Abstract
We report a case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated encephalomyelitis following vector-based vaccination against SARS-CoV-2 that mimicked bacterial meningomyelitis upon initial presentation. A 43-year-old woman who had received a first dose of ChAdOx1 nCoV-19 (Vaxzevria; Astra Zeneca, UK Limited) 9 days earlier presented with subacute sensorimotor paraparesis, urinary retention, headache, meningism, and fever. Clinical findings and cerebrospinal fluid (CSF) features were highly suggestive of bacterial infection; however, despite receiving broad anti-infective treatment alongside with high-dose glucocorticoids, symptoms deteriorated. Imaging findings and the detection of immunoglobulin G against MOG substantiated diagnosis of an anti-MOG associated disorder. Treatment with high-dose intravenous (IV) methylprednisolone and plasma exchange resulted in substantial clinical improvement, which sustained under monthly regimen of IV Tocilizumab at 3-month follow-up. Awareness of this post-vaccinal presentation of a rare autoimmune disorder is important to not miss potential treatment options.
Keywords: autoimmune disorder, COVID-19, encephalomyelitis, myelin oligodendrocyte glycoprotein, vaccination
Introduction
Immunoglobin G (IgG) antibodies against myelin oligodendrocyte glycoprotein (MOG) are associated with autoimmune inflammatory conditions of the central nervous system (CNS). In neuromyelitis optica spectrum disorder (NMOSD), up to 42% of aquaporin 4 (AQP4) antibody–negative patients harbor antibodies against MOG. 1 However, the clinical spectrum of MOG antibody–associated disorders (MOGAD) is broad and goes beyond the phenotype of classical NMOSD with its predominant affection of the optic nerves and spinal cord. Encephalitic presentations involving the supra- and/or the infratentorial brain have been described in adults as well as in pediatric populations, where up to 58% of children with acute disseminated encephalomyelitis (ADEM) are seropositive for MOG IgG.2–4
As in AQP4-positive NMOSD, onset of MOGAD is preceded by acute infection or vaccination in some cases. 5 With regard to the ongoing global pandemic, several cases of MOGAD following SARS-CoV-2 infection have been reported.6–8 There also have been sporadic cases of inflammatory CNS disorders after vaccination against SARS-CoV-2, including a seronegative NMOSD-like presentation in a patient with longstanding stable multiple sclerosis (MS) after a vector-based vaccine, as well as a case of AQP4-positive NMOSD following administration of an inactivated virus vaccine.9,10 However, cases of MOGAD following COVID-19 vaccination have not been described in the literature so far.
Here, we present a case of MOG antibody–associated encephalomyelitis following vaccination with ChAdOx1 nCoV-19 with unusual cerebrospinal fluid (CSF) features that mimicked bacterial meningomyelitis upon initial presentation.
Case description
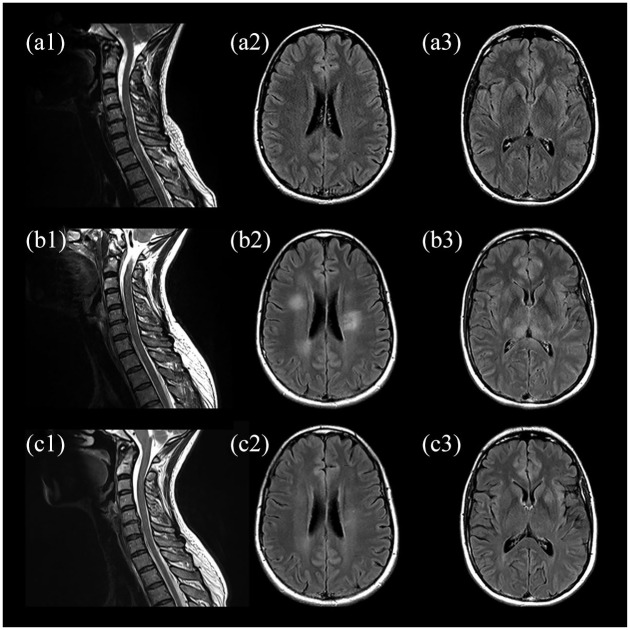
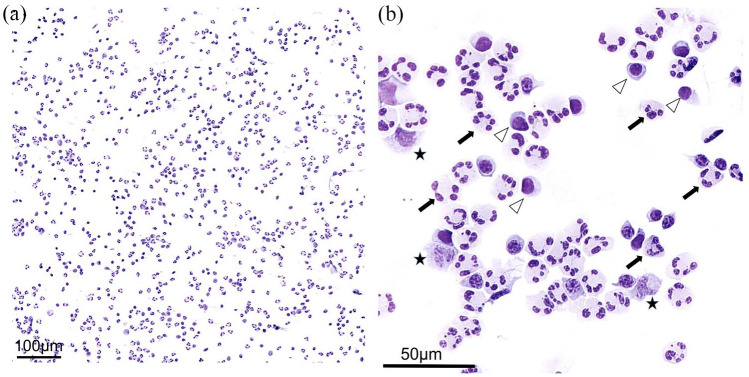
A 43-year-old woman presented to our hospital with sensorimotor paraparesis, urinary retention, and headache for 24 hours. Nine days earlier, she had received a first dose of ChAdOx1 nCoV-19 (Vaxzevria; Astra Zeneca), a vector-based vaccine against SARS-CoV-2. Medical history was unremarkable except for migraine. On admission, the patient had mild paraparesis, hyperreflexia, bilateral positive Babinski sign, a thoracic sensory level (T10), meningism, and fever of 38°C, scoring 5.0 on the Expanded Disability Status Scale (EDSS). Spinal magnetic resonance imaging (MRI) revealed T2 hyperintense lesions involving C6 to T1 as well as T3 and T4, consistent with transverse myelitis (Figure 1(a1)). Despite sporadic T2 hyperintense foci that were interpreted as unspecific, MRI of the brain did not show any abnormalities (Figure 1(a2) and (a3)). Initial laboratory tests showed a white blood cell count of 11.52/nl, and serum C-reactive protein and procalcitonin were not elevated. CSF analysis revealed extensive predominant granulocytic pleocytosis of 545 cells/μl (Figure 2), elevated lactate and CSF protein (4.4 mmol/l and 135 mg/dl, respectively), as well as a reduced CSF to serum glucose ratio (Table 1). Oligoclonal bands were negative and no other immunoglobin abnormalities were detected. An autoimmune disorder was suspected; bacterial meningomyelitis, however, was considered the main differential diagnosis. Broad empiric treatment with 1000 mg of intravenous (IV) methylprednisolone (IVMP) daily alongside with administration of ceftriaxone and ampicillin was initiated. Furthermore, plasma exchange (PLEX) was planned. During the next 5 days, this regimen of antibiotics, cumulative administration of 5 g of IVMP and one session of PLEX resulted in slight clinical improvement. However, on the fifth day after initiation of treatment, the patient developed a stuporous to comatose state with fever over 40°C as well as sensorimotor tetraparesis (EDSS 9.0) requiring monitoring on our intensive care unit (ICU). On a second CSF examination, further increase in granulocytic pleocytosis (720 cells/μl) was detected. The anti-infective medication was escalated to meropenem, and extensive infectious evaluation was performed, including next generation sequencing (NGS) for over 1500 pathogens. All results were negative except for an increased CSF-concentration of Cutibacterium acnes DNA upon initial evaluation (in 912 of 1824 reads), which was not confirmed in a follow-up CSF examination and interpreted as contamination. Early follow-up MRI of the brain showed new T2 hyperintense lesions involving frontal cortex, periventricular space, pulvinar thalamic nuclei, brain stem, and cerebellar peduncles (Figure 1(b2) and (b3)). Spinal lesions were progressive with additional involvement of C3 to C5 (Figure 1(b1)). Accordingly, the anti-infective therapy was discontinued. The diagnostic workup resulted in the discovery of antibodies against MOG in CSF and serum with titers of 1:32 and 1:1000, respectively. Furthermore, serologic testing revealed a slightly increased antinuclear antibody titer of 1:320; however, anti-extractable nuclear antigens tested negative. IgG antibodies against AQP4, glial fibrillary acidic protein (GFAP), N-methyl-d-aspartate (NMDA) receptor, or γ-aminobutyric acid receptor B (GABA B) were neither detectable in CSF nor serum.
Figure 1.
MRI upon admission (a), early follow-up 5 days after admission (b), and follow-up at 3 months (c). Initial sagittal T2-weighted spinal images with hyperintense lesions extending from C6 to T1 as well as T3 and T4 (a1) and no abnormalities on axial fluid-attenuated inversion recovery images of the brain (a2, a3). MRI at 5-day follow-up showing progressive spinal lesions with additional involvement of c3 to c5 (b1) and new hyperintense lesions of the subcortical white matter (b2) and bilateral pulvinar (b3). Partial resolution of former findings in cervical spine (c1), subcortical white matter (c2), and bilateral pulvinar (c3) at 3 months.
Figure 2.
A high-grade granulocyte-dominated granulo-lymphomonocytic pleocytosis is observed. The cell number increase can be well assessed in (a). In (b), neutrophilic granulocytes (exemplary marking with arrows), lymphocytes (exemplary marking with arrowheads), and monocytes (exemplary marking with asterisks) can be well differentiated in the higher magnification. Scale bars: (a) = 100 μm, (b) = 5 0μm.
Table 1.
CSF characteristics and serum MOG antibody titers at initial presentation, early follow-up 5 days after admission, and follow-up at 3 months.
| Initial presentation | Follow-up at 5 days | Follow-up at 3 months | Reference value | |
|---|---|---|---|---|
| CSF WCC (cells/µl) | 545 | 720 | 27 | <5 |
| CSF lactate (mmol/l) | 4.4 | 3.6 | 1.8 | 1.2–2.1 |
| CSF total protein (mg/dl) | 135 | 61 | 50 | 15–45 |
| CSF/serum glucose | 0.5 | 0.6 | 0.6 | >0.5 |
| CSF MOG-IgG-Ab titer | 1:32 | 1:10 | 1:3.2 | N/A |
| Serum MOG-IgG-Ab titer | 1:1000 | 1:320 | 1:320 | <1:10 |
Ab, antibody; CSF, cerebrospinal fluid; IgG, immunoglobin G; MOG, myelin oligodendrocyte glycoprotein; WCC, white cell count.
A diagnosis of MOGAD was made and in addition to further administration of IVMP, treatment with PLEX was continued. Fever remitted soon afterward and neurological symptoms improved. In total, the patient received a cumulative dose of 11 g of IVMP and 7 sessions of PLEX in the hyperacute phase. Nonetheless, symptoms occurred again during tapering of glucocorticoids. Due to the unfavorable course of the disease and to keep the option for further COVID-19 vaccination, a monthly regimen of 400 mg IV Tocilizumab was started in addition to treatment with oral prednisone, and the patient was discharged to a rehabilitation facility. At 3-month follow-up, the patient showed sustained clinical improvement; however, light cerebellar syndrome with dominating intention tremor of the right hand persisted (EDSS 2.0). MRI of the brain revealed partial resolution of findings (Figure 1(c1)–(c3)), and antibodies against MOG still remained positive in CSF and serum (titers 1:3.2 and 1:320, respectively). Notably, the subject exhibited a rather low anti-SARS-CoV-2 serum titer of 75.7 BAU/ml 2 weeks after vaccination. After clinical recovery and under temporary intensification of glucocorticoid treatment (100 mg of prednisone daily for five days), the patient received a second COVID-19 vaccination, this time with an mRNA-based vaccine. As a result, the antibody titer increased up to >2080 BAU/ml, without any relapse of symptoms.
Discussion
To our knowledge, this is the first report on MOG antibody–associated encephalomyelitis following a vector-based vaccine against SARS-CoV-2, and there are several interesting aspects to the presented case: Clinical findings and CSF characteristics were highly suggestive of bacterial meningomyelitis, including meningism, high fever, granulocytic CSF pleocytosis of up to 720 cells/μl, and increased CSF lactate of 4.4 mmol/l. Although Jarius et al. 11 found that granulocytic pleocytosis and increased lactate levels are common in acute MOG antibody–associated encephalomyelitis, the authors emphasized that elevation of these parameters usually occurs at much lower levels as compared with bacterial meningitis. In their large study on CSF findings in MOG antibody–associated encephalomyelitis, less than 2% of cases exceeded a CSF cell count > 300 cells/μl or a lactate level > 4 mmol/l, respectively. Nonetheless, rare cases of NMOSD mimicking bacterial infection on CSF evaluation have been reported.12,13 In addition, fever has also been described as a clinical feature of some MOGAD phenotypes. 14
Imaging findings included an ADEM-like pattern of poorly demarcated, fluffy T2-hyperintensities and longitudinal extensive transverse myelitis (LETM), both common radiological features of MOG antibody–associated encephalomyelitis.5,15 However, these findings developed with delay as compared with the clinical course. Of further note was the presence of a pulvinar sign, a finding that is mostly recognized as a feature of variant Creutzfeldt-Jakob-Disease (vCJD) 16 but has been observed in cases of MOG antibody–associated encephalomyelitis as well.3,17
In the past, some authors have characterized MOGAD as a monophasic, steroid-sensitive condition bearing a rather favorable prognosis.18,19 Some of these findings have been revised by larger case series with longer follow-up that revealed frequent relapses and severe disease courses with reduced steroid responsiveness.5,20 Our patient did partially respond to high-dose IVMP and some symptoms occurred again when glucocorticoids were tapered, which is a common issue in MOGAD.15,21 Nonetheless, there are no established guidelines for long-term immunosuppressive treatment and follow-up data are scarce. Tocilizumab could be a promising long-term treatment option in patients with severe, relapsing MOGAD, and a growing number of favorable treatment responses is being reported.22–25 In particular during the COVID-19 pandemic, an immunomodulation that does not hamper antibody response to vaccination is of advantage. 26 Despite being used almost exclusively as a second- or third-line treatment in MOGAD, the presented case highlights the potential use of Tocilizumab as a safe, effective, and well-tolerated first-line, long-term immunosuppressive treatment option in patients with severe relapsing disease, which has been discussed by other authors as well. 27 Large-scale prospective data would be desirable to provide better guidance on treatment decisions.
Regarding the general safety profile of COVID-19 vaccines in patients with neuroimmunological disorders, observational studies did not find an increased risk for relapse.28–30 Interestingly, the majority of patients in these studies received disease-modifying treatments by the time they were vaccinated, as was the case in our patient when receiving the second mRNA vaccine. These findings suggest a potential relationship between immunotherapy and vaccine tolerability in these patients; however, the underlying mechanisms remain unclear. Nonetheless, considering the potential harm of infection with SARS-CoV-2, patients with neuroimmunological disorders should be encouraged to undergo vaccination.
Conclusion
The present case expands the spectrum of MOG antibody–associated encephalomyelitis to coronavirus vaccinations, and clinicians should be aware of atypical presentations that mimic bacterial infection. Since the individual disease course can be disabling, early diagnosis is of great importance. Tocilizumab might be an option in patients with MOGAD unresponsive to steroids. The use of mRNA-based SARS-CoV-2 vaccines may be considered in patients with vector vaccine–associated MOG antibody–associated encephalomyelitis.
Footnotes
Author contributions: Jordi Kühne Escolà: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Cornelius Deuschl: Investigation; Methodology; Validation; Writing – review & editing.
Andreas Junker: Investigation; Validation; Visualization; Writing – review & editing.
Fabian Dusse: Investigation; Methodology; Validation; Writing – review & editing.
Refik Pul: Investigation; Methodology; Validation; Writing – review & editing.
Christoph Kleinschnitz: Conceptualization; Investigation; Resources; Validation; Writing – review & editing.
Martin Köhrmann: Conceptualization; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Benedikt Frank: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RP has received honoraria for lecturing and travel expenses for attending meetings from Alexion, Bayer Health Care, Biogen, Celgene, Janssen, Merck Serono, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. He has received research funding from Novartis and Teva. The other authors report no conflict.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Patient consent: Written, informed consent was obtained from the patient for publication of case details and related images. Further institutional approval was waived in accordance with local regulations.
ORCID iD: Jordi Kühne Escolà  https://orcid.org/0000-0002-8742-0694
https://orcid.org/0000-0002-8742-0694
Refik Pul  https://orcid.org/0000-0002-8940-9317
https://orcid.org/0000-0002-8940-9317
Benedikt Frank  https://orcid.org/0000-0001-8837-9489
https://orcid.org/0000-0001-8837-9489
Contributor Information
Jordi Kühne Escolà, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Hospital Essen, Essen, Germany.
Cornelius Deuschl, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Andreas Junker, Institute of Neuropathology, University Hospital Essen, Essen, Germany.
Fabian Dusse, Department of Anesthesiology and Intensive Care Medicine, University Hospital Cologne and Faculty of Medicine, University of Cologne, Cologne, Germany.
Refik Pul, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Hospital Essen, Essen, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Hospital Essen, Essen, Germany.
Martin Köhrmann, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Hospital Essen, Essen, Germany.
Benedikt Frank, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
References
- 1. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol 2017; 264: 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamid SHM, Whittam D, Saviour M, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol 2018; 75: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarius S, Kleiter I, Ruprecht K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: brainstem involvement – frequency, presentation and outcome. J Neuroinflammation 2016; 13: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry 2015; 86: 265–272. [DOI] [PubMed] [Google Scholar]
- 5. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters J, Alhasan S, Vogels CBF, et al. MOG-associated encephalitis following SARS-COV-2 infection. Mult Scler Relat Disord 2021; 50: 102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou S, Jones-Lopez EC, Soneji DJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol 2020; 40: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Ruijter NS, Kramer G, Gons RAR, et al. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord 2020; 46: 102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen S, Fan XR, He S, et al. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci 2021; 42: 3537–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helmchen C, Buttler GM, Markewitz R, et al. Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2. J Neurol. Epub ahead of print 15 June 2021. DOI: 10.1007/s00415-021-10647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarius S, Pellkofer H, Siebert N, et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: results from 163 lumbar punctures in 100 adult patients. J Neuroinflammation 2020; 17: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lepur D, Peterkovic V, Kalabric-Lepur N. Neuromyelitis optica with CSF examination mimicking bacterial meningomyelitis. Neurol Sci 2009; 30: 51–54. [DOI] [PubMed] [Google Scholar]
- 13. He M, Gao D, Zhang J, et al. Suspected bacterial meningoencephalomyelitis as the trigger or presentation of neuromyelitis optica spectrum disorder flare. Mult Scler Relat Disord 2019; 30: 38–41. [DOI] [PubMed] [Google Scholar]
- 14. Budhram A, Mirian A, Le C, et al. Unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J Neurol 2019; 266: 2481–2487. [DOI] [PubMed] [Google Scholar]
- 15. Salama S, Khan M, Pardo S, et al. MOG antibody-associated encephalomyelitis/encephalitis. Mult Scler 2019; 25: 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeidler M, Sellar RJ, Collie DA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet 2000; 355: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 17. Kumar N, Graven K, Joseph NI, et al. Case report: postvaccination anti-myelin oligodendrocyte glycoprotein neuromyelitis optica spectrum disorder: a case report and literature review of postvaccination demyelination. Int J MS Care 2020; 22: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 19. van Pelt ED, Wong YY, Ketelslegers IA, et al. Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur J Neurol 2016; 23: 580–587. [DOI] [PubMed] [Google Scholar]
- 20. Cobo-Calvo A, Ruiz A, Maillart E, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 2018; 90: e1858–e1869. [DOI] [PubMed] [Google Scholar]
- 21. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–3138. [DOI] [PubMed] [Google Scholar]
- 22. Hayward-Koennecke H, Reindl M, Martin R, et al. Tocilizumab treatment in severe recurrent anti-MOG-associated optic neuritis. Neurology 2019; 92: 765–767. [DOI] [PubMed] [Google Scholar]
- 23. Novi G, Gastaldi M, Franciotta D, et al. Tocilizumab in MOG-antibody spectrum disorder: a case report. Mult Scler Relat Disord 2019; 27: 312–314. [DOI] [PubMed] [Google Scholar]
- 24. Rigal J, Pugnet G, Ciron J, et al. Off-label use of tocilizumab in neuromyelitis optica spectrum disorders and MOG-antibody-associated diseases: a case-series. Mult Scler Relat Disord 2020; 46: 102483. [DOI] [PubMed] [Google Scholar]
- 25. Schwake C, Hellwig K, Gold R, et al. Reader response: comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol 2020; 88: 430. [DOI] [PubMed] [Google Scholar]
- 26. Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology 2021; 60: 3496–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elsbernd PM, Hoffman WR, Carter JL, et al. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): a case-series and review. Mult Scler Relat Disord 2021; 48: 102696. [DOI] [PubMed] [Google Scholar]
- 28. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler 2021; 27: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Filippo M, Cordioli C, Malucchi S, et al. MRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. Epub ahead of print 18 August 2021. DOI: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- 30. Lotan I, Romanow G, Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult Scler Relat Disord 2021; 55: 103189. [DOI] [PMC free article] [PubMed] [Google Scholar]