Abstract
The purpose of this study was to investigate Achilles tendon (AT) length changes during a series of tasks that involved combinations of higher/lower force, and larger/smaller length changes of the medial gastrocnemius muscle-tendon unit (MTU). We sought to determine if common ultrasound-based estimates of AT length change were consistent with expectations for a passive elastic tendon acting in series with a muscle. We tested 8 healthy individuals during restricted joint calf contractions (high force, low displacement), ankle dorsi-/plantar-flexion (DF/PF) with the foot in the air (low force, high displacement), and heel raises (high force, high displacement). We experimentally estimated AT length change using two ultrasound methods, one based on muscle-tendon junction (MTJ) tracking and one based on muscle fascicle (MF) tracking. Estimates of AT length change were consistent with model expectations during restricted calf contractions, when the MTU underwent minimal length change. However, estimates of AT length changes were inconsistent with model expectations during the ankle DF/PF and heel raise tasks. Specifically, the AT was estimated to shorten substantially, often 10–20 mm, when the ankle plantarflexed beyond neutral position, despite loading conditions in which a passive, stiff spring would be expected to either lengthen (under increasing force) or maintain its length (under low force). These unexpected findings suggest the need for improvements in how we conceptually model and/or experimentally estimate MTU dynamics in vivo during motion analysis studies, particularly when the ankle plantarflexes beyond neutral.
Keywords: muscle-tendon unit, muscle fascicle tracking, muscle-tendon junction tracking, gastrocnemius, series elasticity
INTRODUCTION
The Achilles tendon (AT) is a passive elastic structure that facilitates safe (Konow et al., 2011; Roberts and Azizi, 2010) and economical (Alexander, 2002, 1991; Fukunaga et al., 2001; Lichtwark and Wilson, 2007; Sawicki et al., 2009; Zelik et al., 2014) locomotion, and which informs the development of assistive and rehabilitative interventions (Bregman et al., 2011; Collins and Kuo, 2010). Ultrasound provides a means to non-invasively estimate AT kinematics in vivo during human movement. AT kinematics have also been combined with estimates of AT force to compute tendon energy storage and return, which provides additional insights on the functional benefits of tendons, and their interplay with muscle mechanics (Farris et al., 2011; Farris and Sawicki, 2012; Honert and Zelik, 2016; Lichtwark, 2005). Although these ultrasound-based tendon estimates have been employed for decades, estimating AT kinematics and kinetics in vivo remains challenging to validate (Cronin and Lichtwark, 2013; Finni et al., 2013), which can confound scientific interpretation of movement biomechanics. Critical questions remain, including: which (of several) ultrasound-based estimation methods are most accurate, and under what circumstances do these methods yield reliable estimates of tendon dynamics.
Multiple ultrasound tracking methods have been developed and employed on humans and animals to study the AT and other MTU dynamics. Certain methods estimate the distance from the muscle-tendon junction (MTJ) to the tendon’s distal insertion (Bryant et al., 2008; Hawkins et al., 2009; Hoffrén et al., 2012; Lichtwark, 2005; Lichtwark and Wilson, 2006). Other methods seek to track muscle fascicle (MF) length, then subtract muscle length from estimates of overall muscle-tendon unit (MTU) length (Grieve, 1978; Hawkins and Hull, 1990) to approximate tendon kinematics (Cronin and Lichtwark, 2013; Farris and Sawicki, 2012; Hoang et al., 2007; Hoffrén et al., 2012; Masaki Ishikawa et al., 2005; M. Ishikawa et al., 2005; Lichtwark et al., 2007; Lichtwark and Wilson, 2006; Sakuma et al., 2011). Yet other methods quantify local elongations of the tendon (Chernak and Thelen, 2012; Franz et al., 2015; Franz and Thelen, 2015; Korstanje et al., 2010). While the tracking methods themselves rely on slightly different methodological assumptions, researchers typically map experimental data onto a similar conceptual model of the MTU and adopt a similar set of assumptions when interpreting the results. Several common model assumptions include: the AT acts longitudinally in series with muscle (Bobbert et al., 1986; Hoang et al., 2007; Lichtwark and Wilson, 2007; Zajac, 1989), individual MTUs can be analyzed in isolation (Bobbert et al., 1986), and MTU length is primarily a function of joint angle (Grieve, 1978; Hawkins and Hull, 1990).
A variety of methodological factors can affect ultrasound estimates of tendon dynamics (Cronin and Lichtwark, 2013). Experimental inconsistencies (e.g., in stiffness and hysteresis, Finni et al., 2013) and oddities (e.g., AT estimated to return more energy than it stores, despite being a passive structure, (Sakuma et al., 2011; Zelik and Franz, 2017)) in the published literature suggest that ultrasound estimates may be prone to errors. Ultrasound methods, or model assumptions, may begin to breakdown under different mechanical loading conditions or movement tasks, but this has not explicitly been tested. Therefore, the purpose of this study was to investigate a series of movement tasks that involved combinations of higher or lower MTU force, and larger or smaller MTU length changes of the medial gastrocnemius (MG), to determine if common ultrasound-based estimates of AT kinematics were consistent with expectations for a passive spring-like tendon acting in series with a muscle. In this study, we estimated AT kinematics using two common ultrasound-based tracking methods, MTJ and MF (summarized above, and detailed in Methods); thus, a secondary aim was also to compare the consistency of results between these methods.
METHODS
Subjects
Eight healthy subjects participated (5 M / 3 F, age = 21 ± 2 years, mass = 77 ± 12 kg, height = 1.79 ± 0.04 m). Each subject performed a series of movement tasks while lower-body kinematics and ground reaction forces, as well as B-mode ultrasound of the MG MFs and MG-AT MTJ were collected (Fig. 1). Electromyography (EMG) of the MG, lateral gastrocnemius (LG), soleus (SOL), and tibialis anterior (TA) was also recorded (further detailed in Supplementary Material). All subjects gave informed consent to the protocol, which was approved by the Institutional Review Board at Vanderbilt University.
Fig. 1. Experimental methods.
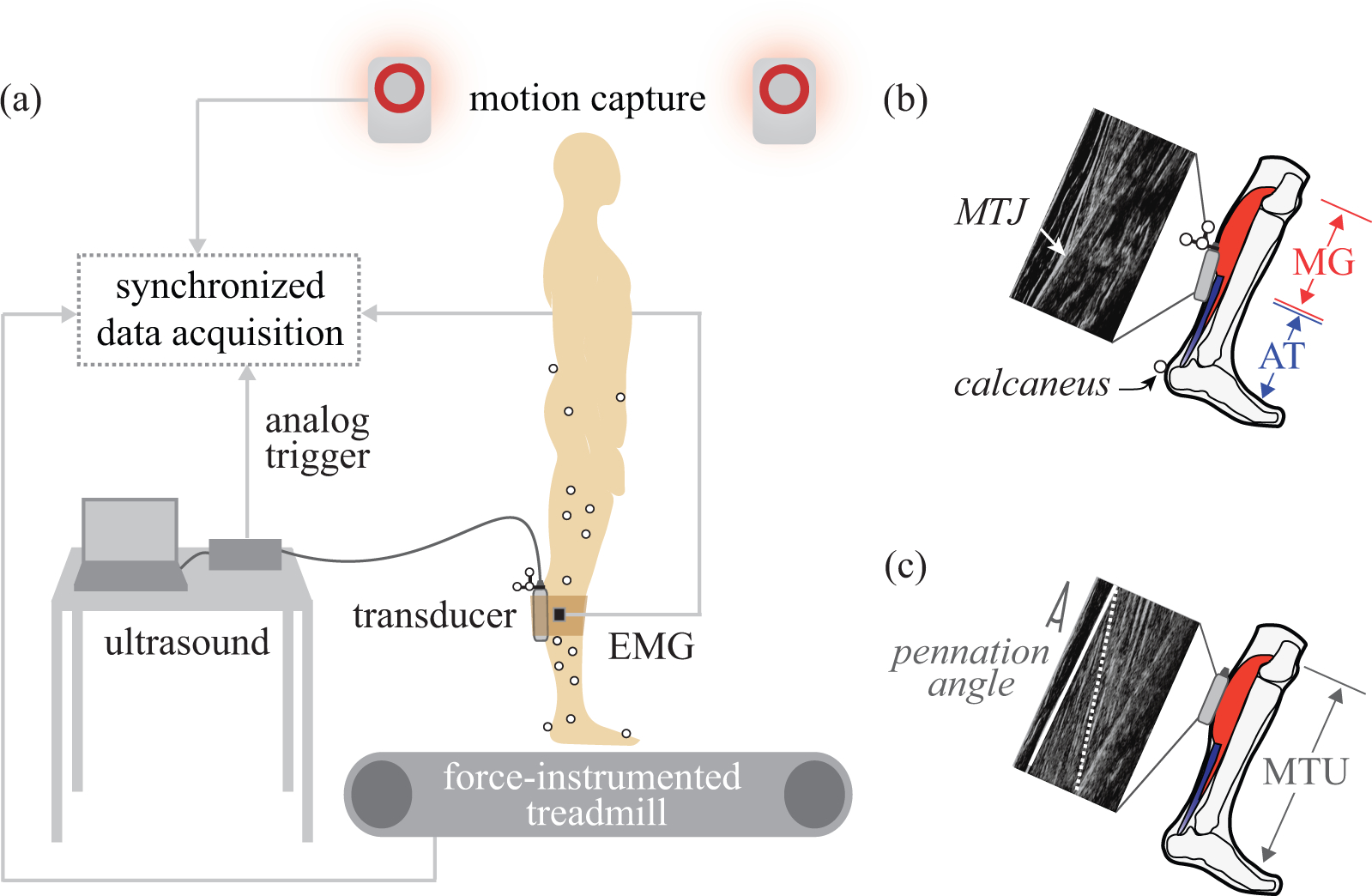
(a) B-mode ultrasound was collected synchronously with motion capture, electromyography (EMG), and ground reaction force data. (b) MTJ tracking. The ultrasound transducer (gray box) was placed over the MTJ of the MG muscle and AT. AT length change was estimated as changes in the straight-line distance from the MTJ to the calcaneus. (c) MF tracking. The transducer was placed over the MG muscle belly. Longitudinal MG length change was calculated from MF length (dotted white line) corrected by the cosine of the pennation angle. Pennation angle was defined as the angle between MF and the superficial fascia (solid white line). MTU length change was estimated from a regression equation based on joint angles.
Experimental Protocol
Subjects performed tasks that involved combinations of high or low force on the MTU, and large or small MTU length changes. The terms “high” or “low” force and “large” or “small” length change are used to signify magnitudes relative to other tasks tested. Restricted joint calf contractions involved high force and small length changes of the MTU (Fig. 2a). Ankle dorsiflexion/plantarflexion (DF/PF) with the foot in the air involved low force and large length changes of the MTU (Fig. 2b). Heel raises involved high force and large length changes of the MTU (Fig. 2c). Prior to data collection, each subject walked ~300 steps to pre-condition their AT (Hawkins et al., 2009). For each task, about 10 cycles were performed to a metronome, paced at 40 beats per minute, to ensure a slow and smooth motion with minimal soft tissue dynamics.
Fig. 2. Experimental tasks.
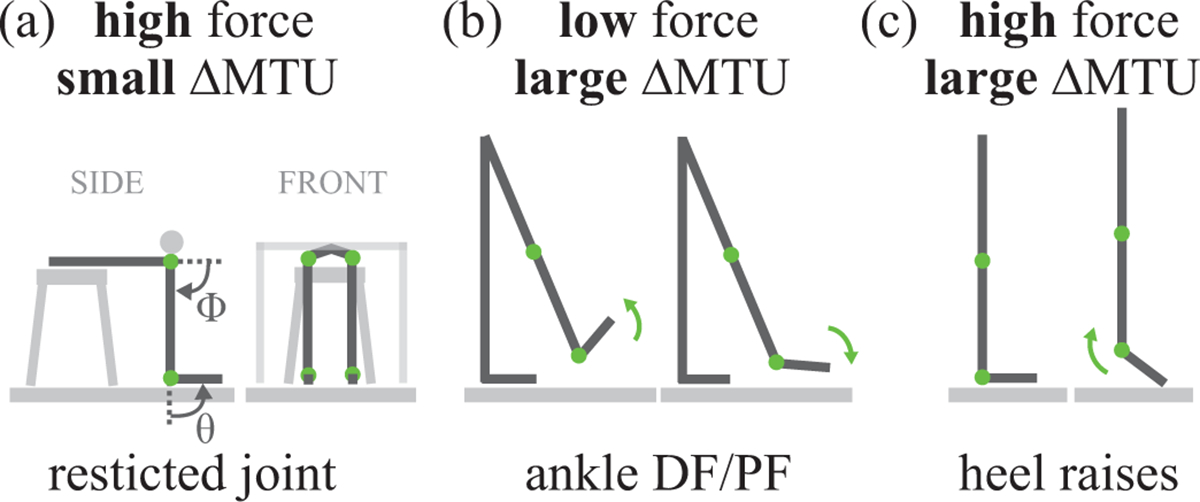
(a) Restricted joint calf contractions, involving high force and small MTU length change (ΔMTU). Subjects were seated on a stool with a rigid bar affixed above their knees, restricting both ankle and knee rotation. The stool and rigid bar were positioned such that the knee was flexed about 90° and the ankle was in the neutral (90°) position. The subject was relaxed, then contracted their calf muscles to push upward against the bar, then returned to a relaxed state. Gray arrows indicate ankle joint angle (θ) and knee joint angle (ϕ) conventions (b) Ankle DF/PF with foot in the air, involving low force and large ΔMTU. Subjects stood on their left foot with their right foot off the ground. Subjects began with their ankle in neutral position, then dorsiflexed their ankle, then fully plantarflexed their ankle, then returned to neutral. (c) Heel raises, involving high force and large ΔMTU. Subjects stood flat-footed with normal posture, then contracted their calf muscles to rise up off their heels, then relaxed to return to the flat-footed posture. Green arrows indicate motion.
Kinematics
Kinematics were collected at 100 Hz (Vicon), then low pass filtered at 6 Hz (3rd order, zero-lag Butterworth) prior to computing joint angles. 4 markers were placed bilaterally on the pelvis, and 2 bilaterally on the greater trochanters. Additional markers were placed unilaterally on the right limb: 4 on each segment (thigh, shank, foot), 2 on the lateral and medial femoral epicondyles, and 2 on the lateral and medial malleoli. Functional joint centers were computed using C-Motion Visual3D software and joint angles were calculated using the convention described in (Hawkins and Hull, 1990); neutral ankle position (foot orthogonal to shank) was defined as 90°, and fully extended knee was defined as 0°. Increasing ankle angles indicated increasing dorsiflexion, and increasing knee angles indicated increasing flexion (Fig. 2a).
Kinetics
Ground reaction forces were collected independently under each foot at 2000 Hz during heel raises only, using a force-instrumented split-belt treadmill (Bertec). Forces were low-pass filtered at 15 Hz (3rd order, zero-lag Butterworth).
Ultrasound
B-mode ultrasound was used to image the MG-AT MTJ or the MG MFs (Fig. 1b,c). Images were collected at approximately 60 Hz in B-mode with a 60 mm field of view and 50 mm depth (Echo Blaster 128, LV7.5/60/128Z-2 transducer, Telemed). Ultrasound data were synchronized with the other measurement modalities via an analog trigger (Fig. 1a), using time stamp data and a synchronization time delay that was quantified in preliminary experiments (similar to Rousseau et al., 2006). The ultrasound transducers were localized (position and orientation) in the lab reference frame using a custom 3D-printed fixture with motion capture markers (Matijevich, 2016). For subjects 1–4, a single ultrasound transducer was positioned to track the MTJ, and each task was performed. Directly afterwards, tasks were repeated with the transducer positioned to track the MG MFs. Prior to data collections on subjects 5–8, a second (identical) ultrasound system was acquired, and for these subjects, MFs and MTJ were imaged simultaneously.
Data Analysis
Muscle, AT and MTU length changes of the MG were estimated using established MTJ tracking methods (Hoffrén et al., 2012; Lichtwark, 2005; Lichtwark and Wilson, 2006), and MF tracking methods (Cronin and Lichtwark, 2013; Farris and Sawicki, 2012; Hoang et al., 2007; Hoffrén et al., 2012; Masaki Ishikawa et al., 2005; M. Ishikawa et al., 2005; Lichtwark et al., 2007; Lichtwark and Wilson, 2006; Sakuma et al., 2011). The MG MTU length change was estimated from a regression equation based on ankle and knee kinematics (Hawkins and Hull, 1990). Via the MTJ tracking method, AT length change was estimated using the straight-line distance from the MTJ (tracked in the ultrasound images, then localized in the motion capture reference frame) to the calcaneus (tracked with motion capture, Fig. 1b). MG muscle length change was then estimated by subtracting AT length change from the overall MTU length change. Via the MF tracking method, MG muscle length change was estimated from MF length changes corrected by pennation angle. AT length change was then estimated by subtracting MG length change from MTU length change (Fig. 1c). See Supplementary Material for further details on these tracking methods. AT force was estimated for the heel raise task using standard inverse dynamics to estimate ankle moment, then assuming a constant AT moment arm to estimate AT force, similar to (Farris and Sawicki, 2012; Honert and Zelik, 2016).
To address the primary aim of this study, AT length change waveforms were qualitatively compared against expectations for a passive tendon acting in series with a muscle (expectations detailed below). Ultrasound and force data were each resampled to 100 Hz to match motion data. Data from each task cycle were normalized to 1000 data points (representing 0–100% cycle). For each task, on a subject-specific basis, data were averaged over five sequential cycles. Muscle, AT and MTU length changes were non-dimensionalized (divided by subject-specific shank length to account for size differences between subjects) before computing inter-subject means and standard deviations. For reporting purposes, length change results were re-dimensionalized by multiplying by average subject shank length (424 mm). Maximum AT lengthening and maximum AT shortening over an average cycle were computed as summary metrics for each task.
To address the secondary aim, we computed the Pearson correlation between average AT length change waveforms estimated from MTJ and MF tracking methods. Correlation coefficients were computed for each task and averaged across subjects
Model Expectations
A common MTU model was used to determine the expected AT behaviors during each task. This simple model is comprised of a passive linear extension spring (representing tendon) acting in series with an actuator (representing muscle). Here we briefly summarize expectations (Fig. 3a). See Supplementary Material for detailed rationale. During the restricted calf contraction (high MTU force, small MTU length change), the AT was expected to lengthen, with magnitude roughly equal to longitudinal muscle shortening. During the ankle DF/PF task (low MTU force, large MTU length change), the AT was expected to exhibit negligible length change. During heel raises (high MTU force, large MTU length change), the AT was expected to lengthen as MTU force increased; albeit with a double-peak length change profile that followed the expected AT loading profile.
Fig. 3. Expected vs. subject-averaged results.
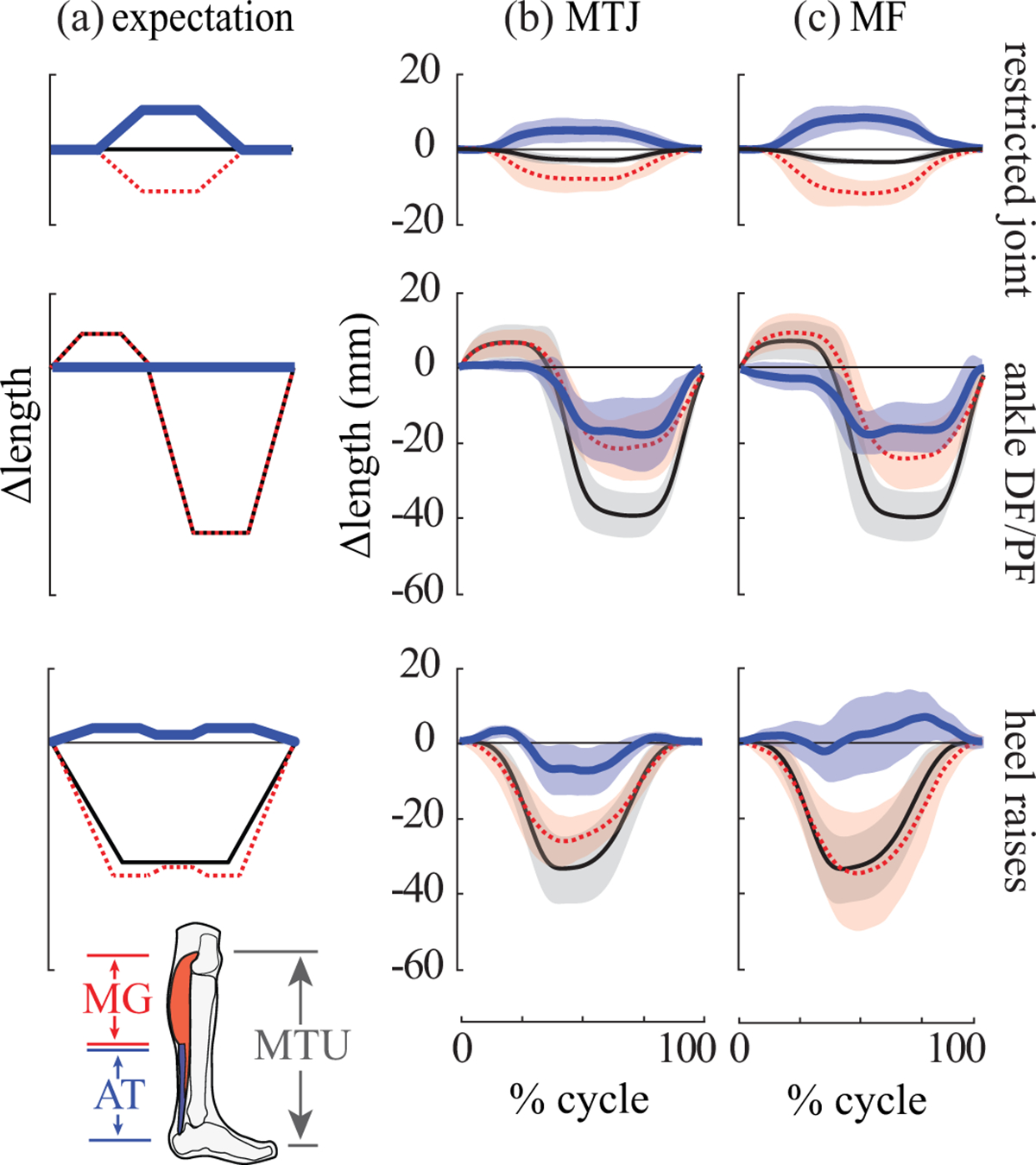
Rows indicate each task. (a) Expected behaviors of the MG muscle (dashed red), AT (thick blue) and MTU (thin black) based on a simple model of the MTU, consisting of a passive extension spring (tendon) acting in series with an actuator (muscle). Expectations are qualitative, so no units are provided on axes. Experimental results from the (b) MTJ tracking method and (c) MF tracking method. Depicted are inter-subject means (lines) and standard deviations (shaded regions). Length change waveforms (in mm) are plotted as a percentage of each movement cycle.
RESULTS
Restricted Joint Calf Contractions
As expected, we estimated AT lengthening and MG muscle shortening for all subjects and both tracking methods (Fig. 3, top row). On average, the AT lengthened a maximum of 5.4 ± 3.1 mm for the MTJ method and 8.4 ± 3.1 mm for the MF method (Table 1, N=7). Muscle shortening magnitude was typically slightly larger than AT lengthening (Fig. 3, top row). One subject was omitted from analysis of this task due to negligible MG activation and length change. AT length changes were strongly correlated between MTJ and MF methods (r = 0.90± 0.07, with min = 0.83, max = 0.99, N=7).
Table 1.
Maximum and minimum AT length changes for each task. Positive values indicate tendon lengthening and negative values indicate tendon shortening compared to length at 0% cycle. Data were non-dimensionalized (divided by subject-specific shank length), then averaged across subjects, and finally re-dimensionalized (multiplied by average shank length across subjects) for reporting purposes. All values are in mm, mean ± standard deviation.
| restricted joint (N=7) | ankle DF/PF (N=8) | heel raises (N=8) | ||||
|---|---|---|---|---|---|---|
| MTJ | MF | MTJ | MF | MTJ | MF | |
| AT max | 5.4 ± 3.1 | 8.4 ± 3.1 | 1.4 ± 1.0 | 1.8 ± 1.9 | 3.4 ± 1.7 | 9.5 ± 7.1 |
| AT min | −0.5 ± 0.4 | −0.4 ± 0.5 | −19.4 ± 8.8 | −19.7 ± 5.6 | −9.3 ± 5.6 | −5.3 ± 5.5 |
Ankle DF/PF with Foot in the Air
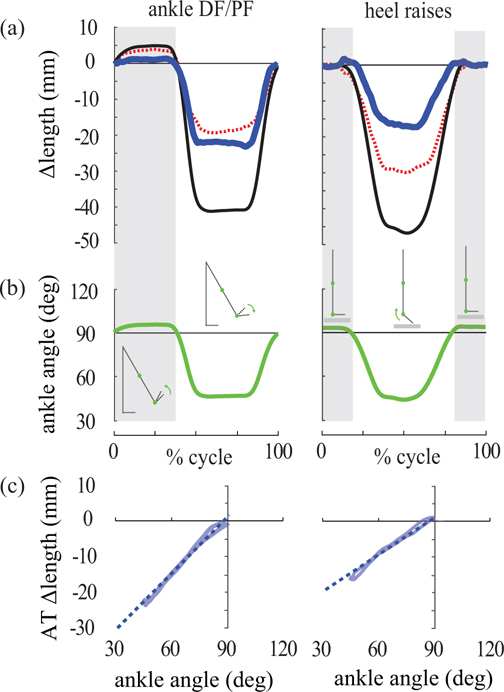
Contrary to expectations, we estimated substantial AT shortening for all subjects (Fig. 3, middle row). Maximum shortening was 19.4 ± 8.8 mm for the MTJ method, and 19.7 ± 5.6 mm for the MF method (Table 1, N=8). AT shortening was roughly proportional to ankle angle for angles <90° (Fig. 4, left column). For ankle angles >90°, AT length changes were small, typically less than a few millimeters, as expected. AT length changes were strongly correlated between MTJ and MF tracking methods (r = 0.91 ± 0.10, with min = 0.71, max = 0.98, N=8).
Fig. 4. AT length change and ankle angle data for a representative subject.
Results for the MTJ tracking method are shown for ankle DF/PF task (left column) and heel raises (right column). (a) AT (thick blue), muscle (dashed red) and MTU (thin black) length changes vs. movement cycle. (b) Ankle angle vs. movement cycle. 90° signifies neutral position, and decreasing angles indicate plantarflexion. (c) AT length change vs. ankle angle plotted only when the ankle was plantarflexed beyond neutral (<90°, white background of (b)). During these periods, the AT shortened proportionally (roughly linearly, dashed line) with decreasing ankle angle.
Heel Raises
For the MTJ method, the AT was estimated to lengthen slightly (3.4 ± 1.7 mm) at the beginning of the movement cycle, and then to shorten substantially (9.3 ± 5.6 mm, Table 1, N=8). This large shortening was inconsistent with the expected behavior of the AT under these loading conditions (Figs. 3, bottom row, S1). For most subjects, we again observed a roughly linear relationship between AT length change and ankle angles for angles <90° (Fig. 4, right column). For the MF method, AT length change estimates varied greatly across subjects. Some subjects exhibited primarily tendon lengthening and others primarily tendon shortening (Fig. S2). Comparing MTJ vs. MF methods, we found high inter-subject variability and that AT length changes were only weakly correlated, on average (r = 0.44 ± 0.45, with min = −0.60, max = 0.80, N=8, Fig. S2).
See Supplementary Material for kinematics and EMG results.
DISCUSSION
We found that AT kinematics were highly inconsistent with model expectations when the MTU underwent large length changes due to ankle plantarflexion beyond neutral. For instance, the AT was estimated to shorten substantially during the ankle DF/PF task (by an average of about 20 mm, Fig. 3, middle row), despite being a stiff passive structure under low force. Although ultrasound-based estimates of AT kinematics were consistent with our model-based expectations when the MTU underwent minimal length changes, scenarios in which the ankle joint is restricted are of limited utility for studying locomotion. The observed incongruence between the experimental estimates of AT length change and the model-based expectations represents a key obstacle to the research field in terms of confidently interpreting tendon function during movement tasks that involve non-negligible ankle plantarflexion. These non-intuitive findings call attention to a pressing need, and important opportunity, to develop improved experimental estimation methods and/or conceptual models of MTU dynamics.
Unexpected Tendon Shortening
The most striking and unexpected observation was that substantial AT shortening was estimated as the ankle plantarflexed beyond the neutral position. At ankle angles <90°, there was a surprisingly linear relationship between ankle angle and AT length change, such that with more plantarflexion the AT shortened proportionally (Fig. 4). This relationship was observed consistently for all subjects during the ankle DF/PF task, and for about half of the subjects during the heel raise task. Our observations were qualitatively consistent with Csapo et. al (2013) who estimated AT shortening of up to 13 mm when the ankle plantarflexed 20° beyond neutral. At ankle angles >90° (i.e., more dorsiflexed), the AT length change estimates in our study were more consistent with model-based expectations (i.e., small length changes under low force, Fig. 3, middle row).
These findings have potential implications for the interpretation of AT function during locomotor tasks that involve substantial ankle plantarflexion. During walking, energy storage occurs primarily at ankle angles >90°, i.e., ankle angles in this study when we observed expected AT elongations. However, energy return occurs over a larger range of motion that includes angles <90° (Farris and Sawicki, 2012; Masaki Ishikawa et al., 2005; Sakuma et al., 2011), i.e., ankle angles when we observed unexpected AT shortening. It may be that AT shortening (and therefore energy return) is overestimated when employing the ultrasound methods described in this study. It is often presumed that the AT accounts for the vast majority of positive ankle work near the end of stance in human gait (e.g., Alexander, 1991; Fukunaga et al., 2001; Hof, 1998; Maganaris and Paul, 2002). However, it has also been documented that common ultrasound-based methods can estimate 2–5 times more AT energy return than energy storage, which is implausible for a passive tendon (Zelik and Franz, 2017). Based on findings our current study, and also observations from other researchers (using separate lines of reasoning and observation, e.g., Nigg, 2010), it may be necessary to further explore the relative contribution of the AT to ankle work generation in human gait.
It is challenging to pinpoint the precise reason(s) for the unexpected AT shortening estimates, though primary source(s) of error may reside in the assumed MTU model and/or experimental methods. Collecting data and mapping results onto a model requires many choices (e.g., which features of the musculoskeletal system to track and how to associate measurements with model features). A simplified model of the MTU must also be assumed to fuse ultrasound, motion and force data. A variety of methodological choices and/or model assumptions could contribute to unexpected AT length change estimates. The impact of these choices may be highly dependent on the task, MTU range of motion or loading pattern. Interestingly, the ankle DF/PF task had consistent results between methods (Fig. 3), suggesting errors may reside in a shared assumption between methods. On the contrary, inconsistent results in the heel raise task (Fig. 3) suggest errors may be in assumptions unique to each method. Unexpected AT shortening in both the ankle DF/PF and the heel raise task (Fig. 4) may further suggest errors in the shared conceptual model. Follow-up studies are needed to unmask the primary culprit(s).
Where Might the Simplified Conceptual Model Go Wrong?
A foundational assumption of the MTU model is that a tendon is loaded in series with a muscle. However, some researchers have argued against this assumption, noting that the actual loading behavior may be complex and highly non-intuitive (Epstein and Herzog, 2003; Herzog and Nigg, 2007). Thus, the 1-dimensional, in-series model may fail to capture important dynamics. Another implicit model assumption is that MTUs can be studied in isolation, ignoring forces from adjacent MTUs. There is some evidence suggesting that muscle loading is borne primarily along individual tendon fascicles (Arndt et al., 2012; Franz and Thelen, 2015). However, there also exist intermuscular interactions via epimuscular linkages (Maas and Finni, 2017), as well as sheet-like aponeuroses and other connective tissues that interconnect the plantarflexors, and thus transverse forces and biaxial strain may affect tendon dynamics (Arellano et al., 2016; Azizi and Roberts, 2009).
Where Might the Experimental Methods Go Wrong?
Each method provides a partial snapshot of the MTU. Various assumptions in relating features of the ultrasound images to actual tissue motion could introduce errors. For the MF method, correcting localized muscle fascicle length change by pennation angle is assumed to provide a reasonable approximation of overall muscle length change, however this may not always be valid (Domire and Challis, 2010; Zatsiorsky, 2012). For the MTJ method, it is assumed that the junction feature tracked in each ultrasound frame is representative of the overall MTJ displacement. However, the MTJ is a complex 3D interweaving of muscle and tendinous tissues (Zatsiorsky, 2012), making it difficult to precisely track the same feature in each frame, or to comprehensively capture tissue dynamics. For both methods, the AT is assumed to have a linear connection between the MTJ and calcaneus, but this assumption begins to break down if the tendon curves due to muscle bulging or wrapping around the calcaneus, or if the tendon becomes slack. See Supplementary Material for extended discussion of these potential confounds and how much of the unexpected tendon shortening they might explain.
MTJ vs. MF Tracking Methods
As evident in Fig. 3, and confirmed via correlation analysis, the MTJ and MF methods yielded similar AT length change estimates during restricted joint calf contraction and ankle DF/PF tasks. However, notable differences in AT length change were observed for most subjects during heel raises (Figs. 3, bottom row, S2). The inconsistent results during the heel raise task (high force, large MTU displacement) suggest that using different methods to estimate AT behavior during other tasks involving high force and large MTU displacement may lead to disparate results. Indeed, prior studies of walking (Zelik and Franz, 2017), running (Lichtwark and Wilson, 2006) and other plantarflexor tasks (Fukutani et al., 2017) provide more direct evidence supporting this implication.
Limitations
This study evaluated the consistency between simple model expectations and experimental estimates of AT length change. We did not collect ground truth tendon length change or force data, as these would require invasive sensor implantation, and non-invasively partitioning individual muscle forces remains a grand challenge in the field. Experimental tasks were carefully selected to avoid the need for precise ground truth measures. Results were interpreted in the context of whether the AT lengthened or shortened under higher or lower loading conditions. Methodological limitations related to the MTJ and MF methods were discussed above and have been well-documented in prior literature (Cronin and Lichtwark, 2013; Zelik and Franz, 2017). For Subjects 1–4, the MFs and MTJs were not imaged simultaneously; therefore the task performance varied slightly.
CONCLUSION
Our ability to correctly infer muscle and tendon function depends on using well-validated methods that can map experimental data onto a model, and employing a model that adequately captures the salient features and dominant dynamics of the physiological MTU being studied. The AT is often conceptualized as an extension spring acting in series with muscle, and ultrasound imaging provides a non-invasive means of peering underneath the skin at these tendon dynamics. However, here we observed simple movement tasks in which the AT was empirically estimated to shorten despite loading conditions in which a passive spring would be expected to either stretch (under increasing force) or maintain its length (under low force). These unexpected findings suggest the need for improvements in how we conceptually model and/or experimentally estimate MTU dynamics in vivo during motion analysis studies, particularly when the ankle plantarflexes beyond neutral.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by funding from the National Institutes of Health (K12HD073945) and from Vanderbilt University. The authors would like to thank Ethan Jones for his assistance with data collection and analysis.
ABBREVIATIONS
- AT
Achilles tendon
- DF/PF
dorsiflexion/plantarflexion
- MF
muscle fascicle
- MG
medial gastrocnemius
- MTJ
muscle-tendon junction
- MTU
muscle-tendon unit
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests to declare.
References
- Alexander RM, 2002. Tendon elasticity and muscle function. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 133, 1001–1011. 10.1016/S1095-6433(02)00143-5 [DOI] [PubMed] [Google Scholar]
- Alexander RM, 1991. Energy-saving mechanisms in walking and running. J. Exp. Biol 160, 55–69. [DOI] [PubMed] [Google Scholar]
- Arellano CJ, Gidmark NJ, Konow N, Azizi E, Roberts TJ, 2016. Determinants of aponeurosis shape change during muscle contraction. J. Biomech 49, 1812–1817. 10.1016/j.jbiomech.2016.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt A, Bengtsson A-S, Peolsson M, Thorstensson A, Movin T, 2012. Non-uniform displacement within the Achilles tendon during passive ankle joint motion. Knee Surg. Sports Traumatol. Arthrosc 20, 1868–1874. 10.1007/s00167-011-1801-9 [DOI] [PubMed] [Google Scholar]
- Azizi E, Roberts TJ, 2009. Biaxial strain and variable stiffness in aponeuroses. J. Physiol 587, 4309–4318. 10.1113/jphysiol.2009.173690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbert MF, Huijing PA, van Ingen Schenau GJ, 1986. A model of the human triceps surae muscle-tendon complex applied to jumping. J. Biomech 19, 887–898. 10.1016/0021-9290(86)90184-3 [DOI] [PubMed] [Google Scholar]
- Bregman DJJ, van der Krogt MM, de Groot V, Harlaar J, Wisse M, Collins SH, 2011. The effect of ankle foot orthosis stiffness on the energy cost of walking: A simulation study. Clin. Biomech 26, 955–961. 10.1016/j.clinbiomech.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, Marshall-Gradisnik S, Payne C, Crossley KM, 2008. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J. Appl. Physiol 105, 1035–1043. 10.1152/japplphysiol.01281.2007 [DOI] [PubMed] [Google Scholar]
- Chernak LA, Thelen DG, 2012. Tendon motion and strain patterns evaluated with two-dimensional ultrasound elastography. J. Biomech 45, 2618–2623. 10.1016/j.jbiomech.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SH, Kuo AD, 2010. Recycling Energy to Restore Impaired Ankle Function during Human Walking. PLOS ONE 5, e9307. 10.1371/journal.pone.0009307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin NJ, Lichtwark G, 2013. The use of ultrasound to study muscle–tendon function in human posture and locomotion. Gait Posture 37, 305–312. 10.1016/j.gaitpost.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Csapo R, Hodgson J, Kinugasa R, Edgerton VR, Sinha S, 2013. Ankle morphology amplifies calcaneus movement relative to triceps surae muscle shortening. J. Appl. Physiol 115, 468–473. 10.1152/japplphysiol.00395.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domire ZJ, Challis JH, 2010. A critical examination of the maximum velocity of shortening used in simulation models of human movement. Comput. Methods Biomech. Biomed. Engin 13, 693–699. 10.1080/10255840903453082 [DOI] [PubMed] [Google Scholar]
- Epstein M, Herzog W, 2003. Aspects of skeletal muscle modelling. Philos. Trans. R. Soc. B Biol. Sci 358, 1445–1452. 10.1098/rstb.2003.1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris DJ, Sawicki GS, 2012. Human medial gastrocnemius force–velocity behavior shifts with locomotion speed and gait. Proc. Natl. Acad. Sci 109, 977–982. 10.1073/pnas.1107972109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris DJ, Trewartha G, Polly McGuigan M, 2011. Could intra-tendinous hyperthermia during running explain chronic injury of the human Achilles tendon? J. Biomech 44, 822–826. 10.1016/j.jbiomech.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Finni T, Peltonen J, Stenroth L, Cronin NJ, 2013. Viewpoint: On the hysteresis in the human Achilles tendon. J. Appl. Physiol 114, 515–517. 10.1152/japplphysiol.01005.2012 [DOI] [PubMed] [Google Scholar]
- Franz JR, Slane LC, Rasske K, Thelen DG, 2015. Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture 41, 192–197. 10.1016/j.gaitpost.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Thelen DG, 2015. Depth-dependent variations in Achilles tendon deformations with age are associated with reduced plantarflexor performance during walking. J. Appl. Physiol 119, 242–249. 10.1152/japplphysiol.00114.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN, 2001. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. Lond. B Biol. Sci 268, 229–233. 10.1098/rspb.2000.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutani A, Misaki J, Isaka T, 2017. Relationship between joint torque and muscle fascicle shortening at various joint angles and intensities in the plantar flexors. Sci. Rep 7, 290. 10.1038/s41598-017-00485-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve DW, 1978. Prediction of gastrocnemius length from knee and ankle joint posture.
- Hawkins D, Lum C, Gaydos D, Dunning R, 2009. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. J. Biomech 42, 2813–2817. 10.1016/j.jbiomech.2009.08.023 [DOI] [PubMed] [Google Scholar]
- Hawkins Hull, M.L., 1990. A Method For Determining Lower Extremity Muscle-Tendon Lengths During Flexion/Extension Movements. [DOI] [PubMed]
- Herzog W, Nigg B, 2007. Biomechanics of the musculo-skeletal system. John Wiley & Sons. [Google Scholar]
- Hoang PD, Herbert RD, Todd G, Gorman RB, Gandevia SC, 2007. Passive mechanical properties of human gastrocnemius muscle–tendon units, muscle fascicles and tendons in vivo. J. Exp. Biol 210, 4159–4168. 10.1242/jeb.002204 [DOI] [PubMed] [Google Scholar]
- Hof AL, 1998. In vivo measurement of the series elasticity release curve of human triceps surae muscle. J. Biomech 31, 793–800. 10.1016/S0021-9290(98)00062-1 [DOI] [PubMed] [Google Scholar]
- Hoffrén M, Ishikawa M, Avela J, Komi PV, 2012. Age-related fascicle–tendon interaction in repetitive hopping. Eur. J. Appl. Physiol 112, 4035–4043. 10.1007/s00421-012-2393-x [DOI] [PubMed] [Google Scholar]
- Honert EC, Zelik KE, 2016. Inferring Muscle-Tendon Unit Power from Ankle Joint Power during the Push-Off Phase of Human Walking: Insights from a Multiarticular EMG-Driven Model. PLOS ONE 11, e0163169. 10.1371/journal.pone.0163169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann G-P, 2005. Muscle-tendon interaction and elastic energy usage in human walking. J. Appl. Physiol 99, 603–608. 10.1152/japplphysiol.00189.2005 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Niemelä E, Komi PV, 2005. Interaction between fascicle and tendinous tissues in short-contact stretch-shortening cycle exercise with varying eccentric intensities. J. Appl. Physiol 99, 217–223. 10.1152/japplphysiol.01352.2004 [DOI] [PubMed] [Google Scholar]
- Konow N, Azizi E, Roberts TJ, 2011. Muscle power attenuation by tendon during energy dissipation. Proc. R. Soc. Lond. B Biol. Sci. rspb20111435. 10.1098/rspb.2011.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korstanje J-WH, Selles RW, Stam HJ, Hovius SER, Bosch JG, 2010. Development and validation of ultrasound speckle tracking to quantify tendon displacement. J. Biomech 43, 1373–1379. 10.1016/j.jbiomech.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Lichtwark G, 2005. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. [DOI] [PubMed]
- Lichtwark GA, Bougoulias K, Wilson AM, 2007. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech 40, 157–164. 10.1016/j.jbiomech.2005.10.035 [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM, 2007. Is Achilles tendon compliance optimised for maximum muscle efficiency during locomotion? J. Biomech 40, 1768–1775. 10.1016/j.jbiomech.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM, 2006. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol 209, 4379–4388. 10.1242/jeb.02434 [DOI] [PubMed] [Google Scholar]
- Maas H, Finni T, 2017. Mechanical Coupling Between Muscle-Tendon Units Reduces Peak Stresses. Exerc. Sport Sci. Rev Publish Ahead of Print. 10.1249/JES.0000000000000132 [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP, 2002. Tensile properties of the in vivo human gastrocnemius tendon. J. Biomech 35, 1639–1646. 10.1016/S0021-9290(02)00240-3 [DOI] [PubMed] [Google Scholar]
- Matijevich E, 2016. Ultrasound Probe Marker Holder. Zelik Lab Biomech. Assist. Technol. [Google Scholar]
- Nigg B, 2010. Biomechanics of sport shoes. University of Calgary. [Google Scholar]
- Roberts TJ, Azizi E, 2010. The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J. Appl. Physiol 109, 396–404. 10.1152/japplphysiol.01272.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Hellier P, Barillot C, 2006. A novel temporal calibration method for 3-D ultrasound. IEEE Trans. Med. Imaging 25, 1108–1112. 10.1109/TMI.2006.877097 [DOI] [PubMed] [Google Scholar]
- Sakuma J, Kanehisa H, Yanai T, Fukunaga T, Kawakami Y, 2011. Fascicle–tendon behavior of the gastrocnemius and soleus muscles during ankle bending exercise at different movement frequencies. Eur. J. Appl. Physiol 112, 887–898. 10.1007/s00421-011-2032-y [DOI] [PubMed] [Google Scholar]
- Sawicki GS, Lewis CL, Ferris DP, 2009. It pays to have a spring in your step. Exerc. Sport Sci. Rev 37, 130. 10.1097/JES.0b013e31819c2df6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE, 1989. Muscle and tendon: properties, models, scaling, and application to biomechnics and motor control. Crit. Rev. Biomed. Eng 17. [PubMed] [Google Scholar]
- Zatsiorsky V, 2012. Biomechanics of Skeletal Muscles. Human Kinetics. [Google Scholar]
- Zelik KE, Franz JR, 2017. It’s positive to be negative: Achilles tendon work loops during human locomotion. PLOS ONE 12, e0179976. 10.1371/journal.pone.0179976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelik KE, Huang T-WP, Adamczyk PG, Kuo AD, 2014. The role of series ankle elasticity in bipedal walking. J. Theor. Biol 346, 75–85. 10.1016/j.jtbi.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.