Abstract
In the province of Quebec, Canada, from 1996 to 1998, 3,650 invasive Streptococcus pneumoniae infections were reported. A total of 1,354 isolates were serotyped and tested for antimicrobial susceptibility. The distribution of serotypes remained stable over the 3 years, with serotypes 14, 6B, 4, 9V, 23F, and 19F accounting for 61% of the isolates. Overall, 90% of isolates were included in the current 23-valent vaccine and 67% were included in the 7-valent conjugate vaccine. We were able to determine that resistance to penicillin and to other antibiotics is increasing.
Streptococcus pneumoniae is a significant bacterial pathogen causing pneumonia, meningitis, otitis media, and bacteremia (17). There is a worldwide increase in the incidence of pneumococcal resistance to several antimicrobial agents (3, 9, 15, 16, 18, 33). In some areas of the United States, as many as 35% of invasive isolates have been reported to be nonsusceptible to penicillin (5, 6). In Canada, prior to 1990, the rates of penicillin resistance were less than 5% (1, 8, 13, 19, 23). During the last decade, reports from Canada have identified increasing rates of nonsusceptibility to penicillin, ranging from 7.8 to 20% for invasive isolates (21, 32). In Quebec, resistant strains, especially those of serotype 23F, as well as multiresistant strains have been identified (14). The Laboratoire de Santé Publique du Québec (LSPQ) initiated, in November 1995, a surveillance program in order to monitor the invasive pneumococcal infections in Quebec and to provide the Ministry of Health with data to be used in establishing pneumococcal vaccination policies. In this article, we report the data obtained from 1996 to 1998.
Since the beginning of this surveillance program, all hospital laboratories (134 in 1996 to 114 in 1998) have reported on a monthly basis the total number of S. pneumoniae strains isolated from normally sterile body fluids, using a questionnaire sent to the LSPQ. The total population of the province of Quebec was estimated at 7,396,742 people in 1996 (28). The surveillance case definition was the isolation of S. pneumoniae in a normally sterile body fluid such as blood or cerebrospinal fluid from patients of any age (one isolate per patient per 14-day period). Twenty-seven hospitals, located throughout major regions of the province and cities, were enrolled as sentinel units and asked to send all of their invasive pneumococcal isolates to the LSPQ. Identification of isolates was confirmed by colony morphology, susceptibility to ethylhydrocuprein, and agglutination with antipneumococcal polysaccharide capsule antibodies by a Quellung reaction (22). Serotyping was performed by the capsular swelling method (22) using antisera from Statens Seruminstitut, Copenhagen, Denmark, in collaboration with Marguerite Lovgreen, National Center for Streptococcus, University of Alberta, Edmonton, Canada. Testing of susceptibility to penicillin G, ceftriaxone and ofloxacin was performed by using the broth microdilution method, and testing of susceptibility to chloramphenicol, erythromycin, rifampin, trimethoprim-sulfamethoxazole (TMP-SMX), and vancomycin was performed by the agar dilution method during the first half of the study and by broth microdilution thereafter. Both methods were performed in accordance with those of the National Committee for Clinical Laboratory Standards (25, 26). Antibiotic powders were purchased from Sigma Chemical Co., St. Louis, Mo. We defined reduced susceptibility as including intermediate and resistant categories and multiple resistance as including reduced susceptibility to three or more classes of antimicrobial agents. Information on age, sex, type of infection, and clinical outcome for each patient at the time the isolate was shipped to the LSPQ was provided by each sentinel unit. Statistical analysis of the data was performed by the chi-square test (34). A P value of <0.05 was considered statistically significant.
During the 3-year period of this study, a total of 3,650 cases of invasive S. pneumoniae infection were reported, for a mean incidence of 16.5 per 100,000 persons annually (15.5 during 1996, 17.3 during 1997, and 16.6 during 1998). Although the total number of cases remained stable over these years (1996, 1,139; 1997, 1,280; 1998, 1,231), the distribution was seasonal, reflecting the influence, during the winter, of the respiratory viral infection season on the risk of acquiring pneumococcal pneumonia.
A total of 1,354 isolates representing 37% of all reported isolates were received at the LSPQ (450 during 1996, 434 during 1997, and 470 during 1998) for analysis. Strains were isolated from blood (93.4%), cerebrospinal fluid (4.1%), or other normally sterile sites (2.5%). Table 1 shows the distribution of the serotypes by age. Overall, 90% of the isolates (1,217 of 1,354) were included in the current 23-valent vaccine and 67% (904 of 1,354) were included in the new 7-valent conjugate vaccine.
TABLE 1.
Distribution of serotypes with respect to the age of the patient and serotype inclusion in the 23-valent vaccine or in the 7-valent conjugated vaccine
| Serotype | No. of patients who were:
|
||||
|---|---|---|---|---|---|
| <2 yr old (n = 242) | 2 to <5 yr old (n = 97) | 5 to <10 yr old (n = 29) | 10 to <18 yr old (n = 20) | ≥18 yr old (n = 966) | |
| Included in the 7- and 23-valent vaccines | |||||
| 4 | 10 | 7 | 3 | 4 | 114 |
| 6B | 53 | 13 | 1 | 1 | 89 |
| 9V | 14 | 2 | 6 | 4 | 101 |
| 14 | 73 | 27 | 4 | 0 | 116 |
| 18C | 17 | 16 | 5 | 0 | 40 |
| 19F | 32 | 7 | 1 | 0 | 43 |
| 23F | 20 | 7 | 3 | 0 | 71 |
| Included in the 23-valent vaccine only | |||||
| 3 | 3 | 0 | 0 | 1 | 66 |
| 7F | 0 | 1 | 0 | 5 | 39 |
| 8 | 0 | 0 | 0 | 0 | 19 |
| 9N | 2 | 0 | 0 | 0 | 27 |
| 11A | 0 | 0 | 0 | 1 | 20 |
| 19A | 5 | 5 | 1 | 0 | 18 |
| 22F | 0 | 2 | 0 | 0 | 50 |
| 33F | 0 | 1 | 0 | 0 | 11 |
| Othersa | 1 | 2 | 1 | 2 | 36 |
| Not included in vaccines | |||||
| 6A | 7 | 3 | 2 | 0 | 41 |
| Othersb | 5 | 4 | 2 | 2 | 65 |
Serotypes 1, 2, 5, 10A, 12F, 15B, 17F, and 20.
Serotypes 7C, 10F, 11B, 13, 15A, 15C, 16F, 18B, 18F, 22A, 23A, 35B, 35F, 38, and 42.
Globally, 25% (342 of 1,354) of the isolates were nonsusceptible to one or more of the antimicrobial agents studied. Table 2 shows the percentage of isolates with reduced susceptibility to each antibiotic tested over the 3 years. Globally, nonsusceptibilities to penicillin, ceftriaxone, chloramphenicol, erythromycin, and TMP-SMX were 11.7, 7.7, 2.7, 8.1, and 20.2%, respectively. Nine isolates were found to be nonsusceptible to ofloxacin during 1998, including eight intermediate isolates (MICs of 4 μg/ml). One isolate showing an ofloxacin MIC of 16 μg/ml was also intermediate to penicillin and resistant to TMP-SMX. All isolates were susceptible to vancomycin. The serotypes of the 158 penicillin-nonsusceptible isolates were those commonly associated with penicillin resistance: 9V (55 isolates), 14 (30 isolates), 23F (27 isolates), 6B (19 isolates), 19A (9 isolates), 6A (9 isolates), 19F (6 isolates), 17F (2 isolates), and 35B (one isolate), with the last being the only one not included in the 23-valent vaccine. Among the 36 penicillin-nonsusceptible isolates recovered from patients under 2 years old, 34 (94%) were included in the 7-valent conjugate vaccine. Among the 79 meningitis cases, five and six isolates were intermediate and resistant to penicillin, respectively, and six isolates were intermediate to ceftriaxone (MIC of 1 μg/ml). There were nine fatal cases of meningitis, all caused by strains susceptible to both antibiotics. During 1998, we observed that the proportion of penicillin-nonsusceptible isolates was significantly higher for patients under 2 years of age than for older patients (25.3 versus 11.2%) (P < 0.01). Also, the proportion of nonsusceptible isolates among young children was found to increase over the years from 7.9 to 25.3% (P < 0.005, chi-square for trend). Multiple resistance patterns were observed in 56 of the 1,354 isolates (4.1%). The isolates were resistant to β-lactams (except for four isolates) and to at least two of the following agents: erythromycin, chloramphenicol, TMP-SMX, and ofloxacin.
TABLE 2.
Antimicrobial susceptibility data for a total of 1,354 pneumococcal isolates in Quebec from 1996 to 1998
| Yr (n) | % Intermediate/% resistant toa:
|
||||||
|---|---|---|---|---|---|---|---|
| PEN | CEF | CHL | ERY | RIF | TMP-SMX | OFL | |
| 1996 (450) | 2.9/6.9 | 6.0/1.1 | 0/2.0 | 0/4.9 | 0/0.2 | 4.7/14.7 | NDb |
| 1997 (434) | 5.3/6.2 | 6.7/0.5 | 0/2.3 | 0/9.5 | 0/0 | 6.2/16.6 | ND |
| 1998 (470) | 5.7/7.9 | 7.9/0.8 | 0/3.8 | 0/10.0 | 0/0 | 5.3/16.4 | 1.7/0.2 |
| Total | 4.7/7.0 | 6.9/0.8 | 0/2.7 | 0/8.0 | 0/0.1 | 5.4/14.8 | 1.7/0.2 |
No isolate was found to be resistant to vancomycin. PEN, penicillin; CEF, ceftriaxone; CHL, chloramphenicol; ERY, erythromycin; RIF, rifampin; OFL, ofloxacin.
ND, not determined.
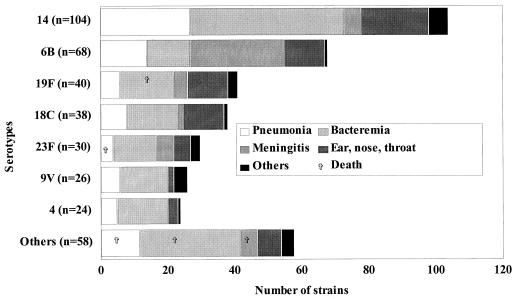
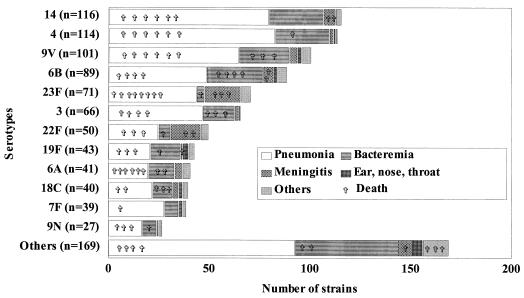
The types of infection and the clinical outcomes with respect to serotypes in both pediatric and adult groups are shown in Fig. 1 and 2. In pediatric patients, bacteremia without a primary focus of infection was most frequent (42%), while pneumonia was predominant (61.5%) in adults. The overall mortality rate was 7.2% (97 of 1,354 patients). This fatality rate, which is lower than the 12% reported in the United States (10), could be explained partly by the fact that the present study used clinical information that was available at the time the isolates were sent to the LSPQ by the sentinel units. Thus, the mortality rate does not include those patients who may have died after the clinical information and isolates were forwarded. However, the mortality rate increased in relation to age, with a rate of 1.3% (5 of 388) in patients under 18 years of age (including 4 under 2 years of age), 7.4% (34 of 458) in patients between 18 and 64 years old, and 11.4% (58 of 508) in those ≥65 years old (P < 0.05).
FIG. 1.
Diagnosis and outcome for 388 patients <18 years old according to serotype.
FIG. 2.
Diagnosis and outcome for 966 patients ≥18 years old according to serotype.
The observed mean incidence rate is lower than that of 24.1/100,000 reported among persons of all ages in the United States during 1998 by the Centers for Disease Control and Prevention (Active Bacterial Core Surveillance Report [http://www.cdc.gov/ncidod/dbmd/abcs]) but is consistent with a rate of 14.7/100,000 reported in metropolitan Toronto and the Peel region in 1995 (J. D Kellner et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-44, 1998).
As observed a decade ago in Quebec (13) and in other parts of Canada (21), the serotype coverage of the 23-valent vaccine remains at least 90% for adult patients and those aged 2 to 18 years. In the event that the 7-valent conjugate vaccine (29, 31) is licensed for use in Canada, 90% of infections occurring among children <2 years of age could potentially be prevented by immunization. Considering that the conjugate 7-valent vaccine has been licensed in the United States for use in children under 10 years of age, 87.2% of cases in this age group would be preventable. If the recommendations were to vaccinate younger children under 5 years of age, 88% of cases would then be preventable.
Since our last report (13), the prevalence of isolates with reduced susceptibility to penicillin increased dramatically, from 1.6% (6 of 468 isolates) to 11.7% (158 of 1 354 isolates). Ten years ago, no fully penicillin-resistant isolates were found, while this study identified 7.0% of such strains. This increase in the number of isolates showing reduced susceptibility to penicillin is consistent with observations in Canada (20, 21, 30) and worldwide (2, 9, 12, 16, 18, 33). The most frequent serotype among penicillin-nonsusceptible isolates was serotype 9V, accounting for 35% of such isolates. A study conducted in Canada from 1991 to 1998 (D. Greenberg et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1030, 1999) concluded that this serotype appeared in Quebec, spreading to Ontario, Alberta, and British Columbia and was related to a Spanish-French clone (20, 24). As reported elsewhere (21, 27, 35), erythromycin resistance has also increased in Quebec, from 0.4% during 1984 to 1986 (13) to 4.9% in 1996 and 10% in 1998 (P < 0.05). The high proportion of isolates with reduced susceptibility to TMP-SMX observed is consistent with Canadian surveys reporting rates of 18.5% (32) and 19.5% (21). The ofloxacin nonsusceptibility rate is similar to that in other Canadian studies reporting <2.0% of strains as nonsusceptible to fluoroquinolones (J. A. Karlowsky, G. G. Zhanel, and D. J. Hoban, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 822, 1999) (7), confirming that resistance to fluoroquinolones is very low in Quebec.
We observed a mortality rate of 11.4% in the elderly, confirming that age by itself is one of the most important factors associated with fatal pneumococcal pneumonia (4, 11, 13).
This study has shown that the formulation of the current 23-valent vaccine and the recently licensed 7-valent conjugate vaccine will cover the great majority of isolates implicated in the respective target groups, supporting the use of vaccination programs as a means to prevent pneumococcal infection.
Since the fall of 1999, in the province of Quebec, the Ministry of Health has funded a vaccination program for persons 2 years old and older with risk factors for severe pneumococcal infections. This program will be extended to the elderly (65 years and older) during the fall of 2000. The implementation of an immunization program with the 7-valent conjugate vaccine must await its licensing for use in Canada.
Our continuing surveillance program will enable us to monitor the impact of new publicly funded antipneumococcal vaccination programs in Quebec.
Acknowledgments
We acknowledge Stéphane Charbonneau, Lucie Cormier, Martial Demers, Robert Langevin, and François Robillard for their technical assistance. We are grateful to Luc Massicotte for the supervision of medium preparation and to Johanne Lefevbre for identification expertise. We also thank all the technologists in hospital microbiology laboratories who sent us pneumococcal strains. We acknowledge Marguerite Lovgren of the National Centre for Streptococcus, University of Alberta, Edmonton, Canada, for her contribution to the serotyping. Finally, we thank Lucie Carrière for secretarial assistance.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Butler J C, Dowell S F, Breiman R F. Epidemiology of emerging pneumococcal drug resistance: implications for treatment and prevention. Vaccine. 1998;16:1693–1697. doi: 10.1016/s0264-410x(98)00132-7. [DOI] [PubMed] [Google Scholar]
- 3.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F the Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's pneumococcal sentinel surveillance system. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 4.Butler J C, Schuchat A. Epidemiology of pneumococcal infections in the elderly. Drugs Aging. 1999;15:S11–S19. doi: 10.2165/00002512-199915001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Geographic variation in penicillin resistance in Streptococcus pneumoniae—selected sites, United States, 1997. Morb Mortal Wkly Rep. 1999;48:656–661. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevention of pneumocccal disease. Morb Mortal Wkly Rep. 1997;46:1–24. [Google Scholar]
- 7.Chen D K, McGeer A, De Azavedo J C, Low D W. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 8.Dixon J M S, Lipinski A E, Graham M E P. Detection and prevalence of pneumococci with increased resistance to penicillin. Can Med Assoc J. 1977;177:1159–1161. [PMC free article] [PubMed] [Google Scholar]
- 9.Doern G V, Pfaller M A, Kugler K, Freeman J, Jones R N. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY Antimicrobial Surveillance Program. Clin Infect Dis. 1998;27:764–770. doi: 10.1086/514953. [DOI] [PubMed] [Google Scholar]
- 10.Feikin D R, Schuchat A, Kolczak M, Barrett N L, Harrison L H, Lefkowitz L, McGeer A, Farley M M, Vugia D J, Lexau C, Stefonek K R, Patterson J E, Jorgensen J H. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am J Public Health. 2000;90:223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fein A M. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28:726–729. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 12.Fluit A C, Jones M E, Schmitz F-J, Acar J, Gupta R, Verhoef J the SENTRY Participants Group. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Can Infect Dis. 2000;30:454–460. doi: 10.1086/313710. [DOI] [PubMed] [Google Scholar]
- 13.Jetté L P, Lamothe F the Pneumococcus Study Group. Surveillance of invasive Streptococcus pneumoniae infection in Quebec, Canada, from 1984 to 1986: serotype distribution, antimicrobial susceptibility, and clinical characteristics. J Clin Microbiol. 1989;27:1–5. doi: 10.1128/jcm.27.1.1-5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetté L, Ringuette P L, Dascal A, Lapointe J-R, Turgeon P. Pneumococcal resistance to antimicrobial agents in the province of Québec, Canada. J Clin Microbiol. 1994;32:2572–2575. doi: 10.1128/jcm.32.10.2572-2575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan S L, Mason E O, Barson W J, Wald E R, Arditi M, Tan T Q, Schutze G E, Bradley J S, Givner L B, Kim K S, Yogev R. Three-year multicenter surveillance of systemic pneumococcal infections in children. Pediatrics. 1998;102:538–545. doi: 10.1542/peds.102.3.538. [DOI] [PubMed] [Google Scholar]
- 16.Kertesz D A, Di Fabio J L, de Cunto Brandileone M C, Castaneda E, Echaniz-Aviles G, Heitmann I, Homma A, Hortal M, Lovgren M, Ruvinsky R O, Talbot J A, Weekes J, Spika J S the PAHO Pneumococcal Surveillance Study Group. Invasive Streptococcus pneumoniae infection in Latin American children: results of the Pan American Health Organization surveillance study. Clin Infect Dis. 1998;26:1355–1361. doi: 10.1086/516350. [DOI] [PubMed] [Google Scholar]
- 17.Klein D L. Pneumococcal disease and the role of conjugate vaccines. Microb Drug Resist. 1999;5:147–157. doi: 10.1089/mdr.1999.5.147. [DOI] [PubMed] [Google Scholar]
- 18.Linares J, Pallares R, Alonso T, Perez J L, Ayats J, Gudiol F, Viladrich P F, Martin R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1980) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 19.Loo V G, Lavellée J, McAlear D, Robson H G. The in-vitro susceptibilities of 326 Streptococcus pneumoniae isolates to nine antimicrobial agents including penicillin and newer quinolones. J Antimicrob Chemother. 1994;33:641–645. doi: 10.1093/jac/33.3.641. [DOI] [PubMed] [Google Scholar]
- 20.Louie M, Louie L, Papia G, Talbot J, Lovgren M, Simor A E. Molecular analysis of the genetic variation among penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae serotypes in Canada. J Infect Dis. 1999;179:892–900. doi: 10.1086/314664. [DOI] [PubMed] [Google Scholar]
- 21.Lovgren M, Spika J S, Talbot J A. Invasive Streptococcus pneumoniae infections: serotype distribution and antimicrobial resistance in Canada, 1992–1995. Can Med Assoc J. 1998;158:327–331. [PMC free article] [PubMed] [Google Scholar]
- 22.Lund E, Henrichsen J. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods Microbiol. 1978;12:241–262. [Google Scholar]
- 23.Mazzulli T, Simor A E, Jaeger R, Fuller S, Low D E. Comparative in vitro activities of several new fluoroquinolones and β-lactam antimicrobial agents against community isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1990;34:467–469. doi: 10.1128/aac.34.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melander E, Ekdahl K, Hansson H B, Kamme C, Laurell M, Nilsson P, Persson K, Söderstrom M, Mölstad S. Introduction and clonal spread of penicillin- and trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae, serotype 9V, in Southern Sweden. Microb Drug Resist. 1998;4:71–78. doi: 10.1089/mdr.1998.4.71. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria which grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing, 7th informational supplement M100-S7. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 27.Oster P, Zanchi A, Cresti S, Lattanzi M, Montagnani F, Cellesi C, Rossolini G M. Patterns of macrolide resistance determinants among community-acquired Streptococcus pneumoniae isolates over a 5-year period of decreased macrolide susceptibility rates. Antimicrob Agents Chemother. 1999;43:2510–2512. doi: 10.1128/aac.43.10.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelletier G. La population du Québec par territoire de CLSC, de DSC et de RSS pour la période 1981 à 2016. Québec, Canada: Ministère de la Santé et des Services Sociaux, Direction Générale de la Planification et de l'Évaluation; 1996. [Google Scholar]
- 29.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 30.Scheifele D, Gold R, Marchessault V, Talbot J the LCDC/CPS Impact Group. Penicillin resistance among invasive pneumococccal isolates at 10 children's hospitals, 1991–1994. Can Commun Dis Rep. 1996;22:157–160. [PubMed] [Google Scholar]
- 31.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 32.Simor A E, Louie M, Low D E the Canadian Bacterial Surveillance Network. Canadian national survey of prevalence of antimicrobial resistance among clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2190–2193. doi: 10.1128/aac.40.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J-H, Lee N Y, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu C-H, Lalitha M K, Thomas K, Perera J, Yee T T, Jamal F, Warsa U C, Vinh B X, Jacobs M R, Appelbaum P C, Pai C H the ANSORP Study Group. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian network for surveillance of resistance of resistant pathogens (ANSORP) study. Clin Infect Dis. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel M R. Le test du Khi-deux. In: Ergas A, Marcotorchino J-F, editors. Théorie et applications de la statistique. 15th ed. Paris, France: McGraw-Hill; 1972. pp. 201–216. [Google Scholar]
- 35.Thornsberry C, Ogilvie P T, Holley H P, Sahm D F. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: a prospective U.S. study. Antimicrob Agents Chemother. 1999;43:2612–2623. doi: 10.1128/aac.43.11.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]