Abstract
NOD-like receptor (NLR), family pyrin domain containing 3 (NLRP3) assembles an intracellular protein complex known as the NLRP3 inflammasome upon sensing certain pathogen products or sterile danger signals. Gain-of-function mutations such as the D301N substitution in NLRP3, which cause its constitutive activation (NLRP3CA) also results in inflammasome assembly. This inflammasome processes pro-interleukin-1 β (pro-IL-1β) and pro-IL-18 into bioactive IL-1β and IL-18, respectively, and cleaves gasdermin D (GSDMD). GSDMD N-terminal fragments form plasma membrane pores that facilitate the secretion of IL-1β and IL-18 and lead to the inflammatory cell death pyroptosis. Accordingly, GSDMD inactivation results in negligible spontaneous inflammation in various experimental models such as in Nlrp3CA/+ mice lacking GSDMD (Nlrp3CA/+;Gsdmd−/− mice). Here, we found that Nlrp3CA/+;Gsdmd−/− mice, when challenged with LPS or TNF-α, still secreted IL-1β and IL-18, indicating inflammasome activation independent of GSDMD. Accordingly, Gsdmd−/− macrophages failed to secrete IL-1β and undergo pyroptosis when briefly exposed to NLRP3 inflammasome activators (LPS and nigericin), but released these cytokines when persistently activated. Sustained NLRP3 inflammasome induced caspase-8/−3 and GSDME cleavage, and IL-1β maturation in vitro in Gsdmd−/− macrophages. Thus, a salvage inflammatory pathway involving caspase-8/−3-GSDME was activated following NLRP3 activation when the canonical NLRP3-GSDMD signaling was blocked. Consistent with genetic data, the active metabolite of FDA-approved disulfiram CuET, which inhibited GSDMD and GSDME cleavage in macrophages, reduced the severe inflammation and tissue damage that occurred in the Nlrp3CA mice. Thus, NLRP3 inflammasome activation overwhelms the protection afforded by GSDMD deficiency, rewiring signaling cascades through mechanisms that include GSDME to propagate inflammation.
ONE SENTENCE SUMMARY
Sustained stimulation of the NLRP3 inflammasome causes inflammation in the absence of GSDMD.
INTRODUCTION
The innate immune receptor NOD-like receptor (NLR), family pyrin domain containing 3 (NLRP3) assembles an intracellular protein complex known as the NLRP3 inflammasome upon sensing plasma membrane perturbations caused by certain microbial products or sterile danger signals (1-3). This inflammasome leads to cell death and inflammatory responses. The macromolecular structure of the inflammasome is formed by NLRP3, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1, which becomes activated when it is recruited to inflammasomes. Caspase-1, which is activated by all the canonical inflammasomes, cleaves inactive pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into bioactive IL-1β and IL-18, respectively (2, 3); it also cleaves gasdermin D (GSDMD), generating N-terminal fragments which oligomerize within the plasma membrane to form pores through which IL-1β and IL-18 are secreted (4-8). Excessive pore formation compromises the integrity of the plasma membrane, causing a lytic form of cell death known as pyroptosis. In some situations, cells can survive GSDMD activation but still release inflammatory cytokines in a GSDMD-dependent process, thus have been termed “hyperactivated” (6). Pyroptosis is a double-edged sword - it releases inflammatory factors that recruit immune cells to sites of pathogenic infection, which attack and phagocytose pathogens, destroy their replication niches and boost adaptive immune responses (9, 10). However, excessive or uncontrolled pyroptosis and inflammatory cytokine release can lead to organ damage, circulatory collapse or even death (11-13).
GSDMD is also cleaved by caspase-11 (mouse ortholog of human caspase-4 and −5) (14), neutrophil elastase and cathepsin G (15-18), caspase-3 (19), and caspase-8 (20, 21). Cleavage by all of these proteases, except caspase-3 which inactivates GSDMD, produces a pore-forming N-terminal fragment. Caspase-3 also cleaves gasdermin E (GSDME, also known as DNFA5), producing active N-terminal fragments whose pore-forming activity promotes pyroptosis in response to apoptotic stimuli (22-24), including chemotherapeutic drugs (25, 26), Yersinia infection (20), glucocorticoid treatment (24), and inflammasome activators in cells lacking GSDMD or caspase-1 (27, 28). GSDME is also a substrate of granzyme B (23, 29). The role of GSDME in cell death may be cell context-dependent as it is seemingly dispensable for pyroptosis in certain cell types or experimental conditions (19, 20, 30-33). The function of GSDME in mediating the release of inflammatory cytokines is less studied. A recent study reported that IL-1α was secreted through GSDME conduits in caspase-1 and −11 deficient macrophages, but pro-IL-1β was neither processed nor released (28). Thus, like GSDMD, cleaved GSDME generates pore-forming fragments that can release cytokines and cause pyroptosis.
Chronic activation of the NLRP3 inflammasome by endogenous host danger-associated molecular patterns and the ensuing excessive production of IL-1β and IL-18 underlie the pathogenesis of several autoimmune and autoinflammatory diseases, including inflammatory bowel disease (34-36) and rheumatoid arthritis (37-39), and contribute to the severity of atherosclerosis (40, 41), gout (42-45), and diabetes (46). Gain-of-function mutations in NLRP3 cause a spectrum of autoinflammatory disorders known as cryopyrin-associated periodic syndromes (CAPS), whose severity is linked to specific NLRP3 mutations. Neonatal-onset multisystem inflammatory disease (NOMID) is the most severe and familial cold autoinflammatory syndrome (FCAS) and Muckle-Wells syndrome (MWS) are less severe (12, 13). Clinical manifestations of CAPS include systemic inflammation, skin lesions, central nervous system symptoms including headache, hearing loss and learning difficulties, and skeletal anomalies (13, 47-51). Mice genetically engineered to express NLRP3 variants that harbor mutations found in CAPS patients (NLRP3CA) develop severe systemic inflammation characterized by excessive secretion of IL-1β and IL-18, multi-organ damage, and premature death (52, 53). Although young NOMID mice lacking the IL-1 receptor do not develop inflammation (54), a persistent low-grade inflammation is reported in FCAS mice and MWS mice with defective IL-1 and IL-18 signaling (55), suggesting that pyroptosis may be the culprit. In support of this view, knocking out GSDMD prevents the pathogenesis of NOMID (56), Familial Mediterranean Fever (FMF) (57), and experimental autoimmune encephalitis (58). However, how these mutant mice with an underlying dysregulated NLRP3 inflammasome activity withstand superimposed inflammatory challenges has not been studied.
CAPS and several other diseases of dysregulated inflammasome activity, including FMF and macrophage activation syndrome which are caused by PYRIN and NLRC4 mutations, respectively, are treated with IL-1 blockers (59). These therapies have significantly improved the quality of life of affected individuals, but the disease does not always resolve (60-63). The underlying mechanisms of resistance are not understood, but lingering low-grade inflammation driven by IL-18 and pyroptosis is suspected. To identify drugs that block the integrating nodes of inflammasome signaling, recent studies screened several safe marketed drugs for anti-GSDMD activity. They identified disulfiram, also known as Antabuse, an FDA-approved drug for the treatment of alcohol addiction, and dimethyl fumarate, also known as Tecfidera used to treat multiple sclerosis, as inhibitors of GSDMD and pyroptosis (58, 64). Both drugs also inhibited LPS-induced IL-1β and IL-18 secretion in vitro and in vivo (65). These observations provide a rationale for evaluating pyroptosis inhibitors in animal inflammasomopathy models.
In this study, we used mice bearing one allele of Nlrp3 with D301N substitution, which induces constitutive activation of NLRP3 (NLRP3CA), and challenged them with inflammatory stimuli (LPS or TNF-α) to understand better the role of GSDMD in promoting inflammation. We found that NLRP3 inflammasome activation caused IL-1 family cytokine secretion and pyroptosis in Gsdmd−/− cells, responses that were associated with caspase-8/−3 and GSDME cleavage. The active metabolite of disulfiram, bis(diethyldithiocarbamate)-copper (CuET), inhibited the cleavage of both GSDMD and GSDME as well as ASC activation, protecting mice from the pathology caused by dysregulated NLRP3 inflammasome. Thus, the inflammatory actions of dysregulated NLRP3 inflammasome involve GSDME in Gsdmd−/− cells and are inhibited by CuET.
RESULTS
Gsdmd deficient mice expressing hyperactive NLRP3 inflammasome aged normally
D301N substitution in NLRP3 imposes conformational changes and constitutive activation of NLRP3 (NLRP3CA); this autosomal dominant mutation ultimately causes an inflammatory disease in Nlrp3CA/+ mice that resembles human NOMID (52, 66). Most Nlrp3CA/+ mice die prematurely around 3 weeks of age due to severe systemic inflammation that damages multiple organs, including the skin, brain, bones, and spleen (52, 66, 67). Gsdmd−/− and Nlrp3CA/+ mice lacking GSDMD (Nlrp3CA/+;Gsdmd−/− mice) grow normally and are indistinguishable from their wild-type (WT) counterparts when monitored for up to 66 days, indicating strong GSDMD-dependence of inflammation in NOMID mice (56). However, mice expressing constitutively activated NLRP3 as a result of L351P substitution that are genetically deficient in Il-1 and Il-18 unexpectedly show signs of lingering inflammation (55). This finding prompted us to monitor Nlrp3CA/+;Gsdmd−/− mice for longer time periods. Mouse survival, white blood cell (WBC) counts, and spleen weight of Nlrp3CA/+;Gsdmd−/− and Gsdmd−/− mice remained indistinguishable at 6 months and 12 months of age (fig. S1A). Accordingly, the architecture of the spleen and the liver was histologically similar between both genotypes (fig. S1, B and C). Thus, Nlrp3CA/+;Gsdmd−/− mice age normally in homeostatic conditions, consistent with our previous study (56).
LPS or TNF-α induced IL-1β and IL-18 secretion in Nlrp3CA/+;Gsdmd−/− but not Gsdmd−/− mice.
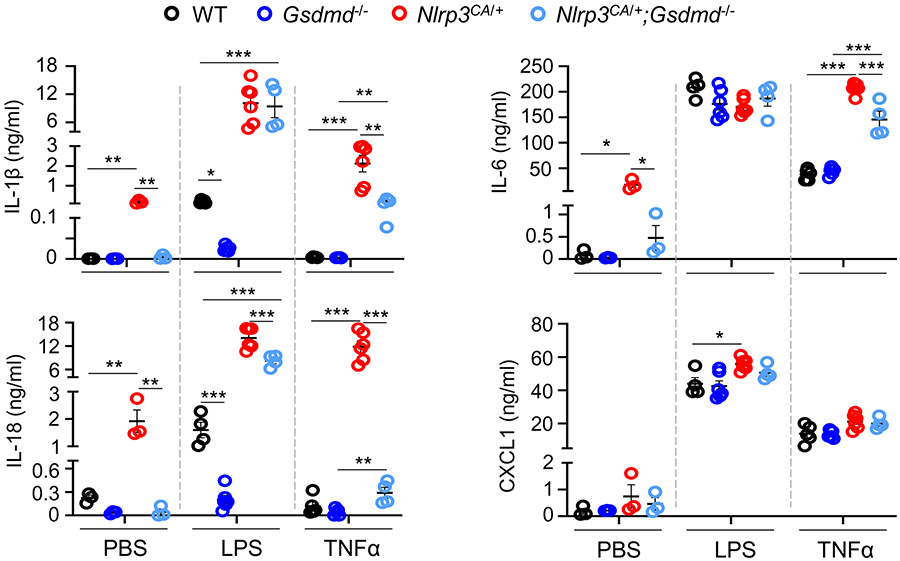
Gsdmd−/− mice, unlike caspase-11−/− mice, are not fully protected from death caused by a lethal dose of LPS (14), suggesting that there might be a GSDMD-independent inflammatory pathway. Baseline blood serum IL-1β, IL-18, and IL-6 were low and comparable in WT and Gsdmd−/− mice but were constitutively increased in Nlrp3CA/+ mice and returned to normal in Nlrp3CA/+;Gsdmd−/− mice as expected (Fig. 1). After LPS challenge, serum IL-1β and IL-18 increased in WT mice, but not in Gsdmd−/− mice. However, in Nlrp3CA/+ mice these cytokine levels were about 10-fold higher than in LPS-stimulated WT mice and surprisingly were not substantially reduced in Nlrp3CA/+;Gsdmd−/− mice. LPS stimulated high serum levels of IL-6 and CXCL1 in all the genotypes. TNF-α injection increased serum IL-1β and IL-18 in Nlrp3CA/+, but not in WT or Gsdmd−/− mice, but this increase was significantly reduced but not eliminated in Nlrp3CA/+;Gsdmd−/− mice. TNF-α also elevated serum IL-6 and CXCL1 in all genotypes, but induced more IL-6 in Nlrp3CA/+ mice which was still above the level in WT mice (Fig, 1). These results indicate that both GSDMD-dependent and GSDMD-independent inflammation is induced in the setting of intense NLRP3 inflammasome activation.
Figure 1. LPS or TNF-α induced IL-1β and IL-18 secretion in by Nlrp3CA/+;Gsdmd−/− but not Gsdmd−/− mice.
Three-month-old WT, Gsdmd−/−, Nlrp3CA/+, and Nlrp3CA/+;Gsdmd−/− mice were injected with 15 mg/kg LPS for 6 hours or 0.5 mg/kg TNF-α for 2 hours. PBS-administrated mice served as controls. N=4-6 mice/group. Serum cytokine levels were measured by V-PLEX Plus Proinflammatory Panel 1 Mouse Kit, except for IL-18, which were assessed by ELISA. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. One-Way ANOVA. LPS, lipopolysaccharide; IL, interleukin; WT, wild type; ca, constitutive activation; TNF-α, tumor necrosis factor α.
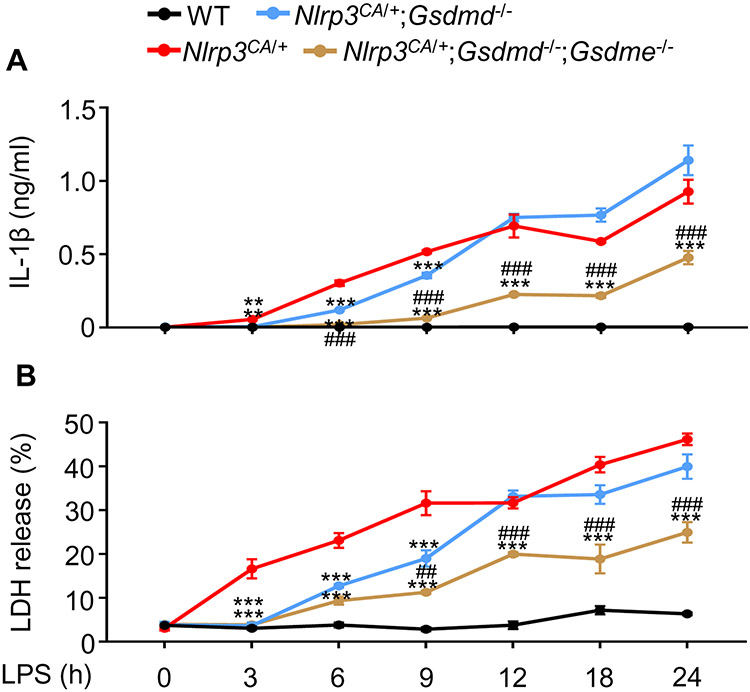
LPS stimulated IL-1β release and GSDME cleavage by Nlrp3CA/+;Gsdmd−/− BMDMs
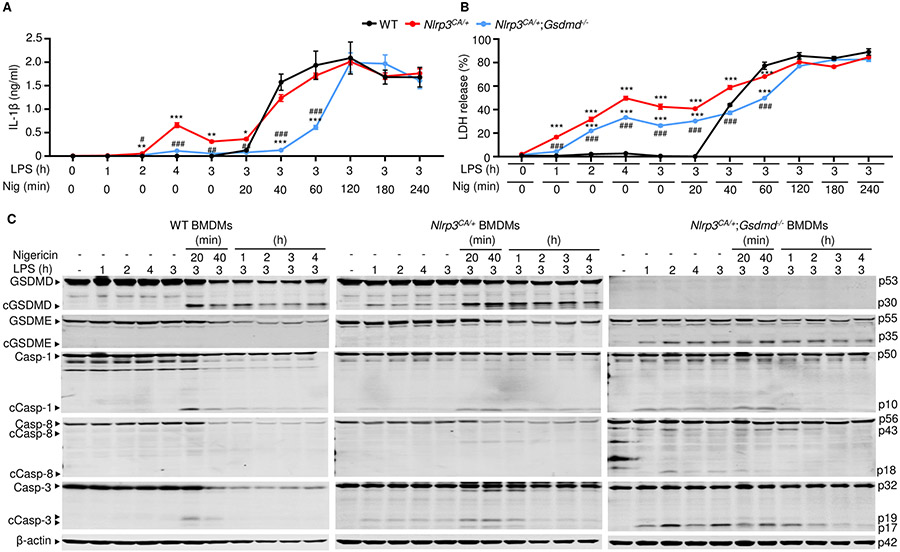
To examine how GSDMD deficiency affects IL-1β secretion in vitro, its levels were measured after treatment of WT, Nlrp3CA/+ or Nlrp3CA/+;Gsdmd−/− bone marrow-derived macrophages (BMDMs) primed with LPS and exposed to nigericin, which induces the assembly of the NLRP3 inflammasome (56). LPS alone induced IL-1β secretion in Nlrp3CA/+ cells (Fig. 2A). Nigericin increased the release of IL-1β by LPS-primed cells in the three genotypes, though this response was delayed in double mutant cells, reaching levels comparable to WT and Nlrp3CA/+ cells by 2 hours. We also assessed cell death by measuring the release of lactate dehydrogenase (LDH) into the cell supernatant. While LPS and nigericin were required for LDH release from WT BMDMs, endotoxin alone induced these responses in Nlrp3CA/+ and Nlrp3CA/+;Gsdmd−/− cells, though this response was attenuated in double mutant cells and enhanced by nigericin in both genotypes (Fig. 2B). While GSDMs are the executioners of pyroptosis, mixed lineage kinase domain-like pseudokinase (MLKL) plays a critical role in the cell death necroptosis, the programmed form of necrosis. MLKL perforates the plasma cell membrane and executes necroptosis upon phosphorylation by receptor interacting serine/threonine kinase 3 (RIPK3) (68, 69). We determined whether MLKL was involved in the responses of Nlrp3CA/+;Gsdmd−/− BMDMs. MLKL was not phosphorylated in Nlrp3CA/+;Gsdmd−/− BMDMs treated with LPS or LPS plus nigericin, (fig. S2), suggesting that the delayed death of these cells was necroptosis-independent. Thus, IL-1β secretion and pyroptosis are attenuated in Nlrp3CA/+;Gsdmd−/− compared to WT and Nlrp3CA/+ BMDMs in response to NLRP3 inflammasome activators. However, these responses are undistinguishable among the three cell types where the NLRP3 inflammasome is persistently activated.
Figure 2. LPS stimulated IL-1β release and GSDME cleavage by Nlrp3CA/+;Gsdmd−/− BMDMs.
BMDMs were expanded in vitro in M-CSF-containing media from bone marrow cells isolated from WT, Nlrp3CA/+, or Nlrp3CA/+;Gsdmd−/− mice. BMDMs were primed with 100 ng/ml LPS for 1, 2, 3, or 4 hours and treated with 15 μM nigericin for 20 minutes, 40 minutes, 1, 2, 3, 4 hours. IL-1β (A) and LDH (B) in the conditioned media were measured by ELISA and by the cytotoxicity detection Kit, respectively. (C) The indicated proteins in the whole cell lysates were analyzed by immunoblotting. Data are mean ± SEM from experimental triplicates and are representative of at least three independent experiments. **P < 0.01; ***P < 0.001; ##P < 0.01; ###P < 0.001. **,***Nlrp3CA/+ or Nlrp3CA/+;Gsdmd−/− compared to WT; ##, ###Nlrp3CA/+;Gsdmd−/− compared to Nlrp3CA/+. One-Way ANOVA. BMDMs, bone marrow-derived macrophages; cCasp, cleaved caspase; cGSDM, cleaved gasdermin; h, hour; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; min, minute; M-CSF, macrophage colony-stimulating factor; WT, wild type; CA, constitutive activation.
To study NLRP3 inflammasome signaling in GSDMD sufficient or insufficient cells, we treated WT, Nlrp3CA/+, and Nlrp3CA/+;Gsdmd−/− BMDMs with LPS in the absence or presence of nigericin. While the combination of LPS and nigericin was required for the formation of ASC specks , a readout of activated inflammasomes (fig. S3) and the cleavage of GSDMD and caspase-1 in WT BMDMs, LPS alone induced these responses in Nlrp3CA/+ and Nlrp3CA/+;Gsdmd−/− cells (Fig. 2C; fig. S3), consistent with the constitutively activated state of NLRP3 (52). However, the combination of LPS and nigericin caused more GSDMD and caspase-1 cleavage than LPS alone in cells with constitutively active NLRP3 (Fig. 2C). Whenever caspase-1 was cleaved in these cells there was a faint caspase-8, −3 cleavage band. Although full length GSDME was easily visualized in WT and Nlrp3CA/+ BMDMs, cleavage of GSDME was not readily detected. However, in Nlrp3CA/+;Gsdmd−/− cells, although basal levels of caspase-8, −3 and GSDME appeared similar to levels in WT or Nlrp3CA/+ BMDMs, cleaved caspase-8 (p18 fragment) and caspase-3 (p17 fragment), which are fully active, were more prominent and GSDME cleavage was readily detected (Fig. 2C). Neither GSDME nor caspase-8, −3 cleavage in Nlrp3CA/+;Gsdmd−/− BMDMs required nigericin. Thus, in Nlrp3CA/+;Gsdmd−/− cells, caspase-8, −3 and GSDME are activated by LPS.
GSDME was involved in IL-1β and IL-18 secretion induced by LPS or TNF-α in Nlrp3CA/+;Gsdmd−/− mice
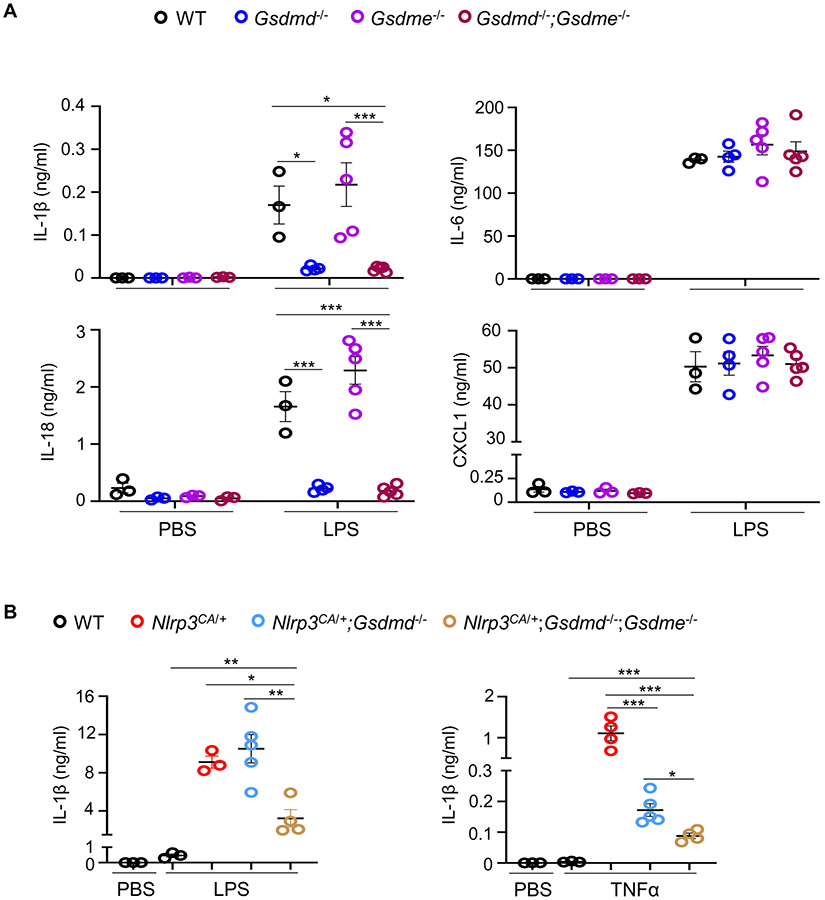
To determine whether GSDMD-independent inflammation in LPS-treated mice might be due to GSDME activation, we first compared baseline and LPS-stimulated blood serum cytokines (IL-1β, IL-18, IL-6, and CXCL1) in Gsdme−/− and Gsdmd−/−;Gsdme−/− animals bearing unmutated Nlrp3. Baseline cytokine levels were comparable between Gsdme−/− and Gsdmd−/−;Gsdme−/− mice and were similar to levels in WT animals (Fig. 3A). After LPS challenge, the IL-1 family cytokines were similarly elevated in Gsdme−/− mice as in WT mice but did not increase in Gsdmd−/−;Gsdme−/− animals. LPS induced elevated IL-6 and CXCL1 in all genotypes. Next, we measured IL-1β levels in response to LPS or TNF-α in WT, Nlrp3CA/+, Nlrp3CA/+;Gsdmd−/−, and Nlrp3CA/+;Gsdmd−/−;Gsdme−/− mice. Nlrp3CA/+;Gsdme−/− mice were not generated since LPS-induced responses were not impaired in Gsdme−/− animals (Fig. 3A). LPS or TNF-α induced IL-1β production in WT, Nlrp3CA/+, and Nlrp3CA/+;Gsdmd−/− mice (Fig. 3B). Notably, IL-1β levels were decreased in Nlrp3CA/+;Gsdmd−/−;Gsdme−/− compared to Nlrp3CA/+;Gsdmd−/− mice. These results suggest that GSDME participates in NLRP3 inflammasome signaling in states of GSDMD deficiency.
Figure 3. GSDME was involved in IL-1β and IL-18 secretion induced by LPS or TNF-α in Nlrp3CA/+;Gsdmd−/− mice.
Three-month-old WT, Gsdme−/−, Gsdme−/− and Gsdmd−/−;Gsdme−/− mice (A) or WT, Nlrp3CA/+, Nlrp3CA/+;Gsdmd−/− and Nlrp3CA/+;Gsdmd−/−;Gsdme−/− mice (B) were injected with 15 mg/kg LPS for 6 hours or 0.5 mg/kg TNF-α for 2 hours. PBS-administrated mice served as controls. N=3-5 mice/group. Serum cytokine levels were measured by V-PLEX Plus Proinflammatory Panel 1 Mouse Kit, except for IL-18, which were assessed by ELISA. Data are mean ± SEM. ***P < 0.001. One-Way ANOVA. LPS, lipopolysaccharide; IL, interleukin; WT, wild type; CA, constitutive activation; TNF-α, tumor necrosis factor α.
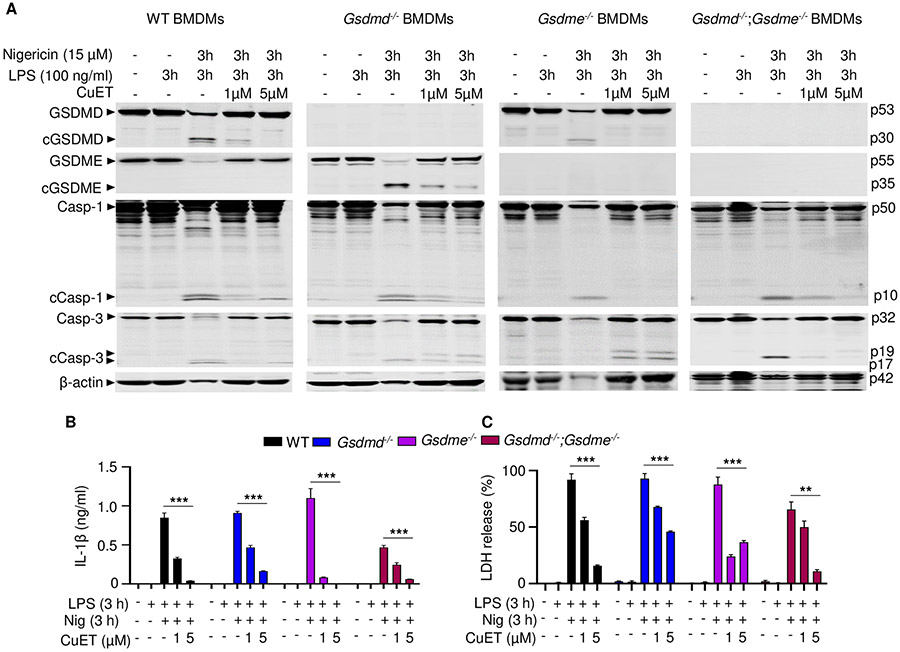
GSDME was cleaved in BMDMs lacking GSDMD and involved in IL-1β and LDH release
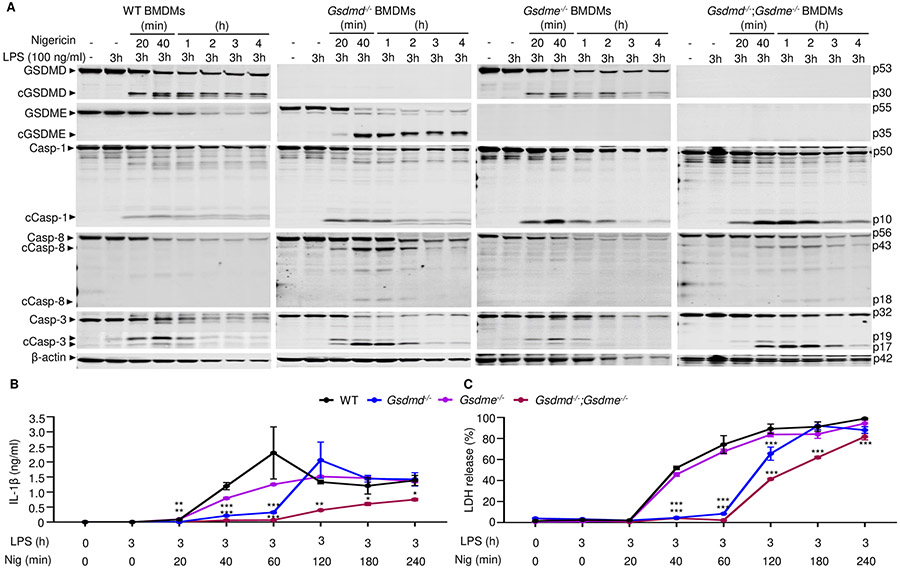
To further examine the impact of GSDMD and GSDME deficiency on LPS plus nigericin induced pyroptosis and IL-1β processing and release in vitro, WT, Gsdmd−/−, Gsdme−/−, and Gsdmd−/−;Gsdme−/− BMDMs lacking constitutively activated NLRP3 were pretreated with LPS for 3 hours and then with nigericin for up to 4 hours. Within 20 minutes of adding nigericin, caspase-1, and −3 were cleaved in all 4 cell lines (Fig. 4A, and fig. S4, A and B). Similar to our findings in Fig. 3A in Nlrp3CA/+ BMDMs, in cells bearing unmutated Nlrp3 treated with LPS and nigericin, GSDMD was cleaved in WT and Gsdme−/− BMDMs. Consistent with the notion that caspase-8 directly activates caspase-3, the catalytically active caspase-8 p18 and caspase-3 p17 fragments were both more prominent in cells lacking GSDMD. GSDME cleavage was detected only in Gsdmd−/− cells. GSDMD and GSDME cleavage was detected at the same time as caspase activation. Since GSDME was not readily cleaved in WT BMDMs, the time-dependent decline of its levels in WT cells was likely secondary to pyroptosis. Thus, GSDME cleavage occurred after NLRP3 inflammasome activation not only in Nlrp3CA/+;Gsdmd−/− BMDMs (Fig. 3A) but also Gsdmd−/− BMDMs.
Figure 4. GSDME was cleaved in Gsdmd−/− BMDMs and involved in IL-1β and LDH release.
BMDMs from bone marrow isolated from WT, Gsdmd−/−, Gsdme−/− or Gsdmd−/−;Gsdme−/− mice were expanded in vitro in M-CSF-containing media. BMDMs were primed with 100 ng/ml LPS for 3 hours and treated with 15 μM nigericin for 20 minutes, 40 minutes, 1, 2, 3, 4 hours. (A) The indicated proteins in the whole cell lysates were analyzed by immunoblotting. IL-1β (B) and LDH (C) in the conditioned media were measured by ELISA and by the cytotoxicity detection Kit, respectively. Data are mean ± SD from experimental triplicates and are representative of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. *,**,***compared to WT. One-Way ANOVA. BMDMs, bone marrow-derived macrophages; cCasp, cleaved caspase; cGSDM, cleaved gasdermin; h, hour; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; min, minute; nig, nigericin; M-CSF, macrophage colony-stimulating factor; WT, wild type.
Next, we examined whether caspase-1 was required to activate caspase-3 and GSDME by treating caspase-1 deficient BMDMs with LPS and nigericin. Consistent with previous reports (70), LPS and nigericin activated caspase-3, and promoted the generation of the GSDMD p10 fragment in Casp1−/− BMDMs, although caspase-3 and GSDMD processing were delayed compared to caspase-1 sufficient cells (fig. S4C). GSDME cleavage was detected in Casp1−/− BMDMs at the same time as fully processed caspase-3. Thus, although the maturation of caspase-1 and GSDMD was unperturbed by GSDME deficiency, NLRP3 inflammasome activation forced GSDME processing in cells lacking either caspase-1 or GSDMD.
We examined the extent to which GSDME mediated IL-1β and LDH release in GSDMD deficient cells. Although LPS-primed Gsdmd−/− BMDMs did not release IL-1β or LDH at early time-points up to 1 hour after nigericin addition, they were as active as WT cells in secreting IL-1β and releasing LDH at later time-points (Fig. 4, B and C) and in dose-dependent manner (fig. S4D). Loss of GSDME did not affect IL-1β and pyroptosis, but these readouts were significantly impaired, but not abrogated, in Gsdmd−/−;Gsdme−/− BMDMs (Fig. 4B). Likewise, LDH release was delayed and IL-1β levels were nearly undetectable in caspase-1−/− BMDMs (fig. S4E), suggesting that cleaved and secreted IL-1β but not intracellular pro-IL-1β was detected in the supernatants of Nlrp3CA/+;Gsdmd−/− (Fig. 3B) or Gsdmd−/− (Fig. 4B) BMDM cultures. GSDME cleavage and IL-1β secretion in Gsdmd−/− and Nlrp3CA/+;Gsdmd−/− BMDMs induced by LPS and nigericin were inhibited by the pan caspase inhibitor, zVAD (fig. S5, A and B), results that were consistent with the reported cleavage of GSDME by caspase-3 (22-24). zVAD failed to consistently inhibited LDH release (fig. S5C), and caspase-11 was not activated in these experimental conditions where LPS was added extracellularly (fig. S6A). As a result, GSDME processing (fig. S6A), IL-1β secretion and LDH release (fig. S6B) were comparable between WT and caspase-11−/− BMDMs.
Finally, we studied the effects of LPS on WT, Nlrp3CA/+, Nlrp3CA/+;Gsdmd−/−, and Nlrp3CA/+;Gsdmd−/−;Gsdme−/− BMDMs. LPS alone did not stimulate IL-1β secretion (Fig. 5A) and LDH release (Fig. 5B) in WT cells, as expected. However, LPS induced IL-1β secretion and LDH release in Nlrp3CA/+; howeverm the inhibition of this response was transient in Nlrp3CA/;Gsdmd−/− BMDMs (up to 9 hours) and it was sustained in Nlrp3CA/+;Gsdmd−/−;Gsdme−/− BMDMs up to 24 hours (Fig. 5A and B). Thus, the release of IL-1β and LDH was persistently inhibited, though not completely eradicated, in Gsdmd−/−;Gsdme−/− BMDMs subjected to sustained NLRP3 inflammasome activation.
Figure 5. GSDME was involved in IL-1β and LDH release by Nlrp3CA/+;Gsdmd−/− BMDMs.
BMDMs were expanded in vitro in M-CSF-containing media from bone marrow cells isolated from WT, Nlrp3CA/+, Nlrp3CA/+;Gsdmd−/−, Nlrp3CA/+;Gsdmd−/−;Gsdme−/− mice. BMDMs were treated with 100 ng/ml LPS for 3, 6, 9, 12, 18 or 24 hours. IL-1β (A) and LDH (B) in the conditioned media were measured by ELISA and by the cytotoxicity detection Kit, respectively. Data are mean ± SEM from experimental triplicates and are representative of at least three independent experiments. **P < 0.01; ***P < 0.001; ##P < 0.01; ###P < 0.001. ***Nlrp3CA/+;Gsdmd−/− or Nlrp3CA/+;Gsdmd−/−;Gsdme−/− compared to Nlrp3CA/+; ###Nlrp3CA/+;Gsdmd−/−;Gsdme−/− compared to Nlrp3CA/+;Gsdmd-/−. One-Way ANOVA. BMDMs, bone marrow-derived macrophages; h, hour; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; WT, wild type; CA, constitutive activation.
CuET inhibited GSDMD, GSDME, and IL-1β maturation and LDH release
Disulfiram and its active metabolite, bis(diethyldithiocarbamate)-copper (CuET), antagonize GSDMD pore-forming activity but have not been reported to inhibit GSDME pore formation (64). To investigate whether these compounds might inhibit GSDMD-independent pyroptosis, WT, Gsdmd−/−, Gsdme−/−, and Gsdmd−/−;Gsdme−/− BMDMs were treated with LPS for 3 hours and then CuET or vehicle for 1 hour followed by an additional 3 hours of incubation with nigericin. In the absence of CuET, GSDMD was cleaved in WT and Gsdme−/− BMDMs (Fig. 6A) and caspase-1 deficient BMDMs (fig. S7A), consistent with the results shown in Fig. 4. While GSDME was cleaved only in Gsdmd−/− cells, caspase-1 and caspase-3 were cleaved in all cell lines. However, although the full-length caspase protein bands sharply decreased, the signals of their cleaved fragments were faint (Fig. 6A), presumably because they might have been released to the extracellular milieu during pyroptosis (71-73), which was maximal in cells treated with nigericin for 2 hours. GSDMD, GSDME, and caspase-1 cleavage was consistently inhibited by CuET, in a dose-dependent manner (Fig. 6A, and fig. S7A). The band for cleaved caspase-3 was more prominent in CuET-treated than untreated WT, Gsdmd−/−, and Gsdme−/− BMDMs. CuET inhibited the release of IL-1β and LDH in all cell genotypes (Fig. 6, B and C, and fig. S7B), but was less efficacious in Gsdmd−/−;Gsdme−/− BMDMs, in which cytokine release and pyroptosis were attenuated (Fig. 6, B and C). Disulfiram and CuET both inhibited IL-1β and LDH release in WT BMDMs with comparable dose response curves (fig. S7C-F). Thus, CuET inhibits the maturation of both GSDMD and GSDME, inhibiting both GSDMD-dependent and -independent pyroptosis and IL-1β release.
Figure 6. CuET inhibited GSDMD, GSDME, and IL-1β maturation and LDH release.
BMDMs were expanded in vitro in M-CSF-containing media from bone marrow cells isolated from WT, Gsdmd−/−, Gsdme−/−, or Gsdmd−/−;Gsdme−/− mice. BMDMs were primed with 100 ng/ml LPS for 3 hours and treated with vehicle or CuET for 1 hour before adding 15 μM nigericin for 3 hours. (A) The indicated proteins in the whole cell lysates were analyzed by immunoblotting. IL-1β (B) and LDH (C) in the conditioned media were measured by ELISA and by the cytotoxicity detection Kit, respectively. Data are mean ± SD from experimental triplicates and are representative of at least three independent experiments. **P < 0.01; ***P < 0.001. Two-Way ANOVA. BMDMs, bone marrow-derived macrophages; cCasp, cleaved caspase; cGSDM, cleaved gasdermin; h, hour; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; min, minute; nig, nigericin; M-CSF, macrophage colony-stimulating factor; WT, wild type.
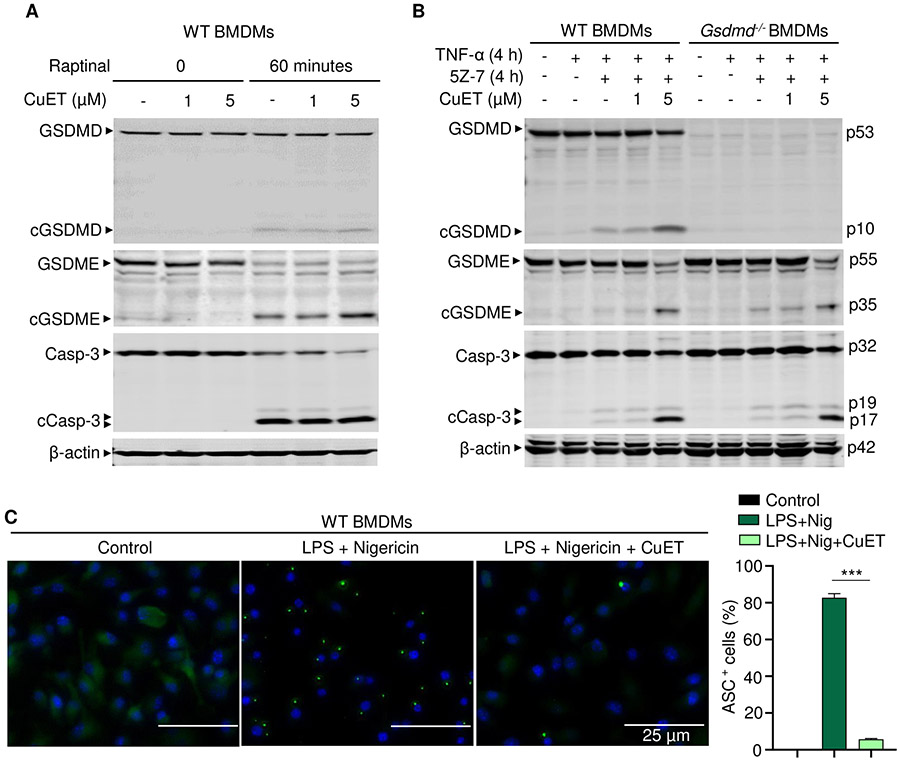
CuET inhibited NLRP3 inflammasome-dependent but not -independent responses
The ability of CuET to inhibit the cleavage of GSDMD and GSDME downstream of caspase-1 and caspase-3, respectively, raises concerns that this compound may act as a pan caspase inhibitor. To determine whether CuET inhibits inflammasome-independent GSDME-maturation, BMDMs were treated with raptinal or TNF-α and 5Z-7-oxozeaenol (5Z-7, a TGF-β-activated kinase-1 inhibitor) in the presence or absence of CuET; both treatments are known to activate caspase-3 and GSDME-dependent pyroptosis independently of any inflammasome (23, 74). CuET did not inhibit the cleavage of GSDMD (to p10 GSDMD), GSDME, or caspase-3 induced by raptinal or TNF-α and 5Z-7 (Fig. 7, A and B, and fig. S8A) and if anything appeared to enhance their cleavage in response to these stimuli (Fig. 7B). To determine why CuET inhibited the cleavage of these proteins in response to NLRP3 inflammasome activation but not apoptotic stimuli, we analyzed its effects on NF-κB and MAPK activation. CuET had no effect on LPS stimulated phosphorylation of IκBα, NF-κB/p65, and p38 MAPK (fig. S8B). We also analyzed CuET effects on LPS plus nigericin-induced inflammasome activation by measuring the formation of ASC specks, a key step in inflammasome assembly and activation, using WT BMDMs expressing fluorescent Asc-citrine. To minimize potential effects of CuET on inflammasome priming signals, the drug was added to LPS-primed cells and immediately followed by adding nigericin. The formation of ASC specks was significantly inhibited by CuET (Fig. 7C). These results suggest that CuET inhibits NLRP3 inflammasome activation, but not GSDME, caspase-3 or LPS-induced NF-κB or MAPK activation.
Figure 7. CuET inhibited NLRP3 inflammasome-dependent but not -independent responses.
BMDMs were expanded in vitro M-CSF-containing media from bone marrow cells isolated from WT or Gsdmd−/− mice. (A) WT BMDMs were pretreated with vehicle or CuET for 1 hour, and were stimulated with vehicle or 10 μM Raptinal for 1 hour. (B) WT and Gsdmd−/− BMDMs were pretreated with vehicle or CuET for 1 hour, and were stimulated with 100 ng/ml TNF-α and 1 μM 5Z-7-oxozeaenol (TAK1 inhibitor) for 4 hours. The indicated proteins in the whole cell lysates were analyzed by immunoblotting (A and B). (C) WT BMDMs from Asc-citrine mice were primed with 100 ng/ml LPS for 3 hours and treated with vehicle or 1 μM CuET for 15 minutes, then with15 μM nigericin for additional 30 minutes. ASC specks were visualized under fluorescence microscopy and quantified using imageJ. Data are mean ± SEM from experimental triplicates and are representative of at least three independent experiments. ***P < 0.001. One-Way ANOVA. BMDMs, bone marrow-derived macrophages; cCasp, cleaved caspase; CuET, bis(diethyldithiocarbamate)-copper; cGSDM, cleaved gasdermin; h, hour; LPS, lipopolysaccharide; nig, nigericin; M-CSF, macrophage colony-stimulating factor; WT, wild type.
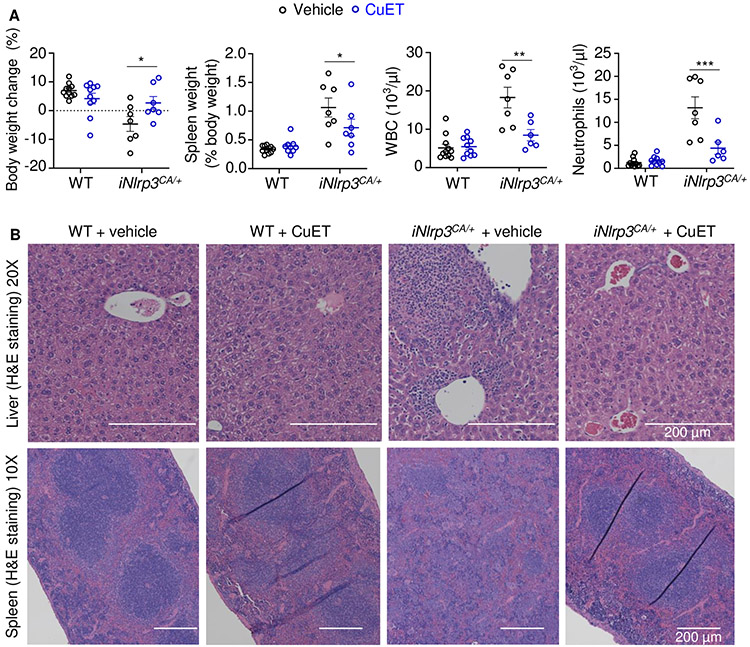
CuET prevented inflammasomopathy in Nlrp3CA/+ mice
Based on its in vitro potency in inhibiting the release of cytokines and pyroptosis, CuET should improve the disease outcomes of Nlrp3CA/+ mice. To test this idea, CuET was intraperitoneally injected to 10-day-old pups, once every 2 days (75). CuET significantly improved the survival rate of Nlrp3CA/+ mice (fig. S9A) and reduced splenomegaly in these mice (fig. S9B). Despite improved survival, treated- Nlrp3CA/+ mice still showed early mortality, likely due to the severe inflammation already activated before treatment was initiated. To control the onset of this possible inflammation, we leveraged the Nlrp3+/fl(D301N);Cre-ERTM model for postnatal inducible activation of the NLRP3CA (iNLRP3CA) inflammasome in adult mice to test the efficacy of prophylactically administrated CuET. Three-month-old mice were injected with tamoxifen 3 times/week for 2 weeks. Vehicle or CuET were injected 2 days before starting tamoxifen and continued 3 times/week for 6 weeks. iNlrp3CA/+ mice all survived but developed cachexia, splenomegaly, leukocytosis and neutrophilia, which were significantly attenuated by CuET (Fig. 8A). CuET prevented inflammation in the liver and the disorganization of splenic architecture in iNlrp3CA/+ mice as assessed by H & E staining and had no apparent effect on these tissues in WT controls (Fig. 8B, and fig. S9C). Although the effects of CuET were not analyzed in Nlrp3CA/+;Gsdmd−/− mice due to the limited number of animals for in vivo studies, we determined its actions on these cells in vitro. We found that CuET inhibited IL-1β secretion and LDH release in WT and Nlrp3CA/+;Gsdmd−/− BMDMs (fig. S10). Thus, our findings show that CuET-treated Nlrp3CA/+ mice phenocopy Nlrp3CA/+;Gsdmd−/−;Gsdme−/−mice, consistent with the ability of this drug to inhibit GSDMD and GSDME cleavage (Fig. S11).
Figure 8. CuET prevented inflammasomopathy in the inducible NLRP3CA/+ (iNLRP3CA/+) model.
Three-month-old mice were injected with tamoxifen once every other day, 3 times a week for 2 weeks. Injections with vehicle or 1 mg/kg body weight CuET started 2 days before the tamoxifen regimen, and were carried out once every other day, 3 times a week for 6 weeks. All injections were given intraperitoneally. N=6-11 mice/group. (A) Body weight change, spleen weight, WBC; and neutrophils. Data are means ± SEM. Two-Way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001. (B) Representative H&E staining of liver and spleen sections. WBC, white blood cell; WT, wild-type; CA, constitutive activation; CuET, bis(diethyldithiocarbamate)-copper; iNLRP3CA/+, inducible NLRP3CA/+.
DISCUSSION
Nlrp3CA/+ (NOMID) mice die perinatally because of severe systemic multi-organ complications driven by excessive blood levels of IL-1β and IL-18 (52, 76). Nlrp3CA/+ mice lacking GSDMD (Nlrp3CA/+;Gsdmd−/− mice) grew and aged normally and are fertile. Consistent with these observations, ablation of GSDMD reduces inflammation and improves disease outcomes in mouse models of NOMID, sepsis, Familial Mediterranean Fever (FMF), and experimental autoimmune encephalitis (14, 56, 57, 77). However, when stressed by exposure to LPS, Nlrp3CA/+;Gsdmd−/− mice unexpectedly secreted high amounts of IL-1β and IL-18. The combination of these inflammatory stimuli with constitutively active NLRP3 overwhelmed the protection provided by GSDMD or caspase-1 deficiency to various extents, rewiring signaling cascades through caspase-3 and GSDME likely to cause pyroptosis (22, 25). These observations suggest that therapeutic inhibition of GSDMD may not be sufficient to prevent complications such as those associated with infections, which have the potential to activate not only GSDMD but also GSDME. This scenario can be tested preclinically by assessing the consequences of infections or injuries in NOMID or FMF mice lacking GSDMD, which can be viewed as animals with an underlying inflammatory condition. Thus, though apparently normal in homeostatic conditions, Nlrp3CA/+;Gsdmd−/− mice are vulnerable to stressful insults.
Both Nlrp3CA/+;Gsdmd−/− BMDMs and Gsdmd−/− BMDMs produced IL-1β and underwent pyroptosis in response to LPS, although these responses were delayed in both cell lines compared to WT cells, occurring only upon sustained exposure to NLRP3 inflammasome activators. The responsiveness of Gsdmd−/− cells to LPS in vitro suggested that exposure of GSDMD-deficient mice to endotoxin for a long period may lead to significant IL-1β and IL-18 secretion. Consistent with this, a small proportion of Gsdmd−/− mice die upon LPS-challenge (14, 64). Pyroptosis and cytokine release by GSDMD or caspase-1 deficient cells positively correlated with caspase-3 and GSDME activation and were significantly suppressed in Nlrp3CA/+;Gsdmd−/−;Gsdme−/− cells and Gsdmd−/−;Gsdme−/− cells, suggesting that caspase-3 activation of GSDME was largely responsible for inflammatory death when NLRP3 was activated but the canonical caspase-1-GSDMD pathway was not available. Consistent with our findings on GSDM complementation, a recent study reported that the TLR1/TLR2 agonist Pam3CSK4 promotes the release of IL-1α by caspase-1 deficient macrophages through GSDME conduits (28). In our study, caspase-3 activation was enhanced by GSDMD or caspase-1 genetic deficiency. We did not explore the mechanism responsible for increased caspase-8 and caspase-3 activation in these settings, but one possibility was that caspase-8 was efficiently recruited to the NLRP3 complex when caspase-1 or GSDMD was absent and very potent at processing caspase-3, which in turn activated GSDME. It is worth noting that there was some residual IL-1β release and pyroptosis in chronically activated Nlrp3CA/+;Gsdmd−/−;Gsdme−/− cells and Gsdmd−/−;Gsdme−/− BMDMs, suggesting that another GSDMD and GSDME independent inflammation pathway may be activated downstream of NLRP3, potentially involving another caspase and/or another gasdermin. From our study it remains uncertain whether cytokines are secreted through GSDME pores in the setting of caspase-1 or GSDMD deficiency or are released when the cell membrane is grossly disrupted and LDH is released, as was recently reported (82, 83). Taken together, these results suggest that macrophages have more than one salvage mechanism to ensure that signals transmitted by inflammasome activators result in inflammation. However, outcomes such as temperature changes and immune cell recruitment to tissues in LPS-treated Nlrp3CA/+;Gsdmd−/−;Gsdme−/− mice were not assessed in this study, but are themes of interest for future investigations.
Drug discovery and repurposing efforts have identified the FDA-approved drugs dimethyl fumarate and disulfiram as GSDMD inhibitors (58, 64). Covalent modification of Cys191 in human and Cys192 in mouse is the proposed main mechanism through which disulfiram inhibits GSDMD pore-forming activity, although dimethyl fumarate likely works through another mechanism (64). Here, we report that the disulfiram metabolite CuET exhibited remarkable efficacy in the Nlrp3CA inflammasomopathy model as it prevented systemic inflammation and the damage to the liver and spleen. Mechanistically, we found that CuET suppressed cytokine secretion and pyroptosis caused by both GSDMD and GSDME. However, CuET inhibited GSDME cleavage and pyroptosis in cells stimulated with NLRP3 inflammasome activators but not apoptotic stimuli. These results ruled out GSDME as the major direct target of CuET inhibitory actions, a view that was consistent with the compound’s ability to inhibit the formation of ASC specks. Although, as far as we are aware, direct inhibition of GSDME pore formation by disulfiram has never been measured, Cys191/192 is not conserved in mouse GSDME and therefore disulfiram is not predicted to be a potent regulator of GSDME. However, as a cysteine-reactive drug, disulfiram has the potential to inactivate other enzymes, including the caspase cysteine proteases and other proteins with reactive cysteines that are modulated by cellular redox status, which include the NLRP3 inflammasome (84). When a potent drug target such as GSDMD is not present, disulfiram may switch to inhibit other less reactive substrates, a scenario that may explain the inhibitory effects of CuET in cell lacking GSDMD and GSDME. However, we currently have limited knowledge on the selectivity profile of CuET, thus future studies will need to investigate how it inhibits ASC polymerization and whether it antagonizes other early steps of inflammasome signaling.
This study revealed that ablation of GSDMD in Nlrp3CA/+ mice did not prevent these animals from producing excessive levels of IL-1β and IL-18 in response to inflammatory challenges. Importantly, the disease severity in Nlrp3CA/+ mice was remarkably reduced by CuET, a drug that interferes directly or indirectly with both GSDMD-dependent and independent inflammation. Our findings suggest that disulfiram might be worth testing in CAPS patients for whom existing therapies that inhibit IL-1 or other inflammatory cytokines do not adequately suppress disease symptoms.
MATERIALS AND METHODS
Study design
The objective of this study was to determine the responses of the seemingly normal Gsdmd−/− mice expressing constitutive active NLRP3 – yet viewed as animals with an underlying inflammatory condition – to inflammatory stimuli (e.g., LPS, TNF-α). To achieve this goal, we used various strategies, including genetic and pharmacological manipulation, biochemical, histology, and immunostaning approaches.
Animals
Casp1−/− were kindly provided by Dr. Thirumala-Devi Kanneganti (St. Jude Children's Research Hospital). Cre-ERTM (B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J) mice and lysozyme M-Cre mice were purchased from The Jackson Laboratory (Sacramento, CA). Nlrp3fl(D301N)/+ mice were kindly provided by Dr. Hal Hoffman (University of California, San Diego), and have been previously described (52, 54, 85). Nlrp3CA/+ mice with constitutive activation of NLRP3 in myeloid cells driven by lysozyme M-Cre have been previously described (54). Cre-ERTM mice and Nlrp3fl(D301N)/+ mice were crossed to generate Nlrp3fl(D301N)/+;Cre-ERTM mice and Cre-ERTM mice. Injection of tamoxifen (i.p., 75 mg/kg body weight; Sigma-Aldrich) to Nlrp3fl(D301N)/+;Cre-ERTM mice and Cre-ERTM mice to yield inducible Nlrp3CA (iNlrp3CA) mice and control mice, respectively, has been previously described (85). Gsdmd−/− mice were kindly provided by Dr. V. M. Dixit (Genentech, South San Francisco, CA). Asc-citrine and Gsdme−/− mice were purchased from The Jackson Laboratory (Sacramento, CA). All mice were on the C57BL6J background and mouse genotyping was performed by PCR. All procedures were approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine in St. Louis.
Administration of drugs, LPS, and TNF-α
Mice were i.p. injected with CuET (TCI America, OR) (1 mg/kg) formulated in sesame oil (0.2 mg/ml) or vehicle, once every other day, 3 times per week. Ten day-old Nlrp3CA/+mice were treated with vehicle or CuET for 9 weeks; 12-week-old iNlrp3CA/+ and control mice were treated with vehicle or CuET 3 times/week for 6 weeks. For LPS challenge experiments, WT, Gsdmd−/−, Gsdme−/−, Gsdmd−/−;Gsdme−/−, Nlrp3CA/+, Nlrp3CA/+;Gsdmd−/− and Nlrp3CA/+;Gsdmd−/−;Gsdme−/− mice were i.p. injected with 15 mg/kg LPS (Escherichia coli O111:B4, Sigma, MO). Mice were i.p. injected with 0.5 mg/kg TNF-α (Biolegend, CA).
Serum cytokine assay
Blood was collected by cardiac puncture 6 hours or 2 hours after LPS or TNF-α challenge, and was allowed to clot at room temperature. Serum obtained after centrifugation at 2,000 x g for 10 minutes was used for determinations of cytokine and chemokine levels by V-PLEX Plus Proinflammatory Panel1 Mouse Kit (Meso Scale Diagnostics, MD), except IL-18, which was analyzed by enzyme linked immunosorbent assay (ELISA) kit (Sigma, MO).
Cell cultures
BMDMs were obtained by culturing mouse bone marrow cells in culture media containing a 1:10 dilution of supernatant from the fibroblastic cell line CMG 14-12 as a source of macrophage colony-stimulating factor (86), a mitogenic factor for BMDMs, for 4-5 days in a 15-cm dish as previously described (85). Briefly, nonadherent cells were removed by vigorous washes with PBS, and adherent BMDMs were detached with trypsin-EDTA and cultured in culture media containing a 1:10 dilution of CMG for various experiments.
For all in vitro pharmacology experiments except otherwise specified, cells were pre-treated with vehicle (0.1% DMSO, final concentration) or inhibitors (in 0.1% DMSO, final concentration) for 1 hour before stimulation with the indicated ligand or ligands. Protein expression was analyzed by ELISA or Western blot as described below. To activate the NLRP3 inflammasome, BMDMs were plated at 104 cells per well on a 96-well plate or 106 cells per well on a 6-well plate overnight. Cells were primed with LPS and then with 15 μM nigericin (AdipoGen) as indicated, and conditioned media were collected for the analysis of IL-1β and LDH.
Histology
Tissue samples were processed as described previously (85). In brief, liver, and spleen were fixed in 10% formalin overnight. Fixed tissues were embedded in paraffin, sectioned at 5-μm thicknesses, and mounted on glass slides. The sections were stained with H&E as described previously (85). Photographs were taken using ZEISS microscopy (Carl Zeiss Industrial Metrology, MN).
Peripheral blood analyses
Mouse blood was collected by cardiac puncture in the EDTA-containing tubes. Complete blood counts were performed by the Washington University School of Medicine as previously described (54).
Western blot analysis
Cell extracts were prepared by lysing cells with RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% NaDOAc, 0.1% SDS, and 1.0% NP-40) plus phosphatase and protease inhibitors (2 mM NaVO4, 10 mM NaF, and 1 mM PMSF) and Complete protease inhibitor cocktail (Roche, CA). For tissue extracts, liver tissues were homogenized and lysed with RIPA buffer containing phosphatase and protease inhibitors. Protein concentrations were determined by the Bio-Rad Laboratories method, and equal amounts of proteins were subjected to SDS-PAGE gels (12%) as previously described (56). Proteins were transferred onto nitrocellulose membranes and incubated with antibodies against GSDMD (1;1,000; Abcam, MA), GSDME (1;1,000; Abcam, MA), caspase-1 (1;1,000; Abcam, MA), caspase-3 (1:1,000; Cell Signaling Technologies, MA), p38 MAPK (1:1,000; Cell Signaling Technologies, MA), β-actin (1:2,000; Santa Cruz Biotechnology, TX), p65/p-p65 (1:1,000; Cell Signaling Technologies, MA), IκBα/p-IκBα (1:1,000; Cell Signaling Technologies, MA), or pMLKL (1:1,000; Cell Signaling Technologies, MA) overnight at 4°C followed by incubation for 1 hour with secondary goat anti-mouse IRDye 800 (Thermo Fisher Scientific, MA) or goat anti–rabbit Alexa Fluor 680 (Thermo Fisher Scientific, MA), respectively. The results were visualized using the Odyssey infrared imaging system (LI-COR Biosciences, NE).
LDH assay and IL-1β ELISA
Cell death was assessed by the release of LDH in conditioned medium using LDH Cytotoxicity Detection Kit (TaKaRa, CA). IL-1β levels in conditioned media were measured by an ELISA kit (eBiosciences, NY).
ASC specks assay
Asc-citrine, WT, and Nlrp3CA/+;Gsdmd−/− BMDMs were plated at 104 cells per well on a 16-well glass plate overnight. Cells were primed with LPS for 3 hours and pretreated or not with CuET for 15 minutes before adding 15 μM nigericin (AdipoGen, CA) for 30 minutes. Cells were washed with PBS and fixed with 4% polyformalin buffer for 10 minutes at room temperature. For immunostaining, WT and Nlrp3CA/+;Gsdmd−/− BMDMs were permeabilized with 0.2% Triton in PBS for 20 minutes, blocked with 0.2% Triton, 1% BSA, and CD61 antibody (1:1000; Alexa Fluor 647, BD, NJ) in PBS for 30 minutes, and were incubated with ASC antibody (1:1000; clone 2EI-7, EMD Millipore, MA) overnight at 4°C in blocking buffer, followed by incubation with secondary antibody (Alexa Fluor 594, Life Technologies) for 30 minutes. Cells were counterstained with Fluoro-gel II containing DAPI (Fluoro-Gel, Fisher Scientific Intl INC, PA). Asc-citrine photographs were taken using ZEISS microscopy (Carl Zeiss Industrial Metrology, MN). ASC immunostaining images were taken using a Leica inverted microscope with a TCS SPEII confocal module and processed using LAS X software (Leica Microsystems Inc, IL). Quantification of ASC specks was carried out using ImageJ.
Statistical analysis
Statistical analysis was performed using the Student’s t test, one-way ANOVA with Tukey’s multiple comparisons test, or two-way ANOVA with Tukey’s multiple comparisons test, Dunnett's multiple comparisons test, or Sidak's multiple comparisons test as well as the Log-rank (Mantel-Cox) test for comparison of survival curves using the GraphPad Prism 8.0 Software. Values are expressed as mean ± SEM or means ± SD, as indicated. *p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We want to thank Dr. Deborah J. Veis for critical reading of this manuscript.
Funding:
This work was supported by NIH/NIAMS AR068972 and AR076758 grants to G.M. Y.A-A. was supported by NIH grants AR049192, AR054326, AR072623 and by a grant #85160 from the Shriners Hospital for Children.
Footnotes
Competing interest: GM is Consultant for Aclaris Therapeutics, Inc. CX and JBM are employees of Aclaris Therapeutics, Inc. They have no additional financial interests. JL is cofounder and SAB member of Ventus Therapeutics and has filed a patent application for the use of disulfiram for inhibiting pyroptosis and inflammation. All other authors declare no competing financial interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper of supplementary materials.
REFERENCES AND NOTES
- 1.Schroder K, Tschopp J, The inflammasomes. Cell 140, 821–832 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Guo H, Callaway JB, Ting JP, Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21, 677–687 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling. Nature reviews. Immunology 16, 407–420 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang D-C, Shao F, Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC, The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44.e36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W.-t., Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, Han J, Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Research 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J, Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man SM, Karki R, Kanneganti T-D, Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological reviews 277, 61–75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Gao W, Shao F, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends in Biochemical Sciences 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL, Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Critical care medicine 45, 253–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman HM, Brydges SD, Genetic and molecular basis of inflammasome-mediated disease. The Journal of biological chemistry 286, 10889–10896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R, Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annual review of immunology 33, 823–874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, Luo HR, Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell reports 22, 2924–2936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, Abbott DW, Wang K, Shao F, Catz SD, Dubyak GR, Pearlman E, N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nature communications 11, 2212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K, Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Science immunology 3, (2018). [DOI] [PubMed] [Google Scholar]
- 18.Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, Benarafa C, Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell reports 27, 3646–3656.e3645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P, Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. Embo j 38, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A, Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proceedings of the National Academy of Sciences of the United States of America 115, E10888–e10897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E, Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science (New York, N.Y.) 362, 1064–1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES, Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature communications 8, 14128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, Sengupta S, Yao Y, Wu H, Lieberman J, Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES, Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nature communications 10, 1689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F, Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, Wang K, Sun X, Zheng J, Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death & Disease 10, 193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y, Miura M, Kinoshita T, Kushiyama H, Sakurai M, Shiroishi T, Suda T, Caspase-1 initiates apoptosis in the absence of gasdermin D. Nature communications 10, 2091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aizawa E, Karasawa T, Watanabe S, Komada T, Kimura H, Kamata R, Ito H, Hishida E, Yamada N, Kasahara T, Mori Y, Takahashi M, GSDME-Dependent Incomplete Pyroptosis Permits Selective IL-1α Release under Caspase-1 Inhibition. iScience 23, 101070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, Xie J, Gao Y, Cheng F, Zhou Y, Zhang Z, Hu Y, Zhang X, Gao Q, Zhang Y, Huang B, Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Science immunology 5, (2020). [DOI] [PubMed] [Google Scholar]
- 30.Tixeira R, Shi B, Parkes MAF, Hodge AL, Caruso S, Hulett MD, Baxter AA, Phan TK, Poon IKH, Gasdermin E Does Not Limit Apoptotic Cell Disassembly by Promoting Early Onset of Secondary Necrosis in Jurkat T Cells and THP-1 Monocytes. Frontiers in immunology 9, 2842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BL, Mirrashidi KM, Stowe IB, Kummerfeld SK, Watanabe C, Haley B, Cuellar TL, Reichelt M, Kayagaki N, ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Scientific reports 8, 3788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilig R, Dilucca M, Boucher D, Chen KW, Hancz D, Demarco B, Shkarina K, Broz P, Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life science alliance 3, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, Vijayaraj S, Lindqvist LM, Bouillet P, Rizzacasa MA, Man SM, Silke J, Masters SL, Lessene G, Huang DCS, Gray DHD, Kile BT, Shao F, Lawlor KE, The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and −7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell reports 25, 2339–2353.e2334 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Shen HH, Yang YX, Meng X, Luo XY, Li XM, Shuai ZW, Ye DQ, Pan HF, NLRP3: A promising therapeutic target for autoimmune diseases. Autoimmunity reviews 17, 694–702 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Zambetti LP, Mortellaro A, NLRPs, microbiota, and gut homeostasis: unravelling the connection. The Journal of pathology 233, 321–330 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Zhen Y, Zhang H, NLRP3 Inflammasome and Inflammatory Bowel Disease. Frontiers in immunology 10, 276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spel L, Martinon F, Inflammasomes contributing to inflammation in arthritis. Immunological reviews 294, 48–62 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Jäger E, Murthy S, Schmidt C, Hahn M, Strobel S, Peters A, Stäubert C, Sungur P, Venus T, Geisler M, Radusheva V, Raps S, Rothe K, Scholz R, Jung S, Wagner S, Pierer M, Seifert O, Chang W, Estrela-Lopis I, Raulien N, Krohn K, Sträter N, Hoeppener S, Schöneberg T, Rossol M, Wagner U, Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nature communications 11, 4243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alippe Y, Mbalaviele G, Omnipresence of inflammasome activities in inflammatory bone diseases. Seminars in immunopathology 41, 607–618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang T, Liu J, Chen X, Zhang L, Pi J, Sun H, Li L, Bauer R, Wang H, Yu Z, Zhang Q, Tomlinson B, Chan P, Zheng X, Morrisey E, Liu Z, Reilly M, Zhang Y, Endothelial Foxp1 Suppresses Atherosclerosis via Modulation of Nlrp3 Inflammasome Activation. Circulation research 125, 590–605 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Grebe A, Hoss F, Latz E, NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circulation research 122, 1722–1740 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Eisenbarth SC, Flavell RA, Innate instruction of adaptive immunity revisited: the inflammasome. EMBO molecular medicine 1, 92–98 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renaudin F, Orliaguet L, Castelli F, Fenaille F, Prignon A, Alzaid F, Combes C, Delvaux A, Adimy Y, Cohen-Solal M, Richette P, Bardin T, Riveline JP, Venteclef N, Lioté F, Campillo-Gimenez L, Ea HK, Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Annals of the rheumatic diseases 79, 1506–1514 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, Stamp LK, Gout. Nature reviews. Disease primers 5, 69 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J, Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Ding S, Xu S, Ma Y, Liu G, Jang H, Fang J, Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, Stein L, Russo R, Goldsmith D, Dent P, Rosenberg HF, Austin F, Remmers EF, Balow JE Jr., Rosenzweig S, Komarow H, Shoham NG, Wood G, Jones J, Mangra N, Carrero H, Adams BS, Moore TL, Schikler K, Hoffman H, Lovell DJ, Lipnick R, Barron K, O’Shea JJ, Kastner DL, Goldbach-Mansky R, De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis and rheumatism 46, 3340–3348 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaki FM, Sridharan R, Pei TS, Ibrahim S, Ping TS, NOMID: the radiographic and MRI features and review of literature. Journal of radiology case reports 6, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill SC, Namde M, Dwyer A, Poznanski A, Canna S, Goldbach-Mansky R, Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA). Pediatric radiology 37, 145–152 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G, Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. American journal of human genetics 71, 198–203 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD, Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature genetics 29, 301–305 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonar SL, Brydges SD, Mueller JL, McGeough MD, Pena C, Chen D, Grimston SK, Hickman-Brecks CL, Ravindran S, McAlinden A, Novack DV, Kastner DL, Civitelli R, Hoffman HM, Mbalaviele G, Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PloS one 7, e35979 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM, Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. The Journal of clinical investigation 123, 4695–4705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Xu CX, Alippe Y, Qu C, Xiao J, Schipani E, Civitelli R, Abu-Amer Y, Mbalaviele G, Chronic inflammation triggered by the NLRP3 inflammasome in myeloid cells promotes growth plate dysplasia by mesenchymal cells. Scientific reports 7, 4880 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGeough MD, Wree A, Inzaugarat ME, Haimovich A, Johnson CD, Peña CA, Goldbach-Mansky R, Broderick L, Feldstein AE, Hoffman HM, TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. The Journal of clinical investigation 127, 4488–4497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, Civitelli R, Abu-Amer Y, Kanneganti TD, Link DC, Mbalaviele G, Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS biology 16, e3000047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanneganti A, Malireddi RKS, Saavedra PHV, Vande Walle L, Van Gorp H, Kambara H, Tillman H, Vogel P, Luo HR, Xavier RJ, Chi H, Lamkanfi M, GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. The Journal of experimental medicine 215, 1519–1529 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, Fitzgerald KA, Succination inactivates gasdermin D and blocks pyroptosis. Science (New York, N.Y.) 369, 1633–1637 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ter Haar N, Lachmann H, Özen S, Woo P, Uziel Y, Modesto C, Koné-Paut I, Cantarini L, Insalaco A, Neven B, Hofer M, Rigante D, Al-Mayouf S, Touitou I, Gallizzi R, Papadopoulou-Alataki E, Martino S, Kuemmerle-Deschner J, Obici L, Iagaru N, Simon A, Nielsen S, Martini A, Ruperto N, Gattorno M, Frenkel J, Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Annals of the rheumatic diseases 72, 678–685 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Anton J, Calvo I, Fernández-Martin J, Gamir ML, Merino R, Jimenez-Treviño S, Sevilla B, Cabades F, Bou R, Arostegui JI, Efficacy and safety of canakinumab in cryopyrin-associated periodic syndromes: results from a Spanish cohort. Clinical and experimental rheumatology 33, S67–71 (2015). [PubMed] [Google Scholar]
- 61.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, Zalewski C, Kim HJ, Bishop R, Hill S, Paul SM, Kicker P, Phillips Z, Dolan JG, Widemann B, Jayaprakash N, Pucino F, Stone DL, Chapelle D, Snyder C, Butman JA, Wesley R, Goldbach-Mansky R, Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis and rheumatism 64, 2375–2386 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, Pinto G, Pagnier A, Bodemer C, Bodaghi B, Tardieu M, Prieur AM, Quartier P, Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis and rheumatism 62, 258–267 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Rigante D, Leone A, Marrocco R, Laino ME, Stabile A, Long-term response after 6-year treatment with anakinra and onset of focal bone erosion in neonatal-onset multisystem inflammatory disease (NOMID/CINCA). Rheumatology international 31, 1661–1664 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, Luo X, Lou X, Bai Y, Wang J, Hollingsworth LR, Magupalli VG, Zhao L, Luo HR, Kim J, Lieberman J, Wu H, FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature immunology 21, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng W, Yang Z, Yue H, Ou Y, Hu W, Sun P, Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free radical biology & medicine 152, 8–17 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, Soroosh P, Watford WT, O’Shea JJ, Kastner DL, Hoffman HM, Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–887 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snouwaert JN, Nguyen M, Repenning PW, Dye R, Livingston EW, Kovarova M, Moy SS, Brigman BE, Bateman TA, Ting JP, Koller BH, An NLRP3 Mutation Causes Arthropathy and Osteoporosis in Humanized Mice. Cell reports 17, 3077–3088 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Garnish SE, Meng Y, Koide A, Sandow JJ, Denbaum E, Jacobsen AV, Yeung W, Samson AL, Horne CR, Fitzgibbon C, Young SN, Smith PPC, Webb AI, Petrie EJ, Hildebrand JM, Kannan N, Czabotar PE, Koide S, Murphy JM, Conformational interconversion of MLKL and disengagement from RIPK3 precede cell death by necroptosis. Nature communications 12, 2211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X, Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Taabazuing CY, Okondo MC, Bachovchin DA, Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell chemical biology 24, 507–514.e504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, Buján S, Couillin I, Brough D, Arostegui JI, Pelegrín P, The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nature immunology 15, 738–748 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmüller W, Latz E, The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nature immunology 15, 727–737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sagoo P, Garcia Z, Breart B, Lemaître F, Michonneau D, Albert ML, Levy Y, Bousso P, In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nature medicine 22, 64–71 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Mihaly SR, Ninomiya-Tsuji J, Morioka S, TAK1 control of cell death. Cell death and differentiation 21, 1667–1676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Legros H, Janin F, Dourmap N, Bonnet JJ, Costentin J, Semi-chronic increase in striatal level of 3,4-dihydroxyphenylacetaldehyde does not result in alteration of nigrostriatal dopaminergic neurones. Journal of neuroscience research 75, 429–435 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Qu C, Bonar SL, Hickman-Brecks CL, Abu-Amer S, McGeough MD, Peña CA, Broderick L, Yang C, Grimston SK, Kading J, Abu-Amer Y, Novack DV, Hoffman HM, Civitelli R, Mbalaviele G, NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29, 1269–1279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Wu Y, Yang D, Wu C, Ma C, Liu X, Moynagh PN, Wang B, Hu G, Yang S, Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. The Journal of experimental medicine 216, 2562–2581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lentz EK, Cherla RP, Jaspers V, Weeks BR, Tesh VL, Role of tumor necrosis factor alpha in disease using a mouse model of Shiga toxin-mediated renal damage. Infection and immunity 78, 3689–3699 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A, Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science (New York, N.Y.) 289, 2350–2354 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biesmans S, Bouwknecht JA, Ver Donck L, Langlois X, Acton PD, De Haes P, Davoodi N, Meert TF, Hellings N, Nuydens R, Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice. BioMed research international 2015, 716920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphey ED, Traber DL, Protective effect of tumor necrosis factor-alpha against subsequent endotoxemia in mice is mediated, in part, by interleukin-10. Critical care medicine 29, 1761–1766 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM, Indirect regulation of HMGB1 release by gasdermin D. Nature communications 11, 4561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O'Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM, NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Tschopp J, Schroder K, NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews. Immunology 10, 210–215 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Wang C, Hockerman S, Jacobsen EJ, Alippe Y, Selness SR, Hope HR, Hirsch JL, Mnich SJ, Saabye MJ, Hood WF, Bonar SL, Abu-Amer Y, Haimovich A, Hoffman HM, Monahan JB, Mbalaviele G, Selective inhibition of the p38α MAPK-MK2 axis inhibits inflammatory cues including inflammasome priming signals. The Journal of experimental medicine 215, 1315–1325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeshita S, Kaji K, Kudo A, Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 15, 1477–1488 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.