Abstract
The COVID-19 pandemic has shaken the world since early 2020 and its health, social, economic, and societal negative impacts at the global scale have been catastrophic. Since the early days of the pandemic, development of safe and effective vaccines was judged to be the best possible tool to minimize the effects of this pandemic. Drastic public health measures were put into place to stop the spread of the virus, with the hope that vaccines would be available soon. Thanks to the extraordinary commitments of many organizations and individuals from around the globe and the collaborative effort of many international scientists, vaccines against COVID-19 received regulatory approval for emergency human use in many jurisdictions in less than a year after the identification of the viral sequence. Several of these vaccines have been in use for some time; however, the pandemic is still ongoing and likely to persist for the foreseeable future. This is due to many reasons including reduced compliance with public health restrictions, limited vaccine manufacturing/distribution capacity, high rates of vaccine hesitancy, and the emergence of new variants with the capacity to spread more easily and to evade current vaccines. Here we discuss the discovery and availability of COVID-19 vaccines and evolving issues around mass vaccination programs.
Keywords: COVID-19, COVID-19 vaccines, SARS-CoV-2, vaccination, vaccine hesitancy
1. Introduction
In December 2019, Chinese authorities notified the World Health Organization (WHO) of a cluster of unusual pneumonia cases in Wuhan, of an unknown viral aetiology. The cause of the cases was subsequently identified as a new type of coronavirus, later named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and the disease caused by this virus was named Coronavirus Disease 2019 (COVID-19) [1]. In March 2020, WHO declared the COVID-19 outbreak a pandemic and warned governments globally to take all the necessary measures to prevent its spread [2]. Due to its high transmission rates, governments and public health agencies worldwide adopted public awareness campaigns and enforced social distancing restrictions, to minimize person-to-person transmission of the virus. Despite many efforts to contain the spread of the disease, including mandatory face mask wearing in public places, cancelling or limiting the number of people at gatherings and implementing lockdowns in many countries, the overall number of COVID-19 cases has exceeded 250 million worldwide with over 5.1 million deaths as of 29 November 2021 [3]. The severity of the pandemic led many scientists worldwide to focus on finding ways to treat the disease. Discovering and developing vaccines to fight COVID-19 has been the main objective of researchers and pharmaceutical companies. This review discusses the key elements of the SARS-CoV-2, COVID-19 disease, vaccines that have been developed very rapidly and are now commercially available and the pipeline of new vaccine candidates that are yet to enter the commercial arena. Even after two years, the COVID-19 pandemic continues as a major public health concern. Thus, providing a contemporary review of currently available and emerging COVID-19 vaccines globally would be a useful addition to the literature on the COVID-19 pandemic.
Key topics that are included in this review include:
Background on COVID-19 pandemic as well as on beta-coronaviruses more generally, clinical overview of COVID-19, traditional and contemporary vaccine discovery platforms, classes of COVID-19 vaccines and key current and emerging COVID-19 vaccination themes like universal availability and affordability, vaccine hesitancy and the outlook for novel COVID-19 vaccines to tackle emerging variants.
This is not the first instance in the 21st century that a new and dangerous coronavirus has made the headlines. The first time that viruses from the coronavirus family shocked the world was in 2003 when a beta-coronavirus (SARS-CoV) caused an outbreak in China and spread to four other countries, causing more than 700 deaths with around 50% mortality in individuals over the age of 60 [4]. In 2012, another coronavirus, MERS-CoV, was identified causing Middle East respiratory syndrome (MERS), a deadly respiratory infection with a mortality rate of approximately 40% [5]. Even though SARS-CoV-2 is not as deadly as previous coronaviruses, it spreads far more efficiently than previous coronaviruses and has become a major global public health concern in only a couple of months.
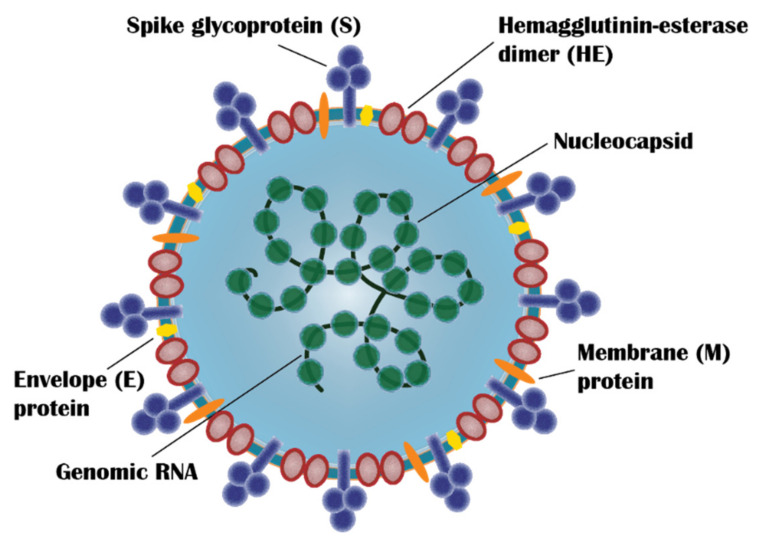
SARS-CoV-2 is a member of the coronavirus family and belongs to the genera, beta-coronaviruses. It shares a similar genome sequence with other well-known beta-coronaviruses, SARS-CoV (~79%) and MERS-CoV (~50%) [6]. It is a single-stranded RNA virus with a genome size of ~30 kb and expresses 29 proteins including four main SARS-CoV-2 proteins: Spike (S) protein, Envelope (E) protein, Membrane (M) protein and Nucleocapsid (N) protein [7] (Figure 1). S protein is the major surface protein of SARS-CoV-2 and is responsible for cell entry [8]. It is a 1273 amino acid trimeric protein with each monomer consisting of two subunits, S1 and S2 [9]. S1 subunit (aa14-685) consists of N-terminal domain, Receptor Binding Domain (RBD) and receptor-binding motif and facilitates the binding of the virus to the host cell receptor. S2 subunit (aa686-1273) comprises of fusion peptide, heptad repat 1 (HR1), heptad repeat 2 (HR2), transmembrane domain and cytoplasm domain, and mediates membrane fusion and entry into the host cell [10,11]. Structurally, S protein is classified as a class 1 viral fusion protein due to its characteristic heptad repeat region and N-terminal or N-proximal fusion peptide and formation of a heterotrimeric six-helix bundle during the membrane fusion process [8]. S protein has also been shown to generate strong immune response and elicit neutralizing antibodies which has led vaccine developers to focus on utilizing not only the S protein itself but also parts of the protein that trigger the immune response [12].
Figure 1.
Schematic drawing of SARS-CoV-2 representing the major virus components: spike glycoprotein (S), hemagglutinin-esterase dimer (HE), membrane protein (M), envelope protein (E), nucleocapsid, and genomic RNA. (Redrawn by the authors based on Figure 2 in Ref. [13]).
2. SARS-CoV-2 Variants
Mutation, alterations in one or several amino acids in the virus genome, can arise as a product of viral replication or natural selection. Although most of the amino acid changes are expected to be insignificant, some mutations yield differences in antigenicity, transmissibility, or virulence, resulting in new viral variants [14]. Throughout the COVID-19 pandemic, the emergence of new SARS-CoV-2 variants has been observed. The USA Centers for Disease Control and Prevention (CDC) has classified these as variants of interest, variants of concern, and variants of high consequence [15].
Variants that are predicted to impact the transmission, diagnostics, treatments, or immune evasion and are responsible for an increasing number of outbreaks are classified as variants of interest. Such a variant of interest may necessitate one or more additional public health actions, such as improved laboratory characterization, increased sequence surveillance, or epidemiological investigations to determine how easily the virus spreads to others, the severity of disease, therapeutic efficacy, and whether currently approved vaccines provide protection. A variant of interest (VOI) is classified as a variant of concern (VOC) if there is evidence for surge in transmissibility, increase in disease severity, and decreased efficacy of therapeutics or vaccines, or failure in its diagnosis. Variants of concern may require escalation of one or more public health responses, such as notification to WHO, efforts to control spread, increased testing, or studies to determine the efficacy of vaccines and treatments against the variant. Additional considerations such as developing new diagnostic tools or modifying treatments or vaccines may be necessary depending on the variant’s characteristics. Compared to previously circulating variants, a variant of high concern has clear evidence that preventative efforts or medical countermeasures (MCMs) are much less effective [15].
As of November 2021, six previous VOI (Epsilon, Eta, Iota, Kappa, Zeta, and Lambda) and three previous VOC (Alpha, Beta, and Gamma) have been designated as Variants Being Monitored (VBM) by CDC. VOI or VOC may be moved to this list if its national and regional prevalence has decreased significantly and sustainably over time, or if other evidence suggests that the variant does not pose a major risk to public health. Mu variant, which was detected in Columbia in January 2021, was designated as VBM in September 2021; no SARS-CoV-2 variants are currently considered as being of high consequences and variants of interest (Table 1). On 26 November 2021, The Technical Advisory Group on SARS-CoV-2 Virus Evolution (TAG-VE) was convened to evaluate a newly detected the SARS-CoV-2 variant B.1.1.529. This variant was designated as a VOC, named Omicron, first reported in South Africa on 24 November 2021. The first confirmed infection with the Omicron variant was identified in a specimen obtained on 9 November 2021 [16].
Table 1.
| WHO Terminology | Pangolin * | S Protein Mutations of Interest | Country of First Detection | Time of First Detection | |
|---|---|---|---|---|---|
| VBM | Epsilon | B.1.427/B.1.429 | L452R, D614G, S13I, W152C, L452R, D614G | United States (California) | September 2020 |
| Eta | B.1.525 | A67V, 69del, 70del, 144del, E484K, D614G, Q677H, F888L | United Kingdom/Nigeria | December 2020 | |
| Iota | B.1.526 | L5F, (D80G*), T95I, (Y144-*), (F157S*), D253G, (L452R*), (S477N*), E484K, D614G, A701V, (T859N*), (D950H*), (Q957R*) | United States (New York) | November 2020 | |
| Kappa | B.1.617.1 | (T95I), G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H | India | December 2020 | |
| Zeta | P.2 | E484K, (F565L*), D614G, V1176F | Brazil | April 2020 | |
| Lambda | C.37 | G75V, T76I, 246-252del, L452Q, F490S, D614G and T859N | Peru | December 2020 | |
| Mu | B.1.621, B.1.621.1 | N/A | Colombia | September 2021 | |
| Alpha | B.1.1.7 | 69del, 70del, 144del, (E484K*), (S494P*), N501Y, A570D, D614G, P681H, T716I, S982A, D1118H (K1191N*) | United Kingdom | September 2020 | |
| Beta | B.1.351 | D80A, D215G, 241del, 242del, 243del, K417N, E484K, N501Y, D614G, A701V | South Africa | May 2020 | |
| Gamma | P.1 | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I | Japan/Brazil | November 2020 | |
| VOC | Delta | B.1.617.2 | T19R, (V70F*), T95I, G142D, E156-, F157-, R158G, (A222V*), (W258L*), (K417N*), L452R, T478K, D614G, P681R, D950N | India | October 2020 |
| Omicron | B.1.1.529 | N/A | South Africa | November 2021 |
* The genetic lineages of SARS-CoV-2 variants are classified using a software tool named PANGOLIN (Phylogenetic Assignment of Named Global Outbreak Lineages) developed by Andrew Rambaut’s laboratory in the United Kingdom. This classification system has since been used by researchers and public health agencies worldwide to monitor the transmission and spread of SARS-CoV-2 [19].
3. Clinical Overview of COVID-19
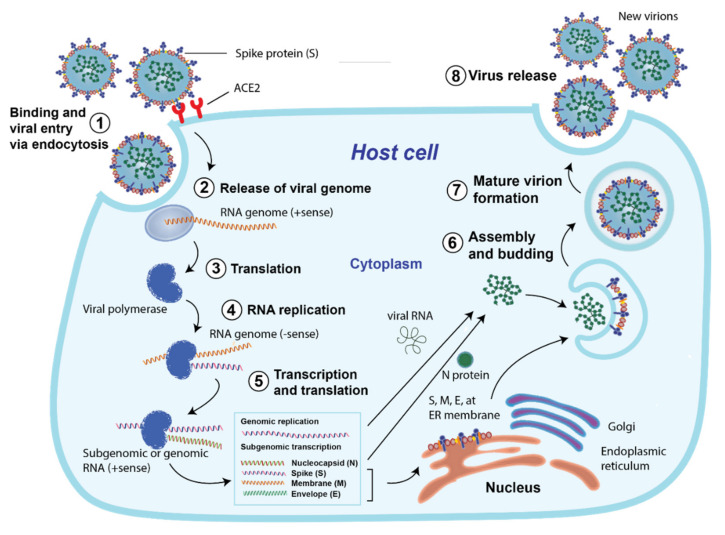
As with other coronaviruses, SARS-CoV-2 uses its transmembrane S protein to enter host cells (Figure 2). It binds with high affinity to human Angiotensin Converting Enzyme 2 (ACE2), ACE2 receptors, which acts as a cellular doorway for SARS-CoV-2 to fuse and enter the target cells [20]. Once this connection is established between the virus and the ACE2 receptor, an endosomal cysteine protease, Transmembrane Serine Protease 2 (TMPRSS2), is activated, and the virus enters the host cell via endocytosis [21]. After the virus enters the cell, it hijacks the cell’s protein machinery and starts making new virus particles which are released from the host cell and infect neighboring healthy cells, causing COVID-19 infection.
Figure 2.
SARS CoV-2 life cycle: (1) Binding and viral entry to the host cell via endocytosis; (2) release of viral genome; (3) translation and (4) replication of RNA genome; (5) transcription and translation of RNA into protein; (6) assembly and budding, followed by (7) mature virion formation; and (8) release of the new virion from the host cell. (Redrawn by the authors based on second figure in Ref. [22]).
COVID-19 is a highly transmissible disease that represents a severe threat to public health, causing mild or moderate symptoms in most infected individuals; in ~ 20% of cases, this can be severe [23]. The virus is primarily transmitted through respiratory droplets (aerosols) from close contact between individuals [24]. The most common method used in the diagnosis of COVID-19 is real-time reverse transcriptase polymerase chain reaction (RT-PCR) of respiratory specimens, including nasal and pharyngeal swab or lung fluids [1]. Common COVID-19 symptoms include cough, shortness of breath, fatigue, fever, and loss of taste and smell, but in some cases it can progress to pneumonia, followed by a critical condition known as acute respiratory distress syndrome (ARDS), which can potentially result in multi-organ failure (MOF) and death [1,25,26,27]. Frequency of developing severe disease increases with age and comorbidities such as hypertension, obesity, and diabetes [28].
Although COVID-19 infection lasts approximately 2 weeks in most individuals and its acute effects fade by the end of the infection, its long-term effects have been demonstrated in some individuals, mainly health care workers who have had significant exposure to the virus [29]. The most common long-term effect that COVID “Long Haulers” suffer from is dyspnea, observed in ~40% of individuals [30,31]. Risk of myocardial inflammation is also substantially increased in patients who have recovered from COVID-19 [32,33]. Other residual negative effects from COVID-19 infection include increased fatigue, muscular weakness, chest pain, chronic kidney disease, neuropsychiatric disorders, cognitive disturbances and increased risk of thromboembolism [34].
Some existing small molecule drugs have been tested against COVID-19 clinically. Due to its demonstrated potent in vitro inhibitory activity against SARS-CoV, antimalarial drug, chloroquine, was proposed as an effective treatment against COVID-19 [35]. A much less toxic derivative of chloroquine, hydroxychloroquine, gained wide interest from researchers in the early days of the pandemic; however, several randomized controlled trials and meta-analyses have clearly indicated that hydroxychloroquine and chloroquine are not associated with improved recovery or reduced mortality rates [36,37]. In light of these results, The USA Food and Drug Administration (FDA) revoked the emergency use authorization for hydroxychloroquine and chloroquine on 15 June 2020 [38].
Antiviral, remdesivir, has been effective in shortening the recovery time, decreasing the mortality rate and lowering respiratory tract infections in hospitalized adults with COVID-19 [39], and it is the only currently approved small molecule drug for use by most health regulatory authorities including European Medicines Agency (EMA) and FDA against COVID-19 [40,41]. Dexamethasone, a corticosteroid, has also shown to increase recovery of patients with severe COVID-19; however, the use of corticosteroids is not recommended in people with non-severe COVID-19 because of their potentially serious adverse effects [42]. Other drugs, recommended by The National Institute of Health (NIH) COVID-19 Treatment Guidelines Panel, to be useful depending on the severity of COVID-19, are baricitinib (an anti-inflammatory biologic for rheumatoid arthritis), tocilizumab (an anti-IL-6R monoclonal immunosuppressive antibody used for autoimmune disorders) and neutralizing SARS-CoV-2 combination monoclonal antibodies, bamlanivimab/etesevimab (Eli Lilly), casirivimab/imdevimab (Regeneron Pharmaceuticals) and sotrovimab (GSK/Vir Biotech) [43] for mild to moderate cases, in particular in the early stages of infection.
Clinical trial data released in October by pharmaceutical company Merck demonstrated that molnupiravir, another antiviral, can reduce the risk of hospitalization or death by around 50% [44]. The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has granted authorization for molnupiravir as the first oral antiviral in treating mild-to-moderate COVID-19 on 4 November 2021 [45].
Pfizer has recently developed an oral small molecule antiviral drug candidate, PF-07321332, with coronavirus-specific protease inhibitory activity and the drug has entered clinical trials (ClinicalTrials.gov Identifier: NCT04756531) [46,47]. Clinical trial data published in November 2021 have shown that PAXLOVIDTM (PF-07321332 and ritonavir combination) has been shown to reduce the risk of hospitalization or death by 89%; however, as of 29 November 2021, it has not been approved by regulatory authorities yet [48]. Even though the advances in novel drug development are promising, they will not be able to help with the fight to stop the transmission of the virus. COVID-19 has been spreading uncontrollably since the beginning of this pandemic, and the history of infectious diseases has shown that successful vaccines against COVID-19 remain the best weapon.
4. Traditional Vaccine Discovery
The beginning of the modern vaccination era is attributed to the English physician Edward Jenner, dating back to 1796, who is considered to have made one of the most significant breakthroughs in public health. Prior to Jenner, however, there was a technique called ‘inoculation’ or ‘engrafting’ that was practiced widely in Asia Minor and Far East [49]. Jenner was not the first scientist to determine that individuals who had contracted cowpox did not develop smallpox infection; however, he was the first to demonstrate that using cowpox pus from an infected person, another individual could be immunized, creating the first vaccine [50]. This discovery has led many researchers around the world to focus their research on vaccination, fostering advancements in the immunology field. Smallpox, one of the deadliest infections in human history, was declared eradicated in 1980 by WHO through worldwide vaccination campaigns, and many infectious diseases such as measles, polio, diphtheria and whooping cough, once seen as a significant threat to the community, are not a public health concern anymore in most parts of the world, thanks to the continuous global vaccination efforts for decades [51].
Historically, developing a safe and effective vaccine has required ~ten years, starting from early preclinical studies and followed by Phase 1–3 human clinical trials and then the arduous authorization process by national and international regulatory bodies [52]. Phase 1 involves determining the efficacy and evaluating the basic safety of the vaccine candidate in a small group (20–200) of healthy individuals [53]. Phase 2 involves a larger sample group with several hundred individuals and aims to collect additional information on safety, immunogenicity, efficacy and appropriate dosing of the vaccine candidate [53]. Phase 3 involves thousands of individuals and assesses the safety and efficacy of the vaccine candidate in larger populations, identifies real-world reactions to the vaccine candidate and determines the effectiveness by comparing vaccinated and unvaccinated groups [53]. If these results collectively demonstrate acceptable safety and efficacy, manufacturers can then submit authorization applications to different regulatory jurisdictions.
5. Classes of COVID-19 Vaccines
From the early days of the pandemic, it was clear to vaccine scientists/researchers, public health agencies and political leaders across the globe that the most effective way to fight the pandemic was to develop effective vaccines. Concerted global coordination and collaboration, and injection of significant financial resources from governments as well as philanthropic individuals and institutions (like the Bill & Melinda Gates Foundation and Wellcome Trust), has led to the first vaccine against COVID-19 being approved for emergency use by MHRA on 2 December 2020 and by FDA on 11 December 2020, less than a year after the pandemic began [54,55]. As of 15 November 2021, 23 vaccines have been authorized or approved for emergency use in at least one country, most receiving Emergency Use Authorization (Table 2 and Appendix A), 122 vaccine candidates are in different phases (1–3) of clinical trials (Appendix B) and 194 vaccine candidates are in pre-clinical development phase [56,57].
Table 2.
Authorized/approved vaccines in at least one country as per 15 November 2021.
| No | Vaccine Name | Status | Developer | Vaccine Type | Efficacy | Dose | Storage | Price (per Dose) | Source |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ComirnatyTM (BNT162b2) | Approved in several countries, emergency use in US, elsewhere | Pfizer-BioNTech Germany-US |
RNA based vaccine | 95% [83] | 2 dose, 3 weeks apart [84] | −70 °C [85] | €19.50 (US$23.15) [86] | [56] |
| 2 | Moderna mRNA-1273 and mRNA-1273.351 | Approved in Switzerland, emergency use in US, elsewhere | ModernaTX, Inc US |
RNA based vaccine | 94.1% [87] | 2 doses, 4 weeks apart [88] | −25 °C [89] | US$25.50 [86] | [56] |
| 3 | AstraZeneca AZD1222 | Approved in Brazil, emergency use in EU, elsewhere | The University of Oxford-AstraZeneca UK |
Viral vector (non-replicating) | 76% [90] | 2 doses, between four and 12 weeks apart [91] | 2–8 °C [85] | US$2.15 (EU), US$5.25 (others) [92] | [56] |
| 4 | ConvideciaTM (Ad5-nCoV) | Approved in China, emergency use in other countries | CanSino Biologics China |
Viral vector (non-replicating) | 65.28% | Single dose | 2–8 °C [93] | US$27.15 (Pakistan) [94] | [56] |
| 5 | Ad26.COV2.S | Emergency use in US, elsewhere | Janssen (Johnson & Johnson) US | Viral vector (non-replicating) | 66.9% [95] | Single dose [95] | 2–8 °C [85] | US$10 [96] | [56] |
| 6 | BBIBP-CorV | Approved in China, Bahrain, UAE, emergency use in other countries | Sinopharm (Beijing) China |
Inactivated virus | 79% [97] | 2 doses, 3 weeks apart [98] | 2–8 °C [99] | US$37.50 (Hungary) [100] | [56] |
| 7 | Inactivated SARS-CoV-2 (vero cell) | Approved in China, Limited use in UAE | Sinopharn + Wuhan Institute of Biological Products China |
Inactivated virus | 72.8% | 2 doses, 3 weeks apart | 2–8 °C | N/A | [56] |
| 8 | CoronaVac | Approved in China, emergency use in other countries | Sinovac China |
Inactivated virus | 51% in Brazil trial, 84% in Turkey trial [99] | 2 doses, 2 weeks apart [99] | 2–8 °C [89] | US$13.60 (Indonesia) [101] | [56] |
| 9 | Sputnik V | Emergency use in Russia, elsewhere | The Gamaleya Research Institute Russia |
Viral vector (non-replicating) | 91.6% | 2 doses, 3 weeks apart | −18 °C [89] | Less than US$10 [102] | [103] |
| 10 | EpiVacCorona | Approved in Turkmenistan, early use in Russia | FBRI Russia |
Protein subunit | N/A | 2 doses, 3 weeks apart | 2–8 °C | US$11 [104] | [103] |
| 11 | ZF2001/RBD-Dimer | Emergency use in China, Uzbekistan | Anhui Zhifei Longcom China |
Protein subunit | N/A | 3 doses, 4 weeks apart | 2–8 °C | N/A | [103] |
| 12 | Soberana 2/Pasteur | Emergency use in Iran, Cuba | Instituto Finlay de Vacunas Cuba |
Protein subunit | 62% two doses, 91.2% with Soberana Plus | 2 doses, 4 weeks apart | 2–8 °C | N/A | [103] |
| 13 | Abdala/CIGB-66 | Emergency use in Cuba | Center for Genetic Engineering and Biotechnology (CIGB) Cuba |
Protein subunit | 92.28% | 3 doses, 2 weeks apart | 2–8 °C | N/A | [103] |
| 14 | Medigen | Emergency use in Taiwan | Medigen Vaccine Biologics Taiwan |
Protein subunit | N/A | 2 doses, 4 weeks apart | 2–8 °C | N/A | [56] |
| 15 | Covaxin® | Emergency use in India, elsewhere | Bharat Biotech India |
Inactivated virus | 77.8% | 2 doses, 4 weeks apart | At least a week at room temperature | US$16.42 (India) [105] | [103] |
| 16 | QazCovid-in® | Early use in Kazakhstan | Research Institute for Biological Safety Problems Kazakhstan |
Inactivated virus | N/A | 1 or 2 doses, 3 weeks apart | 2–8 °C | US$4.7 [106] | [103] |
| 17 | Inactivated (Vero Cells) | Emergency use in China | Shenzhen Kangtai Biological Products Co., Ltd. China |
Inactivated virus | N/A | 2 doses, 4 weeks apart | 2–8 °C | N/A | [103] |
| 18 | COVIran Barekat | Emergency use in Iran | Shifa Pharmed ParsIran | Inactivated virus | N/A | 2 doses | 2–8 °C | N/A | [103] |
| 19 | CoviVac | Early use in Russia | Chumakov Cente Russia |
Inactivated virus | N/A | N/A | 2–8 °C | N/A | [103] |
| 20 | NVX-CoV2373 | Emergency use in Indonesia | Novavax US | Protein subunit | 89.7% | 2 doses, 3 weeks apart | 2–8 °C | US$20.90 (Denmark) [86] | [103] |
| 21 | ZyCoV-D | Emergency use in India | Zydus CadilaIndia | DNA based vaccine | 66.6% | 3 doses, 4 weeks apart | 2–8 °C | N/A | [103] |
| 22 | COVAX-19® | Emergency use in Iran | Vaxine Pty.Ltd/Cinnagen Co. Australia |
Protein Subunit | N/A | 2 doses, 3 weeks apart | N/A | N/A | [103] |
| 23 | Soberana Plus | Emergency use in Cuba | Instituto Finlay de Vacunas Cuba |
Protein subunit | N/A | N/A | N/A | N/A | [103] |
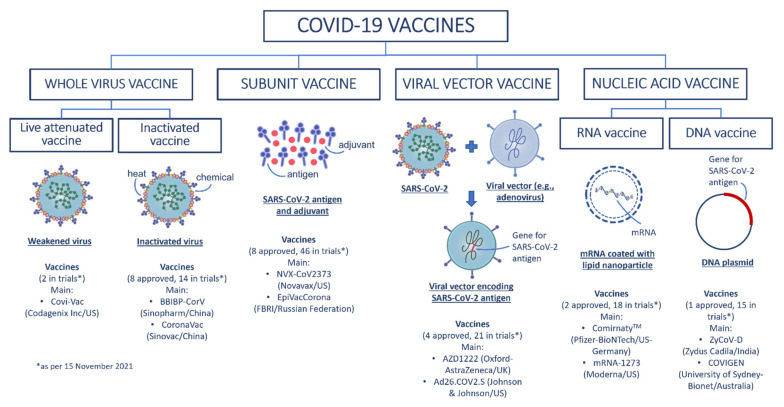
COVID-19 vaccines and vaccine candidates may be categorized into four groups based on their development technology. In addition to traditional vaccine development platforms such as inactivated and live attenuated vaccines, novel methods including nucleic acid vaccines and viral vector vaccines are also being used in the development of COVID-19 vaccines. The four broad groups of COVID-19 vaccines are summarized in Figure 3 followed by a brief description of these vaccine types.
Figure 3.
Schematic summary of the four broad groups of COVID-19 vaccines with examples of current COVID-19 vaccines. (Redrawn by the authors based on second figure in Ref. [60]).
5.1. Whole Virus COVID-19 Vaccines
5.1.1. Inactivated Vaccines
Inactivated vaccines are produced by using heat, radiation or chemicals, such as formaldehyde or β-propiolactone, to break down the viral structure and/or genetic material [58]. These vaccines still contain all parts of the virus, but in an inactive form making the virus unable to cause human disease. Inactivated vaccines are generally considered safe, easy to develop and manufacture and are less immunogenic; as such, they may not induce a strong enough immune response, which would necessitate addition of adjuvants and/or multiple doses [59].
Since the start of the COVID-19 pandemic, inactivated vaccine development has been the preferred vaccine development platform by some companies since it is the most established methodology with easy development and manufacturing of vaccines without compromising safety. As of 15 November 2021, there are 17 inactivated COVID-19 vaccines and/or vaccine candidates being tested in different phases of clinical trials. 8 of these 16 vaccines; Sinopharm-BBIBP-CorV (Sinopharm, Beijing, China), CoronaVac (Sinovac, Beijing, China), Covaxin (Bharat Biotech, Hyderabad, India), Sinopharm-WIBP (Sinopharm, Beijing, China), CoviVac (Chumakov Center, Moscow, Russia), QazVac (Research Institute for Biological Safety Problems, Zhambyl, Kazakhstan), COVIran Barakat (Shifa Pharmed Industrial Co, Kordan, Iran) and another vaccine developed by Shenzhen Kangtai Biological Products, China have been granted emergency use authorization in at least one country [57]. However, only three of these vaccines, Sinopharm-BBIBP-CorV, Sinopharm-WIBP, and CoronaVac, were included in the WHO Emergency Use Listing (EUL) released on 12 November 2021 [61].
Clinical trials of CoronaVac were conducted in more than 10 million individuals from four countries: Chile, Indonesia, Brazil and Turkey. Results from these trials show that the effectiveness of vaccination varies between 50% and 84%; however, the efficacy of vaccine against hospitalization was superior, 85% in Chile and 100% in Brazil and Turkey. Sinopharm-BBIBP-CorV was found to be 78% effective in phase 3 trials that were conducted in approximately 40,000 participants from the United Arab Emirates and Bahrain [62].
According to a survey of 1526 individuals who had CoronaVac, the most common side effect after the vaccination was localized pain at the injection site, which accounted for ~70% of the total reported side effects, that was experienced by 15% of the vaccinated group. Other notable side effects were fatigue, muscle pain and dizziness, which were experienced by 8.3%, 8.1% and 6% of the group, respectively [63]. Of the 35.8 million doses that have been administered since CoronaVac authorization in China by March 2021, the number of severe adverse effects reported was only 49 [64].
5.1.2. Live Attenuated Vaccines
Live attenuated virus vaccines are developed by attenuating the viruses, usually by repeated culturing. These vaccines generate a strong immune response and in most attenuated vaccines immunity is produced with a single dose. This strong immune response, however, can cause unwanted effects resulting in limited use of these vaccines in individuals with compromised immune status. This vaccine development method is the least preferred of the existing methods and as of 15 November 2021, only two COVID-19 vaccine candidates, which are in phase 3 and 1 trials, were developed using this method [65].
5.2. Subunit Vaccines
Unlike traditional platforms, a subunit vaccine only uses a part of the virus, as antigen, to stimulate immune response. This type of vaccine is developed using recombinant proteins or synthetic peptides targeting specific epitope, therefore eliminating the potential risk of pathogenicity and thus minimizing side-effects [65]. Despite such advantages, the ability of subunit vaccines to trigger immune response are lower than those vaccines that contain the entire virus. Thus, multiple doses and adjuvant(s) are often needed [66,67]. Several types of COVID-19 subunit vaccines are being developed: protein subunit vaccines containing specific isolated proteins from virus; polysaccharide vaccines that contain chains of sugar molecules; conjugate subunit vaccines in which a polysaccharide chain is attached to a carrier protein; and virus-like particles (VLPs) which mimic the structure of actual virus particles [68].
The S protein is used as the pivotal target for developing subunit vaccines against SARS-CoV-2, as it plays a key role in receptor binding and serves as the major antigen that triggers the response of protective neutralizing antibodies [69]. As of 15 November 2021, eight protein subunit vaccines against SARS-CoV-2 have been approved for emergency use at least in one country, such as RBD-Dimer, developed by Anhui Zhifei Longcom Biopharmaceutical, that uses a dimeric form of the S protein RBD. EpiVacCorona, developed by Vektor State Research Center of Virology and Biotechnology in Russia, utilizes a synthetic peptide antigen of SARS-CoV-2 containing a carrier protein and an adjuvant (Table 2).
5.3. Viral Vector Vaccines
Viral vectors are viruses that have been genetically engineered to create vaccines. They are completely harmless and are used as carriers to deliver the genetic information which the host cell uses to produce the antigen that initiates the body’s immune response [70]. The concept of using a virus as a vector dates back to 1972 [71]; however, it was not until 2019 that the first viral vector vaccine, ERVEBO® vaccine against Ebola virus, was approved for human use [72]. Several viruses such as Retrovirus, Lentivirus, Cytomegalovirus and Adenovirus have been used as carriers. Adenovirus is the most frequently used viral vector due to its well-established safety profile and inflammatory and immune system triggering effects [70].
This vaccine development platform is one of the most common used technologies for the development of COVID-19 vaccines; a number of vaccines developed with this approach are approved for emergency use in several countries and this method will remain a promising vaccine development pathway for ongoing vaccine research [57]. As of 15 November 2021, 21 viral vector vaccines have entered clinical trials and four of these vaccines; ChAdOx1-S (Oxford-AstraZeneca, Cambridge, UK and Stockholm, Sweden), Sputnik V (Gamaleya Research Institute, Moscow, Russia), Ad26.COV2.S (Johnson & Johnson, New Brunswick, NJ, USA) and Convidecia (CanSino Biologics, Shanghai, China) have been approved for either emergency or full use in different countries [65].
ChAdOx1-S by Oxford-AstraZeneca was approved for emergency use against COVID-19 on 30 December 2020 and in the subsequent 6 months it has become the most widely approved COVID-19 vaccine internationally [57]. Clinical trials have demonstrated that the vaccine is 66% effective after the second dose against COVID-19 infection with no hospital admissions in the vaccinated group [73]. However, it has been associated with venous thromboembolism, coagulation disorders and blood clots. According to a study that examined 280,000 vaccinated individuals, 59 venous thromboembolic events were observed in the vaccinated cohort compared with 30 expected based on the incidence rates in the general population, corresponding to a standardised morbidity ratio of 1.97. The same study also demonstrated that the standardised morbidity ratio for any thrombocytopenia/coagulation disorders was 1.52, indicating an increased risk of around 50% [74]. In some cases, these adverse events have led to several deaths, which has prompted nine countries in Europe to suspend emergency use approval of this vaccine [75]. A recent study demonstrated that ChAdOx1 viral vaccine vector binds to platelet factor 4 (PF4), a protein involved in the pathogenesis of heparin-induced thrombocytopenia (HIT), which could be a major step in discovering the mechanism underlying this rare side effect [76].
5.4. Nucleic Acid COVID-19 Vaccines
Nucleic acid-based vaccines have received much interest in the field of new vaccines, following studies in the early 1990s that plasmid DNA induces an immune response to the plasmid-encoded antigen and a mRNA vaccine was found to be effective as a result of direct gene transfer [47,77,78].
Nucleic acid vaccines use a part of genetic material in the form of DNA (as plasmids) or RNA (as mRNA) which encode and are translated in cells to a specific protein to stimulate immune responses. They have significant benefits over conventional methods in terms of safety (live virus and adjuvant are not required), effectiveness (expressing antigen in situ and mimicking true infection, thus inducing both B and T-cell responses), and high specificity (inducing immune response to the antigen of interest only) [79]. Additionally, it is relatively cheap and requires a shorter time to develop and manufacture this type of vaccine compared to traditional vaccines.
5.4.1. DNA Vaccines
DNA vaccines are formulated in the form of plasmids expressing the specific target protein (antigen). This process requires the intermediary steps of translation of DNA into messenger RNA which carries the specific genetic information (code) to the ribosomes where the protein synthesis takes place [80]. As per 15 November 2021, a DNA vaccine candidate against SARS-CoV-2, ZyCoV-D, has been approved for emergency use in India (Table 2) and several others are being developed and are entering clinical trials (Appendix B), like AG0301-COVID19 (Phase 2/3—AnGes, Tottori, Japan), and Covigenix VAX-001 (Phase 1—Entos Pharmaceuticals Inc, Edmonton, AB, Canada).
5.4.2. RNA Vaccines
RNA vaccine in the form of mRNA involves an intermediate process between DNA and protein translation by ribosomes. Currently, there are two main types of mRNA vaccines being studied: non-replicating mRNA, and self-amplifying RNA. The conventional non-replicating mRNA containing 5′ and 3′ untranslated region (UTRs) work by encoding the protein of interest, whereas the self-amplifying RNA encodes not only the protein/antigen, but also the viral replication machinery enabling the intracellular RNA amplification and large amounts of protein expression [81].
Although they work in similar manner, RNA-based vaccines appear to be more effective and safer than DNA vaccines; injection of RNA poses no (or minimal) risk of disrupting original DNA sequences in cells since it does not need to enter the cell nucleus [82]. The mRNA vaccines have become one of the leading vaccines to be developed against SARS-CoV-2. Two mRNA vaccines: BNT162b2 (Pfizer/BioNTech, New York, NY, USA/Berlin, Germany) and mRNA-1273 (Moderna, Cambridge, MA, USA) have been approved for emergency use worldwide (Table 2). Recently, BNT162b2 (Pfizer/BioNTech, New York, NY, USA/Berlin, Germany) vaccine received full approval from the FDA.
6. COVID-19 Vaccines—Future Outlook
6.1. Combination COVID-19 Vaccines
When faced with evolving safety issues and variable supply and logistical challenges with current COVID-19 vaccines, combination COVID-19 vaccinations have emerged as superior alternatives for providing individuals with the immune protection required. A research study carried out in March 2021 revealed that combining several distinct COVID-19 vaccines increased immune responses in mice [107]. Consecutive immunization with an adenovirus vector vaccine followed by inactivated/recombinant subunit/mRNA vaccine particularly enhanced neutralizing antibody levels and promoted antibody response to primary neutralizing antibodies. Preliminary results of the CombiVacS trial, which included over 600 patients in Spain, are the first to demonstrate the benefits of mixing different coronavirus vaccines [108]. Other studies also support this concept in that combining the Oxford-AstraZeneca vaccine and the Pfizer-BioNTech vaccine induces a stronger immune response compared to two doses of the same vaccine [109,110,111]. However, while no serious side effects have been recorded in mix-and-match trials, some safety concerns remain when combining two distinct vaccines, as each vaccine has its own set of adverse events/side effects.
6.2. Booster COVID-19 Vaccines
SARS-CoV-2 B.1.617.2 variant, also known as the Delta variant, was first detected in India in late 2020; quickly spreading to many countries and becoming the dominant variant by mid-2021. Due to its higher transmissibility, this new strain caused a resurgence in the number of COVID-19 cases, particularly in regions with lower vaccination rates [112,113]. Although existing vaccines like BNT162b2 and ChAdOx1 are effective against this new Delta variant, 88% and 67% respectively, the efficacy of BNT162B2 was reduced from 94% to 88% and from 74% to 67% for ChAdOx1 compared to their respective effectiveness against the earlier alpha variant [114]. Breakthrough infections caused by the Delta variant among vaccinated healthcare workers have also been reported in several countries [115,116]. The resurgence in COVID-19 cases and the possibility of reductions in the protective effects of the vaccines over time [117], combined with the Delta variant’s higher transmissibility and increased risk of infection among vaccinated individuals have prompted health authorities to consider introducing booster vaccines.
Recent studies demonstrated that BNT162b2 booster shot significantly reduces the risk of COVID-19 infection and severe illness [118]. Another study tested seven different vaccines (BNT162b2, ChAdOx1-S, mRNA-1273, NVX-CoV2373, Ad26.COV2.S, CVnCoV and VLA2001) as boosters and showed that all seven of them boosted the immunity in both older and younger populations [119]. A number of countries, such as Israel, UAE, Russia and Turkey, had already commenced administering booster shots to high-risk individuals and healthcare professionals, even before the data were published, with an effort to counteract the decrease in immunity over time by standard 2-dose vaccination [120,121,122]. FDA released a statement on 12 August 2021, authorizing booster vaccines for immunocompromised individuals [123]. Both FDA and EMA have recently approved the use of BNT126b2 and mRNA-1273 as booster vaccines, and as of November 2021, many countries from all the continents around the world are offering their citizens booster shots [120]. WHO on the other hand, is questioning the urgency for booster doses with concerns that such practice would further increase disparities in vaccination rates in many low income countries, and preventing the ultimate international public health goal of high vaccination rates globally [124].
6.3. Mandatory COVID-19 Vaccinations
Another strategy being implemented by several countries and organizations to reduce the spread of COVID-19 is the mandatory vaccination of individuals working in certain sectors such as healthcare, transportation, education and retail. Tajikistan has become the first country to make the COVID-19 vaccines mandatory for all its citizens over 18 years of age [125]. Turkmenistan has been reluctant to share data on COVID-19 cases and deaths since the first days of the pandemic; however, it became the second country to make COVID-19 vaccines mandatory for all its citizens over 18 years of age [126]. Saudi Arabia has imposed mandatory vaccination for all individuals wanting to enter any government or private facility and made full vaccination mandatory for participation in Hajj [127]. Italy, France, Greece and the UK are making it mandatory for certain healthcare workers to be fully vaccinated and are imposing vaccination requirements for many social gatherings and venues including cinemas, bars, clubs and other closed spaces [128]. In the United States, California and New York are the only two states so far to impose a vaccine mandate, requiring all government officials to get fully vaccinated [129]. While these measures have resulted in noticeable increases in vaccination rates, they have also sparked protests around the world, mostly by anti-vaccination activists [130].
7. COVID-19 Vaccination Consideration
7.1. Uniform Vaccine Availability and Affordability
The development, testing and approval of several vaccines in the space of 18 months is an extraordinary scientific achievement and will be remembered as a milestone in the history of pandemics. Within 8 months, nearly 5 billion doses of vaccines have been administered since the authorization of the first vaccine [3]. Although this number is impressive for such a short time-period, it is not an accurate indicator of the global availability of vaccines. The increased movement of individuals globally, the economic and social interdependencies between countries and the ease of travel will make it even harder to contain the spread of the virus unless the global immunity goal is achieved. To reach the global immunization goal, a huge stepwise increase in the number of vaccines is required, in every region, state and country, particularly in areas with low vaccination rates.
COVID-19 Vaccines Global Access (COVAX) is a worldwide initiative co-led by Gavi the Vaccine Alliance, Coalition for Epidemic Preparedness Innovations (CEPI) and WHO, with UNICEF as the delivery partner; COVAX aims to accelerate the development, manufacturing of COVID-19 vaccines and ensure equitable access for every country in the world [131]. High Income Countries such as UAE, Qatar and Israel have been able to vaccinate more than 70% of their population; however, this target declines to below 1% in Low- and Middle-Income Countries such as DR Congo, South Sudan and Yemen [3]. One of the goals of COVAX is to ensure that at least 20% of each country’s population receives vaccines. As of 24 August 2021, COVAX has delivered 215 million COVID-19 vaccines to nearly 100 countries with the aim of providing 1.9 billion doses by the end of 2021 [132,133].
The currently available COVID-19 vaccines cost in the order of US$2 to US$37 per dose (Table 2). Many western countries (like the US, UK and Australia) either offer these vaccines free of charge to their citizens or subsidize such vaccination programs for the public good. Government or third-party payer subsidized vaccination schemes are designed to absorb the cost of COVID-19 vaccines and to encourage greater participation in COVID-19 vaccinations. According to WHO’s report released in April 2021, more than 87% of the of the vaccine doses have gone to either high income or upper-middle income countries [134]. This issue needs to be addressed. From an ethical perspective, regardless of their wealth or economic situation every individual’s access to appropriate basic health care needs to be guaranteed, especially from the perspective of infectious diseases during a global pandemic, since at least a certain percentage of each population needs to be vaccinated to confer herd immunity and to combat a pandemic. In addition, western and other high-income countries have much higher drug budgets and are also able to move funds quickly to COVID-19-related activities (testing, vaccine administration programs and/or providing/expanding ICU facilities) as the pandemic evolves. This is neither possible or feasible in many low- and middle- income countries where unfortunately drug/health budgets are smaller and government subsidies or third-party insurers do not operate in the health market. It is therefore critical for world agencies (like WHO, COVAX and the UN) to find equitable solutions to not only timely availability of COVID-19 vaccines for all countries but also at an affordable price so that herd immunity to COVID-19 is reached in all countries. This is especially critical as new variants of the virus emerge which will require additional vaccines beyond the initial vaccination cycle.
7.2. Vaccine Hesitancy
The Strategic Advisory Group of Experts on Immunization (SAGE) is a working group whose mission is to advise WHO on global policies and strategies concerning all vaccine-preventable diseases and SAGE defines vaccine hesitancy as “delay in acceptance or refusal of vaccination despite availability of vaccination services” [135]. SAGE was established in 1999; however, emergence of vaccine hesitancy and anti-vaccine (anti-vax) movement(s) can be traced to Jenner’s time (18th century), with respect to early smallpox vaccination programs. Factors including religious bigotry (injecting a purulent matter from a lower species to humans), substandard methods used in vaccination and the 1853 Compulsory Vaccination Act being seen as an example of class legislation-initiated public mistrust against vaccination have all given rise to anti-vaccine campaigns [136].
Reluctance to vaccination, in particular essential childhood vaccines like measles, has been rising in recent years [137]. Genuine public discourse about vaccine efficacy and side effects and other safety issues has translated to rising levels of global vaccine hesitancy. Safety of vaccines and vaccination programs have become paramount public health issues that need to be addressed urgently. Major public concerns have arisen for political, religious, socio-economic or philosophic reasons and include, for example, questions about use of cell cultures from human or animal embryo, which are used commonly to grow the virus for vaccine preparation [138,139]. It is worth emphasizing that vaccines undergo rigorous safety and efficacy evaluation and their benefits far outweigh their risks as part of mass vaccination programs. Some infections like measles require high levels of herd immunity to prevent community spread. Achieving such herd immunity is a prerequisite to protecting immune-compromised individuals or others who cannot be vaccinated. Inadequate herd immunity is probably the major reason why recent measle outbreaks have been observed in some tight-knit communities in the US, such as Ultra-orthodox Jewish populations in New York and other US states [140].
The anti-vaccine movement gained significant momentum in the 1990s due mainly to publications that appeared in Lancet that was retracted ~12 years later. Andrew Wakefield, a UK physician reported that measles, mumps and rubella (MMR) vaccine caused autism in eight children [141]. Even though this was hypothesized in a small number of children, Wakefield’s claims attracted widespread interest, first in the UK but then globally, and resulted in parents either refusing or delaying MMR vaccines and sometimes even other vaccines as well. An upsurge in measles in the UK, EU and particularly in the USA coincided with Wakefield’s erroneous claims. Large-scale studies subsequently refuted Wakefield’s claims and proved that vaccines were not causing autism [142].
With the proliferation of social media and its rapid and immediate influence, and with the involvement of public figures (e.g., prominent politicians and Hollywood actors), the anti-vax movement has gained an even stronger hold in some regions of the world and has garnered new followers or at least empowered many to be highly skeptical about vaccine safety [143,144,145]. Currently, there are many online anti-vax campaigns, podcasts, blogs, videos, articles and books [146]. Some “anti-vaxxers” are highly ‘educated’ in non-medical topics and claim that they would like to protect their children from vaccine side-effects. It is unfortunate, from a public health perspective, that many individuals remain skeptical about the effectiveness and safety of vaccines. A more sophisticated education and behavior-change approach is necessary to educate the public about overall vaccine development, how vaccines work, nature of vaccine clinical trials and their public health utility as well as their shortcomings, so individuals can base their decisions regarding vaccination on objective scientific evidence and clear public health messages. The potential harmful effects of vaccine hesitancy on global public health has become so evident that the WHO has listed vaccine hesitancy as one of the top ten threats to global health in 2019 [147].
As a result, immunization of many politicians and other prominent public figures with COVID-19 vaccines has been highly publicized to highlight their overall safety and to ensure greater COVID-19 vaccine uptake. Based on surveys that were conducted in 2020, the acceptance rates of COVID-19 vaccines differ dramatically across countries around the world with the highest rate seen in Ecuador (97%) [148] and the lowest rate in Kuwait (23%) [149]. In a study in healthcare workers, the highest COVID-19 vaccine acceptance rate (94%) was in those working in COVID-19 departments compared to 77% in healthcare professionals working in non-COVID-19 departments [150]. A systematic review [151] has shown that the population intending to get a vaccine has declined as the pandemic has progressed, which is likely to be attributed to both increased exposure to misinformation and concerns over the safety of the vaccines [152,153]. The same study demonstrated that generally being female, younger, of lower income or education status or belonging to an ethnic minority group were associated with increased vaccine reluctance. A systematic review of COVID-19 vaccine hesitancy in the US population has shown a similar result with the highest hesitancy rate being in Black/African Americans and pregnant or breastfeeding women [154]. In the case of COVID-19 vaccines, public health authorities not only have to deal with existing prejudices of anti-vaxxers such as political, religious or cultural beliefs, they also need to convince the public that, with advanced vaccine development technologies and the substantial funding that have been provided to the scientific community, it is now possible to develop safe and effective COVID-19 vaccines in much shorter timeframes without compromising effectiveness and more importantly safety.
8. Conclusions
Despite stepwise technological advancements in public health and medicine, substantial increases in number of well-trained health personnel, facilities and sophisticated medical equipment and increased access to and sharing of up-to-date scientific and medical information in many countries, the COVID-19 pandemic has proven that pandemic preparedness is still a major global issue that needs to be addressed urgently. SARS, MERS, Ebola, and various influenza outbreaks were able to be contained before evolving into global pandemics; however, it is clear from the ongoing COVID-19 pandemic that the world needs to revisit its emergency plans and improve its preparedness for potential similar future outbreaks.
One striking shining light during this pandemic has been the timely development and commercial availability of COVID-19 vaccines. This has largely been possible due to targeted international collaborative research and scientific efforts and availability of significant financial resources from governments, individuals, institutions/international organizations and philanthropists. Although developing COVID-19 vaccines in less than a year has been one of the significant breakthroughs in the history of medical science, with the emergence of new COVID-19 variants, future research needs to specifically focus on vaccines that have the potential to act against new variants to either stop transmission or infection.
Nevertheless, many major issues remain, including uneven availability/distribution of such vaccines across the globe, their affordability and the increase in vaccine hesitancy. These issues will require strong political will and leadership and concerted public health initiatives and messaging if the full potential of COVID-19 vaccines is to be realised for all individuals in all countries irrespective of socio-economic status and cultural and/or religious backgrounds. This is the challenge that lies ahead.
Acknowledgments
R.F.N. acknowledges the Indonesia Endowment Fund for Education (LPDP) for the postgraduate scholarship.
Appendix A
Table A1.
More information on authorized/approved vaccines in at least one country as per 15 November 2021.
| No | Vaccine Name | Age Group | Common Side Effect(s) | Main User–Country |
|---|---|---|---|---|
| 1 | ComirnatyTM (BNT162b2) | 5 years of age and older [84] | Pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, and fever | US, UK, South Korea, Singapore, Saudi Arabia, New Zealand, Japan, Israel, Hungary, Germany, France, Canada, Australia |
| 2 | Moderna COVID-19 VaccinemRNA-1273 and mRNA-1273.351 | 18 years of age and older [88] | Pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever | US, UK, Singapore, France |
| 3 | COVID-19 Vaccine AstraZeneca (AZD1222) | 18 years of age and older [91] | Injection site pain or tenderness, tiredness, headachemuscle pain, fever and chills | UK, South Korea, Saudi Arabia, Phillipines, India, Germany, France, Canada, Brazil, Australia |
| 4 | ConvideciaTM (Ad5-nCoV) | 18 years of age and older [155] | Injection site pain, mild to severe fever (up to grade 3), headache, mild to severe fatigue (up to grade 3), muscle and joint pain, throat pain and cough. | China, Pakistan |
| 5 | Ad26.COV2.S | 18 years of age and older [95] | Arm (pain, redness, swelling), body (tiredness, headache, muscle pain, chills, fever, nausea) | US, South Africa, The Netherlands |
| 6 | BBIBP-CorV | 18 years of age and older [98] | Headaches, fatigue, injection site reactions | China, Hungary, UAE |
| 7 | Inactivated SARS-CoV-2 (vero cell) | 18 years of age and older | Injection site pain, followed by fever, which were mild and self-limiting | China, UAE |
| 8 | CoronaVac | 18 years of age and older [99] | Injection site reactions, fatigue, diarrhea, and muscle pain | China, Brazil, Turkey, Indonesia, Phillipines |
| 9 | Sputnik V | N/A | Headaches, pain at injection site | Russia |
| 10 | EpiVacCorona | N/A | N/A | Turkmenistan, Russia |
| 11 | ZF2001/RBD-Dimer | N/A | Common mild side-effects including injection pain, redness and swelling | China, Uzbekistan |
| 12 | Soberana 2/Pasteur | N/A | Pain and redness at the injection site, general malaise | Iran |
| 13 | Abdala/CIGB-66 | N/A | No serious adverse side effect | Cuba |
| 14 | Medigen | N/A | No vaccine-related serious adverse effects | Taiwan |
| 15 | Covaxin® | 18 years of age and old-er [156] | Fever, headaches, irritability, pain, swelling, or both at the site of injection | India |
| 16 | QazCovid-in® | N/A | No serious side effects | Kazakhstan |
| 17 | Inactivated (Vero Cells) | N/A | N/A | China |
| 18 | COVIran Barekat | N/A | N/A | Iran |
| 19 | CoviVac | N/A | N/A | Russia |
| 20 | NVX-CoV2373 | N/A | N/A | Indonesia, Denmark |
| 21 | ZyCoV-D | N/A | N/A | India |
| 22 | COVAX-19® | N/A | N/A | Iran |
| 23 | Soberana Plus | N/A | N/A | Cuba |
Appendix B
Table A2.
COVID-19 vaccine candidates in phase 1–3 clinical trial as per 15 November 2021 [56].
| No | Vaccine Candidate | Developer | Vaccine Type | Developer Country | Trial Phase | Clinical Trial ID | Country | Enrolment Target | Actual/Estimated Trial Dates | Primary Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gam-COVID-Vac Sputnik V |
Gamaleya Research Institute | Viral vector (non-replicating) | Russia | 3 | NCT04530396 | Russia | 33,758 | 7 September 2020–1 May 2021 | Percentage of trial subjects with COVID-19 developed within 6 months after the first dose |
| 2 | NVX-CoV2373 | Novavax | Protein subunit | US | 3 | NCT04611802 | Mexico, Puerto Rico, US | 33,000 | 27 December 2020–30 June 2023 | Participants with symptoms; reactogenicity incidence and severity; incidence and severity of MAAEs, UnSoAEs, SAEs, AESIs; antibodies to SARS-CoV-2 Nucleoprotein (NP); deaths due to any cause |
| 3 | ZF2001/RBD-Dimer | Anhui Zhifei Longcom | Protein subunit | China | 3 | NCT04646590 | China, Ecuador, Indonesia, Pakistan, Uzbekistan | 29,000 | 16 December 2020–April 2022 | Endpoints of efficacy and safety |
| 4 | CVnCoV | Curevac | RNA based vaccine | Germany | 3 | NCT04652102 | Germany | 36,500 | 14 December 2020–15 May 2022 | Participants with virologically confirmed PCR positive cases of COVID-19 of any severity; participant with MAAEs, SAEs, AESIs |
| 5 | Inactivated (Vero Cells) | Chinese Academy of Medical Sciences | Inactivated virus | China | 3 | NCT04659239 | Brazil, Malaysia | 34,020 | 28 January 2021–July 2022 | Incidence of COVID-19 cases after two-doses of vaccination, the incidence of SoAEs |
| 6 | QazCovid-in® | Research Institute for Biological Safety Problems Kazakhstan | Inactivated virus | Kazakhstan | 3 | NCT04691908 | Kazakhstan | 3000 | 25 December 2020–30 July 2021 | Seroconversion; vaccine immunogenicity versus placebo, frequency of confirmed COVID-19 cases |
| 7 | ZyCoV-D | Zydus Cadila | DNA based vaccine | India | 3 | CTRI/2021/01/030416 | India | 28,216 | N/A | To demonstrate the efficacy of ZyCoV-D in the prevention of virologically confirmed symptomatic COVID-19 cases as compared to placebo |
| 8 | Covaxin | Bharat Biotech | Inactivated virus | India | 3 | NCT04641481 | India | 25,800 | 16 November 2020–December 2022 | First occurrence of virologically confirmed (RT-PCR positive) symptomatic COVID-19 cases |
| 9 | VAT00002: with adjuvant | Sanofi/GSK | Protein subunit | US | 3 | NCT04904549 | US | 37,430 | 26 May 2021–13 January 2023 | Occurrence of symptomatic COVID-19, presence of injection site or systemic reactions, non-serious UnSoAEs, immediate AEs, MAAEs, SAEs, AESIs, and virologically confirmed SARS-CoV-2 infections and/or symptomatic COVID-19 |
| 10 | Inactivated (Vero Cells) | Shenzhen Kangtai Biological Products Co., Ltd. | Inactivated virus | China | 3 | NCT04852705 | China | 28,000 | May 2021–November 2022 | Incidence density of symptomatic COVID-19 cases |
| 11 | FINLAY-FR-2 anti-SARS-CoV-2 Vaccine | Instituto Finlay de Vacunas | Protein subunit | Cuba | 3 | IFV/COR/09 | Cuba | 44,010 | N/A | Virologically confirmed symptomatic COVID-19 infection |
| 12 | EpiVacCorona | FBRI | Protein subunit | Russia | 3 | NCT04780035 | Russia | 3000 | 18 November 2020–September 2021 | The proportion of vaccinated volunteers with no laboratory confirmed symptoms caused by SARS-CoV-2, within 6 months post vaccination versus placebo, the prophylactic efficacy of the vaccine |
| 13 | Recombinant (Sf9 cell) | West China Hospital | Protein subunit | China | 3 | NCT04904471 | China | 40,000 | 1 June 2021–31 December 2022 | Virologically confirmed (polymerase chain reaction [PCR] positive) symptomatic COVID-19 cases at first appearance, regardless of severity; incidence of SAEs, AESIs, MAAEs, SoAEs, UnSoAEs |
| 14 | mRNA vaccine (ARCoV) | Academy of Military Science (AMS), Walvax Biotechnology | RNA based vaccine | China | 3 | NCT04847102 | N/A | 28,000 | 28 May 2021–30 May 2023 | Incidence rate (person-year) of COVID-19 cases, AEs, and SAEs |
| 15 | CIGB-66 | Center for Genetic Engineering and Biotechnology (CIGB) | Protein subunit | Cuba | 3 | RPCEC00000359 | Cuba | 48,000 | N/A | Vaccine efficacy (number of symptomatic COVID-19 subjects with no evidence of previous exposure to viral infection) |
| 16 | VLA2001 | Valneva | Inactivated virus | France | 3 | NCT04864561 | UK | 4000 | 26 April 2021–30 June 2022 | Immune response measured after completion of a 2-dose immunization schedule, as determined by the GMT of SARS-CoV-2-specific neutralizing antibodies, frequency and severity of any AEs |
| 17 | Nanocovax | Nanogen Pharmaceutical Biotechnology | Protein Subunit | Viet Nam | 3 | NCT04922788 | Viet Nam | 13,000 | 7 June 2021–7 August 2022 | Participants who experience a first episode of virologically confirmed case of COVID-19; any severity SAEs, MAAEs; geometric mean of anti-S IgG concentrations; geometric mean of SARS-CoV-2 serum neutralizing titres by plaque reduction neutralization test (PRNT) |
| 18 | ERUCOV-VAC (Turkovac) | Erciyes University | Inactivated virus | Turkey | 3 | NCT04942405 | Turkey | 40,800 | 21 June 2021–31 March 2023 | Protection indexes of two vaccine doses for symptomatic COVID-19, 2 weeks after the second dose of vaccination |
| 19 | ARCT-154 | Arcturus Therapeutics Inc | RNA based vaccine | US | 3 | ISRCTN15779782 | Switzerland | N/A | 3 August 2021–1 September 2023 | Percentage of participants with virologically confirmed COVID-19 |
| 20 | INO-4800 | Inovio Pharmaceuticals | DNA based vaccine | US | 3 | ISRCTN15779782 | Switzerland | N/A | 3 August 2021–1 September 2023 | Percentage of participants with virologically confirmed COVID-19 |
| 21 | SCB-2019 | Clover Biopharmaceuticals/GSK/Dynavax | Protein subunit | Australia | 3 | NCT05012787 | South Africa, Ukraine | 300 | 13 September 2021–16 December 2022 | Participants with AEs, UnSoAEs, SAEs, MAAEs, AESIs, any confirmed relapse of immune-mediated disease |
| 22 | CoVLP | Medicago | Virus like particle | Canada | 3 | NCT04636697 | Canada, US | 900 | 22 November 2021–31 May 2022 | GMTs of the three vaccine lots |
| 23 | COVAX-19® | Vaxine Pty.Ltd/Cinnagen Co. | Protein Subunit | Australia | 2 | IRCT20150303021315N24 | Iran | 16,876 | N/A | Evaluation of COVID-19 incidence |
| 24 | DelNS1-2019-nCoV-RBD-OPT1 | The University of Hong Kong and Xiamen University | Viral vector (Replicating) | Hong Kong | 2 | ChiCTR2100051391 | Hong Kong | N/A | N/A | N/A |
| 25 | BECOV2 | Biological E Limited | Protein subunit | India | 3 | CTRI/2021/08/036074 | India | 2140 | N/A | Immune response measured after completion of 2-dose immunization schedule, as determined by GMT/C |
| 26 | GBP510 | SK Bioscience Co., Ltd. and CEPI | Protein subunit | South Korea | 3 | NCT05007951 | South Korea | 3990 | 30 August 2021–September 2022 | GMT of SARS-CoV-2 neutralizing antibody |
| 27 | COVI-VAC | Codagenix Inc | Live-Attenuated | US | 3 | ISRCTN15779782 | Switzerland | N/A | 3 August 2021–1 September 2023 | Percentage of participants with virologically confirmed COVID-19 |
| 28 | Razi Cov Pars | Razi Vaccine and Serum Research Institute | Protein subunit | Iran | 2 | IRCT20210206050259N3 | Iran | 41,128 | N/A | Occurrence of confirmed symptomatic COVID-19 disease two weeks after the second vaccine dose |
| 29 | AG0301-COVID19 | AnGes + Takarabio + Osaka University | DNA based vaccine | Japan | 2/3 | NCT04655625 | Japan | 500 | 23 November 2020–31 March 2022 | Incidence of treatment-emergent AEs; Immunogenicity |
| 30 | GRAd-COV2 | ReiThera | Viral vector (non-replicating) | Italy | 2/3 | NCT04791423 | Italy | 10,300 | 15 March 2021–30 April 2022 | Participants with symptomatic laboratory confirmed COVID-19, incidence of AEs, SAEs, MAAEs, and AESIs, local and systemic SoAEs, post-treatment GMTs and GMFRs in SARS-CoV-2 S and/or RBD antibodies |
| 31 | UB-612 | Vaxxinity | Protein subunit | US | 2/3 | NCT04683224 | N/A | 7320 | 1 February 2021–22 March 2023 | The incidence of local reactions solicited systemic events, AEs, MAAEs, SAEs and AESIs, change in safety chemistry and hematology blood lab values for assessment of risk in Phase 3, prevention of SARS-CoV-2 infection in adults, change after second dose through to the end of study in antibody titres |
| 32 | GX-19 | Genexine Consortium | DNA based vaccine | South Korea | 2/3 | NCT05067946 | N/A | 14,000 | October 2021–October 2023 | First occurrence of COVID-19 at least 14 days after the second vaccination. Incidence of SoAEs, UnSoAEs, SAEs |
| 33 | rVSV-SARS-CoV-2-S Vaccine | Israel Institute for Biological Research | Viral vector (Replicating) | Israel | 2/3 | NCT04990466 | Israel | 20,000 | 30 September 2021–28 February 2022 | Prevention of Serology-confirmed SARS-CoV-2 infection |
| 34 | COVIran Barekat | Shifa Pharmed Industrial Co | Inactivated virus | Iran | 2/3 | IRCT20201202049567N3 | Iran | 20,000 | N/A | Vaccine efficacy of Shifa-Pharmed inactivated SARS-CoV-2 vaccine |
| 35 | ReCOV | Jiangsu Rec-Biotechnology Co Ltd. | Protein subunit | China | 2/3 | NCT05084989 | N/A | 20,301 | 31 December 2021–31 December 2021 | Number of Participants with Occurrence of COVID-19 cases, AEs, SAEs and AESIs |
| 36 | mRNA-1273.211 | ModernaTX.Inc | RNA based vaccine | US | 2/3 | NCT04927065 | US | 896 | 24 March 2021–22 December 2021 | Vaccine efficacy against SARS-CoV-2 infection; effect of vaccine on peak nasal viral load |
| 37 | AZD2816 | AstraZeneca and The University of Oxford | Viral vector (non-replicating) | UK | 2/3 | NCT04973449 | UK | 2475 | 27 June 2021–15 June 2022 | Safety and tolerability of 1 dose of AZD2816 in seronegative participants previously vaccinated with AZD1222, and 2 doses in unvaccinated seronegative participants |
| 38 | SCTV01C | Sinocelltech Ltd. | Protein Subunit | China | 2/3 | NCT05043311 | N/A | 12,420 | 30 October 2021–1 October 2022 | The incidence of COVID-19 infections |
| 39 | FINLAY-FR-1 | Instituto Finlay de Vacunas Cuba | Protein subunit | Cuba | 2 | IFV/COR/04 | Cuba | 676 | 13 August 2020–11 January 2021 | SAEs, titre of specific anti-RBD IgG antibodies at baseline and 14, 28 and 56 days |
| 40 | LUNAR-COV19/ARCT-021 | Arcturus Therapeutics Inc | RNA based vaccine | US | 2 | NCT04668339 | Singapore, US | 600 | 7 January 2021–30 April 2022 | Local and systemic SoAEs, AEs, SAEs, MAAEs, new onset of chronic disease, abnormal chemistry and hematology values; GMT and GMFR of neutralizing antibody |
| 41 | VXA-CoV2-1 | Vaxart | Viral vector (non-replicating) | US | 2 | NCT05067933 | US | 896 | October 2020–June 2023 | Rate of UnSoAEs, frequency of SAEs and MAAEs |
| 42 | Dendritic cell vaccine AV-COVID-19 | Aivita Biomedical, Inc + Ministry of Health Republic of Indonesia | Viral vector (Replicating) + APC | US | 2 | NCT05007496 | Indonesia | 145 | April 2021–May 2021 | Efficacy based on T-cell-induced immune response |
| 43 | MRT5500 | Sanofi Pasteur | RNA based vaccine | US | 2 | NCT04798027 | US | 333 | 12 March 2021–July 2022 | Presence of immediate AEs, solicited injection site reactions and systemic reactions, UnSoAEs, MAAEs, and AESIs; Presence of out-of-range biological test results, neutralizing antibody titre, seroconversion |
| 44 | SARS-CoV-2 VLP Vaccine | The Scientific and Technological Research Council of Turkey | Virus like particle | Turkey | 2 | NCT04962893 | Turkey | 330 | 26 June 2021–September 2022 | Comparison of efficacy, specific IgG, neutralizing antibody, and cellular immune response |
| 45 | Recombinant SARS-CoV-2 Fusion Protein Vaccine (V-01) | Guangdong Provincial Center for Disease Control and Prevention | Protein subunit | China | 2 | ChiCTR2100045107 | China | 880 | 28 March 2021–30 July 2022 | Positive conversion rate of serum anti-SARS-CoV-2 RBD protein antibody, and its GMT and GMI; positive conversion rate of serum anti-SARS-CoV-2 neutralizing antibody, and its GMI |
| 46 | SCB-2020S | Clover Biopharmaceuticals AUS Pty Ltd. | Protein subunit | Australia | 2 | NCT04950751 | N/A | 150 | August 2021–April 2020 | GMT and GMFR of SARS-CoV-2 neutralising antibodies to B.1.351 variant, proportion of subjects achieving seroconversion of SARS-CoV-2 neutralising antibodies to B.1.351 variant |
| 47 | SC-Ad6-1 | Tetherex Pharmaceuticals Corporation | Viral vector (non-replicating) | US | 2 | NCT05077267 | Germany | 210 | 19 August 2021–1 February 2024 | SARS-CoV-2 neutralizing antibody titers |
| 48 | Recombinant RBD Protein Vaccine | Bagheiat-allah University of Medical Sciences | Protein subunit | Iran | 2 | IRCT20210620051639N2 | Iran | 300 | N/A | IgG antibody against Receptor Binding Domain (RBD) protein |
| 49 | KBP-201 | Kentucky Bioprocessing | Protein subunit | US | 1/2 | NCT04473690 | US | 180 | 31 July 2021–31 July 2022 | Solicited administration site reactions and systemic events |
| 50 | RBD SARS-CoV-2 HBsAg VLP | SpyBiotech + Serum Institute of India | Virus like particle | UK | 1/2 | ACTRN12620000817943 | Australia | 280 | N/A | To assess the immune response, safety and reactogenicity, of a two-dose schedule of two dose amounts of RBD SARS-CoV-2 HBsAg VLP vaccine as compared with two-dose administration of placebo |
| 51 | IMP CoVac-1 | University Hospital Tuebingen | Protein subunit | Germany | 1/2 | NCT04954469 | Germany | 68 | 30 June 2021–31 March 2022 | Safety- Eastern Cooperative Oncology Group (ECOG) status, vital signs, blood chemistry and coagulation, and hematology |
| 52 | LV-SMENP-DC | Shenzhen Geno-Immune Medical Institute | Viral vector (non-replicating) + APC | China | 1/2 | NCT04276896 | China | 100 | 24 March 2020–31 December 2024 | Clinical improvement based on the 7-point scale, lower Murray lung injury score |
| 53 | hAd5-S+N bivalent vaccine | ImmunityBio Inc | Viral vector (non-replicating) | US | 1/2 | NCT04843722 | US | 540 | May 2021–August 2022 | Efficacy: percent of subjects that show an increase in N-reactive T cells |
| 54 | CIGB-669 | Center for Genetic Engineering and Biotechnology (CIGB) | Protein subunit | Cuba | 1/2 | RPCEC00000345 | Cuba | 88 | N/A | Safety: occurrence and intensity of AEs, subjects with seroconversion of anti-RBD IgG antibodies to SARS-CoV-2 |
| 55 | AdCLD-CoV19 | Cellid Co., Ltd. | Viral vector (non-replicating) | South Korea | 1/2 | NCT04666012 | South Korea | 150 | 29 December 2020–April 2022 | Incidence of SoAEs and UnSoAEs |
| 56 | GLS-5310 | GeneOne Life Science Inc | DNA based vaccine | South Korea | 1/2 | NCT04673149 | South Korea | 345 | 23 December 2020–31 December 2022 | Incidence of AEs; GMT of antigen-specific binding antibody titres |
| 57 | S-268019 | Shionogi | Protein subunit | Japan | 1/2 | jRCT2051200092 | Japan | 214 | N/A | AEs, adverse reactions, SAEs, local and systemic reactogenicity SoAEs, GMT of SARS-CoV-2 neutralizing antibody |
| 58 | SARS-CoV-2-RBD-Fc-fusion protein | University Medical Center Groningen + Akston Biosciences Inc. | Protein subunit | The Netherlands | 1/2 | NCT04681092 | The Netherlands | 130 | 12 April 2021–30 June 2021 | Safety / Tolerability (35 days) |
| 59 | COVAC-1 and COVAC-2 | University of Saskatchewan | Protein subunit | Canada | 1/2 | NCT04702178 | Canada | 108 | 10 February 2021–February 2023 | AEs during 28 days after each injection |
| 60 | COVID-eVax | Takis + Rottapharm Biotech | DNA based vaccine | Italy | 1/2 | NCT04788459 | Italy | 160 | 25 February 2021–June 2022 | Local and systemic SoAEs, UnSoAEs, quantitative antibody titres, binding to the specific SARS-CoV-2 antigen, SARS-CoV-2 neutralizing antibody titre, change from baseline in antigen-specific cellular immune responses to SARS-CoV-2, percentage of subjects who seroconverted |
| 61 | Inactivated (NDV based) chimeric vaccine | Mahidol University and Government Pharmaceutical Organization | Inactivated virus | Thailand | 1/2 | NCT04764422 | Thailand | 460 | 20 March 2021–April 2023 | Frequency of reportable local and systemic SoAEs after each vaccination; measurement of changes in hemoglobin, white blood cells, platelet count, creatinine, AST, ALT, bilirubin |
| 62 | VBI-2902a | VBI Vaccines Inc | Virus like particle | Canada | 1/2 | NCT04773665 | Canada | 780 | 15 March 2021–June 2022 | Rate and severity of local and systemic SoAEs, UnSoAEs, MAAEs, SAEs, and laboratory abnormalities; AEs leading to discontinuation of study vaccination |
| 63 | EuCorVac-19 | POP Biotechnologies and EuBiologics Co Ltd. | Protein subunit | South Korea | 1/2 | NCT04783311 | South Korea | 280 | 23 February 2021–January 2023 | Immediate AEs, local and systemic AEs, UnSoAEs, SAEs, AESIs. |
| 64 | DS-5670a | Daiichi Sankyo Co Ltd. | RNA based vaccine | Japan | 1/2 | NCT04821674 | Japan | 152 | 15 March 2021–31 December 2022 | Number of participants reporting treatment-emergent AEs, local and systemic AEs, and SAEs; GMT, GMFR, and SCR of SARS-CoV-2 specific neutralizing antibody |
| 65 | COVIVAC | Institute of Vaccines and Medical Biologicals | Viral vector (non-replicating) | Viet Nam | 1/2 | NCT04830800 | Viet Nam | 420 | 10 March 2021–30 September 2022 | Number and severity of local and systemic SoAEs, UnSoAEs, SAEs, MAAEs, AESIs, and clinically significant hematological and biochemical measurements |
| 66 | Recombinant SARS-CoV-2 Vaccine (CHO Cell) | National Vaccine and Serum Institute | Protein subunit | China | 1/2 | NCT04869592 | China | 3580 | 25 April 2021–25 October 2022 | Incidence and severity of any adverse reactions/events and abnormal blood biochemistry, blood routine, blood coagulation function and urine routine, SAEs, AESIs, GMT of SARS-CoV-2 neutralizing antibody |
| 67 | EXG-5003 | Elixirgen Therapeutics Inc | RNA based vaccine | Japan | 1/2 | NCT04863131 | Japan | 60 | 28 April 2021–31 January 2023 | Number of participants reporting local and systemic AEs |
| 68 | KD-414 | KM Biologics Co Ltd. | Inactivated virus | Japan | 1/2 | jRCT2071200106 | Japan | 210 | N/A | Safety: all adverse events and immunogenicity: neutralizing antibody conversion rate against SARS-CoV-2 |
| 69 | MVA vector expressing stabilized S Protein | German Centre for Infection Research | Viral vector (non-replicating) | Germany | 1/2 | NCT04895449 | Germany | 240 | 1 June 2021–1 March 2022 | Percentage of participants experiencing solicited local or systemic reactogenicity as defined by the study protocol |
| 70 | QazCovac | Research Institute for Biological Safety Problem | Protein subunit | Kazakhstan | 1/2 | NCT04930003 | Kazakhstan | 244 | 15 June 2021–December 2021 | Frequency of AEs for up to 7 and 21 days after immunization. The proportion of volunteers with increased levels of the immune response of specific neutralizing antibody titres using ELISA following the vaccination, compared with placebo |
| 71 | AG0302-COVID19 | AnGes, Inc/Osaka University | DNA based vaccine | Japan | 1/2 | NCT04993586 | Japan | 400 | 29 July 2021–31 December 2021 | Incidence of Treatment-Emergent AEs, immunogenicity |
| 72 | Hipra | Laboratorios Hipra, S.A. | Protein subunit | Spain | 1/2 | NCT05007509 | Spain | 30 | 16 August 2021–September 2022 | Local and systemic SoAEs and UnSoAEs |
| 73 | Versamune-CoV-2FC vaccine | Farmacore Biotecnologia Ltd.a | Protein subunit | Brazil | 1/2 | NCT05016934 | N/A | N/A | 1 November 2021–20 April 2022 | Frequency and severity of local and systemic AEs and AESIs. |
| 74 | ARCT-165 | Arcturus Therapeutics Inc | RNA based vaccine | US | 1/2 | NCT05037097 | Singapore, US | 72 | 30 August 2021–March 2023 | Local and systemic SoAEs, AEs, SAEs, MAAEs, new onset of chronic disease, abnormal chemistry and hematology values; GMT and GMFR of neutralizing antibody |
| 75 | ARCT-021 | Arcturus Therapeutics Inc | RNA based vaccine | US | 1/2 | NCT05037097 | Singapore, US | 72 | 30 August 2021–March 2023 | Local and systemic SoAEs, AEs, SAEs, MAAEs, new onset of chronic disease, abnormal chemistry and hematology values; GMT and GMFR of neutralizing antibody |
| 76 | SII B.1.351, a monovalent (Beta) variant | Novavax | Protein subunit | US | 1/2 | NCT05029856 | Australia | 240 | February 2022–August 2022 | MN50 GMTs to the SARS-CoV-2 B.1.351 (Beta) and B.1.617.2 (Delta), expressed as GMT and SCRs/SRRs. Local and systemic SoAEs, UnSoAEs, MAAEs |
| 77 | SII Bivalent: (ancestral strain and (Beta) variant) | Novavax | Protein subunit | US | 1/2 | NCT05029856 | Australia | 240 | February 2022–August 2022 | MN50 GMTs to the SARS-CoV-2 B.1.351 (Beta) and B.1.617.2 (Delta), expressed as GMT and SCRs/SRRs. Local and systemic SoAEs, UnSoAEs, MAAEs |
| 78 | SII B.1.617.2, monovalent (Delta) variant | Novavax | Protein subunit | US | 1/2 | NCT05029856 | Australia | 240 | February 2022–August 2022 | MN50 GMTs to the SARS-CoV-2 B.1.351 (Beta) and B.1.617.2 (Delta), expressed as GMT and SCRs/SRRs. Local and systemic SoAEs, UnSoAEs, MAAEs |
| 79 | AAV5-RBD-S vaccine (BCD-250) | Biocad | Viral vector (non-replicating) | Russia | 1/2 | NCT05037188 | Russia | 160 | 10 August 2021–December 2022 | Percentage of subjects with ≥ 4 fold rise of serum SARS-CoV-2-specific IgG titer from baseline |
| 80 | CoviVac | Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products | Inactivated virus | Russia | 1/2 | NCT05046548 | Russia | 400 | 3 October 2020–1 October 2021 | Geometric mean titer (GMT) |
| 81 | VB10.2129, encoding RBD | Vaccibody AS | DNA based vaccine | Norway | 1/2 | NCT05069623 | Norway | 160 | 27 October 2021–October 2023 | Local and systemic SoAEs, UnSoAEs, SAEs |
| 82 | VB10.2210 | Vaccibody AS | DNA based vaccine | Norway | 1/2 | NCT05069623 | Norway | 160 | 27 October 2021–October 2023 | Local and systemic SoAEs, UnSoAEs, SAEs |
| 83 | SARS-CoV-2 Protein Subunit Recombinant Vaccine | Biofarma | Protein subunit | Indonesia | 1/2 | NCT05067894 | Indonesia | 780 | November 2021–March 2022 | Safety (phase I) and immunogenicity (phase II) of the SARS-CoV-2 protein subunit recombinant vaccine. |
| 84 | MVA-SARS-2-S | University of Munich | Viral vector (non-replicating) | Germany | 1 | NCT04569383 | Germany | 30 | October 2020–May 2021 | Percentage of participants experiencing solicited local or systemic reactogenicity |
| 85 | LNP-nCoVsaRNA | Imperial College London | RNA based vaccine | UK | 1 | ISRCTN17072692 | UK | 320 | 1 April 2020–31 July 2021 | Solicited local injection site reactions, solicited systemic and laboratory reactions, UnSoAEs, SAEs, titres of neutralizing antibody and IgG |
| 86 | Covid-19/aAPC | Shenzhen Geno-Immune Medical Institute | Viral vector (Replicating) | China | 1 | NCT04299724 | China | 100 | 15 February 2020–31 December 2024 | Frequency of vaccine events and serious vaccine events, proportion of subjects with positive T cell response |
| 87 | AdimrSC-2f | Adimmune Corporation | Protein subunit | Taiwan | 1 | NCT04522089 | Taiwan | 70 | 20 August 2020–20 March 2021 | SoAEs and incidence of abnormal laboratory tests results |
| 88 | Covigenix VAX-001 | Entos Pharmaceuticals Inc | DNA based vaccine | Canada | 1 | NCT04591184 | Canada | 72 | 7 April 2021–August 2022 | Safety of a 2-dose regimen of VAX-001 when doses are given 14 days apart, Mean change from baseline in safety laboratory measures, frequency of treatment-emergent SAEs throughout the study and up to 12 months post-second dose |
| 89 | CORVax | Providence Health & Services | DNA based vaccine | US | 1 | NCT04627675 | US | 36 | 30 December 2020–May 2022 | Toxicity and MAAEs at various time frames |
| 90 | ChulaCov19 | Chulalongkorn University | RNA based vaccine | Thailand | 1 | NCT04566276 | Thailand | 96 | January 2021–June 2021 | Frequency and grade of AEs, reportable local and systemic SoAEs |
| 91 | bacTRL-Spike | Symvivo corporation | DNA based vaccine | Canada | 1 | NCT04334980 | Australia | 24 | 2 November 2020–28 February 2022 | Frequency of adverse events up to 12 months post-vaccination |
| 92 | COH04S1 | City of Hope Medical Center | Viral vector (non-replicating) | US | 1 | NCT04639466 | US | 129 | 11 December 2020–10 November 2022 | Incidence of adverse events for up to 365 days |
| 93 | MF59 | The University of Queensland | Protein Subunit | Australia | 1 | NCT04495933 | Australia | 216 | 13 July 2020–8 November 2021 | Local and systemic SoAEs, UnSoAEs, SAEs, GMT of the serum antibody and Nab response |
| 94 | COVIGEN | The University of Sydney Bionet Co., Ltd. | DNA based vaccine | Australia | 1 | NCT04742842 | Australia | 150 | 15 February 2021–31 December 2022 | Frequency of solicited local and systemic reactogenicity AEs, UnSoAEs, SAEs, MAAEs, change in safety laboratory values from baseline |
| 95 | BBV154 | Bharat Biotech | Viral vector (non-replicating) | India | 1 | NCT04751682 | India | 175 | 1 March 2021–30 November 2021 | Incidence of immediate AEs, local and systemic SoAEs, SAEs, and UnSoAEs |
| 96 | PTX-COVID19-B | Providence Therapeutics Holdings Inc | RNA based vaccine | Canada | 1 | NCT04765436 | Canada | 60 | 14 January 2021–14 February 2022 | Occurrence of AEs after each vaccination, assessments of AEs (days 1-42) and safety (days 1-395), immunogenicity analysis |
| 97 | CoV2 SAM (LNP) | GlaxoSmithKline | RNA based vaccine | UK | 1 | NCT04758962 | US | 40 | 15 February 2021–9 May 2022 | Participants with at least 1 solicited administration site event and solicited systemic event, during 7-day follow-up period, with any UnSoAEs during 30-day follow-up period after each vaccination, and with any hematological and biochemical laboratory abnormality at screening, day 1, 2, 8, 31, 32, and 38. |
| 98 | NBP2001 | SK Bioscience Co Ltd. | Protein subunit | South Korea | 1 | NCT04760743 | South Korea | 50 | 17 December 2020–April 2022 | Occurrence of immediate systemic reactions, local and systemic SoAEs, UnSoAEs, SAEs, MAAEs, and AESIs, GMT and GMFR of IgG and neutralizing antibody to the SARS-CoV-2 |
| 99 | ChAdV68-S and AM-LNP-S | Gritstone Oncology | Viral vector (non-replicating) | US | 1 | NCT04776317 | US | 130 | 25 March 2021–19 September 2022 | Frequency by grade of solicited local reactogenicity AEs, solicited systemic reactogenicity AEs, UnSoAEs, AESIs, clinical safety laboratory AEs by severity grade and SAEs |
| 100 | SpFN COVID-19 Vaccine | Walter Reed Army Institute of Research | Protein subunit | US | 1 | NCT04784767 | US | 72 | 5 April 2021–30 October 2023 | Participants with local and systemic reactions, incidents of treatment-adverse events as assessed by FDA toxicity grading scale, and participants with humoral immune response |
| 101 | FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research | Inactivated virus | Iran | 1 | IRCT20210206050259N1 | Iran | 135 | N/A | Abnormal vital signs and anaphylactic reactions immediately after vaccination, local and systemic AEs within the first week post-vaccination, abnormal laboratory findings |
| 102 | MV-014-212 | Meissa Vaccines Inc | Live-Attenuated | US | 1 | NCT04798001 | US | 130 | 12 April 2021–31 October 2022 | SoAEs, UnSoAEs, SAEs, MAAEs, and change in serum neutralizing antibody titres against vaccine-encoded SARS-CoV-2 S protein |
| 103 | Koçak-19 Inaktif Adjuvanlı COVID-19 Vaccine | Kocak Farma | Inactivatedviral vecviral virus | Turkey | 1 | NCT04838080 | Turkey | 38 | 19 March 2021–20 October 2021 | AEs, from day 0 until the end of follow up period of 6 months |
| 104 | ABNCoV2 | Radboud University | Virus like particle | The Netherlands | 1 | NCT04839146 | The Netherlands | 42 | 11 March 2021–20 December 2021 | Safety: possibly related Grade 3 AEs and SAEs, Immunogenicity endpoint: concentration of ABNCoV2-specific antibodies |
| 105 | HDT-301 | SENAI CIMATEC | RNA based vaccine | Brazil | 1 | NCT04844268 | N/A | 78 | May 2021–July 2022 | Safety and tolerability of two doses of HDT-1 vaccine (day 1, 29 and 57) |
| 106 | Adjuvanted Inactivated Vaccine | The Scientific and Technological Research Council of Turkey | Inactivated virus | Turkey | 1 | NCT04866069 | Turkey | 50 | 25 April 2021–April 2022 | AAEs, local and systemic SoAEs and UnSoAEs |
| 107 | mRNA-1283 | ModernaTX, Inc | RNA based vaccine | US | 1 | NCT04813796 | US | 125 | 11 March 2021–13 April 2022 | Number of participants with solicited local and systemic reactogenicity Adverse Reactions (ARs), UnSoAEs, and MAAEs, AESIs, and SAEs |
| 108 | Recombinant NDV Vectored Vaccine | Laboratorio Avi-Mex | Inactivated virus | Mexico | 1 | NCT04871737 | Mexico | 90 | 20 May 2021–June 2022 | AEs, pregnancy test, urinalysis, oxygen saturation |
| 109 | mRNACOVID-19 Vaccine | Stemirna Therapeutics Co Ltd. and Shanghai East Hospital | RNA based vaccine | China | 1 | ChiCTR2100045984 | China | 30 | 25 March 2021–25 May 2022 | Safety and immunogenicity |
| 110 | CoVepiT | OSE Immunotherapeutic | Protein subunit | Belgium | 1 | NCT04885361 | Belgium | 48 | 26 May 2021–31 March 2022 | The incidence of solicited local and systemic reactogenicity signs and symptoms, UnSoAEs, SAEs, AESIs, subjects with significantly increased CD8+ T cells responding to SARS-CoV-2 |
| 111 | CoV2-OGEN1 | USSF/Vaxform | Protein subunit | US | 1 | NCT04893512 | N/A | 45 | 7 June 2021–15 September 2022 | Safety evaluation of 2-dose vaccination schedule of orally administered CoV2-OGEN1 by following local and systemic adverse events |
| 112 | LNP-nCOV saRNA-02 Vaccine | MRC/UVRI and LSHTM Uganda Research Unit | RNA based vaccine | Uganda | 1 | NCT04934111 | Uganda | 42 | September 2021–August 2022 | Participant with solicited local injection site and systemic reactions, UnSoAEs, SAEs, titre of neutralizing antibody and IgG |
| 113 | Baiya SARS-CoV-2 Vax 1 Vaccine | Baiya Phytopharm Co Ltd. | Protein subunit | Thailand | 1 | NCT04953078 | N/A | 96 | September 2021–November 2022 | Frequency and grade SoAEs and AEs, change in blood pressure, pulse rate, respiratory rate, and physical condition, safety laboratory value |
| 114 | PIV5-SARS CoV-2 | CyanVac LLC | Viral vector (non-replicating) | US | 1 | NCT04954287 | US | 80 | July 2021–November 2022 | SoAEs, UnSoAEs |
| 115 | 202-CoV | Shanghai Zerun Biotechnology, Walvax Biotechnology | Protein subunit | China | 1 | NCT04982068 | China | 144 | 12 July 2021–October 2022 | SoAEs, UnSoAEs |
| 116 | COVIDITY | Scancell Ltd. | DNA based vaccine | UK | 1 | NCT05047445 | South Africa | 40 | 30 September 2021–June 2022 | Safety and tolerability of COVIDITY as assessed by AEs, vital signs, and physical examination |
| 117 | PIKA-adjuvanted vaccine | Yisheng Biopharma | Protein subunit | Singapore | 1 | ACTRN12621001009808 | Australia, New Zealand | 45 | 24 September 2021–13 June 2022 | To assess the safety and tolerability of the PIKA COVID-19 vaccine by monitoring AEs and clinical laboratory tests |
| 118 | SARS-CoV-2 DNA vaccine | The University of Hong Kong; Immuno Cure 3 Limited | DNA based vaccine | Hong Kong | 1 | NCT05102643 | Hong Kong | 30 | November 2021–December 2022 | Reactogenicity and AEs |
| 119 | Ad5-triCoV/Mac or ChAd-triCoV/Mac | McMaster University | Viral vector (non-replicating) | Canada | 1 | NCT05094609 | Canada | 30 | 9 November 2021–30 June 2023 | Number of participants reporting AEs and severity of AEs following vaccination |
| 120 | T-cell priming specific peptides on a gold nanoparticle | Emergex Vaccines | Protein subunit | Switzerland | 1 | NCT05113862 | Switzerland | 26 | December 2021–November 2022 | Local and systemic SoAEs, UnSoAEs, SAEs, AESIs, SAEs, MAAEs, AESIs. GMT and GMFR of Anti-SAS-CoV-2 RBD IgG and neutralizing anti-SARS-CoV-2 |
| 121 | IN-B009 | HK inno.N Corporation | Protein subunit | South Korea | 1 | NCT05113849 | South Korea | 40 | 16 September 2021–February 2023 | Occurrence of IAR, local and systemic AE, UnSoAE, |
MAAEs: Medically Attended Adverse Events, NOCMCs: New-onset Chronic Medical Conditions, SAEs: Serious adverse events, SCR: Seroconversion Rate, SRR: Seroresponse Rate, SoAEs: The solicited adverse events, UnSoAEs: The unsolicited adverse events, TEAE: Treatment Emergent Adverse Event, IAR: Immediate Adverse Reaction.
Author Contributions
B.E., R.F.N., I.R., and V.K. contributed to writing, editing, and formatting the manuscript. V.K. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Virtual Press Conference on COVID-19. [(accessed on 11 March 2020)]. Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2.
- 3.World Health Organization Novel Coronavirus 2019. [(accessed on 29 November 2021)]. Available online: https://covid19.who.int/
- 4.Sørensen M.D., Sørensen B., Gonzalez-Dosal R., Melchjorsen C.J., Weibel J., Wang J., Jun C.W., Huanming Y., Kristensen P. Severe acute respiratory syndrome (SARS): Development of diagnostics and antivirals. Ann. N. Y. Acad. Sci. 2006;1067:500–505. doi: 10.1196/annals.1354.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., Yang C., Xu X.-f., Xu W., Liu S.-w. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 13.Abbasi-Oshaghi E., Mirzaei F., Farahani F., Khodadadi I., Tayebinia H. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): Laboratory, PCR, and chest CT imaging findings. Int. J. Surg. 2020;79:143–153. doi: 10.1016/j.ijsu.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauring A.S., Hodcroft E.B. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) SARS-CoV-2 Variant Classifications and Definitions. [(accessed on 22 November 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- 16.WHO Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. [(accessed on 29 November 2021)]. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 17.Wink P.L., Zempulski Volpato F.C., Monteiro F.L., Willig J.B., Zavascki A.P., Luís Barth A., Martins A.F. First identification of SARS-CoV-2 Lambda (C.37) variant in Southern Brazil. Infect. Control. Hosp. Epidemiol. 2021;9:1–2. doi: 10.1017/ice.2021.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Tracking SARS-CoV-2 Variants. [(accessed on 22 November 2021)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 19.Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonathan K., Kosinski-Collins M., Sundberg E. Coronavirus Structure, Vaccine and Therapy Development. [(accessed on 6 September 2021)]. Available online: https://web.mit.edu/fnl/volume/324/king_etal.html.
- 23.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet. Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nath A. Long-Haul COVID. Neurology. 2020;95:559–560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- 30.Carfì A., Bernabei R., Landi F., for the Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., Doucet L., Berkani S., Oliosi E., Mallart E., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabri A., Kalra A., Kumar A., Alameh A., Adroja S., Bashir H., Nowacki A.S., Shah R., Khubber S., Kanaa’N A., et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw. Open. 2020;3:e2014780. doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elavarasi A., Prasad M., Seth T., Sahoo R.K., Madan K., Nischal N., Soneja M., Sharma A., Maulik S.K., Shalimar, et al. Chloroquine and Hydroxychloroquine for the Treatment of COVID-19: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2020;35:3308–3314. doi: 10.1007/s11606-020-06146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 doi: 10.1101/2020.07.15.20151852. [DOI] [Google Scholar]
- 38.FDA Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. [(accessed on 22 June 2021)]; Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
- 39.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First COVID-19 Treatment Recommended for EU Authorisation. [(accessed on 22 June 2021)]. Available online: https://www.ema.europa.eu/en/news/first-COVID-19-treatment-recommended-eu-authorisation.
- 41.FDA Approves First Treatment for COVID-19. [(accessed on 22 June 2021)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-COVID-19.
- 42.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NIH Therapeutic Management of Adults With COVID-19. [(accessed on 22 June 2021)]; Available online: https://www.covid19treatmentguidelines.nih.gov/management/therapeutic-management/
- 44.Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. [(accessed on 15 November 2021)]. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/
- 45.First Oral Antiviral for COVID-19, Lagevrio (Molnupiravir), Approved by MHRA. [(accessed on 15 November 2021)]; Available online: https://www.gov.uk/government/news/first-oral-antiviral-for-COVID-19-lagevrio-molnupiravir-approved-by-mhra.
- 46.Bartoli A., Gabrielli F., Alicandro T., Nascimbeni F., Andreone P. COVID-19 treatment options: A difficult journey between failed attempts and experimental drugs. Intern. Emerg. Med. 2021;16:281–308. doi: 10.1007/s11739-020-02569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 48.Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 Epic-Hr Study. [(accessed on 15 November 2021)]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-COVID-19-oral-antiviral-treatment-candidate.
- 49.Kayser V.R.I. A Concise History of Vaccines and Vaccination. Hum. Vaccin. Immunother. 2021. in press . [DOI] [PMC free article] [PubMed]
- 50.Willis N.J. Edward Jenner and the eradication of smallpox. Scott. Med. J. 1997;42:118–121. doi: 10.1177/003693309704200407. [DOI] [PubMed] [Google Scholar]
- 51.Tang D.C., DeVit M., Johnston S.A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 52.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H.J.H.M., Osterhaus A.D.M.E. Risk in Vaccine Research and Development Quantified. PLoS ONE. 2013;8:e57755. doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plotkin S., Robinson J.M., Cunningham G., Iqbal R., Larsen S. The complexity and cost of vaccine manufacturing—An overview. Vaccine. 2017;35:4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Regulatory Approval of Pfizer/BioNTech Vaccine for COVID-19. [(accessed on 17 June 2021)]; Available online: https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-COVID-19.
- 55.FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. [(accessed on 21 June 2021)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-COVID-19-issuing-emergency-use-authorization-first-COVID-19.
- 56.WHO COVID-19 Vaccine Tracker and Landscape. [(accessed on 6 August 2021)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-candidate-vaccines.
- 57.The New York Times Coronavirus Vaccine Tracker. [(accessed on 6 August 2021)]. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 58.Sabbaghi A., Miri S.M., Keshavarz M., Zargar M., Ghaemi A. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 2019;29:e2074. doi: 10.1002/rmv.2074. [DOI] [PubMed] [Google Scholar]
- 59.Sanders B., Koldijk M., Schuitemaker H. Inactivated Viral Vaccines. In: Nunnally B.K., Turula V.E., Sitrin R.D., editors. Vaccine Analysis: Strategies, Principles, and Control. Springer; Berlin/Heidelberg, Germany: 2015. pp. 45–80. [Google Scholar]
- 60.Ramesh N. Types of Vaccines Infographics. [(accessed on 6 September 2021)]. Available online: https://sites.bu.edu/covid-corps/projects/science-communication/types-of-vaccines-infographics/
- 61.WHO Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. [(accessed on 28 June 2021)]. Available online: https://www.who.int/teams/regulation-prequalification/eul/COVID-19.
- 62.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J., et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M.-X., Zhang T.-T., Shi G.-F., Cheng F.-M., Zheng Y.-M., Tung T.-H., Chen H.-X. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev. Vaccines. 2021;20:891–898. doi: 10.1080/14760584.2021.1925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinovac Responses to the SAGE Working Group on COVID-19 Vaccines. [(accessed on 21 April 2021)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1.
- 65.Hansson M., Nygren P.A., Ståhl S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000;32:95–107. doi: 10.1042/BA20000034. [DOI] [PubMed] [Google Scholar]
- 66.Christensen D. Vaccine adjuvants: Why and how. Hum. Vaccines Immunother. 2016;12:2709–2711. doi: 10.1080/21645515.2016.1219003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oxford Types of Vaccine. [(accessed on 12 August 2021)]. Available online: https://vk.ovg.ox.ac.uk/vk/types-of-vaccine.
- 68.NIH Vaccine Types. [(accessed on 12 August 2021)]; Available online: https://www.niaid.nih.gov/research/vaccine-types.
- 69.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ura T., Okuda K., Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines. 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson D.A., Symons R.H., Berg P. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1972;69:2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vrba S.M., Kirk N.M., Brisse M.E., Liang Y., Ly H. Development and Applications of Viral Vectored Vaccines to Combat Zoonotic and Emerging Public Health Threats. Vaccines. 2020;8:680. doi: 10.3390/vaccines8040680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., Lidegaard Ø., Tapia G., Gulseth H.L., Ruiz P.L.-D., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wise J. COVID-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 76.Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., Waraich K., Vant J., Wilson E., Truong C.D., et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021;7:eabl8213. doi: 10.1126/sciadv.abl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogel F.R., Sarver N. Nucleic acid vaccines. Clin. Microbiol. Rev. 1995;8:406–410. doi: 10.1128/CMR.8.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodríguez-Gascón A., del Pozo-Rodríguez A., Solinís M.Á. Development of nucleic acid vaccines: Use of self-amplifying RNA in lipid nanoparticles. Int. J. Nanomed. 2014;9:1833–1843. doi: 10.2147/IJN.S39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silveira M.M., Moreira G.M.S.G., Mendonça M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021;267:118919. doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines. 2019;7:37. doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.FDA U.S. Pfizer-BioNTech COVID-19 Vaccine EUA Letter of Authorization Reissued. [(accessed on 5 October 2021)]; Available online: https://www.fda.gov/media/144412/download.
- 84.FDA U.S. Pfizer-BioNTech COVID-19 Vaccine. [(accessed on 18 May 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/pfizer-biontech-COVID-19-vaccine.
- 85.Holm M.R., Poland G.A. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine. 2021;39:457–459. doi: 10.1016/j.vaccine.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kollewe J. COVID-19 Vaccines: The Contracts, Prices and Profits. [(accessed on 12 August 2021)]. Available online: https://www.theguardian.com/world/2021/aug/11/COVID-19-vaccines-the-contracts-prices-and-profits.
- 87.FDA U.S. Moderna COVID-19 Vaccine EUA Letter of Authorization. [(accessed on 18 May 2021)]; Available online: https://www.fda.gov/media/144636/download.
- 88.FDA U.S. Moderna COVID-19 Vaccine. [(accessed on 18 May 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/moderna-COVID-19-vaccine.
- 89.Santos A.F., Gaspar P.D., de Souza H.J.L. Refrigeration of COVID-19 Vaccines: Ideal Storage Characteristics, Energy Efficiency and Environmental Impacts of Various Vaccine Options. Energies. 2021;14:1849. doi: 10.3390/en14071849. [DOI] [Google Scholar]
- 90.Vogel G., Kupferschmidt K. Side effect worry grows for AstraZeneca vaccine. Science. 2021;372:14. doi: 10.1126/science.372.6537.14. [DOI] [PubMed] [Google Scholar]
- 91.Torjesen I. COVID-19: AstraZeneca vaccine is approved in EU with no upper age limit. BMJ. 2021;372:n295. doi: 10.1136/bmj.n295. [DOI] [PubMed] [Google Scholar]
- 92.Dyer O. COVID-19: Countries are learning what others paid for vaccines. BMJ. 2021;372:n281. doi: 10.1136/bmj.n281. [DOI] [PubMed] [Google Scholar]
- 93.McDougallScientific 2021 COVID-19 Vaccines: Summary, Updates and Status. [(accessed on 12 August 2021)]. Available online: https://www.mcdougallscientific.com/wp-content/uploads/2021-COVID-19-Vaccine-and-Clicnial-Trials-Update.pdf.
- 94.Farooq U., Shahzad A. Pakistan to Start Private Imports of CanSino COVID-19 Vaccine for Sale. [(accessed on 12 August 2021)]. Available online: https://www.reuters.com/article/us-health-coronavirus-pakistan-vaccine-idUSKBN2BE0N3.
- 95.WHO The J&J COVID-19 Vaccine: What You Need to Know. [(accessed on 18 May 2021)]. Available online: https://www.who.int/news-room/feature-stories/detail/the-j-j-COVID-19-vaccine-what-you-need-to-know.
- 96.Trefis Team What’s Happening With Johnson & Johnson’s COVID-19 Vaccine? [(accessed on 12 August 2021)]. Available online: https://www.forbes.com/sites/greatspeculations/2021/06/09/whats-happening-with-johnson--johnsons-COVID-19-vaccine/?sh=53842c5f1aed.
- 97.WHO The Sinopharm COVID-19 Vaccine: What You Need to Know. [(accessed on 18 May 2021)]. Available online: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-COVID-19-vaccine-what-you-need-to-know#:~:text=A%20large%20multi%2Dcountry%20Phase,efficacy%20against%20hospitalization%20was%2079%25.
- 98.WHO Evidence Assessment: Sinopharm/BBIBP COVID-19 Vaccine. [(accessed on 18 May 2021)]. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf.
- 99.WHO COVID-19 Vaccine (Vero Cell), Inactivated (Sinopharm) [(accessed on 12 August 2021)]. Available online: https://www.who.int/docs/default-source/coronaviruse/v.3_21195_sinopharm-vaccine-explainer-24.pdf?sfvrsn=e7507eb4_19&download=true.
- 100.Reuters Hungary Publishes Chinese, Russian Vaccine Contracts Amid COVID-19 Surge. [(accessed on 12 August 2021)]. Available online: https://www.reuters.com/article/us-health-coronavirus-hungary-idUSKBN2B30YP.
- 101.Reuters Sinovac Coronavirus Vaccine Offered by Chinese City for Emergency Use Costs $60. [(accessed on 12 August 2021)]. Available online: https://www.reuters.com/article/us-health-coronavirus-china-vaccine-idUSKBN2710UQ.
- 102.Sputnik V. The Cost of One Dose of the Sputnik V Vaccine Will be Less Than $10 for International Market. [(accessed on 11 September 2021)]. Available online: https://sputnikvaccine.com/newsroom/pressreleases/the-cost-of-one-dose-will-be-less-than-10-for-international-markets/
- 103.Zimmer C., Corum J., Wee S.-L. Coronavirus Vaccine Tracker. [(accessed on 15 November 2021)]. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html?
- 104.PrecisionVax EpiVacCorona Vaccine. [(accessed on 11 September 2021)]. Available online: https://www.precisionvaccinations.com/vaccines/epivaccorona-vaccine.
- 105.Kapur M. For All Its “Made in India” Pitch, Covaxin Is the Most Expensive COVID-19 Vaccine in India. [(accessed on 11 September 2021)]. Available online: https://qz.com/india/2019375/why-does-covaxin-cost-more-than-covishield-and-sputnik-in-india/
- 106.Demesinova A. Kazakhstan Vaccine against CVI to be Rolled Out in Late April. [(accessed on 11 September 2021)]. Available online: https://www.kazpravda.kz/en/news/society/kazakhstan-vaccine-against-cvi-to-be-rolled-out-in-late-april.
- 107.He Q., Mao Q., An C., Zhang J., Gao F., Bian L., Li C., Liang Z., Xu M., Wang J. Heterologous prime-boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021;10:629–637. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callaway E. Mix-And-Match COVID Vaccines Trigger Potent Immune Response. Nature. 2021;593:491. doi: 10.1038/d41586-021-01359-3. [DOI] [PubMed] [Google Scholar]
- 109.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hillus D., Schwarz T., Tober-Lau P., Hastor H., Thibeault C., Kasper S., Helbig E.T., Lippert L.J., Tscheak P., Schmidt M.L., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: A prospective cohort study. medRxiv. 2021 doi: 10.1101/2021.05.19.21257334. 2021.2005.2019.21257334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., Abu-Omar A., Schneitler S., Becker S.L., Gärtner B.C., et al. Immunogenicity and reactogenicity of a heterologous COVID-19 prime-boost vaccination compared with homologous vaccine regimens. medRxiv. 2021 doi: 10.1101/2021.06.13.21258859. medRxiv: 2021.06.13.21258859. [DOI] [Google Scholar]
- 112.CDC Delta Variant: What We Know About the Science? [(accessed on 15 August 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html.
- 113.Mahase E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513. doi: 10.1136/bmj.n1513. [DOI] [PubMed] [Google Scholar]
- 114.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stephenson J. COVID-19 Vaccinations in Nursing Home Residents and Staff Give Robust Protection, Though Breakthrough Infections Still Possible. JAMA Health Forum. 2021;2:e211195. doi: 10.1001/jamahealthforum.2021.1195. [DOI] [PubMed] [Google Scholar]
- 117.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., Bula M., Cathie K., Chatterjee K., Dodd K., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Factbox: Countries Weigh Need for Booster COVID-19 Shots. [(accessed on 15 August 2021)]. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/countries-weigh-need-booster-COVID-19-shots-2021-08-05/
- 121.Moscow Begins Booster Vaccine Campaign as Cases Surge. [(accessed on 15 August 2021)]. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/moscow-begins-booster-vaccine-campaign-russias-COVID-19-cases-surge-2021-07-01/
- 122.Turkey Offers COVID-19 Booster Shot After Early Use of Chinese Vaccine. [(accessed on 15 August 2021)]. Available online: https://www.wsj.com/articles/turkey-offers-COVID-19-booster-shot-after-using-chinese-vaccine-in-early-drive-11625144544.
- 123.FDA Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. [(accessed on 15 August 2021)]; Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
- 124.WHO Interim Statement on COVID-19 Vaccine Booster Doses. [(accessed on 16 August 2021)]. Available online: https://www.who.int/news/item/10-08-2021-interim-statement-on-COVID-19-vaccine-booster-doses.
- 125.Tajikistan Declares Mandatory COVID-19 Vaccination. [(accessed on 15 August 2021)]. Available online: https://tass.com/world/1310239.
- 126.Dyer O. COVID-19: Turkmenistan becomes first country to make vaccination mandatory for all adults. BMJ. 2021;374:n1766. doi: 10.1136/bmj.n1766. [DOI] [PubMed] [Google Scholar]
- 127.Saudi Arabia’s Vaccination Rate Spikes as Deadline Approaches. [(accessed on 15 August 2021)]. Available online: https://www.bloomberg.com/news/articles/2021-07-15/saudi-arabia-s-vaccination-rate-spikes-as-deadline-approaches.
- 128.Factbox: Countries Making COVID-19 Vaccines Mandatory. [(accessed on 15 August 2021)]. Available online: https://www.reuters.com/world/countries-make-COVID-19-vaccines-mandatory-2021-07-13/
- 129.California and New York City to Mandate Vaccine for Government Workers. [(accessed on 15 August 2021)]. Available online: https://www.theguardian.com/us-news/2021/jul/26/covid-california-new-york-city-department-of-veterans-affairs-vaccine-mandate.
- 130.Coronavirus: Thousands Protest against Restrictions across Europe. [(accessed on 15 August 2021)]. Available online: https://www.dw.com/en/coronavirus-thousands-protest-against-restrictions-across-europe/a-58627841.
- 131.COVAX—Working for Global Equitable Access to COVID-19 Vaccines. [(accessed on 24 August 2021)]. Available online: https://www.who.int/initiatives/act-accelerator/covax.
- 132.COVAX Vaccine Roll-Out. [(accessed on 24 August 2021)]. Available online: https://www.gavi.org/covax-vaccine-roll-out#country-updates.
- 133.CEPI COVAX: CEPI’s Response to COVID-19. [(accessed on 24 August 2021)]. Available online: https://cepi.net/covax/
- 134.Director-General’s Opening Remarks at the Media Briefing on COVID-19. [(accessed on 4 September 2021)]. Available online: https://www.who.int/director-general/speeches/detail/director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19-9-april-2021.
- 135.MacDonald N.E., Sage Working Group on Vaccine Hesitancy Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 136.Durbach N. ‘They Might As Well Brand Us’: Working-Class Resistance to Compulsory Vaccination in Victorian England. Soc. Hist. Med. 2000;13:45–63. doi: 10.1093/shm/13.1.45. [DOI] [PubMed] [Google Scholar]
- 137.Patel M.K., Orenstein W.A. Classification of global measles cases in 2013–17 as due to policy or vaccination failure: A retrospective review of global surveillance data. Lancet Glob. Health. 2019;7:e313–e320. doi: 10.1016/S2214-109X(18)30492-3. [DOI] [PubMed] [Google Scholar]
- 138.de Figueiredo A., Simas C., Karafillakis E., Paterson P., Larson H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet. 2020;396:898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith T.C. Vaccine Rejection and Hesitancy: A Review and Call to Action. Open Forum Infect. Dis. 2017;4:ofx146. doi: 10.1093/ofid/ofx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zucker J.R., Rosen J.B., Iwamoto M., Arciuolo R.J., Langdon-Embry M., Vora N.M., Rakeman J.L., Isaac B.M., Jean A., Asfaw M., et al. Consequences of Undervaccination—Measles Outbreak, New York City, 2018–2019. N. Engl. J. Med. 2020;382:1009–1017. doi: 10.1056/NEJMoa1912514. [DOI] [PubMed] [Google Scholar]
- 141.Wakefield A.J., Murch S.H., Anthony A., Linnell J., Casson D.M., Malik M., Berelowitz M., Dhillon A.P., Thomson M.A., Harvey P., et al. RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/S0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 142.Taylor B., Miller E., Farrington C., Petropoulos M.-C., Favot-Mayaud I., Li J., Waight P.A. Autism and measles, mumps, and rubella vaccine: No epidemiological evidence for a causal association. Lancet. 1999;353:2026–2029. doi: 10.1016/S0140-6736(99)01239-8. [DOI] [PubMed] [Google Scholar]
- 143.Kennedy J. Populist politics and vaccine hesitancy in Western Europe: An analysis of national-level data. Eur. J. Public Health. 2019;29:512–516. doi: 10.1093/eurpub/ckz004. [DOI] [PubMed] [Google Scholar]
- 144.Germani F., Biller-Andorno N. The anti-vaccination infodemic on social media: A behavioral analysis. PLoS ONE. 2021;16:e0247642. doi: 10.1371/journal.pone.0247642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Zhang E.J., Chughtai A.A., Heywood A., MacIntyre C.R. Influence of political and medical leaders on parental perception of vaccination: A cross-sectional survey in Australia. BMJ Open. 2019;9:e025866. doi: 10.1136/bmjopen-2018-025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kata A. Anti-vaccine activists, Web 2.0, and the postmodern paradigm—An overview of tactics and tropes used online by the anti-vaccination movement. Vaccine. 2012;30:3778–3789. doi: 10.1016/j.vaccine.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 147.WHO Ten Threats to Global Health in 2019. [(accessed on 18 July 2021)]. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 148.Sarasty O., Carpio C.E., Hudson D., Guerrero-Ochoa P.A., Borja I. The demand for a COVID-19 vaccine in Ecuador. Vaccine. 2020;38:8090–8098. doi: 10.1016/j.vaccine.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., Yaseen A., Ababneh N.A., Bakri F.G., Mahafzah A. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines. 2021;9:42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., Srouji S., Sela E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robinson E., Jones A., Lesser I., Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024–2034. doi: 10.1016/j.vaccine.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Daly M., Robinson E. Willingness to vaccinate against COVID-19 in the US: Longitudinal evidence from a nationally representative sample of adults from April–October 2020. medRxiv. 2020 October 20; doi: 10.1101/2020.11.27.20239970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roozenbeek J., Schneider C.R., Dryhurst S., Kerr J., Freeman A.L.J., Recchia G., van der Bles A.M., van der Linden S. Susceptibility to misinformation about COVID-19 around the world. R. Soc. Open Sci. 2020;7:201199. doi: 10.1098/rsos.201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yasmin F., Najeeb H., Moeed A., Naeem U., Asghar M.S., Chughtai N.U., Yousaf Z., Seboka B.T., Ullah I., Lin C.Y., et al. COVID-19 Vaccine Hesitancy in the United States: A Systematic Review. Front. Public Health. 2021;9:770985. doi: 10.3389/fpubh.2021.770985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.WHO Strategic Advisory Group of Experts on Immunization (SAGE) COVID-19 Vaccines Technical Documents. [(accessed on 22 November 2021)]. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/COVID-19-materials.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.