Abstract
Mycotoxicoses in animals are caused by exposure to mycotoxin-contaminated feeds. Disease risk is managed using dietary adsorbing agents which reduce oral bioavailability. The objective of this work was to evaluate the efficacy of three selected yeast products as mycotoxin binders using in vitro and in vivo models. Their capacity to adsorb deoxynivalenol (DON), zearalenone (ZEA), and ochratoxin A (OTA) was evaluated using an in vitro model designed to simulate the pH conditions during gastric passage in a monogastric animal. Results showed that only one product, an enzymatic yeast hydrolysate (YHY) of a novel strain Saccharomyces cerevisiae, adsorbed about 45% of DON in solution. Next, we determined the effect of YHY on oral absorption of a DON, ZEA, and OTA mixture using a toxicokinetic model in swine. Toxicokinetic modeling of the plasma concentration-time profiles of DON, OTA, and zearalenone-glucuronide (ZEA-GlcA) showed that YHY tended to reduce the maximal plasma concentration of OTA by 17%. YHY did not reduce oral bioavailability of OTA, DON, and ZEA-GlcA. Within the context of this experiment, and despite some positive indications from both the in vitro and in vivo models employed, we conclude that the YHY prototype was not an effective agent for multiple mycotoxin adsorption.
Keywords: deoxynivalenol, mycotoxins, swine, toxicokinetic model, yeast hydrolysate
1. Introduction
In the context of food and feed, mycotoxins are toxic secondary metabolites formed by fungi growing on agricultural commodities. Mycotoxin contamination is a serious threat to human and animal health and results in considerable economic losses to global animal husbandry. Mycotoxicoses are diseases caused by exposure to mycotoxin-contaminated feeds. In animal production systems, consumption of mycotoxin-contaminated feed can negatively affect performance through decreased feed efficiency, feed refusal, and reduced weight [1,2].
The Fusarium mycotoxin DON is the most frequently occurring mycotoxin in the temperate regions of North America and Western Europe. Other mycotoxins produced by Fusarium include ZEA and fumonisins (FB1 & 2). OTA produced by Aspergillus and Penicillium species is the main contaminant of cereals and soybeans. OTA prevalence and average concentration were reported highest in South Asia. Aflatoxins (AFB1 & B2), produced by Aspergillus species, are frequently found in cereal grains, such as maize, and are most prevalent in South Asia, South-East Asia, and Southern Europe [3,4]. Mycotoxin exposure induces a host of biological responses in animals, and, whole, some mycotoxins are classified as carcinogenic. DON and ZEA are of particular concern for swine due to their high susceptibility. DON consumption is characterized by immunosuppression and reduction of the barrier function of intestinal epithelium [5]. ZEA is best known for its toxic effect on fertility and reproduction in swine: it induces an estrogenic effect on the animal [6]. FB1 exposure has been implicated with lung pneumonitis and pulmonary oedema [7]. The primary target organ for OTA is the kidney; it is known to induce nephropathy in pigs and poultry [8].
Previous in vitro studies have shown that yeast cells can bind mycotoxins through physical adsorption, ion exchange, and complex binding structures on the cell surface and cell wall polysaccharides (β-D-glycan, glucomannan) have been identified as specific binding sites [9,10,11,12,13,14].
In feed, administration of mycotoxin binders results in adsorption of mycotoxins in the gastrointestinal tract, which should result in reduced absorption into the systemic circulation. However, there is little published data available in the literature on the effect of mycotoxin binders on the in vivo absorption of mycotoxins. In piglets administered an oral mycotoxin-contaminated bolus with or without a binder, the agricultural byproduct white grape pomace reduced significantly urinary mycotoxin biomarkers for AFB1 and ZEA [15]. The European Food Safety Authority (EFSA) stipulates that in vivo testing of mycotoxin binders is necessary to evaluate the efficacy of mycotoxins inactivators. However, in vivo studies where non-specific parameters such as body weight, feed intake, or feed conversion rate are measured exclusively do not meet the prerequisite criteria from EFSA to demonstrate efficacy of these products [16,17]. Toxicokinetic studies, based on absorption, distribution, metabolization, and excretion (ADME) of the mycotoxin, are necessary to evaluate the possible effects on the absorption of the toxin in pigs [18]. Similar approaches have notably been used to assess the impact of mycotoxin binders on the relative oral bioavailability of coccidiostats in pigs and chickens [19,20]. The goal of this work was to evaluate the in vitro efficiency of three yeast products as mycotoxin binders and to evaluate the efficacy of a selected hydrolysate on the oral absorption of DON, OTA, and ZEA using a toxicokinetic model in swine.
2. Results
2.1. In Vitro Adsorption of Mycotoxins by Yeast Products
In order to assess the mycotoxin binding capacity of three selected yeast products, the in vitro adsorption/desorption test was used as described in Sabater et al. [21]. This test was designed to mimic the temperature, pH, and passage time through the stomach and gut of a monogastric animal. Briefly, the mycotoxin adsorption capacity of adsorbents was tested by sequential incubations, each step for 2 h at 37 °C under constant agitation, starting at pH 3.0, and followed by pH 5.0 and 8.5. Table 1 and Supplementary Table S1 show the results of in vitro DON, OTA, and ZEA adsorption by the yeast products at the pH levels tested. The yeast enzymatic hydrolysate YHY was able to adsorb about 45% of DON independent (p = 0.2001) of the pH value of the buffer during incubation steps. In contrast, the yeast autolysate products YA1 and YA2 displayed a very low DON adsorption (<12%), which was not significantly different from the control incubations (negative control, NC) without any adsorbent (p > 0.05). None of the yeast hydrolysates showed significant adsorption of OTA (p ˃ 0.1). Contrary to YHY, the products YA1 and YA2 both showed a statistically significant adsorption (p < 0.0001) in the range of 49.7% to 64.7% of ZEA, which was dependent (p < 0.0001) on pH value of the buffer. Effective adsorption of DON by the new yeast ingredient YHY could reduce the oral bioavailability of DON in farm animals, especially key for swine [5], and therefore YHY was selected for further evaluation of its efficacy. Studies concerning in vitro binding between mycotoxins and adsorbents are not always a reliable predictor of in vivo efficacy [15,22]. Therefore, to evaluate the in vivo efficacy of YHY, we have measured specific toxicokinetic parameters using the oral bolus model developed for swine [23].
Table 1.
Concentration of DON, OTA, and ZEA in supernatants (ng/mL) and percent adsorption (%) of mycotoxins by three yeast product treatment groups in an in vitro model designed to mimic the gastric passage of a monogastric animal. The values are indicated as means ± standard deviation (SD) of three independent experiments *.
| DON | |||||||||
| pH 3.0 | pH 5.0 | pH 8.5 | |||||||
| Treatment | ng/mL | % of NC | p Value | (ng/mL) | % of NC | p Value | ng/mL | % of NC | p Value |
| NC | 990 ± 26 | --- | --- | 992 ± 30 | --- | --- | 1012 ± 70 | --- | --- |
| YHY | 540 ± 5 | 45.5 ± 1.0 | <0.0001 | 538 ± 7 | 45.8 ± 0.8 | <0.0001 | 543 ± 20 | 46.7 ± 1.8 | <0.0001 |
| YA1 | 887 ± 36 | 10.3 ± 1.7 | 0.3133 | 890 ± 5 | 10.0 ± 2.1 | 0.3345 | 903 ± 92 | 11.4 ± 2.1 | 0.2551 |
| YA2 | 876 ± 45 | 11.4 ± 2.4 | 0.2049 | 878 ± 26 | 11.3 ± 1.9 | 0.2049 | 963 ± 90 | 4.8 ± 3.6 | 0.9802 |
| OTA | |||||||||
| NC | 32 ± 6 | --- | --- | 57 ± 8 | --- | --- | 68 ± 6 | ---- | --- |
| YHY | 31 ± 3 | 9.3 ± 8.1 | 1.0000 | 52 ± 3 | 8.3 ± 7.6 | 0.9943 | 60 ± 5 | 12.0 ± 3.6 | 0.8283 |
| YA1 | 25 ± 5 | 21.0 ± 7.6 | 0.9525 | 50 ± 9 | 14.0 ± 13.5 | 0.9525 | 66 ± 5 | 8.3 ± 7.6 | 0.9998 |
| YA2 | 27 ± 8 | 16.3 ± 14.8 | 0.9943 | 52 ± 6 | 8.3 ± 7.6 | 0.9943 | 65 ± 5 | 5.0 ± 4.4 | 0.9998 |
| ZEA | |||||||||
| NC | 275 ± 6 | --- | --- | 280 ± 5 | --- | --- | 376 ± 38 | --- | --- |
| YHY | 264 ± 13 | 4.0 ± 3.5 | 0.9998 | 247 ± 11 | 11.7 ± 2.0 | 0.6119 | 328 ± 45 | 13.0 ± 2.7 | 0.1427 |
| YA1 | 125 ± 9 | 54.3 ± 4.0 | <0.0001 | 120 ± 10 | 57.0 ± 3.1 | <0.0001 | 188 ± 8 | 49.7 ± 5.5 | <0.0001 |
| YA2 | 109 ± 5 | 60.3 ± 0.6 | <0.0001 | 107 ± 16 | 62.0 ± 6.2 | <0.0001 | 133 ± 8 | 64.7 ± 3.2 | <0.0001 |
* Calculated in comparison to control incubations without any adsorbent. p values were calculated with SAS Studio using the actual DON concentration value in assay solutions.
2.2. Toxicokinetic Study
In conjunction with DON, the mycotoxins ZEA and OTA were also included in the in vivo toxicokinetic study, due to their relevance for mycotoxicoses in swine [6,8]. The effect of YHY on specific toxicokinetic parameters and relative oral bioavailability after a single oral bolus containing DON, ZEA, and OTA was determined. The control feed was shown to be contaminated with significant levels of DON, namely 1170 µg/kg feed, whereas the maximum guidance level is 900 µg/kg feed [18]. This indicates that all animals were exposed to the mycotoxin for one week prior to the bolus administration. The animals were deprived from feed 12 h prior to the bolus administration and this resulted in the absence of DON, as well as OTA and ZEN-GlcA, in plasma before bolus administration in all animals including the control group (see below).
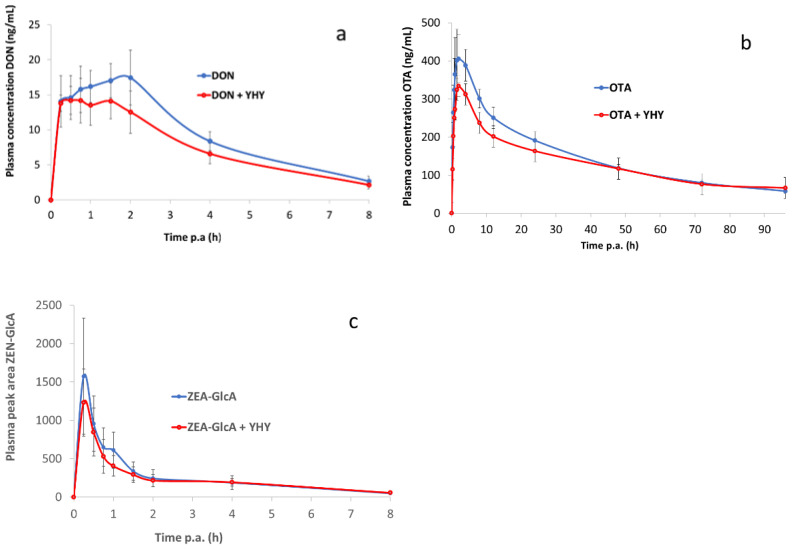
No adverse effects were observed during the animal trial following bolus administration of the three mycotoxins. Figure 1a shows the plasma concentration-time profile of DON following oral administration of DON, whether or not combined with YHY (see Figure S1). Each profile represents the mean of six animals ± standard deviation (SD).
Figure 1.
Plasma concentration-time profile of mycotoxins in piglets after single oral doses of DON (0.05 mg/kg BW) (a), OTA (0.05 mg/kg BW) (b), and ZEA (0.5 mg/kg BW) (c), alone or in combination with yeast hydrolysate YHY (0.1 g/kg BW). Values are presented as mean (n = 6) + SD. p.a: post administration.
The toxicokinetic parameter responses to DON challenge are shown in Table 2 and Table S2. The mean (± SD) AUC0–8 h was 74.57 ± 8.39 h.ng/mL for DON and 60.08 ± 12.49 h.ng/mL for DON combined with YHY. With regard to the absorption phase of DON, a physiologically relevant parameter which occurs in the first 2 h following administration, the mean AUC0–2 h was 30.03 ± 3.87 for 25.76 ± 4.58 for DON combined with YHY. No statistically significant differences for any of the toxicokinetic parameters were observed between groups. The relative oral bioavailability AUC0–8 h and AUC0–2 h for YHY was 80.50% and 85.79%, respectively. The maximum plasma concentration Cmax for DON was numerically reduced by 23% by adsorbent YHY (p = 0.306).
Table 2.
Major toxicokinetic characteristics of DON after single oral bolus administration, with and without the binder YHY. Values are presented as mean (n = 6) ± SD.
| Toxicokinetic Parameters | DON | DON + YHY | p Value |
|---|---|---|---|
| AUC0–8 h (h.ng/mL) | 74.57 ± 8.39 | 60.03 ± 12.58 | 0.462 |
| AUC0–2 h (h.ng/mL) | 30.03 ± 3.87 | 25.76 ± 4.58 | 0.561 |
| Cmax (ng/mL) | 20.17 ± 4.22 | 15.47 ± 1.83 | 0.306 |
| Tmax (h) | 1.13 ± 0.71 | 0.88 ± 0.29 | 0.626 |
| T1/2 el (h) | 2.15 ± 0.10 | 2.39 ± 0.25 | 0.479 |
| ke (1/h) | 0.32 ± 0.01 | 0.30 ± 0.03 | 0.377 |
| Relative F AUC0–8 h (%) | / | 80.50 | |
| Relative F AUC0–2 h (%) | / | 85.79 |
AUC0-t: area under the plasma concentration-time curve from time 0 to 2 or 8 h post administration; Cmax, maximum plasma concentration; Tmax, time at maximum plasma concentration; T1/2 el, elimination half-life; ke, elimination rate constant; and Relative F, relative oral bioavailability.
Figure 1b shows the plasma concentration-time profile of OTA after oral bolus administration (Figure S1). Table 3 shows the derived toxicokinetic results of OTA following oral bolus administration, with or without YHY (Table S3). The mean (±SD) AUC0–96 h of OTA was 14.13 ± 1.48 h.µg/mL for OTA and 12.28 ± 2.06 h.µg/mL for OTA combined with YHY. The timespan of absorption of OTA was longer in comparison with that of DON, as was demonstrated by the Tmax value (2.42 and 1.13 h, respectively). In the first 4 h following administration, the mean (±SD) AUC0–4 h was 1.42 ± 0.24 h.µg/mL for OTA and 1.14 ± 0.25 h.µg/mL for OTA combined with YHY. Moreover, clearance of OTA from the blood was much slower in comparison with DON and ZEA-GlcA, because OTA is reported to bind plasma proteins [24]. This resulted in the observed elimination half-life T1/2 el for OTA of 45.12 h ± 6.61, whereas for DON and ZEA-GlcA, T1/2 el was 2.15 h ± 0.10 (Table 2) and 3.60 h ± 2.00 (Table 4), respectively. In the case of OTA, the relative oral bioavailability AUC0–96 h and AUC0–4 h for YHY was 86.86% and 79.86%, respectively. No statistically significant differences for the toxicokinetic parameters of OTA were observed between the control group and the group receiving YHY, with the exception of a tendency (p = 0.076) for maximum plasma concentration Cmax that was reduced by 17% with the addition of YHY (Table 3).
Table 3.
Major toxicokinetic characteristics of OTA after single oral bolus administration, with and without the binder YHY. Values are presented as mean (n = 6) ± SD.
| Toxicokinetic Parameters | OTA | OTA + YHY | p Value |
|---|---|---|---|
| AUC0–96 h (h.µg/mL) | 14.13 ± 1.48 | 12.28 ± 2.06 | 0.135 |
| AUC0–4 h (h.µg/mL) | 1.42 ± 0.24 | 1.14 ± 0.25 | 0.288 |
| Cmax (ng/mL) | 426.23 ± 85.10 | 353.83 ± 64.21 | 0.076 |
| Tmax (h) | 2.42 ± 1.06 | 2.04 ± 0.65 | 0.853 |
| T1/2 el (h) | 45.12 ± 6.61 | 50.69 ± 12.40 | 0.464 |
| ke (1/h) | 0.016 ± 0.002 | 0.015 ± 0.004 | 0.674 |
| Relative F AUC0–96 h (%) | / | 86.86 | |
| Relative F AUC0–4 h (%) | / | 79.86 |
Abbreviations are as in Table 2.
Table 4.
Major toxicokinetic characteristics of ZEA-GlcA after single oral bolus administration, with and without the binder YHY. Values are presented as mean (n = 6) ± SD.
| Toxicokinetic Parameters | ZEA | ZEA + YHY | p Value |
|---|---|---|---|
| AUC0–8 h (h.peak area/mL) | 2168.20 ± 494.87 | 1917.16 ± 444.98 | 0.985 |
| AUC0–0.5 h (h.peak area/mL) | 505.92 ± 227.89 | 407.48 ± 141.70 | 0.765 |
| Cmax (peak area/mL) | 1627.15 ± 737.12 | 1302.53 ± 485.21 | 0.308 |
| Tmax (h) | 0.33 ± 0.14 | 0.29 ± 0.07 | 0.482 |
| T1/2 el (h) | 3.60 ± 2.00 | 2.93 ± 0.85 | 0.523 |
| ke (1/h) | 0.27 ± 0.12 | 0.27 ± 0.05 | 0.533 |
| Relative F AUC0–8 h (%) | / | 88.42 | |
| Relative F AUC0–0.5 h (%) | / | 80.54 |
Abbreviations are as in Table 2.
Following ZEA administration, all plasma concentrations were below the limit of quantification of the method of 0.5 ng/mL. Therefore, ZEA-GlcA, the major ZEA phase II metabolite, was selected as biomarker in blood plasma for ZEA exposure. To enable the quantification of ZEA-GlcA, ZEA was administered at a relatively high dose of 0.5 mg ZEA/kg BW. In Figure 1c, the chromatographic peak area-time curve is presented of ZEA-GlcA after oral bolus administration of ZEA (Figure S1).
Table 4 shows the results of the most relevant toxicokinetic parameters of ZEA-GlcA after oral bolus administration of ZEA, with and without YHY inclusion (Table S4). The mean (±SD) AUC0–8 h of ZEA-GlcA was 2168.20 ± 494.87 h.peak area/mL for ZEA and 1917.16 ± 444.98 h.peak area/mL for ZEA combined with YHY. ZEA demonstrated a short absorption phase of 0.5 h, and the AUC0–0.5 h of ZEA-GlcA was 505.92 ± 227.89 h.peak area/mL for ZEA and 407.48 ± 141.70 h.peak area/mL for ZEA combined with YHY. The relative oral bioavailability AUC0–8 h and AUC0–0.5 h for YHY was 88.42 and 80.54, respectively. No statistically significant differences or tendencies for any of the toxicokinetic parameters were observed between the treatment groups. Addition of YHY to the oral bolus numerically reduced the maximum plasma concentration Cmax of ZEA-GlcA by 20% (p = 0.308).
3. Discussion
The present work examined the efficacy of the selected yeast product YHY to adsorb the predominant mycotoxins in vitro and in vivo. This bioactive YHY comprises dehydrated enzymatically hydrolyzed yeast cells of a selected strain of S. cerevisiae, which includes the cell wall fraction. The yeast cell wall represents about 30% (w/w) of the weight of the cell. The inner layer is composed of the polysaccharides β-1,3-glucan and highly branched β-1,6-glucan, which together with chitin represents 50–60% of cell wall dry weight and is providing mechanical strength to the wall [11]. The outer cell wall layer is composed of heavily glycosylated mannoproteins, which are involved in cell–cell recognition events and limitation of wall porosity [25,26]. Several studies demonstrated that the yeast cell wall components exhibit many different adsorption sites, as well as different binding mechanisms with mycotoxins such as hydrogen bonds, ionic or hydrophobic interactions, and van der Waals interactions [9,10,11,12,27]. Yeasts and yeast cell wall extracts have shown in vitro adsorption efficacy for a number of mycotoxins, including ZEA, AFB1, T2-toxin, patulin, and OTA [8,11,12,13,28]. However, there are only limited and conflicting reports on DON adsorption by yeasts or by products derived therefrom. It has been reported that DON had an in vitro affinity of around 30% with pure model β-D-glucans at pH 3.0 and 6.0, but no affinity at pH 8.0 [12]. Furthermore, in vitro studies have shown that a commercial preparation of S. cerevisiae mannans has the capacity to adsorb DON [22]. In the latter study, the amount of DON adsorbed in buffers at pH 3 and 7 was between 80 and 90% with a DON and adsorbent concentration of 1–2 µM (0.3–0.6 ppm) and 1 mg/mL, respectively. Additionally, three commercial organic adsorbents (glucomannan, modified yeast cell wall, and esterified glucomannan), tested at 2 mg/mL in an in vitro model with buffer solutions at pH 3 and pH 7, showed 36–56% adsorption of DON (assayed at 0.9 ppm) [29]. In comparison, our study showed a significant (p < 0.0001) in vitro DON adsorption of about 45% carried out in buffers at pH 3, 5, and 8.5 with a concentration of 1 ppm for DON and 2 mg/mL for the adsorbent YHY. The kinetic profile of various trichothecenes, including DON, was investigated during a four-day fermentation with the lager yeast S. pastorianus [30]. The authors reported that during the first 4 h of fermentation, this yeast removed about 13% of the DON from the brewer’s wort. DON removal was reported to be likely due to binding of DON to yeast cells, since after a steady decline over the first 24 h, the DON concentration stabilized at ca. 85% for the remainder of the fermentation period. The manufacturing process of YHY involved a heating and roller drying step, effectively inactivating the processing enzyme and endogenous yeast enzymes, and, hence, suggesting a binding mechanism for DON removal. In the present in vitro study, the stability of the DON-YHY complex was numerically unaffected (p = 0.2001) at a pH scale ranging from 3.0 to 8.5, suggesting the absence of cation exchange mechanism and a more major role of hydrophobic interaction in the binding [14].
The capability of YHY to adsorb DON was further examined using a toxicokinetic model, which indicated that YHY, at a realistic inclusion level corresponding to 2 mg/kg feed, had a limited effect on the absorption of DON, as the maximal plasma concentration and oral bioavailability of DON was only numerically reduced by about 20%. This discrepancy confirms earlier observations of other research groups that in vitro binding between adsorbent and mycotoxins seems to have a limited utility in predicting the in vivo efficacy of binders [15,22].
DON is mainly absorbed in the proximal part of the small intestine by means of passive diffusion [31]. Nevertheless, the present toxicokinetic study showed that a yeast hydrolysate comprising cell wall components was able to reduce the DON plasma concentration in piglets, which, according to the EFSA [18], is the most relevant parameter to evaluate the efficacy of a mycotoxin binder. Additionally, the toxicokinetic study showed that YHY reduced the maximal plasma concentration Cmax for OTA and ZEA-GlcA by 17% and 20%, respectively.
It is important to note that the pig toxicokinetic model system used in this study has limitations. In our study, the SD values for important parameters such as maximal plasma concentration and oral bioavailability for DON, OTA, and ZEA-GlcA were ranging between 10 to 45%, suggesting that the high variability in these measurements limited our ability to determine statistically meaningful differences. Previous studies using the same mycotoxin oral bolus toxicokinetic pig model reported comparable variability for maximal plasma concentration and oral bioavailability values of DON [23,32,33,34].
An additional limitation in the current model was the administration of the DON-contaminated feed one week prior to evaluation of the YHY. During this time, we cannot rule out that the animals mounted a response, e.g., by alteration of the barrier function of intestinal epithelium [5], that would have masked the benefits induced with the introduction of the YHY. It is reported that pigs have the ability to adapt to the presence of DON in the diet [35]. Another study showed that a primary decreased weight gain in pigs fed a DON contaminated diet (1 mg/kg), which is a similar DON contamination level as in our study, in the first week was compensated thereafter [36]. The adaptive mechanism of pigs to DON has not been fully understood. This resistance may be attributed to the effect of DON in the gut and especially its ability to alter the composition of intestinal microbiota [35].
Furthermore, the effect of different dosages of mycotoxin and binder in the oral bolus model was not explored. Future work using this experimental approach merits increased sample size to account for the inherent variability in toxicokinetic evaluations.
It cannot be excluded that the animals receiving YHY experienced a higher-than-expected oral bioavailability of mycotoxins, due to an indirect effect of YHY on intestinal health of the piglets. Evaluation of two mycotoxin detoxifiers that contained yeast cells or esterified glucomannans derived from yeast cell wall S. cerevisiae showed a significantly higher oral bioavailability for DON in broilers for the detoxifying groups compared to the negative control [37]. Furthermore, it was found that a glucomannan mycotoxin binder, in combination with T2-toxin, enhanced the oral absorption of the antibiotic doxycycline in pigs [38]. This observation was confirmed in another study that showed a significant influence of a mycotoxin detoxifying agent, consisting of bentonite-montmorillonite clay with a yeast, on the oral absorption of oxytetracycline in broiler chickens [39]. Although the mechanism is still unclear, it was hypothesized that the mycotoxin binder indirectly promoted intestinal health, changed intestinal immunological parameters, or influenced intestinal mucus production, albeit after a short-term exposure of the animal to the yeast [37]. A recent investigation using the probiotic yeast S. cerevisiae var. boulardii reported that the dietary administration of the probiotic to piglets fed a diet contaminated with DON at 3 mg/kg resulted in significantly lower histological alteration in the intestine, suggesting a better intestinal health, while the effect of DON on plasma metabolome and histological alterations in liver and kidney were attenuated by the yeast. Even if the modes of action involved between a probiotic and mycotoxin binders are likely to be different it appears difficult to conclude that an improved intestinal health would result in an increase oral bioavailability of mycotoxins [40].
Although within the context of this experiment and the described limitations, the YHY prototype was not an effective agent for mycotoxin adsorption, it is important to note that the piglets were administered with a multiple mycotoxin bolus that resembled a feed contamination amount of 1 mg/kg for DON and OTA, and 10 mg/kg for ZEA. This dose was realistic for DON under conventional conditions, whereas for OTA and ZEA, it greatly exceeded (a factor 20 and 100 above EU guidelines, respectively) mycotoxin levels commonly found in feed and raw materials of different geographical origin [4]. Interestingly, our results showed that YHY reduced the absorption of DON in pigs despite the presence of high concentrations of OTA and ZEA, also suggesting that YHY exerted different binding mechanisms for these mycotoxins. The observation of simultaneous binding of mycotoxins by YHY is of particular importance, since animals are generally exposed to multiple mycotoxins present in the feed under field conditions [41].
Additionally, in our study, the influence of feed matrix was not taken into account. Interestingly, it has been suggested that pre-incubation of binder and mycotoxin (as in feed under field conditions) could lead to a more efficient adsorption for B-glucan: i.e., pre-incubation led to a rapid and better reduction of DON absorption in vitro using a Caco-2 cells model [22].
To the best of the authors’ knowledge this is the first study specifically addressing the efficacy of a single yeast hydrolysate ingredient in a toxicokinetic model on the capacity to adsorb multiple mycotoxins in piglets. The outcomes of our toxicokinetic study indicate the need to further improve the experimental model and for a more thorough in vivo evaluation of the efficacy of YHY using natural mycotoxin contaminated feed to evaluate its capability in reducing mycotoxicoses in farm animals under field conditions.
4. Materials and Methods
4.1. Preparation of the Yeast Products
The S. cerevisiae strains tested in vitro were selected based on distinct physiological properties related to yeast cell wall and sterol metabolism (proprietary information). The enzymatic yeast hydrolysate (designated YHY) was prepared from cream of S. cerevisiae strain CNCM I-5405 that was heated to 50 °C, and papain (PromodTM, Biocatalysts, Cardiff, UK) was added. The mixture was incubated for 15 to 20 h at a pH of above 5 for hydrolysis. Next, the mixture was heated for 1 h at a temperature above 70 °C to inactivate all enzyme activity. The pH of the mixture or hydrolysate was then adjusted with NaOH to 6.0, heated to 75 °C for 60 s, and dried by roller drying into powder. The yeast autolysates YA1 and YA2 were prepared from cream of S. cerevisiae strains LYCC6988 and LYCC6382, respectively, without recourse to exogeneous enzymes, and dried by roller drying into powder.
4.2. In Vitro Assessment of pH-Dependent Adsorption/Desorption
All chemicals and solvents used were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were of analytical grade. The adsorption test was performed essentially as described in [21] with DON, ZEA, and OTA concentration in the test buffer of 1, 0.5, and 0.1 ppm. The yeast products were resuspended in PBS solution (CaCl2·2H2O, 1.2 mM; KCl, 2.7 mM; KH2PO4, 1.5 mM; MgCl2·6H2O, 1.1 mM; NaCl, 138 mM; Na2HPO4·2H2O, 8.1 mM; pH = 5.0) to reach a final concentration 2 mg/mL. The pH of the mixtures was adjusted to 3.0 with 1 M HCl and incubated at 37 °C for 2 h under constant agitation to simulate the pH condition during gastric passage in a monogastric animal. After this first incubation step, a sample was taken for further analysis. The incubations were continued in the same flask by raising the pH to pH 5.0 with 1 M NaOH and leaving the incubation mixture for 2 h under constant agitation at 37 °C. After sampling, incubations were continued at a final pH of 8.5. These latter two incubation steps simulate the pH conditions during intestinal passage of a monogastric animal. As a negative control, the PBS solution with mycotoxin and without adsorbent was incorporated in the assay. Samples were immediately centrifuged to separate the binder from the aqueous phase and the supernatants were stored at −20 °C until further analysis by LC-MS/MS. All adsorption tests were carried out in triplicate. The amount of mycotoxin adsorbed by the yeast products was calculated according to the difference between the initial and final concentration of the mycotoxin in the supernatant. Data was analyzed in SAS Studio version 9.4 (SAS Inst. Inc., Cary, NC, USA) using a mixed model with pH and binder as fixed effect. To determine the differences between the binder and pH levels, the Tukey test was used. For all outcomes, statistical significance was declared where p ≤ 0.05.
4.3. Quantification of Mycotoxinsin Supernatants of Adsorption Tests
The mycotoxin concentration in the supernatants was determined by ultra-high performance liquid chromatography with mass spectrometry (LC-MS/MS) (ThermoFischer Scientific Inc., Waltham, MA, USA). For this purpose, 20 µl of an internal standard solution is added to 200 µl of supernatant, mixed by vortex (15 s), and transferred into an autosampler vial. As internal standards were used, 13C15–DON (25 µg/mL), 13C20–OTA (10 µg/mL), and 13C18 –ZEA (25 µg/mL) (Romer Laboratories, Tulln, Austria) in acetonitrile was used. An aliquot of 5 µl was analyzed using a TSQ® Thermo EnduraTM LC-MS/MS equipped with a quaternary, low-pressure mixing Ultimate 3000 pump, a column oven (40 °C ± 1 °C), and a temperature controlled autosampler (ThermoFischer Scientific Inc., Waltham, MA, USA). Chromatographic separation of aliquots was achieved on a reversed phase C18 Ascentis Express column (75 mm × 2.1 mm internal diameter, particle diameter: 2.7 µm), and a guard column of the same type (Sigma-Aldrich, St. Louis, MO, USA). A gradient elution program was performed with 5% (v/v) acetic acid and 0.03854% (w/v) ammonium acetate in water and in methanol. Water, methanol, acetic acid and acetonitrile were of LC-MS grade (VWR International B.V, Amsterdam, The Netherlands). The LC column effluent was interfaced to a TSQ® triple-stage quadrupole mass spectrometer equipped with a heated electronspray ionization (h-ESI) probe (ThermoFischer Scientific Inc., Waltham, MA, USA). The mass spectrometer was operated in the multiple reaction monitoring mode with two ion transitions for each mycotoxin for identification and quantification. LC-MS/MS instrument control and data processing was performed using ThermoScientificTM DionexTM ChromeleonTM Chromatography Data System Version 7.2.9 (Thermo Fischer Scientific Inc., Waltham, MA, USA). The limit of quantification (LOQ) of DON, ZEA, and OTA was 5, 5, and 1 ng/mL, respectively, whereas the limit of detection (LOD) was 2, 2, and 0.5 ng/mL, respectively.
4.4. Animal Study
Twelve female piglets (breed: Hybrid), of 15–20 kg bodyweight (BW), were randomized on arrival in such a way that two groups, each with six animals, were formed with about the same average BW/group. The administration of mycotoxins and the binder YHY to the piglets was as described in [23]. The piglets received commercial pig feed during the acclimatization period (7 days). This feed was also given during the study, except for 12 h prior to bolus administration to 4 h post administration. The feed was given ad libitum. The commercial feed was evaluated on contamination with mycotoxins by a multi-mycotoxin LC-MS/MS method at Primoris (Zwijnaarde, Belgium).
The piglets were administrated a single oral bolus of 0.05 mg DON/kg BW, 0.05 mg OTA/kg BW, and 0.5 mg ZEA/kg BW, by oral gavage using an intragastric tube. This dose resembles a feed contamination amount of 1 mg/kg for DON and OTA, and 10 mg/kg for ZEA. The EU recommendation for maximal tolerable concentration in pig feed for DON, OTA, and ZEA is 0.9, 0.05, and 0.1 mg/kg, respectively [18]. All mycotoxins used in the animal study were purchased from Fermentek® (Jerusalem, Israel). DON (1 mg/mL), OTA (1 mg/mL), and ZEA (10 mg/mL) were dissolved in ethanol and further diluted with tap water to a volume of 10 mL. Six of the twelve piglets received the mycotoxin bolus in combination with the yeast hydrolysate (100 mg/kg BW), resembling an inclusion amount of 2 g/kg feed), suspended in 10 mL of tap water, in a parallel study design. Immediately after administration of the bolus, the intragastric tube was rinsed with 50 mL of tap water. Blood samples (2 mL), drawn from the vena jugularis externa, were taken in EDTA tubes and centrifuged (2851× g, 10 min, 4 °C) to obtain plasma. The timepoints of blood sampling were 0 h (just before administration) and 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h post administration (p.a.). Aliquots (250 µL) of plasma samples were stored at −20 °C until analysis.
4.5. Quantification of Mycotoxins in Plasma
The analysis of DON, OTA, ZEA and the main phase II metabolite of ZEA, namely ZEA-GlcA, in plasma was performed by using a validated UHPLC-MS/MS method [42]. For ZEA-GlcA peak areas were corrected for the internal standard (IS, 13C-ZEA) and results are presented as peak area ratio (=peak area ZEA-GlcA/peak area IS).
4.6. Toxicokinetic Analysis
Toxicokinetic modeling of the plasma concentration-time profiles of DON, OTA, and ZEA-GlcA was done by non-compartmental analysis (Phoenix 8.1, Pharsight Corporation, Sunnyvale, CA, USA). No ZEA was quantifiable in plasma, hence the use of ZEA-GlcA as biomarker for exposure. The following parameters were calculated: area under the curve from time zero to 0.5, 2, 4, 8, and/or 96 h (AUC0–0.5 h, AUC0–2 h, AUC0–4 h, AUC0–8 h or AUC0–96 h); maximal plasma concentration (DON and OTA) or maximal plasma chromatographic peak area (ZEA-GlcA) (Cmax); time at Cmax (Tmax), elimination half-life time (T1/2el); and elimination rate constant (kel).
4.7. Effect of the Mycotoxin Binder on Oral Absorption of the Mycotoxins
The relative oral bioavailability expressed as a percentage, F = ([AUC0–0.5/2/4/8 h or 96 h mycotoxin + binder/AUC0–0.5/2/4/8 h or 96 h mycotoxin]×100), was evaluated as marker for efficacy of the mycotoxin binder. The effect of the mycotoxin binder on the oral absorption of the mycotoxin was evaluated by comparing the above described toxicokinetic parameters between the mycotoxin and mycotoxin plus binder treated piglets. A one-way ANOVA was performed (SPSS, IBM, Armonk, NY, USA), with the LSD-test as post hoc test, to evaluate possible significant differences between groups for each toxicokinetic parameter. Homogeneity of variances was first evaluated using the Levene’s test. When variances were not homogeneous, data were log-transformed. The level of significance was set at p ≤ 0.05.
Acknowledgments
The authors would like to thank Mathias Devreese and John Doelman for critically reading the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins14010007/s1, Table S1: In vitro binding raw data, Figure S1: Mycotoxin-time curves raw data, Table S2 Final parameters toxicokinetic study, Table S3 Final parameters toxicokinetic study, Table S4 Final parameters toxicokinetic study.
Author Contributions
Conceptualization, P.G.B. and M.C.; methodology, P.G.B. and M.C.; software, P.G.B.; validation, P.G.B.; formal analysis, P.G.B.; investigation, P.G.B.; resources, P.G.B. and M.C.; data curation, P.G.B.; writing—original draft preparation, P.G.B.; writing—review and editing, P.G.B. and M.C.; visualization, P.G.B.; supervision, P.G.B.; project administration, P.G.B.; funding acquisition, P.G.B. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study was approved by CER’s Ethical Committee and identified by the number CE/Sante/ET/residus-1. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of CER GROUPE, Marloie, Belgium (study protocol code no 19 131, 21 March 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request; please contact the contributing authors.
Conflicts of Interest
Paul Bruinenberg is employed by Trouw Nutrition and Mathieu Castex by Lallemand SAS. Both companies provide feed additive solutions.
Key Contribution
A novel yeast hydrolysate effectively adsorbed deoxynivalenol in an in vitro model designed to mimic the gastric passage in a monogastric animal.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pierron A., Alassane-Kpembi I., Oswald I.P. Impact of mycotoxins on immune response and consequences for pig health. Anim. Nutr. 2016;2:63–68. doi: 10.1016/j.aninu.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidal A., Mengelers M., Yang S., De Saeger S., De Boevre M. Mycotoxin biomarkers: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018;17:1127–1155. doi: 10.1111/1541-4337.12367. [DOI] [PubMed] [Google Scholar]
- 3.Schatzmayer G., Streit E. Global occurrence of mycotoxins in food and feed chain: Facts and figures. World Mycotoxin J. 2013;6:213–222. doi: 10.3920/WMJ2013.1572. [DOI] [Google Scholar]
- 4.Guerre P. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins. 2016;8:350. doi: 10.3390/toxins8120350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh A., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins. 2015;7:2071–2095. doi: 10.3390/toxins7062071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fink-Gremmels J., Malekinejad H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Technol. 2007;137:326–341. doi: 10.1016/j.anifeedsci.2007.06.008. [DOI] [Google Scholar]
- 7.Voss K.A., Smith G.W., Haschek W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007;137:299–325. doi: 10.1016/j.anifeedsci.2007.06.007. [DOI] [Google Scholar]
- 8.Piotrowska M., Masek A. Saccharomyces cerevisiae cell wall components as tools for ochratoxin A decontamination. Toxins. 2015;7:1151–1162. doi: 10.3390/toxins7041151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouany J.-P., Yiannikouris A., Bertin G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch. Zootech. 2005;8:26–50. [Google Scholar]
- 10.Ringot D., Lerzy B., Bonhoure J.P., Auclair E., Oriol E., Larondelle Y. Effect of temperature on in vitro ochratoxin bisorption onto yeast cell wall derivatives. Process Biochem. 2005;40:3008–3016. doi: 10.1016/j.procbio.2005.02.006. [DOI] [Google Scholar]
- 11.Yiannikouris A., François J., Poughon L., Dussap C.-G., Bertin G., Jeminet G., Jouany J.-P. Alkali extraction of beta-D-glucans from Saccharomyces cerevisiae cell wall and study of their adsorptive properties toward zearalenone. J. Agric. Food Chem. 2004;52:3666–3673. doi: 10.1021/jf035127x. [DOI] [PubMed] [Google Scholar]
- 12.Yiannikouris A., André G., Poughon L., François J., Dussap C.-G., Jeminet G., Bertin G., Jouany J.-P. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules. 2006;7:1147–1155. doi: 10.1021/bm050968t. [DOI] [PubMed] [Google Scholar]
- 13.Faucet-Marquis V., Joannis-Cassan C., Hadjeba-Medjdoub K., Ballet N., Pfohl-Leszkowicz A. Development of an in vitro method for the prediction of mycotoxin binding on yeast-based products: Case of aflatoxin B1, zearalenone and ochratoxin A. Appl. Microbiol. Biotechnol. 2014;98:7583–7596. doi: 10.1007/s00253-014-5917-y. [DOI] [PubMed] [Google Scholar]
- 14.Shetty P.H., Jespersen L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006;17:48–55. doi: 10.1016/j.tifs.2005.10.004. [DOI] [Google Scholar]
- 15.Gambacorta L., Pinton P., Avantaggiato G., Oswald I.P., Solfrizzo M. Grape pomace, an agricultural byproduct reducing mycotoxin absorption: In vivo assessment in pig using urinary biomarkers. J. Food Chem. 2016;64:6762–6771. doi: 10.1021/acs.jafc.6b02146. [DOI] [PubMed] [Google Scholar]
- 16.EFSA Review of Mycotoxin-Detoxifying agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. 8 December 2009. [(accessed on 21 October 2021)]. Available online: http://www.efsa.europa.eu/en/scdocs/scdoc/22e.htm.
- 17.EFSA Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010;8:1693. doi: 10.2903/j.efsa.2010.1693. [DOI] [Google Scholar]
- 18.European Commission COMMISSION RECOMMENDATION of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 2006;L229:7–9. [Google Scholar]
- 19.De Mil T., Devreese M., De Saeger S., Eeckhout M., De Backer P., Croubels S. Influence of mycotoxin binders on the oral bioavailability of doxycycline in pigs. J. Agric. Food Chem. 2016;64:2120–2126. doi: 10.1021/acs.jafc.5b06084. [DOI] [PubMed] [Google Scholar]
- 20.De Mil T., Devreese M., Maes A., De Saeger S., De Backer P., Croubels S. Influence of mycotoxin binders on the oral bioavailability of tylosin, doxycycline, diclazuril, and salinomycin in fed broiler chickens. Poult. Sci. 2017;96:2137–2144. doi: 10.3382/ps/pew503. [DOI] [PubMed] [Google Scholar]
- 21.Sabater-Vilarand M., Malekinejad H., Selman M.H.J., van der Doelen M.A.M., Fink-Gremmels J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia. 2007;163:81–90. doi: 10.1007/s11046-007-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavret S., Laurent N., Videmann B., Mazallou M., Lecoeur S. Assessment of deoxynivalenol (DON) adsorbents and characterization of their efficacy using complementary in vitro tests. Food Addit. Contam. 2010;27:43–53. doi: 10.1080/02652030903013252. [DOI] [PubMed] [Google Scholar]
- 23.Devreese M., Antonissen G., De Backer P., Croubles S. Efficacy of active carbon towards the absorption of deoxynivalenol in pigs. Toxins. 2014;5:2998–3004. doi: 10.3390/toxins6102998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwerdt G., Freudinger R., Silbernagagl S., Gekle M. Ochratoxin A-binding proteins in rat organs and plasma and in different cell lines of the kidney. Toxicology. 1999;135:1–10. doi: 10.1016/S0300-483X(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 25.Kollar R., Reinhold B.B., Petrakova E., Yeh H.J.C., Ashwell G., Drgonova J., Kapteyn J.C., Klis F.M., Cabib E. Architecture of the yeast cell wall: β(1,6)-D-glucan interconnects mannoprotein, β(1,3)-D-glucan, and chitin. J. Biol. Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 26.Manners D.J., Masson A.J., Patterson J.C. The structure of a β(1,3)-D-glucan from yeast cell walls. Biochem. J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiannikouris A., François J., Poughon L., Dussap C.-G., Bertin G., Jeminet G., Jouany J.-P. Adsorption of zearalenone by β-D-glucans in the Saccharomyces cerevisiae cell wall. J. Food Prot. 2004;67:1195–1200. doi: 10.4315/0362-028X-67.6.1195. [DOI] [PubMed] [Google Scholar]
- 28.Freimund S., Sauter M., Rys P. Efficient adsorption of the mycotoxins zearalenone and T-2 toxin on a modified yeast glucan. J. Environ. Sci. Health B Pestic. Food Contam. Agric. Wastes. 2003;38:243–255. doi: 10.1081/PFC-120019892. [DOI] [PubMed] [Google Scholar]
- 29.Kolawole O., Meneely J., Greer B., Chevallier O., Jones D.S., Connoly L., Elliot C. Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins. 2019;11:659. doi: 10.3390/toxins11110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathanail A.V., Gibson B., Han L., Peltonen K., Ollilainen V., Jestoi M., Laitila A. The lager yeast Saccharomyces pastorianus removes and transforms trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016;203:448–455. doi: 10.1016/j.foodchem.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 31.Grenier B., Applegate T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catteuw A., Devreese M., De Baere S., Antonissen G., Ivanova L., Uhlig S., Martens A., De Saeger S., De Boevre M., Croubels S. Investigation of age-related differences in toxicokinetic processes of deoxynivalenol and dexoxynivalenol-3-glucoside in weaned piglets. Arch. Toxicol. 2020;94:417–425. doi: 10.1007/s00204-019-02644-x. [DOI] [PubMed] [Google Scholar]
- 33.Broekaert N., Devreese M., De Mil T., Fraeyman S., Antonissen G., De Baere S., De Backer P., Vermeulen A., Croubels S. Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol in broiler chickens and pigs. J. Agric. Food Chem. 2015;63:8734–8742. doi: 10.1021/acs.jafc.5b03270. [DOI] [PubMed] [Google Scholar]
- 34.Broekaert N., Devreese M., van Bergen T., Schauvliege S., De Boevre M., De Saeger S., Vanhaecke L., Berthiller F., Michlmayr H., Malachová A., et al. In vivo contribution of deoxynivalenol-3-β-D-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017;91:699–712. doi: 10.1007/s00204-016-1710-2. [DOI] [PubMed] [Google Scholar]
- 35.Serviento A.M., Brossard L., Renaudeau D. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behaviour in finishing pigs. J. Anim. Sci. 2018;96:5209–5221. doi: 10.1093/jas/sky378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friend D.W., Trenholm H.L., Elliot J.I., Thompson B.K., Hartin K.E. Effect of feeding vomitoxin-contaminated wheat to pigs. Can. J. Anim. Sci. 1982;62:1211–1222. doi: 10.4141/cjas82-141. [DOI] [Google Scholar]
- 37.Devreese M., Osselaere A., Goossens J., Vandenbroucke V., De Baere S., Eeckhout M., De Baeker P., Croubels S. New bolus models for in vivo efficacy testing of mycotoxin-detoxifying agents in relation to EFSA guidelines, assessed using deoxynivalenol in broiler chickens. Food Addit. Contam. Part A. 2012;29:1101–1107. doi: 10.1080/19440049.2012.671788. [DOI] [PubMed] [Google Scholar]
- 38.Goossens J., Vandenbroucke V., Pasmans F., De Baere S., Devreese M., Osselaere A., Verbrugghe E., Haesebrouck F., De Saeger S., Eeckhout M., et al. Influence of mycotoxin detoxifying agents on oral bioavailability of commonly used antibiotics in pigs. Toxins. 2012;4:281–295. doi: 10.3390/toxins4040281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osselaere A., Devreese M., Watteyn A., Vandenbroucke V., Goossens J., Haetekiet V., Eeckhout M., De Saeger S., De Baere S., De Backer P., et al. Efficacy and safety testing of mycotoxin-detoxifying agents in broilers following EFSA guidelines. Poult. Sci. 2012;91:2046–2054. doi: 10.3382/ps.2012-02245. [DOI] [PubMed] [Google Scholar]
- 40.Alassane-Kpembi M., Canlet C., Tremblay-Franco M., Jourdan F., Chalzaviel M., Pinton P., Cossalter A.M., Achard C., Castex M., Combes S., et al. 1H-NMR metabolomics response to a realistic diet contamination with the mycotoxin deoxynivalenol: Effect of probiotics supplementation. Food Chem. Toxicol. 2020;138:111222. doi: 10.1016/j.fct.2020.111222. [DOI] [PubMed] [Google Scholar]
- 41.Kovalsky P., Kos G., Nährer K., Schwab C., Jenkins T., Schatzmayer G., Sulyok M., Krska R. Co-occurrence of regulated, masked and emerging mycotoxins in finished feed and maize-An extensive survey. Toxins. 2016;8:363. doi: 10.3390/toxins8120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauwers M., De Baere S., Letor B., Rychlik M., Croubels S., Devreese M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and phase II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins. 2019;11:171. doi: 10.3390/toxins11030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request; please contact the contributing authors.