Abstract
One anastomosis gastric bypass (OAGB) is an emerging bariatric procedure, yet data on its effect on the gastrointestinal tract are lacking. This study sought to evaluate the incidence of small-intestinal bacterial overgrowth (SIBO) following OAGB; explore its effect on nutritional, gastrointestinal, and weight outcomes; and assess post-OABG occurrence of pancreatic exocrine insufficiency (PEI) and altered gut microbiota composition. A prospective pilot cohort study of patients who underwent primary-OAGB surgery is here reported. The pre-surgical and 6-months-post-surgery measurements included anthropometrics, glucose breath-tests, biochemical tests, gastrointestinal symptoms, quality-of-life, dietary intake, and fecal sample collection. Thirty-two patients (50% females, 44.5 ± 12.3 years) participated in this study, and 29 attended the 6-month follow-up visit. The mean excess weight loss at 6 months post-OAGB was 67.8 ± 21.2%. The glucose breath-test was negative in all pre-surgery and positive in 37.0% at 6 months (p = 0.004). Positive glucose breath-test was associated with lower reported dietary intake and folate levels and higher vitamin A deficiency rates (p ≤ 0.036). Fecal elastase-1 test (FE1) was negative for all pre-surgery and positive in 26.1% at 6 months (p = 0.500). Both alpha and beta diversity decreased at 6 months post-surgery compared to pre-surgery (p ≤ 0.026). Relatively high incidences of SIBO and PEI were observed at 6 months post-OAGB, which may explain some gastrointestinal symptoms and nutritional deficiencies.
Keywords: gastric bypass, small-intestinal bacterial overgrowth, pancreatic exocrine insufficiency, gut microbiota
1. Introduction
One anastomosis gastric bypass (OAGB), an emerging bariatric surgery (BS) [1,2], employs a long narrow-sleeve gastric tube in conjunction with end-to-side or side-to-side gastrojejunostomy performed 150–200 cm distal to the ligament of Treitz [2,3]. This surgical technique was found to be effective in terms of weight loss and co-morbidities improvements [2]. However, some areas of concern remain, including substantial gastrointestinal (GI) symptoms [4].
Altered bowel anatomy and motility caused by OAGB could create a blind intestinal loop resulting in small-intestinal bacterial overgrowth (SIBO) [5,6,7,8]. SIBO, characterized by the presence of excessive bacteria in the small intestine [9], is related to the presence of symptoms such as bloating, diarrhea, and gas, but it may also be asymptomatic [9,10]. The traditional “gold standard” for diagnosing SIBO is a culture of the intestinal fluid, but this method has several technical and definition hurdles [8,9,11]. The breath test, a non-invasive test that detects the presence of exhaled hydrogen (H2) or methane [9,11] following the ingestion of a carbohydrate substrate, is considered a valid, inexpensive, and safe diagnostic test [12]. Presently, there is no standardized protocol for SIBO assessment in patients who underwent BS [8], and data exploring the prevalence and implications of SIBO following BS are limited [6,13,14,15,16,17,18].
Pancreatic exocrine insufficiency (PEI), a well-known complication after upper GI surgery [19,20,21], refers to an insufficient secretion of pancreatic enzymes and/or sodium bicarbonate that prevents normal digestion [22]. PEI symptoms may include steatorrhea, abdominal pain, and flatulence [19,20,22,23]. Fecal elastase-1 (FE1) serves as a non-invasive marker of pancreatic secretion, and its measurement is considered a relatively reliable diagnostic test [19,20,24]. Notably, pancreatic exocrine function was evaluated only in a limited number of studies among BS patients [19,23,24].
Different studies on various BS techniques have shown that these surgeries induce drastic changes in gut microbiota composition [25,26,27]. Currently, most studies reported on microbiota composition changes following Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), adjustable gastric banding (AGB), and vertical banded gastroplasty (VBG) [25,26,27,28,29]. However, to the best of our knowledge, no study has investigated these changes following OAGB.
Therefore, the primary aim of this study was to evaluate the incidence of SIBO following OAGB and to explore its effect on nutritional deficiencies, GI symptoms, and weight loss. The secondary aims were to evaluate PEI incidence and to elucidate alterations in the gut microbiota composition at 6 months following OAGB.
2. Materials and Methods
2.1. Study Population
A pilot prospective cohort study included 32 patients who underwent primary-OAGB surgery at the Assuta Medical Centers from October 2018 to March 2020. Inclusion criteria were age between 18 and 65 years, body mass index (BMI) ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with co-morbidities [30], and approval of the Assuta Medical Centers BS committee to undergo BS and primary-OAGB surgery. Exclusion criteria included the use of antibiotics [9,12] or probiotics in the preceding month, concomitant use of promotility drugs and laxatives one week prior to each study examination [9,12], uncontrolled mental illness or cognitive deterioration, chronic medical conditions that could interfere with the study (e.g., active cancer, organ-transplant subjects, and advanced kidney disease), treatment with insulin, excessive alcohol consumption, and pregnancy/lactation.
The study was approved by the Assuta Medical Center Institutional Ethics Committee (#0014-18-ASMC), and informed consent was obtained from all participants.
All patients received the standard of care during the study period [30,31]. The manuscript was written according to the STROBE cohort checklist [32].
2.2. Pre- and Post-Surgical Follow-Up Evaluations (Baseline and 6 Months)
2.2.1. Medical History
An interview at both time-points collected data on smoking habits and the use of medications and supplements.
2.2.2. Anthropometric Measurements
Height was measured without shoes, with a wall-mounted stadiometer; weight was measured with a high-capacity weigh-scale; and BMI was calculated and expressed in kg/m2. Waist circumference (WC) was measured twice at the umbilicus level [33], and neck circumference (NC) was measured twice, with head erect and eyes facing forward, at the level of the cricothyroid membrane [34]. Excess weight loss (EWL) percentage was calculated as previously recommended [35].
2.2.3. Glucose Breath-Test to Assess SIBO
The test protocol for the breath-test to assess SIBO was performed in accordance with a recently published North American consensus regarding breath-testing in GI disorders [12], but with slight modifications [36]. The breath-test was initiated by collecting a baseline sample where the H2 levels in patient’s breath were examined by a breath analyzer (Gastro+™ Gastrolyzer®, coVita, Santa Barbara, CA, USA). Then the patients ingested a dose of 50 gr glucose mixed with one cup of water (250 mL) [36]. The participants exhaled into a breath analyzer every 30 min for 2 h, while H2 was measured in expired air. A rise in H2 of ≥20 parts per million (ppm) during the test when compared with the basal value was considered indicative of SIBO [12]. Patients with SIBO were divided to “early risers” or “late risers” (i.e., the rise in H2 of ≥20 ppm occurred within 60 min or after 60 min, respectively). This cutoff was based on the observation that the median oral–cecal transit time was 60 min following RYGB [8].
A trained physician performed all assays, and the preparation for the test was verified for each participant with the research team. The test was performed under standard conditions, including a low-carbohydrate diet the day prior to testing and at least 12 h of fasting [9,12]. On the day of testing, smoking was asked to be avoided, and physical activity was asked to be limited during the testing period [9,12].
2.2.4. Assessment of Gastrointestinal Symptoms
Data on GI symptoms were collected by an interview, and all participants filled in the Irritable Bowel Syndrome Rome III Diagnostic Criteria questionnaire [37].
2.2.5. Biochemical Tests
Each participant underwent biochemical testing following a 12 h fast, including complete blood count, albumin, total protein, transferrin, ferritin, iron, vitamin B12, folic acid, vitamin A, and vitamin D. Nutritional abnormalities were defined as a plasma level below/above the recommended reference range (Table 1).
Table 1.
Characteristics of the study participants at baseline and at 6 months post-surgery.
| Variable a | Baseline (n = 32) |
6 Months Post-Surgery (n = 29) | p-Value |
|---|---|---|---|
| Age (years) | 44.5 ± 12.3 | - | - |
| Sex (%female) | 50 | - | - |
| Marital Status (%) | |||
| Married | 75 | - | - |
| Divorced | 6.3 | - | - |
| Single | 18.8 | - | - |
| Co-morbidities (%) | |||
| Diabetes | 9.4 | - | - |
| IFG | 50 | - | - |
| NAFLD | 84.4 | - | - |
| Dyslipidemia | 59.4 | - | - |
| Hypertension | 21.9 | - | - |
| Sleep Apnea | 15.6 | - | - |
| Hypothyroidism | 3.1 | - | - |
| Gastroesophageal Reflux Disease | 21.9 | - | - |
| Orthopedic Problems | 31.3 | - | - |
| Medication (%) | |||
| Drugs for diabetes | 6.3 | 0 | 0.500 |
| Drugs for dyslipidemia | 12.5 | 3.4 | 0.500 |
| Drugs for Hypertension | 18.8 | 10.3 | 0.500 |
| Anti-aggregation drugs | 6.3 | 3.4 | 1.000 |
| Drugs for hypothyroidism | 3.1 | 3.4 | 1.000 |
| Antacids | 9.4 | 31 | 0.109 |
| Anti-depressive drugs | 3.1 | 3.4 | 1.000 |
| Anthropometrics | |||
| Weight (kg) | 120.8 ± 25.2 | 90.9 ± 20.3 | <0.001 |
| Height (meter) | 1.7 ± 0.1 | - | - |
| BMI (kg/m2) | 41.7 ± 6.6 | 31.4 ± 5.6 | <0.001 |
| WC (cm) | 122.8 ± 14.3 | 100.5 ± 13.9 | <0.001 |
| NC (cm) | 38.7 ± 4.5 | 34.8 ± 3.7 | <0.001 |
| %EWL | NR | 67.8 ± 21.2 | - |
| Lifestyle | |||
| Smoking (%yes) | 6.3 | 6.9 | 1.000 |
| Physical activity (%yes) | 56.3 | 79.3 | 0.180 |
| Physical activity (h/week) | 1.2 ± 1.6 | 2.8 ± 2.3 | 0.017 |
| Dietary intake | |||
| Calories (kcal/day) | 2563.0 ± 979.6 | 1626.7 ± 712.8 | <0.001 |
| Protein (g/day) | 136.7 ± 48.8 | 87.3 ± 40.2 | <0.001 |
| Carbohydrates (g/day) | 226.7 ± 85.2 | 150.0 ± 73.7 | <0.001 |
| Fats (g/day) | 116.8 ± 63.4 | 69.5 ± 38.4 | <0.001 |
| Percent of food intake compared to before surgery | NR | 31.7 ± 14.6 | - |
| No. of dietitian appointments after surgery | NR | 4.3 ± 3.5 | - |
| Participation in support group after surgery (%yes) | NR | 13.8 | - |
| Supplementation (%) | |||
| Multivitamin | 43.8 | 93.1 | <0.001 |
| Calcium | 6.3 | 62.1 | <0.001 |
| Vitamin D | 62.5 | 86.2 | 0.039 |
| Vitamin B12 | 34.4 | 72.4 | 0.007 |
| Iron | 18.8 | 24.1 | 0.375 |
| Folic Acid | 18.8 | 6.9 | 0.250 |
| Biochemical tests ᵇ | |||
| Hemoglobin (g/dL) | 14.0 ± 1.3 | 13.2 ± 1.2 | <0.001 |
| %anemia (<13.5(male), <12(female)) |
12.5 | 28.6 | 0.063 |
| MCV (fL) | 83.3 ± 4.7 | 86.0 ± 5.1 | <0.001 |
| %low values (<80) | 25.0 | 10.7 | 0.063 |
| %high values (>95) | 0 | 0 | NR |
| MCHC (g/dL) | 33.8 ± 0.7 | 33.6 ± 0.7 | 0.632 |
| %low values (<33) | 6.3 | 14.3 | 0.625 |
| %high values (>37) | 0 | 0 | NR |
| Albumin (g/dL) | 4.4 ± 0.3 | 4.2 ± 0.3 | 0.002 |
| %hypoalbuminemia (<3.5) | 0 | 3.6 | 1.000 |
| Total protein (g/dL) | 7.6 ± 0.4 | 7.2 ± 0.4 | <0.001 |
| %low values (<6.3) | 0 | 0 | NR |
| Iron (µg/dL) | 90.3 ± 29.8 | 80.6 ± 25.1 | 0.107 |
| %deficiency (<49 [male], <37 [female]) |
0 | 3.7 | 1.000 |
| Ferritin (ng/mL) | 155.0 ± 138.3 | 154.4 ± 125.3 | 0.564 |
| %deficiency (<22[male], <10[female]) |
0 | 0 | NR |
| Transferrin (mg/dL) | 277.7 ± 37.5 | 238.9 ± 55.7 | 0.001 |
| %low values (<220) | 6.3 | 29.6 | 0.070 |
| %high values (>400) | 0 | 0 | NR |
| Transferrin saturation (%) | 23.8 ± 8.8 | 26.4 ± 14.1 | 0.323 |
| %low values (<20) | 43.8 | 33.3 | 0.289 |
| Folate (ng/mL) | 11.0 ± 5.6 | 12.4 ± 5.7 | 0.285 |
| %deficiency (<2.76) | 0 | 0 | NR |
| Vitamin B12 (pg/mL) | 519.5 ± 233.1 | 534.6 ± 221.9 | 0.725 |
| %deficiency (<239) | 0 | 3.7 | 1.000 |
| Vitamin D (ng/mL) | 25.3 ± 8.6 | 27.5 ± 10.2 | 0.366 |
| %insufficiency (<30) | 78.1 | 63.0 | 0.289 |
| %deficiency (<20) | 25.0 | 18.5 | 1.000 |
| Vitamin A (µg/dL) | - | 42.4 ± 10.1 | - |
| %deficiency (<30) | - | 15.4 | - |
| Quality of life | |||
| VAS QoL | 63.8 ± 18.3 | 81.0 ± 16.0 | <0.001 |
| M-A QoLII score c | 1.1 ± 0.8 | 1.8 ± 0.9 | 0.002 |
| M-A QoLII (%Good/Very good) d | 56.3 | 82.8 | 0.065 |
| GI symptoms (%) | |||
| ROME III score (%positive) e | 9.4 | 20.7 | 0.250 |
| Vomit | 0 | 0 | NR |
| Nausea | 6.3 | 24.1 | 0.125 |
| Regurgitation | 9.4 | 10.3 | 1.000 |
| Hiccups | 9.4 | 31.0 | 0.070 |
| Heartburn | 28.1 | 10.3 | 0.070 |
| Abdominal pain | - | 17.2 | - |
| Flatulence | 12.5 | 58.6 | 0.002 |
| Frequent soft stool | 6.3 | 34.5 | 0.021 |
| No. of feces per day | 1.5 ± 0.8 | 1.7 ± 1.2 | 0.188 |
| ≥3 feces per day | 3.1 | 13.8 | 0.250 |
| Hair loss | 21.9 | 48.3 | 0.016 |
| Glucose breath test (%positive) f,g | 0 | 37.0 | 0.004 |
| PEI (%positive) g,h | 0 | 26.1 i | 0.500 |
Abbreviations: body mass index (BMI), excess weight loss (EWL), gastrointestinal (GI), impaired fasting glucose (IFG), mean cell hemoglobin concentration (MCHC), mean cell volume (MCV), Moorehead–Ardelt Quality of Life Questionnaire II (M-A QoLII); neck circumference (NC), nonalcoholic fatty liver disease (NAFLD), not relevant (NR), pancreatic exocrine insufficiency (PEI), visual analogue scale quality of life (VAS QoL), waist circumference (WC). a Values expressed as the mean ± standard deviation, unless otherwise stated. b n = 32 for this test at baseline and n = 27 for this test at 6 months post-surgery. c A 10-point Likert scale is used for scoring, and its total score ranges from −3 to +3 (very poor to very good quality of life). d Score of 1.1–2 equals “good quality of life”, and 2.1–3 equals “very good quality of life”. e Irritable Bowel Syndrome was considered present when abdominal pain occurred more than 2 to 3 days a month, relieved after defecation, was related to changes in form and frequency of defecation and existed for 6 months or more. f n = 31 for this test at baseline and n = 27 for this test at 6 months post-surgery. g There was no difference in bypassed limb length between patients with positive and negative glucose breath test or FE1 test. h n = 12 for this test at baseline, and n = 23 for this test at 6 months post-surgery. i Four patients were categorized with severe PEI (FE1 < 100 µg/g), and 2 patients with mild-to-moderate PEI (FE1 = 100–200 µg/g).
2.2.6. Quality-of-Life Assessment
Quality of life (QoL) was assessed by the Moorehead–Ardelt QoL Questionnaire II (M-A QoLII), which includes 6 key areas: self-esteem, physical activity, social contacts, satisfaction concerning work, pleasure related to sexuality, and eating behavior [38,39,40]. In addition, patients were asked to rate their overall state-of-health from 0 to 100, using a visual analog scale QoL (VAS QoL), with 100 reflecting the “best imaginable state-of-health” [41].
2.2.7. Dietary Intake Assessment
Dietary intake in the last month before testing was evaluated by using a food frequency questionnaire (FFQ) [42] that was modified for the current study. The nutrient content of the food items was obtained from the Israeli nutritional software “Zameret” [43].
2.2.8. Physical Activity
Data on physical activity in the last month before testing were collected by an interview. Weekly hours spent in physical activity were calculated by the number of training sessions per week × the duration of exercise in hours.
2.2.9. Fecal Sample Collection and Analysis
Stool samples were self-collected in sterile tubes and a regular FLOQSwabs® transport system (COPAN ITALIA spa, Brescia, Italy) given to the participants in advance at baseline and at 6 months post-surgery and stored at −80 °C until processed and analyzed. For the diagnosis of PEI, samples with a concentration of FE1 of >200 µg/g stool were considered normal. Samples that demonstrated the concentrations ranging from 200 to 100 µg/g stool were considered as exhibiting mild-to-moderate PEI, and <100 µg/g stool were considered as exhibiting severe PEI [21]. 16S amplicon sequencing and microbiome analyses were undertaken, including comparisons of relative abundance at the phylum and genus levels, comparisons of alpha and beta diversity, and differential abundance analysis. Fecal-sample collection and analysis are further detailed in Supplementary Materials Methods S1 [44,45,46,47,48,49].
2.3. Statistical Methods
The IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA) was used for statistical analyses. Descriptive statistics were used to describe the distribution of variables associated with characteristics of the study sample. Continuous variables were presented as means ± SD and categorical variables as proportions. Continuous variables that failed the normality test were analyzed by using nonparametric tests.
To compare continuous variables between two time-points, the t-test for dependent groups was performed, and for dichotomous variables, the McNemar test was performed. To test differences in continuous variables between two groups, the independent-samples t-test was performed. For comparison of categorical variables, the Chi-Square test or Fisher’s exact test was performed. The level of significance for all analyses was set at p < 0.05. For the statistical methods used for the comparison of the results of microbiota analyses, see Supplementary Materials Methods S1.
3. Results
3.1. Characteristics of the Study Participants at Baseline and at 6 Months Post-Surgery
Thirty-two patients (50% females) who underwent primary OAGB surgery participated in this study, and 29 of them attended the 6-month follow-up visit (range: 5.6–7.1 months). One patient underwent BS in another hospital, one cancelled the surgery, and one withdrew from the study. Their mean age and BMI pre-surgery were 44.5 ± 12.3 years (range: 18–62 years) and 41.7 ± 6.6 kg/m2 (range: 33.0–62.2 kg/m2), respectively. The mean length of the bypassed limb was 176.3 ± 21.9 cm (range: 150–200 cm).
At 6 months post-surgery, %EWL was 67.8 ± 21.2%, patients reported higher QoL scores, hemoglobin was significantly reduced, patients reported significantly lower dietary intake and higher rates of flatulence, frequent soft stool, and hair-loss when compared to baseline (p ≤ 0.021 for all) (Table 1). The glucose breath-test was negative in all patients at baseline and positive in 10/27 (37.0%) patients at 6 months post-surgery (p = 0.004) (Table 1). Figure 1A,B presents the mean glucose breath-test results at baseline and 6 months post-surgery, respectively.
Figure 1.
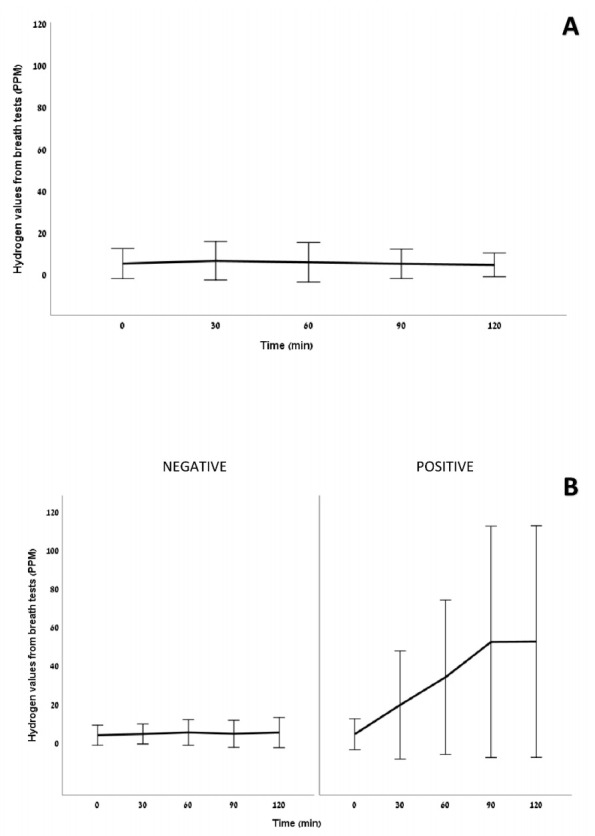
Mean (±SD) glucose breath-test results (A) at baseline and (B) at 6 months post-surgery. Abbreviations: parts per million (PPM); hydrogen (H2). In Figure 1A, H2 values for all at 0 min were 4.6 ± 3.6 (range: 1–15). In Figure 1B, H2 values for all at 0 min were 3.5 ± 3.1 (range: 1–13); a rise in H2 of ≥20 ppm during the test when compared with the basal value considered as indicative of SIBO; Among the patients with a positive glucose breath-test, 6 (60%) were “early risers” (i.e., H2 of ≥20 ppm rise occurred up to 60 min), and 4 (40%) were “late risers” (i.e., H2 of ≥20 ppm rise occurred after 60 min).
The FE1 test was negative for all patients at baseline (n = 12) and positive in 6/23 (26.1%) patients at 6 months post-surgery (p = 0.500) (Table 1).
There were no re-hospitalizations during the study follow-up term, but two patients reported emergency-room visits due to high fever and abdominal pain.
3.2. Comparison between Patients According to Glucose Breath-Test Results at 6 Months Post-Surgery
No significant difference was found between patients with positive and negative glucose breath-tests at baseline. A comparison between patients according to their glucose breath-test results at 6 months post-surgery is presented in Table 2.
Table 2.
Comparison between patients according to their glucose breath-test results at 6 months post-surgery a.
| Variable b | SIBO Positive (n = 10) | SIBO Negative (n = 17) | p-Value |
|---|---|---|---|
| Age (years) | 41.0 ± 9.9 | 48.3 ± 12.8 | 0.133 |
| Sex (%female) | 80 | 41.2 | 0.107 |
| Medication (%) | |||
| Drugs for diabetes | 0 | 0 | NR |
| Drugs for dyslipidemia | 0 | 5.9 | 1.000 |
| Drugs for Hypertension | 0 | 17.6 | 0.274 |
| Anti-aggregates | 0 | 5.9 | 1.000 |
| Drugs for hypothyroidism | 0 | 5.9 | 1.000 |
| Anti-acids | 20 | 35.3 | 0.666 |
| Drugs for depression | 10 | 0 | 0.370 |
| Bypass length (cm) | 175.0 ± 26.4 | 175.3 ± 19.7 | 0.976 |
| Anthropometric measurements | |||
| Weight (kg) | 87.2 ± 15.2 | 93.7 ± 23.8 | 0.449 |
| BMI (kg/m2) | 32.0 ± 5.4 | 31.2 ± 6.1 | 0.755 |
| WC (cm) | 97.2 ± 9.3 | 102.3 ± 16.2 | 0.370 |
| NC (cm) | 33.3 ± 3.2 | 35.5 ± 4.0 | 0.158 |
| %EWL | 64.8 ± 19.6 | 69.4 ± 22.5 | 0.600 |
| Lifestyle | |||
| Smoking (%yes) | 10 | 5.9 | 1.000 |
| Physical Activity (%yes) | 80 | 82.4 | 1.000 |
| Physical Activity (h/week) | 2.7 ± 2.3 | 2.9 ± 2.4 | 0.766 |
| Dietary intake | |||
| Calories (kcal/day) | 1192.9 ± 471.6 | 1908.4 ± 704.1 | 0.009 |
| Protein (g/day) | 65.1 ± 26.9 | 101.3 ± 42.2 | 0.023 |
| Carbohydrates (g/day) | 113.6 ± 52.9 | 175.1 ± 77.3 | 0.036 |
| Fats (g/day) | 49.2 ± 19.3 | 82.1 ± 41.8 | 0.028 |
| Percent of food intake compared to before surgery | 33.5 ± 13.1 | 28.8 ± 13.1 | 0.414 |
| No. of dietitian appointments after surgery | 3.5 ± 1.8 | 4.6 ± 4.4 | 0.675 |
| Participation in support group after surgery (%) | 10 | 17.6 | 1.000 |
| Supplementation (%) | |||
| Multivitamin | 90 | 94.1 | 1.000 |
| Calcium | 60 | 64.7 | 1.000 |
| Vitamin D | 70 | 94.1 | 0.128 |
| Vitamin B12 | 60 | 82.4 | 0.365 |
| Iron | 10 | 29.4 | 0.363 |
| Folic Acid | 0 | 11.8 | 0.516 |
| Biochemical tests | |||
| Hemoglobin (g/dL) | 13.2 ± 0.4 | 13.2 ± 1.5 | 0.928 |
| %anemia (<13.5(male), <12(female)) |
20 | 35.3 | 0.666 |
| MCV (fL) | 88.1 ± 3.2 | 84.9 ± 5.8 | 0.116 |
| %low values (<80) | 0 | 17.6 | 0.274 |
| %high values (>95) | 0 | 0 | NR |
| MCHC (g/dL) | 33.7 ± 0.7 | 33.7 ± 0.7 | 0.891 |
| %low values (<33) | 10 | 11.8 | 1.000 |
| %high values (>37) | 0 | 0 | NR |
| Albumin (g/dL) | 4.2 ± 0.4 | 4.2 ± 0.3 | 0.967 |
| %hypoalbuminemia (<3.5) | 10 | 0 | 0.370 |
| Total protein (g/dL) | 7.2 ± 0.4 | 7.2 ± 0.4 | 0.994 |
| %low values (<6.3) | 0 | 0 | NR |
| Iron (µg/dL) | 75.9 ± 13.4 | 83.3 ± 30.0 | 0.470 |
| %deficiency (<49(male), <37(female)) |
0 | 5.9 | 1.000 |
| Ferritin (ng/mL) | 98.9 ± 80.4 | 187.0 ± 137.2 | 0.077 |
| %deficiency (<22(male), <10(female)) |
0 | 0 | NR |
| Transferrin (mg/dL) | 249.8 ± 60.3 | 232.5 ± 53.7 | 0.477 |
| %low values (<220) | 20 | 35.3 | 0.666 |
| %high values (>400) | 0 | 0 | NR |
| Transferrin saturation (%) | 23.1 ± 6.5 | 28.3 ± 17.0 | 0.364 |
| %low values (<20) | 40 | 29.4 | 0.683 |
| Folate (ng/mL) | 8.4 ± 3.6 | 14.7 ± 5.5 | 0.003 |
| %deficiency (<2.76) | 0 | 0 | NR |
| Vitamin B12 (pg/mL) | 442.1 ± 212.8 | 589.1 ± 214.6 | 0.097 |
| %deficiency (<239) | 10 | 0 | 0.370 |
| Vitamin D (ng/mL) | 25.5 ± 10.2 | 28.7 ± 10.4 | 0.444 |
| %insufficiency (<30) | 60 | 64.7 | 1.000 |
| %deficiency (<20) | 30 | 11.8 | 0.326 |
| Vitamin A (µg/dL) | 38.5 ± 12.9 | 44.9 ± 7.3 | 0.171 |
| %deficiency (<30) | 40 | 0 | 0.014 |
| Quality of life | |||
| VAS QoL | 77.5 ± 19.5 | 84.4 ± 9.2 | 0.317 |
| M-A QoLII score c | 1.7 ± 0.7 | 2.0 ± 0.7 | 0.265 |
| M-A QoLII (%Good/Very good) d | 80 | 88.2 | 0.613 |
| GI symptoms (%) | |||
| ROME III score (%positive) e | 10 | 23.5 | 0.621 |
| Vomit | 0 | 0 | NR |
| Nausea | 20 | 23.5 | 1.000 |
| Regurgitation | 30 | 0 | 0.041 |
| Hiccups | 40 | 23.5 | 0.415 |
| Heartburn | 10 | 11.8 | 1.000 |
| Abdominal pain | 30 | 11.8 | 0.326 |
| Flatulence | 50 | 64.7 | 0.453 |
| Frequent soft stool | 50 | 17.6 | 0.102 |
| No. of feces per day | 1.6 ± 0.7 | 1.8 ± 1.4 | 0.598 |
| ≥3 feces per day | 10 | 17.6 | 1.000 |
| Hair loss | 70 | 35.3 | 0.120 |
| PEI (%positive) f | 22.2 | 23.1 | 1.000 |
Abbreviations: body mass index (BMI), excess weight loss (EWL), gastrointestinal (GI), mean cell hemoglobin concentration (MCHC), mean cell volume (MCV), Moorehead–Ardelt Quality of Life Questionnaire II (M-A QoLII), neck circumference (NC), not relevant (NR), pancreatic exocrine insufficiency (PEI), small intestine bacterial overgrowth (SIBO), visual analogue scale quality of life (VAS QoL), waist circumference (WC). a No significant differences were found between positive SIBO and negative SIBO groups at baseline. b Values expressed as the mean ± standard deviation, unless otherwise stated. c A 10-point Likert scale is used for scoring, and its total score ranges from −3 to +3 (very poor to very good quality of life). d Score of 1.1–2 equals “good quality of life”, and 2.1–3 equals “very good quality of life”. e Irritable Bowel Syndrome was considered present when abdominal pain occurred more than 2 to 3 days a month, relieved after defecation, was related to changes in form and frequency of defecation, and existed for 6 months or more. f n = 22, additional patient had FE1 test but not glucose breath-test.
The reported dietary intake was significantly lower among patients with a positive glucose breath-test (p ≤ 0.036). Lower levels of folate (8.4 ± 3.6 vs. 14.7 ± 5.5 ng/mL, p = 0.003) and higher vitamin A deficiency rates (40 vs. 0%, p = 0.014) were found among the positive glucose breath-test group, although no significant difference in supplement use was found between both groups. Regurgitation was significantly more common among the positive glucose breath-test group (30 vs. 0%, p = 0.041) (Table 2).
3.3. Gut Microbiota Analysis
The phyla Proteobacteria and Verrucomicrobia showed significant increases in relative abundance over time, whereas Firmicutes, Fusobacteria, and Tenericutes showed significant decreases in relative abundance over time for all patients.
Changes in the relative abundance of main phyla over time are presented in Figure 2 and Table 3. Changes in the relative abundance of main genera over time are presented in Figure 3 and Supplementary Materials Table S1. Moreover, significant changes were observed in the differential abundance analysis at the genera level, using LefSe (Supplementary Materials Figure S1 and Table S2).
Figure 2.
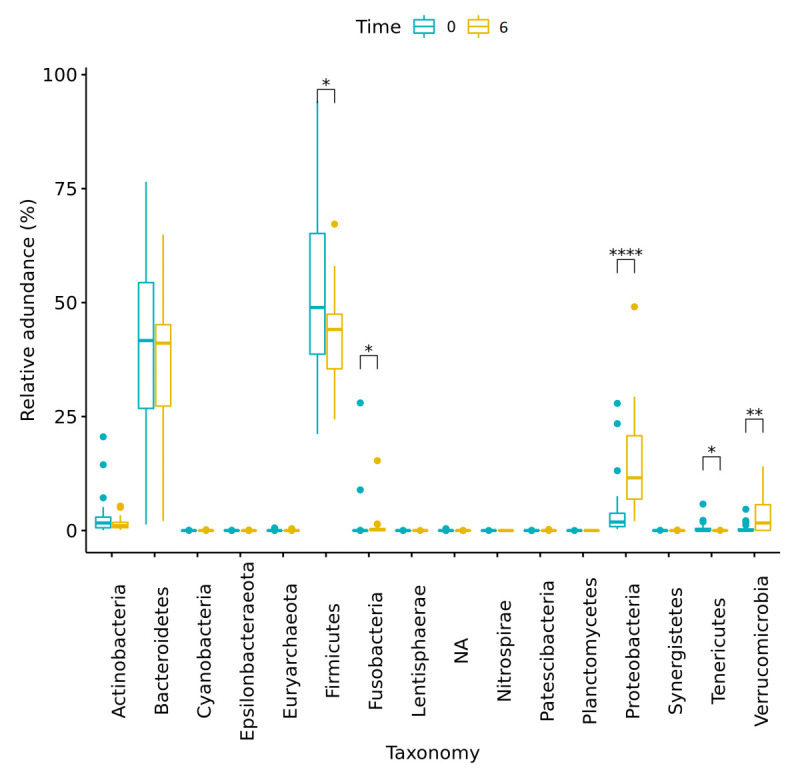
Changes in relative abundances of phyla for all patients (n = 28) from baseline (Time 0) to 6 months post-surgery (Time 6). Significant differences in abundance are marked with an asterisk (* indicates p < 0.05; ** indicates p < 0.005; **** indicates p < 0.00005). NA = sequences that were assigned to kingdom bacteria but were not assigned to a specific phylum.
Table 3.
Changes in relative abundances of phyla for all patients from baseline to 6 months post-surgery (n = 28). Only significant results (p < 0.05) are listed.
| Phyla | Relative Abundance at Baseline (%) | Relative Abundance at 6 Months Post-Surgery (%) | p-Value (FDR Adjusted) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | ||
| Firmicutes | 52.28 | 27.54 | 86.38 | 42.47 | 24.35 | 67.20 | 0.021 |
| Fusobacteria | 1.320 | 0 | 27.99 | 0.87 | 0 | 15.32 | 0.021 |
| Proteobacteria | 4.533 | 0.288 | 27.88 | 14.17 | 2.046 | 49.07 | <0.001 |
| Tenericutes | 0.505 | 0 | 5.802 | 0.003 | 0 | 0.061 | 0.021 |
| Verrucomicrobia | 0.446 | 0 | 4.659 | 3.326 | 0 | 14.07 | 0.002 |
Figure 3.
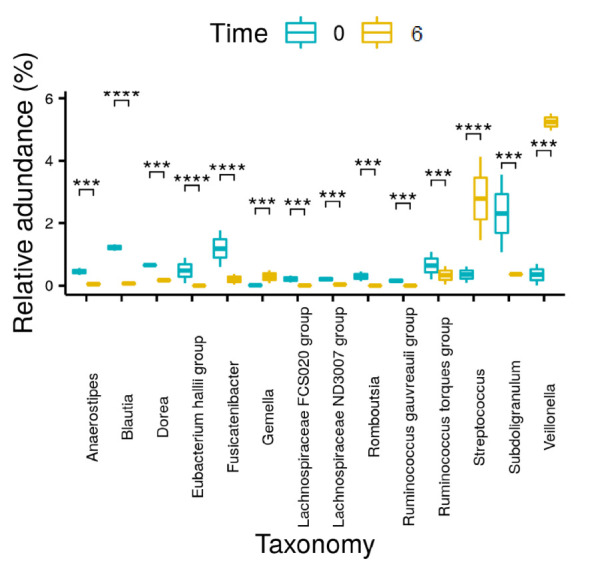
Changes in relative abundances of genera for all patients (n = 28) from baseline (Time 0) to 6 months post-surgery (Time 6). Significant differences in abundance are marked with an asterisk (*** indicates p < 0.0005; **** indicates p < 0.00005). Note that only significant differences in abundance with p < 0.0005 are presented here.
Significant reductions in alpha diversity (Shannon index, p = 0.021) and beta diversity (Bray–Curtis dissimilarity index, p = 0.026) over time were observed among all patients (Figure 4A,B, respectively).
Figure 4.
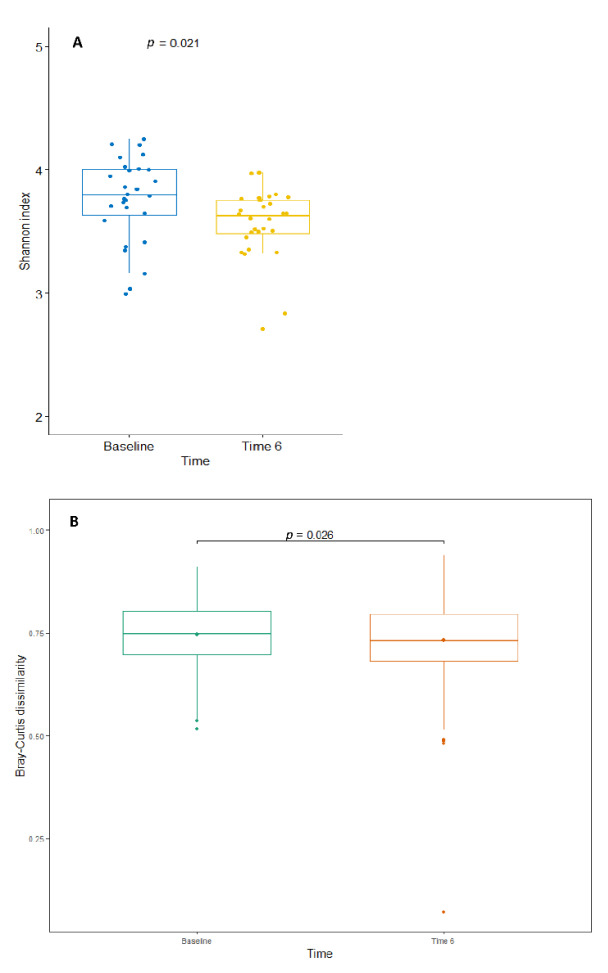
(A) Alpha diversity (using the Shannon index) and (B) beta diversity (using the Bray–Curtis dissimilarity metric) for all patients (n = 28) from baseline (Time 0) to 6 months post-surgery (Time 6).
3.4. Gut Microbiota Analysis According to Glucose Breath-Test Results at 6 Months Post-Surgery
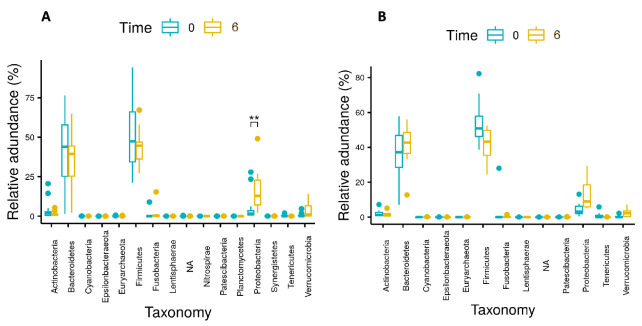
The changes in the relative abundance of main phyla for patients who did not develop SIBO and patients who developed SIBO over time are presented in Figure 5A,B, respectively. Significant changes were observed in the differential abundance analysis at the genera level, using LefSe, for both groups (Supplementary Materials Figures S2 and S3 and Tables S3 and S4). A comparison of the results from the differential abundances at the genera level, using LefSe, for both patient groups is presented in Supplementary Materials Table S5.
Figure 5.
Changes in relative abundances of phyla for (A) patients (n = 17) who did not develop SIBO and (B) patients (n = 10) who developed SIBO from baseline (Time 0) to 6 months post-surgery (Time 6). Significant differences in abundance are marked with an asterisk (** indicates p < 0.005). NA = sequences that were assigned to the kingdom bacteria but were not assigned to a specific phylum.
No significant differences in alpha diversity were observed between SIBO groups at baseline (p = 0.98) and at 6 months post-surgery (p = 0.675). A significant reduction in alpha diversity was observed over time within the group that did not develop SIBO at 6 months post-surgery (Shannon index, p = 0.038), while no significant reduction in alpha diversity over time was observed within the group that developed SIBO at 6 months post-surgery (p = 0.46) (Supplementary Materials Figure S4). Significant differences in beta diversity between SIBO groups were observed at baseline (Unweighted Unifrac, p = 0.033), but not at 6 months post-surgery (p = 0.75). There was a significant reduction in beta diversity over time within the group that did not develop SIBO at 6 months post-surgery (Unweighted Unifrac, p = 0.0018), while no significant change over time was observed within the group that developed SIBO at 6 months post-surgery (p = 0.79) (Supplementary Materials Figure S5). Moreover, the magnitude of change over time was lower among the group that developed SIBO at 6 months post-surgery (Unweighted Unifrac, p = 0.0055) (Supplementary Materials Figure S6).
3.5. Gut Microbiota Analysis of Patients According to FE1 Test at 6 Months Post-Surgery
Significantly higher beta diversity was observed in patients who presented a positive FE1 test at 6 months post-surgery (Unweighted Unifrac, p = 0.0034) (Supplementary Materials Figure S7). However, no difference was noticed for changes in alpha diversity between these groups.
4. Discussion
In the present study, we report the incidence of SIBO and PEI and the corresponding changes in the gut microbiota composition during a 6-month follow-up period after OAGB.
A substantial improvement in anthropometric parameters was found among our participants at 6 months post-surgery, accompanied by a high satisfaction rate from overall state-of-health and QoL. However, more than a third of the patients developed SIBO, and more than a quarter were diagnosed with PEI at 6 months following OAGB. An overlap of both conditions was found in two patients. Moreover, relatively high rates of frequent soft stools and flatulence were reported at 6 months post-surgery.
Data on the prevalence and implications of SIBO following BS are currently scarce [6,13,14,15,16,17,18]. Furthermore, most published studies lacked standardization. Our results are in line with a previous study that found a prevalence of 40% SIBO in patients with a median follow-up of 9.2 months after RYGB, although the prevalence of SIBO was 15% at baseline among these subjects [18], as compared to 0% in the present study. However, two other studies that investigated this phenomenon included only patients with a history of RYGB, OAGB, and SG, with subjective abdominal symptoms, and found much higher rates of SIBO [6,17]. Therefore, it may be reasonable to recommend a routine workup for SIBO in patients with a history of BS in the context of abdominal symptoms [6]. Moreover, the management of SIBO can be challenging following BS [50].
In the current study, there were several significant differences between patients with SIBO when compared to those without SIBO at 6 months post-surgery, including higher vitamin A deficiency rates, lower folate levels, a trend toward lower vitamin B12 levels, higher reported rates of regurgitation, and lower dietary intake among the group that developed SIBO. It is important to mention that no difference was found for these parameters between the groups at baseline, except vitamin A, which was not measured. However, the prevalence of vitamin A deficiency among BS candidates before surgery is presumably low [51]. Moreover, high and comparable adherence to supplementation was found in both groups at 6 months post-surgery. In agreement with our results, established SIBO is commonly related to fat-soluble vitamins and vitamin B12 deficiencies [8,9]. However, in contrast to our findings, SIBO is generally related to excessive folate levels secondary to bacterial synthesis [8,9]. One plausible explanation for our findings can be the trend towards increased folic acid supplementation among the group that did not develop SIBO at 6 months post-surgery. However, folic acid can also be found in multivitamin, iron, and vitamin B12 supplementation.
The adverse nutritional consequences of SIBO may involve multiple factors, including diminished food intake due to the presence of GI symptoms [9]. In the current study, patients who developed SIBO indeed reported significantly lower dietary intake at 6 months post-surgery, but no difference in GI symptoms, expect regurgitation, was reported, and no differences in anthropometric parameters were observed between the groups at 6 months post-surgery.
PEI was found in more than a quarter of the participants at 6 months post-surgery, but in none of them at baseline. This rate is higher than in a previous study of 22 patients one year following RYGB and OAGB that reported a rate of 9.1% PEI according to FE1 [23], but similar to a study of 188 RYGB patients with a mean follow-up of 12.5 months which found a prevalence of 31% PEI according to FE1 [19]. The latter study also found that a shorter biliopancreatic limb length lessened the prevalence of PEI [19]. Here, there was no difference in the bypassed limb length between the PEI and non-PEI groups at 6 months post-surgery. Pancreatic enzyme replacement therapy has been advocated for patients with symptomatic PEI, in addition to the implementation of the needed dietary modifications [21]. However, since the purpose of BS is to achieve weight loss, it is still uncertain what is the optimal therapy in such cases, and more research is currently needed [21], yet one study showed that pancreatic enzyme replacement therapy did not affect weight loss during three months of treatment [52].
The analysis of the gut microbiota in our cohort resulted in several observations. An overall reduction in both the richness and diversity of the microbiota was evident 6 months following OAGB. These results contrast with the current literature regarding alpha diversity changes in the short term, following different types of bariatric procedures [25]. Nonetheless, in the current study, the microbiota composition in terms of similarity between samples increased over time. In addition, significant changes in abundances of specific phyla and genera were observed over time. Proteobacteria, which may be beneficial due to a decrease in systemic inflammation and improved glucose homeostasis [25], and Verrucomicrobia, including Akkermansia, which are negatively related to the desire to eat sweets [26], were significantly increased. Although the Bacteroidetes phylum showed a trend towards decrease, its related genera Bacteroides and Prevotella significantly increased, and Alistipes significantly decreased. Bacteroides and Alistipes are positively related to a reduction of body fat mass and leptin, which affects the reduction of body weight [26,29]. Bacteroides is also related to remission of type 2 diabetes [27]. Actinobacteria showed a trend of decrease; Firmicutes and related genera, including Blautia, Dorea, and Faecalibacterium significantly decreased; and Roseburia and Streptococcus significantly increased. Blautia and Dorea have been suggested to positively correlate with leptin levels [26]. Our study is only partially in line with previous studies of RYGB patients which showed an increase in Bacteroides and Alistipes (Bacteroidetes) and Escherichia (Proteobacteria), and a decrease in Dorea, Blautia, and Roseburia (Firmicutes) and Actinobacteria at 6 months post-surgery [25,26,27,29].
To the best of our knowledge, no studies have yet investigated the associations between SIBO and the microbiota among BS patients. In the current study, for both SIBO and non-SIBO patients, a reduction in alpha diversity was noted; however, these results were significant only for the non-SIBO group and are thus probably unrelated to SIBO.
Patients who had a positive PEI diagnosis at 6 months post-surgery exhibited a significantly higher beta diversity in comparison to non-PEI. This result is in line with a large-scale population-based study that found that exocrine pancreatic function is associated with the microbiota composition and diversity [53]. Nonetheless, the exact mechanisms that link both and the pathophysiological consequences are currently unknown.
The strengths of the current study include its novelty, as this is the first study to examine SIBO, PEI, and microbiota dynamics following OAGB, in depth. Nevertheless, some limitations are noteworthy. First, our pilot study included a small sample size. Thus, the lack of significant differences between groups might be a consequence of its being underpowered. However, the sample power calculated with no-SIBO cases at baseline and 37% over time was 0.999. Second, over-diagnosis of SIBO may occur following gastric bypass surgeries, according to the traditional cutoff time for positive SIBO diagnosis. By this period of time, the carbohydrate substrate may have already reached the colon in some patients [8,17]. However, a majority of the patients who developed SIBO in our cohort were “early risers”, and this is believed to reflect abnormal fermentation of the carbohydrate substrate in the small intestine [7]. Moreover, breath-tests were performed after the ingestion of glucose, which, compared to lactulose, generally shows a single “early” peak of hydrogen excretion [36]. In addition, the FE1 test was not available for all participants for a pair-wise assessment. Therefore, future studies of larger sample size are needed to corroborate these findings with respect to SIBO and PEI in the long-term, following OAGB. Future studies may also include an analysis of inflammatory markers to assess microbiome changes and possible systemic effects.
5. Conclusions
The relatively high incidence rates of SIBO and PEI may partially explain the GI symptoms and nutritional deficiencies found following OAGB. These findings were accompanied by a less diverse and increasingly similar microbiota composition at 6 months post-surgery, coupled with significant changes in abundances of specific phyla and genera over time. These findings reflect on the pathophysiology of the adverse effects of OABG and should allow future guidance on the diagnosis and treatment of GI symptoms following OAGB.
Abbreviations
Adjustable gastric banding (AGB), bariatric surgery (BS), body mass index (BMI), excess weight loss (EWL), fecal elastase-1 (FE1), food frequency questionnaire (FFQ), gastrointestinal (GI), hydrogen (H2), impaired fasting glucose (IFG), mean cell hemoglobin concentration (MCHC), mean cell volume (MCV), Moorehead–Ardelt Quality of Life Questionnaire II (M-A QoLII), neck circumference (NC), nonalcoholic fatty liver disease (NAFLD), one anastomosis gastric bypass (OAGB), pancreatic exocrine insufficiency (PEI), parts per million (PPM), Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), small-intestinal bacterial overgrowth (SIBO), vertical banded gastroplasty (VBG), visual analogue scale quality of life (VAS QoL), waist circumference (WC).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14020304/s1. Methods S1: Supplementary Methods. Figure S1: Differential abundance analysis at the genera level, using LefSe, for all patients from baseline (Time 0) to 6 months (Time 6) post-surgery (n = 28). Figure S2: Differential abundance analysis at the genera level, using LefSe, for patients who did not develop SIBO from baseline (Time 0) to 6 months (Time 6) post-surgery (n = 17). Figure S3: Differential abundance analysis at the genera level, using LefSe, for patients who developed SIBO from baseline (Time 0) to 6 months (Time 6) post-surgery (n = 10). Figure S4: Differences in alpha diversity (using the Shannon Index). Figure S5: Differences in beta diversity (using the Unweighted Unifrac metric). Figure S6: Differences in the delta of change of beta diversity (using the Unweighted Unifrac metric) over time between the group that did not develop SIBO at 6 months post-surgery (no SIBO at T6, n = 17) and the group that developed SIBO at 6 months post-surgery (SIBO at T6, n = 10). Figure S7: Differences in beta diversity (using the Unweighted Unifrac metric) when comparing samples according to FE1 test at 6 months post-surgery. Table S1: Changes in relative abundances of genera for all patients from baseline to 6 months post-surgery (n = 28). Table S2: Differential abundance analysis at the genera level, using LefSe, for all patients from baseline to 6 months post-surgery (n = 28). Table S3: Differential abundance analysis at the genera level, using LefSe, for patients who did not develop SIBO from baseline to 6 months post-surgery (n = 17). Table S4: Differential abundance analysis at the genera level, using LefSe, for patients who developed SIBO from baseline to 6 months post-surgery (n = 10). Table S5: Comparison of the differential abundance analysis at the genera level, using LefSe results, for patients who did not develop SIBO from baseline to 6 months post-surgery (SIBO neg) and patients who did develop SIBO (SIBO pos).
Author Contributions
Conceptualization, S.S.-D., O.K., J.M.-G. and A.S.; methodology, S.S.-D., O.K. and J.M.-G.; validation, J.M.-G.; formal analysis, S.S.-D., O.K., J.M.-G., B.K. and Y.M.; investigation, P.L., S.S.-D., Y.K., N.S., A.R. and D.G.; resources, N.S., A.R. and D.G.; data curation, B.K., O.K., S.S.-D., Y.K. and P.L.; writing—original draft preparation, S.S.-D., O.K. and J.M.-G.; writing—review and editing, A.S., P.L., Y.K., N.S., A.R., D.G., Y.M. and B.K.; visualization, S.S.-D. and Y.M.; supervision, J.M.-G.; project administration, S.S.-D. and O.K.; funding acquisition, S.S.-D. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by an internal grant from Assuta Medical Center. The funder was not involved in the study design, collecting and analyzing of the data, writing of the report, and decision to submit this paper for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Assuta Medical Center (#0014-18-ASMC, 17 May 2018).
Informed Consent Statement
Informed consent was obtained from all study participants.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, J.M.-G., upon reasonable request. The raw sequencing data were deposited in the European Bioinformatics Institute European Nucleotide Archive under accession no. PRJEB47612.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angrisani L., Santonicola A., Iovino P., Vitiello A., Higa K., Himpens J., Buchwald H., Scopinaro N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018;28:3783–3794. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 2.Ramos A.C., Chevallier J.-M., Mahawar K., Brown W., Kow L., White K.P., Shikora S. IFSO (International Federation for Surgery of Obesity and Metabolic Disorders) Consensus Conference Statement on One-Anastomosis Gastric Bypass (OAGB-MGB): Results of a Modified Delphi Study. Obes. Surg. 2020;30:1625–1634. doi: 10.1007/s11695-020-04519-y. [DOI] [PubMed] [Google Scholar]
- 3.Deitel M., Kular K.S. Consensus survey on mini-gastric bypass and one-anastomosis gastric bypass. Ann. Bariatr. Metab. Surg. 2018;1:1001. doi: 10.33582/ann-bariatr-metab-surg/1001. [DOI] [Google Scholar]
- 4.Kessler Y., Adelson D., Mardy-Tilbor L., Ben-Porat T., Szold A., Goitein D., Sakran N., Raziel A., Sherf-Dagan S. Nutritional status following One Anastomosis Gastric Bypass. Clin. Nutr. 2020;39:599–605. doi: 10.1016/j.clnu.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kassir R., Blanc P., Lointier P., Breton C., Debs T., Tiffet O. Laparoscopic Revision of an Omega Loop Gastric Bypass to Treat Afferent Loop Syndrome. Obes. Surg. 2015;25:1976–1978. doi: 10.1007/s11695-015-1805-5. [DOI] [PubMed] [Google Scholar]
- 6.Mouillot T., Rhyman N., Gauthier C., Paris J., Lang A.-S., Lepers-Tassy S., Manfredi S., Lepage C., Leloup C., Jacquin-Piques A., et al. Study of Small Intestinal Bacterial Overgrowth in a Cohort of Patients with Abdominal Symptoms Who Underwent Bariatric Surgery. Obes. Surg. 2020;30:2331–2337. doi: 10.1007/s11695-020-04477-5. [DOI] [PubMed] [Google Scholar]
- 7.Quigley E.M.M. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO) Curr. Gastroenterol. Rep. 2019;21:3. doi: 10.1007/s11894-019-0671-z. [DOI] [PubMed] [Google Scholar]
- 8.Jirapinyo P., Makuvire T.T., Dong W.Y., Chan W.W., Thompson C.C. Impact of Oral-Cecal Transit Time on the Interpretation of Lactulose Breath Tests After RYGB: A Personalized Approach to the Diagnosis of SIBO. Obes. Surg. 2019;29:771–775. doi: 10.1007/s11695-018-3575-3. [DOI] [PubMed] [Google Scholar]
- 9.Adike A., DiBaise J.K. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol. Clin. N. Am. 2018;47:193–208. doi: 10.1016/j.gtc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson J., Minaya M.T., Monegro M., Lebwohl B., Lewis S.K., Green P.H., Stenberg R. Outcome of breath tests in adult patients with suspected small intestinal bacterial overgrowth. Gastroenterol. Hepatol. Bed Bench. 2017;10:168–172. [PMC free article] [PubMed] [Google Scholar]
- 11.Krajicek E.J., Hansel S.L. Small Intestinal Bacterial Overgrowth: A Primary Care Review. Mayo Clin. Proc. 2016;91:1828–1833. doi: 10.1016/j.mayocp.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Rezaie A., Buresi M., Lembo A., Lin H., McCallum R., Rao S., Schmulson M., Valdovinos M., Zakko S., Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorneklett A., Viddal K.O., Midtvedt T., Nygaard K. Intestinal and gastric bypass. Changes in intestinal microecology after surgical treatment of morbid obesity in man. Scand. J. Gastroenterol. 1981;16:681–687. doi: 10.3109/00365528109182030. [DOI] [PubMed] [Google Scholar]
- 14.Lakhani S.V., Shah H.N., Alexander K., Finelli F.C., Kirkpatrick J.R., Koch T.R. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr. Res. 2008;28:293–298. doi: 10.1016/j.nutres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Machado J.D., Campos C.S., Lopes Dah Silva C., Marques Suen V.M., Barbosa Nonino-Borges C., Dos Santos J.E., Ceneviva R., Marchini J.S. Intestinal Bacterial Overgrowth After Roux-en-Y Gastric Bypass. Obes. Surg. 2008;18:139–143. doi: 10.1007/s11695-007-9365-y. [DOI] [PubMed] [Google Scholar]
- 16.Shah H.N., Bal B.S., Finelli F.C., Koch T.R. Constipation in patients with thiamine deficiency after Roux-en-Y gastric bypass surgery. Digestion. 2013;88:119–124. doi: 10.1159/000353245. [DOI] [PubMed] [Google Scholar]
- 17.Andalib I., Shah H., Bal B.S., Shope T.R., Finelli F.C., Koch T.R. Breath Hydrogen as a Biomarker for Glucose Malabsorption after Roux-en-Y Gastric Bypass Surgery. Dis. Markers. 2015;2015:102760. doi: 10.1155/2015/102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabate J.M., Coupaye M., Ledoux S., Castel B., Msika S., Coffin B., Jouet P. Consequences of Small Intestinal Bacterial Over-growth in Obese Patients Before and After Bariatric Surgery. Obes. Surg. 2017;27:599–605. doi: 10.1007/s11695-016-2343-5. [DOI] [PubMed] [Google Scholar]
- 19.Borbély Y., Plebani A., Kröll D., Ghisla S., Nett P.C. Exocrine Pancreatic Insufficiency after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2016;12:790–794. doi: 10.1016/j.soard.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 20.Vujasinovic M., Valente R., Thorell A., Rutkowski W., Haas S.L., Arnelo U., Martin L., Lohr J.M. Pancreatic Exocrine Insufficiency after Bariatric Surgery. Nutrients. 2017;9:1241. doi: 10.3390/nu9111241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh V.K., Haupt M.E., Geller D.E., Hall J.A., Quintana Diez P.M. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017;23:7059–7076. doi: 10.3748/wjg.v23.i39.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips M.E., Hopper A.D., Leeds J.S., Roberts K.J., McGeeney L., Duggan S.N., Kumar R. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021;8:e000643. doi: 10.1136/bmjgast-2021-000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miroslav V., Gregor K., Brane B., Barbara R., Bojan T., Sasa R., Andreja K. Is Pancreatic Exocrine Insufficiency a Cause of Malabsorption in Patients after Bariatric Surgery? JOP J. Pancreas Online. 2016;17:402–405. [Google Scholar]
- 24.Akpinar M.Y., Ozturk D., Murat K., Aksoy E.K., Nazligul Y., Bulus H. Sleeve gastrectomy relieves exocrine pancreatic insufficiency in morbidly obese patients: A prospective case-control study. Prz. Gastroenterol. 2019;14:268–273. doi: 10.5114/pg.2019.84223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debédat J., Clément K., Aron-Wisnewsky J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019;8:229–242. doi: 10.1007/s13679-019-00351-3. [DOI] [PubMed] [Google Scholar]
- 26.Davies N.K., O’Sullivan J.M., Plank L.D., Murphy R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019;15:656–665. doi: 10.1016/j.soard.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Luijten J., Vugts G., Nieuwenhuijzen G.A.P., Luyer M.D.P. The Importance of the Microbiome in Bariatric Surgery: A System-atic Review. Obes. Surg. 2019;29:2338–2349. doi: 10.1007/s11695-019-03863-y. [DOI] [PubMed] [Google Scholar]
- 28.Ejtahed H.S., Angoorani P., Hasani-Ranjbar S., Siadat S.D., Ghasemi N., Larijani B., Soroush A.R. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: A systematic review. Microb. Pathog. 2018;116:13–21. doi: 10.1016/j.micpath.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Magouliotis D.E., Tasiopoulou V.S., Sioka E., Chatedaki C., Zacharoulis D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017;27:1345–1357. doi: 10.1007/s11695-017-2595-8. [DOI] [PubMed] [Google Scholar]
- 30.Bariatric Surgery Criteria of the Ministry of Health. The Ministry of Health Web Site. [(accessed on 19 January 2021)]; Available online: http://www.health.gov.il/hozer/mr33_2013.pdf.
- 31.Sherf Dagan S., Goldenshluger A., Globus I., Schweiger C., Kessler Y., Kowen Sandbank G., Ben-Porat T., Sinai T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017;8:382–394. doi: 10.3945/an.116.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Pinho C.P.S., Diniz A.D.S., De Arruda I.K.G., Leite A., Petribu M.M.V., Rodrigues I.G. Waist circumference measurement sites and their association with visceral and subcutaneous fat and cardiometabolic abnormalities. Arch. Endocrinol. Metab. 2018;62:416–423. doi: 10.20945/2359-3997000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi Y., Fukumoto S., Inaba M., Koyama H., Shoji T., Shoji S., Nishizawa Y. Different Impacts of Neck Circumference and Visceral Obesity on the Severity of Obstructive Sleep Apnea Syndrome. Obes. Silver Spring. 2011;19:276–282. doi: 10.1038/oby.2010.170. [DOI] [PubMed] [Google Scholar]
- 35.Toolabi K., Arefanian S., Golzarand M., Arefanian H. Effects of Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) on Weight Loss and Biomarker Parameters in Morbidly Obese Patients: A 12-Month Follow-Up. Obes. Surg. 2011;21:1834–1842. doi: 10.1007/s11695-011-0525-8. [DOI] [PubMed] [Google Scholar]
- 36.Gasbarrini A., Corazza G.R., Gasbarrini G., Montalto M., di Stefano M., Basilisco G., Parodi A., Usai-Satta P., Satta P.U., Vernia P., et al. Methodology and Indications of H2-Breath Testing in Gastrointestinal Diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009;29((Suppl. 1)):1–49. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 37.Shih D.Q., Kwan L.Y. All Roads Lead to Rome: Update on Rome III Criteria and New Treatment Options. Gastroenterol. Rep. 2007;1:56–65. [PMC free article] [PubMed] [Google Scholar]
- 38.Oria H.E., Moorehead M.K. Updated Bariatric Analysis and Reporting Outcome System (BAROS) Surg. Obes. Relat. Dis. 2009;5:60–66. doi: 10.1016/j.soard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Bergeat D., Lechaux D., Ghaina A., Thibault R., Bouygues V. Postoperative Outcomes of Laparoscopic Bariatric Surgery in Older Obese Patients: A Matched Case-Control Study. Obes. Surg. 2017;27:1414–1422. doi: 10.1007/s11695-016-2517-1. [DOI] [PubMed] [Google Scholar]
- 40.Moorehead M.K., Ardelt-Gattinger E., Lechner H., Oria H.E. The validation of the Moorehead-Ardelt Quality of Life Ques-tionnaire II. Obes. Surg. 2003;13:684–692. doi: 10.1381/096089203322509237. [DOI] [PubMed] [Google Scholar]
- 41.Padwal R.S., Majumdar S.R., Klarenbach S., Birch D.W., Karmali S., McCargar L., Fassbender K., Sharma A.M. Health status, quality of life, and satisfaction of patients awaiting multidisciplinary bariatric care. BMC Health Serv. Res. 2012;12:139. doi: 10.1186/1472-6963-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shai I., Rosner B.A., Shahar D.R., Vardi H., Azrad A.B., Kanfi A., Schwarzfuchs D., Fraser D. Dietary evaluation and attenuation of relative risk: Multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall question-naires: The DEARR study. J. Nutr. 2005;135:573–579. doi: 10.1093/jn/135.3.573. [DOI] [PubMed] [Google Scholar]
- 43.Tzameret—Israeli National Nutrient Database 2015. Ministry of Health Public Health Services Nutrition Division; Jerusalem, Israel: 2015. [Google Scholar]
- 44.Tóth A.Z., Szabó A., Hegyi E., Hegyi P., Sahin-Tóth M. Detection of human elastase isoforms by the ScheBo Pancreatic Elas-tase 1 Test. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G606–G614. doi: 10.1152/ajpgi.00060.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene data-base project: Improved data processing and web-based tools. Nucleic. Acids. Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bokulich N.A., Kaehler B.D., Rideout J.R., Dillon M., Bolyen E., Knight R., Huttley G.A., Gregory Caporaso J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss S., Xu Z.Z., Peddada S., Amir A., Bittinger K., Gonzalez A., Lozupone C., Zaneveld J.R., Vázquez-Baeza Y., Birmingham A., et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. 2017;5:27. doi: 10.1186/s40168-017-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mechanick J.I., Apovian C., Brethauer S., Timothy Garvey W., Joffe A.M., Kim J., Kushner R.F., Lindquist R., Pessah-Pollack R., Seger J., et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obes. Silver Spring. 2020;28:O1–O58. doi: 10.1002/oby.22719. [DOI] [PubMed] [Google Scholar]
- 51.Frame-Peterson L.A., Megill R.D., Carobrese S., Schweitzer M. Nutrient Deficiencies Are Common Prior to Bariatric Surgery. Nutr. Clin. Pract. 2017;32:463–469. doi: 10.1177/0884533617712701. [DOI] [PubMed] [Google Scholar]
- 52.Ozmen M.M., Gundogdu E., Guldogan C.E., Ozmen F. The Effect of Bariatric Surgery on Exocrine Pancreatic Function. Obes. Surg. 2021;31:580–587. doi: 10.1007/s11695-020-04950-1. [DOI] [PubMed] [Google Scholar]
- 53.Frost F., Kacprowski T., Rühlemann M., Bülow R., Kühn J.P., Franke A., Heinsen F.A., Pietzner M., Nauck M., Völker U., et al. Impaired Exocrine Pancreatic Function Associates with Changes in Intestinal Microbiota Composition and Diversity. Gastroenterology. 2019;156:1010–1015. doi: 10.1053/j.gastro.2018.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, J.M.-G., upon reasonable request. The raw sequencing data were deposited in the European Bioinformatics Institute European Nucleotide Archive under accession no. PRJEB47612.