Abstract
Background & Aims:
IL-17A-producing T cells are present in autoimmune cholestatic liver diseases; however, little is known about the contribution of IL-17 to periductal immune responses. Here we investigated the role of IL-17 produced by antigen specific CD8+ T cells in a mouse model of cholangitis and in vitro in human cholangiocyte organoids.
Methods:
K14-OVAp mice express a MHC I restricted ovalbumin peptide sequence (SIINFEKL) on cholangiocytes. Cholangitis was induced by the adoptive transfer of transgenic OVA-specific OT-1 CD8+ T cells that either had OT-1wt or lacked IL-17A/F (OT-1IL17ko). The response of mouse and human cholangiocytes/organoids to IL-17A was assessed in vitro.
Results:
Transfer of OVA-specific OT-1IL17ko cells significantly aggravated periductal inflammation in K14-OVAp recipient mice, compared to transfer of OT-1wt T cells. OT-1IL17ko T cells were highly activated in the liver and displayed increased cytotoxicity and proliferation. IL-17A/F produced by transferred OT-1wt CD8+ T cells induced upregulation of the inhibitory molecule PD-L1 on cholangiocytes, restricting cholangitis by limiting cytotoxicity and proliferation of transferred cells. In contrast, OT-1IL17ko T cells failed to induce PD-L1 on cholangiocytes, resulting in uncontrolled expansion of cytotoxic CD8+ T cells and aggravated cholangitis. Blockade of PD-L1 after transfer of OT-1wt T cells with anti-PD-L1 antibody also resulted in aggravated cholangitis. Using human cholangiocyte organoids, we were able to confirm that IL-17A induces PD-L1 expression in cholangiocytes.
Conclusion:
We demonstrate an important function of IL-17 in restricting cholangitis and protecting from CD8+ T cell mediated inflammatory bile duct injury that was mediated by upregulation of PD-L1 on cholangiocytes. Targeting of IL-17, which is an effective treatment for several autoimmune diseases, for the treatment of cholangitis should be undertaken with caution.
Keywords: Interleukin (IL)-17, Programmed cell death ligand 1 (PD-L1), biliary epithelial cell, cholangitis, CD8
Lay summary
Interleukin 17 (IL-17) is assumed to be a driver of inflammation in several autoimmune diseases, such as psoriasis. IL-17 is also present in inflammatory diseases of the bile duct, but its role in these conditions is not clear, as the effects of IL-17 depend on the context of its expression. We here investigated the role of IL-17 in an experimental autoimmune cholangitis mouse model and we identified an important protective effect of IL-17 on cholangiocytes enabling them to downregulate bile duct inflammation via check point inhibitor PD-L1.
Introduction
Cholangiocytes are epithelial cells that line the intra- and extrahepatic bile ducts and actively modify bile volume and composition. In autoimmune cholestatic liver diseases, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), cholangiocytes are targeted by T cell driven immune attack, leading to cholestasis, ductopenia and finally, to end-stage liver disease1, 2. To date, there is no curative therapy, rendering these diseases major indications for liver transplantation3. Genetic and environmental factors, such as the microbiota in combination with dysregulated adaptive and innate immune responses likely contribute to the pathogenesis of these diseases1, 2, 4, 5.
It has been shown that cholangiocytes are not only passive targets of immune cells, but also actively contribute to the process of periductal inflammation. Cholangiocytes express various Toll-like receptors (TLRs), the ligation of which can induce the recruitment of neutrophils and dendritic cells via CCL2, IL-6, IL-8 and Mip-3a6–8. Senescence associated cytokines add to a pro-inflammatory periductal environment9. Cytokines described to act on cholangiocytes include IFNgamma, IL-1beta, IL-6 and IL-178, 10. Cholangiocytes activated by IFNgamma express chemokines such as CCL2 and CCL20 and surface proteins, including MHC class II, ICAM-1 and VCAM-1, and thus actively participate in antigen presentation and immune cell recruitment11–14.
IL-17 is involved in the pathogenesis of many autoimmune diseases and neutralizing IL-17 has been established as a therapy, e.g. of psoriasis15, 16. However, targeting IL-17 in mucosal disease such as Crohńs disease, has been disappointing17. IL-17 clearly acts in a highly context dependent manner, so it is of paramount importance to gain a better understanding of the role of IL-17, and especially of its most prominent family members IL-17A and IL-17F in mucosal immunology. At mucosal barriers, IL-17 contributes to the protection against extracellular bacterial and fungal pathogens18. We have previously described the localization of IL-17+ T cells around bile ducts of PSC patients and a shift in the balance between IL-17A producing CD4+ T (Th17) cells and regulatory T cells (Tregs)19, 20. Th17 cells may be induced by intestinal microbiota and have recently been shown to aggravate murine cholangitis4. In addition, we recently reported that the monocyte-cholangiocytes interaction contributes to microbiota-induced Th17 differentiation in PSC patients21. Not only Th17 cells, but also IL-17+ CD8+ T (Tc17) cells were reported to accumulate in inflammatory and autoimmune liver diseases11, 22, 23. Compared to conventional cytotoxic T cells, Tc17 cells were described to exhibit higher pro-inflammatory potential, but reduced secretion of granzyme B, perforins and overall cytotoxicity23–25. Auto-reactive, cytotoxic CD8+ T cells are suspected to promote the pathogenesis of PBC26, 27, but little is known about the functional role of Tc17 cells in the context of autoimmune cholestatic diseases.
Increasing evidence suggests that the interaction of inflammatory T cells and cholangiocytes involves IL-17; however, the effects of IL-17 on cholangiocytes remain unclear. In this study, we focused on the role of IL-17A/F produced by CD8+ T cells on cholangiocytes in vitro and murine cholangitis in vivo.
Materials and Methods
Mice
C57BL6/J and OT-1wt mice (C57BL/6-Tg(TcraTcrb)1100Mjb/J) were obtained from Jackson Laboratory, Maine, USA. K14-OVAp mice were kindly provided by Kirsten Hogquist (Minnesota, USA) and IL-17A/F−/− mice by Immo Prinz (Hannover, Germany), both on C57BL6/J background. OT-1IL17ko mice were generated by crossbreeding. All mice were bred and housed under specific pathogen free conditions with 12 h light/dark cycles at the animal care facility of the University Medical Center Hamburg-Eppendorf with access to standard chow diet (1318 rodent diet, Altromin, Germany) and water available ad libitum. The K14-OVAp mouse model was described previously28. Animal care was in accordance with the governmental and institutional guidelines and all experiments comply with the ARRIVE guidelines29 and were approved by the local review board of the State of Hamburg, Germany (G36/16, ORG846 and ORG 979).
Induction of cholangitis in mice
Cholangitis in female K14-OVAp mice was induced as described previously28. Briefly, congenic CD8+ T cells recognizing the ovalbumin peptide expressed on K14-OVAp cholangiocytes were isolated freshly from spleens of female OT-1wt or OT-1IL17ko donors. Cells were isolated using anti-CD8-FITC antibody (BioLegend, Germany) and anti-FITC immunomagnetic beads (Miltenyi Biotec, Germany) according to the manufacturer’s instructions. Female K14-OVAp recipients were injected i.v. with 200,000 OT-1wt or OT-1IL17ko CD8+ donor T cells and sacrificed on day 5 after adoptive T cell transfer. 400 µg of isotype control or anti-PD-L1 antibodies (both BioLegend, Germany) were administered one day before and three days after adoptive T cell transfer by i.p. injection as indicated in respective experiments.
Serum transaminases
Mouse alanine (ALAT) and aspartate (ASAT) serum aminotransferase levels were measured using a Cobas Integra 400 plus System (Roche Diagnostics, Switzerland) at the Institute of Experimental Immunology and Hepatology, University Medical Center Hamburg-Eppendorf.
Cell isolation
Spleen cells and liver infiltrating lymphocytes were isolated as described previously28. To isolate cholangiocytes30, female mouse livers were perfused with 0.5 mg/ml collagenase NB 4G (Nordmark, Germany) in PBS and dissected mechanically. Tissue was digested with collagenase (2.5 mg/ml) in PBS for 20 min shaking at 37 °C and filtered with 100 µm strainer. Remaining tissue was further digested with collagenase (5 mg/ml). Finally, tissue was degraded and filtered using 0.05 % trypsin/EDTA (Thermo Fisher Scientific, Germany) for 10 min shaking at 37 °C. Hepatocytes were sedimented two times at 40 g, and debris was depleted using a 35 % percoll gradient (GE Healthcare, UK) at 900 g for 10 min. For mRNA analysis, cholangiocytes were stained with anti-CD326 (EpCAM) (Miltenyi Biotec, Germany) anti-CD45.1-FITC and anti-CD45.2-FITC (both BioLegend, Germany) antibodies for 20 min and subsequently sorted for FITC-negative/APC-positive cells using a BD FACSAria™ III at the Cytometry and Sorting Core Unit, University Medical Center Hamburg-Eppendorf.
Cell cultivation and stimulation
Isolated lymphocytes from spleen or liver were cultivated in Panserin 401-Medium (PAN Biotech, Germany) supplemented with 1 % Penicillin-Streptomycin (Thermo Fisher Scientific, Germany) in flat bottom 96 well plates. Cells were seeded at 500,000 cells per well and stimulated for 24 h using anti-CD3 and anti-CD28 (each 2 µg/ml, BD Biosciences, Germany).
Primary female mouse derived cholangiocytes were cultivated as described previously31. Cholangiocytes were grown confluent in 24 well plates and stimulated with fresh medium containing 10 ng/ml IFNgamma and/or IL-17A (PeproTech, USA) for 24 h. For co-culture experiments 500,000 freshly isolated splenic derived CD8+ T cells from female OT-1wt and OT-1IL17ko donor mice were added for 48 hours to 70 % confluent primary mouse cholangiocytes isolated from female K14-OVAp donors. Supernatants were harvested and analyzed using ELISA for CCL20, IL-6, IL-17A, IFNgamma (all R&D Systems, USA) and granzyme B (Thermo Fisher Scientific, Germany) and cells were harvested for RNA isolation and qPCR analysis.
3 D Organoids
Cholangiocyte organoids were isolated and cultured as described previously32. In brief, liver tissue was mechanically dissected followed by collagenase digestion for 30–60 minutes. Digested cells were washed repeatedly and debris was depleted using a percoll gradient (GE Healthcare, UK) at 500 g for 30 min. Cells were washed with DMEM (1% FCS, 1% P/S) and mixed with Matrigel-Culturex RGF BME Type2 (Roche, Germany), plated in a 24-well plate, and allowed to polymerize. After polymerization of the Matrigel, cells were cultured with conditioning medium containing 30% Wnt3a, 10% R-spondin, 1% P/S, 1% L-Glut, 2% B27 (Thermo Fisher Scientific, Germany)), 10 mM nicotinamide (Sigma; Germany)), 1 mM N -acetyl cysteine (Sigma, USA),1 % N2 (Thermo Fisher Scientific, Germany)),10 nM Gastrin (Sigma, USA), 50 ng/ml HGF (PeproTech, Germany)), 50 ng/ml EGF (PeproTech, USA), 5 µM TGFbeta inhibitor (Tocris, Germany)), 100 ng/ml FGF10 (PeproTech, Germany)) and 10 µM Forskolin (Tocris, Germany)). Medium was changed to expansion medium (conditioning medium without Wnt3a) after 3 days and changed every 2 days. Cultures were passaged after 7–10 days. For stimulation, organoids were cultured as described above and stimulated after 3 days with fresh expansion medium containing 100 ng/ml IFNgamma and/or IL-17A (both PeproTech, Germany) for 24 h. Organoids were harvested for RNA isolation and qPCR analysis.
Patients
A total of 76 patients who attended the outpatient service of the YAEL Center for Autoimmune Liver Disease of the I. Department of Medicine, University Medical Center Hamburg-Eppendorf (UKE, Hamburg, Germany), were included in the study. IL-17A cytokine secretion of PBMC was analyzed in blood samples of 30 patients with PSC, 26 patients with PBC, 20 patients with AIH and 20 healthy donors. Cholangiocyte organoids were isolated from liver tissue of two patients with alcoholic liver disease (ALD), two patients with non-alcoholic fatty liver disease (NAFLD) and one patient with cirrhosis of unknown origin. No donor organs were obtained from executed prisoners or other institutionalized persons. This study was approved by the Ethics Committee of Hamburg and written informed consent was obtained from all patients and healthy controls (PV4081).
Real-time qPCR
Total RNA was extracted from liver tissue (NucleoSpin® RNA, Macherey-Nagel, Germany) and reverse-transcribed (High capacity cDNA reverse transcription kit, Thermo Fisher Scientific, Germany) according to the manufacturer’s instructions.
For quantitative real-time PCR analysis, mRNA expression of various genes listed in Table 1 was measured using TaqMan™ Fast Advanced Master Mix and TaqMan®Gene Expression Assays (Thermo Fisher Scientific, Germany). Target gene expression was normalized to Hprt expression and the fold-induction was quantified by normalization to control groups using the ΔΔCt method.
Table 1.
TaqMan®Gene Expression Assay IDs of genes used in this study
| Gene | Assay ID | Gene | Assay ID |
|---|---|---|---|
| Ccl2 | Mm00441242_m1 | IL6 | Hs00174131_m1 |
| CCL2 | Hs00234140_m1 | Il10 | Mm00439614_m1 |
| Ccl20 | Mm01268754_m1 | Il12a | Mm00434169_m1 |
| CCL20 | Hs00355476_m1 | Il23a | Mm00518984_m1 |
| Cd274 | Mm03048248_m1 | Krt19 | Mm00492980_m1 |
| CD274 | Hs00204257_m1 | Ly6c | Mm03009946_m1 |
| Col1a1 | Mm00801666_g1 | Ly6g | Mm04934123_m1 |
| Col3a1 | Mm01254476_m1 | Mmp9 | Mm00442991_m1 |
| Cxcl9 | Mm00434946_m1 | Mmp13 | Mm00439491_m1 |
| Cxcl10 | Mm00445235_m1 | Mpo | Mm01298424_m1 |
| Foxp3 | Mm00475162_m1 | Nfkb1 | Mm00476361_m1 |
| Gzmb | Mm00442837_m1 | Pdcd1 | Mm01285676_m1 |
| Hprt | Mm03024075_m1 | Rorc | Mm01261022_m1 |
| HPRT | Hs02800695_m1 | Tgfb1 | Mm01178820_m1 |
| Ifng | Mm01168134_m1 | Tnfa | Mm00443258_m1 |
| Il1b | Mm00434228_m1 | Vcam1 | Mm01320970_m1 |
Flow cytometry
Immunofluorescence surface staining was performed with antibodies to CD3, CD4, CD8, CD25, CD45.1, CD45.2, CD274 (PD-L1), CD279 (PD-1) listed in Table 2 (all BioLegend, USA), and CD326 (EpCAM) (Miltenyi Biotec, Germany). Dead cells were stained with Pacific Orange™ Succinimidyl Ester (Thermo Fisher Scientific, Germany) and excluded from further analysis. For intracellular cytokine staining, cells were restimulated with PMA (10 ng/ml) and ionomycin (1 µg/ml, both Sigma-Aldrich, Germany) in the presence of GolgiPlug™ (1 µl/ml, BD Biosciences, USA) for 3 h. Cells were PFA fixed and perforated in PBS containing 0,5% saponine and 2% BSA and stained for IL-17, GzmB, Ki67 (all BioLegend, USA), IFNgamma and IL-2 (both BD Biosciences, USA). Apoptotic cells were stained with Annexin V-FITC in Annexin V Binding Buffer (both BD Bioscience, USA) according to BD protocols. Flow cytometry was performed using a BD LSR II cytometer (BD Biosciences, USA) and data were analyzed with FlowJo software V10.6.0.
Table 2.
List of antibodies
| Antibody | Fluorochrome | Clone | Company | Order no |
|---|---|---|---|---|
| Murine PD-L1 | - | 10F.9G2 | Biolegend | 124301 |
| Rat IgG2b,kappa | - | RTK4530 | Biolegend | 400601 |
| anti-mouse CD3 | - | 145–2C11 | Biolegend | 100331 |
| anti-mouse CD28 | - | 37.51 | Biolegend | 102112 |
| anti-mouse CD3 | FITC | 17A2 | Biolegend | 100204 |
| anti-mouse CD4 | PE-Dazzle | RM4–5 | Biolegend | 100566 |
| anti-mouse CD8 | V450 | 53–6.7 | BD Bioscience | 560469 |
| anti-mouse CD45.1 | APC | A20 | Biolegend | 110714 |
| anti-mouse CD45.1 | FITC | A20 | Biolegend | 110706 |
| anti-mouse CD45.2 | FITC | 104 | Biolegend | 109806 |
| anti-mouse GzmB | FITC | GB11 | Biolegend | 515403 |
| anti-mouse PD-1 | BV421 | 29F.1A12 | Biolegend | 135217 |
| anti-mouse PD-L1 | PE | MIH5 | eBioscience | 12–5982-82 |
| anti-mouse IFNgamma | AF700 | XMG.1 | Biolegend | 557998 |
| anti-mouse IL-17A | PE | eBio17B7 | eBioscience | 12–7177-81 |
| anti-human CD3 | AF700 | UCHT1 | Biolegend | 300424 |
| anti-human CD4 | PacificBlue | RPA-T4 | Biolegend | 300521 |
| anti-human IL17A | AF647 | BL168 | Biolegend | 512310 |
| anti-human IFNgamma | PE | 4S.B3 | Biolegend | 502509 |
| Murine PD-L1 | - | PD-L1/B7-H1 | R&D | AF1019 |
| CK19 | - | Troma III | DSHB | - |
| goat-anti Rat | Cy5 | - | Invitrogen | A10525 |
| goat-anti Rat | AF546 | - | Invitrogen | A11081 |
Histology
For mouse derived liver tissue formalin-fixed and paraffin-embedded sections were stained with Hematoxilin/Eosin (H&E, Carl Roth, Germany) according to standard procedure. CK19 (Troma III, DSHB, USA), mouse PD-L1 (R&D Systems, Germany) and CD45.1 (BioLegend, Germany) stainings were performed on cryo-frozen liver tissue from K14-OVAp mice.
CK19 and IL-17A RNAscope® staining was carried out on cryo-frozen tissue from K14-OVAp mice. In situ RNA hybridization was performed using the RNAscope® Fluorescent Multiplex Detection Reagent Kit (Advanced Cell Diagnostic, Italy) according to manufactureŕs instruction. Briefly, cryo-frozen tissue was fixated and endogenous peroxidase activity was blocked. Slides were stained with CK19 (Troma III, DSHB, USA) and goat-anti rat-Cy5 (Invitrogen, Germany) followed by an incubation for 2 hours at 40 °C with target C1 probe specific for IL-17A (Mm-Il17a). The signal was amplified and developed with fluorescent label combination (Amp4 B) for fluorescent detection.
For human CD8/ IL-17 double staining formalin-fixed paraffin-embedded human liver sections were dehydrated, followed by antigen retrieval and endogenous peroxidase block. CD8 was stained using mouse anti-human CD8 (Dako, Germany) and anti-mouse HRP polymer. Peroxidase activity was visualized using 3.3’-Diaminobenzidine (DAB). IL-17A was stained using anti-human IL-17A (R&D Systems, Germany) polyclonal rabbit anti-goat IgG (DAKO, Germany) and anti-rabbit AP-complex (Polap kit, Zytomed, Germany). AP was used to visualize IL-17 using the POLAP-Kit (Zytomed Systems, Germany) according to manufactureŕs instructions. Slides were the counterstained with Haemalaun and mounted with mounting media (both Roth, Germany). All pictures were taken with a Biorevo BZ-9000 fluorescence microscope (Keyence, Japan) at RT. Histological scoring was performed by a pathologist in a blinded fashion and liver inflammation was assessed using the modified hepatitis activity index (mHAI)32.
Statistical analysis
Statistical analysis was performed with GraphPad Prism (V.8.4.3) software. Data are presented as mean ± SD. Differences between two groups were assessed for statistical significance using the Mann-Whitney test. Comparisons between more than two groups were performed by ordinary one-way ANOVA (Analysis of Variance) Test and Tukey`s Post Test if not mentioned otherwise. Significance levels are indicated by asterisks: **** indicate p < 0.0001; *** indicate p < 0.001; ** indicate p < 0.01; * indicates p < 0.05.
Results
Antigen-specific CD8+ T cells deficient in IL-17A/F aggravate experimental autoimmune cholangitis
OT-1IL17ko cells were isolated from crossbred mice. To exclude inherent functional differences, the activation status between OT-1 CD8+ T cells proficient and deficient in IL-17A/F was investigated ex vivo. Proliferative capacity, cytokine secretion and cytotoxicity were found to be unaffected by the lack of IL-17A/F. In addition, the expression of inhibitory molecules (such as Tim3), activation marker (CD69, CD25, CD44) or effector memory marker (CCR7, CD62L) were similar on CD8+ T cells isolated from OT-1wt compared to OT-1IL17ko mice (Suppl. Fig. 1+2).
For cholangitis induction, OVA-specific OT-1wt CD8+ T cells or OT-1IL17ko CD8+ T cells were adoptively transferred into K14-OVAp recipient mice that present the MHC-I restricted OVA-peptide SIINFEKL on cholangiocytes28. On day 5 following adoptive T cell transfer, recipient mice showed periportal inflammation and increased levels of serum transaminases compared to PBS treated controls (Fig. 1A, B). The transferred antigen-specific T cells located in the periductal area of recipient livers (Fig. 1C). We observed increased cholangitis severity in mice receiving OT-1IL17ko T cells, with more severe histological inflammation and increased levels of serum transaminases (Fig. 1A, B).
Fig. 1. Lack of IL-17A/F in antigen-specific CD8+ T cells aggravates cholangitis in K14-OVAp animals.
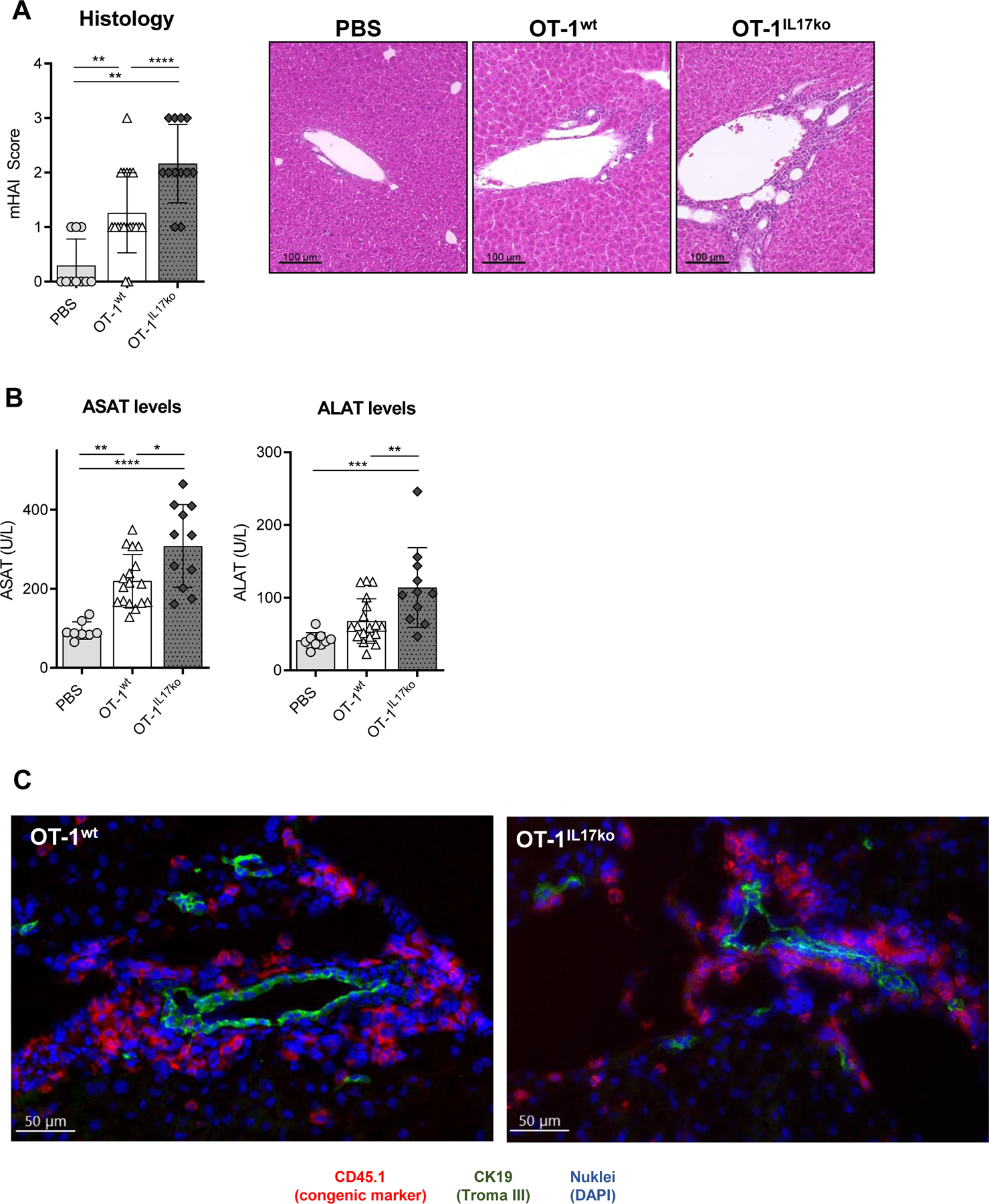
Cholangitis severity in K14-OVAp recipient mice on day 5 after adoptive transfer of OT-1wt or OT-1IL17ko CD8+ T cells: (A) mHAI histological activity index of H&E-stained liver sections and (B) levels of serum transaminases ASAT and ALAT. (C) Representative immunofluorescent staining of congenic cell marker CD45.1 (red, transferred T cells), CK19 (green, cholangiocytes) and nuclei (blue) performed on cryo-sections of K14-OVAp liver after adoptive cell transfer. Data are expressed as mean ± SD; n=10–17, representing pooled data from three independent experiments. Levels of significance: *p≤0,05; **p≤0,01; ***p≤0,001; ****p≤0,0001 (ordinary one-way ANOVA).
Lack of IL-17A/F in antigen-specific CD8+ T cells affects gene expression in cholangiocytes and T cells themselves
We next aimed to elucidate the mechanisms leading to the increase in cholangitis severity induced by OT-1 CD8+ T cells deficient in IL-17A/F. To exclude parenchymal or endothelial cell apoptosis, TUNEL staining of liver sections was performed. This did not reveal any significant differences between the groups (Suppl. Fig. 3). However, transfer of antigen-specific T cells significantly induced the hepatic expression of pro-inflammatory, chemotactic and immune-regulating genes compared to PBS treated controls (Fig. 2A). Importantly, the transfer of OT-1IL17ko CD8+ T cells resulted in significantly increased hepatic mRNA expression of genes regulating T cell recruitment (Vcam1 and Cxcl9), inflammation (Ifng) and cytotoxicity (Gzmb) compared to transfer of OT-1wt CD8+ T cells proficient in IL-17 (Fig. 2B). However, the expression of Ccl20, Il1b and Il6 was significantly reduced. These genes were described to be expressed by cholangiocytes, the target cells of T cell driven inflammation in this model10, 11. To confirm that a direct interaction between CD8+ T cells and cholangiocytes contributes to these findings, primary cholangiocytes were isolated from healthy K14-OVAp mice and co-cultured with OVA-specific OT-1wt or OT-1IL17ko CD8+ T cells (Fig. 2C). Co-culture of OT-1IL17ko CD8+ T cells with primary cholangiocytes resulted in a decreased secretion of CCL20 and IL-6 by cholangiocytes and a greatly increased secretion of T cell-derived IFNgamma. Since this was not a feature of OT-1IL17ko T cells per se, these data pointed to a bidirectional interaction between cholangiocytes and CD8+ T cells dependent on IL-17.
Fig. 2. IL-17A/F-deficiency in OT-1IL17ko CD8+ T cells leads to differential effects on T cell and cholangiocyte activation.
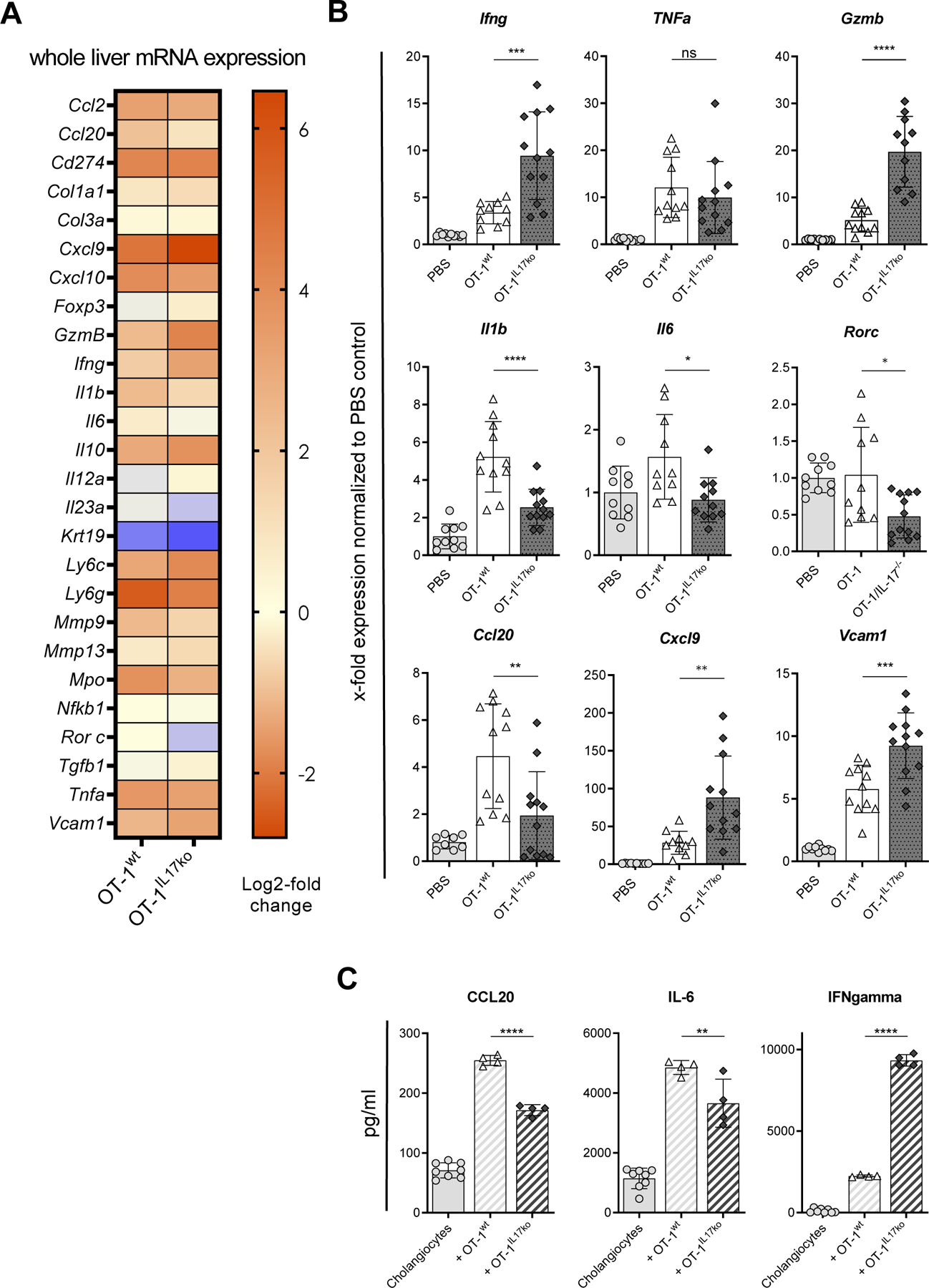
Whole liver mRNA expression analysis of pro-inflammatory, chemotactic and immune-regulating genes from K14-OVAp recipient mice 5 days after transferring OVA-specific OT-1wt cells or OT-1IL17ko cells and PBS as control. (A) Log2-fold change of mRNA expression of all measured genes normalized to PBS treated controls. (B) X-fold mRNA expression of Ifng, Gzmb, Pdcd1, Vcam1, Ccl20, Cxcl9, Il1b and Il6 normalized to PBS treated control mice. (C) Primary cholangiocytes isolated from K14-OVAp mice were cultured alone or in the presence of OT-1wt or OT-1IL17ko CD8+ T cells for 48 h. Chemokine and cytokine secretion were analyzed in supernatants using ELISA. Data are expressed as mean ± SD; n=10–12, representing pooled data from two independent experiments. Levels of significance: *p≤0,05; **p≤0,01; ***p≤0,001; ****p≤0,0001 (ordinary one-way ANOVA).
Antigen-specific CD8+ T cells lacking IL-17A/F show increased proliferation and activation during cholangitis in vivo
We previously demonstrated that liver damage in our model was primarily driven by the transferred antigen-specific OT-1 CD8+ T cells28, 34. In a detailed flow cytometric analysis of liver infiltrating lymphocytes in K14-OVAp animals following transfer of OT-1wt compared to OT-1IL17ko CD8+ T cells we here confirmed that the most prominent differences in T cell populations were observed regarding the frequency of transferred CD8+ T cells by (Suppl. Fig 4). Therefore, we next focused on the phenotype of transferred OT-1wt and OT-1IL17ko T cells during cholangitis. IL-17A/F-deficiency in transferred OT-1IL17ko CD8+ T cells resulted in their increased expression of the cytotoxicity marker granzyme B, whereas high expression of IFNgamma was detected in both IL-17A/F-competent and -deficient OT-1 CD8+ T cells (Fig. 3A). Of note, increased expression of IL-17A in livers of K14-OVAp recipient animals was observed following transfer of OT-1wt CD8+ T cells (Fig. 3B). Confirming our previous findings, the majority of cytokines were produced by the transferred OT-1 CD8+ T cells and not by recruited endogenous T cells (Fig. 3C). Stimulation of MACS-purified T cells confirmed the results observed by flow cytometry (Fig. 3D).
Fig. 3. Enhanced cytotoxicity and cell expansion of OT-1IL17ko CD8+ T cells in experimental cholangitis.
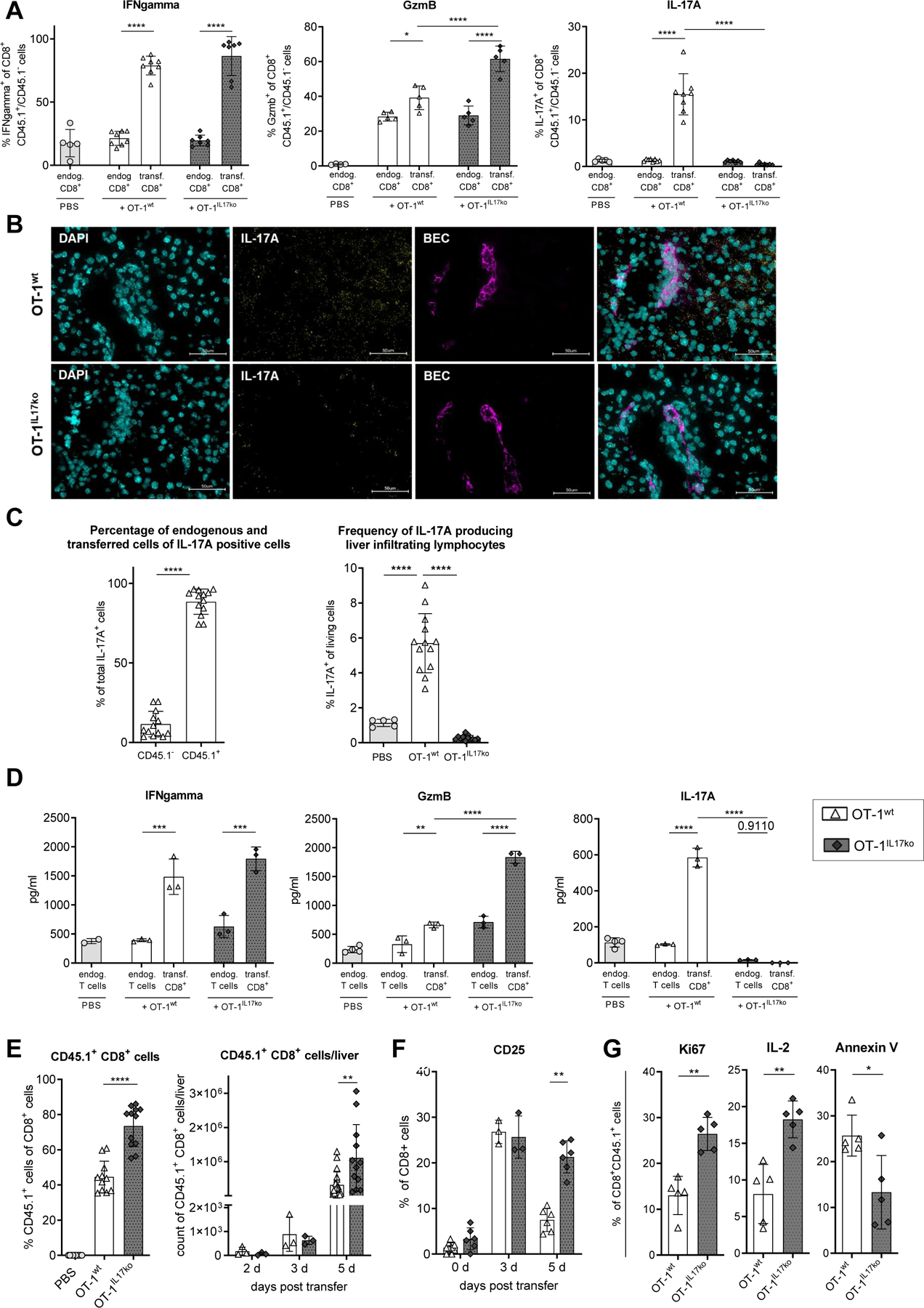
On day 5 after disease induction, transferred OT-1wt or OT-1IL17ko CD8+ T cells were isolated from livers and analyzed. (A) Cytokine expression in endogenous and transferred CD8+ T cells was analyzed by flow cytometry. (B) Immunohistochemical RNA scope staining of IL-17A (yellow) and cholangiocytes (purple) in livers of K14-OVAp animals on day 5 following transfer of OT-1wt or OT-1IL17ko CD8+ T cells. (C) IL-17A expression of total living cells and of total CD45.1− and CD45.1+ (transferred) T cells. (D) Cytokine secretion of stimulated liver infiltrating lymphocytes was quantified by ELISA. Flow cytometry was used to analyze (E) the percentages and total numbers and (F) surface expression of CD25 of OT-1wt and OT-1IL17ko CD8+ T cells within recipient livers. (G) Expression of IL-2, Ki67 and annexin V was analyzed in liver infiltrating OT-1wt and OT-1IL17ko CD8+ T cells using flow cytometry. Data are expressed as mean ± SD; (A), (F), (G) Data from one representative experiment of three independent experiments is shown; (C), (E) n=10–12, representing pooled data from two independent experiments. Levels of significance: *p≤0,05; **p≤0,01; ***p≤0,001; ****p≤0,0001 (ordinary one-way ANOVA (A, C, D, E), multiple t test (E, F), Mann-Whitney U test (G)).
Moreover, significantly increased frequencies and absolute numbers of OT-1 CD8+ T cells were observed in livers of recipient mice on day 5 after transfer of OT-1IL17ko T cells (Fig. 3E). In order to differentiate between increased recruitment and proliferation of transferred T cells, time kinetic experiments were performed. On day 3 following disease induction the numbers of recruited OT-1 CD8+ T cells were not different between cells proficient or deficient in 17A/F and the expression of CD25, the IL-2 receptor alpha chain which is required for T cell proliferation, was similar in both groups. However, on day 5 after cell transfer, IL-17A/F-competent OT-1wt CD8+ T cells showed a marked decrease in expression of CD25, whereas this downregulation was not observed in OT-1IL17ko CD8+ T cells (Fig. 3F). In addition, lack of IL-17A/F in OT-1IL17ko T cells resulted in significantly increased T cell proliferation, indicated by elevated intracellular IL-2 and Ki67 expression and in reduced T cell apoptosis, demonstrated by reduced annexin V staining on day 5 after transfer (Fig. 3G). These results suggested that IL-17 produced by CD8+ T cells served to regulate T cell expansion and contraction in the later phase of inflammation.
IL-17A/F expression by CD8+ T cells induces upregulation of PD-L1 in cholangiocytes
It has previously been shown that cholangiocytes can be activated by pro-inflammatory cytokines. Thus, upon stimulation with IFNgamma, the cytokine highly expressed by transferred OT-1 cells, cholangiocytes upregulated the expression of Ccl2 and Ccl20 mRNA (Suppl. Fig. 5A). Since we now demonstrated that, in our cholangitis model, T cell activation, proliferation and contraction depend on IL-17 and that IL-17 regulates cholangiocyte activation, we next asked how IL-17A/F produced by CD8+ T cells modulates the interaction between T cells and cholangiocytes. The inactivation of effector T cells can be mediated by PD-1/PD-L1 interaction35 and remarkably high PD-1 expression was found on transferred OT-1 CD8+ T cells in the livers of recipient mice independent of their IL-17 expression (Fig. 4A). Thus, we hypothesized that IL-17 might induce the expression of co-inhibitory molecules such as PD-L1 on cholangiocytes. Immunofluorescence staining confirmed the expression of PD-L1 on cholangiocytes in K14-OVAp recipient mice on day 5 after disease induction (Fig. 4B). We next aimed to quantify the expression of PD-L1 on mRNA and protein level and analyzed isolated cholangiocytes from K14-OVAp recipient mice on day 5 after disease induction. After transfer of OT-1wt CD8+ cells competent in IL-17A/F, cholangiocytes showed upregulation of Cd274 mRNA encoding for PD-L1 (Fig. 4C). Significantly lower levels of Cd274 mRNA were found in cholangiocytes targeted by IL-17A/F-deficient OT-1IL17ko CD8+ T cells. At the protein level, flow cytometric analysis confirmed these observations (Fig. 4D). To further determine the effect of IL-17 on the expression of PD-L1 by cholangiocytes, primary mouse cholangiocytes were examined after stimulation with pro-inflammatory cytokines in vitro. IFNgamma, but not IL-17A alone, strongly induced the expression of Cd274 mRNA. However, the expression of Cd274 mRNA in cholangiocytes was highest when cells had been stimulated with a combination of IFNgamma and IL-17A (Fig. 4E; Supp. Fig. 6). In summary, these results show that IL-17 acts as an enhancer of IFNgamma-induced expression of inhibitory PD-L1 in cholangiocytes in vitro and in vivo.
Fig. 4. PD-L1 expression on cholangiocytes is enhanced in the presence of IL-17.
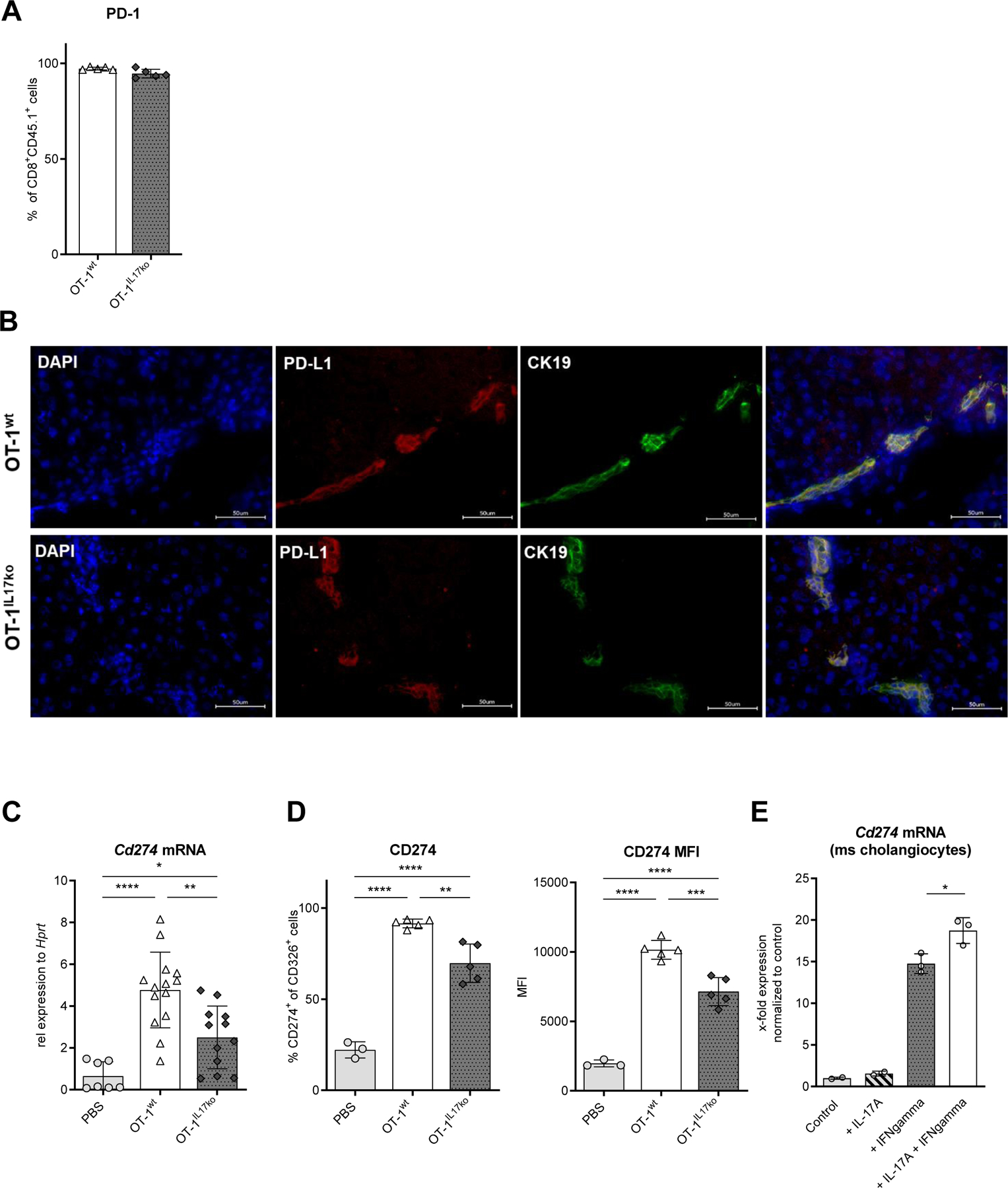
5 days after induction of experimental cholangitis, flow cytometry was used to analyze (A) the surface expression of PD-1 on OT-1wt and OT-1IL17ko CD8+ T cells isolated from liver. (B) Immunofluorescence staining of PD-L1 (red) on cholangiocytes (green) in K14-OVAp mice on day 5 following transfer of OT-1wt and OT-1IL17ko CD8+ T cells. (C) Cd274 mRNA expression and (D) surface expression and MFI of CD274 (PD-L1) on cholangiocytes determined in cholangiocytes isolated on day 5 after cell transfer and cell sorted based on the expression of CD326. Regulation of Cd274 mRNA expression was measured in (E) primary mouse cholangiocytes following cytokine stimulation for 24 h. Data were analyzed using qPCR and normalized to the control group. Bars represent mean ± SD. (A), (D), (E) Data from one representative experiment of three independent experiments is shown; (C) n=10–14, representing pooled data from two independent experiments. Levels of significance: *p≤0,05; **p≤0,01; ***p≤0,001; ****p≤0,0001 (ordinary one-way ANOVA).
Inhibition of PD-L1 signalling aggravates experimental autoimmune cholangitis through uncontrolled T cell expansion
We next aimed to confirm our hypothesis that CD8+ T cell activation and expansion during experimental cholangitis can be regulated via the PD-1/PD-L1 axis. To that end, the development of cholangitis was investigated after adoptive transfer of OT-1wt CD8+ T cells in combination with a blocking antibody to PD-L1. The inhibition of PD-L1-signalling aggravated cholangitis in K14-OVAp recipient animals compared to isotype-treated mice with highly increased histopathological inflammation scores (Fig. 5A) and elevated levels of transaminases (Fig. 5B) on day 5 after adoptive transfer. Analysis of OT-1wt T cells in anti-PD-L1-treated mice showed significantly increased intrahepatic frequencies of transferred antigen-specific CD8+ CD45.1+ T cells (Fig. 5C) with elevated surface expression of CD25 and intracellular expression of IL-2 compared to cells isolated from isotype treated controls (Fig. 5D). Anti-PD-L1 treatment reduced apoptosis of transferred OT-1wt CD8+ T cells, indicated by lower frequencies of annexin V positive OT-1 CD8+ T cells compared to OT-1 CD8+ T cells from isotype-treated mice. Moreover, the expression of pro-inflammatory Ifng and cytotoxic Gzmb mRNA was increased in liver tissue of antibody-treated K14-OVAp mice on day 5 after disease induction (Fig. 5E).
Fig. 5. Inhibition of PD-L1 induces aggravated cholangitis in K14-OVAp recipient animals.
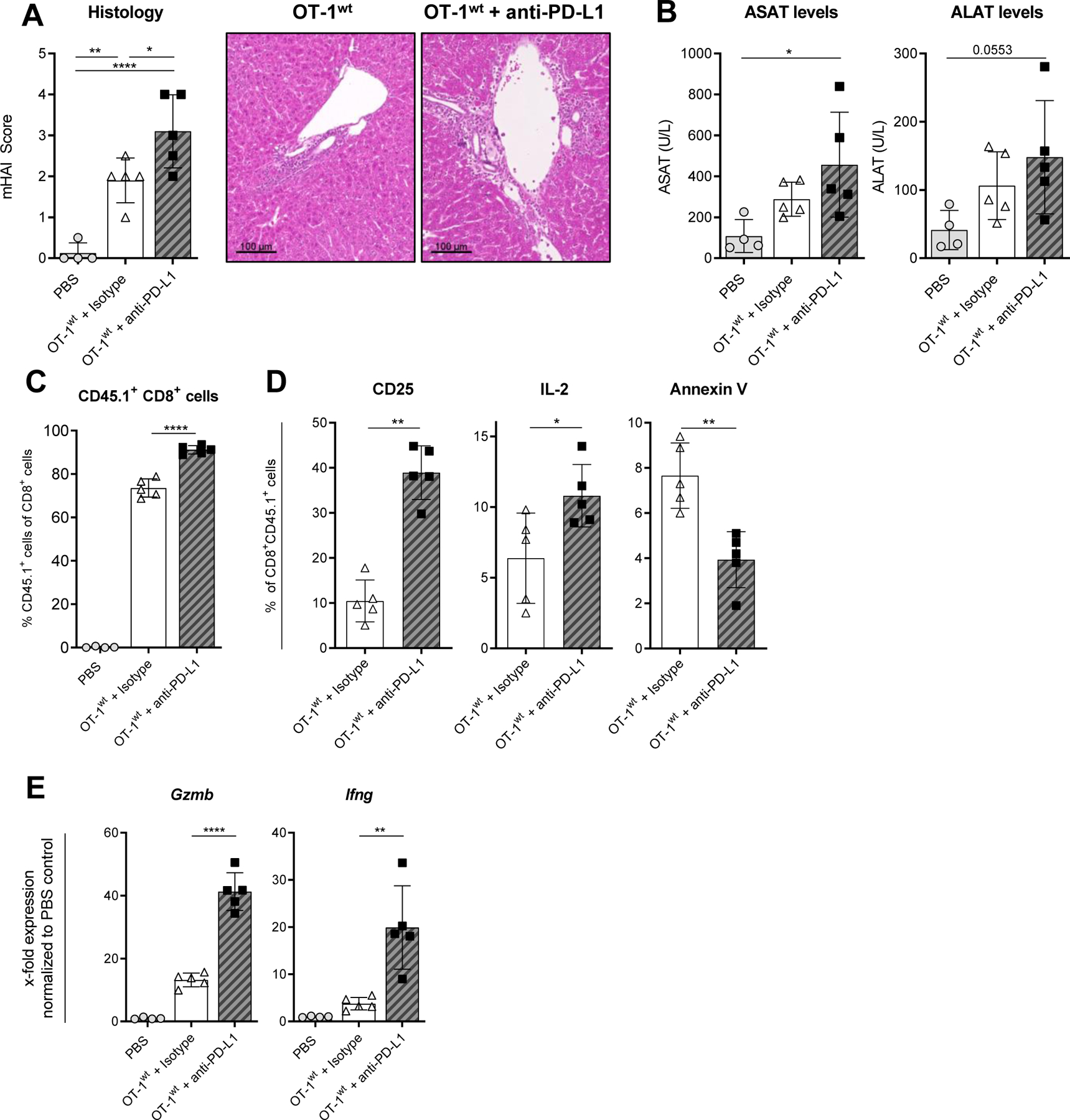
Cholangitis severity was assessed 5 days after adoptive transfer of OT-1wt CD8+ T cells into K14-OVAp mice treated with anti-PD-L1 or isotype-control. (A) mHAI histological activity index of H&E stained liver sections and (B) serum levels of liver transaminases ASAT and ALAT. (C) The percentage of transferred OT-1wt CD8+ CD45.1+ T cells was determined within recipient livers and (D) the expression of CD25, IL-2 and annexin V on OT-1wt CD8+ T cells isolated from livers using flow cytometry. (E) Expression of Gzmb, Ifng and Pdcd1 mRNA was analyzed in K14-OVAp whole liver tissue by qPCR. Data are expressed as mean ± SD; n=4–5, Data from one representative experiment of two independent experiments is shown. Levels of significance: *p≤ 0,05; **p≤ 0,01; ****p≤0,0001 (ordinary one-way ANOVA (A, B, C, E), Mann-Whitney U test (D)).
CD8+ IL-17A producing T cells in patients with cholestatic liver diseases
We next investigated the presence of IL-17A producing CD8+ T cells in human blood and liver from patients with autoimmune cholestatic liver disease. To this end we performed immunohistochemical double-staining for CD8 and IL-17A in human explant liver tissue (Fig. 6A). We observed CD8+ T cells in all analyzed liver sections mainly within portal tracts and liver lobules. Only in the livers of PSC patients we observed CD8+ T cells infiltrating the bile duct epithelial layer and thus close interaction between CD8+ T cells and cholangiocytes. In addition, IL-17A protein expression was detected around bile duct infiltrating CD8+ T cells in PSC. In peripheral blood, we observed significantly increased expression of IL-17A in CD8+ T cells from PSC and PBC patients compared to AIH patients and healthy controls (Fig. 6B). Using human cholangiocyte organoids derived from explant liver tissue, we were able to confirm that the highest expression of CD274 mRNA in cholangiocytes was induced by the combination of IFNgamma and IL-17A (Fig. 6C).
Fig. 6. Presence of CD8+IL17A+ producing T cells in cholestatic liver diseases.
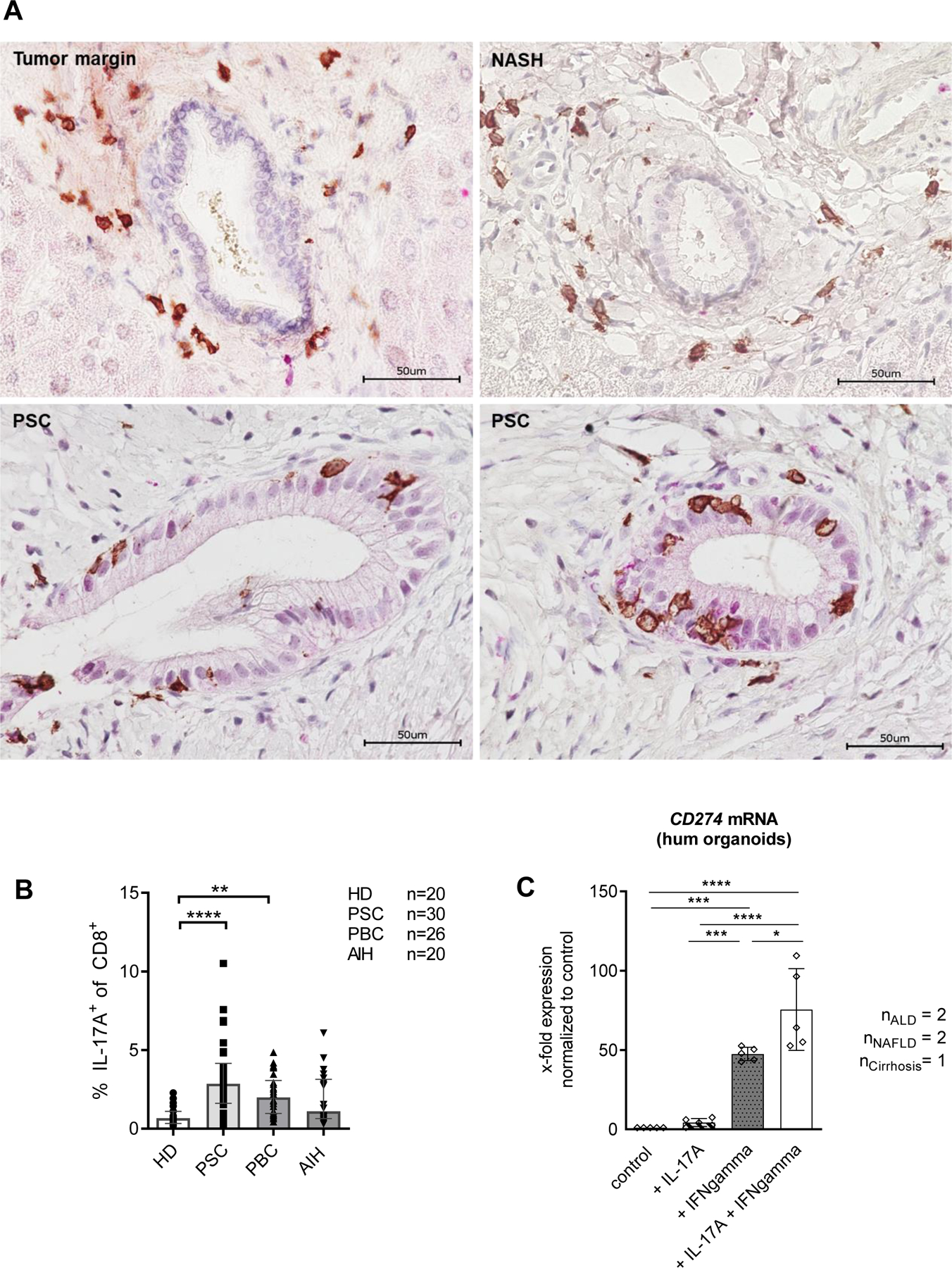
(A) Immunohistochemical double staining for CD8 (brown) and IL-17A (red) in explant liver sections from NASH and PSC, and from unaffected tissue derived from tumour margin. (B) Peripheral blood of patients with PSC (n=30), PBC (n=26), AIH (n=20) and healthy donors (n=20) was stimulated with PMA/Ionomycin for 4 h and analyzed for cytokine production using flow cytometry. (C) CD274 mRNA expression was measured using qPCR in human liver derived organoids generated from ALD (n=2), NAFLD (n=2) and cirrhosis of unknown origin (n=1) following cytokine stimulation for 24 h. (B) Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s Multiple Comparison **p≤0.01, ***p≤0.001. Bars represent mean ± SD. Levels of significance: *p≤0,05; ***p≤0,001; ****p≤0,0001 (ordinary one-way ANOVA).
Discussion
There is no curative treatment for autoimmune cholestatic liver diseases such as PBC and PSC, thus they often progress to end stage liver disease1, 2. Targeted treatment options are therefore urgently needed. PBC and PSC are diseases of the bile ducts, but mucosal immunology of the bile ducts is poorly understood. IL-17 plays a major role in the pathogenesis of several autoimmune diseases outside the gastrointestinal tract15. Even though IL-17-producing T cells have been implicated in PBC and PSC pathogenesis it remains unclear whether IL-17 presents such a therapeutic target due to its highly context-dependent activities, including its antimicrobial functions18. We aimed to dissect the interaction of T cells and cholangiocytes by investigating the effects of IL-17 expressed by CD8+ T cells on cholangiocytes in vitro and in vivo in autoimmune experimental cholangitis.
We previously reported that IL-17+ cells localized around the bile ducts of PSC livers19. Pathogen-induced Th17 differentiation was increased in PSC patients and we recently observed that an increased Th17 differentiation in PSC already occurs in vivo21. The presence of IL-17-producing cells has been associated with a more severe disease phenotype and a harmful role in autoimmune liver diseases11, 36–38. IL-17 is produced by several T cell subsets, including CD8+ T cells. IL-17+ CD8+ T (Tc17) cells have mainly been assigned a pro-inflammatory role, but could also contribute to immune regulation23, 25. The distinct role of IL-17 and Tc17 cells in the pathogenesis of autoimmune liver diseases remains unclear.
In previous work, we described enhanced recruitment of endogenous Th17 cells towards the site of inflammation after disease induction with OT-1wt CD8+ T cells in the K14-OVAp mouse model used in this study28. We hypothesized, that transferred, antigen-specific OT-1wt CD8+ T cells induce initial cholangiocyte activation and subsequent recruitment of endogenous CD4+ T cells to the liver. By inducing cholangitis with antigen-specific OT-1 CD8+ T cells lacking IL-17A/F (OT-1IL17ko), we now demonstrate communication between CD8+ T cells and cholangiocytes. In line with a regulatory function of Tc17 cells23, 25, we observed highly activated and cytotoxic OT-1 T cells in the setting of IL-17A/F deficiency, and increased expansion of these cells.
Mechanistically, CD8+ T cell-derived IL-17 induced the expression of PD-L1 on antigen-presenting cholangiocytes that limited the expansion of self-reactive T cells in cholangitis. PD-L1 presented by APCs is well known to inhibit T cell proliferation after binding to the PD-1 receptor35 and cholangiocytes have been previously described to upregulate expression of PD-L1 and PD-L2 in vitro, protecting themselves by reducing CD8+ T cell cytotoxicity35, 39, 40.
Indeed, imbalanced expression of PD-1/PD-L1 has previously been reported in inflamed livers of PBC and AIH patients41, 42. Moreover, recent case reports on the development of secondary sclerosing cholangitis in patients receiving checkpoint inhibitor treatment highlight the functional importance the PD-L1/PD-1 axis for biliary immune homeostasis43, 44.
Inflammatory cytokines, such as IFNgamma and TNF have already been described as potent inducers of PD-L1 expression45, 46. By activation of STAT347–49 or by its influence on Cd274 mRNA translation46, IL-17 was described to support PD-L1 expression in different epithelial and immune cells. Our in vivo and in vitro data clearly demonstrate that IL-17 significantly enhances the expression of PD-L1 on cholangiocytes, thereby providing mechanistic evidence for the bidirectional communication between CD8+ T cells and cholangiocytes.
We and others have shown that IL-17A induces the expression of Ccl20 mRNA in cholangiocytes in vitro (Suppl. Fig. 6). Secretion of CCL20 by cholangiocytes leads to the recruitment and positioning of CCR6+ Th17 and Tc17 cells around bile ducts11. Furthermore, activated cholangiocytes were shown to promote Th17 and Tc17 differentiation by providing IL-1beta and IL-610. Importantly, we found increased numbers of Tc17 cells in recipient animals after disease induction with IL-17-competent OT-1 CD8+ T cells and the observed reduced cytotoxicity of Tc17 cells may have contributed to the milder inflammation observed in these animals 23–25. Therefore, antigen-specific Tc17 cells seem to engage in a feedback loop with cholangiocytes, which limits auto-reactive T cell expansion in autoimmune experimental cholangitis.
It is tempting to speculate whether IL-17+ T cells seen in PBC and PSC patients also engage in such a feedback loop in human disease. In light of the recently reported pathogenetic role of Th17 cells in PSC, our data underline the need to better define the role of IL-17 in liver inflammation in a cell dependent manner and may help to explain, why broad neutralization of IL-17 may not be effective treatment of cholangitis.
Supplementary Material
Highlights:
IL-17 induces the expression of PD-L1 in mouse and human cholangiocytes
CD8+ T cell-derived IL-17 induces the expression of PD-L1 on antigen-presenting cholangiocytes in an antigen driven, CD8+ T cell mediated experimental cholangitis model
PD-L1 expression by cholangiocytes restricts the expansion of self-reactive T cells in experimental cholangitis
caution may be warranted using broad neutralization of IL-17 for the treatment of T cell mediated cholangitis.
Acknowledgements
We are grateful for excellent technical assistance by Sabrina Kress, Jennifer Wigger Angelika Schmidt, Nina Verse, Marko Hilken, Carsten Rothkegel and Susanne Roscher. We thank Mariangela Amenduni for introduction into organoid cell culture and Elaine Hussey for critical reading. Cell sorting was performed by the flow cytometry core facility of the University Medical Center Hamburg-Eppendorf. We thank Marcial Sebode and Stefan Wolter for providing human material. Mice were kindly provided by Kirsten Hogquist, Minnesota, USA and Immo Prinz, Hannover, Germany. H69 cells were kindly provided by Ulrich Beuers, University of Amsterdam and Douglas M. Jefferson, Tufts University School of Medicine Department of Integrative Physiology and Pathobiology, Boston. Rspo-1 cells were kindly provided by Calvin J Kuo, Stanford University, London. This study was supported by the German Research Foundation (DFG), CRC 841(CS, NG), the YAEL-Foundation and the Helmut and Hannelore Greve Foundation. MS and RF acknowledge the support of the NIH grants DK101528, 5 R01 DK096096–08, and DK034989, Silvio O. Conte Digestive Diseases Research Core Center (Cellular, Molecular, and Clinical/Translational cores), and a grant from PSC Partners Seeking a Cure Foundation (AWD0002203, Proposal ID: 18–004707)
Grant support
Supported by the Deutsche Forschungsgemeinschaft (CRC 841), the YAEL-Foundation and the Helmut and Hannelore Greve Foundation.
Abbreviations
- IL
Interleukin
- PD
programmed cell death protein
- PDL-1
Programmed cell death 1 ligand
- OVA
Ovalbumin
- OT
Ovalbumin transgene
- PSC
primary sclerosing cholangitis
- PBC
primary biliary cholangitis
- TLR
Toll like receptor
- Treg
regulatory T cells
- ALAT
alanine serum aminotransferase
- ASAT
aspartate serum aminotransferase
- ALD
alcoholic liver disease
- NAFLD
non-alcoholic fatty liver disease
- CK
Cytokeratin
- MFI
mean fluorescence intensity
Footnotes
Conflict of interest
The authors do not have anything to disclose.
Data availability statement:
Data are available upon request.
References
- [1].Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis: a comprehensive review. J Hepatol 2017; 67:1298–1323 [DOI] [PubMed] [Google Scholar]
- [2].Hirschfield GM and Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol 2013; 8:303–30 [DOI] [PubMed] [Google Scholar]
- [3].Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol 2012; 57(3):675–88 [DOI] [PubMed] [Google Scholar]
- [4].Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 2019; 4:492–503 [DOI] [PubMed] [Google Scholar]
- [5].Kummen M, Holm K, Anmarkrud JA, Nygard S, Vesterhus M, Hoivik ML et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017; 66: 611–619 [DOI] [PubMed] [Google Scholar]
- [6].Yokoyama T, Komori A, Nakamura M, Takii Y, Kamihira T, Shimoda S et al. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-κB and -MAPK signaling pathways. Liver International 2006; 26(4), 467–476 [DOI] [PubMed] [Google Scholar]
- [7].Chen XM, O’Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol 2005; 175: 7447–7456 [DOI] [PubMed] [Google Scholar]
- [8].Harada K, Shimoda S, Ikeda H, Chiba M, Hsu M, Sato Y et al. Significance of periductal Langerhans cells and biliary epithelial cell-derived macrophage inflammatory protein-3alpha in the pathogenesis of primary biliary cirrhosis. Liver Int 2011; 31:245–53 [DOI] [PubMed] [Google Scholar]
- [9].GUICCIARDI ME, TRUSSONI CE, LARUSSO NF, GORES GJ. The Spectrum of Reactive Cholangiocytes in Primary Sclerosing Cholangitis. Hepatology 2020; 71(2):741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol 2009; 157: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].OO YH, BANZ V, Kavanagh D, Liaskou E, Withers DR, Humphreys E et al. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol 2012; 57(5):1044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH. Intercellular adhesionmolecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut 1993; 34:1245–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morland CM, Fear J, McNab G, Joplin R, Adams DH. Promotion of leukocyte transendothelial cell migration by chemokines derived from human biliary epithelial cells in vitro. Proc Assoc Am Physicians 1997; 109:372–382 [PubMed] [Google Scholar]
- [14].Afford SC, Humphreys EH, Reid DT, Russell CL, Banz VM, Oo Y et al. Vascular cell adhesion molecule 1 expression by biliary epithelium promotes persistence of inflammation by inhibiting effector T-cell apoptosis. Hepatology 2014; 59(5):1932–43 [DOI] [PubMed] [Google Scholar]
- [15].Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm 2017; 2017:3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Langley RG1, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 2014; 371(4):326–38 [DOI] [PubMed] [Google Scholar]
- [17].Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PDR et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61:1693–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 2017; 18(6):612–621 [DOI] [PubMed] [Google Scholar]
- [19].Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013; 58:1084–93 [DOI] [PubMed] [Google Scholar]
- [20].SEBODE M, PEISELER M, Franke B, Schwinge D, Schoknecht T, Wortmann F et al. Reduced FOXP3(+) regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J Hepatol 2014; 60(5):1010–1016 [DOI] [PubMed] [Google Scholar]
- [21].KUNZMANN LK, SCHOKNECHT T, Poch T, Henze L, Stein S, Kriz M et al. Monocytes as potential mediators of pathogen-induced Th17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 2020; doi: 10.1002/hep.31140 [DOI] [PubMed] [Google Scholar]
- [22].AI G, YAN W, Yu H, Xiao F, Xi D, Ma K et al. Soluble Fgl2 restricts autoimmune hepatitis progression via suppressing Tc17 and conventional CD8+ T cell function. J Gene Med 2018; 20(7–8):e3023. [DOI] [PubMed] [Google Scholar]
- [23].BILLERBECK E, KANG YH, Walker L, Lockstone H, Grafmueller S, Fleming V et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA 2010; 107(7):3006–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].LIU SJ, TSAI JP, Shen CR, Sher YP, Hsieh CL, Yeh YC et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6, J. Leukoc.Biol 2007; 82, 354–360 [DOI] [PubMed] [Google Scholar]
- [25].Srenathan U, Steel K, Taams LS. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol Lett 2016; 178:20–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med 2002; 195(1):113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol 2013; 8:303–330 [DOI] [PubMed] [Google Scholar]
- [28].Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G et al. Testosterone Suppresses Hepatic Inflammation by the Downregulation of IL-17, CXCL-9, and CXCL-10 in a Mouse Model of Experimental Acute Cholangitis. J Immunol 2015; 194(6):2522–30 [DOI] [PubMed] [Google Scholar]
- [29].Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010;160: 1577–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schaub JR, Huppert KA, Kurial SNT, Hsu BY, Cast AE, Donnelly B, et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 2018; 557:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Groen PC, Vroman B, Laakso K and LaRusso NF. Characterization and Growth Regulation of a Rat Intrahepatic Bile Duct Epithelial Cell Line under Hormonally Defined, Serum-Free Conditions. Lab Invest 1996; 74(1):303–13 [DOI] [PubMed] [Google Scholar]
- [32].Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 2016; 11(9):1724–43 [DOI] [PubMed] [Google Scholar]
- [33].Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696–699 [DOI] [PubMed] [Google Scholar]
- [34].GLASER F, JOHN C, ENGEL B, HÖH B, Weidemann S, Dieckhoff J et al. Liver infiltrating T cells regulate bile acid metabolism in experimental cholangitis. J Hepatol 2019; 71(4):783–792 [DOI] [PubMed] [Google Scholar]
- [35].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med 2000; 192:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].LAN RY, SALUNGA TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. JAutoimmun 2009; 32(1):43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Longhi MS, Liberal R, Holder B, Robson SC, Ma Y, Mieli-Vergani G, Vergani D. Inhibition of interleukin-17 promotes differentiation of CD25⁻ cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology 2012; 142(7):1526–35.e6 [DOI] [PubMed] [Google Scholar]
- [38].Liberal R, Grant CR, Ma Y, Csizmadia E, Jiang ZG, Heneghan MA et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun 2016; 72:102–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Waeckerle-Men Y; Starke A; Wüthrich RP. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+ cytotoxic T cells. Nephrology, Dialysis, Transplantation 2007; 22(6):1527–1536 [DOI] [PubMed] [Google Scholar]
- [40].Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology 2005; 41: 151–159 [DOI] [PubMed] [Google Scholar]
- [41].Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C et al. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol 2007; 102:302–312 [DOI] [PubMed] [Google Scholar]
- [42].Agina HA, Ehsan NA, Abd-Elaziz TA, Abd-Elfatah GA, Said EM, Sira MM. Hepatic expression of programmed death-1 (PD-1) and its ligand, PD-L1, in children with autoimmune hepatitis: relation to treatment response. Clin Exp Hepatol 2019; 5(3):256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kono M, Sakurai T, Okamoto K, Masaki S, Nagai T, Komeda Y et al. Efficacy and Safety of Chemotherapy Following Anti-PD-1 Antibody Therapy for Gastric Cancer: A Case of Sclerosing Cholangitis. Intern Med 2019; 58(9):1263–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].OGAWA K, KAMIMURA K, Terai S. Antiprogrammed Cell Death-1 Immunotherapy-Related Secondary Sclerosing Cholangitis. Hepatology 2019; 69:914–916 [DOI] [PubMed] [Google Scholar]
- [45].Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep 2017; 19(6):1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, You Z. Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett 2017; 184:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler ME et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008; 105(52):20852–20857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018; 132(11):1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hu Z, Luo D, Wang D, Ma L, Zhao Y, Li L. IL-17 Activates the IL-6/STAT3 Signal Pathway in the Proliferation of Hepatitis B Virus-Related Hepatocellular Carcinoma. Cell Physiol Biochem, 2017; 43(6):2379–2390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.


