Abstract
Dexamethasone (DEX) induces dysregulation of protein turnover, leading to muscle atrophy and impairment of glucose metabolism. Positive protein balance, i.e., rate of protein synthesis exceeding rate of protein degradation, can be induced by dietary essential amino acids (EAAs). In this study, we investigated the roles of an EAA-enriched diet in the regulation of muscle proteostasis and its impact on glucose metabolism in the DEX-induced muscle atrophy model. Mice were fed normal chow or EAA-enriched chow and were given daily injections of DEX over 10 days. We determined muscle mass and functions using treadmill running and ladder climbing exercises, protein kinetics using the D2O labeling method, molecular signaling using immunoblot analysis, and glucose metabolism using a U-13C6 glucose tracer during oral glucose tolerance test (OGTT). The EAA-enriched diet increased muscle mass, strength, and myofibrillar protein synthesis rate, concurrent with improved glucose metabolism (i.e., reduced plasma insulin concentrations and increased insulin sensitivity) during the OGTT. The U-13C6 glucose tracing revealed that the EAA-enriched diet increased glucose uptake and subsequent glycolytic flux. In sum, our results demonstrate a vital role for the EAA-enriched diet in alleviating the DEX-induced muscle atrophy through stimulation of myofibrillar proteins synthesis, which was associated with improved glucose metabolism.
Keywords: dexamethasone, muscle atrophy, essential amino acids, protein turnover, glucose metabolic flux
1. Introduction
Muscle atrophy is defined as the loss of muscle mass and function (including strength and endurance) resulting from decreased protein synthesis and/or increased protein degradation [1,2]. Numerous physiological and pathological conditions, such as prolonged inactivity, cancer cachexia, aging, diabetes, and drug treatments, can cause muscle atrophy [3,4,5]. The various muscle atrophy conditions induced by dysregulation of muscle protein quality cause several unfavorable consequences, including a decrease in quality of life, an increase in mortality, and a dysregulation of the systemic energy homeostasis.
Dexamethasone (DEX) is a synthetic glucocorticoid, a commonly prescribed therapeutic compound for treating inflammatory, autoimmune, cancer, and neuromuscular disorders. Despite its pharmacological benefits in disease conditions, acute and prolonged treatment with DEX induces an imbalance in protein turnover and impaired glucose metabolism, leading to a decrease in both muscle mass and function. DEX treatment decreases muscle weight and inhibits the mTOR pathway, reduces the rate of muscle protein synthesis by 59%, and reduces muscle strength by up to 50% [6,7]. Moreover, DEX treatment is accompanied by insulin resistance in rodents [8,9]. In addition to the mTOR pathway, the ubiquitin-proteasome system (UPS) and autophagy are involved in maintaining protein quality control [10]. The activation of the UPS and autophagy is markedly increased under catabolic conditions, including DEX [11]. In particular, myofibrillar proteins are degraded by the enhanced UPS [12] and the expression of autophagy proteins (e.g., microtubule-associated protein II-light chain 3 (LC3-II), autophagy related 7 (ATG7)) have upregulated by DEX [13,14]. DEX can affect protein quality and quantity by suppressing protein synthesis and activating protein degradation. Furthermore, acute administration of DEX inhibits the metabolic clearance rate of glucose [15], and prolonged DEX treatment increases gluconeogenesis and decreases muscle glucose uptake [16]. Although DEX can be used to treat specific illnesses, an effective means against the DEX-mediated muscle atrophy and its negative impact on metabolism remains unresolved. Therefore, it is important to examine whether alterations occur both in muscle protein turnover and metabolism to better understand and in turn treat the DEX-induced muscle atrophy. However, previous studies have evaluated the effects of nutraceuticals, such as BCAA and omega-3 fatty acids, in vivo [17,18] without examinations on both protein turnover and metabolism.
Amino acids act as building blocks for proteins and produce favorable effects alone or together on health and diseases [19]. Particularly, essential amino acids (EAAs) are the main drivers that induce positive muscle protein balance (i.e., gains in muscle mass) [20,21,22,23] and improve muscle function via affecting various factors, including protein metabolism, mitochondrial biogenesis, and energy metabolism. In humans, it was shown that EAA supplementation for 1 week led to a robust anabolic stimulation in skeletal muscle and enhanced recovery capacity through protecting myofibrillar protein degradation following exercise [24,25]. Additionally, EAAs promote mitochondrial biogenesis and muscle function as well as suppress plasma glucocorticoid concentration [21,26,27]. Furthermore, some EAAs have been demonstrated to stimulate glucose uptake in rodents [28,29,30] during OGTT. Therefore, EAA supplementation has been utilized as a potential nutritional treatment for various muscle atrophy conditions, including aging [31], obesity [32], and osteoporosis [33]. According to the above-mentioned results, it is suggested that EAAs may alleviate muscle atrophy induced by DEX via enhancement of muscle protein synthesis and glucose metabolism. However, no studies have been conducted regarding the potential positive impact on the DEX-mediated dysregulation of muscle protein balance and glucose metabolism.
Therefore, in the present study, we tested hypotheses that a diet rich in EAAs will alleviate muscle atrophy and reverse impaired glucose metabolism induced by dexamethasone treatment. Here we show that an EAA-enriched diet protects muscle from the DEX-induced atrophy via enhancement of muscle protein synthesis and insulin stimulated-glucose metabolism.
2. Results
2.1. The EAA-Enriched Diet Alleviates Loss of Muscle Mass and Strength in Muscle Atrophy by DEX
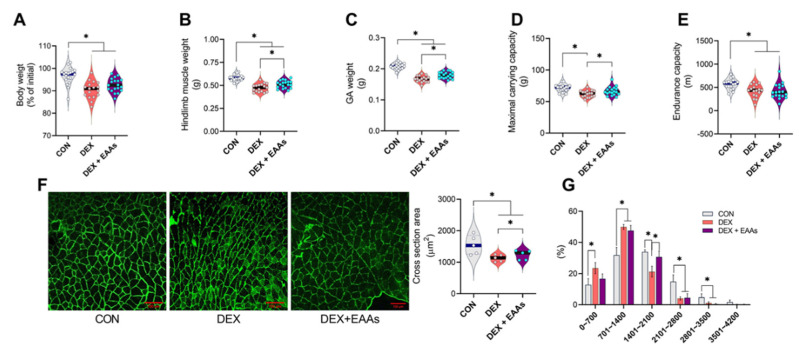
Although DEX is widely used as a treatment for various disease conditions, muscle atrophy is a major side effect of DEX. In contrast, an EAA-enriched diet is known to induce an anabolic response [23,34]. Thus, we examined whether an EAA-enriched diet can prevent the loss of muscle and function induced by DEX. We found that 20 mg/kg DEX daily injection for 10 days significantly decreased body weight in all groups (Figure 1A). The EAA-enriched diet improved muscle mass of total hindlimb (gastrocnemius, soleus, plantaris, extensor digitorum longus, tibialis anterior) and gastrocnemius (GA) in DEX-treated mice (Figure 1B,C). It is well known that an EAA-enriched diet induces not only net muscle protein synthesis (i.e., gains in muscle mass) but also improvement of muscle function. Consistent with changes in muscle mass, we found a significant reduction in cross-sectional area (CSA) of GA muscle by DEX that was partially prevented by the EAA-enriched diet (Figure 1F,G). These results suggest that the EAA-enriched diet can prevent decreases in muscle mass and strength induced by DEX via attenuating decreases in middle-sized fibers. Similarly, we found that DEX decreased the maximal carrying capacity (MCC), which was prevented by EAA-enriched diet (Figure 1D). Contrary to our expectation based on the previous study [26,35], we found that the EAA-enriched diet did not improve endurance capacity or mitochondrial biogenesis (Figure 1E).
Figure 1.
The EAA-enriched diet alleviated the reduction of muscle mass and strength on DEX-induced muscle atrophy. (A) Changes in body weight after 10 days of injection and/or the EAAs diet (n = 17–19). (B) Total hindlimb and (C) GA muscle weight (n = 17–19). (D) Maximal carrying capacity (n = 17–19). (E) Endurance capacity (n = 17–19). (F) Cross-section area in GA muscle (n = 5). (G) Muscle fiber frequency distribution of GA muscle (n = 5). Data are presented as mean ± S.E. * Significant difference between labeled groups (* p < 0.05). CON, control; DEX, dexamethasone; EAAs, essential amino acids; GA, gastrocnemius.
2.2. The EAA-Enriched Diet Increased Myofibrillar Protein Synthesis in DEX Treated Mice
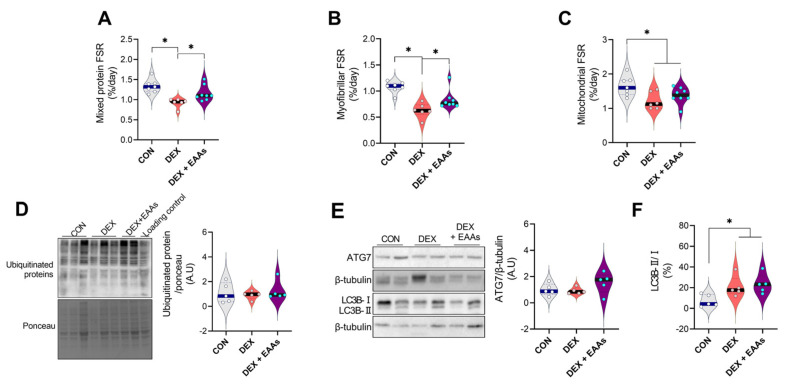
Muscle mass is determined by the balance between rates of protein synthesis and degradation. DEX induces muscle atrophy through the imbalance in protein metabolism resulting from inhibition of protein synthesis rate and/or activation of protein degradation pathways, which are promoted by dysregulation of UPS and autophagy pathway [36,37]. Since the EAA-enriched diet has been shown to increase muscle mass through enhanced muscle protein synthesis rate and decreased protein degradation in normal conditions [38,39], we tested whether the EAA-enriched diet could increase protein synthesis even in a condition in which DEX is continuously injected in mice. As expected, we found that DEX significantly decreased integrated mixed muscle fractional protein synthetic rate (FSR) in GA muscle, and this decrease was prevented by the EAA-enriched diet (Figure 2A). However, Akt/mTORC1 pathway has not differed in all groups (Figure S1A–C). Interestingly, the EAA-enriched diet prevented a decrease in FSR of myofibrillar proteins (Figure 2B). Furthermore, we found that the integrated mixed muscle FSR and myofibrillar FSR of the EAA-enriched diet were not significantly different from CON. However, mitochondrial proteins FSR have not been differed between DEX and EAA-enriched diet (Figure 2C). These results are in accordance with the results on the physical performance tests: we found an improvement in MCC but not in endurance capacity when treated with the EAA-enriched diet (Figure 1C,D). Since UPS and autophagy are major aspects of control of protein breakdown, we identified the ubiquitination of proteins, ATG7 and LC3B. We found that DEX and/or the EAA-enriched diet have not changed the amounts of UPS and ATG7 protein abundance compared to CON (Figure 2D,E). DEX increased the ratio of LC3B-II/I than CON, however, the EAA-enriched diet did not change the ratio of LC3B-II/I protein amount than DEX (Figure 2F). Overall, these data indicate that the EAA-enriched diet protects skeletal muscle atrophy via enhanced muscle protein synthesis rate, (mainly myofibrillar protein synthesis), without changing the protein degradation pathways that DEX promotes.
Figure 2.
The EAA-enriched diet promotes myofibrillar protein synthesis rate but not the protein degradation pathway by DEX treatment in GA muscle. (A) Rates of integrated mixed muscle protein synthesis rate (n = 6–8). (B) Rates of myofibrillar protein synthesis rate (n = 6–8). (C) Mitochondrial protein synthesis rate (n = 6–8). (D) Relative protein expression levels of ubiquitinated proteins (expression levels were normalized by total proteins, n = 5). (E) Relative protein expression levels of ATG7 and (F) the ratio of LC3B-II/I (n = 5). * Significant difference between labeled groups (* p < 0.05). CON, control; DEX, dexamethasone; EAAs, essential amino acids; GA, gastrocnemius; FSR, fractional protein synthetic rate; ATG7, autophagy related7; LC3B, microtubule-associated protein II-light chain 3.
2.3. The EAA-Enriched Diet Improves Insulin Sensitivity and Glucose Uptake in the Presence of DEX Treatment
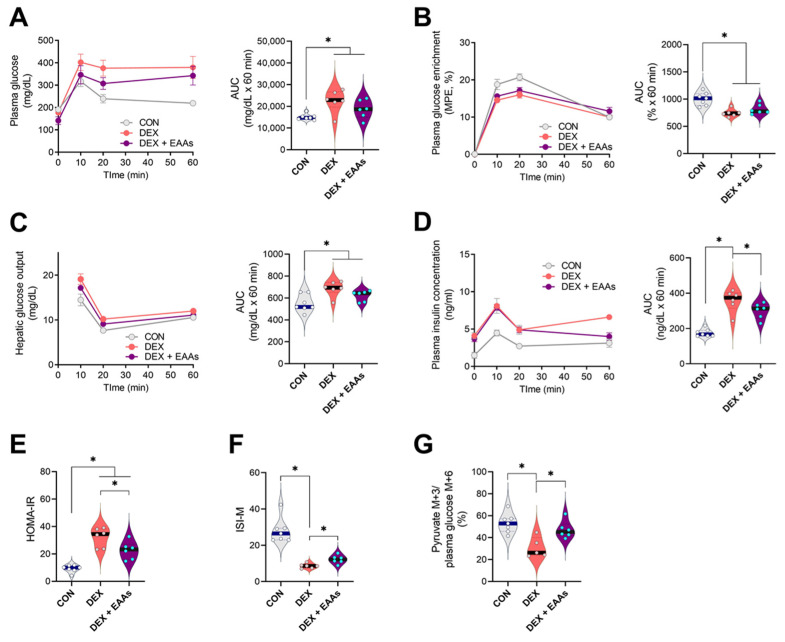
DEX can activate gluconeogenesis and reduce glucose uptake in muscle by inducing insulin resistance [40,41]. DEX could impair regulation of glucose metabolism as a consequence of muscle atrophy, which can be reversed by increased dietary EAAs [27,28]. Thus, we examined the effect of the EAA-enriched diet on glucose metabolism in the presence of DEX using a glucose stable isotope tracer during an oral glucose tolerance test (OGTT). Plasma glucose concentration is regulated by rates of hepatic glucose output and glucose uptake [42]. We first determined that DEX lowered the enrichment of glucose after the OGTT (Figure 3B), reflecting a reduced suppression of hepatic glucose output as compared to CON, and that the EAA-enriched diet did not affect this response (Figure 3C). Interestingly, while DEX increased plasma insulin concentrations and worsened insulin resistance in fasting and postprandial states (HOMA-IR index and ISI-M) compared to CON, the decrease in insulin sensitivity was partially revered by the EAA-enriched diet (Figure 3D–F). We also analyzed pyruvate enrichment (normalized by plasma glucose enrichment) in the GA muscle, which reflected glucose uptake and subsequent glycolytic flux. We found that DEX decreased pyruvate enrichment in the GA muscle compared to CON, which was prevented by the EAA-enriched diet (Figure 3G). Collectively, these findings demonstrated that the EAA-enriched diet prevents the DEX-induced impaired insulin sensitivity and muscle glucose uptake.
Figure 3.
The EAA-enriched diet improved impaired insulin sensitivity and glucose uptake in DEX-induced muscle atrophy. (A) Plasma glucose concentration during the oral glucose tolerance test (OGTT) (n = 6–7). (B) Plasma glucose enrichment during the OGTT (n = 6–7). (C) Hepatic glucose output during the OGTT (n = 6–7). (D) Plasma insulin concentrations during the OGTT (n = 6–7). (E) HOMA-IR (n = 6–7). (F) Matsuda insulin sensitivity index (n = 6–7). (G) GA muscle pyruvate M+3 to plasma glucose M+6 ratio (n = 6–7). Data are presented as mean ± S.E. * Significant difference between labeled groups (* p < 0.05). CON, control; DEX, dexamethasone; EAAs, essential amino acids; GA, gastrocnemius, MPE, mole percent excess; AUC; area under curve; HOMA-IR, homeostatic model assessment of insulin resistance; ISI-M, Matsuda-DeFronzo insulin sensitivity index.
3. Discussion
The current study provides experimental evidence that the EAA-enriched diet alleviates the DEX-induced muscle atrophy by stimulating myofibrillar protein synthesis, and also improves glucose metabolism. The improved insulin sensitivity in the animals receiving the EAA-enriched diet may have been linked to the increased muscle protein synthesis, which minimized the extent of muscle atrophy resulting from DEX. Elucidation of the favorable effect of the EAA-enriched diet on the DEX-induced muscle atrophy model extends potential opportunities for future studies of muscle wasting in clinical conditions that are induced by adverse side effects of drugs.
DEX is used to treat many conditions related to immune function, cancer, and neuromuscular disorders [43,44]. However, chronic treatment with DEX decreases muscle mass and function [45]. Thus, we found that DEX decreased GA muscle mass by approximately 20% (Figure 1B–E). In addition, maximal carrying capacity was reduced by 16%, and endurance capacity was reduced by 26%. It is well known that a diet rich in EAAs induces gains in muscle net protein balance (i.e., gains in muscle mass) in normal [21,22] and pathological conditions [20]. Therefore, we performed the experiment to test whether an EAA-enriched diet could protect the muscle against DEX-induced muscle atrophy. As expected, we found that the EAA-enriched diet improved muscle mass by 5% and strength by 10% even in the condition in which DEX was continuously stimulating muscle atrophy. However, the EAA-enriched diet did not improve endurance capacity and mitochondrial biogenesis (Figure 1E and Figure 2C). Maybe this difference of result has been partly related to differences in the format of the EAA provision. Because of that, it will be a topic of future study (EAA supplement vs. EAA-enriched diet). Importantly, we found that the EAA-enriched diet improved myofibrillar protein synthesis rate that was decreased by DEX treatment without changes in Akt/mTORC1 signaling pathway (Figure S1). It is not uncommon that disparity between rate of muscle protein synthesis and Akt/mTORC1 signaling pathway exists as the signaling is one of many factors, including the availability of EAAs that determine the rate of muscle protein synthesis [46]. In this study, it is also possible that mTORC1 independent processes may regulate protein synthesis [47]. Myofibrillar proteins (actin, myosin, and other muscle contraction-related proteins) are involved in the contraction and compose 50–55% of the total muscle protein [48]. We also anticipated that the EAA-enriched diet would improve endurance capacity, as it has been demonstrated that EAAs may prevent mitochondrial dysfunction and oxidative stress in muscle atrophy conditions [49]. However, we did not observe an enhancement of endurance capacity and mitochondrial protein synthesis rate by the EAA-enriched diet.
At the molecular level, muscle protein mass is regulated by rates of protein synthesis and degradation [50]. DEX has been shown to trigger muscle atrophy by enhanced protein degradation machinery, i.e., UPS and autophagy [35,51,52]. However, despite the promotion of muscle atrophy by DEX in our study, we could not find changes in the amount of ubiquitin protein. This disparity might be due in part to differences in rodent species and tissue sampling times as well as other unknown factors. However, another muscle atrophy mechanism, i.e., autophagy, was increased by DEX as demonstrated by an increase in the ratio of LC3B-II/I [37]. It is an interesting finding that the EAA-enriched diet prevented net muscle protein degradation induced by DEX despite the absence of any change in autophagy, which is believed to be a main driver of the DEX-mediated muscle atrophy [14]. Previous studies have shown that the autophagy pathway can be enhanced to regulate protein quality through facilitating degradation of the aggregated pathogenic protein [53,54]. Thus, an increase in autophagy mechanism may be good or bad, namely, acting as a double-edged sword depending on conditions. However, the effect of the EAA-enriched diet on autophagy in relation to muscle mass and function remains unclear. Thus, further studies are required to find roles placed by autophagy (changes) in the enhancement of muscle function with the EAA-enriched diet.
Skeletal muscle accounts for approximately 80% of the insulin-stimulated glucose uptake [55]. Whole-body glucose metabolism is regulated by the balance between the rate of glucose appearance and the rate of glucose disappearance [56,57,58]. In the normal fed condition, insulin inhibits hepatic glucose output and activates glucose uptake in the peripheral tissues, mainly in the skeletal muscle [59,60]. It is known that DEX induces insulin resistance and contributes to elevated hepatic glucose output and decreased muscle glucose uptake [61]. Thus, it is likely that DEX-induced muscle atrophy plays a role in the progression of metabolic diseases, such as insulin resistance and type 2 diabetes mellitus. In fact, since insulin resistance and dysregulation of glucose uptake can directly induce muscle atrophy [62,63], muscle mass and metabolic function are linked. In this context, it is possible that the improved glucose metabolism in the DEX animals fed the EAA-enriched diet was due to the concurrent increase in muscle mass. However, a further studies exploring mechanisms by which the EAA-diet induces improved insulin sensitivity and muscle glucose uptake are required. Furthermore, it is possible that alternative ways of providing EAAs, such as consumption of EAAs alone between meals, would have different metabolic effects. Lastly, it is important to confirm the results from the present study in clinical outcome trials.
4. Materials and Methods
4.1. Animals Models and Treatments
C57BL/6 mice at the age of 8 weeks were used for all experiments. The mice were randomly divided into three experimental groups: control (CON, n = 19), dexamethasone treat + normal chow (DEX, n = 17), and dexamethasone treat + EAAs chow (DEX + EAAs, n = 19). The same experiment was repeated three times for FSR (n = 6–8), OGTT (n = 6–7), and molecular signaling pathway (n = 5) analysis, and mice were distributed according to each experiment. The mice were maintained at 22 ± 1 °C on a 12-h light/dark cycle, with ad libitum food and water. Normal groups consumed a normal chow (D10001, open-source diets, New Brunswick, NJ, USA) consisting of 21% Kcal from protein, 68% kcal carbohydrate, and 12% kcal fat. To induce increases of EAA intake, EAA-enriched diet groups were fed chow containing twice the EAAs (Table 1, A19071101, open-source diets, New Brunswick, NJ, USA) as normal chow protein. Normal and EAA-enriched chow were matched for total kcal. The body weight was measured at the same time every day. To generate a muscle atrophy model, we injected 20 mg/kg/day dexamethasone (D4902, Sigma-Aldrich, St Louis, MO, USA) in an intraperitoneal manner for 10 days. All mice experiments were approved by the Lee Gil Ya Cancer and Diabetes Institute.
Table 1.
EAA compositions of normal chow and EAA-enriched chow.
| Amino Acids (g/100 g) | Normal Chow (D10001, in Protein) | EAAs Chow (A19071101) |
|---|---|---|
| L-Isoleucine | 0.27 | 0.8 |
| L-Leucine | 0.53 | 1.6 |
| L-Lysine | 0.43 | 1.3 |
| L-Methionine | 0.27 | 0.8 |
| L-Phenylalanine | 0.27 | 0.8 |
| L-Threonine | 0.23 | 0.7 |
| L-Tryptophan | 0.07 | 0.2 |
| L-Valine | 0.3 | 0.9 |
| L-Histidine | 0.17 | 0.5 |
| Total EAAs | 2.54 | 7.6 |
4.2. Muscle Function Tests
Before the maximal carrying capacity test, familiarization exercise sessions without load for 3 days were conducted using a 1-m ladder with rungs that were 15 mm apart and inclined at 85°. During the test, the mice climbed the ladder with 50%, 70%, 90%, and 100% of the body weight, and the load was added by 3 g until the test was completed. The mice rested for 2 min at the top of the ladder apparatus [22]. The sum of the highest load successfully carried and bodyweight was considered as the maximum carrying capacity.
For the exhaustion test, mice were adapted on a treadmill for 2 days before the test. The adaptation involved mice running for 30 min at 8 m/min. At the start of the exhaustion test, mice started running at the speed of 8 m/min. The running speed was increased at 1 m/min every 2 min until mice were exhausted. The criterion of exhaustion was defined as the point at which mice stayed >5 s on the electric shocker without trying to resume running even if pushed [22].
4.3. Immunofluorescence Staining
To measure muscle cross-section area, muscle sections were incubated overnight at 4 °C with 1:500 dilution of rabbit anti-laminin (#L9393, Sigma-Aldrich, St Louis, MO, USA). After washes in PBS, muscle sections were incubated for 1 h with 1:2000 dilution of Alexa Fluor 488-conjugated anti-rabbit IgG (#A-21121, Invitrogen, Waltham, MA, USA). Images were obtained using a confocal microscope (Zeiss LSM 700, Oberkochen, Germany) at the same light setting, exposure time, and magnification. For fiber CSA calculation, ImageJ image analysis software (National Institutes of Health) was used.
4.4. Oral Glucose Tolerance Test
The mice were fasted for 6 h. At the start of the oral glucose tolerance test, the body weight was measured, and a baseline plasma was collected via the tail vein for the determination of fasting glucose and insulin prior to an oral gavage of a 1.5 g/kg body weight glucose (1:1 ratio of [U-13C6]glucose and unlabeled glucose). Plasma was collected at 0, 10, 20, and 60 min post-gavage using capillary tubes. Plasma glucose concentration was measured immediately using a glucometer (GM9, Analox Instruments, Stourbridge, UK). Plasma insulin was determined using an insulin ELISA kit (80-INSMSU-E10, ALPCO, Salem, MA, USA). The homeostasis model assessment IR (HOMA-IR) index and Matsuda insulin sensitivity index were calculated as described in the previous study [64]. The area under the curve (AUC) was calculated for each adjacent time point of plasma glucose concentration, enrichment, hepatic glucose output, and insulin concentration.
4.5. The Isolation of Myofibrillar and Mitochondrial Related Proteins
Differential centrifugation method used to extract myofibrillar and mitochondrial subtractions as previously describe [65]. Briefly, GA muscle was homogenized in a buffer consisting of 550 mM KCl, 5 mM EGTA and 100 mM MOPS and centrifuged at 800× g for 5 min at 4 °C for precipitating myofibrillar fraction. The supernatant was transferred and centrifuged further at 7000× g for 10 min at 4 °C for collecting mitochondrial fraction.
4.6. D2O Labeling and Protein Synthetic Rate Calculation
All mice received an intraperitoneal injection of 35 μL/g body weight of 99%, D2O (DLM-4, Cambridge isotope laboratories, Tewksbury, MA, USA) with 0.9% NaCl at 0 day. To maintain the D2O enrichment in plasma, the mice received drinking water with 8% D2O enrichment for 10 days. Calculations of muscle protein fractional synthesis rate were determined, based on the precursor-produce relation [57,58].
| FSR (%/t) = (MPEAla)/(MPEBW × 3.7) × 100 |
| ks = −ln(1 − FSR)/t |
where FSR is the fractional synthetic rate of muscle, MPE (mole percent excess) is calculated as a tracer to tracee ratio (TTR)/(TTR+1), and MPEAla represents the total 2H-labeling of protein bound alanine. “3.7” represents the potential exchange site of 2H alanine from the body fluid, MPEBW represents the labeling of body fluid and “t” is the labeling period (time). ks is the fractional synthesis rate constant [66].
4.7. Stable Isotope Enrichment Analysis
Body fluid and muscle protein enrichment were analyzed as previously described [21,67]. Briefly, the acetone exchange method was used to measure 2H enrichment in plasma. For the analysis of alanine enrichment, muscle tissue was homogenized with 6% perchloric acid, precipitated at 21,000× g at 4 °C and hydrolyzed into amino acids at 100 °C for 16 h. The free amino acids from the hydrolyzed sample were extracted via cation-exchange resin columns and dried under Speed vac (Savant Instruments, Farmingdale, NY). To investigate glycolytic pathway and TCA cycle metabolic flux in skeletal muscle, muscle tissues were quickly dissected and immediately frozen in liquid nitrogen. GA muscle was homogenized with 70% ACN and 30% distilled water and centrifuged at 14,000× g for 5 min at 4 °C. After centrifuging, the supernatant was transferred to a new tube. The supernatant was dried using Speed Vac. Derivatization for GC-MS was initiated in 50 µL of 2% MOX in pyridine for 90 min at 37 °C. Then, 50 µL of MTBSFTA + 1% TBDMSCIS (#00942, Sigma-Aldrich, St Louis, MO, USA) was added and incubated for 30 min at 60 °C. Lastly, the samples were centrifuged at 14,000× g for 5 min at room temperature and 75 µL of supernatant was transferred to a GC-MS vial.
4.8. Immunoblot Analysis
Immunoblotting was performed as described previously [22]. In brief, GA muscle was denatured in lysis buffer and protein concentration was measured using a BCA assay kit (#A53225, Thermo Fisher Scientific, Waltham, MA, USA). A total protein concentration of 20 µg was resolved by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Primary antibodies, including AKT (#9272), p-AKT (Ser473)(#9271), mTOR (#4844S), p-mTOR (Ser2448)(#2971), rpS6 (#2217), p-rpS6 (Ser235/236)(#4856S), ATG7 (#8558), LC3B (#NB100-2220, Novus Biologicals, CO, USA), ubiquitin (#3933S, Cell Signaling Technology, Danvers, MA, USA), and β-tubulin (#05-661, Merck Millipore, Burlington, MA, USA) were diluted to a 1:1000 concentration ratio and incubated overnight at 4 °C. After an hour of incubation secondary antibody, bands were detected using ECL solution and quantified with ImageJ image analysis software (National Institutes of Health).
4.9. Statistical Analysis
The data were expressed as mean ± S.E and analyzed using GraphPad Prism 9 and SPSS for window version 21.0. Data were analyzed to determine the main effects of interventions groups on variables by one-way ANOVA (followed by Fisher’s least significant difference test). The area under curve (AUC) for the blood glucose concentration, enrichment, insulin concentration during the OGTT was determined using the trapezoidal method. Statistical significance was set at p < 0.05.
5. Conclusions
We have evaluated the effect of an EAA-enriched diet on the DEX-induced muscle loss and function and its impact on glucose metabolism. The EAA-enriched diet protected from the DEX-induced muscle atrophy via enhanced rates of protein synthesis and insulin stimulated-glucose metabolism. These findings suggest a nutraceutical potential for an EAA-enriched diet that may counteract DEX-induced muscle atrophy in clinical settings.
Acknowledgments
We appreciate Hee-joo Kim, Ui Young Yang, and Yewon Chang for their helping experiments. In addition, we also thank th personnel in the Animal Resource Center at Lee Gil Ya Cancer and Diabetes Institute for their sincere support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo12010084/s1. Figure S1: Ratio of phosphorylated Akt/mTORC1 related proteins.
Author Contributions
I.-Y.K. conceptualized the study. Y.K., S.P. and I.-Y.K. designed the study. Y.K., J.L. and J.J. (Jiwong Jang) conducted animal experiments. Y.K. and J.L. performed Western blot and GC-MS analysis. Y.K. and S.P. performed LC-MS analysis. J.J. (Jiyeon Jung) conducted statistical analysis. Y.K. and J.-H.K. prepared tables and figures. Y.K. drafted the manuscript under the guidance of I.-Y.K. S.P., J.J. (Jiwong Jang), J.J. (Jiyeon Jung), J.-H.K., C.S.C. and R.R.W. helped write the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C3005801), the Gachon University research fund of 2020 (GCU-202008430005), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare of Korea (HR14C0001).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Committee on the Ethics of Animal experiments of the Gachon University Lee Gil Ya Cancer and Diabetes Institute (permit number: LCDI-2021-0016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and supplementary material.
Conflicts of Interest
I.-Y.K., S.P., and J.J. (Jiyeon Jung) are shareholders of Myocare., Inc., and Robert Wolfe is a shareholder in Essential Blends, LLC, and the Amino Company, LLC. All others have no potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao R.Y., Li J., Dai Q., Li Q., Yang J. Muscle Atrophy: Present and Future. Adv. Exp. Med. Biol. 2018;1088:605–624. doi: 10.1007/978-981-13-1435-3_29. [DOI] [PubMed] [Google Scholar]
- 2.Kim I.-Y., Park S., Jang J., Wolfe R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020;24:157–165. doi: 10.4235/agmr.20.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers S.K., Lynch G.S., Murphy K., Reid M.B., Zijdewind I. Disease-Induced Skeletal Muscle Atrophy and Fatigue. Med. Sci. Sports Exerc. 2016;48:2307–2319. doi: 10.1249/MSS.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campins L., Camps M., Riera A., Pleguezuelos E., Yébenes J.C., Serra-Prat M. Oral Drugs Related with Muscle Wasting and Sarcopenia. A Review. Pharmacology. 2017;99:1–8. doi: 10.1159/000448247. [DOI] [PubMed] [Google Scholar]
- 5.Evans W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 6.Shah O.J., Kimball S.R., Jefferson L.S. Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2000;278:E76–E82. doi: 10.1152/ajpendo.2000.278.1.E76. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.W., Ku S.-K., Han M.H., Kim K.Y., Kim S.G., Kim G.Y., Hwang H.J., Kim B.W., Kim C.M., Choi Y.H. The administration of Fructus Schisandrae attenuates dexamethasone—Induced muscle atrophy in mice. Int. J. Mol. Med. 2015;36:29–42. doi: 10.3892/ijmm.2015.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruzzin J., Wagman A.S., Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: Defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 9.Hsu F.-L., Huang C.-F., Chen Y.-W., Yen Y.-P., Wu C.-T., Uang B.-J., Yang R.-S., Liu S.-H. Antidiabetic Effects of Pterosin A, a Small-Molecular-Weight Natural Product, on Diabetic Mouse Models. Diabetes. 2013;62:628–638. doi: 10.2337/db12-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W.J., Ye L., Huang W.F., Guo L.J., Xu Z.G., Wu H.L., Yang C., Liu H.F. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016;21:21–29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodine S.C., Latres E., Baumhueter S., Lai V.K.-M., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 12.Thompson M.G., Thom A., Partridge K., Garden K., Campbell G.P., Calder G., Robert M.P. Stimulation of Myofibrillar Protein Degradation and Expression of mRNA Encoding the Ubiquitin-Proteasome System in C2C12 Myotubes by Dexamethasone: Effect of the Proteasome Inhibitor MG-J. Cell Physiol. 1999;181:455–461. doi: 10.1002/(SICI)1097-4652(199912)181:3<455::AID-JCP9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Girón M.D., Vilchez J.D., Shreeram S., Salto R., Manzano M., Cabrera E., Campos N., Edens N.K., Rueda R., López-Pedrosa J.M. β-Hydroxy-β-Methylbutyrate (HMB) Normalizes Dexamethasone-Induced Autophagy-Lysosomal Pathway in Skeletal Muscle. PLoS ONE. 2015;10:e0117520. doi: 10.1371/journal.pone.0117520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng J., Guo Y., Yuan F., Chen S., Yin H., Jiang X., Jiao F., Wang F., Ji H., Hu G., et al. Autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening. Autophagy. 2020;16:451–465. doi: 10.1080/15548627.2019.1628537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneiter P., Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am. J. Physiol.-Endocrinol. Metab. 1998;275:E806–E813. doi: 10.1152/ajpendo.1998.275.5.E806. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein S.P., Wilson C.M., Pritsker A., Cushman S.W. Dexamethasone inhibits insulin-stimulated recruitment of GLUt4 to the cell surface in rat skeletal muscle. Metabolism. 1998;47:3–6. doi: 10.1016/S0026-0495(98)90184-6. [DOI] [PubMed] [Google Scholar]
- 17.Fappi A., Godoy T.S., Maximino J.R., Rizzato V.R., Neves J.D.C., Chadi G., Zanoteli E. The Effects of Omega-3 Fatty Acid Supplementation on Dexamethasone-Induced Muscle Atrophy. Bio. Med Res. Int. 2014;2014:961438. doi: 10.1155/2014/961438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishida H., Ikegami A., Kaneko C., Kakuma H., Nishi H., Tanaka N., Aoyama M., Usami M., Okimura Y. Dexamethasone and BCAA Failed to Modulate Muscle Mass and mTOR Signaling in GH-Deficient Rats. PLoS ONE. 2015;10:e0128805. doi: 10.1371/journal.pone.0128805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhubi-Bakija F., Bajraktari G., Bytyçi I., Mikhailidis D.P., Henein M.Y., Latkovskis G., Latkovskis G., Rexhaj Z., Zhubi E., Banach M. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP) Clin. Nutr. 2021;40:255–276. doi: 10.1016/j.clnu.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Børsheim E., Tipton K.D., Wolf S.E., Wolfe R.R. Essential amino acids and muscle protein recovery from resistance exercise. Am. J. Physiol. Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kim I.-Y., Park S., Smeets E.T.H.C., Schutzler S., Azhar G., Wei J.Y., Ferrando A.A., Wolfe R.R. Consumption of a Specially-Formulated Mixture of Essential Amino Acids Promotes Gain in Whole-Body Protein to a Greater Extent than a Complete Meal Replacement in Older Women with Heart Failure. Nutriens. 2019;11:1360. doi: 10.3390/nu11061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang J., Park S., Kim Y., Jung J., Lee J., Chang Y., Lee S., Park B.-C., Wolfe R., Choi C., et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients. 2021;13:1508. doi: 10.3390/nu13051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S., Church D.D., Azhar G., Schutzler S.E., Ferrando A.A., Wolfe R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020;17:9. doi: 10.1186/s12970-020-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bird S.P., Tarpenning K.M., Marino F.E. Liquid carbohydrate/essential amino acid ingestion during a short-term bout of resistance exercise suppresses myofibrillar protein degradation. Metabolism. 2006;55:570–577. doi: 10.1016/j.metabol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Osmond A.D., Directo D.J., Elam M.L., Juache G., Kreipke V.C., Saralegui D.E., Wildman R., Wong M., Jo E. The Effects of Leucine-Enriched Branched-Chain Amino Acid Supplementation on Recovery After High-Intensity Resistance Exercise. Int. J. Sports Physiol. Perform. 2019;14:1081–1088. doi: 10.1123/ijspp.2018-0579. [DOI] [PubMed] [Google Scholar]
- 26.D’Antona G., Ragni M., Cardile A., Tedesco L., Dossena M., Bruttini F., Caliaro F., Corsetti G., Bottinelli R., Carruba M.O., et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Robinson M.M., Soop M., Sohn T.S., Morse D.M., Schimke J., Klaus K.A., Nair K.S. High insulin combined with essential amino acids stimulates skeletal muscle mitochondrial protein synthesis while decreasing insulin sensitivity in healthy humans. J. Clin. Endocrinol. Metab. 2014;99:E2574–E2583. doi: 10.1210/jc.2014-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi M., Yamaoka I., Fukunaga T., Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2003;312:1111–1117. doi: 10.1016/j.bbrc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Doi M., Yamaoka I., Nakayama M., Sugahara K., Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol.-Endocrinol. Metab. 2007;292:E1683–E1693. doi: 10.1152/ajpendo.00609.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bernard J.R., Liao Y.-H., Hara D., Ding Z., Chen C.-Y., Nelson J.L., John L.I. An amino acid mixture improves glucose tolerance and insulin signaling in Sprague-Dawley rats. Am. J. Physiol. Metab. 2011;300:E752–E760. doi: 10.1152/ajpendo.00643.2010. [DOI] [PubMed] [Google Scholar]
- 31.Coker R.H., Shin K., Scholten K., Johannsen M., Tsigonis J., Kim I.-Y., Schutzler S.E., Wolfe R.R. Essential amino acid-enriched meal replacement promotes superior net protein balance in older, overweight adults. Clin. Nutr. 2019;38:2821–2826. doi: 10.1016/j.clnu.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu L., Li Y., Zhang Q., Zhu L., Ding N., Zhang B., Zhang J., Liu W., Li S. Association between dietary essential amino acids intake and metabolic biomarkers: Influence of obesity among Chinese children and adolescents. Amino Acids. 2021;53:635–644. doi: 10.1007/s00726-021-02970-4. [DOI] [PubMed] [Google Scholar]
- 33.Della Torre S., Benedusi V., Pepe G., Meda C., Rizzi N., Uhlenhaut N.H., Maggi A. Dietary essential amino acids restore liver metabolism in ovariectomized mice via hepatic estrogen receptor α. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-021-27272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond M.J., Dreyer H.C., Pennings B., Fry C.S., Dhanani S., Dillon E.L., Sheffield-Moore M., Volpi E., Rasmussen B. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J. Appl. Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis L.M., E Murphy N., Carrigan C.T., McClung H.L., Pasiakos S.M. Ingesting a Combined Carbohydrate and Essential Amino Acid Supplement Compared to a Non-Nutritive Placebo Blunts Mitochondrial Biogenesis-Related Gene Expression after Aerobic Exercise. Curr. Dev. Nutr. 2017;1:e000893. doi: 10.3945/cdn.117.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillero E., Alamdari N., Lecker S.H., Hasselgren P.O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism. 2013;62:1495–1502. doi: 10.1016/j.metabol.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Troncoso R., Paredes F., Parra V., Gatica D., Vásquez-Trincado C., Quiroga C., Bravo-Sagua R., Lopez-Crisosto C., E Rodriguez A., Oyarzún A.P., et al. Dexamethasone-induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle. 2014;13:2281–2295. doi: 10.4161/cc.29272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Church D.D., Hirsch K.R., Park S., Kim I.-Y., Gwin J.A., Pasiakos S.M., Wolfe R.R., Ferrando A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients. 2020;12:3717. doi: 10.3390/nu12123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleveland B.M., Radler L.M. Essential amino acids exhibit variable effects on protein degradation in rainbow trout (Oncorhynchus mykiss) primary myocytes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019;229:33–39. doi: 10.1016/j.cbpa.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Binnert C., Ruchat S., Nicod N., Tappy L. Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab. 2004;30:321–326. doi: 10.1016/S1262-3636(07)70123-4. [DOI] [PubMed] [Google Scholar]
- 41.Stojanovska L., Rosella G., Proietto J. Evolution of dexamethasone-induced insulin resistance in rats. Am. J. Physiol. Metab. 1990;258:E748–E756. doi: 10.1152/ajpendo.1990.258.5.E748. [DOI] [PubMed] [Google Scholar]
- 42.Sharabi K., Tavares C.D., Rines A.K., Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015;46:21–33. doi: 10.1016/j.mam.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eadie M.J., Brophy T.R., Ohlrich G., Tyrer J.H. Dexamethasone: Pharmacokinetics in neurological patients. Exp. Neurol. 1984;20:107–118. [PubMed] [Google Scholar]
- 44.Xiang Z., Zhou Z., Song S., Li J., Yan R., Wang J., Cai W., Hu W., Zang L., Zhu Z., et al. Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene. 2021;40:5002–5012. doi: 10.1038/s41388-021-01897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuld A., Birkmann S., Beitinger P., Haack M., Kraus T., Dalal M., Holsboer F., Pollmächer T. Low Doses of Dexamethasone Affect Immune Parameters in the Absence of Immunological Stimulation. Exp. Clin. Endocrinol. Diabetes. 2006;114:322–328. doi: 10.1055/s-2006-924255. [DOI] [PubMed] [Google Scholar]
- 46.Kim I.-Y., Deutz N.E.P., Wolfe R.R. Update on maximal anabolic response to dietary protein. Clin. Nutr. 2018;37:411–418. doi: 10.1016/j.clnu.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philp A., Schenk S., Perez-Schindler J., Hamilton D.L., Breen L., Laverone E., Jeromson S., Phillips S.M., Baar K. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. J. Physiol. 2015;593:4275–4284. doi: 10.1113/JP271219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X.D., Holley R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011;10:33–51. doi: 10.1111/j.1541-4337.2010.00137.x. [DOI] [Google Scholar]
- 49.Ruocco C., Segala A., Valerio A., Nisoli E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24:88–95. doi: 10.1097/MCO.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 50.Phillips S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016;13:1–9. doi: 10.1186/s12986-016-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son Y.H., Lee S.-J., Lee K.-B., Lee J.-H., Jeong E.M., Chung S.G., Park S.-C., Kim I.-G. Dexamethasone downregulates caveolin-1 causing muscle atrophy via inhibited insulin signaling. J. Endocrinol. 2015;225:27–37. doi: 10.1530/JOE-14-0490. [DOI] [PubMed] [Google Scholar]
- 52.Desler M.M., Jones S.J., Smith C.W., Woods T.L. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J. Anim. Sci. 1996;74:1265–1273. doi: 10.2527/1996.7461265x. [DOI] [PubMed] [Google Scholar]
- 53.Menzies F.M., Fleming A., Caricasole A., Bento C.F., Andrews S.P., Ashkenazi A., Füllgrabe J., Jackson A., Sanchez M.J., Karabiyik C., et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Limanaqi F., Biagioni F., Gambardella S., Familiari P., Frati A., Fornai F. Promiscuous Roles of Autophagy and Proteasome in Neurodegenerative Proteinopathies. Int. J. Mol. Sci. 2020;21:3028. doi: 10.3390/ijms21083028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honka M.-J., Latva-Rasku A., Bucci M., A Virtanen K., Hannukainen J., Kalliokoski K., Nuutila P. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: A positron emission tomography study. Eur. J. Endocrinol. 2018;178:523–531. doi: 10.1530/EJE-17-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim I.-Y., Park S., Kim Y., Chang Y., Choi C.S., Suh S.-H., Wolfe R.R. In Vivo and In Vitro Quantification of Glucose Kinetics: From Bedside to Bench. Endocrinol. Metab. 2020;35:733–749. doi: 10.3803/EnM.2020.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe R.R., Chinkes D.L. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Wiley-Liss; New York, NY, USA: 2004. [Google Scholar]
- 58.Kim I.-Y., Suh S.-H., Lee I.-K., Wolfe R.R. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp. Mol. Med. 2016;48:e203. doi: 10.1038/emm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A DeFronzo R., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A DeFronzo R., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 61.Tappy L., Randin D., Vollenweider P., Vollenweider L., Paquot N., Scherrer U., Schneiter P., Nicod P., Jéquier E. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J. Clin. Endocrinol. Metab. 1994;79:1063–1069. doi: 10.1210/jcem.79.4.7962275. [DOI] [PubMed] [Google Scholar]
- 62.Katta A., Kundla S., Kakarla S.K., Wu M., Fannin J., Paturi S., Liu H., Addagarla H.S., Blough E.R. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010;299:R1666–R1675. doi: 10.1152/ajpregu.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundell L., Massart J., Altıntaş A., Krook A., Zierath J.R. Regulation of glucose uptake and inflammation markers by FOXO1 and FOXO3 in skeletal muscle. Mol. Metab. 2019;20:79–88. doi: 10.1016/j.molmet.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furugen M., Saitoh S., Ohnishi H., Akasaka H., Mitsumata K., Chiba M., Furukawa T., Miyazaki Y., Shimamoto K., Miura T. Matsuda–DeFronzo insulin sensitivity index is a better predictor than HOMA-IR of hypertension in Japanese: The Tanno–Sobetsu study. J. Hum. Hypertens. 2011;26:325–333. doi: 10.1038/jhh.2011.23. [DOI] [PubMed] [Google Scholar]
- 65.Holm L., Haslund M.L., Robach P., van Hall G., A Calbet J., Saltin B., Lundby C. Skeletal Muscle Myofibrillar and Sarcoplasmic Protein Synthesis Rates Are Affected Differently by Altitude-Induced Hypoxia in Native Lowlanders. PLoS ONE. 2010;5:e15606. doi: 10.1371/journal.pone.0015606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao P.-C., Bergamini C., Fato R., Pon L.A., Pallotti F. Isolation of mitochondria from cells and tissues. Methods Cell Biol. 2020;155:3–31. doi: 10.1016/bs.mcb.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gasier H.G., Fluckey J.D., Previs S.F. The application of 2H2O to measure skeletal muscle protein synthesis. Nutr. Metab. 2010;7:1–8. doi: 10.1186/1743-7075-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in article and supplementary material.