Abstract
Cardiovascular diseases (CVD) are one of the leading causes of morbidity and mortality worldwide. mtDNA (mitochondrial DNA) mutations are known to participate in the development and progression of some CVD. Moreover, specific types of mitochondria-mediated CVD have been discovered, such as MIEH (maternally inherited essential hypertension) and maternally inherited CHD (coronary heart disease). Maternally inherited mitochondrial CVD is caused by certain mutations in the mtDNA, which encode structural mitochondrial proteins and mitochondrial tRNA. In this review, we focus on recently identified mtDNA mutations associated with CVD (coronary artery disease and hypertension). Additionally, new data suggest the role of mtDNA mutations in Brugada syndrome and ischemic stroke, which before were considered only as a result of mutations in nuclear genes. Moreover, we discuss the molecular mechanisms of mtDNA involvement in the development of the disease.
Keywords: cardiovascular diseases, atherosclerosis, mitochondria, coronary artery disease, Brugada syndrome, hypertension, ischemic stroke
1. Introduction
Atherosclerosis is a chronic inflammatory disease of large and medium-sized arteries that causes other dangerous complications, which are collectively defined as CVD (cardiovascular diseases). Atherosclerosis is characterized by the accumulated deposition of lipoproteins and the migration of monocyte and macrophage artery walls, leading to the restriction of circulation and the risk of thrombosis. Despite the continuing investigation for atherosclerosis’s mechanisms and causes, as well as the search for novel drugs and means for improving healthcare and quality of life, atherosclerosis and the subsequent CVD are consistently high around the globe. Currently, atherosclerosis is an important socio-economic problem and is the leading cause of death worldwide [1].
Coronary artery disease (CAD, also known as atherosclerotic heart disease) is the most common heart disease, and is caused by stenosis in coronary arteries and their branches. During the classical genetics period, candidate genes and polymorphism sites were selected for analysis based on our knowledge of the disease’s pathophysiology. Due to the low-throughput technologies and a low number of subjects, many false positive (and false negative) results were common for this period. The next period, with the GWAS (genome-wide association study), has entailed a significant improvement and extension of our knowledge of the genetic background and pathogenesis of many diseases [2]. The application of GWAS to search for CAD risk alleles has led to the identification of new genes and many significant variants, which greatly expand our understanding of the disease, with a total of 164 chromosomal loci identified [3]. Accumulating evidence supports the idea that genetic factors play a crucial role in atherosclerosis pathogenesis; however, the exact underlying mechanisms are still not fully understood. Nevertheless, the analysis of family history and screening for verified gene variants are effective strategies to predict the individual risk of atherosclerosis development [4].
In addition to nuclear gene variants, mitochondrial mutations also play a crucial role in many human diseases, including atherosclerosis and other CVD [5]. The mitochondria produce ATP (adenosine triphosphate) and harmful ROS (reactive oxygen species) as by-products, which are normally effectively neutralized by antioxidants. However, mutated mitochondrial genes unbalance cellular respiration and energy production, leading to extreme ROS production, oxidative damage and mitochondria dysfunction, further triggering cellular damage, apoptosis and cell death [6]. Mitochondria dysfunction is the first step in atherosclerosis development: an altered ROS (reactive oxygen species) level causes vascular EC (endothelial cell) dysfunction and the recruitment of circulating immune cells, initiating immune reactions for further atherosclerotic plaque formation. Thus, mitochondria represent a promising target for pharmaceutical intervention [7]. The pathogenesis of atherosclerosis starts from local endothelial dysfunction and the inflammatory response of the arterial wall. Further, the emerging atherosclerotic lesion site expresses adhesion molecules, lipoproteins (LDL (low-density lipoprotein) is the main fraction) penetrate the arterial wall and cause local accumulation, subsequently leading to plaque formation [8,9].
In this review, we discuss the recently identified mutations in mitochondrial DNA associated with CVD (CAD, hypertension, ischemic stroke and Brugada syndrome), their significance as diagnostic and therapeutic targets. Additionally, molecular mechanisms, linking mtDNA mutations and CVD development and progression are discussed.
2. Mitochondrial Genome Organization, Functions and Dynamics
The mitochondrial genome is represented by a circular double-stranded mtDNA with an approximate length of around 16.5 thousand nucleotides. The mitochondrial genome encodes only 37 genes (13 structural genes encoding subunits of OXPHOS (oxidative phosphorylation complexes)), 22 tRNAs (transport RNAs) and 2 rRNAs (ribosomal RNAs). All other proteins necessary for mitochondrial function, transcription, repair and maintenance, are encoded by a nuclear genome and imported to the mitochondria [10]. Because every organelle contains a different number of mtDNA copies, occurring mutations could be homoplasmic (all mtDNA copies are identical and carry this particular mutation) or heteroplasmic (only some mtDNA copies have the mutation) [11]. Mitochondrial mutations are often associated with human diseases, and they could be inherited from the mother or acquired and accumulated during the individual’s life [12]. The effect of mitochondrial mutations depends on the affected tissue and the heteroplasmy level [13]. A mutant-specific phenotype develops when a certain threshold of mutation heteroplasmy is reached [11]. Because mtDNA mutations are normally masked by functional wild-type copies, the threshold of heteroplasmy is usually high (more than 70%); however, this value could be tissue specific [14].
The most crucial role of the mitochondria is energy production in the form of ATP. There are several steps in ATP synthesis: (1) the conversion of pyruvate and fatty acids into acetyl-CoA; (2) the Krebs cycle uses acetyl-CoA to produce NADH (nicotine amide adenine dinucleotide); (3) electron transfer from NADH to oxygen via the respiratory chain; and (4) ATP synthesis by the membrane ATP synthase [15]. ATP synthesis is accompanied by ROS production, which has some regulatory and signalling roles [16]. However, excessive ROS generation could lead to oxidative damage to biomolecules (mtDNA, proteins, lipids) [17]. Nowadays, excessive ROS and subsequent oxidative damage are recognized as two of the main factors of atherosclerosis pathogenesis [6].
Mitochondria participate in the regulation of Ca2+ homeostasis via their close interaction with ER (endoplasmic reticulum), the main cellular Ca2+ reservoir. The mechanisms of Ca2+ flux in/from the mitochondria is crucial for cellular signalling, neurotransmitter and hormone release, the regulation of mitochondrial membrane potential and respiratory bioenergetics [18]. Additionally, disturbance in the mitochondrial lipid metabolism could activate ER stress and UPR (unfolded protein response) via mitochondria–ER contact sites [19].
The processes of mitochondrial biogenesis, turnover and recycling are directed based on the equal distribution of mitochondria between dividing cells, the maintenance of the healthy and efficient mitochondrial population and the salvage of damaged or ineffective mitochondria, respectively. Mitochondrial turnover consists of cycles of fission (split) and fusion (merge) of dysfunctional or damaged organelles. Separated dysfunctional parts of the mitochondria are further degraded via a specialized form of autophagy—mitophagy. Healthy parts of the mitochondria are fused and continue normal functioning as a part of the mitochondrial network [20].
DNM1L (dynamin 1 like) and FIS1 (fission, mitochondrial 1) are the main genes responsible for mitochondrial fission, and MFN1, MFN2 (mitofusin 1 and 2) and OPA1 (optic atrophy protein 1) are responsible for fusion. The expression of those proteins and the activity of the corresponding proteins are tightly regulated on several levels. Their excessive or inadequate expression/activity would lead to impaired mitophagy, which was shown to be associated with many human diseases [21], including CVDs [22]. However, the importance of mitochondrial turnover mechanisms also suggests their great potential as a therapeutic target. Recently, a mitochondrial fission inhibitor, mdivi1, was used on the mice Drp1+/− model of AAA (abdominal aortic aneurysm), where it helped to prove the key role of mitochondrial fission in AAA development [23].
Mutations in the nuclear genes that encode the respiratory chain subunits and proteins responsible for mtDNA maintenance (replication, transcription and copy number control), biogenesis and dynamics (fission and fusion) could cause the secondary instability of the mitochondrial genome and mitochondria dysfunction. Mitochondrial fragmentation and cardiomyopathy are caused by mutations in OPA1 (responsible for fusion), SLC25A4 (mitochondrial solute carrier family 25 member 4) (responsible for ADP/ATP balance and mtDNA stability) and DRP1 (responsible for fission) (reviewed in [24,25]). Similarly, cardiomyopathies were described for the nuclear encoded genes required for proper OXPHOS forming, including CI assembly factors (NDUFAF1, ACAD9), CIII assembly factors (UQCC3), CIV assembly factors (COA5), CV assembly factors (TMEM70) and other genes (reviewed in [26]).
Mitochondrial diseases are the focus of many researchers. While there are still many unanswered questions, some pioneering treatments and preventive methods are already available [27]. Some major strategies can be defined: (1) enhance the efficacy of the electron transfer chain (with the application of thiamine, coenzyme Q10, idebenone), improve the energy buffer (creatine), cardiolipin protection (elamipretide), NO production (with amino acids arginine and citrulline), antioxidants supplementation (vitamin E, D, C) and others [28]. Additionally, mitochondria transplantation, mitochondrial gene therapies [29] and the application of pharmacological agents to modulate mitochondrial metabolism and functions are under intensive investigation [30].
3. Mitochondrial DNA Mutations
The mutation rate of mtDNA is much higher in comparison to nuclear DNA. There are several mechanisms leading to such an outcome. Firstly, ROS damage is currently recognized as the major driver of mtDNA mutagenesis. Further mutation accumulation leads to more severe mitochondrial dysfunction and even higher ROS production, thus forming a vicious circle [31]. Secondly, the mitochondrial DNA repair system is not so efficient in comparison to the nuclear one [32]. Additionally, mtDNA is not protected by histone proteins, and thus is more accessible for harmful agents. Finally, mutations in polymerase γ (POLG), which is responsible for the replication of mtDNA, is the most common cause of inherited mitochondrial disorders [33].
Further in this section, we analyse the recently identified mtDNA mutations associated with CVDs (CAD (Table 1), hypertension (Table 2), ischemic stroke and Brugada syndrome (Table 3)).
Table 1.
List of mtDNA mutations associated with CAD.
| Mutation | Gene | Other Notes | References | |
|---|---|---|---|---|
| Mt5568 (A > G) | tRNATrp | Iranian CAD Patients | [34] | |
| Mt5711 (T > A) | tRNAAsn | |||
| Mt5725 (T > G) | tRNAAsn | |||
| Mt12308 (A > G) | tRNALeu (CUN) | |||
| Mt16089 (T > C) | D-loop | TG | Association with CVD risk factors in Chinese Han CAD patients | [35] |
| Mt16145 (G > A) | D-loop | TG; LVEF | ||
| Mt16089 (T > C) | D-loop | PC | ||
| Mt14178 (T > C) | MT-ND6 | TC | ||
| Mt215 (A > G) | D-loop | LDLC | ||
| Mt8231 (C > A) | MT-CO2 | Iranian CAD Patients | [36] | |
| Mt8376 (T > A) | MT-ATP8 | |||
| Mt15928 (G > A) | tRNAThr | |||
| Mt5628 (T > C) | tRNAAla | Chinese CAD patients | [37] | |
| Mt681 (T > C) | 12S rRNA | |||
| Mt5592 (A > G) | tRNAAla | |||
| mtDNA4977 Deletion | Alone or in combination with LTL associated with recurrent MACEs and all-cause mortality in Caucasian CAD patients | [38] | ||
| Associated with MACEs and all-cause mortality in Italian CAD patients | [39] | |||
| In combination with low folate level associated with high CAD risk among Chinese diabetic patients | [40] | |||
| Mt15910 (C > T) | tRNAThr | Han Chinese patients withLHON, signs of maternally inherited CHD | [41] | |
Table 2.
List of mtDNA mutations associated with hypertension.
| Mutation | Gene | Other Notes | References |
|---|---|---|---|
| Mt3970 (C > T) | MT-ND1 | Chinese MIEH patients | [50] |
| Mt4048 (G > A) | |||
| Mt4071 (C > T) | |||
| Mt4086 (C > T) | |||
| Mt4164 (A > G) | |||
| Mt4248 (T > C) | |||
| Mt4386 (T > C) | tRNAGln | ||
| Mt4394 (C > T) | |||
| Mt8414 (C > T) | MT-ATP8 | [51] | |
| Mt8701 (A > G) | MT-ATP6 | ||
| Mt8584 (G > A) | |||
| Mt8273_8281del | |||
| Mt8701 (A > G) | MT-ATP6 | A Chinese family with MIEH cases | [52] |
| Mt5587 (T > C) | tRNAAla | [53] | |
| Mt12280 (A > G) | tRNALeu(CUN) | ||
| Mt5512 (A > G) | tRNATrp | [54] | |
| Mt15077 (G > A) | MT-CYB | [55] | |
| Mt15992 (A > G) | tRNAPro | ||
| Mt10410 (T > C) | tRNAArg | [56] | |
| Mt10454 (T > C) | |||
| Mt3253 (T > C) | tRNALeu(UUR) | Chinese Han EH patients | [57] |
| Mt15910 (C > T) | tRNAThr | [58] | |
| Mt5655 (T > C) | tRNAAla | Han Chinese family with EH | [59] |
| Mt4401 (A > G) | Between tRNAMet and tRNAGln | ||
| Mt7471 delC | tRNASer(UCN) | [60] | |
| Mt4467 (C > A) | tRNAMet | [61] | |
| Mt4263 (A > G) | tRNAIle | [62] | |
| Mt15909 (A > G) | tRNAThr | [63] | |
| Mt4363 (T > C) | tRNAGln | [64] | |
| Mt5601 (C > T) | tRNAAla | [65] | |
| Mt4435 (A > G) | tRNAMet |
Table 3.
List of mtDNA mutations associated with Brugada syndrome and ischemic stroke.
| Mutation | Gene | Other Notes | References |
|---|---|---|---|
| Mt4216 (T > C) | MT-ND1 | Associated with the most severe BrS phenotype among Caucasian BrS patients | [72] |
| Mt11251 (A > G) | MT-ND4 | ||
| Mt15452 (C > A) | MT-CYB | ||
| Mt16126 (T > C) | D-loop | ||
| Mt4377 (T > A) | tRNAGln | Associated with BrS in Iranian patients | [73] |
| Mt4407 (G > A) | tRNAMet | ||
| Mt4456 (C > T) | |||
| Mt5580 (T > C) | junction region between tRNATrp and tRNAAla | ||
| m.16145G > A | D-loop | Genetic risk factors for IS | [76] |
| m.16311T > C | |||
| Mt195 (T > C) | D-loop | Protective factors of IS in Chinese patient cohort | [77] |
| Mt311 (C > T) | D-loop | ||
| Mt12338 (T > C) | MT-ND5 |
The mtDNA mutations associated with CAD could be grouped into several main categories, each with different mechanisms of action: (1) mutations in tRNA were predicted to destabilize the base pairing at the affected sites, potentially altering the secondary structure of this tRNA and causing its quicker degradation and the subsequent reduction in mitochondrial protein levels [41]; (2) mutations in the OXPHOS components were shown to reduce ATP synthesis and increase ROS production; and (3) mutations in the D-loop would interrupt the normal mtDNA replication process, resulting in the reduced mtDNA copy number [38]. Interestingly, the majority of identified mtDNA mutations were found to be non-pathogenic or mild; thus, they may not be able to cause a certain pathogenic phenotype development [42]. However, the disease could manifest under the influence of other genetic, nutritional or environmental factors [40].
Hypertension (high blood pressure) is a common public health problem, affecting more than 1.28 billion people worldwide. Hypertension (HTN) can be primary (essential, with no identifiable cause and developing over many years) and secondary, which is caused by various other conditions (kidney, lung diseases, thyroid problems, sleep apnoea and others). HTN is a risk factor for CAD, stroke, heart failure and renal dysfunction [43].
Essential HTN is known as a multifactorial disease, where both environmental and genetic factors are responsible for the physiopathology and severity of the disease manifestation. The role of genetic factors in HTN has a long history being investigated, with studies attributing a tendency to familial aggregation to this disease despite different environmental factors [44]. Recently, several studies have identified multiple mtDNA mutations associated with HTN, thus suggesting its maternal transmission [45,46,47]. A wide-scope study, conducted in 2007, has defined the fraction of mitochondria-mediated cases among hypertensive pedigrees as 35.2% [48]. Interesting, in north China, the number of HNT incidences is much higher than average [49]. Unsurprisingly, the majority of current investigations have been conducted in this ethnic group (Chinese, Chinese Han and Mongolian Chinese) (Table 2); however, in other ethnic groups, HTN incidence may have a different rate and fraction of mitochondria-mediated maternal transmission.
Brugada syndrome (BrS), described in 1992 by the Brugada brothers, is a rate cardiac disorder characterized by a structurally normal heart, but with typical electrocardiogram alterations and a high risk of sudden death. BrS accounts for 4% of all sudden deaths and 20% of sudden deaths in the absence of structural heart disease [66]. Approximately 80% of Brugada patients are male of age 40–45, and symptoms develop during night or daytime rest periods and are often combined with fever. Diagnosis is based on the characteristic electrocardiogram pattern with a cove-shaped ST elevation in leads V1 to V3. The most effective strategy to prevent sudden cardiac death is the application of an implantable cardioverter-defibrillator. However, this approach has many drawbacks for the patient [67].
BrS is a genetically transmitted disease with an autosomal dominant transmission and incomplete penetrance. The SCN5A (sodium voltage-gated channel alpha subunit 5) gene, which encodes the α-subunit of the Na+ channel, is considered to be the main genetic factor responsible for BrS. Mutations in SCN5A are found in 25–30% of BrS patients, with over 300 mutations known for the SCN5A gene being associated with BrS [68,69]. In addition to SCN5A, mutations in 17 other genes are also known to be responsible for a small number of Brugada cases [70], suggesting the co-segregation of different involvement mutations or genetic variants in the clinical manifestation of this disorder.
BrS is considered endemic in Southeast Asian countries, where the number of cases is higher in comparison to the average number (5–20 cases for every 10,000 people worldwide) [71]. There are only two recent reports suggesting the involvement of mtDNA mutations in BrS (Table 3) [72,73] (Table 3). Taking into account that the current genetic monitoring covers only 30% of BrS patients, the identification of additional biomarkers associated with BrS could be particularly beneficial for the early diagnosis of asymptomatic patients.
Ischemic stroke (IS) is a multifactorial disorder characterized by the sudden loss of blood circulation to an area of the brain due to cerebral artery stenosis or occlusion, resulting in brain ischemia, hypoxia or necrosis. Oxidative stress, energy disturbance, excitatory amino acid toxicity, neuroinflammation and nerve cell death are caused by brain ischemia and form a complex network, leading to subsequent cascade damage [74]. Mitochondrial dysfunction is known to be involved in neuronal cell death and oxidative damage in neurodegenerative and CVD via the triggering of several molecular mechanisms, leading to vascular dysfunction [75]. The identified MtDNA variants could be used as diagnostic and IS-predicting biomarkers. Similarly, identified IS-protective MtDNA mutations (Table 3) could be used in future research to better understand the aetiology of IS.
4. Molecular Mechanisms of mtDNA Mutations
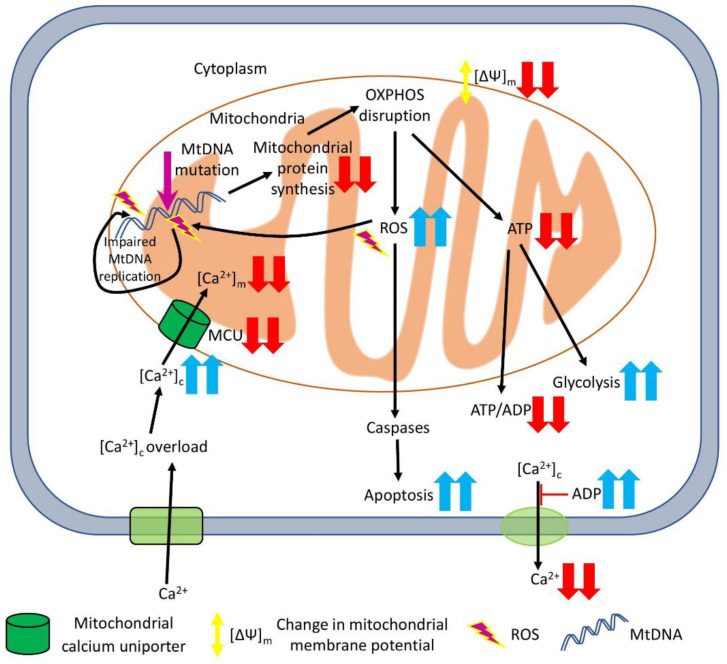
The exact molecular mechanisms connecting mtDNA mutations with different CVD are not fully understood. However, multiple studies suggest that mtDNA mutations disrupt mitochondrial homeostasis, causing a rise in ROS production, dysregulating Ca2+ metabolism and reducing energy synthesis. The molecular pathways affected by mtDNA mutations are briefly summarised in Figure 1.
Figure 1.
Potential pathways affected by mtDNA mutations and associated with CVD. The mtDNA mutations lead to a decreased level of tRNAs and protein synthesis (including proteins involved in mitochondrial OXPHOS), and increased ROS production and, subsequently, mitochondrial oxidative stress and cell apoptosis. High ROS levels tend to cause more mutations in mtDNA during its replication. OXPHOS malfunction leads to lower ATP output and the collapse of the mitochondrial membrane potential, thus changing the ATP/ADP ratio, interrupting normal ion traffic and stimulating glycolysis as an alternative ATP-generation pathway. An impaired Ca2+ metabolism can cause the accumulation of Ca2+ in the cytosol, with low concentrations in mitochondria and extracellular space, which may result in cell swelling and death. Red arrows represent decrease, blue – increase of a particular process/parameter.
Dysfunctions of OXPHOS in the mitochondria lead to abnormal energy metabolism and abnormal changes in the exchange of sodium and calcium, leading to calcium overload in the cytoplasm, the diastolic dysfunction of cardiomyocytes and smooth muscle cells, and promoting blood pressure increase.
4.1. Mutations in tRNA Genes
tRNA genes are normally highly conserved due to their indispensable role in the biosynthesis of mitochondrial proteins. Thus, mutations in tRNA are predicted to decrease tRNA stability and destabilize the base pairing, potentially altering the secondary structure of this tRNA. Previously, those effects have been reported for the Mt10398 A > G mutation in the MT-ND3 gene in hypertension-associated stage renal disease [78].
The cybrid cells carrying the Mt15910 (C > T) mutation (tRNAThr) had 37.5% lower levels of tRNAThr in comparison to healthy control cells, suggesting that the Mt15910 (C > T) mutation speeds up its degradation [41]. Previously, similar results were described for other tRNA mutations, Mt3243 (A > G) tRNALeu(UUR) [79], Mt5655 (T > C) tRNAAla, Mt10003 (T > C) tRNAGly, Mt3253 (T > C) tRNALeu(UUR), Mt7551 (A > G) tRNAAsp and Mt14692 (A > G) tRNAGlu [45]. Subsequently, alterations of the tRNA levels lead to the reduced rate of mitochondrial protein synthesis and a reduction in mitochondrial protein levels in the mutant cells, altered complex I/III activity, electron leakage and a rise in ROS production [41]. Elevated levels of ROS production could lead to the damage of cellular biomolecules and contribute to the disease-related phenotype (discussed in the following section).
However, most likely is that, in addition to the mitochondrial mutations, other nuclear genetic or epigenetic, as well as a combination of environmental and lifestyle factors are responsible for the development of the clinical phenotype of the particular CVD. For example, platelet mitochondrial DNA methylation was proposed as a new biomarker to predict CVD [80,81]. As it was shown, mtDNA methylation could be affected by environmental stress factors (such as air pollution), causing mitochondrial dysfunction and, subsequently, affecting heart functions [82,83]. Similarly, mtDNA methylation could be influenced by a diet supplemented with l-carnitine and trimethylamine-N-oxide (TMAO), which are known to be CVD biomarkers. While the exact mechanism and role of mtDNA methylation are not fully understood, the high TMAO level was associated with a worse lipid profile and an increased risk of major adverse cardio and cerebrovascular events [84]. Dietary antioxidants could also remodel mtDNA methylation patterns and provide a positive effect on the initiation, development and progression of many chronic diseases (such as type 2 diabetes, Alzheimer’s disease, cancer and atherosclerosis) (reviewed in [85]). For more information, we wish to redirect interested readers to the following recent reviews [86,87].
4.2. Mitochondrial Oxidative Stress
Mitochondria are involved in the regulation of apoptosis, cell cycle and cell development, ROS production and cell signal transmission and intracellular Ca2+ homeostasis. However, the major function of mitochondria is energy generation (in the form of ATP), covering approximately 95% of cells’ active demands. This function is vital in energy-consuming cells, such as neurons and cardiomyocytes [88]. Thus, mitochondrial dysfunction and injury may have significant effects on general cell function. Several recent reports suggest that atherosclerosis progression and development are closely related to mitochondrial structure, abnormal function and, especially, energy metabolism. The application of anti-miR33 therapy (miR33 is a known repressor of several energy-metabolism responsible genes, namely, PGC-1α (PPARG coactivator 1 alpha), PDK4 (pyruvate dehydrogenase kinase 4) and SLC25A25 (calcium-binding mitochondrial carrier protein ScaMC-2)) resulted in enhanced mitochondrial respiration and ATP production, the subsequent stimulation of macrophage cholesterol efflux and the reduction of atherosclerosis [89]. Similarly, the application of CoQ10 (coenzyme Q10—one of the mitochondrial respiratory chain components) improved mitochondrial function, inhibited ROS production and enhanced energy metabolism, thus attenuating atherosclerosis [90].
ROS is one of the main factors contributing to oxidative stress in the body and promoting cardiovascular diseases. ROS is a normal cellular component, presenting usually at low concentrations, and is known to maintain vascular integrity by regulating endothelial function and vascular contraction–relaxation. However, under pathological conditions, the ROS levels rise dramatically and could damage vascular endothelial cells and cause endothelial dysfunction. An increase in ROS levels plays a key role in the pathogenesis of CVD through the proliferation and migration of VSMC (vascular smooth muscle cells), the stimulation of inflammation mediator release and an increase in free calcium in endothelial cells. In cardiomyocyte mitochondria, and increased ROS leads to the loss of mtDNA and increased autophagy [91]. Currently, ROS-targeted approaches are the most promising type of anti-atherosclerotic therapies [92].
Several mitochondria mutations have been studied in detail. The tRNAIle mutation at the position Mt4263 (A > G) is involved in malfunctioning respiratory complexes I, III and IV, which are significantly enriched in AUC and/or AUU codons that pair with tRNAIle(GAU). Thus, the resulting oxygen consumption was reduced to 70−80% of normal levels [93]. The Mt15910 (C > T) mutation in tRNAThr, known to be linked to CHD, on the organelle level was associated with a 37.5% reduction in tRNAThr levels and a 25% decrease in mitochondrial translation rates [41]. Further, a damaged mitochondrial respiratory chain leads to a vicious cycle: increased ROS production means a higher rate of mtDNA mutations and cell death.
4.3. Mitochondrial Energy Synthesis
mtDNA mutations and deletions disrupt the normal functioning of the respiration chain and decrease proton flow, thus reducing mitochondrial membrane potential and inhibiting mitochondrial ATP synthesis. A recent study showed that the Mt3253 (T > C) mutation (tRNALeu(UUR)) resulted in the decreased activity of mitochondrial complexes I and V, leading to 66% lower ATP production, reduced membrane potential and the increased production of ROS [57]. Similarly, the Mt10454 (T > C) and Mt10410 (T > C) mutations significantly reduced mitochondrial ATP and membrane potential, increased ROS production and raised the levels of MDA (malondialdehyde) and 8-OhdG (8-Oxo-2’-deoxyguanosine), while levels of SOD (superoxide dismutase) and GSH-Px (glutathione peroxidase) were decreased [56]. Cell lines carrying the Mt4467 (C > A) mutation (tRNAMet) had decreased oxygen consumption, 26.2% lower mitochondrial membrane potential, 26.4% lower ATP level, and 114.5% higher ROS production [61]. The Mt15909 (A > G) mutation (tRNAThr) resulted in the decrease in the overall levels of mitochondrial translation products and ATP production, while ROS generation was increased [63]. Therefore, MtDNA mutations and deletions contribute to oxidative stress and mitochondrial dysfunction, which may be involved in the development and pathogenesis of CVD, in particular, hypertension and atherosclerosis.
4.4. Mitochondrial Ca2+ Regulation
Mitochondria could affect cytosolic Ca2+ levels as a result of both mtDNA mutations and nuclear gene mutations. The direct pathway would affect Ca2+ uptake into the mitochondria via the MCU (mitochondrial calcium uniporter). Cells carrying the Mt4263 (A > G) mutation (tRNAIle) exhibited a lower expression of MCU, which resulted in dysregulated Ca2+ uptake and cytoplasmic Ca2+ overload [62]. Various mutations in nuclear-encoded GARS (glycyl-tRNA synthetase 1) resulted in alterations to the mitochondrial respiratory chain complex subunits, Krebs cycle enzymes, assembly genes and the proteins involved in fatty acid oxidation, thus causing mitochondrial cardiomyopathy. Specifically, mitochondrial calcium metabolism and ER–mitochondria interactions sites were altered, which contributed to the clinical presentations of the inherited neuropathies [94]. Additionally, indirectly, Ca2+ transport depends on the available ATP. MtDNA mutations cause a decrease in ATP synthesis and mitochondrial membrane potential, which could lead to Ca2+ dysregulation, malfunction in smooth muscle and apoptosis [95].
The MCU plays a key role in the transport of Ca2+ between the mitochondria and the sarcoplasmic reticulum in skeletal muscle cells and cardiomyocytes [96]. The knock-out of myocardial cell mitophagy regulating protein BNIP3 (BCL2 interacting protein 3) leads to Ca2+ transport in the ER, mitochondrial injury, a rise in the apoptosis rate and left ventricular myocardial fibrosis [97]. FOXO3a (forkhead box O3a) upregulates BNIP3 expression in normal and stressed cardiomyocytes, with subsequent increases in mitochondrial Ca2+, leading to decreased mitochondrial membrane potential, mitochondrial fragmentation and apoptosis. Therefore, FOXO3a/BNIP3-mediated Ca2+ regulation contributes to mitochondrial dysfunction in heart failure and could be used as a potential therapeutic target [98]. The upregulation of SERCA2a (sarcoendoplasmic reticulum calcium ATPase 2a) facilitated greater depolarization-induced Ca2+ transience and increased endoplasmic reticulum and mitochondria Ca2+ load in spontaneously hypertensive rats stellate neurons [99]. The application of MCU inhibitors was shown to inhibit excessive mitophagy in neurons in the ischemia/reperfusion model, thus suggesting an additional Ca2+-mediated protective mechanism [100].
In total, several molecular mechanisms are associated with mitochondrial Ca2+ homeostasis and the pathogenesis of CVD. The alteration of mitochondrial Ca2+ levels can affect mitochondrial autophagy, fission and fusion, mitochondrial morphology and function, and thus could be used as a target in CVD prevention and treatment.
4.5. MtDNA Copy Number
The mitochondrial DNA copy number (mtDNA-CN) is associated with ATP production and general mitochondrial enzyme activity, and thus could serve as a biomarker of mitochondrial efficiency. In practical applications, this is a low-cost, scalable assay that allows measuring mtDNA levels per cell (usually in peripheral blood cells) in a large number of samples. It is known that mtDNA-CN declines with age and is associated with frailty and all-cause mortality [101,102]. Here, we discuss the association between mtDNA-CN and atherosclerosis.
A recent study has shown an association between the low peripheral blood mtDNA-CN and the severity of CHD (coronary heart disease or coronary atherosclerosis) and increased risks for CHD [103]. Similarly, an evaluation of the association between low mtDNA-CN and PAD (peripheral arterial disease, the most common occurring in leg blood vessels) showed that lower levels of mtDNA-CN resulted in a two-fold higher risk. However, normalized scores were not significant for the mtDNA-PAD pair, while low mtDNA-CN was still linked with all-cause-mortality and prevalent and incident CVD in PAD patients [104]. Other studies have found a strong correlation between the low mtDNA-CN and SCD (sudden cardiac death) and CVD [105,106,107].
Thus, mtDNA-CN could be used as a biomarker to evaluate the risk of CVD development and particular CVD complications. Such biomarkers need to be adjusted for an individual’s variability over time, and also need to account for the difference between different populations and ethnic groups.
5. Conclusions
mtDNA mutations are common among CVD patients. Many CAD and hypertension-related mtDNA mutations have been reported. On the other hand, only a limited number of mtDNA mutations associated with Brugada syndrome and ischemic stroke are known so far. Available data from different studies (cybrid, cellular and animal models) suggest that the described mtDNA mutations affect the mtDNA copy number, general mitochondrial functions, increase ROS generation, decrease the efficiency of mitochondrial protein synthesis and energy production and interrupt normal Ca2+ metabolism and signalling.
The rapid development of sequencing technologies and accumulated knowledge about population-specific and unique mtDNA mutations help to predict the likelihood of disease development in ethnic- and family-specific ways. The investigation of every mtDNA mutation and genome individually would allow us to explore their effects on the metabolism. Respectively, this knowledge could be used for disease-preventing intervention as soon as necessary or the development and administration of personalized treatment. Similarly, the early diagnosis and treatment of mtDNA-mediated CVD would help to reduce the damage of target organs and consequences of the disease, and thus increase the quality of patients’ lives.
CVD represent a complex type of pathology that usually involves nuclear gene interactions, maternal inheritance and environmental factors. Further exploration and investigation of the underlying molecular mechanisms of every mtDNA mutation would help to create cheaper diagnostic tools, more effective treatment and define the effect of traditional risk factors (lifestyle, diet, accompanying illnesses and others).
In total, the genotyping of mtDNA mutations to determine and predict CVD could be a powerful tool for family screening and diagnosis. However, the wide application and successful use of mtDNA testing in clinical practice depend on a standardized approach to interpret mtDNA variability and level of heteroplasmy.
Abbreviations
| AAA | abdominal aortic aneurysm |
| ATP | adenosine triphosphate |
| BrS | Brugada syndrome |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| CVD | cardiovascular diseases |
| ECs | endothelial cells |
| EH | essential hypertension |
| ER | endoplasmic reticulum |
| GWAS | genome-wide association study |
| HTN | hypertension |
| IS | ischemic stroke |
| LDL | low-density lipoprotein |
| LDLC | low-density lipoprotein cholesterol |
| LHON | Leber hereditary optic neuropathy |
| LTL | leucocyte telomere length |
| LVEF | left ventricular ejection fraction |
| MACEs | major adverse cardiovascular events |
| MIEH | maternally inherited essential hypertension |
| mtDNA | mitochondrial DNA |
| mtDNA-CN | mitochondrial DNA copy number |
| NADH | nicotine amide adenine dinucleotide |
| OXPHOS | oxidative phosphorylation complexes |
| PAD | peripheral arterial disease |
| ROS | reactive oxygen species |
| rRNAs | ribosomal RNAs |
| SCD | sudden cardiac death |
| TC | total cholesterol |
| TG | triglyceride |
| tRNAs | transport RNAs |
| UPR | unfolded protein response |
| VSMC | vascular smooth muscle cells |
Author Contributions
S.A.D. and A.N.O. conceptualized the manuscript; S.A.D. wrote the manuscript text; V.N.S., V.A.K. (Victoria A. Khotina), V.A.K. (Vladislav A. Kalmykov), L.M.M. and A.N.O. reviewed the text; V.A.K. (Victoria A. Khotina) and V.N.S. developed the methodology; V.A.K. (Vladislav A. Kalmykov) and L.M.M. completed the formal analysis; A.N.O. obtained funding and supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant # 22-25-00393).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kobiyama K., Ley K. Atherosclerosis: A chronic inflammatory disease with an autoimmune component. Circ. Res. 2018;123:1118–1120. doi: 10.1161/CIRCRESAHA.118.313816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sazonovs A., Barrett J.C. Rare-variant studies to complement genome-wide association studies. Annu. Rev. Genom. Hum. Genet. 2018;19:97–112. doi: 10.1146/annurev-genom-083117-021641. [DOI] [PubMed] [Google Scholar]
- 3.Schunkert H., von Scheidt M., Kessler T., Stiller B., Zeng L., Vilne B. Genetics of coronary artery disease in the light of genome-wide association studies. Clin. Res. Cardiol. 2018;107:2–9. doi: 10.1007/s00392-018-1324-1. [DOI] [PubMed] [Google Scholar]
- 4.Erdmann J., Kessler T., Munoz Venegas L., Schunkert H. A Decade of genome-wide association studies for coronary artery disease: The challenges ahead. Cardiovasc. Res. 2018;114:1241–1257. doi: 10.1093/cvr/cvy084. [DOI] [PubMed] [Google Scholar]
- 5.Wallace D.C. Mitochondrial genetic medicine. Nat. Genet. 2018;50:1642–1649. doi: 10.1038/s41588-018-0264-z. [DOI] [PubMed] [Google Scholar]
- 6.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 7.Veloso C.D., Belew G.D., Ferreira L.L., Grilo L.F., Jones J.G., Portincasa P., Sardão V.A., Oliveira P.J. A Mitochondrial Approach to cardiovascular risk and disease. Curr. Pharm. Des. 2019;25:3175–3194. doi: 10.2174/1389203720666190830163735. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammation in atherosclerosis—No longer a theory. Clin. Chem. 2021;67:131–142. doi: 10.1093/clinchem/hvaa275. [DOI] [PubMed] [Google Scholar]
- 9.Myasoedova V.A., Di Minno A., Songia P., Massaiu I., Alfieri V., Valerio V., Moschetta D., Andreini D., Alamanni F., Pepi M., et al. Sex-specific differences in age-related aortic valve calcium load: A systematic review and meta-analysis. Ageing Res. Rev. 2020;61:101077. doi: 10.1016/j.arr.2020.101077. [DOI] [PubMed] [Google Scholar]
- 10.Basu U., Bostwick A.M., Das K., Dittenhafer-Reed K.E., Patel S.S. Structure, mechanism, and regulation of mitochondrial DNA transcription initiation. J. Biol. Chem. 2020;295:18406–18425. doi: 10.1074/jbc.REV120.011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aryaman J., Johnston I.G., Jones N.S. Mitochondrial heterogeneity. Front. Genet. 2019;9:718. doi: 10.3389/fgene.2018.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulton J., Finsterer J., Yu-Wai-Man P. Genetic counselling for maternally inherited mitochondrial disorders. Mol. Diagn. Ther. 2017;21:419–429. doi: 10.1007/s40291-017-0279-7. [DOI] [PubMed] [Google Scholar]
- 13.Jackson C.B., Turnbull D.M., Minczuk M., Gammage P.A. Therapeutic manipulation of mtDNA heteroplasmy: A shifting perspective. Trends Mol. Med. 2020;26:698–709. doi: 10.1016/j.molmed.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Stewart J.B., Chinnery P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015;16:530–542. doi: 10.1038/nrg3966. [DOI] [PubMed] [Google Scholar]
- 15.Lobo-Jarne T., Ugalde C. Respiratory chain supercomplexes: Structures, function and biogenesis. Semin. Cell Dev. Biol. 2018;76:179–190. doi: 10.1016/j.semcdb.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand A., Duburcq T., Dekeyser T., Neviere R., Howsam M., Favory R., Preau S. Involvement of mitochondrial disorders in septic cardiomyopathy. Oxid. Med. Cell. Longev. 2017;2017:4076348. doi: 10.1155/2017/4076348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belosludtsev K.N., Belosludtseva N.V., Dubinin M.V. Diabetes mellitus, mitochondrial dysfunction and Ca2+-dependent permeability transition pore. Int. J. Mol. Sci. 2020;21:6559. doi: 10.3390/ijms21186559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H.-E., Grant A.R., Simic M.S., Kohnz R.A., Nomura D.K., Durieux J., Riera C.E., Sanchez M., Kapernick E., Wolff S., et al. Lipid biosynthesis coordinates a mitochondrial-to-cytosolic stress response. Cell. 2016;166:1539–1552. doi: 10.1016/j.cell.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Hattab A.W., Suleiman J., Almannai M., Scaglia F. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol. Genet. Metab. 2018;125:315–321. doi: 10.1016/j.ymgme.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Morales P.E., Arias-Durán C., Ávalos-Guajardo Y., Aedo G., Verdejo H.E., Parra V., Lavandero S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol. Asp. Med. 2020;71:100822. doi: 10.1016/j.mam.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Cooper H.A., Cicalese S., Preston K.J., Kawai T., Okuno K., Choi E.T., Kasahara S., Uchida H.A., Otaka N., Scalia R., et al. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc. Res. 2021;117:971–982. doi: 10.1093/cvr/cvaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusecka J., Kaliszewska M., Bartnik E., Tońska K. Nuclear genes involved in mitochondrial diseases caused by instability of mitochondrial DNA. J. Appl. Genet. 2018;59:43–57. doi: 10.1007/s13353-017-0424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yapa N.M.B., Lisnyak V., Reljic B., Ryan M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595:1184–1204. doi: 10.1002/1873-3468.14077. [DOI] [PubMed] [Google Scholar]
- 26.Ghezzi D., Zeviani M. Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem. 2018;62:271–286. doi: 10.1042/EBC20170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell O.M., Gorman G.S., Lightowlers R.N., Turnbull D.M. Mitochondrial diseases: Hope for the future. Cell. 2020;181:168–188. doi: 10.1016/j.cell.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 28.El-Hattab A.W., Zarante A.M., Almannai M., Scaglia F. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 2017;122:1–9. doi: 10.1016/j.ymgme.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park A., Oh M., Lee S.J., Oh K.-J., Lee E.-W., Lee S.C., Bae K.-H., Han B.S., Kim W.K. Mitochondrial transplantation as a novel therapeutic strategy for mitochondrial diseases. Int. J. Mol. Sci. 2021;22:4793. doi: 10.3390/ijms22094793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A., Faccenda D., Campanella M. Pharmacological advances in mitochondrial therapy. EBioMedicine. 2021;65:103244. doi: 10.1016/j.ebiom.2021.103244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalska M., Piekut T., Prendecki M., Sodel A., Kozubski W., Dorszewska J. Mitochondrial and nuclear DNA oxidative damage in physiological and pathological aging. DNA Cell Biol. 2020;39:1410–1420. doi: 10.1089/dna.2019.5347. [DOI] [PubMed] [Google Scholar]
- 32.DeBalsi K.L., Hoff K.E., Copeland W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017;33:89–104. doi: 10.1016/j.arr.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman S., Copeland W.C. POLG-related disorders and their neurological manifestations. Nat. Rev. Neurol. 2019;15:40–52. doi: 10.1038/s41582-018-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidari M.M., Derakhshani M., Sedighi F., Foruzan-Nia S.K. Mutation Analysis of the mitochondrial tRNA genes in iranian coronary atherosclerosis patients. Iran. J. Public Health. 2017;46:1379–1385. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Chen H., Qin M., Liu C., Ma Q., Chen X., Zhang Y., Lai W., Zhang X., Zhong S. Associations of mitochondrial variants with lipidomic traits in a chinese cohort with coronary artery disease. Front. Genet. 2021;12:630359. doi: 10.3389/fgene.2021.630359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heidari M.M., Mirfakhradini F.S., Tayefi F., Ghorbani S., Khatami M., Hadadzadeh M. Novel point mutations in mitochondrial MT-CO2 gene may be risk factors for coronary artery disease. Appl. Biochem. Biotechnol. 2020;191:1326–1339. doi: 10.1007/s12010-020-03275-0. [DOI] [PubMed] [Google Scholar]
- 37.Jia Q., Xu L., Shen J., Wei Y., Xu H., Shi J., Jia Z., Zhao X., Liu C., Zhong Q., et al. Detecting rare variants and heteroplasmy of mitochondrial DNA from high-throughput sequencing in patients with coronary artery disease. Med. Sci. Monit. 2020;26:e925401. doi: 10.12659/MSM.925401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecoli C., Borghini A., Pulignani S., Mercuri A., Turchi S., Picano E., Andreassi M.G. Independent and combined effects of telomere shortening and mtDNA4977 deletion on long-term outcomes of patients with coronary artery disease. Int. J. Mol. Sci. 2019;20:5508. doi: 10.3390/ijms20215508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecoli C., Borghini A., Pulignani S., Mercuri A., Turchi S., Carpeggiani C., Picano E., Andreassi M.G. Prognostic Value of mitochondrial DNA4977 deletion and mitochondrial DNA copy number in patients with stable coronary artery disease. Atherosclerosis. 2018;276:91–97. doi: 10.1016/j.atherosclerosis.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Wang X.-B., Cui N.-H., Liu X., Liu X. Joint effects of mitochondrial DNA4977 deletion and serum folate deficiency on coronary artery disease in type 2 diabetes mellitus. Clin. Nutr. 2020;39:3771–3778. doi: 10.1016/j.clnu.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Liu M., He J., Zhang X., Chen Y., Li H. Maternally inherited coronary heart disease is associated with a novel mitochondrial tRNA Mutation. BMC Cardiovasc. Disord. 2019;19:293. doi: 10.1186/s12872-019-01284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pei H., Peng Q., Lan C., Chi Liu B. Variations in mitochondrial tRNAThr gene may not be associated with coronary heart disease. Mitochondrial DNA. 2016;27:565–568. doi: 10.3109/19401736.2014.905862. [DOI] [PubMed] [Google Scholar]
- 43.Hypertension—Key Facts. WHO Fact Sheets. [(accessed on 23 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension.
- 44.Zinner S.H., Levy P.S., Kass E.H. Familial aggregation of blood pressure in childhood. N. Engl. J. Med. 1971;284:401–404. doi: 10.1056/NEJM197102252840801. [DOI] [PubMed] [Google Scholar]
- 45.Xue L., Wang M., Li H., Wang H., Jiang F., Hou L., Geng J., Lin Z., Peng Y., Zhou H., et al. Mitochondrial tRNA mutations in 2070 chinese han subjects with hypertension. Mitochondrion. 2016;30:208–221. doi: 10.1016/j.mito.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Li Y., Wang X., Ma Q., Zhu C., Li Z., Yin T., Yang J., Chen Y., Guan M. Mitochondrial tRNA mutations in chinese hypertensive individuals. Mitochondrion. 2016;28:1–7. doi: 10.1016/j.mito.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y., Chen X., Li H., Zhu C., Li Y., Liu Y. Mitochondrial genome mutations in 13 subunits of respiratory chain complexes in chinese han and mongolian hypertensive individuals. Mitochondrial DNA Part A. 2018;29:1090–1099. doi: 10.1080/24701394.2017.1407762. [DOI] [PubMed] [Google Scholar]
- 48.Yang Q., Kim S.K., Sun F., Cui J., Larson M.G., Vasan R.S., Levy D., Schwartz F. Maternal influence on blood pressure suggests involvement of mitochondrial DNA in the pathogenesis of hypertension: The framingham heart study. J. Hypertens. 2007;25:2067–2073. doi: 10.1097/HJH.0b013e328285a36e. [DOI] [PubMed] [Google Scholar]
- 49.Weiwei C., Runlin G., Lisheng L., Manlu Z., Wen W., Yongjun W., Zhaosu W., Huijun L., Zhe Z., Lixin J., et al. Outline of the report on cardiovascular diseases in China, 2014. Eur. Heart J. Suppl. 2016;18:F2–F11. doi: 10.1093/eurheartj/suw030. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., You J., Xu C., Gu X. Associations of mitochondrial DNA 3777–4679 region mutations with maternally inherited essential hypertensive subjects in China. BMC Med. Genet. 2020;21:105. doi: 10.1186/s12881-020-01045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y., Gu X., Xu C. Mitochondrial DNA 7908–8816 region mutations in maternally inherited essential hypertensive subjects in China. BMC Med. Genom. 2018;11:89. doi: 10.1186/s12920-018-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y., Gu X., Xu C. A Mitochondrial DNA A8701G mutation associated with maternally inherited hypertension and dilated cardiomyopathy in a chinese pedigree of a consanguineous marriage. Chin. Med. J. 2016;129:259–266. doi: 10.4103/0366-6999.174491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin L., Cui P., Qiu Z., Wang M., Yu Y., Wang J., Sun Q., Zhao H. The mitochondrial tRNAAla 5587T>C and tRNALeu(CUN) 12280A>G mutations may be associated with hypertension in a chinese family. Exp. Ther. Med. 2018;17:1855–1862. doi: 10.3892/etm.2018.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo L., Yuan Y., Bi R. Mitochondrial DNA mutation m.5512A > G in the acceptor-stem of mitochondrial tRNATrp causing maternally inherited essential hypertension. Biochem. Biophys. Res. Commun. 2016;479:800–807. doi: 10.1016/j.bbrc.2016.09.129. [DOI] [PubMed] [Google Scholar]
- 55.Guo H., Guo L., Yuan Y., Liang X.-Y., Bi R. Co-occurrence of m.15992A>G and m.15077G>A is associated with a high penetrance of maternally inherited hypertension in a chinese pedigree. Am. J. Hypertens. 2021;36:96–102. doi: 10.1093/ajh/hpab123. [DOI] [PubMed] [Google Scholar]
- 56.Ding Y., Yu J., Guo Q., Gao B., Huang J. Molecular characterization of two chinese pedigrees with maternally inherited hypertension. J. Gene Med. 2021;23:e3328. doi: 10.1002/jgm.3328. [DOI] [PubMed] [Google Scholar]
- 57.Zhou M., Wang M., Xue L., Lin Z., He Q., Shi W., Chen Y., Jin X., Li H., Jiang P., et al. A hypertension-associated mitochondrial DNA mutation alters the tertiary interaction and function of tRNALeu(UUR) J. Biol. Chem. 2017;292:13934–13946. doi: 10.1074/jbc.M117.787028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai J., Ma Q., Lan Y., Chen Y., Ma S., Li J., Liu C., Fu Z., Lu X., Huang Y., et al. Mitochondrial tRNA mutation and regulation of the adiponectin pathway in maternally inherited hypertension in chinese han. Front. Cell Dev. Biol. 2021;8:623450. doi: 10.3389/fcell.2020.623450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y., Chen X., Huang H., Liu W. The mitochondrial tRNAAla T5655C mutation may modulate the phenotypic expression of tRNAMet and tRNAGln A4401G mutation in a han chinese family with essential hypertension. Int. Heart J. 2017;58:95–99. doi: 10.1536/ihj.16-205. [DOI] [PubMed] [Google Scholar]
- 60.Yang P., Wu P., Liu X., Feng J., Zheng S., Wang Y., Fan Z. Mitochondrial tRNASer(UCN) 7471delC may be a novel mutation associated with maternally transmitted hypertension. Ir. J. Med. Sci. 2020;189:489–496. doi: 10.1007/s11845-019-02143-z. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Li Y., Zhu C., Tian L., Guan M., Chen Y. Mitochondrial biogenesis dysfunction and metabolic dysfunction from a novel mitochondrial tRNAMet 4467 C>A mutation in a han chinese family with maternally inherited hypertension. Sci. Rep. 2017;7:3034. doi: 10.1038/s41598-017-03303-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X., Zhang Y., Xu B., Cai Z., Wang L., Tian J., Liu Y., Li Y. The mitochondrial calcium uniporter is involved in mitochondrial calcium cycle dysfunction: Underlying mechanism of hypertension associated with mitochondrial tRNA Ile A4263G mutation. Int. J. Biochem. Cell Biol. 2016;78:307–314. doi: 10.1016/j.biocel.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Li H., Geng J., Yu H., Tang X., Yang X., Xue L. Mitochondrial tRNAThr 15909A>G mutation associated with hypertension in a chinese han pedigree. Biochem. Biophys. Res. Commun. 2018;495:574–581. doi: 10.1016/j.bbrc.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Dong Z., Lin W., Gao R., Chen C., Xu J. Molecular characterization of a pedigree carrying the hypertension-associated mitochondrial tRNAGln T4363C mutation. Mol. Med. Rep. 2017;16:6029–6033. doi: 10.3892/mmr.2017.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng P., Li S., Liu C., Zha Z., Wei X., Yuan Y. Mitochondrial tRNAAla C5601T mutation may modulate the clinical expression of tRNAMet A4435G mutation in a han chinese family with hypertension. Clin. Exp. Hypertens. 2018;40:595–600. doi: 10.1080/10641963.2017.1411497. [DOI] [PubMed] [Google Scholar]
- 66.Brugada P., Brugada J. Right Bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. J. Am. Coll. Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-J. [DOI] [PubMed] [Google Scholar]
- 67.Gourraud J.-B., Barc J., Thollet A., Le Marec H., Probst V. Brugada syndrome: Diagnosis, risk stratification and management. Arch. Cardiovasc. Dis. 2017;110:188–195. doi: 10.1016/j.acvd.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Chen C., Tan Z., Zhu W., Fu L., Kong Q., Xiong Q., Yu J., Hong K., Fu L. Brugada syndrome with SCN5A mutations exhibits more pronounced electrophysiological defects and more severe prognosis: A meta-analysis. Clin. Genet. 2020;97:198–208. doi: 10.1111/cge.13552. [DOI] [PubMed] [Google Scholar]
- 69.Wijeyeratne Y.D., Tanck M.W., Mizusawa Y., Batchvarov V., Barc J., Crotti L., Bos J.M., Tester D.J., Muir A., Veltmann C., et al. SCN5A mutation type and a genetic risk score associate variably with brugada syndrome phenotype in SCN5A families. Circ: Genom. Precis. Med. 2020;13:e002911. doi: 10.1161/CIRCGEN.120.002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monasky M.M., Micaglio E., Ciconte G., Pappone C. Brugada syndrome: Oligogenic or mendelian disease? Int. J. Mol. Sci. 2020;21:1687. doi: 10.3390/ijms21051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brugada R., Campuzano O., Sarquella-Brugada G., Brugada J., Brugada P. Brugada syndrome. Methodist DeBakey Cardiovasc. J. 2014;10:25–28. doi: 10.14797/mdcj-10-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stocchi L., Polidori E., Potenza L., Rocchi M.B.L., Calcabrini C., Busacca P., Capalbo M., Potenza D., Amati F., Mango R., et al. Mutational analysis of mitochondrial DNA in brugada syndrome. Cardiovasc. Pathol. 2016;25:47–54. doi: 10.1016/j.carpath.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Tafti M.F., Khatami M., Rezaei S., Heidari M.M., Hadadzadeh M. Novel and heteroplasmic mutations in mitochondrial tRNA genes in brugada syndrome. Cardiol. J. 2018;25:113–119. doi: 10.5603/CJ.a2017.0104. [DOI] [PubMed] [Google Scholar]
- 74.Hurford R., Sekhar A., Hughes T.A.T., Muir K.W. Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 2020;20:304–316. doi: 10.1136/practneurol-2020-002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J.-L., Mukda S., Chen S.-D. Diverse roles of mitochondria in ischemic stroke. Redox. Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umbria M., Ramos A., Aluja M.P., Santos C. The role of control region mitochondrial DNA mutations in cardiovascular disease: Stroke and myocardial infarction. Sci. Rep. 2020;10:2766. doi: 10.1038/s41598-020-59631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luan Y., Yang D., Zhang Z., Bie X., Zhao H., Wang Y., Liu Y., Yang S., Zhou B., Xu Y., et al. Association study between genetic variation in whole mitochondrial genome and ischemic stroke. J. Mol. Neurosci. 2021;71:2152–2162. doi: 10.1007/s12031-020-01778-3. [DOI] [PubMed] [Google Scholar]
- 78.Watson B., Khan M.A., Desmond R.A., Bergman S. Mitochondrial DNA mutations in black americans with hypertension-associated end-stage renal disease. Am. J. Kidney Dis. 2001;38:529–536. doi: 10.1053/ajkd.2001.26848. [DOI] [PubMed] [Google Scholar]
- 79.Liu C.-H., Chang C.-H., Kuo H.-C., Ro L.-S., Liou C.-W., Wei Y.-H., Huang C.-C. Prognosis of symptomatic patients with the A3243G mutation of mitochondrial DNA. J. Formos. Med. Assoc. 2012;111:489–494. doi: 10.1016/j.jfma.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Baccarelli A.A., Byun H.-M. Platelet mitochondrial DNA methylation: A potential new marker of cardiovascular disease. Clin. Epigenet. 2015;7:44. doi: 10.1186/s13148-015-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corsi S., Iodice S., Vigna L., Cayir A., Mathers J.C., Bollati V., Byun H.-M. Platelet mitochondrial DNA methylation predicts future cardiovascular outcome in adults with overweight and obesity. Clin. Epigenetics. 2020;12:29. doi: 10.1186/s13148-020-00825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Byun H.-M., Colicino E., Trevisi L., Fan T., Christiani D.C., Baccarelli A.A. Effects of Air Pollution and Blood Mitochondrial DNA Methylation on Markers of Heart Rate Variability. J. Am. Heart Assoc. 2016;5:e003218. doi: 10.1161/JAHA.116.003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janssen B.G., Byun H.-M., Gyselaers W., Lefebvre W., Baccarelli A.A., Nawrot T.S. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. 2015;10:536–544. doi: 10.1080/15592294.2015.1048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bordoni L., Sawicka A.K., Szarmach A., Winklewski P.J., Olek R.A., Gabbianelli R. A pilot study on the effects of l-carnitine and trimethylamine-n-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. Int. J. Mol. Sci. 2020;21:1047. doi: 10.3390/ijms21031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beetch M., Harandi-Zadeh S., Shen K., Lubecka K., Kitts D.D., O’Hagan H.M., Stefanska B. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br. J. Pharmacol. 2020;177:1382–1408. doi: 10.1111/bph.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Westerman K.E., Ordovás J.M. DNA methylation and incident cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care. 2020;23:236–240. doi: 10.1097/MCO.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y., Mei J., Li J., Zhang Y., Zhou Q., Xu F. DNA methylation in atherosclerosis: A new perspective. Evid.-Based Complement Alternat. Med. 2021;2021:6623657. doi: 10.1155/2021/6623657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frazier A.E., Thorburn D.R., Compton A.G. Mitochondrial energy generation disorders: Genes, mechanisms, and clues to pathology. J. Biol. Chem. 2019;294:5386–5395. doi: 10.1074/jbc.R117.809194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karunakaran D., Thrush A.B., Nguygen M.-A., Richards L., Geoffrion M., Singaravelu R., Ramphos E., Shangari P., Ouimet M., Pezacki J.P., et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-MiR33 in atherosclerosis. Circ. Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie T., Wang C., Jin Y., Meng Q., Liu Q., Wu J., Sun H. CoenzymeQ10-induced activation of AMPK-YAP-OPA1 pathway alleviates atherosclerosis by improving mitochondrial function, inhibiting oxidative stress and promoting energy metabolism. Front. Pharmacol. 2020;11:1034. doi: 10.3389/fphar.2020.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y., Rawish E., Nording H.M., Langer H.F. Inflammation in metabolic and cardiovascular disorders-role of oxidative stress. Life. 2021;11:672. doi: 10.3390/life11070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji H., Peng R., Jin L., Ma J., Yang Q., Sun D., Wu W. Recent advances in ROS-sensitive nano-formulations for atherosclerosis applications. Pharmaceutics. 2021;13:1452. doi: 10.3390/pharmaceutics13091452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang S., Li R., Fettermann A., Li Z., Qian Y., Liu Y., Wang X., Zhou A., Mo J.Q., Yang L., et al. Maternally Inherited Essential Hypertension Is Associated With the Novel 4263A>G Mutation in the Mitochondrial tRNAIle gene in a large han chinese family. Circ. Res. 2011;108:862–870. doi: 10.1161/CIRCRESAHA.110.231811. [DOI] [PubMed] [Google Scholar]
- 94.Boczonadi V., Meyer K., Gonczarowska-Jorge H., Griffin H., Roos A., Bartsakoulia M., Bansagi B., Ricci G., Palinkas F., Zahedi R.P., et al. Mutations in glycyl-tRNA synthetase impair mitochondrial metabolism in neurons. Hum. Mol. Genet. 2018;27:2187–2204. doi: 10.1093/hmg/ddy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Modesti L., Danese A., Vitto V.A.M., Ramaccini D., Aguiari G., Gafà R., Lanza G., Giorgi C., Pinton P. Mitochondrial Ca2+ signaling in health, disease and therapy. Cells. 2021;10:1317. doi: 10.3390/cells10061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell. Biol. 2018;19:713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 97.Chaanine A.H., Gordon R.E., Kohlbrenner E., Benard L., Jeong D., Hajjar R.J. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: Mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circ. Heart Fail. 2013;6:572–583. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chaanine A.H., Kohlbrenner E., Gamb S.I., Guenzel A.J., Klaus K., Fayyaz A.U., Nair K.S., Hajjar R.J., Redfield M.M. FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am. J. Physiol.-Heart Circ. Physiol. 2016;311:H1540–H1559. doi: 10.1152/ajpheart.00549.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shanks J., Herring N., Johnson E., Liu K., Li D., Paterson D.J. Overexpression of sarcoendoplasmic reticulum calcium ATPase 2a promotes cardiac sympathetic neurotransmission via abnormal endoplasmic reticulum and mitochondria Ca2+ regulation. Hypertension. 2017;69:625–632. doi: 10.1161/HYPERTENSIONAHA.116.08507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu S., Zheng S., Leng J., Wang S., Zhao T., Liu J. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci. Lett. 2016;628:24–29. doi: 10.1016/j.neulet.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 101.Ashar F.N., Moes A., Moore A.Z., Grove M.L., Chaves P.H.M., Coresh J., Newman A.B., Matteini A.M., Bandeen-Roche K., Boerwinkle E., et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. 2015;93:177–186. doi: 10.1007/s00109-014-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mengel-From J., Thinggaard M., Dalgård C., Kyvik K.O., Christensen K., Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Qual. Life Res. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L.-P., Cheng K., Ning M.-A., Li H.-H., Wang H.-C., Li F., Chen S.-Y., Qu F.-L., Guo W.-Y. Association between peripheral blood cells mitochondrial DNA content and severity of coronary heart disease. Atherosclerosis. 2017;261:105–110. doi: 10.1016/j.atherosclerosis.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Koller A., Fazzini F., Lamina C., Rantner B., Kollerits B., Stadler M., Klein-Weigel P., Fraedrich G., Kronenberg F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J. Intern. Med. 2020;287:569–579. doi: 10.1111/joim.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yue P., Jing S., Liu L., Ma F., Zhang Y., Wang C., Duan H., Zhou K., Hua Y., Wu G., et al. Association between mitochondrial DNA copy number and cardiovascular disease: Current evidence based on a systematic review and meta-analysis. PLoS ONE. 2018;13:e0206003. doi: 10.1371/journal.pone.0206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y., Guallar E., Ashar F.N., Longchamps R.J., Castellani C.A., Lane J., Grove M.L., Coresh J., Sotoodehnia N., Ilkhanoff L., et al. Association between mitochondrial DNA copy number and sudden cardiac death: Findings from the atherosclerosis risk in communities study (ARIC) Eur. Heart J. 2017;38:3443–3448. doi: 10.1093/eurheartj/ehx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ashar F.N., Zhang Y., Longchamps R.J., Lane J., Moes A., Grove M.L., Mychaleckyj J.C., Taylor K.D., Coresh J., Rotter J.I., et al. Association of mitochondrial DNA copy number with cardiovascular disease. JAMA Cardiol. 2017;2:1247–1255. doi: 10.1001/jamacardio.2017.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.