Abstract
We report the generation of monoclonal antibodies against a recombinant 170-kDa subunit of the Gal or GalNAc lectin of Entamoeba histolytica that specifically recognize E. histolytica but not Entamoeba dispar in preserved stool samples. These antibodies do not cross-react with other bowel protozoa, including Entamoeba coli, Giardia lamblia, and Dientamoeba fragilis.
Epidemiologic and molecular research has established that the organism previously known as Entamoeba histolytica actually comprises two genetically distinct but morphologically identical species. The pathogenic species, for which the name E. histolytica has been retained, causes invasive disease. The nonpathogenic species, termed Entamoeba dispar, is a commensal (7, 14). It is important to differentiate between these species in order to avoid unnecessary treatment of E. dispar infections (24, 30).
Traditionally, the diagnosis of E. histolytica infection has depended on the microscopic examination of stool samples. However, this method is labor-intensive and cannot distinguish between E. histolytica and E. dispar (4, 10, 12, 13, 24, 27). Isoenzyme analysis is considered the reference standard for discriminating E. histolytica from E. dispar (1, 10–13, 22, 26, 27). However, this approach is laborious and has remained largely a research tool. Antigen detection assays to detect and/or distinguish E. histolytica from E. dispar are reported to be sensitive and specific (1, 4, 11–13, 15, 18, 25) but require fresh, unpreserved fecal samples. Since the majority of stool specimens submitted for parasite examination are received in fixative, these tests are generally incompatible with standard stool collection procedures in North America.
The 260-kDa galactose- or N-acetyl galactosamine-specific lectin of E. histolytica is an important virulence factor mediating the attachment of amoeba to the intestinal epithelium and contact-dependent cytolysis (19). This lectin, consisting of heavy (170-kDa) and light (31- or 35-kDa) subunits linked by disulfide bonds, is antigenically conserved (20, 21). Although there are shared epitopes between the lectins of E. histolytica and E. dispar, there is only 77 to 85% DNA sequence identity between these molecules (8, 22, 23). In this study, we generated monoclonal antibodies (MAbs) by immunizing mice with a recombinant heavy lectin subunit that had been fixed in the stool preservative, sodium acetate-acetic acid-formalin (SAF). These MAbs specifically recognize fixed E. histolytica trophozoites and were able to detect and distinguish E. histolytica from E. dispar in preserved fecal specimens.
The construction of recombinant Autographa californica nuclear polyhydrosis virus and the purification of the recombinant 170-kDa heavy subunit of the E. histolytica adherence lectin from infected Spodoptera frugiperda (Sf21) cells are described in detail elsewhere (Yau et al., unpublished data). Briefly, the heavy subunit gene of E. histolytica adherence lectin, hgl 2 (from nucleotide 61 to 3800 [29]), was amplified from genomic DNA of strain HM1:IMSS (ATCC 30459; American Type Culture Collection, Rockville, Md.) by PCR and cloned into the baculovirus transfer vector pAcMeH6. Cotransfection in Sf21 cells, plaque selection, and recombinant Autographa californica nuclear polyhydrosis virus propagation were done as recommended (BacPak Baculovirus Expression System; Clonetech, Palo Alto, Calif.). Recombinant protein was immunoaffinity purified with MAb 8A3 (22). The molecular mass of the purified lectin was ∼160 kDa as determined by the mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). The deduced mass (amino acids 11 to 1256) was 140 kDa, suggesting that the recombinant protein was posttranslationally modified.
BALB/c (Charles River, Wilmington, Mass.) mice were immunized with either purified native or SAF-fixed recombinant lectin (four doses of 10 μg of protein/dose with Freund's complete or incomplete adjuvant). Spleens were harvested and cell fusions with NS1 myeloma cells were performed as described previously (9). Culture supernatants from hybridoma clones were tested for antilectin activity by immunofluorescence assay (IFA) on SAF-fixed infected Sf21 cells spotted onto poly-l-lysine (1 mg/ml; Sigma Chemical Co., St. Louis, Mo.)-coated glass slides. Twenty-one clones from mice immunized with fixed recombinant lectin produced MAbs that recognized Sf21 cells expressing the lectin. All clones identified by screening were confirmed by IFA of SAF-fixed E. histolytica trophozoites.
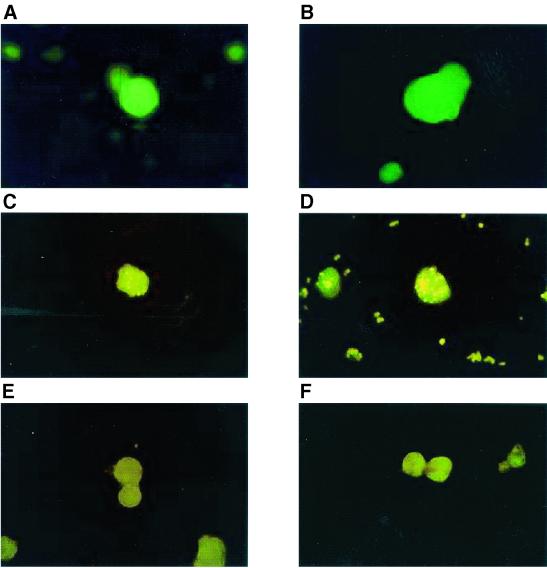
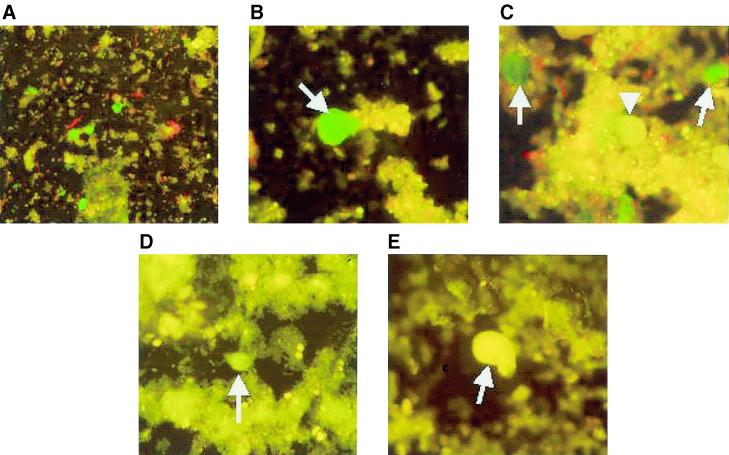
Four clones were selected on the basis of their strong reactivity for E. histolytica trophozoites by IFA and were further evaluated for cross-reactivity with E. dispar. Three of the four MAbs were immunoglobulin M (IgM) (SB2F2, SB4D7, and NL3B3), and one (SB4G11) was shown to be IgG1. All four demonstrated specific bright apple-green fluorescence against fixed E. histolytica trophozoites (Fig. 1A and B), while E. dispar trophozoites (strain CYNO 16 axenically grown [16]) displayed only nonspecific background yellow fluorescence (Fig. 1C and D). Incubation with control irrelevant antibodies of matching isotype (Fig. 1E and F) gave similar background levels of fluorescence. In smears of SAF-preserved stool specimens spiked with E. histolytica, fluorescent green trophozoites could be detected easily by screening at low magnification (Fig. 2A) followed by confirmation of the morphology at higher magnification (Fig. 2B and C). There was no cross-reactivity observed with E. dispar (Fig. 2C), Giardia lamblia (Fig. 2D), Entamoeba coli (Fig. 2E), and Dientamoeba fragilis (data not shown). These results were confirmed using 15 patient samples known to contain E. histolytica or E. dispar (as determined by antigen detection and serology) or other bowel protozoa (data not shown) (24).
FIG. 1.
Representative IFA of E. histolytica (A and B) and E. dispar (C and D) trophozoites with monoclonal antibodies. Results are shown for trophozoites incubated with MAb SB4G11 (A and C), with MAb NL3B3 (B and D), with irrelevant IgG1 (E), and with irrelevant IgM (F). Magnification, ×600 (1-min exposure).
FIG. 2.
IFA of stool specimens with MAb SB4D7. (A) Stool specimen spiked with fixed HM1:IMSS trophozoites under low magnification (×150). (B) Corresponding sample under higher magnification (×600). Arrow, E. histolytica. (C) E. dispar (arrowhead) and E. histolytica (arrow) in stool sample (magnification, ×600), (D) Giardia lamblia (arrow) in stool sample (×600). (E) Entamoeba coli (arrow) in stool sample (magnification, ×600).
There are at least four enzyme-linked immunosorbent assay-based commercially available antigen detection kits. All require fresh, unpreserved stool samples. Results of reconstitution experiments indicate that the detection limit of these tests is ∼100 to 500 trophozoites/ml (11–13, 18, 25). By spiking fixed fecal samples with known concentrations of fixed HM1:IMSS trophozoites, the detection limit of our mAbs by IFA was found to be ∼300 trophozoites/ml, a level comparable to that for previously reported tests. We used an IFA format that may not be an ideal method for automation or for the developing world. Furthermore, IFA methods may result in lower sensitivity, since they will not detect free lectin in stool. Converting the current IFA format to an enzyme-linked immunosorbent assay system or a rapid dipstick assay should improve sensitivity and/or ease of use.
In this report, we present proof-of-concept that MAbs generated against the fixed recombinant heavy subunit of the E. histolytica lectin permit the detection of E. histolytica trophozoites in preserved stool samples. Since most samples submitted for parasite examination are received in fixative, the ability to identify E. histolytica in preserved samples represents a real advantage over the currently available assays, which require fresh, unpreserved fecal specimens. We established these results using cloned ameba isolates, reconstitution experiments in preserved stool samples, and a limited number of patient-derived samples known to contain E. histolytica or E. dispar. However, additional studies examining large numbers of clinical samples will be required in order to confirm the performance characteristics of these reagents.
Acknowledgments
K.C.K is a recipient of a career award from the Ontario Ministry of Health. Y.C.W.Y is funded by a postgraduate fellowship from the Medical Research Council of Canada.
We thank William Petri, Jr. (University of Virginia), and Kris Chadee (McGill University) for the kind gift of MAbs 1G7 and 8A3 and Seiki Kobayahsi for the kind gift of axenically grown E. dispar strain CYNO 16.
REFERENCES
- 1.Abd-Alla M D, Jackson T F, Gathiram V, el-Hawey A M, Ravdin J I. Differentiation of pathogenic Entamoeba histolytica infections from nonpathogenic infections by detection of galactose-inhibitable adherence protein antigen in sera and feces. J Clin Microbiol. 1993;31:2845–2850. doi: 10.1128/jcm.31.11.2845-2850.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acuna-Soto R, Samuelson J, De Girolami P, Zarate L, Millan-Velasco F, Schoolnick G, Wirth D. Application of the polymerase chain reaction to the epidemiology of pathogenic and nonpathogenic Entamoeba histolytica. Am J Trop Med Hyg. 1993;48:58–70. doi: 10.4269/ajtmh.1993.48.58. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre A, Molina S, Blotkamp C, Verveij J, Vinuesa T, Valls M E, Guhl F, Polderman A, Jimenez de Anta M T, Corachan M, Gonzalez-Ruiz A, Frame I A, Warhurst D. Diagnosis of Entamoeba histolytica and Entamoeba dispar in clinical specimens by PCR-SHELA. Arch Med Res. 1997;28:282–284. [PubMed] [Google Scholar]
- 4.Benzeguir A K, Aust Kettis A. Evaluation of an enzyme-immunoassay test kit for diagnosing infections with Entamoeba histolytica. Arch Med Res. 1997;28:276–278. [PubMed] [Google Scholar]
- 5.Britten D, Wilson S M, McNerney R, Moody A H, Chiodini P L, Ackers J P. An improved colorimetric PCR-based method for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in feces. J Clin Microbiol. 1997;35:1108–1111. doi: 10.1128/jcm.35.5.1108-1111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 7.Diamond L S, Clark C G. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40:340–344. doi: 10.1111/j.1550-7408.1993.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 8.Dobson D R, Clark C G, Lockhart L A, Leo B M, Schroeder J W, Mann B J. Comparison of adherence, cytotoxicity and Gal/GalNAc lectin gene structure in Entamoeba histolytica and Entamoeba dispar. Parasitol Int. 1999;46:225–235. [Google Scholar]
- 9.Galfre G, Howe S C, Milstein C, Butcher G W, Howard J C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266:550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Ruiz A, Haque R, Aguirre A, Castanon G, Hall A, Guhl F, Ruiz-Palacios G, Miles M A, Warhurst D C. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J Clin Pathol. 1994;47:236–239. doi: 10.1136/jcp.47.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haque R, Kress K, Wood S, Jackson T F, Lyerly D, Wilkins T, Petri W A., Jr Diagnosis of pathogenic Entamoeba histolytica infection using a stool ELISA based on monoclonal antibodies to the galactose-specific adhesin. J Infect Dis. 1993;167:247–249. doi: 10.1093/infdis/167.1.247. [DOI] [PubMed] [Google Scholar]
- 12.Haque R, Neville L M, Hahn P, Petri W A., Jr Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol. 1995;33:2558–2561. doi: 10.1128/jcm.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Ali I K, Akther S, Petri W A., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson T F. Entamoeba histolytica and Entamoeba dispar are distinct species; clinical, epidemiological and serological evidence. Int J Parasitol. 1998;28:181–186. doi: 10.1016/s0020-7519(97)00177-x. [DOI] [PubMed] [Google Scholar]
- 15.Jelinek T, Peyerl G, Loscher T, Nothdurft H D. Evaluation of an antigen-capture enzyme immunoassay for detection of Entamoeba histolytica in stool samples. Eur J Clin Microbiol Infect Dis. 1996;15:752–755. doi: 10.1007/BF01691966. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Imai E, Tachinbana H, Fujiwara T, Takeuchi T. Entamoeba dispar: cultivation with sterilized Crithidia fasciculata. J Eukaryot Microbiol. 1998;45:3S–8S. doi: 10.1111/j.1550-7408.1998.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 17.Mackenstedt U, Johnson A M. Genetic differentiation of pathogenic and nonpathogenic strains of Entamoeba histolytica by random amplified polymorphic DNA polymerase chain reaction. Parasitol Res. 1995;81:217–221. doi: 10.1007/BF00937112. [DOI] [PubMed] [Google Scholar]
- 18.Mirelman D, Nuchamowitz Y, Stolarsky T. Comparison of use of enzyme-linked immunosorbent assay-based kits and PCR amplification of rRNA genes for simultaneous detection of Entamoeba histolytica and E. dispar. J Clin Microbiol. 1997;35:2405–2407. doi: 10.1128/jcm.35.9.2405-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petri W A, Jr, Smith R D, Schlesinger P H, Murphy C F, Ravdin J I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Investig. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petri W A, Jr, Chapman M D, Snodgrass T, Mann B J, Broman J, Ravdin J I. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989;264:3007–3012. [PubMed] [Google Scholar]
- 21.Petri W A, Jr, Broman J, Healy G, Quinn T, Ravdin J I. Antigenic stability and immunodominance of the Gal/GalNAc adherence lectin of Entamoeba histolytica. Am J Med Sci. 1989;297:163–165. doi: 10.1097/00000441-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Petri W A, Jr, Jackson T F, Gathiram V, Kress K, Saffer L D, Snodgrass T L, Chapman M D, Keren Z, Mirelman D. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to the galactose-specific adherence lectin. Infect Immun. 1990;58:1802–1806. doi: 10.1128/iai.58.6.1802-1806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pillai D R, Britten D, Ackers J P, Ravdin J I, Kain K C. A gene homologous to hgl2 of Entamoeba histolytica is present and expressed in Entamoeba dispar. Mol Biochem Parasitol. 1997;87:101–105. doi: 10.1016/s0166-6851(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 24.Pillai D R, Keystone J S, Sheppard D C, MacLean J D, MacPherson D W, Kain K C. Entamoeba histolytica and Entamoeba dispar: epidemiology and comparison of diagnostic methods in a setting of nonendemicity. Clin Infect Dis. 1999;29:1315–1318. doi: 10.1086/313433. [DOI] [PubMed] [Google Scholar]
- 25.Pillai D R, Kain K C. Immunochromatographic strip-based detection of Entamoeba histolytica-E. dispar and Giardia lamblia coproantigen. J Clin Microbiol. 1999;37:3017–3019. doi: 10.1128/jcm.37.9.3017-3019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sargeaunt P G, Williams J E, Grene J D. The differentiation of invasive and non-invasive Entamoeba histolytica by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg. 1978;72:519–521. doi: 10.1016/0035-9203(78)90174-8. [DOI] [PubMed] [Google Scholar]
- 27.Strachan W D, Chiodini P L, Spice W M, Moody A H, Ackers J P. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet. 1988;i:561–563. doi: 10.1016/s0140-6736(88)91355-4. [DOI] [PubMed] [Google Scholar]
- 28.Summers M D, Smith G E. A manual of methods for baculovirus vectors and insect cell culture procedures. Texas Agriculture Experimental Standards Bulletin no. 1555. Austin, Tex: Texas Department of Agriculture; 1987. [Google Scholar]
- 29.Tannich E, Ebert F, Horstmann R D. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:3248–3252. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh J A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;82:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]