Abstract
Enzymes have played a crucial role in mankind’s challenges to use different types of biological systems for a diversity of applications. They are proteins that break down and convert complicated compounds to produce simple products. Fungal enzymes are compatible, efficient, and proper products for many uses in medicinal requests, industrial processing, bioremediation purposes, and agricultural applications. Fungal enzymes have appropriate stability to give manufactured products suitable shelf life, affordable cost, and approved demands. Fungal enzymes have been used from ancient times to today in many industries, including baking, brewing, cheese making, antibiotics production, and commodities manufacturing, such as linen and leather. Furthermore, they also are used in other fields such as paper production, detergent, the textile industry, and in drinks and food technology in products manufacturing ranging from tea and coffee to fruit juice and wine. Recently, fungi have been used for the production of more than 50% of the needed enzymes. Fungi can produce different types of enzymes extracellularly, which gives a great chance for producing in large amounts with low cost and easy viability in purified forms using simple purification methods. In the present review, a comprehensive trial has been advanced to elaborate on the different types and structures of fungal enzymes as well as the current status of the uses of fungal enzymes in various applications.
Keywords: fungi, enzymes, structure and classification, function, applications
1. Introduction
Enzymes play an important role in lowering the energy of activation and accelerating numerous biological reactions that are crucial in nourishing life without causing any permanent modifications. Enzymes are widely distributed in all forms of living organisms, including animal, plant, and microbial sources. The inability of plant and animal sources to satisfy industrial demands of enzymes directed attention to microbial sources [1]. Microbial enzymes production is faster, cost-effective, scalable, and amenable to genetic manipulations [2]. Among microbial sources, fungi represent an interesting source for industrial enzymes, sharing with more than one has of enzymes in the market [3]. Fungal enzymes are characterized by high production potency, easier purification and separation requirements, especially for filamentous fungi, and efficient catalysis with desired stability against harsh conditions [3]. Furthermore, the uses of fungi in a great number of traditional preparations, such as brewing and baking from ancient periods, offer a safe and firm context for their recent applications [3]. Enzymes have an important role in mankind’s challenges to use biological systems for a wide range of dedications. Currently, fungal enzymes are accounted for more than 50% of the total enzymes market [3]. This huge market share is largely attributed to a few species of Aspergillus, Trichoderma, Rhizopus, and Penicillium genera that fulfill the commercial-scale requirements for enzymes production (Table 1). The fast-growing enzymes market forced the continuous search for novel enzymes producers with desirable industrial characteristics. Recently, mushroom cultivation has represented a promising competitor in enzymes production in terms of higher productivity and lower invested cost [4]. Attributed to the nutritional value and health benefits of the edible mushroom, its global production pace is very fast, recording 8.99 million tons in 2018 [4]. Mushrooms are mostly saprophytic fungi and derive their nutrient from surrounding cellulosic material; hence, lignocellulolytic enzymes are highly characterized in most mushroom cells, including cellulases, β-glucosidases lignin peroxidases, and laccases [5,6]. This diverse enzymatic machine enhanced the ability of mushrooms to grow in diverse low-cost agriculture wastes inspiring their industrial cultivation for nutrition and enzyme production [4].
Table 1.
General application of fungal enzymes in different fields.
| Application | Field | Fungal Name | Enzyme Name | Enzyme Use | Ref. |
|---|---|---|---|---|---|
| Industries | Food and beverage | Aspergillus oryzae, Aspergillus oryzae CCT 3940, and Fusariumculmorum ASP-87 | l-asparaginases | Reduce the acrylamide formation in potato chips or French fries, bakery products, and coffee by degradation of l-asparagine | [7,8,9] |
| Myceliophthora thermophilia | Laccases | Dough conditioner | [10] | ||
| Aspergillus niger DFR-5 | Xylanases | Improve yield and clarity of pineapple juice | [11] | ||
| Aspergillus niger | Pectinases | Improve the quantity of the extracted Orange juice | [12] | ||
| Talaromyces leycettanus | Pectinases | Efficiency in pectin degradation from grape juice | [13] | ||
| Pulp and paper | Pycnoporus cinnabarinus | Laccases | Improve the brightness and strength properties of the pulp | [14] | |
| Trametes villosa | Laccases | Internal sizing of paper by use of laccase and hydrophobic compounds | [15] | ||
| Trichoderma reesei QM9414 | Xylanases | Eco-friendly of biobleaching of Kraft pulp of sugarcane straw | [16] | ||
| Trichoderma viride VKF-3, Fusariumequiseti MF-3, and Aspergillus japonicus MF-1 | Cellulases, xylanases, laccases, and lipases | Treatment enhances the brightness, deinking and reduces the heavy metals in the newspaper pulp | [17] | ||
| Rhizopus oryzae MUCL 28168 and Fusarium solani | Tannases | Detoxification of coffee pulp by reduction of caffeine and tannins | [18] | ||
| Aspergillus niger, Phanerochaete chrysosporium, and Pycnoporus cinnabarinus | Feruloyl esterases, Mn2+-oxidizing peroxidases, and laccases | Decrease the final lignin content of flax pulp and improvement of pulp brightness | [19] | ||
| Textile | Aspergillus niger CKB and Trichoderma reesei ATCC 24449 | Cellulases | Textile waste hydrolysis for recovery of glucose and polyester | [20,21] | |
| Trichoderma longibrachiatum KT693225 | Xylanases | Desizing, bioscouring, and biofinishing of cellulosic fabrics (textile) without adding any additives | [22] | ||
| Aspergillus | Amylases | Desizing of cotton fibers by removal of starch from the surface of textile fibers | [23] | ||
| Candida orthopsilosis | Pectinases | Bioscouring of cotton fibers | [24] | ||
| Aspergillus niger and Penicillium | Glucose oxidases and catalases | Removal of hydrogen peroxide from cotton bioprocessing | [25] | ||
| Chaetomium globosum IMA1 | Lignin peroxidases laccases and manganese peroxidases | Decolorization of the industrial textile effluent | [26] | ||
| Environment | Biodegradation | Irpex lacteus and Pleurotusostreatus | Manganese-peroxidases and laccases | Biodegradation of chlorhexidine and octenidine as antimicrobial compounds used in oral careproducts | [27] |
| Marasmius sp. | Laccases | Degrade lignin by oxidizing the phenolic and non-phenolic compounds to produce dimers, oligomers, and polymers | [28] | ||
| Mucor circinelloides | Lipases, laccases, and peroxidases | Biodegradation of diesel oil hydrocarbons | [29] | ||
| Bioremediation | Penicillium sp. | Enzymatic reduction by the mer operon | The fungal enzyme could detoxify mercury (II) by extracellular sequestration via adsorption and precipitation | [30] | |
| Coriolopsis gallica | Laccases | Bioremediation of pollutants such as bisphenol, diclofenac, and 17-a-ethinylestradiol in real samples from the AQUIRIS wastewater | [31] | ||
| Aspergillus flavus FS4 and Aspergillus fumigates FS6 | Extracellular enzymes | Fungal consortium used for removal of chromium and cadmium | [32] | ||
| Pycnoporus sanguineus | Laccases | Fugal laccase was immobilized on calcium and copper alginate/chitosan beads and used for the removal of 17 a-ethinylestradiol | [33] | ||
| Thermomyces lanuginosus | Chitinases | Biocontrol agent against larvae of Eldana saccharina and fungi of Aspergillus sp., Mucor sp., and Fusarium verticillioides | [34] | ||
| Decolorization | Phanerochaete chrysosporium CDBB 686 | Ligninolytic enzymes | Decolorization of Congo red, Poly R-478, and Methyl green | [35] | |
| Geotrichum candidum | Peroxidases and laccases | Decolorization of methyl orange, Congo red, trypan blue, and Eriochrome black T | [36] | ||
| Coprinopsis cinerea | Laccases | High indigo dye decolorization | [37] | ||
| Trametes sp. SYBC-L4 | Laccases | Decolorization of Congo red, aniline blue, and indigo carmine | [38] | ||
| Phanerochaete chrysosporium | Manganese peroxidase | Decolorization of AO7 or CV pigment | [39] | ||
| Biomedical | Antimicrobial | 32 different isolated fungi identified by morphological characteristics and internal transcribed spacer sequence analysis | Amylases, proteases, pectinases, xylanases, cellulases, chitinases, and lipases | Antimicrobial activity against pathogenic organisms by agar diffusion assays | [40] |
| Aspergillus oryzae and Aspergillus flavipes | Proteases | Production of bioactive peptides from bovine and goat milk and the generated peptides tested against bacteria and fungi | [41] | ||
| Trichoderma harzianum | Chitinases | Degradation of chitosan to form chitosan-oligosaccharides and used as antimicrobial against pathogenic organisms | [42] | ||
| Anticancer | Trichoderma viride AUMC 13021 | Chitinases | Antitumor efficiency of chitinase against different types of cancer cell line | [43] | |
| Trichoderma harzianum | Chitinases | Chitosan-oligosaccharides used as anticancer compounds, which inhibited the growth of cervical cancer cells at concentration of 4 mg/mL and significantly reduced the survival rate of the cells | [42] | ||
| Aspergillus terreus | l-asparaginases | The synthesized zinc oxide conjugated l-asparaginase nanobiocomposite on MCF-7 cell line using MTT assay | [44] | ||
| Antioxidant | Aspergillus flavus | Catalases | Antioxidant system plays a crucial role in fungal development, aflatoxins biosynthesis, and virulence | [45] | |
| Pleurotus columbinus, P. foridanus, Aspergillus fumigatus, and Paecilomyces variotii | Peroxidases and catalases | Production of enzymatic antioxidant from peels of banana, pomegranate, and orange | [46] | ||
| Chytridiomycetes sp. | Ligninases | During biodegradation of lignin, the fungi synthesize bioactive compounds such as mycophenolic acid, dicerandrol C, phenyl acetates, anthrax quinones, benzo furans, and alkenyl phenols that have antioxidant activities | [47,48] |
In general, enzymes are classified under seven classes, including transferases, oxidoreductases, lyases, hydrolases, ligases, isomerases, and translocases [49]. Fungal cells revealed both intracellular and extracellular enzymatic activity. Fungi are considered natural decomposers, and consequently, they are allowed to produce a great number of extracellular enzymes needed for bioconversion of a wide range of substrates and complexes [50]. Other extracellular enzymes may participate in fungal protection against hazardous compounds that exist naturally or result from the substrate hydrolysis process. Commercially important fungal enzymes belong to hydrolases and oxidoreductases. Among extracellular fungal enzymes, laccases and peroxidases, such as manganese peroxidase and lignin peroxidase, represent the two main subclasses of fungal enzymes that have been investigated for the degradation of xenobiotics and removal of harmful phenolic components from industrial environment and wastewater [51]. The current review discusses the different types of enzymes produced by fungi involved in many biotechnological applications. The structure of the fungal enzymes is also discussed. Numerous biotechnological uses of fungal enzymes, including industrial, medical, bioremediation, and environmental applications, are critically reviewed.
2. Fungal Enzymes Structure and Function
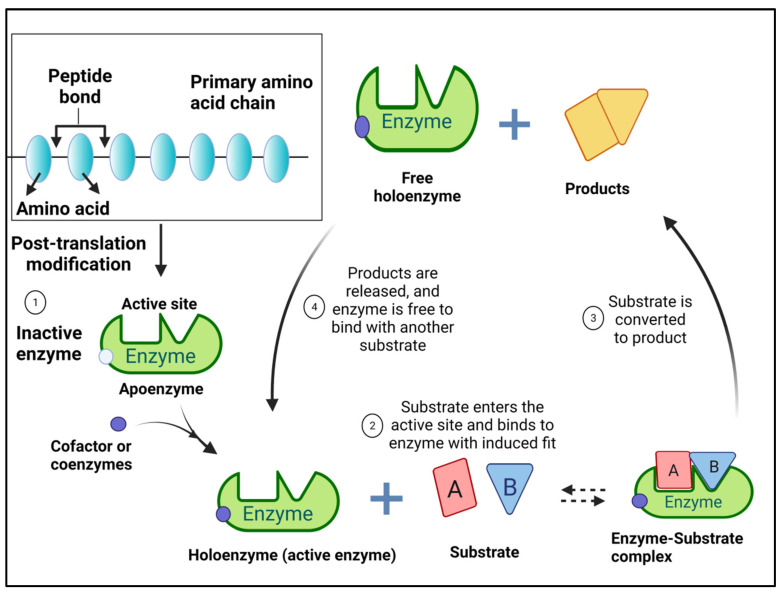
Enzymes are proteins in nature, formed originally from a long chain of amino acids linked together through peptide linkage. Once the primary amino acid chain is formed, subsequent multistep processes take place, collectively known as post-translation modification, where this long chain is modified into its three-dimensional structure (Figure 1). Sequence and nature of the amino acid in various enzymes are varied and have an obvious relationship to enzyme activity and stability. Not all enzyme molecules get involved in the catalytic reaction, but only very few amino acids of (2–4) are called the active site [52], where the remaining part (called Apoenzyme) does not participate directly. Most enzymes are synthesized inactive and activated to holoenzyme (the functional form of an enzyme) with different organic/or inorganic factors. The organic enzyme activators are usually non-proteinaceous molecules known as coenzymes, e.g., vitamins, whereas the inorganic molecules are called cofactors, e.g., zinc, iron, calcium, and copper [52]. The accumulation of enzymes end products may terminate the enzyme activity in a process called feedback inhibition. The feedback enzyme inhibition is a regulatory mechanism initiated by cells to control the amount needed from the enzyme end products [53]. Generally, enzymes are very specific catalysts; however, some enzymes have broad substrate specificity and could work upon multiple substrates. Different theories were proposed to elucidate the enzyme–substrate interaction and recognition, but the most popular one is the lock and key theory proposed by Emil Fischer [54]. This theory elucidates the enzyme and substrate recognition as unique specific complementation between enzyme active site and substrate resembling lock and key. Unfortunately, this theory could not explain the transition state stabilization, where enzyme configuration was altered to fit the proceeding catalytic process [52,55]. To explain these configuration changes in the enzyme active site, a flexible enzyme active site concept was proposed by Koshland, establishing the enzymes Induced Fit Model. In this model, the active site is flexible, where the substrate can modify its orientation to achieve the perfect fit [56]. In Michaelis–Menten’s theory, two phases of catalytic reaction were proposed, where in the first phase, an enzyme-substrate complex was established, and then, the enzyme and products were liberated in the second phase [57].
Figure 1.
Schematic illustration for enzyme structure, activation, and steps of enzyme and substrate interaction.
3. Fungal Enzyme Nomenclature and Classifications
Nowadays, fungal enzymes represent more than one-half of the market enzymes sharing in the different industrial sectors [3]. Due to the vast number of enzymes discovered to date, many criteria were proposed to organize and classify enzymes, including their origin, structure, or substrate. The first rational enzymes nomenclature and classification system were firstly proposed in the 1950s, based upon the enzyme catalytic reaction [58]. The International Union of Pure and Applied Chemistry (IUPAC) adopted the new system, where enzymes are classified into six groups: oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases, and translocases [59]. This classification is a numerically based system where each catalytic reaction (enzymes group) is given a number called commission number (EC number) to specify its enzyme members [52].
3.1. Oxidoreductases (EC 1)
Oxidoreductases are enzymes catalyzing the oxidation/reduction (redox) reaction through the transfer of an electron from donor to an electron acceptor. Generally, redox reactions are numerous in the living cells and included in many vital biological processes such as tricarboxylic acid cycle, glycolysis, amino acid metabolism, and oxidative phosphorylation reactions; hence, oxidoreductases are among the housekeeping enzymes essential for cell’s life and activity. Recently, fungal oxidoreductases were characterized to play a major role in pathogenicity and protection against host defense mechanisms [60]. Yu et al. reported the importance of oxidoreductase-like protein Olp1 in Cryptococcus neoformans for sexual reproduction through enhancing the meiotic division and its essential role in the pathogenicity by protecting the fungal cells from lithium–ion toxicity [60]. Benzoquinone oxidoreductase is another reported fungal virulence factor produced by Beauveria bassiana (Entomopathogenic fungus) to overcome the exposure to benzoquinone from the host cell [61]. To date, more than twenty classes of oxidoreductases have been recognized; however, the most studied and reported oxidoreductases include dehydrogenases (transfer of hydrogen to an electron acceptor), oxygenase (the final electron acceptor is oxygen), and peroxidases (the final electron acceptor is peroxides) [2]. Commercially applied oxygenases include monooxygenases, dioxygenases, and laccases. Fungal laccases are copper-containing oxygenases and play an important role in fungal physiological growth, including sporulation, pigmentation, and aid in plant pathogenesis [62]. Laccases have a high potential to oxidize a wide range of phenolic and non-phenolic compounds, independently of any cofactors [2,62]. The high efficiency of fungal laccases, in addition to cofactor-independence nature, nominated them to several industrial applications such as stain removal [63], paper industry [64], medical applications [65], and biosensor manufacturing [66]. Though microbial laccases were reported from bacteria [67,68], white-rot fungi are the main source of laccases with commercial importance [69]. Peroxidases are oxidoreductases where peroxide compound (H2O2) acts as the final electron acceptor [70]. The majority of peroxidases recognized to date are metal-dependent enzymes, especially iron; however, metal-independent peroxidases were also reported [70]. Two of the most extensively reported peroxidases are lignin peroxidases (LiP) and manganese-peroxidases (MnP). Basidiomycetes, especially white-rot fungi, are characterized by their high ability to degrade lignin attributed to the extracellular production of lignin peroxidases and manganese-peroxidases [71]. Lignin peroxidases (LiP) catalyze the oxidation of a wide range of phenolic compounds; hence, they have several industrial applications [72], in contrast to manganese-peroxidase, which has narrow substrate specificity and low oxidation potential [70].
3.2. Transferases (EC 2)
Transferases catalyze the transfer or exchange of certain groups (amino group) among many compounds [73]. This process has a vital role in creating essential amino acids for protein synthesis in all cells [74]. Some transferases such as glutathione transferase may assist in environmental and oxidative stress tolerance in pathogenic fungi [75]. Glutathione transferases catalyze the reversible transfer (incorporation/elimination) of glutathione group to/from different compounds [76]. Incorporation or elimination of glutathione group resulted in more soluble fewer toxic compounds that can be handled by the fungal cells. Fungal glutathione transferases secreted by fungal cells during host pathogenesis to tolerate oxidative stress created by host and/or toxic wood-derived molecules [77,78]. Fungal glutathione transferases have wide substrate specificity; therefore, they have the potential to detoxify many toxic xenobiotic compounds [76,78,79]. The production of glutathione transferases was reported from many fungi, including Alternaria brassicicola [78], Phanerochaete chrysosporium [80], and Saccharomyces cerevisiae [81]. Another commercially important transferase is fructosyltransferase, which catalyzes the transformation of sucrose into fructooligosaccharides [82]. Fructooligosaccharides are commercially important due to their numerous health benefits as lowering blood pressure and flourishing the growth of beneficial microflora while inhibiting the other pathogenic ones [83]. The sweetness effect and viscous texture of fructooligosaccharides encourage their application in the food industry [82]. Fructosyltransferase production was widely reported from the Aspergillus genus, including Aspergillus oryzae [84], Aspergillus niger [85], Aspergillus aculeatus [86]. However, [82] reported applying Aureobasidium pullulans for fructosyltransferase commercial production.
3.3. Hydrolases (EC 3)
Hydrolases are the most extensively studied groups of enzymes; they catalyze the hydrolysis of their substrate through the addition of water. To date, hydrolases represent the most commercially marketed enzymes due to their wide application in different industrial sectors [3,52,87]. Fungal proteases, amylases, lipases, and cellulases represent the most commercially demanded enzymes [88]; hence, continuous improvement in production efficiency with lowered cost are mandatory [89].
Proteases play an important role in fungal physiology to digest extracellular large peptides and also in defense mechanisms against attaching pathogens [90]. Based upon the amino acid in the enzyme active site, proteases could be categorized into different types, including serine, asparagine, cysteine, aspartic, and metalloproteases [90]. Serine and metalloprotease are the most studied types among all proteases and are usually produced from microbial origins [90]. Fungal proteases are privileged over that of bacteria for easier purification process and less hazardous application [91,92]. Filamentous fungi, especially that of Aspergillus sp., are characterized by their high capacity for protease production [89,93,94]. Other fungal genera also reported for their potency regarding proteases production, including Penicillium sp. [95], Fusarium sp. [96,97], and Pichia farinosa [98].
Amylase is the world premiere in enzyme production for commercial application and was firstly applied medicinally in treating digestive disorders [99]. Amylases could be classified into α, β, and γ-Amylases depending on the attaching site in the starch molecules and the nature of the resulting products. α-Amylases are calcium-dependent metalloenzymes that act randomly on the starchy substrates yielding maltose and maltotriose from amylose or glucose and dextrin from amylopectin [87]. β-Amylases hydrolyze 1,4-glycosidic bonds in the carbohydrate chain, yielding one maltose unit at a time. They are extensively important in plants, especially in the seed ripping process, but they are also reported from the microbial origin [100,101]. γ-Amylases resemble the other two types of amylases in hydrolysis activity toward 1,4-glycosidic linkages, unlike the two forms characterized with 1,6-glycosidic linkages hydrolysis activity and preferring acidic environment pH 3 [87]. Aspergillus niger is considered the potent commercial α-Amylase producer among all filamentous fungi [87]. Many other fungi were reported for their capacity to produce different types of amylases, including Aspergillus oryzae [102], Aspergillus terreus [103], Fusarium solani [104], and Penicillium citrinum [105].
Lipases are a group of hydrolytic enzymes that act by hydrolysis of triacylglycerol yielding fatty acid and glycerol [106]. Lipases also catalyze the reverse reaction by esterification of glycerol and fatty acid. Microbial lipase is produced by many bacteria Bacillus licheniformis [107], Geobacillus thermodenitrificans [108], Pseudomonas aeuriginosa [109], and fungal cells including Aspergillus niger [110,111], Penicillium verrucosum [112], Fusarium solani [113], Arthrographis curvata, and Rhodosporidium babjevae [114]. The remarkable specificity and thermal stability, in addition to the easier recovery procedure of the extracellular fungal lipase over other microbial sources, attracted great attention from the application point of view [115,116,117]. Lipases are implemented in vast commercial applications, including detergents and cosmetics additives, fine chemical production, medical application, paper pitching, leather de-fating, wastewater treatment, and biodiesel production [118,119,120]. The application of lipase in biodiesel production, as an ecofriendly alternative for traditional fuel, intensifies the research in diminishing the production cost and enhancing the enzyme efficiency. In this regard, solid-state fermentation of agricultural wastes represents a step forward cost reduction [110], while protein engineering has been recently applied for enhancing lipase efficiency [121].
The continual necessity for renewable ecofriendly fuel sources intensified the research in cellulose-degrading enzymes. The worldwide abundance of cellulosic material represents a promising ideal source for energy, hence the extensive studies and application of cellulolytic enzymes [48], ranking them among the most worldwide marketing enzyme [122]. Cellulose, hemicellulose, and lignin are the main components of most agricultural wastes representing 40–50%, 25–30%, and 15–20% for cellulose, hemicellulose, and lignin, respectively [48]. Most fungi have the complete enzymatic system (Endoglucanases, Cellobiohydrolases, B-glucosidases, and Xylanases) to degrade this complex cellulosic material for nutrition [123]. Trichoderma reesei is widely applied for the commercial production of cellulases [48], but other fungi also represent potent cellulase producers, including Aspergillus niger [124], Saccharomyces cerevisiae [125], and Aspergillus brasiliensis [126]. Xylan, a complex polysaccharide, is also a major component of hemicellulose; hence, xylanases play an important role in the efficient hydrolysis of plant cellulolytic material [127]. Regarding the diverse and complex structure of Xylan, its hydrolysis required a group of synergistically working enzymes (xylanolytic system) for complete degradation [128]. Filamentous fungi are characterized by the required xylanolytic system for complete xylan degradation, especially that of Trichoderma reesei [128,129], Aspergillus oryzae [130], and Aspergillus flavus [131]. Vast numbers of other hydrolytic enzymes are produced by fungi with wide commercial applications, including fungal phytase and pectinase. Phytase act upon phytic acid (plant inorganic phosphorus), dissolving its content of insoluble inorganic phosphorus makes it available for different physiological processes. Fungal phytase is available for different commercial applications from Aspergillus niger [132]. Pectinase also has important industrial applications, especially in food and paper industries, produced commercially through fungi [133].
The worldwide availability of chitin, representing the second abundant natural polymer next to cellulose, renders chitinases among the most important enzymes [134]. Fungal chitinases belong to hydrolytic enzymes with an extracellular role in chitin decomposition, where intracellularly involved in cell wall lysis and reconstruction in addition to protein deglycosylation [135]. Chitin is one of the main structures in pests’ cell walls and some plant-pathogenic fungi [136]. Hence, chitinases are widely applied in agriculture for plant infection control to overcome the conventional chemical fungi/insecticidal hazardous [137]. In addition, the chitin degradation products (chitosan and chitooligosaccharides) are implemented for various medical applications [134]. Trichoderma viride chitinases were reported with acidoplic, thermostable, and wide chitinolytic activities for commercial applications under the name of Usukizyme [134,138]. Chitinases were reported within various fungal species, including thermostable chitinases (maximum activity between 60 and 55 °C) through Myceliophthora thermophila [139], and Humicola grisea [140] with thermotolerant/mesophilic chitinases (maximum activity between 40 and 37 °C) through Penicillium oxalicum [137] and Trichoderma koningiopsis [141].
3.4. Lyases (EC 4)
Lyases are a group of enzymes that catalyze the addition or elimination reaction. The result of this type of addition or elimination is a new compound with either cyclic structure or new double bonds [73]. Though lyases usually require only one substrate to act in one direction, two substrates are necessary to reverse this reaction. Normally, lyases have very limited substrate specificity; however, the independence of expensive cofactors encourages their commercial applications in many industries. One of the most reported lyases is pectin lyase catalyzes random degradation of pectin through the elimination of water molecules yielding one unite of galacturonan with unsaturated double bonds [142]. Pectin lyase plays a major role in fungal pathogenicity, though degrading the pectic materials holding the plant cell wall together [142]. The role of pectin lyase in fungal pathogenicity was reported in many plant pathogenic fungi, such as Clonostachys rosea [143], Cylindrocarpon destructans [142], and Penicillium canescens [144]. Alginate lyase represents another important fungal lyase; it catalyzes the degradation of alginate to defined oligosaccharides. Alginate lyase was reported in fungi, including terrestrial fungus Aspergillus oryzae [145] and marine fungus Paradendryphiella salina [146].
3.5. Isomerases (EC 5)
Isomerases are a group of enzymes that catalyze the rearrangement of its substrate structure through the interchange of a specific group within the same compound [52]. One of the most widely studied and applied fungal isomerases is glucose/xylose isomerase, representing the third marketing enzyme with protease and amylase [147]. Glucose/xylose isomerase reversely converts D-glucose and D-Xylose into D-fructose and D-Xylulose, respectively [148,149]. Though glucose/xylose isomerase was extensively reported from many bacteria as Bacillus licheniformis [147], Serratia marcescens [150], Caldicellulosiruptor bescii [151], and Streptomyces lividans [152], the fungal enzyme was reported from a few species of Aspergillus genus [153]. Marshall and Kooi reported for the first time the ability of xylose isomerase from Pseudomonas hydrophila to convert D-glucose into D-fructose [154]. Production of high fructose corn syrup through glucose isomerase has wide application in the food industry [148]. With emerging of white biotechnology and biofuel concepts, great attention was directed toward xylose isomerase as a tool to overcome the xylose accumulation issue. The hydrolysis of lignocelluloses is a prerequisite for the growth of Saccharomyces cerevisiae and biofuel production. The monosaccharide in hydrolysate liquor is a mixture of glucose (60–70%) and xylose (30–40%) [155], while Saccharomyces cerevisiae does not have the necessary system to assimilate the xylose [84]. Taking into account that assimilation of xylose in bacteria is one-step process and independent in any cofactor, contrary to that of fungi which is multi-steps and dependent on expensive cofactors [155], many trials were conducted to insert the gene for bacterial xylose isomerase in the genome of Saccharomyces cerevisiae and were first achieved with the xylose isomerase gene from Termus thermophilus [156]. Recently, the expression of xylose isomerase from Clostridium phytofermentans in Saccharomyces cerevisiae greatly enhanced xylose fermentation [157].
3.6. Ligases (EC 6)
Ligases, in general, are those classes of enzymes that catalyze the joining of two compounds through the formation of new bonds. Different types of ligases may be classified according to the nature of bonds established in the new compounds, such as carbon–carbon bonds, carbon–nitrogen bonds, carbon–sulfur bonds, carbon–oxygen bonds, nitrogen–metal bonds, and phosphoric–ester bonds [73]. To date, most of the reported ligases are intracellular, where their main function is modifying the cellular nucleic acid content. The unique structure and mechanism of some fungal ligases represent a promising target for developing new antifungal drugs. The tRNA ligase (Trl1) represents a good candidate for such drugs. The tRNA ligase (Trl1) plays an important role in repairing the RNA breaks in fungal cells [158]. Fungal tRNA ligases (Trl1) were reported to have a similar structure in different pathogenic fungi [159,160]. Most bacteria and mammalian cells have different biochemical mechanism and structure of tRNA ligase from that reported in fungi [160]; therefore, an extensive research directed toward fungal tRNA ligases (Trl1) as a target for new antifungal drugs [160]. Apart from the molecular level, ligases were also reported to have other roles in different fungi. Ubiquitin ligases (E3s) catalyze the ligation of ubiquitin (low molecular weight protein) to the target protein (a process known as ubiquitination). Conjugation of protein to ubiquitin determines its fate inside the cell, whether to be degraded, activated, or relocated [161,162]. Unicellular fungi, including Saccharomyces cerevisiae, represent an ideal model for fully understanding the evolutionary background for the ubiquitination system [163].
4. Application of Fungal Enzymes
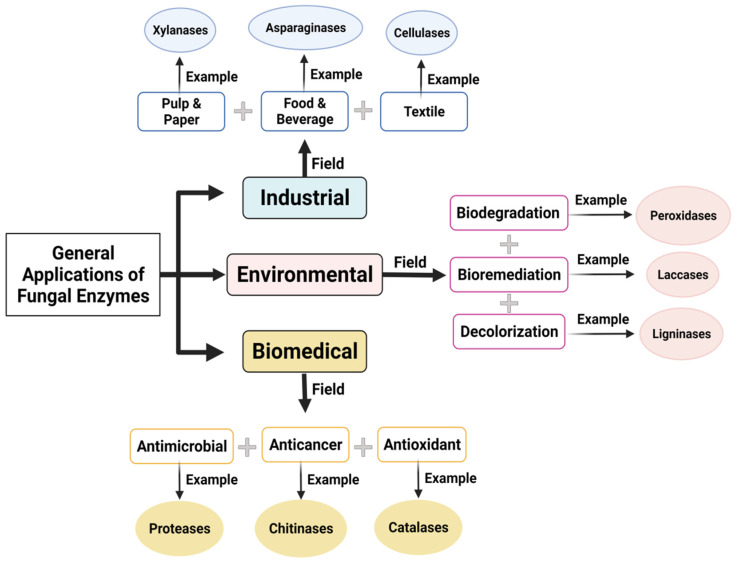
The present review discusses the advancement in fungal enzyme technology for different applications. A comprehensive list of fields, fungal source of enzymes, enzymes names, and the wide range of their application is conceded. Table 1 and Figure 2 give an overview of applications of fungal enzymes in different application fields.
Figure 2.
Schematic diagram of general application of fungal enzymes.
4.1. Industrial Applications
4.1.1. Food and Beverage
Food enzymes are also provided by microbial fermentation (bacteria, fungi, yeasts, actinomycetes, and algae), for which fungal strains are widely used. The application of fungal enzymes in food production offers a promising approach to enhance the preservation and shelf life of foods without affecting the characteristics of the organoleptic and nutritional content of foods [164]. The common fungal enzyme used in food applications is l-asparaginase which is considered GRAS (generally recognized as safe), which is used as a food additive to prevent the formation of acrylamide generated from the reaction between the food components as amino acids and reducing sugars at high temperatures and low humidity. This response often creates desirable color, flavor, and aroma compounds [165,166]. To minimize acrylamide levels in food, l-asparaginase assists with mitigation strategies from two aspects, intervention with the Maillard reaction or by converting l-asparagine into l-aspartic acid (non-toxic) to prevent precursors formation without altering the nutritional value, quality, or taste of the final product [167,168,169]. Single or consortium fungal enzymes were applied to improve the quality, clarity, and yield of fruit juice that ensure consumer appeal [11].
4.1.2. Pulp and Paper
The government’s policies impose sustained pressure on the paper industry to protect the environment from hazards and diseases by replacing the chemical bleaching processes with greener ecofriendly alternatives. Enzymes such as xylanases and laccases obtained from fungi have the ability for bio-bleaching of agriculture wastes-based pulps by cleaving the β-1, 4 backbone of the complex plant cell wall for papermaking [170,171]. Ecofriendly papermaking depends on the two processing, Bio-pulping followed by bio-bleaching. Bio-pulping is the pre-treatment of agriculture-waste-based pulp by lignin-degrading enzymes before the routine pulping process. The process of bio-pulping is technologically feasible and cost-effective, reduces energy costs, and improves paper strength properties [172]. Filamentous fungi, especially members of white-rot fungi are the main source for lignin-degrading enzymes, whoever those with cellulases-free activity are important for the paper industry [173]. Ceriporiopsis subvermispora, Ganoderma australe Phellinus pini, and Phlebia tremellosa are three white-rot fungi with potent cellulases-free lignin-degrading activities suitable for the bio-pulping process where Phanerochaete chrysosporium is the model white-rot fungi with a complete enzymes system for lignin mineralization [174]. Bio-bleaching refers to the complete removal of lignin from bio-pulping wastes by the fungal enzyme to achieve white and bright pulp appropriate for papermaking. Bio-bleaching refers to the complete removal of lignin from bio-pulping wastes by the fungal enzyme to achieve white and bright pulp appropriate for papermaking. In addition to improving paper quality, bio-bleaching reduces the applied chemicals that diminish effluent toxicity and pollution [170]. Xylanases from Fusarium equiseti MF-3 and Talaromyces thermophilus were successfully applied for the bio-pulping process with a significant reduction in the organo-chloro compounds applied in the conventional bleaching process [17,175].
4.1.3. Textile
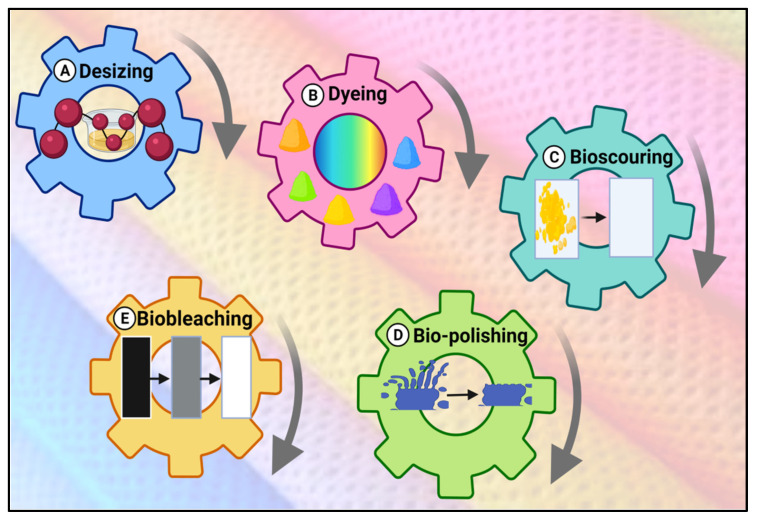
Fungal enzymes play an important role in enhancing textile processing as well as valorizing the waste generated from streams of fibers, textile, and clothing manufacturing processes to value products [176]. Of the 7000 known enzymes, only about 75 are commonly used in textile manufacturing [177]. Xylanases are among the most common enzymes used for their effectiveness in textile processing at several stages, including desizing, bio-scouring (removing waxy material from fibers), suitable for coloration with low conductive fabrics, bio-bleaching, and bio-finishing of cellulosic fabrics to improve the textile quality [22]. The main mechanism for using fungal enzymes in textile processing, removal or degradation of undesirable components such as lignin, pectin, starch, excess H2O2 after bleaching, waxes by xylanases, or polygalacturonases or amylases form a fibers-based textile. Cellulases for cotton remove projecting fibers selectively, and this action is known as bio-polishing. In contrast, the textile wastes contain 35–40% of cellulosic fibers, which is utilized as a carbon sole for the production of bioethanol, biocellulose, etc., after being degraded by cellulases enzyme originated from Trichoderma reesei ATCC 24,449 [20]. Another application of fungal enzymes, which degrade the textile dyes by using Pleurotus ostreatus and Pleurotus eryngii, had the activity of laccase, lignin peroxidase, manganese peroxidase, xylanase, cellulase, and lipase for removal of days after 24 h [178]. The fungal enzymes applications textile is illustrated in Figure 3.
Figure 3.
Fungal enzyme applications for textiles. (A) Desizing agents such as amylases; (B) dyeing and dye diffusers such as proteases; (C) textile processing and bioscouring of cotton fibers such as pectinases; (D) biofinishing and biopolishing such as cellulases; and (E) biobleaching and enzymatic rinse process after reactive dyeing such as peroxidases.
4.2. Environmental Applications
Bioremediation
With the continuous increase in the human being population, production, and consumption, industrial and agricultural wastes emerged as cumulative pressing environmental challenges that threaten human health and welfare. Waste management through fungal enzymes arises as promising solutions toward green and sustainable environments with the potential for generating new valuable products in a less consuming energy approach [179,180]. Currently, polyethylene-based materials (plastic packaging) are heavily consumed commercially attributed to their relatively low cost, and hence environmentally accumulated at a very high rate. Polyethylene-derived material revealed a high resistance for biological bioremediation; therefore, developing an effective approach for polyethylene removal is a pressing necessity from an environmental perspective. In this regard, a mixture of fungal oxidoreductases including laccase, manganese-peroxidase, lignin peroxidase, and unspecified peroxygenase was firstly studied (in silico) for eco-friendly polyethylene degradation [181]. The molecular docking results indicate the importance and essential role of laccase and unspecified peroxygenase in the polyethylene degradation process [181].
In the same regard, the widespread contamination with polyaromatic hydrocarbons from industrial effluents represents a huge challenge for human and animal health. In nature, polyaromatic hydrocarbons usually react to surrounding compounds to form exceptional multi-complex structures, such as aliphatic alkanes/alkenes, or halogenated hydrocarbons, potentially more toxic than the original structures [182]. Long-term exposure to polyaromatic hydrocarbons associated with many health complications included hepatic and renal toxicity and lung function damage, with a high rate of cancer development [183]. To overcome these problems, Aspergillus oryzae and Mucor irregularis were isolated from crude oil and reported as hydrocarbon degradation by secretion of several enzymes such as laccase, manganese peroxidase, and lignin peroxidase [184].
Recently, fungal laccases were widely applied for the bioremediation of synthetic textile dyes [185] by altering the dye molecules into non-colored, safer, and ecofriendly structures. Dyes-based industries (textile, paper, paint, and tannery) usually discharge their wastewater into water systems without any prior treatment and are heavily loaded with many unused synthetic dyes. These dyes imply many serious impacts in the aquatic environment, including increasing water turbidity and inhibiting the growth of some organisms like algae. In addition, most of these dyes are carcinogenic with high hepatocellular and renal toxicity upon long-term exposure [186]. There are two major mechanisms for decolorization of dyes using fungi, which are biosorption and biodegradation. The biosorption is essentially committed for dead cells. In this mechanism, the association of physiochemical interactions such as adsorption, deposition, and ion exchange is capable of decolorization. The biodegradation is particularly committed for living cells because they can produce the lignin-modifying enzymes, laccase, manganese peroxidase, and lignin peroxidase [187]. Fungal species (including Alternaria, Aspergillus, and Cladosporium) are capable of producing extracellular enzymes (lignin peroxidases, manganese peroxidases, and laccases) that can biodegrade complex synthetic dyes (Congo red, Poly R-478, Methyl green, indigo carmine, etc.) which widen the industrial applications of fungal enzymes toward the sustainable environment [35,37,188].
With vast developments in agriculture techniques, the worldwide accumulation of lignocellulosic agricultural wastes also represents a considerable environmental challenge. As discussed above, fungi naturally genetically inherited diverse enzymes (hydrolases and oxidoreductases) for the ecofriendly valorization of cellulosic material into valuable industrial products like bioethanol. Collectively, the application of fungal enzymes revealed dual sustainable benefits from an industrial quality and environmental perspective.
4.3. Biomedical Applications
4.3.1. Antimicrobial
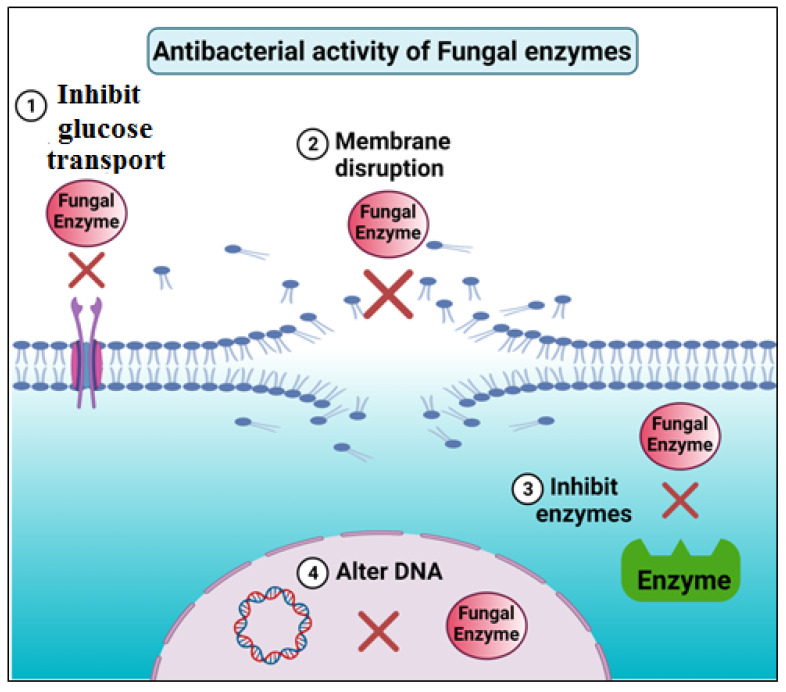
Fungi are well known for the production of antimicrobial agents, industrial enzymes, and microbial biomass. They have evolved the ability to synthesize novel metabolites, including natural products and enzymes, which could be used for novel antimicrobial. The antimicrobial activity of the fungal enzyme may be classified into two categories: direct and indirect antimicrobial agents. First, fungal enzymes such as cellulases, amylases, and lipases were used directly for antimicrobial activity [40], while indirect antimicrobial activity depended on generating intermediate compounds with antimicrobial activity through fungal enzymes [42]. Direct antimicrobial activity includes rupturing the cell membrane, resulting in the loss of intracellular elements necessary for life. Furthermore, fungal enzymes stop DNA synthesis, inhibit essential bacterial enzymes, block bacterial receptors, and inhibit the electron transport chain [189,190], as illustrated in Figure 4. In addition, many fungal enzymes have the ability to inhibit both DNA and RNA viruses. The purified oyster mushroom laccase extracted from Pleurotus ostreatus showed potent antiviral activity against hepatitis C Virus (HCV) [191]. Other fungal enzymes, such as ribonucleases, can exert an alternative antiviral activity against hepatitis B virus (HBV) [192] and human immunodeficiency virus type 1 (HIV-1) [193]. Regarding many fungal crude extracts and their enzymes, the antiviral effect of fungal lectin against HIV was the most investigated virus. El-Maradny et al. [194] indicated that the purified Trichogin protein from Tricholoma giganteum was found to inhibit the HIV reverse transcriptase (RT) enzyme with the lowest IC50 (half maximal inhibitory concentration) value determined to be 83 nM [195], followed by the purified lectin from Russula delica with IC50 value of 0.26 [75] and the purified laccase from Tricholoma mongolicum with IC50 value of 0.62 μM [196]. Lectins bind with viral envelope glycoproteins and inhibit the entry of many viruses, such as HIV, thus inhibiting virus attachment and blocking its entry via inducing the virus conformational rearrangements [197]. Ma et al. [198] revealed that the purified lectin from Agrocybe aegerita was found to be a nontoxic protein and served as an effective adjuvant with influenza vaccine against H9N2 viruses in infected mice model through binding with viral envelope glycoprotein.
Figure 4.
Schematic illustration represents the molecular mechanisms of the main antibacterial effects of fungal enzymes, including 1. alter membrane permeability and inhibition of glucose transport, 2. bacterial membrane damage and disruption, causing cytoplasm leakage, 3. inhibit bacterial enzymes, and 4. inhibit or alter cell division and the DNA replication process.
4.3.2. Anticancer
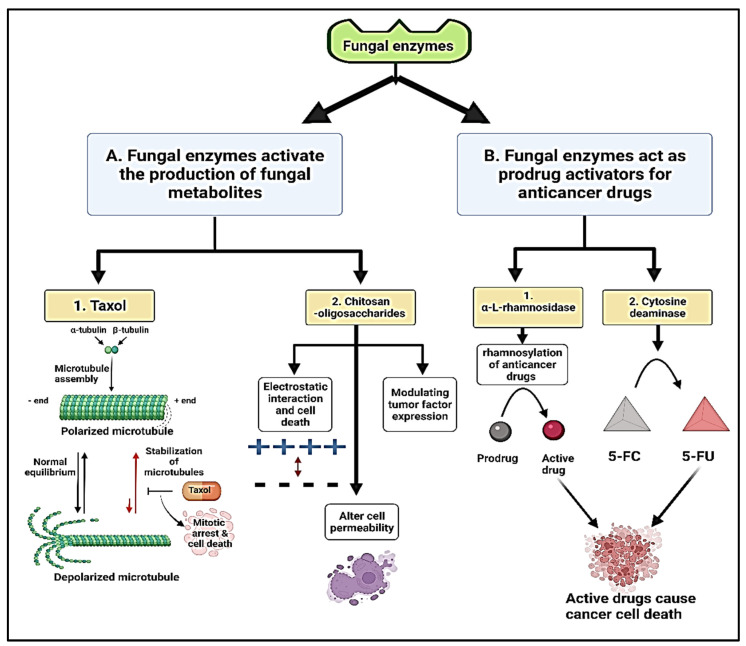
Fungi produce a diverse set of natural products as secondary metabolites, including polyketides, nonribosomal peptides, terpenes, chitosan-oligosaccharides, polyketide–terpene hybrids, and polyketide–nonribosomal peptide hybrids by the action of fungal enzymes. Those secondary metabolites have been a rich source for therapeutics used as antibiotics (penicillin), cholesterol-lowering agents (lovastatin), and immune suppressants (cyclosporine) [199]. Chitosan-oligosaccharides are low-molecular-weight chitosan derivatives with interesting anticancer applications, which are produced from the Trichoderma harzianum chitinolytic system by the action of extracellular enzymes. As anticancer compounds, chitosan-oligosaccharides inhibited the growth of cervical cancer Hela cells at mild concentration and reduced the survival cells rate significantly [42]. The specific mechanism of chitosan preventing tumor growth is unclear, although it might be linked to chitosan electrostatic charges, alterations in tumor cell permeability, and modulation of tumor factor expression, including metalloproteinase-9 or/and vascular endothelial growth factors [200]. Endophytic fungi anticancer toxal (Paclitaxel) production has also been reported from Penicillium aurantiogriseum (NRRL 62431) [201], Cladosporium oxysporum [202], Phoma medicaginis [203] by the action of fungal enzymes. Paclitaxel is the world’s first billion-dollar anticancer drug and is used to treat breast, ovarian, and lung cancer [204]. Taxol was the first microtubule targeting drug to be disclosed in the literature, and its major mode of action was to disrupt microtubule dynamics, resulting in mitotic arrest and cell death [205], as presented in Figure 5. Additionally, various fungal enzymes act as prodrug activators for anticancer drugs [206]. The enzyme α-L-rhamnosidase, derived from Acremonium species, is used in the one-step rhamnosylation of anticancer drugs such as 2′-deoxy-5-fluorouridine, cytosine arabinoside, and hydroxyurea [207]. Surprisingly, these prodrugs only had a lethal impact on breast cancer MDA-MB-231 cells after being exposed to -L-rhamnosidase, showing that they are delivered to tumor locations specifically. The yeast cytosine deaminase enzyme is another model of enzyme-activated prodrug treatment. This enzyme has been studied in the conversion of the non-toxic prodrug 5-fluorocytosine (5-FC) to the cytotoxic 5-fluorouracil (5-FU) [208].
Figure 5.
Schematic illustration represents the mechanism of anticancer effect of the fungal enzymes, 5-fluorocytosine (5-FC), and 5-fluorouracil (5-FU).
4.3.3. Antioxidant
Antioxidants are important compounds that enhance the living organisms to avoid oxidative stress originated by reactive oxygen species, which are harmful compounds, causing damage and altering the structure of the carbohydrates, nucleic acids, lipids, and proteins [209]. Fungi can profoundly participate in the production of antioxidants metabolites, including phenolics, flavonoids, β-glucans, and steroids [210]. The main mechanism of fungal enzyme antioxidants such as catalases, peroxidases, superoxide dismutases, and transferases becomes activated to defend the drastic condition by converting reactive oxygen species into oxygen and water [211]. Phenolic compounds are natural antioxidants and bind to the cellulose matrixes of the plant cell walls [212]. Fungi are the widely promising organisms that produce lignocellulolytic enzymes responsible for plant cell walls degradation [213].
5. Toward Safe, Sustainable Production and Application of Fungal Enzymes
Industrial implementation of the fungal enzymes represents a sustainable, eco-friendly, and energy-saving solution for many environmental and quality aspects compared to the currently applied conventional chemicals/physical approaches [214]. However, some biosafety aspects should be considered in the fungal enzyme applications and productions. Recently, a sensitization toward the fungal enzymes was estimated to be 1–15% among populations, while in the enzyme production workers, the risk increased [215,216]. Bakery and detergent-packaging employees are clear examples of the prevalence of fungal enzymes allergy [216]. From the commercial point of view, the application of genetically modified fungal cells for enzyme production is more reliable and applicable in terms of energy-saving and nutrient consumption [217]; however, the biosafety and regulations for genetically modified enzymes are still challenging. Many criteria govern the application of genetically modified organisms for enzyme production, including genetic stability, absence of any potential virulence, and antibiotic resistance factors [218].
The huge cell mass and byproducts generated during enzyme production through fungi also represent a considerable environmental risk and imply an additional cost for management and control [219]. Several attempts were applied to implement such byproducts as bio-fertilizers [220] or in synthetic dye and heavy metal removal [221]. Nikkilä and his colleagues evaluated the nutritional quality of the aqueous and alkaline treated fungal-biomass resulting as a byproduct from the enzyme industry. The extracted fungal-biomass samples revealed high contents of both polysaccharides and glycoproteins with carbohydrate and amino acid contents of 37.5 and 27.6%, respectively, for alkaline-treated fungal biomass, where the aqueous ex-traction yielded 6.6 and 61.3% of carbohydrate and amino acid contents, respectively [219]. In the same regard, mushroom cultivation at the industrial level produces huge amounts of a byproduct called spent mushroom substrate comprises a mixture of remaining substrate and mushroom mycelia [222]. This byproduct represents a potential challenge for the mushroom growing industry and to the environment. As per literature, there is growing interest in the implementation of the spent mushroom substrate in valuable bioproducts and enzymes synthesis [223]. The enzymes production capacity of six Trichoderma spp. were evaluated using 36% cellulases-hydrolyzed spent mushroom substrate. As a sole substrate, spent mushroom substrate supported several enzymes production through Trichoderma atroviride isolate T42, compared to Aspergillus niger, including β-glucosidases (five isoforms) and twelve isoforms of both endocellulases and xylanases [224].
6. Conclusions and Future Perspectives
In recent times, it has been recognized that the production of microbial enzymes is retaining an advanced topic of innovation. The use of enzymes is still better than the use of chemical processes as they can carry out several reaction types of importance with more considerable efficiency under mild circumstances. Among microbial enzymes, fungal enzymes are at the best locations, owing to their ability to be carried out in large quantities and much faster as well as at affordable costs. Production of the fungal enzyme has had an incredible influence not only on industrial sectors but also on biochemical synthesis processes and healthcare services. The progress of biotechnology in these fields offers different approaches to reduce the cost of enzyme production economically, enhance methods of gene discovery, and decrease the time of enzymes production through accelerating the growth of production hosts. Furthermore, the increased access toward production systems of the fungal enzyme also raises the availability and sustainability of manufacturing dealing with low-value and bulk products. Usage of microbial enzymes, especially fungal enzymes, has significantly established their importance in processing and quality improvement. Rapid progress has happened in enzyme marketing over recent periods, owing to the development of industrial and biotechnological uses. Present developments in protein modeling and engineering, besides advancements in recombination technology, have developed enzyme commercialization and production. Three main applications led to an increase in the marketing of fungal enzymes, including technical, food industry, and animal feed sectors. The technical sector includes enzymes that are used in search and analysis, such as cellulases, proteases, and amylases. Moreover, these enzymes are comprehensively utilized in the textile, leather, starch, detergent, and personal care, as well as paper and pulp industries. The second big market sector is for the use of fungal enzymes in the food industry, such as lipases and pectinases. The third essential one is the feed sector which includes several enzymes such as xylanases, β-glucanases, and phytase. There is no doubt that in the near future, the utilizing of fungal enzymes will be expanded rapidly in several fields such as food processing, bio-pulping, chemical conversions, carbohydrate conversions, cleaning, animal feed, and food additives, as well as used in detoxification and the removal of pollutants from the environment. In addition, in the case of the use of other processes other than enzymatic uses, various industries produce byproducts, including wastes and contaminants, which cause environmental problems and can be harmful to nature. However, the use of fungal enzymes, which break down some particular substrates into amino acids that are useful, biodegradable, and naturally can be easily recycled in the surrounding environment. Additionally, the use of enzymatic processes leads to increasing overall yields and the formation of well-made products with avoiding undesirable byproducts. All these reasons give fungal enzymes advanced importance for enabling many industrial areas to be increased efficiency. Moreover, the usage of fungal enzymes offers the development and production of more friendly main products in the environment and acting via using less raw materials, energy, and water. In addition, the usage of fungal enzymes in many processes leads to the production of less waste and hazardous products. Although there has been a great revolution in exploring novel fungal enzymes, it is also an important demand for assuming a new screening program of novel strains to optimize the enzyme production with new characteristics, as the current identified fungi not more than 5% of the fungal flora. The combination of the modem knowledge of biotechnology and molecular biology can offer a better-quality yield and an efficient approach with novel features.
Author Contributions
H.E.-G.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. A.K.S.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. R.B.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. E.M.R.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. Y.A.E.-M.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. E.M.E.-F.: Conceptualization, formal analysis, data curation, collected literature data, wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guerrand D. Economics of food and feed enzymes: Status and prospectives. In: Nunes C.S., Kumar V., editors. Enzymes in Human and Animal Nutrition: Principles and Perspectives. Academic Press; London, UK: 2018. pp. 487–514. [Google Scholar]
- 2.Singh R., Singh T., Pandey A. Microbial enzymes—An overview. In: Singh R.S., Singhania R.R., Pandey A., Larroche C., editors. Advances in Enzyme Technology. Elsevier; Amsterdam, The Netherlands: 2019. pp. 1–40. [Google Scholar]
- 3.Kango N., Jana U.K., Choukade R. Fungal Enzymes: Sources and Biotechnological Applications. In: Satyanarayana T., Deshmukh S.K., Deshpande M.V., editors. Advancing Frontiers in Mycology & Mycotechnology: Basic and Applied Aspects of Fungi. Springer; Singapore: 2019. pp. 515–538. [Google Scholar]
- 4.Kumla J., Suwannarach N., Sujarit K., Penkhrue W., Kakumyan P., Jatuwong K., Vadthanarat S., Lumyong S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules. 2020;25:2811. doi: 10.3390/molecules25122811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Luz J.M.R., Nunes M.D., Paes S.A., Torres D.P., da Silva M.D.C.S., Kasuya M.C.M. Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz. J. Microbiol. 2012;43:1508–1515. doi: 10.1590/S1517-83822012000400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fen L., Xuwei Z., Nanyi L., Puyu Z., Shuang Z., Xue Z., Pengju L., Qichao Z., Haiping L. Screening of Lignocellulose-Degrading Superior Mushroom Strains and Determination of Their CMCase and Laccase Activity. Sci. World J. 2014;2014:763108. doi: 10.1155/2014/763108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias F.F.G., Junior S.B., Hantao L.W., Augusto F., Sato H.H. Acrylamide mitigation in French fries using native L-asparaginase from Aspergillus oryzae CCT 3940. LWT—Food Sci. Technol. 2017;76:222–229. doi: 10.1016/j.lwt.2016.04.017. [DOI] [Google Scholar]
- 8.Meghavarnam A.K., Janakiraman S. Evaluation of acrylamide reduction potential of l-asparaginase from Fusarium culmorum (ASP-87) in starchy products. LWT. 2018;89:32–37. doi: 10.1016/j.lwt.2017.09.048. [DOI] [Google Scholar]
- 9.Pedreschi F., Mariotti S., Granby K., Risum J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT—Food Sci. Technol. 2011;44:1473–1476. doi: 10.1016/j.lwt.2011.02.004. [DOI] [Google Scholar]
- 10.Renzetti S., Courtin C., Delcour J., Arendt E. Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: Rheological, biochemical and microstructural background. Food Chem. 2010;119:1465–1473. doi: 10.1016/j.foodchem.2009.09.028. [DOI] [Google Scholar]
- 11.Pal A., Khanum F. Efficacy of xylanase purified from Aspergillus niger DFR-5 alone and in combination with pectinase and cellulase to improve yield and clarity of pineapple juice. J. Food Sci. Technol. 2011;48:560–568. doi: 10.1007/s13197-010-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salim D., Anwar Z., Zafar M., Anjum A., Bhatti K.H., Irshad M. Pectinolytic cocktail: Induced yield and its exploitation for lignocellulosic materials saccharification and fruit juice clarification. Food Biosci. 2018;22:154–164. doi: 10.1016/j.fbio.2018.02.005. [DOI] [Google Scholar]
- 13.Li Y., Wang Y., Tu T., Zhang D., Ma R., You S., Wang X., Yao B., Luo H., Xu B. Two acidic, thermophilic GH28 polygalacturonases from Talaromyces leycettanus JCM 12802 with application potentials for grape juice clarification. Food Chem. 2017;237:997–1003. doi: 10.1016/j.foodchem.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Ibarra D., Camarero S., Romero J., Martínez M.J., Martínez A.T. Integrating laccase–mediator treatment into an industrial-type sequence for totally chlorine-free bleaching of eucalypt kraft pulp. J. Chem. Technol. Biotechnol. 2006;81:1159–1165. doi: 10.1002/jctb.1485. [DOI] [Google Scholar]
- 15.Garcia-Ubasart J., Esteban A., Vila C., Roncero M.B., Colom J.F., Vidal T. Enzymatic treatments of pulp using laccase and hydrophobic compounds. Bioresour. Technol. 2011;102:2799–2803. doi: 10.1016/j.biortech.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Campioni T.S., de Jesus Moreira L., Moretto E., Nunes N.S.S., de Oliva Neto P. Biobleaching of Kraft pulp using fungal xylanases produced from sugarcane straw and the subsequent decrease of chlorine consumption. Biomass Bioenergy. 2019;121:22–27. doi: 10.1016/j.biombioe.2018.12.014. [DOI] [Google Scholar]
- 17.Nathan V.K., Rani M.E., Gunaseeli R., Kannan N.D. Enhanced biobleaching efficacy and heavy metal remediation through enzyme mediated lab-scale paper pulp deinking process. J. Clean. Prod. 2018;203:926–932. doi: 10.1016/j.jclepro.2018.08.335. [DOI] [Google Scholar]
- 18.Londoño-Hernandez L., Ruiz H.A., Ramírez T.C., Ascacio J.A., Rodríguez-Herrera R., Aguilar C.N. Biocatalysis and Agricultural Biotechnology Fungal detoxification of coffee pulp by solid-state fermentation. Biocatal. Agric. Biotechnol. 2020;23:101467. doi: 10.1016/j.bcab.2019.101467. [DOI] [Google Scholar]
- 19.Sigoillot C., Camarero S., Vidal T., Record E., Asther M., Pérez-Boada M., Martínez M.J., Sigoillot J.-C., Asther M., Colom J.F., et al. Comparison of different fungal enzymes for bleaching high-quality paper pulps. J. Biotechnol. 2005;115:333–343. doi: 10.1016/j.jbiotec.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Kaur G., Pensupa N., Uisan K., Du C., Yang X., Lin C.S.K. Textile waste valorization using submerged filamentous fungal fermentation. Process. Saf. Environ. Prot. 2018;118:143–151. doi: 10.1016/j.psep.2018.06.038. [DOI] [Google Scholar]
- 21.Hu Y., Du C., Leu S.-Y., Jing H., Li X., Lin C.S.K. Valorisation of textile waste by fungal solid state fermentation: An example of circular waste-based biorefinery. Resour. Conserv. Recycl. 2018;129:27–35. doi: 10.1016/j.resconrec.2017.09.024. [DOI] [Google Scholar]
- 22.Abd El Aty A.A., Saleh S.A., Eid B.M., Ibrahim N.A., Mostafa F.A. Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal. Agric. Biotechnol. 2018;14:129–137. doi: 10.1016/j.bcab.2018.02.011. [DOI] [Google Scholar]
- 23.Aggarwal R., Dutta T., Sheikh J. Extraction of amylase from the microorganism isolated from textile mill effluent vis a vis desizing of cotton. Sustain. Chem. Pharm. 2019;14:100178. doi: 10.1016/j.scp.2019.100178. [DOI] [Google Scholar]
- 24.Aggarwal R., Dutta T., Sheikh J. Extraction of pectinase from Candida isolated from textile mill effluent and its application in bio-scouring of cotton. Sustain. Chem. Pharm. 2020;17:100291. doi: 10.1016/j.scp.2020.100291. [DOI] [Google Scholar]
- 25.Farooq A., Ali S., Abbas N., Fatima G.A., Ashraf M.A. Comparative performance evaluation of conventional bleaching and enzymatic bleaching with glucose oxidase on knitted cotton fabric. J. Clean. Prod. 2013;42:167–171. doi: 10.1016/j.jclepro.2012.10.021. [DOI] [Google Scholar]
- 26.Manai I., Miladi B., El Mselmi A., Smaali I., Ben Hassen A., Hamdi M., Bouallagui H. Industrial textile effluent decolourization in stirred and static batch cultures of a new fungal strain Chaetomium globosum IMA1 KJ472923. J. Environ. Manag. 2016;170:8–14. doi: 10.1016/j.jenvman.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Linhartová L., Michalíková K., Šrédlová K., Cajthaml T. Biodegradability of Dental Care Antimicrobial Agents. Molecules. 2020;25:400. doi: 10.3390/molecules25020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vantamuri A.B., Kaliwal B.B. Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech. 2016;6:189. doi: 10.1007/s13205-016-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchut-Mikolajczyk O., Kwapisz E., Wieczorek D., Antczak T.Z. Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 2015;104:142–148. doi: 10.1016/j.ibiod.2015.05.008. [DOI] [Google Scholar]
- 30.Chang J., Shi Y., Si G., Yang Q., Dong J., Chen J. The bioremediation potentials and mercury(II)-resistant mechanisms of a novel fungus Penicillium spp. DC-F11 isolated from contaminated soil. J. Hazard. Mater. 2020;396:122638. doi: 10.1016/j.jhazmat.2020.122638. [DOI] [PubMed] [Google Scholar]
- 31.Nair R.R., Demarche P., Agathos S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol. 2013;30:814–823. doi: 10.1016/j.nbt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Talukdar D., Jasrotia T., Sharma R., Jaglan S., Kumar R., Vats R., Kumar R., Mahnashi M.H., Umar A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020;20:101050. doi: 10.1016/j.eti.2020.101050. [DOI] [Google Scholar]
- 33.Garcia L., Lacerda M., Thomaz D., de Souza Golveia J., Pereira M., de Souza Gil E., Schimidt F., Santiago M. laccase-alginate-chitosan-based matrix toward 17 α-ethinylestradiol removal. Prep. Biochem. Biotechnol. 2019;49:375–383. doi: 10.1080/10826068.2019.1573195. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M., Puri A.K., Govender A., Wang Z., Singh S., Permaul K. The multi-chitinolytic enzyme system of the compost-dwelling thermophilic fungus Thermomyces lanuginosus. Process. Biochem. 2015;50:237–244. doi: 10.1016/j.procbio.2014.11.008. [DOI] [Google Scholar]
- 35.Sosa-Martínez J.D., Balagurusamy N., Montañez J., Peralta R.A., Moreira R.D.F.P.M., Bracht A., Peralta R.M., Morales-Oyervides L. Synthetic dyes biodegradation by fungal ligninolytic enzymes: Process optimization, metabolites evaluation and toxicity assessment. J. Hazard. Mater. 2020;400:123254. doi: 10.1016/j.jhazmat.2020.123254. [DOI] [PubMed] [Google Scholar]
- 36.Rajhans G., Sen S.K., Barik A., Raut S. Elucidation of fungal dye-decolourizing peroxidase (DyP) and ligninolytic enzyme activities in decolourization and mineralization of azo dyes. J. Appl. Microbiol. 2020;129:1633–1643. doi: 10.1111/jam.14731. [DOI] [PubMed] [Google Scholar]
- 37.Yin Q., Zhou G., Peng C., Zhang Y., Kües U., Liu J., Xiao Y., Fang Z. The first fungal laccase with an alkaline pH optimum obtained by directed evolution and its application in indigo dye decolorization. AMB Express. 2019;9:151. doi: 10.1186/s13568-019-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H.-X., Zhang R.-J., Tang L., Zhang J.-H., Mao Z.-G. In vivo and in vitro decolorization of synthetic dyes by laccase from solid state fermentation with Trametes sp. SYBC-L4. Bioprocess Biosyst. Eng. 2014;37:2597–2605. doi: 10.1007/s00449-014-1237-y. [DOI] [PubMed] [Google Scholar]
- 39.Emami E., Zolfaghari P., Golizadeh M., Karimi A., Lau A., Ghiasi B., Ansari Z. Effects of stabilizers on sustainability, activity and decolorization performance of Manganese Peroxidase enzyme produced by Phanerochaete chrysosporium. J. Environ. Chem. Eng. 2020;8:104459. doi: 10.1016/j.jece.2020.104459. [DOI] [Google Scholar]
- 40.Liu S., Ahmed S., Zhang C., Liu T., Shao C., Fang Y. Diversity and antimicrobial activity of culturable fungi associated with sea anemone Anthopleura xanthogrammica. Electron. J. Biotechnol. 2020;44:41–46. doi: 10.1016/j.ejbt.2020.01.003. [DOI] [Google Scholar]
- 41.Zanutto-Elgui M.R., Vieira J.C.S., do Prado D.Z., Buzalaf M.A.R., de Magalhães Padilha P., de Oliveira D.E., Fleuri L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2018;278:823–831. doi: 10.1016/j.foodchem.2018.11.119. [DOI] [PubMed] [Google Scholar]
- 42.Rafael O.-H.D., Fernándo Z.-G.L., Abraham P.-T., Alberto V.-L.P., Guadalupe G.-S., Pablo P.J. Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydr. Res. 2019;486:107836. doi: 10.1016/j.carres.2019.107836. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Tahon M.A., Isaac G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020;66:32–40. doi: 10.2323/jgam.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Baskar G., Chandhuru J., Fahad K.S., Praveen A.S., Chamundeeswari M., Muthukumar T. Anticancer activity of fungal l-asparaginase conjugated with zinc oxide nanoparticles. J. Mater. Sci. Mater. Med. 2015;26:43. doi: 10.1007/s10856-015-5380-z. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z., Yang M., Bai Y., Ge F., Wang S. Antioxidant-related catalaseCTA1regulates development, aflatoxin biosynthesis, and virulence in pathogenic fungusAspergillus flavus. Environ. Microbiol. 2020;22:2792–2810. doi: 10.1111/1462-2920.15011. [DOI] [PubMed] [Google Scholar]
- 46.El-Katony T.M., El-Dein M.M.N., El-Fallal A.A., Ibrahim N.G., Mousa M. Substrate–fungus interaction on the enzymatic and non-enzymatic antioxidant activities of solid state fermentation system. Bioresour. Bioprocess. 2020;7:28. doi: 10.1186/s40643-020-00316-8. [DOI] [Google Scholar]
- 47.Maitan-Alfenas G.P., Visser E.M., Guimarães V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015;1:44–49. doi: 10.1016/j.cofs.2014.10.001. [DOI] [Google Scholar]
- 48.Gupta V.K., Kubicek C.P., Berrin J.-G., Wilson D.W., Couturier M., Berlin A., Filho E.X., Ezeji T. Fungal Enzymes for Bio-Products from Sustainable and Waste Biomass. Trends Biochem. Sci. 2016;41:633–645. doi: 10.1016/j.tibs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Jeske L., Placzek S., Schomburg I., Chang A., Schomburg D. BRENDA in 2019: A European ELIXIR core data resource. Nucleic Acids Res. 2019;47:D542–D549. doi: 10.1093/nar/gky1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berbee M.L., James T.Y., Strullu-Derrien C. Early Diverging Fungi: Diversity and Impact at the Dawn of Terrestrial Life. Annu. Rev. Microbiol. 2017;71:41–60. doi: 10.1146/annurev-micro-030117-020324. [DOI] [PubMed] [Google Scholar]
- 51.Verma M., Thakur M., Randhawa J., Sharma D., Thakur A., Meehnian H., Jana A. Biotechnological applications of fungal enzymes with special reference to bioremediation. In: Gothandam K.M., Ranjan S., Lichtfouse E., Dasgupta N., editors. Environmental Biotechnology. Springer; Cham, Switzerland: 2020. pp. 221–247. [Google Scholar]
- 52.Gurung N., Ray S., Bose S., Rai V. A Broader View: Microbial Enzymes and Their Relevance in Industries, Medicine, and Beyond. BioMed Res. Int. 2013;2013:329121. doi: 10.1155/2013/329121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Datta P., Gest H. Control of enzyme activity by concerted feedback inhibition. Proc. Natl. Acad. Sci. USA. 1964;52:1004–1009. doi: 10.1073/pnas.52.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemieux R., Spohr U. How Emil Fischer was led to the lock and key concept for enzyme specificity. Adv. Carbohydr. Chem. Biochem. 1994;50:1–20. [PubMed] [Google Scholar]
- 55.Bhatia S. Introduction to enzymes and their applications. In: Bhatia S., editor. Introduction to Pharmaceutical Biotechnology: Enzymes, Proteins and Bioinformatics. IOP Publishing; Bristol, UK: 2018. pp. 1–29. [Google Scholar]
- 56.Koshland D.E. Das Schüssel-Schloß-Prinzip und die Induced-fit-Theorie. Angew. Chem. 1994;106:2468–2472. doi: 10.1002/ange.19941062306. [DOI] [Google Scholar]
- 57.Johnson K.A., Goody R.S. The Original Michaelis Constant: Translation of the 1913 Michaelis–Menten Paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyce S., Tipton K.F. Enzyme Classification and Nomenclature. Encycl. Life Sci. 2013;1:1–11. [Google Scholar]
- 59.arrett A. Enzyme Nomenclature. Recommendations 1992. Eur. J. Biochem. 1995;232:1–6. doi: 10.1111/j.1432-1033.1995.tb20774.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu Q.-K., Han L.-T., Wu Y.-J., Liu T.-B. The Role of Oxidoreductase-Like Protein Olp1 in Sexual Reproduction and Virulence of Cryptococcus neoformans. Microorganisms. 2020;8:1730. doi: 10.3390/microorganisms8111730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pedrini N., Ortiz-Urquiza A., Huarte-Bonnet C., Fan Y., Juárez M.P., Keyhani N.O. Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. Proc. Natl. Acad. Sci. USA. 2015;112:E3651–E3660. doi: 10.1073/pnas.1504552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Góralczyk-Bińkowska A., Jasińska A., Długoński A., Płociński P., Długoński J. Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. PLoS ONE. 2020;15:e0231453. doi: 10.1371/journal.pone.0231453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sayyed R.Z., Bhamare H.M., Sapna, Marraiki N., Elgorban A.M., Syed A., El-Enshasy H.A., Dailin D.J. Tree bark scrape fungus: A potential source of laccase for application in bioremediation of non-textile dyes. PLOS ONE. 2020;15:e0229968. doi: 10.1371/journal.pone.0229968. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Sharma D., Chaudhary R., Kaur J., Arya S.K. Greener approach for pulp and paper industry by Xylanase and Laccase. Biocatal. Agric. Biotechnol. 2020;25:101604. doi: 10.1016/j.bcab.2020.101604. [DOI] [Google Scholar]
- 65.Shin S.K., Hyeon J.E., Joo Y.-C., Jeong D.W., You S.K., Han S.O. Effective melanin degradation by a synergistic laccase-peroxidase enzyme complex for skin whitening and other practical applications. Int. J. Biol. Macromol. 2019;129:181–186. doi: 10.1016/j.ijbiomac.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 66.Sorrentino I., Giardina P., Piscitelli A. Development of a biosensing platform based on a laccase-hydrophobin chimera. Appl. Microbiol. Biotechnol. 2019;103:3061–3071. doi: 10.1007/s00253-019-09678-2. [DOI] [PubMed] [Google Scholar]
- 67.Mehandia S., Sharma S.C., Arya S.K. Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol. Rep. 2020;25:e00413. doi: 10.1016/j.btre.2019.e00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernández-Fernández M., Sanromán M. Ángeles; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013;31:1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Wang J., Chang F., Tang X., Li W., Yin Q., Yang Y., Hu Y. Bacterial laccase of Anoxybacillus ayderensis SK3-4 from hot springs showing potential for industrial dye decolorization. Ann. Microbiol. 2020;70:51. doi: 10.1186/s13213-020-01593-6. [DOI] [Google Scholar]
- 70.Periasamy D., Mani S., Ambikapathi R. White Rot Fungi and Their Enzymes for the Treatment of Industrial Dye Effluents. In: Yadav A., Singh S., Mishra S., Gupta A., editors. Recent Advancement in White Biotechnology through Fungi. Springer; Berlin/Heidelberg, Germany: 2019. pp. 73–100. [Google Scholar]
- 71.Manavalan T., Manavalan A., Heese K. Characterization of Lignocellulolytic Enzymes from White-Rot Fungi. Curr. Microbiol. 2015;70:485–498. doi: 10.1007/s00284-014-0743-0. [DOI] [PubMed] [Google Scholar]
- 72.Erden E., Ucar M.C., Gezer T., Pazarlioglu N.K. Screening for ligninolytic enzymes from autochthonous fungi and applications for decolorization of Remazole Marine Blue. Braz. J. Microbiol. 2009;40:346–353. doi: 10.1590/S1517-83822009000200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manubolu M., Goodla L., Pathakoti K., Malmlöf K. Enzymes as direct decontaminating agents-mycotoxins. Enzym. Hum. Anim. Nutr. Princ. Perspect. 2018:313–330. doi: 10.1016/B978-0-12-805419-2.00016-2. [DOI] [Google Scholar]
- 74.Mehta P., Hale T.I., Christen P. Aminotransferases: Demonstration of homology and division into evolutionary subgroups. JBIC J. Biol. Inorg. Chem. 1993;214:549–561. doi: 10.1111/j.1432-1033.1993.tb17953.x. [DOI] [PubMed] [Google Scholar]
- 75.Mathieu Y., Prosper P., Favier F., Harvengt L., Didierjean C., Jacquot J.-P., Morel-Rouhier M., Gelhaye E. Diversification of Fungal Specific Class A Glutathione Transferases in Saprotrophic Fungi. PLOS ONE. 2013;8:e80298. doi: 10.1371/journal.pone.0080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 77.Rahmanpour S., Backhouse D., Nonhebel H. Induced tolerance of Sclerotinia sclerotiorum to isothiocyanates and toxic volatiles from Brassica species. Plant Pathol. 2009;58:479–486. doi: 10.1111/j.1365-3059.2008.02015.x. [DOI] [Google Scholar]
- 78.Calmes B., Morel-Rouhier M., Bataillé-Simoneau N., Gelhaye E., Guillemette T., Simoneau P. Characterization of glutathione transferases involved in the pathogenicity of Alternaria brassicicola. BMC Microbiol. 2015;15:123. doi: 10.1186/s12866-015-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Madboly L., Ali S.M., El Fakharany E.M., Ragab A.E., Khedr E.G., Elokely K.M. Stress-Based Production, and Characterization of Glutathione Peroxidase and Glutathione S-Transferase Enzymes from Lactobacillus plantarum. Front. Bioeng. Biotechnol. 2020;8:8. doi: 10.3389/fbioe.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morel M., Ngadin A.A., Droux M., Jacquot J.-P., Gelhaye E. The fungal glutathione S-transferase system. Evidence of new classes in the wood-degrading basidiomycete Phanerochaete chrysosporium. Cell. Mol. Life Sci. 2009;66:3711–3725. doi: 10.1007/s00018-009-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcerá A., Barreto L., Piedrafita L., Tamarit J., Herrero E. Saccharomyces cerevisiae cells have three Omega class glutathione S-transferases acting as 1-Cys thiol transferases. Biochem. J. 2006;398:187–196. doi: 10.1042/BJ20060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lateef A., Oloke J., Gueguim-Kana E., Raimi O. Production of fructosyltransferase by a local isolate ofAspergillus nigerin both submerged and solid substrate media. Acta Aliment. 2012;41:100–117. doi: 10.1556/AAlim.41.2012.1.12. [DOI] [Google Scholar]
- 83.Nakakuki T. Development of Functional Oligosaccharides in Japan. Trends Glycosci. Glycotechnol. 2003;15:57–64. doi: 10.4052/tigg.15.57. [DOI] [Google Scholar]
- 84.Cunha J.T., Soares P.O., Romaní A., Thevelein J.M., Domingues L. Biotechnology for Biofuels Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways. Biotechnol. Biofuels. 2019;12:20. doi: 10.1186/s13068-019-1360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mao S., Liu Y., Yang J., Ma X., Zeng F., Zhang Z., Wang S., Han H., Qin H., Lu F. Cloning, expression and characterization of a novel fructosyltransferase from Aspergillus niger and its application in the synthesis of fructooligosaccharides. RSC Adv. 2019;9:23856–23863. doi: 10.1039/C9RA02520K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghazi I., Fernandez-Arrojo L., Garcia-Arellano H., Ferrer M., Ballesteros A.O., Plou F.J. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 2007;128:204–211. doi: 10.1016/j.jbiotec.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Saranraj P., Stella D. Fungal amylase—A review. Int. J. Microbiol. Res. 2013;4:203–211. doi: 10.5829/idosi.ijmr.2013.4.2.75170. [DOI] [Google Scholar]
- 88.Singh S. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier BV; Amsterdam, The Netherlands: 2016. Aspergillus Enzymes for Textile Industry; pp. 191–198. [Google Scholar]
- 89.Chimbekujwo K.I., Ja’Afaru M.I., Adeyemo O.M. Purification, characterization and optimization conditions of protease produced by Aspergillus brasiliensis strain BCW. Sci. Afr. 2020;8:e00398. doi: 10.1016/j.sciaf.2020.e00398. [DOI] [Google Scholar]
- 90.Yike I. Fungal Proteases and Their Pathophysiological Effects. Mycopathologia. 2011;171:299–323. doi: 10.1007/s11046-010-9386-2. [DOI] [PubMed] [Google Scholar]
- 91.Germano S., Pandey A., Osaku C.A., Rocha S.N., Soccol C.R. Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzym. Microb. Technol. 2003;32:246–251. doi: 10.1016/S0141-0229(02)00283-1. [DOI] [Google Scholar]
- 92.Vishwanatha K.S., Rao A.G.A., Singh S.A. Acid protease production by solid-state fermentation using Aspergillus oryzae MTCC 5341: Optimization of process parameters. J. Ind. Microbiol. Biotechnol. 2010;37:129–138. doi: 10.1007/s10295-009-0654-4. [DOI] [PubMed] [Google Scholar]
- 93.El-Shora H.M., Metwally M.A.-A. Production, purification and characterisation of proteases from whey by some fungi. Ann. Microbiol. 2008;58:495–502. doi: 10.1007/BF03175548. [DOI] [Google Scholar]
- 94.De Souza P.M., Bittencourt M.L.D.A., Caprara C.C., de Freitas M., de Almeida R.P.C., Silveira D., Fonseca Y.M., Ferreira Filho E.X., Pessoa Junior A., Magalhães P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015;46:337–346. doi: 10.1590/S1517-838246220140359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benluvankar V., Jebapriya G.R., Gnanadoss J.J. Protease production by Penicillium sp. LCJ228 under solid state fermentation using groundnut oilcake as substrate. Int. J. Life Sci. Pharma Res. 2015;5:12–19. [Google Scholar]
- 96.Pekkarinen A., Mannonen L., Jones B., Niku-Paavola M.-L. Production of Proteases by Fusarium Species Grown on Barley Grains and in Media Containing Cereal Proteins. J. Cereal Sci. 2000;31:253–261. doi: 10.1006/jcrs.2000.0305. [DOI] [Google Scholar]
- 97.Al-Askar A., Abdulkhair W., Rashad Y. Production, Purification and Production, Purification and Optimization of Protease by Fusarium solani under Solid State Fermentation and Isolation of Protease Inhibitor Protein from Rumex vesicarius L. J. Pure Appl. Microbiol. 2014;8:239–250. [Google Scholar]
- 98.Kim J.Y. Isolation of Protease-producing Yeast, Pichia farinosa CO-2 and Characterization of Its Extracellular Enzyme. J. Korean Soc. Appl. Biol. Chem. 2010;53:133–141. doi: 10.3839/jksabc.2010.023. [DOI] [Google Scholar]
- 99.Zakowski J.J., Bruns D.E. Biochemistry of Human Alpha Amylase Isoenzymes. CRC Crit. Rev. Clin. Lab. Sci. 1985;21:283–322. doi: 10.3109/10408368509165786. [DOI] [PubMed] [Google Scholar]
- 100.Amin M., Bhatti H.N., Pervin F. Production, partial purification and thermal characterization of β-Amylase from Fusarium solani in solid state fermentation. J. Chem. Soc. Pakistan. 2008;30:480–485. [Google Scholar]
- 101.Oseni O.A. Activity of β-Amylase in Some Fungi Strains Isolated from Forest Soil in South-Western Nigeria. Br. Biotechnol. J. 2014;4:1–9. doi: 10.9734/BBJ/2014/4686. [DOI] [Google Scholar]
- 102.Silano V., Barat Baviera J.M., Bolognesi C., Cocconcelli P.S., Crebelli R., Gott D.M., Grob K., Lampi E., Mortensen A., Rivière G., et al. Safety evaluation of the food enzyme α-amylase from Aspergillus oryzae (strain DP-Bzb41) EFSA J. 2019;17:e05899. doi: 10.2903/j.efsa.2019.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed N.E., El Shamy A.R., Awad H.M. Optimization and immobilization of amylase produced by Aspergillus terreus using pomegranate peel waste. Bull. Natl. Res. Cent. 2020;44:109. doi: 10.1186/s42269-020-00363-3. [DOI] [Google Scholar]
- 104.Bakri Y., Jawhar M., Arabi M.I.E. Enhanced amylase production by Fusarium solani in solid state fermentation. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2014;57:123–128. doi: 10.52763/PJSIR.BIOL.SCI.57.3.2014.123.128. [DOI] [Google Scholar]
- 105.Shruthi B.R., Achur R.N.H., Nayaka Boramuthi T. Optimized Solid-State Fermentation Medium Enhances the Multienzymes Production from Penicillium citrinum and Aspergillus clavatus. Curr. Microbiol. 2020;77:2192–2206. doi: 10.1007/s00284-020-02036-w. [DOI] [PubMed] [Google Scholar]
- 106.Kanmani P., Aravind J., Kumaresan K. An insight into microbial lipases and their environmental facet. Int. J. Environ. Sci. Technol. 2014;12:1147–1162. doi: 10.1007/s13762-014-0605-0. [DOI] [Google Scholar]
- 107.Bora L. Purification and Characterization of Highly Alkaline Lipase from Bacillus licheniformis MTCC 2465: And Study of its Detergent Compatibility and Applicability. J. Surfactants Deterg. 2014;17:889–898. doi: 10.1007/s11743-013-1517-6. [DOI] [Google Scholar]
- 108.Abdel-Fattah Y., Soliman N.A., Yousef S.M., El-Helow E.R. Application of experimental designs to optimize medium composition for production of thermostable lipase/esterase by Geobacillus thermodenitrificans AZ1. J. Genet. Eng. Biotechnol. 2012;10:193–200. doi: 10.1016/j.jgeb.2012.08.001. [DOI] [Google Scholar]
- 109.Ilesanmi O.I., Adekunle A.E., Omolaiye J.A., Olorode E.M., Ogunkanmi A.L. Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Sci. Afr. 2020;8:e00279. doi: 10.1016/j.sciaf.2020.e00279. [DOI] [Google Scholar]
- 110.Nema A., Patnala S.H., Mandari V., Kota S., Devarai S.K. Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull. Natl. Res. Cent. 2019;43:82. doi: 10.1186/s42269-019-0125-7. [DOI] [Google Scholar]
- 111.Putri D.N., Khootama A., Perdani M.S., Utami T.S., Hermansyah H. Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep. 2020;6:331–335. doi: 10.1016/j.egyr.2019.08.064. [DOI] [Google Scholar]
- 112.Kempka A.P., Lipke N.L., Da Luz Fontoura Pinheiro T., Menoncin S., Treichel H., Freire D.M.G., Di Luccio M., De Oliveira D. Response surface method to optimize the production and characterization of lipase from Penicillium verrucosum in solid-state fermentation. Bioprocess Biosyst. Eng. 2008;31:119–125. doi: 10.1007/s00449-007-0154-8. [DOI] [PubMed] [Google Scholar]
- 113.Geoffry K., Achur R.N. Optimization of novel halophilic lipase production by Fusarium solani strain NFCCL 4084 using palm oil mill effluent. J. Genet. Eng. Biotechnol. 2018;16:327–334. doi: 10.1016/j.jgeb.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El Aamri L., Hafidi M., Scordino F., Krasowska A., Lebrihi A., Orlando M.G., Barresi C., Criseo G., Barreca D., Romeo O. Arthrographis curvata and Rhodosporidium babjevae as New Potential Fungal Lipase Producers for Biotechnological Applications. Braz. Arch. Biol. Technol. 2020;63:1–9. doi: 10.1590/1678-4324-2020180444. [DOI] [Google Scholar]
- 115.Salihu A., Bala M., Bala S.M. Application of Plackett-Burman Experimental Design for Lipase Production by Aspergillus niger Using Shea Butter Cake. ISRN Biotechnol. 2013;2013:718352. doi: 10.5402/2013/718352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fleuri L.F., de Oliveira M.C., de Lara Campos Arcuri M., Capoville B.L., Pereira M.S., Delgado C.H.O., Novelli P.K. Production of fungal lipases using wheat bran and soybean bran and incorporation of sugarcane bagasse as a co-substrate in solid-state fermentation. Food Sci. Biotechnol. 2014;23:1199–1205. doi: 10.1007/s10068-014-0164-7. [DOI] [Google Scholar]
- 117.Mehta A., Bodh U., Gupta R. Fungal lipases: A review. J. Biotech Res. 2017;8:58–77. [Google Scholar]
- 118.Selvakumar P., Sivashanmugam P. Optimization of lipase production from organic solid waste by anaerobic digestion and its application in biodiesel production. Fuel Process. Technol. 2017;165:1–8. doi: 10.1016/j.fuproc.2017.04.020. [DOI] [Google Scholar]
- 119.Gopinath S.C.B., Anbu P., Lakshmipriya T., Hilda A. Strategies to Characterize Fungal Lipases for Applications in Medicine and Dairy Industry. BioMed Res. Int. 2013;2013:154549. doi: 10.1155/2013/154549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chandra P., Enespa, Singh R., Arora P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020;19:169. doi: 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hwang H.T., Qi F., Yuan C., Zhao X., Ramkrishna D., Liu D., Varma A. Lipase-catalyzed process for biodiesel production: Protein engineering and lipase production. Biotechnol. Bioeng. 2014;111:639–653. doi: 10.1002/bit.25162. [DOI] [PubMed] [Google Scholar]
- 122.Meyer V., Andersen M.R., Brakhage A.A., Braus G.H., Caddick M.X., Cairns T.C., De Vries R.P., Haarmann T., Hansen K., Hertz-Fowler C., et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016;3:6. doi: 10.1186/s40694-016-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Várnai A., Mäkelä M.R., Djajadi D.T., Rahikainen J., Hatakka A., Viikari L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 2014;88:103–165. doi: 10.1016/B978-0-12-800260-5.00004-8. [DOI] [PubMed] [Google Scholar]
- 124.Abdullah A., Hamid H., Christwardana M., Hadiyanto H. Optimization of Cellulase Production by Aspergillus niger ITBCC L74 with Bagasse as Substrate using Response Surface Methodology. HAYATI J. Biosci. 2018;25:115. doi: 10.4308/hjb.25.3.115. [DOI] [Google Scholar]
- 125.Amadi O.C., Egong E.J., Nwagu T.N., Okpala G., Onwosi C.O., Chukwu G.C., Okolo B.N., Agu R.C., Moneke A.N. Process optimization for simultaneous production of cellulase, xylanase and ligninase by Saccharomyces cerevisiae SCPW 17 under solid state fermentation using Box-Behnken experimental design. Heliyon. 2020;6:e04566. doi: 10.1016/j.heliyon.2020.e04566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cekmecelioglu D., Demirci A. Production of Cellulase and Xylanase Enzymes Using Distillers Dried Grains with Solubles (DDGS) by Trichoderma reesei at Shake-Flask Scale and the Validation in the Benchtop Scale Bioreactor. Waste Biomass-Valorization. 2020;11:6575–6584. doi: 10.1007/s12649-020-00934-5. [DOI] [Google Scholar]
- 127.Polizeli M.L.T.M., Rizzatti A.C.S., Monti R., Terenzi H.F., Jorge J.A., Amorim D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005;67:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- 128.Silva L., Terrasan C.R.F., Carmona E.C. Purification and characterization of xylanases from Trichoderma inhamatum. Electron. J. Biotechnol. 2015;18:307–313. doi: 10.1016/j.ejbt.2015.06.001. [DOI] [Google Scholar]
- 129.Khalaji A., Sedighi M., Vahabzadeh F. Optimization and Kinetic Evaluation of Acetylxylan Esterase and Xylanase Production by Trichoderma reesei Using Corn Cob Xylan. Environ. Process. 2020;7:885–909. doi: 10.1007/s40710-020-00451-6. [DOI] [Google Scholar]
- 130.Atalla S.M.M., Ahmed N.E., Awad H.M., El Gamal N.G., El Shamy A.R. Statistical optimization of xylanase production, using different agricultural wastes by Aspergillus oryzae MN894021, as a biological control of faba bean root diseases. Egypt. J. Biol. Pest Control. 2020;30:125. doi: 10.1186/s41938-020-00323-z. [DOI] [Google Scholar]
- 131.Nikhil B., Dharmesh A., Priti T. International Journal of Research in Chemistry and Environment Production of Xylanase by Aspergillus flavus FPDN1 on Pearl millet bran: Optimization of Culture Conditions and Application in Bioethanol Production. Int. J. Res. Chem. Environ. 2012;2:204–210. [Google Scholar]
- 132.Corrêa T.L.R., de Araújo E.F. Fungal phytases: From genes to applications. Braz. J. Microbiol. 2020;51:1009–1020. doi: 10.1007/s42770-020-00289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kc S., Upadhyaya J., Joshi D.R., Lekhak B., Chaudhary D.K., Pant B.R., Bajgai T.R., Dhital R., Khanal S., Koirala N., et al. Production, Characterization, and Industrial Application of Pectinase Enzyme Isolated from Fungal Strains. Fermentation. 2020;6:59. doi: 10.3390/fermentation6020059. [DOI] [Google Scholar]
- 134.Mathew G.M., Madhavan A., Arun K.B., Sindhu R., Binod P., Singhania R.R., Sukumaran R.K., Pandey A. Thermophilic Chitinases: Structural, Functional and Engineering Attributes for Industrial Applications. Appl. Biochem. Biotechnol. 2021;193:142–164. doi: 10.1007/s12010-020-03416-5. [DOI] [PubMed] [Google Scholar]
- 135.Hartl L., Zach S., Seidl-Seiboth V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2011;93:533–543. doi: 10.1007/s00253-011-3723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elsoud M.M.A., El Kady E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019;43:59. doi: 10.1186/s42269-019-0105-y. [DOI] [Google Scholar]
- 137.Xie X.-H., Fu X., Yan X.-Y., Peng W.-F., Kang L.-X. A Broad-Specificity Chitinase from Penicillium oxalicum k10 Exhibits Antifungal Activity and Biodegradation Properties of Chitin. Mar. Drugs. 2021;19:356. doi: 10.3390/md19070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Omumasaba C.A., Yoshida N., Ogawa K. Purification and characterization of a chitinase from Trichoderma viride. J. Gen. Appl. Microbiol. 2001;47:53–61. doi: 10.2323/jgam.47.53. [DOI] [PubMed] [Google Scholar]
- 139.Krolicka M., Hinz S.W.A., Koetsier M.J., Joosten R., Eggink G., Van Den Broek L.A.M., Boeriu C.G. Chitinase Chi1 from Myceliophthora thermophila C1, a Thermostable Enzyme for Chitin and Chitosan Depolymerization. J. Agric. Food Chem. 2018;66:1658–1669. doi: 10.1021/acs.jafc.7b04032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar M., Brar A., Vivekanand V., Pareek N. Production of chitinase from thermophilic Humicola grisea and its application in production of bioactive chitooligosaccharides. Int. J. Biol. Macromol. 2017;104:1641–1647. doi: 10.1016/j.ijbiomac.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 141.Baldoni D.B., Antoniolli Z.I., Mazutti M.A., Jacques R.J.S., Dotto A.C., de Oliveira Silveira A., Ferraz R.C., Soares V.B., de Souza A.R.C. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz. J. Microbiol. 2020;51:1897–1908. doi: 10.1007/s42770-020-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sathiyaraj G., Srinivasan S., Kim H.-B., Subramaniyam S., Lee O.R., Kim Y.-J., Yang D.C. Screening and optimization of pectin lyase and polygalacturonase activity from ginseng pathogen Cylindrocarpon Destructans. Braz. J. Microbiol. 2011;42:794–806. doi: 10.1590/s1517-83822011000200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atanasova L., Dubey M., Grujić M., Gudmundsson M., Lorenz C., Sandgren M., Kubicek C.P., Jensen D.F., Karlsson M. Evolution and functional characterization of pectate lyase PEL12, a member of a highly expanded Clonostachys rosea polysaccharide lyase 1 family. BMC Microbiol. 2018;18:178. doi: 10.1186/s12866-018-1310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Semenova M.V., Sinitsyna O.A., Morozova V.V., Fedorova E.A., Gusakov A.V., Okunev O.N., Sokolova L.M., Koshelev A.V., Bubnova T.V., Vinetskii Y.P., et al. Use of a preparation from fungal pectin lyase in the food industry. Appl. Biochem. Microbiol. 2006;42:598–602. doi: 10.1134/S000368380606010X. [DOI] [PubMed] [Google Scholar]
- 145.Singh R.P., Gupta V., Kumari P., Kumar M., Reddy C.R.K., Prasad K., Jha B. Purification and partial characterization of an extracellular alginate lyase from Aspergillus oryzae isolated from brown seaweed. Environ. Boil. Fishes. 2010;23:755–762. doi: 10.1007/s10811-010-9576-9. [DOI] [Google Scholar]
- 146.Pilgaard B., Wilkens C., Herbst F.-A., Vuillemin M., Rhein-Knudsen N., Meyer A.S., Lange L. Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Sci. Rep. 2019;9:12338. doi: 10.1038/s41598-019-48823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nwokoro O. Studies on the production of glucose isomerase by Bacillus licheniformis. Pol. J. Chem. Technol. 2015;17:84–88. doi: 10.1515/pjct-2015-0054. [DOI] [Google Scholar]
- 148.Crueger A., Crueger W. Glucose transforming enzymes. In: Fogarty W.M., Kelly C.T., editors. Microbial Enzymes and Biotechnology. Springer; Dordrecht, The Netherlands: 1990. pp. 177–226. [Google Scholar]
- 149.Bhosale S.H., Rao M.B., Deshpande V.V. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996;60:280–300. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma H.K., Xu C., Qin W. Isolation of Bacterial Strain with Xylanase and Xylose/Glucose Isomerase (GI) Activity and Whole Cell Immobilization for Improved Enzyme Production. Waste Biomass-Valorization. 2021;12:833–845. doi: 10.1007/s12649-020-01013-5. [DOI] [Google Scholar]
- 151.Dai C., Miao T., Hai J., Xiao Y., Li Y., Zhao J., Qiu H., Xu B. A Novel Glucose Isomerase fromCaldicellulosiruptor besciiwith Great Potentials in the Production of High-Fructose Corn Syrup. BioMed Res. Int. 2020;2020:1871934. doi: 10.1155/2020/1871934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rengasamy S., Subramanian M.R., Perumal V., Ganeshan S., Al Khulaifi M.M., Al-Shwaiman H.A., Elgorban A.M., Syed A., Thangaprakasam U. Purification and kinetic behavior of glucose isomerase from Streptomyces lividans RSU. Saudi J. Biol. Sci. 2020;27:1117–1123. doi: 10.1016/j.sjbs.2019.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sayyed R.Z., Shimpi G.B., Chincholkar S.B. Constitutive production of extracellular glucose isomerase by an osmophillic Aspergillus sp. under submerged conditions. J. Food Sci. Technol. 2010;47:496–500. doi: 10.1007/s13197-010-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marshall R.O., Kooi E.R. Enzymatic conversion of D-glucose to D-fructose. Science. 1957;125:648–649. doi: 10.1126/science.125.3249.648. [DOI] [PubMed] [Google Scholar]
- 155.Kwak S., Jin Y.-S. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: A review and perspective. Microb. Cell Factories. 2017;16:82. doi: 10.1186/s12934-017-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lilius S.T.A. Ethanolic Fermentation of Xylose with Saccharomyces cerevisiae Harboring the Thermus thermophilus xylA Gene, Which Expresses an Active Xylose (Glucose) Isomerase. Microb. Cell Factories. 1996;62:4648–4651. doi: 10.1128/aem.62.12.4648-4651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brat D., Boles E., Wiedemann B. Functional Expression of a Bacterial Xylose Isomerase in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009;75:2304–2311. doi: 10.1128/AEM.02522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sidrauski C., Cox J.S., Walter P. tRNA Ligase Is Required for Regulated mRNA Splicing in the Unfolded Protein Response. Cell. 1996;87:405–413. doi: 10.1016/S0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 159.Banerjee A., Ghosh S., Goldgur Y., Shuman S. Structure and two-metal mechanism of fungal tRNA ligase. Nucleic Acids Res. 2018;47:1428–1439. doi: 10.1093/nar/gky1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Remus B.S., Schwer B., Shuman S. Characterization of the tRNA ligases of pathogenic fungi Aspergillus fumigatus and Coccidioides immitis. RNA. 2016;22:1500–1509. doi: 10.1261/rna.057455.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kerscher O., Felberbaum R., Hochstrasser M. Modification of Proteins by Ubiquitin and Ubiquitin-Like Proteins. Annu. Rev. Cell Dev. Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 162.Kulathu Y., Komander D. Atypical ubiquitylation — the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 2012;13:508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 163.Marín I. Origin and evolution of fungal HECT ubiquitin ligases. Sci. Rep. 2018;8:6419. doi: 10.1038/s41598-018-24914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Da Silva R.R. Enzyme technology in food preservation: A promising and sustainable strategy for biocontrol of post-harvest fungal pathogens. Food Chem. 2019;277:531–532. doi: 10.1016/j.foodchem.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 165.Thomas A., Thomas A. Acrylamide—A Potent Carcinogen in Food. Int. J. Sci. Res. 2014;3:177–188. [Google Scholar]
- 166.Cladière M., Camel V. The Maillard reaction and food safety: Focus on acrylamide. Environ. Risques St. 2017;16:31–43. doi: 10.1684/ers.2016.0952. [DOI] [Google Scholar]
- 167.Swanston J. Acrylamide Mitigation in Foods—An Update Acrylamide Mitigation in Foods—An Update. Leatherhead Food Research; Epsom, UK: 2019. [Google Scholar]
- 168.Batool T., Makky E.A., Jalal M., Yusoff M. A Comprehensive Review on l-Asparaginase and Its Applications. Appl. Biochem. Biotechnol. 2015;178:900–923. doi: 10.1007/s12010-015-1917-3. [DOI] [PubMed] [Google Scholar]
- 169.Orabi H.M., El Fakharany E., Abdelkhalek E.S., Sidkey N.M. l-Asparaginase And l-Glutaminase: Sources, Production, And Applications in Medicine and Industry. J. Microbiol. Biotechnol. Food Sci. 2019;9:179–190. doi: 10.15414/jmbfs.2019.9.2.179-190. [DOI] [Google Scholar]
- 170.Walia A., Guleria S., Mehta P., Chauhan A., Parkash J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech. 2017;7:1–12. doi: 10.1007/s13205-016-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh R.S., Singh T., Pandey A. Biomass, Biofuels, Biochemicals: Advances in Enzyme Technology. Elsevier; Amsterdam, The Netherlands: 2019. Microbial enzymes–An overview. [Google Scholar]
- 172.Khonzue P., Laothanachareon T., Rattanaphan N., Tinnasulanon P., Apawasin S., Paemanee A., Ruanglek V., Tanapongpipat S., Champreda V., Eurwilaichitr L. Optimization of Xylanase Production fromAspergillus nigerfor Biobleaching of Eucalyptus Pulp. Biosci. Biotechnol. Biochem. 2011;75:1129–1134. doi: 10.1271/bbb.110032. [DOI] [PubMed] [Google Scholar]
- 173.Kumar A., Gautam A., Dutt D. Bio-pulping: An energy saving and environment-friendly approach. Phys. Sci. Rev. 2020;5 doi: 10.1515/psr-2019-0043. [DOI] [Google Scholar]
- 174.Weng C., Peng X., Han Y. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis. Biotechnol. Biofuels. 2021;14:84. doi: 10.1186/s13068-021-01934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maalej-Achouri I., Guerfali M., Romdhane I.B.-B., Gargouri A., Belghith H. The effect of Talaromyces thermophilus cellulase-free xylanase and commercial laccase on lignocellulosic components during the bleaching of kraft pulp. Int. Biodeterior. Biodegrad. 2012;75:43–48. doi: 10.1016/j.ibiod.2012.04.015. [DOI] [Google Scholar]
- 176.Pensupa N., Leu S., Hu Y., Du C., Liu H., Jing H., Wang H., Lin C. Recent Trends in Sustainable Textile Waste Recycling Methods: Current Situation and Future Prospects. In: Lin C.S.K., editor. Chemistry and Chemical Technologies in Waste Valorization. Springer; Cham, Switzerland: 2017. pp. 189–228. [DOI] [PubMed] [Google Scholar]
- 177.Araújo R., Casal M., Cavaco-Paulo A. Application of enzymes for textile fibres processing. Biocatal. Biotransform. 2008;26:332–349. doi: 10.1080/10242420802390457. [DOI] [Google Scholar]
- 178.Lopes L.S., Vieira N., da Luz J.M.R., Marliane de Cássia S.S., Cardoso W.S., Kasuya M.C.M. Production of fungal enzymes in Macaúba coconut and enzymatic degradation of textile dye. Biocatal. Agric. Biotechnol. 2020;26:101651. doi: 10.1016/j.bcab.2020.101651. [DOI] [Google Scholar]
- 179.Velázquez-Fernández J.B., Muñiz-Hernández S. Bioremediation: Processes, Challenges and Future Prospects. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2014. [Google Scholar]
- 180.Zhang S., Gedalanga P.B., Mahendra S. Advances in bioremediation of 1,4-dioxane-contaminated waters. J. Environ. Manag. 2017;204:765–774. doi: 10.1016/j.jenvman.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 181.Santacruz-Juárez E., Buendia-Corona R.E., Ramírez R.E., Sánchez C. Fungal enzymes for the degradation of polyethylene: Molecular docking simulation and biodegradation pathway proposal. J. Hazard. Mater. 2021;411:125118. doi: 10.1016/j.jhazmat.2021.125118. [DOI] [PubMed] [Google Scholar]
- 182.Steliga T. Role of Fungi in Biodegradation of Petroleum Hydrocarbons. Pol. J. Environ. Stud. 2012;21:471–479. [Google Scholar]
- 183.Sun K., Song Y., He F., Jing M., Tang J., Liu R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021;773:145403. doi: 10.1016/j.scitotenv.2021.145403. [DOI] [PubMed] [Google Scholar]
- 184.Asemoloye M.D., Tosi S., Daccò C., Wang X., Xu S., Marchisio M.A., Gao W., Jonathan S.G., Pecoraro L. Hydrocarbon Degradation and Enzyme Activities of Aspergillus oryzae and Mucor irregularis Isolated from Nigerian Crude Oil-Polluted Sites. Microorganisms. 2020;8:1912. doi: 10.3390/microorganisms8121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanghi R., Dixit A., Verma P., Puri S. Design of reaction conditions for the enhancement of microbial degradation of dyes in sequential cycles. J. Environ. Sci. 2009;21:1646–1651. doi: 10.1016/S1001-0742(08)62468-7. [DOI] [PubMed] [Google Scholar]
- 186.Rápó E., Tonk S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021) Molecules. 2021;26:5419. doi: 10.3390/molecules26175419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karnwal A., Singh S., Kumar V., Sidhu G., Dhanjal D., Datta S., Amin D., Saini M., Singh J. Fungal enzymes for the textile industry. In: Yadav A., Mishra S., Singh S., Gupta A., editors. Recent Advancement in White Biotechnology through Fungi. Springer; Cham, Switzerland: 2019. pp. 459–482. [Google Scholar]
- 188.El Fakharany E., Hassan M.A., Taha T.H. Production and Application of Extracellular Laccase Produced by Fusarium oxysporum EMT. Int. J. Agric. Biol. 2016;18:939–947. doi: 10.17957/IJAB/15.0190. [DOI] [Google Scholar]
- 189.Li K., Guan G., Zhu J., Wu H., Sun Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control. 2019;96:234–243. doi: 10.1016/j.foodcont.2018.09.021. [DOI] [Google Scholar]
- 190.Fuglsang C.C., Johansen C., Christgau S., Adler-Nissen J. Antimicrobial enzymes: Applications and future potential in the food industry. Trends Food Sci. Technol. 1995;6:390–396. doi: 10.1016/S0924-2244(00)89217-1. [DOI] [Google Scholar]
- 191.El Fakharany E., Haroun B.M., Ng T., Redwan E. Oyster Mushroom Laccase Inhibits Hepatitis C Virus Entry into Peripheral Blood Cells and Hepatoma Cells. Protein Pept. Lett. 2010;17:1031–1039. doi: 10.2174/092986610791498948. [DOI] [PubMed] [Google Scholar]
- 192.Li H.-C., Huang E.-Y., Su P.-Y., Wu S.-Y., Yang C.-C., Lin Y.-S., Chang W.-C., Shih C. Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles. PLOS Pathog. 2010;6:e1001162. doi: 10.1371/journal.ppat.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang R., Zhao L., Wang H., Ng T.B. A novel ribonuclease with antiproliferative activity toward leukemia and lymphoma cells and HIV-1 reverse transcriptase inhibitory activity from the mushroom, Hohenbuehelia serotina. Int. J. Mol. Med. 2014;33:209–214. doi: 10.3892/ijmm.2013.1553. [DOI] [PubMed] [Google Scholar]
- 194.El-Maradny Y.A., El-Fakharany E.M., Abu-Serie M.M., Hashish M.H., Selim H.S. Lectins purified from medicinal and edible mushrooms: Insights into their antiviral activity against pathogenic viruses. Int. J. Biol. Macromol. 2021;179:239–258. doi: 10.1016/j.ijbiomac.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 195.He M., Su D., Liu Q., Gao W., Kang Y. Mushroom lectin overcomes hepatitis B virus tolerance via TLR6 signaling. Sci. Rep. 2017;7:5814. doi: 10.1038/s41598-017-06261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang H. Purification and characterization of a laccase from the edible wild mushroom Tricholoma mongolicum. J. Microbiol. Biotechnol. 2010;20:1069–1076. doi: 10.4014/jmb.0912.12033. [DOI] [PubMed] [Google Scholar]
- 197.Barrientos L.G., Matei E., LaSala F., Delgado R., Gronenborn A.M. Dissecting carbohydrate–Cyanovirin-N binding by structure-guided mutagenesis: Functional implications for viral entry inhibition. Protein Eng. Des. Sel. 2006;19:525–535. doi: 10.1093/protein/gzl040. [DOI] [PubMed] [Google Scholar]
- 198.Ma L.-B., Xu B.-Y., Huang M., Sun L.-H., Yang Q., Chen Y.-J., Yin Y.-L., He Q.-G., Sun H. Adjuvant effects mediated by the carbohydrate recognition domain of Agrocybe aegerita lectin interacting with avian influenza H9N2 viral surface glycosylated proteins. J. Zhejiang Univ. Sci. B. 2017;18:653–661. doi: 10.1631/jzus.B1600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li J.W.-H., Vederas J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 200.Liaqat F., Eltem R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018;184:243–259. doi: 10.1016/j.carbpol.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 201.Yang Y., Zhao H., Barrero R.A., Zhang B., Sun G., Wilson I.W., Xie F., Walker K.D., Parks J.W., Bruce R., et al. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL. BMC Genom. 2014;15:69. doi: 10.1186/1471-2164-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raj K.G., Manikandan R., Arulvasu C., Pandi M. Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;138:667–674. doi: 10.1016/j.saa.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 203.Zaiyou J., Li M., Xiqiao H. An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. mairei. Medicine. 2017;96:e7406. doi: 10.1097/MD.0000000000007406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ranjan A., Singh R.K., Singh M. Metabolic versatility of fungi as a source for anticancer compounds. In: Srivastava A.K., Kannaujiya V.K., Singh R.K., Singh D., editors. Evolutionary Diversity as a Source for Anticancer Molecules. Elsevier; London, UK: 2020. pp. 191–207. [Google Scholar]
- 205.Gallego-Jara J., Lozano-Terol G., Sola-Martínez R.A., Cánovas-Díaz M., de Diego Puente T. A Compressive Review about Taxol®: History and Future Challenges. Molecules. 2020;25:5986. doi: 10.3390/molecules25245986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.How C.W., Ong Y.S., Low S.S., Pandey A., Show P.L., Foo J.B. How far have we explored fungi to fight cancer? Semin. Cancer Biol. :2021. doi: 10.1016/j.semcancer.2021.03.009. in press. [DOI] [PubMed] [Google Scholar]
- 207.Xu L., Liu X., Li Y., Yin Z., Jin L., Lu L., Qu J., Xiao M. Enzymatic rhamnosylation of anticancer drugs by an α-l-rhamnosidase from Alternaria sp. L1 for cancer-targeting and enzyme-activated prodrug therapy. Appl. Microbiol. Biotechnol. 2019;103:7997–8008. doi: 10.1007/s00253-019-10011-0. [DOI] [PubMed] [Google Scholar]
- 208.Stolworthy T.S., Korkegian A.M., Willmon C., Ardiani A., Cundiff J., Stoddard B.L., Black M.E. Yeast Cytosine Deaminase Mutants with Increased Thermostability Impart Sensitivity to 5-Fluorocytosine. J. Mol. Biol. 2008;377:854–869. doi: 10.1016/j.jmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aklakur M. Natural antioxidants from sea: A potential industrial perspective in aquafeed formulation. Rev. Aquac. 2016;10:385–399. doi: 10.1111/raq.12167. [DOI] [Google Scholar]
- 210.Butkhup L., Samappito W., Jorjong S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2017;27:193–202. doi: 10.1007/s10068-017-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rafi S., Shoaib A., Awan Z.A., Rizvi N.B., Nafisa, Shafiq M. Chromium tolerance, oxidative stress response, morphological characteristics, and FTIR studies of phytopathogenic fungus Sclerotium rolfsii. Folia Microbiol. 2016;62:207–219. doi: 10.1007/s12223-016-0489-0. [DOI] [PubMed] [Google Scholar]
- 212.Abdel-Azeem M., El-Maradny Y., Othman A., Abdel-Azeem A. Endophytic Fungi as a Source of New Pharmaceutical Biomolecules. In: Abdel-Azeem A.M., Yadav A.N., Yadav N., Sharma M., editors. Industrially Important Fungi for Sustainable Development. Fungal Biology. Springer; Cham, Switzerland: 2021. pp. 115–151. [Google Scholar]
- 213.Filipe D., Fernandes H., Castro C., Peres H., Oliva-Teles A., Belo I., Salgado J.M. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Improv. Lignocellulolytic. 2020;14:78–91. doi: 10.1002/bbb.2073. [DOI] [Google Scholar]
- 214.Arnau J., Yaver D., Hjort C.M. Strategies and Challenges for the Development of Industrial Enzymes Using Fungal Cell Factories. In: Nevalainen H., editor. Grand Challenges in Biology and Biotechnology. Springer; Cham, Switzerland: 2020. pp. 179–210. [Google Scholar]
- 215.Biagini R., MacKenzie B., Sammons D., Smith J., Striley C., Robertson S., Snawder J. Evaluation of the prevalence of anti-wheat-, anti—flour dust-, and anti-alpha amylase-specific IgE antibodies in blood donors. J. Allergy Clin. Immunol. 2003;111:S95. doi: 10.1016/S0091-6749(03)80261-0. [DOI] [PubMed] [Google Scholar]
- 216.Green B.J., Beezhold D.H. Industrial Fungal Enzymes: An Occupational Allergen Perspective. J. Allergy. 2011;2011:682574. doi: 10.1155/2011/682574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nielsen P.H., Oxenbøll K.M., Wenzel H. Cradle-to-Gate Environmental Assessment of Enzyme Products Produced Industrially in Denmark by Novozymes A/S. Int. J. Life Cycle Assess. 2007;12:432–438. doi: 10.1065/lca2006.08.265.1. [DOI] [Google Scholar]
- 218.Sutay Kocabaş D., Grumet R. Evolving regulatory policies regarding food enzymes produced by recombinant microorganisms. GM Crop. Food. 2019;10:191–207. doi: 10.1080/21645698.2019.1649531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nikkilä I., Waldén M., Maina N.H., Tenkanen M., Mikkonen K.S. Fungal Cell Biomass from Enzyme Industry as a Sustainable Source of Hydrocolloids. Front. Chem. Eng. 2020;2:1–12. doi: 10.3389/fceng.2020.574072. [DOI] [Google Scholar]
- 220.Owaid M.N., Abed I.A., Al-Saeedi S.S.S. Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inf. Process. Agric. 2017;4:78–82. doi: 10.1016/j.inpa.2017.01.001. [DOI] [Google Scholar]
- 221.Ayele A., Haile S., Alemu D., Kamaraj M. Comparative Utilization of Dead and Live Fungal Biomass for the Removal of Heavy Metal: A Concise Review. Sci. World J. 2021;2021:5588111. doi: 10.1155/2021/5588111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Phan C.-W., Sabaratnam V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012;96:863–873. doi: 10.1007/s00253-012-4446-9. [DOI] [PubMed] [Google Scholar]
- 223.Rajavat A.S., Rai S., Pandiyan K., Kushwaha P., Choudhary P., Kumar M., Chakdar H., Singh A., Karthikeyan N., Bagul S.Y., et al. Sustainable use of the spent mushroom substrate ofPleurotus floridafor production of lignocellulolytic enzymes. J. Basic Microbiol. 2020;60:173–184. doi: 10.1002/jobm.201900382. [DOI] [PubMed] [Google Scholar]
- 224.Grujić M., Dojnov B., Potočnik I., Duduk B., Vujčić Z. Spent mushroom compost as substrate for the production of industrially important hydrolytic enzymes by fungi Trichoderma spp. and Aspergillus niger in solid state fermentation. Int. Biodeterior. Biodegrad. 2015;104:290–298. doi: 10.1016/j.ibiod.2015.04.029. [DOI] [Google Scholar]