Abstract
Reflectance confocal microscopy (RCM) is a non-invasive imaging method designed to identify various skin diseases. Confocal based diagnosis may be subjective due to the learning curve of the method, the scarcity of training programs available for RCM, and the lack of clearly defined diagnostic criteria for all skin conditions. Given that in vivo RCM is becoming more widely used in dermatology, numerous deep learning technologies have been developed in recent years to provide a more objective approach to RCM image analysis. Machine learning-based algorithms are used in RCM image quality assessment to reduce the number of artifacts the operator has to view, shorten evaluation times, and decrease the number of patient visits to the clinic. However, the current visual method for identifying the dermal-epidermal junction (DEJ) in RCM images is subjective, and there is a lot of variation. The delineation of DEJ on RCM images could be automated through artificial intelligence, saving time and assisting novice RCM users in studying the key DEJ morphological structure. The purpose of this paper is to supply a current summary of machine learning and artificial intelligence’s impact on the quality control of RCM images, key morphological structures identification, and detection of different skin lesion types on static RCM images.
Keywords: dermatology, dermoscopy, in vivo, confocal microscopy, deep learning, artificial intelligence, skin cancer, artifact
1. Introduction
In vivo reflectance confocal microscopy (RCM) has demonstrated, time and again, to be an important imaging tool for dermatologists. This technology offers a non-invasive view of the skin structure similar to histopathology, revealing tissue architecture and individual cells [1,2] down to about 200–250 μm from the skin surface [3]. Even though, at the moment, dermoscopy is the most widespread method for skin cancer screening, RCM has demonstrated significantly higher diagnostic accuracy in several skin tumors [1,4,5,6,7]. RCM has a sensitivity and specificity of more than 70% for identifying skin cancers [8,9,10,11,12,13,14,15], as well as for diagnosing and monitoring the evolution of inflammatory or other skin diseases [8,16]. RCM has also been used for lateral excision margins mapping before the surgical treatment of skin tumors. This technique increases the odds for complete tumor excision, lowering the recurrence rate [17]. Furthermore, when dermoscopy is used in conjunction with RCM, the number of unnecessary biopsies can be reduced [7].
RCM is a suitable bridge between dermoscopy and histopathology, allowing clinicians to do bedside, non-invasive, virtual skin biopsies in real-time [18]. Depending on the source of contrast, imaging can be done with fluorescence or reflectance method. Ex vivo confocal microscopy, in its recently improved version, which includes digital staining, has successfully been used to guide Mohs surgery [19].
RCM images may be difficult to interpret because, unlike dermoscopy and histopathology, there are fewer training programs available for RCM. This method is not included in the regular dermatology training curriculum. Coupled with the morphological nature of RCM, this may lead to subjective variability in confocal-based diagnoses.
Research has shown that a well-coached physician can identify basal cell carcinomas with 92–100% sensitivity and 85–97% specificity [9,12,14,17], and melanomas with 88–92% sensitivity and 70–84% specificity [10], using in vivo RCM imaging. However, those very high figures, can be attained only by doctors who have used this device for five to ten years and have trained to interpret these images efficiently. Several deep learning algorithms have been developed over the last five years to assess RCM images quantitatively [9,20,21].
Machine learning-based algorithms, it is hypothesized, could offer a more quantitative, objective approach [22]. Computer image examination tools for skin lesion monitoring were previously used with dermoscopy images and dermatopathology slides to improve diagnostic accuracy [4,23,24]. Without requiring the clinician’s input, automated diagnostic tools report a score representing observed malignant potential [4]. Several algorithms, including image segmentation, statistical techniques, and artificial intelligence, have been used [8].
This paper aims to show a more detailed overview of the machine learning-based algorithms used for RCM image analysis and diagnostic discrimination. The review brings together the different concerns of confocal image analysis addressed through machine learning, the methods used so far, and offers a clear view of the path ahead.
2. Materials and Methods
A Google Scholar and PubMed search was performed on 10 July 2021 to identify published reviews, clinical trials, and letters to the Editor related to AI use and RCM, without enforcing a time period. The adopted search strategy was identical for both databases and implemented using the keywords: “confocal microscopy”, “artificial intelligence”, “skin”, and “machine learning”. Thus, the authors gathered the latest data reported in the literature about the influence of machine learning algorithms on the RCM images quality control, key morphological structures identification, and detection of different skin lesion types on static RCM images.
3. Results
3.1. Quality Assessment of Reflectance Confocal Microscopy Composite Images (Mosaics)
Even though in vivo RCM is becoming more common in modern dermatological practice, there are instances when the diagnosis is not immediate and the images are evaluated after the patient has left the office. In these cases, the patient must return to the clinic if additional imaging is required, to wit, due to poor quality images being recorded.
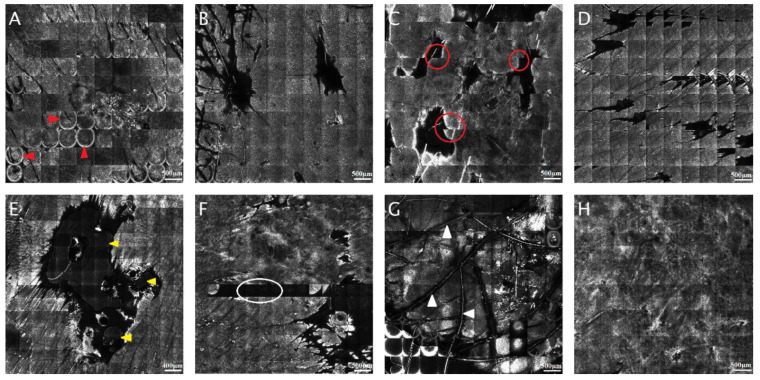
The most frequent artifacts seen in RCM images are relics related to reflection on the surface. That can appear in RCM mosaics due to the presence of corneal layer reflection in the form of hyper-reactive rings, or it can occur as a lighter contour of every single RCM mosaic compared with the rest of the image. Another type of artifact that shows the shifting and misalignment of sole RCM mosaics can be induced by the person’s movement, respiratory movements, talking, or even the heart beating. In the case of papules or nodules, bright or dark relicts appear in the region where the microscope and the skin are not touching [25]. In addition, in some RCM mosaics, air and oil bubbles can be seen commonly stuck by skin creases, hair follicles, or surface unevenness throughout the investigation. Occasionally, several individual images of RCM mosaics are underexposed or completely clear. Sometimes can appear changes in contrast due to the microscope’s automatic intensity control and several curved lines traversing the image, representing hair fibers. These artifacts are widespread, but, as long as they do not affect the entire mosaic rendering it unreadable, the images can still be helpful. Figure 1 shows the most common artifacts that appear in RCM mosaics.
Figure 1.
The most common reflectance confocal microscopy (RCM) mosaic artifacts. (A) Circular hyper-reflective rings caused by corneal layer reflection (red arrowheads). (B) Bright contour of each RCM picture (5 × 5 mm). (C) Sliding of single RCM images (5 × 5 mm); notice how there is no proper alignment of the images on different rows (red circles). (D) Repeating images in the RCM mosaic (5 × 5 mm). (E) Air and oil bubbles (yellow arrowheads). (F) Black individual images (white circle) without useful information in the RCM mosaic (5 × 5 mm). (G) Hair and bright relics (white arrowheads). (H) Correctly captured RCM mosaic. (RCM images courtesy of M.L.).
To prevent this from happening, layers of quality assurance are critical in the clinical setting, at the moment of acquisition to obtain good pictures, as well as at the moment of analysis, to reflect the chances of a good specimen [26]. Automated RCM image quality assessment could also play a major role in ensuring a more streamlined workflow, as the examiner can go through images and cases at a faster pace if mosaics are of consistently good quality. Moreover, less experienced RCM users would also benefit from only looking at high-quality confocal mosaics. This would facilitate recognition of structures and criteria, thus speeding up their learning process.
Bozkurt et al. [27] illustrated the use of the MUNet network to segment RCM images into morphological patterns. This architecture can be comprised of several nested U-Net networks. U-Net is a convolutional neural network (CNN) designed exclusively for segmenting medical pictures. This system relies entirely on a CNN, but its structure has been adapted to perform with a reduced number of pictures and output accurate segmentation [28,29]. In this study, the authors assessed and categorized images of melanocytic skin lesions obtained at the dermal-epidermal junction (DEJ) for six patterns: background, artifacts, meshwork pattern, ringed pattern, clod pattern, and non-specific pattern. The proposed algorithm’s sensitivity and specificity for artifact detection were 79.16% and 97.44%, respectively. Although this outcome appears encouraging, preparing the network entails using a training set as underlying data.
In another research by Kose et al. [26], an identical network was used to design a method for automatically evaluating the quality of RCM mosaics. To recognize and measure unhelpful areas of RCM mosaics, the authors agreed to use the RCM image and the corresponding dermoscopy images. They categorized artifacts, as well as regions outside of lesions, as uninformative areas. The model’s sensitivity was 82% and its specificity was 93%. This research showed that image analysis based on machine learning technology could reliably detect unclear regions in RCM images. It also inferred that implementing machine learning-based picture quality evaluation could be valuable for creating quality requirements, as well for workflow streamlining. This would be achieved firstly during image capture, lowering patient recall due to poor quality images being recorded, and, secondly, during picture analysis, to aid in determining the probability of non-representative selection.
At a later date, a multi-scale MED-Net neural network was presented as an improvement of the MUNet idea by Kose et al. [30]. The MED-Net algorithm was designed to differentiate diagnostically relevant patterns (e.g., meshwork, ring, nested, non-specific) in RCM images at the DEJ of melanocytic lesions from non-lesional and diagnostically uninformative areas. The network was used to divide RCM mosaics into six categories, one of which was artifacts. The system achieved an artifact detection sensitivity of 83% and a specificity of 92%. Although this outcome was somewhat satisfactory, the network still required expert data labeling. Table 1 summarizes the sensitivities and specificities of the various techniques used to identify artifacts in RCM images.
Table 1.
Artifact detection in RCM images using various methods of AI.
| Study | Sensitivity | Specificity |
|---|---|---|
| Bozkurt et al. [27] | 76.16% | 97.44% |
| Kose et al. [26] | 82% | 93% |
| Kose et al. [30] | 83% | 92% |
RCM: reflectance confocal microscopy; AI: artificial intelligence.
Based on a carefully monitored end-to-end machine learning technique, Wodzinski et al. [31] developed a method for evaluating if a provided RCM image has a reasonable quality for subsequent lesion classification. In this deep learning method, all of the different parts were trained simultaneously, rather than sequentially. This study presented an attention-based network to handle the challenge of high-resolution imagery while requiring a tiny group of network variables and minimal inferential complexity. The approach worked across a wide range of skin diseases and represented an important element in the diagnostic process. It could also help collect information directly by notifying the operator about image quality instantaneously throughout the evaluation. The accuracy on the test set was greater than 87% [31].
Although ensuring the quality of acquired RCM images is paramount for diagnostic precision and workflow optimization, assisted interpretation tools, such as automatic, ideally real-time, identification of specific landmarks and structures, are welcome additions. These tools have been in research for some time, closely following in the footsteps of previous research focused on automated analysis of dermoscopy and histopathology images.
3.2. Automated Delineation of the Dermal-Epidermal Junction
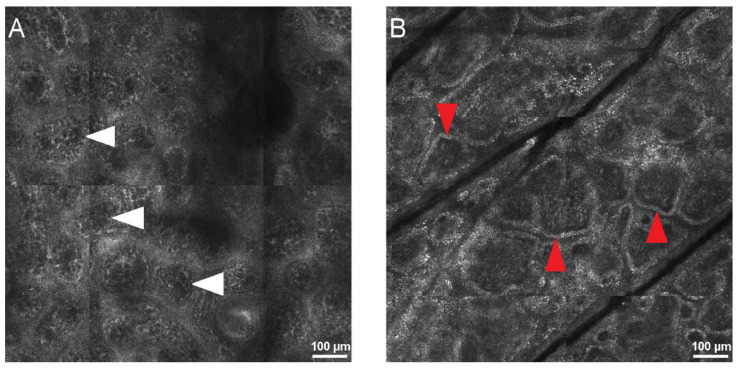
DEJ identification during RCM image analysis is important for diagnosing both skin lesions, such as melanoma or melanoma in situ, type lentigo maligna, and inflammatory skin, such as lupus erythematosus, lichen planus, bullous diseases, and psoriasis. The visual method for identifying the DEJ in RCM images is subject to interpretation, resulting in a wide range of outcomes [22]. Furthermore, while it is easily detected in darker phototypes, the DEJ can be difficult to identify in fair skin and hypo- or depigmented lesions (Figure 2). Techniques based on artificial intelligence can supply an extra analytical view and perform a preliminary examination of acquired RCM images.
Figure 2.
Confocal aspect of the normal dermo-epidermal junction (DEJ) in human skin. (A) RCM mosaic (800 × 800 µm) at the DEJ in Fitzpatrick phototype I skin, showing dermal papillae that are not defined by hyper-reflective rings (white arrowheads). (B) RCM image (800 × 800 µm) at DEJ in Fitzpatrick phototype III skin, revealing bright rings around dermal papillae (red arrowheads) determining the ringed papillae pattern. RCM images courtesy of M.L.
In a paper from 2015, two methods that can autonomously demarcate the DEJ in RCM images of regular skin were tested [22]. To adjust for misalignment caused by patient motion during imaging, this automated approach began by recording the images in every stack sideway. Because these methods depend on changes in regional tile-specific qualities with depth, this step was critical. The technique generated a mean error of 7.9 ± 6.4 μm in a dark complexion, where the contrast was high due to melanin. The algorithm defined the DEJ as a passing area in fair skin, with a median error of 8.3 ± 5.8 μm for the epidermis-to-passing zone limit and 7.6 ± 5.6 μm for the passing zone-to-dermis edge.
Kurugol et al. [32] introduced a hybrid segmentation/classification technique that divides z-stack tiles into uniform segments by adapting the model to dynamic layer patterns, then classify tile segments as epidermis, dermis, and DEJ by structural features. To achieve this, designs from three fields were combined: texture segmentation, pattern classification, and sequence segmentation. The research looked at two various learning circumstances: one for using the same stack for training and testing, and the second for training on one designated stack and testing on a different subject with comparable skin type. The DEJ is distinguishable in both situations with epidermis/dermis misdiagnosis rates below 10% and a mean range from expert-labeled limits of around 8.5 μm.
High melanin backscatter induces the pigmented cells located over the DEJ to have a lighter appearance in hyperpigmented skin. The algorithm operated on small regions to find the peaks of each tile’s smoothed average intensity depth profile to pinpoint these bright areas. The results revealed that this method properly identified the skin phototypes for all 17 examined images. The DEJ recognition algorithm obtained a mean length from the underlying data DEJ of approximately 4.7 μm for dark complexion and nearly 7–14 μm for pale skin [33].
Kose et al. [21] presented a deep learning model that can mimic a clinician’s subjective and graphic process of examining RCM images of the DEJ. The mosaics were divided into regional processing spaces, and the algorithm identified the textural appearance of each space using dense Speeded Up Robust Feature (SURF). These features were used to teach a support vector machine (SVM) classification model to differentiate among meshwork, ring, clod, specific, and background patterns in different skin lesions. The outcomes of 20 RCM images classified by a specialist in RCM reading demonstrated that these patterns could be classified with 55–81% sensitivity and 81–89% specificity.
Hames et al. [34] reported an algorithm for learning a per-pixel fragmentation of a full-depth RCM image with poor surveillance using labels for the whole en-face segments. This algorithm was created and evaluated on a set of data with a total of 16,144 en-face segments. This method correctly identified 85.7% of the testing dataset, and it was capable of recognizing underlying modifications in skin layers depths of aged skin.
3.3. Convolutional Neural Networks and Classification and Regression Trees for Skin Lesion Identification on Static RCM Images
A unique class of algorithms, defined as a neural network, and, more particularly, a version identified as a deep neural network, has been used in most recent advances in diagnostics. Small computer programs called neural networks take in pieces of information and analyze them to produce hypotheses. Neural networks are designed for a specific function utilizing examples with default results. The neural networks then make predictions relying on these samples, which are then assessed for accuracy. The convolutional neural network is the most commonly used deep neural network model for image examination [35].
Artificial intelligence algorithms, such as CNN, have been previously used successfully in dermatology for image analysis [35] and have shown particular promise in medical image classification [31,36,37].
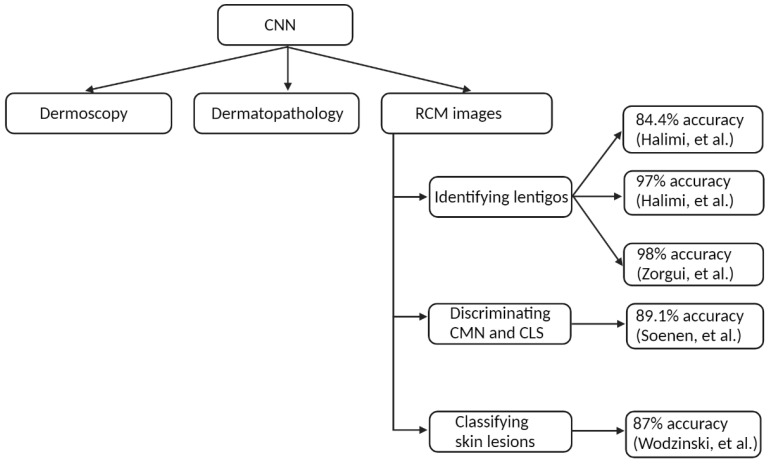
In dermatology, CNN has been extensively tested, with varying degrees of success, to analyze dermoscopy images [38,39,40,41,42,43] and digitized slides in dermatopathology [35,44,45,46,47]. As a natural progression, interest grew in the utility of deep learning in RCM image analysis (Figure 3).
Figure 3.
The use of convolutional neural networks in dermatology. CNN: convolutional neural network; RCM: reflectance confocal microscopy; CMN: congenital melanocytic nevus; CLS: café au lait spot [36,48,49,50,51].
For example, one study [48] aimed to identify and characterize lentigos. RCM images are high-resolution images taken at various skin depths. In this study, the researchers used a double wavelet decomposition technique. The wavelet coefficients were then subjected to quantitative analysis with the help of a SVM to sort RCM pictures into lentigo or clear skin, with an accuracy of 84.4%.
A novel Bayesian technique for both restoration and categorization of RCM pictures was reported by Halimi et al. [49]. This method that distinguished clear skin from lentigo had a 97% accuracy rate. In this paper, the authors offered two RCM lentigo detection approaches that have proven to be complex and difficult to implement in the past, needing manual processes, such as feature selection and data preparation.
Zorgui et al. [36] suggest a novel 3D RCM picture recognition approach for lentigo diagnosis. A CNN InceptionV3 architecture was used to create the technique [52]. The InceptionV3 is a complex and highly developed network with solutions that improve precision and speed without stacking countless layers. This network was trained on 374 pictures and then tested on 54 images. RCM images were classified into healthy and lentigo skin with 98% accuracy. The proposed CNN method has enormous potential and yields very promising results.
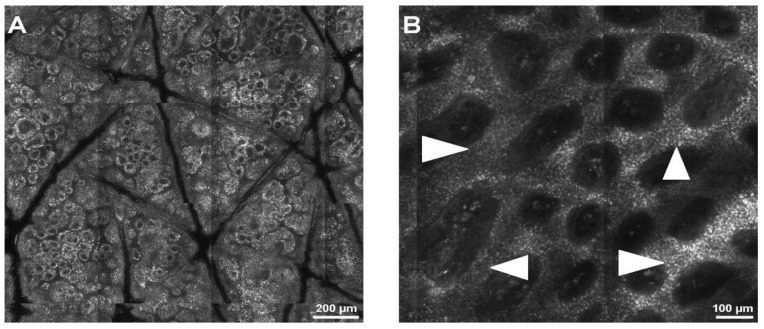
Halimi et al. [53] described a low-complexity recognition technique to determine the skin profoundness where a lentigo might be identified. This approach decomposed the image acquired for each skin layer into multiple resolutions. This method was tested on 45 patients with normal skin and with lentigos from clinical research (2250 images in total), yielding a sensitivity of 81.4% and a specificity of 83.3%. The findings revealed that lentigo could be found at depths of 50 to 60 μm, which corresponds to the DEJ’s average location (Figure 4).
Figure 4.
Normal skin and lentigo appearance during RCM examination. (A) RCM image (1.5 × 1.5 mm) at the dermal-epidermal junction (DEJ), showing normal skin architecture. (B) RCM mosaic (1 × 1 mm) at the DEJ, displaying a lentiginous pattern through thickening of the inter-papillary spaces (white arrowheads), an aspect typical for lentigo. RCM images courtesy of M.L.
Soenen et al. [50] studied the differences between the several types of congenital pigmented macules using RCM images and machine learning techniques. Three experts evaluated the images in a blinded manner. Then, using RCM images at the level of the DEJ, a pre-trained CNN combined with a SVM algorithm was used to identify café au lait spots and congenital melanocytic nevi. Café au lait spots could be distinguished from congenital melanocytic nevi based on unique features on RCM pictures, according to the results. Machine learning can aid pattern recognition and improve the accuracy rate in congenital melanocytic nevus differentiation on RCM images. Furthermore, RCM proves to be a valuable non-invasive diagnostic method in challenging cases and in patients with congenital melanocytic nevi with equivocal dermatoscopic appearances.
Wodzinski et al. [51] proposed a method for classifying skin lesions using RCM mosaics based on a CNN. The ResNet architecture was used to build the proposed network. First, the network was trained on the ImageNet dataset. The dataset contained 429 RCM mosaics grouped into three categories: melanoma, trichoblastic/basal cell carcinoma, and melanocytic nevi (Figure 5), with the diagnosis validated through histology evaluation. The detection precision of the test set was 87%, which was significantly greater than that of participating confocal users. These findings suggest that the presented categorization method could be a helpful technique for noninvasively detecting beginning melanoma.
Figure 5.
Confocal aspect of different skin tumors in reflectance confocal microscopy (RCM) images. (A) RCM mosaic (2 × 2 mm) at the dermal-epidermal junction (DEJ), showing the meshwork pattern of a benign melanocytic nevus. (B) RCM mosaic (2 × 2 mm) at DEJ, showing large, hyper-reflective tumor islands (white arrowheads) connected to the epidermis through tumor cords in a pigmented, superficial basal cell carcinoma. (C) RCM mosaic (2.5 × 2.5 mm), showing complete disarray of the DEJ and massive infiltration by large, round, atypical cells (red arrowheads) in cutaneous melanoma. RCM images courtesy of M.L.
The use of artificial intelligence techniques for evaluating different melanocytic skin lesions on RCM images was investigated by Koller et al. [1]. The ‘Interactive Data Language’ software tool created the image analysis procedure. Features of image analytics were based on transforming the wavelet. Salford Systems’ CART (Classification and Regression Trees) analytic software executed the classification method. CART begins by growing the greatest tree feasible for each tree, then cuts the parts of the tree that do not play an important role in the precision rate up to the base node. The teaching sequence was utilized to create a classification tree initially. The unbiased data was then used to confirm the obtained tree model in a second stage. CART accurately labeled 93.60% of the melanoma and 90.40% of the nevi pictures in the training group. When the whole dataset of pictures was used, the CART tree identified 58.48% of the melanoma and 48.51% of the nevi. The classification achievement of a blinded, objective, and neutral observator extremely skilled in RCM was also studied in all the grouping method test images. The independent clinical dermatologist correctly classified 78.95% of the melanocytic lesions (62.50% of the melanoma and 84.50% of the moles). Future research could benefit from these computerized RCM image recognition techniques [1].
Kaur et al. [54] showed that a CNN trained on a medium-sized image database has poor accuracy. They presented a hybrid deep learning method that trains a deep neural network using traditional text-based feature vectors as input. The dataset contained 1500 images from 15 RCM stacks representing six separate skin layer components. The study showed that this hybrid deep learning approach outperforms CNN in terms of test accuracy, with a score of 82% versus 51% for CNN. They also compared their results to other RCM image recognition algorithms and found this method more accurate.
4. Discussion
Proper analysis of biomedical images is an important element in the diagnostic process of many illnesses recognized through certain images, such as histopathology and radiology [55]. Multiple non-invasive methods, such as RCM, optical coherence tomography (OCT), non-linear optical (NLO) microscopy, multiphoton microscopy (MPM), and dermoscopy, are previously used in dermatology to facilitate the diagnosis of different skin conditions [56].
Compared to microscopy techniques, OCT has the advantage of deeper penetration (several hundred microns), but it is still limited in evaluating cellular morphologic features. These techniques use different contrast modes to identify specific molecular compounds in images [57]. Unlike RCM, NLO penetrates more profound and can distinguish between different elements of the dermal connective tissue [58]. In comparison to RCM, MPM techniques can successfully identify morphological, structural, and even chemical information of both skin cells and extracellular matrix without external labeling. The vascular structures can also be easily observed using fluorescence labeling [59]. These novel techniques are used to assess transdermal drug delivery, disease diagnosis, cancer diagnostic, extracellular matrix abnormality, and hair follicle pathology [60]. In recent years, many studies on lesion diagnosis using dermoscopy images and AI showed a better performance for deep learning methods than dermatologist diagnostic accuracy [61]. The use of dermoscopy in conjunction with RCM could improve the diagnostic accuracy of melanocytic lesions, demonstrating the value of complementary techniques [62,63].
Other non-invasive diagnostic methods used in dermatology other than RCM can see the general architecture of the skin and penetrate three times more profound into the skin than RCM. RCM, on the other hand, provides higher resolution images that can identify different individual cells and their characteristics with three times greater detail. These higher resolution images aid machine-learning algorithms in identifying various skin cells and diagnosing skin disease [64,65,66].
RCM is a promising non-invasive imaging technique that allows the assessment of histological skin and lesion details at the epidermal and upper-dermal levels, thus lowering the need for conventional skin biopsies. Lately, many artificial intelligence algorithms have been developed to provide a more objective approach to RCM image reading. This effort is much needed due to several factors contributing to a relatively high degree of subjectivity and inter-observer variability regarding sensitivity, specificity, and diagnostic accuracy associated with RCM. Machine learning techniques provide great developments, alternatives, and assistance to help dermatologists improve their daily practice.
To date, applications of AI in RCM image analysis have mostly been used to assess the quality of RCM mosaics, identify the DEJ, and help identify and discriminate between different cutaneous tumors. The algorithm types and their utility have been summarized in Table 2.
Table 2.
Artificial intelligence algorithms for reflectance confocal microscopy image analysis.
| Method | Result | Accuracy/Sensitivity and Specificity | |
|---|---|---|---|
| Quality assessment of RCM mosaics | MUNet network MED-Net neural network |
Differentiate diagnostically relevant patterns (meshwork, ring, nested, artifact). | 73% accuracy [27] |
| Estimate the quality of RCM composite images (mosaics). | 87% accuracy [31] | ||
| Automated identification of the DEJ | Texture segmentation, pattern classification, and sequence segmentation | Tile classification for epidermis and dermis in dark skin | 90% accuracy [22] |
| Identifying epidermis | 90% accuracy [32] | ||
| Identifying dermis | 76% accuracy [32] | ||
| SVM classification model | Classifying meshwork, ring, clod, specific, and background patterns | 55–81% sensitivity and 81–89% specificity [21] | |
| Per-pixel segmentation | Identifying DEJ | 85.7% accuracy [34] | |
| Skin lesion identification | CNN | Identifying lentigos | −81.4% sensitivity and 88.8% specificity [48] −96.2% sensitivity and 100% specificity [49] −81.4% sensitivity and 83.3% specificity [51] |
| CART | Identifying melanoma | 93.6% accuracy [1] | |
| Identifying nevi | 90.4% accuracy [1] |
RCM: reflectance confocal microscopy; SVM: support vector machine; DEJ: dermal-epidermal junction; CNN: convolutional neural network; CART: classification and regression trees.
Quality assessment of RCM images is very important for lowering the number of artifacts, shortening evaluation times as the RCM reader does not waste time accessing irrelevant images, as well as reducing the number of patient visits to the clinic due to insufficient or low-quality images. Regarding this, the progress made so far has been promising, with the sole caveat that the networks still require expert data labeling.
It is a well-known fact for confocal users that pinpointing the DEJ on dark skin is simple due to the significant hyper-reflectivity of basal keratinocytes. At the same time, this can prove a difficult task in fair skin due to the lack of the natural source of contrast, melanin. The algorithms proposed in studies directed at the automated DEJ delineation on RCM images could be a valuable training instrument to aid novice RCM readers in learning the key morphological patterns of the dermal-epidermal junction, thus making RCM a more accessible and widely embraced technology in dermatology clinics. Furthermore, this research suggests that automated algorithms could increase objectivity during real-time in vivo RCM examination by quantitatively guiding the DEJ delineation. The advancement of such algorithms could help evaluate unusual morphological features, especially ones located at the DEJ.
All things considered, there are still gaps in artificial intelligence-based RCM image analysis. Clinical studies that demonstrate the value of AI-based RCM image analysis, along with huge sequence differentiating judgment stages, are still lacking.
Several aspects still need to be improved since there is hardly any data on the achievement of AI in identifying atypical melanocytic lesions, such as those on the scalp or mucosal surfaces. The hair follicle, vessels, and air bubbles are all limitations in the segmentation methods, while acral areas have not been thoroughly assessed yet. Additionally, these techniques still do not include amelanotic melanomas.
In addition, the risk of “deskilling” the dermatologist needs to be addressed. Who will be held accountable for the assessment: the doctor or the brand that created the device? What will be done to establish specificity, and what actions are needed to recognize amelanotic tumors?
5. Conclusions
Research showed that most of these artificial intelligence technologies already had reached clinician-level precision in the identification of various skin diseases, and, in some studies, machine accuracy even surpassed them. Thus, further research into the automated RCM image analysis process appears promising. Yet, existing algorithms cannot be used for routine skin cancer screening at this time. As a result, developing a systematic imaging procedure may help to improve the outcomes. Automated image analysis systems provide clinicians with objective and speedy support, while not interfering with the diagnostic process. However, more studies are needed to improve the applicability of automated RCM image analysis in the daily activities of confocalists. Automated image analysis systems may provide great assistance in the decision-making process of RCM examiners looking at skin tumors in the future.
These algorithms need to be further developed and thoroughly validated through large randomized controlled trials. This would lead to improved patient care and safety and an enhancement in dermatologists’ productivity.
Acknowledgments
We would like to thank the Foundation for Cellular and Molecular Medicine for their continuous trust, support and involvement in this research.
Author Contributions
A.M.M., V.M.V. and M.L. contributed to the conception of this paper and performed the preliminary research. All authors participated in the design and structure. A.M.M. and M.L. participated in drafting the manuscript. M.L. and V.M.V. critically revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koller S., Wiltgen M., Ahlgrimm-Siess V., Weger W., Hofmann-Wellenhof R., Richtig E., Smolle J., Gerger A. In vivo reflectance confocal microscopy: Automated diagnostic image analysis of melanocytic skin tumours. J. Eur. Acad. Dermatol. Venereol. 2010;25:554–558. doi: 10.1111/j.1468-3083.2010.03834.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajadhyaksha M., González S., Zavislan J.M., Anderson R.R., Webb R.H. In Vivo Confocal Scanning Laser Microscopy of Human Skin II: Advances in Instrumentation and Comparison With Histology. J. Investig. Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 3.Robic J., Nkengne A., Perret B., Couprie M., Talbot H., Pellacani G., Vie K. Clinical validation of a computer-based approach for the quantification of the skin ageing process of women using in vivo confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2021;35:e68–e70. doi: 10.1111/jdv.16810. [DOI] [PubMed] [Google Scholar]
- 4.Gerger A., Wiltgen M., Langsenlehner U., Richtig E., Horn M., Weger W., Ahlgrimm-Siess V., Hofmann-Wellenhof R., Samonigg H., Smolle J. Diagnostic image analysis of malignant melanoma inin vivoconfocal laser-scanning microscopy: A preliminary study. Ski. Res. Technol. 2008;14:359–363. doi: 10.1111/j.1600-0846.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 5.Kittler H., Pehamberger H., Wolff K., Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–165. doi: 10.1016/S1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerger A., Koller S., Weger W., Richtig E., Kerl H., Samonigg H., Krippl P., Smolle J. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 7.Pellacani G., Cesinaro A.M., Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions–improvement in melanoma diagnostic specificity. J. Am. Acad. Dermatol. 2005;53:979–985. doi: 10.1016/j.jaad.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Mehrabi J.N., Baugh E.G., Fast A., Lentsch G., Balu M., Lee B.A., Kelly K.M. A Clinical Perspective on the Automated Analysis of Reflectance Confocal Microscopy in Dermatology. Lasers Surg. Med. 2021;53:1011–1019. doi: 10.1002/lsm.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nori S., Rius-Díaz F., Cuevas J., Goldgeier M., Jaen P., Torres A., González S. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J. Am. Acad. Dermatol. 2004;51:923–930. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Pellacani G., Guitera P., Longo C., Avramidis M., Seidenari S., Menzies S. The Impact of In Vivo Reflectance Confocal Microscopy for the Diagnostic Accuracy of Melanoma and Equivocal Melanocytic Lesions. J. Investig. Dermatol. 2007;127:2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 11.Lupu M., Tebeica T., Voiculescu V.M., Ardigo M. Tubular apocrine adenoma: Dermoscopic andin vivoreflectance confocal microscopic aspects. Int. J. Dermatol. 2019;58:e210–e211. doi: 10.1111/ijd.14579. [DOI] [PubMed] [Google Scholar]
- 12.Lupu M., Popa I.M., Voiculescu V.M., Boda D., Caruntu C., Zurac S., Giurcaneanu C. A Retrospective Study of the Diagnostic Accuracy of In Vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019;8:449. doi: 10.3390/jcm8040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupu M., Caruntu A., Boda D., Caruntu C. In Vivo Reflectance Confocal Microscopy-Diagnostic Criteria for Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. J. Clin. Med. 2020;9:1987. doi: 10.3390/jcm9061987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupu M., Popa I.M., Voiculescu V.M., Caruntu C. A Systematic Review and Meta-Analysis of the Accuracy of in VivoReflectance Confocal Microscopy for the Diagnosis of Primary Basal Cell Carcinoma. J. Clin. Med. 2019;8:1462. doi: 10.3390/jcm8091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupu M., Caruntu A., Caruntu C., Boda D., Moraru L., Voiculescu V., Bastian A. Non-invasive imaging of actinic cheilitis and squamous cell carcinoma of the lip. Mol. Clin. Oncol. 2018;8:640–646. doi: 10.3892/mco.2018.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupu M., Voiculescu V.M., Vajaitu C., Orzan O.A. In vivo reflectance confocal microscopy for the diagnosis of scabies. BMJ Case Rep. 2021;14:e240507. doi: 10.1136/bcr-2020-240507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupu M., Voiculescu V., Caruntu A., Tebeica T., Caruntu C. Preoperative Evaluation through Dermoscopy and Reflectance Confocal Microscopy of the Lateral Excision Margins for Primary Basal Cell Carcinoma. Diagnostics. 2021;11:120. doi: 10.3390/diagnostics11010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ianoși S.L., Forsea A.M., Lupu M., Ilie M.A., Zurac S., Boda D., Ianosi G., Neagoe D., Tutunaru C., Popa C.M., et al. Role of modern imaging techniques for the in vivo diagnosis of lichen planus. Experimental and therapeutic medicine. Exp. Ther. Med. 2018;17:1052–1060. doi: 10.3892/etm.2018.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilie M.A., Caruntu C., Lupu M., Lixandru D., Tampa M., Georgescu S.-R., Bastian A., Constantin C., Neagu M., Zurac S.A., et al. Current and future applications of confocal laser scanning microscopy imaging in skin oncology (Review) Oncol. Lett. 2019;17:4102–4111. doi: 10.3892/ol.2019.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guitera P., Menzies S.W., Longo C., Cesinaro A.M., Scolyer R.A., Pellacani G. In Vivo Confocal Microscopy for Diagnosis of Melanoma and Basal Cell Carcinoma Using a Two-Step Method: Analysis of 710 Consecutive Clinically Equivocal Cases. J. Investig. Dermatol. 2012;132:2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 21.Kose K., Alessi-Fox C., Gill M., Dy J.G., Brooks D.H., Rajadhyaksha M. Photonic Therapeutics and Diagnostics XII. Volume 9689. International Society for Optics and Photonics; Bellingham, WA, USA: 2016. A machine learning method for identifying morphological patterns in reflectance confocal microscopy mosaics of melanocytic skin lesions in-vivo; p. 968908. [DOI] [Google Scholar]
- 22.Kurugol S., Kose K., Park B., Dy J.G., Brooks D.H., Rajadhyaksha M. Automated Delineation of Dermal–Epidermal Junction in Reflectance Confocal Microscopy Image Stacks of Human Skin. J. Investig. Dermatol. 2015;135:710–717. doi: 10.1038/jid.2014.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkol B., Moss R.H., Stanley R.J., Stoecker W.V., Hvatum E. Automatic lesion boundary detection in dermoscopy images using gradient vector flow snakes. Ski. Res. Technol. 2005;11:17–26. doi: 10.1111/j.1600-0846.2005.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She Z., Liu Y., Damatoa A. Combination of features from skin pattern and ABCD analysis for lesion classification. Ski. Res. Technol. 2007;13:25–33. doi: 10.1111/j.1600-0846.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 25.Wurm E.M.T., Kolm I., Ahlgrimm-Siess V. Reflectance Confocal Microscopy for Skin Diseases. Springer Science and Business Media LLC; Berlin/Heidelberg, Germany: 2011. A Hands-on Guide to Confocal Imaging; pp. 11–19. [Google Scholar]
- 26.Kose K., Bozkurt A., Alessi-Fox C., Brooks D.H., Dy J.G., Rajadhyaksha M., Gill M. Utilizing Machine Learning for Image Quality Assessment for Reflectance Confocal Microscopy. J. Investig. Dermatol. 2020;140:1214–1222. doi: 10.1016/j.jid.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt A., Kose K., Alessi-Fox C., Gill M., Dy J., Brooks D., Rajadhyaksha M. Medical Image Computing and Computer Assisted Intervention–MICCAI 2018, Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Granada, Spain, 16–20 September 2018. Volume 11071. Springer; Cham, Switzerland: 2018. A multiresolution convolutional neural network with partial label training for annotating reflectance confocal microscopy images of skin; pp. 292–299. [Google Scholar]
- 28.Ronneberger O., Fischer P., Brox T. U-Net: Convolutional networks for biomedical image segmentation. arXiv. 20151505.04597 [Google Scholar]
- 29.Shelhamer E., Long J., Darrell T. Fully Convolutional Networks for Semantic Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017;39:640–651. doi: 10.1109/TPAMI.2016.2572683. [DOI] [PubMed] [Google Scholar]
- 30.Kose K., Bozkurt A., Alessi-Fox C., Gill M., Longo C., Pellacani G., Dy J.G., Brooks D.H., Rajadhyaksha M. Segmentation of cellular patterns in confocal images of melanocytic lesions in vivo via a multiscale encoder-decoder network (MED-Net) Med. Image Anal. 2021;67:101841. doi: 10.1016/j.media.2020.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wodzinski M., Pajak M., Skalski A., Witkowski A., Pellacani G., Ludzik J. Automatic Quality Assessment of Reflectance Confocal Microscopy Mosaics using Attention-Based Deep Neural Network. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020;2020:1824–1827. doi: 10.1109/EMBC.2019.8856731. [DOI] [PubMed] [Google Scholar]
- 32.Kurugol S., Dy J.G., Brooks D.H., Rajadhyaksha M. Pilot study of semiautomated localization of the dermal/epidermal junction in reflectance confocal microscopy images of skin. J. Biomed. Opt. 2011;16:036005. doi: 10.1117/1.3549740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurugol S., Dy J.G., Rajadhyaksha M., Gossage K.W., Weissman J., Brooks D.H. Three-Dimensional and Multidimensional Microscopy: Image Acquisition and Processing XVIII. Volume 7904. International Society for Optics and Photonics; Bellingham, WA, USA: 2011. Semi-automated algorithm for localization of dermal/epidermal junction in reflectance confocal microscopy images of human skin; p. 79041A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hames S.C., Ardigo M., Soyer H.P., Bradley A.P., Prow T.W. Anatomical Skin Segmentation in Reflectance Confocal Microscopy with Weak Labels; Proceedings of the 2015 International Conference on Digital Image Computing: Techniques and Applications (DICTA); Adelaide, Australia. 23–25 November 2015. [Google Scholar]
- 35.Puri P., Comfere N., Drage L.A., Shamim H., Bezalel S.A., Pittelkow M.R., Davis M.D., Wang M., Mangold A.R., Tollefson M.M., et al. Deep learning for dermatologists: Part II. Current applications. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zorgui S., Chaabene S., Bouaziz B., Batatia H., Chaari L. Lecture Notes in Computer Science. Volume 12157. Springer; Singapore: 2020. A Convolutional Neural Network for Lentigo Diagnosis; pp. 89–99. [Google Scholar]
- 37.Yamashita R., Nishio M., Do R.K.G., Togashi K. Convolutional neural networks: An overview and application in radiology. Insights Imaging. 2018;9:611–629. doi: 10.1007/s13244-018-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu C., Yang S., Kim W., Jung J., Chung K.Y., Lee S.W., Oh B. Acral melanoma detection using a convolutional neural network for dermoscopy images. PLoS ONE. 2018;13:e0193321. doi: 10.1371/journal.pone.0193321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haenssle H.A., Fink C., Schneiderbauer R., Toberer F., Buhl T., Blum A., Kalloo A., Hassen A.B.H., Thomas L., Enk A., et al. Man against machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann. Oncol. 2018;29:1836–1842. doi: 10.1093/annonc/mdy166. [DOI] [PubMed] [Google Scholar]
- 40.Demyanov S., Chakravorty R., Abedini M., Halpern A., Garnavi R. Classification of dermoscopy patterns using deep convolutional neural networks; Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI); Prague, Czech Republic. 13–16 April 2016; pp. 364–368. [Google Scholar]
- 41.Brinker T.J., Hekler A., Enk A.H., Klode J., Hauschild A., Berking C., Schilling B., Haferkamp S., Schadendorf D., Fröhling S., et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur. J. Cancer. 2019;111:148–154. doi: 10.1016/j.ejca.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Winkler J.K., Fink C., Toberer F., Enk A., Deinlein T., Hofmann-Wellenhof R., Thomas L., Lallas A., Blum A., Stolz W., et al. Association Between Surgical Skin Markings in Dermoscopic Images and Diagnostic Performance of a Deep Learning Convolutional Neural Network for Melanoma Recognition. JAMA Dermatol. 2019;155:1135–1141. doi: 10.1001/jamadermatol.2019.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zafar K., Gilani S.O., Waris A., Ahmed A., Jamil M., Khan M.N., Sohail Kashif A. Skin Lesion Segmentation from Dermoscopic Images Using Convolutional Neural Network. Sensors. 2020;20:1601. doi: 10.3390/s20061601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kent M.N., Olsen T.G., Jackson B.H., Feeser T.A., Moad J.C., Krishnamurthy S., Lunsford D.D., Soans R.E. Diagnostic performance of deep learning algorithms applied to three common diagnoses in dermatopathology. J. Pathol. Inform. 2018;9:32. doi: 10.4103/jpi.jpi_31_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells A., Patel S., Lee J.B., Motaparthi K. Artificial intelligence in dermatopathology: Diagnosis, education, and research. J. Cutan. Pathol. 2021;48:1061–1068. doi: 10.1111/cup.13954. [DOI] [PubMed] [Google Scholar]
- 46.Goceri E. Convolutional Neural Network Based Desktop Applications to Classify Dermatological Diseases; Proceedings of the 2020 IEEE 4th International Conference on Image Processing, Applications and Systems (IPAS); Genova, Italy. 9–11 December 2020; pp. 138–143. [Google Scholar]
- 47.De Logu F., Ugolini F., Maio V., Simi S., Cossu A., Massi D., Nassini R., Laurino M. Italian Association for Cancer Research (AIRC) Study Group Recognition of Cutaneous Melanoma on Digitized Histopathological Slides via Artificial Intelligence Algorithm. Front. Oncol. 2020;10:1559. doi: 10.3389/fonc.2020.01559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halimi A., Batatia H., Le Digabel J., Josse G., Tourneret J.-Y. Statistical modeling and classification of reflectance confocal microscopy images; Proceedings of the 2017 IEEE 7th International Workshop on Computational Advances in Multi-Sensor Adaptive Processing (CAMSAP); Curacao, Dutch Antilles. 10–13 December 2017; pp. 1–5. [Google Scholar]
- 49.Halimi A., Batatia H., Le Digabel J., Josse G., Tourneret J.-Y. An unsupervised Bayesian approach for the joint reconstruction and classification of cutaneous reflectance confocal microscopy images; Proceedings of the 2017 25th European Signal Processing Conference (EUSIPCO); Kos, Greece. 28 August–2 September 2017; pp. 241–245. [Google Scholar]
- 50.Soenen A., Vourc’ H.M., Dréno B., Chiavérini C., Alkhalifah A., Dessomme B.K., Roussel K., Chambon S., Debarbieux S., Monnier J., et al. Diagnosis of congenital pigmented macules in infants with reflectance confocal microscopy and machine learning. J. Am. Acad. Dermatol. 2020;85:1308–1309. doi: 10.1016/j.jaad.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Wodzinski M., Skalski A., Witkowski A., Pellacani G., Ludzik J. Convolutional Neural Network Approach to Classify Skin Lesions Using Reflectance Confocal Microscopy; Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Berlin, Germany. 23–27 July 2019; pp. 4754–4757. [DOI] [PubMed] [Google Scholar]
- 52.Szegedy C., Vanhoucke V., Ioffe S., Shlens J., Wojna Z. Rethinking the inception architecture for computer vision; Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); Las Vegas, NV, USA. 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- 53.Halimi A., Batatia H., Le Digabel J., Josse G., Tourneret J.Y. Wavelet-based statistical classification of skin images acquired with reflectance confocal microscopy. Biomed. Opt. Express. 2017;8:5450–5467. doi: 10.1364/BOE.8.005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur P., Dana K.J., Cula G.O., Mack M.C. Hybrid deep learning for Reflectance Confocal Microscopy skin images; Proceedings of the 2016 23rd International Conference on Pattern Recognition (ICPR); Cancun, Mexico. 4–8 December 2016; pp. 1466–1471. [Google Scholar]
- 55.Pang S., Yu Z., Orgun M.A. A novel end-to-end classifier using domain transferred deep convolutional neural networks for biomedical images. Comput. Methods Programs Biomed. 2017;140:283–293. doi: 10.1016/j.cmpb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Tkaczyk E. Innovations and Developments in Dermatologic Non-invasive Optical Imaging and Potential Clinical Applications. Acta Derm. Venereol. 2017;218:5–13. doi: 10.2340/00015555-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balu M., Zachary C.B., Harris R.M., Krasieva T.B., König K., Tromberg B.J., Kelly K.M. In Vivo Multiphoton Microscopy of Basal Cell Carcinoma. JAMA Dermatol. 2015;151:1068–1074. doi: 10.1001/jamadermatol.2015.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiss N., Fésűs L., Bozsányi S., Szeri F., Van Gils M., Szabó V., Nagy A.I., Hidvégi B., Szipőcs R., Martin L., et al. Nonlinear optical microscopy is a novel tool for the analysis of cutaneous alterations in pseudoxanthoma elasticum. Lasers Med. Sci. 2020;35:1821–1830. doi: 10.1007/s10103-020-03027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuo S., Chen J., Jiang X., Cheng X., Xie S. Visualizing extracellular matrix and sensing fibroblasts metabolism in human dermis by nonlinear spectral imaging. Ski. Res. Technol. 2007;13:406–411. doi: 10.1111/j.1600-0846.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 60.Tsai T.-H., Jee S.-H., Dong C.-Y., Lin S.-J. Multiphoton microscopy in dermatological imaging. J. Dermatol. Sci. 2009;56:1–8. doi: 10.1016/j.jdermsci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Goyal M., Knackstedt T., Yan S., Hassanpour S. Artificial intelligence-based image classification methods for diagnosis of skin cancer: Challenges and opportunities. Comput. Biol. Med. 2020;127:104065. doi: 10.1016/j.compbiomed.2020.104065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maron R.C., Utikal J.S., Hekler A., Hauschild A., Sattler E., Sondermann W., Haferkamp S., Schilling B., Heppt M.V., Jansen P., et al. Artificial Intelligence and Its Effect on Dermatologists’ Accuracy in Dermoscopic Melanoma Image Classification: Web-Based Survey Study. J. Med. Internet Res. 2020;22:e18091. doi: 10.2196/18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tschandl P., Wiesner T. Advances in the diagnosis of pigmented skin lesions. Br. J. Dermatol. 2018;178:9–11. doi: 10.1111/bjd.16109. [DOI] [PubMed] [Google Scholar]
- 64.Boone M., Jemec G.B.E., Del Marmol V. High-definition optical coherence tomography enables visualization of individual cells in healthy skin: Comparison to reflectance confocal microscopy. Exp. Dermatol. 2012;21:740–744. doi: 10.1111/j.1600-0625.2012.01569.x. [DOI] [PubMed] [Google Scholar]
- 65.Patel D.V., McGhee C.N. Quantitative analysis of in vivo confocal microscopy images: A review. Surv. Ophthalmol. 2013;58:466–475. doi: 10.1016/j.survophthal.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Calzavara-Pinton P., Longo C., Venturini M., Sala R., Pellacani G. Reflectance Confocal Microscopy forIn VivoSkin Imaging†. Photochem. Photobiol. 2008;84:1421–1430. doi: 10.1111/j.1751-1097.2008.00443.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.