Abstract
A reverse line blot (RLB) assay was developed to identify different Trichinella genotypes. The RLB assay accomplishes detection and specific identification of the different Trichinella genotypes and relies on hybridization of the amplified 5S ribosomal DNA intergenic spacer regions to specific, membrane-bound oligonucleotide probes. After one single amplification, we were able to detect and genetically identify six sibling species, i.e., T. spiralis, T. britovi, T. nativa, T. murrelli, T. nelsoni, and T. pseudospiralis, and two additional Trichinella genotypes, T6 and T8. Twenty-four Trichinella strains of different genotypes were unequivocally identified evaluated using one simple PCR-based assay based on single larvae. This assay allows the specific identification of Trichinella species without the need to passage larvae in laboratory animals.
Trichinellosis is one of the most important parasitic food-borne zoonotic diseases, caused by the consumption of insufficiently heated or raw meat infected with larvae of the genus Trichinella. In the European Union, monitoring is mandatory, based on the detection of Trichinella larvae during inspection of meat from pigs and horses. Control measures after positive findings consist of withdrawal of infected meat from the food chain. Still, recent outbreaks have been reported in France (1998; www.promedmail.org) and Germany (1998/1999; www.promedmail.org). Outbreaks can result from the consumption of imported meat from countries where the organism is endemic or of locally extensively produced meat infected with sylvatic Trichinella species (8, 22). Today, seven species (five with encapsulated larvae and two with nonencapsulated larvae) and three additional genotypes of undefined level have been identified (20, 21, 24, 30) by biochemical, biological, and molecular studies (5, 6, 7, 9, 10, 13, 14, 16, 25, 26, 33). Of these, Trichinella britovi, T. nativa, T. nelsoni, T. murrelli, T. pseudospiralis, T. papuae, T6, T8, and T9 are predominantly found in wildlife, whereas T. spiralis is able to maintain itself in domestic pigs and horses and is therefore the main source of infections in humans (4, 12). All these Trichinella genotypes are morphologically similar except T. pseudospiralis and T. papuae, which can be identified by the absence of a collagen capsule in the nurse cell during the larval muscle phase (25). It is important to identify single larvae to their genotype level to determine the source of infection, the importance to public health, and the clinical course in humans. Furthermore, the discovery that mixed infections can occur in the same host stresses the need for a test to identify single larvae (23, 24). Until now, many efforts have been made to identify isolated larvae to their specific genotype level. PCR-based assays with specific primer sets have been developed (1, 29, 32, 34). However, for these assays multiple PCRs using different primer sets are sometimes needed, and the assay technique is not always applicable when one or a few single larvae are isolated, due to the lack of a sufficient amount of DNA. Random amplified polymorphic DNA analysis is based on the fast detection of genetic markers using only a single arbitrary primer and can be used without prior sequence information (31). This is one of the first PCR-based methods which is described for the genus Trichinella to identify a single larva at the species level (2, 3). However, in random amplified polymorphic DNA analysis there are problems with the reproducibility between different tests and among different laboratories. Also, if the DNA is partially damaged, the electrophoretic banding pattern will be hard to interpret by comparison to an undamaged specimen, especially for isolates that are closely related (2, 19). It was our aim to develop an easy and applicable method, based on a single PCR, for the identification of different Trichinella genotypes. Liu et al. (17, 18) reported that the 5S rRNA genes in nematodes are highly conserved, while the intergenic spacer regions between the tandemly repeated genes can vary greatly in size and nucleotide composition between closely related organisms. Recently, we used oligonucleotide primers based on the invariant regions of the conserved, rather small 5S rRNA gene to amplify the spacer region. We analyzed the amplified 5S rRNA intergenic region of the different Trichinella genotypes using the cleavage fragment length polymorphism assay (28). It was shown that there was prominent polymorphism within this region between T. spiralis and the sylvatic Trichinella genotypes and minor polymorphism among the sylvatic Trichinella genotypes.
In this study, we analyzed the DNA sequences of the ribosomal intergenic spacer regions of the eight different Trichinella genotypes. Differences in nucleotide composition which were consistent for each genotype were found. The differences in DNA sequence of the 5S rRNA intergenic spacer region were used to develop a reverse line blot (RLB) assay (11, 27) with the goal of detecting and identifying the eight different genotypes of Trichinella based on single larvae. With this new method we were able to simultaneously detect and differentiate six Trichinella species, i.e., Trichinella spiralis, T. britovi, T. nativa, T. murrelli, T. nelsoni, and T. pseudospiralis, and two additional Trichinella genotypes, T6 and T8, on the basis of differences in the 5S rRNA intergenic nontranscribed region. In this paper we show that the RLB assay provides a highly sensitive and specific tool for the unequivocal identification of Trichinella genotypes based on differences in the spacer region of the 5S rRNA genes and can be carried out on single larvae.
MATERIALS AND METHODS
Isolates.
From each of six identified Trichinella species and two additional genotypes, different isolates were used in this study (Table 1). The genotypes of all isolates studied, except one (code Z191), were previously identified at the International Trichinella Reference Center in Rome, Italy (20). All isolates were maintained in laboratory mice by serial passages. Muscle larvae were isolated by artificial digestion in 5 g of pepsin and 30 ml of 2.4 N HCl in 400 ml of water using Trichomatic35 (Foss Electric, Hillerød, Denmark) for 12 min.
TABLE 1.
Trichinella strains used in this study (28)
| No. | Isolatea | Species |
|---|---|---|
| 1 | ISS 3 | T. spiralis (T1) |
| 2 | RIVM (ISS 14) | T. spiralis |
| 3 | MAD83 (ISS 92) | T. spiralis |
| 4 | CO77 (ISS 88) | T. spiralis |
| 5 | ISS 2 | T. britovi (T3) |
| 6 | ISS 324 | T. britovi |
| 7 | ISS 384 | T. britovi |
| 8 | Monegrillo (ISS 89) | T. britovi |
| 9 | ZI91 | T. britovi |
| 10 | ISS 10 | T. nativa (T2) |
| 11 | ISS 296 | T. nativa |
| 12 | ISS 335 | T. nativa |
| 13 | ISS 29 | T. nelsoni (T7) |
| 14 | ISS 232 | T. nelsoni |
| 15 | ISS 103 | T. murrelli |
| 16 | ISS 346 | T. murelli |
| 17 | ISS 456 | Trichinella T6 |
| 18 | ISS 34 | Trichinella T6 |
| 19 | ISS 334 | Trichinella T6 |
| 20 | ISS 124 | Trichinella T8 |
| 21 | ISS 148 | Trichinella T8 |
| 22 | ISS 149 | Trichinella T8 |
| 23 | ISS 13 | T. pseudospiralis (T4) |
| 24 | ISS 470 | T. pseudospiralis |
ISS, Instituto Superiore di Sanità, Rome, Italy; RIVM, National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
DNA isolation from individual larvae.
For isolation of genomic DNA from single larvae, each larva was heated in Tris-HCl (pH 7.6) at 90°C for 10 min and treated with proteinase K (100 μg/ml in a total volume of 10 μl) at 48°C for at least 3 h (3). After inactivation of the proteinase K, 5 μl was amplified by PCR.
Preparation of genomic DNA from Trichinella larvae.
Total genomic DNA was extracted from a pool of 2,000 muscle larvae with the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.), using the plant tissue protocol according to the manufacturers' instructions. Briefly, larvae were homogenized in 600 μl of cell lysis solution for 60 min at 65°C. After RNase treatment proteins were precipitated with 200 μl of protein precipitation solution, followed by DNA precipitation with isopropanol. After a washing with 70% ethanol, DNA was dissolved in 100 μl of distilled water.
PCR.
Primers to amplify the 5S ribosomal DNA (rDNA) intergenic spacer region of Trichinella have been described by Liu et al. (17). A biotin-labeled sense primer (5′-GCGAATTCTTGGATCGGAGACGGCCTG-3′) and antisense primer (5′-GCTCTAGACGAGATGTCGTGCTTTCAACG-3′) were used in the PCR. PCR was optimized for pH and MgCl2 concentration. PCR amplification was carried out on 5 μl of DNA from one individual larva in a total volume of 50 μl on a Perkin Elmer 4800 cycler (28). Briefly, the reaction mixtures contained 1.5 mM MgCl2, 200 μM concentrations of each deoxynucleotide triphosphate, 1.5 U of Taq DNA polymerase (Perkin Elmer), and 25 pmol of each primer and were covered with mineral oil. Thermal cycling conditions were 94°C for 90 s for 1 cycle, 94°C for 30 s, 48°C for 1 min, and 72°C for 1 min for 40 cycles followed by a 10-min extension at 72°C.
DNA sequence analysis.
The PCR products were purified with a QIAquick PCR purification kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer's instructions. The purified products were directly sequenced using 15 pmol of the PCR primers (without the biotin label) with an ABI Prism Bigdye terminator cycle sequencing ready reaction kit (Perkin Elmer, Applied Biosystems, Foster City, Calif.). All reactions were run on an Applied Biosystems (ABI) 373 DNA sequencing apparatus (Perkin Elmer). The DNA sequences of the amplicons were analyzed using DNasis software, version 2.5 (Hitachi Software Engineering Co., Ltd.).
RLB hybridization.
For each Trichinella genotype, a specific oligonucleotide probe with an N-terminal N-(trifluoracetamidohexyl-cyanoethyl, N,N-diisopropyl phosphoramidite)-C6 amino linker (Isogen, Maarsen, The Netherlands) had been designed (Table 2). The oligonucleotides were diluted in 150 μl of 0.5 mM Na2CO3 (pH 8.4) to a final concentration of 50 to 1,000 pmol/μl. The probes were covalently linked to an activated Biodyne C blotting membrane (Pall Biosupport, Ann Arbor, Mich.). The membrane was activated by incubation in 16% 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (Sigma) for 10 min at room temperature, rinsed with distilled water, and applied in a MN45 miniblotter system (Immunetics, Cambridge, Mass.). Each slot of the miniblotter was filled with one of the specific oligonucleotide dilutions and incubated for 1 min at room temperature. After the dilutions were aspirated, the membrane was inactivated in 100 mM NaOH for 10 min at room temperature, followed by washing with 2× SSPE (2× SSPE is 36 mM NaCl, 2 mM NaH2PO4, and 0.2 mM EDTA, pH 7.2)–0.1% sodium dodecyl sulfate (SDS) for 5 min at 60°C. The membrane was replaced in the miniblotter, perpendicular to the previously applied specific oligonucleotides. In 150 μl of 2× SSPE–0.1% SDS, 10 μl of PCR product was heat denatured for 10 min and placed on ice immediately. The products were applied to the miniblotter and incubated for 60 min at 45°C. After aspiration the membrane was washed in 2× SSPE–0.5% SDS twice, for 10 min each time, at 57°C. The membrane was subsequently incubated in a 1:4,000 dilution of streptavidin-peroxidase (Boehringer Mannheim, Mannheim, Germany) in 2× SSPE–0.5% SDS at 42°C for 60 min. The membrane was washed twice, for 10 min each time, in 2× SSPE–0.5% SDS at 42°C, followed by two rinses, 5 min each time, with 2× SSPE at room temperature. Hybridized biotin-labeled PCR products were detected using the ECL detection system according to the procedure of the manufacturer (Amersham). The membrane was exposed to a chemiluminescent detection film (Boehringer Mannheim) for 20 min, and afterwards the film was developed in a Fuji X-ray film processor (Fuji Medical Systems Benelux).
TABLE 2.
Sequences of the oligonucleotide probes used in the RLB assay
| Oligonucleotide | Sequence | Probe location | Tm (°C) | Specificity | Oligonucleotide concn (pmol) |
|---|---|---|---|---|---|
| T | CCAATATCATCGGTGCAGTT | 605–663 | 58.2 | Trichinella spp.a | 100 |
| Ts | TCCTTTTAAGACCACAGTGG | 641–659 | 58.2 | T. spiralis | 1,000 |
| Tbr | TGGTGCATTTTTTCCAATTGTC | 582–603 | 57.7 | T. britovi | 100 |
| Tna | GACATTTGCTAAACGAATCAAC | 662–683 | 57.7 | T. nativa | 100 |
| Tne | GTTTTAGCGAATGTTTAATTTCATA | 99–123 | 57.8 | T. nelsoni | 25 |
| Tmu | TTTTATAACCAGACTGGCCG | 637–656 | 58.2 | T. murrelli | 200 |
| T6 | TTATAACCAAAGTGGCCTAAAATAT | 630–654 | 58.0 | T6 | 400 |
| T8 | ATATCAATTTATGACACTGAACAAG | 418–442 | 58.0 | T8 | 12.5 |
| Tps | TAGTGTTGTAGAAGGATCATCA | 316–336 | 57.7 | T. pseudospiralis | 200 |
Not including T. pseudospiralis.
The membrane can be reused after stripping of the PCR products by incubating the membrane in 100 ml of 1% SDS at 80°C twice for 30 min each time followed by incubation of the membrane in 100 ml of 20 mM EDTA for 15 min at room temperature. After the fluid is discarded, the membrane can be stored in Saran Wrap at 4°C.
Nucleotide sequence accession numbers.
DNA sequences were submitted to GenBank under accession numbers AY009943 to AY009950.
RESULTS
PCR.
The two selected primers amplified a single prominent 5S rDNA intergenic spacer region product of approximately 750 bp for all Trichinella genotypes tested, except for T. pseudospiralis. This species gave a product of 522 bp after amplification (28). The PCR can be applied both to DNA extracts of pooled and to individual larvae.
Determination of the DNA sequences of the 5S rDNA intergenic region.
For each Trichinella genotype, PCR products of the 5S rDNA intergenic regions of two different isolates were directly sequenced from both strands. After alignment of the 5S rRNA intergenic spacer regions of the genotypes T. spiralis, T. britovi, T. nativa, T. nelsoni, T. murrelli, T6, and T8, several differences in the DNA sequences were found. These differences were consistently found in both isolates of each species sequenced (data not shown). The different length of the PCR product of T. pseudospiralis and the large number of differences in the DNA sequence made it impossible to include this species in the multiple alignment.
Development of specific oligonucleotides for RLB assay.
For each genotype a specific oligonucleotide was designed (Table 2), derived from the 5S rDNA intergenic region. The locations of the different probes are shown in Table 2. For the selection of the probes, DNA sequences which showed as many mismatches as possible between the different genotypes were chosen. All of the membrane-bound oligonucleotides had a melting temperature between 57.7 and 58.2°C, which enables simultaneous hybridization. Also, a genus-specific oligonucleotide which can identify seven genotypes other than T. pseudospiralis was developed. The DNA sequence of T. pseudospiralis differed too much from sequences of the other genotypes. For this species, a specific oligonucleotide was developed.
For each oligonucleotide probe, the optimal concentration was empirically determined in such a way that all the specific hybridizations resulted in signals of approximately the same level of intensity. These concentrations are also listed in Table 2.
Specificity of the RLB assay.
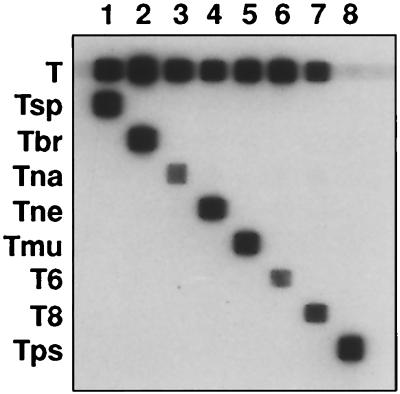
To test the specificity of the RLB assay, the 5S rDNA intergenic region of one reference strain of each of the eight genotypes was amplified. The forward primer TS5F was labeled with biotin to enable direct hybridization to the selected, membrane-bound oligonucleotides. The washing step after the hybridization was performed at 57.0°C. PCR products of the Trichinella strains hybridized with the specific selected oligonucleotides only, without cross-reaction. (Fig. 1). Also, the “catchall” Trichinella control oligonucleotide probe reacted with all the isolates except T. pseudospiralis, which had a deviant sequence. Therefore, a T. pseudospiralis-specific oligonucleotide was included in the assay.
FIG. 1.
RLB assay with 5S rDNA intergenic PCR products of reference strains of each Trichinella genotype. The oligonucleotide probes are in the horizontal lanes, and the PCR products are in the vertical lanes. The oligonucleotides are abbreviated as in Table 2. Lanes: 1, T. spiralis CO77; 2, T. britovi ISS 2; 3, T. nativa ISS 296; 4, T. nelsoni ISS 29; 5, T. murrelli ISS 346; 6, T6 ISS 34; 7, T8 ISS 149; 8, T. pseudospiralis ISS13.
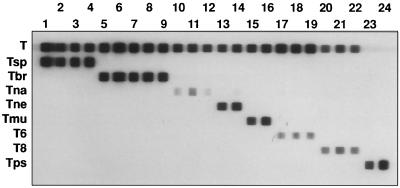
To test the reproducibility of the RLB assay, a total of 24 Trichinella strains of different genotypes were tested in the RLB assay (Table 1). All Trichinella strains of the same genotype gave the same hybridization pattern. Only T. nelsoni strain ISS232 hybridized slightly with the T. nativa oligonucleotide (Fig. 2).
FIG. 2.
RLB assay with 5S rDNA intergenic PCR products of all Trichinella strains, as listed in Table 1. Lane numbers correspond to numbers shown in Table 1. The oligonucleotide probes are in the horizontal lanes, and the PCR products are in the vertical lanes. The oligonucleotides are abbreviated as in Table 2.
Sensitivity of the RLB assay.
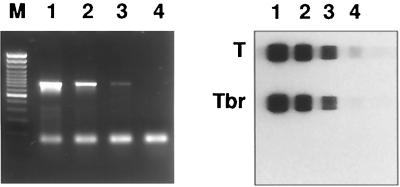
A 10-fold dilution of Trichinella britovi DNA in water was made from a pool of larvae. The 5S rDNA intergenic region was amplified and visualized after agarose gel electrophoresis to determine the detection limit compared with the RLB assay. The RLB assay was more sensitive by at least a factor of 10, as determined by comparing the visibility after gel electrophoresis (Fig. 3).
FIG. 3.
Agarose gel electrophoresis and RLB assay with PCR products of serial dilutions of DNA of T. britovi (1–4). M, 100-bp marker (Fermentas). The RLB assay was performed with the genus-specific Trichinella probe (T). Tbr, species-specific T. britovi probe.
Detection of mixed infections.
To test the ability to detect mixed infections, 10-pg portions of DNA of the following pairs of genotypes were mixed in equal amounts: T. spiralis and T. britovi, T. nativa and T. spiralis, T. britovi and T. nativa, and T. nelsoni and T. nativa. PCR was performed, and the products were used in an RLB assay. For every mixture, we were able to detect both Trichinella genotypes simultaneously without any cross-reactions (Fig. 4).
FIG. 4.
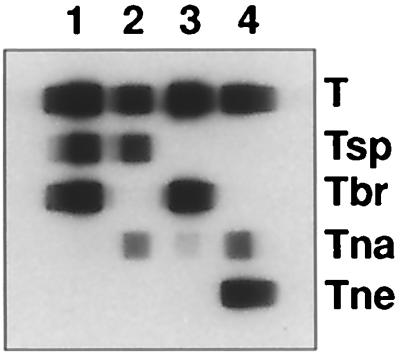
RLB assay with PCR products of mixed DNA of different Trichinella genotypes. Lane 1, PCR products of T. spiralis and T. britovi DNAs; lane 2, PCR products of T. spiralis and T. nativa DNAs. Abbreviations are as in Table 2.
DISCUSSION
We developed an RLB assay (11, 27) to identify and differentiate eight different Trichinella genotypes based on a single PCR assay. The distribution of domestic and sylvatic Trichinella genotypes still constitutes a risk of human trichinellosis (22). For this reason it is important to reduce the risk of infection and to provide more information of the maintenance of different Trichinella genotypes in nature and in different host species. Over the last few years, analysis of different genetic, biochemical, and biological data has contributed to the identification of 10 different genotypes. Of these, eight different genotypes were used in this study. The RLB assay is based on variations in the DNA sequences between 5S rDNA genes within the genus Trichinella. The PCR for the amplification of the 5S rDNA intergenic spacer region, using primers within the conserved region 5S rDNA gene (17, 18), resulted in a single product for all the different genotypes studied. A prominent band of approximately 750 bp was found. Only for the isolates of T. pseudospiralis a smaller product of 522 bp was generated (28). This difference in length of the 5S rRNA intergenic spacer again emphasizes the genetic differences between the nonencapsulated T. pseudospiralis and the other seven encapsulated Trichinella genotypes studied. In addition, after analyses of the DNA sequence of the eight genotypes, no identical sequence in the 5S rRNA intergenic region between T. pseudospiralis and the other genotypes was found. The differences in nucleotide composition of the generated amplicons within the other seven genotypes showed enough variability to develop an RLB assay. Genotype-specific oligonucleotides based on this region were designed and immobilized on a membrane. The oligonucleotides can hybridize specifically with the biotin-labeled PCR products to detect single-base variations under the same hybridization conditions. In this way we were able to distinguish all the Trichinella genotypes tested, even T6 and T. nativa, which are closely related in their allozyme grouping (1, 2), without cross-reactions. Also, a genus-specific Trichinella oligonucleotide, situated in a highly conserved region of the 5S rDNA, was selected. With this oligonucleotide probe, all the Trichinella genotypes tested could be identified, except for T. pseudospiralis, which could be identified only by its specific oligonucleotide probe. The reproducibility of the RLB assay was tested with 24 different strains of the different genotypes. Each strain of the different Trichinella genotypes reacted with its species-specific oligonucleotide probe except for one T. nelsoni strain, which showed some cross-reaction with the T. nativa oligonucleotide probe. More individual T. nelsoni larvae were tested in the RLB assay, and all those larvae gave the same hybridization pattern (data not shown). According to La Rosa and Pozio (15), this isolate was identified as T. nelsoni in the allozyme and molecular analyses. However, this Trichinella strain reacted differently in the Southern blot analysis that distinguishes this isolate from the others of the same species. The cross-reaction could be due to a mutation in one of the intergenic repeats, which causes this slight reaction in the RLB assay.
Overall, the intergenic spacer region of the 5S rRNA genes proved to be a stable genetic marker among the different Trichinella genotypes and could be used to identify these genotypes. To date, other PCR-based methods for detecting and identifying the different Trichinella genotypes have also been described. For the RLB assay, labeled PCR products using only one primer set are necessary, and this enables us to differentiate the genus Trichinella even based on individual muscle larvae. In the case of a new Trichinella genotype, i.e., one not included in the RLB assay, the Trichinella genus-specific probe will be positive while the genotype-specific probes will not give a result. Even these newly isolated Trichinella spp. can be analyzed by sequencing the 5S rDNA intergenic region PCR product. After this DNA sequence is compared and analyzed, a species-specific oligonucleotide probe can be determined and introduced into the RLB assay. Because the primer regions in the 5S rRNA genes are highly conserved, DNA from any possible newly identified Trichinella species can be amplified, sequenced, and introduced into the RLB assay.
T. spiralis and T. britovi have been detected in the same host species in central and southern Europe. Also, a double infection with T. nativa and T. britovi in a raccoon dog has been described (23, 24). This means that mixed infections can occur, and therefore we tested whether the RLB assay can be used to detect and to identify mixed infections. DNA samples of Trichinella species which can occur in the same host were mixed and were equally well amplified. We used DNA samples instead of individual larvae to be sure that the amounts of DNA for the species were equal. With the RLB assay, we were able to identify DNA of mixed genotypes. Theoretically, when different numbers of larvae of each Trichinella genotype are present in muscle samples, competition of the primers can occur. This stresses the importance of using assays which are based on the identification of single larvae from the original host and not using DNA of pools of isolated muscle larvae.
The sensitivity of the RLB assay, depending on the amount of intact DNA in one larva, was tested with a 10-fold serial dilution of the DNA of a single larva. With a decreasing amount of DNA, we were able to generate a specific PCR product to detect the Trichinella DNA. It was clear that only a small amount of DNA was needed to obtain any PCR product. This is in contrast with other tests, where the amount of DNA is very important to get any positive results. The RLB assay was 10-fold more sensitive than agarose gel analysis.
In conclusion, the 5S rRNA intergenic spacer region is a stable marker for the identification and differentiation of Trichinella genotypes in the RLB assay. Only one PCR-based assay is required for the simultaneous detection and identification of eight different genotypes, starting from an individual muscle larva. The RLB assay is easy to perform and can even be used in routine laboratories. The membrane with the covalently linked oligonucleotide probes, after optimization of the concentrations, can be reused several times (11, 27). This enables us to perform the RLB assay within 1 day.
ACKNOWLEDGMENTS
We are grateful to E. Pozio for providing the Trichinella isolates. We thank L. Schouls and I. van der Pol for technical and practical assistance with the RLB assay and J. Vinjé for the technical assistance with the DNA sequencing. We also thank E. Pozio for critically reading the manuscript.
This study was carried out on behalf of the Inspectorate for Health Protection, Commodities and Veterinary Public Health.
REFERENCES
- 1.Appleyard G D, Zarlenga D, Pozio E, Gajadhar A A. Differentiation of Trichinella genotypes by polymerase chain reaction using sequence-specific primers. J Parasitol. 1999;85:556–559. [PubMed] [Google Scholar]
- 2.Bandi C, La Rosa G, Comincini S, Damiani G, Pozio E. Random amplified polymorphic DNA technique for the identification of Trichinella species. Parasitology. 1993;107:419–424. doi: 10.1017/s0031182000067779. [DOI] [PubMed] [Google Scholar]
- 3.Bandi C, La Rosa G, Bardin M G, Daminiani G, Comincini S, Tasciotti L, Pozio E. Random amplified polymorphic DNA fingerprints of the eight taxa of Trichinella and their comparison with allozyme analysis. Parasitology. 1995;110:401–407. doi: 10.1017/s003118200006474x. [DOI] [PubMed] [Google Scholar]
- 4.Bolas-Fernandez F, Wakelin W. Infectivity, antigenicity and host responses to isolates of the genus Trichinella. Parasitology. 1990;100:491–497. doi: 10.1017/s003118200007880x. [DOI] [PubMed] [Google Scholar]
- 5.Boyd D, de Vos T, Klassen G, Dick T. Characterization of the ribosomal DNA from Trichinella spiralis. Mol Biochem Parasitol. 1989;35:67–72. doi: 10.1016/0166-6851(89)90143-6. [DOI] [PubMed] [Google Scholar]
- 6.Bryant C. Molecular variation in Trichinella. Acta Trop. 1993;53:319–330. doi: 10.1016/0001-706x(93)90037-c. [DOI] [PubMed] [Google Scholar]
- 7.Chambers A E, Almond N M, Knight M, Simpson A J G, Parkhouse R M E. Repetitive DNA as a tool for the identification and comparison of nematode variants: application to Trichinella isolates. Mol Biochem Parasitol. 1986;21:113–120. doi: 10.1016/0166-6851(86)90014-9. [DOI] [PubMed] [Google Scholar]
- 8.Dame J B, Murrell K D, Worley D E, Schad G A. Trichinella spiralis: genetic evidence for synanthropic subspecies in sylvatic hosts. Exp Parasitol. 1987;64:195–203. doi: 10.1016/0014-4894(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 9.Fukumoto S, Takechi M, Kamo H, Yamaguchi T. Comparative studies on soluble protein profiles and isozyme patterns of seven Trichinella isolates. Parasitol Res. 1987;73:352–357. doi: 10.1007/BF00531090. [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto S, Nagai D, Yazaki S, Kamo H, Yamaguchi T. The molecular phylogenic tree of the genus Trichinella constructed from isozyme patterns. Parasitol Res. 1988;74:574–580. doi: 10.1007/BF00531637. [DOI] [PubMed] [Google Scholar]
- 11.Gubbels J M, de Vos A P, van der Weide M, Viseras J, Schouls L M, de Vries E, Jongejan F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot. J Clin Microbiol. 1999;37:1782–1789. doi: 10.1128/jcm.37.6.1782-1789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapel C O M, Webster P, Lind P, Pozio E, Henriksen S A, Murrell K D, Nansen P. Trichinella spiralis, T. britovi, and T. nativa: infectivity, larval distribution in muscle and antibody response after experimental infection of pigs. Parasitol Res. 1998;84:264–271. doi: 10.1007/s004360050393. [DOI] [PubMed] [Google Scholar]
- 13.Klassen G R, Thiessen J P, Dick T A. Restriction endonuclease analysis of repetitive sequences in the Trichinella genome: three strain-specific patterns. J Parasitol. 1986;72:772–775. [PubMed] [Google Scholar]
- 14.La Rosa G, Pozio E, Rossi P, Murrell K D. Allozyme analysis of Trichinella isolates from various host species and geographical regions. J Parasitol. 1992;78:641–646. [PubMed] [Google Scholar]
- 15.La Rosa G, Pozio E. Molecular investigation of African isolates of Trichinella reveals genetic polymorphism in Trichinella nelsoni. Int J Parasitol. 2000;30:663–667. doi: 10.1016/s0020-7519(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenfels J R, Murrell K D, Pillitt P A. Comparison of three subspecies of Trichinella spiralis by scanning electron microscopy. J Parasitol. 1983;69:1131–1140. [PubMed] [Google Scholar]
- 17.Liu L X, Blaxter M L, Shi A. The 5S ribosomal RNA intergenic region of parasitic nematodes: variation in size and presence of L1 RNA. Mol Biochem Parasitol. 1996;83:235–239. doi: 10.1016/s0166-6851(96)02753-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu L X, Chi J, Upton M P, Ash L R. Eosinophilic colitis associated with larvae of the pinworm Enterobius vermicularis. Lancet. 1995;346:410–412. doi: 10.1016/s0140-6736(95)92782-4. [DOI] [PubMed] [Google Scholar]
- 19.Penner G A, Bush A, Wise R, Kim W, Domier L, Kasha K, Laroche A, Scoles G, Molnar S J, Fedak G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 1993;2:341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- 20.Pozio E, La Rosa G, Rossi P. Trichinella Reference Centre. Parasitol Today. 1989;5:169–170. [Google Scholar]
- 21.Pozio E, La Rosa G, Murrell K D, Lichtenfels J R. Taxonomic revision of the genus Trichinella. J Parasitol. 1992;78:654–659. [PubMed] [Google Scholar]
- 22.Pozio E. Trichinellosis in the European Union: epidemiology, ecology and economic impact. Parasitol Today. 1998;14:35–38. doi: 10.1016/s0169-4758(97)01165-4. [DOI] [PubMed] [Google Scholar]
- 23.Pozio E, Bandi C, La Rosa G, Järvis T, Miller I, Kapel C M. Concurrent infection with sibling Trichinella species in a natural host. Int J Parasitol. 1995;25:1247–1250. doi: 10.1016/0020-7519(95)00042-z. [DOI] [PubMed] [Google Scholar]
- 24.Pozio E, Serrano F J, La Rosa G, Reina D, Perez-Martin E, Navarrete I. Evidence of potential gene flow in Trichinella spiralis and in Trichinella britovi in nature. J Parasitol. 1997;83:163–166. [PubMed] [Google Scholar]
- 25.Pozio E, Owen I L, La Rosa G, Sacchi L, Rossi P, Corona S. Trichinella papuae n. sp. (Nematoda), a new non-encapsulated species from domestic and sylvatic swine of Papua New Guinea. Int J Parasitol. 1999;29:1825–1839. doi: 10.1016/s0020-7519(99)00135-6. [DOI] [PubMed] [Google Scholar]
- 26.Pozio E, La Rosa G. Trichinella murrelli n. sp: etiological agent of sylvatic trichinellosis in temperate areas of North America. J Parasitol. 2000;86:134–139. doi: 10.1645/0022-3395(2000)086[0134:TMNSEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Rijpkema S G T, Molkenboer M J C H, Schouls L M, Jongejan F, Schellekens J F P. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–3095. doi: 10.1128/jcm.33.12.3091-3095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rombout, Y. B., S. Bosch, W. Homan, and J. W. B. van der Giessen. Genetic diversity within the genus Trichinella as shown by cleavage fragment length polymorphism analysis. J. Helminthol., in press. [DOI] [PubMed]
- 29.Soulé C, Guillou J-P, Dupouy-Camet J, Vallet C, Pozio E. Differentiation of Trichinella isolates by polymerase chain reaction. Parasitol Res. 1993;79:461–465. doi: 10.1007/BF00931583. [DOI] [PubMed] [Google Scholar]
- 30.Wakelin D, Goyal P K. Trichinella isolates: parasite variability and host responses. Int J Parasitol. 1996;26:471–481. doi: 10.1016/0020-7519(96)89377-5. [DOI] [PubMed] [Google Scholar]
- 31.Williams J G, Kubelik A R, Livak K L, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Nagano I, Takahashi Y. The detection of Trichinella with polymerase chain reaction (PCR) primers constructed using sequences of random amplified polymorphic DNA (RAPD) or sequences of complementary DNA encoding excretory-secretory (E-S) glycoproteins. Parasitology. 1998;117:173–183. doi: 10.1017/s0031182098002881. [DOI] [PubMed] [Google Scholar]
- 33.Zarlenga D S, Al-Yaman F, Minchella D J, La Rosa G. A. repetitive DNA probe specific for a North American sylvatic genotype of Trichinella. Mol Biochem Parasitol. 1991;48:131–138. doi: 10.1016/0166-6851(91)90109-j. [DOI] [PubMed] [Google Scholar]
- 34.Zarlenga D S, Chute B M, Martin A, Kapel C M O. A. multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int J Parasitol. 1999;29:1859–1867. doi: 10.1016/s0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]