Abstract
Coccidiosis is an important parasitic disease of poultry with great economic importance. Due to drug resistance issues, the study was conducted to investigate how probiotics (Lactobacillus plantarum or L. plantarum) affected oocysts per gram of feces (OPG), fecal scores, feed conversion ratio (FCR), immunomodulatory effect in terms of the cell-mediated and humoral immune response. Serum chemistry (ALT, AST, LDH, and creatinine) was measured in different treated chicken groups. mRNA expression levels of antioxidant enzymes (SOD 1 and CAT), peptide transporter 1 (PepT 1), and tight junction proteins (ZO and CLDN 1) were also examined in chicken groups infected with Eimeria tenella (E. tenella). Chickens supplemented with L. plantarum 1 × 108 CFU (colony-forming unit) showed an improved cell-mediated and humoral immune response, compared with the control group (p < 0.05). Probiotics also enhanced the performance of antioxidant enzymes, PepT 1, and tight junction proteins, and improved serum chemistry (AST, ALT, and LDH), compared with control-infected, non-medicated chickens. However, no significant difference (p > 0.05) was observed in CLDN 1 expression level and creatinine in all treated chicken groups. These findings demonstrated that probiotics supplementation in the feed can protect the birds against E. tenella infection.
Keywords: Eimeria tenella, probiotics, immunity, gene expression, poultry
1. Introduction
Intestinal diseases of parasitic origin constitute a major problem for domestic poultry and animals worldwide [1,2,3,4]. The phylum Apicomplexa contains numerous well-known obligatory and intracellular parasites of humans and livestock. In these phyla, the genus Eimeria is well known and is the cause of coccidiosis in birds and mammals [5]. Avian coccidiosis is an enteric disease of protozoal origin that causes malabsorption, bloody diarrhea, poor feed conversion ratio, retarded growth, and increased mortality [6,7]. It also enhances the susceptibility of birds/animals to various other enteric diseases, including necrotic enteritis. According to an estimate, annual losses to the poultry industry (USD 3 billion) are a well-established fact [8]. These losses are associated with high morbidity, medicinal expenditure, and birds mortality [9,10]. The chickens are affected by seven species of Eimeria parasite; however, E. tenella is considered the most pathogenic parasite among them. It is associated with the destruction of the cecal epithelium and is also responsible for severe disruption of intestinal homeostasis [3,11].
Live vaccines and various anticoccidial drugs are commonly used to control coccidiosis [2,10]. However, numerous issues are associated with anticoccidial drugs, among which are drug resistance and drug residues in food products in different world regions [2,12]. Due to these problems, alternative strategies for controlling coccidiosis are needed for this age [13]. Probiotics are considered safe and appealing alternatives in controlling enteric pathogens, including Eimeria parasites [14,15]. They can also compensate for the various issues related to the use of anticoccidial vaccines and drugs [3,6].
Probiotics are non-pathogenic microbes that can maintain normal intestinal microbiota and positive activity against intestinal diseases [16]. Probiotics supplementation in the poultry feed enhances the activity of beneficial microbes in the gastrointestinal tract (GIT) [14], increases the performance of digestive enzymes, stimulates the humoral and cell-mediated immunity [4,14], and also neutralizes various enterotoxins [17,18]. Probiotics positively affect the blood parameters, carcass traits, intestinal microflora, growth, and immune functions of the birds [19]. Probiotics can act as anticoccidial agents, with immunomodulators such as improved Toll-like receptor expression [20], cytokine stimulation [21], antibody development [15,22], antioxidant effect [23,24], lower lesion score [4], decreased oocysts shedding [3,25], less fecal score [14], as well as neutralizing various enterotoxins. Based on these results, probiotics have been considered as potential anticoccidial agents; however, there is limited confirmation [26].
The immune system against avian coccidiosis could be categorized as innate and adaptive [27]. The innate immune response (first line of protection) provides the recognition of pathogen-associated molecular patterns (PAMPs) retained by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) [14,28]. These receptors (ligand) contribute to the activation and proliferation of cytokines and immune cells. Macrophages, epithelial cells, dendritic cells, natural killer cells, and heterophils are the main cells that contribute against avian coccidiosis in the innate immune response. However, the adaptive immune response is specific to the antigen. It stimulates the two main kinds of lymphocytes—B cells (immunoglobulins production) and T cells (T-cell receptors), against pathogens, including Eimeria parasites [29]. The objective of the manuscript is to provide a comprehensive explanation of the use of probiotics in chicken farms to combat coccidiosis in the feed and their effects on mRNA gene expression as growth promotor, immunomodulatory, and serum chemistry of chickens.
2. Materials and Methods
2.1. Ethics Statement
All experiments using chickens were carried out in compliance with the recent Chinese Ethical legislation, and special attention was given to the welfare of experimental chickens. The animal protocol was accepted by the Animal Care Committee of Fujian Agriculture and Forestry University (FAFU2021-3-12).
2.2. Parasite Propagation
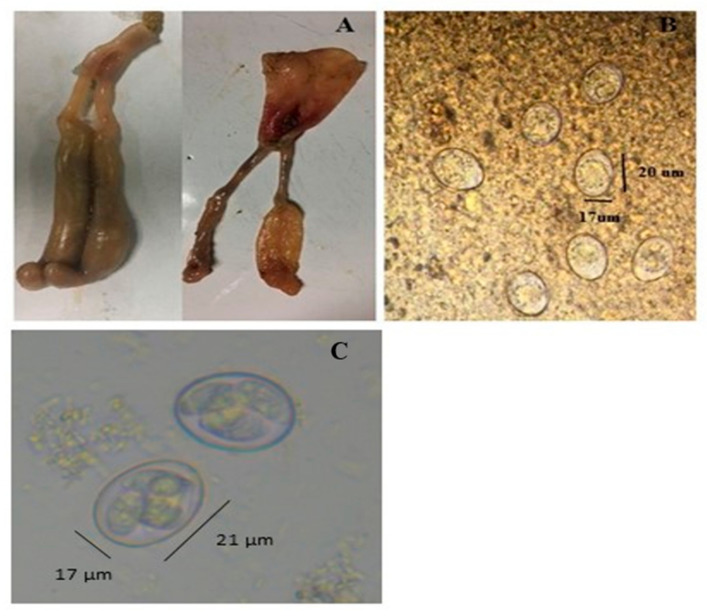
E. tenella (Beijing Strain) was provided by Professor Xun Suo in China Agricultural University, then maintained and propagated in our laboratory with the following steps: First, 200 one-day-old chickens were grown in different cages; feed and water were provided ad libitum in hygienic cage systems. On day 14, chickens were infected with E. tenella (3 × 104 sporulated oocysts/bird) through the oral route. The chicken infected with E. tenella typically showed the cecal lesion (Figure 1A). Unsporulated oocysts were collected (5–9 days of post infection) from infected bird droppings (Figure 1B) and converted to sporulated form (Figure 1C), following Yin et al. [30]. The steps for the collection of un-sporulated and sporulated oocysts were as follows: In detail, on the 5th day of infection, the dropping pan was emptied, and the chickens’ droppings were collected during days 6 to 9 of infection. Infected bird droppings were poured into the clean water and mixed well after filtering the mixture (droppings and water) by using a sieve. The filtrate was centrifuged at 3500 r/min for 5 min, and the supernatant was discarded. Saturated sodium chloride solution (NaCl) was poured into the precipitate and mixed thoroughly and again centrifuged at 3500 r/min for 5 min, after which the supernatant was collected. Then, 3–4 volume of ultrapure water was added to the collected liquid and centrifuged at 3500 r/min for 15 min, and the precipitate was collected. Next, 8–12 times the volume of 2.5% potassium dichromate solution (K2Cr2O7) was added to the precipitate, stirred evenly, and placed in a shaker at 28 °C and 120 rpm speed for 2 days (for unsporulated to sporulated oocysts stage of the parasite). After two days, the sporulation status of coccidial oocysts was observed, the number of oocysts was counted under a microscope using a McMaster chamber, and afterward, they were stored in a refrigerator at 4 °C for later use.
Figure 1.
Collection and propagation of different E. tenella life stages from the infected caecum of the chicken: (A) Eimeria tenella-infected caecum of 14-day old chicken; (B) under the microscope; unsporulated oocysts (from fecal sample of the infected chicken) are denoted by arrows with a width of 17 μm and a length of 20 μm; (C) sporulated oocysts (converted from unsporulated oocysts to sporulated oocysts) observed under the microscope, denoted by arrows with 17 μm width and 21 μm.
2.3. Experimentally Treated Chicken Groups
Chickens were raised in different cages. On day 14 of the trial, chickens (n = 120) were placed into 5 different groups (24 chickens per group) and each group was divided into three cages per treatment (8 chickens per cage). Feed and water were provided ad libitum. During the first week of age, the temperature was maintained at 30–32 °C; however, it was reduced on weekly basis by 2–3 °C and maintained at 23–25 °C through the end of the experiment (42 days). Group PROB6 was infected and supplemented with L. plantarum 1 × 106 CFU in the feed; group PROB7 was infected and supplemented with L. plantarum 1 × 107 CFU in the feed; group PROB8 was infected and supplemented with L. plantarum 1 × 108 CFU in the feed; group NC (infected but no additive control) was the infected, non-medicated group; group Cont (naïve control) was taken as the non-infected and non-medicated control group. Groups excluding group Cont were infected with E. tenella (3 × 104 sporulated oocysts) through the oral route (day 14 of trial). Here, groups NC and Cont were kept as control groups.
2.4. Oocyst per Gram (OPG) and Fecal Score Assessment
The oocyst per gram was assessed on 8, 10, and 13 days post infection of chickens (d.p.i.). Two grams (g) of feces was collected and crushed from each replicate. The crushed fecal sample was placed in a glass beaker (250 mL), and 58–60 milliliter (mL) of saturated sodium chloride (NaCl) solution was added and thoroughly mixed. The prepared mixture was left for 5–10 min before the count, allowing the oocysts to float to the surface. Finally, samples from each replication were collected and checked under the microscope using the McMaster chamber [31]. After 5, 8, and 13 days of E. tenella infection, the anticoccidial potential of all groups was evaluated using the fecal score (from 0–4), following Youn et al. [32].
2.5. Feed Conversion Ratio
The following formula was used to evaluate the feed conversion ratio (FCR) for all experimental groups during 42 days of the research:
| Feed conversation ratio (FCR) = Mean feed consumption/Mean weight |
2.6. Tissue and Serum Collection
On day 8 of post infection, six average birds/group were slaughtered. The cecal samples were collected for mRNA gene expression and kept at −80 °C until further use. On days 7 and 14 after the injection of sheep red blood cells (SRBCs; non-pathogenic T-dependent antigens), blood samples were collected from chicken wing vein for serum extraction, following Adamu et al. [29]. Microplate hemagglutinin assay was used to quantify the anti-SRBC antibody titers. Serum samples were used for the total Ig and IgG against SRBCs.
2.7. Immune Response
2.7.1. Cell-mediated Response to Dinitrochlorobenzene (DNCB)
DNCB test was used to evaluate the delayed-type hypersensitivity reaction, following Blumink et al. [33]. Briefly, the first dose of 0.1 mL (2% DNCB in acetone) was administered on the 4 cm2 area on the skin of the five chickens/group (day 14), followed by the second dose on day 21 of the experiment. Skin thickness was measured using a vernier caliper before and after 24 h of DNCB administration.
2.7.2. Humoral Immune Response to Sheep Red Blood Cells (SRBCs)
Humoral immune responses were analyzed in total immunoglobulin (Ig) and IgG against SRBCs (non-pathogenic T-dependent antigens). Microplate hemagglutinin assay was used to quantify the anti-SRBC antibody titers by following the method in [25]. Briefly, 1 mL SRBCs suspension was injected through the intramuscular route on day 14 (parasite infection day). Serum was collected on days 7 and 14 after SRBC injection. Serum samples were analyzed for total Ig and IgG anti-SRBCs antibodies, and results were shown as geomean titers (GMT).
2.8. Serum Chemistry
Serum chemistry included aspartate aminotransferase (AST) and alanine transferase (ALT). Creatinine and lactate dehydrogenase (LDH) values were measured following the diagnostic kit instructions (Bioengineering Institute, Nanjing, China).
2.9. RNA Extraction, cDNA Preparation, and Determination of mRNA Gene Expression
The total RNA was extracted from the infected caecum using the kit method (Omega Bio-tek, Guangzhou, China). RNA pellets were dissolved in RNase-free water (adequate RNA concentration), and the RNA concentration was measured spectrophotometrically (absorbance at 260/280 nm) and kept at −80 °C until further use. Conversion of mRNA to cDNA was completed following the kit instruction (Yeasen Biotech, Shanghai, China). The mRNA expression levels of antioxidant (SOD, CAT), PepT 1, and tight junction protein (CLDN 1, ZO-1) were measured by qPCR. The primers used in the research are shown in Table 1. SYBR Green Supermix (Bio-Rad, Nanjing, China) was used for qPCR on a Roche Light Cycler 480 Real-Time System. The following were the qPCR conditions: after initial denaturing for 30 s at 95 °C, 40 cycles of 10 s at 95 °C, and 20 s at 60 °C were programmed.
Table 1.
Primers used in this experiment for the assessment of mRNA expression levels.
| Genes | Primer Sequence (5′–3′) | Gene Bank | |
|---|---|---|---|
| CAT | F | GAGGAACCCTCAGACTCATTTG | NM_001031215.2 |
| R | CCATCAGGAATACCACGATCAC | ||
| SOD 1 | F | AGATGGCAGTGGGAAATGAG | NM_205064.1 |
| R | ACTCAAGACAGCAGAGTAGTAATG | ||
| PepT 1 | F | CCCCTGAGGAGGATCACTGTT | KF366603.1 |
| R | CAAAAGAGCAGCAGCAACGA | ||
| CLDN 1 | F | ACTCCTGGGTCTGGTTGGT | AY750897.1 |
| R | CAGGTCAAACAGAGGTACAGG | ||
| ZO 1 | F | CTTCAGGTGTTTCTCTTCCTCCTC | XM_413773 |
| R | CTGTGGTTTCATGGCTGGATC | ||
| β-actin | F | GAGAAATTGTGCGTGACATCA | L08165 |
| R | CCTGAACCTCTCATTGCCA | ||
2.10. Statistical Analysis
Analysis of variance was used to examine data obtained from various parameters. Mean values were compared by Tukey’s test using Statistix software (San Jose, CA, USA). Statistical differences among group means were considered significant at p < 0.05.
3. Results
3.1. Fecal Score and OPG Assessment
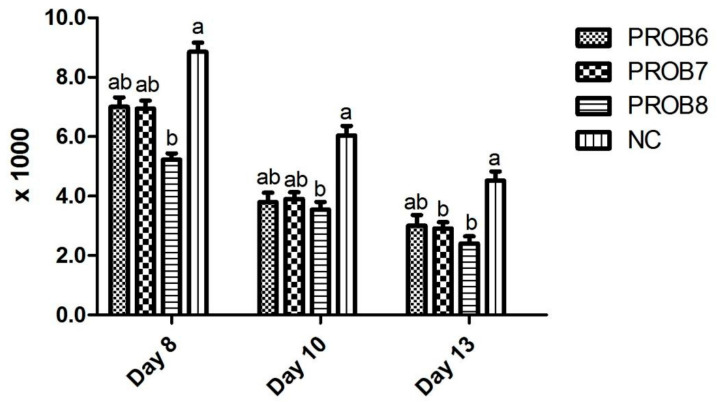
Fecal score and OPG values in chicken groups supplemented with probiotics are shown in Table 2 and Figure 2. A non-significant difference was observed (fecal scores) in L. plantarum supplemented groups (A and B) in feed. However, a significant difference (p < 0.05) was observed in probiotic-supplemented groups (1 × 108 CFU) on 5, 6, and 8 days post infection, compared with the infected, non-medicated control group. OPG was assessed in L. plantarum supplemented groups on 8, 10, and 13 days post infection (Figure 2). Fewer OPG was observed in the probiotic-supplemented group (1 × 108 CFU) in feed than in infected, non-medicated control groups, as shown in Figure 2.
Table 2.
Fecal score in chickens (n = 6) with experimentally induced coccidiosis in different treated groups.
| Groups | Day 5th | Day 6th | Day 8th |
|---|---|---|---|
| PROB6 | 2.50 ± 0.33 b | 2.83 ± 0.27 b | 2.33 ± 0.54 b |
| PROB 7 | 2.33 ± 0.54 b | 2.66 ± 0.14 b | 2.00 ± 0.51 b |
| PROB 8 | 1.67 ± 0.14 c | 1.67 ± 0.15 c | 1.33 ± 0.21 c |
| NC | 3.33 ± 0.15 a | 3.50 ± 0.16 a | 3.33 ± 0.15 a |
| Cont | _ | _ | _ |
Means with different superscripts (a, b, c) inside a column (Table 2) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented in feed; NC = infected, non-medicated treatment group; Cont = non-infected, non-medicated treated group.
Figure 2.
Oocysts per gram (OPG) of feces with experimentally induced coccidiosis in various treatment groups at day 8, 10, and 13 post infection. Means with different superscripts (a, b) are significantly different (p < 0.05). X-axis represents the numbers of different groups, and y-axis shows the numbers of oocyst in the chicken dropping. PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated treatment group; Cont = non-infected, non-medicated group.
3.2. Feed Conversion Ratio
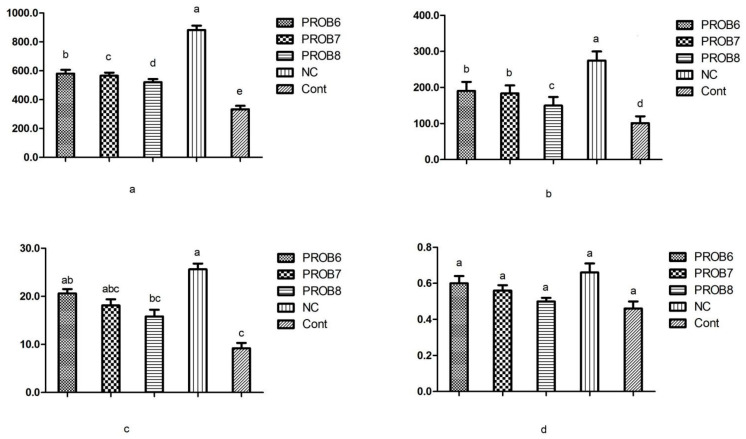
L. plantarum supplemented groups in feed showed a better FCR than the E. tenella infected control group, as shown in Figure 3.
Figure 3.
Feed conversion ratio (FCR) in chickens with experimentally induced coccidiosis in different treated groups. Means with different superscripts (a, b, c, d) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated treatment group; Cont = non-infected, non-medicated treated group.
3.3. Cell-Mediated Immune Response
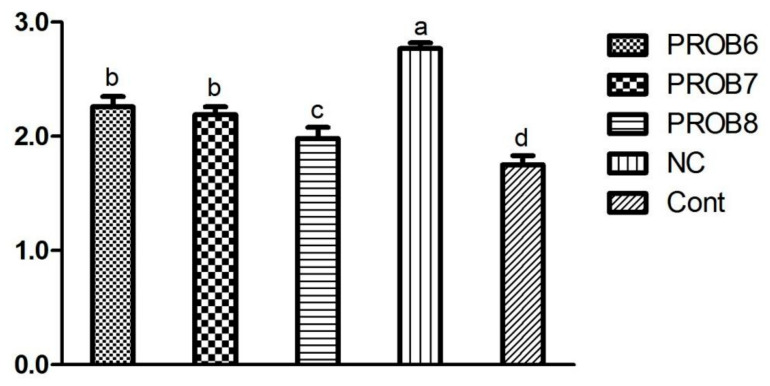
Improved cell-mediated immune response to DNCB was observed in all probiotic-supplemented chicken groups (p ˂ 0.05), compared with that of the infected, non-medicated control group. Among probiotic-supplemented groups, the maximum cell-mediated response was noted in chickens supplemented with 1 × 108 CFU, followed in decreasing order by the groups supplemented with 1 × 107 CFU and 1 × 106 CFU (Figure 4).
Figure 4.
Cell-mediated response to DNCB in different probiotic-supplemented and control groups. Means with different superscripts (a, b, c) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated control group.
3.4. Humoral Immunity
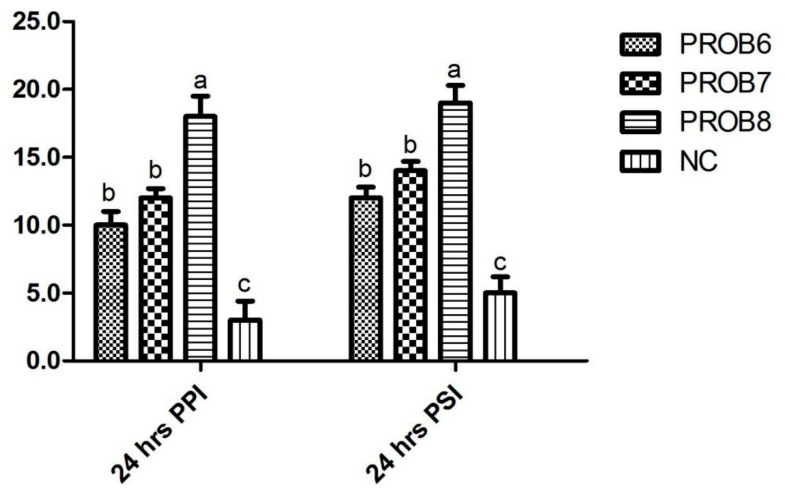
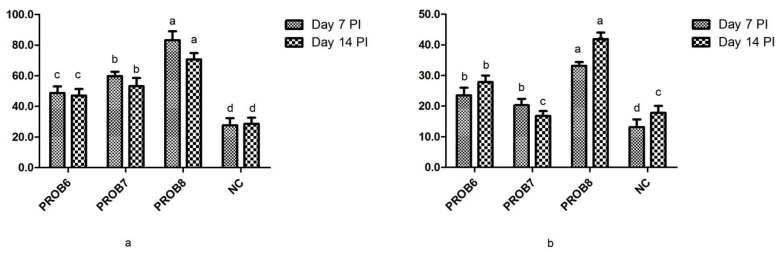
The total antibody titers in probiotic-supplemented (1 × 108 CFU) group C was higher (p ˂ 0.05) than in the infected, non-medicated control group. Among probiotic-supplemented groups, maximum antibody titers were noted in chickens supplemented with 1×108 CFU, followed in decreasing order by the groups supplemented with 1 × 107 CFU and 1×106 CFU. Antibody titers and IgG value of group supplemented with probiotics (1 × 108 CFU in feed) and infected, non-medicated control groups were noted as statistically significant differences (p < 0.05), both at day 7 and 14 of post-SRBC treatment (Figure 5a,b).
Figure 5.
Humoral response to SRBCs in different probiotic-supplemented and control group: (a) total immunoglobulin (Ig) at 7 and 14 days post infection (PI); (b) IgG assessment at 7 and 14 days post infection (PI). Means with different superscripts (a, b, c, d) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated control group.
3.5. Serum Enzyme Levels for Toxicity
Serum chemistry (ALT, AST, and LDH) values were lower in probiotic-supplemented groups. However, AST and LDH values were significantly different (p < 0.05) in all probiotic-supplemented groups in feed, compared with the non-infected, non-medicated, control group (Figure 6).
Figure 6.
Serum chemistry (LDH, AST, ALT, and creatinine) values (a,b,c, and d, respectively) in various treatments of infected chicken groups. Means with different superscripts (a, b, c, d) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated control group; Cont = non-infected, non-medicated control group.
3.6. mRNA Gene Expression
3.6.1. Peptide Transporter 1 (PepT 1) and Antioxidant Enzymes mRNA Expression Levels
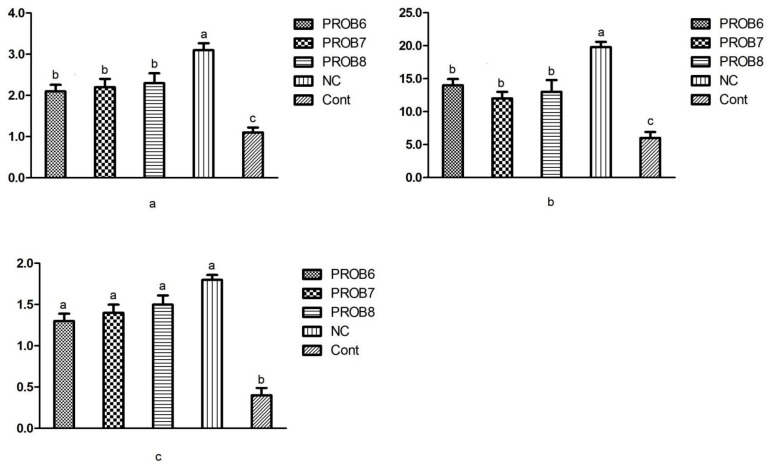
The expression levels of peptide transporter 1 (PepT 1) and alteration in SOD 1 and CAT (antioxidant enzyme) are presented in (Figure 7). Non- infected, non-medicated control group revealed higher mRNA levels of PepT 1 and antioxidant enzymes than the rest of the other groups. In comparison with the infected and untreated birds, probiotic-supplemented birds showed significantly increased (p < 0.05) mRNA levels of PepT 1, SOD 1, and CAT (Figure 7a,b,c).
Figure 7.
mRNA expression levels of antioxidant enzyme and PepT 1: (a) SOD expression level; (b) CAT expression level; (c) PepT 1 expression level. Means with different superscripts (a, b, c) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated treatment group; Cont = non-infected, non-medicated treated group.
3.6.2. Gene Expression Levels of Tight Junction Proteins
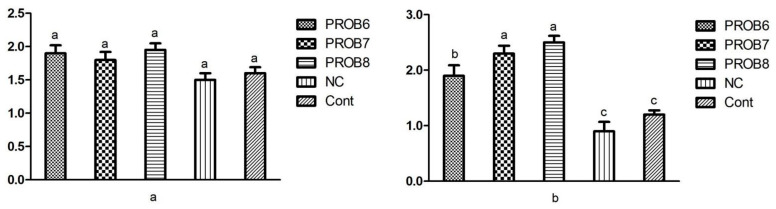
The expression levels of the junctional proteins are represented in Figure 8. A significant upregulation of the mRNA expression levels of ZO 1 was noted in the probiotic-supplemented groups in feed. Moreover, the supplementation with probiotics groups showed a significant (p < 0.05) upregulation of the expression of ZO 1 in comparison with the untreated challenged and untreated unchallenged groups (Figure 8b). However, the expression levels of the CLDN 1 showed no significant difference among various treatments groups (p > 0.05) (Figure 8a).
Figure 8.
mRNA expression levels of tight junction proteins in different treatment groups: (a) CLDN 1 expression level; (b) ZO 1 expression level. Means with different superscripts (a, b, c) are significantly different (p < 0.05). PROB6 = L. plantarum (1 × 106 CFU) supplemented group in feed; PROB7 = L. plantarum (1 × 107 CFU) supplemented group in feed; PROB8 = L. plantarum (1 × 108 CFU) supplemented group in feed; NC = infected, non-medicated treatment group; Cont = non-infected, non-medicated treated group.
4. Discussion
Due to drug-resistant issues, the current study was designed to investigate the antioxidant, immunomodulatory, and growth-promoting activities of probiotics. It evaluated the immune-boosting activities of L. plantarum based on probiotics in humoral and cellular immune responses. The higher cellular immune response to DNCB was observed in probiotic-supplemented groups. Among probiotic-supplemented groups, the maximum cell-mediated response was noted in the 1 × 108 CFU probiotic-supplemented chicken group, as compared with 1 × 107 CFU and 1 × 106 CFU probiotic-supplemented groups. L. plantarum (1 × 108 CFU in feed) showed a significant difference than that of the control group (p < 0.05). The capability of probiotics to stimulate natural killer cells, antigen-specific immune cells, and macrophages could be due to the increased cellular immune response [34]. In this study, antibody titers and IgG values of groups supplemented with probiotics (1 × 108 CFU in feed) and infected, non-medicated control group had notable statistically significant differences (p < 0.05), both at day 7 and 14 of post-SRBC treatment. Probiotics’ immunomodulatory activities can boost T-cell responses, stimulate phagocytosis, and increase Ig secretion [15]. The prominent immune response in the Lactobacillus supplemented groups agrees with a study [35], in which improved antibodies against E. tenella infection in the Pedicoccus-supplemented bird groups were reported.
Avian coccidiosis leads to intestinal damage by free radicals [36]. Further, reactive oxygen species (ROS) lead to necrosis and modification, including imbalance homeostasis in the intestinal tract of infected birds [37]. Even though birds have immune systems against different pathogens, local antioxidant defense systems are insufficient to prevent the pathogen from damage. Therefore, there is an urgent need to offer adequate supplements within the food having an immunomodulatory and antioxidant potential against the pathogen. In this regard, probiotics have potential as antioxidants due to having functions such as upregulation of antioxidase activities through superoxide dismutase (SOD), downregulation of reactive oxygen species (ROS) producing enzymes, and balancing the beneficial intestinal microbes against oxidative destruction [24]. The antioxidant enzymatic activity among various treatments was also examined in the present study. The study results suggested that the treatment with probiotics in the food resulted in an increased mRNA expression of SOD 1 and CAT, compared with the challenged and untreated control group. Previous studies have also reported that probiotics significantly increase the activities of various antioxidative enzymes against microbial infection [14,24]. Various antioxidant enzymes, including SOD 1 and CAT, are considered important in protecting cells against various stresses by degrading hydrogen peroxide and various superoxide anions [38]. Lower SOD activities in the challenged and untreated control group were probably due to higher production of ROS or low antioxidant performance, resulting in cell destruction and death [36].
Proteins associated with tight junctions such as zonula occludins (ZO), claudin (CLDN), and occluding (OCLN) have essential roles in maintaining the epithelial cell barrier of the intestine. This mechanism is crucial for the defense of host intestines from various pathogenic microbes [39]. In the current study, the group challenged with E. tenella but untreated significantly decreased the expression of ZO1 and CLDN1 rather than that of other probiotic-supplemented groups. These results are in agreement with earlier studies showing that the treatment with probiotics alone or in combination with herbs significantly improved the barrier function of intestines by enhancing the functional performance of different junctional proteins [40,41]. Therefore, the supplementation of feed with probiotics can prevent the adverse effects of Eimeria infection on birds’ growth performance and intestinal functions. Probiotics are also associated with enhancing the antioxidant enzymes’ activity, increasing the regulation of pro-inflammatory cytokines, and tight junctional proteins against pathogens [42].
Different genera of yeast and bacteria-based probiotics, such as Saccharomyces, Lactobacillus, Streptococcus, Pedicoccus, and Bifidobacterium, have shown favorable results against pathogens in various animal models [15,16]. Probiotics are bacterial or yeast-based products that improve the microbial balance of intestines and, directly or indirectly, contribute to positive effects on different animals’ production and reproduction potential and immune systems. Mainly, they are used for the treatment of digestive tract problems and respiratory system issues. In the E. tenella challenge, poor FCR and growth performance were possibly due to enormous intestinal damage that occurs with severe infection and causes nutrient malabsorption and homeostasis [6]. Certain nutrients shift from growth to immune response, resulting in a major difference in infected birds’ growth performance from normal birds. Probiotics have essential roles in controlling intestinal diseases [4]. Here, the curative effects of L. plantarum on the pathogenesis of E. tenella were examined by using different parameters such as fecal scores, OPG, and FCR. The positive effects of bacteria and yeast-based probiotics against Eimeria parasites have also been reported in a previous study [15]. Our trial focused on the effects of L. plantarum against the E. tenella challenge, unlike past research. However, supplementation with probiotics does not significantly improve Eimeria infected chicken immunity and body weight [4,43]. Differences may be due to different parameters that may amendment the potential of probiotics, such as probiotics preparation methodologies, the difference in probiotics strains (bacteria, yeast), and probiotics dosage. Other contributors may be environmental stress (temperature), bird age, diet plan, and poultry farm hygienic conditions [43,44].
The oocysts per gram count were considerably lower in chicken groups supplemented with L. plantarum. Corroborating our results, Lactobacillus supplemented broilers shed fewer oocysts than the E. acervulina-infected group [45]. Lower oocysts count may be due to increased production and performance of CD4+ and CD8+ T lymphocytes in the probiotic-supplemented birds [4]. The fewer oocysts and fecal score can also be correlated to probiotics, which may improve intestinal growth by enhancing host immunity, acting as antioxidants, balancing beneficial microbes, and competitive exclusion of pathogens [6,14]. The oocyst reduction, fewer fecal scores in dropping, and improved FCR may be due to improved hemopoietic effects of probiotic-supplemented groups, compared with the infected, non-medicated group, as evinced by a previous study [46].
5. Conclusions
The concept of using probiotics as substitutes for antiparasitic drugs is a promising approach to controlling coccidiosis, as shown by a recent trial. Due to drug resistance, the use of probiotics is of great importance. Anticoccidial effects of probiotics were demonstrated in reducing pathogenic impacts by acting as antioxidants and enhancing host immunity, as well as their essential role in improving the integrity of the digestive route. Further experiments are necessary to examine probiotics exact mode of action with different strains and doses of probiotics. Research evaluating omics (mostly genomics, transcriptomic, proteomics, and metabolomics) and gene expression within immune tissues, microbial profiles, histological discrepancy, and other assessable parameters will further confirm the mode of action of probiotics.
Author Contributions
M.M.: Carried out the experiment, methodology, original draft. G.Y.: Funding acquisition, Project administration, review & editing. Z.Z.: supervised the project, Project administration, Funding acquisition, review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study has been funded by the Natural Science Foundation of China (No. 31872466), grants from the Fujian Science and Technology Department (2021J01083 and 2019N0005), and the Fujian Modern Poultry Industry Technical System Construction Project (2019–2022), a start-up fund for Ziping Zhang (712018R0402), and a special grant for the Leading Talents in Colleges and Universities of Fujian for Ziping Zhang (660160010).
Institutional Review Board Statement
All animal experiments were carried out follow the review and approval of the Committee on Experimental Animal Welfare and Ethics of Fujian Agriculture and Forestry University (FAFU2021-3-12).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macpherson A.J., Harris N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 2.Lin X., Mohsin M., Abbas R.Z., Li L., Chen H., Huang C., Li Y., Goraya M.U., Huang Z., Yin G. Evaluation of immunogenicity and protective efficacy of Eimeria maxima immune mapped protein 1 with EDA adjuvant in chicken. Pak. Vet. J. 2020;40:209–213. doi: 10.29261/pakvetj/2020.043. [DOI] [Google Scholar]
- 3.Mohsin M., Li L., Huang X., Aleem M.T., Habib Y.J., Ismael A. Immunogenicity and Protective Efficacy of Probiotics with EtIMP1C against Eimeria tenella Challenge. Pak. Vet. J. 2021;41:274–278. [Google Scholar]
- 4.Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- 5.Abbas A., Abbas R.Z., Khan M.K., Raza M.A., Mahmood M.S., Saleemi M.K., Hussain T., Khan J.A., Sindhu Z. Anticoccidial effects of Trachyspermum ammi (Ajwain) in broiler chickens. Pak. Vet. J. 2019;39:301–304. doi: 10.29261/pakvetj/2019.056. [DOI] [Google Scholar]
- 6.Ritzi M.M., Abdelrahman W., Van-Heerden K., Mohnl M., Barrett N.W., Dalloul R.A. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 2016;47:1–8. doi: 10.1186/s13567-016-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Huang C., Chen Y., Mohsin M., Li L., Lin X., Huang Z., Yin G. Efficacy of recombinant N-and C-terminal derivative of EmIMP1 against E. maxima infection in chickens. Br. Poult. Sci. 2020;61:518–522. doi: 10.1080/00071668.2020.1759787. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan M., Elmadway R., Lashin A., ELdiarby A. Immunization of lambs against coccidiosis by using ultraviolet irradiated Eimeria oocysts for the first time. Benha. Med. J. 2018;34:246–258. doi: 10.21608/bvmj.2018.44749. [DOI] [Google Scholar]
- 9.Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Chapman H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- 11.Chapman H., Cherry T., Danforth H., Richards G., Shirley M., Williams R. Sustainable coccidiosis control in poultry production: The role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/S0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- 12.Mohsin M., Li Y., Zhang X., Wang Y., Huang Z., Yin G., Zhang Z. Development of CRISPR-CAS9 based RNA drugs against Eimeria tenella infection. Genomics. 2021;113:4126–4135. doi: 10.1016/j.ygeno.2021.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Abbas A., Iqbal Z., Abbas R.Z., Khan M.K., Khan J.A. Immunomodulatory activity of Pinus radiata extract against coccidiosis in broiler chicken. Pak. Vet. J. 2017;37:145–149. [Google Scholar]
- 14.Mohsin M., Abbas R.Z., Yin G., Sindhu Z.-U.-D., Abbas A., Huang Z., Aleem M.T., Saeed Z., Afzal M.Z., Ejaz A. Probiotics as therapeutic, antioxidant and immunomodulatory agents against poultry coccidiosis. World’s Poult. Sci. J. 2021;77:1–15. doi: 10.1080/00439339.2021.1883412. [DOI] [Google Scholar]
- 15.Awais M.M., Jamal M.A., Akhtar M., Hameed M.R., Anwar M.I., Ullah M.I. Immunomodulatory and ameliorative effects of Lactobacillus and Saccharomyces based probiotics on pathological effects of eimeriasis in broilers. Microb. Pathog. 2019;126:101–108. doi: 10.1016/j.micpath.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Ohimain E.I., Ofongo R.T. The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: A review. Int. J. Anim. Vet. Adv. 2012;4:135–143. [Google Scholar]
- 17.Collins M.D., Gibson G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 18.Simmering R., Blaut M. Pro-and prebiotics—The tasty guardian angels? Appl. Microbiol. Biotechnol. 2001;55:19–28. doi: 10.1007/s002530000512. [DOI] [PubMed] [Google Scholar]
- 19.Pourakbari M., Seidavi A., Asadpour L., Martínez A. Probiotic level effects on growth performance, carcass traits, blood parameters, cecal microbiota, and immune response of broilers. An. Da Acad. Bras. Ciências. 2016;88:1011–1021. doi: 10.1590/0001-3765201620150071. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado Galdeano M.C., Lemme Dumit J.M., Thieblemont N., Carmuega E., Weill R., Perdigon G.d.V. Stimulation of Innate Immune Cells Induced by Probiotics: Participation of Toll-Like Receptors. 2015. [(accessed on 4 January 2022)]. Available online: https://www.longdom.org/open-access/stimulation-of-innate-immune-cells-induced-by-probiotics-participation-of-toll-like-receptors-2155-9899-1000283.pdf.
- 21.Azad M., Kalam A., Sarker M., Wan D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018;2018:8063647. doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West C.E., Gothefors L., Granström M., Käyhty H., Hammarström M.L.K., Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr. Allergy Immunol. 2008;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 23.Mishra V., Shah C., Mokashe N., Chavan R., Yadav H., Prajapati J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015;63:3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., Wang Y., Li W. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tewari A., Maharana B. Control of poultry coccidiosis: Changing trends. J. Parasit. Dis. 2011;35:10–17. doi: 10.1007/s12639-011-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Praharaj I., John S.M., Bandyopadhyay R., Kang G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140144. doi: 10.1098/rstb.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakelin D., Rose M.E. Coccidiosis of Man and Domestic Animals. CRC Press; Boca Raton, FL, USA: 2019. Immunity to coccidiosis; pp. 281–306. [Google Scholar]
- 28.Yadav S., Teng P.-Y., Dos Santos T.S., Gould R.L., Craig S.W., Fuller A.L., Pazdro R., Kim W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020;99:5936–5945. doi: 10.1016/j.psj.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillehoj H.S., Lillehoj E.P. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. 2000;44:408–425. doi: 10.2307/1592556. [DOI] [PubMed] [Google Scholar]
- 30.Yin G., Qin M., Liu X., Suo J., Tang X., Tao G., Han Q., Suo X., Wu W. An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 agonist Salmonella typhimurium FliC flagellin. Biochem. Biophys. Res. Commun. 2013;440:437–442. doi: 10.1016/j.bbrc.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 31.Ministry of Agriculture Großbritannien . Manual of Veterinary Parasitological Laboratory Techniques: 160 S.: Ill. HM Stationery Office; London, UK: 1986. 129p [Google Scholar]
- 32.Youn H.-J., Kang Y.-B., Jang D.-H. Effects of γ-irradiation from cobalt-60 on pathogenicity of Eimeria tenella. Korean J. Vet. Res. 1993;33:649–655. [Google Scholar]
- 33.Bleumink E., Nater J.P., Koops H.S., The T. A standard method for DNCB sensitization testing in patients with neoplasms. Cancer. 1974;33:911–915. doi: 10.1002/1097-0142(197404)33:4<911::AID-CNCR2820330404>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Ashraf R., Shah N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014;54:938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- 35.Lee S., Lillehoj H.S., Park D.W., Hong Y.H., Lin J. Effects of Pediococcus-and Saccharomyces-based probiotic (MitoMax®) on coccidiosis in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2007;30:261–268. doi: 10.1016/j.cimid.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Abbas R., Iqbal Z., Mansoor M., Sindhu Z., Zia M., Khan J. Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J. 2013;33:401–407. [Google Scholar]
- 37.Idris M., Abbas R., Masood S., Rehman T., Farooq U., Babar W., Hussain R., Raza A., Riaz U. The potential of antioxidant rich essential oils against avian coccidiosis. World’s Poult. Sci. J. 2017;73:89–104. doi: 10.1017/S0043933916000787. [DOI] [Google Scholar]
- 38.Shen X., Yi D., Ni X., Zeng D., Jing B., Lei M., Bian Z., Zeng Y., Li T., Xin J. Effects of Lactobacillus plantarum on production performance, immune characteristics, antioxidant status, and intestinal microflora of bursin-immunized broilers. Can. J. Microbiol. 2014;60:193–202. doi: 10.1139/cjm-2013-0680. [DOI] [PubMed] [Google Scholar]
- 39.Schneeberger E.E., Lynch R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 40.Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., Zuo W., Li F. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning. J. Anim. Sci. 2019;97:1693–1700. doi: 10.1093/jas/skz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memon F., Yang Y., Lv F., Soliman A., Chen Y., Sun J., Wang Y., Zhang G., Li Z., Xu B. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J. Appl. Microbiol. 2021;131:425–434. doi: 10.1111/jam.14928. [DOI] [PubMed] [Google Scholar]
- 43.Mountzouris K., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- 44.Crittenden R., Bird A.R., Gopal P., Henriksson A., Lee Y., Playne M. Probiotic research in Australia, New Zealand and the Asia-Pacific region. Curr. Pharm. Des. 2005;11:37–53. doi: 10.2174/1381612053382304. [DOI] [PubMed] [Google Scholar]
- 45.Dalloul R.A., Lillehoj H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49:1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M., Mustari A., Salauddin M., Rahman M. Effects of probiotics and enzymes on growth performance and haematobiochemical parameters in broilers. J. Bangladesh Agril. Univ. 2013;11:111–118. doi: 10.3329/jbau.v11i1.18221. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article.