Abstract
The presence of cryptic fliC alleles in the genomes of 120 strains representative of the four Shigella species was investigated. One fragment was obtained by PCR amplification of fliC, with a size varying from 1.2 to 3.2 kbp, depending on the species or serotype. After digestion with endonuclease HhaI, the number of fragments in patterns varied from three to nine, with sizes of between 115 and 1,020 bp. Patterns sharing most of their bands were grouped to constitute an F type. A total of 17 different F types were obtained from all strains included in this study. A unique pattern was observed for each the following serotypes: Shigella dysenteriae 1, 2, 8, and 10 and S. boydii 7, 13, 15, 16, and 17. On the contrary, S. dysenteriae serotype 13 and S. sonnei biotype e were each subdivided into two different F types. S. flexneri serotypes 3a and X could be distinguished from the cluster containing S. flexneri serotypes 1 to 5 and Y. S. flexneri serotype 6 clustered with S. boydii serotypes 1, 2, 3, 4, 6, 8, 10, 11, 14, and 18 and S. dysenteriae serotypes 4, 5, 6, 7, 9, 11, and 12. Two other clusters were outlined: one comprising S. dysenteriae serotypes 3, 12, 13 (strain CDC598-77), 14, and 15 and the other one joining S. boydii serotypes 5 and 9. None of the 17 fliC patterns was found in the fliC HhaI pattern database previously described for Escherichia coli. Overall, this work supports the hypothesis that Shigella evolved from different ancestral strains of E. coli. Moreover, the method outlined here is a promising tool for the identification of some clinically important Shigella strains as well as for confirmation of atypical isolates as Shigella spp.
Shigella species and serotypes, except for Shigella boydii serotype 13, show more than 73% DNA relatedness to Escherichia coli K-12 (4). Because of this close genetic relationship, Shigella strains can be considered E. coli clones which are much less biochemically active, are host restricted, and carry a plasmid encoding invasiveness. However, for historical reasons, nonmotile, anaerogenic Enterobacteriaceae causing dysentery are classified in the genus Shigella, which comprises four species: S. dysenteriae, S. flexneri, S. boydii, and S. sonnei (11).
Since Shigella strains produce neither flagella nor capsular antigens, their antigenic characterization relies exclusively on the properties of their somatic antigens (O antigens) (3, 17). S. dysenteriae, S. boydii, and S. flexneri have been subdivided into 15, 18, and 6 serotypes, respectively. S. flexneri contains two additional variants (X and Y), and serotypes 1 to 5 are further subdivided into subserotypes. Only one serotype has been described for S. sonnei, but strains of this species can be divided into five biotypes.
In contrast to Shigella strains, E. coli strains are often motile and produce a structural complex flagellum consisting of three main structural regions: the basal body, the hook, and the filament (22, 23). The flagellar filament is composed of many thousands of copies of a single protein subunit, flagellin (18), which carries antigenic determinants of the H (flagellar) antigen. The variability of the H antigen is associated with the flagellin amino acid sequence and its structural gene (fliC). The N- and C-terminal portions of flagellin are important for the structure of flagella and are highly conserved (25). The middle region can be quite variable and contains portions of the protein that are surface exposed and H type specific.
Several sequences of the gene encoding flagellin (fliC) are now available (33), allowing restriction of amplified fliC genes to be used for the identification of H types in different bacteria (1, 2, 9, 20, 36, 37).
Recently, Machado et al. (21) demonstrated that HhaI restriction of the amplified fliC gene could be used for a flagellar identification system fitting all E. coli serovars. The correlation between F types (fliC-RFLP types) and H types allowed the deduction of H types from F types. Furthermore, F types of nonmotile isolates could be identified. Actually, nonmotile strains generally possess the structural gene but are unable to build up a functional flagellum (23). In another study, it was found that all E. coli O157:NM strains producing Shiga toxin carried the gene encoding H7 (12, 13).
Although Shigella has long been described as a nonflagellated organism, intact, but cryptic, flagellin genes have been detected for S. flexneri and S. sonnei strains (35). Furthermore, the production of flagella by prototypic strains of all four Shigella species has been demonstrated by electron microscopy (14).
The purposes of this work were to (i) detect the cryptic flagellar gene in all Shigella serotypes, (ii) report on the distribution of flagellar restriction patterns among serotypes, and (iii) compare these patterns with those of E. coli serotypes.
MATERIALS AND METHODS
Bacterial strains.
A total of 120 reference, collection, or clinical strains representing all recognized Shigella serotypes and biotypes were included in this study (Table 1).
TABLE 1.
List of strains and their F types
| F type | Size of the amplified fragment (kbp) | Species and serotype or biotype | Straina | ||
|---|---|---|---|---|---|
| P1 | 2.3 | Shigella dysenteriae 1 | NCDC1007-71 | ||
| Shigella dysenteriae 1 | UE 31127 | ||||
| Shigella dysenteriae 1 | 3116-95 EK1 | ||||
| Shigella dysenteriae 1 | 3125-5 EK1 | ||||
| Shigella dysenteriae 1 | 3090-2 EK1 | ||||
| Shigella dysenteriae 1 | 3095-10 EK1 | ||||
| P2 | 2.2 | Shigella dysenteriae 2 | NCDC4106-65 | ||
| Shigella dysenteriae 2 | UE 9-88 | ||||
| P3 | 1.6 | Shigella dysenteriae 3 | NCDC3690-75 | ||
| Shigella dysenteriae 3 | UE 13-88 | ||||
| Shigella dysenteriae 3 | UE 30-86 | ||||
| Shigella dysenteriae 3 | UE 60-85 | ||||
| Shigella dysenteriae 3 | Polska 38-4064 | ||||
| Shigella dysenteriae 12 | UE 3-89 | ||||
| Shigella dysenteriae 12 | UE 40-85 | ||||
| Shigella dysenteriae 13 | CDC598-77 | ||||
| Shigella dysenteriae 14 | CDC576-83 (E22383) | ||||
| Shigella dysenteriae 15 | CDCE23507 | ||||
| P4 | 1.6 | Shigella dysenteriae 4 | CIP 52-30 | ||
| Shigella dysenteriae 4 | CIP 67-59 | ||||
| Shigella dysenteriae 4 | UE 21-87 | ||||
| Shigella dysenteriae 4 | UE 29-90 | ||||
| Shigella dysenteriae 4 | UE 40-89 | ||||
| Shigella dysenteriae 5 | NCDC853-58 | ||||
| Shigella dysenteriae 5 | CIP 57-42 | ||||
| Shigella dysenteriae 5 | CIP 58-26 | ||||
| Shigella dysenteriae 6 | CIP 52-32 | ||||
| Shigella dysenteriae 6 | UE 16-89 | ||||
| Shigella dysenteriae 7 | NCDC4788-55 | ||||
| Shigella dysenteriae 7 | CIP 67-60 | ||||
| Shigella dysenteriae 7 | CIP 52-123 | ||||
| Shigella dysenteriae 7 | UE 6-87 | ||||
| Shigella dysenteriae 9 | NCDC2860-74 | ||||
| Shigella dysenteriae 11 | NCDC3873-50 | ||||
| Shigella dysenteriae 12 | NCDC3341-55 | ||||
| Shigella boydii 1 | NCDC6310-65 | ||||
| Shigella boydii 1 | UE 47-90 | ||||
| Shigella boydii 1 | UE 52-89 | ||||
| Shigella boydii 2 | NCDC2854-67 | ||||
| Shigella boydii 2 | UE 2-87 | ||||
| Shigella boydii 2 | UE 51-89 | ||||
| Shigella boydii 3 | NCDC67 | ||||
| Shigella boydii 3 | CIP 52-50 | ||||
| Shigella boydii 3 | UE 13-87 | ||||
| Shigella boydii 4 | NCDC871-74 | ||||
| Shigella boydii 4 | UE 42-89 | ||||
| Shigella boydii 6 | NCDC3467-56 | ||||
| Shigella boydii 6 | UE 2162 (Ljublian) | ||||
| Shigella boydii 8 | NCDC3073-50 | ||||
| Shigella boydii 8 | UE 14-88 | ||||
| Shigella boydii 10 | NCDC05-74 | ||||
| Shigella boydii 10 | UE 41-89 | ||||
| Shigella boydii 11 | UE 24-90 | ||||
| Shigella boydii 14 | NCDC2770-51 | ||||
| Shigella boydii 14 | UE 18-86 | ||||
| Shigella boydii 14 | UE 44-89 | ||||
| Shigella boydii 18 | Ewing 10163 | ||||
| Shigella boydii 18 | UE 20-87 | ||||
| Shigella boydii 18 | UE 42-90 | ||||
| Shigella boydii 18 | UE 98-8059 | ||||
| Shigella flexneri 6 | NCDC2924-71 | ||||
| P5 | 1.5 | Shigella dysenteriae 8 | NCDC599-52 | ||
| Shigella dysenteriae 8 | CIP 53-134 | ||||
| Shigella dysenteriae 8 | UE 9-86 | ||||
| P6 | 1.6 | Shigella dysenteriae 10 | NCDC2050-52 | ||
| Shigella dysenteriae 10 | CIP 58-27 | ||||
| Shigella dysenteriae 10 | CIP 58-28 | ||||
| P7 | 2.9 | Shigella dysenteriae 13 | CDC2489-78 | ||
| P8 | 1.6 | Shigella boydii 5 | NCDC5393-79 | ||
| Shigella boydii 5 | UE 88-10 | ||||
| Shigella boydii 9 | NCDC73 | ||||
| Shigella boydii 9 | UE 17-87 | ||||
| P9 | 2.5 | Shigella boydii 7 | NCDC2954-72 | ||
| Shigella boydii 7 | UE 52-54 | ||||
| P10 | 1.6 | Shigella boydii 12 | UE 38-87 | ||
| Shigella boydii 12 | UE 98-0297 | ||||
| Shigella flexneri 1 | NCDC1921-71 | ||||
| Shigella flexneri 1 | CIP 52-37 | ||||
| Shigella flexneri 1a | UE London Sc529 | ||||
| Shigella flexneri 1b | NCDC4173-66 | ||||
| Shigella flexneri 2 | UE 140-80 | ||||
| Shigella flexneri 2 | UE 324-89 | ||||
| Shigella flexneri 2a | NCDC2747-71 | ||||
| Shigella flexneri 2b | NCDC97-68 | ||||
| Shigella flexneri 3 | UE 137-80 | ||||
| Shigella flexneri 3b | NCDC2146 | ||||
| Shigella flexneri 3c | NCDC2479 | ||||
| Shigella flexneri 4 | UE 89-80 | ||||
| Shigella flexneri 4 | UE 303-89 | ||||
| Shigella flexneri 4a | NCDC6603-63 | ||||
| Shigella flexneri 4b | NCDC1242 | ||||
| Shigella flexneri 5 | NCDC6154-61 | ||||
| Shigella flexneri 5a | NCDC1170-74 | ||||
| Shigella flexneri 5b | UE Sc541 | ||||
| Shigella flexneri Y | NTCC 4839 | ||||
| P11 | 1.2 | Shigella boydii 13 | NCDC1610-55 | ||
| Shigella boydii 13 | UE London SL624 | ||||
| P12 | 1.9 | Shigella boydii 15 | NCDC965-58 | ||
| Shigella boydii 15 | CIP 58-19 | ||||
| Shigella boydii 15 | UE Dakar80 | ||||
| P13 | 3.2 | Shigella boydii 16 | NCDC3610-54 | ||
| P14 | 3.2 | Shigella boydii 17 | Ewing 3615-53 | ||
| P15 | 1.6 | Shigella flexneri 3a | NCDC2783-71 | ||
| Shigella flexneri X | UE London 667 | ||||
| P16 | 1.5 | Shigella sonnei a | UE 6-86 | ||
| Shigella sonnei a | UE 11359 | ||||
| Shigella sonnei a | UE 11362 | ||||
| Shigella sonnei a | UE 11363 | ||||
| Shigella sonnei a | UE 98-8725 | ||||
| Shigella sonnei d | CIP 66-4 | ||||
| Shigella sonnei d | UE 60-85 | ||||
| Shigella sonnei e | UE 238-87 | ||||
| Shigella sonnei f | UE 68-89 | ||||
| Shigella sonnei f | UE 155-87 | ||||
| Shigella sonnei g | UE 23-88 | ||||
| Shigella sonnei g | UE 250-88 | ||||
| Shigella sonnei g | UE 285-89 | ||||
| P17 | 1.7 | Shigella sonnei e | UE 55-87 | ||
| Shigella sonnei e | UE 94-2558 | ||||
| NAb | —c | Shigella boydii 12 | NCDC266-59 |
NCDC, National Centers for Disease Control, Atlanta, Ga.; CIP, Collection de l'Institut Pasteur, Institut Pasteur, Paris, France; UE, Unité des Entérobactéries, Institut Pasteur.
NA, not applicable.
—, PCR negative.
Amplification of the fliC gene and restriction of PCR products.
Cell lysis and DNA extraction were done as previously described (16). The amplification of flagellin genes by PCR, digestion of the amplified product with endonuclease HhaI, and electrophoresis for fragment separation were performed following the protocol of Machado et al. (21). Three DNA fragments (101, 210, and 701 bp) obtained by HhaI restriction of the amplified fliC gene from E. coli O4:H5:K3 (strain U4/41) were added to all gel lanes and used as internal fragment size standards (21). Gels were stained with ethidium bromide, and images of band patterns were electronically captured using a charge-coupled device video camera interfaced to a microcomputer (Genomic, Collonges-sous-Saleve, France). Tagged image file format (TIFF) images were transferred to a Macintosh computer (Apple Computers, Cupertino, Calif.) for further analysis.
DNA fragment size determination.
The Taxotron package (Taxolab; Institut Pasteur, Paris, France) was used for searching lanes and bands in the TIFF images and for interpretation of patterns (6).
The algorithm of Schaffer and Sederoff (32) was used to derive an experimental function relating molecular size to electrophoretic migration distances of the standard fragments and to interpolate the sizes of the other restriction fragments.
In pattern comparisons, the percentage of tolerated variation (allowed error) was set to 3.5% for fragment size, indicating that two fragments were considered identical when their sizes did not differ by more than this percentage. Fragments of less than 100 bp were discarded, since error was larger in this range.
Comparison of Shigella restriction patterns against a previously published E. coli database.
The HhaI restriction patterns of fliC (F types) obtained for Shigella in this study were compared with the 62 previously published patterns for E. coli (21).
RESULTS
One fragment was obtained by PCR amplification of fliC from each strain tested, except for the reference strain S. boydii serotype 12 NCDC266-59, which failed to give a PCR product. The size of the amplified fliC fragment varied from 1.2 to 3.2 kbp (Table 1).
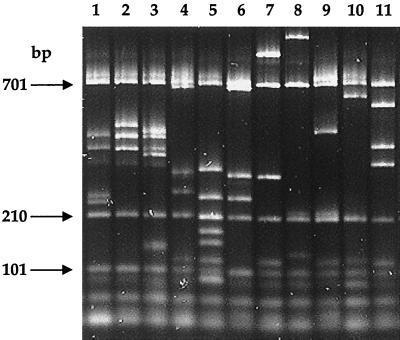
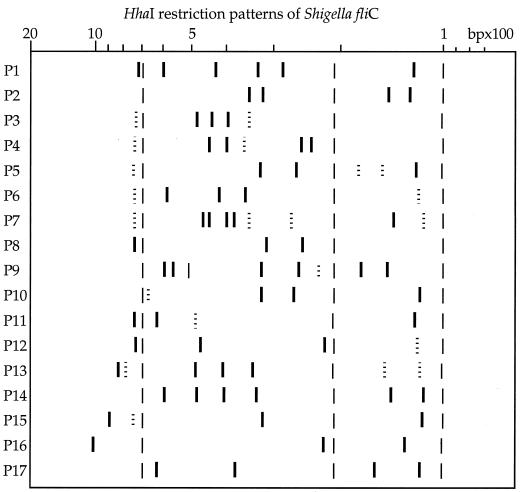
The number of bands in patterns varied from three to nine, with sizes of between 115 and 1,020 bp (Fig. 1 and 2). Patterns sharing most of their bands were grouped to constitute an F type. Seventeen different F types were obtained from all strains. Some F types contained variable bands that were not always present, depending on the strain (bands represented as dotted lines in Fig. 2). Common bands in each F type (thick lines in Fig. 2) allowed F type identification.
FIG. 1.
Restriction length polymorphisms of the Shigella fliC gene after HhaI digestion. Lane 1, F type P4; lane 2, F type P3; lane 3, F type P7; lane 4, F type P10; lane 5, F type P5; lane 6, F type P8; lane 7, F type P15; lane 8, F type P16; lane 9, F type P12; lane 10, F type P11; lane 11, F type P6. Arrows indicate internal marker fragments.
FIG. 2.
Schematic representation showing the different Shigella F types. Dotted lines indicate variable bands that were not always present, depending on the strain. Thin lines indicate internal marker bands (101, 210, and 701 bp).
A unique F type was observed for each of the following serotypes: S. dysenteriae 1, 2, 8, and 10 and S. boydii 7, 13, 15, 16, and 17. On the contrary, S. dysenteriae serotype 13 and S. sonnei biotype e were each subdivided into two different F types. S. flexneri serotypes 3a and X could be distinguished from the cluster containing S. flexneri serotypes 1 to 5 and Y. S. flexneri serotype 6 clustered with S. boydii serotypes 1, 2, 3, 4, 6, 8, 10, 11, 14, and 18 and S. dysenteriae serotypes 4, 5, 6, 7, 9, 11, and 12. Two other clusters were outlined: one comprising S. dysenteriae serotypes 3, 12, 13 (strain CDC598-77), 14, and 15 and the other one joining S. boydii serotypes 5 and 9 (Table 1).
None of the patterns in the present study had been described in the previously published database containing 62 F types of E. coli (21).
DISCUSSION
The notion that flagellar antigens could be an indicator of ancient evolutionary divergences was first suggested by O/rskov et al. (27) to explain the two H types detected among strains of nine enteropathogenic serotypes of E. coli from different origins. In the present study, cryptic fliC was found to be highly conserved among Shigella species, revealing the clonal structure of this genus. Shigella clusters with the same F types are described here, and these data are in agreement with the results of previous studies.
A previous analysis of esterase electrophoretic polymorphisms distinguished five clusters among Shigella serotypes: (i) S. dysenteriae serotype 1, (ii) S. flexneri serotypes 1 to 5, (iii) S. flexneri serotype 6 and S. boydii serotypes 2 and 4, (iv) S. sonnei, and (v) S. boydii serotype 13; these clusters were closely related to those of E. coli (15). These data are in accordance with the results of the present study, except for the close relationship of these clusters to those of E. coli, which was not observed with the method used here.
Clusters of Shigella have been distinguished by ribotyping. In an earlier study, Rolland et al. (31), using a ribotyping system based on restriction with two endonucleases (EcoRI and HindIII), showed that S. sonnei and S. flexneri were easily distinguishable from S. boydii and S. dysenteriae, whereas distinction between S. boydii and S. dysenteriae was less clear. Strains of S. boydii serotype 13, which belong to a discrete DNA hybridization group (5), were clearly distinguished from the other Shigella strains. Rolland et al. (31) could not demonstrate the close taxonomic relationship between S. flexneri serotype 6 strains and S. boydii strains, but this association was proven on the basis of antigenic, chemical, and genetic data (10, 29, 30, 34). Another ribotyping system based on restriction with endonuclease MluI was proposed (8). Results were correlated to serotyping results and, in many cases, the serotypes of clinical strains could be predicted from their ribotypes. Again, some clusters of serotypes were observed within each species. Moreover, S. flexneri serotype 3 and S. boydii serotype 12 had the same ribotype.
Serotyping (28), biotyping (24), and isoenzyme analysis (26) suggested that S. sonnei is genetically homogeneous. However, distinct clones within S. sonnei have been revealed by more discriminating molecular biology techniques (19, 31). Strains UE 94-2558 and UE 55-87 of S. sonnei biotype e had the same MluI ribotype (8). That pattern did not correspond to the one expected for S. sonnei. These two strains were also determined to be different from other S. sonnei strains by MboII restriction of the amplified chromosomal region coding for the enzymes responsible for O-antigen synthesis (rfb-RFLP technique) (7).
The results of this work support the hypothesis that there was no single primordial Shigella ancestral strain (15). Groups of Shigella serotypes may represent different lineages.
However, some clinically important serotypes had unique F types (Table 1), e.g., S. dysenteriae serotype 1. In practical terms, analysis of the HhaI restriction patterns of fliC is a promising tool for the identification of some clinically important Shigella strains as well as for confirmation of atypical isolates as Shigella spp.
REFERENCES
- 1.Akopyanz N, Bukanov N O, Westblom T U, Berg D E. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen Helicobacter pylori. Nucleic Acids Res. 1992;20:6221–6225. doi: 10.1093/nar/20.23.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Guerry P, Trust T J. Distribution and polymorphism of the flagellin genes from isolates of Campylobacter coli and Campylobacter jejuni. J Bacteriol. 1993;175:3051–3057. doi: 10.1128/jb.175.10.3051-3057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansaruzzaman M, Kibriya A K M G, Rahman A, Neogi P K B, Faruque A S G, Rowe B, Albert M J. Detection of provisional serovars of Shigella dysenteriae and designation as S. dysenteriae serotypes 14 and 15. J Clin Microbiol. 1995;3:1423–1425. doi: 10.1128/jcm.33.5.1423-1425.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D J, Fanning G R, Miklos G V, Steigerwalt A G. Polynucleotide sequence relatedness among Shigella species. Int J Syst Bacteriol. 1973;23:1–7. [Google Scholar]
- 5.Brenner D J, Steigerwalt A G, Wathen H G, Gross R J, Rowe B. Confirmation of aerogenic strains of Shigella boydii 13 and further study of Shigella serotypes by DNA relatedness. J Clin Microbiol. 1982;16:432–436. doi: 10.1128/jcm.16.3.432-436.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosch R, Lefevre M, Grimont F, Grimont P A D. Taxonomic diversity of Pseudomonas revealed by computer-interpretation of ribotyping data. Syst Appl Microbiol. 1996;19:541–555. [Google Scholar]
- 7.Coimbra R S, Grimont F, Grimont P A D. Identification of Shigella serotypes by restriction of amplified O-antigen gene cluster. Res Microbiol. 1999;150:543–553. doi: 10.1016/s0923-2508(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 8.Coimbra, R. S., G. Nicastro, P. A. D. Grimont, and F. Grimont. Computer identification of Shigella species by rRNA gene restriction patterns. Res. Microbiol., in press. [DOI] [PubMed]
- 9.Dauga C, Zabrovskaia A, Grimont P A D. Restriction fragment length polymorphism analysis of some flagellin genes of Salmonella enterica. J Clin Microbiol. 1998;36:2835–2843. doi: 10.1128/jcm.36.10.2835-2843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd C E R, Jones D. A numerical taxonomic study of the genus Shigella. J Gen Microbiol. 1982;128:1933–1957. doi: 10.1099/00221287-128-9-1933. [DOI] [PubMed] [Google Scholar]
- 11.Ewing W H. Edwards and Ewing's identification of Enterobacteriaceae. 4th ed. New York, N.Y: Elsevier Publishing Co.; 1986. pp. 135–172. [Google Scholar]
- 12.Fields P I, Blom K H, Hughes J H, Helsel L O, Feng P, Swaminathan B. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol. 1997;25:1066–1070. doi: 10.1128/jcm.35.5.1066-1070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gannon V P J, d'Sousa S, Graham T, King R K, Rahn K, Read S. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli. J Clin Microbiol. 1997;35:656–662. doi: 10.1128/jcm.35.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girón J A. Expression of flagella and motility by Shigella. Mol Microbiol. 1995;18:63–75. doi: 10.1111/j.1365-2958.1995.mmi_18010063.x. [DOI] [PubMed] [Google Scholar]
- 15.Goullet P, Picard B. Differentiation of Shigella by esterase electrophoretic polymorphism. J Gen Microbiol. 1987;133:1005–1017. doi: 10.1099/00221287-133-4-1005. [DOI] [PubMed] [Google Scholar]
- 16.Grimont F, Grimont P A D. Determination of rRNA gene restriction patterns. Methods Mol Biol. 1995;46:181–200. doi: 10.1385/0-89603-297-3:181. [DOI] [PubMed] [Google Scholar]
- 17.Janda J M, Abbott S L. The enterobacteria. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 66–79. [Google Scholar]
- 18.Joys T M. The flagellar filament protein. Can J Microbiol. 1988;34:452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- 19.Karaolis D K R, Lan R, Reeves P R. Sequence variation in Shigella sonnei (Sonnei), a pathogenic clone of Escherichia coli, over four continents and 41 years. J Clin Microbiol. 1994;32:796–802. doi: 10.1128/jcm.32.3.796-802.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilger G, Grimont P A D. Differentiation of phase 1 flagellar antigen types by restriction of the amplified fliC gene. J Clin Microbiol. 1993;31:1108–1110. doi: 10.1128/jcm.31.5.1108-1110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado J, Grimont F, Grimont P A D. Identification of Escherichia coli flagellar types by restriction of amplified fliC gene. Res Microbiol. 2000;151:535–546. doi: 10.1016/s0923-2508(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 22.Macnab R M. Flagella. In: Neidhardt F C, Ingraham J L, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 70–83. [Google Scholar]
- 23.Macnab R M. Genetics and biosynthesis of bacterial flagella. Annu Rev Genet. 1992;26:129–156. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 24.Marranzano M, Giammanco G, d'Hauteville H, Sansonetti P. Epidemiological markers of Shigella sonnei infections: R-plasmid fingerprint, phage-typing and biotyping. Ann Inst Pasteur Microbiol. 1985;136:339–345. doi: 10.1016/s0769-2609(85)80096-x. [DOI] [PubMed] [Google Scholar]
- 25.Namba K, Yamashita I, Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989;342:648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- 26.Ochman H, Whittam T S, Caugant D A, Selander R K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983;129:2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- 27.O/rskov F, Whittam T S, Cravioto A, O/rskov I. Clonal relationships among classic enteropathogenic Escherichia coli (EPEC) belonging to different O groups. J Infect Dis. 1990;162:76–81. doi: 10.1093/infdis/162.1.76. [DOI] [PubMed] [Google Scholar]
- 28.O/rskov I, O/rskov F, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977;41:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovskaya V G, Bondarenko V M. Recommended corrections to the classification of Shigella flexneri on a genetic basis. Int J Syst Bacteriol. 1977;27:171–175. [Google Scholar]
- 30.Petrovskaya V G, Khomenko N A. Proposals for improving the classification of members of the genus Shigella. Int J Syst Bacteriol. 1979;29:400–402. [Google Scholar]
- 31.Rolland K, Lambert-Zechovsky N, Picard B, Denamur E. Shigella and enteroinvasive Escherichia coli strains are derived from distinct ancestral strains of E. coli. Microbiology. 1998;144:2667–2672. doi: 10.1099/00221287-144-9-2667. [DOI] [PubMed] [Google Scholar]
- 32.Schaffer H E, Sederoff R R. Improved estimation of DNA fragment lengths from agarose gels. Anal Biochem. 1981;115:113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- 33.Schoenhals G, Whitfield C. Comparative analysis of flagellin sequences from Escherichia coli strains possessing serologically distinct flagellar filaments with a shared complex surface pattern. J Bacteriol. 1993;175:5395–5402. doi: 10.1128/jb.175.17.5395-5402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timakov V D, Petrovskaya V G, Bondarenko V M, Khomenko N A. Genetic data concerning Shigella flexneri serotypes 5 and 6. Int J Syst Bacteriol. 1972;22:149–154. [Google Scholar]
- 35.Tominaga A, Mahmoud M A-H, Mukaihara T, Enomoto M. Molecular characterization of intact, but cryptic, flagellin genes in the genus Shigella. Mol Microbiol. 1994;12:277–285. doi: 10.1111/j.1365-2958.1994.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 36.Whittam T S, Wolfe M L, Wachsmuth I K, O/rskov F, O/rskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winstanley C, Coulson M A, Wepner B, Morgan J A W, Hart C A. Flagellin gene and protein variation amongst clinical isolates of Pseudomonas aeruginosa. Microbiology. 1996;142:2145–2151. doi: 10.1099/13500872-142-8-2145. [DOI] [PubMed] [Google Scholar]