Abstract
A large proportion of patients with suspected obstructive coronary artery disease (CAD) is found to have ischemia with no obstructive coronary artery disease (INOCA). Based on current evidence, these patients are at increased risk of adverse cardiovascular events, even though they have no obstructive CAD. Importantly, INOCA is associated with recurrent clinical presentations with chest pain, impaired functional capacity, reduced health-related quality of life, and high healthcare costs. Underlying coronary microvascular dysfunction (CMD), through endothelium-dependent and independent mechanisms contribute to these adverse outcomes in INOCA. While non-invasive and invasive diagnostic testing has typically focused on identification of obstructive CAD in symptomatic patients, functional testing to detect coronary epicardial and microvascular dysfunction should be considered in those with INOCA who have persistent angina. Current diagnostic methods to clarify functional abnormalities and treatment strategies for epicardial and/or microvascular dysfunction in INOCA are reviewed.
Keywords: coronary artery disease, ischemic heart disease, functional imaging, atherosclerosis
Introduction
Symptomatic patients who have evidence of myocardial ischemia during stress testing routinely undergo coronary angiography to define coronary anatomy and identify significant obstructive epicardial coronary stenoses. Determining the presence, extent, and severity of obstructive coronary artery disease (CAD) has historically been performed with invasive coronary angiography. However, this approach has been increasingly replaced by non-invasive coronary computed tomography angiography (CCTA) over the past decade. Whereas optimal medical therapy and life-style based recommendations are the cornerstone of management of stable ischemic heart disease (SIHD), the focus on diagnostic testing to detect obstructive CAD is largely because therapy for obstructive lesions is available with revascularization, either with percutaneous coronary intervention (PCI) and stenting or coronary artery bypass grafting (CABG). For patients with obstructive CAD, diagnostic and treatment algorithms are relatively well-defined and evidence-based guidelines are available to clinicians for patient management.1,2
However, there is a large group of symptomatic patients who have myocardial ischemia on routine stress testing but are found to have no obstructed coronary arteries (INOCA).3 Historically, absence of obstructive CAD is typically defined as ≤50% stenosis in any major epicardial coronary vessel; therefore, “no obstructive CAD” should not be interpreted as the absence of CAD. Data show that even in patients with no obstructive CAD evident on coronary angiography, coronary intravascular ultrasound often detects a high plaque burden.4 There are an estimated 3–4 million Americans with INOCA,4 likely a conservative estimate since nonobstructive CAD on angiography is not always systematically reported or coded as a diagnosis. An expert consensus document from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation recently highlighted the importance of INOCA, and the need for larger studies and registries to help improve our understanding and management of this under-diagnosed and challenging condition that is associated with adverse outcomes.5
Mounting evidence indicates that INOCA is not benign; multiple studies have documented an adverse cardiovascular prognosis in this population.4,6 INOCA is associated with an elevated risk of major adverse cardiovascular events (MACE) such as myocardial infarction, stroke, sudden cardiac death, and new heart failure, as well as hospitalizations for heart failure and angina.6–9 Hospitalizations for angina and repeat diagnostic testing are frequent because of recurrent presentations to physician offices and emergency rooms.9 Women are more likely than men to have non-obstructive CAD, but often have adverse cardiac outcomes.10 Although the pathophysiology of INOCA is incompletely understood, a large majority of these patients demonstrate dysfunction of epicardial coronary arteries or the microcirculation when they undergo functional testing during angiography. Indeed, invasive testing often demonstrates a range of abnormalities, including coronary epicardial endothelial dysfunction, coronary microvascular dysfunction (CMD), or enhanced vascular smooth muscle hyperactivity, that can all contribute to limit coronary blood flow during various stimuli such as exercise or mental stress. These abnormalities can be inter-related, and can be further exacerbated by factors such as local changes in shear stress, inflammation, or catecholamines. While there are many factors that contribute to ischemic heart disease (Figure 1), this review focuses on patients with INOCA who have persistent angina without, clear structural or valvular cardiac abnormality. While there is no consensus on the definition of persistent angina, we suggest that in stable out-patients, angina episode at least once a month without obstructive CAD could trigger further evaluation for coronary vascular dysfunction,11 as persistent angina at one-year follow-up in these patients has an adverse prognosis.12 This review focuses on the role of non-invasive and invasive functional testing to detect disorders of coronary vascular function ranging from the epicardial arteries to the microcirculation in symptomatic patients with INOCA.
Figure 1. Mechanisms Contributing to INOCA.
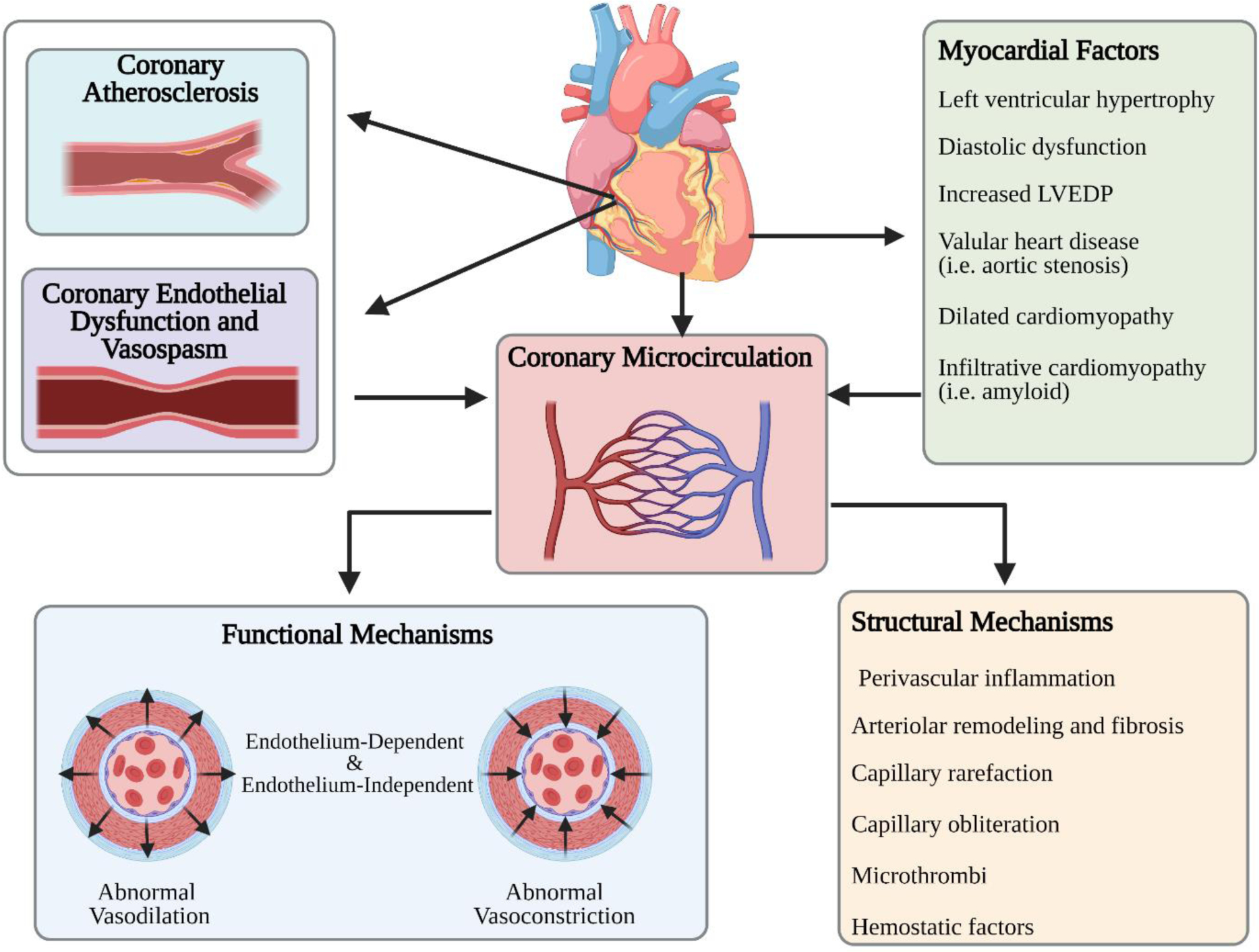
In addition to epicardial atherosclerosis and epicardial coronary vasospasm, CMD is a major cause of abnormal coronary blood flow regulation. CMD-related functional and structural mechanisms contribute to impaired blood flow and ischemia. CAD: coronary artery disease; CMD: coronary microvascular dysfunction; VSMC: vascular smooth muscle cell. Created with BioRender.com.
INOCA Symptoms and Quality of Life (QoL)
Patients with INOCA have a high symptom burden which contributes to poor physical functioning and a reduced QoL.11,13–15 INOCA patients can present with typical and atypical angina symptoms, similar to those with obstructive CAD.15,16 While effort-induced chest discomfort or dyspnea is often elicited in the history of presentation, patients with INOCA often report chest discomfort at rest, which can last for prolonged periods.12,17,18 Patients with INOCA tend to have angina at low hemodynamic workloads and mental stress-related angina, which has implicated abnormal microvascular vasoreactivity.19–27 While some INOCA patients may experience only a single symptom such as chest pain or discomfort, many experience multiple physical and psychological symptoms such as dyspnea, fatigue, headaches, sleep disturbance, somatic pain, anxiety, and depression.28–33 Comorbid psychological factors are highly prevalent in these patients, and psychosocial stress can contribute to and exacerbate angina.12,34–36 In the Women’s Ischemia Syndrome Evaluation (WISE) study, chest pain symptoms were more important determinants of health-related quality of life than the severity of CAD.37
Persistent angina in patients with INOCA may prompt further investigation to assess for functional abnormalities in vascular function, beyond anatomic imaging alone. A detailed review on non-invasive cardiac imaging modalities to assess coronary microvascular dysfunction has recently been published.38 Practical considerations include availability of the test at the individual centers, specific local expertise, and test affordability (i.e. insurance coverage). Invasive coronary functional testing can be pursued for objective evidence of ischemia on a non-invasive stress test, or a Type II myocardial infarction. However, it should be noted that when a patient has unexplained chest pain with equivocal or unrevealing stress testing, it is reasonable to pursue invasive coronary function testing, especially in relatively younger patients who otherwise might not be treated for cardiac risk factor management due to absence of obstructive CAD. While it is unclear why women have more symptoms with INOCA, emerging data indicate that microvascular dysfunction is quite prevalent in both men and women.39,40 Therefore, in symptomatic patients with persistent chest pain, invasive coronary functional testing should be equally pursued in both men and women.
Non-Invasive Functional Testing in INOCA
Stress Echocardiography
Patients with INOCA can have wall motion abnormalities induced by exercise or pharmacologic agents administered during echocardiographic imaging, but may also have a normal stress echocardiogram (STE). In patients with intermediate to high coronary risk, STE has 85% sensitivity and 77% specificity for detection of obstructive CAD.41 Often, CMD may be patchy and/or diffuse, limiting echocardiographic detection. Doppler echocardiography can be used to measure coronary flow velocity reserve (CFVR), and this method has been validated against invasive coronary flow assessment.42 Exercise, dipyridamole, or adenosine stress echo have been used to detect blood flow limitations indicative of CMD.43,44 Contrast echocardiography with microbubble technique can also be used to evaluate myocardial blood flow, but this is not widely utilized.45 Additional information obtained from STE can provide alternative diagnoses for chest pain or dyspnea in symptomatic patients, including diastolic dysfunction, valvular regurgitation, pulmonary hypertension, and ventricular hypertrophy.
Cardiac Positron Emission Tomography (PET)
Cardiac PET is a well-validated and reliable method for quantification of rest/stress myocardial blood flow to assess CMD.46–49 Cardiac PET is preferred in women and those who are obese to reduce non-cardiac tissue artifacts. Myocardial flow reserve (MFR) is defined as the ratio of myocardial blood flow (measured in milliliters/minute/gram of tissue) at peak hyperemia when the coronary vasculature is maximally dilated compared to baseline myocardial blood flow. Normal MFR ranges from 2 to 4, i.e. myocardial blood flow increases 2 to 4 times during peak hyperemia induced by vasodilators such as adenosine.50,51 Reduction in MFR may be due to obstructive epicardial stenosis or due to CMD in a setting of non-obstructive CAD. Either way, a low MFR is associated with a worse prognosis, and a preserved MFR has a high negative predictive value (97%) for excluding ischemia.40,52–57 In 704 patients referred for evaluation of ischemia, those with abnormal MFR had significantly higher cardiac events at 1-year follow up; this included patients with and without obstructive CAD.58 Furthermore, a larger study included more than 2700 subjects showed that global MFR adds an incremental prognostic value beyond the traditional clinical risk factors. In this study, subjects with MFR <1.5 had approximately 6-fold increased risk of cardiac death independent of other risk factors.53 Taqueti et al. reported that in patients with INOCA, an impaired MFR was independently associated with hospitalization for heart failure with preserved ejection fraction (HFpEF).59
Cardiac Magnetic Resonance Imaging (CMRI)
Stress CMRI is a powerful tool for investigation of myocardial causes of persistent chest pain as well as detection of myocardial scar.60 CMRI has superior tissue characterization, no radiation exposure, and ability to detect myocardial edema/fibrosis. In the setting of troponin elevation and no obstructive CAD, CMRI is helpful to rule out myocarditis. Vasodilator stress perfusion CMRI using gadolinium contrast is particularly useful in women, providing higher accuracy than exercise ECG and single photon emission computed tomography (SPECT) MPI.61 Myocardial perfusion reserve index (MPRI) with CMRI is a semi-quantitative method used to estimate coronary blood flow reserve; at experienced centers, MPRI appears to be correlated with CMD detected by invasive coronary functional testing.62 An MPRI cut-off of 1.84 predicted functional abnormalities with sensitivity 73% and specificity 74%.62
Invasive Coronary Functional Testing
When non-invasive testing is unrevealing, but patient continues to have unexplained angina symptoms, invasive coronary functional testing should be pursued and can help diagnose vascular dysfunction (micro- or macro-vascular), due to endothelium- and nonendothelium-dependent pathways. Diagnosing specific vasomotor pathway abnormalities allows for a tailored therapeutic approach and improves angina and quality of life.63 Vasoactive medications such as adenosine, acetylcholine, and nitroglycerin are used to test vascular responses, as well as ECG changes and symptoms.8,64,65 Coronary functional testing can also help with a diagnosis of abnormal cardiac nociception, i.e. pain perception. Patients who report chest pain with contrast or saline injection (even before exposure to any vasoactive medications during functional testing) could have a nociceptive abnormality. Prior studies have reported that women with INOCA have enhanced pain sensitivity and experience more pain with contrast injection during angiography, with right ventricular pacing, and with adenosine infusion, and pain is felt at a lower stimulus intensity compared to obstructive CAD patients.17,66–68 Neuro-cardiac origins of sex-differences in pain perception and differences in cardiac afferent pain signal processing in INOCA remain to be investigated.69 Functional testing is a safe diagnostic procedure when it is performed by trained interventional cardiologists at experienced catheterization laboratories.64 For example, in the WISE study of 293 women who underwent invasive functional testing, there was no mortality related to functional testing; two serious adverse events occurred in 2 women (0.7%), which included coronary artery dissection and myocardial infarction associated with coronary spasm.64 Similarly, others have also reported a low complication rate with intracoronary acetylcholine testing in INOCA patients in the setting of myocardial infarction vs. stable angina.70
Currently, there is no standardized protocol for coronary functional testing, and individual centers perform testing based on the local experience and expertise,71 although the Coronary Artery Vasomotor International Summit (COVADIS) group is working to define terminology and unify protocols.18,72 A decision to pursue functional testing should be considered on an individual basis, weighing the risks and benefits of such testing. Prior authorization for payment by insurance companies is often necessary prior to testing. The European Society of Cardiology guidelines on the management of stable CAD has a Class IIb (level of evidence C) recommendation for coronary functional testing in patients suspected of CMD.73–75
Abnormal hyperemic response to adenosine with a diminished coronary flow reserve (CFR) is associated with MACE (cardiovascular death, myocardial infarction, stroke, and heart failure).7,8 However, it is important to understand that the coronary blood flow response to adenosine infusion is reduced by about a quarter when endothelial nitric oxide is blocked by pretreatment with L-NAME (an L-arginine analogue). Thus, a component of the CFR is secondary to endothelial-mediated dilation. This is important because many studies using adenosine do not test with acetylcholine for endothelial dysfunction. While CFR is a well-accepted prognostic measure in both men and women, it is a combined measure of epicardial and microvascular flow; in the absence of obstructive CAD (with a normal fractional flow reserve), CFR reflects microvascular function, and values that are under ≤2.0 and ≤2.5 are considered abnormal depending on the methodology.76 The index of myocardial resistance (IMR) is a pressure-wire based measure that specifically tests microvascular function and a value >25 is considered abnormal;76–78 IMR provides additional prognostic information when combined with CFR.79 The microcirculation is impacted by cardiac risk factors such as hypertension and diabetes, and it is likely that duration and number of risk factors matter, such that over time, CFR starts to diminish due to endothelial dysfunction and arteriolar remodeling.80–82 Of note, CFR is prognostic beyond traditional cardiac risk factors.53,83,84
Abnormal coronary endothelial function response to acetylcholine testing is also associated with adverse prognosis.7,71,85 Halcox et al86 investigated the relationship between coronary endothelial dysfunction and MACE (defined as death, myocardial infarction, stroke and unstable angina). Subjects were considered to have normal epicardial endothelial function if they dilated in response to intracoronary acetylcholine (15 mcg/min, infused over 2 minutes, 10−6 mol/L). Those who showed epicardial endothelial dysfunction (i.e. any reduction in diameter in response to acetylcholine) had higher rate of adverse events compared to those who had intact endothelial function (13% versus 9.4%, p=0.003). Additionally, subjects with the greatest microvascular vasodilatation in response to acetylcholine infusions (indicating intact microvascular endothelial function) had better cardiovascular outcomes compared to those with less dilatation. The relationship between endothelial dysfunction and adverse outcomes was independent of the severity of CAD.
Impaired coronary blood flow to acetylcholine predicts MACE in women.7 In 224 women with signs and symptoms of ischemia (179 with <50% coronary epicardial artery stenosis), endothelial function was assessed using intracoronary acetylcholine infusion (18.2 mcg/ml, infused over 3 minutes, 10−4 mol/L). A normal response in this study was defined as change in coronary cross-sectional area > 0% (i.e. any vasodilation to acetylcholine was normal). Change in coronary blood flow to acetylcholine was also calculated (a change ≥50% is normal). Two composite outcomes for MACE were evaluated: 1) a 4-component MACE of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, or heart failure hospitalization; and 2) a 3-component MACE of cardiovascular death, nonfatal MI, and nonfatal stroke. For each 10% reduction in coronary blood flow there was a 12% significant increased risk of all-cause mortality (p= 0.038), 11% increase in the 4-component MACE (p=0.006), and 12% increase in the 3-component MACE (p < 0.01). These findings were significant after adjusting for confounding factors such as age and other cardiac risk factors such as hypertension, diabetes, smoking, and hyperlipidemia.7 Among the subset with INOCA, every 10% decrease in coronary blood flow to acetylcholine, there was 23% increase in risk of all-cause mortality (p =0.015), a 16% excess risk for both the 4-component MACE (p=0.001), and 3-component MACE (p=0.003). Additionally, for each 1% decrease in cross sectional area of epicardial coronary artery diameter in response to acetylcholine infusions (indicating abnormal epicardial endothelium-dependent function), there was 5% higher risk of angina hospitalization (p < 0.0001). Similarly, epicardial vasoconstriction to acetylcholine in those with INOCA was associated with higher rates of hospitalization due to angina (p= 0.0002) (Figure 2). Of note, acetylcholine testing protocols vary in various catheterization laboratories in terms of doses of acetylcholine and infusion vs. bolus, which makes it difficult to interpret and combine studies.71 Among symptomatic patients who don’t have visible epicardial spasm, myocardial lactate production in response to acetylcholine may indicate ischemia from microvascular spasm; one approach is to obtain myocardial lactate extraction ratio, calculated with blood collected from the left coronary artery ostium and coronary sinus, to diagnose microvascular spasm.87
Figure 2. Abnormal Coronary Vascular Function in INOCA and Outcomes.
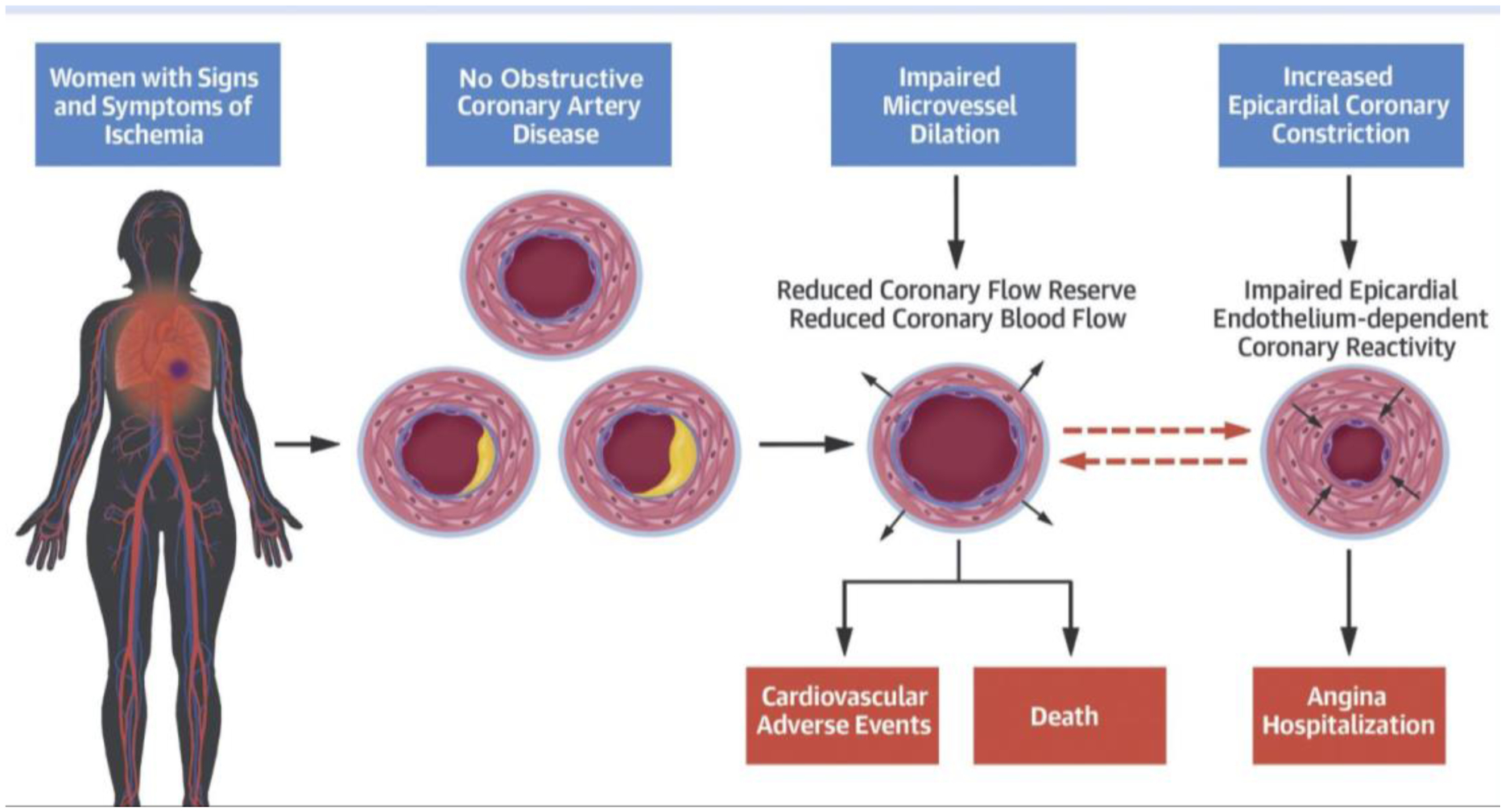
In women with no obstructive coronary artery disease, functional coronary angiography identifies those at higher risk for adverse events and angina hospitalizations. Epicardial coronary vascular reactivity can impact microvascular resistance to flow. A majority of patients with low coronary flow reserve also had abnormal epicardial coronary artery response to acetylcholine, and these pathways are inter-related. Reprinted with Permission from AlBadri et al.7
Therapeutic Strategies in INOCA
Treatment of patients with INOCA should focus on two main goals: 1) anti-atherosclerotic and anti-ischemic strategies to prevent or reduce adverse cardiac event risk and 2) relief of angina to improve physical functioning and quality of life. Therapeutic lifestyle changes and treatment of cardiovascular disease risk factors is the cornerstone of treatment in SIHD. Due to the high prevalence of non-obstructive plaque in INOCA, low dose aspirin and lipid lowering therapy are reasonable. In addition to traditional anti-anginals such as beta-blockers and calcium channel blockers, agents such as ranolazine and ivabradine can be helpful in persistent angina.88 Figure 3 summarizes strategies that can be considered to manage symptoms in CMD, which can be particularly difficult, since the underlying mechanisms of the angina can be diverse, including atheroma, coronary vasospasm, endothelial dysfunction, and abnormal nociception. The landmark Coronary Microvascular Angina (CorMicA) trial demonstrated that medical management that is guided by information from invasive functional testing has a positive impact on angina in patients with INOCA in as little as 6 months.63
Figure 3. Therapeutic Considerations in CMD Management.
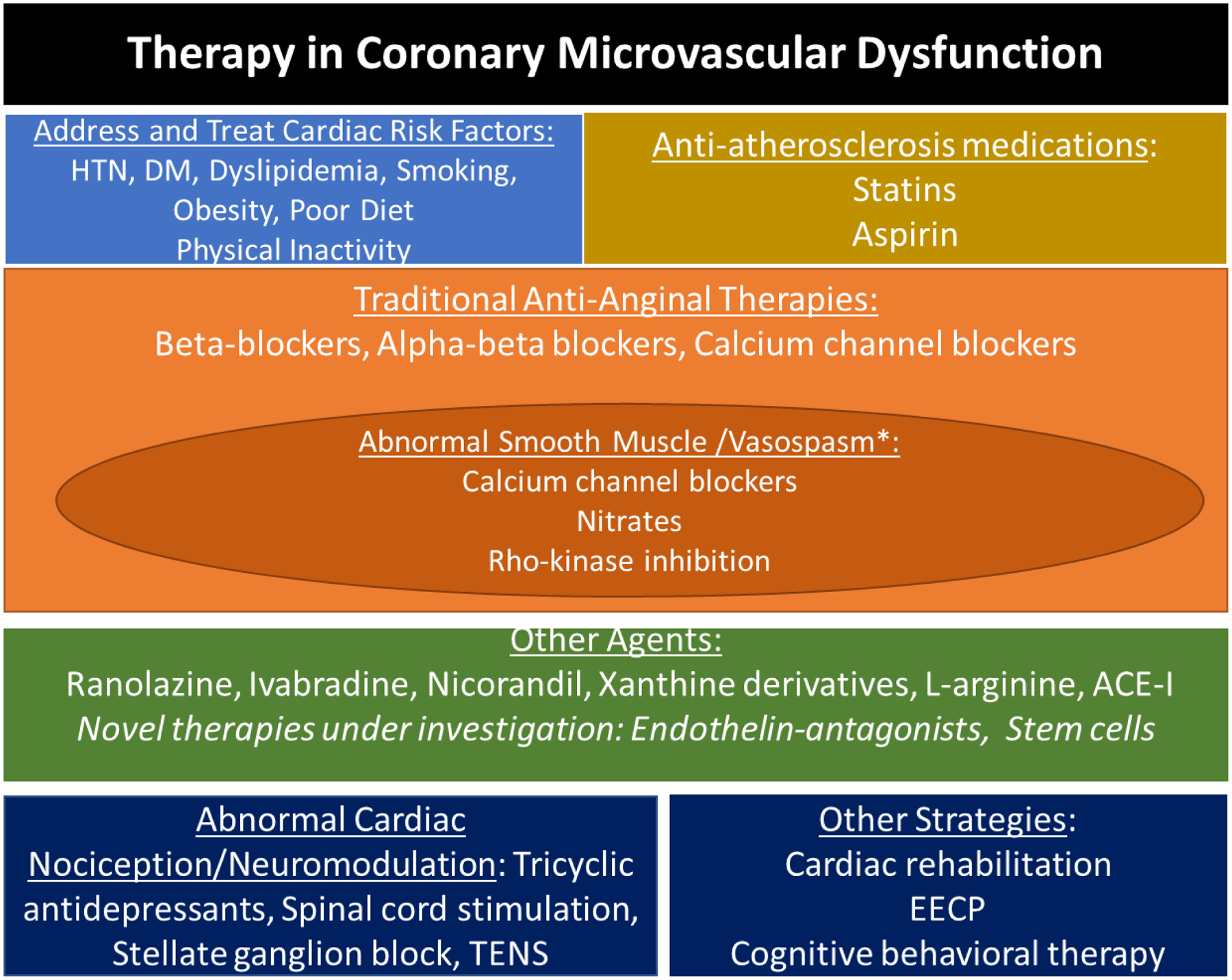
Treatment of CMD and associated abnormal vasoreactivity includes traditional anti-anginal medications to lower myocardial oxygen demand, as well as medications that improve endothelial function. Anti-nociceptive strategies can be helpful given the role of enhanced pain perception described in a subset of CMD subjects. ACE-I: angiotensin converting enzyme-inhibitors; EECP: Enhanced external counter pulsation
*Patients may have overlapping abnormal pathways with low coronary flow reserve + smooth muscle dysfunction.
A systematic review of treatment of patients with INOCA concluded that there is limited data on the efficacy of therapies used.89 Only 8 published articles out of more than 8000 articles on CMD met criteria to evaluate efficacy of therapy for microvascular dysfunction (defined as CFR <2.5).18,89–91 These studies had small sample sizes, thus limiting the power of their conclusions. Nevertheless, the review found that sildenafil, quinapril, estrogen, and transcutaneous electrical nerve stimulation (TENS) application may have some benefits.89 EECP has been shown to improve angina class in CMD patients with refractory angina.92 It has also been shown to have durable effects in improving endothelial function, coronary flow, and vascular inflammation.93 Also, dysregulation of the endothelin system is associated with coronary vascular dysfunction, both of the epicardial vessels and the microcirculation.94,95 A single nucleotide polymorphism (SNP) (rs9349379) enhances circulating concentrations of ET-196 and provides rational for the PRIZE trial (ClinicalTrials.gov: NCT04097314) using zibotentan, a potent selective inhibitor of the endothelin A (ET-A) receptor, in patients with microvascular angina. A pilot study testing autologous intracoronary CD34+ stem cell therapy in INOCA patients showed promise in improving angina and CFR (NCT03508609),97 and this strategy is being tested in a larger trial (NCT04614467).
Intensive medical therapy (IMT) including potent statins in combination with angiotensin converting enzyme inhibitors (ACE-I) or angiotensin-receptor blockers (ARB), at maximally tolerated doses, were found to be effective (improved angina, stress testing, myocardial perfusion) and had beneficial coronary endothelial and microvascular effects in pilot studies.4 The Department of Defense is collaborating with University of Florida to conduct a study of military and civilian women to test the efficacy of IMT in those with INOCA. The ongoing Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD (WARRIOR) is a multicenter, prospective, randomized, blinded outcome evaluation (PROBE design) evaluating IMT vs. usual care in 4,422 symptomatic women with INOCA. The hypothesis is that IMT will reduce MACE by 20% compared to usual care (NCT03417388). The Randomized Evaluation of Beta Blocker and ACEI/ARB Treatment in Myocardial Infarction with No Obstructive Coronary Artery disease (MINOCA-BAT, NCT03686696) is also currently ongoing.
Conclusions
Given our increased understanding of INOCA, clinicians should be aware that absence of significant CAD on anatomic testing does not necessarily indicate a benign prognosis in symptomatic patients with ischemia. Coronary functional testing to detect underlying vascular dysfunction (epicardial and/or microvascular) is helpful for clarifying diagnosis, for prognostication, and for guiding management of INOCA.
Supplementary Material
Highlights.
Patients with evidence of myocardial ischemia and no obstructive coronary artery disease (INOCA) are at increased risk of major cardiovascular events.
Coronary vascular dysfunction, of the epicardial vessels and/or the microcirculation, may contribute to abnormal coronary blood flow and ischemia.
Currently available non-invasive stress testing modalities can help detect abnormal vasodilatory reserve, but invasive testing is needed to assess coronary endothelial and vasomotor function.
Invasive functional coronary angiography can provide a diagnosis and guide therapeutic management in patients with persistent symptoms.
Anti-anginal and anti-atherosclerotic medications are used to manage INOCA, while large, therapeutic trials of outcomes in INOCA are underway.
Acknowledgement
This work was supported by ACC Cardiovascular Disease in Women Committee.
Disclosures:
Mehta, Al-Badri, Quesada, Fleg: none; Volgman: NIH grants, Apple Inc Stock; Bairey Merz: Lecture fees Abbott Diagnostics, Consulting: Sanofi, Board Director: iRhythm; Pepine: none; Shaw: (unpaid) Scientific Advisory Board for Covanos, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the United States Department of Health and Human Services.
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. 2014;64(18):1929–1949. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135(11):1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41(37):3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European heart journal. 2012;33(6):734–744. [DOI] [PubMed] [Google Scholar]
- 7.AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73(6):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39(23):2135–2146. [DOI] [PubMed] [Google Scholar]
- 10.Bairey Merz CN. Women and ischemic heart disease paradox and pathophysiology. JACC Cardiovasc Imaging. 2011;4(1):74–77. [DOI] [PubMed] [Google Scholar]
- 11.Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102(8):571–581. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–1415. [DOI] [PubMed] [Google Scholar]
- 13.Grodzinsky A, Arnold SV, Gosch K, et al. Angina Frequency After Acute Myocardial Infarction In Patients Without Obstructive Coronary Artery Disease. Eur Heart J Qual Care Clin Outcomes. 2015;1(2):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldiwani H, Zaya M, Suppogu N, et al. Angina Hospitalization Rates in Women With Signs and Symptoms of Ischemia But no Obstructive Coronary Artery Disease: A Report from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Heart Assoc. 2020;9(4):e013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handberg E, Johnson BD, Arant CB, et al. Impaired coronary vascular reactivity and functional capacity in women: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47(3 Suppl):S44–49. [DOI] [PubMed] [Google Scholar]
- 16.Jones E, Delia Johnson B, Shaw LJ, et al. Not typical angina and mortality in women with obstructive coronary artery disease: Results from the Women’s Ischemic Syndrome Evaluation study (WISE). Int J Cardiol Heart Vasc. 2020;27:100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon RO 3rd, Quyyumi AA, Schenke WH, et al. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol. 1990;16(6):1359–1366. [DOI] [PubMed] [Google Scholar]
- 18.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 19.McCraty R, Atkinson M, Tiller WA, Rein G, Watkins AD. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol. 1995;76(14):1089–1093. [DOI] [PubMed] [Google Scholar]
- 20.Pagani M, Furlan R, Pizzinelli P, Crivellaro W, Cerutti S, Malliani A. Spectral analysis of R-R and arterial pressure variabilities to assess sympatho-vagal interaction during mental stress in humans. J Hypertens Suppl. 1989;7(6):S14–15. [DOI] [PubMed] [Google Scholar]
- 21.Pagani M, Mazzuero G, Ferrari A, et al. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–51. [PubMed] [Google Scholar]
- 22.Tuininga YS, Crijns HJ, Brouwer J, et al. Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients. Circulation. 1995;92(12):3415–3423. [DOI] [PubMed] [Google Scholar]
- 23.Krantz DS, Kop WJ, Santiago HT, Gottdiener JS. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin. 1996;14(2):271–287. [PubMed] [Google Scholar]
- 24.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. [DOI] [PubMed] [Google Scholar]
- 25.Krantz DS, Hedges SM, Gabbay FH, et al. Triggers of angina and ST-segment depression in ambulatory patients with coronary artery disease: evidence for an uncoupling of angina and ischemia. Am Heart J. 1994;128(4):703–712. [DOI] [PubMed] [Google Scholar]
- 26.Sestito A, Maccallini A, Sgueglia GA, et al. Platelet reactivity in response to mental stress in syndrome X and in stable or unstable coronary artery disease. Thromb Res. 2005;116(1):25–31. [DOI] [PubMed] [Google Scholar]
- 27.Markovitz JH, Matthews KA. Platelets and coronary heart disease: potential psychophysiologic mechanisms. Psychosom Med. 1991;53(6):643–668. [DOI] [PubMed] [Google Scholar]
- 28.Rutledge T, Reis SE, Olson M, et al. History of anxiety disorders is associated with a decreased likelihood of angiographic coronary artery disease in women with chest pain: the WISE study. J Am Coll Cardiol. 2001;37(3):780–785. [DOI] [PubMed] [Google Scholar]
- 29.Rutledge T, Reis SE, Olson M, et al. Depression is associated with cardiac symptoms, mortality risk, and hospitalization among women with suspected coronary disease: the NHLBI-sponsored WISE study. Psychosom Med. 2006;68(2):217–223. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed B, Bairey Merz CN, McClure C, et al. Migraines, angiographic coronary artery disease and cardiovascular outcomes in women. Am J Med. 2006;119(8):670–675. [DOI] [PubMed] [Google Scholar]
- 31.Juelsgaard P, Ronnow Sand NP. Somatic and social prognosis of patients with angina pectoris and normal coronary arteriography: a follow-up study. Int J Cardiol. 1993;39(1):49–57. [DOI] [PubMed] [Google Scholar]
- 32.Asbury EA, Creed F, Collins P. Distinct psychosocial differences between women with coronary heart disease and cardiac syndrome X. Eur Heart J. 2004;25(19):1695–1701. [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Li SB, Zhao LS, et al. Relationship between obstructive sleep apnea and coronary microcirculatory function among patients with cardiac syndrome X. Coron Artery Dis. 2014;25(1):35–39. [DOI] [PubMed] [Google Scholar]
- 34.Stubbs B, Vancampfort D, Veronese N, et al. Depression and pain: primary data and meta-analysis among 237 952 people across 47 low- and middle-income countries. Psychol Med. 2017;47(16):2906–2917. [DOI] [PubMed] [Google Scholar]
- 35.Szpakowski N, Qiu F, Masih S, Kurdyak P, Wijeysundera HC. Economic Impact of Subsequent Depression in Patients With a New Diagnosis of Stable Angina: A Population-Based Study. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold SV, Spertus JA, Ciechanowski PS, et al. Psychosocial modulators of angina response to myocardial ischemia. Circulation. 2009;120(2):126–133. [DOI] [PubMed] [Google Scholar]
- 37.Olson MB, Kelsey SF, Matthews K, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24(16):1506–1514. [DOI] [PubMed] [Google Scholar]
- 38.Mathew RC, Bourque JM, Salerno M, C K. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc Imaging. 2020;13(7):1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Mehta PK, Eshtehardi P, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter Cardiovasc Interv. 2020. [DOI] [PubMed] [Google Scholar]
- 40.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280(10):913–920. [DOI] [PubMed] [Google Scholar]
- 42.Caiati C, Montaldo C, Zedda N, et al. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol. 1999;34(4):1193–1200. [DOI] [PubMed] [Google Scholar]
- 43.Galiuto L, Sestito A, Barchetta S, et al. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99(10):1378–1383. [DOI] [PubMed] [Google Scholar]
- 44.Mygind ND, Michelsen MM, Pena A, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J Am Heart Assoc. 2016;5(3):e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004;11(2):215–221. [DOI] [PubMed] [Google Scholar]
- 46.Camici PG. Positron emission tomography and myocardial imaging. Heart. 2000;83(4):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia EV. Are absolute myocardial blood flow PET measurements ready for clinical use? J Nucl Cardiol. 2014;21(5):857–858. [DOI] [PubMed] [Google Scholar]
- 48.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–1653. [DOI] [PubMed] [Google Scholar]
- 49.Rimoldi OE, Camici PG. Positron emission tomography for quantitation of myocardial perfusion. J Nucl Cardiol. 2004;11(4):482–490. [DOI] [PubMed] [Google Scholar]
- 50.Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol. 1990;16(3):586–595. [DOI] [PubMed] [Google Scholar]
- 51.Czernin J, Muller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88(1):62–69. [DOI] [PubMed] [Google Scholar]
- 52.Cortigiani L, Rigo F, Gherardi S, et al. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106(12):1703–1708. [DOI] [PubMed] [Google Scholar]
- 53.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naya M, Murthy VL, Taqueti VR, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103(5):626–631. [DOI] [PubMed] [Google Scholar]
- 57.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. [DOI] [PubMed] [Google Scholar]
- 59.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39(10):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuruvilla S, Kramer CM. Coronary microvascular dysfunction in women: an overview of diagnostic strategies. Expert Rev Cardiovasc Ther. 2013;11(11):1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greulich S, Bruder O, Parker M, et al. Comparison of exercise electrocardiography and stress perfusion CMR for the detection of coronary artery disease in women. J Cardiovasc Magn Reson. 2012;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ford TJ, Corcoran D, Oldroyd KG, et al. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Angina (CorMicA) stratified medicine clinical trial. Am Heart J. 2018;201:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5(6):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Widmer RJ, Samuels B, Samady H, et al. The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;14(16):1694–1702. [DOI] [PubMed] [Google Scholar]
- 66.Lagerqvist B, Sylven C, Waldenstrom A. Lower threshold for adenosine-induced chest pain in patients with angina and normal coronary angiograms. Br Heart J. 1992;68(3):282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frobert O, Arendt-Nielsen L, Bak P, Funch-Jensen P, Peder Bagger J. Pain perception and brain evoked potentials in patients with angina despite normal coronary angiograms. Heart. 1996;75(5):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cannon RO 3rd. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54(10):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta PK, Bess C, Elias-Smale S, et al. Gender in cardiovascular medicine: chest pain and coronary artery disease. Eur Heart J. 2019;40(47):3819–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Probst S, Seitz A, Martinez Pereyra V, et al. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared to patients with stable angina and unobstructed coronary arteries. Eur Heart J Acute Cardiovasc Care. 2020:2048872620932422. [DOI] [PubMed] [Google Scholar]
- 71.Zaya M, Mehta PK, Merz CN. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol. 2014;63(2):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565–2568. [DOI] [PubMed] [Google Scholar]
- 73.Task Force M, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. [DOI] [PubMed] [Google Scholar]
- 74.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):3097–3137. [DOI] [PubMed] [Google Scholar]
- 75.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(17):2212–2241. [DOI] [PubMed] [Google Scholar]
- 76.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 77.Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107(25):3129–3132. [DOI] [PubMed] [Google Scholar]
- 78.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113(17):2054–2061. [DOI] [PubMed] [Google Scholar]
- 79.Lee JM, Jung JH, Hwang D, et al. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67(10):1158–1169. [DOI] [PubMed] [Google Scholar]
- 80.McCallinhart PE, Sunyecz IL, Trask AJ. Coronary Microvascular Remodeling in Type 2 Diabetes: Synonymous With Early Aging? Front Physiol. 2018;9:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pries AR, Badimon L, Bugiardini R, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015;36(45):3134–3146. [DOI] [PubMed] [Google Scholar]
- 82.Neglia D, Fommei E, Varela-Carver A, et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J Hypertens. 2011;29(2):364–372. [DOI] [PubMed] [Google Scholar]
- 83.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation. 2017;136(24):2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JM, Choi KH, Hwang D, et al. Prognostic Implication of Thermodilution Coronary Flow Reserve in Patients Undergoing Fractional Flow Reserve Measurement. JACC Cardiovasc Interv. 2018;11(15):1423–1433. [DOI] [PubMed] [Google Scholar]
- 85.Seitz A, Gardezy J, Pirozzolo G, et al. Long-Term Follow-Up in Patients With Stable Angina and Unobstructed Coronary Arteries Undergoing Intracoronary Acetylcholine Testing. JACC Cardiovasc Interv. 2020;13(16):1865–1876. [DOI] [PubMed] [Google Scholar]
- 86.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi J, Suda A, Yasuda S, Shimokawa H. Measurement of Myocardial Lactate Production for Diagnosis of Coronary Microvascular Spasm. J Vis Exp. 2021(175). [DOI] [PubMed] [Google Scholar]
- 88.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8(2):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gropler RJ, Siegel BA, Lee KJ, et al. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med. 1990;31(11):1749–1756. [PubMed] [Google Scholar]
- 91.Rahman H, Demir OM, Ryan M, et al. Optimal Use of Vasodilators for Diagnosis of Microvascular Angina in the Cardiac Catheterization Laboratory. Circ Cardiovasc Interv. 2020;13(6):e009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kronhaus KD, Lawson WE. Enhanced external counterpulsation is an effective treatment for Syndrome X. Int J Cardiol. 2009;135(2):256–257. [DOI] [PubMed] [Google Scholar]
- 93.Luo C, Liu D, Wu G, et al. Effect of enhanced external counterpulsation on coronary slow flow and its relation with endothelial function and inflammation: a mid-term follow-up study. Cardiology. 2012;122(4):260–268. [DOI] [PubMed] [Google Scholar]
- 94.Halcox JP, Nour KR, Zalos G, Quyyumi AA. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res. 2001;89(11):969–976. [DOI] [PubMed] [Google Scholar]
- 95.Lerman A, Holmes DR Jr., Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92(9):2426–2431. [DOI] [PubMed] [Google Scholar]
- 96.Ford TJ, Corcoran D, Padmanabhan S, et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merz CN HT, Wei J, Corban M, Joung S, Kotynski C, Lewis M, Schumacher A, Bartel R, Sietsema WK, Losordo DW, Lerman A. Clinical Trial of Autologous CD34 Cell Therapy for Treatment of Coronary Microvascular Dysfunction in Patients With Angina and Non-Obstructive Coronary Arteries. Circulation. 2019;140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


