Abstract
The nematicidal properties of Trichoderma species have potential for developing safer biocontrol agents. In the present study, 13 native Trichoderma strains from T. citrinoviride, T. ghanense (2 strains), T. harzianum (4), T. koningiopsis, T. simmonsii, and T. virens (4) with nematicidal activity were selected and cultured in potato dextrose broth to obtain a culture filtrate (CF) for each. Each CF was partitioned with ethyl acetate to obtain organic (EA) and residual filtrate (RF) fractions, which were then tested on second-stage juveniles (J2s) of the nematodes Meloidogyne javanica and M. incognita in a microdilution assay. The most lethal strains were T. harzianum Th43-14, T. koningiopsis Th41-11, T. ghanense Th02-04, and T. virens Th32-09, which caused 51–100% mortality (%M) of J2s of both nematodes, mainly due to their RF fractions. Liquid chromatography–diode array detector-electrospray-high resolution mass spectrometry analysis of the most-active fractions revealed sesquiterpene and polyketide-like metabolites produced by the four active strains. These native Trichoderma strains have a high potential to develop safer natural products for the biocontrol of Meloidogyne species.
Keywords: ethyl acetate fraction, HRMS dereplication, Trichoderma ghanense, Trichoderma harzianum, Trichoderma koningiopsis, Trichoderma virens
1. Introduction
Fungi belonging to the Trichoderma genus are cosmopolitan species, with 488 species identified [1]. Several of these species have been widely studied as biocontrol agents against phytopathogenic fungi [2] and nematodes [3,4], insects [5], and weeds [6], and as plant growth promoters [7,8]. The nematicidal potential of Trichoderma species is increasingly being harnessed to develop new and safer biocontrol agents against parasitic nematodes such as Globodera pallida, Heterodera avenae, Meloidogyne incognita, M. javanica, M. hapla, and Pratylenchus brachyurus [8,9]. In particular, Meloidogyne root-knot nematodes are considered the most harmful because they can affect a wide range of crops, causing production losses between 25% to 50% and millions of dollars. They thus continue to be controlled mainly with synthetic agrochemicals despite recognized problems for the environment and organisms [10,11,12] because of with lack of safer products and eco-friendly and holistic strategies. As harmful synthetic chemicals are withdrawn from the market, the search for alternatives such as crop rotation, resistant plant varieties, and biological control agents or their derivatives to control nematodes has intensified [4,11,13,14] Trichoderma species that have lethal effects against Meloidogyne species include T. harzianum, T. koningii, T. koningiopsis, T. longibrachiatum, T. citrinoviride and T. viride [15,16,17] against M. incognita; T. hamatum, T. harzianum, T. koningii, T. koningiopsis, and T. viridae against M. javanica [15,18,19]; T. asperellum, T. harzianum, T. viride and T. viride on M. hapla [9]; and T. harzianum against M. enterolobii [20].
The main mechanisms of action known for Trichoderma species are antibiosis, competition for space and nutrients, mycoparasitism, and induction of defense mechanisms. The antibiosis involves the production and secretion of secondary and primary metabolites that inhibit the growth and development of root-knot nematodes [19,21,22]. Regarding the production and secretion of metabolites, approximately 390 non-volatile metabolites have been identified from Trichoderma spp. [2,23], but only a few, such as gliotoxin, gliovirin, heptelidic acid, and viridin identified from T. virens [3,24], have been reported to have nematicidal activity. Therefore, a systematic bio-guided screening of Trichoderma species is a promising option to find novel nematicidal products.
During our ongoing bioprospecting studies in search of natural agrochemical products in the Yucatán península (Mexico), our group isolated 56 native Trichoderma species from soils. The foregoing is in response to the need to develop nematicides based on native species adapted to the areas where they are intended to be applied, with the knowledge of the active metabolites produced by fungal strains. These strains were tested for control of M. incognita, and 29 of these strains affected the viability of eggs and second-stage juveniles (J2s) in vitro and acted as plant growth promoters [25,26,27]. Moreover, these active Trichoderma strains decreased the severity of nematode damage on tomato and improved yields in the greenhouse [18,25].
In the present study, 13 Trichoderma strains from Yucatán were selected due to their activity against phytopathogenic fungi, nematodes, and as plant growth promoters. All selected Trichoderma strains were cultured in the liquid media potato dextrose broth. From each fungal strain, the culture filtrate, ethyl acetate fraction, and residual filtrate fraction were obtained and tested against the J2s of M. incognita and M. javanica. The chemical profiles of the most-active filtrates or fractions were analyzed by liquid chromatography– diode array detector-electrospray-high resolution mass spectrometry (LC-DAD-ESI-HRMS).
2. Materials and Methods
2.1. Trichoderma Strains
The 13 Trichoderma spp. strains, obtained from cultivated and non-cultivated soil in Yucatán state in Mexico [25,26] and supplied by the Phytopathology Laboratory of Tecnológico Nacional de México, Campus Conkal (Table 1), belonged to six Trichoderma species: T. citrinoviride (Th33-58), T. ghanense (Th02-04, Th26-52), T. harzianum (Th02-01, Th20-07, Th43-14 and Th33-59), T. koningiopsis Th41-11, T. simmonsii Th09-06; and T. virens (Th05-02, Th27-08, Th32-09, and Th43-13). The 5.8S-ITS regions of each strain were sequenced previously and are available in the GenBank database [27,28]. The fungal strains were reactivated in Petri dishes with potato dextrose agar (PDA, Dibico, Edo. Mex., MX) and incubated at 25 ± 2 °C, 12/12 h of light/dark for 8 days (Table 1, Figure 1).
Table 1.
Trichoderma strains selected and their biological activity.
| Trichoderma Species | Key | GenBank Number |
Activity | Place of Collection |
|---|---|---|---|---|
| T. citrinoviride Bissett 1984 | Th33-58 | MF078653 | A/B | Ticul |
| T. ghanense Yoshim. Doi, Y. Abe & Sugiy 1987 | Th02-04 | MF078652 | A/B | Tizimín |
| Th26-52 | MF078651 | A/B | Tahdziú | |
| T. harzianum Rifai 1969 | Th02-01 | MF952887 | A/B | Tizimín |
| Th20-07 | MF078650 | A | Tzucacab | |
| Th43-14 | MF078649 | A | San Felipe | |
| Th33-59 | MF078648 | A/B | Ticul | |
| T. koningiopsis Samuels, Carm. Suárez & H.C. Evans 2006 | Th41-11 | MF952888 | A/B | Sanahcat |
| T. simmonsii P. Chaverri, F.B. Rocha, Samuels, Degenkolb & Jaklitsch 2015 | Th09-06 | MF078647 | A/B | Dzidzantun |
| T. virens (J.H. Mill., Giddens & A.A. Foster) Arx 1987 | Th05-02 | MF952889 | A/B | Dzilam González |
| Th27-08 | MF078646 | A | Chacsinkin | |
| Th32-09 | MF078645 | A | Oxkutzcab | |
| Th43-13 | MF078644 | A | San Felipe |
A: Meloidogyne incognita antagonist; B: plant growth promoter.
Figure 1.
Cultures of the 13 study strains of Trichoderma on potato dextrose agar incubated at 25 ± 2 °C, 12 h light/12 h dark for 8 days.
Liquid Culture of Trichoderma Strains
The strains were grown in potato dextrose broth (PDB), which was made by adding 200 g of potato fragmented in distilled water at the boiling point (1000 mL) for 15 min, then filtered and 20 g of dextrose (Difco, Baltimore, MD, USA) added. A volume of 200 mL of the medium was deposited in Roux bottles and sterilized in an autoclave at 121 °C, 15 lb pressure, for 15 min. For each Trichoderma strain, cultured in PDA (8 days), a mycelial disk (7 mm diameter) was added to the medium in each of three Roux bottles. PDB without a Trichoderma strain was used as a control (blank). Three replicates of these cultures were incubated at 25 ± 2 °C, 12/12 h light/dark for 31 days. The mycelium was then removed from the culture broth by filtration through a double layer of cheesecloth. Each culture filtrate (CF), designated as 100% concentration, was then diluted with distilled water to 50% and 25% concentrations. The pH of 5 mL of the 100% CF was measured, then stored at 4 ± 2 °C until organic extraction (1–3 days).
2.2. Preparation of Fractions from Culture Filtrates
Each CF was liquid–liquid extracted with ethyl acetate three times (2:1, 1:1, 1:1 v/v), obtaining an ethyl acetate (EA) and residual filtrate (RF) fractions. The EA fraction was dried over sodium sulfate (Merck, New Jersey, USA), and the solvent was vacuum-evaporated at 40 °C in a rotary evaporator (IKA model RV-10, Staufen, Germany). The residual solvent in the RFs was also removed by evaporation, and the residue was designated as 100% concentration. The PDB control was processed the same way. All EAs were stored at 4 °C, and the RF fractions were frozen.
2.3. Nematicidal Bioassay
2.3.1. Nematode Inoculum
The population of M. incognita was obtained from the Tecnológico Nacional de México/ campus Conkal, Yucatán, México (30 ± 2 °C, 90% relative humidity) and M. javanica from the Instituto de Ciencias Agrarias, CSIC in Madrid, Spain (25 ± 1 °C, 70% relative humidity). Both nematodes were maintained on tomato plants (variety Marmanded) growing in pots in a greenhouse. Egg masses were collected from infested tomato roots and incubated for 72 h in sterile distilled water at 25 ± 2 °C for M. javanica and 30 ± 2 °C for M. incognita. The hatched J2s of Meloidogyne were adjusted to a final concentration of 100 J2 nematodes/100 μL distilled water to test CFs and RFs fractions (aqueous samples) and to 100 J2 nematodes/95 μL of distilled water solution to test EA samples [29,30].
2.3.2. Sample Preparation and Nematicidal Bioassay
Aqueous samples (100 µL) of a serial dilution (100, 50, or 25%) of either the CF or RF samples of a Trichoderma strain or a blank were deposited in wells of 96-well plates with U-bottom (BD Falcon, San Jose, CA, USA). The J2s suspended in distilled water (100 μL) that had been filtered through a 25 µm mesh screen were then transferred into each well. The negative controls consisted of CF or RF blanks, distilled water (DW), and 100 J2s. EA samples (5 µL) dissolved in DMSO:0.6%-Tween 20 (DT) were transferred to each well containing nematode suspension (95 µL), with a final concentration of 1 µg/µL. In this case, the negative control consisted of blank extracts, a mixture of water-DT 95:5 (WDT), and 100 J2s. Four replicates for each treatment were performed. The experimental plates were sealed with parafilm to prevent evaporation and maintained at the same conditions described above for egg masses in the dark [29,31].
After 72 h, immobile and rigid J2s that lacked intestinal contents were counted as dead, using a binocular microscope, and expressed as the percentage of juvenile mortality (%M). The nematicidal activity data were corrected using Schneider–Orelli’s formula [32]. A completely randomized design was used, and means were compared using the Scott-Knott test (p ≤ 0.05) in the statistical package Infostat Ver. 2018 [33].
2.4. Liquid Chromatography-Diode Array Detector-Electrospray-High Resolution Mass Spectrometry
The active EA and RF fractions were freeze-dried (Labconco FreeZone 2.5, model 7670520, Houston, TX, USA) and dissolved to 1% w/v with methanol, then 3 µL was injected onto a C8 column (Zorbax SB, 2.1 × 30 mm) in an Agilent 1200 liquid chromatograph (LC, Santa Clara, CA, USA) coupled to an Agilent diode array detector and a Bruker Maxis HR-QTOF mass detector (HRMS Bruker GmbH, Bremen, Germany). The samples were separated at 40 °C with a flow of 300 μL/min. The mobile phase was a mixture of water–acetonitrile 90:10 v/v 0.01% trifluoroacetic acid and 1.3 mM ammonium formate (solvent A) and 10:90 v/v 0.01% trifluoroacetic acid and 1.3 mM ammonium formate (solvent B). The gradient was from 10% to 100% of solvent B in 6 min, maintained in 100% B for 2 min, then returned to 10% B for 2 min [34]. Mass spectra (50 to 2000 m/z) in the positive mode were acquired, and components detected were compared against the MEDINA Microbial Dereplication Databases (with approximately 900 known bioactive molecules), the Chapman & Hall Dictionary of Natural Products (v25.1, CRC Press, Boca Raton, FL, USA), and a database available in the literature.
3. Results
3.1. Nematicidal Activity
The lethality of the CFs of the 13 species of Trichoderma against M. incognita differed (p ≤ 0.05) from that of the negative controls at the 100% concentration (Figure 2, Table 2). All the CFs were mortal to M. incognita at 100% concentration, except for that of T. citrinoviride (M = 62%) after 72 h. The most mortal CFs at 50% concentration against M. incognita after 72 h were from T. ghanense Th02-04 (M = 100%), T. virens Th27-08 (M = 71%), and T. harzianum Th43-14 (M = 66%). The activity of the CFs decreased to M < 50%) at 25% dilution, so they were considered non-nematicidal at this dilution. Against J2s of M. javanica, however, only CFs from T. ghanense Th02-04, T. harzianum Th20-07, and T. virens Th27-08 (M values of 85–99%) were effective at 100% concentration after 72 h (Table 2). The nematicidal activity of the CFs at 50% and 25% dilution against M. javanica did not differ from the DW. Therefore, the 100% concentration CF from T. ghanense Th02-04 was the most effective against both nematodes (M = 99–100%).
Figure 2.
Second-stage juveniles (J2s) of Meloidogyne incognita in (A) control in distilled water, (B) dead and living in culture filtrate of Trichoderma virens Th27-08.
Table 2.
Mortality of second-stage juveniles of Meloidogyne spp. after 72 h exposure to culture filtrate (CF), ethyl acetate (EA), or residual filtrate (RF) fractions from tropical Trichoderma strains.
| Trichoderma Species | Strains | Mean Percentage Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meloidogyne incognita | Meloidogyne javanica | ||||||||||
| CF | EA | RF | CF | EA | RF | ||||||
| 100% | 50% | 1 µg/µL | 100% | 50% | 25% | 100% | 1 µg/µL | 100% | 50% | ||
| T. citrinoviride | Th33-58 | 62 b | 21 c | 30 c | 100 a | 66 c | 13 c | 15 d | 8 b | 100 a | 1 h |
| T. ghanense | Th02-04 | 100 a | 100 a | 20 d | 100 a | 63 c | 24 c | 99 a | 0 c | 100 a | 16 e |
| Th26-52 | 100 a | 23 c | 22 d | 100 a | 100 a | 20 c | 3 f | 11 b | 100 a | 63 b | |
| T. harzianum | Th02-01 | 100 a | 42 c | 0 e | 100 a | 23 d | 15 c | 4 f | 0 c | 98 a | 34 d |
| Th20-07 | 100 a | 6 d | 0 e | 98 a | 66 c | 13 c | 97 a | 0 c | 7 e | 0 h | |
| Th43-14 | 100 a | 66 b | 51 b | 100 a | 100 a | 57 b | 45 c | 66 a | 93 b | 4 g | |
| Th33-59 | 100 a | 21 c | 30 c | 100 a | 72 b | 22 c | 9 e | 9 b | 10 a | 0 h | |
| T. koningiopsis | Th41-11 | 100 a | 38 c | 54 b | 100 a | 100 a | 82 a | 7 e | 1 c | 93 b | 51 c |
| T. simmonsii | Th09-06 | 100 a | 6 d | 37 c | 100 a | 96 a | 53 b | 6 e | 0 c | 14 d | 0 h |
| T. virens | Th05-02 | 100 a | 22 c | 18 d | 100 a | 19 d | 18 c | 8 e | 0 c | 100 a | 10 f |
| Th27-08 | 100 a | 71 b | 0 e | 100 a | 100 a | 64 b | 85 b | 9 b | 100 a | 94 a | |
| Th32-09 | 100 a | 20 c | 100 a | 100 a | 100 a | 83 a | 7 e | 3 c | 86 c | 5 g | |
| Th43-13 | 100 a | 23 c | 31 c | 100 a | 86 b | 49 c | 7 e | 8 b | 95 b | 0 h | |
| Control | PDB | 0 c | 0 d | 0 e | 0 b | 0 d | 0 c | 0 f | 0 c | 0 f | 0 h |
| DW/WDT * | 0 c | 0 d | 0 e* | 0 b | 0 d | 0 c | 0 f | 0 c* | 0 f | 0 h | |
| Blank | 0 c | 0 d | 0 e | 0 b | 0 d | 0 c | 0 f | 0 c | 0 f | 0 h | |
| SD | 0.2 | 125 | 5.7 | 0.5 | 15.2 | 12.9 | 2.4 | 1.4 | 2.6 | 2.4 | |
PDB: potato dextrose broth, DW: distilled water, WDT *: water-DMSO–0.6% Tween 20 (95:5); Blank: unfermented medium. SD: standard deviation, calculated from the analysis of variance for treatments (CFs, EA, RFs from each strain). Values with different letters within a column differed significantly in Scott–Knott test (p ≤ 0.05).
After the ethyl acetate extraction of the CFs, the activity of all the RF fractions differed greatly (p ≤ 0.05) from that of the negative control. All the residual filtrates generated from the ethyl acetate extraction of the CFs of the 13 strains were mortal against M. incognita, and all but those from T. harzianum Th20-07 and T. simmonsii Th09-06 were mortal against M. javanica (Table 2). The highest mortality against J2s of M. incognita at 25% dilution of the RFs after 72 h was caused by T. koningiopsis Th41-11 (M = 82%) and T. virens Th32-09 (M = 83%). Against J2s of M. javanica, the highest mortality was achieved with 50% dilutions of the RFs from T. ghanense Th26-52 (M = 63%), T. koningiopsis Th41-11 (M = 51%) and T. virens Th27-08 (M = 94%). After a 72 h exposure to EA fractions, the most active against M. incognita were from T. harzianum Th43-14 (M = 51%), T. koningiopsis Th41-11 (M = 54%), and T. virens Th32-09 (M = 100%) at 1 µg/µL; the EA fraction from T. harzianum Th43-14 strain was the only EA fraction active (M = 66%) against M. javanica at 1 µg/µL (Table 2).
In general, against both nematodes, the CFs and their RF and EA fractions from T. harzianum Th43-14 and T. virens Th27-08 were the most active. In addition, the RF fraction from CF of T. koningiopsis induced J2s mortality of both nematodes at the lowest dilution tested.
3.2. Identification of Components in Active Fractions from Trichoderma Strains by LC-DAD-ESI-HRMS
The results obtained from analyses of the liquid chromatograms and ultraviolet and high-resolution mass spectrometry data of the RF fraction from T. harzianum Th43-14, T. ghanense Th26-52, and T. virens Th27-08 and the EA fraction of T. koningiopsis are shown in Table 3, Table 4, Table 5 and Table 6 No significant differences were observed between the chromatograms from the active strains T. ghanense Th02-04 and the T. virens Th32-09 and negative control unfermented culture medium.
Table 3.
Dereplicated metabolites from residual filtrate fraction of Trichoderma harzianum Th43-14.
| No. | Rt (min) |
UV (nm) |
[M + H]+ m/z | Molecular Formula | Putative Metabolite | |
|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||
| 1 | 0.88 | 200, 215 | 317.1599 | 317.1595 | C15H24O7 | unknown |
| 2 | 1.35 | 205, 210 | 299.1492 | 299.1489 | C15H22O6 | 3,7,8,15-Scirpenetetrol |
| 3 | 2.05 | 200, 220 | 249.1488 | 249.1485 | C15H20O3 | Illudin M |
| 4 | 2.69 | 200, 220 | 255.1591 | 255.1591 | C14H22O4 | unknown |
| 5 | 2.86 | 200, 220 | 283.1541 | 283.1540 | C15H22O5 | 3,4,15-Scirpenetriol |
| 6 | 3.09 | 200, 218, 270 | 309.1692 | 309.1697 | C17H24O5 | Naematolin |
| 7 | 3.66 | 200, 220 | 553.3000 | 553.3007 | C29H44O10 | unknown |
| 8 | 3.84 | 200, 220 | 251.1644 | 251.1642 | C15H22O3 | Trichodermol |
No.: Component number; Rt: Retention time; UV: Ultraviolet.
Table 4.
Dereplication metabolites from ethyl acetate fraction of Trichoderma ghanense Th26-52.
| No. | Rt (min) |
UV (nm) |
[M + H]+ m/z | Molecular Formula | Putative Metabolite | |
|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||
| 1 | 0.63 | 200, 218 | 261.0966 | 261.0969 | C11H16O7 | unknown |
| 2 | 0.86 | 218, 230, 300 | 168.0656 | 168.0655 | C8H9NO3 | unknown |
| 3 | 1.22 | 205, 290 | 155.0700 | 155.0703 | C8H10O3 | Unknown |
| 4 | 2.16 | 200, 220, 310 | 169.0493 | 169.0495 | C8H8O4 | atrichodermone D |
| 5 | 2.40 | 200, 210, 240 | 225.0756 | 225.0758 | C11H12O5 | Unknown |
| 6 | 2.64 | 200, 230, 270 | 235.1328 | 235.1329 | C14H18O3 | Unknown |
| 7 | 2.89 | 200, 220, 280 | 195.1379 | 195.1380 | C12H18O2 | 6-heptyl-2H-pyron-2-one |
No.: Component number; Rt: Retention time; UV: Ultraviolet.
Table 5.
Dereplicated metabolites from ethyl acetate fraction of Trichoderma koningiopsis Th41-11.
| No. | Rt (min) |
UV (nm) |
[M + H]+ m/z | Molecular Formula | Putative Metabolite | |
|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||
| 1 | 2.81 | 200, 255 | 281.1750 | 281.1747 | C16H24O4 | Koninginin L |
| 2 | 3.04 | 200, 255 | 281.1749 | 281.1747 | C16H24O4 | Koninginin T |
| 3 | 3.68 | 200, 260 | 283.1906 | 283.1904 | C16H26O4 | Koninginin B |
No.: Component number; Rt: Retention time; UV: Ultraviolet.
Table 6.
Dereplicated metabolites from residual fractions of Trichoderma virens Th27-08.
| No. | Rt (min) |
UV (nm) |
[M + H]+ m/z | Molecular Formula | Putative Metabolite | |
|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||
| 1 | 0.78 | 205, 240, 290 | 207.0653 | 207.0657 | C11H10O4 | unknown |
| 2 | 1.00 | 210, 225, 295, | 225.0757 | 225.0762 | C11H12O5 | sepedonin |
| 3 | 1.57 | 205, 240, 290 | 207.0652 | 207.0657 | C11H10O4 | unknown |
| 4 | 3.35 | 215, 255, 300 | 277.1435 | 277.1438 | C16H20O4 | unknown |
No.: Component number; Rt: Retention time; UV: Ultraviolet.
The compounds were tentatively identified by taking into account the species of the producing strain, the UV spectrum, and the molecular formula assigned through analysis of the protonated and ammonium adducts of each molecule, helped in some cases by the presence of dimers and dehydration products, and through searches of in the internal MEDINA database of HRMS spectra, dictionary of natural products and other natural product databases.
3.2.1. Metabolites from Residual Filtrate Fraction of Trichoderma harzianum Th43-14
The chromatogram of the RF fraction from T. harzianum Th43-14 showed eight main components (Figure 3, Table 3). Five of these were tentatively identified as sesquiterpenes according to their UV and HRMS data. Component 5 (Rt = 2.86 min) presented in its HRMS an ammonium adduct (m/z 300.1807), a protonated molecular ion at m/z 283.1541, indicative of a molecular formula C15H22O5 (calc. for C15H23O5+, 283.1540), which was tentatively assigned as 3,4,15-scirpenetriol. The HRMS of component 2 (Rt = 1.35 min) exhibited an ammonium adduct (m/z 316.1758), and a protonated molecular ion at m/z 299.1492 consistent with the molecular formula C15H22O6 (calc. C15H23O6+, 299.1489) and was putatively identified as 3,7,8,15-scirpenetetrol. All compounds tentatively identified (2, 5, and 8) have the same structural skeleton.
Figure 3.
UV210 nm chromatograms of residual filtrate fraction from Trichoderma harzianum Th43-14 and unfermented culture medium. 1: Not identified (C15H24O7), 2: 3,7,8,15-scirpenetetrol, 3: illudin M, 4: not identified (C14H22O4), 5: 3,4,15-scirpenetriol, 6: naematolin, 7: not identified (C29H44O10), 8: trichodermol.
The HRMS spectrum of the minor component 3 (Rt = 2.05 min) displayed several protonated fragments (m/z, 231.1383, 221.1544, 213.1272) and a protonated molecular ion at m/z 249.1488 in agreement with the molecular formula C15H22O6 (calc. for C15H23O6+, 249.1489) and was putatively identified as illudin M. The HRMS spectrum of another minor component (6, Rt = 3.09 min) revealed a protonated molecular ion at m/z 249.1488 and was given a molecular formula C15H20O3 (calc. for C15H21O3+, 249.1485) and tentatively identified as naematolin.
The largest peaks were from two unknown molecules (1 and 4), which according to their protonated molecular adducts (m/z 317.1599 and 255.1591, respectively), ammonium (m/z 334.1866 and 272.186, respectively), and [2M+ H]+ (m/z 650.3387 and 509.3114, respectively) ions, have molecular formulae of C15H24O7 (Rt = 0.88 min) and C14H22O4 (Rt = 2.69 min). In addition, another non-identified minor component (7, Rt = 2.16 min) was assigned a molecular formula of C29H44O10 (Rt = 3.66 min) based on the analysis of its protonated (m/z 553.3000) and ammonium adducts (m/z 570.3270).
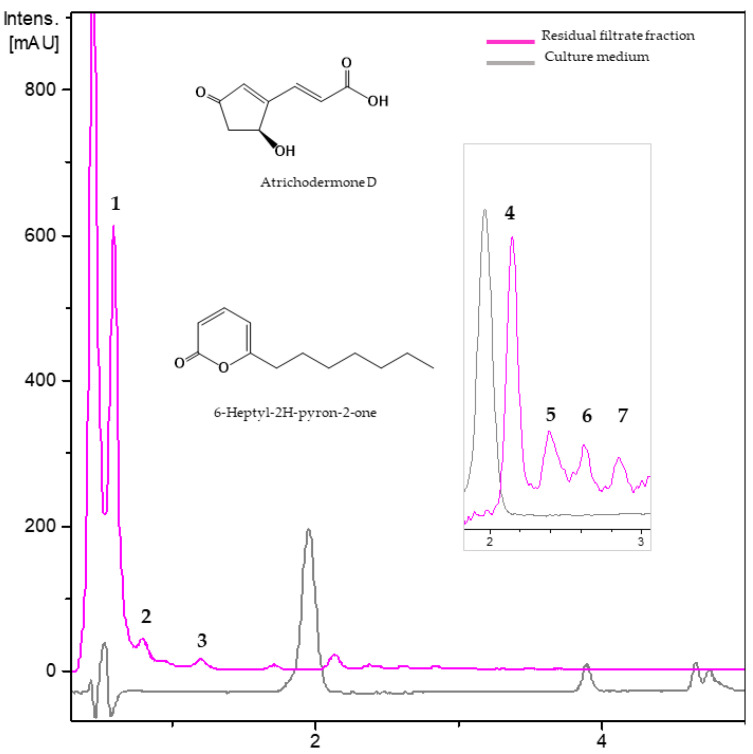
3.2.2. Metabolites from Residual Filtrate Fraction of Trichoderma ghanense Th26-52
Seven components were detected in the chromatogram of the residual fraction of T. ghanense Th26-52 (Figure 4, Table 4). The most abundant component, 1 (Rt = 0.63 min) displayed in its HRMS spectrum an ammonium adduct (m/z 278.1233) and a protonated molecular ion at m/z 261.0966, indicative of a molecular formula of C11H16O7 (calc. C11H16O7+, 261. 0973); this polar metabolite could not be identified. The HRMS of component 4 (Rt = 2.16 min) with a protonated molecular ion at m/z 169.0493 has a molecular formula C8H8O4 (calc. C8H9O4+, 169.0500) and was putatively identified as atrichodermone D. The analysis of the HRMS data of the minor component 7 (Rt = 2.89 min) displayed an ammonium adduct (m/z 212.1640), and a molecular protonated ion at m/z 195.1379 supporting the molecular formula C12H18O2 (calc. C12H19O2+, 195.1384) and tentatively assignment as the unsaturated lactone 6-heptyl-2H-pyron-2one. The other four unknown components included small metabolites with the molecular formulae of C8H9NO3 (Rt = 0.86 min), C8H10O3 (Rt = 1.22 min), C11H12O5 Rt = 2.40 min), and C14H18O3 (Rt = 2.64 min) according to their UV and HRMS data (Table 4).
Figure 4.
UV210 nm chromatograms of residual filtrate fraction from Trichoderma ghanense Th26-52 and the unfermented culture medium. 1: Not identified (C11H16O7), 2: not identified C8H9NO3, 3: not identified C8H10O3, 4: atrichodermone D, 5: Not identified (C11H12O5), 6: not identified (C14H18O3). 7: 6-heptyl-2H-pyron-2-one.
3.2.3. Metabolites from Ethyl Acetate Fraction of Trichoderma koningiopsis Th41-11
The chromatogram of the ethyl acetate extract from T. koningiopsis Th41-11 (Figure 5) showed three components, which tentatively were assigned as koninginin isomers. The HRMS of component 1 (Rt = 2.81 min) and 2 (Rt = 3.04 min) exhibited similar dehydration adducts (m/z 263.1642 245.1535) and protonated adducts (m/z 281.1750 and 281.1749, respectively), indicative of a molecular formula of C16H24O4 (calc. for C16H25O4+, 281.1752) for both molecules. Component 3 (Rt = 3.68 min) in its HRMS data showed dehydrated species (m/z 265.1796, 247.1668, 237.1847), a dimer adduct ([2M+H]+, m/z 565.3759), and a protonated molecular ion at m/z 283.1906 in accordance with a molecular formula of C16H26O4 (calc. for C16H27O4+, 283.1908). An extensive search of the literature based on spectral data and previous reports led to their tentatively identification as koninginin L (1), T (2), and B (3) (Table 5).
Figure 5.
UV210 nm chromatogram of residual filtrate fraction from Trichoderma koningiopsis Th41-11 and the unfermented culture medium. 1: Koninginin T, 2: koninginin L, 3: koninginin B.
3.2.4. Metabolites from Residual Filtrate Fraction of Trichoderma virens Th27-08
The chromatogram of RF fraction of T. virens Th27-08 displayed four components not present in the blank sample (Figure 6, Table 6). Component 3, eluting at Rt of 1.00 min exhibited an ammonium adduct (m/z 242.1020), dehydration fragments (m/z 207.0652, 191.1424), and a protonated ion at m/z 225.0757 indicative of a molecular formula of C11H12O5 (calc. for C11H13O5+, 225.0762). Based on HRMS and UV data, compound 1 was tentatively identified as sepedonin. The major (1) and two minor components (2 and 4) were not identified, and molecular formulae were assigned as C11H10O4 (Rt = 0.78 and 1.57 min) and C16H20O4 (Rt = 3.35) based on their protonated HRMS ion [M+H]+ and additionally supported by its ammonium and dimer adducts.
Figure 6.
UV210 nm chromatogram of residual filtrate fraction from Trichoderma virens Th27-08 and the unfermented culture medium. 1: Not identified (C11H10O4), 2: sepedonin, 3: not identified (C11H10O4), 4: not identified (C16H20O4).
4. Discussion
The results of this study complement our previous discoveries on native Trichoderma strains with nematicidal properties against two Meloidogyne species as part of our continuing work to find and develop safer biocontrol agents. Herein, we demonstrated that 92% of the screened tropical Trichoderma strains (all 13 except T. simmonsii) are mortal to J2s of Meloidogyne javanica and confirmed that the CFs and the EA or RF fractions of all strains were highly mortal to M. incognita. T. koningiopsis UFSMQ40 [12], T. harzianum Th6, JX1614550 [18,19,35], and Trichoderma sp. EF1671 [5] have been shown to have nematicidal effects against J2s of M. javanica, but our report is the first to demonstrate the nematicidal activity of T. citrinoviride, T. ghanense, and T. virens against M. javanica.
The mortality data revealed that M. javanica was less sensitive than M. incognita to the CFs and EA fraction from Trichoderma strains. This differential sensitivity behavior of both Meloidogyne species against extracts, compounds, or fungal strains has been previously reported. For example, fungus Arthobortys sp. MVD18 caused less mortality against M. javanica (92.9%) than against M. incognita (99.0%) after a 48 h exposure. However, two Trichoderma sp. strains (KAV2 and KAV3) were more active against M. javanica than M. incognita [36]. Acetic acid and hexanoic acid yielded an EC50 of 162.4 and 339.3 µg/mL, respectively, against M. javanica but EC50 of 38.3 and 41.1 µg/mL against M. incognita, respectively, after 1 day [37,38]. Sensitivity differences between different species and even different populations of the same species have been attributed to the habitat and environmental conditions to which the organisms are exposed. Other saprophytic fungi have been reported to have low nematicidal effects against J2s of M. javanica; for example, a CF of Arthrobotrys oligospora, A. conoides, and Hypocrea lixii (sexual state of T. harzianum) at 100% concentration [16,39] caused from 16.14 to 64.5% mortality. An EA extract from Trichoderma sp. EFI 671, however, was not active [5].
The present study is also the first nematicidal bio-guided fractionation of the CFs from Trichoderma species and screening the organic and residual aqueous fractions against J2s. After the fractionation, 69% of the RF fractions from the non-nematicidal CFs had a higher mortal effect on M. javanica. This effect could be attributed to antagonistic action between metabolites and the concentration of the metabolites in the RFs of the strains. By contrast, the nematicidal effect of the CF from T. harzianum strain Th20-07 was not confirmed, suggesting that enzymes were mainly involved in the nematicidal activity of strain Th20-07 and were subsequently denatured by the solvent during the fractionation. The antibiosis produced by chitinase and protease action from Trichoderma species has been widely described. For example, an enzymatic filtrate obtained from T. koningiopsis UFSMQ40 is mortal to J2s of M. incognita (90.4% mortality) and M. javanica (63.2%) after 24 h of exposure [15]. Chitinase (51.42 U/mL) and protease (4.27 U/mL) from T. harzianum ITCC 6888 caused high mortality (93%) against M. incognita [40].
On the other hand, in our study, the highest lethal activity against both Meloidogyne species was found for T. koningiopsis Th41-11, T. harzianum Th43-14, T. virens Th27-08, and the two T. ghanense strains tested. The most investigated of these species has been T. harzianum for its properties as a biological control agent and its metabolites [2,4,14]. Other in vitro studies of CFs from T. harzianum strains grown in PDB have shown a significant mortal effect on the J2s of M. incognita. For example, CFs from T. harzianum strains ThU, JX1614550 and Th.6 caused a mortality of 33, 64.5 and 75%, respectively, at 100% concentrations after 72 h [19,41,42].
The residual fraction of T. harzianum Th43-14 revealed a mixture of illudin, naematolin, 4β-scirpenol (syn. trichodermol and roridin C), 3,4,15-scirpenetriol, and 3,7,8,15-scirpenetetrol were tentatively identified based on UV and MS data. Scirpenes have a trichothecene skeleton with a characteristic 9-double-bond and C12-C13 epoxide in their structure and are potent inhibitors of protein synthesis [43,44]. Semisynthetic derivatives of trichodermol have potent cytotoxic and antifungal activity against the fungus Ceratocystiopsis crassivaginata [45,46]. This metabolite has been isolated from several Trichoderma species, and 3,4,15-scirpenetriol has been isolated from Fusarium equiseti, F. roseum, and F. sporotrichiella, whereas 3,7,8,15-scirpenetetrol has only been isolated from F. graminearum [47]. Illudin M, isolated from several Omphalatus species and from Granulobasidium vellereum, has no activity against M. incognita or Caenorhabditis elegans at 100 µg/mL, but it has high antitumor and antimicrobial activities [48,49,50]. Naematolin was isolated from Hypholoma fasciculare (syn. Naematoloma fasciculare), a poisonous basidiomycete [51]. This caryophyllenediol derivative has antitumor, antiviral, and weak antibacterial properties [52,53] and reduced 97% of bloodstream forms of Trypanosoma cruzi at 250 µL/mL [54].
The two studied strains of T. ghanense (Th02-04 and Th26-52) were active on both Meloidogyne species. The data obtained from the UV and ESIMS spectra of its RF fraction allowed us to tentatively identify atrichodermone D and 6-heptyl-2H-pyron-2-one in the RF fraction of T. ghanense Th26-52. To date, metabolites detected from T. ghanense include the phenolics catechin, ferulic acid, and cinnamic acid from the CF of two strains from different areas in India [55]. Atrichodermone D is a cyclopentenone previously reported only from T. atroviride and has no known biological activity [56]. 6-Heptyl-2H-pyran-2-one is an alkyl pyrone isolated from T. koningii strain IMI-308475 and from T. asperellum and T. viride, but no nematicidal activity was reported [57,58]. Related lactones from Trichoderma species include 6-(1-heptenyl)-2-pyran-2-one, 6-(3-hydroxypent-1-en-1-yl)-2H-pyran-2-one and 6-pentyl-2H-pyran-2-one. 6-(3-Hydroxypent-1-en-1-yl)-2H-pyran-2-one was isolated from T. koningiopsis QA3 and has strong antibacterial activity against Micrococcus luteus [59]. 6-Pentyl-2H-pyran-2-one is a recognized plant growth promoter and produced by several Trichoderma species [60].
From the results of the present study, T. koningiopsis Th41-11 is another promising strain that produces nematicidal metabolites. The LC chromatogram of its EA fraction revealed small amounts of koninginins B, L, and T, tentatively identified based on UV, ESIHRMS data analyses, and comparisons with the literature. Koninginin B is a bicyclic polyketide that has also been reported from T. koningii [61], T. neokoningin [62], and T. applanatum [63]. Koninginin L and T are tricyclic polyketides with an oxygen bridge (C7 and C9) at C2 and an alcohol group at C4. Koninginins B, L, and T from T. koningiopsis QA3 and YIM PH30002 were recently reported to be weakly antibacterial [64,65,66].
Among the four T. virens strains studies, only Th27-08 had activity against both nematodes, with the RF fraction achieving the highest mortality. In the LC chromatogram of the RF fraction, we detected sepedonin, a tropolone first isolated from Sepedonium chrysospermum Fries (teleomorph Hyphomyces chrysospermus Tul.) and later from S. ampullosporum, S. chalcipori, S. microspermum, and S. chrysospermum and having antimicrobial activity against several bacterial and fungal pathogens [67]. The artifact anhydrosepedonin (C11H10O4) is produced during the isolation process due to the instability of sepedonin [68,69]. We additionally detected three unknown metabolites in the RF fraction of T. virens Th27-08. Other metabolites previously reported from T. virens include cathequin, caffeic acid, ferulic acid, and 33 other non-volatile metabolites [2,55,70,71]. Our report of sepedonin is thus a new contribution to the chemical composition of this species.
Except for illudin M, the metabolites reported from our native Trichoderma species were not previously tested on nematodes [48]. The nematicidal efficacy of the metabolites tentatively identified from Trichoderma species herein studied are likely due to the alcohol or carboxylic acid groups in their structure. Ntalli et al. [38] reported that acetic acid and hexanoic acid, furfurol (syn. furfuryl alcohol), and furfural paralyzed J2s an EC50/1 h of 1–100 µg/mL or less after 24 h, and the alcohols and aldehyde were more effective than the organic acids. They also demonstrated that acetic acid damages the cuticle and, the nuclei of pseudocoel cells and vacuolizes the cytoplasm of M. incognita, while (E)-2-decenal, and undecanone induced malformation of somatic muscles of the nematodes [72].
In general, few metabolites with nematicidal properties have been isolated from Trichoderma species; some of these are acetic acid, gliotoxin, trichorzianine, viridin [3], trichodermin [73], and cyclonerodiol [65,74]. Therefore, more studies should focus on bioassay-guided isolation and characterization of metabolites responsible for nematicidal activity in the fractions from Trichoderma strains. In addition, studies to optimize the production of the extracts with the most promising active compounds, evaluate their efficacy in greenhouses, and assess their toxicity on plants and beneficial organisms must be carried out before the compounds can be evaluated in the field and further developed as safe bionematicide products.
5. Conclusions
The present contribution enriches our knowledge of the nematicidal potential of 13 tropical Trichoderma species isolated from the soils of Yucatán state. The most effective species against M. incognita and M. javanica were T. ghanense strains Th02-04 and Th26-52, T. harzianum Th43-14, T. koningiopsis Th41-11, and T. virens Th27-08. The LC-DAD-ESIMS chemical profiles of the residual filtrate fractions and ethyl acetate fractions of culture filtrate from Trichoderma spp. revealed the presence of novel metabolites for the genus and others with molecular formulas not found in natural products databases. These results highlight the ability of Trichoderma strains to produce bioactive secondary metabolites that could be developed to manage M. incognita and M. javanica. However, more studies are needed to determine activity and potential phytotoxicity in plant applications and doses for effective, efficient biocontrol effect.
Acknowledgments
The authors thank Irma L. Medina-Baizabal and library technical personal for technical support. This research was supported by the projects CONACyT-IDRC-CIESAS No. CEAR2019-01, TECNM 10759.21P, and PID2019-106222RB-C31 project of Spanish MCIN/AEI/10.13039/501100011033.
Author Contributions
Design and supervision of the study, J.C.-A. and M.G.-A.; fungal cultures, organic extraction and nematicidal bioassays, F.A.M.-K.; academic support and supervision of the nematicidal bioassays, M.F.A. and J.C.-A.; LC-DAD-ESI-HRMS data acquisition, analyses, interpretation, J.M., F.R. and M.G.-A.; financial support of the project, J.M.T.-S. and M.G.-A.; project coordination, J.C.-A.; writing and review of the manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
Consejo Nacional de Ciencia y Tecnología (CONACYT)- International Development Research Centre (IDRC) financed project No. CEAR2019-01; Ministerio de Ciencia e innovación/ Agencia Estatal de Investigación (MCIN/AEI) financed project 10.13039/501100011033, and Tecnológico Nacional de México (TECNM) financed project 10759.21P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Index Fungorum. [(accessed on 20 November 2021)]. Available online: http://www.indexfungorum.org/Names/Names.asp.
- 2.Li M.F., Li G.H., Zhang K.Q. Non-volatile metabolites from Trichoderma spp. Metabolites. 2019;9:58. doi: 10.3390/metabo9030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G., Zhang K., Xu J., Dong J., Liu Y. Nematicidal substances from fungi. Recent Pat. Biotechnol. 2007;1:212–233. doi: 10.2174/187220807782330165. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad G., Khan A., Khan A.A., Ali A., Mohhamad H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Ant. Leeuw. 2021;114:885–912. doi: 10.1007/s10482-021-01577-9. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik N., Díaz C.E., Chhipa H., Julio L.F., Andrés M.F., González-Coloma A. Chemical composition of an aphid antifeedant extract from an endophytic fungus Trichoderma sp. EFI671. Microorganisms. 2020;8:420. doi: 10.3390/microorganisms8030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid A., Ali S. Herbicidal activity of culture filtrates of Trichoderma spp. against two problematic weeds of wheat. Nat. Prod. Res. 2011;25:730–740. doi: 10.1080/14786419.2010.528757. [DOI] [PubMed] [Google Scholar]
- 7.Baazeem A., Almanea A., Manikandan P., Alorabi M., Vijayaraghavan P., Abdel-Hadi A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi. 2021;7:331. doi: 10.3390/jof7050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TariqJaveed M., Farooq T., Al-Hazmi A.S., Hussain M.D., Rehman A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021;183:107626107626. doi: 10.1016/j.jip.2021.107626. [DOI] [PubMed] [Google Scholar]
- 9.Braithwaite M., Clouston A., Minchin R., Yardley J., Nieto-Jacobo M.F., Mendoza-Mendoza A., Steyaert J., Hill R., Marshall J., Stewart A. The density-dependent effect of initial nematode population levels on the efficacy of Trichoderma as a bio-nematicide against Meloidogyne hapla on tomato. Australas. Plant Pathol. 2016;45:473–479. doi: 10.1007/s13313-016-0432-5. [DOI] [Google Scholar]
- 10.Cardona-Piedrahita L.F., Castaño-Zapata J., Ceballos-Aguirre N. Respuesta de quince introducciones de tomate cherry (Solanum lycopersicum L.) al nemátodo nodulador (Meloidogyne spp. Goeldi) e identificación de las especies. Rev. Acad. Colomb. Cienc. Exactas. Fis. Nat. 2016;40:450–460. doi: 10.18257/raccefyn.365. [DOI] [Google Scholar]
- 11.Abd-Elgawad M.M.M., Askary T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest. Control. 2018;28:74. doi: 10.1186/s41938-018-0080-x. [DOI] [Google Scholar]
- 12.Hassan M.A., Pham T.H., Shi H., Zheng J. Nematodes threats to global food security. Acta Agric. Scand, Sect. B-Soil Plant Sci. 2013;63:420–425. doi: 10.1080/09064710.2013.794858. [DOI] [Google Scholar]
- 13.Collange B., Navarrete M., Peyre G., Mateille T., Tchamitchian M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Protect. 2011;30:1251–1262. doi: 10.1016/j.cropro.2011.04.016. [DOI] [Google Scholar]
- 14.Degenkolb T., Vilcinskas A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. App. Microbiol. Biotechnol. 2016;100:3799–3812. doi: 10.1007/s00253-015-7233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldoni D.B., Antoniolli Z.I., Mazutti M.A., Jacques R.J.S., Dotto A.C., de Oliveira Silveira A., Ferraz R.C., Soares V.B., de Souza A.R.C. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz. J. Microbiol. 2020;51:1897–1908. doi: 10.1007/s42770-020-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan H., Yao M., Wang H., Zhao D., Zhu X., Wang Y., Liu X., Duan Y., Chen L. Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol. 2020;20:1–11. doi: 10.1186/s12866-020-01984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan R.A.A., Najeeb S., Mao Z., Ling J., Yang Y., Li Y., Xie B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms. 2020;8:401. doi: 10.3390/microorganisms8030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi S.A., Ruqqia A., Sultana V., Ara J., Ehteshamul-Haque S. Nematicidal potential of culture filtrates of soil fungi associated with rhizosphere and rhizoplane of cultivated and wild plants. Pak. J. Bot. 2012;44:1041–1046. [Google Scholar]
- 19.Elgorban A.M., Abdel-Wahab M.A., Bahkali A.H., Al-Sum B.A. Biocontrol of Meloidogyne javanica on tomato plants by Hypocrea lixii (the Teleomorph of Trichoderma harzianum) Clean-Soil Air Water. 2014;42:1464–1469. doi: 10.1002/clen.201300430. [DOI] [Google Scholar]
- 20.Jindapunnapat K., Chinnasri B., Kwankuae S. Biological control of root-knot nematodes (Meloidogyne enterolobii) in guava by the fungus Trichoderma harzianum. J. Dev. Sustain. Agric. 2013;8:110–118. [Google Scholar]
- 21.Vinale F., Sivasithamparam K., Ghisalberti E.L., Marra R., Barbetti M.J., Li H., Woo S.L., Lorito M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant. Pathol. 2008;72:80–86. doi: 10.1016/j.pmpp.2008.05.005. [DOI] [Google Scholar]
- 22.Yan Y., Mao Q., Wang Y., Zhao J., Fu Y., Yang Z., Pen X., Zhang M., Bai B., Liu A., et al. Trichoderma harzianum induces resistance to root-knot nematodes by increasing secondary metabolite synthesis and defense-related enzyme activity in Solanum lycopersicum L. Biol. Control. 2021;158:104609. doi: 10.1016/j.biocontrol.2021.104609. [DOI] [Google Scholar]
- 23.Reino J.L., Guerrero R.F., Hernández-Galán R., Collado I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008;7:89–123. doi: 10.1007/s11101-006-9032-2. [DOI] [Google Scholar]
- 24.Bansal R., Pachauri S., Gururajaiah D., Sherkhane P.D., Khan Z., Gupta S., Banerjee K., Kumar A., Mukherjee P.K. Dual role of a dedicated GAPDH in the biosynthesis of volatile and non-volatile metabolites-novel insights into the regulation of secondary metabolism in Trichoderma virens. Microbiol. Res. 2021;253:126862. doi: 10.1016/j.micres.2021.126862. [DOI] [PubMed] [Google Scholar]
- 25.Candelero D.J., Cristóbal A.J., Reyes R.A., Gamboa A.M.M., Ruíz S.E., Tun S.J.M. Trichoderma spp. promotoras del crecimiento en plántulas de Capsicum chinense Jacq. y antagónicas contra Meloidogyne incognita. Phyton. Int. J. Exp. Bot. 2015;84:113–119. doi: 10.32604/phyton.2015.84.113. [DOI] [Google Scholar]
- 26.Cristóbal-Alejo J., Cetz-Chi J.I., Tún-Suárez J.M., Moo-Koh F.A., Peraza-Luna F.A., Candelero-De la Cruz J. Filtrados fúngicos de Trichoderma con actividad nematicida contra Meloidogyne incognita (Kofoid & White) Chitwood. Rev. Prot. Veg. 2018;33:1–8. [Google Scholar]
- 27.Moo-Koh F.A., Cristóbal-Alejo J., Reyes-Ramírez A., Tun Suárez J.M., Gamboa-Angulo M. Identificación molecular de aislados de Trichoderma spp. y su actividad promotora en Solanum lycopersicum L. Investig. Cienc. 2017;75:5–11. doi: 10.33064/iycuaa201771335. [DOI] [Google Scholar]
- 28.Moo Koh F.A., Cristóbal Alejo J., Reyes Ramírez A., Tun Suárez J.M., Gamboa Angulo M., Islas-Flores I.R. Incompatibilidad interespecífica de especies de Trichoderma contra Meloidogyne incognita en Solanum lycopersicum. Sci. Fungorum. 2018;47:37–45. doi: 10.33885/sf.2018.47.1191. [DOI] [Google Scholar]
- 29.Andrés M.F., González-Coloma A., Sanz J., Burillo J., Sainz P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012;11:371–390. doi: 10.1007/s11101-012-9263-3. [DOI] [Google Scholar]
- 30.Cristóbal-Alejo J., Tun-Suárez J.M., Moguel-Catzin S., Marbán-Mendoza N., Medina-Baizabal L., Simá-Polanco P., Peraza-Sanchez S.R., Gamboa-Angulo M.M. In vitro sensitivity of Meloidogyne incognita to extracts from native yucatecan plants. Nematropica. 2006;36:89–97. [Google Scholar]
- 31.Julio L.F., González-Coloma A., Burillo J., Diaz C.E., Andrés M.F. Nematicidal activity of the hydrolate byproduct from the semi industrial vapor pressure extraction of domesticated Artemisia absinthium against Meloidogyne javanica. Crop Prot. 2017;94:33–37. doi: 10.1016/j.cropro.2016.12.002. [DOI] [Google Scholar]
- 32.Schneider-Orelli O. Entomologisches Praktikum: Einführung in die land-und. Forstwirtschaftliche Insektenkunde. Aarau Sauerländer; Aarau, Switzerland: 1947. pp. 1–237. [Google Scholar]
- 33.Di Rienzo J.A., Casanoves F., Balzarini M.G., Gonzalez L., Tablada M., Robledo C.W. InfoStat Versión. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba; Cordobá, Argentina: 2020. [(accessed on 12 July 2021)]. Available online: http://www.infostat.com.ar. [Google Scholar]
- 34.Martín J., Crespo G., González-Menéndez V., Pérez-Moreno G., Sánchez-Carrasco P., Pérez-Victoria I., Ruiz-Pérez L.M., González-Packanowska D., Vicente F., Genilloud O., et al. MDN-0104, an antiplasmodial betaine lipid from Heterospora chenopodii. J. Nat. Prod. 2014;77:2118–2123. doi: 10.1021/np500577v. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui I.A., Shaukat S.S. Trichoderma harzianum enhances the production of nematicidal compounds in vitro and improves biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett. Appl. Microbiol. 2004;38:169–175. doi: 10.1111/j.1472-765X.2003.01481.x. [DOI] [PubMed] [Google Scholar]
- 36.Migunova V., Sasanelli N., Kurakov A. Effect of microscopic fungi on larval mortality of the root-knot nematodes Meloidogyne incognita and Meloidogyne javanica. Biol. Integ. Control Plant Pathog. IOBC-WPRS Bull. 2018;133:27–31. [Google Scholar]
- 37.Ntalli N., Menkissoglu-Spiroudi U., Doitsinis K., Kalomoiris M., Papadakis E.N., Boutsis G., Dimou M., Monokrousos N. Mode of action and ecotoxicity of hexanoic and acetic acids on Meloidogyne javanica. J. Pest Sci. 2020;93:867–877. doi: 10.1007/s10340-020-01193-y. [DOI] [Google Scholar]
- 38.Ntalli N.G., Vargiu S., Menkissoglu-Spiroudi U., Caboni P. Nematicidal carboxylic acids and aldehydes from Melia azedarach fruits. J. Agric. Food Chem. 2010;58:11390–11394. doi: 10.1021/jf1025345. [DOI] [PubMed] [Google Scholar]
- 39.Nouranim S.L., Mohammadi-Goltapeh E., Safaie N., Javaran M.J., Pourjam E., Shams-Bakhsh M., Afshar F.J. The effects of Arthrobotrys oligospora and Arthrobotrys conoides culture filtrates on second stage juvenile mortality and egg hatching of Meloidogyne incognita and Meloidogyne javanica. J. Crop Prot. 2015;4:667–674. [Google Scholar]
- 40.Babu B.V., Kamra A., Paul S., Devi T.P. Antibiosis and egg parasitization in root-knot nematode, Meloidogyne incognita by indigenous isolates of Trichoderma harzianum rifai, 1969 in relation to chitinase and protease levels. Indian J. Nematol. 2019;49:187–192. [Google Scholar]
- 41.Tiwari S., Pandey S., Chauhan P.S., Pandey R. Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind. Crops Prod. 2017;97:292–301. doi: 10.1016/j.indcrop.2016.12.030. [DOI] [Google Scholar]
- 42.Sellami S., Benttoumi N., Berrahia S., Boureghda H. Evaluation of antagonistic activity of Trichoderma spp. against Meloidogyne incognita. Acta Phytopathol. Entomol. Hung. 2017;52:177–184. doi: 10.1556/038.52.2017.023. [DOI] [Google Scholar]
- 43.Nielsen K.F., Gräfenhan T., Zafari D., Thrane U. Trichothecene Production by Trichoderma brevicompactum. J. Agric. Food. Chem. 2005;53:8190–8196. doi: 10.1021/jf051279b. [DOI] [PubMed] [Google Scholar]
- 44.Schollenberger M., Drochner W., Müller H.-M. Fusarium toxins of the scirpentriol subgroup: A review. Mycopathologia. 2007;164:101–118. doi: 10.1007/s11046-007-9036-5. [DOI] [PubMed] [Google Scholar]
- 45.Ayer W.A., Miao S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can. J. Chem. 1993;71:487–493. doi: 10.1139/v93-069. [DOI] [Google Scholar]
- 46.Barúa J.E., de la Cruz M., de Pedro N., Cautain B., Hermosa R., Cardoza R.E., Gutiérrez S., Monte E., Vicente F., Collado I.G. Synthesis of trichodermin derivatives and their antimicrobial and cytotoxic activities. Molecules. 2019;24:3811. doi: 10.3390/molecules24203811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kononenko G.P., Soboleva N.A., Leonov A.N. 3, 7, 8, 15-Tetrahydroxy-12, 13-epoxytrichothec-9-en in a culture of Fusarium graminearum. Chem. Nat. Comp. 1990;26:218–220. doi: 10.1007/BF00607551. [DOI] [Google Scholar]
- 48.Mayer A., Anke H., Sterner O. Omphalotin, A new cyclic peptide with potent nematicidal activity from Omphalotus olearius I. Fermentation and biological activity. Nat. Prod. Lett. 1997;10:25–32. doi: 10.1080/10575639708043691. [DOI] [Google Scholar]
- 49.McMorris T.C., Anchel M. Fungal metabolites. The structures of the novel sesquiterpenoids illudin-S and -M. J. Am. Chem. Soc. 1965;87:1594–1600. doi: 10.1021/ja01085a031. [DOI] [PubMed] [Google Scholar]
- 50.Nord C., Menkis A., Broberg A. Cytotoxic illudane sesquiterpenes from the fungus Granulobasidium vellereum (Ellis and Cragin) Jülich. J. Nat. Prod. 2015;78:2559–2564. doi: 10.1021/acs.jnatprod.5b00500. [DOI] [PubMed] [Google Scholar]
- 51.Ito Y., Kurita H., Yamaguchi T., Sato M., Okuda T. Naematolin, a new biologically active substance produced by Naematoloma fasciculare (Fr.) Karst. Chem. Pharm. Bull. 1967;15:2009–2010. doi: 10.1248/cpb.15.2009. [DOI] [PubMed] [Google Scholar]
- 52.Abraham W.R. Bioactive sesquiterpenes produced by fungi: Are they useful for humans as well? Curr. Med. Chem. 2001;8:583–606. doi: 10.2174/0929867013373147. [DOI] [PubMed] [Google Scholar]
- 53.Al-salihi S.A.A., Dao T.T., Williams K., Bailey A.M., Foster G.D. The biogenetic origin of the biologically active naematolin of Hypholoma species involves an unusual sesquiterpene synthase. Mol. Biotechnol. 2019;61:754–762. doi: 10.1007/s12033-019-00199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inchausti A., Yaluff G., Rojas de Arias A., Torres S., Ferreira M.E., Nakayama H., Schinini A., Lorenzen K., Anke T., Fournet A. Leishmanicidal and trypanocidal activity of extracts and secondary metabolites from basidiomycetes. Phytother. Res. 1997;11:193–197. doi: 10.1002/(SICI)1099-1573(199705)11:3<193::AID-PTR68>3.0.CO;2-R. [DOI] [Google Scholar]
- 55.Singh A., Sarma B.K., Singh U.P., Singh R., Singh H.B., Singh K.P. Metabolite profiling can assist variability analysis in Trichoderma species. Arch. Phytopathol. Pflanzenschutz. 2011;44:1697–1702. doi: 10.1080/03235408.2010.516086. [DOI] [Google Scholar]
- 56.Zhou P., Wu Z., Tan D., Yang J., Zhou Q., Zeng F., Zhang M., Bie Q., Chen C., Xue Y., et al. Atrichodermones A–C, three new secondary metabolites from the solid culture of an endophytic fungal strain, Trichoderma atroviride. Fitoterapia. 2017;123:18–22. doi: 10.1016/j.fitote.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Simon A., Dunlop R.W., Ghisalberti E.L., Sivasithamparam K. Trichoderma koningii produces a pyrone compound with antibiotic properties. Soil Biol. Biochem. 1988;20:263–264. doi: 10.1016/0038-0717(88)90050-8. [DOI] [Google Scholar]
- 58.Wickel S.M., Citron C.A., Dickschat J.S. 2H-Pyran-2-ones from Trichoderma viride and Trichoderma asperellum. Eur. J. Org. Chem. 2013;2013:2906–2913. doi: 10.1002/ejoc.201300049. [DOI] [Google Scholar]
- 59.Shi X.-S., Meng L.-H., Li X., Wang D.-J., Zhou X.-W., Du F.-Y., Wang B.-G., Li X.-M. Polyketides and terpenoids with potent antibacterial activities from the Artemisia argyi-derived fungus Trichoderma koningiopsis QA-3. Chem. Biodivers. 2020;17:e2000566. doi: 10.1002/cbdv.202000566. [DOI] [PubMed] [Google Scholar]
- 60.Mazzei P., Vinale F., Woo S.L., Pascale A., Lorito M., Piccolo A. Metabolomics by proton high-resolution magic-angle-spinning nuclear magnetic resonance of tomato plants treated with two secondary metabolites isolated from Trichoderma. J. Agric. Food Chem. 2016;64:3538–3545. doi: 10.1021/acs.jafc.6b00801. [DOI] [PubMed] [Google Scholar]
- 61.Cutler H.G., Himmelsbach D.S., Yagen B., Arrendale R.F., Jacyno J.M., Cole P.D., Cox R.H. Koninginin B: A biologically active congener of koninginin A from Trichoderma koningii. J. Agric. Food Chem. 1991;39:977–980. doi: 10.1021/jf00005a035. [DOI] [Google Scholar]
- 62.Zhou X.X., Li J., Yang Y.H., Zeng Y., Zhao P.J. Three new koninginins from Trichoderma neokongii 8722. Phytochem. Lett. 2014;8:137–140. doi: 10.1016/j.phytol.2014.03.004. [DOI] [Google Scholar]
- 63.Chen L., Wu G.-W., Liu D., Zhuang W.-Y., Yin W.-B. Trichodermatides E and F from fungus Trichoderma applanatum. J. Asian Nat. Prod. Res. 2019;21:659–665. doi: 10.1080/10286020.2018.1465051. [DOI] [PubMed] [Google Scholar]
- 64.Shi X.S., Wang D.J., Li X.M., Li H.L., Meng L.H., Li X., Yan P., Wang X.Z., Wang B.G. Antimicrobial polyketides from Trichoderma koningiopsis QA-3, an endophytic fungus obtained from the medicinal plant Artemisia argyi. RSC Adv. 2017;7:51335–51342. doi: 10.1039/C7RA11122C. [DOI] [Google Scholar]
- 65.Shi X.S., Li H.L., Li X.M., Wang D.J., Li X., Meng L.H., Zhou X.W., Wang B.G. Highly oxygenated polyketides produced by Trichoderma koningiopsis QA-3, an endophytic fungus obtained from the fresh roots of the medicinal plant Artemisia argyi. Bioorg. Chem. 2020;94:103448. doi: 10.1016/j.bioorg.2019.103448. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y.L., Hu B.Y., Qian M.A., Wang Z.H., Zou J.M., Sang X.Y., Li L., Luo D.L., Zhao L.X. Koninginin W, a new polyketide from the endophytic fungus Trichoderma koningiopsis YIM PH30002. Chem. Biodivers. 2021;18:e2100460. doi: 10.1002/cbdv.202100460. [DOI] [PubMed] [Google Scholar]
- 67.Nagao K., Yoshida N., Iwai K., Sakai T., Tanaka M., Miyahara T. Production of sepedonin by Sepedonium chrysospermum NT-1 in submerged culture. Environ. Sci. 2006;13:251–256. [PubMed] [Google Scholar]
- 68.Divekar P.V., Raistrick H., Dobson T.A., Vining L.C. Studies in the biochemistry of microorganisms: Part 117. Sepedonin, a tropolone metabolite of Sepedonium chrysospermum Fries. Can. J. Chem. 1965;43:1835–1848. doi: 10.1139/v65-241. [DOI] [Google Scholar]
- 69.Quang D.N., Schmidt J., Porzel A., Wessjohann L., Haid M., Arnold N. Ampullosine, a new isoquinoline alkaloid from Sepedonium ampullosporum (Ascomycetes) Nat. Prod. Commun. 2010;5:869–872. doi: 10.1177/1934578X1000500609. [DOI] [PubMed] [Google Scholar]
- 70.Hu Z., Tao Y., Tao X., Su Q., Cai J., Qin C., Ding W., Li C. Sesquiterpenes with phytopthogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J. Agric. Food Chem. 2019;67:10646–10652. doi: 10.1021/acs.jafc.9b04053. [DOI] [PubMed] [Google Scholar]
- 71.Shi X.S., Meng L.H., Li X.M., Li X., Wang D.J., Li H.L., Zhou X.W., Wang B.G. Trichocadinins B–G: Antimicrobial cadinane sesquiterpenes from Trichoderma virens QA-8, an endophytic fungus obtained from the medicinal plant Artemisia argyi. J. Nat. Prod. 2019;82:2470–2476. doi: 10.1021/acs.jnatprod.9b00139. [DOI] [PubMed] [Google Scholar]
- 72.Yang Z.S., Li G.H., Zhao P.J., Zheng X., Luo S.L., Li L., Niu X.M., Zhang K.Q. Nematicidal activity of Trichoderma spp. and isolation of an active compound. World J. Microbiol. Biotechnol. 2010;26:2297–2302. doi: 10.1007/s11274-010-0410-y. [DOI] [Google Scholar]
- 73.Zhou Y.M., Ju G.L., Xiao L., Zhang X.F., Du F.Y. Cyclodepsipeptides and sesquiterpenes from marine-derived fungus Trichothecium roseum and their biological functions. Mar. Drugs. 2018;16:519. doi: 10.3390/md16120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ntalli N., Ratajczak M., Oplos C., Menkissoglu-Spiroudi U., Adamski Z. Acetic acid, 2-undecanone, and (E)-2-decenal ultrastructural malformations on Meloidogyne incognita. J. Nematol. 2016;48:248–260. doi: 10.21307/jofnem-2017-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.