Abstract
Rapid identification of possible vascular compromise in free flap reconstruction to minimize time to reoperation improves achieving free flap salvage. Subjective clinical assessment, often complemented with handheld Doppler, is the golden standard for flap monitoring; but this lacks consistency and may be variable. Non-invasive optical methods such as near-infrared spectroscopy (NIRS) and hyperspectral imaging (HSI) could facilitate objective flap monitoring. A systematic review was conducted to compare NIRS with HSI in detecting vascular compromise in reconstructive flap surgery as compared to standard monitoring. A literature search was performed using PubMed and Embase scientific database in August 2021. Studies were selected by two independent reviewers. Sixteen NIRS and five HSI studies were included. In total, 3662 flap procedures were carried out in 1970 patients using NIRS. Simultaneously; 90 flaps were performed in 90 patients using HSI. HSI and NIRS flap survival were 92.5% (95% CI: 83.3–96.8) and 99.2% (95% CI: 97.8–99.7). Statistically significant differences were observed in flap survival (p = 0.02); flaps returned to OR (p = 0.04); salvage rate (p < 0.01) and partial flap loss rate (p < 0.01). However, no statistically significant difference was observed concerning flaps with vascular crisis (p = 0.39). NIRS and HSI have proven to be reliable; accurate and user-friendly monitoring methods. However, based on the currently available literature, no firm conclusions can be drawn concerning non-invasive monitoring technique superiority
Keywords: free flap, near-infrared spectroscopy, hyperspectral imaging, flap failure, flap loss, tissue oxygenation, non-invasive monitoring
1. Introduction
One of the most feared complications in reconstructive flap surgery is flap failure as a consequence of microvascular thrombosis. Usually, vascular compromise occurs within 48 h after surgery [1,2]. Achieving free flap salvage is improved by rapid identification of possible complications to minimize time to reoperation [3]. In theory, the ideal method of monitoring would be continuous, non-invasive, sensitive enough to detect vascular compromise instantly, sufficiently reliable to make specialized nursing care dispensable, easy to use, harmless to the patient and flap, applicable to all types of flaps, and inexpensive [4,5,6,7].
Monitoring traditionally consists of the subjective assessment of skin color, capillary refill time, temperature and tissue turgor. Frequently, techniques such as handheld Doppler ultrasound, implantable Doppler probes, temperature probes and color duplex sonography are used in conjunction. However, differences in level of clinical experience in free flap monitoring of medical staff influences the consistency of recordings and increases variability. Additionally, these methods are labour intensive, performed intermittently, and one is not clearly superior to another [8,9,10,11,12]. Therefore, more objective methods are desired for flap monitoring.
Near-infrared spectroscopy (NIRS) is a non-invasive continuous bedside monitoring technique of flap tissue oxygenation that could potentially live up to this demand. Selective absorption of near-infrared light during transmission through the tissue by oxygen-dependent chromophores (hemoglobin) is measured. The percentage of saturated hemoglobin (StO2) is calculated based on the ratio of oxygenated (HbO2) and deoxygenated (Hb) hemoglobin. StO2 is associated with tissue oxygenation determined by the balance between oxygen delivery and consumption. Therefore, it indirectly reflects the status of tissue perfusion [7,11,13,14]. NIRS can be used to monitor buried flaps as long as the thickness of the overlying skin does not exceed the maximum depth range of the sensor used [11]. However, measuring tissue oxygenation intraoperatively is currently not possible, since sterile sensors are not available.
Another promising method that can be applied to assess the quality of tissue perfusion is hyperspectral imaging (HSI). HSI is a non-invasive, contactless monitoring technique that combines the principles of imaging and spectroscopy. The technique processes the optical properties of the flap area in a wavelength spectrum from visual to near-infrared light. Consequently, a three-dimensional data set is acquired. HSI provides objective, precise, reproducible and relevant information about 4 parameters in tissue perfusion measurements. The cutaneous and subcutaneous oxygenation patterns are analyzed with hemoglobin oxygenation (StO2) and Near-infrared Perfusion index (NPI or NIR (PI)), measuring the superficial hemoglobin oxygen saturation with a penetration depth of consecutively 1 mm and of 3–5 mm. Tissue Hemoglobin Index (THI) displays the distribution of hemoglobin in the flap microcirculation. Tissue Water Index (TWI) provides information concerning water content and distribution in the flap [15,16,17,18,19,20]. Despite the measurement not being continuous, this monitoring technique enables the assessment of flap viability intraoperatively.
This review aims to compare NIRS with HSI in detecting vascular compromise in reconstructive flap surgery compared to standard monitoring.
2. Materials and Methods
This literature review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The PRISMA checklist is provided in Appendix B. The review was registered prospectively on Prospero (receipt number 274,196; formal approval is pending). A systematic literature search was performed by two reviewers independently (AL/AS) utilizing the National library of medicine (PubMed) database and Embase scientific database (via OvidSP). The literature search was completed in August 2021. The search was performed separately for both databases. Various medical subject heading (MeSH) terms combined with free search terms were used as depicted in Table 1. Studies conducted other than in humans, reviews and studies published in languages other than Dutch, English, German, French and Spanish were excluded from this review. A detailed search query is provided in Appendix B. For the selection of the studies included in this study the Population, Intervention, Comparison, Outcome and Study Design (PICOS) approach was used. After removal of the duplicates, eligibility of the remaining articles was primarily determined by screening based on title. Subsequently, studies were screened based on the abstract. Remaining studies were screened by reading the full text; those that did not answer the research question of this review were excluded. In case of disagreement between the two reviewers AL/AS a third researcher (RS) was consulted.
Table 1.
Search strategy.
| Category | MeSH Term | Free Search Term |
|---|---|---|
| #1: Population | Surgical flaps, or mastectomy, or perforator flap | Free flap OR Free tissue flap OR Surgical flaps OR Mastectomy OR Free tissue transfer flaps OR Perforator flap OR Mastectomy skin flap OR Mastectomy flap |
| #2: Intervention | Spectroscopy, near infrared, or hyperspectral imaging, or spectroscopies | Near infrared spectroscopy OR Noninvasive flap monitoring OR Flap monitoring OR Nirs OR Hyperspectral imaging OR Hsi OR Tissue oximetry OR Tivita tissue system OR Tivita OR Near infrared spectroscopies OR Near infrared spectrometry OR Near infrared spectrometries OR Spectrometries, near infrared OR Nir spectroscopies OR Nir spectroscopy |
| #3: Comparators | Venous insufficiency, or surgical wound dehiscence | Flap loss OR Partial flap loss OR Ischemia OR Necrosis OR Venous congestion OR Venous insufficiency OR Post operative complication OR surgical wound dehiscence |
2.1. Data Extraction
From the included studies, the following information was extracted: the surname of the first author, country of origin, year of publication, study design, study period, researched monitoring tool, monitoring protocol, study objective, number of patients, number of flaps, age, sex, Body Mass Index (BMI), flap survival, monitoring control technique, bilateral flaps, flap weight, mean ischemia time, types of flaps, vascular disease, diabetes mellitus, smoking, radiotherapy, chemotherapy, prior abdominal surgery, use of inotropes, decisive monitoring tool, warning value, flaps with vascular crisis, flaps returned to OR, salvage rate, average time to discharge, total flap loss rate, partial flap loss rate, sensitivity and specificity.
2.2. Data Synthesis
Systematic review methodology and standard summary statistics overall were used to summarize available evidence. Study-level data was analyzed using meta-regression using a random-effects model. The analysis was performed in R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) with the ‘meta’ package. Meta-regression was carried out for the following outcomes: flap survival, flaps with vascular crisis, flaps returned to OR, salvage rate and partial flap loss. Because of significant methodological and statistical heterogeneity between the included studies, further meta-analytic methods were not applied.
3. Results
3.1. Literature Search
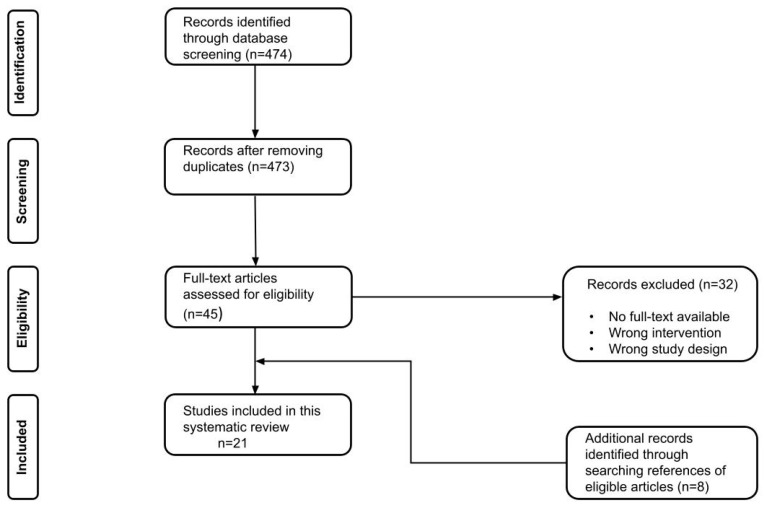
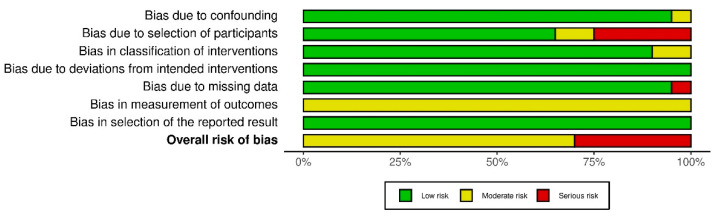
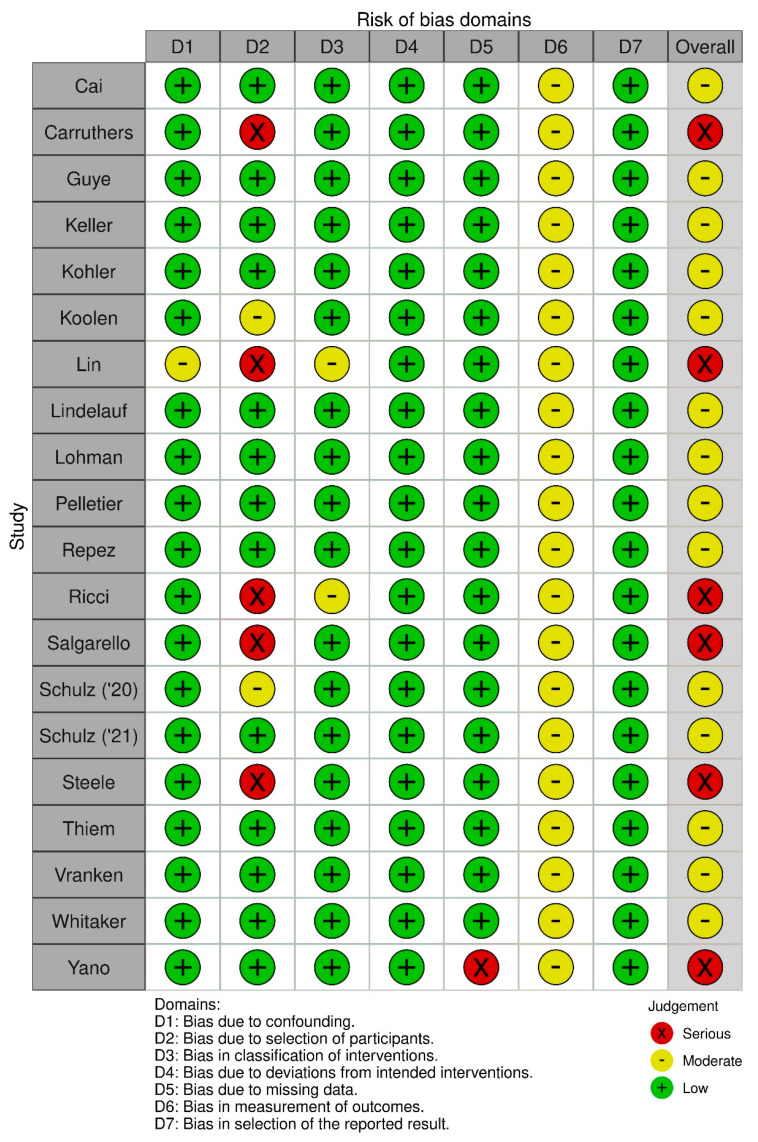
In total, twenty one of the 428 studies that were found with our search strategy qualified for inclusion in this study, see Figure 1. All twenty-one studies were single center studies, except one. Eleven studies were performed in Europe, two in Asia, and eight in the USA during the period from January 2004 to January 2020. Sixteen studies reported on the use of NIRS to detect flap failure and five studies reported on the use of HSI to prevent flap failure. Twenty studies had a cohort study design. Most of the included studies had a prospective design; ten of the NIRS and three of the HSI studies. Seven studies reported retrospectively collected data: six of the NIRS and one of the HSI studies. One HSI study was a case report. No randomized trials were identified. According to the ROBINS-I, the risk of bias assessment of the observational studies is presented in Figure 2 and Figure A1. The bias assessment of one case report was carried out with the Newcastle-Ottawa Scale [20]. Among these studies, inclusion criteria were comparable (Table A1)
Figure 1.
Flow chart of the included studies.
Figure 2.
Summary of the risk of bias assessment of the included observational studies according to the ROBINS-I.
3.2. Overall Flap Surgery Patient Profiles
Data of 2686 patients extracted from 21 studies who underwent flap surgery and were consequently monitored were analyzed (Table 2). The flaps were monitored with either NIRS in 1970 (73.3%), HSI in 90 (3.4%) or standard monitoring alone in 626 (23.3%) patients. The devices used for NIRS monitoring were ViOptix in eight (T.Ox 6, ODIsey 1), Inspectra in three (M325 1, M650 2), INVOS in two (5000C 1, 5100C 1), TSNIR-3 in one and TOS-96/TOS-OR in one study. For HSI TIVITA was used in four and ImSpector V8E in one study. The control monitoring technique consisted of clinical examination in twenty and indocyanine green (ICG) fluorescence [21] imaging in one. Control monitoring was carried out in conjunction with Doppler in eleven studies and with ICG imaging in one. The average/median ages in the included studies are depicted in Table 2. Females accounted for 91.8% (1861/2027) overall; 19.3% (11/57) of the HSI and 93.9% (1850/1970) of the NIRS study population. Gender data weren’t described in one HSI study [22], age wasn’t described in two studies [22,23]. Data on body mass index (BMI) was reported by nine NIRS studies. 3662 flaps were monitored with NIRS in 2759 (75.3%), HSI in 90 (2.5%) or standard monitoring alone in 813 (22.2%) flaps. The overall flap survival was 98.8% (95% CI: 97.1–99.5); HSI and NIRS flap survival respectively were 92.5% (95% CI: 83.3–96.8) and 99.2% (95% CI: 97.8–99.7). This difference was statistically significant (p = 0.02).
Table 2.
Baseline characteristics of included population (n = 2686) and outcomes.
| Author | Country | Year of Publication | Study Type | Study Period | Researched Monitoring Tool (model) | Objective | Patients (N) | Flaps (N) | Age (years) |
Female (N, %) | Body Mass Index (kg/m2) | Flap Survival (%) | Monitoring Control Technique |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cai [30] | China | 2007 | Prospective | Na | NIRS (TSNIR-3) first 24 h every 4 h, 2 per day in the following 5 days | Test sensibility and precision | 41 | 41 | 42 (14–73) | 11 (26.8) | Na | 97.6 | CE |
| Carruthers [25] | USA | 2019 | Retrospective | 24 months | NIRS (T.Ox ViOptix) until discharge |
Reduce monitoring time | 196 | 301 | 50.7 ± 8.3 | 196 (100) | 30.7 ± 5.5 | 100 | CE, pD every hour first 12 h, every 4 h |
| Guye [31] | France | 2017 | Prospective | 7 months | NIRS (InSpectra Model 650) | Reassess risk factors for free flap complications | 40 | 40 | NC 53.3 ± 13.6, C 58 ± 15.9 | 18 (45) | C 26 ± 1.7, NC 22 ± 0.7 | 90 | CE |
| Keller [23] | USA | 2009 | Prospective | Jan 2005– Jan 2008 | NIRS (T.Ox ViOptix) for 36 h |
Continuation of an earlier preliminary study | 145 | 208 | Na | 145 (100) | Na | 100 | CE, hD; hourly for first night, every 2 h for the next 36 h |
| Koolen [26] | USA | 2016 | Retrospective | Feb 2004–Jun 2008, Jun 2008–Feb 2014 | Control, NIRS (T.Ox ViOptix) for 72 h | Compare standard monitoring with NIRS | Co 288, Ni 451 | Co 380, Ni 670 | Co 47.7 ± 7.9, Ni 49.9 ± 8.5 | Co 288 (100), Ni 451 (100) | Co 26.9 ± 5, Ni 28.9 ± 5.6 | Co 57.7, Ni 96.6 | CE |
| Lin [32] | USA | 2010 | Retrospective | Jan 2004–Dec 2007, Jan 2008–Dec 2010 | Control, NIRS (T.Ox ViOptix) for 72 h | Compare monitoring with and without tissue oximetry | Co 288, Ni 164 | Co 380, Ni 234 | Co 47.69 ± 8.44, Ni 49.85 ± 7.88 |
Co 288 (100), Ni 164 (100) | Na | Co 97.1, Ni 99.6 | CE, hD; every 15min first hr, every 30 min second hr, every hour for next 10 h. Surgical resident every 4 h CE. |
| Lindelauf [24] | NL | 2021 | Prospective | Na | NIRS (FORE-SIGHT MC-2030) for 24 h | Confirm the usefulness of NIRS in postoperative monitoring | 30 | 42 | 51 ± 13 | 30 (100) | 27.5 ± 4.3 | 100 | CE, Doppler according to hospital protocol |
| Lohman [8] | USA | 2013 | Prospective | Jan 2006–Feb 2007 | NIRS (ViOptix) for 72 h |
Determine the most useful method | 38 | 38 | 38.5 (21–84) | 27 (71.1) | Na | 100 | CE, hD, hourly;iD |
| Pelletier [33] | USA | 2011 | Prospective | Aug 2006–Jan 2010 | NIRS (ViOptix ODIsey) for 72 h |
Evaluate the costs of autologous free tissue breast reconstruction | 50; ICU 25, Floor 25 | 54 | ICU 49.4 (31–67), Floor 49 (28–75) | 50 (100) | ICU 27.9 (19.5–43), Floor 28.5 (21.8–36.3) | 98 | CE, hD; ICU every hour, Floor every 4–6 h |
| Repez [11] | Slovenia | 2007 | Prospective | Aug 2004–Sep 2005 | NIRS (InSpectra Model 325) for 72 h | Ascertain whether NIRS could be trustworthy | 48 | 50 | 47 (31–64) | 48 (100) | 26 (22–35) | 94 | CE hourly for 72 h |
| Ricci [34] | USA | 2017 | Retrospective | May 2008–Aug 2014 | NIRS (ViOptix T.Ox) for 72 h |
Earlier transfer of patients to a standard surgical inpatient floor | 595 | 900 | 50.3 ± 8.6 | 595 (100) | 28.8 ± 5.6 | 99.7 | CE first 24 h, hD; every 15 min for first hr, every 30 min for second hr, every hr for the next 22 h |
| Salgarello [35] | Italy | 2018 | Retrospective | Jan 2015–Jan 2016 | NIRS (INVOS 5100C) for 48 h |
Identify patient- and flap related variables that can affect rSO2 | 45 | 45 | 52.6 (34–69) | 45 (100) | ** | 100 | ICG imaging |
| Steele [36] | USA | 2011 | Retrospective | Jan 2007–May 2010 | Control, NIRS (ViOptix T.Ox) for 4.5d average | Examine outcomes using a tissue oximeter | Co 50, Ni 63 | Co 53, Ni 75 | Co 57.6 (11–85), Ni 58 (17–89) | Co 18 (36), Ni 29 (46) | Na | Co 90.6, Ni 98.7 | CE, hD, hourly for 48 h, then every 2 h for the following 48 h, then every 4 h * |
| Vranken [27] | NL | 2017 | Prospective | Na | NIRS (INVOS 5000C) for 24 h |
Suitability for the assessment of tissue perfusion | 29 | 29 | 50 ± 10 | 29 (100) | 26.4 ± 3.3 | 100 | CE, Doppler ultrasonography |
| Whitaker [37] | UK | 2012 | Prospective | Na | NIRS (InSpectra Model 650) for 72 h |
Investigate NIRS technology | 10 | 10 | 46 (28–61) | 10 (100) | Na | 90 | CE, hD (hourly), capillary bleeding (25 gauge needle) |
| Yano [28] | Japan | 2020 | Prospective | Sep 2011–Jan 2016 | NIRS (TOS-96/TOS-OR) for 72 h |
Investigate the feasibility of perioperative NIRS monitoring | 25 | 25 | 63.5 (39–85) | 2 (8) | Na | 100 | CE, ICG imaging |
| Calin [20] | Romania | 2017 | Case report | Na | HSI (ImSpector V8E) 0, 2, 4, 24 and 48 h postoperatively | Assess value as a monitoring tool | 1 | 1 | 61 | 0 | Na | 100 | CE |
| Kohler [29] | Germany | 2021 | Prospective | Mar 2019–Jan 2020 | HSI (TIVITA) at t0(0), t1(16–28), t2(39–77) hrs postoperatively | Show the superiority of HSI | 22 | 22 | 55 (26–92) | 5 (22.7) | Na | 81.8 | CE, Doppler ultrasound every 2 h within 24 h, every 4 h until 72 h postoperatively |
| Schulz (‘20) [38] | Germany | 2020 | Retrospective | Dec 2017–Apr 2018 | HSI (TIVITA) for 7 days |
Evaluate HSI as a monitoring method for pedicled flaps | 16 | 16 | 58 (25–78) | 2 (12.5) | Na | 93.8 | Na |
| Schulz (‘21) [39] | Germany | 2021 | Prospective | Jul 2017–Sep 2018 | HSI (TIVITA) for 7 days |
Investigate HSI as a method for free flap monitoring | 18 | 18 | 54 (24–87) | 4 (22.2) | Na | 94.4 | CE |
| Thiem [22] | Germany | 2020 | Prospective | Na | HSI (TIVITA) at t1(0), t2(0–1), t3(4–8), t4(8–12), t5(12–24), t6(24–48), t7(>48) | Feasibility of HSI for objective and reproducible monitoring | 33 | 33 | Na | Na | Na | 97 | CE 72 h |
Co = control, Ni = NIRS, C = complication, NC = No complication, CE = clinical examination, hD = handheld Doppler, pD = pencil Doppler, iD = implantable Doppler, Na = not available, * Implantable Doppler was used in a few patients whose flaps were completely buried, ** Salgarello et al. BMI 18.5–24.9; N = 24, BMI 25–29.9; N = 11, BMI > 30; N = 10.
3.3. Flap-Related Characteristics
Data depicting flap related characteristics were reported inconsistently, except for types of flaps. Therefore, substantial amount of data was not available. Flap types, ischemia time, vascular disease, Diabetes Mellitus, smoking, radiotherapy and chemotherapy are described in Table 3. In one study 14 (47%) patients received (neo)-adjuvant therapy prior to surgery consisting of immunotherapy, endocrine therapy, radiation therapy, chemotherapy or a combination of these [24]. Prior abdominal surgery is described in two studies: 52 (26.5%) [25] and 214 (56.5%) in the control group, 356 (53.1%) in the NIRS group [26]. None of the included studies described use of inotropes.
Table 3.
Flap-related characteristics of included flaps (n = 3662).
| Author | Mean Ischaemia Time (min) |
Types of Flaps (N) | Vascular Disease (N, %) |
DM (N, %) |
Smoker (N, %) |
XRT (N, %) |
Chemo (N, %) |
|---|---|---|---|---|---|---|---|
| Cai [30] | Na | Fibular 41 | Na | Na | Na | Na | Na |
| Carruthers [25] | Na | DIEP 301 (111 delayed, 36.9%) | Na | 8 (4.1) | 9 (4.6) | 78 (25.9) | Na |
| Guye [31] | NC 74 ± 4.5, C 70 ± 6.8 |
Fibular 15, Radial 20, gastro-omental 5 | 5 (12.5) | 5 (12.5) | Na | 9 (22.5) | Na |
| Keller [23] | Na | DIEP 197, SIEA 1, SGAP 10 | Na | Na | Na | Na | Na |
|
Koolen [26]
|
Na | Co; DIEP 336, SIEA 15, Free TRAM 9, SGAP 20 Ni; DIEP 646, SIEA 1, Free TRAM 3, SGAP 20 |
CAD Co 1 (0.3), Ni 5 (0.7) | Co 8 (2.1), Ni 28 (4.2) | Co 30 (7.9), Ni 85 (12.7) | Co 105 (27.6), Ni 235 (35.1) | Co 157 (41.4), Ni 379 (58.2) |
|
Lin [32]
|
Na | Co; DIEP 336, SIEA 15, SGAP 20, Free TRAM 9 Ni; DIEP 222, SGAP 9, Free TRAM 3 |
Na | Na | Na | Na | Na |
| Lindelauf [24] | 42 (35–51) | DIEP 42 (17 secondary) | Na | Na | 2 (7) | Na | Na |
| Lohman [8] | Na | DIEP 18, ALT 15, MS-TRAM 5 | Na | Na | Na | Na | Na |
| Pelletier [33] | ICU 86.7 (46–157), Floor 78.5 (48–138) | DIEP 21, DIEP + DIEP 1, DIEP/SIEV 2, DIEP + SIEA 3, SIEA 9, Free TRAM 3, Free MS-TRAM 11 | 0 | Floor 1, ICU 0 | Floor 1, ICU 0 | Floor 12, ICU 12 | Floor 11, ICU 12 |
| Repez [11] | Na | DIEP 37 (13 secondary), SIEA 5, SGAP 8 (5 secondary) | 0 | 1 (2) | 7 (14) | Na | Na |
| Ricci [34] | Na | DIEP 872, SIEA 2, SGAP 23, TRAM 3 | CAD 5 (<0.1) | 32 (3.6) | 89 (9.9) | 265 (29.4) | 414 (46) |
| Salgarello [35] | Na | DIEP 45 | Na | Na | Na | Na | Na |
|
Steele [36]
|
Na | Co; DIEP 5, ALT 7, Fibular 5, LD 3, Scapula osteocutaneous 1, Free TRAM 14, Radial 14, gracilis 4 Ni; DIEP 26, ALT 20, Fibular 8, Free TRAM 2, MS-TRAM 4, Radial 15 |
Na | Na | Na | Na | Na |
| Vranken [27] | 48 ± 12 | DIEP 29 | Na | Na | Na | Na | Na |
| Whitaker [37] | Na | DIEP 10 | Na | Na | Na | Na | Na |
| Yano [28] | Na | FJG 25 | Na | Na | Na | 6 (24) | 20 (80) |
| Calin [20] | Na | Fasciocutaneous sural flap 1 | 1 (100) | 1 (100) | Na | Na | Na |
| Kohler [29] | Na | DIEP 3, ALT 11, LD 4, Scapula osteocutaneous 1 (parascapular), MS2-TRAM 2, Rectus abdominis 1 (18 with, 4 without skin island) | PAD 4 (18.2), CAD 3 (13.6) | 5 (22.7) | 4 (18.2) | Na | Na |
| Schulz (‘20) [38] | Na | Suralis 3, LD 5, Radial 2, gastrocnemius 2, TFL 1, Foucher 1, MCPA 1, Crossfinger 1 | Na | Na | Na | Na | Na |
| Schulz (‘21) [39] | Na | ALT 10, LD 8 | PAD 7 (38.9), CAD 5 (27.8) | 8 (44.4) | 12 (66.7) | Na | Na |
| Thiem [22] | Na |
25 FF; ALT 3, Radial 12, Osteocutaneous fibula 4, Osteocutaneous scapular 3, Unknown 3 8 PF; PM 3, LD 2, NL 1, LSS 2 |
Na | Na | Na | Na | Na |
Co = control, Ni = NIRS, C = complication, NC = no complication, DIEP = Deep inferior epigastric artery perforator, ALT = Anterolateral thigh, SIEA = Superficial inferior epigastric artery, SGAP = superior gluteal artery perforator, LD = Latissimus Dorsi, TRAM = Transverse rectus abdominis myocutaneous, MS2 = Muscle sparing type 2, TFL = Tensor fascia lata, MCPA = metacarpal arteries, FJG = Free jejunal graft, PM = Pectoralis major, NL = Nasolabial, LSS = Large scale scalp rotation, PAD = peripheral artery disease, CAD = coronary artery disease, DM = Diabetes Mellitus, XRT = radiation therapy, FF = Free flap, PF = Pedicled flap, Na = not available.
3.4. Detection of Flap Failure
In at least nine out of sixteen studies, NIRS was the first tool indicating flap failure. Data regarding the first monitoring tool to detect complication was not provided by five studies. A faster detection with standard monitoring was observed in one study and with ICG imaging or standard monitoring in another compared with NIRS [27,28]. HSI was the first tool to indicate a vascular crisis in at least two out of five studies [22,29]; the other three didn’t provide data concerning the first tool to detect flap complication (Table 4). Time to detection was not mentioned in any of the included studies. The cut-off value for detection of flap complication was mentioned in the majority of studies. Proposed warning values for specific flap monitoring models according to recent studies and parameters to distinguish venous congestion from arterial occlusion are indicated in Table 5. Overall, 6.0% (95% CI: 4.0–8.9) of flaps had vascular crisis. Flaps monitored using HSI presented with vascular crisis in 10.0% (95% CI: 5.3–18.1) and using NIRS in 5.5% (95% CI: 3.4–8.8). This difference was not statistically significant (p = 0.39). Overall, 6.3% (95% CI: 4.3–9.1) of flaps were returned to the OR. In the HSI studies 12.7% (95% CI: 5.5–26.7) and in the NIRS studies 5.6% (95% CI: 3.8–8.2) of flaps were returned to the OR. This difference was statistically significant (p = 0.04). Salvage rate, the percentage of flaps with vascular crisis that could be saved, overall was 81.1% (95% CI: 65.1–90.8). HSI salvage rate was 22.2% (95% CI: 5.6–57.9) and NIRS salvage rate was 88.3% (95% CI: 80.1–93.4). This difference was statistically significant (p < 0.01). Average time to discharge was mentioned in 6 studies and is depicted in Table 4. Partial loss rate overall was 0.57% (95% CI: 0.13–2.52), for HSI 6.64% (0.44–53.36) and for NIRS 0.60% (95% CI: 0.19–1.89). This difference was statistically significant (p < 0.01). Sensitivity and Specificity were described in 13 studies.
Table 4.
Detection of flap complication.
| Author | Decisive Monitoring | Warning Value | Description | Flaps with Vascular Crisis (N, %) |
Flaps Returned to OR (N, %) |
Salvage Rate (%) |
Average Time to Discharge (days) | Total Loss Rate (N, %) |
Partial Loss Rate (N, %) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cai [30] | NIRS | rSO2 70% | Anastomosis vein again, intraoral infection day 7, necrosis 1 | venous 1 (2.4) | 1 (2.4) | 0 | Na | 1 (2.4) | 0 | 100 | 100 |
| Carruthers [25] | NIRS (5 microvascular) | rSO2 | Microvascular 5 (3 immediate reconstructions, 2 delayed cases), Nonvascular 9 (1 positive margin required reexcision, 8 hematoma) | 5 (1.7); venous congestion 3, arterial thrombus 2 | 14 (4.7) | 100 | 3.4 ± 1.1 | 0 | 0 | Na | Na |
| Guye [31] | Na | Na | Venous thrombosis 2, Partial or total necrosis of the flap 8 ( arterial thrombosis 3) | venous thrombosis 2 (5) | Na | Na | Na | 4 (10) | 4 (10) | Na | Na |
| Keller [23] | NIRS | ΔStO2/Δt ≥ 20%/h sustained >30 min | Hematoma, superficial vein thrombosis and vein kink 1, Deep vein thrombosis 2, Arterial thrombosis 2 | 5 (2.4); venous 3, arterial 2 | 5 (2.4, 1 triple) | 100 | Na | 0 | 0 | 100 | 100 |
| Koolen [26] | Na | Co Na, Ni 20-point drop in 1 h OR absolute reading <30% | Na | Na | Co 26 (6.8), Ni 29 (4.3) | Co 57.7, Ni 96.6 | Na | Co 11(2.9), Ni 1(0.1) | Co 8 (2.1) Ni 7 (1) | Co Na, Ni 96.5 | Co Na, Ni 99.8 |
| Lin [32] | Co CE/hD, Ni NIRS | Co Na, Ni 20-point drop in 1 h OR absolute reading <30% | Co; Na, Ni; Venous thrombosis resulted in total loss 1 |
Co 26 (6.8), Ni 16 (6.8) | Co 26 (6.8), Ni 16 (6.8) | Co 57.7, Ni 93.8 | Na | Co 11 (2.9), Ni 1 (0.43) | Co 8 (2.1), Ni 4 (1.7) | Co Na, Ni 100 | Co Na, Ni 100 |
| Lindelauf [24] | Na | Na | Minor complication 13, Major complication 5 (debridement for fat necrosis 1, arterial kinking 1, evacuation hematoma 1, insufficient perfusion resulting in partial loss 1, venous kinking 1) | 3 (7.1) | 5 (12) | 100 | no/minor c 5 [4,5] | 0 | 1 (2.4) | 100 | 100 |
| Lohman [8] | NIRS ( in ⅘) | StO2 ≤30% | Hematomas 2, Venous thrombosis 1, Venous kinking and clotting 1, Venous clotting 1 | 4 (13.2); 3 venous, 1 arteriovenous |
5 (13.2) | 100 | Na | 0 | 0 | 100 | 100 |
| Pelletier [33] | NIRS | StO2 <30% OR StO2 >20%/h drop for 30 min | Venous thrombosis 3, No reoperation 1 | 4 (8); ICU venous 3, Floor 1 | 3 (6) | 75 | Na | ICU 1 (2) | 0 | 100 | 100 |
| Repez [11] | NIRS | StO2 <50% of initial value | Venous thrombosis 8, Arterial thrombosis 2 | 10 (20); arterial 2, venous 11 | 10 (20, 1 twice, 1 triple) | 70 | Na | 3 (6) | 0 | 100 | 100 |
| Ricci [34] | Na | 20-point drop in 1 h OR absolute reading <30% | Venous thrombosis, pedicle kinking or hematoma causing compression 25, Arterial thrombosis or kinking 6, Arteriovenous thrombus 1 | 32 (3.6); venous 25, arterial 6, combined 1 | 32 (3.6, 16 within 24 h) | 90.6 | Na | 3 (<0.1) | 10 (1.1) | 96.5 | 99.8 |
| Salgarello [35] | Na | rSO2 ≤30% OR drop rate in rSO2 ≥ 20% | Na | 0 | 0 | Na | Na | 0 | 0 | Na | Na |
| Steele [36] | Co CE/hD, Ni NIRS | Co Na, Ni StO2 ≤40% OR drop rate StO2 ≥15%/h | Ni; Arterial thrombosis resulting in total loss 1 Hematomas with venous congestion/thrombosis 3 Vascular pedicle kinked during the inset and closure 2 Arterial spasm 1 |
Co 5 (9.4), Ni 7 (9.3) | Co 4 (7.5), Ni 3 (4) | Co 0, Ni 85.7 | Co 14.5, Ni 10.7 | Co 5 (9.4), Ni 1 (1.3) | 0 | Co Na, Ni 100 | Co Na, Ni 100 |
| Vranken [27] | CE/Doppler | Proposed; enlarged ΔStO2 ≥ 38%, decreased StO2 ≤ 43% | StO2 43%; Second anastomosis 1, StO2 44%; partial necrosis (day 5) 1 | 2 (6.9); venous congestion 1 | 2 (7) | 100 | 5 | 0 | 1 (3.4) | Na | Na |
| Whitaker [37] | NIRS | StO2/THI ≤50% of starting value | Venous thrombosis requiring revision anastomosis 3, Minor debridement (after 3–5 days) 2, Evacuation hematoma; flap loss 1 | 4 (40); venous 3 | 3 (30) | 75 | 6–13 | 1 (10) | 0 | 100 | 100 |
| Yano [28] | ICG/CE | Proposed; rSO2 < 55% | Subcutaneous hematoma (detachment anastomosis 3 weeks later) 1, Anastomosis revision; suspected inadequate venous drainage 1 | venous 1 (4) | 1 (4) | 100 | Na | 0 | 0 | Na | Na |
| Calin [20] | Na | Na | Na | 0 | 0 | Na | Na | 0 | 0 | Na | Na |
| Kohler [29] | HSI | Proposed; StO2 <40% and NIR <40 | Venous thrombosis 4 | venous 4 (18.2) | 6 (27.3) | 33.3 | Nr 12 ± 6.6, partial 11.5 ± 2.1, Cr 30 ± 14.5 | 4 (18.2) | 2 (9.1) | 100 | 100 |
| Schulz (‘20) [38] | Na | venous value change; THI 43% → 57%, StO2 45 → 31%, NIR 43 → 25%, TWI 33 → 24% | Minor complication (e.g. wound edge necrosis) 15, Venous congestion radial flap resulting in loss 1 | venous congestion 1 (6.3) | Na | 0 | Na | 1 (6.3) | Na | Na | Na |
| Schulz (‘21) [39] | Na | Proposed; venous THI ≥53%, NIR ≤25%, TWI ≤43%, StO2 ≤22% arterial drop of StO2 ≤3%, THI ≤3% | Arterial embolism resulting in flap loss 1, partial flap necrosis caused by local impaired perfusion 9 | arterial 1 (5.6) | 1 (5.6, triple) | 0 | Na | 1 (5.6) | 9 (50) | Na | Na |
| Thiem [22] | HSI | Proposed; StO2 <45%, NIR <25% | Venous thrombosis 2, Arterial occlusion 1 | 3 (9.1); venous 2, arterial 1 | 3 (9.1, all FF) | 33 | Na | 2 (6.1) | 0 | 100 | 100 |
Co = control, Ni = NIRS, c = complication, Nr = no revision, Cr = complete revision, Na = not available, CE = clinical examination, hD = handheld Doppler, rSO2 = regional oxygen saturation, StO2 = hemoglobin oxygenation, NIR = Near-infrared Perfusion index, THI = tissue hemoglobin index, TWI = tissue water index. ∆ = Delta.
Table 5.
Proposed warning values for vascular crisis, parameters indicative of vascular crisis and parameters to distinguish venous from arterial crisis using NIRS versus HSI.
| Technique | Model | Proposed Warning Value | Vascular Crisis | Venous Congestion | Arterial Occlusion |
|---|---|---|---|---|---|
| NIRS | ViOptix [23] (ViOptix Inc., Fremont, Ca, USA) InSpectra [11] (Hutchinson Technology Inc., Hutchinson, Mn, USA) |
rSO2 ≤ 30% OR drop rate in rSO2 ≥ 20% StO2 < 50% of initial value |
HbO2, StO2 drop Hb rise |
HbT rise | HbT drop |
| HSI | TIVITA [22,39] (Diaspective Vision GmbH, Am Salzhaff, Germany) |
Venous THI ≥ 53%, NIR ≤ 25%, TWI ≤ 43%, StO2 ≤ 22% Arterial Drop of StO2 ≤ 3%, THI ≤ 3% |
StO2, NIR low | THI high | THI low |
NIRS = near-infrared spectroscopy, HIS = hyperspectral imaging, rSO2 = regional oxygen saturation, THI = tissue hemoglobin index, NIR = Near-infrared Perfusion index, TWI = tissue water index, StO2 = hemoglobin oxygenation, HbO2 = oxygenated hemoglobin, Hb = deoxygenated hemoglobin, HbT = total tissue hemoglobin concentration.
4. Discussion
Flap loss is a severe and feared complication after free tissue transfer in reconstructive microsurgery. Alongside the clinical assessment to detect signs of flap failure (either partial or total flap loss) in the early postoperative phase, objective monitoring of free flaps is expedient [11,40]. The ideal monitoring technique most importantly is objective, but also reliable, accurate, sensitive, continuous and user friendly, as defined by Creech and Miller [4]. NIRS and HSI are two different non-invasive monitoring methods that meet (almost) all criteria as described and have also proven to be suitable for detection of vascular compromise [9,23,37]. This study provides a systematic review in which a comparison between NIRS and HSI is made in detecting vascular compromise in reconstructive flap surgery compared to standard monitoring.
For NIRS, several commercial devices are available, such as FORE-SIGHT (Edwards Lifesciences, Irvin, CA, USA), INVOS (Medtronic, Minneapolis, MN, USA), EQUANOXTM (Nonin Medical Inc., Plymouth, MN, USA) and ViOptix (ViOptix Inc., Fremont, Ca, USA). With these devices tissue oxygenation is measured continuously using non-invasive sensors, which need to be applied on the skin in the area of interest. Despite its proven added value in detection of vascular compromise, the technique is only implemented in 5% of the DIEP-flap procedures in clinical practice [8,9,10]. Recently, more research has been performed on implementing HSI to monitor flap viability after free flap surgery. Although data on the use of HSI in the clinical setting is scarce, several studies concluded HSI to be reliable and accurate [22,26,29,38]. In addition, in a recent study by Thiem et al. HSI showed to be able to detect malperfusion of flaps before clinical monitoring [41]. Measuring tissue oxygenation with this imaging modality is discontinuous but contactless: no sensors need to be applied on the skin.
A lack of knowledge concerning the interpretation of values presented by the different devices could be an explanation for the low percentage in daily clinical use of NIRS measurements. Manufacturers use different algorithms to assess the tissue saturation values, apply different fixed ratios between arterial to venous blood volume and incorporate varying number and different wavelengths of near-infrared light [42]. Furthermore, they develop sensors with different transmitter-receiver spacing, resulting in different penetration depths, which also affects estimation and calculation of rStO2 [43]. Hence, it is difficult to define universal cut-off values necessitating prompt intervention [24,44]. In the included studies, most research was performed using the ViOptix device. For this particular device, Keller defined a threshold for rStO2 of an absolute value below 30% as predictive values for detection of vascular compromise [23]. For HSI, the diversity in used devices is currently limited. In all HSI observational studies included in this review, the Tivita system (Diaspective Vision GmbH, Am Salzhaff, Germany) was used for tissue oxygenation measurement. For this device no general cut-off values are defined yet, but most studies concluded a StO2 value below 30% to be an indication for circulatory compromise for which intervention would be recommended and justified [22,29,38,45]. Using continuous NIRS, measurement changes in tissue oxygenation can be monitored over time. A decrease of 20% from baseline for more than 60 minutes in duration is considered to be an indication for a lack in tissue perfusion. By HSI this continuity in monitoring is unfortunately not possible. Nevertheless, using HSI it has recently been shown feasible to detect circulatory compromise before standard clinical detection [41]. Therefore, both monitoring methods can be used to detect vascular compromise in the early postoperative period.
Implementing NIRS as a monitoring tool is less labor intensive for the medical staff. Because measurements are continuous, only one member of the team needs to be trained in performing the measurements. When values decrease below a certain threshold, this member receives a text message stating an extra clinical examination of the flap needs to be performed [40,46]. For using HSI extra medical staff needs to be trained before using the device, because photos need to be taken on different time-points during the day in a standardized manner.
Since sensors need to be used to measure tissue oxygenation with NIRS, not all flaps can be monitored with NIRS. For example, when using the FORE-SIGHT system a flap dimension of at least 50 mm by 30 mm was necessary for proper sensor placement [24]. Furthermore, these sensors are not sterile. Therefore, measuring saturation can only be performed in the postoperative phase. These could also be reasons for the scarce implementation of NIRS in clinical practice. With HSI being a contactless measurement, all types of flaps (e.g., fascio-cutaneous, muscle, intestinal) can be included. For example, a probe fixation of NIRS for an intraoral flap is difficult, although the contactless measurement by HSI may be suitable for intraoral flap monitoring. On the other hand, a buried flap monitoring would be difficult by a contactless way. Furthermore, without applying sensors on the skin, the HSI technique is friendlier for the patient and more importantly, the measurements can also be performed during surgery.
With HSI four different parameters (StO2, rStO2, THI, TWI) are measured. When these parameters are combined it is possible to determine whether the observed changes in values are caused by an arterial inflow or a venous outflow track problem [18,29,38]. For monitoring free flaps this could be of added value. When using NIRS, this distinction can only be made with a few devices. For example, with the ViOptix, which is unavailable in Europe. Therefore, the number of available devices in this area are limited.
The limited use of NIRS could also be due to the fact that implementing this technique comes with a price [9,26]. Implementing tissue oximetry costs $16,500 for the device and $150 per sensor according to Smit et al. In a different study, costs up to $30,000 for a device and $700–$1200 for a sensor are documented. Nevertheless, by implementing NIRS in standard protocol, vascular compromise could be detected in an early phase. Total flap loss could potentially be prevented and consequently duration of hospital stays shortened, resulting in a decrease of $1350–1700 per DIEP-flap procedure [33,34,47]. The costs for an HSI device are approximately $40,000 [38]. Initially implementing HSI would be more expensive than NIRS, but in the long term it could be more cost effective because no extra costs are required for buying the single use sensors. However, literature concerning cost effectiveness of HSI as a monitoring tool for flap viability is currently not available.
A limitation of the current literature study is the amount and quality of the included studies. In this review 21 studies were included. Sixteen reported on NIRS (n = 1970 patients) and five reported on HSI (n = 90 patients). All studies were observational cohort studies; accordingly, the average risk of bias was moderate. For this reason, randomized clinical trials with a larger patient population comparing the two monitoring techniques are mandatory. Moreover, defining solid cut-off values and performing an up-to-date cost-effectiveness evaluation regarding NIRS and HSI are required.
In conclusion, the authors believe that NIRS and HSI can have an added value in the detection of flap failure in the early postoperative phase. Both techniques have proven to be reliable, accurate and user-friendly monitoring methods, but do not (yet) replace the gold standard of clinical flap assessment. Based on the currently available literature, no firm conclusions can be drawn on which technique would be superior as an adjunct tool in free flap monitoring.
Acknowledgments
Not applicable.
Appendix A
Figure A1.
Risk of Bias assessment of the observational studies according to the ROBINS-I.
Newcastle-Ottawa scale; Calin 6 stars (Selection ***, Comparability *, Exposure **).
Table A1.
Inclusion criteria of the studies.
| Author | Inclusion Criteria |
|---|---|
| Cai [30] | All patients undergoing autogenous mandibular reconstruction by vascular fibular flap transplantation. |
| Calin [20] | Chosen for case report after receiving informed consent. |
| Carruthers [25] | All patients who underwent microsurgical breast reconstruction with free DIEP flaps over 24 consecutive months. |
| Guye [31] | Patients undergoing resection of a cervicofacial tumour and immediate reconstruction with a free flap. |
| Keller [23] | Patients undergoing autologous tissue perforator free flap breast reconstruction. |
| Kohler [29] | Patients aged 18 and older who underwent soft tissue reconstruction using a free flap between March 2019 and January 2020 and had given informed consent. |
| Koolen [26] | All immediate and delayed autologous microsurgical free tissue transfers for breast reconstruction from February of 2004 to February of 2014. |
| Lin [32] | All patients undergoing microsurgical breast reconstruction between 2004 and 2010 at Beth Israel Deaconess Medical Center were identified. |
| Lindelauf [24] | Female patients undergoing unilateral or bilateral, immediate or delayed DIEP flap breast reconstructive surgery at one university medical center were included. |
| Lohman [8] | Consecutive patients with free flaps - including external skin paddles - performed between January 2006 and February 2007 were monitored. |
| Pelletier [33] | Any patient scheduled to undergo unilateral autologous free tissue breast reconstruction. |
| Repez [11] | Consecutive free flap autologous breast reconstruction. |
| Ricci [34] | All autologous microsurgical free tissue transfers for breast reconstruction from May 2008 until August 2014. |
| Salgarello [35] | Consecutive patients undergoing breast reconstruction with DIEP flap. |
| Schulz (‘20) [38] | All patients undergoing soft tissue reconstruction and who had given informed consent. |
| Schulz (‘21) [39] | All patients who underwent free tissue transfer at our department from July 2017 to September 2018 were eligible for inclusion. |
| Steele [36] | The author’s microsurgical cases between January 2007 and May 2010. |
| Thiem [22] | Patients with either free or pedicled flaps for reconstruction in the oro-maxillofacial area were included. |
| Vranken [27] | Female patients undergoing unilateral secondary DIEP-flap surgery were included. |
| Whitaker [37] | All women who were undergoing autologous breast reconstruction following mastectomy, aged between 18 and 65 years old. |
| Yano [28] | Consecutive patients who underwent reconstructive surgery using FJG following the resection of cancer of the pharynx or cervical esophagus. |
Appendix B
Figure A2.
PRISMA checklist.
Table A2.
PubMed search strategy.
| Category | Query |
|---|---|
| #1: Population | ((((((((((Free flap) OR (Free tissue flap)) OR (Surgical flaps)) OR (Mastectomy)) OR (Free tissue transfer flaps)) OR (Perforator flap)) OR (Mastectomy skin flap)) OR (Mastectomy flap)) OR ("surgical flaps"[MeSH Terms])) OR ("mas-tectomy"[MeSH Terms])) OR ("perforator flap"[MeSH Terms]) |
| #2: Intervention | (((((((((((((((((Near infrared spectroscopy) OR (Non invasive flap monitoring)) OR (Flap monitoring)) OR (Nirs)) OR (Hyperspectral imaging)) OR (Hsi)) OR (Tissue oximetry)) OR (Tivita tissue system)) OR (tivita)) OR (Near infrared spectroscopies)) OR (Near infrared spectrometry)) OR (Near infrared spectrometries)) OR (Spectrometries, near in-frared)) OR (Nir spectroscopies)) OR (Nir spectroscopy)) OR (“spectroscopy, near infrared”[MeSH Terms])) OR (“hy-perspectral imaging”[MeSH Terms])) OR (Spectroscopies[MeSH Terms]) |
| #3: Comparators | (((((((((Flap loss) OR (Partial flap loss)) OR (Ischemia)) OR (Necrosis)) OR (Venous congestion)) OR (Venous insuffi-ciency)) OR (Post operative complication)) OR (surgical wound dehiscence)) OR ("venous insufficiency"[MeSH Terms])) OR ("surgical wound dehiscence"[MeSH Terms]) |
| #1, #2 and #3: PIC | #1 AND #2 AND #3 |
Author Contributions
Conceptualization, A.G.S., A.A.M.A.L. and R.M.S.; methodology, A.G.S. and A.A.M.A.L.; software, A.G.S.; validation, A.G.S., A.A.M.A.L. and R.M.S.; formal analysis, A.G.S. and S.M.J.v.K.; investigation, A.G.S. and A.A.M.A.L.; resources, A.G.S. and A.A.M.A.L.; data curation, A.G.S.; writing—original draft preparation, A.G.S. and A.A.M.A.L.; writing—review and editing, A.G.S., A.A.M.A.L., S.M.J.v.K., R.R.W.J.v.d.H. and R.M.S.; visualization, A.G.S. and A.A.M.A.L.; supervision, R.M.S. and R.R.W.J.v.d.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen K.-T., Mardini S., Chuang D.C.-C., Lin C.-H., Cheng M.-H., Lin Y.-T., Huang W.-C., Tsao C.-K., Wei F.-C. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast. Reconstr. Surg. 2007;120:187–195. doi: 10.1097/01.prs.0000264077.07779.50. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo D.A., Jones C.S. The role of emergent exploration in free-tissue transfer: A review of 150 consecutive cases. Plast. Reconstr. Surg. 1990;86:492–498. doi: 10.1097/00006534-199009000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Mirzabeigi M.N., Wang T., Kovach S.J., Taylor J.A., Serletti J.M., Wu L.C. Free flap take-back following postoperative microvascular compromise: Predicting salvage versus failure. Plast. Reconstr. Surg. 2012;130:579–589. doi: 10.1097/PRS.0b013e31825dbfb7. [DOI] [PubMed] [Google Scholar]
- 4.Creech B., Miller S. Evaluation of circulation in skin flaps. In: Grabb W.C., Myers M., editors. Skin Flaps. Volume 21 Little, Brown; Boston, MA, USA: 1975. [Google Scholar]
- 5.Sloan G.M., Sasaki G.H. Noninvasive monitoring of tissue viability. Clin. Plast. Surg. 1985;12:185–195. doi: 10.1016/S0094-1298(20)31689-8. [DOI] [PubMed] [Google Scholar]
- 6.Yuen J.C., Feng Z. Monitoring free flaps using the laser Doppler flowmeter: Five-year experience. Plast. Reconstr. Surg. 2000;105:55–61. doi: 10.1097/00006534-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Keller A. Noninvasive tissue oximetry for flap monitoring: An initial study. J. Reconstr. Microsurg. 2007;23:189–197. doi: 10.1055/s-2007-974655. [DOI] [PubMed] [Google Scholar]
- 8.Lohman R.F., Langevin C.-J., Bozkurt M., Kundu N., Djohan R. A prospective analysis of free flap monitoring techniques: Physical examination, external Doppler, implantable Doppler, and tissue oximetry. J. Reconstr. Microsurg. 2013;29:051–056. doi: 10.1055/s-0032-1326741. [DOI] [PubMed] [Google Scholar]
- 9.Smit J.M., Zeebregts C.J., Acosta R., Werker P.M. Advancements in free flap monitoring in the last decade: A critical review. Plast. Reconstr. Surg. 2010;125:177–185. doi: 10.1097/PRS.0b013e3181c49580. [DOI] [PubMed] [Google Scholar]
- 10.Colwell A.S., Craft R.O. Near-infrared spectroscopy in autologous breast reconstruction. Clin. Plast. Surg. 2011;38:301–307. doi: 10.1016/j.cps.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Repež A., Oroszy D., Arnež Z.M. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J. Plast. Reconstr. Aesthetic Surg. 2008;61:71–77. doi: 10.1016/j.bjps.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Machens H.G., Mailaender P., Rieck B., Berger A. Techniques of postoperative blood flow monitoring after free tissue transfer: An overview. Microsurgery. 1994;15:778–786. doi: 10.1002/micr.1920151107. [DOI] [PubMed] [Google Scholar]
- 13.Irwin M., Thorniley M., Dore C., Green C. Near infra-red spectroscopy: A non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br. J. Plast. Surg. 1995;48:14–22. doi: 10.1016/0007-1226(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 14.Thorniley M.S., Sinclair J., Barnett N., Shurey C., Green C. The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery. Br. J. Plast. Surg. 1998;51:218–226. doi: 10.1054/bjps.1997.0145. [DOI] [PubMed] [Google Scholar]
- 15.Lu G., Fei B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014;19:010901. doi: 10.1117/1.JBO.19.1.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz A.F., Vane G., Solomon J.E., Rock B.N. Imaging spectrometry for earth remote sensing. Science. 1985;228:1147–1153. doi: 10.1126/science.228.4704.1147. [DOI] [PubMed] [Google Scholar]
- 17.Grambow E., Dau M., Holmer A., Lipp V., Frerich B., Klar E., Vollmar B., Kämmerer P.W. Hyperspectral imaging for monitoring of perfusion failure upon microvascular anastomosis in the rat hind limb. Microvasc. Res. 2018;116:64–70. doi: 10.1016/j.mvr.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Holmer A., Marotz J., Wahl P., Dau M., Kämmerer P.W. Hyperspectral imaging in perfusion and wound diagnostics–methods and algorithms for the determination of tissue parameters. Biomed. Eng. Biomed. Tech. 2018;63:547–556. doi: 10.1515/bmt-2017-0155. [DOI] [PubMed] [Google Scholar]
- 19.Kulcke A., Holmer A., Wahl P., Siemers F., Wild T., Daeschlein G. A compact hyperspectral camera for measurement of perfusion parameters in medicine. Biomed. Eng. Biomed. Tech. 2018;63:519–527. doi: 10.1515/bmt-2017-0145. [DOI] [PubMed] [Google Scholar]
- 20.Calin M.-A., Coman T., Parasca S.V., Bercaru N., Savastru R.S., Manea D. Hyperspectral imaging-based wound analysis using mixture-tuned matched filtering classification method. J. Biomed. Opt. 2015;20:046004. doi: 10.1117/1.JBO.20.4.046004. [DOI] [PubMed] [Google Scholar]
- 21.Pruimboom T., Schols R.M., Van Kuijk S.M., Van der Hulst R.R., Qiu S.S. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst. Rev. 2020;4:CD013280. doi: 10.1002/14651858.CD013280.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiem D.G., Frick R.W., Goetze E., Gielisch M., Al-Nawas B., Kämmerer P.W. Hyperspectral analysis for perioperative perfusion monitoring—a clinical feasibility study on free and pedicled flaps. Clin. Oral Investig. 2021;25:933–945. doi: 10.1007/s00784-020-03382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann. Plast. Surg. 2009;62:538–543. doi: 10.1097/SAP.0b013e3181a47ce8. [DOI] [PubMed] [Google Scholar]
- 24.Lindelauf A.A., Vranken N., Schols R.M., Bouman E.A., Weerwind P.W., van der Hulst R.R. Exploring personalized postoperative non-invasive tissue oximetry in DIEP flap breast reconstruction. Eur. J. Plast. Surg. 2021:1–9. doi: 10.1007/s00238-021-01873-7. [DOI] [Google Scholar]
- 25.Carruthers K.H., Tiwari P., Yoshida S., Kocak E. Inpatient flap monitoring after deep inferior epigastric artery perforator flap breast reconstruction: How long is long enough? J. Reconstr. Microsurg. 2019;35:682–687. doi: 10.1055/s-0039-1693454. [DOI] [PubMed] [Google Scholar]
- 26.Koolen P.G., Vargas C.R., Ho O.A., Ibrahim A., Ricci J.A., Tobias A.M., Winters H.A., Lin S.J., Lee B.T. Does increased experience with tissue oximetry monitoring in microsurgical breast reconstruction lead to decreased flap loss? The learning effect. Plast. Reconstr. Surg. 2016;137:1093–1101. doi: 10.1097/01.prs.0000481071.59025.82. [DOI] [PubMed] [Google Scholar]
- 27.Vranken N., Weerwind P., Van Onna M., Bouman E., Van der Hulst R. Non-invasive tissue oximetry following unilateral DIEP-flap reconstruction: A pilot evaluation. JPRAS Open. 2017;12:59–65. doi: 10.1016/j.jpra.2017.01.008. [DOI] [Google Scholar]
- 28.Yano A., Orihashi K., Yoshida Y., Kuriyama M. Near-infrared spectroscopy for monitoring free jejunal flap. J. Plast. Reconstr. Aesthetic Surg. 2021;74:108–115. doi: 10.1016/j.bjps.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Kohler L.H., Köhler H., Kohler S., Langer S., Nuwayhid R., Gockel I., Spindler N., Osterhoff G. Hyperspectral Imaging (HSI) as a new diagnostic tool in free flap monitoring for soft tissue reconstruction: A proof of concept study. BMC Surg. 2021;21:1–9. doi: 10.1186/s12893-021-01232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Z.-g., Zhang J., Zhang J.-g., Zhao F.-y., Yu G.-y., Li Y., Ding H.-s. Evaluation of near infrared spectroscopy in monitoring postoperative regional tissue oxygen saturation for fibular flaps. J. Plast. Reconstr. Aesthetic Surg. 2008;61:289–296. doi: 10.1016/j.bjps.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Guye M.-L., Motamed C., Chemam S., Leymarie N., Suria S., Weil G. Remote peripheral tissue oxygenation does not predict postoperative free flap complications in complex head and neck cancer surgery: A prospective cohort study. Anaesth. Crit. Care Pain Med. 2017;36:27–31. doi: 10.1016/j.accpm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Lin S.J., Nguyen M.-D., Chen C., Colakoglu S., Curtis M.S., Tobias A.M., Lee B.T. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast. Reconstr. Surg. 2011;127:1080–1085. doi: 10.1097/PRS.0b013e31820436cb. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier A., Tseng C., Agarwal S., Park J., Song D. Cost analysis of near-infrared spectroscopy tissue oximetry for monitoring autologous free tissue breast reconstruction. J. Reconstr. Microsurg. 2011;27:487–494. doi: 10.1055/s-0031-1284234. [DOI] [PubMed] [Google Scholar]
- 34.Ricci J.A., Vargas C.R., Ho O.A., Lin S.J., Tobias A.M., Lee B.T. Evaluating the use of tissue oximetry to decrease intensive unit monitoring for free flap breast reconstruction. Ann. Plast. Surg. 2017;79:42–46. doi: 10.1097/SAP.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 35.Salgarello M., Pagliara D., Rossi M., Visconti G., Barone-Adesi L. Postoperative monitoring of free DIEP flap in breast reconstruction with near-infrared spectroscopy: Variables affecting the regional oxygen saturation. J. Reconstr. Microsurg. 2018;34:383–388. doi: 10.1055/s-0038-1636527. [DOI] [PubMed] [Google Scholar]
- 36.Steele M.H. Three-year experience using near infrared spectroscopy tissue oximetry monitoring of free tissue transfers. Ann. Plast. Surg. 2011;66:540–545. doi: 10.1097/SAP.0b013e31820909f9. [DOI] [PubMed] [Google Scholar]
- 37.Whitaker I.S., Pratt G.F., Rozen W.M., Cairns S.A., Barrett M.D., Hiew L.Y., Cooper M.A., Leaper D.J. Near infrared spectroscopy for monitoring flap viability following breast reconstruction. J. Reconstr. 2012;28:149–154. doi: 10.1055/s-0031-1296030. [DOI] [PubMed] [Google Scholar]
- 38.Schulz T., Marotz J., Stukenberg A., Reumuth G., Houschyar K.S., Siemers F. Hyperspectral imaging for postoperative flap monitoring of pedicled flaps. Handchir. Mikrochir. Plast. Chir. Organ. Der Dtsch. Arb. Fur Handchir.:Organ. Der Dtsch. Arb. Fur Mikrochir. Der Peripher. Nerven Und Gefasse Organ. Der V. 2020;52:316–324. doi: 10.1055/a-1167-3089. [DOI] [PubMed] [Google Scholar]
- 39.Schulz T., Leuschner S., Siemers F., Marotz J., Houschyar K., Corterier C.C. Assessing flap perfusion after free tissue transfer using hyperspectral imaging (HSI) Eur. J. Plast. Surg. 2021:1–10. doi: 10.1007/s00238-021-01784-7. [DOI] [Google Scholar]
- 40.Keller A. Noninvasive tissue oximetry. Clin. Plast. Surg. 2011;38:313–324. doi: 10.1016/j.cps.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Thiem D.G., Römer P., Blatt S., Al-Nawas B., Kämmerer P.W. New Approach to the Old Challenge of Free Flap Monitoring—Hyperspectral Imaging Outperforms Clinical Assessment by Earlier Detection of Perfusion Failure. J. Pers. Med. 2021;11:1101. doi: 10.3390/jpm11111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshitani K., Kawaguchi M., Tatsumi K., Kitaguchi K., Furuya H. A comparison of the INVOS 4100 and the NIRO 300 near-infrared spectrophotometers. Anesth. Analg. 2002;94:586–590. doi: 10.1097/00000539-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Bevan P.J. Should cerebral near-infrared spectroscopy be standard of care in adult cardiac surgery? Heart Lung Circ. 2015;24:544–550. doi: 10.1016/j.hlc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Hyttel-Sorensen S., Hessel T.W., Greisen G. Peripheral tissue oximetry: Comparing three commercial near-infrared spectroscopy oximeters on the forearm. J. Clin. Monit. Comput. 2014;28:149–155. doi: 10.1007/s10877-013-9507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jafari-Saraf L., Wilson S.E., Gordon I.L. Hyperspectral image measurements of skin hemoglobin compared with transcutaneous PO2 measurements. Ann. Vasc. Surg. 2012;26:537–548. doi: 10.1016/j.avsg.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Ricci J.A., Vargas C.R., Lin S.J., Tobias A.M., Taghinia A.H., Lee B.T. A novel free flap monitoring system using tissue oximetry with text message alerts. J. Reconstr. Microsurg. 2016;32:415–420. doi: 10.1055/s-0036-1582264. [DOI] [PubMed] [Google Scholar]
- 47.Lindelauf A.A., Vranken N.P., Rutjens V.G., Schols R.M., Heijmans J.H., Weerwind P.W., van der Hulst R.R. Economic Analysis of Noninvasive Tissue Oximetry for Postoperative Monitoring of Deep Inferior Epigastric Perforator Flap Breast Reconstruction: A Review. Surg. Innov. 2020;27:534–542. doi: 10.1177/1553350620942985. [DOI] [PMC free article] [PubMed] [Google Scholar]