Abstract
The purpose of this review is to summarize the current acquiredknowledge of Candida overgrowth in the intestine as a possible etiology of autism spectrum disorder (ASD). The influence of Candida sp. on the immune system, brain, and behavior of children with ASD isdescribed. The benefits of interventions such as a carbohydrates-exclusion diet, probiotic supplementation, antifungal agents, fecal microbiota transplantation (FMT), and microbiota transfer therapy (MTT) will be also discussed. Our literature query showed that the results of most studies do not fully support the hypothesis that Candida overgrowth is correlated with gastrointestinal (GI) problems and contributes to autism behavioral symptoms occurrence. On the one hand, it was reported that the modulation of microbiota composition in the gut may decrease Candida overgrowth, help reduce GI problems and autism symptoms. On the other hand, studies on humans suggesting the beneficial effects of a sugar-free diet, probiotic supplementation, FMT and MTT treatment in ASD are limited and inconclusive. Due to the increasing prevalence of ASD, studies on the etiology of this disorder are extremely needed and valuable. However, to elucidate the possible involvement of Candida in the pathophysiology of ASD, more reliable and well-designed research is certainly required.
Keywords: autism, Candida albicans, candidiasis and autism, gastrointestinal, probiotics, fecal microbiota transplantation, microbiota transfer therapy
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, which occurs in early childhood and persists into adulthood, and is manifested by a high level of social interaction insufficiency, language impairment, and repetitive behavior [1]. The etiology of autism has not been clearly defined yet. However, maternal infection during pregnancy [2,3]; maternal obesity and diabetes [4]; excessive early childhood vaccination [5,6]; heavy metal toxicity [7,8]; severe infection in the first 2 years of life [9]; involvement of bacteria, viruses and fungi [10,11]; long-term exposure to antibiotics in early life [12,13]; intestinal microbial dysbiosis [14,15]; gastrointestinal (GI) problems [16,17]; leaky gut syndrome [18,19]; allergies [20,21]; immune dysfunction [22,23,24]; neuroinflammation [25,26]; developmental abnormalities of the nervous system [27]; neurotransmitter imbalances (serotonin, dopamine, GABA, noradrenaline) [28]; metabolic factors deficiency [29,30]; genetic background [31,32,33]; environmental factors [34] and oxidative stress [35] may be involved in the development of autism. Moreover, the estimated prevalence of ASD is higher in males than in females (ratio 3:1) [36]. The higher autistic phenotypes in males compared with females could be attributed to the protective effect of estrogen, the higher diversity and predominance of probiotics in females, the lower liability of females to develop leaky gut, neuroinflammation, and excitotoxicity [37]. Children with ASD are very selective eaters (“picky eaters”), and most of them show aversions to specific food colors, textures, smells, or other foods’ characteristics [38]. Indeed, children with ASD are at increased risk of a broad spectrum of concomitant medical issues; the most prevalent are sleeping problems, epilepsy, immune dysregulation, disruption of gut microbial balance (dysbiosis), and GI disturbances [39,40]. Dysbiosis refers to an imbalance in the microbial community of the human body. In this case, pathogenic bacteria can outnumber the beneficial ones, leading to complicated disorders in the host GI tract [41]; therefore, small intestinal bacterial overgrowth (SIBO) is one of the consequences [42]. Diet-derived carbohydrates that are not fully digested in the upper gut are metabolized by bacteria in the human large intestine. These non-digestible carbohydrates influence microbial fermentation and total bacterial number in the colon [43]. High growth rates of Clostridium histolyticum, Clostridium perfringens, and Sutterella; a high ratio of Escherichia/Shigella; and a low ratio of Bacteroidetes/Firmicutes were generally related to GI problems [44,45,46], while the relative abundance of Desulfovibrio, Clostridium spp., and Bacteroides vulgatus were associated with behavior disorders [47,48,49]. In turn, the studies on gut mycobiome are still in their infancy; numerous sources reported its potential role in host homeostasis and disease development [50]. The actual role of gut fungal microbiota in ASD children has not been clearly elucidated and more studies are needed [51,52]. In this paper, the current knowledge acquired on Candida overgrowth in the intestine of children with ASD is summarized based on several electronic databases and hand-searched references. Moreover, the yeast influence on the immune system, brain, and behavior of children with ASD is also described. The benefits of interventions such as a carbohydrates exclusion diet, probiotic supplementation, antifungal agents, fecal microbiota transplantation (FMT), and microbiota transfer therapy (MTT) are also discussed.
2. Literature Search Strategy
The Scopus, PubMed, and Google Scholar databases were searched for articles. Search terms included “autism and Candida”, “ASD and Candida”, “autism and candidiasis” and “Candida metabolites and autism”. References from reviews concerning Candida sp. in ASD were searched for additional articles and case reports. A manual search was also conducted based on citations in the published literature.
2.1. Inclusion and Exclusion Criteria
Selection criteria excluded articles that examined bacterial gut microbiota in ASD, animal studies, and review articles. Additionally, publications in languages other than English were excluded.
2.2. Study Selection
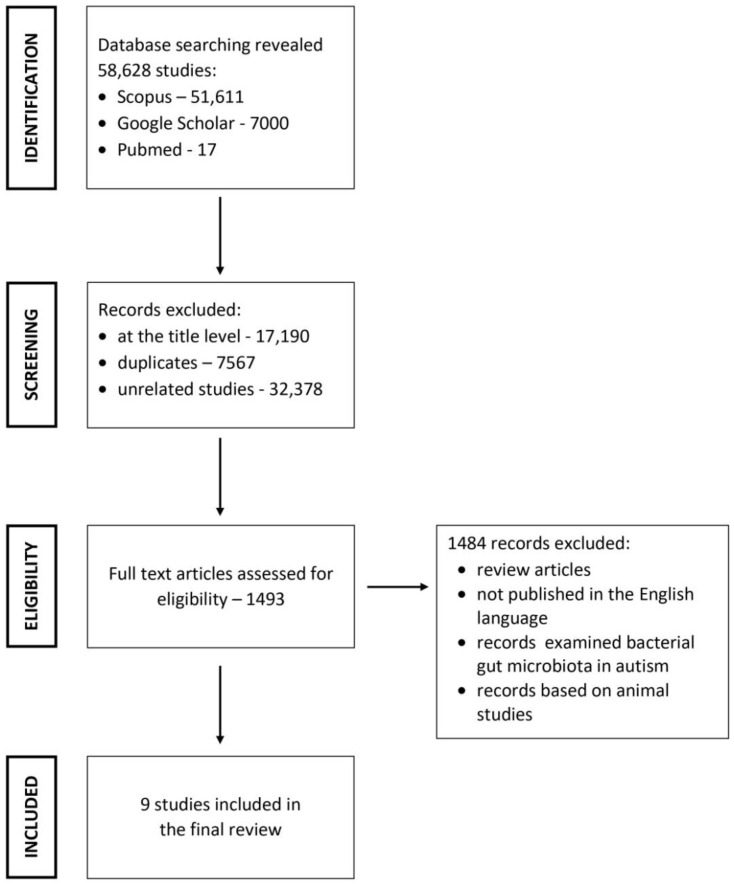
Overall, 58,628 articles were found via the search of the databases. Of these, 57,135 articles were excluded at the title level, as well as duplicates and unrelated articles. Further, 1484 articles were excluded for not meeting the inclusion criteria. Finally, nine articles were used for the review (Figure 1).
Figure 1.
Search strategy used to identify relevant articles.
3. Candida and Their Metabolites Isolated from Stool, Urine, andBlood Samples from Children with ASD
The detection of clinically significant intestinal fungi growth is challenging. The early diagnosis of fungi infection remains problematic due to the poor sensitivity and specificity of current diagnostic modalities. Advances in sequencing technologies hold promise in addressing these shortcomings and for improved fungi detection and identification. Candida albicans germ tube antibody assay, mannan-Ag and mannan-Ab PCR, histopathology, culture, serum beta-glucan contrasted with methylene blue are successfully used [53]. The most popular tests used to diagnose Candida overgrowth in the gut of children with ASD (stool, urine, blood) are based on culture method, microscopic examination with staining, determination of fungi metabolites or immunoglobulins, etc., but none of these tests is completely reliable; therefore, the correct detection of the infection of candidiasis in ASD children is difficult [54].
3.1. Candida Isolated from Stool Samples from Children with ASD
Some studies showed that stools observed in ASD children, in comparison with those from healthy children, have different gut fungal communities [52,55]. Many studies showed that Candida sp. was isolated more frequently from stools from autistic patients than healthy ones (Table 1).
Table 1.
Candida sp. in stool samples from children with ASD.
| Study Groups | Candida sp. Identified in a Stool Samples | Statistical Significance (ASD vs. Control) |
Impact on Children with ASD | Ref. |
|---|---|---|---|---|
|
Candida | Not statistically significant |
|
[55] |
|
||||
|
||||
|
C. parapsilosis | No data |
|
[56] |
|
||||
|
C. glabrata
C. parapsilosis |
Not statistically significant |
|
[57] |
| C. tropicalis |
|
|||
| C. albicans | ||||
| C. krusei | ||||
|
Candida | Not statistically significant |
|
[58] |
|
||||
|
Candida | Partially significant |
|
[46] |
|
Candida | Statistically significant |
|
[59] |
|
||||
|
||||
|
||||
|
||||
|
Candida | Not statistically significant |
|
[60] |
|
C. albicans C.krusei C. glabrata |
No data |
|
[61] |
|
Candida | Not statistically significant |
|
[62] |
Legends: M—male;F—female;ATEC—Autism Treatment Evaluation Checklist; CARS—Childhood Autism Rating Scale; GI—gastrointestinal.
The genus Candida was identified up to twice as frequently in ASD children than in the control population [46]. However, Kantarcioglu et al. [61] reported the presence of Candida sp. in a large percentage of ASD patients (81.4%) compared to controls (19.6%). Emam et al. [63] also showed an increased rate of infection by yeast in the autisticgroup (81.9%) versus the control group (28%). Moreover, there was a significant relationship between autistic children and the heavy growth of C. albicans in stool cultures [63]. Similarly, El-Shouny et al. [64] found a heavy growth of yeast in the autistic group in comparison with the control group. Iovene et al. [59] also identified aggressive forms (pseudohyphae) of Candida spp. in the stools from 57% ASD children and not in age-matched healthy controls. The overgrowth of Candida sp. may lead to the morphological transition from budding yeast to hyphae, as well as biofilm formation; this is a key determinant in the C. albicans pathogenesis [65,66]. The ability of C. albicans to attach and disseminate from the GI tract is associated with its capacity to undergo a morphological transition from yeast to hyphae, which allows the organism to attach, invade and perpetuate the disease [67,68].
Unfortunately, although the abundance of the fungal genus Candida sp. was greater in autistic children than neurotypical subjects, the statistical differences in most studies were not significant [46,55,57,58,60,62] or researchers did not provide this data [56,61]. Furthermore, the methodology and degree of detection of Candida sp. in the stool from children with ASD affects results and statistical significance. Some literature data show that yeast were more commonly observed microscopically and only rarely observed by culture in the autistic or control groups, but the difference between these two groups was also not significant [60].
The most commonly isolated Candida sp. from stool samples of children with diagnosed or suspected ASD were: C. albicans (57.4%), fluconazole-resistant C. krusei (19.8%) and C. glabrata (14.8%) [61]. Moreover, C. albicans was the most prevalent species in both groups, while C. krusei and C. glabrata were only isolated in ASD samples [61]. Additionally, Iovene et al. [59] showed that C. albicans was the most frequently isolated (16 times/27 total cases) yeast from ASD patients. In turn, Ahmed et al. [57] showed that C. glabrata (43.1%) was the most commonly isolated from ASD patients, followed by C. parapsilosis(19.6%), C. tropicalis (17.7%), C. albicans (9.8%), and C. krusei (9.8%). El-shouny et al. [64] reported that C. krusei was the most commonly isolated, while Colombo et al. [69], stated that the nonalbicans Candida sp., such as C. krusei, C. tropicalis, were observed in their ASD cases.
Some studies attempted to link the presence of Candida sp. to GI dysfunction and the severity of autism symptoms (Table 1). The strong correlation of Candida sp. with GI symptoms and autism severity indicates that children with more severe autism are likely to have more severe GI symptoms and vice versa [46,60]. However, most of the studies showed that increased colonization with Candida sp. did not affect the severity of symptoms in ASD children and was not correlated with GI symptoms [55,57,58,59,62]. Moreover, the latest data showed that mycobiome dysbiosis was more pronounced in neurodevelopmental disorders, such as ASD, than in GI disorders [70]. Interestingly, probiotic intake, diet, and antibiotic exposure had a greater effect on fungal abundances than bacterial abundances, suggesting that the presence of strong interactions between dysbiosis caused by a disruption in the microbiota homeostasis and neurodevelopmental disorders, while GI disorders seemed to be associated rather with an imbalance in the bacterial community.
3.2. Candida Metabolites Isolated from Urine Samplesfrom Children with ASD
Some studies showed that fungi growing in the gut produced many metabolites, which affect the behavior of ASD children [52,55]. Candida sp. growing in the gut release many metabolites, such as arabinose [56,71], D-arabinitol [72,73], and tartaric acid [71], which are thought to contribute to autistic behaviors. Unfortunately, the association of urinary arabinose as a biomarker of intestinal yeast overgrowth is questionable. This simple sugar (aldose) is a substrate from dietary carbohydrates that, under the anaerobic conditions in the human intestinal tract, are reduced (consumed) by rapidly growing yeast to arabinitol (five-carbon sugar alcohol). This is the biochemical rationale that arabinitol, but not arabinose, is characteristic of yeast growth [74,75,76]. Furthermore, it was shown that arabinose had a little or marginal impact on C. albicans growth [77], and arabinose was never reported to be a metabolic product of any strain of yeast or fungus [78]. Moreover, some investigators report the ratio of D- to L-arabinitol as a biomarker of yeast overgrowth [72], but the enantiomer, L-arabinitol, is not produced by yeast but comes from the diet [74]. However, with proper calibration procedures, the measurement of D-arabinitol concentrations without the calculation of the D/L ratio is at least as sensitive as the ratio method for the detection of yeast overgrowth [74]. It was also shown that elevated levels of D-arabinitol in urine are a positive indication of Candida overgrowth, even if invasive candidiasis is not present [74]. Similarly, tartaric acid can be a substrate for the growth of fungi [79,80] but no literature data showed that any type of yeast or fungus could produce tartaric acid as a metabolite. To summarize, significant amounts of arabinose or tartaric acid found in urine do not confirm evidence of yeast overgrowth.
3.3. Candida Isolated from Blood Samples from Children with ASD
Some studies confirm the presence of filamentous fungi on blood culture or increased levels of immunoglobulins (Ig)s that target fungal antigen in the blood of ASD children. Markova et al. [81] observed cell-wall-deficient variants from the life-cycle of filamentous fungi of Candida parapsilosis, Cryptococcus albidus and Rhodotorulamucilaginosa, as well increased IgG, IgM, and IgA proving the presence of Aspergillus fumigatus in the blood of almost all autistic children in the study. The anti-Candida IgG was found in about half of the ASD children with GI dysfunction [82]. This suggests that dysbiosis could occur even with the absence of GI symptoms. Moreover, the production of IgG antibodies indicates the current or previous overgrowth of Candida sp. [83]. Therefore, the test for anti-Candida IgG does not clearly explain whether Candida sp. is present in the gut of the children with ASD at this moment, and it is not known if the treatment with antifungal drugs should be initiated. Additionally, blood cultures were assumed to be positive for Candida sp. in only 24 to 60% of cases [84].
4. The Influence Candida Overgrowth on the Immune System, Brain, and Behavior of ASD Children—Mechanisms of Action
In the 1980s some researchers suggested that yeasts are playing a significant role in behavior and learning problems in ASD children [85,86]. It was noted that excessive and long-term exposure to antibiotics (e.g., middle ear infection) led to gut dysbiosis and caused the overgrowth of the yeast C. albicans in the intestinal tract. Candida released many toxins into the bloodstream, which disrupted the immune system and couldinfluence the functioning of the brain, contributing to autistic behaviors. However, not all children with ASD have an overgrowth of Candida sp. in the gut; therefore, it is difficult to conclude that Candida causes autism.
4.1. The Dysregulation of the Immune System
Many autism susceptibility genes are localized in the immune system and are related to immune/infection pathways. They are enriched in the host–pathogen interactions of microbes (bacteria, viruses, and fungi) and to the genes regulated by bacterial toxins, mycotoxins, and Toll-like receptor ligands [87]. C. albicans can cause lesion formation in the gut by degrading the protective mucin layer through the action of mucolytic enzymes [88]. Simultaneously, some fatty acid metabolites secreted by gut bacterial flora modulate C. albicans germination [89] and hyphal growth through the target of the rapamycin (TOR) signaling pathway [90], which leads to adverse effects on the host via proinflammatory cytokines secretion [91]. Candida sp. colonization in the intestinal tract delays healing of inflammatory lesions [92]. These effects may create a vicious cycle where low-level inflammation promotes fungal colonization and fungal colonization promotes further inflammation. The cell wall of commensal fungi, such as S. cerevisiae and C. albicans, contains various ligands (e.g., β-glucans and chitin) for the receptors of innate immune cells [93]. The most important pathogen recognition receptors are C-type lectin receptors such as Dectin-1 and Dectin-2, mainly expressed in dendritic cells and macrophages to recognize commensal fungi [94]. After the ligand is linked to receptors, nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPKs) are activated via the CARD9-BCL10-MALT1 complex, which modulates pro-inflammatory cytokines and reactive oxygen species (ROS) production, finally restricting the growth of fungi [94]. It was shown that fungi such as C. albicans, S. cerevisiae, and A. fumigatus can modulate cytokine expression, particularly IL-6 [95]. The GI Candida colonization is associated with elevated levels of the pro-inflammatory cytokine IL-17 and enhanced inflammation [96]. It was also found that β-glucan derived from C. albicans can stimulate canonical inflammasomes of innate immune cells, leading to the cleavage of proinflammatory cytokines, especially IL-1β and IL-18 [97]. Moreover, it was shown that changes in IL-1β/IL-10 ratios and monocyte cytokine profiles under β-glucan-stimulated cultures indicated that changes in innate immune responses were not limited to Toll-like receptor (TLR) pathways [24]. It was also observed that autistic children with GI disease had myeloperoxidase (MPO) deficiency resulting in the exaggeration of an inflammatory response [98]. The production of MPO is the main way for neutrophils to be involved in antifungal defense, and so its deficiency may be associated with the increased fungal infection seen in many of these children. In vitro studies showed that C. albicans, C. krusei, C. stellatoidea, and C. tropicalis cannot be killed by MPO-deficient polymorphonuclear leucocytes [99]. This leads to the conclusion that killing fungi may be difficult, depending on the severity of the MPO deficiency; however, bacterial killing may not necessarily be a problem for patients with MPO deficiency [100]. On the contrary, the latest research found that serum MPO activity was significantly higher in ASD cases than in the control subjects [101]. Neutrophil levels and neutrophil/lymphocyte ratio (NLR) were also higher in ASD groups compared with healthy children [102]. Moreover, NLR significantly correlated with social interaction problems in ASD children. These findings implicated MPO as an important therapeutic target in the treatment of inflammatory conditions [103]. Therefore, curing inflammation could improve the difficult behavior and social interactions in children with ASD.
Some fungi produce toxins that regulate host immune responses. Aspergillus fumigatus releases gliotoxin to suppress the immune system. Gliotoxin is an inhibitor of T-cell activation and macrophage phagocytosis and induces apoptosis in monocytes and monocyte-derived dendritic cells [104,105]. Aspergillus-induced immune suppression in children can lead to secondary polymicrobial invasion of opportunistic bacteria and other fungal species, such as C. parapsilosis or Cryptococcus albidus. Additionally, isolates of C. albicans produce gliotoxins [106], immunotoxins [107,108], candidalysin [109,110], and farnesol [111] to regulate host immune responses. Moreover, candidalysin utilizes NLRP3 inflammasome-dependent cytolysis to evade phagocytic clearance [112,113], while farnesol acts as a vital virulence factor to impair the ability of immature DCs (iDC) to induce T cell differentiation and the expression of pro-inflammatory cytokines, therebyaffecting pro-inflammatory and Th1 responses [114]. Notably, fungi also secrete prostaglandins (PGs) or convert exogenous arachidonic acid into PGs [115,116] to affect the functions of phagocytes, which contribute to the continuous colonization of C. albicans [117]. Therefore, scientists suggest that C. albicans overgrowth in intestines causes higher toxin levels which are thought to contribute to autistic behaviors [118].
4.2. The Influence on Brain and Behavior in ASD Children
Some research suggested that Candida sp. are cancausenot only severe, long-term disruptions of the immune system but also attack the brain [63]. Candida has evolved the capacity to cross the blood–brain barrier and adhere to brain tissue [119,120]. MRI imaging using the extracellular vascular contrast agent Gd-DTPA demonstrated that the integrity of the blood–brain barrier is lost during disseminated candidiasis and both yeast and filamentous forms of the pathogen were found in the meninges and brain parenchyma in mice [121]. Moreover, it was shown that intra-amniotic exposure to C. albicans (107 colony-forming units) caused severe systemic inflammatory and neuroinflammatory responses, illustrated by a robust increase in plasma IL-6 concentrations and concomitant white matter injury in the fetal ovine brain [122]. The cerebrospinal fluid cultures were positive for C. albicans in the majority of the 3-day, C. albicans-exposed animals, whereas no positive cultures were present in the 5-day, C. albicans-exposed and fluconazole-treated animals. Although C. albicans was not detected in the brain parenchyma, a neuroinflammatory response in the hippocampus and white matter was seen and was characterized by increased microglial and astrocyte activation. These neuroinflammatory changes were accompanied by structural white matter injury. Unfortunately, intra-amniotic fluconazole reduced fetal mortality but did not attenuate neuroinflammation and white matter injury. Immune system alterations in ASD may be implicated in the severity of behavioral impairment and other developmental outcomes. The study analyzing cytokines concentration in human blood samples showed that the concentration of IL-1β was most significantly increased in ASD patients compared with the healthy controls [123]. The studies on animal models showed that IL-1β may be responsible for the induction of some disorders which commonly accompany ASD, including reduced melatonin secretion [124] and cognitive dysfunction [125]. It is well known that C. albicans infection influences the secretion of IL-1 family cytokines, which are associated with acute and chronic inflammation and are essential for the innate response to infection, particularly stimulating IL-1α/β and IL-33 synthesis [126]. This may partly explain why Candida infections can cause or worsen the disorders associated with ASD.
Reichelt and Knivsberg [127] hypothesized that the overgrowth of C. albicans can be correlated with autism because yeast cells produce ammonia (NH3), which in combination with propionic acid, presents in the GI tract and canbe converted to β-alanine (C3H9NO2). β-alanine is structurally similar to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA, C4H9NO2), which is present in higher quantities in autistic patients. The final structure for β-alanine is almost identical to GABA, except for an additional carbon atom present in GABA. The β-alanine can cross the blood–brain barrier and canbe used in the brain as a partial antagonist, blocking the receptor sites for GABA, thus facilitating the production of more GABA to achieve equilibrium [118]. An excess of GABA [128] and reduced GABAA receptors in brain regions [129] was proposed as a possible contributor to autism. Unfortunately, confirmation of this hypothesis through a biochemical analysis of the reaction between propionic acid and ammonia in the context of the human body, as well as further research in terms of the dependency of β-alanine and GABA in the brain patients with autism, should be conducted.
Researchers report that Candida sp. may increaseintestinal production of serotonin (5-hydroxytryptamine, 5-HT) at the expense of a lower synthesis in the brain (due to consumption of its precursor tryptophan), leading to hyperserotoninemia and behavioral outcomes in ASD children [15]. Elevated whole-blood serotonin or hyperserotonemia were present in more than 25% of ASD children [130]. On the contrary, plasma 5-HT levels in autistic mothers and their ASD children were significantly lower than in mothers of normal children and differed between autistic children and their fathers and siblings [131]. Low maternal plasma 5-HT may be a risk factor for autism through effects on fetal brain development [131], and at least affect social and stereotyped behavior, sleep problems, aggression, and anxiety in ASD children [132]. Moreover, it is known that the altered metabolism of the serotonin precursor, tryptophan, occurs in autism and epilepsy, a disease very often diagnosed in children with ASD [133].
5. Treatment
Some interventions, i.e., diet, probiotic supplementation, antifungal agents, FMT, and MTT could alter the gut microbiota and improve behavioral symptoms among ASD patients [134,135].
5.1. Diet Intervention against Candida Overgrowth
Children with autism are picky eaters, and their diet is usually limited to a very narrow range of foods depending on their type, texture, or appearance [136]. These children prefer starchy and fatty foods, simple carbohydrates, snacks, and processed foods over fruits, vegetables, and proteins (meat, fish, or eggs) [137,138]. It was also shown that a carbohydrate-rich diet correlates positively with the abundance of gut Candida [139], while diets with high levels of protein correlate negatively with the abundance of Candida in healthy volunteers [140]. Carbohydrates, including glucose and mannose, had the largest impact on C. albicans growth and significantly increased the growth of yeast by more than 1000% in comparison with controls [77]. Furthermore, mannitol, sorbitol, xylose, adonitol, and xylitol also significantly increased the growth of C. albicans (100–200%) compared with vehicle control. Other metabolites including raffinose, arabinose, trehalose, lactose, galactinol, galactitol, and arabitol had a little or marginal impact on C. albicans growth [77]. Therefore, carbohydrates reduction is one of the main elements of anti-candida diets andbecame very popular over the last few decades. An anti-candida diet involves reducing or avoiding added sugars (including honey, jam, and candy), highly-refined carbohydrates (especially any products made with flour), red and cured meats, and dairy products [141]. These diets were suggested for their potential benefits to ASD children, but to date, strong empirical evidence of their effect on Candida growth and gut health is lacking. There is insufficient information about their efficacy to make recommendations for their use. No clinical trials to date examined these treatments for ASD.
5.2. Probiotic Intervention against Candida Overgrowth
Processed sugars and antibiotics lead to an imbalance in intestinal microbes as well as an increase in the growth of yeast. Therefore, the use of probiotics was suggested as a treatment for ASD children to reduce GI disturbances and the overgrowth of Candida [142,143,144]. Indeed, probiotics may improve behavioral symptoms in children with ASD [72]. Unfortunately, only two studies focused on determining the effect of probiotics on the growth of Candida sp. in the GI tract. The prospective study was conducted for up to 12 months on preterm newborns (249 objects) treated with Lactobacillus reuteri (ATCC 55730; fivedrops daily; 1 × 108 CFU) or Lactobacillus rhamnosus (ATCC 53103; one capsule daily; 6 × 109 CFU), and reported the reduction in Candida in stools, as well as lower GI symptoms after L. reuteri administration [142]. Moreover, infants treated with probiotics showed a statistically significant lower incidence of abnormal neurological outcomes than the control group. Unfortunately, the number of invasive Candida infections between the control and probiotic groups was not statistically different. The use of probiotics appears to be effective in the prevention of GI colonization by Candida, but not necessarily in the protection from mycotic infections. The oral treatment with Lactobacillus acidophilus (strain Rosell-11, 5 × 109 CFU) for 2 months (twice daily) reduced Candida colonization in the intestines of 22 children with ASD (45% of participants were on a “sugar-free diet”) [72]. The probiotic supplementation led to a significant decrease in D-arabinitol and the ratio of D-/L-arabinitol in the urine of children with autism, as well as a significant improvement in the ability of ASD children to concentrate and carry out orders [72]. However, while the level of concentration ability and carrying out of orders, was improved in autistic children, the response to emotions was not varied. This study provides only suggestive, not conclusive, evidence regarding the efficacy of probiotics on GI and behavioral symptoms among ASD patients. There is insufficient information regarding the efficacy to make recommendations for their use.
The therapeutic potentials of probiotics in ASD disorder remains controversial [143]. It was shown that some Lactobacillus sp. produce a small molecule(1-acetyl-β-carboline, 1-ethoxycarbonyl-β-carboline) that blocks the C. albicans yeast-to-filament transition and biofilm formation, an important virulence trait through Yak1, a DYRK1-family kinase [144]. The study byMurzyn et al. [145] documented the effect of capric acid produced by S. boulardii in inducing adhesion, yeast-to-hyphae transition, and biofilm formation. Probiotics may manipulate intestinal microbial communities and suppress the growth of pathogens by inducing the host’s production of β-defensin and IgA. Probiotics may be able to fortify the intestinal barrier by maintaining tight junctions and inducing mucin production. Probiotic-mediated immunomodulation may occur through the mediation of cytokine secretion through signaling pathways such as NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAPK (mitogen-activated protein kinase), which can also affect proliferation and differentiation of immune cells (such as T cells) or epithelial cells. Gut motility may be modulated through the regulation of pain receptor expression and the secretion of neurotransmitters [146].
5.3. Antifungal Agents against Candida Overgrowth
The detection of high levels of Candida during diagnosis generally requires specific anti-Candida treatment. Nystatin and/or fluconazole are the most often used to restore the proper balance of microbiota or to treat Candida overgrowth in the intestines of children with ASD [61]. It was shown that all C. albicans isolated from the stoolsofchildren with ASD were sensitive to nystatin, fluconazole, and voriconazole, while non-albicans Candida showed different sensitivity patterns to the tested antifungals [57]. These results indicate that nystatin, fluconazole, or voriconazole cannot be used as empiric treatment for children with Candida overgrowth, but instead a culture of stool samples followed by identification and sensitivity testing should be mandatory for the treatment of ASD patients. Children with ASD after oral nystatin treatment at a dose of 100,000 units, 4 times a day for 70 days improved significantly with regard to the childhood autism rating scale, an indicator of the severity of autism [147]. The concentration of several urine markers for Candida growth in the intestine (5-hydroxymethyl 2-furoic acid, furan-2,5-dicarboxylic acid, and arabinose) of autistic children decreased after antifungal treatment [147]. Moreover, the parents and teachers of autistic children observed a decreased hyperactivity, increased eye contact and vocalization, better sleep patterns and concentration, increased imaginative play, reduced stereotypical behaviors such as spinning objects, and better academic performance. Unfortunately, nystatin was never proven to be safe when taken for months or years, and no studies support its use in the treatment of autistic behaviors [148]. Some research demonstrates that combination therapy with antimycotic agents and probiotics showed efficacy in the prevention of GI colonization by Candida sp. and in reducing GI symptoms [142]. Infants with Candida infections, who treated with antimycotic agents (liposomal Amphotericin B at the initial dose of 1 mgkg−1 per day, with a gradual increase up to a maximum of 6 mgkg−1 per day, 7 days) and probiotic supplementation (L. reuteri or L. rhamnosus),experienced fast clinical improvements, with fewer days of treatment in both probiotic groups compared with the control group [142].
On the other hand, antibiotic-treatment-induced gut metabolome and microbiome alterations increase the susceptibility to C. albicans colonization and morphogenesis in the GI tract [77]. The alterations at the level of gut metabolites resulting from antibiotics treatment correlate with the increased growth and hyphae formation of C. albicans in the GI tract [68,77]. Moreover, fungal species, for example Saccharomyces boulardii, C. albicans, and S. cerevisiae may secrete molecules, such as farnesol, fusel alcohols, tyrosol, and fatty acids, which are autoregulatory molecules for the growth of yeast [149]. These molecules enable fungal cells to regulate adhesion, yeast-to-hyphae transition, and biofilm formation themselves, which in turn facilitate the colonization, invasion, and dissemination of the host. Therefore, understanding numerous signals that regulate C. albicans growth and morphogenesis would provide an insight into the balance between commensalism and invasive fungal infection in the GI tract [77].
5.4. Fecal Microbiota Transplantation (FMT) and Microbiota Transfer Therapy (MTT) against Candida Overgrowth
The potential use of FMT and itsmodified protocol, MTT, is considered a promising therapy in the treatment of microbiota dysbiosis and GI disorders in ASD children [150,151]. Some research showed that commensal bacteriain the intestine of adult mice, especially the Bacteroidetes and Firmicutes species, are major resisters for C. albicans colonization [152], while Escherichia coli super-infection promotes C. albicans virulence under certain circumstances [153]. Therefore, it seems that modulating the gut microbiome could alleviate GI symptoms and ASD-related behaviors, but the efficacy of FMT and MTT in ASD is poorly understood and questionable. Only three studies describe the effects of FMT and MTT on children with autism [151,154,155]. An open-label, randomized waitlist-controlled trial confirmed the efficacy and safety of FMT for patients with ASD [154]. The application of FMT (fresh fecal suspension from one anonymous healthy donor), via colonoscopy and gastroscopy under anesthesia three times every 3 months, statistically improved behavioral ASD symptoms (CARS) and shifted the microbiome of ASD patients to a healthy state [154]. MTT-modifiedFMT therapy consisting of 2-week vancomycin treatment followed by a bowel cleanse, and then high dose FMT for 1–2 days and 7–8 weeks of daily maintenance doses, as well as a stomach-acid suppressantadministered for 10weeks to children with ASD, showed an 80% reduction in GI symptoms and a slow but steady improvement in core ASD symptoms [155]. The 2 years after MTT treatment stopped, the authors of the publication verified the long-term effects of these therapies on ASD children [151]. Significant improvements both in GI and behavior symptoms since the end of treatment were noted. Changes in gut microbiota persisted for two years, including overall community diversity and relative abundances of Bifidobacteria and Prevotella. Unfortunately, none of them described the effect of these therapies on Candida overgrowth in the GI tract and a possible improvement in the behavior of children with autism. Only one study showed a decrease in Candida and a positive outcome on intestinal inflammation post-FMT in meliorated ulcerative colitis [156]. While FMT appears to be beneficial for patients with meliorated ulcerative colitis, it is not known whether it would also work for the treatment of autistic children with Candida overgrowth.
Furthermore, the FDA’s 2020 statement about FMT warns against the use of the colon microbiota transplant procedure due to serious infections caused by multidrug-resistant organisms from the fecal suspension of donors (FDA In Brief: FDA warns about the potential risk of serious infections caused by multidrug-resistant organisms related to the investigational use of Fecal Microbiota for Transplantation). Additionally, due to the possibility of transmission of infectious diseases with transplanted material (e.g., implantation of cancer cells of colon carcinoma [157] or other cancerogenic factors, such as HPV virus [158]), thescreening for pathogens and other risks should be performed before the FMT and MTT procedure. However, these few studies do not provide information about the fecal material characteristic, its donors, methods of its preparation, and standardization. Therefore, treating with FMT and MTT can be quite risky. At this moment, in clinical practice, FMT was only used in the treatment of recurrent Clostridium difficile (rCDIs) [159] and restricted to patients not responding positively to standard treatment procedures [160]. Clinical trials to increase the understanding of the benefits of FMT and TMM in autism are still needed.
6. Conclusions
It is important to realize that the etiology of autism is still unknown. Although, some interventions may be effective in alleviating some symptoms and improving skills that may help autistic persons lead more productive lives, so far, people suffering from ASD do not have access to an effective cure or a completely effective therapy. Therefore, new scientific discoveries regarding the etiology of ASD and the potential treatment options for this disorder are enthusiastically received by people with autism and their care providers. Candida overgrowth in the intestines is suggested to be one of many possible causes of autism. Unfortunately, the few studies available do not lead to clear conclusions. The presence of Candida sp. in stool samples from children with ASD and healthy controls in most studies are not significant (6/9), or no data are available (2/9) (Table 1). No significant differences between groups do not allow a confirmation that Candida overgrowth refers to children with autism or that it may cause autism. The size of the groups (test vs. control) is too small (ranging from 1 to 58 ASD children), which probably affects the statistical insignificance of the results. Moreover, the results of most studies do not fully support the hypothesis that Candida overgrowth is correlated with GI problems and affects ASD behavioral symptoms. Furthermore, it would seem that modulatingthe microbiota composition in the gut may decrease Candida overgrowth, help reduce GI problems and autism symptoms, but evidence from human studies suggesting beneficial effects of a sugar-free diet, probiotic supplementation, FMT and MTT treatment in ASD is limited and inconclusive. Current data should not encourage the use of these modalities in the therapy of ASD, and making such changes to the nutrition of autistic children could have a potentially negative impact on their development. Surely, furtherdetailed and better-designed clinical studies are required to elucidate the possible involvement of Candida in the pathophysiology of ASD.
Author Contributions
Conceptualization, A.H.; Methodology, A.H. and A.P.H.; Writing—Original Draft Preparation, A.H.; Writing—Review and Editing, A.P.H.; Visualization, A.H. and A.P.H.; Supervision, A.P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riglin L., Wootton R.E., Thapar A., Livingston L., Langley K., Collishaw S., Tagg J., Smith G.D., Stergiakouli E., Tilling K.M., et al. Variable emergence of autism spectrum disorder symptoms from childhood to early adulthood. Am. J. Psychiatry. 2021;178:752–760. doi: 10.1176/appi.ajp.2020.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerbo O., Qian Y., Yoshida C., Grether J.K., Van de Water J., Croen L.A. Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2015;45:4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S., Zhao S., Dalman C., Karlsson H., Gardner R. Association of maternal diabetes with neurodevelopmental disorders: Autism spectrum disorders, attention-deficit/hyperactivity disorder and intellectual disability. Int. J. Epidemiol. 2021;50:459–474. doi: 10.1093/ije/dyaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amsyari F., Mukarromah N. Reconsideration of early childhood vaccination (an epidemiological study on relationships between vaccination and autism) Folia Med. Indones. 2003;39:102–106. [Google Scholar]
- 6.Blaylock R.L. The danger of excessive vaccination during brain development. Med. Veritas. 2008;5:1727–1741. [Google Scholar]
- 7.Skogheim T.S., Weyde K.V.F., Engel S.M., Aase H., Surén P., Øie M.G., Biele G., Reichborn-Kjennerud T., Caspersen I.H., Horning M., et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021;152:106468. doi: 10.1016/j.envint.2021.106468. [DOI] [PubMed] [Google Scholar]
- 8.Kaur I., Behl T., Aleya L., Rahman M.H., Kumar A., Arora S., Akter R. Role of metallic pollutants in neurodegeneration: Effects of aluminium, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021;28:8989–9001. doi: 10.1007/s11356-020-12255-0. [DOI] [PubMed] [Google Scholar]
- 9.Rosen N.J., Yoshida C.K., Croen L.A. Infection in the first 2 years of life and autism spectrum disorders. Pediatrics. 2007;119:e61–e69. doi: 10.1542/peds.2006-1788. [DOI] [PubMed] [Google Scholar]
- 10.Gondalia S.V., Palombo E.A., Knowles S.R., Austin D.W. Gastrointestinal microbiology in autistic spectrum disorder: A review. Rev. Med. Microbiol. 2010;21:44–50. doi: 10.1097/MRM.0b013e32833a3dc9. [DOI] [Google Scholar]
- 11.Bransfield R.C. Preventable cases of autism: Relationship between chronic infectious diseases and neurological outcome. Pediatric Health. 2009;3:125–140. doi: 10.2217/phe.09.5. [DOI] [Google Scholar]
- 12.Kovtun A.S., Averina O.V., Alekseeva M.G., Danilenko V.N. Antibiotic resistance genes in the gut microbiota of children with autistic spectrum disorder as possible predictors of the disease. Microb. Drug Resist. 2020;26:1307–1320. doi: 10.1089/mdr.2019.0325. [DOI] [PubMed] [Google Scholar]
- 13.Champagne-Jorgensen K., Kunze W.A., Forsythe P., Bienenstock J., McVey Neufeld K.A. Antibiotics and the nervous system: More than just the microbes? Brain Behav. Immun. 2019;77:7–15. doi: 10.1016/j.bbi.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Nitschke A., Deonandan R., Konkle A.T. The link between autism spectrum disorder and gut microbiota: A scoping review. Autism. 2020;24:1328–1344. doi: 10.1177/1362361320913364. [DOI] [PubMed] [Google Scholar]
- 15.Srikantha P., Mohajeri M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019;20:2115. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Settanni C.R., Bibbò S., Ianiro G., Rinninella E., Cintoni M., Mele M.C., Cammarota G., Gasbarrini A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Exp. Rev. Gastroenterol. Hepatol. 2021;15:599–622. doi: 10.1080/17474124.2021.1869938. [DOI] [PubMed] [Google Scholar]
- 17.Lasheras I., Seral P., Latorre E., Barroso E., Gracia-García P., Santabárbara J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatry. 2020;47:101874. doi: 10.1016/j.ajp.2019.101874. [DOI] [PubMed] [Google Scholar]
- 18.De Magistris L., Familiari V., Pascotto A., Sapone A., Frolli A., Iardino P., Carteni M., De Rosa M., Francavilla R., Riegler G., et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol.Nutr. 2010;5:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 19.Muthuirulan P. Leaky Gut Syndrome: Mystery Illness Triggered by Candida albicans. J. Nutr. Health Food Eng. 2016;4:448–449. doi: 10.15406/jnhfe.2016.04.00133. [DOI] [Google Scholar]
- 20.Jyonouchi H. Food allergy and autism spectrum disorders: Is there a link? Curr. Allergy Asthma. Rep. 2009;9:194–201. doi: 10.1007/s11882-009-0029-y. [DOI] [PubMed] [Google Scholar]
- 21.Jyonouchi H. Autism spectrum disorders and allergy: Observation from a pediatric allergy/immunology clinic. Exp. Rev. Clin. Immunol. 2010;6:397–411. doi: 10.1586/eci.10.18. [DOI] [PubMed] [Google Scholar]
- 22.Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristiano C., Lama A., Lembo F., Mollica M.P., Calignano A., MattaceRaso G. Interplay between peripheral and central inflammation in autism spectrum disorders: Possible nutritional and therapeutic strategies. Front. Physiol. 2018;9:184. doi: 10.3389/fphys.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jyonouchi H., Geng L. Associations between monocyte and T cell cytokine profiles in autism spectrum disorders: Effects of dysregulated innate immune responses on adaptive responses to recall antigens in a subset of ASD children. Int. J. Mol. Sci. 2019;20:4731. doi: 10.3390/ijms20194731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matta S.M., Hill-Yardin E.L., Crack P.J. The influence of neuroinflammation in autism spectrum disorder. Brain Behav. Immun. 2019;79:75–90. doi: 10.1016/j.bbi.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Siniscalco D., Schultz S., Brigida A.L., Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018;11:56. doi: 10.3390/ph11020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doernberg E., Hollander E. Neurodevelopmental disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016;21:295–299. doi: 10.1017/S1092852916000262. [DOI] [PubMed] [Google Scholar]
- 28.El-Ansary A. GABA and glutamate imbalance in autism and their reversal as novel hypothesis for effective treatment strategy. Autism Dev. Disord. 2020;18:46–63. doi: 10.17759/autdd.2020180306. [DOI] [Google Scholar]
- 29.Márquez-Caraveo M.E., Ibarra-González I., Rodríguez-Valentín R., Ramírez-García M.Á., Pérez-Barrón V., Lazcano-Ponce E., Vela-Amieva M. Brief report: Delayed diagnosis of treatable inborn errors of metabolism in children with autism and other neurodevelopmental disorders. J. Autism Dev. Disord. 2021;51:2124–2131. doi: 10.1007/s10803-020-04682-2. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Gutierrez E., Narbad A., Rodríguez J.M. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front. Neurosci. 2020;14:1072. doi: 10.3389/fnins.2020.578666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guissart C., Latypova X., Rollier P., Khan T.N., Stamberger H., McWalter K., Cho M.T., Kjaergaard S., Weckhuysen S., Lesca G., et al. Dual molecular effects of dominant RORA mutations cause two variants of syndromic intellectual disability with either autism or cerebellar ataxia. Am. J. Hum. Genet. 2018;102:744–759. doi: 10.1016/j.ajhg.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melke J. Autism: Which genes are involved. Clin. Neuropsychiat. 2008;5:62–69. [Google Scholar]
- 33.Williams E.L., Casanova M.F., Switala A.E., Li H., Qiu M. Transposable elements occur more frequently in autism-risk genes: Implications for the role of genomic instability in autism. Translat. Neurosci. 2013;4:172–202. doi: 10.2478/s13380-013-0113-6. [DOI] [Google Scholar]
- 34.Risch N., Hoffmann T.J., Anderson M., Croen L.A., Grether J.K., Windham G.C. Familial recurrence of autism spectrum disorder: Evaluating genetic and environmental contributions. Am. J. Psychiatry. 2014;171:1206–1213. doi: 10.1176/appi.ajp.2014.13101359. [DOI] [PubMed] [Google Scholar]
- 35.Eshraghi R.S., Deth R.C., Mittal R., Aranke M., Kay S.I.S., Moshiree B., Eshraghi A.A. Early disruption of the microbiome leading to decreased antioxidant capacity and epigenetic changes: Implications for the rise in autism. Front. Cell Neurosci. 2018;12:256. doi: 10.3389/fncel.2018.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loomes R., Hull L., Mandy W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psych. 2017;56:466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 37.El-Ansary A., Bhat R.S., Zayed N. Gut microbiome and sex bias in autism spectrum disorders. Curr. Behav. Neurosci. Rep. 2020;7:22–31. doi: 10.1007/s40473-020-00197-3. [DOI] [Google Scholar]
- 38.Dhaliwal K.K., Orsso C.E., Richard C., Haqq A.M., Zwaigenbaum L. Risk factors for unhealthy weight gain and obesity among children with autism spectrum disorder. Int. J. Mol. Sci. 2019;20:3285. doi: 10.3390/ijms20133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguera M. Human gut microbiome metabolism and autism spectrum disorder. J. Agric. Life Sci. 2020;7:2. doi: 10.30845/jals.v7n2a3. [DOI] [Google Scholar]
- 40.McElhanon B.O., McCracken C., Karpen S., Sharp W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 41.Gondalia S.V., Palombo E.A., Knowles S.R., Cox S.B., Meyer D., Austin D.W. Molecular characterization of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012;5:419–427. doi: 10.1002/aur.1253. [DOI] [PubMed] [Google Scholar]
- 42.Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J. Gastroenterol. 2015;21:6817–6819. doi: 10.3748/wjg.v21.i22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flint H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012;70:S10–S13. doi: 10.1111/j.1753-4887.2012.00499.x. [DOI] [PubMed] [Google Scholar]
- 44.Parracho H.M., Bingham M.O., Gibson G.R., McCartney A.L. Differences between gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 45.Williams B.L., Hornig M., Buie T., Bauman M.L., Cho Paik M., Wick I., Bennett A., Jabado O., Hirschberg D.L., Lipkin W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strati F., Cavalieri D., Albanese D., De Felice C., Donati C., Hayek J., Jousson O., Leoncini S., Renzi D., Calabro A., et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5:24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finegold S.M., Dowd S.E., Gontcharova V., Liu C., Henley K.E., Wolcott R.D., Youn E., Summanen P.H., Granpeesheh D., Dixon D., et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Finegold S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses. 2011;77:270274. doi: 10.1016/j.mehy.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Plaza-Díaz J., Gómez-Fernández A., Chueca N., Torre-Aguilar M.J.D.L., Gil Á., Perez-Navero J.L., Flores-Rojas K., Martin-Borreguero P., Solis-Urra P., Ruiz-Ojeda F.J., et al. Autism spectrum disorder (ASD) with and without mental regression is associated with changes in the fecal microbiota. Nutrients. 2019;11:337. doi: 10.3390/nu11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin V.K., Yong V.C., Chong P.P., Amin Nordin S., Basir R., Abdullah M. Mycobiome in the gut: A multiperspective review. Med. Inflamm. 2020;2020:9560684. doi: 10.1155/2020/9560684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erdogan A., Rao S.S. Small intestinal fungal overgrowth. Curr. Gastroenterol. Rep. 2015;17:16. doi: 10.1007/s11894-015-0436-2. [DOI] [PubMed] [Google Scholar]
- 52.Zou R., Wang Y., Duan M., Guo M., Zhang Q., Zheng H. Dysbiosis of gut fungal microbiota in children with autism spectrum disorders. J. Autism Dev. Disord. 2021;51:267–275. doi: 10.1007/s10803-020-04543-y. [DOI] [PubMed] [Google Scholar]
- 53.Tiew P.Y., Mac Aogain M., Ali N.A.T.B.M., Thang K.X., Goh K., Lau K.J., Chotirmall S.H. The mycobiome in health and disease: Emerging concepts, methodologies and challenges. Mycopathologia. 2020;185:207–231. doi: 10.1007/s11046-019-00413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pappas P.G., Rex J.H., Sobel J.D., Filler S.G., Dismukes W.E., Walsh T.J., Edwards J.E. Guidelines for treatment of candidiasis. Clin. Inf. Dis. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 55.Patel J., Kang D.W., Adams J., Krajmalnik-Brown R. Undergraduate Thesis. Honors College at Arizona State University; Tempe, AZ, USA: 2018. Analysis of Yeast and Fungi in Children with ASD vs. Neurotypical Controls. [Google Scholar]
- 56.Shaw W., Baptist J., Geenens D. Immunodeficiency, gastrointestinal Candidiasis, wheat and dairy sensitivity, abnormal urine arabinose, and autism: A case study. N. Am. J. Med. Sci. 2010;3:1. doi: 10.7156/v3i1p001. [DOI] [Google Scholar]
- 57.Ahmed S.A.S., Meheissen M.A., Azouz H.G., Ashry M.H., Roshdy Y.S., Gad H.A., Ibrahim A.E. Study of Candida species in stool of children with autism spectrum disorders in Alexandria, Egypt. Microbiol. Res. J. Int. 2017;18:1–10. doi: 10.9734/MRJI/2017/32117. [DOI] [Google Scholar]
- 58.Gondalia S.V., Palombo E.A., Knowles S.R., Austin D.W. Faecal microbiota of individuals with autism spectrum disorder. E-J. Appl. Psychol. Clin. Soc. Issues. 2010;6:24–29. doi: 10.7790/ejap.v6i2.213. [DOI] [Google Scholar]
- 59.Iovene M.R., Bombace F., Maresca R., Sapone A., Iardino P., Picardi A., Marotta R., Schiraldi C., Siniscalco D., Serra N., et al. Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia. 2017;182:349–363. doi: 10.1007/s11046-016-0068-6. [DOI] [PubMed] [Google Scholar]
- 60.Adams J.B., Johansen L.J., Powell L.D., Quig D., Rubin R.A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;16:11–22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kantarcioglu A.S., Kiraz N., Aydin A. Microbiota–gut–brain axis: Yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia. 2016;181:1–7. doi: 10.1007/s11046-015-9949-3. [DOI] [PubMed] [Google Scholar]
- 62.Jendraszak M., Gałęcka M., Kotwicka M., Regdos A., Pazgrat-Patan M., Andrusiewicz M. Could selected gut microorganisms be diagnostic biomarkers for autism spectrum disorders? Study based on a commercial microbiota test. Res. Square. 2021:1–19. doi: 10.21203/rs.3.rs-396991/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emam A.M., Mamdouh E., Abdelrahim S. Candida albicans infection in autism. J. Am. Sci. 2012;8:739–744. [Google Scholar]
- 64.El-Shouny W.A., Ismail S., Elzawawy N., Hegazy S. Efficacy of herbal control of the yeasts isolated from autistic children. GJBAHS. 2016;5:65–73. [Google Scholar]
- 65.Lo H.J., Kohler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 66.Carlisle P.L., Banerjee M., Lazzell A., Monteagudo C., López-Ribot J.L., Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc. Natl. Acad. Sci. USA. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toenjes K.A., Munsee S.M., Ibrahim A.S., Jeffrey R., Edwards J.E., Jr., Johnson D.I. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 2005;49:963–972. doi: 10.1128/AAC.49.3.963-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bar-Yosef H., Vivanco Gonzalez N., Ben-Aroya S., Kron S.J. Chemical inhibitors of Candida albicans hyphal morphogenesis target endocytosis. Sci. Rep. 2017;7:5692. doi: 10.1038/s41598-017-05741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colombo A.L., Padovan A.C.B., Chaves G.M. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011;24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laswi I., Shafiq A., Al-Ali D., Burney Z., Pillai K., Salameh M., Mhaimeed N., Zakaria D., Chaari A., Yousri N.A., et al. A comparative pilot study of bacterial and fungal dysbiosis in neurodevelopmental disorders and gastrointestinal disorders: Commonalities, specificities and correlations with lifestyle. Microorganisms. 2021;9:741. doi: 10.3390/microorganisms9040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaw W., Kassen E., Chaves E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin. Chem. 1995;41:1094–1104. doi: 10.1093/clinchem/41.8.1094. [DOI] [PubMed] [Google Scholar]
- 72.Kałużna-Czaplińska J., Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrients. 2012;28:124–126. doi: 10.1016/j.nut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Noto A., Fanos V., Barberini L., Grapov D., Fattuoni C., Zaffanello M., Casanova A., Fenu G., De Giacomo A., De Angelis M., et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J.Matern.-Fetal Neonatal Med. 2014;27:46–52. doi: 10.3109/14767058.2014.954784. [DOI] [PubMed] [Google Scholar]
- 74.Lord R.S., Burdette C.h.K., Bralley J.A. Urinary markers of yeast overgrowth. Integ. Med. 2004;3:24–29. [Google Scholar]
- 75.Becker J., Boles E. A modified Saccharomyces cerevisiae strain that consumes L-Arabinose and produces ethanol. Appl. Environ. Microbiol. 2003;69:4144–4150. doi: 10.1128/AEM.69.7.4144-4150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richard P., Verho R., Putkonen M., Londesborough J., Penttila M. Production of ethanol from L-arabinose by Saccharomyces cerevisiae containing a fungal L-arabinose pathway. FEM Yeast Res. 2003;3:185–189. doi: 10.1016/S1567-1356(02)00184-8. [DOI] [PubMed] [Google Scholar]
- 77.Gutierrez D., Weinstock A., Antharam V.C., Gu H., Jasbi P., Shi X., Dirks B., Krajmalnik-Brown R., Maldonado J., Guinan J., et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2020;96:fiz187. doi: 10.1093/femsec/fiz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lord R.S. Urinary markers of intestinal yeast. Townsend Lett. Dr. Patients. 2003;245:96–97. [Google Scholar]
- 79.Fonseca A. Utilization of tartaric acid and related compounds by yeasts: Taxonomic implications. Can. J. Microbiol. 1992;38:1242–1251. doi: 10.1139/m92-205. [DOI] [PubMed] [Google Scholar]
- 80.Fonseca A., Fell J.W., Kurtzman C.P., Spencer-Martins I. Candida tartarivorans sp. nov., an anamorphic ascomycetous yeast with the capacity to degrade L(+)- and meso-tartaric acid. Int. J. Syst.Evol. Microbiol. 2000;50:389–394. doi: 10.1099/00207713-50-1-389. [DOI] [PubMed] [Google Scholar]
- 81.Markova N. Dysbiotic microbiota in autistic children and their mothers: Persistence of fungal and bacterial wall-deficient L-form variants in blood. Sci. Rep. 2019;9:13401. doi: 10.1038/s41598-019-49768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hughes H.K., Ashwood P. Anti-Candida albicans IgG antibodies in children with autism spectrum disorders. Front. Psychiatry. 2018;9:627. doi: 10.3389/fpsyt.2018.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janeway C.J., Travers P., Walport M. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science; New York, NY, USA: 2001. The Distribution and Functions of Immunoglobulin Isotypes. [Google Scholar]
- 84.Rabalais G.P., Samiec T.D., Bryant K.K., Lewis J. Invasive candidiasis in infants weighing more than 2500 grams at birth admitted to a neonatal intensive care unit. Pediatr. Infect. Dis. J. 1996;15:348–352. doi: 10.1097/00006454-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Rimland B. Candida-caused autism. Autism Res. Rev.Inern. 1988;2:3. [Google Scholar]
- 86.Crook W.G. Yeast can affect behavior and learning. Acad.Ther. 1984;19:517–526. doi: 10.1177/105345128401900501. [DOI] [Google Scholar]
- 87.Carter C.J. Autism genes and the leukocyte transcriptome in autistic toddlers relate to pathogen interactomes, infection and the immune system. A role for excess neurotrophic sAPPα and reduced antimicrobial Aβ. Neurochem. Int. 2019;126:36–58. doi: 10.1016/j.neuint.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Colina A.R., Aumont F., Deslauriers N., Belhumeur P., de Repentigny L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Inf. Immun. 1996;64:4514–4519. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noverr M.C., Huffnagle G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004;72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.García C., Tebbji F., DaigneaultMLiu N.N., Kohler J.R., Allen-Vercoe E., Sellam A. The human gut microbial metabolome modulates fungal growth via the TOR signalling pathway. mSphere. 2017;2:e00555-17. doi: 10.1128/mSphere.00555-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliev I.D., Leonardi I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017;17:635–646. doi: 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumamoto C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011;14:386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wheeler M.L., Limon J.J., Underhill D.M. Immunity to commensal fungi: Detente and disease. Annu. Rev. Pathol. 2017;12:359–385. doi: 10.1146/annurev-pathol-052016-100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roth S., Ruland J. Caspase recruitment domain-containing protein 9 signalling in innate immunity and inflammation. Trends Immunol. 2013;34:243–250. doi: 10.1016/j.it.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Czakai K., Leonhardt I., Dix A., Bonin M., Linde J., Einsele H., Kurzai O., Loeffler J. Kruppel-like Factor 4 modulates interleukin-6 release in human dendritic cells after in vitro stimulation with Aspergillus fumigatus and Candida albicans. Sci. Rep. 2016;6:27990. doi: 10.1038/srep27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conti H.R., Gaffen S.L. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moorlag S., Roring R.J., Joosten L.A.B., Netea M.G. The role of the interleukin-1 family in trained immunity. Immunol. Rev. 2018;281:28–39. doi: 10.1111/imr.12617. [DOI] [PubMed] [Google Scholar]
- 98.Russo A.J., Krigsman A., Jepson B., Wakefield A. Low serum myeloperoxidase in autistic children with gastrointestinal disease. Clin. Exp. Gastroenterol. 2009;2:85–94. doi: 10.2147/CEG.S6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehrer R.I., Cline M.J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: The role of myeloperoxidase in resistance to Candida infection. J. Clin.Investig. 1969;48:1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 1998;76:676–681. doi: 10.1007/s001090050267. [DOI] [PubMed] [Google Scholar]
- 101.Ceylan M.F., Hesapcioglu S.T., Yavas C.P., Senat A., Erel O. Serum ischemia-modified albumin levels, myeloperoxidase activity and peripheral blood mononuclear cells in autism spectrum disorder (ASD) J. Autism Dev. Disord. 2012;51:2511–2517. doi: 10.1007/s10803-020-04740-9. [DOI] [PubMed] [Google Scholar]
- 102.Topal Z., Tufan A.E., Karadag M., Gokcen C., Akkaya C., Sarp A.S., Bahsi I., Kilinc M. Evaluation of peripheral inflammatory markers, serum B12, folate, ferritin levels and clinical correlations in children with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) Nordic. J. Psychiatry. 2021:1–8. doi: 10.1080/08039488.2021.1946712. [DOI] [PubMed] [Google Scholar]
- 103.Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Stanzani M., Orciuolo E., Lewis R., Kontoyiannis D.P., Martins S.L., St John L.S., Komanduri K.V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood. 2005;105:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- 105.Hubmann R., Hilgarth M., SchnablSPonath E., Reiter M., Demirtas D., Sieghart W., Valent P., Zielinski Ch Jager U., Shehata M. Gliotoxin is a potent NOTCH 2 transactivation inhibitor and efficiently induces apoptosis in chronic lymphocytic leukaemia (CLL) cells. Br. J. Haematol. 2013;160:618–629. doi: 10.1111/bjh.12183. [DOI] [PubMed] [Google Scholar]
- 106.Shah D., Larsen B. Clinical isolates of yeast produce a gliotoxin-like substance. Mycopathologia. 1991;116:203–208. doi: 10.1007/BF00436836. [DOI] [PubMed] [Google Scholar]
- 107.Podzorski R., Herron M., Fast D., Nelson R. Pathogenesis of candidiasis immunosuppression by cell wall mannan catabolites. Arch. Surg. 1989;124:1290–1294. doi: 10.1001/archsurg.1989.01410110044009. [DOI] [PubMed] [Google Scholar]
- 108.Witkin S.S. Defective immune responses in patients with recurrent candidiasis. Infect. Med. 1985:129–132. [Google Scholar]
- 109.Ho J., Yang X., Nikou S.A., Kichik N., Donkin A., Ponde N.O., Richardson J.P., Gratacap R.L., Archambault L.S., Zwirner C.P., et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019;10:2297. doi: 10.1038/s41467-019-09915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naglik J.R., Gaffen S.L., Hube B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019;52:100–109. doi: 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hornby J.M., Jensen E.C., Lisec A.D., Tasto J.J., Jahnke B., Shoemaker R., Dussault P., Nickerson K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kasper L., Konig A., Koenig P.A., Gresnigt M.S., Westman J., Drummond R.A., Lionakis M.S., Gross O., Ruland J., Naglik J.R., et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018;9:4260. doi: 10.1038/s41467-018-06607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogiers O., Frising U.C., Kucharikova S., Jabra-Rizk M.A., van Loo G., Van Dijck P., Wullaert A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. MBio. 2019;10:e02221-18. doi: 10.1128/mBio.02221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonhardt I., Spielberg S., Weber M., Albrecht-Eckardt D., Bläss M., Claus R., Barz D., Scherlach K., Hertweck C., Löffler J., et al. The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity. mBio. 2015;6:e00143-15. doi: 10.1128/mBio.00143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noverr M.C., Phare S.M., Toews G.B., Coffey M.J., Huffnagle G.B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim Y.G., Udayanga K.G., Totsuka N., Weinberg J.B., Nunez G., Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2) Cell Host Microbe. 2014;15:95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan T.G., Lim Y.S., Tan A., Leong R., Pavelka N. Fungal symbionts produce prostaglandin E2 to promote their intestinal colonization. Front. Cell Infect. Microbiol. 2019;9:359. doi: 10.3389/fcimb.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burrus C.J. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med. Hypothesis. 2012;79:784–785. doi: 10.1016/j.mehy.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 119.Denaro F.J., López-Ribot J.L., Chaffin W.L. Adhesion of Candida albicans to brain tissue of Macaca mulatta in an ex vivo assay. Infect. Immun. 1995;63:3438–3441. doi: 10.1128/iai.63.9.3438-3441.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jong A.Y., Stins M.F., Huang S.H., Chen S.H.M., Kim K.S. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect Immun. 2001;69:4536–4544. doi: 10.1128/IAI.69.7.4536-4544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Navarathna D.H., Munasinghe J., Lizak M.J., Nayak D., McGavern D.B., Roberts D.D. MRI confirms loss of blood–brain barrier integrity in a mouse model of disseminated candidiasis. NMR Biomed. 2013;26:1125–1134. doi: 10.1002/nbm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ophelders D.R., Gussenhoven R., Lammens M., Küsters B., Kemp M.W., Newnham J.P., Payne M.S., Kallapur S.G., Jobe A.H., Zimmermann L.J., et al. Neuroinflammation and structural injury of the fetal ovine brain following intra-amniotic Candida albicans exposure. J. Neuroinflamm. 2016;13:1–12. doi: 10.1186/s12974-016-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Masi A., Quintana D.S., Glozier N., Lloyd A.R., Hickie I.B., Guastella A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry. 2015;20:440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- 124.Herman A.P., Bochenek J., Król K., Krawczyńska A., Antushevich H., Pawlina B., Herman A., Romanowicz K., Tomaszewska-Zaremba D. Central Interleukin-1β suppresses the nocturnal secretion of melatonin. Med. Inflamm. 2016;2016:2589483. doi: 10.1155/2016/2589483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skelly D.T., Griffin E.W., Murray C.L., Harney S., O’Boyle C., Hennessy E., Dansereau M.A., Nazmi A., Tortorelli L., Rawlins J.N., et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatry. 2019;24:1533–1548. doi: 10.1038/s41380-019-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Griffiths J.S., Camilli G., Kotowicz N.K., Ho J., Richardson J.P., Naglik J.R. Role for IL-1 family cytokines in fungal infections. Front. Microbiol. 2021;12:633047. doi: 10.3389/fmicb.2021.633047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reichelt K., Knivsberg A. The possibility and probability of a gut-to-brain connection in autism. Am. Clin. Psychiatry. 2009;21:205–211. [PubMed] [Google Scholar]
- 128.Dhossche D., Applegate H., Abraham A., Maertens P., Bland L., Bencsath A., Martinez J. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: Stimulus for a GABA hypothesis of autism. Med. Sci. Monitor. 2002;8:PR1–PR6. [PubMed] [Google Scholar]
- 129.Fatemi S.H., Reutiman T.J., Folsom T.D., Thuras P.D. GABA A receptor downregulation in brains of subjects with autism. J. Autism Dev. Disord. 2009;39:223. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muller C.L., Anacker A.M., Veenstra-Vander Weele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016;321:24–41. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Connors S.L., Matteson K.J., Sega G.A., Lozzio C.B., Carroll R.C., Zimmerman A.W. Plasma serotonin in autism. Pediatr. Neurol. 2006;35:182–186. doi: 10.1016/j.pediatrneurol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 132.Cook E.H., Leventhal B.L. The serotonin system in autism. Curr. Opin. Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 133.Marler S., Ferguson B.J., Lee E.B., Peters B., Williams K.C., McDonnell E., Macklin E.A., Levitt P., Gillespie C.H., Anderson G.M., et al. Brief report: Whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J. Autism Dev. Disord. 2016;46:1124–1130. doi: 10.1007/s10803-015-2646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Doenyas C. Dietary interventions for autism spectrum disorder: New perspectives from the gut-brain axis. Physiol. Behav. 2018;194:577–582. doi: 10.1016/j.physbeh.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 135.Yang J., Fu X., Liao X., Li Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2020;2020:113471. doi: 10.1016/j.psychres.2020.113471. [DOI] [PubMed] [Google Scholar]
- 136.Volkert V.M., Vaz P.C.M. Recent studies on feeding problems in children with autism. J. Appl.Behav. Anal. 2010;43:155–159. doi: 10.1901/jaba.2010.43-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharp W.G., Berry R.C., McCracken C., Nuhu N.N., Marvel E., Saulnier C.A., Klin A., Jones W., Jaquess D.L. Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. J. Autism Dev. Disord. 2013;43:2159–2173. doi: 10.1007/s10803-013-1771-5. [DOI] [PubMed] [Google Scholar]
- 138.Vissoker R.E., Latzer Y., Gal E. Eating and feeding problems and gastrointestinal dysfunction in Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2015;12:10–21. doi: 10.1016/j.rasd.2014.12.010. [DOI] [Google Scholar]
- 139.Lam S., Zuo T., Ho M., Chan F.K.L., Chan P.K.S., Ng S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019;50:1159–1171. doi: 10.1111/apt.15523. [DOI] [PubMed] [Google Scholar]
- 140.Pan C., Hoffmann C., Dollive S., Grunberg S., Chen J., Li H., Wu G.D., Lewis J.D., Bushman F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PloS ONE. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jacobs G., Kjaer J. Beat Candida through Diet: A Complete Dietary Programme for Suffers of Candidiasis. Random House; New York, NY, USA: 2012. [Google Scholar]
- 142.Romeo M.G., Romeo D.M., Trovato L., Oliveri S., Palermo F., Cota F., Betta P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011;31:63–69. doi: 10.1038/jp.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu J., Wan G.B., Huang M.S., Agyapong G., Zou T.L., Zhang X.Y., Liu Y.W., Song Y.Q., Tsai Y.C., Kong X.J. Probiotic therapy for treating behavioral and gastrointestinal symptoms in autism spectrum disorder: A systematic review of clinical trials. Curr. Med. Sci. 2019;39:173–184. doi: 10.1007/s11596-019-2016-4. [DOI] [PubMed] [Google Scholar]
- 144.MacAlpine J., Daniel-Ivad M., Liu Z., Yano J., Revie N.M., Todd R.T., Stogios P.J., Sanchez H., O’Meara T.R., Tompkins T.A., et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021;12:6151. doi: 10.1038/s41467-021-26390-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murzyn A., Krasowska A., Stefanowicz P., Dziadkowiec D., Łukaszewicz M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE. 2010;5:e12050. doi: 10.1371/journal.pone.0012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hemarajata P., Versalovic J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaw W., Kassen E., Chaves E. Assessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometry. Clin. Pract. Altern. Med. 2000;1:15–26. [Google Scholar]
- 148.Zimmerman B., Weber E. Candida and “20th-century disease”. CMAJ Can. Med. Assoc. J. 1985;133:965. [PMC free article] [PubMed] [Google Scholar]
- 149.Shareck J., Belhumeur P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryotic Cell. 2011;10:1004–1012. doi: 10.1128/EC.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan Q., Orsso C.E., Deehan E.C., Kung J.Y., Tun H.M., Wine E., Madsen K.L., Zwaigenbaum L., Haqq A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021;14:1820–1836. doi: 10.1002/aur.2560. [DOI] [PubMed] [Google Scholar]
- 151.Kang D.W., Adams J.B., Coleman D.M., Pollard E.L., Maldonado J., McDonough-Means S., Caporaso J.G., Krajmalnik-Brown R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 2019;9:5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan D., Coughlin L.A., Neubauer M.M., Kim J., Kim M.S., Zhan X., Simms-Waldrip T.R., Xie Y., Hooper L.V., Koh A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Akagawa G., Abe S., Yamaguchi H. Mortality of Candida albicans-infected mice is facilitated by superinfection of Escherichia coli or administration of its lipopolysaccharide. J. Infect. Dis. 1995;171:1539–1544. doi: 10.1093/infdis/171.6.1539. [DOI] [PubMed] [Google Scholar]
- 154.Zhao H., Gao X., Xi L., Shi Y., Peng L., Wang C., Zou L., Yang Y. Mo1667 fecal microbiota transplantation for children with an autism spectrum disorder. Gastrointest. Endoscop. 2019;89:AB512–AB513. doi: 10.1016/j.gie.2019.03.857. [DOI] [Google Scholar]
- 155.Kang D.W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A., Khoruts A., Geis E., Maldonado J., McDonough-Means S., et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017;5:1–16. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leonardi I., Paramsothy S., Doron I., Semon A., Kaakoush N.O., Clemente J.C., Faith J.J., Borody T.J., Mitchell H.M., Colombel J.F., et al. Fungal trans-kingdom dynamics linked to responsiveness to fecal microbiota transplantation (FMT) therapy in ulcerative colitis. Cell Host Microbe. 2020;27:823–829. doi: 10.1016/j.chom.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mullish B.H., Quraishi M.N., Segal J.P., McCune V.L., Baxter M., Marsden G.L., Moore D.J., Colville A., Bhala N., Iqbal T.H., et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67:1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 158.Cheng Y.W., Phelps E., Ganapini V., Khan N., Ouyang F., Xu H., Khanna S., Tariq R., Friedman-Moraco R.J., Woodworth M.H., et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am. J. Transplant. 2019;19:501–511. doi: 10.1111/ajt.15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan X., Johnson S. Fecal microbiota transplantation (FMT) for C. difficile infection, just say ‘No’. Anaerobe. 2019;60:102092. doi: 10.1016/j.anaerobe.2019.102092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.