Abstract
The hair follicle (HF) is an exquisite skin appendage endowed with cyclical regenerative capacity; however, de novo follicle formation does not naturally occur. Consequently, patients suffering from extensive skin damage or hair loss are deprived of the HF critical physiological and/or aesthetic functions, severally compromising skin function and the individual's psychosocial well‐being. Translation of regenerative strategies has been prevented by the loss of trichogenic capacity that relevant cell populations undergo in culture and by the lack of suitable human‐based in vitro testing platforms. Here, we provide a comprehensive overview of the major difficulties associated with HF regeneration and the approaches used to overcome these drawbacks. We describe key cellular requirements and discuss the importance of the HF extracellular matrix and associated signaling for HF regeneration. Finally, we summarize the strategies proposed so far to bioengineer human HF or hair‐bearing skin models and disclose future trends for the field.
Keywords: dermal papilla cells, epithelial cells, extracellular matrix, hair follicle, in vitro models, signaling
1. THE NEEDS AND DIFFICULTIES OF HUMAN HAIR FOLLICLE REGENERATION
Hair is a characteristic and unique trait of mammals with key physiological functions. Depending on the species and body distribution, the hair follicle (HF) has critical roles in thermoregulation, physical protection, immunosurveillance, relaying sensory perception and as a reservoir of stem cells that are necessary for both homeostasis and response to injury. Moreover, HF has an important aesthetic role for humans, being deeply involved in the individuals' nonverbal communication and well‐being. Thereby, hair loss is invariably associated with a decline in skin function and, depending on the affected area, a profound psychological impact on patients. 1
The HF is a complex skin appendage that undergoes lifelong cyclical remodeling, 2 which is reminiscent of the hair embryonic development and equally dependent on different stem cells and well‐orchestrated epithelial–mesenchymal interactions (EMIs). 3 , 4 , 5 However, and unlike what happens in some other mammals, in humans no additional HFs are naturally formed after birth. 6 When the hair growth machinery is compromised, such as in full‐thickness skin defects or hair disorders, HFs cannot regenerate on their own. In case of extensive injuries, such as severe burns and chronic wounds, the skin loses its regenerative power and autologous grafting is prevented by the shortage of donor skin. Skin substitutes could be considered the ideal treatment, but current products only replicate the epidermal and dermal layers and have limited regenerative capacity, preventing appendages reformation. 1 Also for hair loss disorders, such as the highly prevalent androgenetic alopecia, there are very few treatments none of which are very effective. Hair growth‐promoting drugs have an unpredictable and limited effect and the only effective treatment requires autologous transplantation of HFs from the healthy occipital area to the affected site, a procedure to which not all patients are electable and that implies the creation of multiple donor sites.
Pioneering studies using rat vibrissa dermal papilla (DP), an inductive mesenchymal structure present at the base of the HF, demonstrated that it was possible to promote HF neogenesis with the transplantation of DP. 7 , 8 Ever since the inductive nature of dissociated murine DP (mDP) and dermal sheath (DS, another mesenchymal compartment of the HF) cells was also demonstrated in combination with receptive epithelium cells. 9 However, it was only after 30 years from the first experiments with murine cells that the inductive nature of intact human DS 10 , 11 and DP 12 was also demonstrated.
While these discoveries have opened possibilities in the development of human HF regenerative strategies, these have been hampered by successive challenges. Comparably to murine, human DP (hDP) cells readily lose their intrinsic inductive properties when removed from their native environment and cultured in vitro. 13 Indeed, successful human HF regenerative strategies have been hampered by the difficulty in preserving the trichogenic capacity of key mesenchymal and epithelial cells after isolation and/or expansion, both indispensable to obtain clinically relevant cell numbers for biomedical applications.
Along the years, murine models and cells have allowed gaining critical cellular and molecular knowledge on HF embryonic development, postnatal cyclical growth and even regeneration. Nonetheless, there are fundamental interspecies differences that need to be considered. Rodents have different types of hair, some of them highly specialized (e.g., vibrissa), that display a regular distribution pattern and grow in a synchronized way. In contrast, human HFs act independently, have different hair cycle duration and are sensitive to androgens. 14 Therefore, given the expected low animal‐to‐human translational success rate, human‐dedicated approaches and study platforms in which human HF development, growth and regeneration can be studied remain unfulfilled needs.
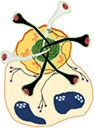
This review starts by contextualizing the basic mechanisms underlying HF morphogenesis and cycling and by describing its constituents, including the resident cellular populations, their niches and main functions. Next, relevant aspects associated with the creation of a HF regenerative microenvironment in vitro are discussed. The use of relevant mesenchymal and epithelial cells, the maintenance of their key properties, the importance of a supportive extracellular matrix (ECM) and the existing knowledge of HF‐related signaling events are highlighted (Figure 1). The strategies attempted so far to bioengineer human HF models, or skin models capable of sustaining HF formation in vitro, are then described, emphasizing their achievements and limitations. Finally, general conclusions are drawn and future lines of investigation and important considerations for the field are put in perspective.
FIGURE 1.
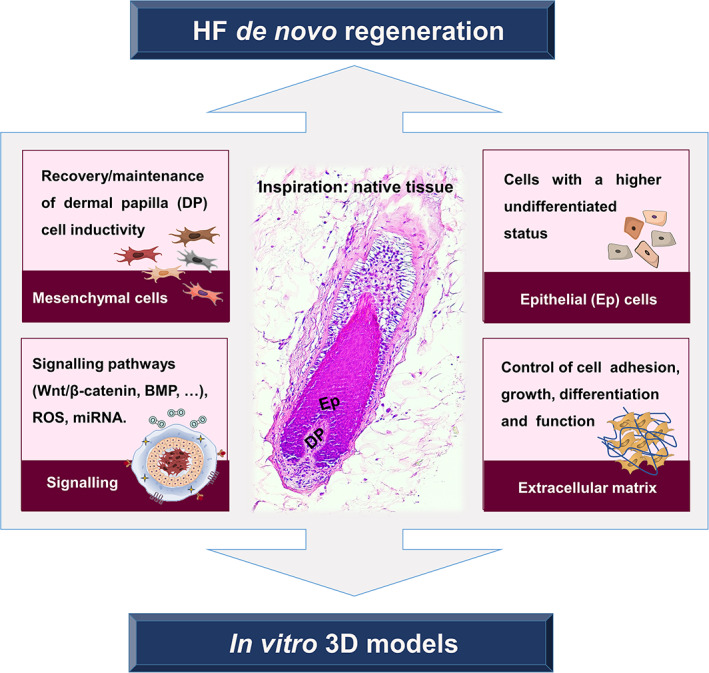
Elements to consider for the recreation of a hair follicle (HF) regenerative microenvironment
2. BIOLOGY OF THE HUMAN HF
2.1. Embryonic development
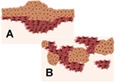
Depending on their embryonic origin, stem cells can differentiate into the epithelial (ectoderm), mesenchymal (mesoderm) or melanocyte (ectoderm derived neural‐crest cells) lineages that constitute the HF. The HF originates from neuroectodermal–mesodermal interactions (Figure 2(a)), which in humans start by week 12 of the fetal development when a yet unknown signal, considered to arise from the dermis, causes the formation of spaced thickenings in the ectoderm‐derived epithelium, the hair placodes 15 (Figure 2(b)). At this point, mesenchymal cells located below the stabilized placodes receive epidermal signals to start to aggregate into dermal condensates (Figure 2(c)), establishing the location of the future HF. 16 Under highly coordinated bidirectional ectoderm–mesoderm interactions, that are triggered by a second dermal signal, the epithelium forms the hair peg (Figure 2(d)) that invades the dermis and eventually surrounds and encloses the dermal condensate. During this process, ectoderm‐derived progenitors give rise to epithelial stem cells (EpSCs) that stay located in the upper part of the incipient HF, in the bulge, providing rapidly dividing cells that allow the downward epithelial movement. 17 In a parallel process, ectoderm‐derived neural crest cells differentiate into melanocyte stem cells, which will reside in the hair bulge and act as the source of the melanocytes that take part in the pigmentary unit. 2 By the time epithelial cells engulf the dermal condensate, it differentiates into a specialized mesenchymal population, the DP 3 , 15 , 18 forming, together with the migrating melanocytes, the hair bulb. Along this process, another population of mesoderm‐derived cells surrounds the incipient HF, from the bulge level down to the stalk, bordering the DP and forming the DS, also known as the connective tissue layer. The hair bulb becomes fully mature when it reaches the dermis base and is encased in a layer of dermis‐derived adipose tissue, known as the dermal white adipose tissue (dWAT). 19 Lineage tracing studies in mice demonstrate that this adipose layer forms independently from the subcutaneous tissue, being originated from a common precursor with the dermal fibroblast lineage, and generates mature adipocytes after HF development. 20 , 21 At this point, the DP is fully established as a permanent part of the HF providing the inductive signals necessary to complete HF formation. 15 , 18 A well‐controlled epidermal–mesenchymal constant intercommunication (Figure 2(e)) will then be responsible for lifelong hair growth, generating the hair shaft, the part of the HF that is projected beyond the skin and becomes visible on the body surface.
FIGURE 2.
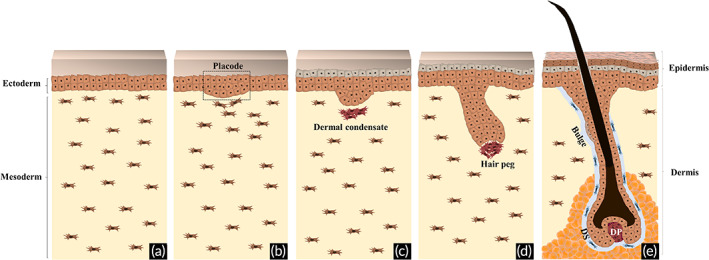
Hair follicle embryonic development. (a) The epithelial and mesenchymal components of the hair follicle (HF) are derived, respectively, from the ectoderm and mesoderm germ layers. (b) Initially, a dermal signal derived from mesodermal cells promotes the formation of spaced thickenings of epidermal progenitors, known as placodes. (c) In response, an epithelial signal stimulates the dermal cells to cluster below the placode, giving rise to the dermal condensate. (d) After a second dermal signal, placode cells start to proliferate and invade the dermis, originating the hair peg. (e) After continuous downward proliferation, the epithelial cells eventually engulf the dermal condensate, which develops into the dermal papilla (DP), allowing the establishment of epithelial–mesenchymal interactions that will further promote the proliferation and differentiation of the epithelial cells into the different structural layers of the mature HF, ultimately leading to the formation of the hair fiber
2.2. Anatomical components
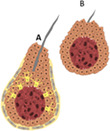
The HF is a singular entity that, together with the sebaceous gland (SG) and arrector pili muscle (APM) forms the pilosebaceous unit 22 (Figure 3(a)). Anatomically, the HF can be divided into different areas: the infundibulum, isthmus, suprabulbar region and bulb. The infundibulum is in the upper part of the HF, extending from the interfollicular epidermis (IFE) to the sebaceous duct, while the isthmus is located between the SG and the APM, accommodating the bulge. Some literature also refers to the upper part of the isthmus as a separated compartment termed junctional zone. 23 Below the isthmus lies the suprabulbar area, followed by the hair bulb, the lowest portion of the HF and the one responsible for its active growing.
FIGURE 3.
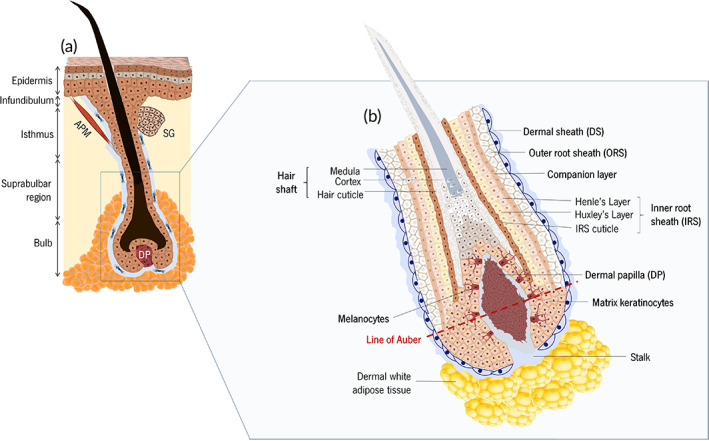
Anatomic representation of the (a) pilosebaceous unit and (b) lower hair follicle
The hair bulb is divided into two compartments separated by the fictional line of Auber. The DP, the mitotically active epithelial cells that colonize the matrix area (commonly referred to as matrix keratinocytes) and the neural‐crest derived melanocytes are present below this line (Figure 3(b)). The two latter are the respective offspring of keratinocytes and melanocytes progenitors that previously migrated from the bulge through the outer root sheath (ORS), a layer of proliferative epithelial cells that extends down to the hair bulb and divides locally. 24 , 25 Above Auber's line lies the offspring of matrix keratinocytes, which differentiated due to the action of factors released by DP, 26 generating an upward movement through the follicle lumen.
2.3. Cycling
Conventionally, a hair growth cycle is divided into three phases: anagen (growing phase), catagen (transition phase), and telogen (resting phase). 18 During each cycle, the lower part of the HF self‐regenerates, while the upper region, from the bulge level up, remains constant. 18 , 27 During anagen, and under highly regulated EMIs, the hair shaft erupts from the skin and the hair fiber grows. In pigmented hairs, this stage is also characterized by the production of melanin by melanocytes and its transfer to the cortical and medullar keratinocytes of the growing hair shaft, making it visible. 28
After anagen, which in humans can last between 2 and 8 years, 29 the mature HF entries into catagen. In this destructive phase, generally lasting for 4–6 weeks, 29 the activity of matrix epithelial cells and melanocytes gradually slows down eventually stopping with the cells entering in an apoptotic state. 2 As a consequence, the bulb size decreases dramatically and the DP, which was deeply rooted in the dWAT, 19 regresses and moves upward to the dermis as the remaining epithelial strand regresses. This leads to the formation of the hair germ, constituted by bulge‐derived keratinocytes and other follicular stem cells, localized between the bulge and the DP. 30 , 31 At this stage, melanin production ceases, 28 remaining absent through the 2–3 months 29 of telogen. Likewise, the DP remains in a quiescent state close to the HF stem cells during telogen. 30
The shedding of the club hair (telogen hair shaft), initially thought to be a passive process, is now known to be a controlled process and thus considered an independent phase of the hair cycle, the exogen. 32 After exogen, the HF can initiate a new cycle and regenerate its lower portion by entering in a new anagen phase, which requires the activation of the hair germ. 22 , 30 Then, the HF epithelium lengthens downward, pushing down the DP and reestablishing the different epithelial layers. At the end, the new hair bulb, necessary to produce a new hair shaft, is reconstituted. 30
2.4. Stem cell niches
The unique ability of the HF to self‐regenerate cyclically relies on different stem cell populations, located in distinct follicular niches, which coordinate or provide the necessary cellular material for the continuous turnover and replacement of differentiated cells.
The bulge is a particularly privileged structure, comprising multipotent stem cells that can give rise to different HF lineages. In fact, the majority of the clonogenic cells in the HF are derived from this area. EpSCs reside in the bulge as long‐lived quiescent cells 33 and, as previously mentioned, their offspring is responsible for reconstituting the lower HF apparatus and the derived epithelial layers upon anagen onset. 34 Moreover, bulge EpSCs can also replenish extrafollicular cellular compartments, including the IFE and SG. 35 , 36 Melanocyte stem cells also make their permanent residence in the HF bulge and sub‐bulge region, after migrating from the perinatal dermis and epidermis. 37 In an analogous way to what happens with EpSCs, melanocyte stem cells give rise to transit‐amplifying melanocyte progenitors, which proliferate and migrate along with the ORS, differentiating into the melanin‐producing melanocytes that during anagen coinhabit the hair bulb with matrix keratinocytes. 37 , 38 These die by apoptosis throughout catagen, being afterward replenished by the bulge melanocyte stem cells in the following cycle. 39 Other neural crest‐like multipotent adult stem cells have been suggested to reside within the bulge, since murine 40 , 41 and human 42 , 43 cells derived from this area demonstrated the capacity to differentiate in vitro into both neural and mesodermal progeny, including cells from the adipogenic, myofibroblastic, glial, myogenic, and neuronal lineages. Nevertheless, in vivo lineage tracing or functional assays still need to be carried out to confirm the existence and role of these populations in humans.
The bulge is classically considered the HF stem cell reservoir, but during the past decade, evidence collected from murine has demonstrated the presence of epidermal stem cell populations among other HF regions, including the infundibulum, SG, junctional zone, and isthmus. 22 , 44 , 45 Under homeostatic conditions, each subcompartment generally produces a subset of epidermal cells but, upon injury, the different subpopulations can also contribute to a broader range of epidermal lineages. 46 Although markers of those subpopulations have not been described or confirmed so far in humans, they are most likely contributing to the stem cell heterogeneity in the pilosebaceous unit and maintenance of the different epithelial regions.
Along the hair cycle, the DP suffers volume and cell number fluctuations 47 while DS regresses during catagen. 48 Although both DP and DS are known to hold dermal stem cells, 48 , 49 DP cells rarely display proliferative features. 47 Different studies showed that stem cells present in the DS possess the capacity both to regenerate a new DS and supply cells to the DP whenever necessary. 11 , 48 , 50 However, this relation may be more elaborated, since DP cells can themselves contribute to DS renewal during anagen, 51 suggesting cellular interchangeability and cellular plasticity. 47 The future identification of distinctive stemness‐related markers and their link to the mesenchymal progeny of the HF might allow to further understand this dynamic and/or potentially identify other critical niches and functions.
3. CELLULAR REQUIREMENTS FOR HF REGENERATION
The continuous reconstitution of the HF and its growth machinery after each cycle is dependent on elementary EMIs, implying the need to combine inductive mesenchymal cells and responsive epithelial cells as building blocks in HF regenerative strategies.
3.1. Mesenchymal cells
Although the hair mesenchyme is composed of both the DP and DS compartments, DP cells are considered the prime inductive players given their role in the initiation and control of hair formation and growth. 5 , 52 The inductive capacity of the peribulbar portion of the DS is sufficient to replace the DP and to regenerate new HFs both in rodents 10 , 53 and humans. 11 However, dissociated rat 54 DS cells commonly fail to induce hair growth, suggesting that DS‐to‐DP cell transition may be lost upon culture and in the absence of influence from the adjacent epithelium.
The current major challenge associated with the application of hDP cells for HF regenerative purposes is the loss of their inductive phenotype after 2D culture. 13 , 55 Therefore, the use of hDP cells for the recreation of the HF microenvironment relies on the recovery and/or maintenance of their native properties in vitro. Up to now, the gold‐standard strategy to preserve hDP cell inductivity is to artificially promote their native intercellular organization and aggregation into 3D spheroids, which partially restores DP cell features 56 and molecular signature. 13 Depending on the size of the spheroid, oxygen (O2) diffusion might be progressively decreased and whether this play a role in rescuing hDP cell properties in vitro has to be further understood. Cell culture is typically performed under atmospheric O2 levels (21% O2, normoxia) but in vivo physiological levels usually range between 1 and 11% O2. 57 Comparably to cells cultured under normoxia, hDP cells cultured in 2% O2 showed a significant increase in alkaline phosphatase (ALP) activity, a recognized DP cell inductive marker, 50 , 58 suggesting improved hair inductive capacity. This was confirmed in vivo, where DP spheroids formed from hDP cells cultured under 2% O2 were able to sustain hair neogenesis, unlike cells cultured under atmospheric oxygen levels. 59
Others have considered DP and bulbar epithelial cells proximity and signaling during anagen, to design new approaches capable of rescuing or preserving native DP cell key features in vitro. We have recently demonstrated that the conditioned medium collected from human skin‐derived keratinocytes (KCs‐CM) improved the inductivity and self‐aggregation capacity of hDP cells, while supporting a secretome profile that matches the one displayed by these cells during anagen. 60 Moreover, cells cultured with KCs‐CM displayed improved ALP activity compared to hDP cells cultured as spheroids and were capable of reforming HF‐ and SG‐like structures in vivo, suggesting that this could be a superior and simple strategy to improve hDP cell trichogenic capacity. 60 Interestingly, the supplementation of hDP cells culture with 1α,25‐dihydroxyvitamin D3 (VD3), a compound identified in KCs‐CM, also leads to improved ALP activity. The analysis of possible mechanisms of action revealed that VD3 significantly upregulated Wnt‐10b and TGF‐β2 gene expression in a dose‐dependent manner. However, Wnt inhibition did not hinder VD3 effects on ALP activity. Moreover, as TGF‐β2 upregulation was also maintained and TGF‐β2 signaling was demonstrated to be crucial to hair folliculogenesis and maturation in animal models, 61 it was suggested that VD3 beneficial effects might involve this growth factor pathway.
Despite these discoveries, strategies that directly target HF signaling pathways known to be downregulated in 2D culture, such as Wnt/β‐catenin, bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) 55 have been more commonly explored to revert the loss of DP cell inductivity in culture. Adding Wnt‐1a to the culture medium of hDP cells restores the expression of hair induction‐related genes, namely versican, lymphoid enhancer‐binding factor 1 (LEF1), patched homolog‐1, and GLI‐Kruppel family member GLI‐1, previously inhibited with dihidrotestosterone. 62 Also, human cell treatment with Wnt‐3a and Wnt‐7b enriched extracellular vesicles improved versican, ALP and β‐catenin protein expression and LEF1, vascular endothelial growth factor (VEGF) and keratinocyte growth factor (KGF) transcripts. 63 An alternative strategy to improve Wnt/β‐catenin activation in hDP cells comprises blocking glycogen synthase kinase‐3, a key enzyme responsible for the degradation of cytoplasmic β‐catenin. So far, molecules successfully used to this end include valproic acid and 6‐bromoindirubin‐3′‐oxime (BIO). Treatment with valproic acid improved ALP activity and quantity in cultured hDP cells, concomitantly with the upregulation of β‐catenin. 64 In turn, BIO has been mainly used in combination with other biomolecules as a strategy to target multiple signaling pathways. An optimized combination of BIO with recombinant BMP2 and basic FGF (bFGF) maintained the expression of hDP signature genes and improved their ALP activity. 55 Increased ALP activity and quantity were also confirmed with the supplementation of the culture medium only with BIO and bFGF. 65 A combined approach showed that VD3, BMP6—a key molecule in maintaining mice DP cell inductive capacity 66 —and valproic acid further enhanced versican expression and ALP activity in comparison to Wnt‐3a treatment. 67
Another pathway more recently associated with hair growth control is the Janus kinase/Signal transducers and activator of transcription (JAK/STAT), whose pharmacological inhibition has been demonstrated to promote robust hair regrowth in patients with alopecia areata, an autoimmune hair‐loss disorder. 68 The impact of JAK/STAT inhibitors on the inductive capacity of hDP cells was showed when tofacitinib‐treated hDP spheroids displayed improved HF induction rate than those treated with ruxolitinib, which performed similarly to nontreated spheroids. 69 The treatment with tofacitinib upregulated TGF‐β2, BMP6, and LEF1 expression 69 while ruxolitinib improved β‐catenin gene expression but decrease TGF‐β2 expression. 70 This suggests a TGF‐β2 mediated hair growth after inhibition of the JAK/STAT signaling pathway.
Despite the different rationales and strategies that have been explored so far for the recovery of the inductive phenotype of hDP cells, further studies will be needed to select or develop the most promising one(s).
3.2. Epithelial cells
Epithelial cells isolated both from follicular and interfollicular sources possess the capacity to differentiate into follicular epithelium under proper DP cell signaling. Among the follicular sources already described are the bulge, 35 , 71 ORS, 72 , 73 , 74 or hair bulb. 75 Besides, skin epithelial cells have also been successfully combined with DP cells to regenerate HFs, even when isolated from glabrous skin sources such as foreskin. 76
The differentiation level of the epithelial cells, however, deeply affects their response to inductive dermal cell stimuli and consequently, their trichogenic capacity. K15 positive cells from the skin of adult mice support HF formation when co‐transplanted with neonatal mouse dermal cells, but the same is not observed when the whole epidermal population is used. 35 This was also demonstrated with human epithelial cells from neonatal skin that, in combination with mDP cells, led to the recreation of a higher number of HF‐like structures than adult skin cells, which lose their trichogenicity after the second passage. 76 Indeed, few studies demonstrated de novo HF formation from dissociated human cells and most of them report the use of hDP cells with the skin of mouse embryos 77 or embryonic 78 /newborn 79 , 80 skin epithelial cells. To date, entirely human HF regeneration was only succeeded when using epithelial cells from fetal origin 81 , 82 or neonatal/children skin. 58 , 83 While these keratinocytes display higher competence than adult cells, 74 the use of newborn cells for regenerative purposes cannot be completely dissociated from ethical concerns. The use of adult cells with stem cell properties, however, can represent a viable alternative. We have recently demonstrated that human IFE epithelial cells with stem‐like features, isolated by fluorescence‐activated cell sorting based on their α6‐integrinbri/CD71dim expression, were capable of supporting the formation of immature HFs and SGs when co‐transplanted with hDP cells in mice, 84 thus representing a promising epithelial cell source for HF regeneration.
Besides preserving epithelial cells undifferentiated state, it is also most advantageous to maintain or promote the trichogenic capacity of the cells in culture. Studies with human cells showed that a follicular‐fate and the trichogenic capacity of keratinocytes can be promoted by coculture with inductive dermal cells. The expression of the epidermal differentiation markers keratin 1 and profillagrin was downregulated in bulge‐derived keratinocytes indirectly cocultured with hDP cells, whereas the expression of the companion layer‐specific markers K75 (formerly known as K6hf) significantly increased, demonstrating that the cells engage in a follicular‐type of differentiation under hDP cell stimuli. 85 The upregulation of keratins predominantly or exclusively expressed in the HF epithelium, namely K6, K16, K17, and K75, in high passage rat ORS keratinocytes expanded in 3T3 feeders was also reported after coculture with DP cells from the same species. 74 Moreover, keratinocytes cocultured with DP cells were able to support the formation of new HFs, which was not observed when only clonal expansion in feeders was performed. Similar results were observed when human ORS were grafted with newborn mDP cells. 86 More recently, follicular keratinocytes cocultured with a CD49fhigh dermal subpopulation in Matrigel, both from newborn mice, coled the formation of bud‐like structures with an improved expression of Sox9 and ectodysplasin A receptor (Edar), a marker of the developing hair placode. 87 Nevertheless, in vivo demonstration or translation of those results to human cells has not been performed so far.
Others have shown that the enrichment of mouse IFE cells in the bulge markers integrin α6+/CD34+ improves cellular trichogenicity, as demonstrated by the increased number of follicles formed after co‐grafting with freshly isolated neonatal mouse fibroblasts. 88
Overall, the literature demonstrates that different epithelial cell sources may be used for HF regeneration but those with higher undifferentiated status and clonogenic capacity exhibit improved hair regenerative efficiency. Moreover, maintaining in culture the characteristic signaling receptiveness of follicular keratinocytes, or predisposing nonfollicular undifferentiated cells toward a follicular fate, may prove beneficial in improving trichogenic capacity of epithelial cells in culture.
4. HF‐RELATED SIGNALING
The events regulating human hair morphogenesis and cycling are still largely unknown. However, it is well‐established that HF formation and growth involves different conserved signaling pathways including, but not limited to, Wnt/β‐catenin, Sonic hedgehog (Shh)/PDGF‐A, FGF, BMP, TGF‐β, and Edar/nuclear factor‐kappa B (NF‐κB) pathways. Although the dissection of the mechanisms regulating HF is beyond the scope of this review (for more details please see References 3, 4, 5, 15, and 89), the knowledge generated so far regarding each one of these signaling pathways, may present opportunities for HF regenerative strategies.
The Wnt/β‐catenin signaling pathway is highly involved in hair development, growth and cycling. 90 , 91 Studies in mice showed that the activation of this pathway, and β‐catenin stabilization, direct epidermal cells toward a follicular phenotype. 92 , 93 Transient β‐catenin activation in mice epidermis promotes HF neogenesis and induces anagen in follicles in the telogen phase, by improving cell proliferation and outgrowth both from the epidermis and existing ORS. 93 Wnt/β‐catenin signaling is also critical in the de novo HF formation during wound healing, which reproduces the HF embryonic development. 92 In vitro, β‐catenin activation and nuclear translocation were linked to the upregulated expression of follicular‐specific epithelial markers in keratinocytes cocultured with DP cells, both for human 85 and rat cells. 74 Thus, the role of different Wnt proteins in promoting epithelial cell differentiation has been addressed. Recombinant Wnt‐10b, for example, was shown to be necessary to initiate HF development in mouse embryonic skin organ cultures. Moreover, hair formation was observed only when mice neonatal epithelial cells and fibroblasts were co‐grafted with a fibroblast cell line transfected to produce Wnt‐10b. 94 In vitro, Wnt‐10b supplementation, contrarily to Wnt‐3a, Wnt‐5a, and Wnt‐11, promoted advanced follicular differentiation of primary keratinocytes from mice skin, suppressing their proliferation and inducing the expression of late differentiation markers, including cortex (mHa5 and mHb5) 95 and IRS (AE13 and AE15) 96 specific markers. Moreover, as previously mentioned, Wnt ligands also seem to maintain hDP cell inductivity in vitro. Thereby, Wnt ligands may be used as a strategy to direct keratinocytes follicular differentiation and preserve the identity of DP cells in vitro.
NF‐κB activation through Eda (ectodysplasin)/Edar signaling is also acknowledged as crucial for HF morphogenesis, namely for placode formation, stabilization, and patterning. 97 , 98 , 99 Eda‐A1 is expressed in the epidermis and hair placodes whereas Eda‐A2 is only expressed after, in the hair bulb epithelium. 100 Probably for this reason, Eda‐A1 seems to be required for ectodermal differentiation, as shown by the inhibition of hair development after mutation of the Eda‐A1 isoform and its receptor. 101 Nevertheless, both Eda‐A1 and Eda‐A2 isoforms are capable of enhancing the number of epidermal invaginations in skin organ cultures, despite the superior effect of Eda‐A2. 100 Conversely, postnatal Eda signaling is linked to HF cycle control, particularly in promoting catagen onset and progression. 97 , 102 Exploration of possible mechanisms in vitro demonstrated that Eda‐A2 stimulates apoptosis in cultured human ORS keratinocytes and hDP cells, 102 and that Eda‐A2 receptor is upregulated in balding hDP cells compared to cells from the corresponding nonbalding areas. 103 Although the role of Eda/Edar signaling has been mainly explored in rodents, its capacity to trigger HF morphogenesis might prove advantageous if explored for human regenerative strategies.
In the mature HF, the activation of BMP signaling is crucial to promote HF differentiation and maturation, 104 but it needs to be initially counteracted for anagen entry. 105 Noggin, a protein that neutralizes BMP, is exclusively produced in the HF mesenchyme and knockout experiments in mice showed it to be necessary to stimulate postnatal hair induction. 106 On the other hand, transgenic mice expressing noggin are characterized by excessive proliferation of matrix keratinocytes and prevention of hair shaft maturation, 104 clearly demonstrating that a tight spatiotemporal control between BMPs and their inhibitors need to be carefully achieved for their effective use in the recreation of functional hairs.
PDGF‐A and Shh are required to promote DP development and HF maturation. 107 , 108 , 109 PDGF‐A is expressed in mouse embryonic epidermis and developing HF epithelium and acts directly in receptors present in the underlying mesenchyme, promoting their proliferation and the normal development of all skin mesenchymal compartments: DP, DS, and dermis. 107 Thus, PDGF‐A deficiency results in dermal hypoplasia and impaired DP and DS formation, with animals exhibiting a scarce and thinner hair coat. Shh is required to drive DP formation from dermal condensates, the reason why its deficiency blocks hair formation in mice at an early stage. 107 , 108 , 109 Postnatal PDGF‐A ligands are still required to maintain DP cell function and receptor deficiency results in a progressive depletion of the HF dermal stem cell pool 110 and disturbs both dermal connective tissue development and hair canal formation. 111 Also, local injection of PDGF ligands in mice skin induces anagen, which was associated with an upregulation of Shh, Wnt‐5a, and LEF1 at the injection sites. 112 From the TGF‐β family, only the TGF‐β2 isoform is indispensable to promote hair development, playing a specific role in hair morphogenesis induction that is not shared with other isoforms. 113 TGF‐β2 treatment in mouse embryonic skin significantly increased the number of hair buds and their stage of development, whereas TGF‐β1 reduced it. 113 In vitro, TGF‐β2 secretion by hDP cells significantly increases when these are cultured with KCs‐CM, which is associated with an improvement of these cells' inductive capacity. 61 Indeed, when TGF‐β2 signal was inhibited in mice, HF formation and maturation were significantly impaired. 61 TGF‐β1, in turn, acts as a negative regulator of keratinocytes proliferation, promoting instead their differentiation and even apoptosis, being commonly appointed as a catagen regulator in murine. 114 Considering this evidence, it is reasonable to conclude that both PDGF‐A/Shh and TGF‐β2 hold great potential to be used in HF regenerative strategies.
Different elements of the FGF family can also have quite distinct hair regulatory effects. FGF7, also known as KGF, is an important mitogenic factor for keratinocytes and is normally secreted within the DP, contributing to the proliferation of the hair germ cells and activation of a new hair cycle in mice. Its expression increases in telogen but, on its own, is not enough to promote anagen. 30 In turn, topical application of FGF1, FGF2, and FGF10 in telogenic mice was shown to promote hair growth by inducing early anagen and prolonging its duration. 115 A similar role was observed for FGF18, whose subcutaneous injection in mice uncovered a potential role in promoting telogen‐to‐anagen transition. 116 Contrariwise, FGF5 regulates catagen onset in human HF organ cultures. Mutations in this growth factor are associated with increased HF lengthening in humans. 117 In vitro studies demonstrated that FGF2 increases the proliferation of both hDP and DS cells 118 and, interestingly, the addition of FGF2 to terminally differentiated human epidermal keratinocytes promoted their dedifferentiation toward an early proliferative. 119 This increase in the proliferative capacity of both cell types might explain their capacity to induce anagen. Besides the modulation of hair‐related pathways, there are other signaling molecules known to impact hair development and growth. Although classically associated with oxidative stress, reactive oxygen species (ROS) at physiological levels play an important role in hair formation. Bulbar epithelial cells produce high amounts of ROS during hair growing 120 and studies using human ex vivo HFs organ cultures proved that transient endogenous ROS production promotes HF stem cell activation and anagen entry, which was associated with the activation of Wnt transcriptional factors. 121 Corroborating these observations, Hamanaka et al. 122 showed that mice genetically manipulated to prevent mitochondrial ROS generation in basal epithelium displayed impaired development of epidermis, HF and SG. This suggests that both the modulation of different FGFs and the transitory increase of ROS production may represent alternative strategies to promote anagen and hair growth. Yet, studies demonstrating their influence or possible impact during HF development or regeneration are lacking.
MicroRNA (miRNA), involved in the posttranscriptional genetic control of multiple biological processes, also play a role in controlling HF organogenesis and postnatal growth (reviewed in Reference 123). For example, miRNA‐203 targets human and mouse p63, a transcriptional factor highly expressed in the innermost basal keratinocytes, and its upregulation is associated with a decrease of the clonogenic capacity of human keratinocytes. 124 This explains why its expression is intensified in differentiating cells of the HF. 125 Similarly, the antiproliferative miRNA‐24 was shown to block the keratinocyte stemness regulator transcription factor 3, compromising the normal process of HF differentiation in mice. 126 In turn, miRNA‐125b is implicated in stem cells maintenance and its sustained expression, in engineered transgenic mice, blocks hair growth by repressing hair stem cell differentiation. 127 Noteworthy, miRNA‐31 is associated with HF cycle progression, increasing during anagen and decreasing afterward, and administration of an antisense inhibition was associated with hair shaft defects and ORS hyperplasia in mice. 128 Exploration of the underlying mechanisms showed that miRNA‐31 acted by targeting the expression of structural keratins in keratinocytes. 128 Although the incorporation of relevant miRNA in HF regenerative strategies is still theoretical, increased understanding of the involved mechanisms is expected to take miRNA‐based strategies closer to control HF cell behavior.
5. THE IMPORTANCE OF THE ECM
In addition to the critical homotypic or heterotypic cell–cell communication, cell–ECM interactions in the HF are crucial not only to maintain the specific cell phenotype and role within the individual compartments but are also involved in physiological (hair cycle) and pathophysiological (hair associated disorders) environment alterations. 129 The epithelial and mesenchymal compartments of the human HF are separated by a highly specialized ECM basement membrane rich in collagen IV, fibronectin, and laminin. 130 The hDP ECM also has a prominent expression of these basement membrane elements, comparably to the nonfollicular dermis, along with collagen III, collagen I, versican, and glycosaminoglycans. 131 , 132 , 133
The basement membrane ECM is particularly relevant for epithelial cells differentiation state. Like in the skin, HF basal keratinocytes engagement in a differentiation pathway is associated with the loosening of their interaction with the basement membrane, while the maintenance of the epidermal reservoir is ensured by anchored stem cells. 134 ORS keratinocytes interact with the basement membrane via α2β1, α3β1, and α6β4 integrins, which are differentially expressed along the HF epithelium, most likely reflecting their differentiation along the HF. 135 Collagen XVII (COL17A1) is highly expressed in bulge EpSCs and represents a specific stem‐cell anchoring protein required to maintain epithelial and melanocyte stem cell quiescence. 136 COL17A1‐null mice suffer from premature hair loss and hair graying, which is associated with bulge depletion of EpSCs and also of melanocytes stem cells, which do not express COL17A1 but directly adhere to EpSCs, being equally lost in the process. In humans, mutations in this protein lead to atrophic hair loss associated with generalized atrophic benign epidermolysis bullosa. 137
The HF ECM is also a key player in the regulation of HF development, hair cycle and signaling. The disruption of the DP ECM and the basal lamina by dispase, a fibronectinase, and Type IV collagenase, inhibited bulb growth in rat vibrissae ex vivo cultures. This confirms the importance of the ECM in supporting hair growth and that the paracrine signaling between epithelial and mesenchymal compartments is not sufficient. 138 Laminin‐511 is prominently expressed in the basement membrane of the hair germ. Its blockage in embryonic human scalp xenografts induces alopecia, suggesting a role in hair development. 139 Indeed, mice lacking laminin‐511 have impaired hair development, contained fewer hair germs and exhibit a defective basement membrane compared to wild type, features that can be reverted with exogenous laminin‐511 treatment. 139 Moreover, laminin‐511 is required for the expression of noggin and downstream regulators, which are necessary for mDP maturation during HF development. 140
Decorin, expressed in the HF epithelium and ECM of the DP, has been suggested to play a role in anagen maintenance. 141 , 142 This is confirmed by the higher expression of this proteoglycan during the anagen phase, and by the accelerated anagen onset in follicles at telogen phase after injection of human decorin in mice, and delayed transition to catagen in those at the anagen stage. 142 Moreover, decorin‐knockout mice showed shortened anagen, as well as downregulated β‐catenin signaling. In vitro results showed that upregulation of β‐catenin in ORS cells overexpressing decorin occurs together with LEF1 and Wnt‐10b. 141 This effect was also associated with increased proliferation and migration of human ORS keratinocytes in culture. 141
A similar impact could be attributed to hyaluronic acid owing to the enhanced ORS growth observed in a human hair germ model in vitro. 67 Hyaluronic acid, fibronectin, and the proteoglycans aggrecan and biglycan stimulate hDP cell proliferation in vitro. 67 Further, the motility of rat vibrissae DP cells is diminished on collagen IV, laminin, and collagen I coated surfaces, 143 while human melanocytes migration is enhanced on collagen IV, but not on fibronectin or laminin. 144 Interestingly, both RAD16‐I peptide, 145 a synthetic peptide that shares structural similarity with the integrin receptor‐binding site RGD present in several ECM proteins, 146 and collagen type IA 78 hydrogels improved ALP activity and versican expression in hDP cells, suggesting an effect on their inductive potency by these ECM‐replacing matrices in vitro. In turn, the ECM derived from hDP cells supported enhanced tyrosinase activity in melanocytes, 147 a rate‐limiting enzyme for melanin production. This effect was higher than the one observed in melanocytes cultured onto an hDP cell feeder layer or with hDP cell‐CM, further demonstrating the vital role of the ECM that other signaling mediators cannot replace.
ECM molecules can also sequester and act as a repository of soluble growth factors, protecting them from degradation and regulating their activity and presentation to adjacent cells. 148 Although no explicit reports are studying or exploring this specific capacity within the human HF, studies performed with ECM elements known to be present in the HF may provide relevant clues. Fibronectin, for example, has a high affinity to growth factors of the VEGF, PDGF and FGF families and, to a lesser extent, to TGF‐β. 149 Likewise, fibrin, vitronectin, collagens, and proteoglycans are capable of differently bind VEGFs, BMPs, hepatocyte growth factors (HGFs) and TGF‐β. 148 , 149 As previously discussed, PDGFs, TGF‐βs, and FGFs have important roles in the control of HF development and/or hair growth. Moreover, VEGF is also required for the reformation of the perifollicular vascularization upon anagen entry 150 and HGF, a potent mitogenic factor produced by DP cells, stimulates bulbar epithelial cells proliferation. 151 The ECM can regulate the distribution of growth factors either in the presence of cell‐mediated forces by the action of enzymes, such as metalloproteinases, allowing a spatiotemporal control of growth factor release, 148 and empowering the ECM with regulatory capacity.
Altogether, these studies highlight the importance of the HF ECM components in balancing epithelial cell growth and differentiation, modulating HF development and EMIs, and influencing cell‐specific function and properties, including the inductive properties of DP cells.
6. DEVELOPMENTS IN THE BIOENGINEERING OF HUMAN HF MODELS
Unquestionably, the development of HF regenerative strategies would benefit from the existence of reliable and representative in vitro models allowing, in a standardized way, to test new bioactive compounds to modulate hair growth or to study the biological and molecular events regulating hair morphogenesis and growth.
Over the years, the development of in vitro models of the human HF was looked at from different perspectives. From simple systems where 3D cultured epithelial cells and DP cells were assembled in close proximity, to models that mimicked the spatial organization of the cells within the HF bulb and, more recently, to more elaborate platforms in which HF recreation was viewed not as an isolated structure but instead integrated with the skin. Table 1 summarizes the HF models developed so far using cells of human origin, from the simplest to the more complex, highlighting their features (in vitro) and HF regeneration ability (in vivo).
TABLE 1.
Reported bioengineered human HF or hair‐bearing skin in vitro models
| In vitro structures | Strategy | In vitro experimental findings | In vivo outcomes | Ref. |
|---|---|---|---|---|
|
Epidermoid cyst‐like spheroids
|
DP cells were:
|
Independently of their location, DP cells accelerated epithelial cells proliferation. Both epithelial cell types formed aggregates (larger in ORS cells) but these displayed an epidermis‐type of stratification toward the center instead of a follicle‐type of development. | NA | 152 |
|
“Folliculoid sandwiches”
|
Dermal fibroblasts were mixed with collagen I and covered on top by: A) Matrigel network with DP cells, where ORS cells were seeded on top (layered system) B) A Matrigel network were both DP cells and ORS were encapsulated (mixed system). |
The cells remained in close contact and ORS keratinocytes were capable of proliferating and forming epithelial aggregates rather than generating an epidermis‐like stratified epithelia. | NA | 153 |
|
HF‐like structures
|
DP or DS cells were encapsulated in a collagen I gel and different epithelial cells—IFE keratinocytes, ORS keratinocytes (superior or inferior anatomical location) or matrix keratinocytes—were seeded on top. | The combination of both ORS and DS cells led the formation of structures with inward oriented epithelial concentric layers, which was not reported for DP cells. | Simple HF‐like structures mainly observed when DP cells and superior ORS keratinocytes were co‐transplanted. | 72 |
|
Folliculoid microsphere
|
DP cells and ORS keratinocytes were encapsulated in a matrix mixture of collagen I and Matrigel (4:1 ratio) and casted as small droplets. | Small aggregates of DP cells expressing versican or ORS cells positively staining for K6 and displaying proliferative properties were observed, but a HF type of organization was not observed. | NA | 154 |
|
Follicular DP structures
|
DP cells and IFE keratinocytes were encapsulated in different domains of water‐soluble chitin (polycation), separated by a sodium alginate solution (polyanion) and brought together to form an insoluble fibrous hydrogel by interfacial polyelectrolyte complexation. | DP cells self‐assembled into aggregates, while the adjacent epithelial layers remained in close contact, but only partially surrounding the DP‐spheroids. | Formation of rudimentary HF‐like structures. | 155 |
|
Unpigmentated fiber‐producing structures
|
A) DP aggregates were coated with basement membrane proteins (collagen IV, laminin and fibronectin) and ORS keratinocytes and melanocytes were added to the culture afterward. | Formation of organoids in which DP cells were surrounded by concentric epithelial layers, including a K15 and a trichohyalin‐positive layers. Melanocytes remained in close proximity with DP cells and the formation of unpigmented hair‐like fibers was observed. | NA | 73 |
| B) DP cells spheroids were formed on top of Matrigel and matrix keratinocytes were posteriorly added. | Formation of structures capable of generating a colorless hair‐like fiber. | NA | 156 | |
|
HF organoid model
|
DP‐spheroids were encapsulated in a silk‐gelatin hydrogel and cocultured with both HF stem cells and HF keratinocytes. In a biomaterial‐free approach, DP‐spheroids were directly cultured with both epithelial cells. | Formation of an in vitro HF‐organoid model in which cellular proliferation and the expression of DP cell signature genes versican (ALP, BMP4, and β‐catenin) were significantly higher with the hydrogel support. | NA | 157 |
|
Tubular structures
|
DP cells were embedded in collagen I hydrogels and IFE keratinocytes seeded on top. Alternatively, DP cells were replaced by their conditioned medium in the culture. | Both DP cells and their conditioned medium induced the projection of keratinocytes into the collagen matrix, forming tubular structures that resembled hair germ formation during embryogenesis. | NA | 158 |
|
Epidermal invaginations or folliculoid structures
|
A) DP cells were cultured in porcine acellular dermal matrices and follicular keratinocytes enriched in stem cells were posteriorly seeded on the opposite side. | Constructs with DP cells lead to a better stratified epidermis with a higher number of proliferative basal cells and epidermal invaginations. | Presence of embryonic hair bud‐like structures which expressed the companion layer marker K6hf, demonstrating commitment to the follicular lineage. | 159 |
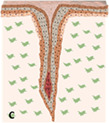
|
B) DP spheroids were incorporated between layers of fibroblast‐embedded collagen I hydrogels, which were then used to produce standard reconstructed skin. | DP spheroids within dermal equivalents stimulated epidermal downward movement and dermis invasion. These epidermal invaginations were composed of an inner K15‐positive layer, an outer K10‐positive layer and displayed a basement membrane rich in collagen IV and laminin. | NA | 160 |
|
C) DP spheroids surrounded by IFE keratinocytes were embedded in collagen‐based dermal equivalents and microscopy‐guided laser ablation was used to create a hole from the surface of the construct up to the incorporated multicellular aggregates. | The created channel guided keratinocytes migration and downward movement toward the aggregates with DP cells, which led the formation of folliculoid structures that recapitulated the HF microarchitecture. | NA | 161 |
|
HF generation within reconstructed skin
|
3D‐printed molds were used to produce dermal equivalents with microwells of controlled dimensions, inside which DP cells (transfected or not with an LEF1 vector) spontaneously formed spheroids. Neonatal keratinocytes were then seeded over the dermal constructs. | The keratinocytes encased the DP cell‐spheroids, forming an epithelial column that initiated HF‐specific differentiation, expressing hair lineage markers and occasionally forming colorless hair fibers. DP cells overexpressing LEF‐1 synergistically improved HF differentiation and hair shaft formation. Dermal vascularization was observed in the constructs used for in vivo implantation. | Vascularized constructs induced the formation of unpigmented hairs, but DS reformation in the grafts was not observed. | 83 |
|
Hair‐bearing skin organoid
|
Human embryonic (WA25) or induced pluripotent (DSP–GFP) stem cell lines were used to form aggregates and cultured with 2% Matrigel, bFGF, BMP4, and a TGF‐β inhibitor. Afterward, a BMP inhibitor and bFGF were added to the culture. The resultant organoid structures were culture and matured over 140 days. | Independently of the cell line used organoids acquired a bipolar organization, with a chondral tail and an epidermal cyst head, which developed in complete stratified skin with HFs. The recreated HFs featured all the cellular layers (except the medullar layer), were embedded in a lipid‐rich dermis, in which hairs were associated with SGs and a neuronal network. The number of HFs was higher in WA25 organoids, while a higher number of pigmented hairs were observed in the DSP–GFP organoids. | Organoids without the cartilaginous tail were engrafted in nude mice. In 55% of the xenografts, hair shaft growing perpendicular to the skin surface was observed. | 162 |
Abbreviations: ALP, alkaline phosphatase; bFGF, basic FGF; BMP, bone morphogenetic protein; DP, dermal papilla; DS, dermal sheath; FGF, fibroblast growth factor; HF, hair follicle; IFE, interfollicular epidermis; LEF1, lymphoid enhancer‐binding factor 1; ORS, outer root sheath.
The first attempts to construct these HF models were focused on creating 3D systems where the close contact between epithelial and DP cells was reestablished. These constructs depicted simplified spatial organizations and were elementary in what concerns the recapitulation of the HF structure. They involved the coculture of the cells in Matrigel, collagen I or other matrices with different spatial arrangements. 72 , 152 , 153 , 154 , 155 Despite the recreation of the signaling in these systems, the epithelial cells often formed cyst‐like spheroidal structures and a follicular‐type differentiation was not observed or confirmed. Therefore, their functionality was short and their use might be restricted to testing simple features, such as the effect of hair growth modulatory agents in epithelial cell proliferation or the expression of specific markers expression, more closely resembling coculture systems than in vitro models of HF.
More complex approaches allowed to create in vitro models in which unpigmented hair fiber‐like structures were formed. In one method DP, spheroids formed on top of Matrigel were directly cocultured with matrix keratinocytes 156 while, in a slightly more elaborated strategy, DP cell aggregates were coated with basement membrane molecules (collagen IV, laminin, fibronectin) and only then cultured with matrix keratinocytes and melanocytes. 73 The formation of “microfollicles” with a proper topological cellular arrangement in these models represented a substantial improvement in the fulfillment of criteria steps toward HF formation. In another endeavor, a simple in vitro DP organoid model, characterized by enhanced expression of inductive markers and epithelial proliferation, was developed. 157 Here, DP spheroids were encapsulated in silk‐gelatin hydrogels together with normal HF keratinocytes and HF stem cells, to mimic the HF bulb cellular environment.
Instead of bioengineering HFs as a single independent structure, others have developed or incorporated HFs in skin constructs, better reproducing the native tissue and, thereby, improving the model functionality and translational value. In a first approach, DP cells' paracrine signaling was explored for the promotion of epidermal keratinocytes organization and formation of tubular epidermal invaginations in an organotypic skin model. Both DP cell‐ and fibroblast‐laden collagen matrices were permissive to this effect although the latter still required the presence of DP cells‐conditioned medium in the culture. 158 Similarly, epithelial invasion of the dermis was demonstrated when DP cells and follicular keratinocytes enriched in stem cells were seeded in a porcine acellular dermal matrix. 159 Also, a more robust epidermal protrusion directed toward DP cells was observed when DP spheroids were incorporated within the dermal equivalents of a standard organotypic skin model. 160 While epithelial invagination and formation of an epithelial column are required for HF morphogenesis, DP spheroids rapidly dissociate when placed within collagen matrices, 83 which might be compromising DP cell inductivity and model progression. Bearing this in mind, we were able to create a more complex skin model with folliculoid appendages by incorporating, within the dermal equivalent layers, aggregates with compartmentalized DP cells and keratinocytes. 161 Microscopy‐guided laser ablation was used to fabricate a channel connecting the constructs' surface and the aggregates, to support epithelial cell migration. Follicle‐like structures with differenced concentric epithelial layers and mimicking the hair bulb architecture were obtained, though a hair shaft was not formed. In another strategy where DP spheroids integrity was maintained, the creation of a skin model with follicular units capable of generating an unpigmented hair shaft was accomplished. 83 Here, micromolding was used to create, in fibroblast‐populated collagen I hydrogels, defined microwells in which DP spheroids were formed in situ. Keratinocytes were then seeded on top, engulfing the spheroids and differentiating into proper follicular layering over the control of the inductive DP cells. Hair shafts were only formed occasionally in these models thus, to further enhance DP cell inductivity, a subpopulation of LEF1 overexpressing DP cells was also used, significantly improving HF induction efficiency and the expression of hair lineage‐specific genes.
However, the most complete in vitro recreation of human HF achieved so far was developed entirely using pluripotent stem cell lines. In a quite distinct and successful approach, hair‐bearing skin organoids akin to 18‐week human fetal skin were produced. The formation of a cyst‐like skin organoid was attained from aggregates of pluripotent stem cells and using a step‐by‐step modulation of the TGF‐β, BMP, and FGF signaling pathways, to promote ectoderm and cranial‐neural crest induction. 162 The formation of pigmented HFs within the skin organoids was observed after a 4/5‐month culture period. Importantly, the skin organoids also display a stratified epidermis and a dermis with SGs and neural innervation, linked to adjacent fat tissue. This study clearly demonstrates the relevance of selective modulation of hair development signaling pathways, as well as the advantage of reconstructing embryonic events guiding the natural development of the skin and associated appendages. Still, the long preparation time and associated costs represent considerable disadvantages comparably to the use of dissociated primary cells.
7. CONCLUSION AND FUTURE TRENDS
The need for human HF regenerative strategies, and in vitro models has been mostly nourished by the growing demonstration that murine and human skin and HFs are dissimilar.
The evidence has highlighted the importance of combining inductive DP cells and immature epithelial cells with a high proliferative capacity for the recreation of the HF cellular compartments, pushing efforts toward the maintenance of the trichogenic capacity of human cells in vitro. Even so, human hair regeneration promoted entirely from human adult cells is yet to be achieved. In truth, the literature concerning hair regeneration continues to be dominated by murine cell studies or chimeric human–murine combinations, instead of purely human approaches. On the other hand, poor success may also be partially explained by the lack of integrative approaches that go beyond the segregated exploitation of strategies to improve either the inductivity of DP cells or the competence of epithelial cells. In the future, comparative studies capable of identifying the most promising trichogenic combinations may allow significant developments in the field.
Meanwhile, other mesenchymal and epithelial human cell sources may be considered for HF regeneration. Human bone marrow or umbilical cord‐derived cells were able to induce HF formation after co‐transplantation with human ORS keratinocytes in mice and to promote hair bulb reformation in an ex vivo assay with transected human HFs. 163 Furthermore, human adipose‐derived stem cells overexpressing PDGF‐A, Sox2, and β‐catenin showed higher ALP expression levels than the nontransfected counterparts or hDP cells 164 thus representing a potential and easily available, mesenchymal cell source for HF reformulation. In an era of stem cell engineering, the use of iPSCs, from available and more easily reprogrammable autologous epithelial and mesenchymal sources, may also rapidly expand and led to important breakthroughs, such as the recently reported hair‐bearing skin organoids. 162 Nonetheless, some constraints should be first overcome, such as the differentiation of the iPSCs toward the phenotypes of interest. We still have a long road ahead to achieve this goal, but the work by Lee et al. 162 provide hope for iPSCs future applications in the HF regenerative field.
The most complex and functional HF models achieved so far comprise the whole skin with HF appendage reformation. Besides HF integration in a supportive biomimetic skin, the inclusion of other HF specialized cell populations, such as neuronal cells and melanocytes or melanocyte precursors in the models are necessary to bioengineer functional and aesthetically acceptable hairs. Also, the macroenvironment outside of the HF should be considered, particularly the adipose tissue given its regulatory role in hair development and growth. Mature HFs are deeply rooted in the dWAT and previous studies demonstrated that intradermal adipogenesis is intimately coupled with the HF stem cell activity and cycle progression. 165 Immature adipocytes in the dWAT are necessary for follicular stem cell activation, while mature adipocytes promote follicular differentiation by inhibiting bulge activity, 166 supporting the relevance of the adipose tissue for HF regenerative strategies.
Besides the cellular counterparts, the HF microenvironment constitutes, by itself, a challenge when bioengineering HFs. The ECM, in particular, has a key role in directing hair growth and the maintenance of cell function, which also varies with physiological HF cycling. Despite the knowledge generated on this subject, its translation has been limited and bioengineered HF models continue to be elementary and lacking an appropriate recreation of the complexity of the ECM or signaling molecules involved in hair control. Most strategies rely on the encapsulation of heterotypic hDP/epithelial cell combinations in ECM‐based hydrogels or their incorporation in the organotypic skin model; however, the adoption of advanced biofabrication techniques could improve our HF biomimicry capacity. The use of 3D‐bioprinting and associated layer‐by‐layer deposition of cells and respective ECM component, in particular, hold the promise to accurately recapitulate the 3D‐architecture and milieu of the HF, along with its cell–cell and cell–ECM interactions. This explains why commercial companies such as the L'Oreal/Poietis partnership or Organovo, Inc have announced their intention to bioprint HFs for in vitro testing applications. The latter even released a patent for the 3D‐bioprinting of HFs in recipient skin 167 but failed to promote neohair formation. Nevertheless, the application of this addictive manufacturing technology by researchers working in the hair restoration field is still taking its first steps. In the future, successful outcomes will be intimately connected with the development of ECM biomimetic cell‐laden bioinks and the maintenance of the native properties of the cells in culture.
Ultimately, the techniques and knowledge generated when pursuing HF recreation in vitro also hold potential for regenerative purposes. Companies like RepliCel and HairClone have been working on the concept of rejuvenating miniaturized/resting follicles by, respectively, injecting autologous DS or DP cells in the patients' scalp to produce thicker terminal hair shafts. The former published a phase I/IIa study in patients with androgenic alopecia but, so far, they were only able to temporarily improve hair density and diameter. 168 Alternatively, although only theoretical for now, the use of small amounts of donor HF samples to isolate, propagate and even genetically manipulate relevant cell populations in vitro would be ideal. These could then be used to promote HF de novo formation in situ, or to bioengineer HFs, allowing the production of ready‐to‐use follicular units that could be transferred back to the patient for hair restoration. Another important milestone would be the incorporation of HF‐like structures in skin substitutes, or the inclusion of cellular and/or bioactive clues that could sustain HF regeneration after implantation, also contributing to their functionality. Additionally, and depending on the cellular populations used and their stemness, a variable degree of self‐regeneration capacity could be granted to the skin substitutes, making them more likely to reach long‐term use and representing a more effective treatment for patients with severe skin damage.
Future research may even provide knowledge to reprogram the resident skin cells of the patient and recreate hair morphogenic events in situ. The complexity and spatiotemporal controlled nature of the HF signaling interactions imply that this type of intervention would most likely require the modulation of multiple signaling pathways and to be addressed in a timely manner, making it less likely to succeed compared to autologous cell‐based therapies. Nonetheless, there are conditions where simpler signaling modulation with inductive factors may prove sufficient to promote hair restoration or cycle reactivation in preexisting HFs. For example, hair miniaturization and loss, observed in common baldness, have been associated with a defective conversion of bulge stem cells into proliferative progenitor cells, 169 representing a prime example of a condition that can eventually be reversed by pharmacological intervention. Also, the knowledge that during wound 92 and increased β‐catenin activity 170 neofollicle formation can occur from the IFE, and not from adjacent bulge stem cells, encourage further exploration of these mechanisms in humans.
New and more effective treatment options for hair loss disorders are a major target of the pharmaceutical and cosmetic industry. Similarly, skin substitutes with increased functionality have long been a pressing requirement for effective cutaneous wound healing. The work performed in the last years in the bioengineering of purely human HFs in vitro and, particularly in the knowledge regarding HF regeneration have paved the way for future approaches with a high potential to result in clinically applicable solutions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Carla M. Abreu: Conceptualization; data curation; visualization; writing‐original draft; writing‐review and editing. Alexandra P. Marques: Conceptualization; funding acquisition; project administration; validation; visualization; writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10235.
ACKNOWLEDGMENTS
This work was supported by the consolidator grant “ECM_INK" (ERC‐2016‐COG‐726061) and by the FCT/MCTES (Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior) through the PD/59/2013, PD/BD/113800/2015 (C. M. A.) and IF/00945/2014 (A. P. M.) grants.
Abreu CM, Marques AP. Recreation of a hair follicle regenerative microenvironment: Successes and pitfalls. Bioeng Transl Med. 2022;7(1):e10235. 10.1002/btm2.10235
Funding information Consolidator grant “ECM_INK”, Grant/Award Number: ERC‐2016‐COG‐726061; Fundação para a Ciência e a Tecnologia, Grant/Award Numbers: PD/59/2013, PD/BD/113800/2015, IF/00945/2014
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Mahjour SB, Ghaffarpasand F, Wang H. Hair follicle regeneration in skin grafts: current concepts and future perspectives. Tissue Eng Part B Rev. 2012;18(1):15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider MR, Schmidt‐Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132‐R142. [DOI] [PubMed] [Google Scholar]
- 3. Sennett R, Rendl M. Mesenchymal‐epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8):917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt‐Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27(3):247‐261. [DOI] [PubMed] [Google Scholar]
- 5. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal–epithelial interactions in the hair follicle. PLoS Biol. 2005;3(11):e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jönsson EH, Bendas J, Weidner K, et al. The relation between human hair follicle density and touch perception. Sci Rep. 2017;7(1):2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliver RF. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J Embryol Exp Morphol. 1966;15(3):331‐347. [PubMed] [Google Scholar]
- 8. Oliver RF. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. Development. 1970;23(1):219‐236. [PubMed] [Google Scholar]
- 9. Yang C‐C, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57(1):2‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jahoda CAB, Oliver RF, Reynolds AJ, Forrester JC, Horne KA. Human hair follicle regeneration following amputation and grafting into the nude mouse. J Invest Dermatol. 1996;107(6):804‐807. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds AJ, Lawrence C, Cserhalmi‐Friedman PB, Christiano AM, Jahoda CA. Trans‐gender induction of hair follicles. Nature. 1999;402(6757):33‐34. [DOI] [PubMed] [Google Scholar]
- 12. Jahoda CAB, Oliver RF, Reynolds AJ, et al. Trans‐species hair growth induction by human hair follicle dermal papillae. Exp Dermatol. 2001;10(4):229‐237. [DOI] [PubMed] [Google Scholar]
- 13. Higgins CA, Chen JC, Cerise JE, Jahoda CAB, Christiano AM. Microenvironmental reprogramming by three‐dimensional culture enables dermal papilla cells to induce de novo human hair‐follicle growth. Proc Natl Acad Sci U S A. 2013;110(49):19679‐19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh JW, Kloepper J, Langan EA, et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136(1):34‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 16. Paus R, Muller‐Rover S, van der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113(4):523‐532. [DOI] [PubMed] [Google Scholar]
- 17. Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3(1):33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8(2):55‐61. [DOI] [PubMed] [Google Scholar]
- 19. Driskell RR, Jahoda CAB, Chuong C‐M, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23(9):629‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CAB. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One. 2013;8(3):e59811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzales KAU, Fuchs E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell. 2017;43(4):387‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcin CL, Ansell DM. The battle of the bulge: re‐evaluating hair follicle stem cells in wound repair. Exp Dermatol. 2017;26(2):101‐104. [DOI] [PubMed] [Google Scholar]
- 24. Legue E. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132(18):4143‐4154. [DOI] [PubMed] [Google Scholar]
- 25. Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126(7):1459‐1468. [DOI] [PubMed] [Google Scholar]
- 26. Mesler AL, Veniaminova NA, Lull MV, Wong SY. Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep. 2017;19(4):809‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449‐494. [DOI] [PubMed] [Google Scholar]
- 28. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malkud S. Telogen effluvium: a review. J Clin Diagnostic Res. 2015;9(9):WE01‐WE03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greco V, Chen T, Rendl M, et al. A two‐step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4(2):155‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins CA, Westgate GE, Jahoda CAB. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J Invest Dermatol. 2009;129(9):2100‐2108. [DOI] [PubMed] [Google Scholar]
- 33. Purba TS, Haslam IS, Poblet E, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays. 2014;36(5):513‐525. [DOI] [PubMed] [Google Scholar]
- 34. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104(2):233‐245. [DOI] [PubMed] [Google Scholar]
- 35. Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411‐417. [DOI] [PubMed] [Google Scholar]
- 36. Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46(2):81‐89. [DOI] [PubMed] [Google Scholar]
- 37. Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem‐cell fate determination. Nature. 2002;416(6883):854‐860. [DOI] [PubMed] [Google Scholar]
- 38. Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9(6):304‐310. [DOI] [PubMed] [Google Scholar]
- 39. Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apotosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111(6):941‐947. [DOI] [PubMed] [Google Scholar]
- 40. Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin‐positive, keratin‐negative hair‐follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005;102(15):5530‐5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sieber‐Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231(2):258‐269. [DOI] [PubMed] [Google Scholar]
- 42. Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130(5):1227‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clewes O, Narytnyk A, Gillinder KR, Loughney AD, Murdoch AP, Sieber‐Blum M. Human epidermal neural crest stem cells (hEPI‐NCSC)—characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev. 2011;7(4):799‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Solanas G, Benitah SA. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol. 2013;14(11):737‐748. [DOI] [PubMed] [Google Scholar]
- 45. Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13(4):471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science. 2014;346(6212):937‐940. [DOI] [PubMed] [Google Scholar]
- 47. Tobin DJ, Gunin A, Magerl M, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme during the hair growth cycle: implications for growth control and hair follicle transformations. J Investig Dermatol Symp Proc. 2003;8(1):80‐86. [DOI] [PubMed] [Google Scholar]
- 48. Rahmani W, Abbasi S, Hagner A, et al. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev Cell. 2014;31(5):543‐558. [DOI] [PubMed] [Google Scholar]
- 49. Vapniarsky N, Arzi B, Hu JC, Nolta JA, Athanasiou KA. Concise review: human dermis as an autologous source of stem cells for tissue engineering and regenerative medicine. Stem Cells Transl Med. 2015;4(10):1187‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121(6):1267‐1275. [DOI] [PubMed] [Google Scholar]
- 51. Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa‐type fibres are specified. Development. 1992;115(4):1103‐1109. [DOI] [PubMed] [Google Scholar]
- 52. Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124(8):1179‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development. 1992;116(3):563‐571. [DOI] [PubMed] [Google Scholar]
- 54. Jahoda CAB, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101(4):584‐590. [DOI] [PubMed] [Google Scholar]
- 55. Ohyama M, Kobayashi T, Sasaki T, Shimizu A, Amagai M. Restoration of the intrinsic properties of human dermal papilla in vitro. J Cell Sci. 2012;125(Pt 17):4114‐4125. [DOI] [PubMed] [Google Scholar]
- 56. Higgins CA, Richardson GD, Ferdinando D, Westgate GE, Jahoda CAB. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19(6):546‐548. [DOI] [PubMed] [Google Scholar]
- 57. Carreau A, el Hafny‐Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15(6):1239‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thangapazham RL, Klover P, Wang JA, et al. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal‐epidermal composites. J Invest Dermatol. 2014;134(2):538‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng M, Jang Y, Choi N, et al. Hypoxia improves hair inductivity of dermal papilla cells via nuclear NADPH oxidase 4‐mediated reactive oxygen species generation. Br J Dermatol. 2019;181(3):523‐534. [DOI] [PubMed] [Google Scholar]
- 60. Abreu CM, Cerqueira MT, Pirraco RP, Gasperini L, Reis RL, Marques AP. Rescuing key native traits in cultured dermal papilla cells for human hair regeneration. J Adv Res. 2021;30:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inoue K, Aoi N, Yamauchi Y, et al. TGF‐β2 is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med. 2009;13(11–12):4643‐4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dong L, Hao H, Xia L, et al. Treatment of MSCs with Wnt1a‐conditioned medium activates DP cells and promotes hair follicle regrowth. Sci Rep. 2015;4(1):5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rajendran RL, Gangadaran P, Seo CH, et al. Macrophage‐derived extracellular vesicle promotes hair growth. Cell. 2020;9(4):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee S‐H, Yoon J, Shin SH, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. 2012;7(4):e34152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamauchi K, Kurosaka A. Inhibition of glycogen synthase kinase‐3 enhances the expression of alkaline phosphatase and insulin‐like growth factor‐1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch Dermatol Res. 2009;301(5):357‐365. [DOI] [PubMed] [Google Scholar]
- 66. Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle‐inductive properties. Genes Dev. 2008;22(4):543‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kalabusheva E, Terskikh V, Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte‐dermal papilla interactions: impact of hyaluronic acid. Stem Cells Int. 2017;2017:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jabbari A, Sansaricq F, Cerise J, et al. An open‐label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch‐type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138(7):1539‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK‐STAT signaling promotes hair growth. Sci Adv. 2015;1(9):e1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim JE, Lee YJ, Park HR, Lee DG, Jeong KH, Kang H. The effect of JAK inhibitor on the survival, anagen re‐entry, and hair follicle immune privilege restoration in human dermal papilla cells. Int J Mol Sci. 2020;21(14):5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toyoshima K, Asakawa K, Ishibashi N, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3(1):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu J‐J, Zhu T‐Y, Lu Y‐G, et al. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch Dermatol Res. 2006;298(4):183‐190. [DOI] [PubMed] [Google Scholar]
- 73. Lindner G, Horland R, Wagner I, Ataç B, Lauster R. De novo formation and ultra‐structural characterization of a fiber‐producing human hair follicle equivalent in vitro. J Biotechnol. 2011;152(3):108‐112. [DOI] [PubMed] [Google Scholar]
- 74. Chan CC, Fan SM, Wang WH, Mu YF, Lin SJ. A two‐stepped culture method for efficient production of trichogenic keratinocytes. Tissue Eng Part C Methods. 2015;21(10):1070‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nilforoushzadeh M, Jameh ER, Jaffary F, et al. Hair follicle generation by injections of adult human follicular epithelial and dermal papilla cells into nude mice. Cell J. 2017;19(2):259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ehama R, Ishimatsu‐Tsuji Y, Iriyama S, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127(9):2106‐2115. [DOI] [PubMed] [Google Scholar]
- 77. Qiao J, Zawadzka A, Philips E, et al. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen Med. 2009;4(5):667‐676. [DOI] [PubMed] [Google Scholar]
- 78. Kageyama T, Yan L, Shimizu A, Maruo S, Fukuda J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials. 2019;212:55‐63. [DOI] [PubMed] [Google Scholar]
- 79. Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132(1):237‐239. [DOI] [PubMed] [Google Scholar]
- 80. Lin B, Miao Y, Wang J, et al. Surface tension guided hanging‐drop: producing controllable 3D spheroid of high‐passaged human dermal papilla cells and forming inductive microtissues for hair‐follicle regeneration. ACS Appl Mater Interfaces. 2016;8(9):5906‐5916. [DOI] [PubMed] [Google Scholar]
- 81. Wu X, Scott L, Washenik K, Stenn K. Full‐thickness skin with mature hair follicles generated from tissue culture expanded human cells. Tissue Eng Part A. 2014;20(23–24):3314‐3321. [DOI] [PubMed] [Google Scholar]
- 82. Yoshida Y, Soma T, Matsuzaki T, Kishimoto J. Wnt activator CHIR99021‐stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle‐enriched human skin. Biochem Biophys Res Commun. 2019;516(3):599‐605. [DOI] [PubMed] [Google Scholar]
- 83. Abaci HE, Coffman A, Doucet Y, et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9(1):5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Abreu CM, Pirraco RP, Reis RL, Cerqueira MT, Marques AP. Interfollicular epidermal stem‐like cells for the recreation of the hair follicle epithelial compartment. Stem Cell Res Ther. 2021;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roh C, Tao Q, Lyle S. Dermal papilla‐induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics. 2005;19(2):207‐217. [DOI] [PubMed] [Google Scholar]
- 86. Bak SS, Kwack MH, Shin HS, Kim JC, Kim MK, Sung YK. Restoration of hair‐inductive activity of cultured human follicular keratinocytes by co‐culturing with dermal papilla cells. Biochem Biophys Res Commun. 2018;505(2):360‐364. [DOI] [PubMed] [Google Scholar]
- 87. Yang Z, Ma S, Cao R, et al. CD49fhigh defines a distinct skin mesenchymal stem cell population capable of hair follicle epithelial cell maintenance. J Invest Dermatol. 2020;140(3):544‐555.e9. [DOI] [PubMed] [Google Scholar]
- 88. Chacon‐Martinez CA, Klose M, Niemann C, Glauche I, Wickstrom SA. Hair follicle stem cell cultures reveal self‐organizing plasticity of stem cells and their progeny. EMBO J. 2017;36(2):151‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rishikaysh P, Dev K, Diaz D, Qureshi W, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014;15(1):1647‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643‐653. [DOI] [PubMed] [Google Scholar]
- 91. Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β‐catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533‐545. [DOI] [PubMed] [Google Scholar]
- 92. Ito M, Yang Z, Andl T, et al. Wnt‐dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316‐320. [DOI] [PubMed] [Google Scholar]
- 93. Celso CL. Transient activation of β‐catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131(8):1787‐1799. [DOI] [PubMed] [Google Scholar]
- 94. Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Promotion of hair follicle development and trichogenesis by Wnt‐10b in cultured embryonic skin and in reconstituted skin. Biochem Biophys Res Commun. 2006;345(2):581‐587. [DOI] [PubMed] [Google Scholar]
- 95. Ouji Y, Yoshikawa M, Moriya K, Nishiofuku M, Matsuda R, Ishizaka S. Wnt‐10b, uniquely among Wnts, promotes epithelial differentiation and shaft growth. Biochem Biophys Res Commun. 2008;367(2):299‐304. [DOI] [PubMed] [Google Scholar]
- 96. Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Wnt‐10b promotes differentiation of skin epithelial cells in vitro. Biochem Biophys Res Commun. 2006;342(1):28‐35. [DOI] [PubMed] [Google Scholar]
- 97. Botchkarev VA, Fessing MY. Edar signaling in the control of hair follicle development. J Investig Dermatol Symp Proc. 2005;10(3):247‐251. [DOI] [PubMed] [Google Scholar]
- 98. Mustonen T, Ilmonen M, Pummila M, et al. Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development. 2004;131(20):4907‐4919. [DOI] [PubMed] [Google Scholar]
- 99. Laurikkala J, Pispa J, Jung H‐S, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129(10):2541‐2553. [DOI] [PubMed] [Google Scholar]
- 100. Yan M, Wang LC, Hymowitz SG, et al. Two‐amino acid molecular switch in an epithelial morphogen that regulates binding to two distinct receptors. Science. 2000;290(5491):523‐527. [DOI] [PubMed] [Google Scholar]
- 101. Srivastava AK. Ectodysplasin‐A1 is sufficient to rescue both hair growth and sweat glands in Tabby mice. Hum Mol Genet. 2001;10(26):2973‐2981. [DOI] [PubMed] [Google Scholar]
- 102. Kwack MH, Kim JC, Kim MK. Ectodysplasin‐A2 induces apoptosis in cultured human hair follicle cells and promotes regression of hair follicles in mice. Biochem Biophys Res Commun. 2019;520(2):428‐433. [DOI] [PubMed] [Google Scholar]
- 103. Kwack MH, Jun MS, Sung YK, Kim JC, Kim MK. Ectodysplasin‐A2 induces dickkopf 1 expression in human balding dermal papilla cells overexpressing the ectodysplasin A2 receptor. Biochem Biophys Res Commun. 2020;529(3):766‐772. [DOI] [PubMed] [Google Scholar]
- 104. Kulessa H, Turk G, Hogan BLM. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19(24):6664‐6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Botchkarev VA, Botchkareva NV, Roth W, et al. Noggin is a mesenchymally derived stimulator of hair‐follicle induction. Nat Cell Biol. 1999;1(3):158‐164. [DOI] [PubMed] [Google Scholar]
- 106. Botchkarev VA, Botchkareva NV, Nakamura M, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15(12):2205‐2214. [DOI] [PubMed] [Google Scholar]
- 107. Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF‐A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126(12):2611‐2621. [DOI] [PubMed] [Google Scholar]
- 108. Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 109. St‐Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8(19):1058‐1069. [DOI] [PubMed] [Google Scholar]
- 110. González R, Moffatt G, Hagner A, et al. Platelet‐derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self‐renewal. npj Regen Med. 2017;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Takakura N, Yoshida H, Kunisada T, Nishikawa S, Nishikawa S‐I. Involvement of platelet‐derived growth factor receptor‐α in hair canal formation. J Invest Dermatol. 1996;107(5):770‐777. [DOI] [PubMed] [Google Scholar]
- 112. Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43(2):105‐115. [DOI] [PubMed] [Google Scholar]
- 113. Foitzik K, Paus R, Doetschman T, Paolo GP. The TGF‐β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212(2):278‐289. [DOI] [PubMed] [Google Scholar]
- 114. Foitzik K, Lindner G, Mueller‐Roever S, et al. Control of murine hair follicle regression (catagen) by TGF‐β1 in vivo. FASEB J. 2000;14(5):752‐760. [DOI] [PubMed] [Google Scholar]
- 115. Lin W, Xiang L‐J, Shi H‐X, et al. Fibroblast growth factors stimulate hair growth through β‐catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kawano M, Komi‐Kuramochi A, Asada M, et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124(5):877‐885. [DOI] [PubMed] [Google Scholar]
- 117. Higgins CA, Petukhova L, Harel S, et al. FGF5 is a crucial regulator of hair length in humans. Proc Natl Acad Sci U S A. 2014;111(29):10648‐10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Katsuoka K, Schell H, Hornstein OP, Wessel B. Epidermal growth factor and fibroblast growth factor accelerate proliferation of human hair bulb papilla cells and root sheath fibroblasts cultured in vitro. Br J Dermatol. 1987;116(3):464‐465. [DOI] [PubMed] [Google Scholar]
- 119. Sun X, Fu X, Han W, Zhao Y, Liu H, Sheng Z. Dedifferentiation of human terminally differentiating keratinocytes into their precursor cells induced by basic fibroblast growth factor. Biol Pharm Bull. 2011;34(7):1037‐1045. [DOI] [PubMed] [Google Scholar]
- 120. Lemasters JJ, Ramshesh VK, Lovelace GL, et al. Compartmentation of mitochondrial and oxidative metabolism in growing hair follicles: a ring of fire. J Invest Dermatol. 2017;137(7):1434‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Calvo‐Sánchez MI, Fernández‐Martos S, Montoya JJ, Espada J. Intrinsic activation of cell growth and differentiation in ex vivo cultured human hair follicles by a transient endogenous production of ROS. Sci Rep. 2019;9(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hamanaka RB, Glasauer A, Hoover P, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6(261):ra8‐ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Andl T, Botchkareva NV. MicroRNAs (miRNAs) in the control of HF development and cycling: the next frontiers in hair research. Exp Dermatol. 2015;24(11):821‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lena AM, Shalom‐Feuerstein R, di Val Cervo PR, et al. miR‐203 represses “stemness” by repressing ΔNp63. Cell Death Differ. 2008;15(7):1187‐1195. [DOI] [PubMed] [Google Scholar]
- 125. Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing “stemness”. Nature. 2008;452(7184):225‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Amelio I, Lena AM, Bonanno E, Melino G, Candi E. miR‐24 affects hair follicle morphogenesis targeting Tcf‐3. Cell Death Dis. 2013;4(11):e922‐e922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self‐renewal and early lineage commitment. Cell Stem Cell. 2011;8(3):294‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mardaryev AN, Ahmed MI, Vlahov NV, et al. Micro‐RNA‐31 controls hair cycle‐associated changes in gene expression programs of the skin and hair follicle. FASEB J. 2010;24(10):3869‐3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Watt FM. Engineered microenvironments to direct epidermal stem cell behavior at single‐cell resolution. Dev Cell. 2016;38(6):601‐609. [DOI] [PubMed] [Google Scholar]
- 130. Messenger AG, Elliott K, Westgate GE, Gibson WT. Distribution of extracellular matrix molecules in human hair follicles. Ann N Y Acad Sci. 1991;642:253‐262. [DOI] [PubMed] [Google Scholar]
- 131. Messenger AG, Elliott K, Temple A, Randall VA. Expression of basement membrane proteins and interstitial collagens in dermal papillae of human hair follicles. J Invest Dermatol. 1991;96(1):93‐97. [DOI] [PubMed] [Google Scholar]
- 132. Jahoda CA, Mauger A, Bard S, Sengel P. Changes in fibronectin, laminin and type IV collagen distribution relate to basement membrane restructuring during the rat vibrissa follicle hair growth cycle. J Anat. 1992;181(Pt 1):47‐60. [PMC free article] [PubMed] [Google Scholar]
- 133. Soma T, Tajima M, Kishimoto J. Hair cycle‐specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39(3):147‐154. [DOI] [PubMed] [Google Scholar]
- 134. Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21(15):3919‐3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Commo S, Bernard BA. The distribution of α2β1, α3β1 and α6β4 integrins identifies distinct subpopulations of basal keratinocytes in the outer root sheath of the human anagen hair follicle. Cell Mol Life Sci. 1997;53(5):466‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8(2):177‐187. [DOI] [PubMed] [Google Scholar]
- 137. McGrath JA, Gatalica B, Christiano AM, et al. Mutations in the 180–kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat Genet. 1995;11(1):83‐86. [DOI] [PubMed] [Google Scholar]
- 138. Link RE, Paus R, Stenn KS, Kuklinska E, Moellmann G. Epithelial growth by rat vibrissae follicles in vitro requires mesenchymal contact via native extracellular matrix. J Invest Dermatol. 1990;95(2):202‐207. [DOI] [PubMed] [Google Scholar]
- 139. Li J. Laminin‐10 is crucial for hair morphogenesis. EMBO J. 2003;22(10):2400‐2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gao J, DeRouen MC, Chen C‐H, et al. Laminin‐511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22(15):2111‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhou L, Jing J, Wang H, Wu X, Lu Z. Decorin promotes proliferation and migration of ORS keratinocytes and maintains hair anagen in mice. Exp Dermatol. 2018;27(11):1237‐1244. [DOI] [PubMed] [Google Scholar]
- 142. Jing J, Wu X, Li Y, Cai S‐Q, Zheng M, Lu Z‐F. Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exp Dermatol. 2014;23(7):486‐491. [DOI] [PubMed] [Google Scholar]
- 143. Young T‐H, Tu H‐R, Chan C‐C, et al. The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell–substratum adhesivity and cell motility. Biomaterials. 2009;30(28):5031‐5040. [DOI] [PubMed] [Google Scholar]
- 144. Morelli JG, Yohn JJ, Zekman T, Norris DA. Melanocyte movement in vitro: role of matrix proteins and integrin receptors. J Invest Dermatol. 1993;101(4):605‐608. [DOI] [PubMed] [Google Scholar]
- 145. Betriu N, Jarrosson‐Moral C, Semino CE. Culture and differentiation of human hair follicle dermal papilla cells in a soft 3D self‐assembling peptide scaffold. Biomolecules. 2020;10(5):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang S, Gelain F, Zhao X. Designer self‐assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005;15(5):413‐420. [DOI] [PubMed] [Google Scholar]
- 147. Buffey JA, Messenger AG, Taylor M, Ashcroft ATT, Westgate GE, Neil SMAC. Extracellular matrix derived from hair and skin fibroblasts stimulates human skin melanocyte tyrosinase activity. Br J Dermatol. 1994;131(6):836‐842. [DOI] [PubMed] [Google Scholar]
- 148. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor‐binding domain. FASEB J. 2010;24(12):4711‐4721. [DOI] [PubMed] [Google Scholar]
- 150. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF‐mediated angiogenesis. J Clin Invest. 2001;107(4):409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shimaoka S, Tsuboi R, Jindo T, et al. Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J Cell Physiol. 1995;165(2):333‐338. [DOI] [PubMed] [Google Scholar]
- 152. Limat A, Breitkreutz D, Hunziker T, et al. Outer root sheath (ORS) cells organize into epidermoid cyst‐like spheroids when cultured inside Matrigel: a light‐microscopic and immunohistological comparison between human ORS cells and interfollicular keratinocytes. Cell Tissue Res. 1994;275(1):169‐176. [DOI] [PubMed] [Google Scholar]
- 153. Havlickova B, Biro T, Mescalchin A, Arenberger P, Paus R. Towards optimization of an organotypic assay system that imitates human hair follicle‐like epithelial‐mesenchymal interactions. Br J Dermatol. 2004;151(4):753‐765. [DOI] [PubMed] [Google Scholar]
- 154. Havlickova B, Bíró T, Mescalchin A, et al. A human folliculoid microsphere assay for exploring epithelial—mesenchymal interactions in the human hair follicle. J Invest Dermatol. 2009;129(4):972‐983. [DOI] [PubMed] [Google Scholar]
- 155. Lim TC, Leong MF, Lu H, et al. Follicular dermal papilla structures by organization of epithelial and mesenchymal cells in interfacial polyelectrolyte complex fibers. Biomaterials. 2013;34(29):7064‐7072. [DOI] [PubMed] [Google Scholar]
- 156. Miao Y, Sun YB, Liu BC, Jiang JD, Hu ZQ. Controllable production of transplantable adult human high‐passage dermal papilla spheroids using 3D matrigel culture. Tissue Eng Part A. 2014;20(17–18):2329‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gupta AC, Chawla S, Hegde A, et al. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J Cell Physiol. 2018;233(11):9015‐9030. [DOI] [PubMed] [Google Scholar]
- 158. Chermnykh ES, Vorotelyak EA, Gnedeva KY, et al. Dermal papilla cells induce keratinocyte tubulogenesis in culture. Histochem Cell Biol. 2010;133(5):567‐576. [DOI] [PubMed] [Google Scholar]
- 159. Leirós GJ, Kusinsky AG, Drago H, et al. Dermal papilla cells improve the wound healing process and generate hair bud‐like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med. 2014;3(10):1209‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Vahav I, Broek LJ, Thon M, et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J Tissue Eng Regen Med. 2020;14(6):761‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Abreu CM, Gasperini L, Lago MEL, Reis RL, Marques AP. Microscopy‐guided laser ablation for the creation of complex skin models with folliculoid appendages. Bioeng Transl Med. 2020;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lee J, Rabbani CC, Gao H, et al. Hair‐bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582(7812):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yoo B‐Y, Shin Y‐H, Yoon H‐H, Seo Y‐K, Song K‐Y, Park J‐K. Application of mesenchymal stem cells derived from bone marrow and umbilical cord in human hair multiplication. J Dermatol Sci. 2010;60(2):74‐83. [DOI] [PubMed] [Google Scholar]
- 164. Choi N, Choi J, Kim JH, et al. Generation of trichogenic adipose‐derived stem cells by expression of three factors. J Dermatol Sci. 2018;92(1):18‐29. [DOI] [PubMed] [Google Scholar]
- 165. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. WO2018089750A1—bioprinted hair follicles and uses thereof. 2019. https://patents.google.com/patent/WO2018089750A1/en. Accessed May 5, 2021
- 168. Tsuboi R, Niiyama S, Irisawa R, Harada K, Nakazawa Y, Kishimoto J. Autologous cell–based therapy for male and female pattern hair loss using dermal sheath cup cells: a randomized placebo‐controlled double‐blinded dose‐finding clinical study. J Am Acad Dermatol. 2020;83(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 169. Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200‐rich and CD34‐positive hair follicle progenitor cells. J Clin Invest. 2011;121(2):613‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Silva‐Vargas V, Lo Celso C, Giangreco A, et al. β‐catenin and hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9(1):121‐131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.