Abstract
Immunosuppression regimens used in solid organ transplant have evolved significantly over the past 70 years in the United States. Early immunosuppression and targets for allograft success were measured by incidence and severity of allograft rejection and 1-year patient survival. The limited number of agents, infancy of human leukocyte antigen (HLA) matching techniques and lack of understanding of immuno-reactivity limited the early development of effective regimens. The 1980s and 1990s saw incredible advancements in these areas, with acute rejection rates halving in a short span of time. However, the constant struggle to achieve the optimal balance between under- and overimmunosuppression is weaved throughout the history of transplant immunosuppression. The aim of this paper is to discuss the different eras of immunosuppression and highlight the important milestones that were achieved while also discussing this in the context of rational agent selection and regimen design. This discussion sets the stage for how we can achieve optimal long-term outcomes during the next era of immunosuppression, which will move from universal protocols to patient-specific optimization.
Keywords: immunosuppression, solid
1 |. HISTORIC PERSPECTIVE (1954–2010)
The first successful human-to-human transplant occurred on December 23, 1954, in the United States, when Dr. Murray performed a living donor kidney transplant from one identical twin brother to his sibling. It is now known that this innovation was successful because the brothers had identical human leukocyte antigen (HLA) types and thus allograft recognition in the recipient as non-self was not possible.1,2 This marked what most consider the start of the modern era of organ transplantation.2 Our knowledge of the recipient’s immune response to mismatched HLA from the donor organ grew rapidly, largely driven by the work from Terasaki, Calne, and Startzl.2 This led to numerous achievements in the years to follow, both within kidney transplantation and beyond; successful solid organ transplants for heart (1963),3 lung (1964),3 pancreas (1966),4 and liver (1968)5 occurred in the years that followed Dr. Murray’s report.1,2 Over the next 50 years, remarkable surgical and medical advances ensued within this fledgling field, transforming organ transplantation from what most considered “experimental” in the 1950s and 1960s to becoming the gold-standard treatment option of end-organ diseases.2 This first section will present a general review of the innovations that have occurred regarding the development and implementation of immunosuppression regimens between in the early 1960s to 2010.
1.1 |. Induction immunosuppression
Initially, antibody preparations targeted against human lymphocytes (either polyclonal or monoclonal lymphocyte depleting therapies) were not utilized as prophylaxis induction; rather, they were primarily given to treat rejection episodes, which were common in the 1970s, before the advent of potent maintenance oral immunosuppression.2,6–8 In 1977, Thomas and colleagues published results of a randomized controlled trial in 71 kidney transplant recipients, demonstrating induction therapy with rabbit antilymphocyte globulin (rATG) improved graft survival from 42% in controls to 78% in the intervention arm at a mean of 18 months posttransplant (p < 0.05).9 However, in these early studies using ATG preparations for induction in combination with azathioprine with or without steroids, there were mixed results; several studies failed to demonstrate significant reductions in rejection rates or improved graft survival.10–13 In the 1980s, cytolytic antibody induction using either polyclonal preparations (rATG and horse ATG [hATG]) or monoclonal therapy (muromonab-CD3) demonstrated reductions in acute rejection, with some studies demonstrating improved 1-year graft survival as well.14,15 As potent maintenance immunosuppression regimens took hold in transplantation, the widespread use of induction therapy diminished in the 1980s through the mid-1990s. This is true not only for kidney transplant recipients, but also within liver, heart, and lung transplantations.16
In the mid to late 1990s, the use of antibody induction therapy in kidney transplantation began to reemerge as a common modality used to prevent rejection and improve outcomes.7,12,15–17 This was driven by several innovative therapies gaining US Food and Drug Administration (FDA) approval, particularly anti-CD25 antibodies (basiliximab and daclizumab), which were tested in large-scale studies demonstrating safety and efficacy. The anti-CD25 antibody therapies represented a new class of agents that, for the first time, were not cytolytic, but rather blunted the activation and propagation of T-helper cells. These agents were considered less potent than cytolytic therapies (rATG, hATG, and muromonab-CD3), but also safer and better tolerated with a lower risk of infections, particularly cytomegalovirus (CMV).13,16,18,19 There were several pivotal trials demonstrating promising outcomes. The study by Kahan and colleagues20 (Table 1), which compared basiliximab to placebo with all receiving cyclosporine and prednisone maintenance immunosuppression, reduced rejection rates from 49% in the control arm to 35% in the intervention arm. Graft survival was excellent in both arms, whereas CMV infections were slightly higher in the control arm, attributed to steroid boluses to treat the rejections.19 Basiliximab therapy also demonstrated lower rejection rates with triple maintenance immunosuppression (cyclosporine, prednisone, and azathioprine or mycophenolate) with similar rates of infection and adverse drug events, as compared with placebo.16 Although daclizumab demonstrated similar results with both 2 and 5-dose regimens, in 2009, it was withdrawn from the market for use in transplantation by Roche, related to a business decision.16,21
TABLE 1.
Select pivotal RCTs from 1970 to 2010 in kidney transplantation
| Citation | Year | Regimen(s) | Antibody induction | End point | Rejection rate | Graft survival | CMV infection | CMV prophylaxis |
|---|---|---|---|---|---|---|---|---|
| Hakala et al30 | 1983 | CyA + Pred vs. AZA + Pred | None | 1 year | 10% vs. 45% | 91% vs. 56%* | 10% vs. 0% | None |
| Sollinger37 | 1995 | CyA + MMF + Pred vs. CyA + AZA + Pred | ATG | 6 months | 23% vs. 45%* | 95% vs. 91% | 24% vs. 21% | None |
| Pirsch et al34 | 1997 | Tac + AZA + Pred vs. CyA + AZA + Pred | ATG | 1 year | 31% vs. 46%* | 91% vs. 88% | 20% vs. 19% | None |
| Brennan et al18 | 1999 | CyA + AZA + Pred | rATG vs. hATG | 1 year | 4% vs. 25%* | 98% vs. 96% | 13% vs. 33%* | Ganc |
| Kahan et al20 | 1999 | CyA + Pred | Bas vs. placebo | 1 year | 35% vs. 49%* | 95% vs. 93% | 7% vs. 9% | NR |
| Miller et al38 | 2000 | Tac + MMF + Pred vs. Tac + AZA + Pred | ATG | 1 year | 9% vs. 32%* | 95% vs. 95% | 7% vs. 5% | Per center |
| Vitko et al40 | 2004 | CyA + Evr + Pred vs. CyA + MMF + Pred | None | 1 year | 21% vs. 24% | 92% vs. 91% | 5% vs. 20%* | Per center |
| Mendez et al41 | 2005 | Tac + Sir + Pred vs. Tac + MMF + Pred | Per center for DGF | 1 year | 13% vs. 11% | 91% vs. 94% | NR | Per center |
| Vincenti et al42 | 2010 | Bel + MMF + Pred vs. CyA + MMF + Pred | Bas | 1 year | 19% vs. 7%* | 95% vs. 93% | 7% vs. 9% | Per center |
Abbreviations: ATG, antithymocyte globulin; AZA, azathioprine; Bas, basiliximab; Bel, belatacept; CMV, cytomegalovirus; CyA, cyclosporine; DGF, delayed graft function; Evr, everolimus; Ganc, ganciclovir; hATG, horse antithymocyte globulin; MMF, mycophenolate; MP, methylprednisolone; NR, not reported; Pred, prednisone; rATG, rabbit antithymocyte globulin; RCT, randomized controlled trial; Sir, sirolimus; Tac, tacrolimus.
p < 0.05.
The late 1990s also saw the dramatic rise in rATG use for induction therapy in the United States. This agent was long-approved in Europe, but only received FDA approval for use in the United States in 1998. Promising data were produced from several randomized controlled trials (RCTs). The most notable was the study by Brennan and colleagues (Table 1), which demonstrated a 4% 1-year acute rejection rate with rATG induction, as compared with 25% in those receiving hATG. Graft survival was similar, but CMV infection rates were two-thirds lower in the rATG arm. All received cyclosporine, azathioprine, and prednisone maintenance therapy.18 The bottom panel of Figure 122,23 displays induction therapy use of hATG, rATG, and basiliximab from 1995 to 2004 in kidney transplantation. By the end of this 10-year period, the majority of kidney recipients were receiving some form of antibody induction; whereas the use of hATG fell out of favor, rATG became the predominant agent of choice, followed by basiliximab and daclizumab (before withdrawal from the market).19,21,22 This trend continues today, with the vast majority of kidney transplant recipients receiving antibody induction therapy.23 Antibody induction therapy in other solid organ transplant recipients differs substantially, both historically and in current practice. Very few liver recipients receive induction therapy, whereas heart and lung recipients receive less than kidneys, but more than livers. Anti-CD25 agents are not commonly used in thoracic transplantation, and the agent of choice was, and continues to be, rATG.15,24–26
FIGURE 1.
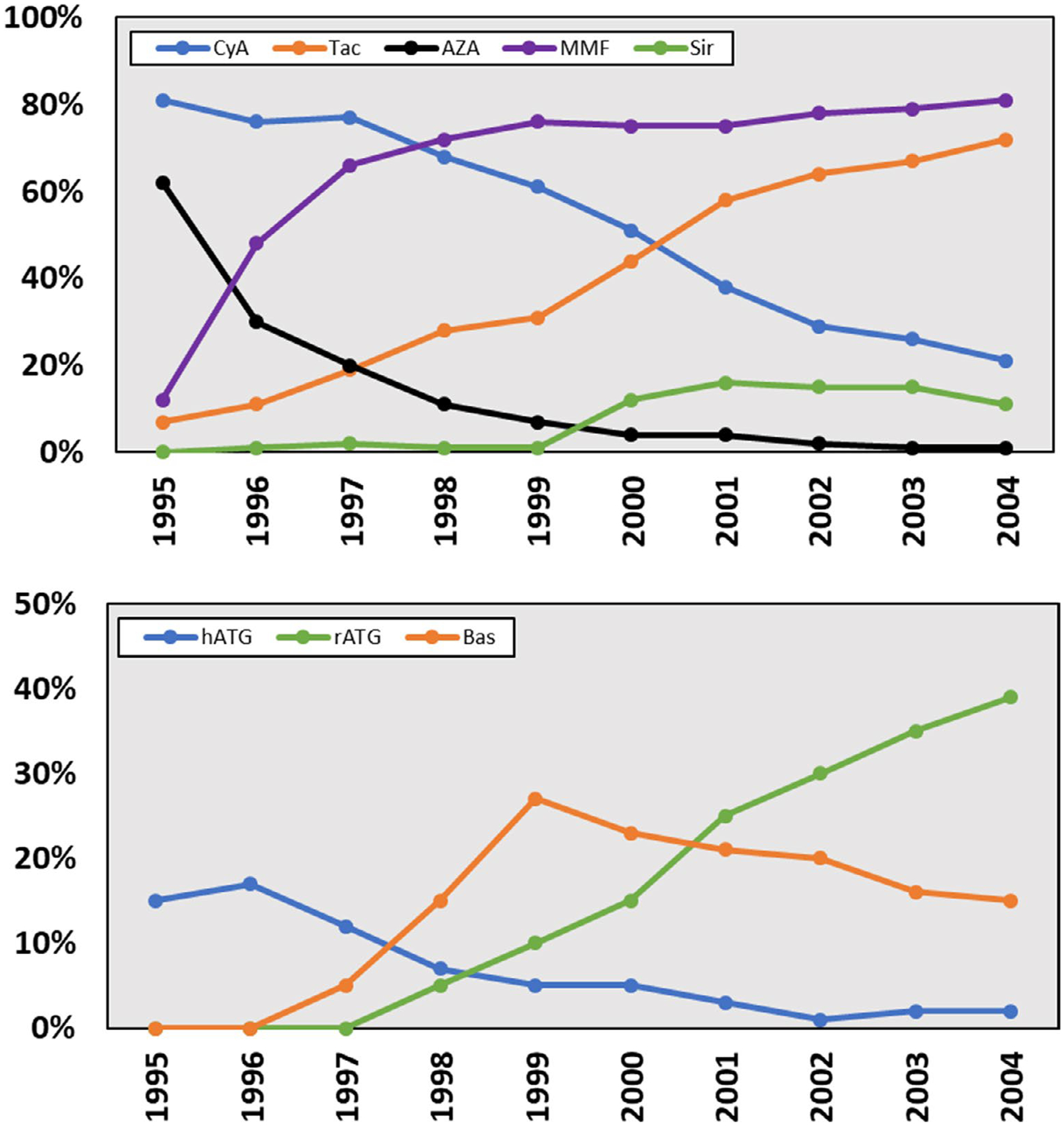
Maintenance (top panel) and induction (bottom panel) immunosuppression utilization in US Kidney Transplant Recipients 1995 to 2004.22,24–26 AZA, azathioprine; Bas, basiliximab; CyA, cyclosporine; hATG, horse antilymphocyte globulin; hATG, horse antithymocyte globulin; MMF, mycophenolate; rATG, rabbit antilymphocyte globulin; rATG, rabbit antithymocyte globulin; Sir, sirolimus; Tac, tacrolimus
1.2 |. Maintenance immunosuppression
Before the arrival of cyclosporine in the late 1970s, maintenance immunosuppression consisted of azathioprine and corticosteroids. Splenectomy, radiation, and chemotherapeutic agents were also used to enhance the marginal efficacy of these agents.2 One-year rejection rates were high, reported anywhere from 40% to 60%; graft survival was poor, with 1-year rates reported from 20% to 80%, depending on the study and transplant type. Transplantation was considered experimental due to these poor outcomes.2,5 In 1978, Calne and colleagues27 reported the first use of cyclosporine in human transplantation; reporting results of use in 7 kidney recipients with excellent outcomes. These encouraging results led to the completion of 2 large-scale multicenter trials; both demonstrated dramatic improvements in 1-year acute rejection rates and graft survival. Acute rejection was reduced in half, whereas graft survival increased from 50% to 60% to nearly 80%.28,29 The study by Hakala and colleagues30 produced some of the most impressive results (Table 1). This study compared cyclosporine and prednisone with azathioprine and prednisone in 41 kidney recipients and demonstrated a 35% absolute reduction in 1-year acute rejection and graft loss rates (10% vs. 45% for acute rejection and 91% vs. 56% for graft survival, respectively).30 Following this, studies in heart, lung, and liver transplantation produced more impressive results.31 Cyclosporine emerged as a remarkable advancement in the field of transplantation, achieving outcomes that most considered infeasible just years before. It became the gold-standard treatment in all of solid organ transplantation until the early 2000s when it was replaced by tacrolimus.31
In the 1990s, tacrolimus emerged as a potent alternative to cyclosporine. This research was spearheaded by Starzl and Fung in liver transplantation. Tacrolimus (FK506) demonstrated remarkable efficacy in reversing liver transplant rejection and prolonging graft survival in those refractory to cyclosporine.32 A large body of experience from Pittsburgh led to the common use of this agent in liver transplantation, followed by use in kidney, then in later years, heart and lung transplantation.33 In a large-scale RCT published by Pirsch and colleagues in 1997, tacrolimus was compared with cyclosporine in kidney recipients also receiving azathioprine, prednisone, and ATG induction. Rejection rates were 15% lower in the tacrolimus arm (31% vs. 46%), whereas graft survival (91% vs. 88%) and CMV infection (20% vs. 19%) were similar.34 These studies were followed by additional multicenter RCTs demonstrating reduced rejection rates with tacrolimus-based therapy, as compared with cyclosporine.32,35 Based on these trials, tacrolimus utilization took hold in transplantation. By 1999, it was utilized in more than 30% of US kidney transplant recipients and by 2001 it overtook cyclosporine as the predominant calcineurin inhibitor (CNI, top panel of Figure 1).22,23 Routine use in liver recipients outpaced kidney transplantation; whereas tacrolimus use in thoracic transplantation lagged.22,23,25 However, by 2009, tacrolimus was used in greater than 90% of all organ transplant recipients.24,26
Another major advance in maintenance immunosuppression therapy coincided with tacrolimus development. Mycophenolate, an antiproliferative specific to lymphocyte production, was studied for use in organ transplantation in the mid-1990s; first in kidney transplantation and later in liver, heart, and lung transplantations.36 Sollinger and colleagues published results of a multicenter RCT comparing mycophenolate with azathioprine in 1995 (Table 1). All received cyclosporine, prednisone, and ATG induction. Six-month rejection rates were 22% lower in the mycophenolate arm (23% vs. 45%), whereas graft survival and CMV infections were similar.37 In 2000, Miller and colleagues conducted a similar RCT, using tacrolimus in place of cyclosporine (Table 1). Rejection rates were 9% in the mycophenolate arm, as compared with 32% in the azathioprine arm. For the first time in a large-scale RCT, 1-year rejection rates were less than 10% and graft survival was 95% in both arms.38 These results are considered the benchmark to which contemporary kidney transplant outcomes are now measured. Similar improvements in efficacy, as compared with azathioprine, were later achieved in liver, heart, and lung transplantations.36 Based on these data, mycophenolate use across the United States rapidly expanded in the late 1990s. By 1997, it overtook azathioprine as the predominant adjunct agent and was utilized in 80% of kidney transplant recipients by 2000 (see top panel of Figure 1).22,24–26
In the late 1990s, the mammalian target of rapamycin (mTOR) inhibitors, sirolimus and everolimus, were studied in kidney transplantation. When initially used with cyclosporine, results were mixed; rejection rates tended to be lower, but toxicities were common.39,40 Later, in 2005, Mendez and colleagues demonstrated low rejection rates when used in combination with tacrolimus, as compared with mycophenolate and tacrolimus.41 However, due to several significant issues, including impaired wound healing, proteinuria, and edema, mTOR therapy has never been commonly utilized as maintenance therapy in transplantation (top panel of Figure 1).22,24–26,39 Toward the end of the 2000s, belatacept therapy was assessed for use in kidney transplantation. This is the first antibody used as chronic maintenance immunosuppression in transplantation. It is a costimulatory blocker, inhibiting the second signal required for T-cell activation. Vincenti and colleagues published the results of the BENEFIT study in 2010 (Table 1), which compared belatacept with cyclosporine; all received mycophenolate, prednisone, and basiliximab induction. One-year rejection rates were higher in the belatacept group (19% vs. 7%), whereas graft survival and CMV infections were similar. Importantly, graft function was improved in the belatacept group, attributed to the lack of nephrotoxicity with this agent, as compared with cyclosporine.42,43 This study perhaps represented a paradigm shift in transplantation; accepting higher acute rejection rates with non-CNI-based regimens to avoid the plethora of debilitating drug toxicities. Belatacept use, both within kidney and other organ transplant types, has yet to become widespread. However, use has grown as clinicians continue to attempt to strike the balance between reducing rates of acute rejection while minimizing the long-term sequelae of immunosuppression toxicities.44,45
1.3 |. The balance between under and overimmunosuppression
From the advent of cyclosporine in the early 1980s through the mid to late 1990s, the primary goal of immunosuppression therapy was to reduce acute rejection rates to prolong allograft survival. During this period, remarkable advances in therapies produced dramatic reductions in acute rejection and improvements in 1-year graft survival. Figure 2 displays 1-year acute rejection rates in kidney transplant recipients from 1993 to 2010; demonstrating three distinct phases. Before 1996, rejection rates were mostly stagnant, hovering between 30% and 50%. The 4-year period from 1997 to 2000 demonstrated a dramatic reduction in rejection from 40% to 20%; this coincided with substantial increases in the use of potent immunosuppression, including tacrolimus, mycophenolate, and antibody induction across US-based transplant centers (Figure 1). From 2000 to 2010, the third phase represented a prolonged slow decline in 1-year acute rates from 20% to less than 10%, which is now considered the contemporary standard.20 Rejection rates in other organ transplant types followed similar trends.24–26 Long-term graft survival also improved, but not to the same extent.17,44,46–48 This is attributed to allograft injury and chronic toxicities from CNI, as well as graft loss from death due to infections and malignancies resulting from overimmunosuppression. The dogma during this period was to use immunosuppression to dial down rejection rates as low as humanly possible, making rejection a rare event; the inevitable consequence was that most transplant recipients were chronically overimmunosuppressed. Starting in the mid-2000s, transplant clinicians attempted to strike a better balance between keeping rejection rates low while minimizing the impact of overimmunosuppression and drug toxicities, with mixed results.42,49 By 2010, death with a functioning allograft was the most common cause of graft loss, driven primarily by infections, cardiovascular events, and malignancies. These are well-known long-term complications of immunosuppressive therapy, particularly with CNI-based regimens.47,49 Thus, although we have achieved very low acute rejection rates at 1-year posttransplantation, this has likely led to a state of net overimmunosuppression in the majority of our transplant recipients leading to suboptimal long-term outcomes.
FIGURE 2.
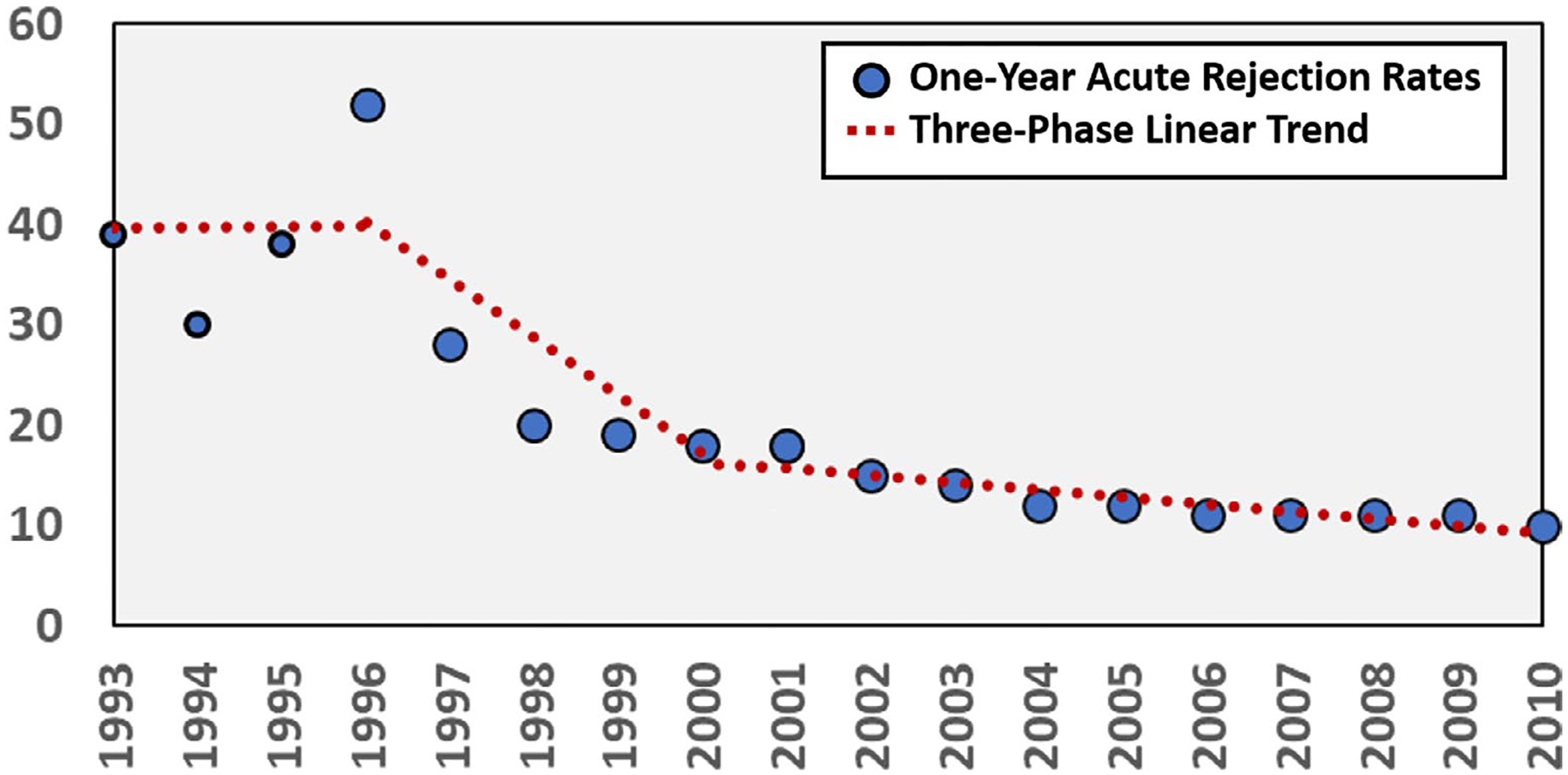
One-year acute rejection rates in kidney transplant recipients from 1993 to 2010 demonstrated a 3-phase trend
2 |. LANDSCAPE CHANGES WITH REGULATION (2011–PRESENT)
Throughout the latter part of the 2000s, the transplant community was transformed by a new set of regulations published by the Centers for Medicare and Medicaid Services (CMS) requiring transplant centers to adhere to minimum volume and 1-year patient and outcome requirements to receive payment. This in conjunction with the provision in most third-party payor coverage plans, which states that the transplant center must be CMS certified for beneficiaries to receive a transplant at the center created an environment of calculated risk.50 Selection criteria for both recipients and donors became tighter especially if centers were experiencing outcome anomalies that could lead to flagging and loss of CMS coverage. The intended beneficial consequences of minimum outcomes requirements meant that sicker patients were being denied transplantation based on their center’s performance and more organs were being discarded secondary to quality.50 This past decade also saw dramatic changes in organ allocation with the intent to improve access and equity of organ access (see Figure 3).
FIGURE 3.
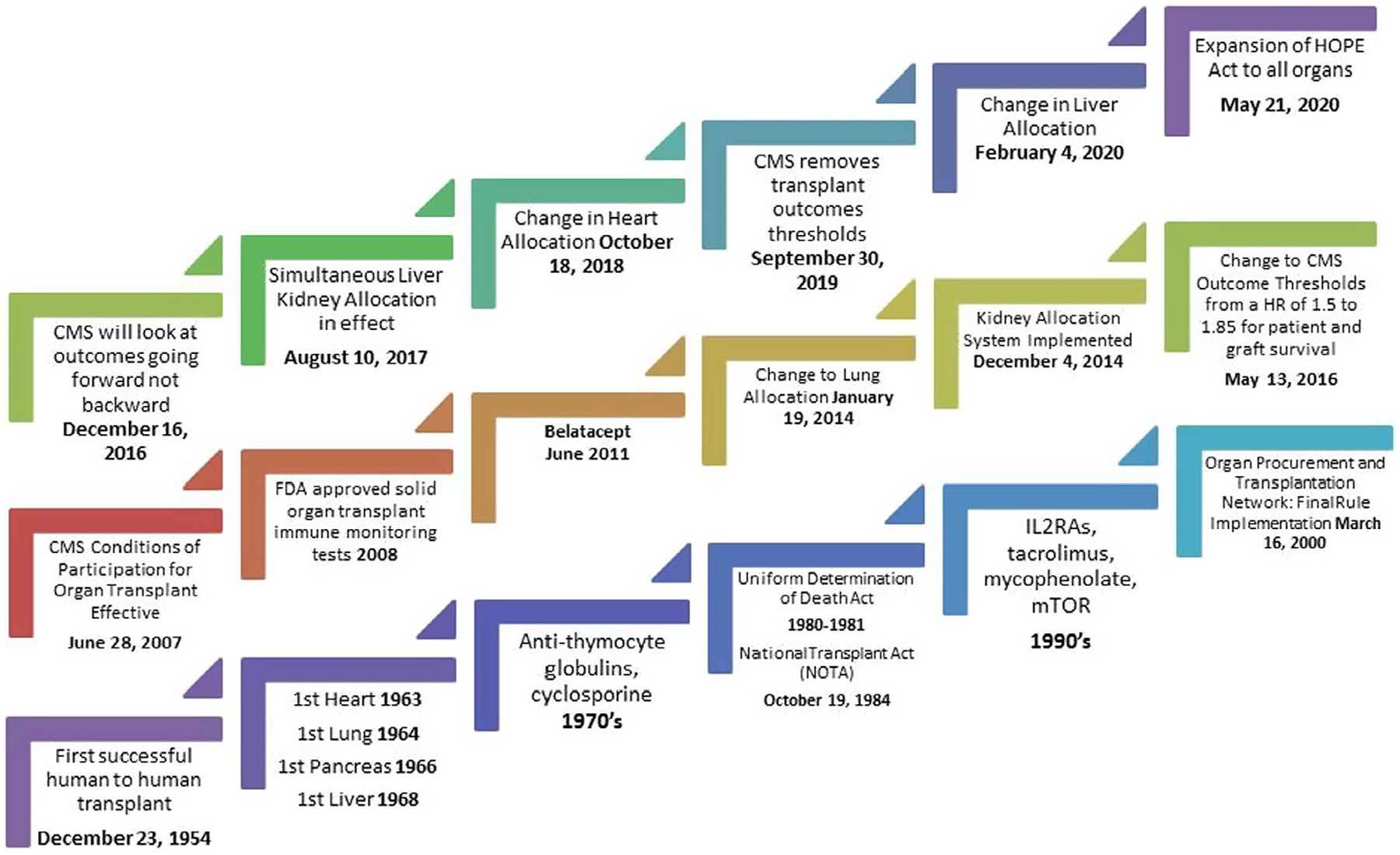
Solid organ transplant. A brief selected history lesson of significant events based on review of the literature, which outline major regulatory and immunosuppression milestones that have impacted outcomes. This figure does not include the major milestones of organ preservation or associated technology or the extensive advances related to infection over the past decades. Regulatory milestones can be accessed through www.cmc.gov/ (accessed June 9, 2020); https://www.organdonor.gov/about/facts-terms/history.html (accessed August 4, 2020). CMS, Centers for Medicare and Medicaid Services; FDA, US Food and Drug Administration; HOPE, Heart Outcomes Prevention Evaluation; mTOR, mammalian target of rapamycin
After 12 years, the minimum volume and outcome requirements were removed in September of 2019 for CMS reapproval of transplant centers but the impact of the deregulation by presidential executive order is unknown.50 Oversite of outcomes and patients safety still resides with the United Network for Organ Sharing (UNOS), as per their contract with the Organ Procurement and Transplantation Network of The US Department of Health and Human Services.50
3 |. CURRENT INDUCTION
The approaches to induction therapy after 2010 and beyond was specifically aimed at maintenance immunosuppression optimization to decrease the risks of underimmunosuppression (e.g., rejection) and overimmunosuppression (e.g., infection) balanced with mitigation of concurrent medication-related adverse effects (e.g., hyper-glycemia) and organ dysfunction (e.g., renal failure) at the time of transplantation. Several strategies were used through the last decade, specifically strategies to reduce steroid exposure, CNI minimization or avoidance, overcoming immunologic barriers, and expanding the donor pool. The collective goal of these strategies (Table 2) is to promote long-term patient and allograft survival beyond the traditional 1-year benchmark. The recognition and impact of polyomavirus in the renal allograft was also realized during this decade as a potential marker of overimmunosuppression.51 Additional advances during this time include the advent of direct acting antivirals for hepatitis C and more treatment options for CMV and fungal infections. There was also the development of more novel maintenance immunosuppression strategies and immune monitoring, all of which have allowed for broader use of donors and the acceptance of more challenging recipients.
TABLE 2.
Current perspective induction strategies – select pivotal trials
| Pivotal trial | Organ | Induction | Design | Design | Outcome |
|---|---|---|---|---|---|
| Hanaway et al (INTAC)59 | Kidney | A vs. IL2RA low risk BPAR A vs. rATG high risk BPAR kidney | CSWD day 5 + MMF + FK | Prospective, open label, risk stratified, multicenter RCT | 1 yr BPAR: A vs. IL2RA (3% vs. 20%, p < 0.001), A vs. rATG (10% vs. 13%, p = 0.58) |
| Thomusch etal (HARMONY)90 | Kidney | IL2RA and rATG low risk | Group A: IL2RA + C + MMF + prolonged release FK, group B: IL2RA or group C: rATG + CSWD + prolonged release FK+ MMF | Prospective, open label, multicenter, parallel arm | 1 year BPAR: group A:11.2%, B: 10.6%, C: 9.9%, p=NS between groups |
| Lange et al64 | Liver | IL2RA | CNI + MMF + CS, groups divided based on renal function recovery | Retrospective, single center | IL2RA may allow delay in CNI |
| Best et al61 | Liver | IL2RA, rATG | Meta-analysis | 23 studies included | Graft failure, patient death |
| Nozohoor et al62 | Heart | IL2RA vs. ATG vs. None | IL2RA vs. ATG vs. None | ISHLT registry | All cause mortality: ATG or no induction better than IL2RA, p = 0.001 |
| Jaksch et al91 | Lung | A vs. ATG | A + reduced FK + MMF + low CS vs. ATG + FK+MMF + CS | Prospective, open label, single center | No difference in 1 and 2 years patient and graft survival |
Abbreviations: A, alemtuzumab; BPAR, biopsy proven acute rejection; CNI, tacrolimus or cyclosporine; CS, corticosteroids; CSWD, corticosteroid withdrawal; EC-MPS, enteric coated mycophenolate acid sodium; FK, tacrolimus; IL2RA, interleukin-2 receptor antagonist; ISHLT, International Society for Heart and Lung Transplants; Low risk, low immunologic risk (e.g., low panel reactive antibody [PRA]); MMF, mycophenolate mofetil; rATG, rabbit antithymocyte globulin; RCT, randomized controlled trial.
The current induction trends are illustrated in Figure 4 based on 2018 Organ Procurement and Transplant Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) annual report, which is based on transplants completed by December 2018.52 The data demonstrate an increase in T-cell depleting induction in all represented organ groups except the liver.52 Induction analysis in the various organ groups mainly focuses on trends seen in the SRTR data, the limitation in the large analysis is incomplete reporting from transplant centers at discharge and follow-up. In addition, definitions for some variables can be subjective, for example, symptomatic peripheral vascular disease in the kidney transplant model, which is defined as “demonstrated intermittent claudication, diminished peripheral pulses, or other signs and symptoms of peripheral vascular disease” per the Transplant Information Electronic Data Interchange (TIEDI) glossary. Analysis of the data in aggregate then becomes challenging and unfortunately provides limited direction. Whitson and colleagues recently published their analysis of the Standard Transplant Analysis and Research (STAR) files for heart transplant recipients transplanted since 1987 and were not able to demonstrate a difference in survival among the induction agents used.53 These limitations allow for only the identification of signals and trends.
FIGURE 4.
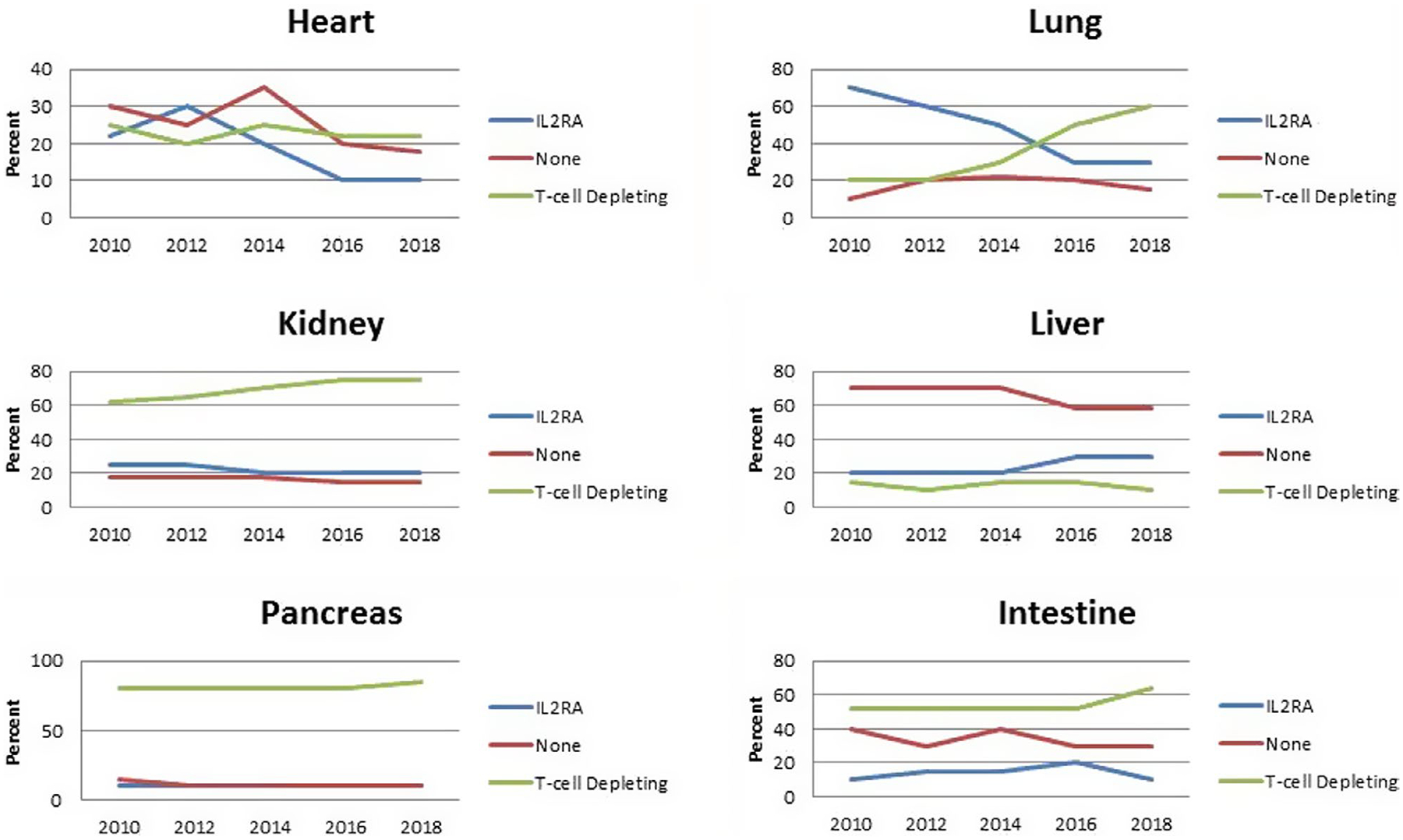
Scientific Registry of Transplant Recipients (SRTR) reported current induction trends adapted from 2018 Annual Data Report. Scientific Registry of Transplant Recipients http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx (accessed June 3, 2020)
Most multicenter RCTs for induction therapy exist in the kidney transplant population secondary to the numbers needed to power the study adequately. The last 10 years have brought forth several pooled analyses. This decade began the use of alemtuzumab in select centers. Alloway and colleagues recently published a systematic review of induction therapy in kidney transplant recipients specifically focusing on rATG versus interleukin 2 receptor subunit alpha (IL2RA), alemtuzumab, or none.54 This pooled analysis revealed that rATG had a slight advantage in the composite end point of biopsy proven acute rejection, graft loss, death, or loss to follow-up compared with other induction strategies.54 Follow-up in this study was limited to 1 year posttransplant and therefore did not assess the long-term risk of infection and malignancy and also did not consider the maintenance immunosuppression regimens, which may have varied between studies. The predominance of studies using alemtuzumab for induction, however, was focused on steroid minimization or withdrawal. Hill and colleagues published their meta-analysis on induction therapy for kidney transplant recipients taking into consideration the maintenance immunosuppression in their analysis of outcomes.55 Alemtuzumab was concluded to have similar outcomes to rATG in the setting of early corticosteroid withdrawal or minimization for 1 year acute rejection rates, however, did not provide any benefit with 1-year patients survival.55 The Cochrane group also evaluated IL2RA for kidney transplant recipients compared with placebo and other induction strategies and found some benefits in reducing acute rejection but no survival benefit in pooled analysis.56 Despite the lack of true guidance from pooled analyses, some general considerations can be extracted from single center trials, which will set the stage for more individualized regimens going into the next era.
Early corticosteroid minimization and avoidance has been desirable specifically in the kidney transplant population in the setting of tacrolimus-based regimens to decrease the incidence and severity of new onset diabetes after transplant, cardiovascular disease, wound healing, mood issues, and weight gain.57 Double-blind placebo controlled trials demonstrate that in order to mitigate the risk of rejection within the first year, T-cell depleting induction must be used but removal of steroids does not necessarily reduce the risk metabolic abnormalities, such as new onset diabetes after transplantation.58,59
Underlying kidney dysfunction and immunologic barriers at the time of transplant have led to an increase in the use of induction therapy in non-kidney transplant recipients. Interestingly, none of the induction therapies are approved for use in nonrenal organs but several studies have used them to achieve maintenance optimization in the last decade. The majority of data available in nonrenal organs evaluates the use of IL2RA versus rATG, whereas there is limited data for the use of alemtuzumab.60 Recently a meta-analysis performed by Best and colleagues61 articulated the flaws of current induction data in liver transplantation, mainly short follow-up, various end points, and definitions and small denominators to draw conclusions. Registry data have also been evaluated in lung and heart transplantation; however, these analyses alone often do not account for: (a) the individual nuances of induction decisions (i.e., renal failure), (b) changes in allocation based on disease severity, (c) maintenance immunosuppression, and (d) or other comorbidities that would limit the use of certain agents (i.e., malignancy).60,62 T-cell depleting induction definitely plays a role in nonrenal organ transplant recipients at high risk for acute rejection, specifically those with circulating donor-specific antibodies.63 In addition, using induction therapy with IL2RA or rATG may allow for delayed initiation of CNI in the setting of renal dysfunction for nonrenal organs and there is significant evidence to support this.63,64
Center specific assessment of outcomes through the Quality Assurance/Performance Improvement process is essential for optimizing regimens for each patient. There is a paucity of data truly looking at dosing and duration opportunities in extremes of body weight populations, and our older and pediatric patients.
3.1 |. Maintenance immunosuppression in the modern era
For the last decade, the transplant community has shifted its attention toward improving long-term outcomes that have historically been sacrificed in part from the sequelae of maintenance immunosuppression and overimmunosuppression while striving to achieve low rejection rates. Although the gold standard has remained tacrolimus, mycophenolate, and corticosteroids across all solid organ transplant groups, the modern era has brought a more tailored approach to immunosuppression.23,65–69 Transplant teams have grown more multidisciplinary to develop individualized and precise drug regimens not only based on donor, recipient, and transplant characteristics, but also utilizing distinct biomarkers to evaluate immune function and allograph injury and using novel immunosuppressant combinations when necessary. The last decade has also pushed the limits of immunosuppression minimization with lower tacrolimus trough goals, steroid-free regimens, and dual CNI-free therapies.
3.2 |. Rejection
In the latest OPTN/SRTR data report published in January 2020, overall 1-year acute rejection rates from 2016 to 2017 were lowest post-kidney transplant at 7.5% and continues to be highest in our intestinal transplant patients at nearly 40%.23,65–69 Despite historically low rejection rates across all solid organ transplant populations, acute rejection episodes continue to be an important issue affecting both patient and allograft survival.
3.3 |. T-cell-mediated rejection in the modern era
Little has changed for the treatment of T-cell-mediated rejection since the late 1990s when rATG was introduced. Although few robust studies exist for all transplant types, decades of clinical practice have demonstrated that the conventional approach to the treatment of acute cellular rejection (ACR) consists of pulse doses of corticosteroids and rATG for severe or nonsteroid responsive rejection. The optimal dose and duration of steroids and rATG for the treatment of ACR is not well-defined. The study that led to the approval of rATG for the treatment of acute rejection in renal transplantation used 1.5 mg/kg/day for 7–14 days70 (≥ 10.5 mg/kg cumulative dose); however, recognition of the profound and sustained T-cell depletion of rATG has led to substantially lower cumulative doses for the treatment of cellular rejection in modern practice.
3.4 |. Antibody-mediated rejection in the modern era
The favorable responses typically seen with ACR treatment are often not mirrored in the setting of antibody-mediated rejection (AMR). Understanding the role of antibodies and allograft injury has led to significant advances in the detection of HLA antibodies and the diagnosis of AMR. The precipitous and deleterious effects of AMR on long-term outcomes have made anti-HLA donor specific antibodies (DSAs) and AMR a significant focus over the last decade. The optimal treatment for AMR posttransplantation has yet to be defined and is largely due to the low quality of published data stemming from small, heterogeneous populations with mixed therapeutic interventions delivered at various stages of allograft injury. The mainstays of conventional therapy for the treatment of AMR has been plasma exchange, intravenous immunoglobulin (IVIG), and glucocorticoids. The anti-CD20 monoclonal antibody, rituximab, as well as proteasome inhibitors, such as bortezomib and carfilzomib, have also been used in clinical practice despite published data providing mixed results.71–74 With the crucial role of the complement system on AMR pathophysiology, the monoclonal antibody directed at C5, eculizumab, has also been tried for both the treatment and prevention of AMR. Although promising results were shown for the prevention of early AMR in HLA-incompatible renal transplant recipients75–78 and in a small study for the treatment of early AMR, long-term outcomes have shown no difference in chronic AMR or graft loss compared with historical controls.79,80 Novel therapeutic approaches to AMR have recently been reviewed81 and a summary of various agents are included in Table 3.
TABLE 3.
Novel therapies for prevention and treatment of AMR
| Citation, year | Organ | Condition | N | Study design and findings |
|---|---|---|---|---|
| TCZ (IL-6 inhibitor) | ||||
| Vo et al 201592 | Kidney | DES | 10 | Patients unresponsive to IVIG + rituximab were treated with IVIG 2 g/kg days 0 and 30 + TCZ 8 mg/kg day 15 then monthly × 6 months Five patients (50%) were transplanted. No AMR at 6-month protocol biopsies. DSA (number and strength) were reduced with TCZ |
| Choi et al 201793 | Kidney | cAMR and TG | 36 | Patients unresponsive to IVIG + rituximab ± plasma exchange received TCZ 8 mg/kg monthly × 6–25 months TCZ-treated patients had significant reductions in DSAs and stabilization of renal function at 2 years |
| Berinert and Cinryze (C1 esterase inhibitors [C1-INH]) | ||||
| Vo et al 201592 | Kidney | DES | 20 | After IVIG +rituximab ± plasma exchange, patients enrolled 1:1 to C1-INH (20 IU/kg/dose) or placebo intraoperatively, then twice weekly for 7 doses No study drug related adverse effects, less DGF with C1-INH (1 vs. 4), AMR during study (0 C1-INH vs. 1 placebo), AMR after study (2 C1-INH vs. 2 placebo), C1q+ DSAs reduced in 2 C1-INH patients |
| Viglietti et al 201694 | Kidney | AMR | 6 | Patients unresponsive to IVIG + rituximab + plasmapheresis received C1-INH (Berinert; 20 IU/kg) × 3 days, then twice weekly + IVIG 2 g/kg every 4 weeks × 6 months All patients showed improvement in eGFR from inclusion to month-6 (38.7 ± 17.9 to 45.2 ± 21.3 ml/min/1.73 m2; p = 0.028). There was a significant decrease in the number of patients regarding C4d deposition rate and DSA C1q status pre- and post-C1-INH |
| Montgomery et al 201695 | Kidney | AMR | 18 | Patients enrolled 1:1 to C1-INH (20,000 units divided every other day over 2 weeks) or placebo + IVIG + plasmapheresis ± anti-CD20 No difference between groups on day 20 pathology or graft survival. C1-INH group demonstrated a trend toward sustained improvement in renal function compared with placebo. No TG shown in the 7 C1-INH patients with 6-mo protocol biopsies (compared with 3/7 with TG in placebo group) |
| Imlifidase (IgG-degrading enzyme of Streptococcus pyogenes [IdeS]) | ||||
| Jordan et al 201796 | Kidney | DES | 25 | Highly sensitized (mean cPRA 95%) patients from an HLA-incompatible donor received IdeS 0.24 mg/kg (US) or 0.25 or 0.5 mg/kg (Sweden) 4–6 h pretransplant Total IgG and HLA antibodies were eliminated in all patients at time of transplant. Hyperacute rejection and graft loss occurred in 1 patient due to non-HLA IgM and IgA antibodies. Ten patients (40%) experienced AMR 2 weeks-5 months posttransplant, but all responded to treatment |
| Lonze et al 201897 | Kidney | DES | 7 | Highly sensitized (cPRA 98%−100%) with positive crossmatches received IdeS All crossmatches became negative post-IdeS allowing for transplant. Three patients (43%) had rebound DSAs and AMR that responded to treatment |
Abbreviations: AMR, antibody-mediated rejection; C1-INH, C1 esterase inhibitor; cAMR, chronic antibody-mediated rejection; CLAD, chronic lung allograft dysfunction; cPRA, calculated panel reactive antibody; DES, desensitization; DGF, delayed graft function; DSA, donor specific antibodies; eGFR, estimated glomerular filtration rate; IdeS, investigational device exemption; IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; TCZ, tocilizumab; TG, transplant glomerulopathy.
Given the detrimental effects of the production of de novo DSAs (dnDSA), acute and chronic AMR, and the grim treatment options for such, consideration has been made to the dissection of maintenance regimens and their interplay with DSA development82 and AMR. It is known that dnDSA are often the product of underimmunosuppression due to provider-directed weaning, patient nonadherence, or genetic variability in drug metabolism. Ascending from a period of overimmunosuppression, we are once again met with the need to be hypervigilant in the modern era of minimal immunosuppression as we strive to achieve the optimal balance. Belatacept has been under recent evaluation for the prevention of DSA originating from the BENEFIT and BENEFIT-EXT studies that demonstrated that DSA development at 7 years posttransplant was significantly lower with belatacept compared with cyclosporine-based immunosuppression.43,83 A recent post hoc analysis further detailed this data showing that in BENEFIT, 1.4%, 3.5%, and 12.1% of belatacept more intense-treated, belatacept less intense-treated, and cyclosporine-treated patients, respectively, developed dnDSAs and corresponding values in BENEFIT-EXT were 3.8%, 1.1%, and 11.2%.84 In addition, belatacept-based immunosuppression was associated with numerically lower mean fluorescence intensity compared with cyclosporine-treated patients in those with dnDSAs. In 2020, a brief communication described a significant reduction in HLA class I antibodies with the use of belatacept compared with a control group maintained on the gold standard immunosuppression in 72 highly sensitized (calculated panel reactive antibody [cPRA] ≥ 98%) kidney transplant recipients.85 Although belatacept-treated patients also had a significant reduction in cPRA compared with controls, there was no difference in HLA class II antibodies.
Antibody-mediated rejection is challenging and is limited by unique cases and few RCTs. Recently, the kidney community published some guidance with an expert opinion paper.86
4 |. FUTURE DIRECTIONS
Clinicians are eager to move beyond the traditional early 1-year outcomes and optimize long-term outcomes in organ transplantation. This necessitates individualization of posttransplant immunosuppression regimens. The continual need to balance between over- and underimmunosuppression and prevent or mitigate adverse effects will remain an Achilles heel until we have better mechanisms to assess functional immune status or true tolerance can be achieved.87 The One study,88 perhaps provides a glimpse into the next decade of research and immunosuppression avoidance. Investigators evaluated the use of cell-based medicinal products to facilitate the use of tacrolimus monotherapy in kidney transplant recipients. This multicenter, international pooled data trial was a combined look at six nonrandomized trials compared with a large group on standard immunosuppression. These products (on day −7 and day +10) were combined with basiliximab induction and prednisolone tapered off by week 15, mycophenolate mofetil 2 g daily tapered to 1.5 g on day 14 and off on day 365; tacrolimus was started 4 days before surgery and gradually reduced to a goal of 3–6 ng/ml over 9 months. Analysis of the 104 patients enrolled demonstrated a 12% rate of biopsy proven rejection in the standard group at 60 months versus 16% in the patients who received immunotherapy (n = 38). Infections were significantly lower in the immunotherapy group; however, immunosuppression in the reference group varied significantly.88 The One study, along with other similar research, is still in its infancy, but will likely be an addition or replacement to the traditional dogmatic approach of one size fits all. These findings are specifically exciting for organ groups that experience high rates of infection, such as lung transplant recipients. In the meantime, more widespread use of biomarkers that improve our ability to assess immune function or immunologic risk are beginning to be used in clinical practice. Commercially available assays, such as AlloMap or TruGraf may provide less invasive methods to determine immunologic risk and likelihood of immune-mediated allograft injury. Donor derived cell-free DNA (Allosure and Prospera), a noninvasive marker of allograft injury, has also been used to determine the risk of rejection or other causes of allograft injury. The use of these less-invasive tests has demonstrated mixed results and their true utility in solid organ transplant is still being elucidated.89
To truly optimize long-term outcomes, individualized immunosuppression in the next decade needs to be coupled with continued work to improve posttransplant comorbidity management and reduce medication nonadherence. In order to mitigate the limited supply of donor organs, there is a need to ensure that both the patient and allograft maintain long-term function. This will prevent death with a functioning graft or early graft loss requiring retrains-plantation. The transplant community needs to continue to find new ways to simplify immunosuppression regimens, improve follow-up monitoring, and reduce the financial hardships transplantation often induces. Follow-up care using telehealth and remote patient monitoring has recently demonstrated successes, particularly during this most recent coronavirus disease 2019 (COVID-19) pandemic. Allowing these innovative practices to flourish requires that the COVID-19 payment system implemented through the Coronavirus Aid, Relief, and Economic Security Act to continue indefinitely. Only time will tell if this will occur. Regardless, if historical trends continue, the next several decades will undoubtedly be a time of great innovation in solid organ transplantation.
Footnotes
CONFLICT OF INTEREST
L.J.B. is a member of the Speakers Bureau for Veloxis Pharmaceuticals. All other authors declare no conflicts of interest.
REFERENCES
- 1.Murray JE, Merrill JP, Harrison JH. Kidney transplantation between seven pairs of identical twins. Ann Surg. 1958;148:343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker CF, Markmann JF. Historical overview of transplantation. Cold Spring Harb Perspect Med. 2013;3:a014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy JD. The first lung transplant in man (1963) and the first heart transplant in man (1964). Transplant Proc. 1999;31:25–29. [DOI] [PubMed] [Google Scholar]
- 4.White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373:1808–1817. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614S–636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosimi AB, Burton RC, Colvin RB, et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. [DOI] [PubMed] [Google Scholar]
- 7.Helderman JH, Bennett WM, Cibrik DM, Kaufman DB, Klein A, Takemoto SK. Immunosuppression: practice and trends. Am J Transplant. 2003;3:41–52. [DOI] [PubMed] [Google Scholar]
- 8.Perico N, Remuzzi G. Prevention of transplant rejection. Drugs. 1997;54:533–570. [DOI] [PubMed] [Google Scholar]
- 9.Thomas F, Thomas J, Flora R, Mendez-Picon G, Peace K, Lee HM. Effect of antilymphocyte-globulin potency on survival of cadaver renal transplants: prospective randomised double-blind trial. Lancet. 1977;310:671–674. [DOI] [PubMed] [Google Scholar]
- 10.Bell PR, Blamey RW, Briggs JD, et al. Medical research council trial of antilymphocyte globulin in renal transplantation. A multicenter randomized double-blind placebo controlled clinical investigation. Transplantation. 1983;35:539–545. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen A, Sødal G, Flatmark A, Thorsby E. Antilymphocyte globulin in cadaveric renal transplantation–a controlled trial. Scand J Urol Nephrol. 1977;42:94–96. [PubMed] [Google Scholar]
- 12.Novick AC, Braun WE, Steinmuller D, Buszta C, Greenstreet R, Kiser W. A controlled randomized double-blind study of antilymphoblast globulin in cadaver renal transplantation. Transplantation. 1983;35:175–179. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HE, Ackman CF, Horowitz I. Canadian clinical trial of antilymphocyte globulin in human cadaver renal transplantation. Can Med Assoc J. 1976;115:1205–1208. [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SL. Ten years of orthoclone OKT3 (Muromonab-CD3): a review. J Transpl Coord. 1996;6:109–121. [DOI] [PubMed] [Google Scholar]
- 15.Nashan B Antibody induction therapy in renal transplant patients receiving calcineurin-inhibitor immunosuppressive regimens: a comparative review. BioDrugs. 2005;19:39–46. [DOI] [PubMed] [Google Scholar]
- 16.Mahmud N, Klipa D, Ahsan N. Antibody immunosuppressive therapy in solid-organ transplant. mAbs. 2010;2:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6:1111–1131. [DOI] [PubMed] [Google Scholar]
- 18.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of thymoglobulin versus atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. [DOI] [PubMed] [Google Scholar]
- 19.Chapman TM, Keating GM. Basiliximab. Drugs. 2003;63:2803–2835. [DOI] [PubMed] [Google Scholar]
- 20.Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. Transplantation. 1999;67:276–284. [DOI] [PubMed] [Google Scholar]
- 21.Roche discontinues Zenapax injection [Internet]. MPR. 2009. [cited 2020 May 27]. Available from https://www.empr.com/home/news/roche-discontinues-zenapax-injection/ [Google Scholar]
- 22.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant. 2013;13:11–46. [DOI] [PubMed] [Google Scholar]
- 23.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. 2020;20:20–130. [DOI] [PubMed] [Google Scholar]
- 24.Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 annual data report: heart. Am J Transplant. 2013;13:119–148. [DOI] [PubMed] [Google Scholar]
- 25.Kim WR, Stock PG, Smith JM, et al. OPTN/SRTR 2011 annual data report: liver. Am J Transplant. 2013;13:73–102. [DOI] [PubMed] [Google Scholar]
- 26.Valapour M, Paulson K, Smith JM, et al. OPTN/SRTR 2011 annual data report: lung. Am J Transplant. 2013;13:149–177. [DOI] [PubMed] [Google Scholar]
- 27.Calne RY, White DJ, Evans DB, et al. Cyclosporin A in cadaveric organ transplantation. Br Med J Clin Res Ed. 1981;282:934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Multicentre Trial Group. Cyclosporin in cadaveric renal transplantation: one-year follow-up of a multicentre trial. Lancet. 1983;322(8357):986–989. [PubMed] [Google Scholar]
- 29.The Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med. 1986;314:1219–1225. [DOI] [PubMed] [Google Scholar]
- 30.Hakala TR, Starzl TE, Rosenthal JT, Shaw B, Iwatsuki S. Cadaveric renal transplantation with cyclosporin-A and steroids. Transplant Proc. 1983;15:465–470. [PMC free article] [PubMed] [Google Scholar]
- 31.Hesselink DA, Gregoor PJHS, Weimar W. The use of cyclosporine in renal transplantation. Transplant Proc. 2004;36:S99–S106. [DOI] [PubMed] [Google Scholar]
- 32.Fung JJ, Starzl TE. FK506 in solid organ transplantation. Ther Drug Monit. 1995;17:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus. Drugs. 2003;63:1247–1297. [DOI] [PubMed] [Google Scholar]
- 34.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation. 1997;63:977–983. [DOI] [PubMed] [Google Scholar]
- 35.Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177–1187. [DOI] [PubMed] [Google Scholar]
- 36.Srinivas TR, Kaplan B, Meier-Kriesche H-U. Mycophenolate mofetil in solid-organ transplantation. Expert Opin Pharmcother. 2003;4:2325–2345. [DOI] [PubMed] [Google Scholar]
- 37.Sollinger H. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225–232. [DOI] [PubMed] [Google Scholar]
- 38.Miller J, Mendez R, Pirsch JD, Jensik SC. Safety and efficacy of tacrolimus in combination with mycophenolate mofetil (MMF) in cadaveric renal transplant recipients. Transplantation. 2000;69:875–880. [DOI] [PubMed] [Google Scholar]
- 39.Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15:303–319. [DOI] [PubMed] [Google Scholar]
- 40.Vítko S, Margreiter R, Weimar W, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004;78:1532–1540. [DOI] [PubMed] [Google Scholar]
- 41.Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation. 2005;80:303–309. [DOI] [PubMed] [Google Scholar]
- 42.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A Phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535–546. [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. [DOI] [PubMed] [Google Scholar]
- 44.Black CK, Termanini KM, Aguirre O, Hawksworth JS. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez CP, Patel N, Mardis CR, Meadows HB, Taber DJ, Pilch NA. Belatacept in solid organ transplant: review of current literature across transplant types. Transplantation. 2018;102:1440–1452. [DOI] [PubMed] [Google Scholar]
- 46.Knoll G Trends in kidney transplantation over the past decade. Drugs. 2008;68:3–10. [DOI] [PubMed] [Google Scholar]
- 47.Marcén R Immunosuppressive drugs in kidney transplantation. Drugs. 2009;69:2227–2243. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Tilea A, Gillespie B, et al. Understanding trends in kidney function 1 year after kidney transplant in the United States. J Am Soc Nephrol. 2017;28:2498–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parasuraman R, Abouljoud M, Jacobsen G, Reddy G, Koffron A, Venkat KK. Increasing trend in infection-related death-censored graft failure in renal transplantation. Transplantation. 2011;91:94–99. [DOI] [PubMed] [Google Scholar]
- 50.Schold JS. The evolving role of regulatory reporting on patient and donor selection in organ transplantation. Curr Opin Organ Transplant. 2020;25:158–162. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of practice. Clin Transplant. 2019;33:e13528. [DOI] [PubMed] [Google Scholar]
- 52.2018 Annual Data Report. Scientific Registry of Transplant Recipients. Available from http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx. Accessed 6/3/2020.
- 53.Whitson BA, Kilic A, Lehman A, et al. Impact of induction immunosuppression on survival in heart transplant recipients: a contemporary analysis of agents. Clin Transplant. 2015;29:9–17. [DOI] [PubMed] [Google Scholar]
- 54.Alloway RR, Woodle ES, Abramowicz D, et al. Rabbit anti-thymocyte globulin for the prevention of acute rejection in kidney transplantation. Am J Transplant. 2019;19(8):2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webster AC, Ruster LP, McGee R, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010;2010:CD003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prashar R, Venkat KK. Immunosuppression minimization and avoidance protocols: when less is not more. Adv Chronic Kidney Dis. 2016;23:295–300. [DOI] [PubMed] [Google Scholar]
- 58.Pirsch JD, Henning AK, First MR, et al. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15:1982–1990. [DOI] [PubMed] [Google Scholar]
- 59.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. [DOI] [PubMed] [Google Scholar]
- 60.Whitson BA, Lehman A, Wehr A, et al. To induce or not to induce: a 21st century evaluation of lung transplant immunosuppression’s effect on survival. Clin Transplant. 2014;28:450–461. [DOI] [PubMed] [Google Scholar]
- 61.Best LMJ, Leung J, Freeman SC, et al. Induction immunosuppression in adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:CD013203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nozohoor S, Stehlik J, Lund LH, Ansari D, Andersson B, Nilsson J. Induction immunosuppression strategies and long-term outcomes after heart transplantation. Clin Transplant. 2020;9:e13871. [DOI] [PubMed] [Google Scholar]
- 63.Zuckermann A, Schulz U, Deuse T, et al. Thymoglobulin induction in heart transplantation: patient selection and implications for maintenance immunosuppression. Transpl Int. 2015;28:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lange NW, Salerno DM, Sammons CM, Jesudian AB, Verna EC, Brown RS. Delayed calcineurin inhibitor introduction and renal outcomes in liver transplant recipients receiving basiliximab induction. Clin Transplant. 2018;32(12):e13415. [DOI] [PubMed] [Google Scholar]
- 65.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2018 annual data report: pancreas. Am J Transplant. 2020;20:131–192. [DOI] [PubMed] [Google Scholar]
- 66.Kwong A, Kim KR, Lake JR, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant. 2020;20:193–299. [DOI] [PubMed] [Google Scholar]
- 67.Smith JM, Weaver T, Skeans MA, et al. OPTN/SRTR 2018 annual data report: intestine. Am J Transplant. 2020;20:300–339. [DOI] [PubMed] [Google Scholar]
- 68.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 annual data report: heart. Am J Transplant. 2020;20:340–426. [DOI] [PubMed] [Google Scholar]
- 69.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 annual data report: lung. Am J Transplant. 2020;20:s427–s508. [DOI] [PubMed] [Google Scholar]
- 70.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. [DOI] [PubMed] [Google Scholar]
- 71.Ensor CR, Yousem SA, Marrari M, et al. Proteasome inhibitor carfilzomib-based therapy for antibody-mediated rejection of the pulmonary allograft: use and short-term findings. Am J Transplant. 2017;17:1380–1388. [DOI] [PubMed] [Google Scholar]
- 72.Eskandary F, Regele H, Baumann L, et al. A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol. 2018;29:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreso F, Crespo M, Ruiz JC, et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized double-blind clinical trial. Am J Transplant. 2018;18:927–935. [DOI] [PubMed] [Google Scholar]
- 74.Sautenet B, Blancho G, Büchler M, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016;100:391–399. [DOI] [PubMed] [Google Scholar]
- 75.Glotz D, Russ G, Rostaing L, et al. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am J Transplant. 2019;19:2865–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marks WH, Mamode N, Montgomery RA, et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: a randomized trial. Am J Transplant. 2019;19:2876–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. [DOI] [PubMed] [Google Scholar]
- 78.Tan EK, Bentall A, Dean PG, Shaheen MF, Stegall MD, Schinstock CA. Use of eculizumab for active antibody-mediated rejection that occurs early post-kidney transplantation: a consecutive series of 15 cases. Transplantation. 2019;103:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant. 2015;15:1293–1302. [DOI] [PubMed] [Google Scholar]
- 80.Schinstock CA, Bentall AJ, Smith BH, et al. Long-term outcomes of eculizumab-treated positive crossmatch recipients: allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant. 2019;19:1671–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jordan SC, Ammerman N, Choi J, et al. Novel therapeutic approaches to allosensitization and antibody-mediated rejection. Transplantation. 2019;103:262–272. [DOI] [PubMed] [Google Scholar]
- 82.OʼLeary JG, Samaniego M, Crespo Barrio M, et al. The influence of immunosuppressive agents on the risk of de novo donor-specific HLA antibody production in solid organ transplant recipients. Transplantation. 2016;100:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Durrbach A, Pestana JM, Florman S, et al. Long-term outcomes in Belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant. 2016;16:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bray RA, Gebel HM, Townsend R, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018;18:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parsons P, Zahid A, Bumb S, et al. The impact of belatacept on third-party HLA alloantibodies in highly sensitized kidney transplant recipients. Am J Transplant. 2020;20:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 expert consensus from the transplantion society working group. Transplantation. 2020;104:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ezekian B, Schroder PM, Freischlag K, Yoon J, Kwun J, Knechtle SJ. Contemporary strategies and barriers to transplantation tolerance. Transplantation. 2018;102:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawitzki B, Harden PN, Reinke P, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet. 2020;395:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thongprayoon C, Vaitla P, Craici IM, et al. The use of donor-derived cell-free DNA for assessment of allograft rejection and injury status. J Clin Med. 2020;9:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomusch O, Wiesener M, Opgenoorth M, et al. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (harmony): an open-label, multicentre randomised controlled trial. Lancet. 2016;388:3006–3016. [DOI] [PubMed] [Google Scholar]
- 91.Jaksch P, Ankersmit J, Scheed A, et al. Alemtuzumab in lung transplantation: an open-label, randomized, prospective single center study. Am J Transplant. 2014;14:1839–1845. [DOI] [PubMed] [Google Scholar]
- 92.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the interleukin-6 receptor–specific humanized monoclonal (tocilizumab)+ intravenous immunoglobulin in difficult to desensitize patients. Transplantation. 2015;99:2356–2363. [DOI] [PubMed] [Google Scholar]
- 93.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti–interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 94.Viglietti D, Gosset C, Loupy A, et al. C1 inhibitor in acute antibody-mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: a pilot study. Am J Transplant. 2016;16:1596–1603. [DOI] [PubMed] [Google Scholar]
- 95.Montgomery RA, Orandi BJ, Racusen L, et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am J Transplant. 2016;16:3468–3478. [DOI] [PubMed] [Google Scholar]
- 96.Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377:442–453. [DOI] [PubMed] [Google Scholar]
- 97.Lonze BE, Tatapudi VS, Weldon EP, et al. Ides (imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high-strength donor-specific antibody. Ann Surg. 2018;268:488–496. [DOI] [PubMed] [Google Scholar]


