Abstract
Data from several cohorts of coronavirus disease 2019 (COVID-19) suggest that the most common comorbidities for severe COVID-19 disease are the elderly, high blood pressure, and diabetes; however, it is not currently known whether the previous use of certain drugs help or hinder recovery. This study aims to explore the association of previous hospitalisation use of medication on the mortality of COVID-19 disease. A retrospective case-control from two hospitals in Madrid, Spain, included all patients aged 18 years or above hospitalised with a diagnosis of COVID-19. A Propensity Score matching (PSM) analysis was performed. Confounding variables were considered to be age, sex, and the number of comorbidities. Finally, 3712 patients were included. Of these, 687 (18.5%) patients died (cases). The 22,446 medicine trademarks used previous to admission were classified according to the ATC, obtaining 689 final drugs; all of them were included in PSM analysis. Eleven drugs displayed a reduction in mortality: azithromycin, bemiparine, budesonide-formoterol fumarate, cefuroxime, colchicine, enoxaparin, ipratropium bromide, loratadine, mepyramine theophylline acetate, oral rehydration salts, and salbutamol sulphate. Eight final drugs displayed an increase in mortality: acetylsalicylic acid, digoxin, folic acid, mirtazapine, linagliptin, enalapril, atorvastatin, and allopurinol. Medication associated with survival (anticoagulants, antihistamines, azithromycin, bronchodilators, cefuroxime, colchicine, and inhaled corticosteroids) may be candidates for future clinical trials. Drugs associated with mortality show an interaction with the underlying conditions.
Keywords: coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hospitalisation, mortality, previous medication, risk factor, propensity score matching analysis
1. Introduction
Data from several cohorts of coronavirus disease 2019 (COVID-19) suggest that the most common comorbidities for severe COVID-19 disease are the elderly, high blood pressure, and other comorbidities; however, it is not currently known whether certain drugs help or hinder recovery [1,2,3,4,5,6,7]. There is evidence that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) binds to the cellular receptor for angiotensin-converting enzyme 2 (ACE2) to inoculate itself. The ACE2 receptor is expressed in a high percentage of lung cells. It is also visible in other extrapulmonary tissues, such as the endothelium, heart, kidney, and intestine, which casts doubt on the use of drugs that may impact the renin-angiotensin-aldosterone axis by increasing the probability of acquiring infection. Paradoxically, blocking the renin-angiotensin pathway can attenuate the damage that depends on the expression of ACE2 [8]. Likewise, the use of thiazolidinediones or nonsteroidal anti-inflammatory drugs (NSAIDs) has been associated between mortality from COVID-19 and ACE-inhibitors (ACEI) or angiotensin receptor blockers (ARB) after adjustment for a range of potential confounders [9,10]. Other studies reached similar conclusions or showed beneficial effects [11,12]. In addition, other medications could act as a protector against infection or its severity. Several reviews have proposed candidates to study in clinical trials [13,14,15]. As newer interventions will take months or years to develop, to detail the pool of existing therapeutic options, the principles behind their use to treat COVID-19, current application, and adverse effects could offer candidates for clinical trials [16].
The study aimed to explore the association of previous hospitalisation and use of medication with the mortality of patients hospitalised in the cohort of patients with COVID-19 of La Paz University Hospital and La Princesa University Hospìtal (Madrid, Spain).
2. Results
2.1. Case-Control Results
2.1.1. Characteristics of the Patients
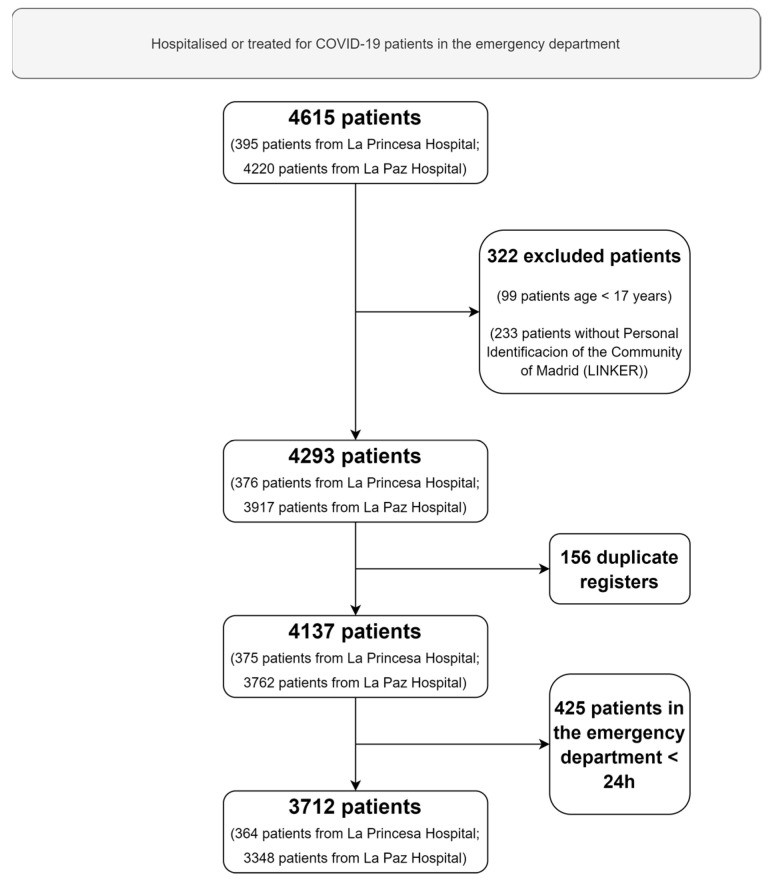
A total of 3712 patients were consecutively hospitalised or treated for COVID-19 in the emergency department for more than 24 h (Figure 1). The mean age of the patients was 62 years, and 1930 (52.0%) of the patients were women. Arterial hypertension (40.7%) followed by dyslipidemia (31.5%), chronic heart disease (18.5%), and diabetes mellitus (17.7%) were the most frequent comorbidities of the patients. Of these, 687 (18.5%) patients died (cases). Table 1 shows the characteristics of patients on admission and the complications during hospitalisation.
Figure 1.
Flow chart of patients included.
Table 1.
Characteristics on admission and complications during hospitalisation.
| [ALL] n = 3712 n (%) |
Case (Deceased) n = 687 n (%) |
Control (Live Discharges) n = 3025 n (%) |
OR | p.ratio | p.overall | |
|---|---|---|---|---|---|---|
| Sex | <0.001 | |||||
| Man | 1777 (47.9%) | 419 (61.0%) | 1358 (44.9%) | Ref. | Ref. | |
| Woman | 1930 (52.0%) | 268 (39.0%) | 1662 (54.9%) | 0.52 [0.44;0.62] | <0.001 | |
| ‘Missing’ | 5 (0.13%) | 0 (0.00%) | 5 (0.17%) | 0.00 [0.00;3.55] | 0.261 | |
| Age, years (mean (SD)) | 62.0 [48.0;78.0] | 83.0 [75.0;88.0] | 57.0 [44.0;72.0] | 1.10 [1.09;1.11] | <0.001 | <0.001 |
| Arterial hypertension | <0.001 | |||||
| No | 2190 (59.0%) | 223 (32.5%) | 1967 (65.0%) | Ref. | Ref. | |
| Yes | 1511 (40.7%) | 462 (67.2%) | 1049 (34.7%) | 3.88 [3.25;4.66] | <0.001 | |
| ‘Missing’ | 11 (0.30%) | 2 (0.29%) | 9 (0.30%) | 1.96 [0.20;9.55] | 0.406 | |
| Diabetes mellitus | <0.001 | |||||
| No | 3043 (82.0%) | 459 (66.8%) | 2584 (85.4%) | Ref. | Ref. | |
| Yes | 656 (17.7%) | 225 (32.8%) | 431 (14.2%) | 2.94 [2.42;3.56] | <0.001 | |
| ‘Missing’ | 13 (0.35%) | 3 (0.44%) | 10 (0.33%) | 1.69 [0.30;6.59] | 0.433 | |
| Non-complicated diabetes mellitus | <0.001 | |||||
| No | 3225 (86.9%) | 536 (78.0%) | 2689 (88.9%) | Ref. | Ref. | |
| Yes | 421 (11.3%) | 134 (19.5%) | 287 (9.49%) | 2.34 [1.85;2.95] | <0.001 | |
| ‘Missing’ | 66 (1.78%) | 17 (2.47%) | 49 (1.62%) | 1.74 [0.93;3.10] | 0.062 | |
| Complicated diabetes mellitus | <0.001 | |||||
| No | 3545 (95.5%) | 623 (90.7%) | 2922 (96.6%) | Ref. | Ref. | |
| Yes | 100 (2.69%) | 45 (6.55%) | 55 (1.82%) | 3.84 [2.50;5.85] | <0.001 | |
| ‘Missing’ | 67 (1.80%) | 19 (2.77%) | 48 (1.59%) | 1.86 [1.02;3.24] | 0.031 | |
| Dislipemia | <0.001 | |||||
| No | 2526 (68.0%) | 334 (48.6%) | 2192 (72.5%) | Ref. | Ref. | |
| Yes | 1168 (31.5%) | 350 (50.9%) | 818 (27.0%) | 2.81 [2.36;3.34] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 3 (0.44%) | 15 (0.50%) | 1.31 [0.24;4.67] | 0.641 | |
| Obesity | 0.001 | |||||
| No | 3190 (85.9%) | 564 (82.1%) | 2626 (86.8%) | Ref. | Ref. | |
| Yes | 459 (12.4%) | 102 (14.8%) | 357 (11.8%) | 1.33 [1.04;1.70] | 0.021 | |
| ‘Missing’ | 63 (1.70%) | 21 (3.06%) | 42 (1.39%) | 2.33 [1.30;4.06] | 0.003 | |
| Chronic heart disease | <0.001 | |||||
| No | 3007 (81.0%) | 399 (58.1%) | 2608 (86.2%) | Ref. | Ref. | |
| Yes | 686 (18.5%) | 283 (41.2%) | 403 (13.3%) | 4.59 [3.80;5.54] | <0.001 | |
| ‘Missing’ | 19 (0.51%) | 5 (0.73%) | 14 (0.46%) | 2.33 [0.65;6.90] | 0.130 | |
| Chronic lung disease | <0.001 | |||||
| No | 3475 (93.6%) | 619 (90.1%) | 2856 (94.4%) | Ref. | Ref. | |
| Yes | 216 (5.82%) | 60 (8.73%) | 156 (5.16%) | 1.77 [1.28;2.44] | <0.001 | |
| ‘Missing’ | 21 (0.57%) | 8 (1.16%) | 13 (0.43%) | 2.84 [1.02;7.42] | 0.030 | |
| Chronic obstructive pulmonary disease | <0.001 | |||||
| No | 3434 (92.5%) | 588 (85.6%) | 2846 (94.1%) | Ref. | Ref. | |
| Yes | 260 (7.00%) | 95 (13.8%) | 165 (5.45%) | 2.79 [2.11;3.67] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 4 (0.58%) | 14 (0.46%) | 1.38 [0.33;4.42] | 0.556 | |
| Asthma | 0.040 | |||||
| No | 3489 (94.0%) | 656 (95.5%) | 2833 (93.7%) | Ref. | Ref. | |
| Yes | 205 (5.52%) | 26 (3.78%) | 179 (5.92%) | 0.63 [0.40;0.96] | 0.024 | |
| ‘Missing’ | 18 (0.48%) | 5 (0.73%) | 13 (0.43%) | 1.66 [0.46;4.99] | 0.347 | |
| Neurological chronic disease | <0.001 | |||||
| No | 3331 (89.7%) | 543 (79.0%) | 2788 (92.2%) | Ref. | Ref. | |
| Yes | 363 (9.78%) | 139 (20.2%) | 224 (7.40%) | 3.18 [2.51;4.03] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 5 (0.73%) | 13 (0.43%) | 1.97 [0.55;5.93] | 0.218 | |
| Mild liver disease | 0.342 | |||||
| No | 3601 (97.0%) | 663 (96.5%) | 2938 (97.1%) | Ref. | Ref. | |
| Yes | 95 (2.56%) | 19 (2.77%) | 76 (2.51%) | 1.11 [0.63;1.87] | 0.680 | |
| ‘Missing’ | 16 (0.43%) | 5 (0.73%) | 11 (0.36%) | 2.01 [0.55;6.31] | 0.216 | |
| Moderate or severe liver disease | 0.043 | |||||
| No | 3662 (98.7%) | 671 (97.7%) | 2991 (98.9%) | Ref. | Ref. | |
| Yes | 35 (0.94%) | 11 (1.60%) | 24 (0.79%) | 2.04 [0.90;4.36] | 0.063 | |
| ‘Missing’ | 15 (0.40%) | 5 (0.73%) | 10 (0.33%) | 2.23 [0.60;7.18] | 0.167 | |
| Chronic kidney disease | <0.001 | |||||
| No | 3435 (92.5%) | 549 (79.9%) | 2886 (95.4%) | Ref. | Ref. | |
| Yes | 260 (7.00%) | 134 (19.5%) | 126 (4.17%) | 5.59 [4.27;7.31] | <0.001 | |
| ‘Missing’ | 17 (0.46%) | 4 (0.58%) | 13 (0.43%) | 1.62 [0.38;5.26] | 0.407 | |
| GF < 30: | <0.001 | |||||
| No | 137 (3.69%) | 65 (9.46%) | 72 (2.38%) | Ref. | Ref. | |
| Yes | 104 (2.80%) | 57 (8.30%) | 47 (1.55%) | 1.34 [0.78;2.31] | 0.261 | |
| ‘Missing’ | 3471 (93.5%) | 565 (82.2%) | 2906 (96.1%) | 0.22 [0.15;0.31] | <0.001 | |
| Solid malignant disease | <0.001 | |||||
| No | 3304 (89.0%) | 546 (79.5%) | 2758 (91.2%) | Ref. | Ref. | |
| Yes | 387 (10.4%) | 135 (19.7%) | 252 (8.33%) | 2.71 [2.14;3.42] | <0.001 | |
| ‘Missing’ | 21 (0.57%) | 6 (0.87%) | 15 (0.50%) | 2.02 [0.64;5.54] | 0.167 | |
| Haematological chronic disease | <0.001 | |||||
| No | 3481 (93.8%) | 615 (89.5%) | 2866 (94.7%) | Ref. | Ref. | |
| Yes | 211 (5.68%) | 67 (9.75%) | 144 (4.76%) | 2.17 [1.58;2.96] | <0.001 | |
| ‘Missing’ | 20 (0.54%) | 5 (0.73%) | 15 (0.50%) | 1.55 [0.44;4.52] | 0.400 | |
| Rheumatological disease | <0.001 | |||||
| No | 3284 (88.5%) | 569 (82.8%) | 2715 (89.8%) | Ref. | Ref. | |
| Yes | 412 (11.1%) | 113 (16.4%) | 299 (9.88%) | 1.80 [1.41;2.29] | <0.001 | |
| ‘Missing’ | 16 (0.43%) | 5 (0.73%) | 11 (0.36%) | 2.17 [0.59;6.80] | 0.175 | |
| HIV infection | 0.668 | |||||
| No | 3671 (98.9%) | 678 (98.7%) | 2993 (98.9%) | Ref. | Ref. | |
| Yes | 21 (0.57%) | 4 (0.58%) | 17 (0.56%) | 1.04 [0.25;3.20] | 0.905 | |
| ‘Missing’ | 20 (0.54%) | 5 (0.73%) | 15 (0.50%) | 1.47 [0.42;4.28] | 0.455 | |
| Malnutrition | 0.002 | |||||
| No | 3670 (98.9%) | 671 (97.7%) | 2999 (99.1%) | Ref. | Ref. | |
| Yes | 15 (0.40%) | 8 (1.16%) | 7 (0.23%) | 5.10 [1.61;16.6] | 0.003 | |
| ‘Missing’ | 27 (0.73%) | 8 (1.16%) | 19 (0.63%) | 1.88 [0.71;4.52] | 0.152 | |
| Dementia | <0.001 | |||||
| No | 3482 (93.8%) | 565 (82.2%) | 2917 (96.4%) | Ref. | Ref. | |
| Yes | 212 (5.71%) | 118 (17.2%) | 94 (3.11%) | 6.48 [4.82;8.72] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 4 (0.58%) | 14 (0.46%) | 1.47 [0.35;4.72] | 0.489 | |
| Mental illness | <0.001 | |||||
| No | 3327 (89.6%) | 587 (85.4%) | 2740 (90.6%) | Ref. | Ref. | |
| Yes | 365 (9.83%) | 96 (14.0%) | 269 (8.89%) | 1.67 [1.28;2.15] | <0.001 | |
| ‘Missing’ | 20 (0.54%) | 4 (0.58%) | 16 (0.53%) | 1.17 [0.28;3.63] | 0.752 | |
| Non-severe mental illness, type | 0.001 | |||||
| 1 | 224 (6.03%) | 62 (9.02%) | 162 (5.36%) | Ref. | Ref. | |
| 2 | 110 (2.96%) | 23 (3.35%) | 87 (2.88%) | 0.69 [0.38;1.22] | 0.184 | |
| 3 | 25 (0.67%) | 8 (1.16%) | 17 (0.56%) | 1.23 [0.44;3.19] | 0.643 | |
| ‘Missing’ | 3353 (90.3%) | 594 (86.5%) | 2759 (91.2%) | 0.56 [0.41;0.78] | <0.001 | |
| Severe mental illness | 0.288 | |||||
| No | 3561 (95.9%) | 663 (96.5%) | 2898 (95.8%) | Ref. | Ref. | |
| Yes | 132 (3.56%) | 19 (2.77%) | 113 (3.74%) | 0.74 [0.42;1.21] | 0.218 | |
| ‘Missing’ | 19 (0.51%) | 5 (0.73%) | 14 (0.46%) | 1.56 [0.44;4.61] | 0.399 | |
| Severe mental illness, type | 0.017 | |||||
| 1 | 74 (1.99%) | 5 (0.73%) | 69 (2.28%) | Ref. | Ref. | |
| 2 | 22 (0.59%) | 4 (0.58%) | 18 (0.60%) | 3.02 [0.54;15.7] | 0.146 | |
| 3 | 36 (0.97%) | 10 (1.46%) | 26 (0.86%) | 5.21 [1.46;21.4] | 0.005 | |
| ‘Missing’ | 3580 (96.4%) | 668 (97.2%) | 2912 (96.3%) | 3.17 [1.29;10.1] | 0.005 | |
| Charlson Comorbidity Index | 2.00 [0.00;5.00] | 5.00 [4.00;7.00] | 2.00 [0.00;4.00] | 1.57 [1.51;1.63] | <0.001 | <0.001 |
GF < 30, glomerular filtration lower than 30 mL/min; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; NR, normal range; OR, odds ratio; p.ratio, p-value in the logistic regression; p.overall, p ratio of t.
2.1.2. Propensity Score Matching Analysis
The 22,446 medicine trademarks used previous to admission for COVID-19 infection in the case-control, were classified according to ATC obtaining 689 final drugs, all of them were included in PSM analysis. Of these, 20 demonstrated a statistically significant difference for mortality (p < 0.05). Eleven of them demonstrated reduction in mortality (Table 2): enoxaparin, bemiparine, oral rehydration salts, azithromycin, cefuroxime, ipratropium bromide, mepyramine theophylline acetate, budesonide-formoterol fumarate, loratadine, colchicine, and salbutamol sulphate. Eight drugs demonstrated an increase in mortality: digoxin, folic acid, mirtazapine, linagliptin, enalapril, atorvastatin, allopurinol, and acetylsalicylic acid (Table 3).
Table 2.
Final drug PSM analysis. Lower and upper limits of the 95% confidence interval for the odds ratio. The final substances significantly associated with survival.
| Final Drug | ATC Code | Drug p-Value | OR | Lower Limit 95% CI | Upper Limit 95% CI | Power |
|---|---|---|---|---|---|---|
| ENOXAPARINE | B01AB05 | <0.001 | 0.11 | 0.06 | 0.21 | <0.001 |
| BEMIPARINE | B01AB12 | <0.001 | 0.18 | 0.08 | 0.37 | 0.585 |
| ORAL REHYDRATION SALTS (GLUCOSE, POTASSIUM CHLORIDE, SODIUM CHLORIDE, TRISODIUM CITRATE) | A07CA91 | <0.001 | 0.15 | 0.03 | 0.53 | 0.591 |
| AZITHROMYCIN | J01FA10 | 0.002 | 0.46 | 0.26 | 0.78 | 0.517 |
| CEFUROXIME | J01DC02 | 0.011 | 0.26 | 0.06 | 0.83 | 0.553 |
| IPRATROPIUM BROMIDE | R03BB01 | 0.006 | 0.48 | 0.27 | 0.84 | 0.516 |
| MEPYRAMINE THEOPHYLLINE ACETATE | R03DA12 | 0.015 | 0.00 | 0.00 | 0.85 | 0.853 |
| BUDESONIDE, FORMOTEROL FUMARATE | R03AK07 | 0.013 | 0.51 | 0.28 | 0.90 | 0.514 |
| LORATADINE | R06AX13 | 0.022 | 0.20 | 0.02 | 0.93 | 0.576 |
| COLCHICINE | M04AC01 | 0.022 | 0.20 | 0.02 | 0.93 | 0.576 |
| SALBUTAMOL SULPHATE | R03AC02 | 0.039 | 0.62 | 0.37 | 0.99 | 0.508 |
OR: odds ratio; p-value: statistical significance.
Table 3.
Final drug PSM analysis. Lower and upper limits of the 95% confidence interval for the odds ratio. The final substances significantly associated with mortality.
| Final Drug | ATC Code | Drug p-Value | OR | Lower Limit 95% CI | Upper Limit 95% CI | Power | Interactions p < 0.05 |
|---|---|---|---|---|---|---|---|
| DIGOXIN | C01AA05 | 0.011 | 3.81 | 1.20 | 15.84 | 0.553 | Chronic heart disease |
| FOLIC ACID | B03BB01 | 0.001 | 2.32 | 1.36 | 4.08 | 0.520 | Malnutrition Pregnancy |
| MIRTAZAPINE | N06AX11 | 0.001 | 2.17 | 1.32 | 3.65 | 0.517 | Mental illness |
| LINAGLIPTIN | A10BH05 | 0.025 | 2.12 | 1.05 | 4.52 | 0.519 | Chronic kidney disease |
| ENALAPRIL | C09BA02 | 0.012 | 1.93 | 1.12 | 3.39 | 0.513 | Chronic kidney disease |
| ATORVASTATIN | C10AA05 | 0.002 | 1.52 | 1.16 | 2.01 | 0.505 | Dislipemia |
| ALLOPURINOL | M04AA01 | 0.030 | 1.42 | 1.02 | 1.99 | 0.504 | Solid malignant disease |
| ACETYLSALICYLIC ACID | B01AC06 | 0.038 | 1.31 | 1.01 | 1.71 | 0.502 | Chronic heart disease Diabetes, dislipemia, obesity |
OR: odds ratio; p-value: statistical significance.
Supplementary Tables S1–S3 show the ATC codes and the results of the PSM analysis of the final drugs, subgroups, and groups, respectively.
3. Discussion
This study explores the association between previous use of medications and the mortality of patients hospitalised due to COVID-19. A positive association has been found with azithromycin, bemiparine, budesonide-formoterol fumarate, cefuroxime, colchicine, enoxaparin, ipratropium bromide, loratadine, mepyramine theophylline acetate, oral rehydration salts, and salbutamol sulphate. On the other hand, the analysis shows a risk associated with acetylsalicylic acid, allopurinol, atorvastatin, digoxin, enalapril, folic acid, linagliptin, and mirtazapine. However, the results should be interpreted with great caution as propensity score matching cannot assess and balance all the factors that come into play in the clinical management of patients and that may be present in the circumstances of the study. Thus, propensity score matching analyses may omit, due to nonrecognition, the effects of several clinically significant, but not considered, factors that can affect the outcomes of the analyses being reported, causing them to possibly be misleading, or at best hypothesis-generating [17]. However, a drug-morbidity interaction analysis has been performed in the propensity score matching. The results demonstrated an interaction effect in the propensity score analysis that could explain the association of its use with the risk of death from COVID-19 disease.
3.1. Final Substances Significantly Associated with Mortality
3.1.1. Digoxin
Atrial arrhythmias are common among hospitalised COVID-19 patients and are independently associated with increased mortality [18]. Several in vitro and in vivo studies have discovered the antiviral and anti-inflammatory properties of digoxin by inhibiting the entry of the coronavirus into cells and suppressing the cytokine storm [19,20]. However, in 2015, three independent meta-analyses raised concerns about the association of digoxin treatment with an increased risk of mortality in patients with atrial fibrillation (AF) and heart failure (HF) [21,22,23]. A 2019 update confirmed that the use of digoxin is associated with higher mortality in patients with AF or HF [24]. These data are consistent with the risk results found in this study could be due to the interaction with chronic heart disease.
3.1.2. Folic Acid
Systemic inflammation, immune system impairment, and sarcopenia could act as crucial factors linking nutritional status and the course and outcome of COVID-19 [25]. Current data suggest that pregnant women have similar disease course and outcomes compared to nonpregnant people; however, pregnant women may have increased risk of hospitalisation and intensive care unit (ICU) admission. Among patients who develop severe and critical disease, major maternal morbidity and mortality have been described including cardiomyopathy, mechanical ventilation, extracorporeal membrane oxygenation, and death [26]. These data are consistent with the risk results found in this study and could be due to the interaction with malnutrition and pregnancy.
3.1.3. Mirtazapine
We cannot explain the risk associated with mirtazapine outside of the risk of the sedative effects of this drug, especially in comorbid elderly patients [27,28,29]. Nevertheless, patients with mental illness are at high risk for SARS-CoV-2 infection and COVID-19-related death. Behavioural changes associated with cognitive deterioration increase the SARS-CoV-2 infection risk, and severe medical conditions and delayed treatment increase the COVID-19-related mortality risk in patients with mental illness [30].
3.1.4. Linagliptin
Inflammation is implicated in the development and severity of COVID-19 infection, as well as in the pathophysiology of diabetes. Diabetes is also recognised as a considerable risk factor for COVID-19 morbidity and mortality. Furthermore, some inflammatory markers [i.e., C-reactive protein (CRP), interleukin-6 (IL-6), and ferritin] were reported as solid predictors of worse outcomes in COVID-19 positive patients. The same biomarkers have been associated with poor glycemic control. However, linagliptin can cause side effects, such as acidosis, dehydration, kidney problems, hypoglycemia, and increased cholesterol in the blood [31]. Chronic kidney disease, associated with linagliptin in the PSM interaction analysis, seems to be associated with enhanced risk of COVID-19 mortality. The disease course and outcomes in COVID-19 patients are associated with baseline estimated glomerular filtration rate [32].
3.1.5. Enalapril
There is some evidence from retrospective trials suggesting that using an ACEI or an ARB in patients with hypertension who were hospitalised for COVID-19 was associated with similar or lower mortality rates, as compared with patients who were not taking a drug from either class prior to infection [33]. A systematic review of observational studies and trials until 4 May 2020 that examined the association and effects of ACEIs or ARBs on risk for SARS-CoV-2 infection and COVID-19 disease severity in adults found high-certainty evidence, suggesting that ACEI or ARB use is not associated with more severe COVID-19 disease [34]. A critical confounder in retrospective studies was revealed in data on patients with COVID-19. Approximately 50% of the patients who had been prescribed ACE inhibitors or ARBs discontinued the medication when they were hospitalised [35]. Hypertension increases COVID-19 severity due to underlying endothelial dysfunctions and coagulopathy. COVID-19 might augment the hypertensive complications due to the down-regulation of ACE2 [36]. The benefit of using ACEIs or ARBs in the treatment of hypertensive patients with COVID-19 is now being explored in randomised clinical trials. Underlying diseases, such as chronic kidney disease associated with enalapril use, could also explain this increased risk [29].
3.1.6. Atorvastatin
Several studies have observed a decrease in total cholesterol, LDL-C, and HDL-C levels in patients with COVID-19 infections. In most studies, the decrease in LDL-C and/or HDL-C was more profound with a greater severity of illness. LDL-C and HDL-C levels were inversely correlated with C-reactive protein (CRP) levels, i.e., the lower the LDL-C or HDL-C level, the higher the CRP levels. Patients with low HDL-C levels on admission to the hospital were at an increased risk of developing a severe disease compared to patients with high HDL-C levels [37]. Patients with various infections (gram-positive bacterial, gram-negative bacterial, viral, tuberculosis, and parasites) have similar alterations in plasma lipid levels. Specifically, total cholesterol, LDL-C, and HDL-C levels are decreased, while plasma triglyceride levels may be elevated or inappropriately normal for the poor nutritional status [38]. One needs to be aware that certain drugs that are used to treat COVID-19 infections may interact with lipid lowering drugs. Remdesivir is metabolised by the CYP3A4 pathway, so it would be advisable to avoid statins that are also metabolised by this pathway (atorvastatin, simvastatin, and lovastatin) [39]. With the antiretroviral drugs (lopinavir/ritonavir), the use of low dose rosuvastatin therapy is recommended [40]. Tocilizumab interferes with both the CYP3A4 and CYP2C9 pathways of metabolism, and therefore it is recommended to temporarily suspend treatment with statins [41].
3.1.7. Allopurinol
A study to assess gout management during the COVID-19 pandemic through surveys demonstrated that gout flares were common: 63% had ≥1 gout flare monthly; 11% underwent emergency room/urgent care; and 2% were hospitalised with gout flares. Between 41% and 56% of respondents reported more difficulty with gout management and functional status related to COVID-19, this could explain the detrimental effect of allopurinol in this study [42]. The use of allopurinol associated with cancer treatment in this study could also explain the increased risk, as these patients are susceptible to serious clinical adverse events and increased mortality from COVID-19 infection, as well as morbidity and mortality from its underlying malignancy [43].
3.1.8. Acetylsalicylic Acid
The results of a meta-analysis of three retrospective observational studies suggested that there is no protective effect of aspirin on mortality from COVID-19. Studies likely suggest that aspirin does not reduce mortality in these patients, although patients taking aspirin tended to have more risk factors for severe COVID-19 infection (e.g., advanced age, pre-existing coronary artery disease, diabetes mellitus, etc.) [44]. Morbidity appears to be a greater factor than aspirin use.
3.2. Final Substances Significantly Associated with Survival
3.2.1. Enoxaparine and Bemiparine
SARS-CoV-2 infection has been linked to a higher risk of mortality compared to influenza, which is mainly due to severe secondary diseases, such as acute respiratory distress syndrome (ARDS). In turn, ARDS is characterised by an acute inflammation and excessive coagulation cascade activity, raising the vulnerability for venous thromboembolic events. In accordance with previous studies, our study outlines that anti-coagulation may constitute a promising tool for treating SARS-CoV-2, reducing both the cytokine storm and the risk for thrombotic complications [45].
3.2.2. Oral Rehydration Salts
We do not know the mechanism for the protection of oral rehydration with salts beyond the protective effect against dehydration.
3.2.3. Azithromycin
Azithromycin presents in vitro activity against SARS-CoV-2 and could act at different points of the viral cycle. Its immunomodulatory properties include the ability to downregulate cytokine production, maintain epithelial cell integrity, and prevent lung fibrosis. Azithromycin use was associated with a reduction in mortality and days on a ventilator in other viral infections. These properties could be beneficial throughout the COVID-19 infection. However, the evidence of its use is scarce and of low quality. Azithromycin has been assessed in retrospective observational studies mainly in combination with hydroxychloroquine, which has shown no benefit [46]. This macrolide presents a well-known safety profile. Upcoming clinical trials will determine the role of azithromycin in COVID-19 (including the stage of the disease where it offers the maximal benefits and the effect of its combination with other drugs).
3.2.4. Cefuroxime
Cefuroxime is a second-generation cephalosporin antibiotic. It has broad-spectrum activity and is commonly used for the treatment of both upper and lower respiratory tract infections, Lyme disease, and genitourinary tract infections. Several studies have reported cefuroxime as a potential inhibitor of three essential SARS-CoV-2 proteins; main protease, RNA-dependent RNA polymerase, and ACE2-Spike complex [47,48,49]. Further in vitro and in vivo studies are required to evaluate the potential of cefuroxime for COVID-19.
3.2.5. Inhaled Glucocorticoids and Bronchodilators
Multiple early reports of patients admitted to the hospital with COVID-19 demonstrated that patients with chronic respiratory disease were significantly under-represented in these cohorts. We hypothesised that the widespread use of inhaled glucocorticoids among these patients was responsible for this finding and tested if inhaled glucocorticoids would be an effective treatment for early COVID-19 [50]. An open-label, parallel-group, phase 2, randomised controlled trial (Steroids in COVID-19; STOIC) of inhaled budesonide, compared with usual care, in adults within seven days of the onset of mild COVID-19 symptoms found that early administration of inhaled budesonide reduced the likelihood of needing urgent medical care and reduced recovery time after early COVID-19. The number of patients that needed to treat with inhaled budesonide to reduce COVID-19 deterioration was eight. Clinical recovery was one day shorter in the budesonide group compared with the usual care group (median seven days [95% CI 6 to 9] in the budesonide group vs. eight days [7 to 11] in the usual care group; log-rank test p = 0.007). Early administration of inhaled budesonide reduced the likelihood of needing urgent medical care and reduced recovery time after early COVID-19 [51]. The positive association of ipratropium bromide, mepyramine theophylline acetate, and salbutamol could be related to the rest of maintenance asthma and chronic obstructive pulmonary disease (COPD) medicines.
3.2.6. Loratadine
A study of in vitro severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike pseudotyped viral infection experiments indicated that histamine H1 antagonists loratadine and desloratadine could prevent the entry of the pseudotyped virus into ACE2-overexpressing HEK293T cells and demonstrated that desloratadine was more effective. Molecular docking results elucidated that loratadine and desloratadine could bind to ACE2 on the interface of the SARS-CoV-2-binding area [52].
3.2.7. Colchicine
Colchicine has been observed to help reduce inflammation in several inflammatory diseases. This study aims to analyse the efficacy of colchicine administration and outcomes of COVID-19. A recent systematic review and meta-analysis on COVID-19 and colchicine treatment found in a total of eight studies with 5778 COVID-19 patients included that colchicine was associated with an improvement of outcomes of COVID-19 [OR 0.43 (95% CI 0.34–0.55), p < 0.00001, I2 = 0%, fixed-effect modelling] and its subgroup, which was comprised of reduction from severe COVID-19 [OR 0.44 (95% CI 0.31–0.63), p < 0.00001, I2 = 0%, fixed-effect modelling] and reduction in the mortality rate of COVID-19 [OR 0.43 (95% CI 0.32–0.58), p < 0.00001, I2 = 0%, fixed-effect modelling] [53]. More randomised clinical trials are still needed to confirm the results of this study.
3.3. Strengths and Limitations
One strength of the study is the use of computerised databases, prescription, and administration of the study patients. Lack of adherence to treatment was controlled using a prescription–administration database. However, over-the-counter use is occasionally recorded and this could lead to a misclassification of the exposure. However, in this health area, non-prescription purchases are limited to paracetamol 500 mg, ibuprofen 400 mg, antacids, lotions and creams, anti-hemorrhoids, eye drops, vitamin preparations and some mucolytics, anti-cold, and antihistamines [54]. In addition to the design limitations of the type of analysis already addressed, other limitations are the retrospective nature of the study; however, the chosen outcome, mortality, is robust and clinically relevant. This study aimed to explore the association of drug use and prescription-administration data before hospitalisation, with mortality from COVID-19 disease due to any indication of appropriate or inappropriate use; thus, the adequacy of use has not been established in the study. Most medications identified as a risk had an associated risk morbidity in the drug-morbidity interaction analysis. With respect to beneficial medications, we cannot affirm that the treatment “protects” from infection, but that it improves the outcome. We think that it is irrelevant to be able to differentiate between improvement due to treatment of symptoms (protopathic bias), or improvement due to the reduction of viral load that translates into milder symptoms.
4. Materials and Methods
4.1. Study Design and Population
The retrospective case-control from two hospitals in Madrid, Spain, La Paz University Hospital and La Princesa University Hospital, included all patients, 18 years or older, hospitalised in the wards (or emergency department) with a diagnosis of COVID-19 and who either died or were discharged, from March to May 2020. Patients discharged from the emergency department after less than 24 h were not considered hospitalised and were not included in this analysis. Deaths were categorised as cases and survivors as controls. This study was approved by the Research Ethics Committee (PI-4072), by the Spanish Agency of Medicines and Medical Devices (HUL-AIN-2020-01), and registered in the EU PAS Register (EUPAS34331). Informed consent was not sought due to the emergency situation and because the data collection was retrospective. The program complied with the Spanish Personal Data Protection Law [55].
COVID-19 infection was diagnosed by a positive reverse transcription-polymerase chain reaction (RT-PCR) obtained from nasopharyngeal and oropharyngeal specimens. RT-PCR was determined using a commercial and in-house method in the microbiology department of the hospitals. Case inclusion criteria were hospitalisation for COVID-19 infection and being at least 18 years of age.
4.2. Clinical Data Collection
An electronic case record form (eCRF) was used to collect hospital emergency ward patients with suspected or confirmed cases of COVID-19. It was a modified version of the WHO/International Severe Acute Respiratory and Emerging Infection Consortium CRF for severe acute respiratory infections [56]. The eCRF includes 386 variables grouped into demographic, medical history, infection-exposure history, clinical symptoms, complications, evolution, medications, laboratory results, and imaging features. The results of the first 2226 adult patients of the cohort were published elsewhere [57]. These data were collected from electronic medical records (DXC-HCIS HCIS3_10_6_st30_SERMAS_V1_HULP_P9—Healthcare Information System) by a volunteer team of resident doctors and senior medical students. Laboratory results were automatically extracted from the Integrated Laboratory System (LABTrack; TrackHealth, Woolloomooloo, Australia) of the hospitals. Real-time RT-PCR results were automatically extracted from the Laboratory Information System (Microb Dynamic; Spain) of the microbiology Departments. Medications were automatically extracted from the electronic prescription systems (EPSs) from primary care and confirmed with the electronic dispensation system (MUP; General Directorate for Healthcare Coordination of the Madrid Health Service, Madrid, Spain). All the CRFs were monitored and the data curated in the Central Clinical Research Unit (UCICEC), Service of Clinical Pharmacology of La Paz University Hospital, and validated by the study’s scientific committee. The medications were coded using the World Health Organization’s Anatomical Therapeutic Chemical (ATC) classifications [58]. The code of the drug bank and the link were also added to the final substances [59].
4.3. Variables and Exposure
The principal independent variable was exposure to medication in the last month previous to hospitalisation due to COVID-19 infection. The medication under evaluation was the drug the patient was exposed to until admission for COVID-19 infection. Exposure to a drug was defined as the patient’s current prescription until just before hospital admission, with dispensing verified in the period. The dependent variables were age, sex, and number of morbidities (chronic heart disease, arterial hypertension, chronic obstructive pulmonary disease, asthma, other chronic lung diseases, chronic kidney disease, chronic neurological disease, solid malignant disease, chronic hematological disease, obesity, diabetes mellitus, rheumatological disease, dementia, dyslipemia, malnutrition, and severe mental illness).
4.4. Analysis
A case-control propensity score matching (PSM) analysis was performed to estimate the effect of the medication used previous to admission on the mortality of patients hospitalised for COVID-19. Confounding variables were considered to be age, sex, and the number of comorbidities. The odds ratio adjusted for covariables was estimated using a multiple logistic regression model. PSM analysis of the chemical pharmacological subgroup and the pharmacological therapeutic subgroup of ATC classification was also performed. The interactions of statistical significant final drugs with the underlying pathologies were also considered in the PSM analysis. Statistical calculations were performed under the statistical environment R, using RStudio editor (version 3.4.0) [60,61].
5. Conclusions
In conclusion, this study provides an exploratory association of the previous use of medications with the mortality of patients hospitalised due to COVID-19. Medication associated with survival (anticoagulants, antihistamines, azithromycin, bronchodilators, cefuroxime, colchicine, and inhaled corticosteroids) may be candidates for future clinical trials. Drugs associated with mortality show an interaction with the underlying conditions.
Acknowledgments
The authors are grateful to all the members of the COVID@HULP Working Group. The authors would like to thank Morote.net for their editing of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph15010078/s1. Table S1: Propensity score matching analysis of other final drugs. Lower and upper limits of the 95% confidence interval for the odds ratio, Table S2: Propensity score matching the analysis of the subgroup of ATC. Lower and upper limits of the 95% confidence interval for the odds ratio, Table S3: Propensity score matching analysis of the main therapeutic group. Lower and upper limits of the 95% confidence interval for the odds ratio, Complete list of the members of the COVID@HULP Working Group and other collaborators from Hospital Universitario de la Princesa.
Author Contributions
Conceptualisation, J.M.V. and E.R.; methodology, F.A.-S. and A.M.B.; software, M.J.G.; validation, G.F.J. and I.C.; formal analysis, G.M.-A., L.D.G. and P.Z.; investigation, J.R.A., C.S.F., J.M., J.G.R. and J.R.V.F.; resources, A.M.B.; data curation, G.F.J. and I.C.; writing—original draft preparation, E.R.; writing—review and editing, A.M.B. and F.A-S.; visualisation, F.A-S.; supervision, A.J.C. and J.F.; project administration, E.R.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of La Paz University Hospital (protocol code PI-4072, 19 March 2020l) registered in the EU PAS Register (EUPAS34331).
Informed Consent Statement
Informed consent was not sought due to the emergency situation and because the data collection was retrospective. The program complied with the Spanish Personal Data Protection Law.
Data Availability Statement
This project is a sub-analysis of the COVID@HULP cohort (DOI: 10.3390/jcm9061733). The data presented in this study are available on request from the corresponding author. The data are not publicly available due to this is a sub-analysis of a cohort of patients containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Safford M.M. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020. Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Peng Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Zhong N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. [(accessed on 29 June 2021)];Intensive Care Med. 2020 46:586–590. doi: 10.1007/s00134-020-05985-9. Available online: http://link.springer.com/10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hochman J.S. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarcho J.A., Ingelfinger J.R., Hamel M.B., D’Agostino R.B., Sr., Harrington D.P. Inhibitors of the Renin-Angiotensin-Aldosterone System and COVID-19. N. Engl. J. Med. 2020;382:2462–2464. doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Li H. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bean D.M., Kraljevic Z., Searle T., Bendayan R., Pickles A., Folarin A., Dobson R.J. Treatment with AVE-inhibitors is associated with less severe disease with SARS-COVID-19 infection in a multi-site UK acute Hospital Trust. MedRxiv. :2020. doi: 10.1101/2020.04.07.20056788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 15.Khan S., Siddique R., Shereen M.A., Ali A., Liu J., Bai Q., Xue M. Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 2020;58:e00187-20. doi: 10.1128/JCM.00187-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rishita P., Thommana M.V., Ruiz M.B., Ayna S. Therapeutic Options for COVID-19: A Review. Cureus. 2020;12:e10480. doi: 10.7759/cureus.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiffel J.A. Propensity Score Matching: The ‘Devil is in the Details’ Where More May Be Hidden than You Know. Am. J. Med. 2020;133:178–181. doi: 10.1016/j.amjmed.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 18.Peltzer B., Manocha K.K., Ying X., Kirzner J., Ip J.E., Thomas G., Liu C.F., Markowitz S.M., Lerman B.B., Safford M.M., et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020;12:3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard B.S., Blanco J.C., Pollard J.R. Classical Drug Digitoxin Inhibits Influenza Cytokine Storm, with Implications for COVID-19 Therapy. In Vivo. 2020;34:3723–3730. doi: 10.21873/invivo.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho J., Lee Y.J., Kim J.H., Kim S.I., Kim S.S., Choi B.S., Choi J.H. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci. Rep. 2020;10:16200. doi: 10.1038/s41598-020-72879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vamos M., Erath J.W., Hohnloser S.H. Digoxin-associated mortality: A systematic review and meta-analysis of the literature. Eur. Heart J. 2015;36:1831–1838. doi: 10.1093/eurheartj/ehv143. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.Q., Zhang R., Chen M.T., Wang Q.S., Zhang Y., Huang X.H., Wang J., Yan J.H., Li Y.G. Digoxin Is Associated with Increased All-Cause Mortality in Patients with Atrial Fibrillation Regardless of Concomitant Heart Failure: A Meta-Analysis. J. Cardiovasc. Pharmacol. 2015;66:270–275. doi: 10.1097/FJC.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang A.J., Lv Y.N., Zhong H.L., Wen J.H., Wei X.H., Peng H.W., Zhou J., Liu L.L. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am. J. Cardiol. 2015;115:901–9066. doi: 10.1016/j.amjcard.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Vamos M., Erath J.W., Benz A.P., Lopes R.D., Hohnloser S.H. Meta-Analysis of Effects of Digoxin on Survival in Patients with Atrial Fibrillation or Heart Failure: An Update. Am. J. Cardiol. 2019;123:69–74. doi: 10.1016/j.amjcard.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Fedele D., De Francesco A., Riso S., Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition. 2021;81:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson J.L., Nguyen L.M., Noble K.N., Aronoff D.M. COVID-19-related disease severity in pregnancy. Am. J. Reprod. Immunol. 2020;84:e13339. doi: 10.1111/aji.13339. [DOI] [PubMed] [Google Scholar]
- 27.Spanish Agency for Medicines and Health Products Technical Data Sheet Mirtazapina Cinfa®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/67068/FT_67068.html.
- 28.Spanish Agency for Medicines and Health Products Technical Data Sheet Qudix®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/70169/FT_70169.html.
- 29.Coupland C., Dhiman P., Morriss R., Arthur A., Barton G., Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seon J.Y., Kim S., Hong M., Lim M.K., Oh I.H. Risk of COVID-19 diagnosis and death in patients with mental illness: A cohort study. Epidemiol. Psychiatr. Sci. 2021;30:e68. doi: 10.1017/S2045796021000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spanish Agency for Medicines and Health Products Technical Data Sheet Trajenta®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/11707004/FT_11707004.html.
- 32.Kilis-Pstrusinska K., Akutko K., Braksator J., Dancewicz A., Grosman-Dziewiszek P., Jamer T., Juszczyńska K., Konikowska K., Koruba M., Pupek M., et al. Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. J. Clin. Med. 2021;10:5522. doi: 10.3390/jcm10235522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., Laredo L., Laosa O., Centeno-Soto G.A., Ángeles Gálvez M., et al. MED-ACE2-COVID-19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackey K., King V.J., Gurley S., Kiefer M., Liederbauer E., Vela K., Sonnen P., Kansagara D. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann. Intern. Med. 2020;173:195–203. doi: 10.7326/M20-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriram K., Insel P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020;177:4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batiha G.E., Gari A., Elshony N., Shaheen H.M., Abubakar M.B., Adeyemi S.B., Al-Kuraishy H.M. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021;11:200121. doi: 10.1016/j.ijcrp.2021.200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., Grossman A., Hershman J.M., Hofland J., et al. Endotext [Internet] MDText.com, Inc.; South Dartmouth, MA, USA: 2020. Lipid and Lipoprotein Levels in Patients with COVID-19 Infections. [Google Scholar]
- 38.Sammalkorpi K., Valtonen V., Kerttula Y., Nikkilä E., Taskinen M.R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–865. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 39.Spanish Agency for Medicines and Health Products Technical Data Sheet Veklury®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/1201459002/FT_1201459002.html.
- 40.Spanish Agency for Medicines and Health Products Technical Data Sheet Kaletra®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/01172006/FT_01172006.html.
- 41.Spanish Agency for Medicines and Health Products Technical Data Sheet Roactemra®. [(accessed on 29 June 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/108492007/FT_108492007.html#4-datos-cl-nicos.
- 42.Singh J.A., Edwards N.L. Gout management and outcomes during the COVID-19 pandemic: A cross-sectional internet survey. Ther. Adv. Musculoskelet Dis. 2020;12:1759720X20966124. doi: 10.1177/1759720X20966124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madan A., Siglin J., Khan A. Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020;9:9205–9218. doi: 10.1002/cam4.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salah H.M., Mehta J.L. Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19. Am. J. Cardiol. 2021;142:158–159. doi: 10.1016/j.amjcard.2020.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paar V., Wernly B., Zhou Z., Motloch L.J., Hoppe U.C., Egle A., Lichtenauer M. Anti-coagulation for COVID-19 treatment: Both anti-thrombotic and anti-inflammatory? J. Thromb. Thrombolysis. 2020;51:226–231. doi: 10.1007/s11239-020-02212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Echeverría-Esnal D., Martin-Ontiyuelo C., Navarrete-Rouco M.E., Cuscó M.D.-A., Ferrández O., Horcajada J.P., Grau S. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti Infect. Ther. 2021;19:147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 47.Durojaiye A.B., Clarke J.-R.D., Stamatiades G.A., Wang C. Repurposing cefuroxime for treatment of COVID-19: A scoping review of in silico studies. J. Biomol. Struct. Dyn. 2020;39:4547–4554. doi: 10.1080/07391102.2020.1777904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elfiky A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: An in silico perspective. J. Biomol. Struct. Dyn. 2021;39:3204–3212. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Khafaji K., Al-Duhaidahawi D., Taskin Tok T. Using integrated computational approaches to identify safe and rapid treatment for SARS-CoV-2. J. Biomol. Struct. Dyn. 2021;39:3387–3395. doi: 10.1080/07391102.2020.1764392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicolau D.V., Bafadhel M. Inhaled corticosteroids in virus pandemics: A treatment for COVID-19? Lancet Respir. Med. 2020;8:846–847. doi: 10.1016/S2213-2600(20)30314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramakrishnan S., Nicolau D.V., Jr., Langford B., Mahdi M., Jeffers H., Mwasuku C., Krassowska K., Fox R., Binnian I., Glover V., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou Y., Ge S., Li X., Wang C., He H., He L. Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis. Chem. Interact. 2021;338:109420. doi: 10.1016/j.cbi.2021.109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hariyanto T.I., Halim D.A., Jodhinata C., Yanto T.A., Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2021;48:823–830. doi: 10.1111/1440-1681.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royal Decree 1090/2015, of 4 December, Regulating Clinical Trials with Medicinal Products, Ethics Committees for Investigation with Medicinal Products and the Spanish Clinical Studies Registry. [(accessed on 13 December 2021)]. Available online: https://www.aemps.gob.es/legislacion/espana/investigacionClinica/docs/Royal-Decree-1090-2015_4-December.pdf.
- 55.Organic Law 3/2018 of 5 December 2018, on the Protection of Personal Data and Guarantee of Digital Rights. 2018. [(accessed on 20 December 2021)]. Available online: https://noticias.juridicas.com/base_datos/Laboral/632849-lo-3-2018-de-5-dic-proteccion-de-datos-personales-y-garantia-de-los-derechos.html.
- 56.Novel Coronaviris (nCoV) Acute Respiratory Infection Clinical Characterisation Data Tools nCoV Case Records form Version 1.3. [(accessed on 16 April 2020)]. Adapted from Sprint Sari Case Report Form by ISARIC. 24 February 2020. Available online: https://media.tghn.org/medialibrary/2020/03/ISARIC_COVID-19_CRF_V1.3_24Feb2020.pdf.
- 57.Borobia A.M., Carcas A.J., Arnalich F., Álvarez-Sala R., Monserrat-Villatoro J., Quintana M. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J. Clin. Med. 2020;9:1733. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ATC/DDD Index 2020 Updated 16 December 2019. [(accessed on 4 September 2020)]. Available online: https://www.whocc.no/atc_ddd_index/>.
- 59.Drugbank Pharmaceutical Knowledge Base that is Enabling Major Advances across the Data-Driven Medicine Industry. [(accessed on 4 September 2020)]. Available online: https://www.drugbank.ca/drugs.
- 60.R Foundation . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: [(accessed on 29 June 2021)]. Available online: https://www.R-project.org/ [Google Scholar]
- 61.RStudio Team . RStudio, PBC; Boston, MA, USA: 2021. [(accessed on 29 June 2021)]. RStudio: Integrated Development Environment for R. Available online: http://www.rstudio.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This project is a sub-analysis of the COVID@HULP cohort (DOI: 10.3390/jcm9061733). The data presented in this study are available on request from the corresponding author. The data are not publicly available due to this is a sub-analysis of a cohort of patients containing information that could compromise the privacy of research participants.