Abstract
Family history of cancer (FHC) is a hallmark of cancer risk and an independent predictor of outcome, albeit with uncertain biologic foundations. We previously showed that FHC-high patients experienced prolonged overall (OS) and progression-free survival (PFS) following PD-1/PD-L1 checkpoint inhibitors. To validate our findings in patients with NSCLC, we evaluated two multicenter cohorts of patients with metastatic NSCLC receiving either first-line pembrolizumab or chemotherapy. From each cohort, 607 patients were randomly case–control matched accounting for FHC, age, performance status, and disease burden. Compared to FHC-low/negative, FHC-high patients experienced longer OS (HR 0.67 [95% CI 0.46–0.95], p = 0.0281), PFS (HR 0.65 [95% CI 0.48–0.89]; p = 0.0074) and higher disease control rates (DCR, 86.4% vs 67.5%, p = 0.0096), within the pembrolizumab cohort. No significant associations were found between FHC and OS/PFS/DCR within the chemotherapy cohort. We explored the association between FHC and somatic DNA damage response (DDR) gene alterations as underlying mechanism to our findings in a parallel cohort of 118 NSCLC, 16.9% of whom were FHC-high. The prevalence of ≥ 1 somatic DDR gene mutation was 20% and 24.5% (p = 0.6684) in FHC-high vs. FHC-low/negative, with no differences in tumor mutational burden (6.0 vs. 7.6 Mut/Mb, p = 0.6018) and tumor cell PD-L1 expression. FHC-high status identifies NSCLC patients with improved outcomes from pembrolizumab but not chemotherapy, independent of somatic DDR gene status. Prospective studies evaluating FHC alongside germline genetic testing are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01226-2.
Keywords: Family history of cancer, DDR genes, NSCLC, Pembrolizumab, Immune checkpoint inhibitors, Immunotherapy
To the editor,
Pathogenic germline mutations affecting the DNA damage response and repair (DDR) genes are among the few underlying known mechanisms of inherited cancer susceptibility. However, clear inheritable defects like those leading to Lynch syndrome (LS) [1] and hereditary breast-ovarian cancer syndrome (HBOC) [2], explain only a limited part of the family history of cancer (FHC) usually seen in clinic. Acknowledging the immune-sensitive phenotype of cancers related to DDR genes defects [3], we postulated that FHC may be linked to immunotherapy efficacy and demonstrated that a high burden of FHC (FHC-high) is an independent, tumour-agnostic predictor of prolonged overall survival (OS) and progression-free survival (PFS) in a large cohort treated with PD-1/PD-L1 checkpoint inhibitors [4], a finding that led us to hypothesize that the underlying mechanism may relate to pathogenetic DDR genes alterations. To investigate whether FHC correlates with outcomes from immunotherapy in non-small cell lung cancer (NSCLC), we designed this study including two large, matched cohorts of patients with metastatic NSCLC treated with either first-line pembrolizumab (PD-L1 tumor expression ≥ 50%) or chemotherapy [5–9].
Detailed study methodology is provided as supplementary material. Overall, 167/890 (18.7%) and 88/740 (11.9%) patients were excluded from the pembrolizumab and chemotherapy cohorts, due to missing FHC data, resulting in 723 and 652 patients, respectively. FHC data was collected as previously described and patients were categorized as FHC-high and FHC-low/negative (Fig. 1). Patients’ characteristics are summarized in Additional file 1: Table S1. None of the baseline characteristics were significantly associated with FHC categories in either the pembrolizumab or the chemotherapy cohorts (Additional file 1: Table S2). Additional file 1: Table S3 and Fig. S1 provide detailed FHC information for the 49 FHC-high patients from the pembrolizumab cohort. Lung cancer was the most frequently reported malignancy, without specific family clusters. Cases/controls were randomly paired on the basis of the FHC, age, ECOG-PS, and burden of disease, and 607 patients from the pembrolizumab and chemotherapy cohorts were perfectly paired.
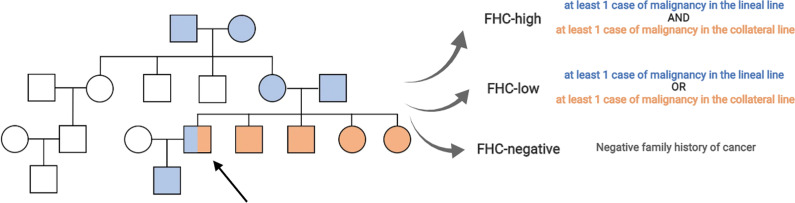
Fig. 1.
Family history data collection. All oncological disease with malignant potential, both hematological and solid, were screened. Lineal line (descendants or ascendants) and collateral line (non-descentants/ascendants e.g., brothers/sisters) were screened till the second degree (grandparents for lineal line and brothers/sisters for the collateral line). Patients were categorized as follow: FHC-high (in case of at least one cancer diagnosis in both lineal and collateral family lines), FHC-low (in case of at least one cancer diagnosis in either the lineal or collateral line) and FHC-negative (Fig. 1). On the basis of our previous findings (Ref. [4]), FHC-high was considered the group of interest for all analyses
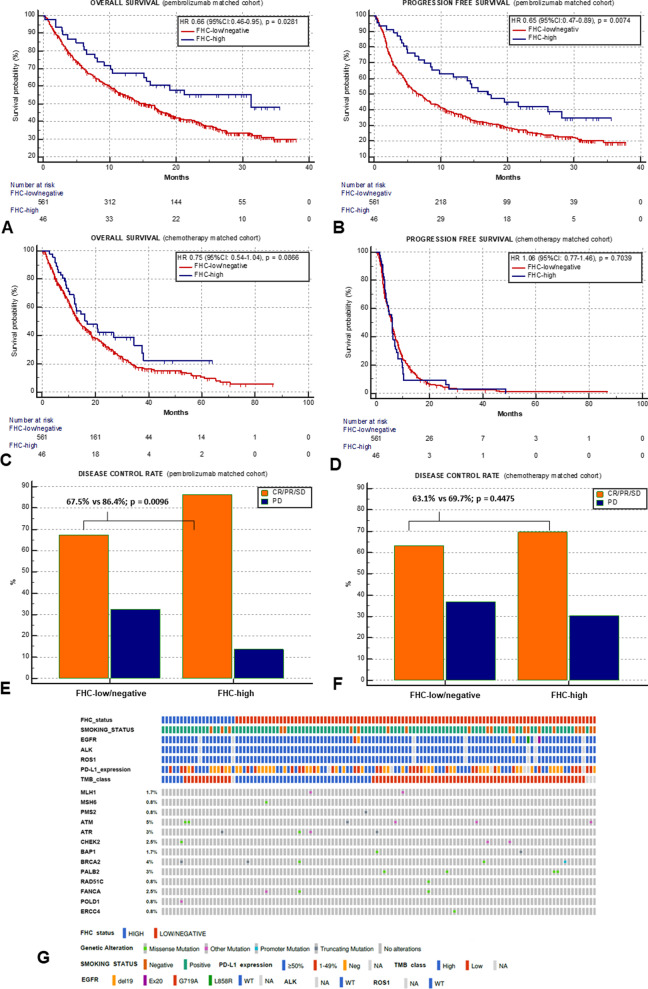
As compared to FHC-low/negative patients, FHC high achieved a significantly longer OS (31.3 vs. 15.3 months; HR = 0.67 [95% CI 0.46–0.95], p = 0.0281; Fig. 2A), and PFS (17.2 vs. 6.5 months; HR = 0.65 [95% CI 0.48–0.89]; p = 0.0074; Fig. 2B) and a higher disease control rate (DCR) (86.4% vs 67.5%, p = 0.0096; Fig. 2E), within the pembrolizumab cohort. On the contrary, no significant associations were found between FHC and OS (16.9 vs 13.8 months; p = 0.0866; Fig. 2C), PFS (5.9 vs. 5.0 months; p = 0.7039; Fig. 2D), DCR (69.7% vs. 63.1%; p = 0.4475; Fig. 2F) within the chemotherapy cohort . Additional file 1: Table S4 and Fig. S2A–F summarize all the univariable analyses according to the FHC across the entire pembrolizumab and chemotherapy cohorts. The pooled multivariable analysis including both cohorts, with and without the interaction term between the FHC and therapeutic modality (immunotherapy vs chemotherapy) is reported in Additional file 1: Table S5. Of note, a statistically significant interaction was found (p = 0.0170) with respect to PFS, highlighting a differential effect of FHC depending on treatment modality.
Fig. 2.
Clinical outcomes analysis according to the FHC across the pembrolizumab and chemotherapy matched cohorts. Median OS and PFS of the entire pembrolizumab cohort were 15.4 months (95% CI 12.8–17.3; 421 events) and 6.9 months (95% CI 5.8–7.9; 523 events), respectively, whilst for the chemotherapy cohort were 14.4 months (95% CI 12.9–16.6; 466 events) and 5.9 months (95% CI 5.3–6.3; 594 events), respectively. The median follow-up was 23.3 months (95% CI 21.8–38.0) for the pembrolizumab cohort and 38.4 months (95% CI 33.1–86.7) for the chemotherapy cohort. Kaplan–Meier survival estimates for OS; pembrolizumab cohort (A) FHC-high: 31.3 months (95% CI 15.2–31.3, 21 events) vs FHC-low/negative: 15.3 months (95% CI 12.8–17.5, 327 events), p = 0.0281; chemotherapy cohort (C) FHC-high: 16.9 months (95% CI 12.1–34.5, 29 events) vs FHC-low/negative: 13.8 months (95% CI 12.3–15.8, 408 events), p = 0.0866. Kaplan–Meier survival estimates for PFS; pembrolizumab cohort (B) FHC-high: 17.2 months (95% CI 8.6–28.1, 28 events) vs FHC-low/negative: 6.5 months (95% CI 5.4–28.3, 405 events), p = 0.0074; chemotherapy cohort (D) FHC-high: 5.9 months (95% CI 3.9–6.9, 44 events) vs FHC-low/negative: 5.0 months (95% CI 5.3–6.4, 5090 events), p = 0.7039. DCR; pembrolizumab cohort (E) FHC-high: 86.4% (95% CI 61.1–118.5) vs FHC-low/negative: 67.5% (95% CI 60.5–75.1), p = 0.0096; chemotherapy cohort (F) FHC-high: 69.7% (95% CI 44.1–104.5) vs FHC-low/negative: 63.1% (95% CI 56.0–70.1), p = 0.4475. (G) OncoPrint plot summarizing relevant baseline clinic-pathologic characteristics and the DDR genes profile of the parallel cohort. Patients are clustered according to the FHC status (first row) and in the upper section the smoking status, common actionable biomarkers (including EGFR, ALK and ROS-1), the PD-L1 tumour expression and the TMB category (with a cut off of ≥ vs < 10 mutations/megabase) are reported. The mutational status and its prevalence of selected DDR genes is reported with different colours according to the mutation’s type. Made with cBioPortal oncoprinter, available at: https://www.cbioportal.org/oncoprinter. FHC, family history of cancer; OS, overall survival; PFS, progression free survival; DCR, disease control rate
We used a parallel cohort of 118 patients with NSCLC (20 FHC-high, 16.9% and 98 FHC-low/negative, 83.1%) to explore the implication of somatic DDR gene alterations in explaining FHC-driven benefit. Using the FoundationOne CDx assay we focused on 24 genes among the 324 detectable cancer-related alterations derived from a reference panel defined by Ricciuti et al. [10]. The prevalence of ≥ 1 DDR gene mutation was 20% (4/20) and 24.5% (24/74) for FHC-low/negative and FHC-high patients (p = 0.6684). Baseline characteristics and DDR gene profiles are summarized in Fig. 2G. No association between FHC and tumor mutational burden/PD-L1 expression was found (Additional file 1: Fig. S3A-B).
This study identifies FHC-high patients as a subgroup characterised by increased benefit to pembrolizumab, strengthening the putative role FHC as a predictive correlate of benefit following PD-1 inhibition. To elucidate the underlying mechanisms, we focused on somatic DDR defects, since in NSCLC they have been already established as independent predictors of response/survival to PD-1/PD-L1 inhibitors [10], whereas the FHC has found a partial role only in the context of early detection/screening [11, 12], and has never been comprehensively evaluated in advanced patients, probably because of no solid linkage to hereditary syndromes [13, 14]. However, the distribution of DDR defects was not enriched in FHC-high patients, highlighting the complexity of the mechanisms involved, that may go beyond single-hit germline tumour-suppressor genes mutations, as we are used to see in HBOC and LS.
Of note, we did not have PD-L1 expression data for the chemotherapy group, and we were not able to match the clinical cohorts according to the PD-L1 tumor expression. However, we did not find any association between the FHC and the PD-L1 status in either the pembrolizumab or the parallel exploratory cohorts, but we can assume that only 30% of the chemotherapy recipients had a high PD-L1 status [15]. Although our study acknowledges several limitations mainly coming from its design and risk of recalling bias, we provided informative evidence in the context of first-line immunotherapy of NSCLC, confirming that FHC-high patients achieve better outcomes to single-agent pembrolizumab, a finding that requires prospective studies incorporating germline and somatic mutational screening in immunotherapy recipients.
Supplementary Information
Additional file 1. Supplementary materials.
Acknowledgments
Alessio Cortellini is supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). David J Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and from the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG Grant ID 25697) and acknowledges support by the NIHR Imperial Biomedical Research Centre (BRC), the Imperial Experimental Cancer Medicine Centre (ECMC) and the Imperial College Tissue Bank.
Abbreviations
- DDR
DNA damage response and repair
- LS
Lynch syndrome
- HBOC
Hereditary breast-ovarian cancer syndrome
- FHC
Family history of cancer
- OS
Overall survival
- PFS
Progression-free survival
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- NSCLC
Non-small cell lung cancer
- HR
Hazard ratio
- DCR
Disease control rate
Authors' contributions
All authors contributed to the publication according to the ICMJE guidelines for the authorship (study conception and design, acquisition of data, analysis, and interpretation of data, drafting of manuscript, critical revision). All authors read and approved the submitted version of the manuscript (and any substantially modified version that involves the author's contribution to the study). Each author has agreed both to be personally accountable for his own contribution and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All alive patients provided written, informed consent to participate. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. The study was approved by the respective local ethical committees on human experimentation of each institution, after previous approval by the coordinating center (Comitato Etico per le province di L’Aquila e Teramo, verbal N.15 del 28 November 2019).
Consent for publication
Not applicable.
Competing interests
Dr. Alessio Cortellini received speaker fees and grant consultancies by Astrazeneca, MSD, BMS, Roche, Novartis. Dr. Sebastiano Buti received honoraria as speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca, and Novartis. Dr. Raffaele Giusti received speaker fees and grant consultancies by Astrazeneca and Roche. Dr. Joachim GJV Aerts reports receiving commercial research grants from Amphera and Roche, holds ownership interest (including patents) in Amphera BV, and is a consultant/advisory board member for Amphera, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, MSD, and Roche. Dr. Alex Friedlaender received grant consultancies by Roche, Pfizer, Astellas, and BMS. Dr. Francesca Mazzoni received grant consultancies by MSD and Takeda. Dr. Rita Chiari received speaker fees by BMS, MSD, Takeda, Pfizer, Roche, and Astrazeneca. Dr Carlo Genova received speaker fees/grant consultancies by Astrazeneca, BMS, Boehringer-Ingelheim, Roche, and MSD. Dr. Marco Russano received honoraria for scientific events by Roche, Astrazeneca, BMS, MSD and Boehringer Ingelheim. Dr. Emilio Bria received speaker and travel fees from MSD, Astra-Zeneca, Pfizer, Helsinn, Eli-Lilly, BMS, Novartis, and Roche; grant consultancies by Roche and Pfizer. Dr. Alfredo Addeo received grant consultancies by Takeda, MSD, BMJ, Astrazeneca, Roche and Pfizer. Dr. Massimo Di Maio received research funding from Tesaro-GlaxoSmithKline; acted in a consulting/advisory role for Novartis, Pfizer, Eisai, Takeda, Janssen, Astellas, Roche, AstraZeneca. Dr. Marcello Tiseo received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen, Merck. He also received institutional research grants from Astra-Zeneca, Boehringer Ingelheim. Dr. Gian Paolo Spinelli received advisory board/editorial collaboration fees from Novartis, Servier, Teva; Bayer; Genetic, Epionpharma. Dr. David J Pinato received lecture fees from ViiV Healthcare, Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, Astra Zeneca; received research funding (to institution) from MSD, BMS. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cerretelli G, Ager A, Arends MJ, et al. Molecular pathology of Lynch syndrome. J Pathol. 2020;250(5):518–531. doi: 10.1002/path.5422. [DOI] [PubMed] [Google Scholar]
- 2.Lee K, Seifert BA, Shimelis H, et al. Clinical validity assessment of genes frequently tested on hereditary breast and ovarian cancer susceptibility sequencing panels. Genet Med. 2019;21(7):1497–1506. doi: 10.1038/s41436-018-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamberti G, Andrini E, Sisi M, et al. Targeting DNA damage response and repair genes to enhance anticancer immunotherapy: rationale and clinical implication. Future Oncol. 2020;16(23):1751–1766. doi: 10.2217/fon-2020-0215. [DOI] [PubMed] [Google Scholar]
- 4.Cortellini A, Buti S, Bersanelli M, et al. Evaluating the role of FAMIly history of cancer and diagnosis of multiple neoplasms in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: the multicenter FAMI-L1 study. Oncoimmunology. 2020;9(1):1710389. doi: 10.1080/2162402X.2019.1710389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50% Cancer Immunol Immunother. 2020;69(11):2209–2221. doi: 10.1007/s00262-020-02613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortellini A, Friedlaender A, Banna GL, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020 doi: 10.1016/j.cllc.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Cortellini A, Circuity B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403. doi: 10.1136/jitc-2020-001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortellini A, Cannita K, Tiseo M, et al. Post-progression outcomes of NSCLC patients with PD-L1 expression ≥ 50% receiving first-line single-agent pembrolizumab in a large multicentre real-world study. Eur J Cancer. 2021;148:24–35. doi: 10.1016/j.ejca.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Cortellini A, Di Maio M, Nigro O, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9(4):e002421. doi: 10.1136/jitc-2021-002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricciuti B, Recondo G, Spurr LF, et al. Impact of DNA damage response and repair (DDR) gene mutations on efficacy of PD-(L)1 immune checkpoint inhibition in non-small cell lung cancer. Clin Cancer Res. 2020;26(15):4135–4142. doi: 10.1158/1078-0432.CCR-19-3529. [DOI] [PubMed] [Google Scholar]
- 11.Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523–1531. doi: 10.1016/S1470-2045(17)30597-1. [DOI] [PubMed] [Google Scholar]
- 12.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Alencar VTL, Formiga MN, de Lima VCC. Inherited lung cancer: a review. Ecancermedicalscience. 2020;14:1008. doi: 10.3332/ecancer.2020.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jove M, Gausachs M, Bosch-Barrera J, et al. Prospective study of germline and somatic alterations for early onset lung cancer patients (EOLUNG MASTER protocol) J Clin Oncol. 2019;37(15_suppl):T9122. doi: 10.1200/JCO.2019.37.15_suppl.TPS9122. [DOI] [Google Scholar]
- 15.Dietel M, Savelov N, Salanova R, et al. Real-World prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–179. doi: 10.1016/j.lungcan.2019.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary materials.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.