Abstract
Ubiquinol-cytochrome c reductase core protein 1 (UQCRC1) is an indispensable component of mitochondrial complex III. It plays a key role in cardioprotection and maintaining mitochondrion function. However, the exact role of UQCRC1 in maintaining cardiac function has not been reported by in vivo models. Also, the exact biological functions of UQCRC1 are far from fully understood. UQCRC1+/− mice had decreased both mRNA and protein expression of UQCRC1 in the left ventricular myocardia, and these mice had reduced tolerance to acute exhaustive exercise including decreased time and distance with higher apoptosis rate, higher expression level of cleaved CASPASE 3, and higher ratio of cleaved PARP1 to full-length PARP1. Moreover, UQCRC1 knockdown led to increased LV interventricular septal thicknesses both at systole and diastole, as well as decreased LV volume both at end-systole and end-diastole. Finally, UQCRC1 gene disruption resulted in mitochondrial vacuolation, fibril disarrangement, and more severe morphological and structural changes in mitochondria after acute exhaustive exercise. In conclusion, UQCRC1 contributes to cardiac tolerance to acute exhaustive exercise in mice, and it may be an essential component of complex III, playing a crucial role in maintaining cardiac functions.
Keywords: UQCRC1, acute exhaustive exercise, cardiac function, mitochondrial complex III, myocardium, cardioprotection
Impact statement
UQCRC1, an essential subunit of mitochondrial complex III, may play a crucial role in maintaining life and organ functions. Knockout of Uqcrc1 leads to mice embryonic death and knockdown of Uqcrc1 results in poor brain function. Mutations of Uqcrc1 are closely associated with some severe diseases. Also, UQCRC1 may be closely correlated with several types of cancer including colorectal cancer, pancreatic cancer. Of note, it has been proved that UQCRC1 has a protective effect on both myocardia and brain ischemic injury. However, there has been no evidence so far for the cardioprotective roles of UQCRC1 from in vivo models. Here, we found that UQCRC1+/− mice had decreased tolerance to acute exhaustive exercise due to poor cardiac function and myocardial including mitochondrial structural damage, offering new proof for the crucial role of UQCRC1 in maintaining cardiac functions in in vivo models, and also providing a new insight into its biological functions.
Introduction
Ubiquinol-cytochrome c reductase core protein 1 (UQCRC1) is an indispensable component of the mitochondrial complex III (also named as bc1 complex). Although it has been proved to be involved in many biological processes in several fields including oncology, ischemia injury, and metabolism-related diseases, the exact functions of UQCRC1 are far from fully understood.1–6
Increasing evidence shows that UQCRC1 may play a crucial part in maintaining life and organ functions. Knockout of Uqcrc1 leads to mice embryonic death and knockdown of Uqcrc1 results in poor brain function. 6 Also, several studies have found that mutations of Uqcrc1 are closely associated with some severe diseases including malignant pleural mesothelioma and autosomal dominant parkinsonism with polyneuropathy.7–9 In addition, UQCRC1 has also been discovered to play a crucial role in oncology, where, among other things, it may be closely related to lymph node metastasis and poor prognosis in colorectal cancer, serve as a biomarker in clear cell renal cell carcinoma, and act as a therapeutic target for pancreatic cancer.10–12
In the setting of cardioprotection, it has been shown by several studies that UQCRC1 plays a protective role in cardiomyocyte cultures. Ischemia/reperfusion injury may decrease UQCRC1 expression, 13 but a cardioprotective reagent may increase its expression. 14 Moreover, we have shown in previous studies that overexpression of UQCRC1 attenuates mimic ischemia/reperfusion injury and that knockdown of UQCRC1 has the opposite effects in cultured cardiac cells.15,16 However, the exact functions of UQCRC1 are far from fully understood.
Mitochondrion has been widely thought to play a vital part in cardioprotection, and it has been considered as the common target for several cardioprotective effects, such as ischemic preconditioning, pharmacological preconditioning, and postconditioning.17–19 Accumulating evidence shows that UQCRC1 expression changes may directly affect mitochondria structure and function. Early studies discovered that overexpression of UQCRC1 may enhance mitochondrial complex III activity, 20 but its expression may decrease along with mitochondria dysfunction. 19 Our recent study further shows that knockdown of Uqcrc1 reduces both the formation and activity of complex III in mouse brain. 6 Also, another study found that tryptophan oxidation of UQCRC1 causes large structural changes of complex III in a myodegeneration mouse model. 21
Given the cardioprotective role of UQCRC1 and if UQCRC1 is an indispensable component of the mitochondrion, the common target for cardioprotection, it is reasonable to believe that UQCRC1 may take part in determining cardiac function in myocardial injury. However, the role of UQCRC1 in maintaining cardiac function has not been reported using in vivo models.
Therefore, this study aimed to further explore the exact functions of UQCRC1 in in vivo models. We postulated that UQCRC1 may be an indispensable component in maintaining mitochondria structure, and that disruption of UQCRC1 expression might impair cardiac functions. Here, we found that UQCRC1+/− mice had decreased both mRNA and protein expression of UQCRC1 in the left ventricular myocardia. These mice had reduced tolerance to acute exhaustive exercise due to poor cardiac function and myocardial structure, including mitochondrial structural damage.
Materials and methods
Animals
The animal protocol was approved by the Institutional Animal Ethics Committee of Army Medical University (Chongqing, China). All animal experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23) revised in 2011. About 96 male mice at 6 to 8 weeks old, weighing about 20–25 g, were used for the current study.
Generation and genotyping of Uqcrc1 knockdown mice
F1 heterozygous Uqcrc1 mice were generated and genotyped as recently described. 6 Briefly, a Lac-Neo cassette was inserted to replace partial gene sequence of Uqcrc1 between exon 3 and exon 4. The genomic DNA from mice tails was extracted using the Quick Genotyping Assay Kit for Mouse Tail (Bimake, China). The primers of F1 (5′-AGGATCTCCTGTCATCTCACCTTGCTCCTG-3′) and R1 (5′-AAGAACTCGTCAAGAAGGCGATAGAAGGCG-3′) were designed to confirm the inserted Neo cassette. The primers of F2 (5′-GGGATGTGGAGCCAGAATCAACAAC-3′) and R2 (5′-CCAGAGCTACCCAACAGCCTATCTT-3′) were used to identify the original gene sequence of Uqcrc1 that should be replaced by the Neo cassette. The PCR program was set as 94 °C for 5 min, 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 35 s per cycle for 35 cycles and then 72 °C for 10 min.
Acute exhaustive exercise
Similar to the previous study, 22 mice aged 6 to 8 weeks were acclimated for 2 days prior to the fatigue task, 3 times per day for 10 min at each time on a rotary fatigue meter (Shangdong Academy of Medical Science, China). From the third day on, fatigue was induced by voluntary running on the rotary fatigue meter for 7 consecutive days. Mice were encouraged to run by electric shocks (1.5 mA for 3 s) till fatigue, which is defined as stopping to rest for 5 times in 5 minutes.
TUNEL assay
As previously described, 23 myocardial apoptosis rate was detected using a One Step TUNEL apoptosis assay kit (Beyotime, China). TUNEL staining (green fluorescence) was utilized to label apoptotic cell nuclei and DAPI staining (blue fluorescence) was utilized to label the total cardiomyocyte nuclei. The stained samples were photographed under a confocal fluorescence microscope (Leica, Germany).
Western blotting
Left ventricular myocardia of mice were harvested immediately after AEE to detect the expression of apoptosis related proteins. Briefly, equal amounts of protein were loaded per lane and separated by 8% or 12% gels. After transferring to membranes, the membranes were blocked for 1 h, then incubated with the primary antibodies overnight at 4 °C including PARP1 monoclonal antibody (Proteintech, USA, 1:10,000 dilution), cleaved CASPASE 3 Polyclonal Antibody (Proteintech, USA, 1:1000 dilution) and GAPDH Antibody HRP-60004 (Proteintech, USA, 1:10000 dilution). Secondary antibodies for PARP1 and cleaved CASPASE 3 were incubated for 2 h at room temperature. The intensity of protein bands was quantified by Image J. The relative expression level was normalized to the level of GAPDH in the same sample.
Transmission electron microscopy
Left ventricular myocardia of mice were harvested under anesthesia and fixed in 2.5% glutaraldehyde in phosphate buffer (0.1 M; pH 7.4) overnight at 4 °C. Samples were postfixed for another 2 h in 2% osmic acid in 0.1 M phosphate buffer, followed by gradient dehydration in acetone (50%, 70%, 90%, and 100%, respectively), then were embedded in epoxy resins. Sections were detected under a transmission electron microscope (JEM-1400PLUS, Japan) after co-stained with uranyl acetate and lead citrate.
Transthoracic echocardiography
As described previously, 24 transthoracic 2 D-guided M-mode echocardiography was performed on anesthetized mice using a Vevo 2100 Imaging System (VisualSonics Inc., Canada) with a 13–24 MHz scan probe (MS250) by an experienced ultrasonographic technician. The cardiac indexes such as LV fractional shortening (%FS) and ejection fraction (%EF) were calculated automatically by the echocardiographic system.
Total RNA extraction and real-time PCR
Total RNA from left ventricular myocardia of mice was isolated using RNAiso Plus reagent (Takara, Japan). Reverse transcription PCR was performed using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan) for cDNA synthesis. Primers were used as follows: Uqcrc1, F3 (5′-CAGTGTCTCCCGAGTGTATG-3′) and R3 (5′-GGTCACGTTGTCTGGGTTAG-3′). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal reference [F4 (5′-AATGTGTCCGTCGTGGATCT-3′) and R4 (5′-GGTCCTCAGTGTAGCCCAAG-3′)].
Statistical analysis
Data are presented as mean ± SD. Data were analyzed using the t test or one-way or two-way repeated measures analysis of variance followed by the Tukey test. Statistical significance was regarded as P < 0.05 based upon two-tailed hypothesis testing.
Results
Uqcrc1 knockdown mice had reduced mRNA and protein of UQCRC1 in their left ventricular myocardia
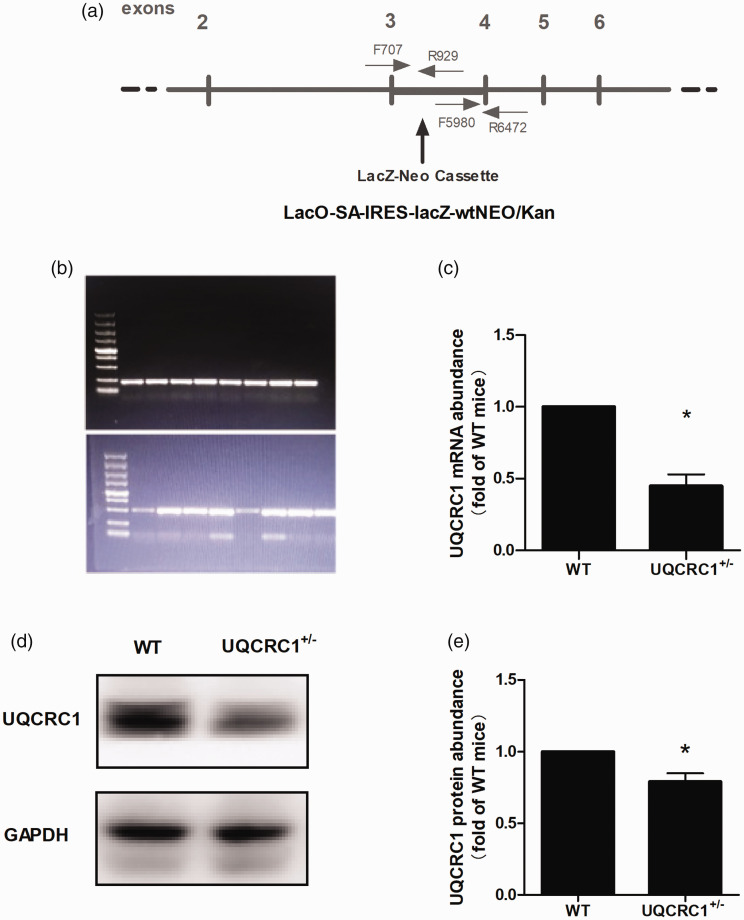
As we reported previously, 6 the PCR products were expected as follows: single band of 493 bp for the homozygous UQCRC1−/− mice, single band of 223 bp for wild-type mice, and both bands of 493 and 223 bp for the heterozygous UQCRC1+/− mice (Figure 1). 6 A total of 96 male mice at 6 to 8 weeks old were genotyped. Since Uqcrc1 knockout resulted in embryonic death, 6 no homozygous UQCRC1−/− mice were found as expected, confirming the observation again that UQCRC1 may play a crucial part in maintaining life and knockout of UQCRC1 is not viable. As shown in Figure 1, the UQCRC1+/− mice (mice with UQCRC1 knockdown) had reduced mRNA and protein of UQCRC1 in their left ventricular myocardia.
Figure 1.
Knockdown of Uqcrc1 in mice. (a) Schematic diagram of UQCRC1 mutation. (b) Identification of UQCRC1 mutation. (c) UQCRC1 mRNA expression from left ventricular myocardia analyzed by real-time PCR (n = 4). (d and e) UQCRC1 protein expression from left ventricular myocardia analyzed by Western blotting (n = 3). WT, wild type. *P <0.05.
Uqcrc1 knockdown mice had decreased tolerance to acute exhaustive exercise
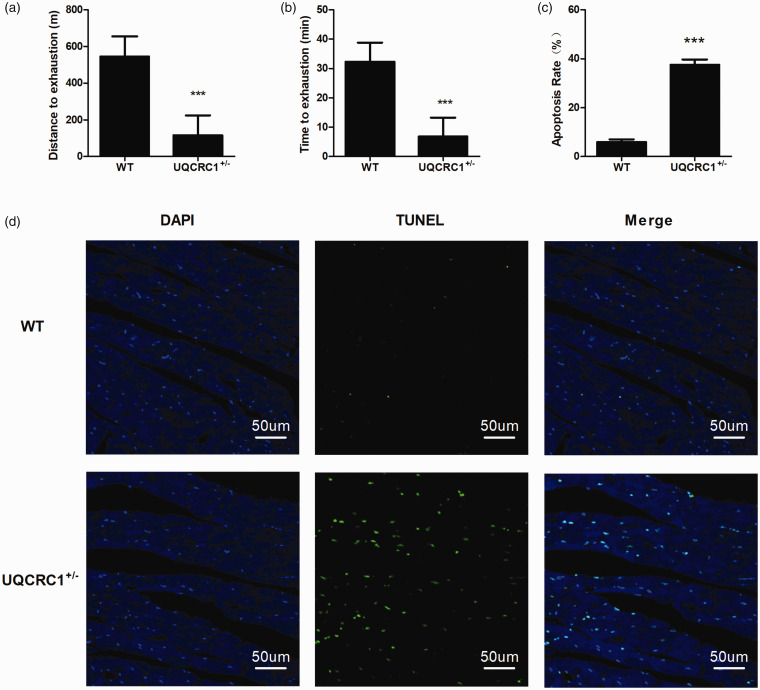
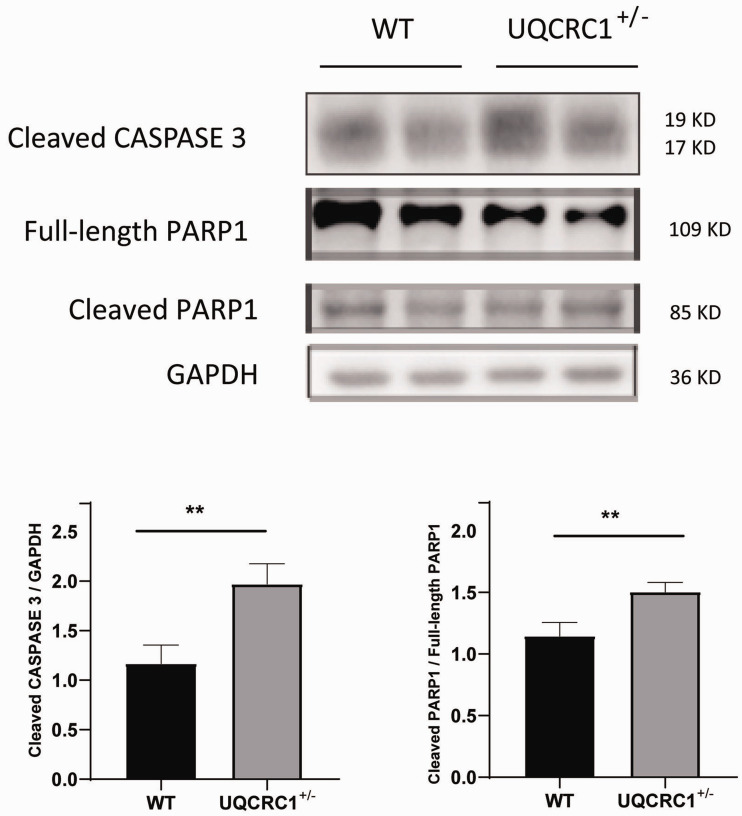
To examine whether Uqcrc1 knockdown had any cardiac functional consequence in the heterozygous UQCRC1+/− mice, these mice were subjected to acute exhaustive exercise. Compared with the wild-type group, both the distance and time to exhaustion in the UQCRC1+/− group were significantly shorter (Figure 2(a) and (b)). In addition, the average apoptosis rate of myocardial cells in isolated hearts in the UQCRC1+/− group was markedly higher after AEE than the wild-type group (Figure 2(c) and (d)). Furthermore, UQCRC1 knockdown resulted in an increase of cleaved CASPASE 3, as well as caused an increased ratio of cleaved PARP1 to full-length PARP1 after AEE (Figure 3).
Figure 2.
Performance in AEE and apoptosis analysis after AEE (n = 5). (a and b) Average time and distance to exhaustion for each group. (c and d) Apoptosis analysis in isolated mice hearts by TUNEL after AEE. ***P < 0.001. (A color version of this figure is available in the online journal.)
Figure 3.
Western blotting analysis of cleaved CASPASE 3 and PARP1 after AEE. WT, wild type; n = 4 for the WT group, n = 3 for the UQCRC1+/− group; **P < 0.01.
Uqcrc1 knockdown mice had decreased cardiac function
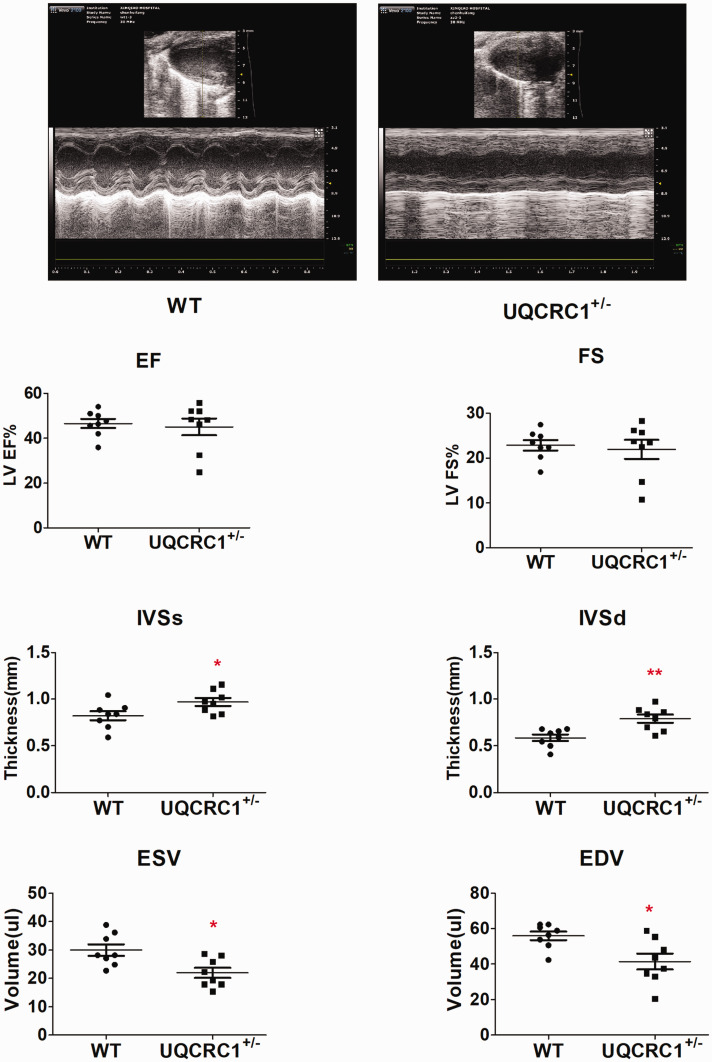
To investigate the possible reasons for changes of tolerance to acute exhaustive exercise, we measured the cardiac function of wild-type and UQCRC1+/− mice using transthoracic echocardiography under anesthesia (Figure 4). No significant difference was detected in left ventricular (LV) fractional shortening (FS) and ejection fraction (EF) between the wild-type and UQCRC1+/− mice. However, compared to wild-type mice, UQCRC1 knockdown leads to increased LV interventricular septal thicknesses both at systole and diastole (IVSs and IVSd), as well as decreased LV volume both at end-systole and end-diastole (ESV and EDV), and this may be a possible reason for no significant difference in LV EF.
Figure 4.
Cardiac measurement by transthoracic echocardiography (n = 8). WT, wild type; LV, left ventricular; EF, ejection fraction; FS, fractional shortening; IVSs and IVSd, interventricular septal thicknesses at systole and diastole; EDV, end-diastolic volume; ESV, end-systolic volume. *P< 0.05; **P< 0.01.
Uqcrc1 gene disruption affected myocardial structure
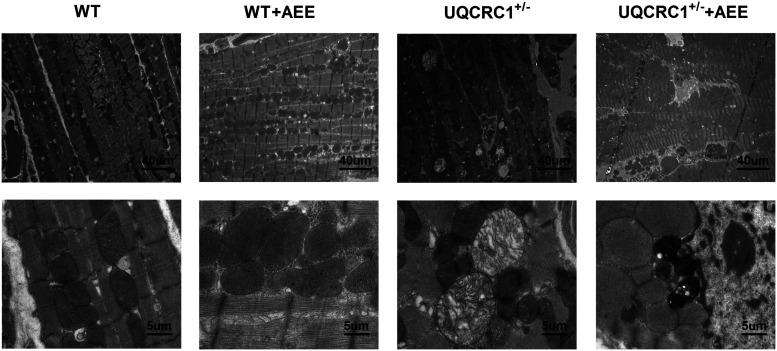
To figure out the possible structural changes in UQCRC1-disruption cardiomyocytes, cardiomyocytes from untreated mice and those subjected to AEE were examined using transmission electron microscopy (Figure 5). In untreated mice, we found evident fibril disarrangement and mitochondrial vacuolation in the UQCRC1+/− group, compared to the wild type. After AEE, the ultrastructural changes of the myocardium showed edema, swelling and dissolution of mitochondrial cristae both in wild type and UQCRC1+/− mice. Notably, more evidently severe structural damage was observed in UQCRC1+/− mice with an increase of lysosomes, disorderly fibril organization, even condensate and fragmental myofibrils in some cardiomyocytes.
Figure 5.
Typical ultrastructural changes of left ventricular cardiomyocytes. Three sections per specimen from three mice per group were examined under transmission electron microscopy, and typical images are shown.
Discussion
In the present study, we demonstrated that UQCRC1 contributes to cardiac tolerance to acute exhaustive exercise in mice, offering new proof for the crucial role of UQCRC1 in maintaining cardiac functions in in vivo models, and also providing a new insight into its biological functions.
The incidence of human mitochondrial disorders is 1:10,000 live births. Sole complex III deficiencies are among the least frequently diagnosed mitochondrial disorders. 25 Clinical symptoms may range from isolated myopathy to severe multi-systemic disorders with early death and disability. 25 Complex III mutations may directly cause enzymes dysfunction on the respiratory chain in human, which may further lead to several diseases, such as fetal bradycardia, hypertrophic cardiomyopathy, histiocytoid cardiomyopathy, ischemic cardiomyopathy, benign congenital myopathy, ventricular septal defect, and progressive exercise muscle intolerance.25–34 Of note, the latest studies have found that mitochondrial UQCRC1 mutations are closely associated with some severe diseases including malignant pleural mesothelioma and autosomal dominant parkinsonism with polyneuropathy.7–9
In particular, our recent study shows that Uqcrc1 knockout leads to embryonic death, implying a crucial role of UQCRC1 in maintaining life. 6 Moreover, knockdown of Uqcrc1 in mice results in reduced brain tolerance to ischemia injury and poor ability in learning and memory, while mutations of Uqcrc1 also cause autosomal dominant parkinsonism with polyneuropathy,6,7 suggesting a crucial role of UQCRC1 in maintaining brain functions.
In the present study, we found that mice with Uqcrc1 disruption (heterozygous mice) had decreased both mRNA and protein expression of UQCRC1 in the left ventricular myocardia. These mice had decreased tolerance to acute exhaustive exercise, poor cardiac function (typically increased LV interventricular septal thicknesses both at systole and diastole, as well as decreased LV volume both at end-systole and end-diastole) and myocardial, including mitochondrial, structural damage, suggesting a crucial role of UQCRC1 in maintaining cardiac functions.
Mitochondrion has been widely thought to play a vital part in cardioprotection, and it has been considered as the common target for several cardioprotective effects, such as ischemic preconditioning, pharmacological preconditioning and postconditioning.17–19 Accumulating evidence shows that changes of UQCRC1 expression may directly influence mitochondria structure and function, which has been proved to be necessary to maintain mitochondrial membrane potential, complex III activity, and ATP production.6,15,16 Any pathophysiological change or disruption of these processes may cause tissues or organs very vulnerable to injury,4,5,35 which may be a mechanism for the decreased cardiac tolerance to AEE in UQCRC1+/− mice. Furthermore, disruption of one Uqcrc1 allele leads to myocardial including mitochondrial, structural damage, resulting in increased LV interventricular septal thicknesses both at systole and diastole, as well as decreased LV volume both at end-systole and end-diastole, and this may directly influence blood and energy supply and may also explain the decreased cardiac tolerance to AEE. These findings indicate a crucial role of UQCRC1 in maintaining cardiac functions, along with previous findings that Uqcrc1 knockout is lethal and the crucial role of UQCRC1 in maintaining brain functions, suggesting that UQCRC1 is an indispensable component of complex III and plays a crucial part in maintaining life and organ functions. Of course, future studies are necessary to further verify this suggestion.
In conclusion, our findings suggest that UQCRC1 contributes to cardiac tolerance to acute exhaustive exercise in mice, offering new proof for the crucial role of UQCRC1 in maintaining cardiac functions in in vivo models, and also providing a new insight into its biological functions, which may be an indispensable component of mitochondria complex III, and playing a crucial role in maintaining life and organ functions.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Xiaotang Fan for guidance of experiments design and thank Prof. Kebing Zhang for advice in experimental methods.
AUTHORS’ CONTRIBUTIONS: All authors took part in the design of the study, review of the manuscript, and data analysis; TTY and HFC carried out the experiments and wrote the manuscript; JZ, ZHL, and YL supplied main reagents and animals; ZXW, TTP, and MY contributed to data analysis; HL conceived the project and supervised the study.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [Grant no. 81070094] and Natural Science Foundation of Chongqing [Grant no. CSTC2019jcyj-zdxmX0001].
ORCID iD: Tingting Yi https://orcid.org/0000-0001-6803-4272
References
- 1.Schulte U, Arretz M, Schneider H, Tropschug M, Wachter E, Neupert W, Weiss H. A family of mitochondrial proteins involved in bioenergetICS and biogenesis. Nature 1989; 339:147–9 [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GG, Lee S, Christiano AM, Chung-Honet LC, Cheng W, Katchman S, Uitto J, Greenspan DS. Complete coding sequence, intron/exon organization, and chromosomal location of the gene for the core I protein of human ubiquinol-cytochrome c reductase. J Biol Chem 1993; 268:21113–9 [PubMed] [Google Scholar]
- 3.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y, Consortium F, I RGERGP, Team II. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature 2002; 420:563–73 [DOI] [PubMed] [Google Scholar]
- 4.Xia D, Esser L, Tang WK, Zhou F, Zhou Y, Yu L, Yu CA. Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochim Biophys Acta 2013; 1827:1278–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Vizarra E, Zeviani M. Nuclear gene mutations as the cause of mitochondrial complex III deficiency. Front Genet 2015; 6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan W, Li J, Xu W, Li H, Zuo Z. Critical role of UQCRC1 in embryo survival, brain ischemic tolerance and normal cognition in mice. Cell Mol Life Sci 2019; 76:1381–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CH, Tsai PI, Lin HY, Hattori N, Funayama M, Jeon B, Sato K, Abe K, Mukai Y, Takahashi Y, Li Y, Nishioka K, Yoshino H, Daida K, Chen ML, Cheng J, Huang CY, Tzeng SR, Wu YS, Lai HJ, Tsai HH, Yen RF, Lee NC, Lo WC, Hung YC, Chan CC, Ke YC, Chao CC, Hsieh ST, Farrer M, Wu RM. Mitochondrial UQCRC1 mutations cause autosomal dominant parkinsonism with polyneuropathy. Brain 2020; 143:3352–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagano M, Ceresoli LG, Zucali PA, Pasello G, Garassino M, Grosso F, Tiseo M, Soto Parra H, Zanelli F, Cappuzzo F, Grossi F, De Marinis F, Pedrazzoli P, Gnoni R, Bonelli C, Torricelli F, Ciarrocchi A, Normanno N, Pinto C. Mutational profile of malignant pleural mesothelioma (MPM) in the phase II RAMES study. Cancers 2020; 12:2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CH, Chen PL, Tai CH, Lin HI, Chen CS, Chen ML, Wu RM. A clinical and genetic study of early-onset and familial parkinsonism in Taiwan: an integrated approach combining gene dosage analysis and next-generation sequencing. Mov Disord 2019; 34:506–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Wubulikasimu G, Zhao X, Wang C, Liu R, Wang L, Zhu X, Chen Z. UQCRC1 downregulation is correlated with lymph node metastasis and poor prognosis in CRC. Eur J Surg Oncol 2019; 45:1005–10 [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Li M, Gan Y, Jiang S, Qiao J, Zhang W, Fan Y, Shen Y, Song Y, Meng Z, Yao M, Gu J, Zhang Z, Tu H. Mitochondrial protein UQCRC1 is oncogenic and a potential therapeutic target for pancreatic cancer. Theranostics 2020; 10:2141–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellinger J, Gromes A, Poss M, Bruggemann M, Schmidt D, Ellinger N, Tolkach Y, Dietrich D, Kristiansen G, Muller SC. Systematic expression analysis of the mitochondrial complex III subunits identifies UQCRC1 as biomarker in clear cell renal cell carcinoma. Oncotarget 2016; 7:86490–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HB, Cadete VJ, Sawicka J, Wozniak M, Sawicki G. Effect of the myosin light chain kinase inhibitor ML-7 on the proteome of hearts subjected to ischemia-reperfusion injury. J Proteomics 2012; 75:5386–95 [DOI] [PubMed] [Google Scholar]
- 14.Wong R, Aponte AM, Steenbergen C, Murphy E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol 2010; 298:H75–H91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi T, Wu X, Long Z, Duan G, Wu Z, Li H, Chen H, Zhou X. Overexpression of ubiquinol-cytochrome c reductase core protein 1 may protect H9c2 cardiac cells by binding with zinc. BioMed Res Int 2017; 2017:1314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long Z, Duan G, Li H, Yi T, Wu X, Chen F, Wu Z, Gao Y. Ubiquinol-cytochrome c reductase core protein 1 may be involved in delayed cardioprotection from preconditioning induced by diazoxide. PloS One 2017; 12:e0181903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 2007; 1767:1007–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol 2011; 3:186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibanuma M, Inoue A, Ushida K, Uchida T, Ishikawa F, Mori K, Nose K. Importance of mitochondrial dysfunction in oxidative stress response: a comparative study of gene expression profiles. Free Radic Res 2011; 45:672–80 [DOI] [PubMed] [Google Scholar]
- 20.Kriaucionis S, Paterson A, Curtis J, Guy J, Macleod N, Bird A. Gene expression analysis exposes mitochondrial abnormalities in a mouse model of Rett syndrome. Mol Cell Biol 2006; 26:5033–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unni S, Thiyagarajan S, Srinivas Bharath MM, Padmanabhan B. Tryptophan oxidation in the UQCRC1 subunit of mitochondrial complex III (ubiquinol-cytochrome C reductase) in a mouse model of myodegeneration causes large structural changes in the complex: a molecular dynamics simulation study. Sci Rep 2019; 9:10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D, Chen K, Li J, Fang Z, Pang H, Yin Y, Rong X, Guo J. Gut microbiota combined with metabolomics reveals the metabolic profile of the normal aging process and the anti-aging effect of FuFang Zhenshu TiaoZhi (FTZ) in mice. Biomed Pharmacother 2020; 121:109550. [DOI] [PubMed] [Google Scholar]
- 23.Li ZL, Hu J, Li YL, Xue F, Zhang L, Xie JQ, Liu ZH, Li H, Yi DH, Liu JC, Wang SW. The effect of hyperoside on the functional recovery of the ischemic/reperfused isolated rat heart: potential involvement of the extracellular signal-regulated kinase 1/2 signaling pathway. Free Radic Biol Med 2013; 57:132–40 [DOI] [PubMed] [Google Scholar]
- 24.Jia W, Jian Z, Li J, Luo L, Zhao L, Zhou Y, Tang F, Xiao Y. Upregulated ATF6 contributes to chronic intermittent hypoxia-afforded protection against myocardial ischemia/reperfusion injury. Int J Mol Med 2016; 37:1199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusic M, Schottmann G, Feichtinger RG, Du C, Scholz C, Wagner M, Mayr JA, Lee CY, Yepez VA, Lorenz N, Morales-Gonzalez S, Panneman DM, Rotig A, Rodenburg RJT, Wortmann SB, Prokisch H, Schuelke M. Bi-Allelic UQCRFS1 variants are associated with mitochondrial complex III deficiency, cardiomyopathy, and alopecia totalis. Am J Hum Genet 2020; 106:102–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, Smeitink JA. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun 2000; 275:63–8 [DOI] [PubMed] [Google Scholar]
- 27.Castro-Gago M, Eiris J, Pintos E, Rodrigo E, Blanco-Barca O, Campos Y, Arenas J. [ Benign congenital myopathy associated with a partial deficiency of complexes I and III of the mitochondrial respiratory chain. Rev Neurol 2000; 31:838–41. [PubMed] [Google Scholar]
- 28.Johns DR, Neufeld MJ. Cytochrome b mutations in Leber hereditary optic neuropathy. Biochem Biophys Res Commun 1991; 181:1358–64 [DOI] [PubMed] [Google Scholar]
- 29.Andreu AL, Checcarelli N, Iwata S, Shanske S, DiMauro S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr Res 2000; 48:311–14 [DOI] [PubMed] [Google Scholar]
- 30.Marin-Garcia J, Hu Y, Ananthakrishnan R, Pierpont ME, Pierpont GL, Goldenthal MJ. A point mutation in the cytb gene of cardiac mtDNA associated with complex III deficiency in ischemic cardiomyopathy. Biochem Mol Biol Int 1996; 40:487–95 [DOI] [PubMed] [Google Scholar]
- 31.Miyake N, Yano S, Sakai C, Hatakeyama H, Matsushima Y, Shiina M, Watanabe Y, Bartley J, Abdenur JE, Wang RY, Chang R, Tsurusaki Y, Doi H, Nakashima M, Saitsu H, Ogata K, Goto Y., Matsumoto N. Mitochondrial complex III deficiency caused by a homozygous UQCRC2 mutation presenting with neonatal-onset recurrent metabolic decompensation. Hum Mutat 2013; 34:446–52 [DOI] [PubMed] [Google Scholar]
- 32.Tegelberg S, Tomasic N, Kallijarvi J, Purhonen J, Elmer E, Lindberg E, Nord DG, Soller M, Lesko N, Wedell A, Bruhn H, Freyer C, Stranneheim H, Wibom R, Nennesmo I, Wredenberg A, Eklund EA, Fellman V. Respiratory chain complex III deficiency due to mutated BCS1L: a novel phenotype with encephalomyopathy, partially phenocopied in a Bcs1l mutant mouse model. Orphanet J Rare Dis 2017; 12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ardissone A, Granata T, Legati A, Diodato D, Melchionda L, Lamantea E, Garavaglia B, Ghezzi D, Moroni I. Mitochondrial complex III deficiency caused by TT. C19 defects: report of a novel mutation and review of literature. JIMD Rep 2015; 22:115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuppen HA, Fehmi J, Czermin B, Goffrini P, Meloni F, Ferrero I, He L, Blakely EL, McFarland R, Horvath R, Turnbull DM, Taylor RW. Long-term survival of neonatal mitochondrial complex III deficiency associated with a novel BCS1L gene mutation. Mol Genet Metab 2010; 100:345–8 [DOI] [PubMed] [Google Scholar]
- 35.Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999; 79:1431–568 [DOI] [PubMed] [Google Scholar]