Abstract
In this study, we focused on the isolation and structural characterization of polysaccharides from a basidiocarp of polypore fungus Ganoderma resinaceum. Polysaccharide fractions were obtained by successive extractions with cold water at room temperature (20 °C), hot water under reflux (100 °C), and a solution of 1 mol L−1 sodium hydroxide. The purity of all fractions was controlled mainly by Fourier transform infrared (FTIR) spectroscopy, and their composition and structure were characterized by organic elemental analysis; neutral sugar and methylation analyses by gas chromatography equipped with flame ionization detector (GC/FID) and mass spectrometry detector (GC/MS), respectively; and by correlation nuclear magnetic resonance (NMR) spectroscopy. The aqueous extracts contained two main polysaccharides identified as a branched O-2-β-d-mannosyl-(1→6)-α-d-galactan and a highly branched (1→3)(1→4)(1→6)-β-d-glucan. Mannogalactan predominated in the cold water extract, and β-d-glucan was the main product of the hot water extract. The hot water soluble fraction was further separated by preparative anion exchange chromatography into three sub-fractions; two of them were identified as branched β-d-glucans with a structure similar to the corresponding polysaccharide of the original fraction. The alkaline extract contained a linear (1→3)-α-d-glucan and a weakly branched (1→3)-β-d-glucan having terminal β-d-glucosyl residues attached to O-6 of the backbone. The insoluble part after all extractions was identified as a polysaccharide complex containing chitin and β-d-glucans.
Keywords: Ganoderma resinaceum, wood decay fungi, polysaccharides, spectroscopy
1. Introduction
Wood decay polypore fungi of the genus Ganoderma, some species of which have long been cultivated used in traditional oriental medicine [1,2,3,4], are widespread in warm regions and are often found in parks and gardens [5]. These fungi have thick, corky fruiting bodies (basidiocarps), which are capable of growing on affected dead or living trees at the site of injury and causing white rot. They decompose lignocellulosic complex of the wood using a number of lignin-modified enzymes, including laccases, lignin peroxidase, and manganese-dependent peroxidase [6,7]. As a result, the wood loses its hardness, strength, and mass. The destruction of lignin leads to a lightening of the color of the wood [8,9].
Fungi of genus Ganoderma produce secondary metabolites such as proteins, polysaccharides [10,11,12,13,14,15,16,17,18], terpenoids [14,16,17,19,20], polyphenols [21,22], steroids, alkaloids, and many others with various biological effects [1,23,24,25,26]. Due to these properties, metabolites of Ganoderma are used in functional foods, food supplements, or cosmetics [27,28,29].
Unlike the low molecular weight metabolites mentioned above, in higher fungi including Ganoderma, most polysaccharides, as well as some proteins and glycoproteins, primarily play the role of structural components of the cell walls of spores, mycelium, and fruiting bodies, where they are interconnected and form a layered three-dimensional basis [30,31,32]. To isolate individual polysaccharides from the fungal material, it is necessary to break numerous durable bonds between them. For this purpose, extraction with various solvents and reagents under appropriate conditions is usually used [33]. Before proceeding with the isolation of polysaccharides, the crushed basidiocarp is first washed with organic solvents to remove lipids and other low molecular weight substances [18]. Hot water extraction is commonly used for obtaining polysaccharides from basidiocarp and other parts of mushrooms Ganoderma [34,35]. This method does not require any special reagents and makes it possible to obtain a water-soluble polysaccharide fraction most suitable for use. Heating up to the boiling point usually allows the separation of heteropolysaccharides and some glucans from the cell walls of fungi, often mixed with proteins and glycoproteins. In some cases, however, extractions are used first with cold and then with hot water to separate the individual water-soluble fractions [36]. Acidic, saline, and alkaline solutions are also used for the extraction of fungal polysaccharides [37,38]. Here, multiple extractions are often used under moderate to high conditions to gradually destroy the cell walls from the periphery to the inside and thus obtain polysaccharide fractions with different solubility [18]. In addition to the above, recently, several advanced methods, such as hot compressed water, subcritical or two-phase extraction, microwave heating, ultrasound, enzymes, and their combinations have been used to more effectively destroy cell walls and thus increase the yield of fungal polysaccharides [39,40,41,42,43,44,45].
The crude polysaccharide fractions are subjected to further purification steps, including the removal of proteins by hydrolysis with proteolytic enzymes or precipitation using various reagents [46]. At this stage, mild conditions should be preferred to avoid partial degradation of the polysaccharides. The final purification step includes dialysis and preparative chromatography, usually a combination of anion exchange and gel filtration methods [18]. Then, the purity, composition, molecular weight, and structure of the obtained polysaccharides are analyzed by appropriate spectroscopic and separation methods [33,47,48].
In general, linear and branched d-glucans of α- and β- and mixed α,β-anomeric configurations are very common in fungi including Ganoderma, while cellulose or (1→4)-β-d-glucan are not found in these organisms [30]. Some heteropolysaccharides of various structures and composition are also found in Ganoderma, but mainly in the water-soluble part [49,50,51]. These include, for example, heterogalactans [52,53] and heteromannans [54]. In contrast, chitin is present in fungi as rigid component of cell walls in complex with d-glucans [34].
The most studied water and alkali-extracted polysaccharides have been isolated from Ganoderma lucidum, also known as Linzhi or Reishi, which has been used for centuries in Asian medicine [11,14,16,55,56,57]. These polysaccharides were shown to be mainly glucans of various structures, including (1→3)(1→6)-β-d-glucan, β-(1→3)-glucan, and α-(1→4)-glucan. The minor structural monosaccharides were found to be xylose, arabinose, galactose, mannose, and fucose. Various polysaccharides of Ganoderma have positive health effects such as immunomodulatory [44,58,59], antimicrobial [14,15], antiviral [60,61], prebiotic [62], hypoglycemic [63,64,65,66], antioxidant [14,15], anti-inflammatory [51,67], and antitumor [15,17,51,67] effects. Biological activities of fungal polysaccharides are closely related to the sugar composition, branching, and molecular weight, which in turn are closely related to the isolation procedure [47,68].
On the other hand, the polysaccharides of other species of the genus Ganoderma have been insufficiently studied. Moreover, species such as G. resinaceum may be interesting as sources of various biologically active polysaccharides, structurally different from the polysaccharides of G. lucidum, since certain species of this genus show a certain specificity in biochemical composition [59,69]. In this regard, it may be promising to grow some unconventional species of the genus Ganoderma to obtain not only polysaccharides, but also other biologically active substances, such as terpenoids or polyphenols. Partially, the mentioned species G. resinaceum is quite widespread in Central Europe, common in parks and forests, and is currently considered promising for experimental cultivation.
Therefore, this work is devoted to the isolation, structural analysis, and composition of polysaccharide-rich fractions obtained from Ganoderma resinaceum basidiocarp, originated from the Czech Republic. These fractions were purified by chemical and enzymatic treatments enzymes, and further separated by ion exchange chromatography. Composition and structure of obtained polysaccharides were characterized by spectroscopic, chromatographic, and other analytical methods.
2. Materials and Methods
2.1. Materials
Dried and homogenized basidiocarp from the fungus Ganoderma resinaceum was found in the town park in Kralupy nad Vltavou, Middle Bohemian region, Czech Republic. This basidiocarp (Figure 1a,b) was identified by Dr. Michal Tomšovský (Mendel University in Brno, Czech Republic), who is a specialist in mycology of forest wood decay fungi, and the specimen was deposited in the Herbarium of Moravian Museum (BRNM), Brno, Czech Republic. This raw material was dried in air and homogenized to a particle size of 0.5 mm using the IKA homogenizer (IKA Werke, Staufen, Germany).
Figure 1.
Part of basidiocarp of G. resinaceum used in this work: (a) top view; (b) bottom view.
Chemicals:
Distilled water, ethanol (University of Chemistry and Technology Prague, Czech Republic).
Methanol, hexane, acetone, dichloromethane, acetanhydride, hydrochloric acid, sulfuric acid, sodium chloride, sodium hydroxide, hydrogen peroxide (PENTA s.r.o., Prague, Czech Republic).
Sodium borohydride, copper(II) chloride, pepsin from porcine gastric mucosa, formic acid, trifluoroacetic acid, m-hydroxybiphenyl, methyl iodide, 1-methylimidazole, 2-deoxy-d-glucose (Sigma-Aldrich, Saint Louis, MO, USA).
Pronase from Streptomyces griseus (Roche Holding AG, Basel, Switzerland).
Dimethyl sulfoxide (ThermoFisher Scientific, Waltham, MA, USA).
Potassium bromide for IR spectroscopy (Merck, KGaA, Darmstadt, Germany).
2.2. Preparative Procedures
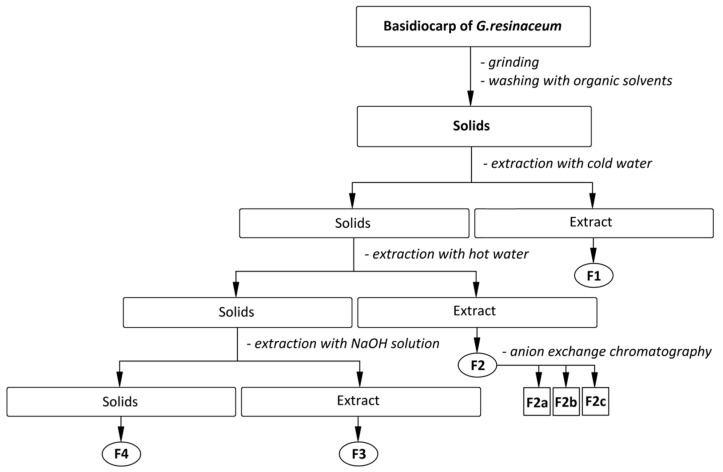
Before the extraction of polysaccharides, according to the isolation scheme (Figure 2), lipids, pigments, and other small molecules were removed from the homogenized raw material by subsequent washing of the milled material with hexane followed by 0.2 mol L−1 HCl in 80% aqueous ethanol (acidified ethanol), 80% aqueous ethanol until neutral reaction, and finally, by 96% ethanol and acetone to remove water residues. Solids after washing with an acidic ethanol mixture were re-homogenized, subjected to recurrent extraction with cold water (20 °C), followed by hot water under reflux (100 °C), and then with a solution of 1 mol L−1 aqueous NaOH [68].
Figure 2.
The scheme of isolation of the polysaccharidic fractions F1, F2, F3, and F4 and sub-fractions F2a–c from basidiocarps of G. resinaceum.
Extraction with cold water was performed four times; 50 g of homogenized dry solids and 1500 mL of distilled water were used. The extraction was carried out on a magnetic stirrer at 350 rpm, and the average stirring time was 6 h. The solids were then separated, filtered, and used for another extraction cycle under the same conditions. The extracts were filtrated, combined, and evaporated in a RVO 400 vacuum evaporator (Ingos s.r.o, Prague, Czech Republic) at 65 °C and 40 mBar. The concentrated extract was freeze-dried on Alpha 1–4 LSC lyophilizer (Pragolab s.r.o., Prague, Czech Republic) yielding fraction F1, which was further successively washed in the same way as the raw material and dried in air. Further purification was performed using the combination of proteolytic enzymes (pepsin and pronase) to remove proteins. The sample dissolved in 100 mL of 0.01 mol L−1 aq. HCl was incubated with pepsin (100 mg) for 12 h and then the enzyme was inactivated by heating to 90 °C for 30 min. The product was then precipitated in an excess of 96% ethanol (3:1 v/v); successively washed with 80% ethanol, 96% ethanol, and acetone; dried in air; and homogenized.
The insoluble part after extractions with cold water was used for further extractions with hot water under reflux (100 °C). The extraction was performed in three portions five times in distilled water (1000 mL each portion) for approximately 8 h. The extracts were used for obtaining of purified fraction F2 by the same way as described above for F1; further purification was performed using anion exchange preparative chromatography [36,70]. Chromatographic fractionation of F2 (66.67 mg) led to obtaining of three sub-fractions assigned as F2a, F2b, and F2c.
The insoluble part after extractions with hot water was used for two successive extractions in 1 mol L−1 aqueous NaOH (700 mL) at 4 °C. The extract was further precipitated in three volumes of 96% ethanol and stored at 4 °C for 12 h. The precipitate was centrifuged and neutralized by washing with the acidic ethanol, then successively washed with 80% ethanol, 96% ethanol, and acetone, and dried in air. The dark-colored precipitate was treated with of 3% H2O2 in weak alkaline solution (pH ~ 8) to remove colorant residues, and then precipitated with three volumes of 96% ethanol. The precipitate was again neutralized, washed, and dried in the same manner, and finally homogenized in an oscillating mill MM 301 (Retsch GmbH, Haan, Germany) yielding fraction F3. The remaining insoluble part after all of the extractions (F4) was prepared by the same way as F3, but the treatment with 3% H2O2 took place several times in suspension because of the insolubility of F4 under alkaline conditions.
2.3. Preparative Chromatography
The fraction F2 (66.67 mg mL−1) was used for further fractionation by preparative anion exchange chromatography performed on an Omnifit column, 44 cm high and 1.5 cm in diameter, packed with DEAE-Sepharose Fast-Flow gel (GE Healthcare, Chicago, IL, USA). The flow rate was 1 mL/min. The gradient was 0–2 mol L−1 aq. NaCl. The sample was dissolved in 1 mL of distilled water. The eluted fractions were collected using a Gilson FC 203B fraction collector (Gilson Inc., Middleton, WI, USA) with the required number of tubes and with an Ecom DG 3014 vacuum degasser (ECOM spol. s.r.o., Chrastany u Prahy, Czech Republic) driven by a Gilson MP3 pump (Gilson Inc., Middleton, WI, USA). One hundred and ten tubes were collected, and the amount of total carbohydrates in each was estimated according to the phenol sulfuric acid assay (PSA).
2.4. Analytical Methods
2.4.1. Phenol-Sulfuric Acid Assay
The phenol-sulfuric acid assay is a rapid colorimetric reaction for the determination of total carbohydrates in samples. PSA was used after preparative chromatography. In 200 μL of each sample (tube—preparative chromatography), 5% phenol solution and 1 mL of 96% sulfuric acid were added. Each tube was thoroughly shaken on an IKA Vortex 2 tube shaker (IKA Werke, Staufen, Germany) tube shaker and then left to stand for 20 min at laboratory temperature. Then, 250 μL of each sample (tube) was transferred to a micro-titer plate and the absorbance was measured at 490 nm on an Epoch TM 2 microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA). The presence of carbohydrates in the sample was then demonstrated by the yellow gold color of the reaction mixture [71]. Chromatograms, calibration curve, and calculations were processed using Excel software (Microsoft, Redmond, WA, USA).
2.4.2. Organic Elemental Analysis
Organic elemental analysis of C, H, N, and S was performed on an Elementar Vario EL III instrument (Elementar Analysensysteme GmbH, Langenselbold, Germany). The manufacturer determined the precision of the method for the simultaneous analysis of 5 mg of the 4-aminobenzene sulfonic acid standard in the CHNS module at <0.1% abs. for each element. The results of the analysis include all combustible sulfur, both organic and inorganic (S2−, SO42−), as well as all combustible carbon, organically bound and inorganically bound (CO32−). The hydrogen content found is affected by the moisture of the supplied sample.
2.4.3. Monosaccharide Composition and Linkages
Quantitative determination of neutral sugars in all polysaccharide fractions obtained was carried out using their alditol acetates [72] by gas chromatography on a Trace GC Ultra (ThermoFisher Scientific, Waltham, MA, USA) equipped with a Restek RT-2330-NB column (0.32 mm × 105 m), a temperature program of 80 °C (12 min)–160 °C (8 °C min−1) – 250 °C (4 °C min−1, 25 min at 250 °C) – 265 °C (20 °C min−1, 10 min at 265 °C), and a flow rate of helium of 1 mL min−1.
The gas chromatograph was coupled to a TSQ Quantum XLS mass spectrometer (GC/MS) (ThermoFisher Scientific, Waltham, MA, USA) with EI ionization 70 eV and mass range of 50 to 650 amu with a scan time of 1 s [73]. Sugar linkage analysis of F2 was performed using the methods of Ciucanu and Purdie [74,75]. The permethylated products were hydrolyzed with 90% HCOOH at 100 °C for 1 h and then with 2 mol L−1 TFA at 120 °C for 1 h, followed by reduction with NaBD4 and acetylation. The partially methylated alditol acetates were analyzed by GC/MS.
The content of uronic acids in the hot water extract was measured by photometry with m-hydroxybiphenyl at 520 nm [76].
2.4.4. FTIR Spectroscopy
The FTIR spectra of the raw material and all fractions obtained were measured on a Nicolet 6700 FTIR spectrometer in KBr pellets under the given conditions. The wavelength range was 400–4000 cm−1, with a resolution of 2 cm−1, with 64 scans. Vibration spectra were recorded and processed using Omnic 8.0 software (ThermoFisher Scientific, Waltham, MA, USA). The raw spectra were exported in ASCII format to Origin 6.0 software (OriginLab, Northampton, MA, USA) for further processing (smoothing, baseline correction) and calculation of the average spectra.
2.4.5. FT Raman Spectroscopy
FT Raman spectra of selected fractions (range 100–4000 cm−1, 1000 scans, resolution 4 cm−1) were recorded on Nicolet iS50 with the FT Raman module (ThermoFisher Scientific, Waltham, MA, USA), Nd:YAG laser (λex = 1064 nm, power 500 mW), CaF2 beam splitter and InGaAs detector. Vibration spectra were recorded and processed using Omnic 9.0 software (ThermoFisher Scientific, Waltham, MA, USA). The raw spectra were exported in ASCII format to Origin 6.0 software (OriginLab, Northampton, MA, USA) for further processing (smoothing, baseline correction), calculation of the average spectra.
2.4.6. NMR Spectroscopy
The selected fractions were analyzed on a Bruker Avance III omet 500 MHz NMR spectrometer (Bruker, Billerica, MA, USA). The water-soluble fractions (F1, F2) were dissolved in D2O for NMR and measured at 20 °C and 80 °C; alkali soluble fraction (F3) was dissolved in Me2SO-d6 and measured at the same temperatures. NMR spectra were processed using MestReNova 10.0 software (Mestrelab Research, Santiago de Compostela, Spain).
3. Results and Discussion
3.1. Yields of Isolation
The yields of the subsequent isolated fractions are summarized in Table 1. The mass yield of the obtained products of successive extractions gradually increased from 0.37% w/w for F1 and was maximum for the insoluble part F4 (15.59% w/w). As a result, the total yield of the entire product after purification was 28.86% w/w, which is less than a third of the initial mass of ground basidiocarp. The extraction yields with hot water (2.33% w/w) and alkaline solution (10.57% w/w) were slightly higher than that previously reported for G. lucidum polysaccharides extracted under optimized conditions, i.e., 1.45% w/w and 8.30% w/w, respectively [34,38].
Table 1.
Yields and composition of the fractions obtained.
| Fraction | Extraction Medium | Yield (% w/w) | Composition |
|---|---|---|---|
| F1 | Cold water | 0.37 | Polysaccharides, proteins |
| F2 | Hot water | 2.33 | Polysaccharides |
| F3 | 1 mol L−1 NaOH | 10.57 | Polysaccharides |
| F4 | Insoluble residues | 15.59 | Polysaccharides |
| Total | All fractions | 28.86 | Polysaccharides, proteins |
3.2. Organic Elemental Composition
Amounts of organic elements in the fractions obtained from G. resinaceum by successive extractions with cold water, hot water, and 1 mol L−1 aqueous NaOH, which were further purified, are summarized in Table 2. Carbon and hydrogen are among the basic skeletal elements of all polysaccharides. The fractions also showed the presence of the nitrogen, maximal for F1 (2.44% w/w), and significantly lower amounts of sulfur; both are derived mainly from proteins. These results are consistent with similar studies [45] that analyzed water extracts of G. lucidum. The fractions F2 and F3 thus contained less nitrogen and sulfur compared to F1, which indicates that most proteins are readily soluble in cold water and removed in this way. In the case of F4, the nitrogen is also derived from chitin, which is also a component of Ganoderma cell walls [77].
Table 2.
Amounts of organic elements (% w/w) in the fractions obtained from basidiocarps of G. resinaceum.
| Fraction | % N | % C | % H | % S |
|---|---|---|---|---|
| F1 | 2.44 | 39.47 | 6.25 | 0.13 |
| F2 | 0.70 | 39.43 | 6.92 | 0.04 |
| F3 | 0.21 | 39.53 | 7.13 | 0.21 |
| F4 | 2.08 | 39.47 | 6.44 | 0.07 |
3.3. Monosaccharide Composition and Linkage
Composition of neutral monosaccharides in the fractions is summarized in Table 3. Among neutral sugars, glucose predominated in all of the fractions, especially in F3 and F4. Glucans of various structure predominate in fungal cell walls [47], so it is not surprising that the glucose content in all fractions, with the exception of F1, exceeded 85 mol % and even reached about 92 mol % in the water-insoluble fractions. In contrast, in the fraction F1, glucose (35.6 mol %) was found together with comparable amounts of galactose (26.9 mol %) and mannose (26.5 mol %). This fraction also contained the highest percentage of fucose (6.7 mol %) and xylose (4.3 mol %). Since mannose and galactose were found in almost equimolar proportions, these sugars are possibly interconnected in the form of mannogalactan (or galactomannan) polysaccharide in a mixture with d-glucans. A similar monosaccharide composition was achieved with cold water extraction of Pleurotus ostreatus basidiocarps [36], where the presence of branched mannogalactan was proved by methylation analysis and correlation NMR. Mannose, fucose, and xylose were found as minor sugars in various proportions (less than 5 mol % each) in all of the other fractions analyzed. Galactose was also found in the fraction F2, but at content lower than 2%, and the glucose content prevailed. This means that the dominant polysaccharide of this fraction is d-glucan. This fraction also contained 5.5% w/w of uronic acids, as determined by photometry. A similar composition was previously determined for Pleurotus ostreatus and G. lucidum polysaccharides obtained by microwave-assisted extraction [78]. Aqueous extracts of fungal cell walls usually contain the most labile structural elements of the outer layer, and these are primarily heteropolysaccharides, although some glucans may also be present. However, the water-soluble glucans are preferably released later during the hot water extraction [79], so most of the heteropolysaccharides, mainly mannogalactans, were collected by cold water extraction. Subsequent extractions with alkaline solutions usually lead to the release of mainly glucans of various structures. A similar relationship between fungal glucans and mannogalactans was previously observed for various multistep extraction protocols. As described for the G. lucidum basidiocarps [34], successive isolation steps including hot 0.9% NaCl, hot water, and alkaline extractions resulted in an increase in glucose, while the contributions of galactose and mannose were decreased, with branching also declining from high to almost negligible.
Table 3.
Molar ratio (%) of monosaccharides in the fractions obtained from basidiocarps of G. resinaceum.
| Fraction | Fuc | Man | Glc | Gal | Xyl |
|---|---|---|---|---|---|
| F1 | 6.7 | 26.5 | 35.6 | 26.9 | 4.3 |
| F2 | 3.5 | 4.2 | 86.5 | 1.9 | 3.9 |
| F3 | 2.0 | 2.7 | 92.2 | 0.0 | 3.1 |
| F4 | 3.6 | 0.0 | 92.4 | 0.0 | 4.0 |
Sugar linkage analysis made for the more pronounced water-soluble fraction F2 revealed about 13 types of sugar derivatives, suggesting a complex chemical structure of the polysaccharides (Table 4). This fraction contained five major types of the fragments in descending order: 1,3-linked, terminal, 1,4-linked, 1,3,6-linked, and 1,6-linked glucosyl units. These fragments indicate the presence of different glucans, possibly a mixture of branched (1→3)(1→6)-β-d-glucan and non-branched amylose-like (1→4)-α-d-glucan. The 1,6-linked glucosyl units can form chains of non-branched (1→6)-β-d-glucan or be part of side chains in the branched β-d-glucan. Fragments of mannose, galactose, fucose, and xylose were also found, but their proportion was too low to judge the inter-connection between them and predict the structure of the corresponding heteropolysaccharides. Obviously, the composition and linkages in polysaccharides obtained from fungi of the genus Ganoderma will depend on the type and method of extraction. For example, methylation analysis of polysaccharide isolated from G. atrum consisted mainly of 1,3-linked, terminal, 1,3,6-linked, 1,4-linked, and 1,6-linked glucosyl units (from 21% to 12% each), but also contained smaller amounts of galacturonic acid, mannosyl and other glucosyl units [80]. In contrast, two polysaccharides GLC-1 and GLC-2 isolated from G. lucidum were defined as galactoglucan and glucan, respectively; both composed of 1,6- and 1,3-linked glucosyl units and GLC-1 also contained 1,6-linked galactosyl residues [81]. In any case, hot water extractable polysaccharides from Ganoderma are likely to be highly branched and may contain other carbohydrates besides glucose.
Table 4.
Sugar linkage analysis of F2.
| Sugar Derivative | Ratio (mol %) | Mode of Linkage |
|---|---|---|
| 2,3,4,6-Me4-Man | Traces | Manp-(1→ |
| 2,3,4,6-Me4-Glc | 22.1 | Glcp-(1→ |
| 2,4-Me2-Fuc | 1.1 | →3)-Fucp-(1→ |
| 2,3,4,6-Me4-Gal | 0.5 | Galp-(1→ |
| 2,3-Me2-Xyl | 0.2 | →4)-Xyl-(1→ |
| 2,4,6-Me3-Glc | 26.8 | →3)-Glcp-(1→ |
| 2,3,4-Me3-Glc | 12.8 | →6)-Glcp-(1→ |
| 2,3,6-Me3-Glc | 17.6 | →4)-Glcp-(1→ |
| 2,3,4-Me3-Gal | 1.6 | →6)-Galp-(1→ |
| 2,6-Me2-Man | 0.4 | →3,4)-Manp1→ |
| 2,4-Me2-Glc | 14.2 | →3,6)-Glcp-(1→ |
| 2,3-Me2-Man | 1.9 | →4,6)-Manp-(1→ |
| 3,4-Me2-Man | 0.6 | →2,6)-Manp-(1→ |
3.4. Preparative Chromatography
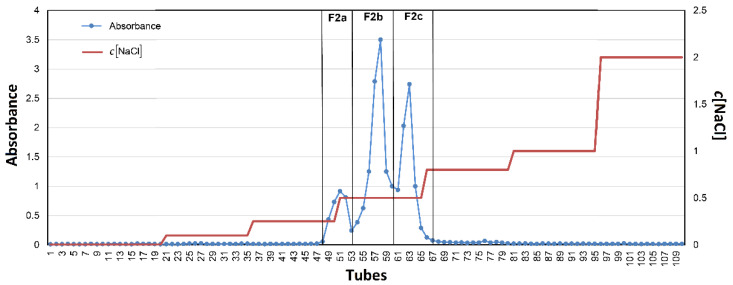
A chromatogram describing the fractionation of F2 is represented in Figure 3. According to the record, the tubes were merged into three sub-fractions, one minor (tubes 47–51) and two major ones (tubes 52–60 and 62–70), assigned as F2a, F2b, and F2c, respectively. All three fractions were dialyzed (1000 Da cut off) and then lyophilized. The yields of these sub-fractions compared to F2 are summarized in Table 5.
Figure 3.
Anion exchange preparative chromatography of F2.
Table 5.
Preparative chromatography of F2.
| Fraction/Sub-Fraction | Weight (mg) | Yield (% w/w) | |
|---|---|---|---|
| F2 | Enter | 66.67 | |
| F2a | Minor | 0.13 | 0.19 |
| F2b | Major | 32.54 | 48.81 |
| F2c | Major | 11.73 | 17.59 |
3.5. Vibrational Spectra
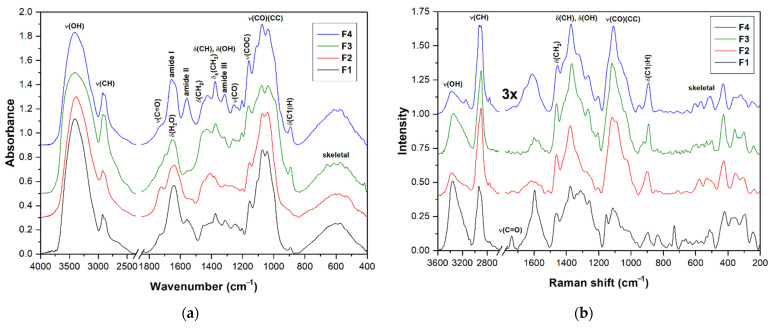
FTIR and FT Raman spectra of the fractions F1–F4 are shown in Figure 4a,b. The assignment of vibrational bands is given according to the literature [45,48,82,83,84,85]. The spectra of all fractions showed several intense overlapping IR bands in the range of 950–1200 cm−1, mainly CC and CO stretching vibrations of the pyranose rings, proving the presence of polysaccharides as the main component of the samples analyzed. An IR band around 1157 cm−1 can be assigned to the stretching vibration of the COC glycosidic bonds. Several Raman bands at 1417, 1276, 1205, 1116, 1093, and 1029 cm−1 are also characteristic for polysaccharides. A weak IR/Raman band around 897 cm−1 from C1H is present in all spectra and is characteristic of β-anomeric unit configurations, which could indicate β-glucans and, in the case of insoluble part F4, also chitin. Additional IR bands and shoulders that can be assigned to β-glucans were found near 1375, 1315, 1157, 1073, and 1040 cm−1 [84]. The FTIR spectrum of F4 has three bands at 1656 cm−1 (amide I), 1557 cm−1 (amide II), and 1317 cm−1 (amide III) that indicate the presence of chitin. In the case of the F1, the IR bands of amide vibrations around 1648 and 1546 cm−1 were assigned to proteins [86]. The presence of chitin and proteins in the mentioned fractions was also confirmed by the significant nitrogen content (see Table 2).
Figure 4.
FTIR (a) and FT Raman (b) spectra of the fractions F1, F2, F3, and F4 obtained from basidiocarp of G. resinaceum.
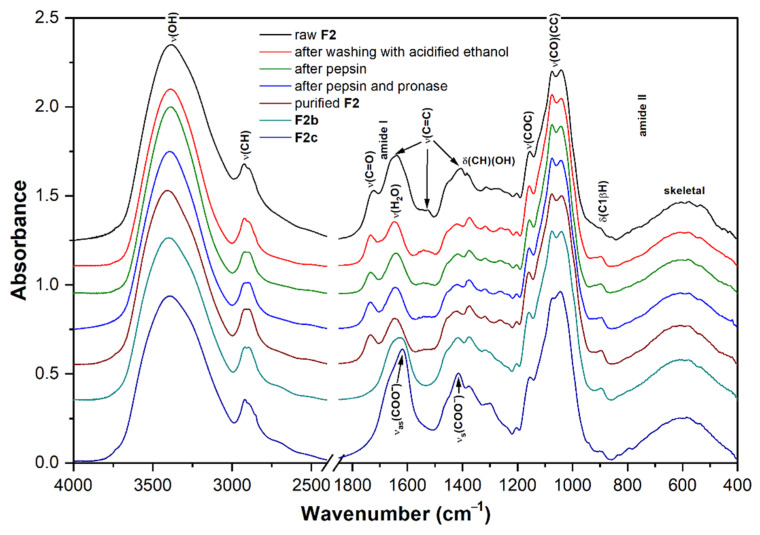
The spectra of the lyophilized raw fraction F2 and the products of its successive purification including sub-fractions F2b and F2c obtained by anionic preparative chromatography are shown in Figure 5. In all these spectra, the intense highly overlapped bands at 950–1200 cm−1 confirmed the predominance of polysaccharides. Phenolic compounds and other small molecules were removed by washing with 0.2 mol L−1 HCl in ethanol, which was confirmed by a decrease in a number of bands related to stretching vibrations of C=C bonds. To remove protein residues, a two-step enzymatic hydrolysis with pepsin and pronase was used. This is because pepsin is capable of cleaving long polypeptide chains into fragments that are still quite large and thus cannot be completely removed. Therefore, for a more complete hydrolysis of proteins, other proteases should be used after pepsin to cleave these fragments into smaller peptides and free amino acids that can be easily removed. The change in the protein content in the fraction can be traced to the intensity of the bands of amide vibrations at about 1647 cm−1 (amide I) and 1540 cm−1 (amide II) [86]. It should be noted here that the amide I band is not well suitable for the detection of the reminder protein due to overlap with the pronounced scissor vibrations of bound water, and therefore, it is better to use the less intense amide II band for this purpose. As a result, after all purification procedures, the band of amide II became insignificant, which confirms the effective removal of proteins. On the other hand, the two vibration bands of the carboxyl groups at 1740 cm−1 (C=O stretching) and 1240 cm−1 (CO stretching) remained unchanged after purification, so these groups are most likely part of polysaccharides, possibly in the form of uronic acids [87]. The spectra of sub-fractions did not contain these two bands; instead, the presence of two bands near 1610 cm−1 and 1405 cm−1, which were assigned to the antisymmetric and symmetric stretching vibrations of the carboxylate anions, respectively, confirmed that the sub-fractions, especially F2c, probably contain uronic acids, but in the form of a salt [87]. These two carboxylate bands were not strongly pronounced in the FTIR spectrum of F2b; therefore, this sub-fraction contained fewer uronic acids. The spectra of both sub-fractions have several bands at 1375, 1315, 1155, 1075, 1040, and 895 cm−1 characteristic for β-anomeric glucose units and, therefore, β-glucans [65,66,67,68,69].
Figure 5.
FTIR spectra of the successively purified fraction F2 and derived sub-fractions F2b and F2c.
3.6. NMR Spectra
The assignment of the proton and carbon signals of the main sugar units of the fractions F1, F2, and F3 is summarized in Table 6. The assignment was made using semi-empirical calculations of Casper software (Future Systems Solutions, Inc., USA) [88] and using the literature [47,48,89].
Table 6.
The assignment of proton and carbon signals (ppm) of the main sugar units for the fractions F1, F2, and F3 [30,66,67,73].
| Fraction | Unit | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | O2H | O4H | O6H | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1, F2 | A | →6)-α-Galp-(1→ | 4.95 | 3.82 | 3.85 | 4.02 | 4.14 | 3.68; 3.85 | |||
| 98.8 | 68.9 | 70.3 | 70.4 | 69.5 | 67.3 | ||||||
| A’ | →6)-α-Galp-(1→ | 5.00 | 3.83 | 3.85 | 4.02 | 4.14 | 3.68; 3.85 | ||||
| 98.8 | 68.9 | 70.3 | 70.4 | 69.5 | 67.3 | ||||||
| B | β-Manp-(1→2 | 5.10 | 4.04 | 3.68 | 3.60 | 3.33 | 3.70; 3.87 | ||||
| 102.9 | 70.5 | 74.2 | 68.1 | 76.5 | 61.9 | ||||||
| B’ | β-Manp-(1→2 | 5.02 | 4.03 | 3.68 | 3.60 | 3.33 | 3.68; 3.85 | ||||
| 102.9 | 70.5 | 74.2 | 68.1 | 76.5 | 61.9 | ||||||
| C | →2,6)-α-Galp-(1→ | 5.08 | 3.92 | 4.00 | 4.02 | 4.14 | 3.68; 3.85 | ||||
| 101.6 | 78.48 | 67.7 | 70.4 | 69.5 | 67.3 | ||||||
| C’ | →2,6)-α-Galp-(1→ | 5.22 | 4.06 | 3.99 | 4.02 | 4.14 | 3.68; 3.85 | ||||
| 101.2 | 78.90 | 67.8 | 70.4 | 69.5 | 67.3 | ||||||
| D | β-Glcp-(1→ | 4.47 | 3.30 | 3.48 | 3.38 | 3.61 | 3.78; 3.94 | ||||
| 103.6 | 74.1 | 76.7 | 70.6 | 76.2 | 61.8 | ||||||
| D’ | →6)-β-Glcp-(1→ | 4.49 | 3.34 | 3.48 | 3.37 | 3.51 | 3.85; 4.15 | ||||
| 103.6 | 74.3 | 76.7 | 70.6 | 76.5 | 69.3 | ||||||
| E | →3)-β-Glcp-(1→ | 4.73 | 3.54 | 3.74 | 3.48 | 3.65 | 3.70; 3.87 | ||||
| 103.2 | 73.4 | 85.3 | 69.2 | 79.6 | 61.8 | ||||||
| E’ | →3,6)-β-Glcp-(1→ | 4.52 | 3.50 | 3.70 | 3.41 | 3.72 | 3.85; 4.15 | ||||
| 103.1 | 76.5 | 85.3 | 70.6 | 74.2 | 69.3 | ||||||
| F | →4)-β-Glcp-(1→ | 4.71 | 3.39 | 3.63 | 3.63 | 3.60 | 3.78; 3.94 | ||||
| 103.2 | 76.6 | 76.5 | 79.7 | 76.2 | 61.8 | ||||||
| F’ | →4)-β-Glcp-(1→ | 4.68 | 3.37 | 3.62 | 3.63 | 3.60 | 3.78; 3.94 | ||||
| 103.3 | 74.5 | 76.5 | 79.7 | 76.2 | 61.8 | ||||||
| G | →4)-α-Glcp-(1→ | 5.34 | 3.60 | 3.92 | 3.53 | 3.80 | 3.87 | ||||
| 100.3 | 71.5 | 73.3 | 78.8 | 72.6 | 60.7 | ||||||
| H | →3)-α-Fucp-(1→ | 4.12 | 1.20 | ||||||||
| 16.2 | |||||||||||
| F3 | A | →3)-β-Glcp-(1→ | 4.76 | 3.56 | 3.73 | 3.48 | 3.51 | 3.73; 3.96 | 5.12 | 4.66 | 4.54 |
| 103.6 | 73.4 | 86.9 | 69.1 | 79.1 | 61.7 | ||||||
| B | →3)-β-Glcp-(1→ | 5.31 | 3.67 | 3.88 | 3.67 | 4.07 | 3.76; 3.88 | 5.07 | 4.67 | ||
| 100.3 | 71.3 | 83.7 | 69.0 | 72.8 | 61.6 | ||||||
| C | β-Glcp-(1→6 | 4.47 | 3.26 | 3.44 | 3.34 | 3.38 | 3.71; 3.96 | 4.63 | |||
| 103.6 | 73.9 | 76.9 | 71.2 | 77.2 | 61.7 | ||||||
| D | →3,6)-β-Glcp-(1→ | 4.76 | 3.53 | 3.72 | 3.57 | 3.77 | 3.83; 4.32 | 4.70 | |||
| 103.6 | 73.7 | 86.9 | 69.1 | 76.8 | 69.3 | ||||||
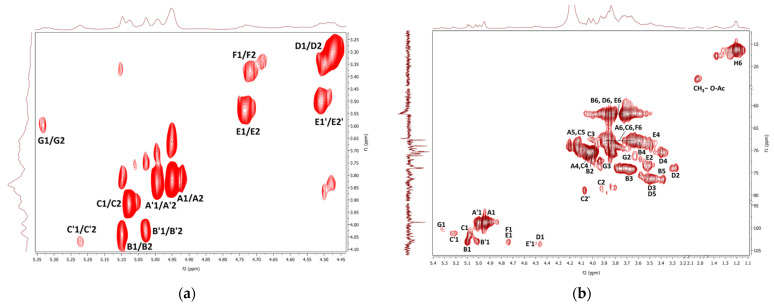
The COSY NMR spectrum of the fraction F1 (Figure 6a) showed several H1α/H2 cross peaks assigned to, 1,6-linked (A, A’) and 1,2,6-linked α-d-galactosyl (C, C’), terminal β-d-mannosyl (B, B’), and 1,4-linked α-d-glucosyl (G) units. These units probably come from two polysaccharides, i.e., branched O-2-β-d-mannosyl-(1→6)-α-d-galactan, and, in smaller amounts, amylose-like (1→4)-α-d-glucan. In contrast to mannogalactans previously described for mushrooms of genus Pleurotus [36,90,91], this mannogalactan obtained from G. resinaceum was not methylated at the O-3 position of the backbone galactosyl units because no signals of OCH3 groups were found. Moreover, several H1β/H2 cross peaks were assigned to terminal β-d-glucosyl (D), 1,6-linked β-d-glucosyl (D’), 1,3-linked β-d-glucosyl (E), 1,3,6-linked β-d-glucosyl (E’), and 1,4-linked β-d-glucosyl (F, F’) units; all of them are probably parts of a highly branched (1→3)(1→4)(1→6)-β-d-glucan. The HMQC spectrum (Figure 6b) shows the signals that indicate the presence of the CHOH, CHOR, CH2OH, and CH2OR’ residues of the above polysaccharides. The HMQC signal of CH3 at 1.20 ppm/16.2 ppm and COSY cross peak at 1.20 ppm/4.13 ppm arose from the α-fucosyl units (H). The HMQC signal at 2.02 ppm/22.9 ppm indicated a small amount of O-acetyl groups. Therefore, according to the relative intensities of the resonance signals and previous analyses mentioned above, F1 contains branched mannogalactan as the main product, lower content of branched β-glucan, and a negligible amount of α-glucan. Water-soluble heterogalactan has been previously isolated from the fruiting bodies of Ganoderma atrum [52]. As in the case of the current study, this polysaccharide had a backbone of 1,6-linked α-d-galactosyl units, but in this case, terminal α-l-fucosyl and α-d-mannosyl units and oligosaccharide fragments can act as side chains attached to the O-6 position of some of the backbone units.
Figure 6.
1H, 1H COSY (a) and 1H, 13C HMQC (b) NMR spectra of the fraction F1.
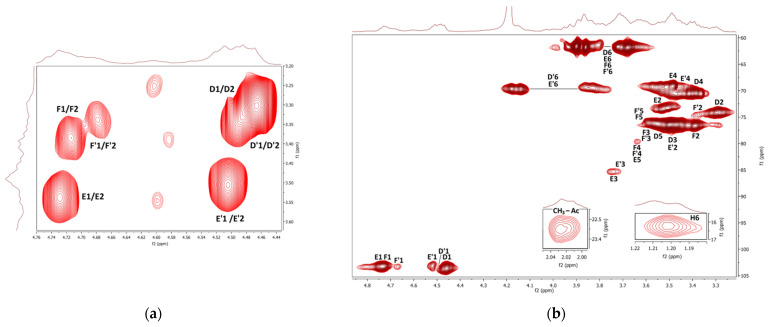
The COSY and HMQC NMR spectra of the fraction F2 are shown in Figure 7a,b. As in the case of F1, branched (1→3)(1→6)-β-d-glucan is represented here by 1,3-linked, 1,4-linked, 1,6-linked, 1,3,6-linked, and terminal β-d-glucosyl units (D–F). Furthermore, the presence of mannogalactan was proved by the weak signals of 1,6-linked and 1,2,6-linked α-d-galactosyl and terminal β-d-mannosyl units (A–C). A weak HMQC signal at 2.03 ppm/22.96 ppm was assigned to CH3 of O-acetyl groups. In addition, 1,4-linked α-d-glucosyl (G) and α-l-fucosyl (H) units were also detected. In general, branched β-glucan predominated in this fraction, while α-glucan and mannogalactan were present to a lesser extent. Water-soluble highly branched β-glucans of similar structure have been previously described for some Ganoderma species [18]; these polysaccharides were isolated from fruiting bodies, and crowing culture of mycelium [92] and spores [93,94]. For example, water-soluble β-d-glucan was previously isolated from basidiocarps of Ganoderma resinaceum [95]. It was a highly branched polysaccharide that contained 1,3-linked β-d-glucosyl units in the backbone, partially substituted mainly by the side chains of 4-O-substituted β-d-glucosyl units at the O-6 on average for every two residues of the main chain.
Figure 7.
1H, 1H COSY (a) and 1H, 13C HMQC (b) NMR spectra of the fraction F2.
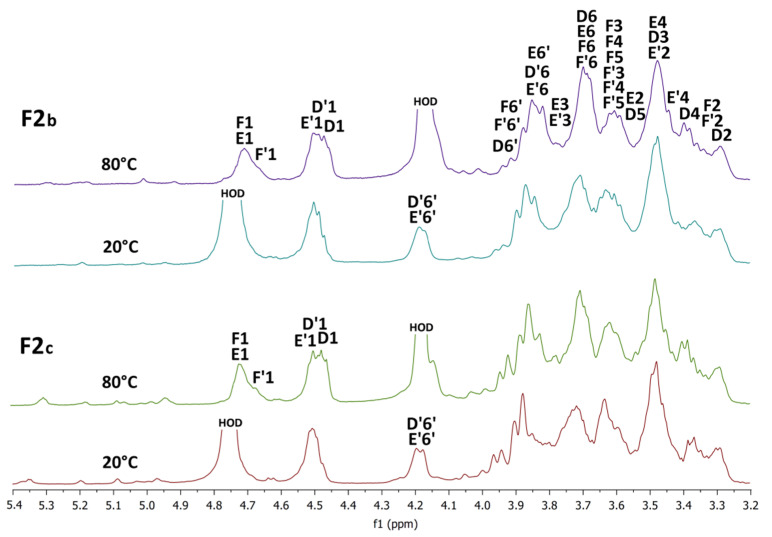
The 1H NMR spectra of the F2b and F2c sub-fractions were measured in D2O at 20 °C and 80 °C and are shown in Figure 8. For both fractions, when measured at 20 °C, an intense HOD signal overlapped the H1β resonances, and when measured at 80 °C, this intense signal shifted upfield and already overlapped the H6 resonances. The spectra of both sub-fractions are very similar to the spectrum of the initial fraction F2, and only minor differences were observed between them. Within the fractions, there is certainly a different ratio between the signals of individual glucosyl residues. For example, the signals of terminal β-d-glucosyl units D were more pronounced in the case of sub-fraction F2c, so this product should have a higher degree of branching.
Figure 8.
1H NMR spectra of the sub-fractions F2b and F2c measured in D2O at 20 °C and 80 °C.
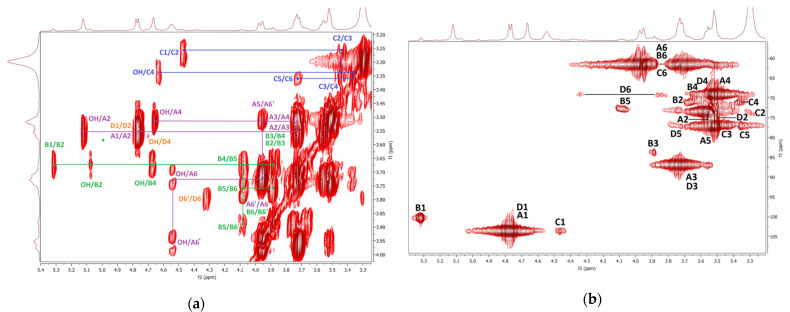
The COSY and HMQC NMR spectra of the fraction F3 are represented in Figure 9. The spectra were measured in Me2SO-d6, so there is no proton exchange between the hydroxylic groups and the solvent, and the COSY spectra reveal both CH and OH proton signals (Figure 9a). The assignment of the proton and carbon signals of the main sugar units is shown in Table 6. The H1/H2 and OH/CH cross peaks with different intensities found in the COSY spectrum were assigned to the three main glucosyl units. The signals of 1,3-linked β-d-glucosyl units (A) were the most pronounced; the less intense signals were assigned to the 1,3-linked α-d-glucosyl (B), terminal β-glucosyl (C), and 1,3,6-linked β-d-glucosyl (D) residues. The signals of units A were also the most intense in the HMQC spectrum (Figure 9b). These units are evidently inter-connected into the (1→3)-β-d-glucan backbone that can be slightly branched at O-6 of the units D (branching points) by the terminal β-d-glucosyl units C. Slightly branched (1→3)(1→6)-β-d-glucan was isolated by alkaline extraction from the fruiting bodies of Ganoderma japonicum [96]. In this polysaccharide, only every 30th unit of the backbone was a branching point carrying a terminal β-d-glucosyl attached at the O-6 position. In contrast, the units B are rather linked to each other, yielding the (1→3)-α-d-glucan, which is less pronounced in F3. Pure α-d-glucan of a similar structure has been previously isolated from Ganoderma lucidum by alkaline extraction [89]. The alkaline extract from mycelium of Ganoderma tsugae contained the mixture of (1→3)-α-d-glucan and mannoxylan [97].
Figure 9.
1H, 1H COSY (a) and 1H, 13C HMQC (b) NMR spectra of the fraction F3.
4. Conclusions
In this work, polysaccharide fractions of G. resinaceum extracted from dried fruiting bodies were used for a deeper investigation. Four polysaccharidic fractions, including the insoluble part, were obtained from the basidiocarp by successive extractions with cold water, hot water, and alkaline solution. The cold water extract was defined as a mixture of three polysaccharides: branched O-2-β-d-mannosyl-(1→6)-α-d-galactan, branched (1→3)(1→6)-β-d-glucan, and branched (1→6)(1→2)-β-d-galactan. The hot water extract consisted mainly of branched (1→3)(1→6)-β-d-glucan and linear (1→4)-α-d-glucan, but also contained a small amount of (1→2)(1→6)-β-d-galactan. Two sub-fractions of this extract were obtained by preparative chromatography; both contained branched (1→3)(1→6)-β-d-glucans, but differed in the degree of branching and in the presence of uronic acids. The alkaline extract consisted mainly of (1→3)-β-d-glucan slightly branched at O-6 by terminal β-glucose, and a small amount of (1→3)-α-d-glucan was also found in this fraction. Therefore, the β-d-glucans obtained by alkaline extraction were less branched than the water-soluble β-d-glucans. The solid residues after all of the extractions were defined as the complex of two main cell wall polysaccharides, i.e., chitin and β-d-glucan. The high content of d-glucans of various structures with potential immunomodulatory activity in basidiocarps of Ganoderma resinaceum, described in this study, makes this species promising for cultivation in order to obtain these polysaccharides, as well as other potentially biologically active substances for the preparation of functional food products or nutritional supplements that can support health and prevent the development of various diseases.
Author Contributions
Conceptualization and methodology, R.B. and A.S.; software, R.B. and A.S.; formal analysis and validation, R.B., L.T., L.S. and P.C.; investigation, R.B., L.T., L.S. and P.C.; resources, R.B., P.K., I.J. and A.S.; data curation, R.B., A.S. and L.T.; writing—original draft preparation, review and editing, R.B., P.K., I.J. and A.S.; supervision, A.S. and J.Č.; project administration, R.B. and A.S.; funding acquisition, P.K. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of the Czech Republic (project QK1910209), Specific University Research of UCT Prague (project numbers 21-SVV/2019, 21-SVV/2020, and 21-SVV/2021) and Slovak Grant Agency VEGA (Grant no. 2/0054/22).
Acknowledgments
Authors would like to thank Michal Tomšovský (Mendel University in Brno, Czech Republic) for his help with the identification and placement of the fungus basidiocarp sample in the herbarium of the Moravian Museum (BRNM), Brno, Czech Republic.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang L., Li J.Q., Zhang J., Li Z.M., Liu H.G., Wang Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020;10:42084–42097. doi: 10.1039/D0RA07219B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wińska K., Mączka W., Gabryelska K., Grabarczyk M. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules. 2019;24:4075. doi: 10.3390/molecules24224075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hapuarachchi K.K., Elkhateeb W.A., Karunarathna S.C., Cheng C.R., Bandara A.R., Kakumyan P., Cheng C.R., Bandara A.R., Kakumyan P., Hyde K.D., et al. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere. 2018;9:1025–1052. doi: 10.5943/mycosphere/9/5/6. [DOI] [Google Scholar]
- 4.Rai M.K., Gaikwad S., Nagaonkar D., dos Santos C.A. Current advances in the antimicrobial potential of species of genus Ganoderma (higher Basidiomycetes) against human pathogenic microorganisms. Int. J. Med. Mushrooms. 2015;17:921–932. doi: 10.1615/IntJMedMushrooms.v17.i10.20. [DOI] [PubMed] [Google Scholar]
- 5.Beck T., Gáper J., Šebesta M., Gáperová S. Host preferences of wood-decaying fungi of the genus Ganoderma in the urban areas of Slovakia. Ann. Univ. Paedagog. Crac. Studia Nat. 2018;3:22–37. doi: 10.24917/25438832.3.2. [DOI] [Google Scholar]
- 6.Zhou X.W., Cong W.R., Su K.Q., Zhang Y.M. Ligninolytic enzymes from Ganoderma spp.: Current status and potential applications. Crit. Rev. Microbiol. 2013;39:416–426. doi: 10.3109/1040841X.2012.722606. [DOI] [PubMed] [Google Scholar]
- 7.De Souza Silva C.M.M., De Melo I.S., De Oliveira P.R. Ligninolytic enzyme production by Ganoderma spp. Enzym. Microb. Technol. 2005;37:324–329. doi: 10.1016/j.enzmictec.2004.12.007. [DOI] [Google Scholar]
- 8.Murugesan K., Nam I., Kim Y., Chang Y. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enz. Microb. Technol. 2007;40:1662–1672. doi: 10.1016/j.enzmictec.2006.08.028. [DOI] [Google Scholar]
- 9.Deflorio G., Johnson C., Fink S., Schwarze F.W.M.R. Decay development in living sapwood of coniferous and deciduous trees inoculated with six wood decay fungi. For. Ecol. Manag. 2008;255:2373–2383. doi: 10.1016/j.foreco.2007.12.040. [DOI] [Google Scholar]
- 10.Xu Z.T., Chen X.U., Zhong Z.F., Che L.D., Wang Y.T. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011;39:15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 11.Seweryn E., Ziała A., Gamian A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients. 2021;13:2725. doi: 10.3390/nu13082725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat Z.A., Wani A.H., War J.M., Bhat M.Y. Mayor bioactive properties of Ganoderma polysaccharides. A review. Asian J. Pharm. Clin. Res. 2021;14:11–24. doi: 10.22159/ajpcr.2021.v14i3.40390. [DOI] [Google Scholar]
- 13.Zhang J., Liu Y., Tang Q., Zhou S., Feng J., Chen H. Polysaccharide of Ganoderma and its bioactivities. In: Lin Z., Yang B., editors. Ganoderma and Health. Advances in Experimental Medicine and Biology. Volume 1181. Springer; Singapore: 2019. pp. 107–134. [DOI] [PubMed] [Google Scholar]
- 14.Cör D., Knez Ž., Knez Hrnčič M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules. 2018;23:649. doi: 10.3390/molecules23030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira I.C., Heleno S.A., Reis F.S., Stojkovic D., Queiroz M.J.R., Vasconcelos M.H., Sokovic M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry. 2015;114:38–55. doi: 10.1016/j.phytochem.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Benkeblia N. Ganoderma lucidum polysaccharides and terpenoids: Profile and health benefits. J. Food Nutr. Diet. 2015;1:1–6. doi: 10.19104/jfnd.2015.101. [DOI] [Google Scholar]
- 17.Camargo M.R., Kaneno R. Antitumor properties of Ganoderma lucidum polysaccharides and terpenoids. Annu. Rev. Biomed. Sci. 2011;13:1–8. [Google Scholar]
- 18.Nie S., Zhang H., Li W., Xie M. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydr. Diet. Fibre. 2013;1:10–20. doi: 10.1016/j.bcdf.2013.01.001. [DOI] [Google Scholar]
- 19.Liang C., Tian D., Liu Y., Li H., Zhu J., Li M., Xin M., Xia J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019;174:130–141. doi: 10.1016/j.ejmech.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Peng X., Qiu M. Meroterpenoids from Ganoderma species: A review of last five years. Nat. Prod. Bioprosp. 2018;8:137–149. doi: 10.1007/s13659-018-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu E.A., Choi J.H., Seong G.U., Chung S.K. Isolation of polyphenol compounds from Ganoderma lucidum and pancreatic lipase inhibitory activities. J. Korean Soc. Food Sci. Nutr. 2020;49:28–34. doi: 10.3746/jkfn.2020.49.1.28. [DOI] [Google Scholar]
- 22.Cho J.H., Lee J.Y., Lee M.J., Oh H.N., Kang D.H., Jhune C.S. Comparative analysis of useful β-glucan and polyphenol in the fruiting bodies of Ganoderma spp. J. Mushroom. 2013;11:164–170. doi: 10.14480/JM.2013.11.3.164. [DOI] [Google Scholar]
- 23.Baby S., Johnson A.J., Govindan B. Secondary metabolites from Ganoderma. Phytochemistry. 2015;114:66–101. doi: 10.1016/j.phytochem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Sharma C., Bhardwaj N., Sharma A., Tuli H.S., Batra P., Beniwal V., Gupta G.K., Sharma A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019;17:100268. doi: 10.1016/j.hermed.2019.100268. [DOI] [Google Scholar]
- 25.Ahmad M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018;107:507–519. doi: 10.1016/j.biopha.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Paterson R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Kiss A., Mirmazloum I., Naár Z., Némedi E. Supplementation of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes) extract enhanced the medicinal values and prebiotic index of hungarian acacia honey. Int. J. Med. Mushrooms. 2019:21. doi: 10.1615/IntJMedMushrooms.2019032897. [DOI] [PubMed] [Google Scholar]
- 28.Prasad S., Rathore H., Sharma S., Yadav A.S. Medicinal mushrooms as a source of novel functional food. Int. J. Food Sci. Nutr. Diet. 2015;4:221–225. [Google Scholar]
- 29.Perera P.K., Li Y. Mushrooms as a functional food mediator in preventing and ameliorating diabetes. Funct. Foods Health Dis. 2011;1:161–171. doi: 10.31989/ffhd.v1i4.133. [DOI] [Google Scholar]
- 30.Gow N.A., Latge J.P., Munro C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectrum. 2017;5 doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Free S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- 32.Latgé J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 33.Ren Y., Bai Y., Zhang Z., Cai W., Del Rio Flores A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules. 2019;24:3122. doi: 10.3390/molecules24173122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Ma Z., Zhang L., Fang Y., Jiang F., Phillips G.O. Structure and chain conformation of water-soluble heteropolysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2011;86:844–851. doi: 10.1016/j.carbpol.2011.05.031. [DOI] [Google Scholar]
- 35.Pan K., Jiang Q., Liu G., Miao X., Zhong D. Optimization extraction of Ganoderma lucidum polysaccharides rides and its immunity and antioxidant activities. Int. J. Biol. Macromol. 2013;55:301–306. doi: 10.1016/j.ijbiomac.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Baeva E., Bleha R., Lavrova E., Sushytskyi L., Čopíková J., Jablonsky I., Klouček P., Synytsya A. Polysaccharides from basidiocarps of cultivating mushroom Pleurotus ostreatus: Isolation and structural characterization. Molecules. 2019;24:2740. doi: 10.3390/molecules24152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J.K., Ding Z.C., Gao X., Wang Y.Y., Yang Y., Wu D., Zhang H.N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohydr. Polym. 2018;193:373–382. doi: 10.1016/j.carbpol.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Huang S.Q., Li J.W., Wang Z., Pan H.X., Chen J.X., Ning Z.X. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice. Molecules. 2010;15:3694–3708. doi: 10.3390/molecules15053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leong Y.K., Yang F.C., Chang J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021;251:117006. doi: 10.1016/j.carbpol.2020.117006. [DOI] [PubMed] [Google Scholar]
- 40.Chikari F., Han J., Wang Y., Ao W. Synergized subcritical-ultrasound-assisted aqueous two-phase extraction, purification, and characterization of Lentinus edodes polysaccharides. Process Biochem. 2020;95:297–306. doi: 10.1016/j.procbio.2020.03.009. [DOI] [Google Scholar]
- 41.Lin Y., Zeng H., Wang K., Lin H., Li P., Huang Y., Zhou S., Zhang W., Chen C., Fan H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019;136:305–315. doi: 10.1016/j.ijbiomac.2019.06.064. [DOI] [PubMed] [Google Scholar]
- 42.Kang Q., Chen S., Li S., Wang B., Liu X., Hao L., Lu J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019;124:1137–1144. doi: 10.1016/j.ijbiomac.2018.11.215. [DOI] [PubMed] [Google Scholar]
- 43.Do D.T., Lam D.H., Nguyen T., Phuong Mai T.T., Phan L.T.M., Vuong H.T., Nguyen D.V., Linh N.T., Hoang M.N., Mai T.P., et al. Utilization of response surface methodology in optimization of polysaccharides extraction from Vietnamese Red Ganoderma lucidum by ultrasound-assisted enzymatic method and examination of bioactivities of the extract. Sci. World J. 2021;2021:7594092. doi: 10.1155/2021/7594092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang S., Ning Z. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int. J. Biol. Macromol. 2010;47:336–341. doi: 10.1016/j.ijbiomac.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Matsunaga Y., Wahyudiono, Machmudah S., Sasaki M., Goto M. Hot compressed water extraction of polysaccharides from Ganoderma lucidum using a semibatch reactor. Asia-Pac. J. Chem. Eng. 2014;9:125–133. doi: 10.1002/apj.1752. [DOI] [Google Scholar]
- 46.Zeng X., Li P., Chen X., Kang Y., Xie Y., Li X., Xie T., Zhang Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019;126:867–876. doi: 10.1016/j.ijbiomac.2018.12.222. [DOI] [PubMed] [Google Scholar]
- 47.Synytsya A., Novak M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013;92:792–809. doi: 10.1016/j.carbpol.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 48.Synytsya A., Novak M. Structural analysis of glucans. Ann. Transl. Med. 2014;2:17. doi: 10.3978/j.issn.2305-5839.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y., Ou X., Yang J., Bi S., Peng B., Wen Y., Song L., Li C., Yu R., Zhu J. Structural characterization and biological activities of a novel polysaccharide containing N-acetylglucosamine from Ganoderma sinense. Int. J. Biol. Macromol. 2020;158:1204–1215. doi: 10.1016/j.ijbiomac.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Yi P., Li N., Wan J.B., Zhang D., Li M., Yan C. Structural characterization and antioxidant activity of a heteropolysaccharide from Ganoderma capense. Carbohydr. Polym. 2015;121:183–189. doi: 10.1016/j.carbpol.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 51.Chuang C.M., Wang H.E., Chang C.H., Peng C.C., Ker Y.B., Lai J.E., Chen K.C., Peng R.Y. Sacchachitin, a novel chitin-polysaccharide conjugate macromolecule present in Ganoderma lucidum: Purification, composition, and properties. Pharm. Biol. 2013;51:84–95. doi: 10.3109/13880209.2012.711840. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H., Nie S.P., Yin J.Y., Wang Y.X., Xie M.Y. Structural characterization of a heterogalactan purified from fruiting bodies of Ganoderma atrum. Food Hydrocol. 2014;36:339–347. doi: 10.1016/j.foodhyd.2013.08.029. [DOI] [Google Scholar]
- 53.Nara K., Kato Y. Structural characterization of a heterogalactan from antler-shaped Ganoderma lucidum. J. Appl. Glycosci. 2015;62:149–151. doi: 10.5458/jag.jag.JAG-2015_009. [DOI] [Google Scholar]
- 54.Tel-Çayan G., Muhammad A., Deveci E., Duru M.E., Öztürk M. Isolation, structural characterization, and biological activities of galactomannans from Rhizopogon luteolus and Ganoderma adspersum mushrooms. Int. J. Biol. Macromol. 2020;165:2395–2403. doi: 10.1016/j.ijbiomac.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Lai L., Yang D. Rheological properties of the hot-water extracted polysaccharides in Ling-Zhi (Ganoderma lucidum) Food Hydrocol. 2007;21:739–746. doi: 10.1016/j.foodhyd.2006.09.009. [DOI] [Google Scholar]
- 56.Lu J., He R., Sun P., Zhang F., Linhardt R.J., Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 57.Sohretoglu D., Huang S. Ganoderma lucidum polysaccharides as an anti-cancer agent. Anti-Cancer Agents Med. Chem. 2018;18:667–674. doi: 10.2174/1871520617666171113121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren L., Zhang J., Zhang T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021;340:127933. doi: 10.1016/j.foodchem.2020.127933. [DOI] [PubMed] [Google Scholar]
- 59.Hennicke F., Cheikh-Ali Z., Liebisch T., Maciá-Vicente J.G., Bode H.B., Piepenbring M. Distinguishing commercially grown Ganoderma lucidum from Ganoderma lingzhi from Europe and East Asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry. 2016;127:29–37. doi: 10.1016/j.phytochem.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Eo S.K., Kim Y.S., Lee C.K., Han S.S. Antiherpetic activities of various protein bound polysaccharides isolated from Ganoderma lucidum. J. Ethnopharmacol. 1999;68:175–181. doi: 10.1016/S0378-8741(99)00086-0. [DOI] [PubMed] [Google Scholar]
- 61.Eo S.K., Kim Y.S., Lee C.K., Han S.S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. Ethnopharmacol. 2000;72:475–481. doi: 10.1016/S0378-8741(00)00266-X. [DOI] [PubMed] [Google Scholar]
- 62.Khan I., Huang G., Li X., Leong W., Xia W., Hsiao W.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods. 2018;41:191–201. doi: 10.1016/j.jff.2017.12.046. [DOI] [Google Scholar]
- 63.Liu Y., Li Y., Zhang W., Sun M., Zhang Z. Hypoglycemic effect of inulin combined with Ganoderma lucidum polysaccharides in T2DM rats. J. Funct. Foods. 2019;55:381–390. doi: 10.1016/j.jff.2019.02.036. [DOI] [Google Scholar]
- 64.Xiao C., Wu Q., Xie Y., Tan J., Ding Y., Bai L. Hypoglycemic mechanisms of Ganoderma lucidum polysaccharides F31 in db/db mice via RNA-seq and iTRAQ. Food Funct. 2018;9:6495–6507. doi: 10.1039/C8FO01656A. [DOI] [PubMed] [Google Scholar]
- 65.Xiao C., Wu Q.P., Cai W., Tan J.B., Yang X.B., Zhang J.M. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch. Pharm. Res. 2012;35:1793–1801. doi: 10.1007/s12272-012-1012-z. [DOI] [PubMed] [Google Scholar]
- 66.Ma H.T., Hsieh J.F., Chen S.T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry. 2015;114:109–113. doi: 10.1016/j.phytochem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Joseph S., Sabulal B., George V., Antony K.R., Janardhanan K.K. Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum. Acta Pharm. 2011;61:335–342. doi: 10.2478/v10007-011-0030-6. [DOI] [PubMed] [Google Scholar]
- 68.Huang X.J., Nie S.P. The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 2015;6:3205–3217. doi: 10.1039/C5FO00678C. [DOI] [PubMed] [Google Scholar]
- 69.Chen B., Ke B., Ye L., Jin S., Jie F., Zhao L., Wu X. Isolation and varietal characterization of Ganoderma resinaceum from areas of Ganoderma lucidum production in China. Sci. Hortic. 2017;224:109–114. doi: 10.1016/j.scienta.2017.06.002. [DOI] [Google Scholar]
- 70.Sushytskyi L., Lukáč P., Synytsya A., Bleha R., Rajsiglová L., Capek P., Pohl R., Vannucci L., Čopíková J., Kaštánek P. Immunoactive polysaccharides produced by heterotrophic mutant of green microalga Parachlorella kessleri HY1 (Chlorellaceae) Carbohydr. Polym. 2020;246:116588. doi: 10.1016/j.carbpol.2020.116588. [DOI] [PubMed] [Google Scholar]
- 71.Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S.I., Lee Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Englyst H.N., Cummings J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1984;109:937–942. doi: 10.1039/an9840900937. [DOI] [PubMed] [Google Scholar]
- 73.Jansson P.E., Kenne L., Liedgren H., Lindberg B., Lonngren J. A practical guide to the methylation analysis of carbohydrates. Chem. Commun. (Stockholm Univ.) 1976;8:1–75. [Google Scholar]
- 74.Ciucanu I., Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984;131:209–217. doi: 10.1016/0008-6215(84)85242-8. [DOI] [Google Scholar]
- 75.Purdie T., Irvine J.C. The Alkylation of Sugars. Chem. Soc. Trans. 1903;83:1021–1037. doi: 10.1039/CT9038301021. [DOI] [Google Scholar]
- 76.Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 77.Mokhtari-Hosseini Z.B., Hatamian-Zarmi A., Mohammadnejad J., Ebrahimi-Hosseinzadeh B. Chitin and chitosan biopolymer production from the Iranian medicinal fungus Ganoderma lucidum: Optimization and characterization. Prep. Biochem. Biotechnol. 2018;48:662–670. doi: 10.1080/10826068.2018.1487847. [DOI] [PubMed] [Google Scholar]
- 78.Smiderle F.R., Morales D., Gil-Ramírez A., Jesus L.I., Gilbert-Lopez B., Iacominy M., Soler-Rivas C. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-d-glucans from mushrooms. Carbohydr. Polym. 2017;156:165–174. doi: 10.1016/j.carbpol.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 79.Gao X., Qi J., Ho C.T., Li B., Mu J., Zhang Y., Hu H., Mo W., Chen Z., Xie Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020;249:116874. doi: 10.1016/j.carbpol.2020.116874. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Li W.J., Nie S.P., Chen Y., Wang Y.X., Xie M.Y. Structural characterisation of a novel bioactive polysaccharide from Ganoderma atrum. Carbohydr. Polym. 2012;88:1047–1054. doi: 10.1016/j.carbpol.2012.01.061. [DOI] [Google Scholar]
- 81.Li J., Gu F., Cai C., Hu M., Fan L., Hao J., Yu G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020;143:806–813. doi: 10.1016/j.ijbiomac.2019.09.141. [DOI] [PubMed] [Google Scholar]
- 82.Bekiaris G., Tagkouli D., Koutrotsios G., Kalogeropoulos N., Zervakis G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods. 2020;9:535. doi: 10.3390/foods9040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzaga M.L.C., Menezes T.M., de Souza J.R.R., Ricardo N.M., Soares S.D.A. Structural characterization of β-glucans isolated from Agaricus blazei Murill using NMR and FTIR spectroscopy. Bioact. Carbohydr. Diet. Fibre. 2013;2:152–156. doi: 10.1016/j.bcdf.2013.10.005. [DOI] [Google Scholar]
- 84.Synytsya A., Míčková K., Synytsya A., Jablonsky I., Speváček J., Erban V., Kovářiková E., Čopíková J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009;76:548–556. doi: 10.1016/j.carbpol.2008.11.021. [DOI] [Google Scholar]
- 85.Galichet A., Sockalingum G.D., Belarbi A., Manfait M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Let. 2001;197:179–186. doi: 10.1111/j.1574-6968.2001.tb10601.x. [DOI] [PubMed] [Google Scholar]
- 86.Grdadolnik J. Saturation effects in FTIR spectroscopy: Intensity of amide I and amide II bands in protein spectra. Acta Chim. Slov. 2003;50:777–788. [Google Scholar]
- 87.Synytsya A., Čopíková J., Matějka P., Machovič V.J. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003;54:97–106. doi: 10.1016/S0144-8617(03)00158-9. [DOI] [Google Scholar]
- 88.Jansson P.E., Kenne L., Widmalm G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H-and 13C-NMR data. Carbohydr. Res. 1989;188:169–191. doi: 10.1016/0008-6215(89)84069-8. [DOI] [PubMed] [Google Scholar]
- 89.Chen J., Zhou J., Zhang L., Nakamura Y., Norisuye T. Chemical structure of the water-insoluble polysaccharide isolated from the fruiting body of Ganoderma lucidum. Polym. J. 1998;30:838–842. doi: 10.1295/polymj.30.838. [DOI] [Google Scholar]
- 90.Smiderle F.R., Olsen L.M., Carbonero E.R., Marcon R., Baggio C.H., Freitas C.S., Santos A.R.S., Torri G., Gorin P.A.J., Iacomini M. A 3-O-methylated mannogalactan from Pleurotus pulmonarius: Structure and antinociceptive effect. Phytochemistry. 2008;69:2731–2736. doi: 10.1016/j.phytochem.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Rosado F.R., Carbonero E.R., Claudino R.F., Tischer C.A., Kemmelmeier C., Iacomini M. The presence of partially 3-O-methylated mannogalactan from the fruit bodies of edible basidiomycetes Pleurotus ostreatus ‘florida’ Berk. and Pleurotus ostreatoroseus Sing. FEMS Microbiol. Lett. 2003;221:119–124. doi: 10.1016/S0378-1097(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 92.Amaral A.E.A., Carbonero E.R., Simao R.C.G., Kadowaki M.K., Sassaki G.L., Osaku C.A., Gorin P.A.J., Iacomini M. An unusual water-soluble b-glucan from the basidiocarp of the fungus Ganoderma resinaceum. Carbohydr. Polym. 2008;72:473–478. doi: 10.1016/j.carbpol.2007.09.016. [DOI] [Google Scholar]
- 93.Bao X., Liu C., Fang J., Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001;332:67–74. doi: 10.1016/S0008-6215(01)00075-1. [DOI] [PubMed] [Google Scholar]
- 94.Sone Y., Okuda R., Wada N., Kishida E., Misaki A. Structures and antitumor activities of the polysaccharides isolated from fruiting body and the crowing culture of mycelium of Ganoderma lucidum. Agric. Biol. Chem. 1985;49:2641–2653. [Google Scholar]
- 95.Dong Q., Wang Y., Shi L., Yao J., Li J., Ma F., Ding K. A novel water-soluble β-d-glucan isolated from the spores of Ganoderma lucidum. Carbohydr. Res. 2012;353:100–105. doi: 10.1016/j.carres.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 96.Ukai S., Yokoyama S., Hara C., Kiho T. Structure of an alkali-soluble polysaccharide from the fruit body of Ganoderma japonicum Lloyd. Carbohydr. Res. 1982;105:237–245. doi: 10.1016/S0008-6215(00)84971-X. [DOI] [Google Scholar]
- 97.Peng Y., Zhang L., Zhang Y., Xu X., Kennedy J.F. Solution properties of water-insoluble polysaccharides from the mycelium of Ganoderma tsugae. Carbohydr. Polym. 2005;59:351–356. doi: 10.1016/j.carbpol.2004.10.004. [DOI] [Google Scholar]