Key Points
Question
Can fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) predict response and immunological changes in patients receiving neoadjuvant immunotherapy for oral cavity squamous cell cancer (OCSCC)?
Findings
In this prospective phase 2 study of 27 patients with OCSCC, there were no correlations between changes in FDG uptake and pathologic primary tumor response or baseline CD8+ T-cell infiltrates. Patients developed new FDG-avid cervical lymph nodes after neoadjuvant immunotherapy and systemic immune responses on imaging were observed.
Meaning
Important early patterns of FDG uptake after neoadjuvant immunotherapy for OCSCC did not reflect progressive disease and suggest that surgical plans should not be altered in this setting.
Abstract
Importance
Neoadjuvant immunotherapy is a novel approach with the potential to improve outcomes for patients with oral cavity squamous cell cancer (OCSCC). Adverse events of varying severity are reported with immunotherapy, and a biomarker to predict response would be clinically useful to avoid toxic effects in those unlikely to benefit.
Objective
To correlate changes on fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) scans with primary tumor pathologic response and immunologic biomarkers in patients with OCSCC receiving neoadjuvant immunotherapy.
Design, Setting, and Participants
This was a retrospective analysis of serial FDG-PET/CT scans obtained prospectively as part of a phase 2 open-label randomized clinical trial investigating neoadjuvant immunotherapy in patients with untreated OCSCC between 2016 and 2019. Included were a total of 29 patients from a single academic medical center with untreated OCSCC (≥T2, or clinically node positive) randomized 1:1 to receive neoadjuvant therapy with single agent nivolumab or combination nivolumab and ipilimumab followed by surgery and standard of care adjuvant therapy.
Interventions
The interventions in this study were FDG-PET/CT scans before (T0) and after (T1) preoperative immunotherapy.
Main Outcomes and Measures
Data collected from FDG-PET/CT scans included maximum standardized uptake value (SUVmax) of primary OCSCC and cervical lymph nodes (LNs) at T0 and T1 and new LN uptake and uptake consistent with radiologic immune-related adverse events (irAEs) at T1. Primary OCSCC pathologic response reported as percentages of viable vs nonviable tumor. The number of CD8+ cells/mm2 was determined in the primary tumor biopsy specimen and at surgery.
Results
There was no correlation between pathologic response and change in SUVmax in the primary OCSCC between T0 and T1. Out of 27 total participants, 13 had newly FDG-avid ipsilateral LNs at T1, most negative on pathology. A total of 9 had radiologic irAEs, most commonly sarcoid-like LN (7 of 27). No correlations were found between primary OCSCC SUVmax at T0 and CD8+ T-cell number in the primary tumor biopsy, and no correlations were found between primary OCSCC SUVmax at T1 and CD8+ T-cell number in the primary tumor at surgery.
Conclusions and Relevance
There were no correlations between changes in FDG uptake after neoadjuvant immunotherapy and pathologic primary tumor response. Importantly, newly FDG-avid ipsilateral LNs following neoadjuvant immunotherapy were commonly observed but did not represent progressive disease or indicate pathologically disease positive nodes in most cases. These findings argue against altering surgical plans in this setting and suggest that the role of FDG-PET/CT may be limited as an early imaging biomarker for predicting pathologic response to preoperative immunotherapy for OCSCC.
Trial Registration
ClinicalTrials.gov Identifier: NCT02919683
This study assesses whether there are changes on fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography scans in patients with oral cavity squamous cell cancer receiving neoadjuvant immunotherapy.
Introduction
Oral cavity squamous cell carcinomas (OCSCCs) are aggressive head and neck cancers with poor outcomes for patients. Despite multimodality treatment, typically with surgery then adjuvant radiation or chemoradiation, 5-year survival in the US is less than 50% for patients with advanced cases, highlighting the need for new therapies and strategies to improve outcomes. For patients with metastatic OCSCC, treatment paradigms have recently shifted owing to the advent of immunotherapy. Presently, the immune checkpoint inhibitors pembrolizumab and nivolumab are approved for treatment of metastatic or recurrent OCSCC with improved overall survival compared with chemotherapy alone.1,2,3
Preoperative systemic therapy may improve surgical outcomes, lessen overall morbidity, reduce metastatic disease risk, and provide a framework for studying biomarkers. Great interest in neoadjuvant immunotherapy regimens for OCSCC has emerged owing to the benefit seen in the recurrent and metastatic disease settings.4 However, immune-related adverse events (irAEs) occur with variable frequency.5 Biomarkers to predict response to preoperative immunotherapy would be clinically useful to avoid toxic effects in those unlikely to benefit.
Fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) is standard of care for staging OCSCC and is used routinely to assess treatment response. This imaging method shows potential for assessing tumor response to immunotherapy, but data are mixed due to confounding by inflammatory cell infiltration.6 Imaging with FDG-PET/CT has been shown to identify irAEs, often prior to biochemical changes, symptoms, or changes on anatomic imaging.7,8 In preoperative immunotherapy settings, the role of FDG-PET/CT is beginning to be explored.9,10,11,12 In a phase 2 open-label randomized clinical trial conducted at the Dana-Farber Cancer Institute/Brigham and Women’s Hospital (NCT02919683), we demonstrated the feasibility of administering immunotherapy for untreated OCSCC before surgery and observed pathologic response rates of 54% to 73%.13
Imaging with FDG-PET/CT at baseline and after 3 weeks of preoperative immunotherapy was performed, with 52% of participants showing decreased FDG uptake in the primary tumor. A large fraction of participants also had increasing FDG uptake in lymph nodes (LNs) after preoperative immunotherapy, but without pathologic LN involvement at surgery performed shortly afterwards, suggesting immunotherapy-induced inflammation.13 In the present study, we expanded the FDG-PET/CT analyses for assessing response to preoperative immunotherapy and correlated the FDG-PET/CT findings with primary tumor pathologic response and immunologic biomarker changes.
Methods
Study Population and Trial Design
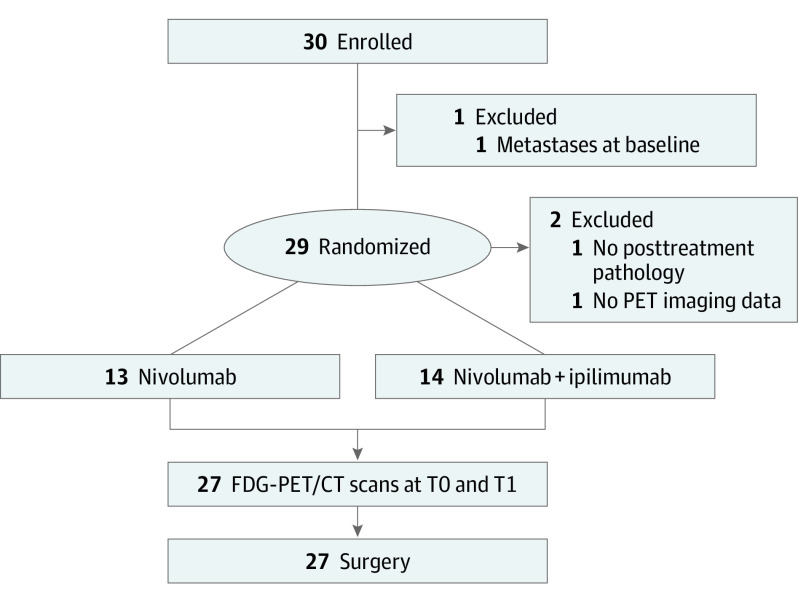
This was a retrospective analysis of serial FDG-PET/CT scans obtained prospectively in a phase 2 open-label randomized clinical trial investigating preoperative immunotherapy in patients with untreated OCSCC (NCT02919683).13 Participants were randomized 1:1 to receive 3 weeks of preoperative therapy with single-agent nivolumab or combination immunotherapy with nivolumab and ipilimumab followed by surgery and standard of care adjuvant therapy based on pathologic findings at surgery (Figure 1). The trial followed Declaration of Helsinki principles and Good Clinical Practice and was approved by the Dana-Farber Harvard Cancer Center institutional review board. All participants gave informed written consent.
Figure 1. Study Schema and Participants.
Abbreviation: FDG-PET/CT, fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography.
Major eligibility criteria were age 18 years or older, pathology-confirmed surgically resectable OCSCC with clinical stage T2 or higher and/or evidence of LN involvement by clinical exam or imaging, and Eastern Cooperative Oncology Group performance status 1 or less. Key exclusion criteria were distant metastatic disease, prior immunotherapy for cancer, prior head and neck radiation, and active or prior documented autoimmune disease requiring systemic treatment.
Acquisition of FDG-PET/CT Scans and Image Interpretation
Imaging from FDG-PET/CT scans was obtained before starting (T0) and after completing preoperative therapy (T1, 3-4 weeks) prior to surgery. Of 27 total participants, 22 T0 and all 27 T1 FDG-PET/CT scans were performed at Dana-Farber Cancer Institute/Brigham and Women’s Hospital. A total of 5 baseline FDG-PET/CT scans performed at outside institutions were reviewed and deemed of acceptable quality for inclusion.
Imaging with FDG-PET/CT performed at Dana-Farber Cancer Institute/Brigham and Women’s Hospital followed institutional protocols. Participants fasted for 4 to 6 hours and had blood glucose levels lower than 200 mg/dL prior to intravenous injection of FDG. Imaging with FDG-PET/CT was performed from either the vertex or base of skull to the mid-thighs after a planned 60-minute uptake time. Computed tomography was obtained first for attenuation correction without oral or intravenous contrast (3.75 to 5 mm axial slice thickness). Three-dimensional PET emission data were obtained (GE Discovery DMI, GE Discovery DSTE/VCT64, GE Discovery DRX/VCT64, and Siemens Biograph 16 Hi-Rez) and reconstructed using ordered subset expectation maximization iterative reconstruction methods.
Fellowship-trained nuclear medicine physicians (H.J., H.S.) reviewed FDG-PET/CT in consensus for abnormal FDG uptake within the primary OCSCC and cervical LN. Abnormal FDG uptake was defined as increased FDG uptake greater than background, not consistent with normal anatomy or physiology, and in a pattern definitely or probably owing to OCSCC. At T0 and T1, primary OCSCC and cervical LN maximum standardized uptake values (SUVmax) were determined using Hermes software (Hermes Medical Solutions, Stockholm, Sweden). After preoperative immunotherapy (T1), the presence or absence of FDG uptake in new cervical LNs and in patterns consistent with radiologic irAEs were recorded. Primary tumor (long axis) and LN size (short axis) were measured on axial CT images from PET/CT scans.
Surgery and Pathology
Per protocol design, surgery was performed based on the original planned resection margins without consideration of immunotherapy response. Primary tumor pathologic response was assessed by a head and neck pathologist blinded to treatment and reported as percentages of viable vs nonviable tumor. The number of CD8+ cells/mm2 in the baseline primary tumor biopsy and post-treatment surgery resection specimens were determined using multiplex immunofluorescence as previously described.13
Statistical Analysis
Absolute SUVmax change was calculated as: Δ = T1 SUVmax – T0 SUVmax. Percentage SUVmax change was calculated as: Δ/T0 SUVmax × 100%. Relationships between 2 continuous variables were evaluated by Pearson correlation coefficient r (90% CI). Effect sizes measuring associations between a binary group variable and dichotomized response variables were estimated by the differences in percentages of responders (90% CI) between 2 groups. Effect sizes by Wilcoxon rank sum test (ESwrs [90% CI]) were the estimated difference of the location parameters for comparing continuous variables between 2 groups. Effect sizes by Wilcoxon signed rank test (ESwsr [90% CI]) were pseudo-median estimates for the distribution of T1 to T0 to compare continuous variables measured at paired T0 and T1.
Results
Study Participants and FDG-PET/CT Scans
A total of 30 participants were enrolled in the phase 2 clinical trial (Figure 1). Of these, 3 were excluded: post-treatment pathology was not available because surgery was deferred (n = 1), distant metastases at baseline (n = 1), and no PET imaging data (n = 1). The remaining 27 participants (16 male, 11 female, median age 66, range 39-81 years) completed all the study requirements and were included in this analysis. Baseline characteristics were published in the main clinical phase 2 trial report.13 A total of 13 participants were randomized to receive preoperative nivolumab and 14 received preoperative nivolumab and ipilimumab. At surgery, median percent nonviable tumor (% response) was 10% (range 5%-95%).
Imaging with FDG-PET/CT was obtained between April 2017 and July 2019. T0 FDG-PET/CT scans were performed a median of 14 days (range 6-20 days) prior to starting preoperative immunotherapy, and T1 scans were performed a median of 3 days before surgery (range 1-16 days). Differences between technical parameters between T0 and T1 FDG-PET/CT scans were not clinically meaningful (eTable 1 in the Supplement).
Primary OCSCC FDG-PET/CT Findings vs Pathologic Response
Primary OCSCC tumors were visualized on the T0 FDG-PET/CT scans for 26 of the 27 patients and had a median SUVmax of 9.95 (range 4.5-37.3). A total of 24 measurable tumors on CT had a median tumor size of 2.2 cm (range 1.7-3.1 cm). There was no linear correlation between pathologic response at surgery and primary tumor SUVmax at T0 (r = −0.17 [90% CI, –0.47 to 0.16], Figure 2A) or T1 (r = −0.26 [90% CI, –0.54 to 0.06], Figure 2B). There were also no correlations between pathologic response at surgery and absolute (r = −0.02 [90% CI, –0.34 to 0.31], Figure 2C) or percentage change (r = 0.02 [90% CI, –0.30 to 0.34], Figure 2D) in primary tumor SUVmax between T0 and T1. The lack of correlation in these parameters held true across both treatment cohorts (eTable 2 in the Supplement).
Figure 2. Primary OCSCC FDG-PET/CT Findings vs Pathologic Response.
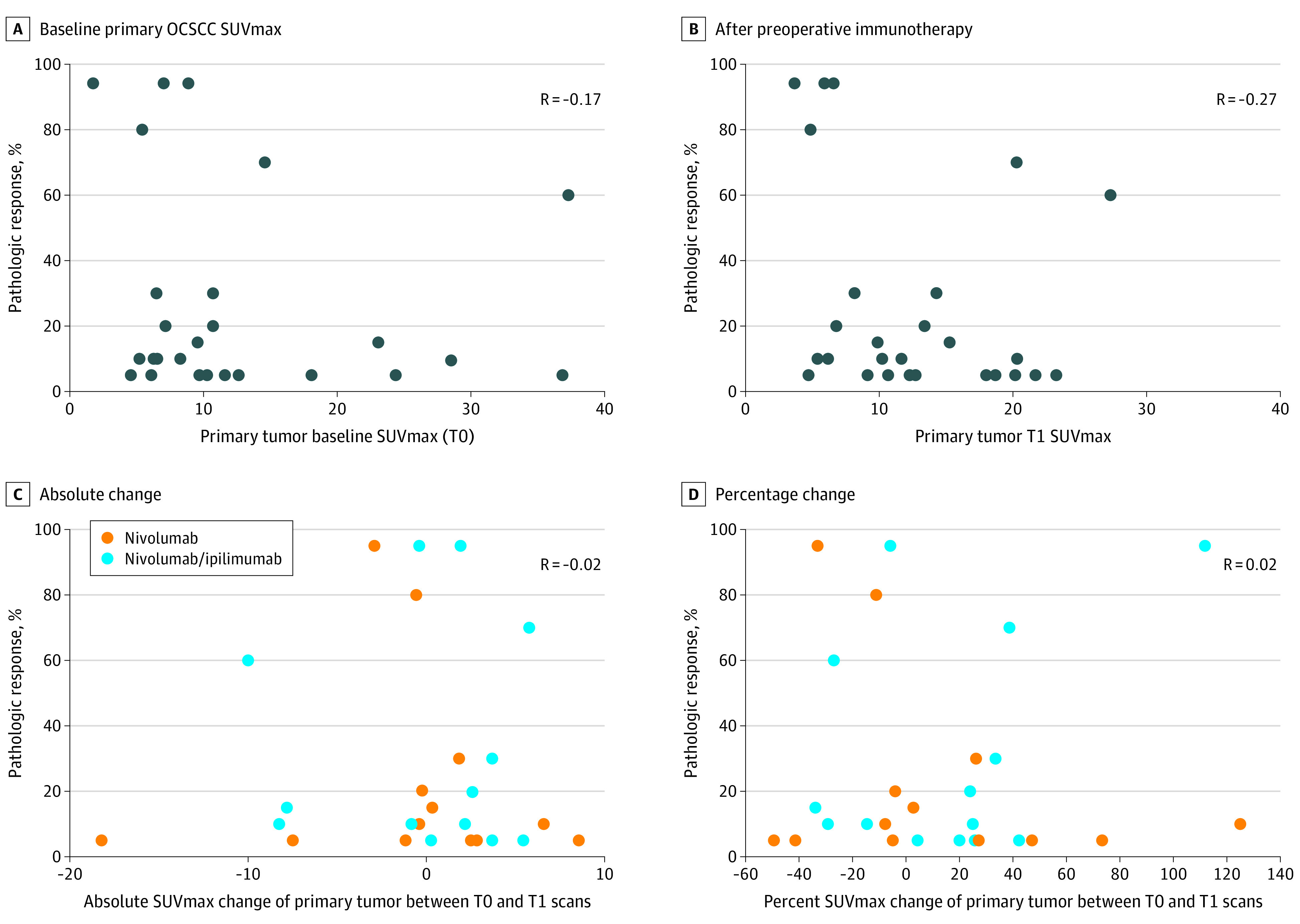
No correlations were found between pathologic response and baseline primary oral cavity squamous cell cancer (OCSCC) maximum standardized uptake value (SUVmax) (A) and primary OCSCC SUVmax after preoperative immunotherapy (B). No correlations were found for absolute (C) or percentage change (D), in primary tumor SUVmax between T0 and T1.
Cervical LN FDG-PET/CT Findings vs Pathology
At T0, 16 participants had abnormal ipsilateral cervical LN FDG uptake suggesting metastatic disease. None had contralateral LN disease. Median SUVmax of the most FDG-avid metastatic ipsilateral LNs at T0 was 5 (range 2.8-18.3). On the T1 scan, 13 of 27 participants had 18 newly FDG-avid ipsilateral cervical LNs (median 1 per participant, range 1-2); 8 with positive LN at T0 and 5 without positive LN at T0. Newly FDG-avid LNs were found at levels 1-5, most commonly at levels 2 and 3. Median SUVmax of the most intense newly FDG-avid ipsilateral LNs per participant was 3.1 (range 1.4-7.9). For 8 of these 13 participants, surgical dissections of cervical levels that included newly FDG-avid LNs at T1 were negative for malignancy. A total of 2 participants did not have dissection of cervical levels that included their newly FDG-avid LNs. In 1 participant a newly FDG-avid level 5 LN was not included in the neck dissection, but another newly FDG-avid LN at level 3 had negative pathology. A total of 2 participants had newly FDG-avid LNs with at least 1 node in the corresponding level dissection testing positive for metastases. The SUVmax of these 2 newly FDG-avid ipsilateral LNs at T1 were the highest (SUVmax 6.3 and 7.9).
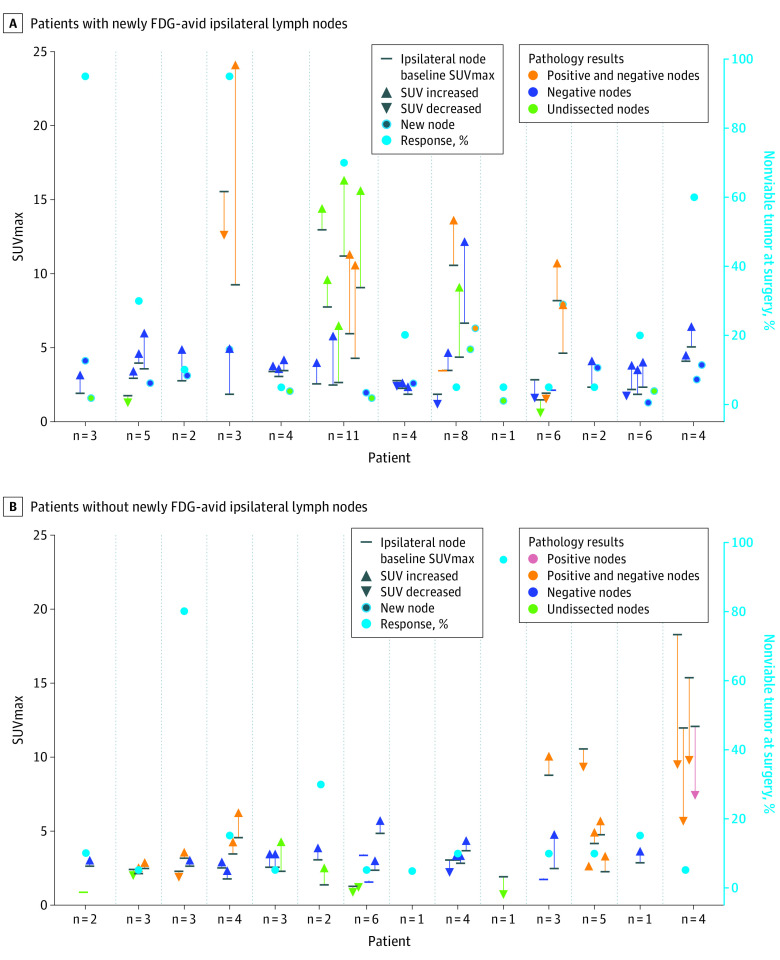
Figure 3 summarizes SUVmax changes of ipsilateral LNs between T0 and T1 compared with pathologic response in primary OCSCC and the pathology results from neck dissection in participants with (Figure 3A) vs without (Figure 3B) newly FDG-avid ipsilateral LNs at T1. More and larger changes in SUVmax of LN between scans were observed in participants with newly FDG-avid ipsilateral LNs compared with those without.
Figure 3. Changes in Ipsilateral Nodal SUVmax and Association With Primary Tumor Response and Lymph Node Pathology.
Changes of maximum standardized uptake value (SUVmax) of ipsilateral lymph nodes between T0 and T1 compared with pathologic response in the primary oral cavity squamous cell cancer (OCSCC) and pathology results from the neck dissection in participants with (A) vs without (B), without newly fluoro-[18F]-deoxy-2-D-glucose (FDG)-avid ipsilateral lymph nodes at T1. More and larger changes in lymph nodes between the scans were observed in participants with newly FDG-avid ipsilateral nodes compared with those without.
We explored the association of newly FDG-avid ipsilateral LNs with dichotomized pathologic response in primary OCSCC (Table). The frequency of participants with newly FDG-avid ipsilateral LNs was similar (50% vs 47%) comparing those with below 10% response to those with 10% or higher response in primary OCSCC. A higher percentage of participants with newly FDG-avid ipsilateral LNs achieved 50% or higher response in primary OCSCC tumor (31%) compared with those without newly FDG-avid ipsilateral LNs (14%); the difference in percentages of responders was 16.5% (90% CI, –9.6% to 42.6%, Table).
Table. Primary Tumor Response vs Newly FDG-Avid Ipsilateral Lymph Nodes.
| Newly FDG-avid ipsilateral lymph nodes at T1 | Primary tumor response, No. (%) | |||||
|---|---|---|---|---|---|---|
| <10% | ≥10% | Effect size,a % (90% CI) | <50% | ≥50% | Effect size,a % (90% CI) | |
| Yes (n = 13) | 5 (38.5) | 8 (61.5) | –2.7 (–33.3 to 27.9) | 9 (69.2) | 4 (30.8) | 16.5 (–9.6 to 42.6) |
| No (n = 14) | 5 (35.7) | 9 (64.3) | 12 (85.7) | 2 (14.3) | ||
Estimates the difference in percentages of responders for Yes or No.
A total of 9 participants (33%) had 16 newly FDG-avid contralateral cervical LNs at T1 (median SUVmax 2.4, range 0.8-5.8). These 9 participants with newly FDG-avid contralateral LNs included 8 of the 13 participants with newly FDG-avid ipsilateral LNs, while 1 participant had only newly FDG-avid contralateral LNs. Only 1 of these 9 participants underwent contralateral neck dissection at the level of the newly FDG-avid LNs with negative pathology. The distribution of patients with newly FDG-avid LNs at T1 between the 2 treatment cohorts was more in the nivolumab plus ipilimumab arm: 9 ipsilateral, 7 contralateral, and 6 bilateral, compared with 4, 2, and 2, respectively, in the nivolumab arm that corresponded to 19% (90% CI, –7% to 39%), 28% (90% CI, –5% to 46%), and 25% (90% CI, –10% to 45%) above chance.
Immune-Related Adverse Events After Neoadjuvant Immunotherapy
The T1 FDG-PET/CT suggested radiologic irAEs in 9 of 27 participants. The most common was sarcoid-like lymphadenopathy (n = 7, in combination with thyroiditis in 3 and colitis in 1), thyroiditis (n = 5), and colitis (n = 1). A total of 4 of the 5 participants with suspected thyroiditis at T1 had a clinical thyroid-related irAE (hyperthyroidism: n = 2 grade 1, n = 1 grade 2; hypothyroidism: n = 1 grade 2). One participant with thyroiditis and sarcoid-like lymphadenopathy on T1 FDG-PET/CT had a clinical autoimmune disorder probably related to nivolumab. The radiology-suspected colitis was unconfirmed.
No association was found between primary tumor pathologic response and visualization of radiologic irAEs on T1 FDG-PET/CT (Figure 4A). On the T1 scan, 5 of 9 participants with radiologic irAEs also had newly FDG-avid ipsilateral cervical LNs, including 5 of 7 with sarcoid-like lymphadenopathy. Of the 9 with radiologic irAEs, 5 of were in the nivolumab plus ipilimumab arm.
Figure 4. Visualization of Radiologic irAEs vs SUVmax Changes in Primary OCSCC and Ipsilateral Cervical Lymph Nodes.
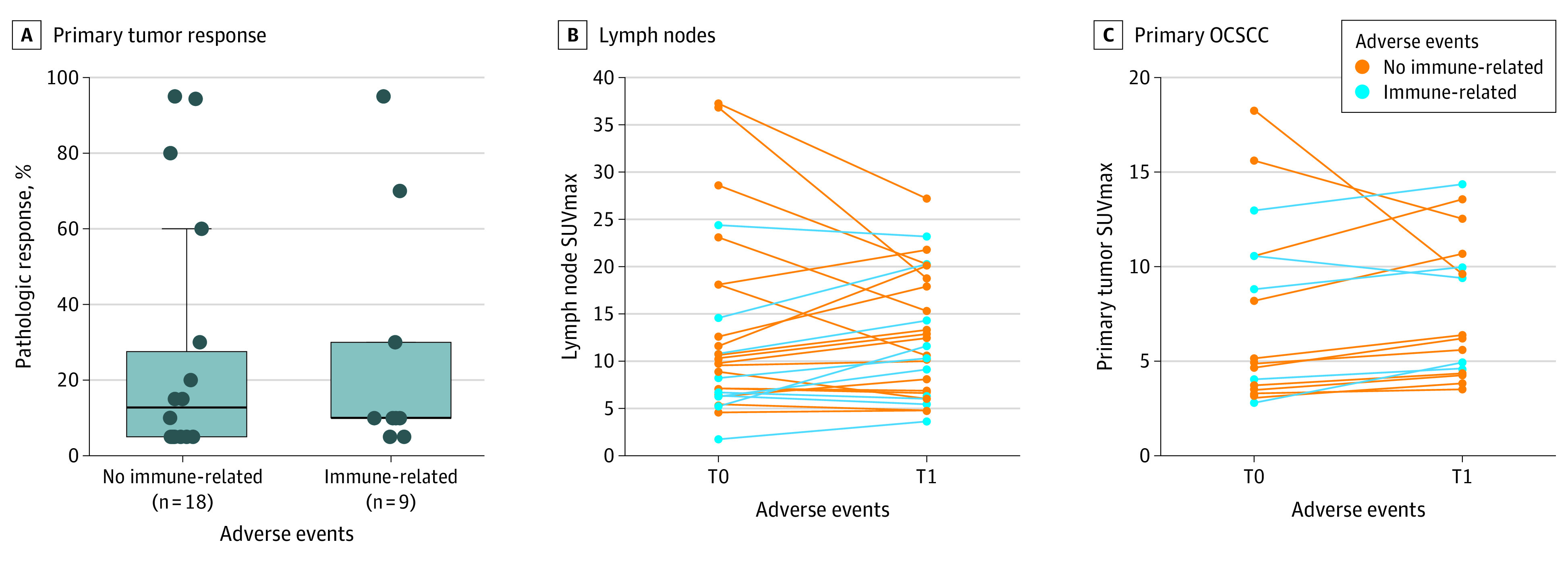
A, Nο correlation was found between pathologic response in primary tumor and visualization of radiologic immune-related adverse effects (irAEs) on the T1 fluoro-[18F]-deoxy-2-D-glucose positron emission tomography/computed tomography (FDG-PET/CT). B, Change in maximum standardized uptake value (SUVmax) of the most FDG-avid metastatic cervical lymph nodes between T0 and T1 was not different between participants with (median 14%, range −11% to 75%) and without radiologic irAEs (median 20%, range −48% to 35%, ESwrs = −5% [90% CI, –20% to 45%] for with or without) after preoperative immunotherapy. C, Primary oral cavity squamous cell cancer (OCSCC) SUVmax increased more in participants with (median 34%, range −5 to 47%) vs without (median −1%, range −29 to 25%, ESwrs = 30% [90% CI, 6% to 67%] for with or without) radiologic irAEs on T1 FDG-PET/CTs.
Percent change in SUVmax of the most FDG-avid metastatic cervical LNs between T0 and T1 was similar between participants with radiologic irAEs (median 14%, range −11% to 75%) and without radiologic irAEs (median 20%, range −48 to 35%), ESwrs = −5% (90% CI, –20% to 45%) for with and without after preoperative immunotherapy (Figure 4B). Percent change in primary OCSCC SUVmax was higher in participants with radiologic irAEs compared with those without radiologic irAEs on T1 FDG-PET/CT (median 34%, range −5 to 47% vs median −1%, range −29 to 25%, ESwrs = 30% [90% CI, 6% to 67%] for with or without, Figure 4C).
Correlation of CD8 and FDG Uptake
Neither SUVmax of the primary OCSCC, nor the most FDG-avid metastatic ipsilateral cervical LN, was linearly correlated with CD8+ T-cell number at T0 or T1 (eFigure in the Supplement). Although percent change in CD8+ T-cells in primary OCSCC between baseline biopsy specimen and postimmunotherapy surgical specimen did not correlate linearly with percent SUVmax change between T0 and T1 scans, 1 participant with a considerably large percent increase in SUVmax also had a large percent increase in CD8+ T cells.
Discussion
This is the first preoperative OCSCC clinical trial to include serial FDG-PET/CT scans before and after starting immunotherapy.13 The context of the trial was ideal for studying FDG-PET/CT as an imaging biomarker of response because all patients proceeded to surgery with histopathology.
We did not observe correlations between change in primary OCSCC FDG uptake from baseline to early ( ~ 3 weeks) after starting preoperative immunotherapy and pathologic response at surgery in either the nivolumab or nivolumab plus ipilimumab treatment arms. We did not observe an early increase in tumor FDG uptake after starting preoperative immunotherapy (or “flare phenomenon”) due to infiltration of immune effectors cells in responding tumors as previously reported in 2 studies of melanoma.14,15 The lack of correlation may be related to the complexity of the whole tumor composition at the early time point, including viable and nonviable tumor cells, fibrosis, necrosis, and immune effector cells. Han et al16 recently demonstrated unique gene signatures in the tumor microenvironment of head and neck squamous cell cancer based on high and low FDG uptake in 21 patients, highlighting this complexity.
We did not observe a correlation between CD8+ T-cell number in the primary OCSCC and primary tumor FDG uptake level on the T0 or T1 FDG-PET/CT scans. In contrast, Togo et al17 reported an association between a lower number of CD8+ T cells and higher FDG uptake in 50 patients with untreated OCSCC undergoing surgery. Using a cut-off of SUVmax 11.4, sensitivity was 50% and specificity 91% for identifying low CD8+ cell tumors. Except for very high uptake, substantial overlap of FDG uptake between CD8+ low vs high tumor groups limits its use for characterization of most individual tumors.
Furthermore, while the level of FDG uptake is known to be well correlated with viable cell number,18 aerobic glycolysis also drives immune-cell activation and differentiation.19,20 The presence of both cell types in the tumor and its microenvironment could potentially explain this lack of correlation and negate the ability of FDG-PET/CT to differentiate responders vs nonresponders early after starting immunotherapy. Higher densities of tumor-infiltrating lymphocytes (TILs) have been associated with better outcomes in head and neck squamous cell cancers,21,22 but CD8+ cells represent a subset of TILs, and alone might not be an optimal tissue biomarker. More specific radiotracers targeting CD8+ cells and activated T cells, ie, 89Zr-Df-IAB22M2C, 18F-AraG, and Granzyme B, are promising and under investigation.23,24,25
One of the most striking and clinically relevant findings we observed was the high percentage of newly FDG-avid ipsilateral (48%) and contralateral (24%) cervical LNs on T1 FDG-PET/CT scans. A total of 24 participants had new or increasing ipsilateral FDG-avid LNs included in the planned initial resection, and in 20, pathology was negative for tumor. Contralateral neck dissection was generally not performed; however, no participant progressed in the contralateral neck after a median follow up of 14.2 months. Increased FDG uptake in the LN was most likely related to immune response and should not be interpreted as progressive disease or used in this setting to alter surgical plans. The level of FDG uptake in the LN was wide (SUVmax range 0.6-7.9) and less helpful than the overall pattern and unlikelihood of developing metastatic disease in such a short interval. This study was not designed to determine an SUVmax threshold for discriminating whether a newly FDG-avid LN was metastatic or inflammatory after starting neoadjuvant immunotherapy for OCSCC. The 2 newly FDG-avid LNs positive for malignant neoplasm had SUVmax greater than 6; however, any threshold should be viewed with caution. These preliminary data could help inform the design of future trials.
Interestingly, 5 of the 9 participants with radiologic irAEs also had newly FDG-avid ipsilateral cervical LN, including 5 of 7 with new sarcoid-like lymphadenopathy. Additionally, most participants with newly FDG-avid LNs at T1 received combination immunotherapy, suggesting a potential synergistic effect of the agents on systemic inflammation. This combined pattern suggesting a systemic immune response was very helpful for interpretation, providing some reassurance that the new LNs are, most likely, from the systemic immune response and not progressive disease.
One-third of participants had evidence of irAEs visualized on FDG-PET/CT, which is unsurprising given the wide incidence range reported with immunotherapy across agents and cancer types. Possible treatment-related adverse events were reported in 72% of participants in the present study.13 The most common radiologic irAE on FDG-PET/CT was sarcoid-like lymphadenopathy, which is typically occult on physical exam and not reported by participants. Although FDG-PET/CT can identify the onset of clinically relevant irAEs prior to clinical or biochemical evidence, clinical and radiologic irAEs were identified temporally close together likely owing to the trial calendar for data collection. Outside a clinical trial setting, this is important because some irAEs can result in poor outcome or long-term sequelae without prompt treatment.
We did not find an association between the presence of irAEs on imaging and pathologic tumor response in the present study cohort. Interestingly, we did observe a larger increase in primary OCSCC SUVmax at T1 scan in patients with imaging irAEs compared with those without. This may indicate overall immune activation but, given the lack of correlation with pathologic tumor response across all parameters evaluated, the clinical meaning remains uncertain. Data are mixed in other malignant neoplasms, with some studies demonstrating associations of irAEs with response26,27,28,29,30 and others not, including a meta-analysis of a limited number of studies.31
Limitations
This study had several limitations, notably the small participant number. However, prospective inclusion of FDG-PET/CT scans in the study design led to standardization of imaging time points and correlation to histopathology and tissue biomarkers. Possible differences in response between the 2 treatment cohorts was a potential limitation, but given that the goal of this study was to compare changes in FDG uptake to pathology, and the reported efficacy outcomes between the arms were similar, this was not considered a major limitation. As discussed in the main clinical study,13 the lack of a validated end point for clinically meaningful response to preoperative immunotherapy was also a limitation. We chose to compare FDG uptake and changes of FDG uptake to pathologic response as a continuous variable; pathologic response has been increasingly associated with favorable clinical outcome in patients treated with neoadjuvant immunotherapy.32 Finally, heterogeneity of T-cell infiltration within the primary tumor and sampling bias from a small biopsy are potential limitations for the comparison of CD8+ cell number and tumor FDG uptake at baseline.
Conclusions
In conclusion, this is the first report of early FDG-PET/CT assessment after starting preoperative immunotherapy for OCSCC. We identified important early patterns of FDG uptake that frequently do not reflect progressive disease, namely the development of new ipsilateral and contralateral LNs. The presence of irAEs on imaging in combination with the new LN added confidence to the interpretation of an immune-related systemic response, rather than progressive disease. These findings argue against altering surgical plans in this setting. Finally, the lack of FDG uptake correlation with CD8+ tumor cell number alone highlights the complexity of measuring tumor response early after preoperative immunotherapy initiation and suggests a limited role for early FDG-PET/CT as an imaging biomarker for predicting pathological response to preoperative immunotherapy for OCSCC.
eFigure. Correlation of CD8 and FDG Uptake
eTable 1. FDG-PET/CT Technical Parameters (median, range)
eTable 2. Correlation between change of primary tumor SUVmax and pathologic response (%) in two treatment cohorts
References
- 1.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res. 2019;25(19):5743-5751. doi: 10.1158/1078-0432.CCR-18-2641 [DOI] [PubMed] [Google Scholar]
- 5.Kim PH, Suh CH, Kim HS, et al. Immune checkpoint inhibitor with or without radiotherapy in melanoma patients with brain metastases: a systematic review and meta-analysis. Korean J Radiol. 2021;22(4):584-595. doi: 10.3348/kjr.2020.0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharmacologic intervention, part 1: the role of 18F-FDG PET/CT. J Nucl Med. 2020;61(7):943-950. doi: 10.2967/jnumed.119.234278 [DOI] [PubMed] [Google Scholar]
- 7.Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46(1):238-250. doi: 10.1007/s00259-018-4171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35(2):424-437. doi: 10.1148/rg.352140121 [DOI] [PubMed] [Google Scholar]
- 9.Marandino L, Capozza A, Briganti A, et al. Secondary analysis of PURE-01: role of FDG-PET/CT in evaluating lymph node involvement of patients with muscle invasive bladder cancer (MIBC) receiving neoadjuvant pembrolizumab and radical cystectomy (RC). J Clin Oncol. 2020;38(6_suppl):445-445. doi: 10.1200/JCO.2020.38.6_suppl.445 [DOI] [Google Scholar]
- 10.Zuur L VJ, Elbers JB, Krijgsman O, Qiao X, et al. LBA40 Neoadjuvant nivolumab and nivolumab plus ipilimumab induce (near-) complete responses in patients with head and neck squamous cell carcinoma: the IMCISION trial Abstract. Ann Onc. 2020;31(4):S1169. doi: 10.1016/j.annonc.2020.08.2270 [DOI] [Google Scholar]
- 11.Marandino L, Capozza A, Bandini M, et al. Incidence and clinical impact of inflammatory fluorodeoxyglucose positron emission tomography uptake after neoadjuvant pembrolizumab in patients with organ-confined bladder cancer undergoing radical cystectomy. Eur Urol Focus. . 2021;7(5):1092-1099. doi: 10.1016/j.euf.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Marandino L, Capozza A, Bandini M, et al. [18F]Fluoro-deoxy-glucose positron emission tomography to evaluate lymph node involvement in patients with muscle-invasive bladder cancer receiving neoadjuvant pembrolizumab. Urol Oncol. 2021;39(4):235.e15-235.e21. doi: 10.1016/j.urolonc.2020.09.035 [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6(10):1563-1570. doi: 10.1001/jamaoncol.2020.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang B, Huang A, Chang C, et al. Evaluation of the anti-PD-flare response in patinets with advanced melanoma using FDG PET/CT imaging and hematologic biomarkers. J Nucl Med. 2019;60(1):1270.30737300 [Google Scholar]
- 15.Cho SY, Lipson EJ, Im HJ, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58(9):1421-1428. doi: 10.2967/jnumed.116.188839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, Oh JS, Kim JS. Immune microenvironment of the gene signature reflecting the standardised uptake value on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in head and neck squamous cell carcinoma. Ann Nucl Med. 2021;35(1):65-75. doi: 10.1007/s12149-020-01537-9 [DOI] [PubMed] [Google Scholar]
- 17.Togo M, Yokobori T, Shimizu K, et al. Diagnostic value of 18F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8+tumour-infiltrating lymphocytes in oral squamous cell carcinoma. Br J Cancer. 2020;122(11):1686-1694. doi: 10.1038/s41416-020-0820-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashi K, Clavo AC, Wahl RL. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med. 1993;34(3):414-419. [PubMed] [Google Scholar]
- 19.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5(11):844-852. doi: 10.1038/nri1710 [DOI] [PubMed] [Google Scholar]
- 20.Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015;6:1. doi: 10.3389/fimmu.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balermpas P, Michel Y, Wagenblast J, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501-509. doi: 10.1038/bjc.2013.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpathiou G, Casteillo F, Giroult JB, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8(12):19310-19322. doi: 10.18632/oncotarget.14242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larimer BM, Bloch E, Nesti S, et al. The effectiveness of checkpoint inhibitor combinations and administration timing can be measured by granzyme B PET imaging. Clin Cancer Res. 2019;25(4):1196-1205. doi: 10.1158/1078-0432.CCR-18-2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with 89Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61(4):512-519. doi: 10.2967/jnumed.119.229781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronald JA, Kim BS, Gowrishankar G, et al. A PET imaging strategy to visualize activated T cells in acute graft-versus-host disease elicited by allogenic hematopoietic cell transplant. Cancer Res. 2017;77(11):2893-2902. doi: 10.1158/0008-5472.CAN-16-2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237-247.e1. doi: 10.1016/j.cllc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 27.Ishihara H, Takagi T, Kondo T, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019;37(6):355.e21-355.e29. doi: 10.1016/j.urolonc.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Kim HI, Kim M, Lee SH, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. 2017;7(1):e1375642. doi: 10.1080/2162402X.2017.1375642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada N, Kawazoe H, Takechi K, et al. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther. 2019;41(1):59-67. doi: 10.1016/j.clinthera.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785-792. doi: 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 31.Ouwerkerk W, van den Berg M, van der Niet S, Limpens J, Luiten RM. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res. 2019;29(5):453-464. doi: 10.1097/CMR.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021;27(2):301-309. doi: 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Correlation of CD8 and FDG Uptake
eTable 1. FDG-PET/CT Technical Parameters (median, range)
eTable 2. Correlation between change of primary tumor SUVmax and pathologic response (%) in two treatment cohorts