Abstract
Nanotechnology plays a significant role in the field of medicine and in drug delivery, mainly due to the major limitations affecting the conventional pharmaceutical agents, and older formulations and delivery systems. The effect of nanotechnology on healthcare is already being felt, as various nanotechnology applications have been developed, and several nanotechnology-based medicines are now on the market. Across many parts of the world, nanotechnology draws increasing investment from public authorities and the private sector. Most conventional drug-delivery systems (CDDSs) have an immediate, high drug release after administration, leading to increased administration frequency. Thus, many studies have been carried out worldwide focusing on the development of pharmaceutical nanomedicines for translation into products manufactured by local pharmaceutical companies. Pharmaceutical nanomedicine products are projected to play a major role in the global pharmaceutical market and healthcare system. Our objectives were to examine the nanomedicines approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in the global market, to briefly cover the challenges faced during their development, and to look at future perspectives. Additionally, the importance of nanotechnology in developing pharmaceutical products, the ideal properties of nanocarriers, the reasons behind the failure of some nanomedicines, and the important considerations in the development of nanomedicines will be discussed in brief.
Keywords: nanotechnology, pharmaceutical nanomedicines, nanoparticles, drug delivery
1. Introduction
Nanotechnology and nanosciences are commonly seen as providing a significant advantage to many areas of study and applications [1]. Nanotechnology means the production and use of materials, equipment, and systems in nanoscale, which is an intermediate range between atoms and the molecular scale with the important prerequisite that at least one dimension is in the nanometer length [2,3]. The effect of nanotechnology on healthcare is already being felt, as different nanotechnology ideas have been developed, and several nanotechnology-based medicines are now available in the market. Across many parts of the world, nanotechnology draws expanded investments from public authorities and the private sector [2].
Many studies have been undertaken to discover and improve new medicines that have the ability to target disease sites much more precisely. Nanotechnology allows therapeutic agents to reach the target site of disease by overcoming their limitations [4]. Many drugs have limitations when it comes to the route of administration due to issues surrounding solubility, permeability, and bioavailability, leading to poor pharmacokinetics. The goal is thus to develop a dosage form with a proper pharmacokinetics delivery system. Nanoparticle-based drugs can either be therapeutic agents themselves or act as a carrier for transporting different therapeutic agents to certain parts of the body.
Nanoparticle-based drugs display a promising approach to obtaining desirable drug-specific properties by manipulating the biopharmaceutical and pharmacokinetical properties of the molecule. Reducing the undesirable toxicity from nonspecific distribution, improving patient adherence, and providing beneficial clinical findings are the main advantages of using nanotechnology in improving disease targeting by therapeutic agents using nanotechnology to deliver such agents with indirect minimizing of the burden on the healthcare system. In general, the development of new medicines and therapies that are focused on nanotechnology is driven by the need to build therapies that are less toxic and cheaper than conventional treatments.
Since 1989, the number of approved nano-based pharmaceutical applications and products has significantly increased [1]. Over the last 20 years, around 80 nanomedicine products have been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for marketing (Table 1). This shows the importance of nanotechnology in the field of drug delivery. Several nanomedicines have been used to increase effectiveness and reduce adverse reactions through changes in the efficacy, safety, and physicochemical and pharmacokinetic/pharmacodynamic characteristics of the original medicines [5].
Table 1.
List of globally marketed nanomedicines approved by the FDA and the EMA *.
| Type | Trade Name | Company | Date of Approval | Active Ingredients | Indication |
|---|---|---|---|---|---|
| Nanocrystals | Emend® | Merk & Co. Inc. | FDA (2003) | aprepitant | antiemetic drug [54,55] |
| Ivemend® | Merk & Co. Inc. | FDA, EMA (2008) | fosaprepitant dimeglumine (prodrug of aprepitant) | antiemetic drug [56] | |
| Ostim® | Osartis GmbH & Co. | FDA (2004) | calcium hydroxyapatite | bone-grafting material [57] | |
| Rapamune® | Wyeth Pharmaceuticals Inc. (a subsidiary of Pfizer Inc.) | EMA (2001), FDA (2010) | sirolimus (rapamycin) | prevents rejection of kidney transplants [58] (immunosuppressant) | |
| Rapamune® | Wyeth Pharmaceuticals Inc. (a subsidiary of Pfizer Inc.) | FDA (2015) | sirolimus (rapamycin) | a rare progressive lung disease [58] (lymphangioleiomyomatosis) | |
| Vitoss® | Orthovita Inc. | FDA (2003) | β-tricalcium phosphate | bone-grafting material [59] | |
| Ritalin LX® | Novartis | FDA (2002) | methylphenidate | attention deficit hyperactivity disorder (ADHD) [60] in children | |
| Avinza® | Pfizer Pharmaceuticals | FDA (2002) | morphine sulfate | psychostimulant [61] | |
| Focalin XR® | Novartis | FDA (2008) | dexamethylphenidate HCl | ADHD in children [62] | |
| Invega® | Janssen Pharmaceuticals | FDA (2009) | paliperidone | schizophrenia [63] | |
| Invega Sustenna® | Janssen Pharmaceuticals | FDA (2009) | paliperidone Palmitate | schizophrenia [64] | |
| Megace ES® | Par Pharmaceuticals | FDA (2005) | megestrol acetate | antianorexic [65] | |
| NanOss® | RTI Surgical | FDA (2005) | hydroxyapatite | bone substitute [66] | |
| EquivaBone® | Zimmer Biomet | FDA (2009) | hydroxyapatite | bone substitute [67] | |
| OsSatura® | Isotis Othobiologics Inc. | FDA (2003) | hydroxyapatite | bone substitute | |
| Epaxal® | Crucell Berna Biotech | EMA (1993) | inactivated hepatitis A virus vaccine | prevents hepatitis A infection [68] | |
| Zanaflex® | Acorda | FDA (2002) | tizanidine HCl | muscle relaxant [69] | |
| Ryanodex® | Eagle pharm | FDA (2014) | dantrolene sodium | malignant hyperthermia [70] | |
| TriCor® | Abbott Laboratories | FDA (2004) | fenofibrate | antihyperlipidemia [71] | |
| Lipid-based Nanoparticles | Doxil® | Johnson & Johnson | FDA (1995), EMA (1996) | doxorubicin (adriamycin) | metastatic ovarian cancer, HIV-associated Kaposi’s sarcoma (KS) [72] |
| Lipodox® | Sun Pharma Global FZE | FDA (2013) | doxorubicin hydrochloride | metastatic ovarian cancer, HIV-associated KS [73] | |
| DaunoXome® | Galen Ltd. | FDA, EMA (1996) | daunorubicin | cancers and HIV-associated KS [74] | |
| Onivyde® | Merrimack Pharmaceuticals | FDA (2015) | irinotecan | metastatic pancreatic cancer [75] | |
| DepoCyt® | Pacira Pharmaceuticals | EMA (2002), FDA (2007) | cytarabine | lymphomatous meningitis [76] | |
| Myocet® | Teva Pharmaceutical Industries Ltd. | EMA (2000) | doxorubicin hydrochloride | breast cancer [77,78] | |
| Caelyx® | Janssen Pharmaceuticals | EMA (1996) | doxorubicin | breast cancer, ovarian cancer, HIV-associated KS [79,80] | |
| Mepact® | Takeda France SAS | EMA (2009) | mifamurtide | osteogenic sarcoma [81] | |
| Marqibo® | Talon Therapeutics | FDA (2012) | vincristine | Philadelphia chromosome-negative chronic myelogenous leukemia in adult patients [82,83] | |
| Onpattro® | Alnylam | FDA & EMA (2018) | patisiran | hereditary transthyretin (TTR) mediated amyloidosis [84,85] | |
| Lipusu® | FDA (2016) | paclitaxel | breast cancer, non-small-cell lung cancer (NSCLC) [86] | ||
| AmBisome® | NeXstar Pharmaceuticals | EMA (1990), FDA (1997) | amphotericin B | antifungal drug [87] | |
| Vyxeos® | Jazz Pharmaceutics | FDA (2017), EMA (2018) | daunorubicin and cytarabine | acute myeloid leukemia [88] | |
| Abelcet® | Defiante Farmaceutica | FDA (1995) | amphotericin B | antifungal drug [87] | |
| DepoDur® | SkyePharma | FDA (2004), EMA (2006) | liposomal morphine sulphate | postoperative analgesia [89] | |
| Curosurf® | Chiesi | FDA (1999) | poractant alfa | respiratory distress syndrome (RDS) [90,91] | |
| Zevalin® | Bayer Pharma | FDA (2002) Disc. * EMA (2004) |
90Y-ibritumomab tiuxetan | lymphoma [92] | |
| Inflexal® | Crucell Berna Biotech | EMA (1997) | inactivated influenza virus vaccine | prevents influenza infection [93,94] | |
| Pfizer-BioNTech Vaccine | Pfizer Pharmaceuticals | FDA (2020) | mRNA vaccine | prevents COVID-19 infection [95,96] | |
| Moderna COVID-19 Vaccine | ModernaTX Inc. | FDA (2020) | mRNA vaccine | prevents COVID-19 infection [96,97] | |
| Visudyne® | QLT Phototherapeutics | FDA & EMA (2000) | photosensitizer (PS), benzoporphyrin | choroidal neovascularization caused by wet age-related macular degeneration [87] | |
| Polymer-based Nanoparticles | Cimzia® | UCB | FDA (2008), EMA (2009) | IgG Fab’ fragment that specifically recognizes and binds to TNF-α | rheumatoid arthritis [98], Crohn’s disease [99], psoriatic arthritis [100], and ankylosing spondylitis [101] |
| Apealea® | Oasmia Pharmaceutical AB | EMA (2018) | paclitaxel | ovarian cancer, peritoneal cancer, fallopian tube cancer [102] | |
| Adagen® | Enzon Pharmaceuticals Inc. | FDA (1990) | adenosine deaminase (ADA) | adenosine deaminase (ADA)-severe combined immunodeficiency disorder [103] | |
| Neulasta® | Amgen, Inc. | FDA (2002) | filgrastim | febrile neutropenia, consequent infections arising due to lack of neutrophils [104] | |
| Oncaspar® | Enzon Pharmaceuticals Inc. | FDA (1994), EMA (2016) | L-asparaginase | acute lymphoblastic leukemia, chronic myelogenous leukemia [105] | |
| Genexol-PM® | Lupin Ltd. | FDA (2007) | paclitaxel | breast cancer [106] | |
| Pegasys® | Genentech USA, Inc | FDA, EMA (2002) | recombinant human alfa-2a interferon | hepatitis C [107], hepatitis B [108] | |
| Diprivan® | Fresenius Kabi | FDA (1989), EMA (2001) | propofol | (sedative-hypnotic agent) used in surgeryto induce relaxation before and during general anesthesia | |
| Somavert® | Pfizer Pharmaceuticals | EMA (2002), FDA (2003) | analog of human growth hormone (acts as an antagonist of GH receptors) | acromegaly [109] | |
| Macugen® | Pfizer Pharmaceuticals | FDA (2004) | pegatinib sodium | choroidal neovascularization caused by wet age-related macular degeneration [110] | |
| Mircera® | Vifor | EMA (2007), FDA (2018) | epoetin β (EPO) (EPO is a genetically recombinant form of erythropoietin) | anemia [111] | |
| PegIntron® | Merk & Co. Inc. | EMA (2000), FDA (2001) | alpha interferon (INF) molecule | hepatitis C [112] | |
| Krystexxa® | Savient Pharmaceuticals | FDA (2010) | pegloticase is a recombinant porcinelike uricase | refractory chronic gout [113] | |
| Plegridy® | Biogene | FDA (2014) | recombinant IFN-β | relapsing remitting multiple sclerosis (RRMS) in adult patients [114] | |
| Adynovate® | Baxalta US Inc. | FDA (2015) | coagulation factor VIII | hemophilia A [115] | |
| Copaxone®/FOGA | Teva Pharmaceutical Industries Ltd. | FDA (1996), EMA (2016) | glatiramer acetate | multiple sclerosis (MS) [116,117] | |
| Eligard® | Tolmar Pharmaceuticals Inc. | FDA (2002) | leuprolide acetate | prostate cancer [118] | |
| Renagel® | Sanofi | FDA (2000) | sevelamer carbonate | hyperphosphatemia caused by chronic kidney disease (CKD) [119] | |
| Renagel®/Renvela® | Genzyme | EMA (2007) | sevelamer HCL | hyperphosphatemia caused by CKD [120] | |
| Restasis® | Allergan | FDA (2003) | cyclosporine | chronic dry eye [121] | |
| Rebinyn® | NovoNordisk | FDA (2017) | recombinant DNA-derived coagulation FIX | hemophilia B [122,123] | |
| Estrasorb™ | Novavax, Inc. | FDA (2003) | estradiol (17β-estradiol) hemihydrate | moderate vasomotor symptoms due to menopause [124] | |
| Zilretta® | Flexion Therapeutics | FDA (2017) | triamcinolone acetonide | knee osteoarthritis [125] | |
| Dendrimer-based Nanoparticles | VivaGel® BV | Starpharma | FDA (2015) | astodrimer sodium | anti-infective for prevention of recurrent bacterial vaginosis (BV) [126] |
| Protein-based Nanoparticles | Abraxane® | Celgene Pharmaceutical Co. Ltd. | FDA (2005, 2012, 2013), EMA (2008) | paclitaxel | approved by the FDA for treatment of metastatic breast cancer [127] (2005), lung cancer [128] (2012), and metastatic pancreatic adenocarcinoma [129] (2013) |
| Ontak® | Eisai | FDA (1999) | diphtheria toxin | leukemia, T-cell lymphoma [130,131] | |
| Inorganic Nanoparticles | Feraheme™ | AMAG Pharmaceuticals | FDA (2009) | ferumoxytol | anemia [132,133] |
| Venofer® | Luitpold Pharm | FDA (2000) | iron sucrose | iron deficiency in CKD [134] | |
| Dexferrum® | American Regent | FDA (1996) | iron dextran | iron deficiency in CKD [135] | |
| Ferinject® | Vifor | FDA, EMA (2013) | iron carboxymaltose colloid | iron deficient anemia [136] | |
| Ferrlecit® | Sanofi-Aventis | FDA (1999), EMA (2013) | sodium ferric gluconate | iron deficiency in CKD [137] | |
| Hensify® | Nanobiotix | EMA (2019) | hafnium oxide nanoparticles | locally advanced squamous cell carcinoma [138] | |
| Infed® | Actavis Pharma | FDA (1992) | iron dextran | iron deficiency in CKD [139] | |
| Feridex®/Endorem® | AMAG Pharma | FDA (1996) Disc. * 2008 [140] | SPION-dex | imaging agent [141] | |
| GastroMARK™/Umirem® | Mallinckrodt Inc. | FDA (2009)Disc. * 2012 [140] | SPION-silicone | imaging agent [141,142] |
* Disc.: discontinued.
In this review, we discuss the importance of nanotechnology in developing pharmaceutical products, the ideal properties of nanocarriers, the reasons behind the failure of some nanomedicines, and the important considerations in the development of nanomedicines. Our objectives were to sum up the different approved nanomedicines on the global market, to briefly cover the challenges faced during the development of nanomedicines, and to look at the future prospects.
2. The Importance of Nanotechnology in Developing Pharmaceutical Medicines
Nanotechnology plays a significant role in the field of medicine and in drug delivery, mainly due to the major limitations and problems affecting conventional pharmaceutical agents, and older formulations and delivery systems [1]. Poor delivery to the target site is one of the inefficiencies of certain currently available medicines [3]. For this reason, drug delivery in therapeutics is a significant factor. Most conventional drug-delivery systems (CDDSs) have an immediate, high drug release after administration, leading to increased administration frequency [1]. Misuse is one of the drawbacks associated with increased administration frequency that can lead to drug toxicity. It is also a major challenge for pharmaceutical companies in developing new medicines, as drug solubility in the CDDSs tends to be low, hence affecting efficacy [1,3]. Moreover, low drug stability is one of the major limitations of using conventional pharmaceutical agents, as the CDDSs in some dosage forms are not sufficient to protect the active pharmaceutical ingredients (APIs) against biological fluids in the body. Accordingly, solving all previous issues related to drug delivery would help in improving quality of life and the healthcare system, and in reducing healthcare costs. A reduction in overall healthcare costs is one of the beneficial features of nanomedicines. There are pressures in many countries to reduce overall healthcare costs. Thus, many studies have been carried out worldwide focusing on the development of pharmaceutical nanomedicines, translating to products being manufactured by local pharmaceutical companies.
The above-mentioned issues related to CDDSs can be successfully resolved by applying nanotechnology to the development of pharmaceutical medicines. The use of nanoparticles (NPs) in drug production has multiple benefits compared with the use of CDDSs, such as: (1) delivering therapeutic agents more specifically to the targeted tissue and reducing total dose and potential toxic side effects; (2) improving the stability of the APIs after administration and thus their bioavailability; (3) demonstrating better safety and efficacy; (4) releasing drugs at a constant rate over a desired timescale; (5) allowing passive targeting and the accumulation of drugs in malignant tumors and other pathological sites through the enhanced permeability and retention (EPR) effect; and (6) nanopharmaceutical products can be far cheaper than conventional ones [1].
3. Ideal Properties of Nanoparticle Delivery Systems
Nanoparticles are colloidal particles, ranging from 1 to 1000 nm, and are mainly composed of different macromolecules in which the therapeutic drugs can be adsorbed, entrapped, or covalently attached. In the field of drug development, nanoparticles provide significant advantages that can be attributed to their physicochemical properties in order to achieve controlled release characteristics [6]. Controlling the product life cycle through reducing dosing frequency creates a potential opportunity that can be achieved by designing nanoparticles with controlled release for drugs that go off-patent. Numerous factors impact the effectiveness of nanoparticles in drug delivery, such as physical and biological stability, good component tolerability, the simplicity of the manufacturing method, ease of manufacturing process scale-up, the convenience of freeze-drying, and sterilization [6]. There are some properties of nanoparticles that are important for drug-delivery application and should be taken into consideration during the development of nanomedicines:
Nanoparticles should be characterized by high drug capacity and exhibit a very low possibility of immediate release of the therapeutic agent.
Nanoparticles should have the ability to be combined with ligands for targeted drug delivery.
Nanoparticles should be stable enough to pass through the biological barriers with respect to their physicochemical properties, such as size, size distribution, zeta potential, etc.
Therapeutic agents should be completely released from nanoparticles at an optimal rate that is based on the formulation design.
Nanoparticles should be biocompatible, biodegradable, and nonimmunogenic.
Organic solvents and toxic ingredients should be excluded from the manufacturing process.
All components of the formulation should be safe, affordable and commercially available.
Simplicity, affordability, and ease of scaling up are the main characteristics that should be included in the manufacturing process.
Nanoparticle formulation should have the ability to be involved in different processes during the manufacturing process, such as lyophilization, sterilization, drying, blending, granulation, compression, capsule filling, and packaging.
Nanoparticles should be stable in storage.
Biocompatibility, biodegradability, and nonimmunogenicity are the most essential characteristics that should be applied to nanoparticle delivery systems. These characteristics are being studied extensively for nanocarriers to have efficient drug delivery with improved bioavailability and reduced side effects. To ensure high biosafety for in vivo applications, an ideal drug nanocarrier should have high biocompatibility and good biodegradability. Many inorganic drug nanocarriers, such as metals, metal oxides, and carbon-based materials, are difficult to degrade, which can result in toxicity and limit clinical applications. Lipid-based nanoparticles and polymer-based nanoparticles are the most useful nanocarriers for drug delivery in most global approved nanomedicines (Table 1), due to their biocompatibility, lack of intrinsic toxicity, and improved drug-loading capacity. In addition, these nanocarriers may provide special protection for therapeutic agents against environmental degradation. The surfaces of liposomes and micelles can also be functionalized to improve pharmacokinetic profiles of therapeutic agents.
4. Challenges in the Development of Pharmaceutical Nanomedicines
The field of pharmaceutical nanotechnology has seen enormous growth and progress over the last 20 years. Particular attention has been paid to the field of nanomedicine, as it promised to revolutionize medical care through more efficient, less toxic, and more intelligent therapies that can be targeted to the site of disease [7]. Many nanomedicines have been developed successfully and approved for clinical use, with significant effort from both academia and the biopharmaceutical industries [1,8]. However, the field of nanomedicine is still in its infancy with few success stories, as various types of challenges are faced during the development of pharmaceutical nanomedicines. These challenges can be categorized as follows.
4.1. Challenges in Drug Delivery across Different Biological Barriers
Drug-loaded nanoparticles have been developed for different routes of administration, such as nasal, oral, transdermal, ocular, and parenteral [9,10,11,12,13]. There are several biological barriers that prevent NPs from reaching their desired disease sites successfully, which mainly depends on the targeted organ and the route of administration [7,14]. Oral administration is the most preferred route for drugs and bioactive molecules. However, the low solubility, stability, and bioavailability of many drugs are the main challenges for oral administration [15]. It is difficult to deliver some drugs through the gastrointestinal tract (GIT), as they become inactive in the GIT before being absorbed, mainly due to the harsh conditions in the GIT system, including the wide range of pH across the GIT and the presence of large numbers of degrading enzymes. Furthermore, the mucus layers that cover epithelial surfaces are significant barriers to the penetration of NPs through the gut epithelium, as the rapid secretion and shedding of GIT mucus can significantly limit the effectiveness of nanoparticulate drug-delivery systems [16,17].
Generally, the local delivery of therapeutic agents has fewer barriers than systemic delivery. The systemic delivery of therapeutic agents, especially genes, proteins, and peptides, faces significant challenges in traveling from the site of administration to the site of action [14]. Briefly, after therapeutic agents reach the bloodstream following intravenous administration, they travel through the circulatory system, and hence accumulate in nontargeted organs of the reticuloendothelial system (RES), including the liver, lungs, and spleen. In general, biotherapeutic agents aggregate with proteins in the serum, are degraded by endogenous enzymes, and are taken up by nontargeted cells such as phagocytes [14,18,19]. This increases the systemic elimination of biotherapeutic agents, resulting in a short plasma half-life after intravenous administration [14].
One of the most essential defense mechanisms in the central nervous system (CNS) is the blood–brain barrier (BBB). Drug movement through the BBB is mainly limited by the occurrence of tight junctions, rather than large fenestrations, between endothelial cells [20]. Only small molecules, including water, gases, and other lipid-soluble compounds, can penetrate passively through the BBB. Conversely, transports of large molecules with high electric charges, polarity, and hydrophilicity, such as glucose, amino acids, and most drugs by active routes of transportation, will rely on specific proteins [21]. The delivery and release of therapeutic agents into the brain is a challenging area. Therefore, NPs that have been developed and applied to cross the BBB mainly include polymeric NPs, such as poly(butylcyanoacrylate) (PBCA) [22], poly(lactic-co-glycolic acid) (PLGA) and poly(lactic acid) (PLA) NPs [23], liposomes [24], and inorganic composites such as gold, silver, and zinc oxide NPs [25,26,27].
Another critical challenge is addressing the difficulty of delivering therapeutic drugs into solid tumors, as wide areas of tumors may not be well permeated. Tumor vasculature in some cases is highly heterogeneous in distribution and more permeable, but large tumor areas may have little perfusion [28,29]. The poor perfusion of blood in solid tumors can be explained by five major abnormal physical and physiological properties: (a) a solid tumor mass compresses the blood vessels, resulting in a minimization of the drug supply to several tumor regions; (b) immature vasculature with high viscous and geometric resistances and low pressure gradients allow the blood in a tumor to slow down and heterogeneously limit drug supply; (c) high metabolic consumption rate of glucose resulted in significant production of lactate and H+ that leads to low pH media, (d) nonfunctional lymphatics and highly permeable blood vessels cause high hydrostatic pressure in tumors, stopping the continuous movement of drugs from blood vessels into tumor tissue; and (e) the dense structure of the cells and the interstitial matrix acts as a steric barrier for the diffusion of therapeutic agents [30]. Therefore, the essential pharmacokinetic steps that must be performed for the delivery of drugs into tumor cells include transport and metabolism in cells, vascular transport, and interstitial transport [30]. For example, the high concentration of H+ in the tumor can diffuse into adjacent cells, resulting in an acidic microenvironment for neighboring normal cells. Accordingly, some nanomedicines have been developed recently to target the pH of tumors through activation of prodrug, or drug release from nanocarriers by low pH of the tumor or using drugs that raise the pH of the acidic tumor [31].
4.2. Challenges in the Formulation, Characterization, and Manufacturing of Nanomedicines
Nanomedicines are likely to be three-dimensional constructs with multiple components in ideal spatial arrangements. Therefore, slight changes in the method or composition of these constructs can influence the dynamic superposition of the components [7]. Highly reproducible manufacturing processes for nanomedicines can be achieved by understanding the physicochemical characterization of the components, understanding the critical components and their interactions, and identifying the main features and their performance relationships [7].
4.2.1. Physicochemical Characterization of Nanoparticle Components
The ways nanoparticles act in vitro and in vivo depend on several physicochemical characteristics, including size and size distribution, surface morphology, surface chemistry, surface charge, surface adhesion, steric stabilization, drug-loading efficiency, drug release kinetics, and the hemodynamic properties of the nanoparticles [7]. Nanoparticles have been developed and adapted to deliver different kinds of therapeutic agents, including small-molecule drugs, peptides, proteins, nucleic acids, and genes. Liposomes, polymers, proteins, micelles, dendrimers, quantum points, nanoshells, nanocrystals, gold nanoparticles, paramagnetic nanoparticles, and carbon nanotubes are all nanoparticles used in drug delivery [32]. Each system varies greatly in its architecture and has special key characteristics that may help to improve the performance of nanoparticles in particular indications. However, it is crucial to identify the appropriate nanoparticle parameters for particular indications. Going one way may fix a specific problem, but may also lead to another [7]. Doxil® is a good example to illustrate how the physicochemical properties of nanoparticles can affect pharmacokinetics. Non-PEGylated liposomes, containing doxorubicin, are extremely affinous to the RES and are easily removed from circulation due to a process called “opsonization”, while PEGylated liposomes can minimize their affinity to the RES and hence decrease the uptake of liposomes by macrophages significantly. Doxil® is a PEGylated liposomal doxorubicin that shows a long half-life, an increased concentration of tumor drugs, an improvement in antitumor effectiveness, and fewer side effects than non-PEGylated ones.
4.2.2. Analysis and Characterization of Nanoparticle Formulation
The identification of proper analytical tests to complete the characterization of nanoparticles may be one of the most challenging areas of nanomedicine development. As nanomedicines have a complex nature compared with conventional pharmaceutical products, and each component of nanomedicines has a specific function, a more advanced testing level should be applied to fully characterize nanoparticles to ensure that they have all the desired properties for the intended therapeutic purpose [7]. Several proposed nanomedicines have biological components, such as proteins or nucleic acids [33,34], that may be sensitive to conditions in the manufacturing process and, hence, compositional changes may occur from processing in some cases [7]. These biological components may not be the active pharmaceutical ingredients in nanomedicines, but they may have a role to play in targeting certain cells or in distributing the active components in the body. Thus, suitable analytical tests should be applied to these components in order to obtain a full characterization, and they cannot be regarded as an inactive excipient due to their important role in the effectiveness or the protection of the drug [7]. The better the understanding of the components of nanomedicines in the early stages of development, the more likely an effective reproducible manufacturing process will be achieved [7].
4.2.3. Scale-Up and Manufacturing
In terms of pharmaceutical development, the successful scale-up and production of nanomedicines presents difficulties and challenges. Traditional drug production does not normally create nanometer-scale, three-dimensional multicomponent systems, a fact that presents several barriers to the scale-up of nanomedicines. As most pharmaceutical nanoparticles are complex in nature with multiple components in ideal spatial arrangements, a thorough understanding of the critical components and their interactions is important for identifying the key characteristics of the product. The early identification of these characteristics in the development of nanomedicines will assist in the choice of a suitable large-scale manufacturing method to establish the critical process steps and analytical criteria that will ensure the reproducibility of the product [7].
The formulation of nanoparticles usually requires the use of organic solvents, sonication, high-speed homogenization, milling, emulsification, crosslinking, evaporation of organic solvents, filtration, centrifugation, and lyophilization. At an early stage of development in the laboratory, it is helpful to consider, on a “small scale”, which method may be most beneficial for the scaling-up of the product [7]. A sterile process of manufacturing nanomedicines that uses a special route of administration will face challenges, depending on the size and composition of the particles. One of these challenges is that the risk of nanoparticles being damaged by sterilization techniques such as gamma irradiation or autoclaving will increase, especially when biological materials are involved [35,36,37]. The sterilization of nanoparticles through conventional sterile filters may not be an issue if the structure of the particles is flexible, as with liposomes, especially if their particle size is well below 220 nm. For hard-structure nanoparticles, such as polymeric, metal, silica, and others, sterilization through filters may be the only choice. However, large particle-size distribution and a particle size closer to 220 nm may result in great filtration difficulties due to the small pore size of standard filtration membranes. Accordingly, enormous amounts of active ingredients could be lost on filtration if the average particle size is not well below 220 nm [7]. Although aseptic manufacturing is often a suitable choice, it can be quite complicated, especially for a multistage process involving handling and the transfer of materials in a sterile environment.
Environmental safety is a further problem for the manufacturing of nanoparticles. Nanoparticles can spread in the air during the handling of dry materials, which leads to deposition of the nanoparticles in the lung causing pulmonary toxicity [38,39]. Therefore, extreme caution is required during the manufacturing of nanomedicines. In addition, sufficient personal protective equipment is essential during manufacturing, as some nanoparticles are able to penetrate the skin barrier, making dermal exposure a potential risk [38]. Nanoparticles that are manufactured entirely in the liquid environment, mostly similar to the standard manufacturing of pharmaceutical liquids, may have a significantly lower environmental impact.
4.2.4. Challenges in the Regulation of Nanomedicine Development
In comparison to conventional pharmaceutical products, in which a single active agent is normally used, most pharmaceutical nanoparticles are complex in nature, with multiple components and heterogenous structures, where more than one component can affect the pharmacological behavior of the active ingredient. Due to this complexity, the regulation of nanomedicines may face several obstacles [7,40]. Currently, new medicines based on nanoparticles are evaluated by the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other agencies using a case-by-case approach under the traditional framework of benefit/risk analysis [7,41,42]. In general, there is a lack of standards in the evaluation of nanomedicines, as they are a unique category of therapeutic agents [7].
Generally, pharmaceutical products are regulated by the FDA under two main laws: the Federal Food, Drug, and Cosmetic Act (FDCA), which covers all chemically synthesized drugs and devices; and the Public Health Service Act (PHSA), which covers biologically derived therapeutic products [40,41]. The definitions and policies of these laws differ among three product areas based on whether the product includes a chemical action mode (drug), mechanical action mode (device), or biological source [41]. The FDA has not published any particular guidance for nanomedicines until recently, as they are categorized under complex products with multiple components. In other words, the FDA makes no categorical assessment of nanomedicines as being safe or harmful, and will continue to consider the particular characteristics of individual products. In December 2016, provisions for transparency and consistency in FDA procedures were established for classifying and evaluating combination products. A combination product is defined as a product that contains a mix of three product areas: a drug and a device; a drug and a biologic; a device and a biologic; or all three types of products [41]. Nanomedicines are categorized by the FDA as combination products, assigned by the traditional regulatory route and supplemented with special requirements to assure safety and efficacy. For example, paclitaxel and doxorubicin nanoformulations have been approved by the FDA as new cancer drugs, classified as combination products. Due to the debate on the adequacy of current regulatory frameworks and procedures, wider concerns have been raised about the inherent risks of nanotechnology and products containing nanoparticles, including nanoparticle toxicity, the unintended effects of nanoparticles’ ability to cross the BBB, and the long-term effects of nanoparticles [41,43,44]. Accordingly, the FDA has issued one draft and five final guidance documents discussing the use of nanotechnology in FDA-regulated products, including nanomedicines. All six guidelines encourage manufacturers to consult the company before marketing their products.
Nanomedicines are categorized according to the EMA into biological and nonbiological medicines [45]. More comprehensive study beyond plasma concentration measurement is required for biological and nonbiological nanomedicines. A step-by-step comparison of the nanomedicine with a reference medicine is required, including bioequivalence, safety, quality, and efficacy, which will lead to therapeutic equivalence [42,46]. The biological framework can sometimes be considered as the basis for the regulation of nonbiological complex drugs (NBCDs), as there are certain features in common: the structure is not fully characterized, and the in vivo activity is dependent on the process of manufacture, which means that comparability needs to be made throughout the life cycle, as with biological nanomedicines [42,47]. In the field of nanomedicine, the EMA has already published several reflection papers. These papers are used to provide advice to developers in the processing of marketing authorization applications for new nanomedicines and nanosimilars [42].
As we have recently entered the era of generic nanomedicines, both generic drug manufacturers and drug regulators face real challenges in defining a framework for the evaluation of generic nanomedicines to demonstrate that they are bioequivalent to the branded ones and have the same physicochemical properties, as well as being safe and effective [7,42,48]. The connections between the physicochemical properties of nanoparticles and their clinical pharmacokinetics (PK) and safety are poorly understood, and traditional animal models may not be adequate for the proper extrapolation and prediction of the biodistribution and toxicity of nanoparticles in humans. This is particularly important when a novel drug based on nanoparticles is compared with conventional formulations, and when a generic approved nanomedicine is evaluated against a product of innovation. The similar outcomes in general PK and toxicity studies, or a simple comparison of the composition of drug products, cannot be used to assume the bioequivalence of generic and innovator nanomedicines.
5. Considerations in Nanomedicine Development
5.1. Chemistry, Manufacturing, and Controls (CMC) Considerations
Nanomedicines are more complex than conventional drugs, as they face CMC challenges during product development and in the later stage of manufacturing scale-up. Thus, the first step in developing nanomedicine is to determine practicability through understanding the makeup and structure of the early formulation to prove the principle in the research. This will ensure that the formulation can be reproducible during confirmatory studies, as well as ensure its future safety and efficacy in clinical trials [4]. It is essential that the early nanomedicine candidates have adequate physical, chemical, and functional characterization. Analytical techniques such as nuclear magnetic resonance (NMR), mass spectrometry (MS), chromatography, etc., must be used to identify the chemical structure of each component involved in nanoparticle formulation [4]. The physicochemical properties of the early formulated sample; i.e., particle size, zeta potential, pH, viscosity, and purity, also need to be established and understood. The biological functions of nanoparticles are required to be characterized and investigated to provide acceptable levels of confidence.
Moreover, the potential commercial-scale manufacturing of nanomedicine products that are easily reproducible at a reasonable cost is very important. For this reason, the CMC developers should understand the early stages of nanomedicine synthesis and assess whether the chemicals and the processing can be used and performed on an industrial scale. Cytotoxic compounds and complex processing may be achievable on a laboratory scale, but may be expensive and challenging on an industrial scale [4]. The manufacturing process of nanomedicines is quite complex, and so minimizing the batch-to-batch variability of nanomedicine formulations is a major challenge. Thus, the quality of the product, which impacts the strength, purity, safety, and efficacy of nanomedicines, is essential for successful development and commercialization. Being aware of the quality of the starting materials and understanding the processing conditions for preparing nanomedicine formulations allow the developer to control the manufacturing successfully on an industrial scale [4].
5.2. Economic Considerations
The amount of investment required to fund the development and scaling-up of nanomedicine production needs to be considered. The overall risk of CMC development will need to be factored into the investment profiles used for analyzing potential nanomedicine programs against other development portfolios. Instrumentations, manufacturing equipment, and other facilities may be costed for some companies, therefore these facilities need to be involved in the investment strategies of nanomedicine development aligned with clinical requirements [4].
5.3. Regulatory Considerations
Early FDA consultations regarding nanomedicine development will help in understanding the scientific and regulatory issues relevant to the product, and in addressing questions concerning the safety, effectiveness, public health impact, and regulatory status of the product [4]. Therefore, nanomedicine development should follow the usual pathways and processes of drug development using a suitable evaluation framework. In Europe, the EMA has established an Expert Working Group, and has released some reflection papers for particular nanomedicines in order to provide guidance to developers in the preparation of marketing authorization applications [4,42]. However, it is not clear whether the existing regulatory frameworks will pose challenges in the future for more innovative nanotechnology.
6. Developmental Nanomedicines
Pharmaceutical nanomedicine products are projected to play a major role in the global pharmaceutical market and healthcare system. Since 1995, around 70 nanomedicine products have been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for marketing [1,5,8,49,50,51,52] (Table 1), and double this number are available in clinical trials. Many reports demonstrate that the number of medications based on nanotechnology are increasing annually. Every year, new nanomedicines of previously approved drugs enter clinical trials to investigate their improvement with regard to efficacy compared with conventional formulations [53]. This is due to the rapid growth in research and development (R&D) and the high market demand, which shows the importance of nanotechnology in the field of drug delivery. However, the majority of nanomedicines approved to date have shown a reduced toxicity rather than improved efficacy [53]. A variety of nanoparticle-based drugs have entered the market and are used daily by many patients (Table 1). These products are from various companies worldwide, and indicate the success of nanomedicines as therapeutic agents.
Since 1989, 78 nanomedicines have been approved and have entered the global market. Of these nanomedicines, 66 have been approved by the FDA, and 31 have been approved by the EMA. Both the FDA and the EMA have shared in the approval of 20 nanomedicines globally, while other nanomedicines have an approval from one side (FDA: 43 nanomedicines; EMA: 12 nanomedicines). Since 2010, focus on the development of nanomedicines and the number of marketed nanomedicines have significantly increased due to the resulting healthcare system benefits. The globally marketed nanomedicines can be classified as nanocrystals, lipid-based nanoparticles, polymer-based nanoparticles, dendrimer-based nanoparticles, protein-based nanoparticles, or inorganic nanoparticles (Table 1).
Inorganic nanoparticles represent many pharmaceutical carriers that can be used for the cellular delivery of various drugs. Inorganic nanoparticles, such as metal, carbon nanotubes, calcium phosphate, iron oxide, silica, and quantum dot nanoparticles are very attractive for use in drug delivery due to their dual function as diagnostic platforms and as therapeutic carriers [140]. Chemical or biological modifications may be applied to inorganic nanoparticles to meet the ideal requirements for cellular delivery, such as biocompatibility, high charge density, and site-specific delivery [140]. Inorganic nanoparticles exhibit good stability over broad ranges of temperature and pH and are not subject to microbial attack. Despite these obvious benefits, only a small number of inorganic nanoparticle systems have reached the clinic due to slow dissolution and lack of biodegradation, which are the main disadvantages of inorganic nanoparticles, especially for long-term administration [140]. Organic carriers are carbon-based materials that are generally characterized by their biocompatibility, lack of intrinsic toxicity, and improved drug-loading capacity. In these carriers, therapeutic agents are often trapped or bound within the matrix. Organic carriers are mainly classified into three groups, which are lipid-based vectors, polymer-based vectors, and dendrimers. These carriers may provide special protection for therapeutic agents against environmental degradation The surfaces of liposomes and micelles can also be functionalized to improve pharmacokinetic profiles of therapeutic agents.
Cancer is one of the most significant diseases in the world, according to a 2015 WHO factsheet, with 14 million new cases in 2012 and 8.2 million cancer-related deaths [143]. Therefore, new and effective cancer medicines are urgently needed to control the high mortality. Anticancer nanomedicines have made dramatic advancements in the past several decades with the rapid progress of nanotechnologies in medicine. Researchers in academia and pharmaceutical industries have developed several nanoparticles that have the ability to deliver therapeutic and diagnostic agents to tumors selectively to increase the accumulation of chemotherapeutic agents within the tumor [144,145,146]. Different strategies can be used to target the tumor, including: passive targeting based on the enhanced permeability and retention (EPR) effect; active targeting directed by tumor-specific moieties; and stimuli-responsive tumor targeting [147]. Passive targeting is a result of the EPR effect, a phenomenon that leads nanoparticles to accumulate in the tumor tissues due to the leaky nature of the tumor vasculature [148]. Passive EPR targeting and particle-size control were the basic principles of first-generation cancer nanomedicines, such as Doxil, which were simple lipid vesicles shielded with polyethylene glycol (PEG) to avoid opsonization by the cells of the reticuloendothelial system, hence preventing an immune response and prolonging circulation time. Doxil is a pegylated liposomal doxorubicin used to treat different kinds of cancer, including metastatic ovarian cancer and AIDS-related Kaposi’s sarcoma while reducing the drug’s side effects, which can be toxic to various parts of the body, particularly the skin and the heart. Doxorubicin was developed by encapsulating an 80–90 nm size unilamellar liposome coated with PEG [149,150]. Two different mechanisms of doxorubicin have been confirmed to act on cancer cells: intercalation into DNA and the disruption of topoisomerase II-mediated DNA repair, and the generation of free radicals, resulting in damage to cellular membranes, DNA, and proteins [151]. However, doxorubicin has been known to cause severe heart problems, such as heart failure, occurring upon or after treatment [149].
The second strategy for targeting tumors is active targeting, which is based on the interaction between ligand-coated nanoparticles and tumor markers. This strategy of targeting results in an increased accumulation of nanoparticles at the target site or an enhanced cellular uptake of nanoparticles by expressing the target receptor [4]. Abraxane is an example of an active-targeting nanomedicine that was approved by the FDA for the treatment of metastatic breast cancer in 2005, lung cancer in 2012, and advanced pancreatic cancer in 2013. In this product, human albumin is conjugated to paclitaxel in order to deliver it in the form of nanoparticles (130 nanometers in diameter). Biodegradability, lack of toxicity, and immunogenicity make albumin an ideal carrier for drug delivery by causing a potential uptake by cells in tumor and inflamed tissue. The bioavailability of albumin-bound paclitaxel (Abraxane) in the tumor is facilitated by the gp60 receptor (albondin)-mediated pathway in the endothelial cell walls of tumors, which binds to albumin with a high affinity [152,153,154]. The accumulation of Abraxane at the tumor site is also facilitated by SPARC (secreted protein, acidic and rich in cysteine), overexpressed in multiple types of tumors, including breast, prostate, gastric, lung, and kidney. Thus, it should be evident that the response of Abraxane will be enhanced with an increased SPARC level in the tumor [149].
Another innovative strategy for targeting is stimuli-responsive tumor targeting, which focuses on nanoparticles that can be triggered to release their contents on exposure to external stimuli such as heat, light, or ultrasound. ThermoDox is a good example of a stimuli-responsive targeting nanomedicine; it was developed by Celsion Corporation in partnership with Duke University for the treatment of liver cancer. ThermoDox is a temperature-sensitive doxorubicin-pegylated liposome that is able to release the contents of the drug at the target site by elevating the temperature to 39–42 °Celsius via the application of radiofrequency [149]. Moreover, the accumulation of ThermoDox nanoparticles in tumors increases as the blood vessels within the tumors become leaky due to the local application of hyperthermia [149]. Tissue-specific ligand-coated nanoparticles and stimuli-controlled nanoparticle release are the next-generation cancer nanomedicines to target tumors and increase the accumulation of the drug in the tumor.
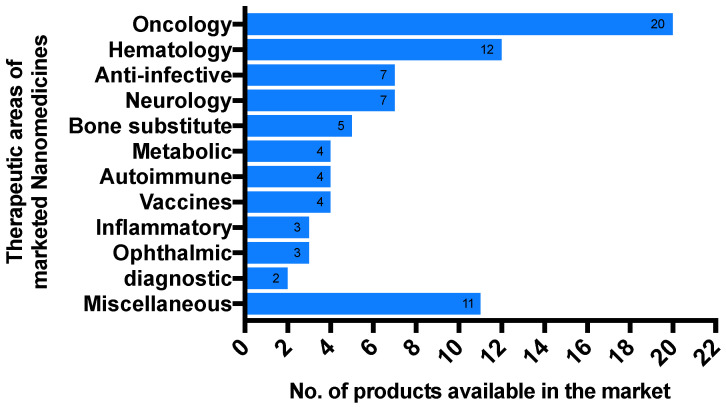
Although more anticancer nanomedicines are currently available on the market than any other drug classes, many formulations are also being marketed for other indications, including autoimmune conditions, metabolic disorders, ophthalmic conditions, neurological diseases, hematological disorders, inflammatory diseases, and others [53] (Figure 1). The majority of the developed nanomedicines have already proven successful use with liposomes and polymers as incorporated NPs [53]. Furthermore, there is a clear trend in the movement of NPs from simple to complex multicomponent drug-delivery systems [154]. These complex systems can involve functions such as controlled release and active targeting to allow nanomedicines to improve PK, efficacy, and safety. While most FDA-approved nanomedicines are based on passive targeting via the EPR effect, some next-generation nanomedicines use active-targeting approaches in clinical trials [4,147,155] (Table 2).
Figure 1.
List of globally marketed nanomedicines.
Table 2.
Selected nanomedicines that are in phase III clinical trials.
| Type | Proprietary Name | Company | Indication | Active Ingredients | NCT Number | Status | Outcome |
|---|---|---|---|---|---|---|---|
| Lipid-based Nanoparticles | ThermoDox | Celsion | hepatocellular carcinoma [156] | doxorubicin | NCT00617981 | completed | Positive: ThermoDox increased intratumoral concentration of doxorubicin under external hyperthermia induction by 3.7 times compared with ThermoDox without hyperthermia induction |
| EndoTAG-1 | SynCore Biotechnology | breast cancer | paclitaxel | NCT03002103 | ongoing | - | |
| pancreatic adenocarcinoma [157] | paclitaxel | NCT03126435 | ongoing | - | |||
| Allovectin-7® | Vical | melanoma | VCL-1005 plasmid | NCT00395070 | completed | Nothing has been mentioned | |
| Tecemotide | Merk KGaA | non-small-cell lung cancer | MUC1 antigen | NCT00409188 | completed | Negative: the administration of Tecemotide after chemoradiotherapy compared with placebo showed no significant difference in overall survival for all patients with stage III NSCLC | |
| MAGE-A3 + AS15 | GSK | melanoma | human melanoma-associated antigen A3 protein | NCT00796445 | terminated | Negative: MAGE-A3 immunotherapeutic for use in melanoma has been stopped, as it was not efficacious | |
| non-small-cell lung cancer | NCT00480025 | terminated | Negative: MAGE-A3 immunotherapeutic for use in NSCLC has been stopped because it did not increase disease-free survival compared with placebo | ||||
| MM-302 | Merricmack Pharmaceutical | breast cancer | doxorubicinhydrochloride | NCT02213744 | terminated | Negative: MM-302 did not demonstrate benefits over the control | |
| Taxoprexin | Luitpold Pharmaceuticals | melanoma | paclitaxel | NCT00087776 | completed | Nothing has been mentioned | |
| non-small-cell lung cancer | NCT00243867 | completed | Nothing has been mentioned | ||||
| Nanocort | Enceladus in collaboration with Sun Pharma Global | rheumatoid arthritis | prednisolone | NCT02534896 | terminated | Nothing has been mentioned | |
| Polymer-based Nanoparticles | Nanoplatin | NanoCarrier | advanced solid tumors, lung, biliary, bladder, or pancreatic cancers | cisplatin | NCT02043288 | completed | Nothing has been mentioned |
| CRLX101 | Cerulean | ovarian, renal cell, small cell lung, or rectal cancers | Cyclodextrin-Camptothecin | NCT00163319 | completed | Nothing has been mentioned | |
| NC-6004 | NanoCarrier | pancreatic cancer | cisplatin | NCT02043288 | completed | Nothing has been mentioned | |
| NKTR-102 | Nektar Therapeutics | breast cancer brain metastases (BCBM) | irinotecan | NCT02915744 | completed | Positive: there was a significant improvement in survival of patients with BCBM | |
| NK-105 | NanoCarrier | breast cancer | paclitaxel | NCT01644890 | completed | Negative: the progression-free survival, which was the primary outcome measure, was not met | |
| CT-2103 | CTI BioPharma | ovarian, peritoneal, or fallopian tube cancer | paclitaxel | NCT00108745 | ongoing | - | |
| non-small-cell lung cancer | NCT00054210 | terminated | Nothing has been mentioned | ||||
| Inorganic nanoparticles | NBTXR3 | Nanobiotix | sarcoma | hafnium-oxide nanoparticle | NCT02379845 | ongoing | - |
| Other | Livatag | Onxeo | hepatocellular carcinoma | doxorubicin | NCT01655693 | ongoing | - |
A good example of an active-targeting nanomedicine that has been in clinical trials is MAGE-A3 + AS15, an immunotherapeutic product that is given to patients with carcinomic tumors after the removal of the tumor [158]. Several tumor antigens are encoded by genes of the MAGE-A family that are expressed in various tumor types, such as melanoma [159,160], non-small-cell lung cancer [161], bladder cancer [162,163], and liver cancer [164]. However, these antigens are not expressed in normal tissues, except male germline cells or placenta [165]. Therefore, antigen-based active immunotherapy is an attractive way to fight against cancer, as it has the potential to prepare the immune system of the patient to eliminate the tumor cells and to prolong their lives. Different studies have claimed that the MAGE-A3 protein alone without an immunostimulant has shown limited immunologic and clinical responses [166,167]. A novel immunostimulant, which is a combination of QS21, monophosphoryl lipid A, and CpG7909 (a TLR-9 agonist) in a liposomal formulation (AS15), was associated with a more robust cellular and humoral response in phase II clinical trials compared with other immunostimulants. Therefore, this AS15 immunostimulant has been chosen for further advanced clinical trials [158]. Two phase III clinical trials proceeded with MAGE-A3 + AS15 for melanoma and non-small-cell lung cancer, but both have been terminated, as MAGE-A3 + AS15 was not efficacious and did not increase disease-free survival compared with the placebo (Table 2).
7. Potential Causes for the Failure of Some Nanomedicines
Although the pharmaceutical companies that develop nanomedicines obtain funding from venture capital, capital markets, and partnerships with industries, clinical failure of these products may occur and result in product terminations and business liquidation [147]. Here, we would like to briefly cover the most common causes of nanomedicine clinical trial termination or discontinuation from the market. Nanomedicine toxicity in clinical trials is one of the most common reasons related to clinical failure that usually occurs in phase I trials. For example, the MRX34 nanomedicine, manufactured by Mirna Therapeutics, Austin, TX, USA, was terminated in 2016 in a phase I clinical trial, as one-fifth of patients experienced severe immune-related adverse events [147]. In 2019, due to cumulative neuropathy observed in phase I trials, Merrimack Pharmaceuticals ended the development of MM-310, which was an antibody-directed nanotherapeutic [147]. In addition, the choice of drug carrier is critical in terms of how it works and what the physicochemical properties are. BIND-014, an anticancer treatment for head and neck cancer, failed in a phase II clinical trial (NCT02479178), possibly due to the wrong choice of payload [147].
The right selection of patient is a critical step in successful clinical trials. For instance, involving patients with heterogenous cancer diseases in clinical trials that are established to investigate the safety and efficacy of anticancer treatments could contribute to poor clinical outcomes. Therefore, the development of innovative approaches to control in vivo monitoring of the distribution and transport of nanoparticles will provide a good reference for all researchers in this field [147].
The high-quality production of nanoparticles with reproducibility to meet high manufacturing practice standards is a major challenge in nanomedicine development. Thus, there are some cases of FDA-approved nanomedicines that were discontinued from the market because of manufacturing issues. In 2017, the production of DepoCyt, an injectable nanomedicine used for treating lymphomatous meningitis, was discontinued by Pacira Pharmaceuticals due to unknown technical problems in its manufacturing process [147].
8. Future of Nanomedicines
Nanotechnology is already starting to have an impact on the healthcare system. The effect of nanotechnology on healthcare is already being felt, as different nanotechnology ideas have been developed, and several nanotechnology-based medicines are now on the market. Increasing the progress and interest in the field of nanotechnology from the last two decades is predicted for future positive developments in nanomedicines. Therefore, the future of nanomedicines is likely to follow two paths. One path has been already established, where nanomedicines are mainly and still developed for cancer indications; this can be seen in Figure 1, as most nanomedicines currently available in the market are anticancer. Another path is developing nanomedicines to target different diseases other than cancers, which is demonstrated by the recent approvals of Patisiran/ONPATTRO (the first FDA-approved RNAi therapeutic) and VYXEOS (a nanoparticle capable of delivering synergistic ratios of two drugs). More developments along the second path may be required to increase application of nanomedicines to those diseases that cannot be effectively addressed with CDDSs. For instance, nanomedicine in medical diagnosis will enable detection and examination of tissues in more detail: at the cellular, subcellular, and molecular levels, using particular devices [158]. Such a diagnosis would guide the proper treatment, and is called “Personalized Medicine”. Furthermore, to get maximal benefits from the nanoparticle-based strategy for drug delivery, in vitro and in vivo studies need to be further pursued to reach an understanding the behaviors of nanoparticles to accelerate nanomedicines through clinical development, and then provide them to the patients who need them.
Across many parts of the world, nanotechnology draws increasing investment from public authorities and the private sector [2]. In 2016, the global market size of nanomedicine reached USD 138.8 billion, and by 2025, it is projected to hit USD 350.8 billion. This shows the importance of nanotechnology in the field of drug delivery. Reducing undesirable toxicity from nonspecific distribution and improving patient adherence are the main advantages of using nanotechnology to deliver therapeutic agents, with an indirect reduction in the burden on the healthcare system.
Abbreviations
| Word | Abbreviation |
| Conventional drug delivery systems | CDDSs |
| Food and Drug Administration | FDA |
| European Medicines Agency | EMA |
| Active pharmaceutical ingredients | APIs |
| Nanoparticles | NPs |
| Enhanced permeability and retention | EPR |
| Gastrointestinal tract | GIT |
| Reticuloendothelial system | RES |
| Central nervous system | CNS |
| Poly(butylcyanoacrylate) | PBCA |
| Poly(lactic-co-glycolic acid) | PLGA |
| Poly(lactic acid) | PLA |
| Federal Food, Drug, and Cosmetic Act | FDCA |
| Public Health Service Act | PHSA |
| Nonbiological complex drugs | NBCDs |
| Pharmacokinetics | PK |
| Chemistry, manufacturing, and controls | CMC |
| Nuclear magnetic resonance | NMR |
| Mass spectrometry | MS |
| Research and development | R&D |
| World Health Organization | WHO |
| Polyethylene glycol | PEG |
Funding
This research received no external funding.
Conflicts of Interest
The author declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farjadian F., Ghasemi A., Gohari O., Roointan A., Karimi M., Hamblin M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine. 2019;14:93–126. doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thassu D., Pathak Y., Deleers M. Nanoparticulate Drug-Delivery Systems: An Overview. Nanopart. Drug Deliv. Syst. 2007;9:31. doi: 10.1201/9781420008449-1. [DOI] [Google Scholar]
- 3.Jain K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pr. 2008;17:89–101. doi: 10.1159/000112961. [DOI] [PubMed] [Google Scholar]
- 4.Havel H., Finch G., Strode P., Wolfgang M., Zale S., Bobe I., Youssoufian H., Peterson M., Liu M. Nanomedicines: From Bench to Bedside and Beyond. AAPS J. 2016;18:1373–1378. doi: 10.1208/s12248-016-9961-7. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y.H., Han H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018;48:43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majuru S., Oyewumi M.O. Nanotechnology in Drug Development and Life Cycle Management. Nanotechnol. Drug Deliv. 2009:597–619. doi: 10.1007/978-0-387-77668-2_20. [DOI] [Google Scholar]
- 7.Desai N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ventola C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 9.Alami-Milani M., Zakeri-Milani P., Valizadeh H., Salehi R., Jelvehgari M. Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran. J. Basic Med. Sci. 2017;21:153–164. doi: 10.22038/IJBMS.2017.26590.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong C.Y., Al-Salami H., Dass C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release. 2017;264:247–275. doi: 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Goyal R., Macri L.K., Kaplan H.M., Kohn J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release. 2016;240:77–92. doi: 10.1016/j.jconrel.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernocchi B., Carpentier R., Betbeder D. Nasal nanovaccines. Int. J. Pharm. 2017;530:128–138. doi: 10.1016/j.ijpharm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Ragelle H., Riva R., Vandermeulen G., Naeye B., Pourcelle V., Le Duff C.S., D’Haese C., Nysten B., Braeckmans K., De Smedt S., et al. Chitosan nanoparticles for siRNA delivery: Optimizing formulation to increase stability and efficiency. J. Control. Release. 2014;176:54–63. doi: 10.1016/j.jconrel.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Lu Z., Wientjes M.G., Au J.L.-S. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cone R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Suk J.S., Lai S.K., Boylan N.J., Dawson M.R., Boyle M.P., Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine. 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S.-D., Huang L. Gene therapy progress and prospects: Non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- 20.Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Zheng W., Aschner M., Ghersi-Egea J.-F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003;192:1–11. doi: 10.1016/S0041-008X(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koffie R.M., Farrar C., Saidi L.-J., William C., Hyman B.T., Spires-Jones T.L. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA. 2011;108:18837–18842. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergoni A.V., Tosi G., Tacchi R., Vandelli M.A., Bertolini A., Costantino L. Nanoparticles as drug delivery agents specific for CNS: In vivo biodistribution. Nanomed. Nanotechnol. Biol. Med. 2009;5:369–377. doi: 10.1016/j.nano.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Spuch C., Navarro C. Liposomes for Targeted Delivery of Active Agents against Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease) J. Drug Deliv. 2011;2011:469679. doi: 10.1155/2011/469679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y., Dai Q., Morshed R.A., Fan X., Wegscheid M.L., Wainwright D., Han Y., Zhang L., Auffinger B., Tobias A.L., et al. Blood-Brain Barrier Permeable Gold Nanoparticles: An Efficient Delivery Platform for Enhanced Malignant Glioma Therapy and Imaging. Small. 2014;10:5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trickler W.J., Lantz S.M., Murdock R.C., Schrand A.M., Robinson B.L., Newport G.D., Schlager J.J., Oldenburg S.J., Paule M.G., Slikker W., et al. Silver Nanoparticle Induced Blood-Brain Barrier Inflammation and Increased Permeability in Primary Rat Brain Microvessel Endothelial Cells. Toxicol. Sci. 2010;118:160–170. doi: 10.1093/toxsci/kfq244. [DOI] [PubMed] [Google Scholar]
- 27.Grabrucker A.M., Rowan M., Garner C.C. Brain-Delivery of Zinc-Ions as Potential Treatment for Neurological Diseases: Mini Review. Drug Deliv. Lett. 2011;1:13–23. doi: 10.2174/2210304x11101010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang S.H., Wientjes M.G., Lu D., Au J.L. Drug Delivery and Transport to Solid Tumors. Pharm. Res. 2003;20:1337–1350. doi: 10.1023/A:1025785505977. [DOI] [PubMed] [Google Scholar]
- 29.Sriraman S.K., Aryasomayajula B., Torchilin V.P. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2:e29528. doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan V.P., Stylianopoulos T., Boucher Y., Jain R.K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu. Rev. Chem. Biomol. Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Lin Y., Gillies R.J. Tumor pH and Its Measurement. J. Nucl. Med. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandhiya S., Dkhar S.A., Surendiran A. Emerging trends of nanomedicine-an overview. Fundam. Clin. Pharmacol. 2009;23:263–269. doi: 10.1111/j.1472-8206.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Wartchow C.A., Danthi S.N., Shen Z., Dechene N., Pease J., Choi H.S., Doede T., Chu P., Ning S. A novel antiangiogenesis therapy using an integrin antagonist or anti–Flk-1 antibody coated 90Y-labeled nanoparticles. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1215–1227. doi: 10.1016/j.ijrobp.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Farokhzad O.C., Cheng J., Teply B.A., Sherifi I., Jon S., Kantoff P., Richie J.P., Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Özcan I., Bouchemal K., Segura-Sánchez F. Effects of sterilization techniques on the PEGylated poly (γ-benzyl-L-glutamate) (PBLG) nanoparticles. Acta Pharm. Sci. 2009;11:7945. [Google Scholar]
- 36.Bozdag S., Dillen K., Vandervoort J., Ludwig A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D,L-lactide-glycolide) nanoparticles. J. Pharm. Pharmacol. 2010;57:699–707. doi: 10.1211/0022357056145. [DOI] [PubMed] [Google Scholar]
- 37.Kempner E.S. Effects of High-Energy Electrons and Gamma Rays Directly on Protein Molecules. J. Pharm. Sci. 2001;90:1637–1646. doi: 10.1002/jps.1114. [DOI] [PubMed] [Google Scholar]
- 38.Nel A., Xia T., Mädler L., Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 39.Song Y., Li X., Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 40.Mühlebach S. Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv. Drug Deliv. Rev. 2018;131:122–131. doi: 10.1016/j.addr.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Paradise J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethic. 2019;21:E347–E355. doi: 10.1001/amajethics.2019.347. [DOI] [PubMed] [Google Scholar]
- 42.Soares S., Sousa J., Pais A., Vitorino C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018;6:360. doi: 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resnik D.B., Tinkle S.S. Ethical issues in clinical trials involving nanomedicine. Contemp. Clin. Trials. 2007;28:433–441. doi: 10.1016/j.cct.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bawa R., Johnson S. The Ethical Dimensions of Nanomedicine. Med. Clin. N. Am. 2007;91:881–887. doi: 10.1016/j.mcna.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Hussaarts L., Mühlebach S., Shah V.P., McNeil S., Borchard G., Flühmann B., Weinstein V., Neervannan S., Griffiths E., Jiang W., et al. Equivalence of complex drug products: Advances in and challenges for current regulatory frameworks. Ann. N. Y. Acad. Sci. 2017;1407:39–49. doi: 10.1111/nyas.13347. [DOI] [PubMed] [Google Scholar]
- 46.Astier A., Pai A.B., Bissig M., Crommelin D.J., Flühmann B., Hecq J.-D., Knoeff J., Lipp H.-P., Morell-Baladrón A., Mühlebach S. How to select a nanosimilar. Ann. N. Y. Acad. Sci. 2017;1407:50–62. doi: 10.1111/nyas.13382. [DOI] [PubMed] [Google Scholar]
- 47.Schellekens H., Stegemann S., Weinstein V., De Vlieger J.S.B., Flühmann B., Mühlebach S., Gaspar R., Shah V.P., Crommelin D.J.A. How to Regulate Nonbiological Complex Drugs (NBCD) and Their Follow-on Versions: Points to Consider. AAPS J. 2013;16:15–21. doi: 10.1208/s12248-013-9533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tinkle S., McNeil S.E., Mühlebach S., Bawa R., Borchard G., Barenholz Y., Tamarkin L., Desai N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014;1313:35–56. doi: 10.1111/nyas.12403. [DOI] [PubMed] [Google Scholar]
- 49.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad M., Lambe U.P., Brar B., Shah I., Manimegalai J., Ranjan K., Rao R., Kumar S., Mahant S., Khurana S.K., et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018;97:1521–1537. doi: 10.1016/j.biopha.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 51.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein K., Stolk P., De Bruin M.L., Leufkens H., Crommelin D., De Vlieger J. The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: Observations and recommendations. Eur. J. Pharm. Sci. 2019;133:228–235. doi: 10.1016/j.ejps.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 53.Caster J., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed. Nanobiotechnol. 2017;9:e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 54.Andrews P., Kovacs M., Watson J. The anti-emetic action of the neurokinin1 receptor antagonist CP-99,994 does not require the presence of the area postrema in the dog. Neurosci. Lett. 2001;314:102–104. doi: 10.1016/S0304-3940(01)02269-8. [DOI] [PubMed] [Google Scholar]
- 55.Müller R.H., Junghanns J.-U.A.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008;3:295–309. doi: 10.2147/IJN.S595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnock-Jones K.P. Fosaprepitant Dimeglumine: A Review in the Prevention of Nausea and Vomiting Associated with Chemotherapy. Drugs. 2016;76:1365–1372. doi: 10.1007/s40265-016-0627-7. [DOI] [PubMed] [Google Scholar]
- 57.Huber F.-X., Belyaev O., Hillmeier J., Kock H.-J., Huber C., Meeder P.-J., Berger I. First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste OSTIM® in human cancellous bone. BMC Musculoskelet. Disord. 2006;7:50. doi: 10.1186/1471-2474-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayan R., Pednekar A., Bhuyan D., Gowda C., Nayak U.Y. A top-down technique to improve the solubility and bioavailability of aceclofenac: In vitro and in vivo studies. Int. J. Nanomed. 2017;12:4921–4935. doi: 10.2147/IJN.S141504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marya S., Ariyanayagam T., Chatterjee B., Toms A.P., Crawford R. A Prospective Study of the Efficacy of Vitoss (Beta Tricalcium Phosphate) as a Bone Graft Substitute for Instrumented Posterolateral Lumbar Fusions. Spine J. 2017;17:S23. doi: 10.1016/j.spinee.2016.12.071. [DOI] [Google Scholar]
- 60.Diller L.H. The Run on Ritalin: Attention Deficit Disorder and Stimulant Treatment in the 1990s. Häst. Cent. Rep. 1996;26:12. doi: 10.2307/3528571. [DOI] [PubMed] [Google Scholar]
- 61.Caldwell J.R., Rapoport R.J., Davis J.C., Offenberg H.L., Marker H.W., Roth S.H., Yuan W., Eliot L., Babul N., Lynch P.M. Efficacy and Safety of a Once-Daily Morphine Formulation in Chronic, Moderate-to-Severe Osteoarthritis Pain: Results from a Randomized, Placebo-Controlled, Double-Blind Trial and an Open-Label Extension Trial. J. Pain Symptom Manag. 2002;23:278–291. doi: 10.1016/S0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 62.Chavez B., Sopko M.A., Ehret M.J., Paulino R.E., Goldberg K.R., Angstadt K., Bogart G.T. An Update on Central Nervous System Stimulant Formulations in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Ann. Pharmacother. 2009;43:1084–1095. doi: 10.1345/aph.1L523. [DOI] [PubMed] [Google Scholar]
- 63.Janssen P. DailyMed—INVEGA-paliperidone tablet, extended release. Daily Med. USA Natl. Libr. Med. 2012;14:45–49. [Google Scholar]
- 64.Janssen P. Invega Sustenna. Food Drug Adm. 2009;11:160407. [Google Scholar]
- 65.Musaji N. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int. J. Nanomed. 2009;4:185–192. doi: 10.2147/IJN.S6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Epstein N. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg. Neurol. Int. 2015;6:318–322. doi: 10.4103/2152-7806.159380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langston J.R., DeHaan A.M., Huff T.W. Staged total hip arthroplasty in a patient with hip dysplasia and a large pertrochanteric bone cyst. Arthroplast. Today. 2016;2:57–61. doi: 10.1016/j.artd.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke P.D., Adams P., Ibáñez R., Herzog C. Rate, intensity, and duration of local reactions to a virosome-adjuvanted vs. an aluminium-adsorbed hepatitis A vaccine in UK travellers. Travel Med. Infect. Dis. 2006;4:313–318. doi: 10.1016/j.tmaid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Kaddar N., Vigneault P., Pilote S., Patoine D., Simard C., Drolet B. Tizanidine (Zanaflex) J. Cardiovasc. Pharmacol. Ther. 2011;17:102–109. doi: 10.1177/1074248410395020. [DOI] [PubMed] [Google Scholar]
- 70.Wappler F. S1-Leitlinie maligne Hyperthermie. Der Anaesthesist. 2018;67:529–532. doi: 10.1007/s00101-018-0462-1. [DOI] [PubMed] [Google Scholar]
- 71.Alagona P. Fenofibric acid: A new fibrate approved for use in combination with statin for the treatment of mixed dyslipidemia. Vasc. Heal. Risk Manag. 2010;6:351–362. doi: 10.2147/VHRM.S6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barenholz Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 73.Smith J.A., Mathew L., Burney M., Nyshadham P., Coleman R.L. Equivalency challenge: Evaluation of Lipodox® as the generic equivalent for Doxil® in a human ovarian cancer orthotropic mouse model. Gynecol. Oncol. 2016;141:357–363. doi: 10.1016/j.ygyno.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 74.Petre C., Dittmer D.P. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007;2:277–288. [PMC free article] [PubMed] [Google Scholar]
- 75.Drummond D.C., Noble C.O., Guo Z., Hong K., Park J.W., Kirpotin D. Development of a Highly Active Nanoliposomal Irinotecan Using a Novel Intraliposomal Stabilization Strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 76.Glantz M.J., Jaeckle K.A., Chamberlain M.C., Phuphanich S., Recht L., Swinnen L.J., Maria B., LaFollette S., Schumann G.B., Cole B.F., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999;5:11. [PubMed] [Google Scholar]
- 77.Balazsovits J.A.E., Mayer L.D., Bally M.B., Cullis P.R., McDonell M., Ginsberg R.S., Falk R.E. Analysis of the effect of liposome encapsulation on the vesicant properties, acute and cardiac toxicities, and antitumor efficacy of doxorubicin. Cancer Chemother. Pharmacol. 1989;23:81–86. doi: 10.1007/BF00273522. [DOI] [PubMed] [Google Scholar]
- 78.Batist G., Ramakrishnan G., Rao C.S., Chandrasekharan A., Gutheil J., Guthrie T., Shah P., Khojasteh A., Nair M.K., Hoelzer K., et al. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared With Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. J. Clin. Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 79.Tejada-Berges T., Granai C., Gordinier M., Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev. Anticancer. Ther. 2002;2:143–150. doi: 10.1586/14737140.2.2.143. [DOI] [PubMed] [Google Scholar]
- 80.Franco Y.L., Vaidya T.R., Ait-Oudhia S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer Targets Ther. 2018;10:131–141. doi: 10.2147/BCTT.S170239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frampton J.E. Mifamurtide. Pediatr. Drugs. 2010;12:141–153. doi: 10.2165/11204910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 82.Johnston M.J., Semple S.C., Klimuk S.K., Edwards K., Eisenhardt M.L., Leng E.C., Karlsson G., Yanko D., Cullis P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta (BBA) Biomembr. 2006;1758:55–64. doi: 10.1016/j.bbamem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Krishna R., Webb M.S., Onge G.S., Mayer L.D. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 2001;298 [PubMed] [Google Scholar]
- 84.Weng Y., Xiao H., Zhang J., Liang X.-J., Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019;37:801–825. doi: 10.1016/j.biotechadv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y.Y. Approval of the first-ever rnai therapeutics and its technological development history. Prog. Biochem. Biophys. 2019;46:249. doi: 10.16476/j.pibb.2018.0249. [DOI] [Google Scholar]
- 86.Zhou H., Yan J., Chen W., Yang J., Liu M., Zhang Y., Shen X., Ma Y., Hu X., Wang Y., et al. Population Pharmacokinetics and Exposure–Safety Relationship of Paclitaxel Liposome in Patients with Non-small Cell Lung Cancer. Front. Oncol. 2021;10:01731. doi: 10.3389/fonc.2020.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lister J. Amphotericin B Lipid Complex (Abelcet®) in the treatment of invasive mycoses: The North American experience. Eur. J. Haematol. 1996;56:18–23. doi: 10.1111/j.1600-0609.1996.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 88.Krauss A.C., Gao X., Li L., Manning M.L., Patel P., Fu W., Janoria K.G., Gieser G., Bateman D.A., Przepiorka D., et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019;25:2685–2690. doi: 10.1158/1078-0432.CCR-18-2990. [DOI] [PubMed] [Google Scholar]
- 89.Hartrick C.T., Manvelian G. Sustained-release epidural morphine (DepoDurTM): A review. Today’s Ther. Trends. 2004;22:33. [Google Scholar]
- 90.Van Helden H., Kuijpers W., Langerwerf P., Langen R., Haagsman H., Bruijnzeel P. Efficacy of Curosurf in a rat model of acute respiratory distress syndrome. Eur. Respir. J. 1998;12:533–539. doi: 10.1183/09031936.98.12030533. [DOI] [PubMed] [Google Scholar]
- 91.Gharehbaghi M.M., Yasrebi S. Comparing the Efficacy of two Natural Surfactants, Curosurf and Alveofact, in Treatment of Respiratory Distress Syndrome in Preterm Infants. Int. J. Women’s Health Reprod. Sci. 2014;2:245–248. doi: 10.15296/ijwhr.2014.36. [DOI] [Google Scholar]
- 92.Rizzieri D. Zevalin® (ibritumomab tiuxetan): After more than a decade of treatment experience, what have we learned? Crit. Rev. Oncol. 2016;105:5–17. doi: 10.1016/j.critrevonc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Glück R., Mischler R., Finkel B., Que J., Cryz S., Scarpa B. Immunogenicity of new virosome influenza vaccine in elderly people. Lancet. 1994;344:160–163. doi: 10.1016/S0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- 94.Conne P., Gauthey L., Vernet P., Althaus B., Que J.U., Finkel B., Glück R., Cryz S.J. Immunogenicity of trivalent subunit versus virosome-formulated influenza vaccines in geriatric patients. Vaccine. 1997;15:1675–1679. doi: 10.1016/S0264-410X(97)00087-X. [DOI] [PubMed] [Google Scholar]
- 95.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_. [DOI] [PubMed] [Google Scholar]
- 97.Hinton D.M. Moderna COVID-19 Vaccine EUA Letter of Authorization. US Food Drug Adm. 2020;18:564. [Google Scholar]
- 98.Connock M., Tubeuf S., Malottki K., Uthman A., Round J., Bayliss S., Meads C., Moore D. Certolizumab pegol (CIMZIA®) for the treatment of rheumatoid arthritis. Heal. Technol. Assess. 2010;14:1–10. doi: 10.3310/hta14suppl2-01. [DOI] [PubMed] [Google Scholar]
- 99.Schreiber S. Certolizumab pegol for the treatment of Crohn’s disease. Ther. Adv. Gastroenterol. 2011;4:375–389. doi: 10.1177/1756283X11413315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mease P.J., Fleischmann R., Deodhar A., Wollenhaupt J., Khraishi M., Kielar D., Woltering F., Stach C., Hoepken B., Arledge T., et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann. Rheum. Dis. 2014;73:48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landewé R., Braun J., Deodhar A., Dougados M., Maksymowych W.P., Mease P.J., Reveille J.D., Rudwaleit M., Van Der Heijde D., Stach C., et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borgå O., Lilienberg E., Bjermo H., Hansson F., Heldring N., Dediu R. Pharmacokinetics of Total and Unbound Paclitaxel After Administration of Paclitaxel Micellar or Nab-Paclitaxel: An Open, Randomized, Cross-Over, Explorative Study in Breast Cancer Patients. Adv. Ther. 2019;36:2825–2837. doi: 10.1007/s12325-019-01058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stephan J., Vlekova V., Le Deist F., Blanche S., Donadieu J., Basile G.D.S., Durandy A., Griscelli C., Fischer A. Severe combined immunodeficiency: A retrospective single-center study of clinical presentation and outcome in 117 patients. J. Pediatr. 1993;123:564–572. doi: 10.1016/S0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 104.Sheridan W., Fox R., Begley C., Maher D., McGrath K., Juttner C., To L., Szer J., Mostyn G. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 105.Harris J.M., Chess R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 106.Kim J., Shin S. Cost-Effectiveness of Genexol-PM for Treating Metastatic Breast Cancer. J. Breast Cancer. 2010;13:104–110. doi: 10.4048/jbc.2010.13.1.104. [DOI] [Google Scholar]
- 107.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Gonçales F.L., Häussinger D., Diago M., Carosi G., Dhumeaux D., et al. Peginterferon Alfa-2a plus Ribavirin for Chronic Hepatitis C Virus Infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 108.Lau G.K.K., Piratvisuth T., Luo K.X., Marcellin P., Thongsawat S., Cooksley G., Gane E., Fried M.W., Chow W.C., Paik S.W., et al. Peginterferon Alfa-2a, Lamivudine, and the Combination for HBeAg-Positive Chronic Hepatitis B. N. Engl. J. Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 109.Leonart L.P., Tonin F.S., Ferreira V.L., Fernandez-Llimos F., Pontarolo R. Effectiveness and safety of pegvisomant: A systematic review and meta-analysis of observational longitudinal studies. Endocrine. 2018;63:18–26. doi: 10.1007/s12020-018-1729-7. [DOI] [PubMed] [Google Scholar]
- 110.Penman A.D., Crowder K.W., Watkins W.M. Pegaptanib for Neovascular Age-Related Macular Degeneration. Stud. Every Ophthalmol. 2020;76:239–244. doi: 10.1093/med/9780190050726.003.0039. [DOI] [Google Scholar]
- 111.McGahan L. Continuous erythropoietin receptor activator (Mircera) for renal anemia. Issues Emerg. Health Technol. 2008;11:1544. [PubMed] [Google Scholar]
- 112.Jacobson I.M., Brown R.S., Freilich B., Afdhal N., Kwo P.Y., Santoro J., Becker S., Wakil A.E., Pound D., Godofsky E., et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: A randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 113.Nyborg A.C., Ward C., Zacco A., Chacko B., Grinberg L., Geoghegan J.C., Bean R., Wendeler M., Bartnik F., O’Connor E., et al. A Therapeutic Uricase with Reduced Immunogenicity Risk and Improved Development Properties. PLoS ONE. 2016;11:e0167935. doi: 10.1371/journal.pone.0167935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaplin S., Gnanapavan S. Plegridy for the treatment of RRMS in adults. Prescriber. 2015;26:29–31. doi: 10.1002/psb.1387. [DOI] [Google Scholar]
- 115.Turecek P.L., Romeder-Finger S., Apostol C., Bauer A., Crocker-Buqué A., Burger D.A., Schall R., Gritsch H. A world-wide survey and field study in clinical haemostasis laboratories to evaluate FVIII: C activity assay variability of ADYNOVATE and OBIZUR in comparison with ADVATE. Haemophilia. 2016;22:957–965. doi: 10.1111/hae.13001. [DOI] [PubMed] [Google Scholar]
- 116.Grewal I.S. Emerging Protein Biotherapeutics. CRC Press; Boca Raton, FL, USA: 2009. [Google Scholar]
- 117.Melamed-Gal S., Loupe P., Timan B., Weinstein V., Kolitz S., Zhang J., Funt J., Komlosh A., Ashkenazi N., Bar-Ilan O., et al. Physicochemical, biological, functional and toxicological characterization of the European follow-on glatiramer acetate product as compared with Copaxone. Neurol. Sci. 2018;12:19–30. doi: 10.1016/j.ensci.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berges R. Eligard®: Pharmacokinetics, Effect on Testosterone and PSA Levels and Tolerability. Eur. Urol. Suppl. 2005;4:20–25. doi: 10.1016/j.eursup.2005.04.001. [DOI] [Google Scholar]
- 119.Slatopolsky E.A., Burke S.K., Dillon M.A. RenaGel®, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. Kidney Int. 1999;55:299–307. doi: 10.1046/j.1523-1755.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- 120.Swanson B.J., Limketkai B.N., Liu T.-C., Montgomery E., Nazari K., Park J.Y., Santangelo W.C., Torbenson M.S., Voltaggio L., Yearsley M.M., et al. Sevelamer Crystals in the Gastrointestinal Tract (GIT) Am. J. Surg. Pathol. 2013;37:1686–1693. doi: 10.1097/PAS.0b013e3182999d8d. [DOI] [PubMed] [Google Scholar]
- 121.Hwang S.-J., Kang H., Cha K.-H., Cho W., Park J., Park H.J., Sun B.K., Hyun S.-M. Cyclosporine A micellar delivery system for dry eyes. Int. J. Nanomed. 2016;11:2921–2933. doi: 10.2147/IJN.S107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ezban M., Hermit M.B., Persson E. FIXing postinfusion monitoring: Assay experiences with N9-GP (nonacog beta pegol; Refixia®; Rebinyn®) Haemophilia. 2019;25:154–161. doi: 10.1111/hae.13671. [DOI] [PubMed] [Google Scholar]
- 123.Nielsen F.S., Schmidt A.S., Kristensen A.K., Nielsen A.D., Kristensen B.K., Palm L. Characterisation of recombinant factor IX before and after GlycoPEGylation. Int. J. Pharm. 2020;588:119654. doi: 10.1016/j.ijpharm.2020.119654. [DOI] [PubMed] [Google Scholar]
- 124.Buster J. Transdermal menopausal hormone therapy: Delivery through skin changes the rules. Expert Opin. Pharmacother. 2010;11:1489–1499. doi: 10.1517/14656561003774098. [DOI] [PubMed] [Google Scholar]
- 125.Rai M.F., Pham C.T. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018;40:67–73. doi: 10.1016/j.coph.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Madan R.P., Dezzutti C., Rabe L., Hillier S.L., Marrazzo J., McGowan I., Richardson B.A., Herold B.C., The Microbicide Trials Network Biomedical Sciences Working Group and the MTN 004 Protocol Team Soluble Immune Mediators and Vaginal Bacteria Impact Innate Genital Mucosal Antimicrobial Activity in Young Women. Am. J. Reprod. Immunol. 2015;74:323–332. doi: 10.1111/aji.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009;4:99–105. doi: 10.2147/IJN.S3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan D.-M., Lv Y.-L., Yao Y.-W., Miao X.-H., Wang Q., Xiao X.-W., Yin J., Shi Y., Shi M.-Q., Zhang X.-W., et al. Efficacy and safety of Abraxane in treatment of progressive and recurrent non-small cell lung cancer patients: A retrospective clinical study. Thorac. Cancer. 2012;3:341–347. doi: 10.1111/j.1759-7714.2012.00113.x. [DOI] [PubMed] [Google Scholar]
- 129.Choi M., Al-Hajeili M., Azmi A. Nab-paclitaxel: Potential for the treatment of advanced pancreatic cancer. Onco. Targets Ther. 2014;7:187–192. doi: 10.2147/OTT.S40705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mann B.S., Johnson J.R., Cohen M.H., Justice R., Pazdur R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 131.Duvic M. Bexarotene and DAB389IL-2 (Denileukin Diftitox, ONTAK) in Treatment of Cutaneous T-Cell Lymphomas: Algorithms. Clin. Lymphoma. 2000;1:S51–S55. doi: 10.3816/CLM.2000.s.010. [DOI] [PubMed] [Google Scholar]
- 132.Coyne D.W. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin. Pharmacother. 2009;10:2563–2568. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- 133.Schwenk M.H. Ferumoxytol: A New Intravenous Iron Preparation for the Treatment of Iron Deficiency Anemia in Patients with Chronic Kidney Disease. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010;30:70–79. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 134.MacDougall I.C., Comin-Colet J., Breymann C., Spahn D.R., Koutroubakis I.E. Iron Sucrose: A Wealth of Experience in Treating Iron Deficiency. Adv. Ther. 2020;37:1960–2002. doi: 10.1007/s12325-020-01323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hood S., O’Brien M., Higgins R. The safety of intravenous iron dextran (Dexferrum) during hemodialysis in patients with end stage renal disease. Nephrol. Nurs. J. 2000;27:1122. [PubMed] [Google Scholar]
- 136.Lim E.-A., Sohn H.-S., Lee H., Choi S.-E. Cost-utility of ferric carboxymaltose (Ferinject®) for iron-deficiency anemia patients with chronic heart failure in South Korea. Cost Eff. Resour. Alloc. 2014;12:19. doi: 10.1186/1478-7547-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fütterer S., Andrusenko I., Kolb U., Hofmeister W., Langguth P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD) J. Pharm. Biomed. Anal. 2013;86:151–160. doi: 10.1016/j.jpba.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Germain M., Caputo F., Metcalfe S., Tosi G., Spring K., Åslund A.K., Pottier A., Schiffelers R., Ceccaldi A., Schmid R. Delivering the power of nanomedicine to patients today. J. Control. Release. 2020;326:164–171. doi: 10.1016/j.jconrel.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Auerbach M., Pappadakis J.A., Bahrain H., Auerbach S.A., Ballard H., Dahl N.V. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am. J. Hematol. 2011;86:860–862. doi: 10.1002/ajh.22153. [DOI] [PubMed] [Google Scholar]
- 140.Anselmo A., Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015;17:1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y.-X., Hussain S., Krestin G. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 142.Gil P.R., Hühn D., del Mercato L.L., Sasse D., Parak W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 2010;62:115–125. doi: 10.1016/j.phrs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 143.Kumar R., Dalvi S.V., Siril P.F. Nanoparticle-Based Drugs and Formulations: Current Status and Emerging Applications. ACS Appl. Nano Mater. 2020;3:4944–4961. doi: 10.1021/acsanm.0c00606. [DOI] [Google Scholar]
- 144.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 145.Schroeder A., Heller D.A., Winslow M.M., Dahlman J.E., Pratt G.W., Langer R., Jacks T., Anderson D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer. 2011;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 146.Farokhzad O.C., Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 147.He H., Liu L., Morin E.E., Liu M., Schwendeman A. Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019;52:2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- 148.Prabhakar U., Maeda H., Jain R.K., Sevick-Muraca E.M., Zamboni W., Farokhzad O.C., Barry S.T., Gabizon A., Grodzinski P., Blakey D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pillai G. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJ Pharm. Pharm. Sci. 2014;1:13. doi: 10.15226/2374-6866/1/2/00109. [DOI] [Google Scholar]
- 150.Pillai G., Ceballos-Coronel M.L. Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Med. 2013;1:13759. doi: 10.1177/2050312113513759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gordon A.N., Granai C., Rose P.G., Hainsworth J., Lopez A., Weissman C., Rosales R., Sharpington T. Phase II Study of Liposomal Doxorubicin in Platinum- and Paclitaxel-Refractory Epithelial Ovarian Cancer. J. Clin. Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 152.Von Hoff D.D., Ramanathan R.K., Borad M.J., Laheru D.A., Smith L.S., Wood T.E., Korn R.L., Desai N., Trieu V., Iglesias J.L., et al. Gemcitabine Plusnab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. J. Clin. Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Desai N., Trieu V., Yao Z., Louie L., Ci S., Yang A., Tao C., De T., Beals B., Dykes D., et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 154.Desai N., Trieu V., Yao R., Labao E., De T., Soon-Shiong P. 601 Increased transport of nanoparticle albumin-bound paclitaxel (ABI-007) by endothelial gp60-mediated caveolar transcytosis: A pathway inhibited by Taxol. Eur. J. Cancer Suppl. 2004;2:182. doi: 10.1016/S1359-6349(04)80609-8. [DOI] [Google Scholar]
- 155.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 156.Dou Y., Hynynen K., Allen C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release. 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 157.Chen L.-T., Su M.-H. EndoTAG-1 plus gemcitabine versus gemcitabine alone in patients with measurable locally advanced and/or metastatic adenocarcinoma of the pancreas failed on FOLFIRINOX treatment ( NCT03126435) J. Clin. Oncol. 2020;38:TPS4669. doi: 10.1200/JCO.2020.38.15_suppl.TPS4669. [DOI] [Google Scholar]
- 158.Kruit W.H., Suciu S., Dreno B., Mortier L., Robert C., Chiarion-Sileni V., Maio M., Testori A., Dorval T., Grob J.-J., et al. Selection of Immunostimulant AS15 for Active Immunization With MAGE-A3 Protein: Results of a Randomized Phase II Study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 2013;31:2413–2420. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 159.Brasseur F., Rimoldi D., Liénard D., Lethé B., Carrel S.S., Arienti F., Suter L.L., Vanwijck R.R., Bourlond A.A., Humblet Y., et al. Expression of MAGE genes in primary and metastatic cutaneous melanoma. Int. J. Cancer. 1995;63:375–380. doi: 10.1002/ijc.2910630313. [DOI] [PubMed] [Google Scholar]
- 160.Eynde B.J.V.D., van der Bruggen P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997;9:684–693. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 161.Zielinski M., Laskowski U., Bieselt R., Reck M., Strausz J., Perdeus J., Park K., Atanackovic D., Debois M., Debruyne C. 9021 Preliminary results of MAGE-A3 expression and baseline demographic data from MAGRIT, a large phase III trial of MAGE-A3 ASCI (Antigen-Specific Cancer Immunotherapeutic) in adjuvant NSCLC. Eur. J. Cancer Suppl. 2009;7:511–512. doi: 10.1016/S1359-6349(09)71734-3. [DOI] [Google Scholar]
- 162.Patard J.-J., Brasseur F., Gil-Diez S., Radvanyi F., Marchand M., François P., Abi-Aad A., Van Cangh P., Abbou C.C., Chopin D., et al. Expression ofmage genes in transitional-cell carcinomas of the urinary bladder. Int. J. Cancer. 1995;64:60–64. doi: 10.1002/ijc.2910640112. [DOI] [PubMed] [Google Scholar]
- 163.Sharma P., Shen Y., Wen S., Bajorin D.F., Reuter V.E., Old L.J., Jungbluth A.A. Cancer-Testis Antigens: Expression and Correlation with Survival in Human Urothelial Carcinoma. Clin. Cancer Res. 2006;12:5442–5447. doi: 10.1158/1078-0432.CCR-06-0527. [DOI] [PubMed] [Google Scholar]
- 164.Luo G., Huang S., Xie X., Stockert E., Chen Y.-T., Kubuschok B., Pfreundschuh M. Expression of cancer-testis genes in human hepatocellular carcinomas. Cancer Immun. 2002;2:111. [PubMed] [Google Scholar]
- 165.Jungbluth A.A., Busam K.J., Kolb D. Expression of MAGE-antigens in normal tissues and cancer. Int. J. Cancer. 2000;85:43. doi: 10.1002/(SICI)1097-0215(20000215)85:4<460::AID-IJC3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 166.Kruit W.H., van Ojik H.H., Brichard V.G., Escudier B., Dorval T., Dréno B., Patel P., van Baren N., Avril M.-F., Piperno S., et al. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int. J. Cancer. 2005;117:596–604. doi: 10.1002/ijc.21264. [DOI] [PubMed] [Google Scholar]
- 167.Atanackovic D., Altorki N.K., Cao Y., Ritter E., Ferrara C.A., Ritter G., Hoffman E.W., Bokemeyer C., Old L.J., Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc. Natl. Acad. Sci. USA. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]