Abstract
Background
As immunity against SARS-COV-2 wanes following first and second doses of vaccination, a third dose is administered in several countries around the world. Similarly to the first doses, risks related to vaccination and humoral immune response in patients with multiple sclerosis (MS) need to be assessed.
Objective
Characterize safety and humoral immune response following the third dose of COVID-19 vaccination in a large cohort of MS patients.
Methods
We assessed the safety of the third dose of the BNT162b2-COVID-19 mRNA vaccination in adult MS patients and evaluated SARS-CoV-2 IgG response.
Results
Two hundred and eleven adult MS patients received a third dose of BNT162b2 COVID-19 vaccination. Median follow up time was 66 days from vaccine administration (IQR 54–84). The frequency of any adverse event was 54.5%, with the most common reported adverse events being fatigue, local pain at the injection site, fever and muscle or joint pain. Transient increase in MS symptoms was reported in 3.8% of patients, none of them requiring treatment. The rate of acute relapses treated with IV steroids was 3.3%.
In a sub-group of 55 patients, 20 untreated and 35 treated with vaccination-safe disease-modifying treatments, SARS-CoV-2 IgG levels increased 21-fold (median ± SD 21.6 ± 53.05).
Conclusions
The third dose of COVID-19-BNT162b2 vaccine proved safe for MS patients, with no increased risk of relapse activity. Untreated patients and patients treated with vaccination-safe disease-modifying treatments show significant increase in SARS-CoV-2 IgG levels following the third dose of vaccination.
Keywords: Multiple sclerosis, COVID-19, Vaccination, Adverse events, Acute relapse, Immune response, Third booster dose
1. Introduction
The COVID-19 pandemic has been posing a global public health challenge for the last two years. While strategies including social distancing, face masks, and quarantines have been implemented to eradicate the SARS-CoV-2 virus, thus far vaccination proved the most effective tool, providing protection against infection, severe disease, and death [1].
In recent studies, we reported the Pfizer m-RNA COVID-19 vaccine to be safe for patients with multiple sclerosis (MS), with no increased risk for disease activity for up to 4 months following the last vaccine dose [2,3]. Our findings were later validated in several reports from across the world [[4], [5], [6], [7], [8], [9]].
Despite the effectiveness of the vaccine, a waning of the humoral immune response in healthy subjects was reported in studies from Israel within 6 months [10,11]. In addition, an outbreak of SARS-COV-2 infection was reported in fully vaccinated individuals that showed decreased neutralizing antibody titers [12]. As a result, in July 2021, the administration of a third booster dose of vaccination was approved and performed in Israel, resulting in reduced rates of infection and a decrease in the occurrence of severe morbidity [13].
As patients with MS present a unique population concerning immune response, the adverse event profile of the booster vaccine must be carefully assessed. In the current study, we report on the safety profile of the third booster vaccination in a large cohort of fully vaccinated MS patients, with a particular emphasis on its effect on disease activity.
2. Methods
2.1. COVID-19 vaccination
MS patients who previously received two intramuscular injections were administered a third dose of the vaccine, at least 6 months apart from the second dose. The booster injection contained 30 μg of Pfizer BNT162b2 (0.3 ml volume).
2.2. Study design
An observational prospective study.
2.3. Data collection
MS patients were contacted via multiple communication methods or questioned upon a standard medical visit at the MS center. Patients were systematically queried regarding the occurrence of any adverse events using a constructed questionnaire including the severity and duration of symptoms. In the event of possible relapse or sustained neurological worsening patients underwent neurological examination by a neurologist to verify or exclude the event. Post vaccination relapse was defined as the onset of new neurological symptoms or worsening of existing ones that persisted for at least 48 h combined with the presence of objective findings in the clinical neurological examination with an increase in the Expanded Disability Status Scale (EDSS) score. Patients receiving B-cell depletion therapies were prioritized to receive the third booster and more frequently contacted to closely follow their response to the booster vaccination. The cut-off date for safety evaluations and relapse assessments was set to October 21st, 2021.
Demographic, clinical, and disease-modifying treatments (DMTs) data were obtained from electronic health records using the Sheba MS Computerized Database Registry.
2.4. Immunoassay for detection of anti-SARS-CoV-2 IgG
Humoral SARS-COV-2 IgG response was assessed in a subgroup of patients either untreated or treated with vaccination-safe DMTs before and after the third vaccine dose. Vaccination-safe DMTs were defined as medications reported to elicit protective humoral antibody response following PfizerBNT162b2 vaccination [14]. B-cell depletion therapies were excluded from the humoral response analysis as various reports demonstrated diminished IgG responses following vaccination under these treatments [15,16].
Immunoassay for the detection of SARS-CoV-2 IgG antibodies was performed using anti-SARS-CoV-2 QuantiVac ELISA IgG (Euroimmun, Lubeck, Germany) based on the S1 domain of the spike protein. Cut-off for positive IgG level was determined as >35.2 binding antibody units (BAU)/ml. The test has a sensitivity of 93.2% % and a specificity of 100%. Correlation with neutralization tests was reported to be 98.2% (https://www.euroimmun.de/en/).
2.5. Ethics
The study was approved by Sheba Medical Center Institutional Review Board. Each patient record was coded anonymously to ensure confidentiality during statistical analyses.
Patients included in the sub-analysis of humoral IgG response signed written informed consent.
The study was approved by Sheba Medical Center Institutional Review Board (approval number Sheba.SMC-8182-21).
2.6. Statistical analysis
Categorical variables were described as frequency and percentage and continuous variables were reported by their median and interquartile range (IQR). Descriptive data analyses were performed using Python software (version 3.0). Differences between groups were evaluated by the chi-square test for categorical variables and by the unpaired t-test for continuous variables.
3. Results
3.1. Patient population
A total of 211 MS patients, 62.0% females, 74.8% treated with various DMTs, received the third BNT162b2 vaccine injection. Demographic and clinical data of vaccinated MS patients are presented in Table 1 .
Table 1.
Demographic and clinical data of vaccinated MS patients.
| Study Population (n = 211) | |
|---|---|
| Duration of follow-up at data cut-off date, days | |
| Median | 66 |
| 25–75 IQR | 54–84 |
| Gender, n (%) | |
| Female | 131 (62.0%) |
| Male | 80 (37.9%) |
| Age group, n (%) | |
| 18–55 years | 121 (57.3%) |
| >55 years | 90 (42.6%) |
| Disease duration, years | |
| Median | 16.65 |
| 25–75 IQR | 9.48–25.85 |
| Disability by EDSS, n (%) | |
| ⩽3.0 | 93 (44.1%) |
| 3.5–5.5 | 50 (23.7%) |
| ⩾6.0 | 68 (32.2%) |
| Disease type, n (%) | |
| CIS | 7 (3.3%) |
| RRMS | 126 (59.7%) |
| SPMS | 42 (19.9%) |
| PPMS | 36 (17.0%) |
| IMD treatment, n (%) | |
| Untreated | 53 (25.1%) |
| Beta-interferons (1a and 1b) | 6 (0.3%) |
| Glatiramer acetate | 2 (0.9%) |
| Teriflunomide | 19 (9.0%) |
| Dimethyl fumarate | 9 (4.3%) |
| Natalizumab | 17 (8.0%) |
| Fingolimod | 25 (11.8%) |
| Ocrelizumab | 65 (30.8%) |
| Alemtuzumab | 4 (1.9%) |
| Cladribine | 7 (3.3%) |
| Rituximab | 1 (0.5%) |
| IVIg | 3 (1.4%) |
EDSS: Expanded Disability Status Scale; IQR: interquartile range; IMD: immunomodulatory drug; IVIg: intravenous immunoglobulin immunoglobulin; CIS: Clinically Isolated Syndrome; RRMS: Relapsing Remmiting Multiple Sclerosis; SPMS: Secondary Progressive Multiple Sclerosis; PPMS: Primary Progressive Multiple Sclerosis.
3.2. Safety
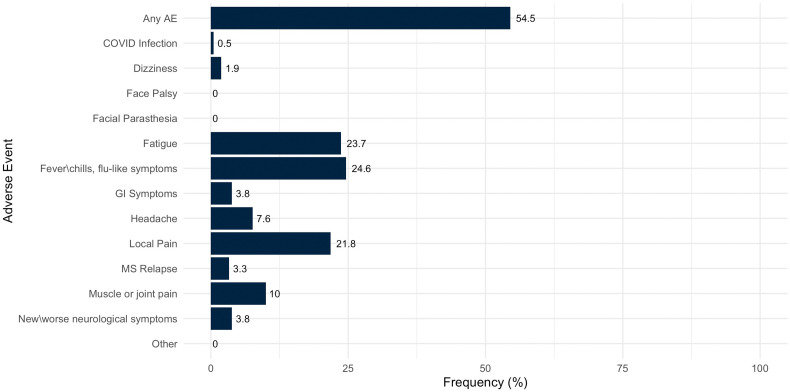
No events of anaphylaxis or life-threatening response occurred immediately following vaccination. The safety profile reported within a median of 66 days (IQR 54–84) was similar to the adverse events reported following the first and second doses. The frequency of patients with any adverse event was 54.5%. The most common reported adverse events were fatigue, local pain at the injection site, fever, and muscle or joint pain. Headache and dizziness were reported in 7.6% and 1.9% of patients respectively. No events of facial palsy or tingling were recorded. One patient (0.5%) developed infection with SARS-CoV-2 within 62 days following the booster vaccination; this patient was under B-cell depletion therapy (last dosing 28 days prior to COVID-19 infection, absolute lymphocyte count of 570 cells/ml3) and had no IgG humoral immune response following the first two doses.
The list of adverse events reported by MS patients following the third dose of the COVID-19 vaccine is presented in Table 2 and demonstrated in Fig. 1 .
Table 2.
Adverse events reported by MS patients following the third dose of the COVID-19 vaccine.
| Study Population (n = 211) | |
|---|---|
| Any adverse events, n(%) | 115 (54.5%) |
| Fatigue | 50 (23.7%) |
| Pain at the injection site | 46 (21.8%) |
| Fever\chills, flu-like symptoms | 52 (24.6%) |
| Muscle or joint pain | 21 (10%) |
| Headache | 16 (7.6%) |
| Infection with SARS-CoV-2 after vaccination | 1 (0.5%) |
| New or worsening neurological symptomatology | 8 (3.8%) |
| GI symptoms | 8 (3.8%) |
| Dizziness | 4 (1.9%) |
| Infection with SARS-CoV-2 after vaccination | 1 (0.4%) |
| Faial tingling\paresthesia | 0 (0%) |
| Facial palsy | 0 (0%) |
| Acute MS Relapse, n(%)* | 7 (3.3%) |
| Time to relapse, days | |
| Median | 34 |
| Range | 14–67 |
Fig. 1.
Adverse events reported by MS patients following a third dose of BNT162b2 COVID-19 vaccination. Numbers beside the bars represent the percentage of participants who reported the specified adverse event.
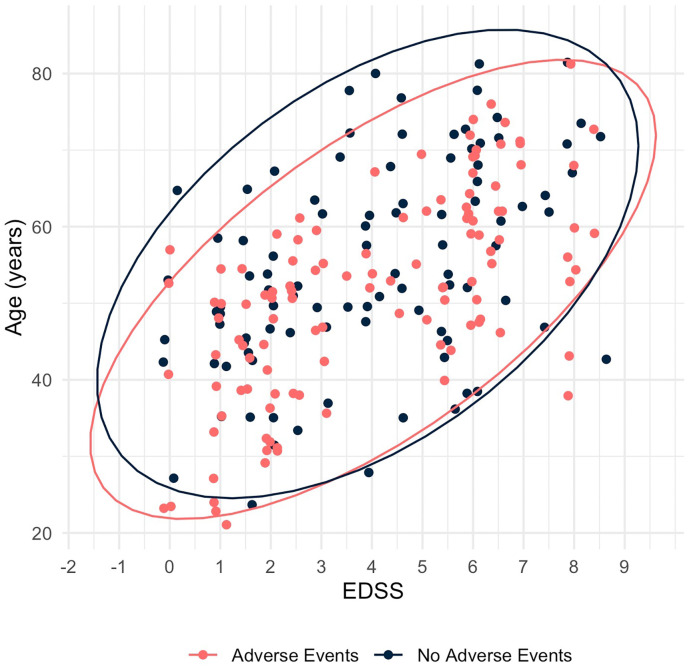
Similar to our findings following the first and second doses of vaccination, the rate of adverse events was higher in younger patients (18 to 55 years), patients suffering from less severe disability (EDSS ≤3.0), and in those treated with DMTs. However, following the third dose, this difference did not reach statistical significance (p = 0.133, p = 0.356, and p = 0.357, respectively). The distribution of the occurrence of any adverse event across age and EDSS is presented in Fig. 2 .
Fig. 2.
Distribution of the occurrence of any adverse event reported by MS patients following a third dose of BNT162b2 COVID-19 vaccination, across age and EDSS.
3.3. MS worsening
Worsening of MS symptomatology defined as a transient increase in MS symptoms following vaccination was reported in 3.8% of patients after the third vaccination dose, compared with 4.8% of patients, reported after the second vaccination dose. The occurrence was short-term and included increased fatigue, facial tingling, dizziness, and general weakness that resolved without treatment within a few days.
3.4. Acute relapses
Within the first 30 days following the third vaccine dose, three patients presented with an acute severe relapse, consisting of 1.4% of the study population; after a median follow-up of 66 days, a total of 7 patients (3.3%) presented with an acute severe relapse. The median time to acute relapse was 34 days (range 14–67 days) from the third vaccine administration. All acute relapses were treated with high-dose intravenous methylprednisolone for five consecutive days.
3.5. Anti-SARS-CoV-2 IgG humoral immune response
Anti-SARS-CoV-2 IgG humoral immune response was assessed at least twice in 55 vaccinated MS patients (26.0% of the cohort), before and after the administration of the third vaccine dose. This sub-group included 42 females, mean ± SD age 51.5 ± 13.0 years; 20 untreated, and 35 treated with vaccination-safe DMTs as follows: cladribine (5), dimethyl fumarate (5), natalizumab (8), teriflunomide (13), diroximel fumarate (1), glatiramer acetate (1), intravenous immunoglobulins (2).
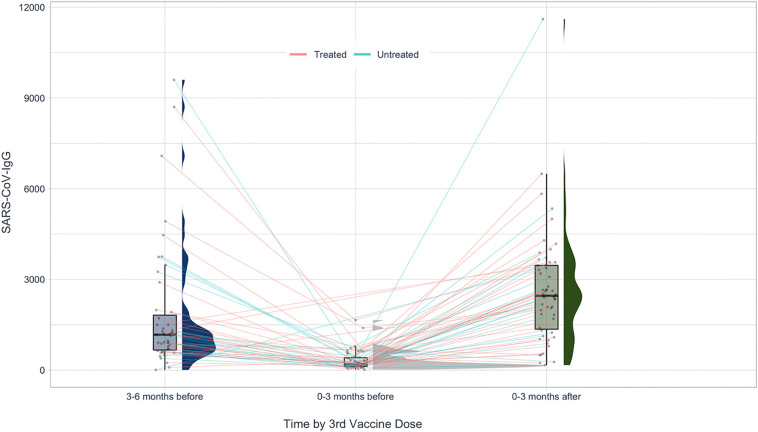
Spike IgG titers (BAU/ml) following the third vaccine dose were heterogeneous among subjects, median 2461, (25 IQR-75 IQR) 1355–3464, range 167.8–11,607. Time from third vaccine dose to IgG test was median 1.5 months, range 0.1–2.8 months. Anti-SARS-CoV-2 IgG levels following the third vaccine dose did not differ between age groups (<50 years, n=32; and ≥50 years, n=23, p = 0.51). Additionally, there was no difference in IgG levels between untreated patients and patients treated with vaccination-safe DMTs (p = 0.56).
Following the third vaccine dose, on average, IgG levels increased 21-fold (median ± SD 21.6 ± 53.05).
In three patients we observed a decrease in IgG levels following the third vaccine dose, compared with their previous result. In one patient, this was due to early testing of IgG titers, performed within three days of vaccine administration. Two additional patients performed only one IgG test between the second and third dose of vaccination, thus making it challenging to observe the trend. Their test was performed approximately four months prior to receiving the third dose, suggesting there was further decrease of IgG titers close to the administration of the third dose.
Fig. 3 demonstrates the paired assessment of circulating SARS-CoV-2 antibody titers following the second and third vaccine doses.
Fig. 3.
Paired SARS-CoV-2 humoral IgG response prior to and following the third vaccine dose in untreated MS patients, and in patients treated with DMTs shown to have no effect on vaccination.
Change in IgG antibody level prior to and following the third booster dose in each group of DMT is presented in Supplementary Table 1.
4. Discussion
In the current study, we explored the safety profile of a third dose of the Pfizer vaccine against SARS-CoV-2 in 2-dose fully vaccinated MS patients.
As the administration of a third dose of the vaccine is not yet common practice in most countries, our study provides important data regarding adverse events in the MS population. Comparing the results acquired in this study we demonstrated that the adverse event profile was similar to that we have reported following the first and second vaccine doses. However, in contrast to our previous findings, age, level of disability, and treatment with DMTs did not significantly affect the frequency of adverse events.
It is important to differently define subjects that receive a booster vaccine dose from subjects that receive a third dose. A booster vaccine suggests that an immune response was generated following the previous vaccine dose, while a third dose does not take into consideration whether an immune response was previously elicited. In our cohort, one patient developed a COVID-19 infection following administration of a third dose. It became evident that immunologically, this subject did not develop SARS-COV-2 IgG humoral response despite receiving three vaccine doses. Treatment with a B-cell depletion medication although not impairing adaptive T-cell response, rendered the patient at risk for COVID-19 infection, as it was recently reported that among fully vaccinated health care workers, the occurrence of SARS-CoV-2 infection correlated with lower neutralizing antibody titers during the peri-infection period [17]. We therefore suggest that assessing IgG humoral response is crucial for determining the vaccination status of immune-compromised patients to evaluate the necessity or redundancy of additional vaccine doses.
Concerning increased disease activity following the third vaccination, we found that the rate of acute relapses within the first 30 days was similar to the rate we reported following the second dose [2]. No events of facial palsy or facial tingling were recorded, and a mere 3.8% of patients reported transient neurological symptomatology, none of them requiring treatment.
We conclude that the third dose of the COVID-19 Pfizer mRNA vaccine is not associated with an increased rate of adverse events or elevated risk of disease activity in MS patients.
Anti-SARS-CoV-2 IgG analysis in a subgroup of 55 patients either untreated or treated with medication shown to have no effect on vaccination in previous doses, revealed a 21-fold increase in IgG titers over time. This finding demonstrates the waning of immunity following the first and second vaccine dose, as well as the efficiency of the third dose in precipitating antibody formation in MS patients. Our data provide longitudinal information regarding the effect of the third dose of the COVID-19 vaccine, and provide a concrete evidence on how beneficial a third dose is based on humoral IgG response. However, further long-term follow-up is of great importance to fully evaluate the safety and lasting efficacy of the third booster dose in preventing infection and severe symptomatology. In addition, to fully portray the complete immune response, analysis of both T-cell and B-cell responses in MS patients is essential. Long term follow up is particularly important to evaluate the durability of both humoral and cellular responses.
As several countries now consider nationwide administration of the third dose of vaccination, we believe our data to be of value to clinicians assessing the risks and benefits for MS patients following the third booster vaccination.
The following is the supplementary data related to this article.
Change in SARS-COV-2 IgG levels prior to and following *Pfizer BNT162b2 COVID-19 third booster vaccination in DMT treated MS patients, N = 35.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare there is no conflict of interest.
Acknowledgements
We thank Ms. Shany Tomer, Mrs. Yamit Titngi, Mrs. Ortal Fyorentino, Mrs. Nurit Amsalem, Mrs. Hagit Zabari, Ms. Neta Sella, Ms. Hen Eisikovitz, Ms. Maria Didkin, and Mrs. Sue Mayust for their outstanding technical help in collecting the data.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 Dec 10;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. J. 2021 Apr 15;135245852110034 doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreyer-Alster S., Dolev M., Menascu S., Zohar D.N., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M., Achiron A. Paper Presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2021 Oct 13–15. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by May 2021. Vienna, Austria. [Google Scholar]
- 4.Chou S., Kaviani K., Ropero B., Russman K., Jobe A., Becker D. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. Incidence of multiple sclerosis relapses and pseudo-relapses following mRNA COVID-19 vaccination. [Google Scholar]
- 5.Lotan I., Wilf-Yarkoni A., Friedman Y., Stiebel-Kalish H., Steiner I., Hellmann M.A. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis: early experience from a tertiary multiple sclerosis center in Israel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sbragia E., Olobardi D., Giovanni N., Lapucci C., Boffa G., Capello E., Cellerino M., Laroni A., Sticchi L., Inglese M. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. Vaccinations in patients with multiple sclerosis: a real-world, single-center, experience. [Google Scholar]
- 7.Ciampi E., Galleguillos L., Pelayo C., Guzman J., Garcia L., Jürgensen L., Guzman I., Uribe-San-Martin R., Soler B., Carcamo C. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. Safety and seroconversion rates of mRNA and inactivated vaccines against SARS-CoV-2 among patients with multiple sclerosis: A multicentric observational study. [Google Scholar]
- 8.Alonso R., Leguizamón F., Silva B.A., Galleguillos Goiry L., Eizaguirre M.B., Rodríguez R., Sosa M., Carballido S., Cruchet V., de Jong-Martis A., Giachello S., Henestroza P., Ferrandina F., Bauer J., Carrá A., Garcea O., Chertcoff A. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. Safety of COVID-19 vaccines in patients with multiple sclerosis from Latin America. [Google Scholar]
- 9.Oreja-Guevara C., Ramirez C.I., Alba-Suarez E., Gomez-Estevez I., Bullón-Sanchez C., Castro Hernandez M., Martinez-Perez E., Díaz-Díaz J. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Austria; Vienna: 2021 Oct 13–15. COVID-19 vaccination and humoral immune response in people with multiple sclerosis. [Google Scholar]
- 10.Achiron A., Mandel M., Dreyer-Alster S., Harari G., Gurevitch M. Humoral SARS-COV-2 IgG decay within 6 months in BNT162b2 mRNA COVID-19 healthy vaccinees: the need for a booster vaccine dose? Eur. J. Int. Med. 2021 Oct 27;94:105–107. doi: 10.1016/j.ejim.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. 2021 Oct 6;395:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021 Jul 28;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., Huppert A. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N. Engl. J. Med. 2021 Oct 7;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achiron A., Mandel M., Dreyer-Alster S., Harari G., Dolev M., Menascu S., Magalashvili D., Flechter S., Givon U., Guber D., Sonis P. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J. Neuroimmunol. 2021 Oct 9:577746. doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 Sep 14:1–2. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak F., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Bystrup A., Byg K.E., Johansen I.S., Mittl K., Rowles W., Mcpolin K. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Multiple Scler. Relat. Disorders. 2021 Nov 1;56 doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021 Oct 14;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in SARS-COV-2 IgG levels prior to and following *Pfizer BNT162b2 COVID-19 third booster vaccination in DMT treated MS patients, N = 35.