Key Points
Question
Is substantial reduction in elective radiotherapy dose and volume associated with adequate locoregional tumor control in patients with human papillomavirus–associated oropharyngeal carcinoma treated with definitive chemoradiotherapy?
Findings
In this cohort study, reduced radiotherapy volume and dose of 30 Gy to the elective regions with concurrent chemotherapy in 276 patients with locally advanced human papillomavirus–positive oropharyngeal carcinoma was associated with a 24-month locoregional control rate of 97.0% and overall survival of 95.1%.
Meaning
These findings suggest that major de-escalation of radiotherapy in elective regions was feasible while maintaining locoregional tumor control.
This cohort study summarizes 2-year follow-up of patients with human papillomavirus–associated oropharyngeal carcinoma who received radiotherapy to subclinical regions at a reduced dose and target volume of 30 Gy.
Abstract
Importance
Several de-escalation strategies for human papillomavirus (HPV)–associated oropharyngeal carcinoma (OPC) have focused on deintensifying gross disease treatment. Reduction of radiotherapy dose and target volume to subclinical regions may achieve good clinical outcomes with favorable patient quality of life (QOL).
Objective
To determine outcomes from a systematic approach of reducing radiotherapy dose and target volume to the elective treatment regions in patients with HPV-associated OPC undergoing concurrent chemoradiotherapy (CCRT).
Design, Setting, and Participants
This retrospective cohort study included 276 consecutive patients with HPV-positive OPC receiving CCRT from March 1, 2017, to July 31, 2019. Data were analyzed from February 23 to September 13, 2021.
Interventions
Elective nodal and subclinical regions received 30 Gy of radiotherapy in 15 fractions, followed by a cone down of 40 Gy in 20 fractions to gross disease for a total dose of 70 Gy. The high retropharyngeal nodal basins in the node-negative neck and bilateral levels IB and V basins were omitted.
Main Outcomes and Measures
Patients were followed up to evaluate locoregional control as the primary outcome and distant metastasis–free survival, progression-free survival, and overall survival as secondary outcomes. Quality-of-life data were obtained at each visit when feasible.
Results
Among the 276 patients included in the analysis, the median age was 61 (range, 36-87) years; 247 (89.5%) were men; and 183 (66.3%) had less than 10 pack-years of smoking history. Most patients (251 [90.9%]) were White. Overall, 87 (31.5%) had cT3-cT4 disease and 65 (23.5%) had cN2-cN3 disease per the 8th edition of the American Joint Committee on Cancer Staging Manual. One hundred seventy-two patients (62.3%) completed 300-mg/m2 high-dose cisplatin therapy. During a median follow-up of 26 (range, 21-32) months, 8 patients developed locoregional recurrence, including 7 at the primary site or gross nodes that received a total dose of 70 Gy and 1 with a persistent node not previously identified as gross disease that received a total dose of only 30 Gy. The 24-month locoregional control was 97.0%; progression-free survival, 88.0%; distant metastasis–free survival, 95.2%; and overall survival, 95.1%. During treatment, 17 patients (6.2%) required a feeding tube. At 24 months, most of the QOL composite scores (jaw-related problems, pain, social contact, eating, speech, and swallow) were comparable or superior to baseline measures except for senses, dry mouth, muscular tension, and cognitive functioning, which improved over time but remained marginally worse than baseline.
Conclusions and Relevance
This cohort study found that the evaluated de-escalation strategy for elective regions showed favorable clinical outcomes and QOL profiles. Long-term follow-up data will help affirm the efficacy of this strategy as a care option for treating HPV-associated OPC with primary CCRT.
Introduction
Patients with human papillomavirus (HPV)–associated oropharyngeal cancer (OPC) constitute most patients with OPC in the US.1 Preclinical and clinical studies2,3 have demonstrated enhanced sensitivity to chemoradiotherapy in these patients. The standard 7-week chemoradiotherapy course, although effective, leads to considerable treatment-related toxic effects. Standard radiotherapy dose and field for head and neck cancers often included treatment of large elective volumes to cumulative doses of approximately 50 to 60 Gy, whereas grossly evident disease on imaging received a total dose of 70 Gy. These doses were derived from data generated in the era of 3-dimensional conformal radiation,4,5,6,7 and the elective volumes typically targeted regional nodal basins and tissues adjacent to the gross tumor suspected of harboring microscopic disease based on surgical patterns of spread.8
Several clinical trials9,10,11,12,13,14,15 have shown that in patients with select HPV-positive OPC, radiation dose to gross disease can be safely reduced, typically by 10 to 16 Gy. However, many trials did not evaluate radiation dose reduction in elective regions systematically, and a wide range of elective doses were used. Even with the contemporary intensity-modulated radiotherapy technique, elective nodal radiotherapy still contributes significantly to acute and long-term toxic effects to critical organs.16,17,18 Studies of patterns of treatment failure in patients with OPC have shown that most locoregional recurrences occur in the high-dose radiation fields, indicating that it might not be necessary to treat the elective regions with doses of 50 to 60 Gy.19,20,21 Because the effectiveness of elective treatment is measured by failure rate in the treatment field, instead of a strict threshold effect for the treatment of gross disease, past radiation dose range for microscopic disease might not be applicable in the context of modern imaging technology.22,23 Results from 2 recent prospective trials of 40-Gy elective neck volumes24,25 showed uncompromised locoregional control, regardless of anatomical site of disease and HPV status.
Beginning in 2017, we systematically started treating the subclinical regions that have historically been included in the elective treatment volume to a dose of 30 Gy and spared selected negative neck, retropharyngeal, level 1b, and level V nodal basins.26,27,28,29 Herein, we summarize the 2-year follow-up outcomes of patients treated using this regimen.
Methods
Patient Population
Consecutive patients with newly diagnosed, previously untreated HPV-positive OPC receiving definitive concurrent chemoradiotherapy (CCRT) with cisplatin or other chemotherapy from March 1, 2017, to July 31, 2019, were included in this cohort study. The new radiotherapy regimen was not intended for patients treated with concurrent cetuximab. The HPV diagnosis was confirmed by both p16 immunohistochemistry staining and HPV-RNA in situ hybridization. Patients who underwent excisional biopsy of the primary site or lymph nodes with residual gross tumor burden after diagnostic biopsy were included. The institutional review board at Memorial Sloan Kettering Cancer Center approved this study with a waiver of informed consent for a retrospective analysis of patient data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Radiotherapy Dose and Volume Reduction
There were only 2 dose levels for most of the radiotherapy plans: 30 Gy to the elective treatment regions (15 fractions of 2 Gy), followed by a cone down of 40 Gy for a total of 70 Gy to all sites of gross disease, as described previously.29 We used positron emission tomography and computed tomography (CT), CT with contrast if no contraindication, and contrast magnetic resonance imaging to delineate the gross primary tumor and nodal volumes treated with a dose of 70 Gy (GTV70_primary and GTV70_nodes, respectively). We then expanded via anatomical routes of spread of GTV70_primary, typically around 5-mm margins, to derive the planning target volume (PTV70_primary). The PTV70 of gross nodal disease (PTV70_nodes) was obtained by a concentric expansion of 3 to 5 mm from GTV70_nodes. There was no clinical target volume expansion around GTV70_primary or GTV70_nodes. The clinical target volume of 30 Gy (CTV30) encompassed all regions of potential subclinical disease spread (retropharyngeal, retrostyloid, and levels II to IV for node-positive neck; levels II to IV for node-negative neck if receiving radiotherapy). With daily kilovoltage or cone-beam CT imaging, our planning target volume of 30-Gy (PTV30) margins of the elective regions were reduced to 2- to 3-mm expansion from CTV30. We also ensured that PTV30 was at least 1.3 cm from the GTV70_primary. Patients were considered for ipsilateral nodal radiotherapy if they had a well-lateralized T1 tonsil primary tumor with 1 to 2 ipsilateral involved lymph nodes with a diameter of less than 3 cm each. In some instances, indeterminate nodes or areas immediately adjacent to the gross primary disease were treated with a dose of 50 Gy per physician discretion. Treatment techniques were intensity-modulated radiotherapy (256 patients) or intensity-modulated proton therapy (20 patients). Select examples of target volume delineation are shown in the eFigure in the Supplement.
Follow-up Disease Status Assessment
All patients were followed up per our institutional standard of care, typically every 3 months in the first year after completion of CCRT, every 4 to 6 months in the second to fifth year, and yearly thereafter. At these visits, the following assessments were performed: evaluations of toxic effects and history and physical examinations. Evaluations with positron emission tomography–CT, CT, and/or magnetic resonance imaging were typically performed at 3 to 4 months after CCRT and repeated yearly thereafter to year 2 or year 3.
Quality-of-Life Measures
All patients were asked to complete the Gothenburg Trismus Questionnaire (GTQ) at each visit per our departmental routine practice. The GTQ is a comprehensive, self-administered, trismus-specific questionnaire with different aspects of objective measures of 13 items and the following 3 domains: jaw-related problems, eating limitation, and muscular tension. There are an additional 8 single items.30 The GTQ was validated against the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck Module (EORTC QLQ H&N35) as well as the EORTC QLQ Core Questionnaire 30 (EORTC QLQ-C30).30,31 In addition to the GTQ, 54 of the 276 patients also enrolled in a prospective trial of adaptive radiotherapy planning32 during their treatment course to account for disease response and temporal variations in anatomy, with quality of life (QOL) evaluated at each visit using the EORTC QLQ H&N35 and EORTC QLQ-C30.33,34,35
Statistical Analyses
Data were analyzed from February 23 to September 13, 2021. The primary outcome of interest was 24-month locoregional control (LRC). Secondary outcomes included progression-free survival, distant metastasis–free survival, overall survival, and QOL. We used the Kaplan-Meier method to calculate the cumulative incidence of each event and survival rates with death as a competing risk. We evaluated factors associated with LRC using multivariable Cox proportional hazards regression models. All analyses were performed with SAS, version 9.4 (SAS Institute Inc) and SPSS, version 26 (IBM Corporation).
The GTQ was scored by calculating a mean for each domain, which was then transformed to a scale ranging from 0 to 100, where a higher score indicated greater perceived dysfunction.30 For ease of comparison and analysis, the 8 single items were grouped into 4 categories: facial pain, facial pain impact, jaw limitation, and jaw limitation impact. Composite scores of each category were calculated accordingly. Similar to GTQ, composite scores of EORTC QLQ-HN 35 and EORTC QLQ-C30 were calculated according to the EORTC scoring manuals.33,34,35,36
Results
Patient Characteristics and Radiotherapy
Table 1 shows the baseline patient and clinical characteristics for the 276 participants. The median age of the cohort was 61 (range, 36-87) years. Two hundred forty-seven patients were men (89.5%) and 29 were women (10.5%). We included self-reported patient race and ethnicity in the analysis to show the distribution of different racial and ethnic groups in our cohort. Eight patients (2.9%) were Asian, 10 (3.6%) were Black, 1 (0.4%) was Hispanic, 251 (90.9%) were White, and 6 (2.2%) were of unknown race and ethnicity. Half of the patients (135 [48.9%]) were never smokers; 183 (66.3%) had a smoking history of less than 10 pack-years. There was a wide range of disease-stage distribution in the cohort; 87 patients (31.5%) had cT3-cT4 disease and 65 (23.5%) had cN2-cN3 disease per the 8th edition of the American Joint Committee on Cancer Staging Manual. Most patients (259 [93.8%]) had a primary site at either the base of the tongue or the tonsil. Most patients (213 [77.2%]) received high-dose cisplatin, and 172 of 213 patients (80.7%; 62.3% overall) were able to complete a cumulative dose of 300 mg/m2. Other commonly used chemotherapy regimens included weekly carboplatin with paclitaxel (Taxol) or fluorouracil. The new radiotherapy regimen was not intended for patients treated with concurrent cetuximab; however, 5 patients who received concurrent cetuximab were incidentally treated with this regimen and were included in the analysis.
Table 1. Clinical Characteristics of the Study Cohort.
| Characteristic | Dataa |
|---|---|
| Follow-up duration, median (range), mo | 26 (1-47) |
| Age, median (range), y | 61 (36-87) |
| Sex | |
| Male | 247 (89.5) |
| Female | 29 (10.5) |
| Race and ethnicity | |
| Asian | 8 (2.9) |
| Black | 10 (3.6) |
| Hispanic | 1 (0.4) |
| White | 251 (90.9) |
| Unknown | 6 (2.2) |
| Smoking, pack-years | |
| <10 | 183 (66.3) |
| ≥10 | 93 (33.7) |
| Primary site | |
| Base of tongue | 127 (46.0) |
| Tonsil | 132 (47.8) |
| Unknown primary/other | 17 (6.2) |
| T category (AJCC-8) | |
| X | 11 (4.0) |
| 1 | 69 (25.0) |
| 2 | 109 (39.5) |
| 3 | 52 (18.8) |
| 4 | 35 (12.7) |
| N category (AJCC-8) | |
| 0 | 12 (4.3) |
| 1 | 199 (72.1) |
| 2 | 60 (21.7) |
| 3 | 5 (1.8) |
| Overall stage (AJCC-8) | |
| I | 160 (58.0) |
| II | 77 (27.9) |
| III | 39 (14.1) |
| Systemic therapy | |
| Cisplatin | |
| High dose | 213 (77.2) |
| Weekly | 8 (2.9) |
| Carboplatin/paclitaxel (Taxol) | 20 (7.2) |
| Carboplatin/fluorouracil | 14 (5.1) |
| Other | 21 (7.6) |
| Disease status at last follow-up | |
| Alive with disease | 6 (2.2) |
| Dead with disease | 10 (3.6) |
| No evidence of disease | 260 (94.2) |
| Locoregional recurrence | |
| No | 268 (97.1) |
| Yes | 8 (2.9) |
| Distant metastasis | |
| No | 257 (93.1) |
| Yes | 19 (6.9) |
| Vital status | |
| Alive | 259 (93.8) |
| Dead | 17 (6.2) |
Abbreviation: AJCC-8, American Joint Committee on Cancer Staging Manual, 8th edition.
Unless otherwise indicated, data are expressed as number (%) of patients. Percentages have been rounded and may not total 100.
All 276 patients were prescribed a radiation dose of 30 Gy to the elective and subclinical regions and cone down to 70 Gy to the gross disease. In 87 patients (31.5%), indeterminate nodes or areas immediately adjacent to the gross disease were treated with a dose of 50 Gy. Most patients who received an intermediate dose of 50 Gy were treated within the first year after implementing the new dosing regimen before physicians gradually became more comfortable treating the entire elective regions with 30 Gy.
Clinical Outcomes
The median follow-up for surviving patients was 26 (range, 1-47) months. The overall 24-month LRC was 97.0% for the entire cohort, 88.0% for progression-free survival, 95.2% for distant metastasis–free survival, and 95.1% for overall survival (Figure 1). Eight patients (2.9%) developed locoregional recurrence; of these, 7 (87.5%) had disease recurrence in the primary site and/or gross disease in nodes that received a full 70-Gy dose, and 1 patient had persistent gross disease in a node that was not previously identified and received only 30 Gy. One patient with locoregional recurrence also developed a synchronous distant recurrence. Five patients had neck-only recurrence; all these patients underwent successful salvage treatment by neck dissection and were still alive without evidence of disease at the time of last follow-up. There was otherwise no recurrence in the subclinical fields receiving a dose of 30 Gy or in the omitted elective nodal regions. Nineteen patients (6.9%) developed distant-only recurrence.
Figure 1. Survival Outcomes of the Study Cohort From Start of Radiotherapy.
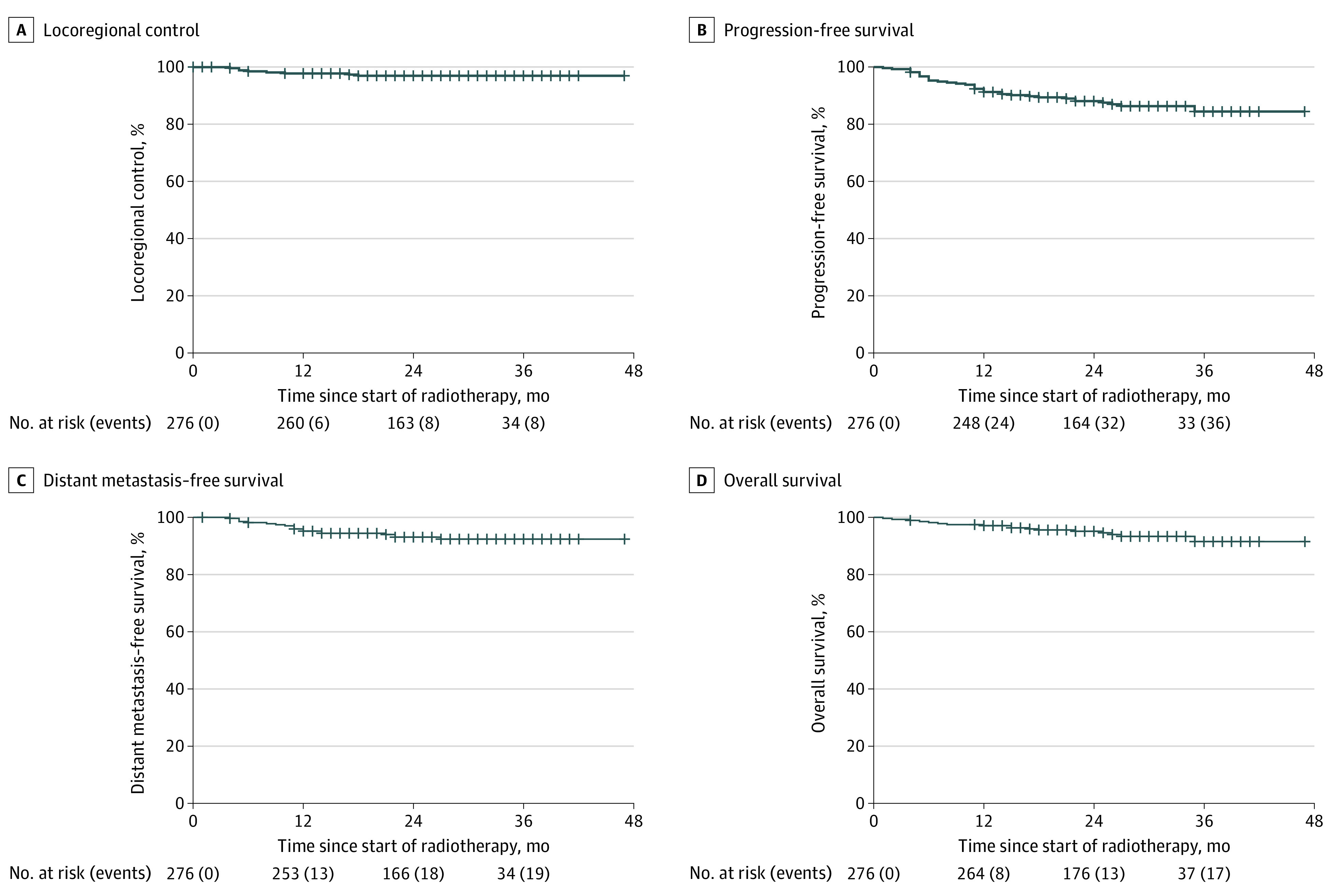
We then performed multivariable Cox proportional hazards regression analyses to evaluate factors associated with LRC. Of all variables included in the proportional hazards regression model (age, disease site, sex, race and ethnicity, smoking history, T category, N category, and chemotherapy), only advanced N category was associated with worse LRC (hazard ratio, 3.4 [95% CI, 1.1-10.6]; P = .03). There was no difference in LRC between patients receiving high-dose cisplatin and weekly cisplatin or other chemotherapy combinations (hazard ratio, 0.8; 95% CI, 0.2-3.5; P = .71). The only patient with treatment failure in the 30-Gy subclinical region received a cumulative cisplatin dose of a 300-mg/m2 bolus.
Toxic Effects and Patient-Reported Outcomes
During treatment, 17 patients (6.2%) had a feeding tube placement, with a median duration of feeding tube dependance of 5 (range, 1-23) months. Four patients had a feeding tube in place at the time of death; 2 of these had locoregional disease recurrence within 6 months after CCRT with feeding tubes still in place and died shortly after, 1 died of complications from treatment, and 1 died of unknown cause. No feeding tube was placed prophylactically in the study cohort.
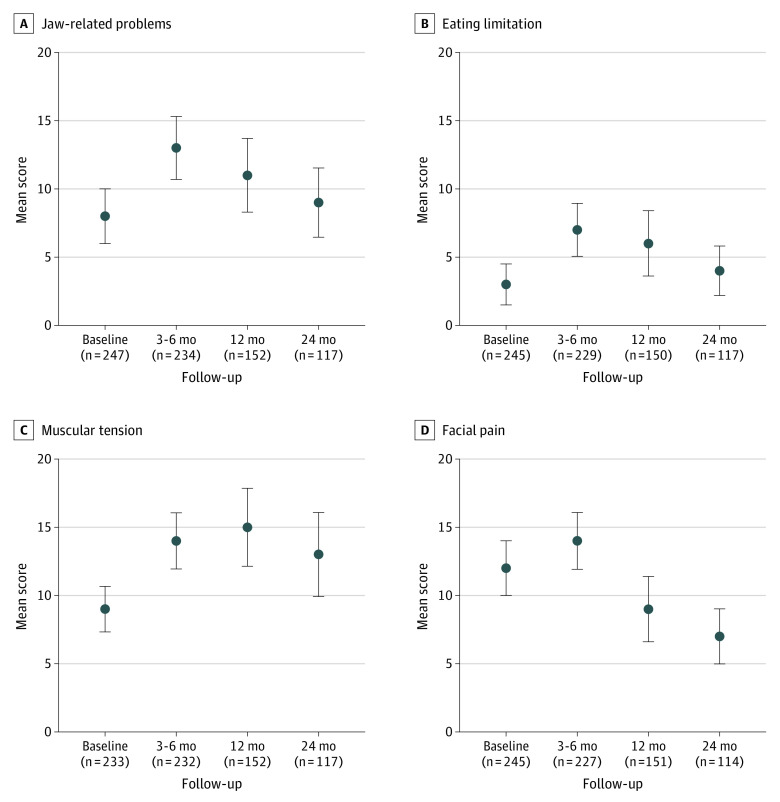
We analyzed GTQ data collected at baseline and at 3 to 6 months, 12 (±3) months, and 24 (±3) months after radiotherapy completion. Figure 2 shows the mean GTQ composite scores of the study participants by symptom domain. Compared with baseline, all domain scores worsened at 3 to 6 months after radiotherapy but improved steadily over time. At 24 months, facial pain scores were notably better than baseline (mean, 7 [95% CI, 5-9] vs 12 [95% CI, 10-14]). All other scores were comparable to baseline, except for the muscular tension scores, which remained marginally worse (mean, 13 [95% CI, 10-16] vs 9 [95% CI, 7-11]).
Figure 2. Patient-Reported Outcomes Measured by Gothenburg Trismus Questionnaire Domains.
Error bars indicate 95% CI.
In addition to GTQ scores, 54 of the 276 patients were also enrolled in a prospective adaptive radiotherapy trial32 with QOL metrics evaluated at baseline and follow-up visits by EORTC QLQ H&N35 and EORTC QLQ-C30 scores, as shown in Figure 3. Dry mouth and global function scores exhibited the most variations after treatment. At 24 months, most of the QOL composite scores (pain, social contact, social eating, speech, and swallow) were comparable or superior to baseline measures except for senses (taste), dry mouth, and cognitive functioning.
Figure 3. Patient-Reported Outcomes Measured by the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) Scores.
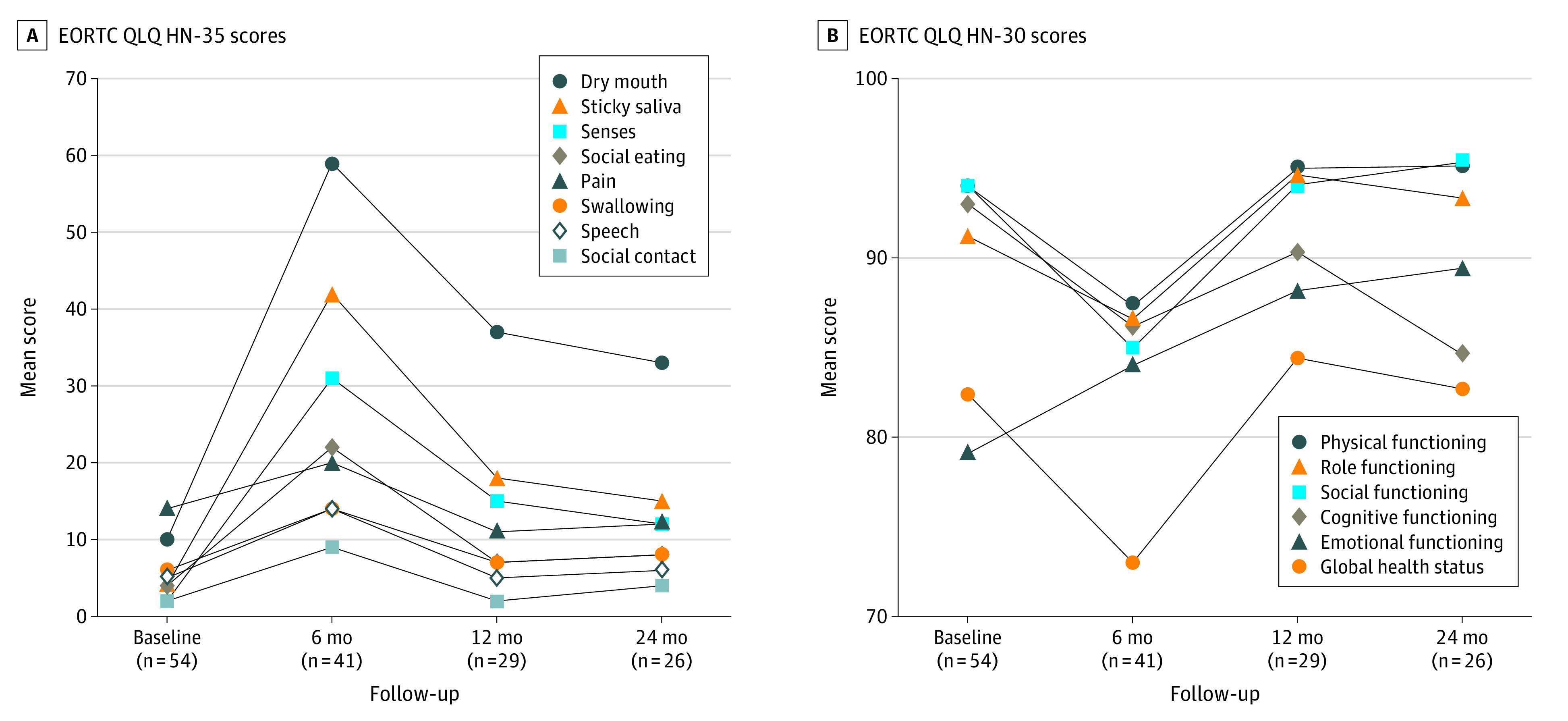
Higher scores indicate greater perceived dysfunction for EORTC QLQ HN-35 but better global functional status for EORTC QLQ-30. EORTC QLQ H&N35 indicates Head and Neck Module; EORTC QLQ-C30, Core Questionnaire 30.
Discussion
In this retrospective cohort study, we demonstrated uncompromised disease control and favorable toxic effect profiles using a systematic approach to reduce radiation dose and volume to elective nodal regions in patients with HPV-positive OPC undergoing CCRT. Despite 31.5% of patients having cT3-cT4 disease, the 2-year LRC, progression-free survival, and overall survival rates remained high. Only 1 patient had a locoregional failure in the elective fields treated with a 30-Gy dose. Only 17 patients (6.2%) required feeding tube placement during and after completion of chemoradiotherapy. Most patients were able to receive a full 3 cycles of high-dose cisplatin. The QOL analysis of the study cohort showed that most of the composite scores were comparable or even superior to baseline measures.
Clinical and toxic effects outcomes from this analysis are equivalent or superior to those of other similar trials,9,10,11,12,13,14,15,24,25 many of which used a lower dose to gross disease but a higher dose to elective regions, as summarized in Table 2. Many of the comparable trials also had wider radiotherapy elective nodal coverage and target volume margins. For example, the NRG HN002 trial for p16-positive OPC9 used a reduced dose of 60 Gy to gross disease but used a dose of 54 Gy to high-risk subclinical sites and 44 to 48 Gy to low-risk nodal levels, with mandatory coverage of ipsilateral neck nodal levels Ib and V and a total of 0.8- to 1.0-cm expansion from GTV to PTV. Similarly, the dose de-escalation trial for p16-positive OPC conducted by Chera et al14 also used a dose of 60 Gy to gross disease and 54 Gy to subclinical nodal volumes with coverage of ipsilateral nodal levels Ib and V. The high dose and volume to the elective nodal regions might have in part contributed to the elevated feeding tube rates of 21.7% and 34%, respectively, in these studies. Notably, our cohort had the lowest rate of feeding tube use among patients treated with definitive CCRT.
Table 2. Radiation Doses to Gross Tumor and Subclinical Regions in Published Trials of Treatment Deintensification.
| Source | Dose to gross tumor/tumor bed, Gy | Dose to subclinical regions, Gy | Outcome, % of patients | ||
|---|---|---|---|---|---|
| 2-y LRC | 2-y PFS | Use of feeding tube | |||
| Gillison et al,13 2019 (RTOG 10-16) | 70 | 50-56 | 95 | 86 | 61.5 |
| Yom et al,9 2021 (NRG HN 002) | 60 | 48-54 | 96.7 | 90.5 | 21.7 |
| Chera et al,14 2019 (University of North Carolina) | 60 | 54 | 95 | 86 | 34 |
| Sher et al,24 2021 (University of Texas Southwestern) | 70 | 40 | 88 | 81 | 33 |
| Deschuymer et al,25 2020 (Belgium) | 70 | 40 | 76.4 | 61.3 | NA |
| Ma et al,12 2019 (Mayo Clinic [postoperative]) | 30-36 (Twice daily) | 30-36 (Twice daily) | 96.2 | 91.15 | 0.9 |
| Chen et al,15 2017 (University of California, Davis [induction chemotherapy]) | 54 | 43 | 93 | 92 | 7 |
| Seiwert et al,10 2019 (OPTIMA [induction chemotherapy]) | 45-75 | 30-54 | 98 | 94.5 | 29 |
| Marur et al,11 2017 (ECOG 13-08 [induction chemotherapy]) | 54 | 51.3 | NA | 80 | NA |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LCR, locoregional control; NA, not applicable; PFS, progression-free survival; RTOG, Radiation Therapy Oncology Group.
Other studies have investigated modest reduction of the radiation dose or omission of elective microscopic regions. A pooled analysis of patients with HPV-positive and HPV-negative cancer found that elective nodal volumes of 40 Gy did not result in any treatment failures within the elective targets among cohorts with HPV-positive disease.37 In the postoperative setting, doses of 30 to 36 Gy delivered twice daily with concurrent chemotherapy were shown to be adequate in the surgical bed as well as elective regions for HPV-positive tumors.12 Two prospective trials using an elective radiation dose of 40 Gy24,25 also showed uncompromised LRC, regardless of the anatomical site of disease and HPV status. The study by Deschuymer et al25 included 200 patients with nonmetastatic squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx who received primary or adjuvant radiotherapy with or without concurrent systemic therapy. All patients received a dose of 70 Gy to gross disease and were randomized to elective nodal volumes of either 40 Gy or 50 Gy. With a median follow-up of 7.6 years, only 2 regional recurrences occurred in the patients receiving 40 Gy, the same as that in the group receiving 50 Gy. Of note, 32% of the study population did not receive systemic therapy, and it was unclear whether those who had regional recurrence in the 40-Gy group received concurrent systemic therapy. Another phase 2 single-arm study by Sher et al24 investigated an elective nodal dose of 40 Gy in 72 patients with OPC or laryngeal cancer (90% received CCRT). With a median follow-up of 25 months, there were no solitary elective nodal recurrences. The study had comparable QOL trends in EORTC QLQ H&N35 and QLQ-C30 scores akin to those of our present cohort, although 33% their patients required feeding tube placement during treatment owing to weight loss or dysphagia. The results of our study also showed similar trends but overall more favorable EORTC QLQ H&N35 swallowing function scores compared with that of the RTOG (Radiation Therapy Oncology Group) 1016 study.13
Our institution has used a systematic approach of radiotherapy de-escalation since March 2017 for all patients with HPV-positive OPC undergoing CCRT, with further reduction of the elective radiation dose to 30 Gy, based on historical data on HPV-related cancers and modern HPV-specific OPC trials.38,39 The classic report by Nigro et al38 showed that most patients with squamous cell carcinoma of the anal canal achieved a complete clinical or pathologic response after preoperative chemoradiotherapy to 30 Gy, suggesting that even some gross disease could be eradicated with this dose. In a pilot study conducted at our institution using functional positron emission tomography imaging response with a hypoxia tracer,39 patients were selected to receive a de-escalated radiation dose of 30 Gy to gross disease. All 14 patients treated per protocol had either a complete or partial pathologic response and maintained LRC at 2 years.
The control of subclinical metastasis has a shallow linear dose-response curve, reflecting interpatient heterogeneity in occult metastatic tumor burden.22 The commonly used subclinical dose in the range of 50 to 60 Gy was based on historical radiobiological data from limited imaging capability for detecting occult metastasis.22,23 Because our ability to discriminate between gross disease and benign lymph nodes has improved with high-quality imaging, the results of our study and others mentioned above highlight that a significantly lower dose for elective volumes at approximately 30 Gy with concurrent chemotherapy can be sufficient for patients with HPV-positive OPC. Therefore, the elective radiotherapy dose of 50 to 60 Gy, or a modestly reduced dose of 40 Gy, likely resulted in overtreatment. In addition, studies from multiple institutions have demonstrated that it is safe to omit the uninvolved ipsilateral level Ib and V nodal regions20,26,27,40,41,42 as well as the contralateral high retropharyngeal regions in uninvolved neck.28,43 Thus, we are confident that a dose of 30 Gy is sufficient for eradiating microscopic disease in our study population. However, whether it is safe to further reduce the elective radiation dose to below 30 Gy or to omit some elective nodal volumes completely remains unknown. It is also unclear whether the same principle can be applied to both HPV-positive and -negative tumors.
Limitations
Some limitations of this study are worth mentioning. This was a single-institution, nonrandomized, retrospective cohort study. Results from this study have yet to be validated in multi-institutional, prospective settings to confirm reproducibility and generalizability. One patient had a previously unidentified gross node that did not receive a full 70 Gy of radiotherapy, indicating that with this approach of significantly reduced elective dose, one must be vigilant in target delineation to include small-volume gross disease that might be eradicated by the commonly used elective dose in the range of 50 to 60 Gy. Of note, 31.5% of the patients did receive an intermediate dose of 50 Gy for indeterminate nodes or other small areas of concern per the treating physician’s discretion, and this flexibility in dosing has worked well for treatment planning while still keeping most of the elective regions dosed to 30 Gy.
Conclusions
In this retrospective cohort study with a median follow-up of 26 months, the systematic de-escalation of radiotherapy to 30 Gy with reduction of elective volumes irradiated was associated with minimal toxic effects. With longer term follow-up data to validate the results, this regimen can be safely adapted for treating a wide range of patients with HPV-associated OPC undergoing primary CCRT.
eFigure. Select Examples of Target Volume Delineation
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107(2):242-246. doi: 10.1016/j.radonc.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart JR, Fajardo LF. Dose response in human and experimental radiation-induced heart disease: application of the nominal standard dose (NSD) concept. Radiology. 1971;99(2):403-408. doi: 10.1148/99.2.403 [DOI] [PubMed] [Google Scholar]
- 5.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7-16. doi: 10.1016/S0360-3016(00)00663-5 [DOI] [PubMed] [Google Scholar]
- 6.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(2):145-153. doi: 10.1016/S1470-2045(11)70346-1 [DOI] [PubMed] [Google Scholar]
- 7.Sher DJ, Adelstein DJ, Bajaj GK, et al. Radiation therapy for oropharyngeal squamous cell carcinoma: executive summary of an ASTRO evidence-based clinical practice guideline. Pract Radiat Oncol. 2017;7(4):246-253. doi: 10.1016/j.prro.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405-409. doi: 10.1016/S0002-9610(05)80554-9 [DOI] [PubMed] [Google Scholar]
- 9.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002). J Clin Oncol. 2021;39(9):956-965. doi: 10.1200/JCO.20.03128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiwert TY, Foster CC, Blair EA, et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus–positive oropharyngeal cancer. Ann Oncol. 2019;30(2):297-302. doi: 10.1093/annonc/mdy522 [DOI] [PubMed] [Google Scholar]
- 11.Marur S, Li S, Cmelak AJ, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx— ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490-497. doi: 10.1200/JCO.2016.68.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma DJ, Price KA, Moore EJ, et al. Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus-associated oropharynx squamous cell carcinoma. J Clin Oncol. 2019;37(22):1909-1918. doi: 10.1200/JCO.19.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40-50. doi: 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chera BS, Amdur RJ, Green R, et al. Phase II trial of de-intensified chemoradiotherapy for human papillomavirus-associated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2019;37(29):2661-2669. doi: 10.1200/JCO.19.01007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18(6):803-811. doi: 10.1016/S1470-2045(17)30246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3)(suppl):S58-S63. doi: 10.1016/j.ijrobp.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy V, Gurram L, Kannan S, et al. Elective nodal dose of 60 Gy or 50 Gy in head and neck cancers: a matched pair analysis of outcomes and toxicity. Adv Radiat Oncol. 2017;2(3):339-345. doi: 10.1016/j.adro.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rancati T, Schwarz M, Allen AM, et al. Radiation dose–volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys. 2010;76(3)(suppl):S64-S69. doi: 10.1016/j.ijrobp.2009.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garden AS, Dong L, Morrison WH, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):941-947. doi: 10.1016/j.ijrobp.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Sanguineti G, Califano J, Stafford E, et al. Defining the risk of involvement for each neck nodal level in patients with early T-stage node-positive oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2009;74(5):1356-1364. doi: 10.1016/j.ijrobp.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 21.Daly ME, Le QT, Maxim PG, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2010;76(5):1339-1346. doi: 10.1016/j.ijrobp.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31(2):353-359. doi: 10.1016/0360-3016(94)00354-N [DOI] [PubMed] [Google Scholar]
- 23.Marks LB. A standard dose of radiation for “microscopic disease” is not appropriate. Cancer. 1990;66(12):2498-2502. doi: [DOI] [PubMed] [Google Scholar]
- 24.Sher DJ, Pham NL, Shah JL, et al. Prospective phase 2 study of radiation therapy dose and volume de-escalation for elective neck treatment of oropharyngeal and laryngeal cancer. Int J Radiat Oncol Biol Phys. 2021;109(4):932-940. doi: 10.1016/j.ijrobp.2020.09.063 [DOI] [PubMed] [Google Scholar]
- 25.Deschuymer S, Nevens D, Duprez F, et al. Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma; update of the long-term tumor outcome. Radiother Oncol. 2020;143:24-29. doi: 10.1016/j.radonc.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Gutiontov S, Leeman J, Lok B, et al. Cervical nodal level V can safely be omitted in the treatment of locally advanced oropharyngeal squamous cell carcinoma with definitive IMRT. Oral Oncol. 2016;58:27-31. doi: 10.1016/j.oraloncology.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam M, Riaz N, Kannarunimit D, et al. Sparing bilateral neck level IB in oropharyngeal carcinoma and xerostomia outcomes. Am J Clin Oncol. 2015;38(4):343-347. doi: 10.1097/COC.0000000000000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leeman JE, Gutiontov S, Romesser P, et al. Sparing of high retropharyngeal nodal basins in patients with unilateral oropharyngeal carcinoma treated with intensity modulated radiation therapy. Pract Radiat Oncol. 2017;7(4):254-259. doi: 10.1016/j.prro.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai CJ, McBride SM, Riaz N, Lee NY. Reducing the radiation therapy dose prescription for elective treatment areas in human papillomavirus-associated oropharyngeal carcinoma being treated with primary chemoradiotherapy at Memorial Sloan Kettering Cancer Center. Pract Radiat Oncol. 2019;9(2):98-101. doi: 10.1016/j.prro.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 30.Johnson J, Carlsson S, Johansson M, et al. Development and validation of the Gothenburg Trismus Questionnaire (GTQ). Oral Oncol. 2012;48(8):730-736. doi: 10.1016/j.oraloncology.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson M, Karlsson T, Finizia C. Further validation of the Gothenburg Trismus Questionnaire (GTQ). PLoS One. 2020;15(12):e0243805. doi: 10.1371/journal.pone.0243805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov . Adaptive Radiotherapy for Head and Neck Cancer. NCT03096808. Accessed September 21, 2021. https://clinicaltrials.gov/ct2/show/NCT03096808
- 33.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–H&N35. J Clin Oncol. 1999;17(3):1008-1019. doi: 10.1200/JCO.1999.17.3.1008 [DOI] [PubMed] [Google Scholar]
- 34.Sherman AC, Simonton S, Adams DC, Vural E, Owens B, Hanna E. Assessing quality of life in patients with head and neck cancer: cross-validation of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Head and Neck Module (QLQ-H&N35). Arch Otolaryngol Head Neck Surg. 2000;126(4):459-467. doi: 10.1001/archotol.126.4.459 [DOI] [PubMed] [Google Scholar]
- 35.López-Jornet P, Camacho-Alonso F, López-Tortosa J, Palazon Tovar T, Rodríguez-Gonzales MA. Assessing quality of life in patients with head and neck cancer in Spain by means of EORTC QLQ-C30 and QLQ-H&N35. J Craniomaxillofac Surg. 2012;40(7):614-620. doi: 10.1016/j.jcms.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 36.EORTC QLQ-C30 Scoring Manual . 2018; https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf.
- 37.Nevens D, Duprez F, Daisne JF, et al. Recurrence patterns after a decreased dose of 40Gy to the elective treated neck in head and neck cancer. Radiother Oncol. 2017;123(3):419-423. doi: 10.1016/j.radonc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 38.Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51(10):1826-1829. doi: [DOI] [PubMed] [Google Scholar]
- 39.Riaz N, Sherman E, Pei X, et al. Precision radiotherapy: reduction in radiation for oropharyngeal cancer in the 30 ROC trial. J Natl Cancer Inst. 2021;113(6):742-751. doi: 10.1093/jnci/djaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Daly ME, Farwell DG, et al. Level IB nodal involvement in oropharyngeal carcinoma: implications for submandibular gland-sparing intensity-modulated radiotherapy. Laryngoscope. 2015;125(3):608-614. doi: 10.1002/lary.24907 [DOI] [PubMed] [Google Scholar]
- 41.Mohindra P, Urban E, Pagan JD, et al. Selective omission of level V nodal coverage for patients with oropharyngeal cancer: clinical validation of intensity-modulated radiotherapy experience and dosimetric significance. Head Neck. 2016;38(4):499-505. doi: 10.1002/hed.23924 [DOI] [PubMed] [Google Scholar]
- 42.Sanguineti G, Pai S, Agbahiwe H, et al. HPV-related oropharyngeal carcinoma with overt level II and/or III metastases at presentation: the risk of subclinical disease in ipsilateral levels IB, IV and V. Acta Oncol. 2014;53(5):662-668. doi: 10.3109/0284186X.2013.858825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi: 10.1002/cncr.28938 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Select Examples of Target Volume Delineation