Abstract
During pregnancy and lactation, considerable factors that affect the maternal microbiome are associated with the advancement of numerous diseases, which can potentially affect offspring health. Probiotics have shown potential for the maintenance of microbiota homeostasis of mothers in this period. The specific objective of this study was to investigate whether the application of Akkermansia muciniphila (A. muciniphila) during pregnancy and lactation impacts maternal and offspring health. Here we show that dams fed with A. muciniphila is safe, enhances the intestinal barrier and alters gut microbiota composition and diversity at the end of lactation, including the significant enrichment of A. muciniphila and Ruminococcus_1 in offspring from probiotic-fed dams. However, compared with the control group, the fecal metabolites of the A. muciniphila group only changed slightly. Additionally, A. muciniphila supplementation did not significantly increase the abundance of A. muciniphila in the fecal microbiota of offspring mice. Compared with the control group, the fecal metabolic profile of three-week-old offspring of mice fed with A. muciniphila were significantly changed, containing the D-glutamine and D-glutamate metabolism pathways. These results provided evidence that A. muciniphila supplementation in mice during pregnancy and lactation is safe and seemed to have a more beneficial effect on dams. In the future, using probiotics to regulate maternal microbiomes during pregnancy and lactation could be shown to have a more lasting and beneficial effect.
Keywords: A. muciniphila, pregnancy, lactation, intestinal barrier, fecal metabolites
1. Introduction
Knowledge on gut microbiota has greatly expanded over the last few years, and growing evidence from various epidemiological studies suggests that allergy, obesity, metabolic syndrome, and other chronic diseases in adulthood are related to the disruption of gut microbiota early in life. A recent study showed that children who were prescribed antibiotics early in life (within the first 2 years) were at increased risk of developing conditions such as asthma in the future [1]. At birth, native microbes quickly and intensively adapt to and take over the gut of a newborn, and these early colonizers can significantly affect gut microbiota development via a “priority effect,” and the taxonomic and functional characteristics of the gut microbiota at birth can persist even after environmental changes [2]. The maternal microbiome during pregnancy or lactation is the dominant force affecting the gut microbiota of the offspring. The maternal microbiome may be transmitted directly to the baby vertically through the gut, vagina, and breast milk [3], or they may indirectly guide the establishment of neonatal microbiota and immune development via their metabolites [4,5]. Population-based cohort studies showed that the alpha diversity of the maternal fecal microbiota during the third trimester of pregnancy can predict the brain and behavioral development of the child at 2 years of age [6]. A new study from an Australian prebirth cohort has suggested a notable correlation between the existence of Prevotella copri in mothers’ gut during pregnancy and the risk of allergic disease in their offspring. Furthermore, increasing the abundance of P. copri in the guts of pregnant mothers may help prevent food allergies in children [7]. Oral probiotics have many benefits for human health [8,9,10,11]. Some probiotics or mixed probiotics can be used to interfere with the microbiota of mothers, which may have beneficial effects on the offspring.
The colon mucus layer is a heavily O-glycosylated mucin layer that is tightly attached to the intestinal epithelium. This layer plays a vital role in preventing the gut microbiota from activating intestinal immune cells, which may lead to inflammation and cancer. The main component of intestinal mucus is MUC2, produced primarily by goblet cells in the distal colon. A. muciniphila, a probiotic with great application potential, is common in the gastrointestinal (GI) tract of mammals and can regulate mucin secretion [12]. Studies have presented evidence that A. muciniphila supplementation can repair the ethanol-induced disruption of intestinal barrier integrity, increase the thickness of intestinal mucus, and increase the expression of tight junction proteins [13]. Moreover, A. muciniphila administration extended crypt length, increased the number of goblet cells, and improved colitis in mice fed a low-cellulose diet [14]. In addition, previous studies have shown that A. muciniphila can improve metabolic function as well as prevent high-fat-diet-induced obesity [15] and type I and type II diabetes [16,17,18]. A novel bacterial strain, A. muciniphila WB-STR-0001, has been shown to be safe, well-tolerated, and able to help control postprandial glucose in patients with type 2 diabetes [19].
A. muciniphila is present in the GI tract of 1-month-old infants (at the earliest), whereafter it colonizes steadily within the first year after birth, eventually accounting for approximately 1–4% of the microbial community in healthy adults [20]. Furthermore, A. muciniphila has the ability to metabolize oligosaccharides in breast milk, which may contribute to microbiome establishment and immune maturation in the early life of infants [21]. A new study found that betaine intake during lactation increased betaine content in breast milk, resulting in a temporary increase in the ratio of A. muciniphila in early life, decreased adiposity, and improved glucose homeostasis in the offspring in adulthood [22]. A. muciniphila treatment successfully prevented hippocampus-dependent learning and memory deficits in mice fed high-fat diet early in life [23]. Therefore, increasing the abundance of A. muciniphila may be a promising strategy to enhance infant health during early life.
Many studies have investigated the influence of probiotic supplementation during pregnancy in women with obesity/diabetes. A randomized controlled clinical trial found that probiotic yoghurts, including Lactobacillus and Bifidobacterium, improved glucose metabolism in overweight and obese pregnant women [24]. In addition, administration of specific probiotic strains has been reported to exert beneficial effects in the offspring, such as preventing allergic disease [25] and infantile colic [26]. However, most studies have focused on Lactobacillus and Bifidobacterium or mixtures of the two strains. Thus, it remains unclear whether A. muciniphila has a positive effect on maternal and fetal health. In light of this, this study aimed to investigate the effects of A. muciniphila intake during pregnancy and lactation on the productive performance of mothers, as well as to monitor the physiological growth indices, changes in microbiota composition, and metabolites of the mothers and their offspring.
2. Materials and Methods
2.1. Bacterial Strains and Preparation
A. muciniphila MucT (ATCC BAA-835) was obtained from the American Type Culture Collection (ATCC). The strain was cultured as described previously [21]. The bacterial culture medium was brain–heart infusion medium (Qingdao Hope Bio-Technology Company, Qingdao, China) supplemented with hog gastric mucin (0.25% w/v Type II; Sigma-Aldrich, St. Louis, MO, USA). The bacterial cells were grown under anaerobic conditions at 37 °C for 18 h, and were enriched by centrifugation (6000× g, 10 min) and washed with normal saline. Finally, the bacterial sludge was resuspended in normal saline at the required final concentration.
2.2. Animals
Male and female C57BL/6 mice (8 weeks of age) were purchased from Charles River Laboratory Animal Technology Co., Ltd. (Beijing, China) and then housed in the Laboratory Animal Center of the Department of Food Science and Technology, Jiangnan University, Wuxi, China. The animals were housed under specific pathogen-free conditions. Male and female mice were randomly selected and mated for 24 h, with a mating ratio of 1:1. After mating (P1), all female mice were individually housed and monitored daily until birth. After observation and recording of the changes in mouse weight and body shape, the female mice confirmed not to be pregnant were again caged with male mice to ensure that there were 10 pregnant female mice in each group. The day of birth was the first day of the offspring (B1). It was guaranteed that each lactating female mouse fed 5–8 pups, which were allowed free access to breast milk. At 3 weeks of age, the pups were weaned, separated from their mother, and housed in populations of five per cage.
2.3. Experimental Design
All animals were randomized into two groups: (1) CON group and (2) AKK group. Each group consisted of 10 mothers and their respective 5–8 pups. Except for 3 days before and after birth, mothers in the AKK group were fed A. muciniphila (108–109 CFU/day) by oral gavage for 6 weeks, including 3 weeks of gestation and 3 weeks of lactation. The CON group, i.e., the control group, received normal saline under the same conditions. The samples of mothers and pups collected at different time points are shown in Figure 1A. Maternal and offspring body weight was monitored weekly. Feces from mothers were collected twice during pregnancy (P1, P17) and once on the 21st day of lactation (D21). Feces from pups were collected after birth (B7, B14, B21, B28, and B42). After weaning, all mothers were sacrificed. At each time point (B7, B14, B21, B28 and B42), 8–10 mice from 3–10 different cages in each group were sacrificed. The colon and ileum of each mouse were quickly removed for further analysis.
Figure 1.
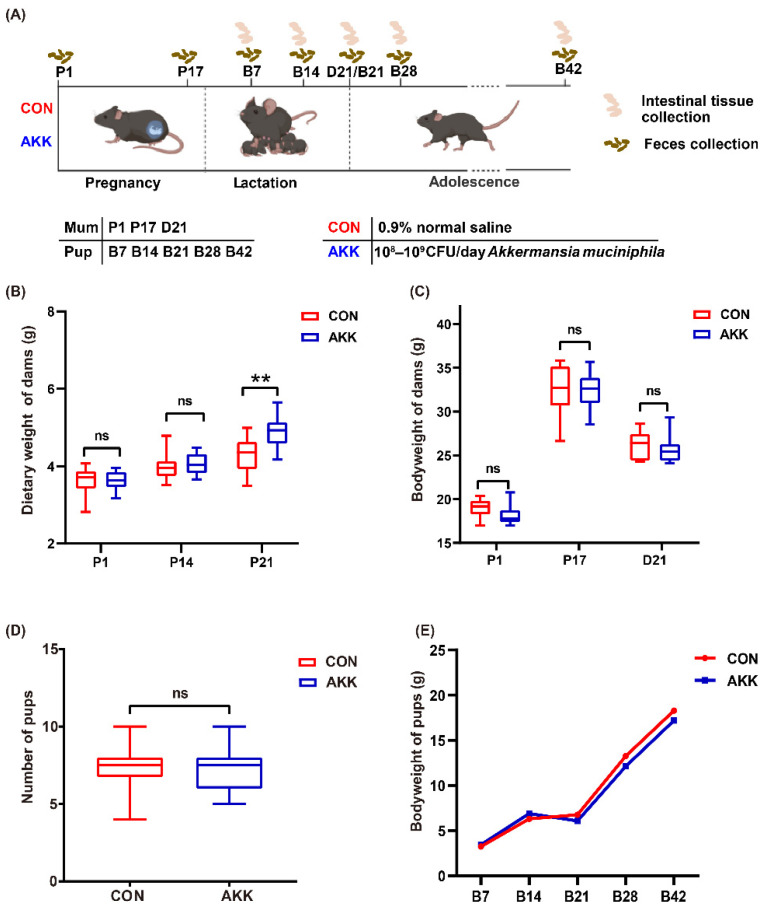
A. muciniphila supplementation had no significant effect on the basic physiological indexes of mouse dams and pups. (A) Experimental design: Mothers were fed A. muciniphila or 0.9% normal saline starting from the first day of pregnancy (P1) to the end of lactation (D21). Starting from postnatal day 7 (B7), pups were sacrificed at regular intervals for sample collection. All mice were fasted and weighed before sacrifice. (B) Dietary weight of dams. (C) Bodyweight of dams during pregnancy and lactation. (D) The number of pups in each group. (E) Bodyweight of pups from day 7 to day 42 after birth. ns: no significant difference between the two groups, ** p < 0.01 ns: not significant difference.
2.4. Histology
The colon and ileum of mice were excised and fixed in 10% neutral buffered formalin or Carnoy’s fluid. Tissue sections were embedded and stained with hematoxylin and eosin (H and E) or periodic acid-Schiff (PAS), as described previously [27]. All images were captured using a Leica BA410E microscope. Crypt height and the number of goblet cells were quantified by a blinded investigator.
2.5. Total RNA Extraction and qPCR Analysis
Total tissue RNA was extracted from colonic and ileal tissues and was reverse-transcribed into complementary DNA using the HiScript® III RT SuperMix R333-01 for qPCR (+gDNA wiper) kit (Vazyme Biotech Co., Ltd., Nanjing, China). qPCR was performed with SYBR green (iTaq™ Universal SYBR® Green SuperMix; Bio-Rad Laboratories Co., Ltd., Shanghai, China) on a real-time PCR system (CFX384 Real-Time PCR Detection System; Bio-Rad Laboratories Co., Ltd., Shanghai, China), using β-actin as a housekeeping gene. Primer sequences are listed in Supplementary Material Table S1.
2.6. Fecal Sample Collection and Genomic DNA Extraction
Fecal samples from each mouse were collected rapidly in a sterile tube and stored at −80 °C until genomic DNA extraction. Genomic DNA was obtained using the Fast DNA Spin Kit for Feces (MP Biomedicals, Carlsbad, CA, USA). Its quantity and quality were assessed using a NanoPhotometer®N50 (Implen, Inc., Westlake Village, CA, USA). The V3–V4 regions of the 16S rRNA gene were amplified using the primers 341F/806R. Amplicons were then purified using a DNA Gel/PCR Purification Miniprep Kit (Biomiga, Hangzhou, China). The purified DNA was added to an equimolar solution and pooled. For library preparation, the DNA samples were sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA).
The paired-end sequences were denoised and merged, and chimeras were removed and filtered using the DADA2 plugin. Quality-controlled data were analyzed using the QIIME 2 pipeline [28]. Taxonomic sequence classification was performed using the classifier trained on the V4 515–806 bp regions of the 16S rRNA gene sequences from the Silva rRNA reference database. Analysis of the structure of gut microbiota was performed using the QIIME 2 software and calculated using the R software.
2.7. Detection of A. muciniphila by qPCR
The ratio of A. muciniphila in stool samples was determined by qPCR, as described previously [29]. Bacterial DNA with a known colony-forming unit value was extracted using a TIANamp Bacteria DNA kit (TIANGEN, Beijing, China), and a standard curve of A. muciniphila was established with a range of 102 to 1010 cells. Amplification was conducted using a real-time PCR system (CFX384 Real-Time PCR Detection System; Bio-Rad Laboratories). The absolute abundance of A. muciniphila in each stool sample was calculated based on the Cq value of the standard curve. All assays were performed twice. Fecal sample DNA was extracted as described in Section 2.6.
2.8. Untargeted Metabolomics Analysis
Fecal metabolites were analyzed as previously described [30,31]. Fecal pellets (60 mg) from mothers (P1, P17, and D21) and pups (B21 and B42) were collected directly into individual 1.5-mL centrifuge tubes and homogenized with 600 μL of methanol/acetonitrile/deionized water mixture (2:1:1, v/v/v, precooled to −20 °C), and then centrifuged at 4 °C (12,000× g, 10 min). The supernatant (400 μL) was concentrated, dried for 2 h under vacuum, and reconstituted with 200 μL of methanol/deionized water (4:1, v/v). Next, the samples were vortexed and centrifuged as described above. Using a 0.22-μm membrane filter, the supernatant was filtered into a sample bottle for further analysis. To prepare quality control (QC) samples, 2 μL of each sample was placed in the same tube and mixed. Methanol (80%) was used as the blank control sample.
Metabolites were detected using a Dionex Ultimate-3000 ultrahigh-performance liquid chromatography (UPLC) system (Thermo Fisher Scientific, Waltham, MA, USA) coupled with a high-resolution Q-ExactiveOrbitrap mass spectrometer system in the full scan mode (Thermo Fisher Scientific, Waltham, MA, USA). An Acquity HSS T3 column (2.1 mm × 100 mm, 1.8 µm; Waters, MA, USA) was used for UPLC separation, at a flow rate of 0.3 mL/min and injection volume of 2 µL. The chromatographic and mass spectrometric conditions are listed in Table S2.
Metabolomics data were analyzed using the Compound Discoverer 3.2 software (Thermo Fisher Scientific) as described previously [31]. The orthogonal partial least-squares discriminant analysis (OPLS-DA) model was used to identify the metabolic features that represented the difference between the two groups. Metabolic features with variable importance in projection (VIP, VIP > 1, and p < 0.05) were reserved as potential differential biomarkers. Metabolic pathway analysis based on the identified metabolites was conducted using MetaboAnalyst 5.0.
2.9. Statistical Analysis
The GraphPad Prism 8 software (version 8.2.1 Windows version, GraphPad Software, San Diego, CA, USA) and charting tools in Hiplot (https://hiplot.com.cn, accessed on 8 October 2021), a comprehensive web platform for scientific data visualization, were used for data processing. Statistical comparisons were conducted using one-way or two-way analysis, followed by Tukey’s test or unpaired t-test. The data for each treatment are shown as the mean ± SEM. Statistical significance was considered at * p < 0.05, ** p < 0.01, and *** p < 0.001. “ns” means no significant difference between the two groups.
3. Results
3.1. Effects of A. muciniphila Supplementation on the Basic Physiological Indexes of Mothers and Pups
There was no significant reduction in dietary weight and body weight between A. muciniphila-fed and placebo-fed dams (Figure 1B,C). The amount of food intake was measured weekly during pregnancy, and our analysis showed that the AKK group had significantly increased food intake before birth (P21) than the CON group (4.29 ± 0.44 vs. 4.89 ± 0.49 for the CON group vs. the AKK group, respectively, p = 0.002) (Figure 1B). As the numbers of pups per litter were not always the same, and these pups had started ingesting solid foods in the later lactation period, the amount of maternal food intake during lactation was not recorded. By the time before birth (P17), dams in both groups weighed approximately 32 g. As the lactation period ended, maternal weight decreased (Figure 1C). In addition, A. muciniphila supplementation had no adverse effects on gestational outcomes. All female mice delivered naturally, and the number of offspring was not affected by A. muciniphila supplementation (Figure 1D). Over time, the pups grew normally and gained weight, with no significant difference between the two groups at any time point (Figure 1E).
3.2. Effects of A. muciniphila Supplementation on the Intestinal Barrier of Mothers
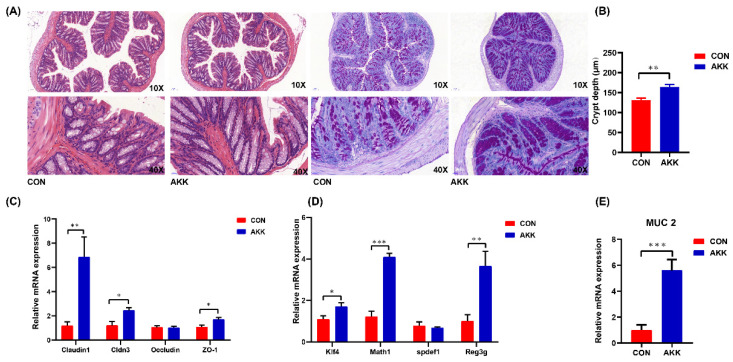
A. muciniphila supplementation during pregnancy and lactation markedly enhanced the intestinal barrier of mothers (D21). Compared with those in the CON group, the number of goblet cells and crypt depth were significantly increased in the AKK group (Figure 2A,B). Analysis of tight junction (TJ) mRNA expression indicated that A. muciniphila-fed mothers had higher mRNA expression levels of claudin 1, claudin 3, and ZO 1 than mothers in the CON group. Furthermore, occludin mRNA expression was not affected (Figure 2C), whereas mucin 2 (MUC2) mRNA expression was increased (Figure 2E). The mRNA expression levels of genes related to goblet cells, including Krüppel-like factor 4 (Klf4) and Math1, showed similar significant changes. However, Spedf mRNA expression was not significantly altered in the AKK group compared with that in the CON group (Figure 2D). Reg3g gene expression in the AKK group was significantly upregulated compared with that in the CON group (Figure 2D). Overall, these results suggest that A. muciniphila supplementation during pregnancy and lactation enhances the intestinal barrier.
Figure 2.
Effects of A. muciniphila supplementation during pregnancy and lactation on the intestinal barrier of mouse dams. (A) Colonic tissues of mothers at the end of lactation (D21) were observed by hematoxylin-eosin (left) and periodic acid-Schiff staining (right). (B) Crypt depth of at least 100 villi or crypts per mice was detected. (C,D) mRNA expression of genes associated with the intestinal barrier. (E) mRNA expression of mucin 2 (MUC2). * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Maternal A. muciniphila Supplementation Did Not Affect the Intestinal Barrier of Offspring
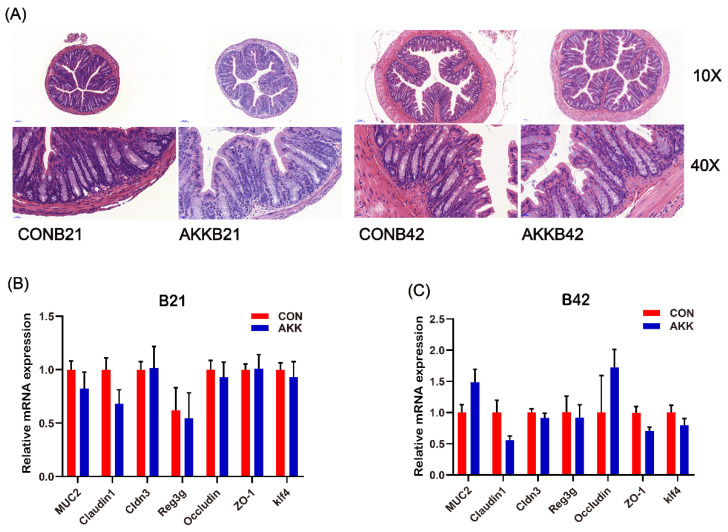
To investigate whether A. muciniphila supplementation in mothers affects the intestinal barrier of their offspring, offspring mice were observed for up to 42 days. In the CON group, the depth of intestinal crypts and the mRNA expression of genes related to the intestinal barrier increased rapidly with time, and the time of weaning (B21) was a key turning point (Figure S1). Furthermore, comparison of the colon sections of offspring on days 21 and 42 (B21 and B42) after birth showed that A. muciniphila supplementation did not affect the development of intestinal tissue structure (Figure 3A). No difference was observed in the expression of TJ genes and the number of goblet cells in pups, and MUC2 expression showed the same trend in the AKK group at B21 and B42 (Figure 3B). Comparisons between the two groups at other time points showed the same conclusion (Figure S2A,B).
Figure 3.
Effects of A. muciniphila supplementation during pregnancy and lactation on the intestinal barrier of mouse offspring. (A) Representative image of hematoxylin–eosin-stained colon tissue of offspring on days 21 and 42 (B21 and B42) after birth. The mRNA expression of genes related to the intestinal barrier of offspring at B21 (B) and B42 (C) in different groups.
3.4. Effects of A. muciniphila Supplementation on the Gut Microbiota Composition of Mothers
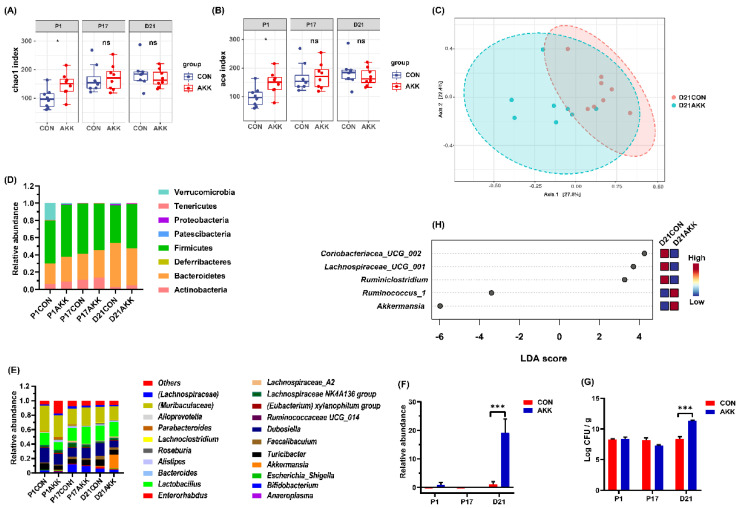
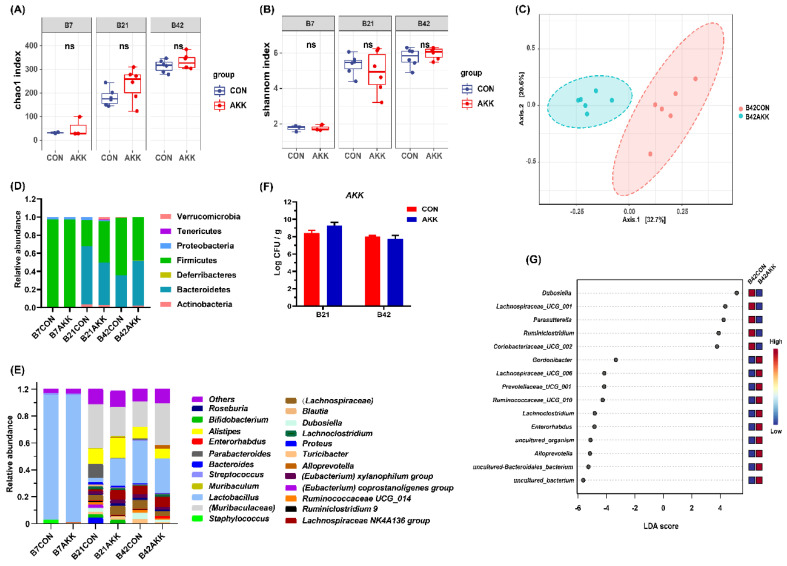
The gut microbiota compositions of mothers at P1, P17, and D21 were analyzed. At the beginning of pregnancy (P1), namely the first day of A. muciniphila supplementation, the community richness index (Chao1 and ACE indices) of pregnant mice was significantly increased (Figure 4A,B), but the community diversity index showed no significant change (Figure S3). As pregnancy progressed, the gut microbiota of the mothers tended to be stable. The AKK group did not display any significant differences in richness and diversity indices. Dramatic differences in the β-diversity of gut microbiota were only observed at D21 (Figure 4C; Bray–Curtis index, p < 0.006). As the mice underwent pregnancy and lactation, the structure of their gut microbiota changed (Figure 4D,E). At the phylum level, the abundance of Actinobacteria first increased and then decreased (Figure 4D; 0.05 vs. 0.13 vs. 0.09, P1 vs. P17, p = 0.014; P17 vs. D21 p = 0.85, in the CON group), whereas the relative abundance of Bacteroidetes gradually decreased (Figure 4D; 0.43 vs. 0.32 vs. 0.29, P1 vs. P17, p = 0.13; P17 vs. D21 p = 0.96, in the CON group). A. muciniphila supplementation shifted the composition of gut microbiota and increased the relative abundance and absolute concentration of A. muciniphila in maternal feces at D21 (Figure 4F,G; p < 0.001, p < 0.001). Furthermore, supplementation with probiotics increased the relative abundance of Ruminococcus_1, but decreased the ratios of Coriobacteriaceae UCG-002, Lachnospiraceae UCG-001, and Ruminiclostridium at D21 (Figure 4H; p < 0.05).
Figure 4.
A. muciniphila supplementation results in favorable alterations in maternal gut microbiota. (A) The Chao1 index of mother mice. (B) The ACE index of mother mice. (C) PCoA indicating the β-diversity of gut microbiota in mother mice. Major changes in bacterial phyla (D) and genera (E) in mother mice. (F) A. muciniphila relative abundance in maternal feces. (G) A. muciniphila absolute concentration in maternal feces. (H) LEfSe analysis of maternal gut microbiota at D21. * p < 0.05, *** p < 0.001, ns: not significant difference.
3.5. Effects of A. muciniphila Supplementation on the Gut Microbiota Composition of Offspring
To determine whether A. muciniphila supplementation to mothers during pregnancy and lactation also affected the composition of gut microbiota of their offspring, stool analysis was conducted by 16S rRNA gene sequencing. We focused on three time points: the seventh day of birth (B7), day after weaning (B21), and post-pubertal phase (B42). There was no significant difference in the α-diversity of gut microbiota between the offspring of the two groups at any time point (Figure 5A). Furthermore, β-diversity analysis results showed that microbial signatures were distinctly clustered in different group at B42 (Figure 5B; Bray-Curtis Index, p < 0.03), but not at the other time points (Figure S4a,b). The composition of intestinal microbiota of the offspring of A. muciniphila-fed mice was not significantly different from that of the CON group on the 21st day after birth, but its composition was similar to that on day 42 (Figure 5D,E). The relative abundance of A. muciniphila in the offspring of the AKK group at day 21 was higher than that in the CON group, but the difference was not significant (Figure S4c). The results of qPCR analysis for A. muciniphila in pup fecal samples were similar to the sequencing results, indicating that there was no significant difference in the abundance of A. muciniphila in the feces of 21- and 42-day-old pups between the two groups (Figure 5F). LEfSe analysis showed that at B42, the offspring of mothers receiving A. muciniphila had decreased abundance of Dubosiella, Lachnospiraceae_UCG_001, and Parasutterella, and increased abundance of Gordonibacter, Lachnospiraceae_UCG_006, and Prevotellaceae_UCG_001, compared with the offspring of the CON group (Figure 5G; p < 0.05).
Figure 5.
A. muciniphila supplementation results in favorable alterations in the gut microbiota of pups. (A) The Chao1 index of pups. (B) The Shannon index of pups. (C) PCoA describing the β-diversity clustering of the gut microbiota of pups. Major changes in bacterial phyla (D) and genera (E) in pups. (F) A. muciniphila absolute concentration in maternal feces. (G) LEfSe analysis of maternal gut microbiota at D21. ns: not significant difference.
3.6. Fecal Metabolites of Mothers and Pups Are Modified by A. muciniphila Supplementation
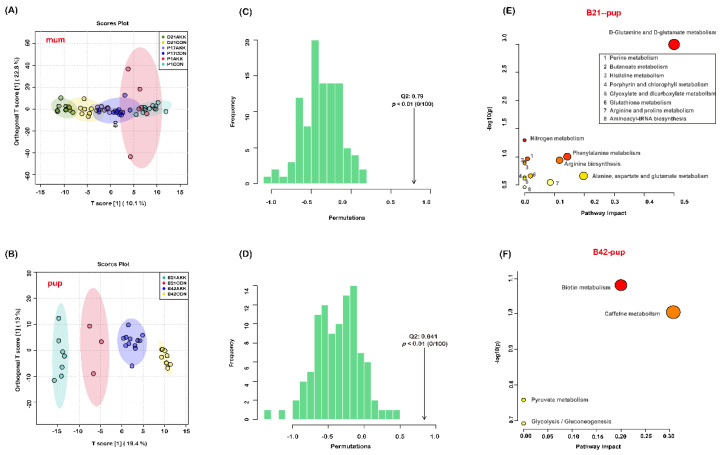
To further analyze the effects of A. muciniphila supplementation on the intestinal metabolites of mothers and pups, untargeted metabolomics analysis was conducted to identify changes in metabolites in mouse feces. After data filtering using the CD software, 448 and 449 different metabolites under the POS and NEG ion modes were successfully identified in the feces of dams and pups, respectively (Tables S3 and S4). OPLS-DA was applied to discriminate the differential features in the stool samples of mothers and pups. The results showed a segregation between mothers in the CON and AKK groups only at D21 (Figure 6A). However, significant clustering was observed among pups at different time points (Figure 6B). Permutation test further validated the good predictability of OPLS-DA (Figure 6C,D). Furthermore, univariate analysis was performed to identify important differential metabolites between the two groups. A total of one, six, and nine metabolites were identified as differential biomarkers (log2(FC) > 1 and p < 0.05) in mothers of the AKK group at P1, P17, and D21, respectively, and 19 and 23 metabolites were identified as differential biomarkers (log2(FC) > 1, p < 0.05) in pups of the AKK group at B21 and B42, compared with the CON group at the corresponding time points (Table 1). Metabolic pathway analysis using MetaboAnalyst showed that the metabolites with significant changes at B21 are involved in 13 metabolic pathways, and that A. muciniphila had the greatest effects on the D-glutamine and D-glutamate metabolism pathways (impact = 0.5), which were significant (p < 0.05) (Figure 6E). At B42, the altered metabolites were related to biotin metabolism, caffeine metabolism, pyruvate metabolism, and glycolysis/gluconeogenesis (p > 0.05) (Figure 6F). The differential metabolites in the other groups could not be associated with relevant pathways.
Figure 6.
Effects of A. muciniphila supplementation during pregnancy and lactation on the fecal metabolites of mothers and pups. OPLS-DA score plots of fecal metabolites of mothers (A) and pups (B) at different time points. The distribution of test statistic (Q2) and p value for permutation test of (C,D). Pathway analyzes of the differential metabolites in pups at B21 (E) B42 (F).
Table 1.
Effects of A. muciniphila on fecal metabolomics.
| Group | Metabolites | Change 1 | log2(FC) | −log10(p) | |
|---|---|---|---|---|---|
| mum | P1 | 2,4-quinolinediol | − | 5.0125 | 2.5454 |
| P17 | quercetin | − | 3.0601 | 1.7229 | |
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-4-one | − | 2.6156 | 2.4021 | ||
| N-({(1S,4S,6S)-4-[2-(4-Acetyl-1-piperazinyl)-2-oxoethyl]-6-isopropyl-3-methyl-2-cyclohexen-1-yl}methyl)-3-fluorobenzamide | −1.2058 | 2.0271 | |||
| 5(Z),8(Z),11(Z)-eicosatrienoic acid methyl ester | − | 1.4851 | 1.3538 | ||
| 3-amino-2-phenyl-2H-pyrazolo[4,3-c]pyridine-4,6-diol | − | 1.4208 | 1.53 | ||
| 2,4-quinolinediol | − | 1.3326 | 1.6166 | ||
| D21 | chalconaringenin | − | 2.2558 | 4.4939 | |
| chrysin | − | 2.2671 | 3.9032 | ||
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-4-one | − | 4.0824 | 3.7509 | ||
| kaempferol | − | 4.2064 | 3.6954 | ||
| apigenin | − | 1.9089 | 2.7149 | ||
| quercetin | − | 3.0535 | 2.0839 | ||
| 3-methoxy-5,7,3’,4’-tetrahydroxy-flavone | − | 1.2944 | 2.0068 | ||
| 1-(3-hydroxy-3-methylpent-4-en-1-yl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol | + | −1.6698 | 1.5552 | ||
| N-({(1S,4S,6S)-4-[2-(4-Acetyl-1-piperazinyl)-2-oxoethyl]-6-isopropyl-3-methyl-2-cyclohexen-1-yl}methyl)-3-fluorobenzamide | + | −1.4211 | 1.499 | ||
| pup | B21 | ferulic acid | − | 1.3433 | 2.3336 |
| 2-deoxyadenosine | − | 1.7339 | 2.1811 | ||
| 1,2,3-propanetricarboxylic acid | + | −3.4132 | 1.9936 | ||
| dihomo-γ-linolenic acid ethyl ester | + | −1.1179 | 1.9883 | ||
| 4-dodecylbenzenesulfonic acid | + | −1.1009 | 1.9561 | ||
| N-ethylglycine | + | −1.9219 | 1.9212 | ||
| adenine | − | 1.3701 | 1.8602 | ||
| trigonelline | − | 1.8588 | 1.7411 | ||
| apigenin | − | 1.1448 | 1.6694 | ||
| 13Z,16Z-docosadienoic Acid | + | −1.3788 | 1.5585 | ||
| D-pyroglutamic acid | + | −1.0427 | 1.4999 | ||
| L-glutamic acid | + | −1.6019 | 1.4835 | ||
| isobutyric acid | − | 1.6023 | 1.4738 | ||
| stachydrine | + | −1.4942 | 1.433 | ||
| phenylacetaldehyde | − | 1.2711 | 1.3859 | ||
| 1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4(1H,3H)-dione | − | 1.0677 | 1.3794 | ||
| acamprosate | + | −5.1411 | 1.354 | ||
| L-carnitine | + | −1.4233 | 1.354 | ||
| (1S,19R)-8,10-dioxa-4,17-diazaheptacyclo[15.4.3.01,18 04,19 05,13 07,11 014,19]tetracosa-5(13),6,11,22-tetraen-3-one | + | −1.0226 | 1.3446 | ||
| B42 | acamprosate | − | 4.3948 | 6.7137 | |
| NP-003553 | − | 1.262 | 5.2985 | ||
| NP-015980 | + | −1.8399 | 5.1508 | ||
| L-lactic acid | − | 1.1072 | 4.1074 | ||
| methyl indole-3-acetate | − | 1.3097 | 3.0721 | ||
| tridecylic acid | − | 2.6194 | 2.7511 | ||
| ursolic acid | + | −1.0904 | 2.7069 | ||
| 1-methylxanthine | + | −1.9701 | 2.4263 | ||
| 1-benzyl-3-[(1S,4S)-4-{3,5-dimethyl-1-[4-(2-methyl-2-propanyl)phenyl]-1H-pyrazol-4-yl}-2-cyclopenten-1-yl]urea | + | −1.7189 | 2.3518 | ||
| N-ethylglycine | − | 1.0197 | 2.3183 | ||
| 3-methylxanthine | + | −1.4299 | 1.9015 | ||
| indole-3-lactic acid | + | −1.2901 | 1.8742 | ||
| 5-[({(2R,4S,5R)-5-[1-methyl-3-(2-thienyl)-1H-pyrazol-5-yl]-1-azabicyclo[2.2.2]oct-2-yl}methyl)amino]-5-oxopentanoic acid | + | −3.698 | 1.8261 | ||
| salicylic acid | + | −1.6365 | 1.7769 | ||
| biotin | + | −1.0759 | 1.7525 | ||
| 4-(methylthio)-6-phenyl-2-(3-pyridyl)pyrimidine-5-carbonitrile | + | −1.6808 | 1.6331 | ||
| taurochenodeoxycholic Acid _sodium salt_ | + | −1.1365 | 1.5416 | ||
| (4aS,5R,6S,8aS)-5-[(3E)-5-methoxy-3-methyl-5-oxopent-3-en-1-yl]-5,6,8a-trimethyl-3,4,4a,5,6,7,8,8a-octahydronaphthalene-1-carboxylic acid | + | −1.2552 | 1.5266 | ||
| 4-fluoro-N-{[(1S,4S,6S)-6-isopropyl-3-methyl-4-(2-{[2-(4-morpholinyl)ethyl]amino}-2-oxoethyl)-2-cyclohexen-1-yl]methyl}benzamide | + | −1.0308 | 1.4497 | ||
| 1,2,3-propanetricarboxylic acid | − | 1.0805 | 1.4491 | ||
| cytosine | − | 1.0388 | 1.4034 | ||
| tropine | − | 1.8667 | 1.3217 | ||
| N-desmethyltramadol | + | −1.8015 | 1.3146 | ||
1 “−”: Compared with the CON group, the abundance of this metabolite at this time point in the AKK group is lower, and “+” means higher.
4. Discussion
Numerous perinatal factors, including diet, antibiotics, stress, different labor types, and feeding methods, have been reported to affect maternal gut microbiota during pregnancy and lactation, affecting offspring immune development and health status in later life [32,33]. Several mechanisms of maternal bacteria in influencing offspring health have been identified. These include mother-to-infant vertical microbial transmission [34], exposure of the fetoplacental unit to bacteria [35], passing of microbial metabolites to the fetus through the umbilical cord [36], and immunoglobulin-related transplacental transport of gut bacterial components. Therefore, pregnancy and lactation can be considered the window period affecting the microbiome establishment and lifelong health of the offspring. This study investigated the effect of A. muciniphila supplementation on mothers and pups.
We observed that A. muciniphila supplementation increased maternal food intake before birth (P21) but did not affect maternal bodyweight and pregnancy outcome. Constant intake of A. muciniphila resulted in increased mucus secretion, intestinal goblet cell number, and expression of genes associated with the intestinal barrier (e.g., Claudin-1, Cldn3, and ZO-1) in mother mice at the end of lactation. These effects were accompanied by a change in the bacterial community structure and a transient increase in the ratio of A. muciniphila in maternal gut microbiota. Moreover, A. muciniphila intake slightly changed maternal fecal metabolites during pregnancy and lactation. However, A. muciniphila supplementation during pregnancy and lactation did not significantly affect the growth and intestinal barrier of offspring mice, but disturbed the microbial community structure and metabolism, as indicated by increased abundance of Dubosiella, Lachnospiraceae_UCG_001, and Parasutterella, as well as decreased abundance of Gordonibacter, Lachnospiraceae_UCG_006, and Prevotellaceae_UCG_001 at B42.
Our results suggest that A. muciniphila supplementation to pregnant and lactating mice is safe and does not lead to any side effects on mothers or pups. Similarly, supplementation of Lactobacillus fermentum CECT5716 did not modify the body weight, body mass index, and Lee index of dams and pups [37]. Most clinical trials also support the safety of probiotics supplementation before, during, and after pregnancy [38,39,40]. A. muciniphila is one of the most common microbes in the mammalian gut. At present, A. muciniphila WB-STR-0001 has been successfully applied as a probiotic medical food to improve the symptoms of type 2 diabetes [41]. In summary, the results of this study add to the evidence that supplementation of the probiotic strain A. muciniphila at appropriate doses during pregnancy and lactation is safe.
As a physical and immune defense, the intestinal barrier plays an important role in combating microbes, food antigens, and environmental toxins. As an intestinal commensal bacterium, A. muciniphila exerts its beneficial effects, at least in part, by improving the intestinal barrier function. In adult obese mice, daily oral administration of A. muciniphila for approximately 1 month increased the number of ileal goblet cells and the expression of intestinal barrier markers, thereby reducing systemic inflammation and improving metabolic health [42,43,44]. A. muciniphila has been implicated in the control of host mucus turnover [15]. The results of 16 sRNA sequencing and qPCR analyses showed that the abundance of A. muciniphila in female mice at D21 increased significantly after continuous A. muciniphila supplementation during pregnancy and lactation. Recently, several studies have indicated that elevation in the abundance of A. muciniphila cells leads to increased number of goblet cells [45]. Similarly, our data showed that 6 weeks of A. muciniphila supplementation increased the number of goblet cells and the expression of MUC2 in mother mice. MUC2 is a major component of the intestinal mucus that is essential to protect the gastrointestinal tract.
TJ proteins are also important for maintaining the integrity of the intestinal barrier [46]. Numerous evidence has shown that decreased expression and translocation of TJ proteins might cause intestinal damage. In the current study, A. muciniphila significantly increased the mRNA expression of claudin-1, Cldn3, and ZO-1, but not that of occludin in mother mice. Furthermore, A. muciniphila administration during pregnancy and lactation led to the upregulation of the regenerating family member 3 gamma (Reg3g) gene, which is essential for defense against the colonization of Gram-positive bacteria, distribution of intestinal mucus, and immune response to pathogens [30]. Thus, A. muciniphila administration enhanced the gut barrier function of mother mice.
Our results showed that the intestinal microbiota diversity and richness in dams were disturbed only at the beginning of A. muciniphila supplementation and reached a stable state in the later period. At D21, A. muciniphila supplementation not only increased the abundance of A. muciniphila but also affected the relative abundance of other bacteria (Figure 4). A previous study on the effect of liraglutide in improving nonalcoholic fatty liver disease in mice showed a similar relationship between the abundances of A. muciniphila and these bacteria; liraglutide significantly increased the ratio of A. muciniphila whilst decreasing the abundance of Bacteroides, Lachnospiraceae_UCG-001, Lachnospiraceae_NK4A136_group, Klebsiella, Anaerotruncus, and Ruminiclostridium [47]. A controlled clinical trial showed that orange juice increased the abundance of Lactobacillus spp., A. muciniphila spp., and Ruminococcus spp. and simultaneously improved glycemia and lipid profiles [48]. A higher abundance of Ruminococcus_1 was associated with improved polycystic ovary syndrome [49]. Thus, A. muciniphila supplementation may exert beneficial changes in the intestinal microbiota structure of mother mice. Moreover, there were significantly different clusters of intestinal metabolites in mother mice at day D21.
In contrast to the results in maternal mice, A. muciniphila supplementation caused minor changes in the gut microbiota of offspring mice, without a significant increase in the abundance of A. muciniphila. As there is a trace amount of oxygen in the neonatal gut, the first colonizers in the intestine are mainly the typical facultative anaerobes Lactobacillaceae and Enterobacteriaceae. With the consumption of oxygen, obligate anaerobes, including Bifidobacterium, Bacteroides, and Clostridium, gradually colonize the intestines of infants [50]. A. muciniphila is a strictly anaerobic bacterium, making it difficult to pass from mother to infant through breastfeeding. In a clinical study, mothers were administered probiotic milk containing LGG, Bb-12, and La-5 from 36 weeks of gestation to 3 months postpartum with breastfeeding, but only LGG led to a transient colonization in the infants at 10 days and 3 months of age [51]. This result proves that different probiotic strains have different vertical transmission abilities from mother to child. It is possible that the strain used in this study cannot spread vertically. This may be because A. muciniphila did not significantly affect the intestinal microbiome of offspring; thus, there was no significant difference in intestinal barrier development between pups in the two groups. Interestingly, in our study, PCoA results revealed that the intestinal microbiota of the two groups of pups showed significantly different clustering on day 42 after birth, and the relative abundance of some bacterial species was changed. This suggests that longer monitoring of mice may be needed, and the effects of A. muciniphila on offspring require a longer time to manifest.
Microbial molecules (such as LPS) and metabolites (such as retinoids, short-chain fatty acids, and secondary bile acids) in the gut of pregnant mothers can penetrate the placental barrier and directly affect the fetus [36]. The maternal microbiome regulates maternal metabolites and fetal brain metabolites, such as trimethylamine-N-oxide and imidazole propionate, promoting the growth of embryonic thalamic axons in vitro [52]. With this in mind, we also analyzed the fecal metabolites of mothers and pups. Our findings showed that probiotic supplementation slightly affected the fecal metabolism of mother mice. However, the fecal metabolic profile of the offspring mice changed more dramatically in comparison. The D-glutamine and D-glutamate metabolism pathways changed significantly in the offspring at B21 (impact = 0.5). Azagra-Boronat et al. revealed that probiotic supplementation in pregnant and lactating mothers might affect their offspring by inducing a lower proportion of palmitic acid and higher proportions of linoleic and α-linolenic acids in breast milk [37,53]. It is possible that A. muciniphila supplementation affects metabolites in breast milk.
Overall, A. muciniphila supplementation during pregnancy and lactation appeared to have a greater beneficial effect on the mothers than on the offspring, such as strengthening the intestinal barrier and improving the structure of the gut microbiota. A. muciniphila supplementation during pregnancy and lactation in mice did not directly increase the abundance of this bacteria in the offspring’s intestines. Yet, it is true that increasing the abundance of A. muciniphila in the intestine in the early stages of life may have some physiological significance. Genistein supplementation to mother mice fed a high-fat diet also induced a significant enrichment of the relative abundance of A. muciniphila in the intestines of their offspring, while improving glucose homeostasis and insulin sensitivity in offspring [54]. Therefore, it is necessary to further explore the significance of increasing the abundance of A. muciniphila and how to increase it in early life. Although this study showed that A. muciniphila was safe to be taken during pregnancy, our results were limited to animals; hence, further clinical evaluation is required to examine the potential safety risks for mothers and infants.
5. Conclusions
In summary, A. muciniphila is safe for use in mice during pregnancy and breastfeeding. Administration of A. muciniphila significantly strengthened the intestinal barrier of mother mice by increasing the colonic mucosa crypt depth, number of goblet cells, and mRNA expression of MUC2 and Reg3g, which are TJ protein-related genes. These improvements might be correlated with changes in the diversity and composition of the intestinal microbiota of dams, increased relative abundance of A. muciniphila and Ruminococcus_1, as well as decreased proportions of Coriobacteriaceae UCG-002, Lachnospiraceae UCG-001, and Ruminiclostridium in the maternal gut. Fecal metabolite analysis of dams revealed no significant results. Additionally, A. muciniphila supplementation caused only small disturbances in the gut microbiota and did not significantly increase the abundance of A. muciniphila in the offspring. Compared to that in the CON group, the fecal metabolic profile of offspring in the AKK group was significantly changed at B21. Therefore, probiotic intervention during pregnancy and lactation may improve gut microbial composition and promote health in mothers. It may be possible to design some diet-related substances for dams that could increase A. muciniphila abundance and provide long-lasting health benefits in offspring mice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14020390/s1, Table S1: Primer sequences for qPCR analyses, Table S2: Instrument conditions for the analysis of metabolomics samples, Table S3: All fecal metabolites in mums, Table S4: All fecal metabolites in pups, Figure S1: The mRNA expression of genes related to the intestinal barrier of mouse offspring in the CON group, Figure S2: Effects of A. muciniphila supplementation during pregnancy and lactation on the intestinal barrier of mouse offspring, Figure S3: A. muciniphila supplementation results in favorable alterations in maternal gut microbiota, Figure S4: A. muciniphila supplementation results in favorable alterations in the gut microbiota of pups.
Author Contributions
Conceptualization, Y.Q., L.Y., H.Z. and Q.Z.; data curation, Y.Q. and L.Y.; formal analysis, Y.Q. and L.Y.; funding acquisition, F.T. and Q.Z.; investigation, Y.Q.; methodology, Y.Q. and Q.Z.; project administration, F.T. and W.C.; resources, J.Z., W.C. and Q.Z.; software, Y.Q. and L.Y.; supervision, F.T., J.Z., W.C. and Q.Z.; validation, Y.Q., F.T., J.Z. and H.Z.; visualization, Y.Q., L.Y. and H.Z.; writing—original draft, Y.Q. and Q.Z.; Writing—review and editing, Y.Q. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangsu Province [BK20200084]; The National Natural Science Foundation of China [No. 32001665]; the Natural Science Foundation of Jiangsu Province [BE2021623]; and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Institutional Review Board Statement
All experimental procedures were conducted in accordance with the institutional guidelines for the care and use of laboratory animals and were approved by the Ethical Committee of Jiangnan University, China. (JN.No20201030c1300430 (281)). Every effort was made to maximize the well-being of mice, minimize their suffering, and minimize the number of animals used. When performing experiments on pregnant and newborn mice, we ensured gentle and careful handling, and noise interference in the environment was minimized throughout the feeding period.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aversa Z., Atkinson E.J., Schafer M.J., Theiler R.N., Rocca W.A., Blaser M.J., LeBrasseur N.K. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 2021;96:66–77. doi: 10.1016/j.mayocp.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W., Sun Z., Ma C., Zhang J., Ma C., Zhao Y., Wei H., Huang S., Zhang H. Exposure to soil environments during earlier life stages is distinguishable in the gut microbiome of adult mice. Gut Microbes. 2021;13:1830699. doi: 10.1080/19490976.2020.1830699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S., Ryan C.A., Boyaval P., Dempsey E.M., Ross R.P., Stanton C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020;28:28–45. doi: 10.1016/j.tim.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 4.You L., Zi Y., Xue Z., Xiaokun G., Guangyu H., Lin S., Zhuo L., Guixia W. The effects of gut microbiota on metabolic outcomes in pregnant women and their offspring. Food Funct. 2018;9:4537–4547. doi: 10.1039/c8fo00601f. [DOI] [PubMed] [Google Scholar]
- 5.García-Mantrana I., Selma-Royo M., González S., Parra-Llorca A., Martínez-Costa C., Collado M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;11:962–978. doi: 10.1080/19490976.2020.1730294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson S.L., O’Hely M., Jacka F.N., Ponsonby A.L., Symeonides C., Loughman A., Collier F., Moreno-Betancur M., Sly P., Burgner D., et al. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine. 2021;68:103400. doi: 10.1016/j.ebiom.2021.103400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuillermin P., O’Hely M., Collier F., Allen K., Tang M., Harrison L., Carlin J., Saffery R., Ranganathan S., Sly P., et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020;11:1452. doi: 10.1038/s41467-020-14552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhushan I., Sharma M., Mehta M., Badyal S., Sharma V., Sharma I., Singh H., Sistla S. Bioactive compounds and probiotics—A ray of hope in COVID-19 management. Food Sci. Hum. Well. 2021;10:131–140. doi: 10.1016/j.fshw.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu M., Xuan S., Wang Z. Oral microbiota: A new view of body health. Food Sci. Hum. Well. 2019;8:8–15. doi: 10.1016/j.fshw.2018.12.001. [DOI] [Google Scholar]
- 10.Zhang C., Gong W., Li Z., Gao D., Gao Y. Research progress of gut flora in improving human wellness. Food Sci. Hum. Well. 2019;8:102–105. doi: 10.1016/j.fshw.2019.03.007. [DOI] [Google Scholar]
- 11.Yao M., Qv L., Lu Y., Wang B., Berglund B., Li L. An update on the efficacy and functionality of probiotics for the treatment of non-alcoholic fatty liver disease. Engineering. 2021;7:679–686. doi: 10.1016/j.eng.2020.01.017. [DOI] [Google Scholar]
- 12.Derrien M., Collado M.C., Ben-Amor K., Salminen S., Vos W.D.J.A., Microbiology E. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microb. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grander C., Adolph T.E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D.V., Grabherr F., Gerner R.R., Pfister A., et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y., Hwang S.W., Kim S., Lee Y.S., Kim T.Y., Lee S.H., Kim S., Yoo H., Kim E.N., Kweon M.-N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes. 2020;11:944–961. doi: 10.1080/19490976.2020.1730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anhê F.F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T.V., Garofalo C., Moine Q., Desjardins Y., Levy E., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 17.Ellekilde M., Krych L., Hansen C.H.F., Hufeldt M.R., Dahl K., Hansen L.H., Sørensen S.J., Vogensen F.K., Nielsen D.S., Hansen A.K. Characterization of the gut microbiota in leptin deficient obese mice—Correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014;96:241–250. doi: 10.1016/j.rvsc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Hansen C.H.F., Krych L., Nielsen D.S., Vogensen F.K., Hansen L.H., Sørensen S.J., Buschard K., Hansen A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 19.Perraudeau F., McMurdie P., Bullard J., Cheng A., Cutcliffe C., Deo A., Eid J., Gines J., Iyer M., Justice N., et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diab. Res. Care. 2020;8:e001319. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado M.C., Derrien M., Isolauri E., De Vos W.D., Salminen S.J.A., Microbiology E. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the Elderly. Appl. Environ. Microb. 2007;73:7767. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostopoulos I., Elzinga J., Ottman N., Klievink J.T., Belzer C. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci. Rep. 2020;10:14330. doi: 10.1038/s41598-020-71113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribo S., Sánchez-Infantes D., Martinez-Guino L., García-Mantrana I., Ramon-Krauel M., Tondo M., Arning E., Nofrarías M., Osorio-Conles Ó., Fernández-Pérez A., et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci. Transl. Med. 2021;13:eabb0322. doi: 10.1126/scitranslmed.abb0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Zhong Z., Wang B., Xia X., Yao W., Huang L., Wang Y., Ding W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology. 2019;44:2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asgharian H., Homayouni-Rad A., Mirghafourvand M., Mohammad-Alizadeh-Charandabi S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: A randomized controlled clinical trial. Eur. J. Nutr. 2020;59:205–215. doi: 10.1007/s00394-019-01900-1. [DOI] [PubMed] [Google Scholar]
- 25.Kallio S., Kukkonen A.K., Savilahti E., Kuitunen M. Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin. Exp. Allergy. 2019;49:506–515. doi: 10.1111/cea.13321. [DOI] [PubMed] [Google Scholar]
- 26.Pourmirzaiee M.A., Famouri F., Moazeni W., Hassanzadeh A., Hajihashemi M. The efficacy of the prenatal administration of Lactobacillus reuteri LR92 DSM 26866 on the prevention of infantile colic: A randomized control trial. Eur. J. Pediatr. 2020;179:1619–1626. doi: 10.1007/s00431-020-03641-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim S., Shin Y.C., Kim T.Y., Kim Y., Kweon M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13:1892441. doi: 10.1080/19490976.2021.1892441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotech. 2019;37:1091. doi: 10.1038/s41587-019-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demirci M., Tokman H.B., Uysal H.K., Demiryas S., Karakullukçu A., Saribas S., Cokugras H., Kocazeybek B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopath. 2019;47:365–371. doi: 10.1016/j.aller.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Li N., Yan F., Wang N., Song Y., Yue Y., Guan J., Li B., Huo G. Distinct gut microbiota and metabolite profiles induced by different feeding methods in healthy Chinese infants. Front. Microbiol. 2020;11:714. doi: 10.3389/fmicb.2020.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Z., Ross R., Stanton C., Hou B., Zhao J., Zhang H., Yang B., Chen W. Lactobacillus casei CCFM1074 alleviates collagen-induced arthritis in rats via balancing Treg/Th17 and modulating the metabolites and gut microbiota. Front. Immunol. 2021;12:680073. doi: 10.3389/fimmu.2021.680073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fouhy F., Watkins C., Hill C.J., O’Shea C.A., Nagle B., Dempsey E.M., O’Toole P.W., Ross R.P., Ryan C.A., Stanton C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019;10:1517. doi: 10.1038/s41467-019-09252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul H.A., Bomhof M.R., Vogel H.J., Reimer R.A. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 2016;6:20683. doi: 10.1038/srep20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratsika A., Codagnone M.C., O’Mahony S., Stanton C., Cryan J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients. 2021;13:423. doi: 10.3390/nu13020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014;6:237. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganal-Vonarburg S., Hornef M., Macpherson A. Microbial—Host molecular exchange and its functional consequences in early mammalian life. Science. 2020;368:604–607. doi: 10.1126/science.aba0478. [DOI] [PubMed] [Google Scholar]
- 37.Azagra-Boronat I., Tres A., Massot-Cladera M., Franch N., Castell M., Guardiola F., Pérez-Cano F., Cells M.R.-L.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells. 2020;9:575. doi: 10.3390/cells9030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.Y., Kwon J.H., Ahn S.H., Lee S.I., Han Y.S., Choi Y.O., Lee S.Y., Ahn K.M., Ji G.E. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo-controlled trial. Pediat. Allergy Immun. 2010;21:e386–e393. doi: 10.1111/j.1399-3038.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 39.Boyle R.J., Ismail I.H., Kivivuori S., Licciardi P.V., Robins-Browne R.M., Mah L.J., Axelrad C., Moore S., Donath S., Carlin J.B., et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy. 2011;66:509–516. doi: 10.1111/j.1398-9995.2010.02507.x. [DOI] [PubMed] [Google Scholar]
- 40.Navarro-Tapia E., Sebastiani G., Sailer S., Toledano L.A., Serra-Delgado M., García-Algar Ó., Andreu-Fernández V. Probiotic supplementation during the perinatal and infant period: Effects on gut dysbiosis and disease. Nutrients. 2020;12:2243. doi: 10.3390/nu12082243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Wang Y., Ni Y., Cheung C.K.Y., Lam K.S.L., Wang Y., Xia Z., Ye D., Guo J., Tse M.A., et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31:77–91. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 43.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 44.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-Talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stichelen O.V., Rother K.I., Hanover J. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front. Microbiol. 2019;10:1360. doi: 10.3389/fmicb.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He C., Deng J., Hu X., Zhou S., Wu J., Xiao D., Darko K., Huang Y., Tao T., Peng M., et al. Vitamin A inhibits the action of LPS on intestinal epithelial barrie function and tight junction proteins. Food Funct. 2019;10:1235–1242. doi: 10.1039/C8FO01123K. [DOI] [PubMed] [Google Scholar]
- 47.Qian L., Beiyu C., Lixin Z., Xin X., Xin W., Ziming A., Li S., Hu Y.Y., Feng Q. Liraglutide modulates gut microbiome and attenuates nonalcoholic fatty liver in db/db mice. Life Sci. 2020;261:118457. doi: 10.1016/j.lfs.2020.118457. [DOI] [PubMed] [Google Scholar]
- 48.Fidélix M., Milenkovic D., Sivieri K., Cesar T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020;11:1599–1610. doi: 10.1039/C9FO02623A. [DOI] [PubMed] [Google Scholar]
- 49.Li T., Zhang T., Gao H., Liu R., Gu M., Yang Y., Cui T., Lu Z., Yin C. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021;41:101886. doi: 10.1016/j.redox.2021.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson R.C., Manges A.R., Finlay B.B., Prendergast A.J. The Human microbiome and child growth—First 1000 days and beyond. Trends Microbiol. 2019;27:131–147. doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Dotterud C.K., Avershina E., Sekelja M., Simpson M.R., Rudi K., Storrø O., Johnsen R., Øien T. Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J. Pediatr. Gastr. Nutr. 2015;61:200–207. doi: 10.1097/MPG.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 52.Vuong H., Pronovost G., Williams D., Coley E., Siegler E., Qiu A., Kazantsev M., Wilson C., Rendon T., Hsiao E. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azagra-Boronat I., Tres A., Massot-Cladera M., Franch À., Castell M., Guardiola F., Pérez-Cano F., Rodríguez-Lagunas M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects breast milk composition. J. Dairy Sci. 2020;103:2982–2992. doi: 10.3168/jds.2019-17384. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L., Xiao X., Zhang Q., Zheng J., Li M., Yu M., Wang X., Deng M., Zhai X., Li R. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front. Endocrinol. 2018;9:516. doi: 10.3389/fendo.2018.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.