Abstract
Introduction
Coronavirus disease of 2019 (COVID-19) has resulted in millions of cases worldwide. As the pandemic has progressed, the understanding of this disease has evolved.
Objective
This first in a two-part series on COVID-19 updates provides a focused overview of the presentation and evaluation of COVID-19 for emergency clinicians.
Discussion
COVID-19, caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), has resulted in significant morbidity and mortality worldwide. Several variants exist, including a variant of concern known as Delta (B.1.617.2 lineage) and the Omicron variant (B.1.1.529 lineage). The Delta variant is associated with higher infectivity and poor patient outcomes, and the Omicron variant has resulted in a significant increase in infections. While over 80% of patients experience mild symptoms, a significant proportion can be critically ill, including those who are older and those with comorbidities. Upper respiratory symptoms, fever, and changes in taste/smell remain the most common presenting symptoms. Extrapulmonary complications are numerous and may be severe, including the cardiovascular, neurologic, gastrointestinal, and dermatologic systems. Emergency department evaluation includes focused testing for COVID-19 and assessment of end-organ injury. Imaging may include chest radiography, computed tomography, or ultrasound. Several risk scores may assist in prognostication, including the 4C (Coronavirus Clinical Characterisation Consortium) score, quick COVID Severity Index (qCSI), NEWS2, and the PRIEST score, but these should only supplement and not replace clinical judgment.
Conclusion
This review provides a focused update of the presentation and evaluation of COVID-19 for emergency clinicians.
Keywords: Coronavirus-2019, COVID-19, Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2
1. Introduction
Coronavirus disease of 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), has resulted in a global pandemic [[1], [2], [3], [4]]. The initial outbreak in late 2019 consisted of 27 patients with pneumonia in Wuhan, Hubei Province, China [1,2]. The virus spread rapidly around the world and was initially declared a pandemic on March 11, 2020 [[1], [2], [3]]. Recent variants, including the Delta and Omicron variants, have resulted in significant increases in cases [4]. As of December 31, 2021, over 287 million cases have occurred worldwide, with over 5.4 million deaths [4]. In the United States, there have been over 54.5 million confirmed cases and over 825,000 deaths [4]. This pandemic has resulted in significant challenges worldwide, and our understanding of this disease continues to evolve. This paper is the first in a two-part narrative review that will provide a focused update on the presentation and evaluation of COVID-19 for emergency clinicians.
2. Methods
A literature review of PubMed and Google Scholar databases was performed for articles up to December 31, 2021, using the keywords ‘COVID’ OR ‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘coronavirus’ for this narrative review. The authors included retrospective and prospective studies, systematic reviews and meta-analyses, clinical guidelines, and other narrative reviews. Commentaries and letters were also included. The literature search was restricted to studies published or translated into English. Authors reviewed all relevant articles and decided which studies to include for the review by consensus, with focus on emergency medicine-relevant articles, including guidelines. A total of 194 resources were selected for inclusion in this review.
3. Discussion
3.1. Virology and variants
SARS-CoV-2 is an enveloped positive-stranded RNA virus, which binds to the angiotensin-converting enzyme 2 (ACE2) receptor and enters cells via the coronavirus spike proteins 1 and 2 [1,3,5]. These spike proteins possess multiple cleavage sites, which may increase the pathogenicity of the virus [1,3,[5], [6], [7]]. Gradual accumulation of small genetic changes in these viral spike proteins results in antigenic drift and different variants. There are three types of variant classifications, stratified as those of interest, those of concern, and those of high consequence [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]] (Table 1 ). The current variants of greatest concern include the Delta (B.1.617.2 lineage) and the Omicron variant (B.1.1.529 lineage) [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]].
Table 1.
| Variant Classification | Definition | Specific Variants |
|---|---|---|
| Variant of interest | Predicted to affect transmission, diagnosis, treatment | – |
| Evidence of increased transmission, outbreak clusters | ||
| Variant of concern | Attributes of variant of interest | B.1.1.7 (Alpha) – first isolated in United Kingdom, 50% increased transmission, may increase mortality |
| Evidence of increased transmissibility and/or disease severity | B.1.351 (Beta) – first isolated in South Africa, increased immune evasiveness, 50% increased transmission | |
| B.1.617.2 (Delta) – first isolated in India, likely 50% more transmissible than Alpha, may evade full vaccination and increase rate of infection, likely increases mortality | ||
| B.1.427 and B.1.429 (Epsilon) – first isolated in California, 20% increased risk of transmissibility | ||
| P.1 (Gamma) – first isolated in Brazil/Japan, likely increased disease transmissibility and severity | ||
| B.1.526 (Iota) – first isolated in New York, likely increased transmissibility but not more severe disease | ||
| B.1.1.529 (Omicron) - first isolated in South Africa, present in over 90 countries, predominant strain in U.S., over 50 mutations in spike protein | ||
| Variant of high consequence | Attributes of variant of concern | None as of December 31, 2021 |
| Evidence of more severe infection, increased hospitalization, decreased vaccine and treatment efficacy, failure of diagnostics |
Viral particles are present in respiratory droplets, aerosols, blood, ocular secretions, urine, and stool, but it is primarily spread through direct person-to-person respiratory transmission [[16], [17], [18],[20], [21], [22], [23], [24], [25], [26]]. The virus is spread from the mouth and nose as droplets and smaller aerosolized particles, which may become airborne and travel past 6 f. [17,18,20]. Of note, over 50% of viral transmission occurs in those with no symptoms, and viral shedding may occur 3 days before the onset of symptoms [20,27]. With symptom onset, viral load peaks [20,27]. While contaminated surfaces are not thought to be a significant cause of transmission, infection may still occur if a person touches their eyes, nose, or mouth with contaminated hands. The incubation period is typically 4 to 5 days, though it ranges from 1 to 14 days [13,[28], [29], [30]]. The initial estimate of the reproductive number, or R0, of the virus was 4.7–6.6 (the number of expected cases originating from a single infected individual) [29]. With early infection control and vaccines, this number decreased to approximately 1.5 [30]. The Delta variant is significantly more transmissible, with an estimated R0 ranging between 6 and 7 [13,28]. The Omicron variant is even more transmissible, approximately 3.2 times that compared to the Delta variant and has a 3-day doubling time [31,32]. Transmission after 7–10 days of symptoms is highly unlikely [28].
The Delta variant is associated with higher viral loads, up to 1000 times higher than other strains, with earlier and prolonged shedding [11,19,28]. It is also associated with higher rates of hospitalization and mortality [11,19,28]. One study found the Delta variant was associated with higher odds of oxygen requirement, need for intensive care unit (ICU) admission, or death (adjusted odds ratio [aOR] 4.90, 95% [confidence interval] CI 1.43–30.78) [19]; other studies have found approximately double the risk of hospitalization [33,34]. A study of over 40,000 patients infected with COVID-19 also found a higher risk of hospitalization associated with the Delta variant, with an adjusted hazard ratio of 2.26 (95% CI 1.32–3.89) [34]. Evidence suggests vaccinated patients with the Delta variant have similar nasopharyngeal viral loads compared to non-vaccinated patients, even though they demonstrate fewer or even no symptoms [11].
The most recent variant of concern, B.1.1.529, also known as Omicron, was identified in South Africa on November 9, 2021, and reported to the World Health Organization (WHO) on November 24, 2021 [14,15]. The WHO declared it a variant of concern on November 26, 2021 [14]. By November 28, 2021, the variant had been identified in South Africa, Belgium, Botswana, Hong Kong, Israel, Italy, Netherlands, and the United Kingdom. By December 25, 2021, the variant was present in over 90 countries and made up over 58% of all new infections in the United States [32]. The Omicron variant possesses over 50 mutations and has quickly become the predominant strain in many countries [14,15,32,35]. While prior infection with SARS-CoV-2 was thought to provide an estimated 80% reduction in infection with other strains, evidence suggests the Omicron variant is associated with increased ability to evade immunity from prior infection [32,[36], [37], [38], [39], [40]]. Fortunately, recent literature suggests vaccinated patients have strong protection against severe illness from the Omicron variant [[41], [42], [43]]. While two vaccine doses without a booster demonstrate less effectiveness in preventing infection with the Omicron variant compared with other strains, data suggest two doses still reduce the risk of severe disease [[41], [42], [43]]. Data are controversial whether the Omicron variant is associated with reduced disease severity [[43], [44], [45]]. Several reports announced a decrease in disease severity, with one finding a 29% reduction in hospitalization rate in those infected with the Omicron variant [[43], [44], [45]]. A report released on December 22, 2021, found a 20–25% reduced risk of any hospitalization and 40–45% reduced risk of multiday hospitalization [45]. Further studies are underway evaluating vaccine efficacy and the risk of severe disease associated with the Omicron variant.
3.2. Disease severity
COVID-19 infection is generally divided into symptomatic and asymptomatic, with symptomatic cases further categorized as critical, severe, and non-severe [2,46] (Table 2 ). The majority of patients have mild disease (over 80%) [2,3,[46], [47], [48]-33]. Clinically asymptomatic infection rates may approximate 33% of those testing positive for COVID-19 based on one meta-analysis, but this number varies [20,47,48]. However, literature suggests severe disease (defined as hypoxia or > 50% lung involvement) can occur in over 15% of patients and critical disease (consisting of respiratory failure, multiorgan injury, or shock) in up to 5%, though this depends on the patient population [[1], [2], [3],26,48,49]. More recently a third category of pre-symptomatic proposes that as many as half of these persons who do not declare symptoms at the time of positive testing develop symptoms later [47,50,51].
Table 2.
| Classification | Consideration |
|---|---|
| Critical | Acute respiratory distress syndrome, sepsis, septic shock, or other conditions requiring life-sustaining therapies (mechanical ventilation or vasopressor therapy) |
| Severe | Oxygen saturation < 90%, signs of severe respiratory distress (accessory muscle use, unable to speak in full sentences) *The 90% threshold is not definitive and should only be used as a component of the whole clinical picture |
| Non-severe | Any patient not meeting criteria for critical or severe |
Reported rates of hospitalization, mechanical ventilation, and mortality vary significantly due to several variables including patient age, healthcare and testing availability, and containment measures, among others. Initial studies suggested high rates of hospitalization and mortality, but with current therapies and vaccination, risks of hospitalization, mechanical ventilation, and mortality have declined [1,2,20,[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]]. Early in the pandemic, overall mortality rates for admitted patients reached 20%, but in those admitted to the ICU, mortality approximated 40% [1,2,20,[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]]. As the pandemic has progressed, ICU survival rates have improved from 58% to 80% [62]. Of those hospitalized with COVID-19, up to 35% require admission to an ICU [20,48,52]. More recent literature suggests the case fatality rate is under 2% in all patients with COVID-19, though this depends on age [20,48,57]. In those over age 60 years this rises to 6.4%, in those over age 80 years it is over 13%, and in those over age 90 years mortality is over 25% [57].
Several factors are associated with worse prognosis in patients with COVID-19. Risk factors for severe disease include age > 75 years, diabetes, cancer, history of transplant, hypertension, and prior cardiac or pulmonary disease [23,26,52,[63], [64], [65], [66], [67]]. Obesity is associated with increased mortality and need for intubation, independent of other factors including race, sex, and other comorbidities, especially in patients less than 65 years of age [68,69]. One study suggested mortality was four-fold higher in patients with a body mass index (BMI) > 45 [69]. Heart failure is associated with longer hospital length of stay, increased need for intubation and ventilation, and mortality [70]. Unfortunately, the Delta variant is also associated with worse outcomes, including need for hospitalization, ICU admission, and mortality [33,71]. Other poor prognostic factors include an initial oxygen saturation < 88%, lymphopenia, thrombocytopenia, acute kidney injury, elevated lactate dehydrogenase, C-reactive protein (CRP) > 200 mg/L, D-dimer >2500 ng/mL, elevated troponin, and ferritin >2500 ng/mL [23,26,52,63].
3.3. Clinical presentation
Approximately 98% of patients who develop symptoms will do so within 12 days of viral exposure [3,20]. Although symptomatic COVID-19 patients exhibit a variety of signs and symptoms, most present with fever, changes in taste and/or smell, myalgias, and respiratory tract symptoms such as cough [[1], [2], [3],20,52,[72], [73], [74], [75], [76]]. However, there are no clinical features with high enough specificity to reliably differentiate COVID-19 from other infections for diagnosis [75]. Literature suggests that the most common symptoms include cough (60–86%), shortness of breath (53–80%), and change in taste or smell disturbance (64–80%) [[72], [73], [74], [75], [76]]. Fever can be present in approximately half of patients at the time of initial presentation depending on the study, but overall, literature suggests 20–99% of patients experience fever during the course of the disease [1,2,18,23,49,53,72]. Literature varies on the definition of fever, with temperature thresholds as low as 37.1C [75,77,78].
Viral pneumonia, hypoxemic respiratory failure, and acute respiratory distress syndrome (ARDS) may result from COVID-19, with hypoxemic respiratory failure the most common reason for ICU admission [[1], [2], [3],20,23,60,74,75]. Bacterial or fungal co-infections affect up to 8% of patients [79]. These are a major source of morbidity and mortality; in one study, half of those who died experienced a secondary infection [23,80,81]. Bacterial respiratory infections most commonly include Streptococcus pneumoniae, Klebsiella pneumoniae, and Haemophilus influenzae, though other infections such as pulmonary aspergillosis and mucormycosis may also occur [[80], [81], [82], [83], [84], [85], [86], [87]]. Mucormycosis has primarily been documented in India, with diabetes and steroid treatment the most common risk factors [[84], [85], [86], [87]]. Concurrent viral infections are also common, with one study finding 20.7% of patients with COVID-19 infected with at least one other virus [82].
3.4. Extrapulmonary presentations and complications
3.4.1. Cardiac
As the pandemic has progressed, a variety of extrapulmonary effects have been uncovered. Myocardial disease, manifested by dysrhythmias, acute coronary syndrome (ACS), heart failure, and myocarditis [22,[88], [89], [90]-76], occur in over 20% of patients admitted to the ICU with COVID-19 [22]. Dysrhythmias include AV blocks, bradycardia, and supraventricular and ventricular tachycardias [22,[88], [89], [90]]. Torsades de pointes may occur due to QT prolongation. The QT interval can be prolonged due to electrolyte changes (diarrhea, dehydration), systemic inflammation, and comorbidities (preexisting cardiac disease) [88,91,92]. ACS is likely associated with severe inflammation [[88], [89], [90], [91], [92], [93], [94], [95], [96]]. This may result in plaque rupture and ST elevation myocardial infarction [[93], [94], [95]]. Cardiomyopathy, heart failure, and myocarditis can also occur, with acute left heart failure occurring in 23% of patients [23,88,90,92]. Left heart failure is associated with increased mortality and was present in 52% of those who died in one study [23]. Right heart failure is more likely associated with lung injury and ARDS, as well as the hyperinflammatory state, thrombotic events, and viral damage [96,97]. Literature suggests right ventricular dilation occurs in 20–31% of cases [96,97]. Myocarditis is more likely in those with heart failure or shock who have no prior history of cardiac disease [88,90]. Myocarditis was the cause of death in 7% of patients and contributed to one third of deaths in one study [23]. However, cardiac involvement is likely more common than originally thought. One study of patients with mild to moderate COVID-19 who underwent cardiac magnetic resonance imaging found abnormal findings in 78%, most commonly cardiac inflammation [98], and a second study found 56% of patients had cardiac inflammation and edema [99]. Other studies suggest cardiac involvement is present even in patients with minimal or no symptoms [100].
3.4.2. Neurologic
Neurologic effects associated with the disease vary, ranging from mild illness to severe manifestations such as stroke. Up to 80% of patients experience neurologic symptoms during the course of the disease [101]. Mild symptoms such as headache and dizziness affect up to 40% of patients during acute illness, which may increase as the illness progresses [[101], [102], [103]]. Changes in smell and taste are common. This may be the first symptom in approximately one-third of patients [102,103]. Up to 80% of patients will experience a change in taste or smell during the course of illness, and almost half will have complete loss of taste or smell [72,75,103,104]. Literature suggests severe neurologic complications occur in a significant number of patients with COVID-19, including seizure, encephalopathy, and cerebral ischemia [105]. Mental status changes due to encephalopathy are more common in older patients with COVID-19 and associated with worse outcomes [101,105], with delirium occurring in up to 55% of critically ill patients with COVID-19 [106]. Meningoencephalitis may occur due to direct central nervous system (CNS) invasion or systemic inflammation due to COVID-19. Immune-mediated complications such as Guillain-Barre Syndrome and myasthenia gravis have been reported [107]. Cerebral ischemia due to stroke may occur in up to 6% of critically ill patients with COVID-19 and can present as small or large vessel occlusion; however, the risk of cerebrovascular accident (CVA) is less than 1% in other populations with COVID-19 [[108], [109], [110]]. CVA most commonly occurs in the first several weeks after symptom development [[108], [109], [110]]. While rare, cerebral venous thrombosis has also been documented and is associated with high mortality rate in COVID-19 [111,112]. These patients can present with headache, seizure, focal neurologic deficit, or altered mental status. Acute disseminated encephalomyelitis and posterior reversible encephalopathy syndrome have also been reported. Finally, patients are at risk of psychiatric complications such as mood disorders, anxiety, insomnia, and psychotic disorders [103,113].
3.4.3. Gastrointestinal
Gastrointestinal (GI) symptoms are common, with up to one-third of patients with COVID-19 presenting first with GI symptoms [24,114,115]. Nausea and vomiting may be present in up to two-thirds of patients with COVID-19 [115]. Approximately 40% of patients with COVID-19 will have loss of appetite, and up to 50% will have diarrhea [24,115]. Abdominal pain is less common, occurring in less than 10% [24,115]. In critically ill patients, acute liver injury, cholecystitis, pancreatitis, ileus, pseudo-obstruction, and mesenteric ischemia may occur [116].
3.4.4. Dermatologic
Dermatologic manifestations of COVID-19 occur in 0.4–20% of cases but are often non-specific consisting of erythema or urticaria-like lesions on the trunk or, less frequently, the extremities [74,[117], [118], [119], [120], [121]]. Similarly, small case series and reports describe livedo reticularis, vesicular eruptions, maculopapular lesions, and areas of thickened erythema resembling chilblains [[117], [118], [119], [120], [121]]. New pernio-like lesions are also suggestive of COVID-19 [74,118].
3.4.5. Hematologic/thrombotic
Hematologic issues including thrombotic complications are common in critically ill patients with COVID-19. This risk is thought to be associated with systemic inflammation [[122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132]]. Initial studies suggested patients with COVID-19 were at a high risk of venous thromboembolic event (VTE), with rates of VTE reaching 31% in critically ill patients [122,[124], [125], [126]]. More recent studies have found that the overall risk of VTE, including pulmonary embolism (PE), in patients with COVID-19, no matter the severity of illness, is lower than initially suspected (< 1%), though the risk remains higher than the general non-COVID-19 population [132]. A second international study found COVID-19 was not an independent risk factor for PE, with 15% in the pandemic era and 15% of patients in the prepandemic era experiencing PE [132]. Routine evaluation for PE in all COVID-19 patients is not recommended [132]. Evaluation for PE should be considered in patients with other risk factors for PE; those with hypoxia, tachycardia, or hypotension out of proportion to clinical evaluation; sudden decompensation; or those whose symptoms are not explained by chest radiograph.
3.5. ED evaluation
3.5.1. SARS-CoV-2 testing
Evaluation in the ED setting primarily focuses on identification of COVID-19 and assessment for severe illness and end-organ injury [[1], [2], [3]]. Identification of COVID-19 infection continues to rely heavily on nucleic acid amplification tests (NAAT) for SARS-CoV-2 in nasopharyngeal specimens, with reverse transcription polymerase chain reaction (RT-PCR) assays comprising common methods recommended by the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), and Infectious Diseases Society of American (IDSA) [[1], [2], [3],20,74,[133], [134], [135], [136], [137], [138], [139]]. Initial concerns for suboptimal sensitivities of RT-PCR as low as 70% have been attributed to oropharyngeal sampling and poor sampling technique, while accuracy has significantly improved with nasal vestibular and middle turbinate swabs or the use of saliva [[133], [134], [135], [136], [137], [138], [139], [140]]. Sensitivity may approach 100% when there is 500–5000 viral RNA/mL [135]. However, timing of testing can have significant impact on RT-PCR test characteristics, with the highest sensitivity at 2–3 days after symptom onset and the lowest sensitivity immediately after exposure [20,138].
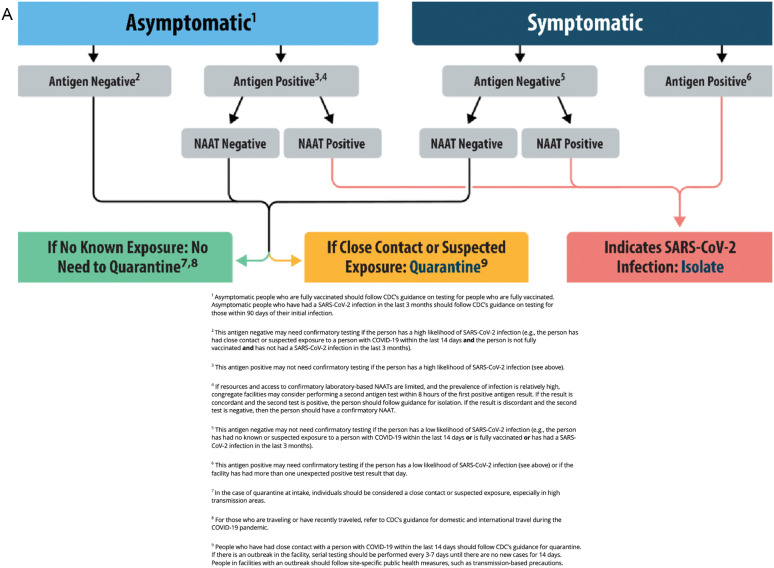
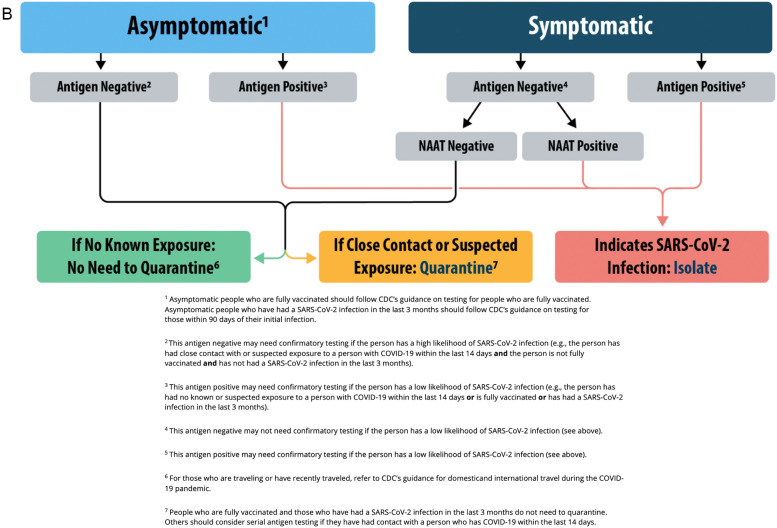
Although RT-PCR can be performed in a condensed timeline, the sheer volume of tests performed in major centers has prompted examination of shorter-turnaround point-of-care testing, such as rapid SARS-CoV-2 antigen (Ag) testing to detect viral proteins. This process utilizes a nasal turbinate swab with a simple qualitative indicator when reagent is applied, with results available in approximately 15 min compared to the 60–120 min required for NAAT [[140], [141], [142], [143], [144], [145]]. Despite generally high specificity, SARS-CoV-2 Ag testing demonstrates highly variable sensitivity (31–93%), likely due to varying viral loads, and it has less sensitivity at lower viral loads [20,133,137,[140], [141], [142], [143], [144], [145]]. Most SARS-Cov-2 Ag tests demonstrate higher sensitivities with a lower cycle threshold (Ct), which describes the number of PCR cycles required to detect the virus [[141], [142], [143], [144]]. SARS-CoV-2 Ag tests demonstrate higher sensitivities for those with a PCR Ct of ≤25, compared to those with higher Ct values [133,137,[140], [141], [142], [143], [144], [145]]. Due to this variability, SARS-CoV-2 Ag testing can be considered for rapidly screening patients who appear highly symptomatic with suspected or confirmed exposure. Current society guidelines recommend that SARS-CoV-2 Ag testing be employed in isolation only when NAAT is not available, or as screening for patients in conjunction with NAAT for definitive diagnosis (Fig. 1 ) [20,140,143]. Limited evaluation of SARS-CoV-2 Ag tests against Omicron variant infections do not demonstrate a significant decrease in overall sensitivity for symptomatic individuals [146,147].
Fig. 1.
CDC Antigen Testing Algorithm [134,140]. 1a) Testing for Congregate Living Settings. 1b) Testing for Community Settings. From https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html#previous
3.5.2. Other laboratory testing
Patients with normal vital signs who appear well do not require laboratory assessment other than SARS-CoV-2 testing. However, additional laboratory testing can assist with risk stratification in determining the need for admission [[1], [2], [3],20]. A complete blood count (CBC) can provide several markers of interest. For example, the CBC may reveal lymphopenia (absolute lymphocyte count <1.0 × 109/L), which can be found in up to half of patients overall and 83% of hospitalized patients, portending an increased risk for the combined outcome of severe disease course and mortality compared to those with normal levels (76% vs. 26%, p < 0.001) [20,38,[148], [149], [150], [151], [152], [153], [154], [155]]. Lymphopenia tends to worsen with the severity of COVID-19 symptoms and is associated with an increased risk for respiratory failure (odds ratio [OR] 2.69, p < 0.001) [[152], [153], [154], [155]]. Total leukocyte counts are variable but generally remain within a normal range of 4–10 × 109/L, though median values for more severe patients are more commonly below this lower limit at 3.7–3.9 × 109/L [18,20,151,155]. Mild thrombocytopenia (platelet count <150 × 109/L) is seen in 12–33% of patients overall, with a higher proportion found in those with more severe illness (57.7% vs. 31.6%) [18,20,149,151,153,155].
Chemistry and liver function testing can be obtained to evaluate for organ injury and other complications in COVID-19 patients. Serum creatinine has been found to be elevated in less than 10% of patients, although one study found elevated creatinine in 28.8% of patients [18,[154], [155], [156], [157], [158], [159]]. Common electrolyte derangements found in COVID-19 include hyponatremia (20.4–50%) and hypokalemia (15.1–62%), with most exhibiting mild depletion (Na 130–135 mmol/L, K 3.0–3.5 mmol/L) [53,[156], [157], [158], [159]]. Lower sodium levels have been associated with more severe illness (139 mmol/L vs. 136 mmol/L, p < 0.0001) [53,157,160]. Those with severe hypokalemia (< 3 mmol/L) are also more likely to have severe COVID-19 (p < 0.001) [157,158]. Hyperglycemia is found in approximately half of patients, and in patients with diabetes, a venous blood gas panel should be considered to evaluate for COVID-19-triggered diabetic ketoacidosis [153,161,162]. Lactate dehydrogenase (LDH) is elevated (>250 U/L) in 27–92% of COVID-19 patients [18,20,149,[151], [152], [153], [154], [155]].
Liver function tests demonstrate statistically significant elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in 10–28% and 9–36% of patients overall, respectively; however, at this time the clinical significance of this is not well established [20,148,153,155]. Scattered case reports suggest COVID-19 can present as acute hepatitis, while small studies show elevated AST and ALT in COVID-19 with preexisting hepatitis B infection [163,164]. However, there is no clear correlation at this time.
As in most inflammatory processes, inflammatory markers including CRP and erythrocyte sedimentation rate (ESR) tend to be elevated in COVID-19. This includes significant CRP elevation (≥10 mg/L) in 46–99% COVID-19 patients [18,20,53,149,155]. ESR is elevated (> 15 mm/h) in 24–96% of patients, albeit with less supporting studies relative to CRP [53,149,153,155]. CRP ≥10 mg/L demonstrates increased risk of respiratory failure (OR 5.91) and thrombotic events (OR 2.7) in COVID-19 patients, while ESR > 40 mm/h shows similar association with thrombosis (OR 2.64) in isolated studies [127,151,152].
Potential thrombotic complications may prompt evaluation for fibrinogen and coagulation studies, with serum D-dimer often elevated. While studies vary on D-dimer levels to define elevation, 46% of COVID-19 patients demonstrate levels >0.5 mg/L and 36% have levels >1.5 mg/L [20,127,149,153,165]. The predictive ability of elevated D-dimer for thrombotic events in these patients increases proportionally to the elevation, with increasing risk from 1 to 2.5 mg/L (OR 1.75) to >2.5 mg/L (OR 4.40), 5–10 mg/L (OR 5.55) and ≥ 10 mg/L (OR 7.09) [[127], [128], [129], [130]]. The degree of D-dimer elevation is also associated with severity of illness, with higher levels associated with more severe disease [155]. Less often, prolonged prothrombin (PT) and activated partial thromboplastin (aPTT) times are found in approximately 5–11% and 6–26% of patients, respectively, without significant correlation between abnormal levels and illness severity or bleeding complications [20,53,119,120,153,155].
3.5.3. Imaging
Chest radiography is generally employed for initial evaluation of COVID-19 patients with respiratory symptoms, although studies reveal significant variation in the frequency of their findings [[1], [2], [3],18,[166], [167], [168], [169], [170], [171], [172]]. A normal chest X-ray (CXR) may be found in a significant proportion of patients with COVID-19 (5.6–53.6%), with 10.9% later progressing to abnormal findings on subsequent plain films [18,[166], [167], [168], [169], [170], [171], [172]]. The most common abnormal CXR findings include peripheral consolidations (5.3–88.9%) or ground glass opacities (14.1–63.1%), with the latter described as hazy increased attenuation with reticular consolidation [[166], [167], [168], [169], [170], [171], [172]] (Fig. 2 ). Patients most commonly have bilateral lung involvement (up to 76%) [[166], [167], [168], [169], [170], [171], [172]]. Computed tomography (CT) of the chest without contrast demonstrates improved sensitivity for detecting lung abnormalities (pooled sensitivity 87.9% to 90.6%) and should be considered in COVID-19 patients with severe respiratory symptoms despite unremarkable CXR [166,169,[171], [172], [173], [174]] (Fig. 3 ). Up to 10% of patients can have a normal CT. A classification system has been proposed with 4 stages [173,174]. Stage 1 includes ground glass appearance (days 0–4), stage 2 is an increased crazy-paving pattern (days 5–8), stage 3 consists of consolidation (days 9–13), and stage 4 is gradual resolution (days ≥14) [174].
Fig. 2.
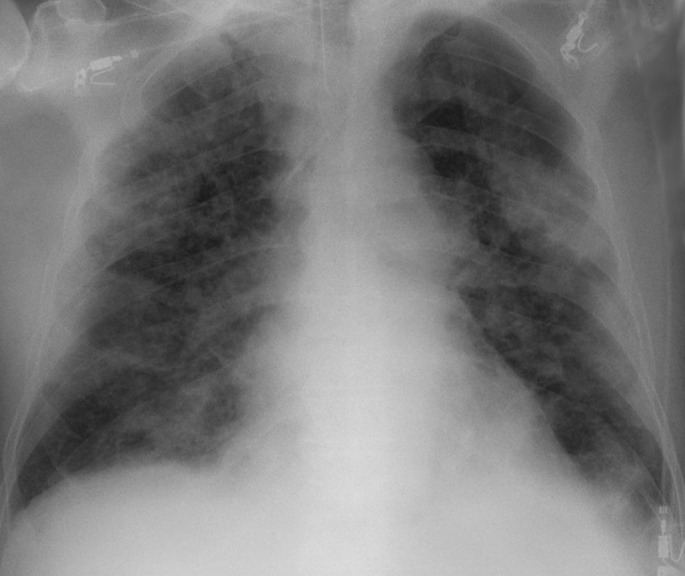
Chest x-ray with bilateral ground-glass opacities From: https://commons.wikimedia.org/wiki/File:COVID-19_Pneumonie_-_82m_Roe_Thorax_ap_-_001.jpg
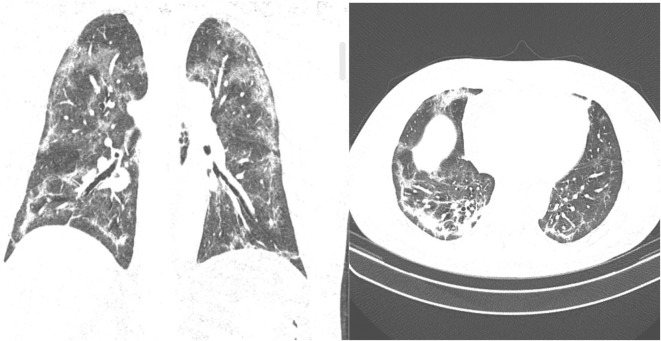
Fig. 3.
Chest CT with bilateral, multilobar areas of airspace consolidations leaving ground-glass opacities, with some subpleural parenchymal bands already emerging. From https://radiopaedia.org/cases/covid-19-pneumonia-158?lang=us
Lung ultrasound (LUS) is another tool in the evaluation of COVID-19 patients. A prospective cohort study found that LUS possessed a sensitivity of 94.4% for diagnosis and was able to identify false-negative cases on PCR [175]. A meta-analysis reported moderately high sensitivity (86.4%) but low specificity (54.6%) [172]. LUS findings in patients with mild-to-moderate respiratory symptoms include abnormal pleural thickening and sliding, B-lines, skip lesions, and small areas of patchy consolidation, with the posterior lung fields most commonly affected (Fig. 4 ) [[175], [176], [177], [178], [179], [180], [181], [182], [183]]. B-line progression with enhanced consolidation suggests increasing disease severity and should raise concern for a potential need for enhanced respiratory support [176,177,179].
Fig. 4.
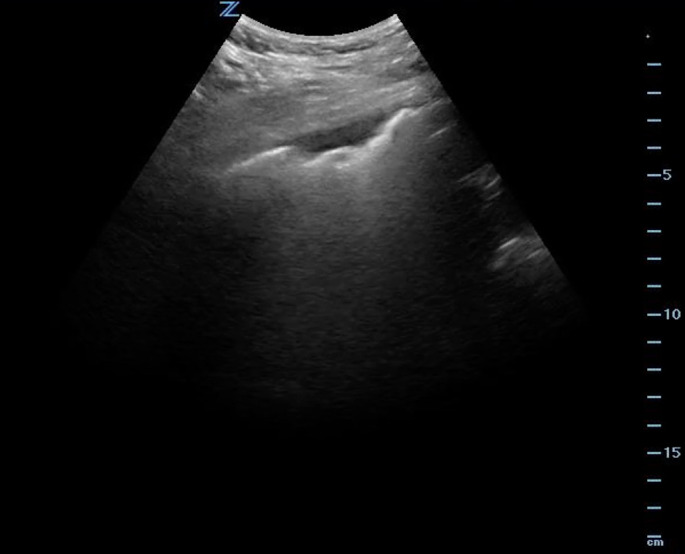
Ultrasound demonstrating an irregular pleural line with a subpleural consolidation in a patient with COVID-19.
3.6. Risk scores
Over 20 prognostic scoring systems have been created for COVID-19. This review will not discuss all of these scores. Many of these scoring tools are a combination of clinical, laboratory, and imaging findings [[184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194]], and unfortunately, many of these have proven unreliable in the clinical setting. These scores are primarily derived from homogenous, small populations of patients, and many lack extensive validation or are too complex for clinical use [184,185]. However, several demonstrate utility when used appropriately. When utilized, these scores should supplement clinical decision making, but they cannot replace clinical judgment [194].
One of the most robust and validated scores to predict mortality in patients with COVID-19 is the 4C (Coronavirus Clinical Characterisation Consortium) score. The 4C score has been validated in several settings in over 57,000 patients and considers age, sex, comorbidities, respiratory rate, oxygen saturation on room air, Glasgow Coma Scale, blood urea nitrogen, and CRP (Table 3 ) [186,187]. The derivation receiver-operator characteristic (ROC) curve was 0.79, with 0.77 in the validation cohort (ROC 0.5 suggests no discrimination, 0.7–0.8 suggests an acceptable level of discrimination, and greater than 0.8 is considered excellent) [186,187].
Table 3.
4C Score
| Variable | Points |
|---|---|
| Age in years | |
| < 50 | 0 |
| 50–59 | 2 |
| 60–69 | 4 |
| 70–79 | 6 |
| ≥80 | 7 |
| Sex at birth | |
| Female | 0 |
| Male | 1 |
| Number of comorbidities | |
| 0 | 0 |
| 1 ≥2 |
1 2 |
| Respiratory rate in breaths per minute | |
| <20 | 0 |
| 20–29 | 1 |
| ≥30 | 2 |
| Peripheral oxygen saturation on room air | |
| ≥92% | 0 |
| <92% | 2 |
| Glasgow coma scale | |
| 15 | 0 |
| <15 | 2 |
| Urea/BUN | |
| Urea <7 mmol/L or BUN <19.6 mg/dL | 0 |
| Urea 7–14 mmol/L or BUN 19.6–39.2 mg/dL | 1 |
| Urea >140 mmol/L or BUN >39.2 mg/dL | 3 |
| CRP | |
| < 50 mg/L | 0 |
| 50–99 mg/L | 1 |
| ≥100 mg/L | 2 |
| Score | Mortality |
| 0–3 = Low risk | 1.2–1.7% |
| 4–8 = Intermediate risk | 9.1–9.9% |
| 9–14 = High risk | 31.4–34.9% |
| ≥15 = Very high risk | 61.5–66.2% |
The quick COVID Severity Index (qCSI) is an effective physiological risk score that can be used at the bedside. It was initially created to determine risk of progressing to respiratory failure and critical illness within 24 h of hospital admission and is comprised of respiratory rate, pulse oximetry, and supplemental oxygen flow rate (Table 4 ) [188]. The initial ROC curve was 0.81, with other studies suggesting the score may be similar to the NEWS score and outperform other risk scores such as 4C and CURB-65 [188,189].
Table 4.
qCSI
| Variable | Points |
|---|---|
| Respiratory rate in breaths per minute | |
| ≤22 | 0 |
| 23–28 | 1 |
| >28 | 2 |
| Peripheral oxygen saturation on room air | |
| >92% | 0 |
| 89–92% | 2 |
| ≤88% | 5 |
| O2 flow rate, L/min | |
| ≤2 | 0 |
| 3–4 | 4 |
| 5–6 | 5 |
| Score | Risk of critical illness at 24h |
| 0–3 = Low risk | 4% |
| 4–6 = Low-intermediate risk | 30% |
| 7–9 = High-intermediate risk | 44% |
| 10–12 = High risk | 57% |
NEWS2 is another physiologic score comprised of respiratory rate, saturation, systolic blood pressure, pulse, consciousness, and temperature [[190], [191], [192]] (Table 5 ). This score demonstrates area under receiver operating characteristic (AUROC) curves of 0.78 for determining who will deteriorate over 24 h and for in-hospital mortality [192].
Table 5.
NEWS2
| Variable | Points |
|---|---|
| Respiratory rate in breaths per minute | |
| ≤8 | 3 |
| 9–11 | 1 |
| 12–20 | 0 |
| 21–24 | 2 |
| ≥25 | 3 |
| SpO2 (on room air or supplemental) | |
| ≤91% 92–93% |
3 2 |
| 94–95% | 1 |
| ≥96% | 0 |
| SpO2 (if patient has hypercapnic respiratory failure) | |
| ≤83% | 3 |
| 84–85% | 2 |
| 86–87% | 1 |
| 88–92%, ≥93% on room air | 0 |
| 93–94% on supplemental oxygen | 1 |
| 95–96% on supplemental oxygen | 2 |
| ≥97% on supplemental oxygen | 3 |
| Oxygen | |
| Supplemental oxygen | 2 |
| Room air | 0 |
| Temperature | |
| ≤35.0 °C (95 °F) | 3 |
| 35.1–36.0 °C (95.1–96.8 °F) | 1 |
| 36.1–38.0 °C (96.9–100.4 °F) | 0 |
| 38.1–39.0 °C (100.5–102.2 °F) | 1 |
| ≥39.1 °C (102.3 °F) | 2 |
| Systolic BP, mm Hg | |
| ≤90 | 3 |
| 91–100 | 2 |
| 101–110 | 1 |
| 111–219 | 0 |
| ≥220 | 3 |
| Pulse, beats per minute | |
| ≤40 | 3 |
| 41–50 | 1 |
| 51–90 | 0 |
| 91–110 | 1 |
| 111–130 | 2 |
| ≥131 | 3 |
| Consciousness | |
| Alert | 0 |
| New onset confusion, responds to voice or pain, or unresponsive | 3 |
| Score | |
| 0–4 = Low risk | |
| 3 in any individual parameter = Low-medium risk | |
| 5–6 = Medium risk | |
| ≥7 = High risk | |
The PRIEST score, comprised of respiratory rate, oxygen saturation, heart rate, systolic blood pressure, temperature, alertness, inspired oxygen, sex, age, and performance status, reported a c-statistic of 0.80, with a score > 4 demonstrating a 98% sensitivity and 34% specificity to predict adverse events [193] (Table 6 ).
Table 6.
PRIEST score
| Variable | Points |
|---|---|
| Age in years | |
| 16–49 | 0 |
| 50–65 | 2 |
| 66–80 | 3 |
| >80 | 4 |
| Sex | |
| Female | 0 |
| Male | 1 |
| Respiratory rate in breaths per minute | |
| ≤8 | 3 |
| 9–11 | 1 |
| 12–20 | 0 |
| 21–24 | 2 |
| ≥25 | 3 |
| Peripheral oxygen saturation | |
| >95% | 0 |
| 94–95% | 1 |
| 92–93% | 2 |
| <92% | 3 |
| Heart rate, beats per minute | |
| ≤40 | 3 |
| 41–50 | 1 |
| 51–90 | 0 |
| 91–110 | 1 |
| 111–130 | 2 |
| ≥131 | 3 |
| Systolic BP, mm Hg | |
| ≤90 | 3 |
| 91–100 | 2 |
| 101–110 | 1 |
| 111–130 | 0 |
| ≥130 | 3 |
| Temperature | |
| ≤35.0 °C (95 °F) | 3 |
| 35.1–36.0 °C (95.1–96.8 °F) | 1 |
| 36.1–38.0 °C (96.9–100.4 °F) | 0 |
| 38.1–39.0 °C (100.5–102.2 °F) | 1 |
| ≥39.1 °C (102.3 °F) | 2 |
| Alertness | |
| Alert | 0 |
| Confused or not alert | 3 |
| Inspired oxygen | |
| Air | 0 |
| Supplemental oxygen | 2 |
| Performance status | |
| Unrestricted normal activity Limited strenuous activity, can do light activity |
0 1 |
| Limited activity, can self-care | 2 |
| Limited self-care | 3 |
| Bed/chair bound, no self-care | 4 |
4. Conclusions
COVID-19 has resulted in millions of infections and deaths. The disease is primarily spread by respiratory droplets and aerosols. Several variants have arisen, including the Delta (B.1.617.2 lineage) and Omicron (B.1.1.529 lineage) variants. Most patients experience a mild infection with upper respiratory symptoms, fever, and change in taste/smell, but some may develop severe infection with respiratory failure and end organ injury. Aside from the respiratory system, SARS-CoV-2 can affect the cardiovascular, neurologic, gastrointestinal, and dermatologic systems. Evaluation includes identification of COVID-19 and assessment for end organ injury. Several risk scores may assist in prognostication.
CRediT authorship contribution statement
Brit Long: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Brandon M. Carius: Writing – review & editing, Writing – original draft. Stephen Y. Liang: Writing – review & editing, Writing – original draft, Supervision. Summer Chavez: Writing – review & editing, Conceptualization. William J. Brady: Writing – review & editing, Conceptualization. Alex Koyfman: Writing – review & editing, Conceptualization. Michael Gottlieb: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of Competing Interest
None
Acknowledgements
All authors conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
References
- 1.World Health Organization WHO coronavirus 2019 (COVID-19) pandemic. Available at. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Centers for Disease Control and Prevention. COVID-19. Available at. https://www.cdc.gov/coronavirus/2019-ncov/index.html Accessed August 21, 2021.
- 3.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2021 Jun;44:220–229. doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins University and Medicine Coronavirus Resource Center. Available at. https://coronavirus.jhu.edu Accessed August 21, 2021.
- 5.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SARS-CoV-2 variant classifications and definitions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html#Consequence Updated July 13, 2021. Accessed July 19, 2021.
- 7.Centers for Disease Control and Prevention What you need to know about variants. November 27, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant.html Accessed November 27, 2021.
- 8.Davies N.G., Abbott S., Barnard R.C., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer C. The New York Times; 2021. The New COVID Variant in the UK. Updated March 6. Accessed July 19, 2021. [Google Scholar]
- 10.Hart R. Forbes; 2021. Pfizer Shot Much Less Effective Against Delta, Israel Study Shows — Here'’s What You Need To Know About Variants And Vaccines. Published July 6. Accessed July 30, 2021. [Google Scholar]
- 11.Dyer O. Covid-19: delta infections threaten herd immunity vaccine strategy. BMJ. 2021:374. doi: 10.1136/bmj.n1933. n1933. Published 2021 Aug 2. [DOI] [PubMed] [Google Scholar]
- 12.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant [published online ahead of print, 2021 Jul 21] N Engl J Med. 2021 doi: 10.1056/NEJMoa2108891. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty K., Mannell M., Naqvi O., et al. SARS-CoV-2 B.1.617.2 (Delta) variant COVID-19 outbreak associated with a gymnastics facility - Oklahoma, April-may 2021. MMWR Morb Mortal Wkly Rep. 2021 Jul 16;70(28):1004–1007. doi: 10.15585/mmwr.mm7028e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available at. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern Accessed December 26, 2021.
- 15.Centers for Disease Control and Prevention CDC Statement on B.1.1529 (Omicron variant). Available at. https://www.cdc.gov/media/releases/2021/s1126-B11-529-omicron.html Accessed December 26, 2021.
- 16.Zhu N., Zhang D., Wang W., China Novel Coronavirus Investigating and Research Team, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Rio C., Malani P.N. COVID-19-new insights on a rapidly changing epidemic. JAMA. 2020;323(14):1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 18.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong S.W.X., Chiew C.J., Ang L.W., et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) [published online ahead of print, 2021 Aug 23] Clin Infect Dis. 2021 doi: 10.1093/cid/ciab7. ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiersinga W.J., Rhodes A., Cheng A.C., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 21.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 May 12;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. published correction appears in JAMA. 2021 Mar 16;325(11):1113. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung K.S., Hung I.F.N., Chan P.P.Y., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020 Jul;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colavita F., Lapa D., Carletti F., et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020 Aug 4;173(3):242–243. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 27.Byambasuren O., Cardona M., Bell K., et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus [published online ahead of print, 2021 Aug 9] J Travel Med. 2021 doi: 10.1093/jtm/taab124. taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanche S., Lin Y.T., Xu C., et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunzler D., Sehgal A.R. Time-varying COVID-19 reproduction number in the United States. Preprint medRxiv. 2020 doi: 10.1101/2020.04.10.20060863. 2020.04.10.20060863. Published 2020 Apr 15. [DOI] [Google Scholar]
- 31.Investigation of SARS-CoV-2 variants: technical briefings. December 23, 2021. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings Accessed December 27, 2021.
- 32.COVID Data Tracker. Centers for Disease Control and Prevention https://covid.cdc.gov/covid-data-tracker/#variant-proportions Accessed December 27, 2021.
- 33.Sheikh A., McMenamin J., Taylor B., Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twohig K.A., Nyberg T., Zaidi A., et al. COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022 Jan;22(1):35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization Enhancing Readiness for Omicron (B.1.1.529): Technical Brief and Priority Actions for Member States. December 10, 2021. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states Accessed on December 13, 2021.
- 36.Hall V.J., Foulkes S., Charlett A., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) The Lancet. 2021 Apr 17;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. The Lancet. 2021 Mar 27;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossler A., Riepler K., Bante D., et al. 2021. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. UNPUBLISHED. Accessed on December 13, 2021. [DOI] [Google Scholar]
- 39.Pulliam J.R.C., van Schalkwyk C., Govender N., et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. MedRixv. 2021 doi: 10.1101/2021.11.11.21266068. Accessed December 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelm A., Widera M., Grikscheit K., et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. Medrixv. 2021 doi: 10.1101/2021.12.07.21267432. Accessed December 24, 2021. [DOI] [Google Scholar]
- 41.Collie S., Champion J., Moultrie H., Bekker L.G., Gray G. Effectiveness of BNT162b2 vaccine against Omicron Variant in South Africa. N Engl J Med. 2021 Dec 29 doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Discovery Health releases at-scale real-world analysis of Omicron outbreak; including collaboration with the SA Medical Research Council (SAMRC) to analyse vaccine effectiveness. December 14, 2021. https://www.discovery.co.za/corporate/health-insights-omicron-outbreak-analysis Accessed December 28, 2021.
- 43.Tshwane District Omicron Variant Patient Profile - Early Features. https://www.samrc.ac.za/news/tshwane-district-omicron-variant-patient-profile-early-features Accessed on December 06, 2021.
- 44.Report 49 - Growth, population distribution and immune escape of Omicron in England. Imperial College London. December 16, 2021. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-49-Omicron/ Accessed December 28, 2021.
- 45.Report 50 - Hospitalisation risk for Omicron cases in England. Imperial College London. December 22, 2021. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-50-Severity-Omicron/ Accessed December 30, 2021.
- 46.Rochwerg B., Agarwal A., Siemieniuk R.A., Agoritsas T., Lamontagne F., Askie L., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 47.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic : a systematic review. Ann Intern Med. 2021;174(5):655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epidemiology Working Group for NCIP Epidemic Response Chinese Center for Disease Control and Prevention. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauer S.A., Grantz K.H., Bi Q., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93(2):820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottlieb M., Sansom S., Frankenberger C., Ward E., Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago. Illinois Acad Emerg Med. 2020;27(10):963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA. 2020 May 26;323(20):2098] JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziehr D.R., Alladina J., Petri C.R., et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auld S.C., Caridi-Scheible M., Blum J.M., et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin A.T., Hanage W.P., Owusu-Boaitey N., et al. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020 Dec;35(12):1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cates J., Lucero-Obusan C., Dahl R.M., et al. Risk for in-hospital complications associated with COVID-19 and influenza - veterans health administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020 Oct 23;69(42):1528–1534. doi: 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie Y., Bowe B., Maddukuri G., Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020 Dec 15;(371) doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lentz S., Roginski M.A., Montrief T., et al. Initial emergency department mechanical ventilation strategies for COVID-19 hypoxemic respiratory failure and ARDS. Am J Emerg Med. 2020;38(10):2194–2202. doi: 10.1016/j.ajem.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verma A.A., Hora T., Jung H.Y., et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ. 2021 Mar 22;193(12):E410–E418. doi: 10.1503/cmaj.202795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis J.M., McGovern A.P., Vollmer S.J., Mateen B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: a National Cohort Study, march to June 2020. Crit Care Med. 2021 Feb 1;49(2):209–214. doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;(369) doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunningham J.W., Vaduganathan M., Claggett B.L., et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020 Sep 9;181(3):379–381. doi: 10.1001/jamainternmed.2020.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 3;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., et al. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020 Sep 10;17(9) doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson M.R., Geleris J., Anderson D.R., et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection : a retrospective cohort study. Ann Intern Med. 2020;173(10):782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tartof S.Y., Qian L., Hong V., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alvarez-Garcia J., Lee S., Gupta A., et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76(20):2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisman D., Tuite A. Progressive increase in virulence of novel Sars-CoV-2 variants in Ontario. Canada medRxiv Preprint posted online July. 2021;12 doi: 10.1101/2021.07.05.21260050. Accessed August 10, 2021. [DOI] [Google Scholar]
- 72.Peyrony O., Marbeuf-Gueye C., Truong V., et al. Accuracy of emergency department clinical findings for diagnosis of coronavirus disease 2019. Ann Emerg Med. 2020;76(4):405–412. doi: 10.1016/j.annemergmed.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kornitzer J., Johnson J., Yang M., et al. A systematic review of characteristics associated with COVID-19 in children with typical presentation and with multisystem inflammatory syndrome. Int J Environ Res Public Health. 2021;18(16):8269. doi: 10.3390/ijerph18168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpenter C.R., Mudd P.A., West C.P., et al. Diagnosing COVID-19 in the emergency department: a scoping review of clinical examinations, laboratory tests, imaging accuracy, and biases. Acad Emerg Med. 2020;27(8):653–670. doi: 10.1111/acem.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Struyf T., Deeks J.J., Dinnes J., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020 Jul 7;7(7) doi: 10.1002/14651858.CD013665. CD013665. Update in: Cochrane Database Syst Rev. 2021 Feb 23;2:CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chua A.J., Charn T.C., Chan E.C., Loh J. Acute olfactory loss is specific for COVID-19 at the emergency department. Ann Emerg Med. 2020;76(4):550–551. doi: 10.1016/j.annemergmed.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bielecki M., Crameri G.A.G., Schlagenhauf P., et al. Body temperature screening to identify SARS-CoV-2 infected young adult travellers is ineffective. Travel Med Infect Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng C., Wang L., Chen X., et al. A novel artificial intelligence-assisted triage tool to aid in the diagnosis of suspected COVID-19 pneumonia cases in fever clinics. Ann Transl Med. 2021;9(3):201. doi: 10.21037/atm-20-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rawson T.M., Moore L.S.P., Zhu N., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 Dec 3;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou P., Liu Z., Chen Y., et al. Bacterial and fungal infections in COVID-19 patients: a matter of concern. Infect Control Hosp Epidemiol. 2020;41(9):1124–1125. doi: 10.1017/ice.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu X., Ge Y., Wu T., et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai C.C., Yu W.L. COVID-19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect. 2021;54(1):46–53. doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ismaiel W.F., Abdelazim M.H., Eldsoky I., et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis [published online ahead of print, 2021 May 14] Am J Otolaryngol. 2021;42(6):103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honavar S.G. Code mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69(6):1361–1365. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Kholy N.A., El-Fattah A.M.A., Khafagy Y.W. Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity [published online ahead of print, 2021 May 19] Laryngoscope. 2021 doi: 10.1002/lary.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sen M., Lahane S., Lahane T.P., et al. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021 Feb;69(2):244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020 Jul;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [published correction appears in JAMA Cardiol. 2020 Jul 1;5(7):848] JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Jul 1;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 91.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142(1):7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 92.Ranard L.S., Fried J.A., Abdalla M., et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13(7) doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahmud E., Dauerman H.L., Welt F.G.P., et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP) J Am Coll Cardiol. 2020;76(11):1375–1384. doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stefanini G.G., Montorfano M., Trabattoni D., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Argulian E., Sud K., Vogel B., et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13(11):2459–2461. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) [published correction appears in JAMA Cardiol. 2020 Nov 1;5(11):1308] JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection [published correction appears in JAMA Cardiol. 2021 Jan 1;6(1):123] JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liotta E.M., Batra A., Clark J.R., et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020 Nov;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beltrán-Corbellini Á., Chico-García J.L., Martínez-Poles J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020;27(9):1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tong J.Y., Wong A., Zhu D., et al. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020 Jul;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 105.Frontera J.A., Sabadia S., Lalchan R., et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96(4):e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pun B.T., Badenes R., Heras La Calle G., et al. COVID-19 Intensive Care International Study Group. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021 Mar;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toscano G., Palmerini F., Ravaglia S., et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mao L., Wang M., Chen S., et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study medRxiv Preprint posted online February. 2020;25 doi: 10.1101/2020.02.22.20026500. Accessed April 2, 2020. [DOI] [Google Scholar]
- 109.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.AHA/ASA Stroke Council Leadership Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke. 2020;51(6):1910–1912. doi: 10.1161/STROKEAHA.120.030023. [DOI] [PubMed] [Google Scholar]
- 111.Al-Mufti F., Amuluru K., Sahni R., et al. Cerebral venous thrombosis in COVID-19: a New York Metropolitan Cohort Study. AJNR Am J Neuroradiol. 2021 Jul;42(7):1196–1200. doi: 10.3174/ajnr.A7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tu T.M., Goh C., Tan Y.K., et al. Cerebral venous thrombosis in patients with COVID-19 infection: a case series and systematic review. J Stroke Cerebrovasc Dis. 2020;29(12) doi: 10.1016/j.jstrokecerebrovasdis.2020.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taquet M., Geddes J.R., Husain M., et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silva F.A.F.D., Brito B.B., Santos M.L.C., et al. COVID-19 gastrointestinal manifestations: a systematic review. Rev Soc Bras Med Trop. 2020 Nov 25;(53) doi: 10.1590/0037-8682-0714-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020 Aug;272(2):e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 118.Gottlieb M., Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38(9):1715–1721. doi: 10.1016/j.ajem.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tammaro A., Adebanjo G.A.R., Parisella F.R., et al. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020 Jul;34(7):e306–e307. doi: 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li H., Zhao Y., Zhou L., Hu J. Cutaneous, skin histopathological manifestations and relationship to COVID-19 infection patients. Dermatol Ther. 2020 Nov;33(6) doi: 10.1111/dth.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A., et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83(1):e61–e63. doi: 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jenner W.J., Kanji R., Mirsadraee S., et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;51(3):595–607. doi: 10.1007/s11239-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shah A., Donovan K., McHugh A., et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24(1):561. doi: 10.1186/s13054-020-03260-3. Published 2020 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miró Ò., Jiménez S., Mebazaa A., et al. Spanish Investigators on Emergency Situations TeAm (SIESTA) network. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 2021 Aug 31;42(33):3127–3142. doi: 10.1093/eurheartj/ehab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Freund Y., Drogrey M., Miró Ò., et al. Improving Emergency Care FHU Collaborators. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: the PEPCOV International Retrospective Study. Acad Emerg Med. 2020 Sep;27(9):811–820. doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 133.Yamamoto K., Ohmagari N. Microbiological testing for coronavirus disease 2019. JMA J. 2021;4(2):67–75. doi: 10.31662/jmaj.2021-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hanson K.E., Caliendo A.M., Arias C.A., et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing [published online ahead of print, 2021 Jan 22] Clin Infect Dis. 2021 doi: 10.1093/cid/ciab048. ciab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yohe S. 2021. How good are COVID-19 diagnostic PCR tests? College of American Pathologists. Accessed May 7, 2021. [Google Scholar]
- 136.Li Y., Yao L., Li J., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamayoshi S., Sakai-Tagawa Y., Koga M., et al. Comparison of rapid antigen tests for COVID-19. Viruses. 2020;12(12):1420. doi: 10.3390/v12121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khandker S.S., Nik Hashim N.H.H., Deris Z.Z., Shueb R.H., Islam M.A. Diagnostic accuracy of rapid antigen test kits for detecting SARS-CoV-2: a systematic review and meta-analysis of 17,171 suspected COVID-19 patients. J Clin Med. 2021;10(16):3493. doi: 10.3390/jcm10163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanson K.E., Altayar O., Caliendo A.M., et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: antigen testing [published online ahead of print, 2021 Jun 23] Clin Infect Dis. 2021 doi: 10.1093/cid/ciab557. ciab557. [DOI] [PubMed] [Google Scholar]
- 141.Strömer A., Rose R., Schäfer M., et al. Performance of a point-of-care test for the rapid detection of SARS-CoV-2 antigen. Microorganisms. 2020;9(1):58. doi: 10.3390/microorganisms9010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayer J., Kasapic D., Zemmrich C. Real-world clinical performance of commercial SARS-CoV-2 rapid antigen tests in suspected COVID-19: a systematic meta-analysis of available data as of November 20, 2020. Int J Infect Dis. 2021;108:592–602. doi: 10.1016/j.ijid.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dinnes J., Deeks J.J., Berhane S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3(3) doi: 10.1002/14651858.CD013705.pub2. CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brümmer L.E., Katzenschlager S., Gaeddert M., et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PLoS Med. 2021;18(8) doi: 10.1371/journal.pmed.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toptan T., Eckermann L., Pfeiffer A.E., et al. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J Clin Virol. 2021 Feb;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deerain J., Druce J., Tran T., et al. Assessment of the analytical sensitivity of ten lateral flow devices against the SARS-CoV-2 omicron variant [published online ahead of print, 2021 Dec 22] J Clin Microbiol. 2021 doi: 10.1128/jcm.02479-21. jcm0247921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferré V.M., Peiffer-Smadja N., Visseaux B., et al. Omicron SARS-CoV-2 variant: what we know and what we don’t [published online ahead of print, 2021 Dec 10] Anaesth Crit Care Pain Med. 2021;41(1):100998. doi: 10.1016/j.accpm.2021.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80(6):441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan L., Wang Q., Zhang D., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study [published correction appears in Signal Transduct Target Ther. 2020 Apr 29;5(1):61] Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-0159-1. 33 Published 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.González-Flores J., García-Ávila C., Springall R., et al. Usefulness of easy-to-use risk scoring systems rated in the emergency department to predict major adverse outcomes in hospitalized COVID-19 patients. J Clin Med. 2021;10(16):3657. doi: 10.3390/jcm10163657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bartoletti M., Giannella M., Scudeller L., et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicentre cohort study (PREDI-CO study) Clin Microbiol Infect. 2020;26(11):1545–1553. doi: 10.1016/j.cmi.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Russo A., Gentilini Cacciola E., Borrazzo C., et al. Clinical characteristics and outcome of patients with suspected COVID-19 in emergency department (RESILIENCY study II) Diagnostics (Basel) 2021;11(8):1368. doi: 10.3390/diagnostics11081368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pourbagheri-Sigaroodi A., Bashash D., et al. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020 Nov;510:475–482. doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De Carvalho H., Richard M.C., Chouihed T., et al. Electrolyte imbalance in COVID-19 patients admitted to the emergency department: a case-control study [published online ahead of print, 2021 Jan 23] Intern Emerg Med. 2021:1–6. doi: 10.1007/s11739-021-02632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen D., Li X., Song Q., et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aggarwal S., Garcia-Telles N., Aggarwal G., et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7(2):91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 160.Xiang T., Liu J., Xu F., Cheng N., Liu Y., Qian K. Analysis of clinical features of 49 patients with new type of coronavirus pneumonia in Jiangxi. Chinese Journal of Respiratory and Critical Care. 2020;19:154–160. [Google Scholar]
- 161.Singh B., Kaur P., Patel P., et al. COVID-19 and diabetic ketoacidosis: a single center experience. Cureus. 2021;13(1) doi: 10.7759/cureus.13000. e13000. [Published 2021 Jan 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wander P., Epstein M., Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115(6):941–942. doi: 10.14309/ajg.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu R., Zhao L., Cheng X., et al. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2021;41(4):720–730. doi: 10.1111/liv.14774. [DOI] [PubMed] [Google Scholar]
- 165.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong H.Y.F., Lam H.Y.S., Fong A.H., et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2):E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cozzi D., Albanesi M., Cavigli E., et al. Chest X-ray in new coronavirus disease 2019 (COVID-19) infection: findings and correlation with clinical outcome. Radiol Med. 2020;125(8):730–737. doi: 10.1007/s11547-020-01232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vancheri S.G., Savietto G., Ballati F., et al. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;30(11):6161–6169. doi: 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sadiq Z., Rana S., Mahfoud Z., Raoof A. Systematic review and meta-analysis of chest radiograph (CXR) findings in COVID-19 [published online ahead of print, 2021 Jul 27] Clin Imaging. 2021;80:229–238. doi: 10.1016/j.clinimag.2021.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jacobi A., Chung M., Bernheim A., Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Borakati A., Perera A., Johnson J., Sood T. Diagnostic accuracy of X-ray versus CT in COVID-19: a propensity-matched database study. BMJ Open. 2020;10(11):e042946. doi: 10.1136/bmjopen-2020-042946. [Published 2020 Nov 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Islam N., Ebrahimzadeh S., Salameh J.-P., et al. 2021. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database of Systematic Reviews. Issue 3. Art. No.: CD013639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adams H.J.A., Kwee T.C., Yakar D., et al. Chest CT imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020;158(5):1885–1895. doi: 10.1016/j.chest.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pivetta E., Goffi A., Tizzani M., et al. Lung ultrasonography for the diagnosis of SARS-CoV-2 pneumonia in the emergency department. Ann Emerg Med. 2021;77(4):385–394. doi: 10.1016/j.annemergmed.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Soldati G., Smargiassi A., Inchingolo R., et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39(7):1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020;75(8):1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peng Q.Y., Wang X.T., Zhang L.N. Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel coronavirus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kulkarni S., Down B., Jha S. Point-of-care lung ultrasound in intensive care during the COVID-19 pandemic. Clin Radiol. 2020;75(9):710.e1–710.e4. doi: 10.1016/j.crad.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Y., Wang S., Liu Y., et al. 2020. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical Novel Coronavirus Pneumonia (COVID-19). SSRN Preprint posted online February 28. Accessed March 20, 2020. [Google Scholar]
- 181.Buonsenso D., Piano A., Raffaelli F., et al. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pneumoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 182.Peng Q.Y., Wang X.T., Zhang L.N., Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel coronavirus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Poggiali E., Dacrema A., Bastoni D., et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295(3):E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wynants L., Van C., Collins G., et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369 doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liang W., Liang H., Ou L., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Knight S., Ho A., Pius R., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score. BMJ. 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Covino M., De Matteis G., Burzo M.L., et al. GEMELLI AGAINST COVID-19 Group. predicting in-hospital mortality in COVID-19 older patients with specifically developed scores. J Am Geriatr Soc. 2021 Jan;69(1):37–43. doi: 10.1111/jgs.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haimovich A., Ravindra N., Stoytchev S., et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442–453. doi: 10.1016/j.annemergmed.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rodriguez-Nava G., Yanez-Bello M.A., Trelles-Garcia D.P., et al. Performance of the quick COVID-19 severity index and the Brescia-COVID respiratory severity scale in hospitalized patients with COVID-19 in a community hospital setting. Int J Infect Dis. 2021 Jan;102:571–576. doi: 10.1016/j.ijid.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gidari A., De S., Sabbatini S., Francisci D. Predictive value of National Early Warning Score 2 (NEWS2) for intensive care unit admission in patients with SARS-CoV-2 infection. Infect Dis (Lond) 2020;52(10):698–704. doi: 10.1080/23744235.2020.1784457. [DOI] [PubMed] [Google Scholar]
- 191.Myrstad M., Ihle-Hansen H., Tveita A., et al. National Early Warning Score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from Covid-19 – a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2020;28(1):66. doi: 10.1186/s13049-020-00764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gupta R.K., Marks M., Samuels T.H.A., et al. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study. Eur Respir J. 2020;56(6):2003498. doi: 10.1183/13993003.03498-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goodacre S., Thomas B., Sutton L., et al. Derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19: the PRIEST observational cohort study. PLoS One. 2021 Jan 22;16(1) doi: 10.1371/journal.pone.0245840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu F., Sun X., Zhang Y., et al. Evaluation of the risk prediction tools for patients with coronavirus disease 2019 in Wuhan, China: a single-centered, retrospective. Observational Study Crit Care Med. 2020;48(11):e1004–e1011. doi: 10.1097/CCM.0000000000004549. [DOI] [PMC free article] [PubMed] [Google Scholar]