Since the World Health Organisation declared the COVID-19 pandemic on March 11, 2020, various restrictive measures (e.g., prohibition of group gatherings, compulsory use of masks) have been introduced globally to limit the dissemination of SARS-CoV-2, indirectly resulting in a reduced circulation of other respiratory viral pathogens and low incidence of acute respiratory infections (ARIs) during the cold season [1]. After the wide-scale implementation of vaccination campaigns, easing of the restrictive measures against COVID-19 began worldwide in spring 2021. This led to an off-season resurgence of respiratory syncytial virus (RSV) infections in several countries around the world, including the United States, France and Japan [2], [3], [4], most likely as a consequence of the immunologic gap caused by the lack of circulation of endemic respiratory viruses in the 2020–2021 winter season [5]. As Europe approached the 2021–2022 winter season, we wondered whether a more severe resurgence not only of RSV but also of other seasonal viruses would affect Italy. To evaluate this question, we retrospectively analysed the results of 1975 respiratory samples (nasopharyngeal swabs and aspirates, tracheal aspirates) collected from 1975 paediatric outpatients (0–18 years old) as per routine admission procedure at the Emergency Department of Bambino Gesù Children's Hospital in Rome between January 2019 and October 2021. Only children (i) presenting with symptoms of an ARI requiring hospitalisation or (ii) <12 months of age and presenting with respiratory distress, loss of appetite, hyporeactivity or vomiting were included in the analysis.
To provide a comprehensive overview of the current ARI epidemiology, our analysis was not limited to RSV but included several other common viral pathogens, identified by multiplex Reverse transcriptase-polymerase chain reaction (RT-PCR) assays. The Allplex Respiratory Panel Assays (Seegene, Seoul, South Korea) was used to test the majority of samples collected between January 2019 and October 2021 (n = 1235); the BioFire FilmArray Respiratory Panel: bioMérieux Clinical Diagnostics, Salt Lake City, Utah, United States 2.1 was used to test the remaining 740 samples collected between May 2021 and October 2021. Because the two assays slightly differ in the targets analysed, we focused on the 13 viruses included in both (Fig. 1 ).
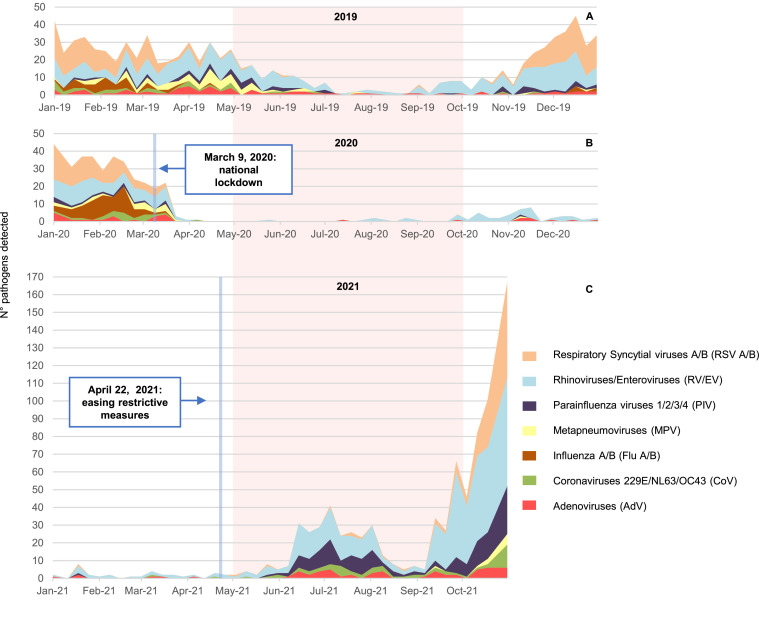
Fig. 1.
Number of different viral pathogens detected in (a) January–December 2019, (b) January–December 2020 and (c) January–October 2021. The pink area highlights the three periods of five months (May–September) with the highest temperature, and the white area highlights the months with the lowest temperature (January–April and October–December). Between April 2020 and April 2021, the number of tests was lower than in the other periods due to the limited number of outpatients admissions with the clinical characteristics described in the text. Note the different scale of the y axis in (c) compared to (a) and (b)
Weekly ARI cases were analysed in the period 1 January–31 October 2021 (Fig. 1C) and compared with 1 January 1–31 December 2020 (Fig. 1B) and 1 January –31 December 2019 (Fig. 1A) as references.
Our analysis revealed an extensive epidemic of paediatric ARIs in 2021 (Fig. 1C) that started in June, with a first peak of cases in July, and reached the maximum in October 2021.
This epidemic is anomalous in terms of timing and aetiology. As shown in the analysis of 2019’s ARI outbreaks (Fig. 1A), seasonal viruses stop circulating in May and are nearly absent from June until October. When comparing the relative frequencies of viral isolations in October 2021 vs. October 2019, RSV was much more frequently identified among patients with the abovementioned clinical characteristics: 104 cases (34% of the total) in October 2021 vs. only 3 in the same month in 2019 (10.7% of the total; P = 0.012). The parainfluenza viruses (72 cases in 2021, 23% of the total, vs. only 1 case in 2019; P = 0.015) and rhinoviruses/enteroviruses (223 cases in 2021 vs. only 26 in 2019; P = 0.019) followed the same trend.
Of note, there were two viruses co-present in about 30% of all positive cases and three in 7%, highlighting the increased circulation of these viruses in 2021.
Our results point towards a striking and out-of-season resurgence of common respiratory viruses, as well as an anomalous and rapid increase in severe RSV infections, most likely associated with the easing of COVID-19 restrictions (22 April 2021). Indeed, only 75 ARIs were detected in 167 children tested in the period from April 2020 to April 2021, which corresponded to the duration of SARS-CoV-2 restrictions in Italy. In addition, the classic winter surge of seasonal infections was missing (Fig. 1B).
In summary, other respiratory viruses, and not SARS-CoV-2, proved to be the predominant cause of the ongoing epidemic outbreak, as the latter was detected in very few cases (about 2%; 12 of 604) between May and October 2021 (data not shown).
Finally, exceptional circulation of non–SARS-CoV-2 viruses can be foreseen in the upcoming winter season without compulsory masking and social distancing and with widening SARS-CoV-2 vaccination coverage; these viruses will probably be the main cause of respiratory symptoms in the paediatric population. Our results can provide the epidemiologic background needed to ensure effective epidemiologic surveillance and preventive measures (i.e., adequate influenza vaccination campaign) to avoid further anomalous outbreaks and to rationalize the use of molecular and antigenic tests for SARS-CoV-2.
Acknowledgments
Acknowledgements
The authors thank Dr. Agata Helena Kowalska for her comments, which were very helpful in improving the manuscript.
Competing interests
There are no conflicts of interest for all authors. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethical approval
Not required.
Editor: Stefania Stefani
References
- 1.Feng L, Zhang T, Wang Q, Xie Y, Peng Z, Zheng J, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat Commun. 2021;12:3249. doi: 10.1038/s41467-021-23440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z, Pitzer VE, Shapiro ED, Bont LJ, Weinberger DM. Estimation of the timing and intensity of reemergence of respiratory syncytial virus following the COVID-19 pandemic in the US. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delestrain C, Danis K, Hau I, Behillil S, Billard M, Krajten L, et al. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr Pulmonol. 2021;56:3669–3673. doi: 10.1002/ppul.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis. 2021;27:2969–2970. doi: 10.3201/eid2711.211565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R, Pettoello-Mantovani M, Somekh E, Levy C. European pediatric societies call for an implementation of regular vaccination programs to contrast the immunity debt associated to coronavirus disease 2019 pandemic in children. J Pediatr. 2021 doi: 10.1016/j.jpeds.2021.11.061. :S0022-3476(21)01159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]