. Mp.Cr increased urine volume, at the doses of 100, 300 (P < .01) and 500 mg/kg (P < .001), indicating diuretic effect. The standard diuretic drug, frusemide (10 mg/kg) also increased the urine output (P < .001). In addition to the increase in the urine output, Mp.Cr and similarly, frusemide increased urinary excretion of Na+ and K+ as compared to vehicle control group.
Figure 5.
The bar diagrams showing the effects of the crude extract of Mentha piperita (Mp.Cr) and furosemide on (a) urine volume/100 g/6 h, (b) urinary Na+ and (c) urinary K+ excretion.The results are analyzed using 1 way ANOVA and the values shown are the mean ± SEM of 6 observations and are compared with the control group. The values are considered non-significant if P > .05, significant if *P < .05, more significant if **P < .01 and highly significant if ***P < .001.
Models of Urolithiasis
Percent Change in Body Weight
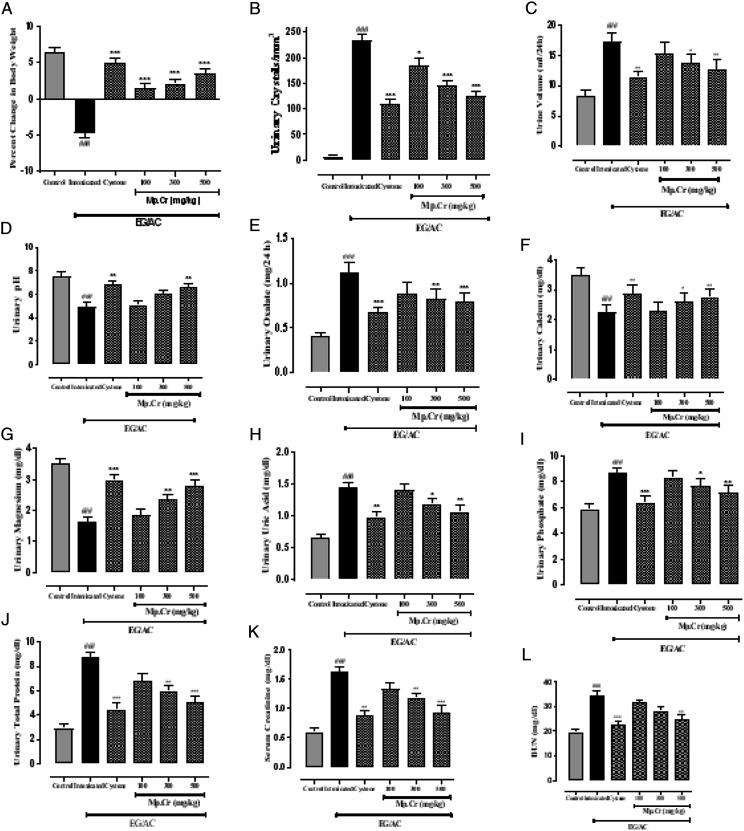
After the intoxication, urolithiatic group showed significant reduction in percent change in body weight as compared to the control group. Significant improvement in percent change in body weight was observed, at all the doses of Mp.Cr (100, 300 and 500 mg/kg) as well as cystone (500 mg/kg), shown in Figure 6 (for prophylactic model) and Table 2 (for curative model).
Figure 6.
The graphs showing effects on (a) percent change in body weight, (b) urinary crystal count, (c) urinary volume/100 g/24 h, (d) urinary pH, (e) urinary magnesium (f) urinary uric acid (g) urinary calcium (h) urinary oxalate (i) urinary phosphate, (j) urinary total protein (k) serum creatinine and (l) serum BUN levels after consumption of AC/EG, along with the different doses of Mp.Cr and cystone on 21st day in prophylactic model of urolithiasis. (Values are expressed as: mean ± SEM, levels of significance: *P < .05, **P < .01, ***P < .001 vs intoxicated; ###P < .001 vs control).
Table 2.
The Effects of Mp.Cr on Various Parameters in Albino Rats using EG/AC-Induced Curative Urolithiasis Model.
| Day | Control | Intoxicated | Cystone (500 mg/kg) | Mp.Cr | ||||
|---|---|---|---|---|---|---|---|---|
| 100 mg/kg | 300 mg/kg | 500 mg/kg | ||||||
| Percent change in b.wt | 21st | 6.87 ± .51 | −8.46 ± .59 | −8.11 ± .66 | −7.76 ± .59 | −7.9 ± .86 | −8.47 ± .61 | |
| 35th | 7.07 ± .61 | 1.7 ± .53 | 5.4 ± .51*** | 2.57 ± .36*** | 3.17 ± .58*** | 4.75 ± .7*** | ||
| Crystal count (/mm3) | 21st | 5.16 ± 0.7 | 240.2 ± 7.32 | 224.7 ± 7.11 | 233 ± 7.1 | 238.3 ± 7.31 | 238.3 ± 9.03 | |
| 35th | 4.83 ± .74 | 233.3 ± 6.67 | 137.7 ± 8.04*** | 184.8 ± 12.95** | 159.3 ± 11.31** | 149.7 ± 12.35*** | ||
| Urinary Parameters (Urine/24 h) | Urine vol. (ml/24 h) | 21st | 9.3 ± 1.23 | 15.6 ± 1.1 | 16.1 ± 1.24 | 17.08 ± 1.13 | 16.63 ± 1.4 | 16.15 ± 1.5 |
| 35th | 9.73 ± 1.01 | 15.37 ± 1.43 | 11.87 ± .99** | 15.65 ± 1.34 | 14.02 ± 1.71* | 12.63 ± 1.02** | ||
| pH | 21st | 7.52 ± .21 | 4.99 ± .34 | 5.2 ± .27 | 5 ± .24 | 5.38 ± .36 | 5.18 ± .24 | |
| 35th | 7.22 ± .35 | 5.03 ± .23 | 7.08 ± .29** | 5.56 ± .33 | 6.56 ± .24 | 6.99 ± .24** | ||
| Oxalate (mg) | 21st | .38 ± .05 | 1.68 ± .13 | 1.66 ± .12 | 1.712 ± .08 | 1.720 ± 0.1 | 1.54 ± .13 | |
| 35th | .37 ± .08 | 1.67 ± .21 | .67 ± .09*** | .97 ± .07* | .95 ± .2** | .84 ± .11*** | ||
| Calcium (mg) | 21st | 3.21 ± .28 | 2.08 ± .22 | 2.09 ± .34 | 1.68 ± .21 | 1.74 ± .29 | 2.11 ± .23 | |
| 35th | 3.31 ± .27 | 2.06 ± .22 | 2.65 ± .25** | 1.89 ± .21 | 1.93 ± .19 | 2.45 ± .19** | ||
| Magnesium (mg) | 21st | 3.78 ± .29 | 1.86 ± .23 | 1.66 ± .17 | 1.48 ± .18 | 1.49 ± .21 | 1.42 ± .16 | |
| 35th | 3.73 ± .35 | 1.89 ± .21 | 2.94 ± .24*** | 2.03 ± .17** | 2.5 ± .13** | 2.83 ± .21*** | ||
| Uric acid (mg) | 21st | .65 ± .03 | 1.41 ± .08 | 1.52 ± 0.1 | 1.47 ± .09 | 1.45 ± .11 | 1.49 ± 0.1 | |
| 35th | .64 ± .05 | 1.41 ± .08 | .96 ± .07** | 1.25 ± .05 | 1.16 ± .07* | 1.04 ± .07** | ||
| Phosphate (mg) | 21st | 6.13 ± .41 | 8.58 ± 0.4 | 8.9 ± .41 | 8.25 ± .55 | 8.78 ± .35 | 9.01 ± .27 | |
| 35th | 5.82 ± .32 | 8.54 ± .27 | 6.22 ± .51*** | 7.66 ± .28 | 6.84 ± .27* | 6.5 ± .3** | ||
| Total protein (g) | 21st | 3.85 ± .51 | 8.97 ± 0.4 | 8.96 ± .67 | 8.76 ± .53 | 8.89 ± .49 | 9.11 ± .43 | |
| 35th | 4.03 ± .48 | 8.71 ± 0.5 | 5.36 ± .4*** | 7.46 ± .39 | 6.21 ± .61* | 5.82 ± .64** | ||
| Serum parameters | Creatinine (mg/dl) | 21st | .63 ± .06 | 1.53 ± .08 | 1.44 ± .11 | 1.34 ± .06* | 1.32 ± .06** | 1.35 ± .08 |
| 35th | .6 ± .07 | 1.55 ± .08 | .78 ± .08*** | 1.18 ± .03 | 1.11 ± .09** | .93 ± .06*** | ||
| BUN (mg/dl) | 21st | 18.46 ± 0.8 | 35.97 ± 1.28 | 32.25 ± 1.67 | 34.13 ± 1.37 | 31.79 ± 2.05 | 34.39 ± 1.64 | |
| 35th | 18.24 ± 0.7 | 35.72 ± 1.26 | 21.42 ± .93** | 29.58 ± 1.19 | 25.15 ± 1.85* | 24.07 ± 1.81** | ||
Urinary Parameters
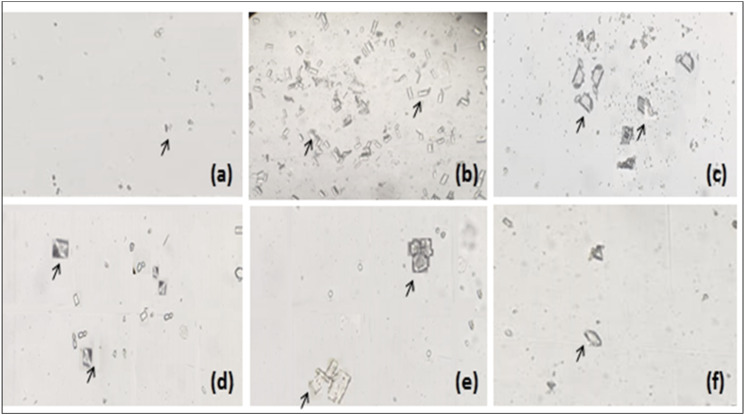
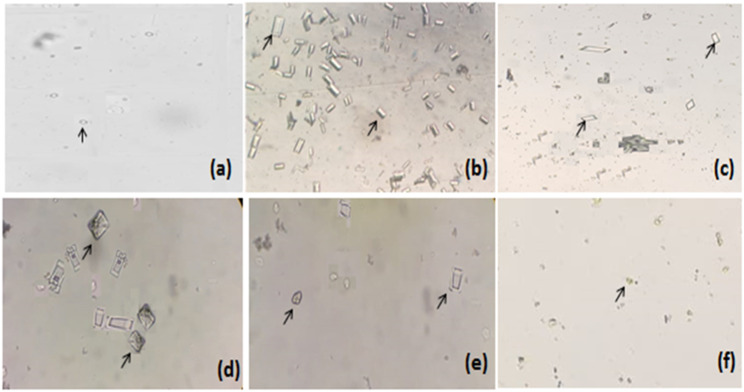
At the end of both models, changes in various urinary parameters were observed to determine the preventive (Figure 6) and curative effects (Table 2) of Mp.Cr. In the preventive model, significantly abundant and visibly larger urinary crystals were observed in diseased group as compared to vehicle control group, whereas a co-treatment with Mp.Cr (100, 300 and 500 mg/kg) reduced the crystal size as well as decreased urinary crystal count; that is, 185.3 ± 14.39/mm3, 145.2 ± 9.34/mm3 and 125.3 ± 9.52/mm3 in dose-dependent manner (Figures 6 and 7). In curative model, various doses of Mp.Cr and cystone at 35th day also showed highly significant reduction in crystal count as summarized in Figure 8. In both models, urine output was observed to be higher in stone forming group as compared to vehicle control group. Mp.Cr, at the dose of 500 mg/kg, reduced the urine volume significantly (Figure 6 and Table 2). Reduction in urinary pH was observed in urolithic group as compared to vehicle control group (7.29 ± .45). The simultaneous (preventive) and post induction (curative) treatment with Mp.Cr significantly neutralized the acidic pH. In parallel with crystalluria, oxalate excretion was also significantly enhanced (P < .01**) in stone forming rats whereas Ca++ excretion was decreased. Mp.Cr at the dose of 300 mg/kg and 500 mg/kg prevented the changes in urinary oxalate (P < .01**) and Ca++ (P < .05*) contents but the protective effects were insignificant at 100 mg/kg. In prophylactic and curative models, decrease in urinary magnesium concentration was observed in lithogenic group which was significantly increased in Mp.Cr and cystone treated groups. In both (prophylactic and curative) models, the levels of uric acid, phosphate and total protein were abnormally increased in diseased group and the treatment with Mp.Cr and cystone showed significant reduction in the levels.
Figure 7.
Representative images of urinary crystals as observed under light microscope (magnification 10 X), Prophylactic model of urolithiasis; (a) control, (b) intoxicated, (c) cystone (500 mg/kg), Mp.Cr; (d) 100 mg/kg, (e) 300 mg/kg and (f) 500 mg/kg, arrow (→)showing urinary crystals.
Figure 8.
Representative images of urinary crystals as observed under light microscope (magnification 10 X), Curative model of urolithiasis; (a) control, (b) intoxicated, (c) cystone (500 mg/kg), Mp.Cr; (d) 100 mg/kg, (e) 300 mg/kg and (f) 500 mg/kg, arrow (→) showing urinary crystals.
Serum Parameters
In both (prophylactic and curative) models, serum creatinine and serum blood urea nitrogen (BUN) levels in urolithic group were highly significantly (P < .001***) increased as compared to the vehicle control group. Mp.Eo, at all the doses; ie 100, 300 and 500 mg/kg and cystone (500 mg/kg) normalized creatinine and BUN levels in dose-dependent manner (Figure 6 and Table 2).
Kidney Homogenate Analysis
In both the models, after 21 days of intoxication in the lithogenic group, increase in MDA levels and decrease in levels of antioxidants; such as glutathione (GSH) and superoxide dismutase (SOD) were observed as compared to control group. Mp.Cr, at all the doses (100, 300 and 500 mg/kg), normalized the levels of MDA, GSH and SOD (Table 3).
Table 3.
The Effects of Mp.Cr on the Levels of MDA, GSH and SOD in Ethylene Glycol-Induced Models of Urolithiasis.
| Control (DW 5 ml/kg; p.o.) |
Intoxicated (Lithogenic Water; EG/AC) | Cystone (500 mg/kg; p.o.) | Mp.Cr (p.o.) | |||
|---|---|---|---|---|---|---|
| 100 mg/kg | 300 mg/kg | 500 mg/kg | ||||
| Prophylactic Model | ||||||
| MDA (nmol/mg protein) | .49 ± .11 | 4.67### ± .56 | 1.04*** ± 0.2 | 2.18*** ± .25 | 1.84*** ± .39 | 1.56*** ± .36 |
| GSH (nmol/mg protein) | 19.67 ± 1.19 | 11.12### ± .32 | 19.21*** ± 1.19 | 15.17* ± .83 | 17.18*** ± .53 | 17.92*** ± 0.9 |
| SOD (U/mg protein) | 7.79 ± 0.5 | 2.3### ± .32 | 6.16*** ± .54 | 2.39ns ± .37 | 4.58* ± 0.5 | 5.58** ± .77 |
| Curative Model | ||||||
| MDA (nmol/mg protein) | .61 ± .09 | 4.28### ± .58 | 1.2*** ± .33 | 1.83*** ± .27 | 1.68*** ± 0.4 | 1.41*** ± .36 |
| GSH (nmol/mg protein) | 20.67 ± 1.9 | 11.62### ± .52 | 19.21*** ± 1.19 | 15.5ns ± .55 | 16.35* ± .85 | 18.08** ± .79 |
| SOD (U/mg protein) | 7.22 ± .51 | 2.53### ± .45 | 3.79*** ± 0.7 | 5.23ns ± 0.5 | 5.81* ± .52 | 6.25** ± 0.6 |
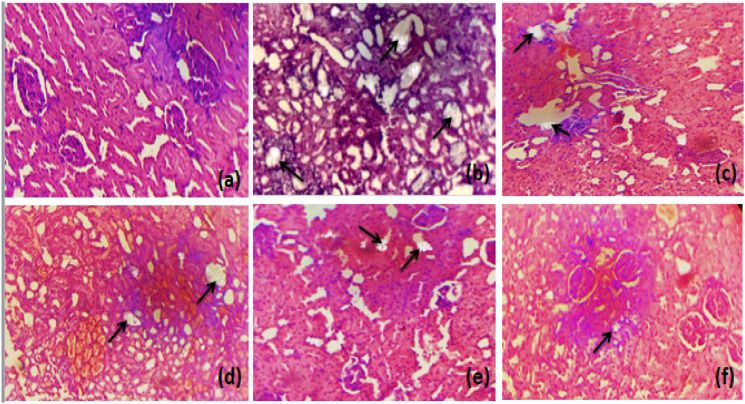
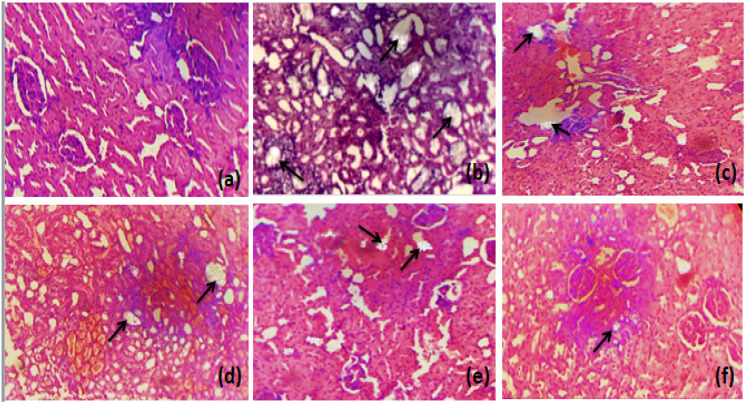
Histological Examination
Microscopic examination of histological slides (kidney section) showed markedly dilated renal tubules and structural disorganization accompanied by inflammation and enlarged interstitial spaces in the entire kidney of the untreated group as compared to control group. Treatment with different doses of Mp.Cr (100, 300 and 500 mg/kg) reduced inflammation, improved the renal epithelial membrane integrity and normalized the interstitial spaces between the cells. Mp.Cr at the dose of 500 mg/kg was found to be highly effective against CaOx oxalate-induced epithelial injury, as shown in Figures 9 and 10.
Figure 9.
The histological slides of kidney section observed under polarized light microscope (100 X) after Hematoxylin & Eosin staining, prophylactic urolithiasis model (a) control, (b) intoxicated, Mp.Cr; (c) 100 mg/kg, (d) 300 mg/kg, (e) 500 mg/kg and (f) cystone (500 mg/kg), arrow (→) showing crystal deposition.
Figure 10.
The histological slides of kidney section observed under polarized light microscope (100 X) after Hematoxylin & Eosin staining, curative urolithiasis model (a) control, (b) intoxicated, Mp.Cr; (c) 100 mg/kg, (d) 300 mg/kg, (e) 500 mg/kg and (f) Cystone (500 mg/kg), arrow (→) showing crystal deposition.
Discussion
The preliminary phytochemical and HPLC analysis of Mp.Cr identified chlorogenic acid, epicatechin, quercetin, gallic acid, epigallocatechin, syringic acid, kaempferol and caffeic acid. The confirmation of these secondary metabolites in peppermint forms a positive correlation regarding its significant potential use in urolithiasis. As shown in present study, Mp.Cr has high total phenolic and flavonoid content, flavonoids have been reported to reduce experimentally induced calculogenesis in rats through multiple pathways; altering urinary stone-forming composition, preventing renal oxidative stress and inflammatory injury. 21 Terpenes are well-known to preserve spasmolytic, calcium channel blocking activity, 22 antioxidant and diuretic effects. 23 Saponins are recognized for inhibiting crystallization by disaggregating the accumulation of mucoproteins, markers of crystallization. 24 However, the influence of other phytochemical constituents accounting for the anti-lithiatic activities cannot be neglected.
In vitro model was designed to study the key events involved in crystallization; nucleation, aggregation and growth. Heterogeneous nucleation begins when the supersaturated solution containing urinary ions and macromolecules form loose clusters and that may increase in size with addition of new components. Once the nucleus is anchored on epithelial surface, crystal aggregation begins that eventually leads to crystal growth. In in vitro crystallization assay, Mp.Cr promoted the formation of COD crystals rather than COM crystals in spontaneously crystallizing solution of CaOx. Mp.Cr significantly inhibited CaOx crystal aggregation, nucleation and growth; these in vitro assays give a quick estimate for antiurolithic activity, crystal modifying activity, and possible mechanism of action. However, these in vitro studies cannot be easily and safely extrapolated for the therapeutic effects as the pathogenesis of urolithiasis and the biological system is complicated. 1 Therefore, in vivo urolithiasis models are to better understand the mechanism of kidney stone formation and investigate the antiurolithic potential of Mp.Cr.
The calculogenesis can be induced either by ethylene glycol (EG) alone, or in combination with ammonium chloride (AC). 25 The hepatic enzymes (glycolate oxidase) metabolize ethylene glycol to oxalic acid, which combine with calcium ion in the renal tubular epithelium to form CaOx crystals. 26 Males are more prone to develop kidney stones than females, as testosterone is linked directly with hepatic glycolate oxidase that increase oxalate production by liver. 27 In the current study, as consistent with some previous reports,4,21,28 percent change in body weight was decreased in EG/AC-treated group which was significantly increased when the animals were treated with different doses of Mp.Cr. Polyuria and acidic urinary pH was observed in intoxicated group as compared to control group, may be due to impaired renal function that cause decrease water reabsorption. Mp.Cr neutralized acidic pH of urine and decreased urinary output, although urine volume remained greater than that of the control group which can be ascribed to the intrinsic diuretic potential of peppermint, as confirmed from the present study. Mp.Cr decreased the urinary crystal count, reduced the colic and sharp cramping pain, as peppermint oil is reported for its potent anti-spasmodic effects that is due to the interference of menthol with the movement of calcium ions (Ca++) across cell membrane. 29 Urinary obstruction due to large crystals resulted in decreased glomerular filtration and subsequent accumulation of nitrogenous wastes such as creatinine and blood urea nitrogen. In our study, the impairment of renal function was clearly depicted from elevated levels of serum creatinine and BUN in intoxicated group as compared to control group, which has been restored by Mp.Cr and the values of creatinine and BUN at highest dose (500 mg/kg) were statistically comparable to standard drug, cystone.
Urinary calcium excretion was decreased in intoxicated rats with a seemingly negative correlation between urinary calcium and oxalate excretion. According to previous studies, urinary calcium concentration may be lowered in lithogenic rats due to the retention of calcium salts or calcium binding to other urinary macromolecules. 2 It has been reported that hyperoxaluria play a significant role in renal inflammation and stone formation and has about 15-times greater effect than urinary calcium. 30 Hyperoxaluria acts in several ways; that is, disturbing membrane potential, deteriorating the membrane surface to increase crystal binding, increasing production of free radical species involve in renal inflammation, promoting mitochondrial dysfunction which finally induces cellular injury to renal epithelium. 31 Peppermint, due to its antioxidant potential, as depicted from current study, reduces hyperoxaluria and crystal-associated oxidative stress thereby reducing renal epithelial injury.
Magnesium is normally present in urine as inorganic inhibitor of crystallization. Magnesium complexes with oxalate in urine and reduce the supersaturation of CaOx crystals and as a consequence reduces the precipitation and growth of crystals. 30 Peppermint is enriched in magnesium; 32 therefore, the crystallization inhibitory potential of Mp.Cr, as seen from decreased urinary crystal count and crystal size, may be due to the presence of magnesium.
Increase in phosphorous excretion was observed in intoxicated group, as already reported in lithogenic rats. Elevated phosphate levels along with hyperoxaluria caused increased gene expression and synthesis of molecules involved in inflammation and tissue remodeling thus provide appropriate environment for crystallization; by forming calcium phosphate stones which epitaxially induces CaOx deposition. 33 Treatment with Mp.Cr lowered the excretion of phosphorus and reduces the risk of renal stones.
The increase in urinary uric acid excretion was observed in lithogenic rats. Uric acid interferes with CaOx crystal solubility and reduces the crystallization inhibitory activity of glycosaminoglycans by binding to it. 34 In the present study, Mp.Cr lowered uric acid levels and reduces the risk of calculogenesis.
The present study showed increased protein excretion in urolithic rats. Proteinuria reflects proximal tubular dysfunction. 35 Administration of Mp.Cr had profound effects on minimizing the protein excretion and thus might have inhibited the nidus formation for crystal nucleation and aggregation.
Elevated levels of lipid peroxide; such as malondialdehyde (the biomarker of lipid peroxidation) and reduced levels of GSH and SOD were observed in lithogenic group. Cells normally consist of scavenging system; non-enzymatic and enzymatic to limit free radical production. Decrease in antioxidants or overproduction of free radicals, result in epithelial damage, leading to crystal attachment to renal tubular epithelium and kidney stone formation. Mp.Cr normalized the levels of MDA and antioxidants; such as GSH and SOD, possibly due to free radical scavenging activity.
Microscopic examination of kidney section of urolithic rats showed irregular epithelial lining, increased interstitial space and dilated proximal tubules along with interstitial inflammation that might be attributed to hyperoxaluria. Treatment with Mp.Cr improved the renal tubular integrity, prevented crystal attachment and retention thus prevented urolithiasis.
Conclusions
The present study demonstrated the prophylactic and curative potential of Mp.Cr against urolithiasis, as it provides scientific credence for the folkloric claim against kidney stone disease. The antiurolithiatic property of Mp.Cr is possibly mediated through combination of crystal inhibitory, antioxidant, anti-inflammatory, spasmolytic and diuretic effects. Mp.Cr also ameliorates urinary and serum biochemistry thus provides a safer and economic alternative for the prevention and cure of kidney stone disease.
Acknowledgments
The authors acknowledge HEC (Higher Education Commission), Pakistan for support through NRPU project, Number: 6300, in providing research animals.
Footnotes
Declaration of Conflicting Interests: The authors declare no conflict of interest with respect to the authorship, research and publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ayesha Jamshed https://orcid.org/0000-0001-7321-8754
References
- 1.Khan A, Bashir S, Khan SR. Antiurolithic effects of medicinal plants: Results of in vivo studies in rat models of calcium oxalate nephrolithiasis-a systematic review. Urolithiasis. 2021;49:95-122. [DOI] [PubMed] [Google Scholar]
- 2.Panigrahi PN, Dey S, Sahoo M, Choudhary SS, Mahajan S. Alteration in oxidative/nitrosative imbalance, histochemical expression of osteopontin and antiurolithiatic efficacy of xanthium strumarium (L.) in ethylene glycol induced urolithiasis. Biomed Pharmacother. 2016;84:1524-1532. [DOI] [PubMed] [Google Scholar]
- 3.Wesson JA, Worcester EM, Kleinman JG. Role of anionic proteins in kidney stone formation: interaction between model anionic polypeptides and calcium oxalate crystals. J Urol. 2000;163:1343-1348. [DOI] [PubMed] [Google Scholar]
- 4.Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122:106-116. [DOI] [PubMed] [Google Scholar]
- 5.Vanachayangkul P, Byer K, Khan S, Butterweck V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine. 2010;17:653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan SR, Canales BK, Dominguez-Gutierrez PR. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat Rev Nephrol. 2021;17:417-433. [DOI] [PubMed] [Google Scholar]
- 8.Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedro RN, Aslam AU, Bello JO, et al. Nutrients, vitamins, probiotics and herbal products: an update of their role in urolithogenesis. Urolithiasis. 2020;48:285-301. [DOI] [PubMed] [Google Scholar]
- 10.Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum schrad. & wendl. (solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2012;144:160-170. [DOI] [PubMed] [Google Scholar]
- 11.Gholamipourfard K, Salehi M, Banchio E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. South Afr J Bot. 2021;141:183-195. [Google Scholar]
- 12.Ahmed S, Khatri MS, Hasan MM. Plants of family lamiaceae: A promising hand for new antiurolithiatic drug development. World J Pharm Pharmaceut Sci. 2017;6:90-96. [Google Scholar]
- 13.Mahendran G, Rahman LU. Ethnomedicinal, phytochemical and pharmacological updates on peppermint ( mentha × piperita l.)-a review. Phytother Res. 2020;34:2088-2139. [DOI] [PubMed] [Google Scholar]
- 14.Rasheed HMF, Rasheed F, Qureshi AW, Jabeen Q. Immunostimulant activities of the aqueous methanolic extract of leptadenia pyrotechnica, a plant from cholistan desert. J Ethnopharmacol. 2016;186:244-250. [DOI] [PubMed] [Google Scholar]
- 15.Javed F, Jabeen Q, Aslam N, Awan AM. Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of indigofera argentea burm. f. J Ethnopharmacol. 2020;259:112966. [DOI] [PubMed] [Google Scholar]
- 16.Alimi H, Hfaiedh N, Bouoni Z, et al. Antioxidant and antiulcerogenic activities of opuntia ficus indica f. inermis root extract in rats. Phytomedicine. 2010;17:1120-1126. [DOI] [PubMed] [Google Scholar]
- 17.Mosquera DMG, Ortega YH, Quero PC, Martínez RS, Pieters L. Antiurolithiatic activity of Boldoa purpurascens aqueous extract: An in vitro and in vivo study. J Ethnopharmacol. 2020;253:112691. [DOI] [PubMed] [Google Scholar]
- 18.Jabeen Q, Bashir S, Lyoussi B, Gilani AH. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J Ethnopharmacol. 2009;122:123-130. [DOI] [PubMed] [Google Scholar]
- 19.Das M, Malipeddi H. Antiurolithiatic activity of ethanol leaf extract of Ipomoea eriocarpa against ethylene glycol-induced urolithiasis in male Wistar rats. Indian J Pharmacology. 2016;48:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain Lodhi A, Ahmad FD, Furwa K, Madni A. Role of oxidative stress and reduced endogenous hydrogen sulfide in diabetic nephropathy. Drug Des Dev Ther. 2021;15:1031-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Jin J, Li X, et al. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-L-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis. 2018;46:231-241. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Hernandez N, Ponce-Monter H, Medina JA, Joseph-Nathan P. Spasmolytic effect of constituents from Lepechinia caulescens on rat uterus. J Ethnopharmacol. 2008;115:30-35. [DOI] [PubMed] [Google Scholar]
- 23.Ramamoorthy J, Venkataraman S, Meera R, Chiristina A, Chidambaranathan N. Physio-phytochemical screening and diuretic activity of leaves of Pavetta indica Linn. J Pharmaceut Sci Res. 2010;2:506-512. [Google Scholar]
- 24.Gürocak S, Küpeli B. Consumption of historical and current phytotherapeutic agents for urolithiasis: A critical review. J Urol. 2006;176:450-455. [DOI] [PubMed] [Google Scholar]
- 25.Khan A, Khan SR, Gilani AH. Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res. 2012;40:671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SR. Animal models of kidney stone formation: An analysis. World J Urology. 1997;15:236-243. [DOI] [PubMed] [Google Scholar]
- 27.Fan J, Glass MA, Chandhoke PS. Effect of castration and finasteride on urinary oxalate excretion in male rats. Urol Res. 1998;26:71-75. [DOI] [PubMed] [Google Scholar]
- 28.Bashir S, Gilani AH, Siddiqui AA, et al. Berberis vulgarisroot bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res. 2010;24:1250-1255. [DOI] [PubMed] [Google Scholar]
- 29.Heghes SC, Vostinaru O, Rus LM, Mogosan C, Iuga CA, Filip L. Antispasmodic effect of essential oils and their constituents: A review. Molecules. 2019;24(9):1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981-986. [PubMed] [Google Scholar]
- 31.Jonassen JA, Cao LC, Honeyman T, Scheid CR. Intracellular events in the initiation of calcium oxalate stones. Nephron Exp Nephrol. 2004;98:e61-e64. [DOI] [PubMed] [Google Scholar]
- 32.Arzani A, Zeinali H, Razmjo K. Iron and magnesium concentrations of mint accessions (Mentha spp.). Plant Physiol Biochem. 2007;45:323-329. [DOI] [PubMed] [Google Scholar]
- 33.Selvam R, Kalaiselvi P, Govindaraj A, Bala Murugan V, Sathish Kumar AS. Effect of A. lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89-93. [DOI] [PubMed] [Google Scholar]
- 34.Kalaiselvi P, Udayapriya KL, Selvam R. Uric acid-binding proteins in calcium oxalate stone formers and their effect on calcium oxalate crystallization. BJU Intern. 1999;83:919-923. [DOI] [PubMed] [Google Scholar]
- 35.Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013-1018. [DOI] [PubMed] [Google Scholar]