Key Points
Question
Are benefits and harms of prostate cancer screening associated with the use of cholesterol-lowering statin drugs?
Findings
In this post hoc subgroup analysis of a cohort of 78 606 men in the Finnish Randomized Study of Prostate Cancer Screening, prostate-specific antigen screening was associated with a lower incidence of advanced prostate cancer regardless of statin use but was associated with a smaller increase in detection of low-grade tumors among statin users. The association between screening and prostate cancer mortality did not differ by statin use.
Meaning
In this study, prostate-specific antigen–based prostate cancer screening was associated with less overdiagnosis of low-risk cancer among statin users, with similar mortality outcomes as in nonusers.
This post hoc subgroup analysis of a cohort from a population-based randomized clinical trial investigates the outcomes of prostate-specific antigen screening for prostate cancer among men who concomitantly use statin drugs.
Abstract
Importance
Prostate-specific antigen (PSA) screening for prostate cancer has resulted in a slight reduction in prostate cancer mortality but also a concomitant overdiagnosis of low-risk tumors. Prostate-specific antigen levels are affected by use of cholesterol-lowering statin drugs, but the association of statin use with PSA screening performance is unknown.
Objective
To investigate whether statin use was associated with outcomes of a randomized PSA-based prostate cancer screening intervention.
Design, Setting, and Participants
This post hoc subgroup analysis of a cohort from a population-based randomized clinical trial used data from the population-based Finnish Randomized Study of Prostate Cancer Screening, which randomized men to PSA screening or routine care from March 1, 1996, to December 31, 1999, with follow-up continuing until December 31, 2015. The population included all men aged 55 to 67 years at baseline and residing in the Tampere or Helsinki districts of Finland. Information on statin purchases from 1996 to 2009 was obtained from a national prescription registry. Eligible men were identified from the population registry of Finland. Prevalent prostate cancer cases at baseline were excluded. Data were analyzed from January 1, 2019 to March 31, 2021.
Interventions
Three invitations for PSA screening at 4-year intervals from 1996 to 2007 vs routine care.
Main Outcomes and Measures
Risk for prostate cancer overall, high-risk disease, and prostate cancer mortality in the screening group vs the control group as an intention-to-treat analysis. The analysis was stratified by statin use.
Results
The study comprised 78 606 men (median age, 59 years [range, 55-67 years]) with statin purchase data available. Although PSA screening was associated with increased prostate cancer incidence among statin nonusers (screening vs control, 11.2 vs 8.6 per 1000 person-years); rate ratio [RR], 1.31; 95% CI, 1.24-1.38), no similar increase in incidence was observed among statin users (6.9 vs 5.9 per 1000 person-years; RR, 1.02; 95% CI, 0.95-1.10; P < .001 for interaction). Incidence of low-risk (Gleason score 6) and localized tumors was lower among statin users, whereas detection of tumors with a Gleason score of 8 to 10 was similar. Screening was associated with a lower incidence of metastatic tumors regardless of statin use.
Conclusion and Relevance
In this post hoc subgroup analysis of a cohort from a population-based randomized clinical trial, PSA screening among statin users was associated with a decreased incidence of advanced prostate cancer that was similar among statin nonusers, but with less increase in detection of low-grade localized tumors in statin users than in nonusers. These findings suggest that statin use does not materially compromise benefits of PSA-based screening.
Introduction
Systematic screening for prostate cancer (PCa) has resulted in overdiagnosis of low-risk tumors and only a modest reduction in PCa mortality.1,2 Screening is considered beneficial for early detection of clinically significant PCa and decreasing PCa mortality.2 The Finnish Randomized Study of Screening for Prostate Cancer (FinRSPC), the largest component of the European Randomized Study of Screening for Prostate Cancer, reported no statistically significant benefit in PCa mortality by systematic prostate-specific antigen (PSA)-based screening.3 However, the benefit became clearer after a minimum of 3 screenings.4 Currently, the harms of systematic PSA-based PCa screening are considered to exceed the benefits by causing unnecessary diagnoses of clinically insignificant cancers.1
Serum PSA is influenced by benign conditions such as prostatic inflammation.5 Some commonly used medications, including statins, influence PSA level.6 Statins inhibit endogenous cholesterol production7 and block protein prenylation, potentially influencing PCa cell proliferation and migration.8 Statins may lower PSA by reducing intraprostatic inflammation or by inhibiting androgen signaling.9 Reduction in PSA levels among statin users has been observed in many studies.10,11,12,13,14 However, not all studies report a PSA reduction.15 In a randomized, placebo-controlled clinical trial,16 short-term, high-dose atorvastatin lowered PSA levels compared with placebo in men with high-grade PCa, but not overall. Another trial17 with a small single intervention group reported a nonsignificant 12% decrease in PSA after fluvastatin intervention. The possible influence of statins on PSA levels could result from improved accuracy of PSA screening in detection of clinically relevant cancer owing to lower PSA levels among statin users, with fewer prostate biopsies for borderline PSA elevations and a reduction in detection of clinically irrelevant cancers. On the other hand, lower PSA levels could presumably also lead to delayed detection of PCa, causing cancers to be diagnosed more often at an advanced stage.
The purpose of this study was to investigate the association of PSA-based screening on PCa incidence and mortality separately among statin users and nonusers.
Methods
This post hoc subgroup analysis of a cohort from the FinRSPC population included 80 458 men. The population characteristics have been described before.2,3,4 The study protocol was reviewed and approved by the ethics committee of the Pirkanmaa Hospital District. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
During the period from March 1, 1996, to December 31, 1999, all men aged 55, 59, 63, or 67 years at baseline and living in the Pirkanmaa or Helsinki districts were identified in the Finnish Population Registry and randomized to the screening or control groups. Men with prevalent PCa diagnosis at baseline were excluded. The remaining men were randomized into 2 groups: the screening group (31 872 men) and the control group (48 295 men). Men in the screening group gave written informed consent for participation, whereas men in the control group were followed via national registries using routinely collected data; thus, informed consent was not required according to Finnish regulations.
The screening group was invited to consecutive PSA screenings at 4-year intervals. Screening invitations stopped at PCa diagnosis, emigration from the study area, or at the age of 71 years. If PSA levels were greater than or equal to 4 ng/mL, men were invited to their local urological clinic for digital rectal examination, prostate ultrasonography, and prostate biopsy. Information on opportunistic PSA tests outside of the screening protocol was obtained from leading local laboratories. Tests for PSA that were outside the screening protocol were attended by 36 149 men.
Information on PCa diagnoses was obtained from the population-based nationwide Finnish Cancer Registry. Information about the date of diagnosis; Gleason grade; and tumor, node, metastasis (TNM) stage (both available for more than 99% of participants) was collected from medical records. Additional information about weight and height was collected by sending a questionnaire to men in the screening group along with the third-round screening invitations. Calculated body mass index was available for 11 698 men (14.9% of the total study cohort). Information about socioeconomic status and employment was obtained from Statistics Finland and was available for 77 072 men (98%). Finland has a largely homogenous White population; as a result, this was the only race available for inclusion in this study.
Information about PCa deaths was obtained from the cause-of-death registry maintained by Statistics Finland (license No. TK-53-1330-18), which was collected from mandatory death certificates. Information on deaths and PCa cases was available until December 31, 2015. Records contained information about the causes and dates of death. The FinRSPC cause-of-death committee evaluated the accuracy of PCa deaths recorded by the cause-of-death registry, regardless of the randomization group. Accuracy of all PCa deaths that occurred between 1996 and 2003 were evaluated based on medical records. Records of PCa deaths were found to be 97% accurate.3
Statin Use
Finnish citizens are entitled to reimbursements for purchases of physician-prescribed medication as part of the national health insurance provided by the Social Insurance Institution of Finland.18 All reimbursed purchases are recorded in the national prescription database. The study population was linked to the database to obtain information about medication purchases during the 1996 to 2009 period. The data were available for 78 606 men, 98% of the FinRSPC study population. Only medication use before PCa diagnosis or by December 31, 2009 (men free of PCa), was included in the analysis. Later medication use was not included, as the screening intervention stopped by December 31, 2008. Information about medication purchases included the date of each purchase, dose, number of doses, and number of packages purchased. The registry does not record over-the-counter medication purchases or drugs administered during hospitalization. Statins are available in Finland by prescription only; thus, they are comprehensively recorded by the registry.
Dose response by yearly amount of statin use was evaluated by stratifying the analysis of the association of screening with PCa incidence and mortality by tertiles of intensity of statin use (ie, the mean annual purchased amount of defined daily doses of statins). The defined daily dose is the drug-specific mean dose per day for a drug used for its main indication in adults.19 Intensity of statin use was calculated by dividing cumulative defined daily doses of all statin purchases with cumulative number of years of usage. Doses were divided by tertiles (low = mean <0.65 defined dose/d; medium = mean 0.65-1.08 defined dose/d; high = mean >1.08 defined dose/d).
Statistical Analysis
Median PSA values among men in the screening group were calculated separately for statin users and nonusers. The Mann-Whitney U test was used to evaluate statistical significance of the difference between users and nonusers.
Prostate cancer incidence and mortality were analyzed as randomized, comparing men in the screening group with those in the control group to assess rate ratios (RRs) and 95% CIs for PCa incidence and PCa-specific mortality. The intention-to-treat principle was used in the analyses, that is, men randomized to the screening group were analyzed in this group regardless of whether they actually participated in the screening. Analyses were performed separately among statin users and nonusers. Only statin use before PCa diagnosis was included in the analysis.
Poisson regression was used to analyze the association of screening on PCa incidence and mortality. Men in the control group were used as the reference. To ensure equal length of follow-up for the entire study population, the common closing date was December 31, 2012, for men randomized in 1996; December 31, 2013, for men randomized in 1997; December 31, 2014, for men randomized in 1998; and December 31, 2015, for men randomized in 1999. The maximum follow-up time was 17 years. Follow-up time continued until PCa diagnosis, PCa death, emigration from the study area, or upon reaching the common closing date. The risk of PCa was analyzed overall, by Gleason score (categorized as Gleason 6, 7, or 8-10) and by TNM stage (categorized as localized disease; T1-T3aNx/0Mx/0 or advanced T3b-T4, all N1, all M1 cases). In total, 962 men were lost to follow-up owing to emigration from Finland.
The role of opportunistic PSA testing outside the systematic screening protocol was evaluated in sensitivity analyses limited to men with at least 1 recorded PSA measurement before the first FinRSPC screening. Thus, the subgroup aimed to eliminate the difference in opportunistic testing by statin use.
All reported P values were 2-sided. The threshold for statistical significance was P ≤ .05. Graph compilation for the figure creation for PSA distribution and Poisson regression analyses were performed using Stata software, version 16.0 (StataCorp). Data were analyzed from January 1, 2019, to March 31, 2021.
Results
Population Characteristics
The study population included 78 606 men. In the screening group, 12 059 men (38%) were statin ever-users; in the control group, 19 567 (41%) were statin ever-users. The median age at randomization was 59 years (range, 55-67 years) in both trial arms. A higher percentage of statin users were employed compared with nonusers in both the screening and control groups. Statin users were more likely to participate in the first screening and had more opportunistic screening tests taken outside the study protocol compared with nonusers. However, the proportions of men who tested positive and who were compliant with undergoing prostate biopsy were slightly lower among statin users than nonusers (Table 1).
Table 1. Descriptive Characteristicsa.
| Characteristic | Screening group (n = 30 336) | Control group (n = 48 270) | ||
|---|---|---|---|---|
| Statin users | Statin nonusers | Statin users | Statin nonusers | |
| No. of participants (n = 30 336) | 12 059 | 18 277 | 19 567 | 28 703 |
| Person-years | 179 441 | 231 251 | 293 675 | 369 184 |
| Baseline characteristics | ||||
| Age at randomization, median (IQR), y | 59 (55-63) | 59 (55-63) | 59 (55-63) | 59 (55-63) |
| Duration of follow-up until PCa diagnosis, median (IQR), y | 17 (13-19) | 17 (9-19) | 17 (14-19) | 17 (9-18) |
| Duration of follow-up until death, median (IQR), y | 18 (16-19) | 17 (12-19) | 18 (17-19) | 17 (11-19) |
| BMI, median (IQR)b | 27.4 (24.3-29.7) | 26.4 (23.9-28.4) | NA | NA |
| Employed, No. (%)c | 5885 (48.8) | 8421 (46.1) | 9433 (48.2) | 13 249 (46.2) |
| Screening characteristics | ||||
| Median (IQR) PSA level, ng/mL | 1.18 (0.68-2.14) | 1.28 (0.74-2.41) | NA | NA |
| Compliance to screening 1st round, No. (%) | 8970 (74.4) | 11 814 (65.0) | NA | NA |
| Positive screening result, No. (%) | 619 (5.1) | 1358 (7.4) | NA | NA |
| Screen-positive men who subsequently had prostate biopsy, No. (%) | 578 (93.4) | 1299 (95.7) | NA | NA |
| Proportion of men with any opportunistic PSA screening before 1st study screening, No. (%) | 476 (3.9) | 689 (3.8) | 671 (3.4) | 880 (3.1) |
| Outcomes | ||||
| PCa diagnoses during follow-up, No. (%) | 1072 (8.9) | 2600 (14.3) | 1718 (8.8) | 3172 (11.1) |
| PCa rate per 1000 person-years | 6.0 | 11.2 | 5.9 | 8.6 |
| PCa mortality, No. (%) | 75 (0.6) | 212 (1.2) | 125 (0.6) | 372 (1.3) |
| PCa mortality rate per 1000 person-years | 0.4 | 0.9 | 0.4 | 1.0 |
| All deaths, No. (%) | 2933 (24.3) | 5846 (32.0) | 4652 (23.8) | 9764 (34.0) |
| Death rate per 1000 person-years | 16.3 | 25.3 | 15.8 | 26.4 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; PCa, prostate cancer; PSA, prostate-specific antigen.
Study population of 78 606 men from the Finnish Randomized Study of Screening for Prostate Cancer.
BMI available for 11 698 men in the screening group.
Men who were employed, students, or entrepreneurs.
PSA Distribution and Cumulative Incidence of PCa and Mortality by Statin Use
Statin users had lower median PSA values (1.18 ng/mL [IQR, 0.68-2.14 ng/mL]) than nonusers (1.28 ng/mL [IQR, 0.74-2.41 ng/mL]; P < .001). The distribution of PSA values followed a similar pattern among statin users and nonusers (Table 1; eFigure 1 in the Supplement).
Overall, PCa cases detected among men in the screening group were more often Gleason 6 tumors (incidence of Gleason 6 tumors was 5.4 of 1000 person-years and 3.4 of 1000 person-years in the screening group and the control group, respectively) and less often metastatic compared with PCa cases (incidence of metastatic PCa was 0.75 of 1000 person-years and 0.89 of 1000 person-years in the screening group and the control group, respectively) arising in the control group (eTable 1 in the Supplement). In both study groups, the proportion of metastatic PCa cases was lower among statin users (ie, 6% vs 8% of cases detected in the screening group among users and nonusers of statins, respectively; in the control arm, proportions were 9% vs 13%).
Cumulative PCa incidence increased along with time in both FinRSPC trial groups, although the influence of 3 screening rounds was visible among men in the screening group (Figure 1). Incidence was lower among statin users in both trial groups, although it increased at the same pace over time regardless of statin use (overall PCa incidence in the screening group was 6.0 of 1000 person-years and 11.2 of 1000 person-years among statin users and nonusers, respectively; control group, 5.9 of 1000 person-years and 8.6 of 1000 person-years, respectively).
Figure 1. Association of Screening With Cumulative Prostate Cancer Incidence and Mortality, Stratified by Statin Use .
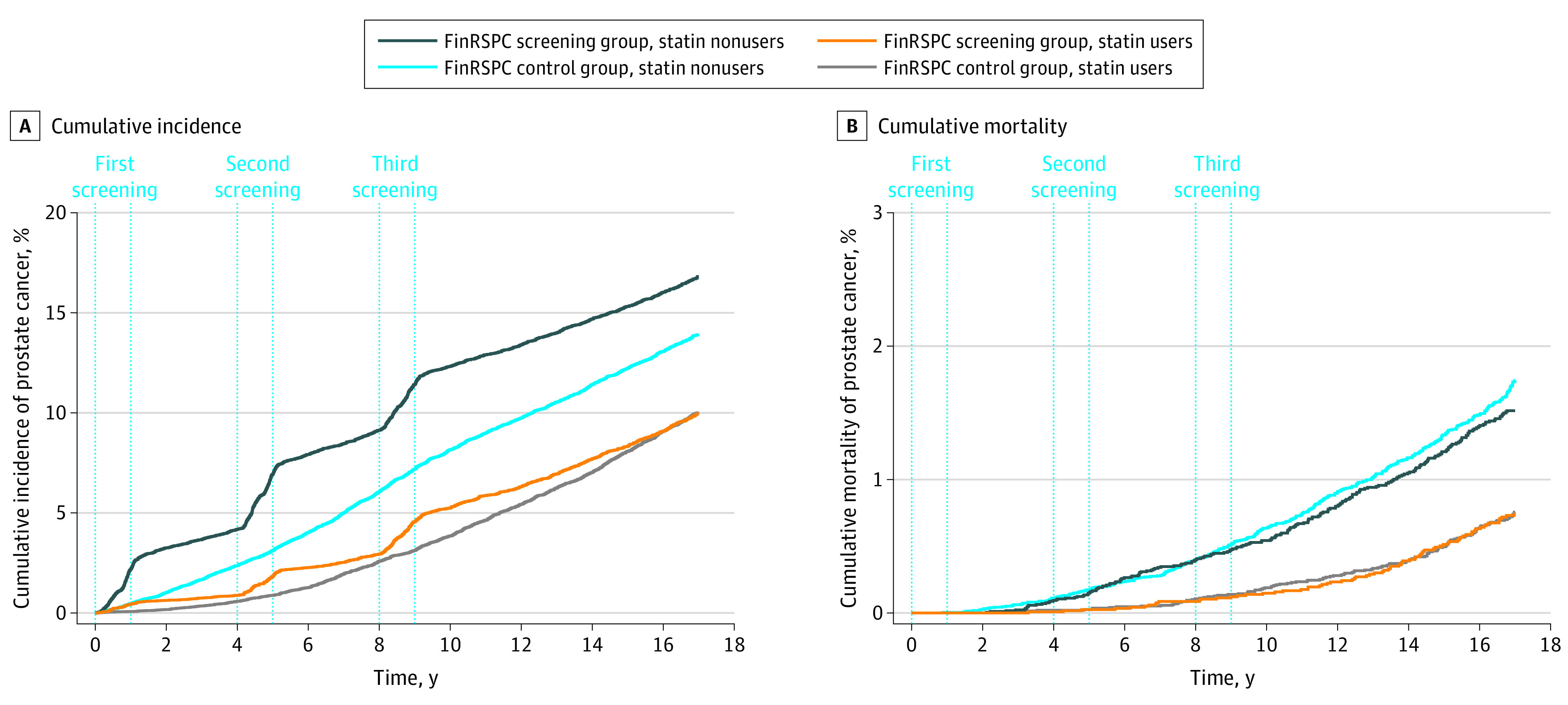
Study population of 78 606 men from the Finnish Randomized Study of Screening for Prostate Cancer (FinRSPC). Cumulative incidence (A) and cumulative mortality (B) of prostate cancer.
Prostate cancer mortality remained lower among statin users than nonusers in both FinRSPC trial groups (Figure 1). Also, accumulation of PCa deaths was slower among statin users regardless of the trial group. In nonusers, PCa mortality curves differed in favor of the screening group after 10 years of follow-up. Among statin users, no such difference between trial groups was observed. Cumulative PCa mortality 15 years after randomization for statin nonusers was 0.76 of 1000 person-years in the screening group and 0.84 of 1000 person-years in the control group, whereas among statin users, mortality in the screening and control groups was 0.32 of 1000 person-years and 0.31 of 1000 person-years, respectively.
Association of Screening With Overall PCa Risk and Risk of Clinically Insignificant Cancers by Statin Use
In total, 8562 cases of PCa were diagnosed. In the screening group, PCa rate per 1000 person-years was higher among statin nonusers in both the screening and control group (11.2 per 1000 person-years and 8.6 per 1000 person-years, respectively) compared with statin users (6.0 per 1000 person-years and 5.9 per 1000 person-years, respectively). Opportunistic PSA tests were more common among statin users in both the screening group and the control group (Table 1).
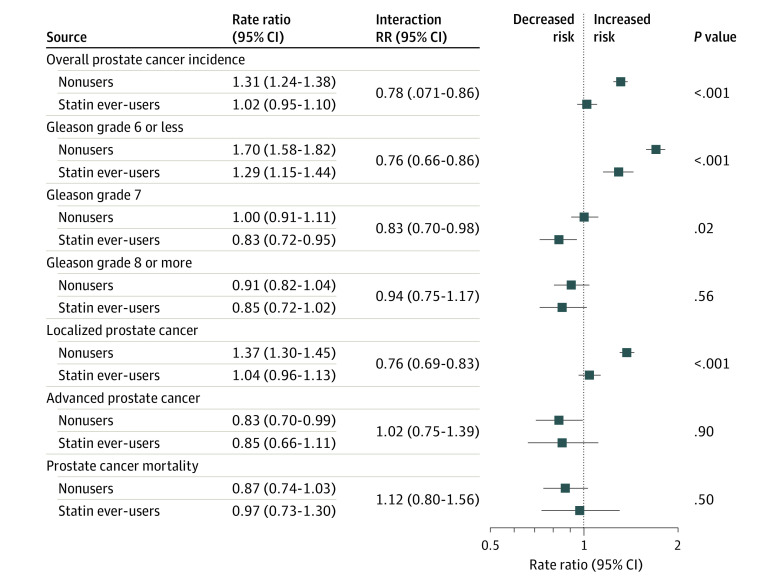
Screening increased overall PCa incidence among nonusers (RR, 1.31; 95% CI, 1.24-1.38), whereas among statin users, screening did not increase the risk for PCa overall (RR, 1.02; 95% CI, 0.95-1.10; P < .001 for interaction) (Table 1, Figure 2; eTable 2 in the Supplement).
Figure 2. Association of Screening With Various Outcomes, Stratified by Statin Use.
Incidence of prostate cancer overall and clinically nonsignificant disease as defined by Gleason score; tumor, node, metastasis (TNM) stage; and mortality. Study population of 78 606 men from the Finnish Randomized Study of Screening for Prostate Cancer.
The screening group had an increased risk for Gleason 6 tumors compared with the control group regardless of statin use, but this difference was smaller among statin users. For localized tumors, the screening group saw an increased risk among statin nonusers compared with the control group, but not among statin users. The screening group had an increased risk for Gleason 7 cancers among nonusers, but statin users in the screening group had a lower risk for Gleason 7 cancer compared with users in the control group (1.9 vs 2.2 per 1000 person-years; RR, 0.83; 95% CI, 0.72-0.95; P = .02 for interaction) (Figure 2; eTable 2 in the Supplement).
Association of Screening With Risk of Aggressive and Advanced PCa and PCa-Specific Mortality
Overall, statin use did not modify the association of screening with Gleason scores 8 to 10 and advanced PCa (Figure 2; eTable 2 in the Supplement). Screening had no statistically significant association with PCa mortality (RR, 0.87; 95% CI, 0.73-1.03; P = .50) (Figure 2; eTable 3 in the Supplement). Statin use did not significantly modify this risk association, and the point estimate was close to unity among statin users.
Contamination by Opportunistic PSA Testing
The incidence of opportunistic PSA testing outside the systematic FinRSPC screening protocol was evaluated by stratifying the analysis to men with at least 1 recorded PSA test apart from the FinRSPC testing or no opportunistic screening tests at all before the first screening. Among men with 1 or more opportunistic screening tests, screening was associated with an increased detection of PCa among nonusers compared with statin users (28.4 vs 16.2 per 1000 person-years; RR, 1.72; 95% CI, 1.39-2.12 and 11.8 vs 7.2 per 1000 person-years; RR, 1.66; 95% CI, 1.20-2.30; respectively).
The findings were similar among men with no opportunistic screening tests, suggesting that association modification by statin use was not related to differences in opportunistic testing (Table 2).
Table 2. Association of Screening With Prostate Cancer Incidence Overall and Prostate Cancer–Specific Mortality Among Statin Users and Nonusers by 1 or More or No Opportunistic Prostate-Specific Antigen–Tests Takena.
| Statin use status | PCa incidence overall by study group (Ref = control group) | PCa mortality by study group (Ref = control group) | ||||||
|---|---|---|---|---|---|---|---|---|
| One or more opportunistic PSA tests | No opportunistic PSA tests | One or more opportunistic PSA tests | No opportunistic PSA tests | |||||
| RR (95% CI) | P value for interaction | RR (95% CI) | P value for interaction | RR (95% CI) | P value for interaction | RR (95% CI) | P value for interaction | |
| Nonuser of statins | 1.72 (1.39-2.12) | NA | 1.28 (1.21-1.35) | NA | 1.39 (0.71-2.73) | NA | 0.83 (0.70-0.99) | NA |
| Any use of statins | 1.66 (1.20-2.30) | .82 | 0.99 (0.92-1.07) | <.001 | 3.87 (1.23-12.1) | .13 | 0.86 (0.64-1.17) | .86 |
| <0.65 defined dose/d | 1.95 (0.20-3.16) | .65 | 0.92 (0.81-1.04) | <.001 | 5.94 (1.26-28.0) | .09 | 0.65 (0.39-1.06) | .33 |
| 0.65-1.08 defined dose/d | 1.88 (1.01-3.50) | .82 | 1.07 (0.93-1.22) | .01 | NAb | NA | 0.88 (0.53-1.45) | .87 |
| >1.08 defined dose/d | 1.22 (0.65-2.29) | .27 | 1.00 (0.86-1.16) | .003 | 0.62 (0.06-6.88) | .55 | 1.29 (0.71-2.34) | .16 |
Abbreviations: NA, not available; PCa, prostate cancer; PSA, prostate-specific antigen; Ref, reference; RR, rate ratio.
Study population of 78 606 men from the Finnish Randomized Study of Screening for Prostate Cancer.
Convergence not achieved.
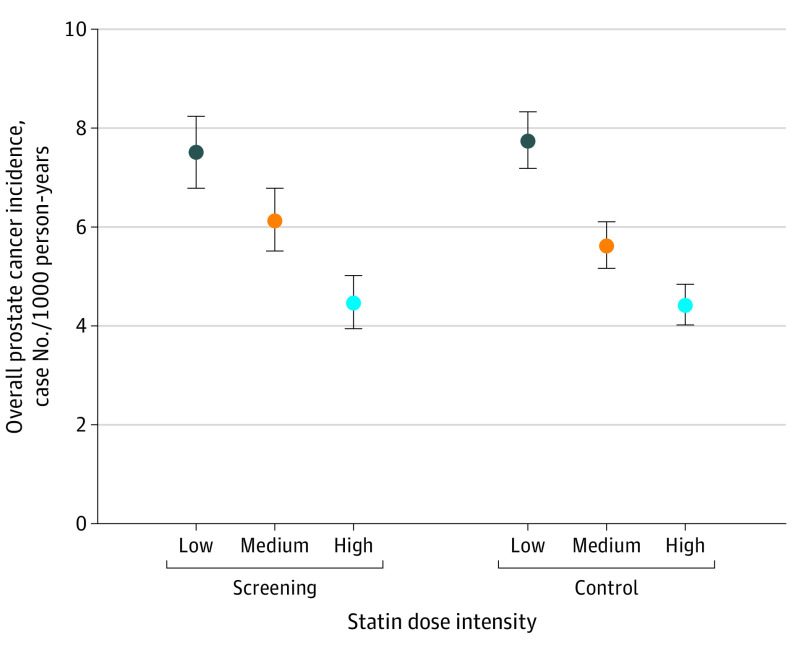
Dose Response
Crude PCa incidence decreased in inverse correlation with intensity of statin use similarly in both trial groups (Figure 3). However, the association of screening on PCa incidence and mortality among statin users remained similar regardless of intensity of usage (eTable 4 in the Supplement).
Figure 3. Prostate Cancer Incidence Among Statin Users by Statin Dose.
Study population of 78 606 men from the Finnish Randomized Study of Screening for Prostate Cancer. Doses divided by tertiles (low = mean <0.65 defined doses/d; medium = mean 0.65-1.08 defined doses/d; high = mean >1.08 defined doses/d). The error bars represent the 95% CIs.
Discussion
Our subgroup analysis of a cohort from the population-based FinRSPC found that, although PSA-based screening had a similar association with incidence of advanced PCa among statin users and nonusers, screening was not associated with an increase in the incidence of low-grade PCa among statin users. Therefore, PSA-based screening may cause less harm among statin users while the benefits remain similar. A nonsignificant reduction in PCa mortality was found among statin nonusers, and no difference was observed in men using statins. To our knowledge, this is the first study to explore outcomes of a randomized PSA-based screening intervention in men using statins.
Statins inhibit 3-hydroxy-3-methylglutal-coenzyme A reductase, thus inhibiting cholesterol biosynthesis.20 Previously suggested direct antitumor effects of statins include inhibition of cell proliferation, inflammation, angiogenesis, invasion, and metastasis, as well as induction of apoptosis and autophagy of PCa cells.9 Cholesterol is essential in the synthesis of androgens that promote PCa growth and PSA secretion.21,22 The fact that other cholesterol-lowering medications have been associated with a lower PCa risk supports an association mediated by reduced serum cholesterol.23
Statin users had a lower median PSA level in our study, concordant with some previous studies.24 One possible mechanism is hemodilution of PSA in men with overweight or obesity.25,26 People with dyslipidemia are 60% to 70% more likely to be obese.27 Statins may also reduce local inflammation in the prostate, which also would lower PSA.28 In vitro models have suggested that statins impair androgen receptor signaling by reducing androgen receptor activity and expression, which results in decreased PSA secretion, cell growth inhibition, and induction of apoptosis.29,30 In a randomized placebo-controlled trial,16 short-term high-dose atorvastatin intervention lowered PSA levels compared with placebo in men with high-grade PCa, but not overall. Another clinical study17 reported a nonsignificant 12% reduction in PSA after fluvastatin intervention.
The possible association of statins with PSA levels may be the result of improved accuracy of PSA screening in the detection of clinically relevant cancer owing to lower PSA levels among statin users with fewer biopsies for borderline PSA elevations and a reduction in detection of clinically irrelevant cancers. A reduction in detection of low-grade tumors among statin users is probably due to lower PSA levels. This possibility is supported by the lower proportion of men who screened positive on PSA testing among statin users. The proportion of men who underwent opportunistic PSA testing outside the trial was larger among statin users in both trial groups. Also, compliance with referrals to prostate biopsy among men who screen positive on PSA testing was slightly lower in statin users. Statin use may be associated with health-conscious behavior and increased health care service use.31 This association could have created a bias toward the null when estimating the outcomes of systematic PSA-based screening in this group.
Participation in opportunistic PSA testing before the first screening did not modify the association between the lower incidence of PCa and screening. The proportion of men who screened positive on PSA testing was only 2% lower among statin users than nonusers in the screening group. Thus, statin use did not necessitate corrections in thresholds for screening positivity or prostate biopsy.
Differences in screening outcomes by statin use did not depend on intensity of statin use. This finding suggests that the difference may not have been caused by statin use directly but rather by systematic differences between statin users and nonusers, or alternatively by factors not related to statin dose. Serum cholesterol is also heavily influenced by other factors, such as dietary fat intake. Therefore, the extent of cholesterol lowering at a given dose varies between individuals, and the association between statins and cholesterol level is not necessarily dose-dependent.
Several previous studies have estimated statin use and prostate cancer risk. Some studies found a decreased overall risk of PCa among statin users,32,33,34,35 whereas others report opposite results.36,37,38,39 Most studies have reported a lower risk of advanced prostate cancer in statin users than nonusers.40 In our study, screening was associated with a decreased incidence of advanced PCa in both statin users and nonusers. Therefore, statin users and nonusers may both benefit from screening, even if the risk of advanced PCa is lower among users.
Strengths and Limitations
The strengths of our study were derived from the large study population undergoing the randomized screening intervention, with an intention-to-treat analysis that showed results by assigned intervention group. The data on medication usage were exceptionally detailed and available for the entire population-based study cohort. Information about cancer cases, Gleason grade, and metastatic status was comprehensive and reliable. The Finnish population is of largely homogenous European ancestry, which minimizes bias by race. A long follow-up is essential considering the slow progression of PCa. Another important strength is the use of registry-based information on opportunistic PSA tests outside of systematic screening protocol, which allowed for the evaluation of potential contamination.
One limitation is the homogenous Finnish population of European ancestry included in the study population; therefore, the results of this study may not be generalizable to other racial or ethnic groups.
Conclusions
In this post hoc subgroup analysis of a cohort from a population-based randomized clinical trial, PSA-based screening was similarly associated with decreased incidence of advanced PCa among both statin users and nonusers. However, there was less of an increase in detection of low-grade localized tumors in statin users than in nonusers. The PSA screening group saw a reduction in PCa mortality among nonusers of statins, although that reduction was nonsignificant; there was no reduction among statin users.
Prostate cancer screening was associated with less overdiagnosis of low-risk tumors among statin users. Further studies should clarify the association of statin use and reduction in cholesterol levels with PSA screening in men with and without prostate cancer.
eFigure. Distribution of PSA Values by Statin Use Before Prostate Cancer Diagnosis
eTable 1. Descriptive Characteristics Information About Gleason Grades and Metastatic Status by Statin Use
eTable 2. Effect of PSA-Based Screening on Incidence of Prostate Cancer Overall and Clinically Nonsignificant Disease as Defined by Gleason Score and TNM Stage
eTable 3. Effect of Screening on Prostate Cancer–Specific Mortality Among Users and Nonusers of Statins
eTable 4. Prostate Cancer Incidence Among Statin Users by Statin Dose
References
- 1.Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi: 10.1056/NEJMms1914228 [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators . Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981-990. doi: 10.1056/NEJMoa1113135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilpeläinen TP, Tammela TL, Malila N, et al. Prostate cancer mortality in the Finnish randomized screening trial. J Natl Cancer Inst. 2013;105(10):719-725. doi: 10.1093/jnci/djt038 [DOI] [PubMed] [Google Scholar]
- 4.Pakarainen T, Nevalainen J, Talala K, et al. The number of screening cycles needed to reduce prostate cancer mortality in the Finnish section of the European Randomized Study of Prostate Cancer (ERSPC). Clin Cancer Res. 2019;25(2):839-843. doi: 10.1158/1078-0432.CCR-18-1807 [DOI] [PubMed] [Google Scholar]
- 5.Umbehr MH, Gurel B, Murtola TJ, et al. Intraprostatic inflammation is positively associated with serum PSA in men with PSA <4 ng ml(-1), normal DRE and negative for prostate cancer. Prostate Cancer Prostatic Dis. 2015;18(3):264-269. doi: 10.1038/pcan.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieder C, Norum J, Geinitz H. Impact of common medications on serum total prostate-specific antigen levels and risk group assignment in patients with prostate cancer. Anticancer Res. 2011;31(5):1735-1739. [PubMed] [Google Scholar]
- 7.Zhang Q, Dong J, Yu Z. Pleiotropic use of statins as non-lipid-lowering drugs. Int J Biol Sci. 2020;16(14):2704-2711. doi: 10.7150/ijbs.42965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawe DE, Mahmud S. Biologic and epidemiologic evidence assessing if statins prevent prostate cancer. Can J Urol. 2017;24(6):9081-9088. [PubMed] [Google Scholar]
- 9.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46691. doi: 10.1371/journal.pone.0046691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cyrus-David MS, Weinberg A, Thompson T, Kadmon D. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173(6):1923-1925. doi: 10.1097/01.ju.0000158044.94188.88 [DOI] [PubMed] [Google Scholar]
- 11.Murtola TJ, Tammela TL, Määttänen L, et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int J Cancer. 2010;127(7):1650-1659. doi: 10.1002/ijc.25165 [DOI] [PubMed] [Google Scholar]
- 12.Nordström T, Clements M, Karlsson R, Adolfsson J, Grönberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer. 2015;51(6):725-733. doi: 10.1016/j.ejca.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100(21):1511-1518. doi: 10.1093/jnci/djn362 [DOI] [PubMed] [Google Scholar]
- 14.Mener DJ, Cambio A, Stoddard DG, Martin BA, Palapattu GS. The impact of HMG-CoA reductase therapy on serum PSA. Prostate. 2010;70(6):608-615. doi: 10.1002/pros.21095 [DOI] [PubMed] [Google Scholar]
- 15.Mondul AM, Selvin E, De Marzo AM, Freedland SJ, Platz EA. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001-2004. Cancer Causes Control. 2010;21(5):671-678. doi: 10.1007/s10552-009-9494-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murtola TJ, Syvälä H, Tolonen T, et al. Atorvastatin versus placebo for prostate cancer before radical prostatectomy—a randomized, double-blind, placebo-controlled clinical trial. Eur Urol. 2018;74(6):697-701. doi: 10.1016/j.eururo.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 17.Longo J, Hamilton RJ, Masoomian M, et al. A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. 2020;23(4):630-637. doi: 10.1038/s41391-020-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martikainen J, Rajaniemi S. Drug Reimbursement Systems in EU Member States, Iceland and Norway. The Social Insurance Institution; 2002. [Google Scholar]
- 19.World Health Organization. Defined daily dose (DDD). Accessed October 18, 2021. https://www.who.int/tools/atc-ddd-toolkit/about-ddd
- 20.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425-430. doi: 10.1038/343425a0 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368(6):576-577. [DOI] [PubMed] [Google Scholar]
- 22.Murtola TJ, Syvälä H, Pennanen P, et al. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS One. 2012;7(6):e39445. doi: 10.1371/journal.pone.0039445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rompay MI, Solomon KR, Nickel JC, Ranganathan G, Kantoff PW, McKinlay JB. Prostate cancer incidence and mortality among men using statins and non-statin lipid-lowering medications. Eur J Cancer. 2019;112:118-126. doi: 10.1016/j.ejca.2018.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Fung KZ, Freedland SJ, Hoffman RM, Tang VL, Walter LC. Statin medications are associated with a lower probability of having an abnormal screening prostate-specific antigen result. Urology. 2014;84(5):1058-1065. doi: 10.1016/j.urology.2014.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103(5):1092-1095. doi: 10.1002/cncr.20856 [DOI] [PubMed] [Google Scholar]
- 26.Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275-2280. doi: 10.1001/jama.298.19.2275 [DOI] [PubMed] [Google Scholar]
- 27.Feingold KR, Anawalt B, Boyce A, et al. Obesity and Dyslipidemia. MDText.com, Inc; 2000-2020. [PubMed] [Google Scholar]
- 28.Bañez LL, Klink JC, Jayachandran J, et al. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):722-728. doi: 10.1158/1055-9965.EPI-09-1074 [DOI] [PubMed] [Google Scholar]
- 29.Yokomizo A, Shiota M, Kashiwagi E, et al. Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate. 2011;71(3):298-304. doi: 10.1002/pros.21243 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Egger M, Plattner R, Klocker H, Eder IE. Lovastatin causes diminished PSA secretion by inhibiting AR expression and function in LNCaP prostate cancer cells. Urology. 2011;77(6):1508.e1-1508.e7. doi: 10.1016/j.urology.2010.12.074 [DOI] [PubMed] [Google Scholar]
- 31.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348-354. doi: 10.1093/aje/kwm070 [DOI] [PubMed] [Google Scholar]
- 32.Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, Egawa S. The impact of hypertriglyceridemia on prostate cancer development in patients aged ≥60 years. BJU Int. 2012;109(4):515-519. doi: 10.1111/j.1464-410X.2011.10358.x [DOI] [PubMed] [Google Scholar]
- 33.Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010;105(9):1222-1225. doi: 10.1111/j.1464-410X.2009.09007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2226-2232. doi: 10.1158/1055-9965.EPI-07-0599 [DOI] [PubMed] [Google Scholar]
- 35.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103(11):885-892. doi: 10.1093/jnci/djr108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platz EA, Tangen CM, Goodman PJ, et al. Statin drug use is not associated with prostate cancer risk in men who are regularly screened. J Urol. 2014;192(2):379-384. doi: 10.1016/j.juro.2014.01.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer. 2011;47(6):819-830. doi: 10.1016/j.ejca.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: a metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123(4):899-904. doi: 10.1002/ijc.23550 [DOI] [PubMed] [Google Scholar]
- 39.Coogan PF, Kelly JP, Strom BL, Rosenberg L. Statin and NSAID use and prostate cancer risk. Pharmacoepidemiol Drug Saf. 2010;19(7):752-755. doi: 10.1002/pds.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan P, Wei S, Tang Z, et al. LDL-lowering therapy and the risk of prostate cancer: a meta-analysis of 6 randomized controlled trials and 36 observational studies. Sci Rep. 2016;6:24521. doi: 10.1038/srep24521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Distribution of PSA Values by Statin Use Before Prostate Cancer Diagnosis
eTable 1. Descriptive Characteristics Information About Gleason Grades and Metastatic Status by Statin Use
eTable 2. Effect of PSA-Based Screening on Incidence of Prostate Cancer Overall and Clinically Nonsignificant Disease as Defined by Gleason Score and TNM Stage
eTable 3. Effect of Screening on Prostate Cancer–Specific Mortality Among Users and Nonusers of Statins
eTable 4. Prostate Cancer Incidence Among Statin Users by Statin Dose