Abstract
Comprehensive sampling of the carbonate system in estuaries and coastal waters can be difficult and expensive because of the complex and heterogeneous nature of near-shore environments. We show that sample collection by community science programs is a viable strategy for expanding estuarine carbonate system monitoring and prioritizing regions for more targeted assessment. ‘Shell Day’ was a single-day regional water monitoring event coordinating coastal carbonate chemistry observations by 59 community science programs and seven research institutions in the northeastern United States, in which 410 total alkalinity (TA) samples from 86 stations were collected. Field replicates collected at both low and high tides had a mean standard deviation between replicates of 3.6 ± 0.3 μmol kg−1 (σmean ± SE, n = 145) or 0.20 ± 0.02%. This level of precision demonstrates that with adequate protocols for sample collection, handling, storage, and analysis, community science programs are able to collect TA samples leading to high-quality analyses and data. Despite correlations between salinity, temperature, and TA observed at multiple spatial scales, empirical predictions of TA had relatively high root mean square error >48 μmol kg−1. Additionally, ten stations displayed tidal variability in TA that was not likely driven by low TA freshwater inputs. As such, TA cannot be predicted accurately from salinity using a single relationship across the northeastern US region, though predictions may be viable at more localized scales where consistent freshwater and seawater endmembers can be defined. There was a high degree of geographic heterogeneity in both mean and tidal variability in TA, and this single-day snapshot sampling identified three patterns driving variation in TA, with certain locations exhibiting increased risk of acidification. The success of Shell Day implies that similar community science based events could be conducted in other regions to not only expand understanding of the coastal carbonate system, but also provide a way to inventory monitoring assets, build partnerships with stakeholders, and expand education and outreach to a broader constituency.
Keywords: ocean acidification, coastal acidification, total alkalinity, community science, citizen science, NECAN
1. Introduction
Ocean and coastal acidification (OCA) has emerged during the last decade as a topic of serious concern, because of its impacts on marine organisms and coastal economies [1, 2]. There is a strong scientific consensus about the drivers and projections of ocean acidification in the open ocean, but the dynamics of acidification in coastal ecosystems are less clear. In addition to absorption of carbon dioxide, the coastal carbonate system is driven by a number of factors including freshwater discharge, stratification, water residence time, eutrophication, biogeochemical processes, and upwelling [3–11]. Natural biogeochemical cycling, which can be strengthened by eutrophication, combined with site-specific differences in tidal flushing and water residence time, leads to large spatio-temporal variations in seawater carbonate system parameters [11–17].
Many drivers of OCA are strongly localized and are likely determined by characteristics specific to both watersheds and estuaries, rendering regional generalizations of OCA conditions difficult. As a result, the OCA research community has identified the need for additional monitoring to better understand the drivers of the coastal carbonate system and quantify localized risk of future OCA [18–21]. Monitoring conditions across multiple spatial and temporal scales is important for developing models that inform management and for identifying and prioritizing opportunities for mitigation and adaptation (e.g. [22–24]; see also state OCA Action Plans).
OCA risk assessments for coastal regions involve comprehensive analyses of current and potential future biogeochemical conditions and prediction of the ecological consequences of OCA, combined with knowledge of societal impacts within specific estuaries (e.g. [2, 25, 26]). Marine calcifiers are particularly at risk from OCA, and mollusks at most life stages are sensitive to reduced carbonate mineral saturation state (Ω) and pH (e.g. [27, 28]). Previous studies have consistently shown negative effects of OCA on critical shellfish life history stages, including fertilization, shell formation, and larval development (e.g. [18], and references therein, [29–31]). Reduced shell strength and growth, increased mortality, and altered behavior have been shown for juveniles and adults of some species, although responses in laboratory experiments have been variable, and both species and sub-population specific [18, 32–38]. Because of the strong potential sensitivity of mollusks to OCA and limited mobility within a coastal estuary, areas with significant wild shellfish populations and aquaculture operations are candidate locations for enhanced monitoring and determination of localized drivers of the carbonate system.
Total alkalinity (TA), a measure of the ability of a solution to resist a change in pH, is one of four parameters that describes the seawater carbonate system. Sample collection is straightforward due to the lack of sensitivity of TA to gas exchange, and sample storage over short periods of time does not require inhibition of biological activity with poisoning agents such as mercuric chloride [39, 40]. In the absence of biological processes, TA is also conservative with salinity, and relationships between salinity and TA have been used in the monitoring of coastal acidification [41]. TA can be a useful indicator of marine ecosystems’ vulnerability to acidification pressure from various CO2 sources; however, TA is not typically monitored by community science organizations because of financial and analytical barriers. The widespread adoption of community science for water quality monitoring has overcome these hurdles for other parameters, e.g. temperature, salinity, dissolved oxygen, and nutrients [42–45]. In addition, community science has proven to be important for public outreach, engagement, education, and adoption of practices that expand and promote environmental stewardship. Many monitoring organizations have also been vocal advocates of the development and implementation of management solutions to improve coastal water quality such as garnering support for nutrient pollution regulation, upgrades to wastewater treatment facilities, and expansion of sewer networks. Expanding site-specific monitoring programs to include observations of coastal carbonate chemistry may be a capacity-building step toward public education and the implementation of local management actions to reduce the drivers of acidification [22, 24, 46, 47].
We carried out ‘Shell Day’ on 22 August 2019, as a synoptic water monitoring event coordinating coastal TA observations among community science programs and research institutions from Long Island Sound (LIS) to Downeast Maine. To our knowledge, this study represents the first large-scale set of synchronous measurements of salinity and TA along the northeastern United States coast. The sampling was motivated by two years of outreach and capacity-building activities by the Northeast Coastal Acidification Network aimed at training community-based water monitoring programs in methods to measure carbonate chemistry parameters [40, 47], an effort to be detailed in a companion manuscript, Gassett et al [48]. Shell Day had three major goals: (a) to evaluate the efficacy of community science for TA monitoring; (b) to assess geographic heterogeneity in mean and tidal variability in TA; and (c) to determine if a regional relationship between salinity, temperature, and TA could be used to estimate TA. This manuscript describes the successes, shortcomings, and uncertainties in achieving these goals.
2. Methods
2.1. Site selection and sampling design
Minimum requirements for participation in Shell Day were the capacity to measure water temperature and salinity; some organizations used thermometers and refractometers, and others used multiparameter datasondes or handheld units. Fifty-nine water monitoring organizations participated, collecting samples from 86 stations. Sampling stations were chosen by individual monitoring organizations, with suggested criteria for choosing locations including: stations with long sampling records, proximity to wild shellfish populations, shellfish aquaculture operations or hatcheries, stations with relatively easy access to facilitate repetitive sampling, and/or stations with large variability in salinity.
An Environmental Protection Agency (EPA) approved quality assurance project plan was developed along with a datasheet, sampling protocol, training video, and a webinar tutorial to instruct community scientists on a standardized sampling protocol (all available at necan.org/shellday). Surface water samples were collected in pre-cleaned and labeled borosilicate glass or HDPE bottles provided to participating organizations. Samples were collected at low, mid, and high tides at each station to assess the tidal variation of TA. Samples were collected directly from the water body or using common sampling devices such as buckets, Van Dorn samplers, or Niskin bottles. Temperature, salinity, and other water characteristics were measured either directly in the water body at the targeted depth of sample collection (handheld units and multiparameter datasondes) or from the water collected in a container (refractometers). To assess the consistency of the sampling protocol, sample handling, and sample storage, field duplicates were collected at both low and high tides. Water samples were placed on ice and stored in the dark upon collection and either returned to the lab on the day of collection or stored overnight on ice until samples could be returned to the nearest laboratory. Upon delivery to a laboratory, water samples were either analyzed immediately or fixed by laboratory staff with saturated mercuric chloride to inhibit biological activity and analyzed over several weeks. Participants were also asked to provide metadata such as location (upper/mid/lower estuary), proximity to wild shellfish populations or aquaculture operations (yes/no/unknown), and other information such as salinity instrument type and date of last calibration. In order to ensure safety of volunteers collecting water samples, participants were notified during the pre-sampling training webinar about inclement weather plans, and no volunteers handled hazardous laboratory materials.
Spatial data layers from the Northeast Ocean Data Portal (NODP) on commercial aquaculture operations [49] and shellfish habitat suitability [50] were used to corroborate participant responses and identify other sampling stations located within 1 km of wild shellfish populations and aquaculture operations.
2.2. Sample processing
Seven laboratories analyzed samples for TA via automated open-cell Gran titration (Method 1: [51]) or modified single-point titration (Method 2: [52]) (table 1). Each laboratory used certified reference material (CRM) from Dr A G Dickson’s laboratory at the Scripps Institute of Oceanography to standardize measurements. Although an inter-laboratory comparison would increase the confidence in and comparability of our results, such comparison was beyond the scope of this study. TA data were quality controlled by each laboratory based on instrument performance, laboratory replicates, and analyses of CRM. Data were excluded from analyses if the standard deviation between field duplicates was greater than 1% of the mean. Reported salinity was converted from practical salinity to absolute salinity using the Gibbs Seawater Matlab toolbox [53]. A subset of 11 samples had salinities verified on a benchtop salinometer (Guildline Portasal).
Table 1.
List of laboratory facilities and instruments. Samples were analyzed for TA via automated open-cell gran titration (1) or modified single-point titration (2).
| Partnering laboratory | Titrator brand | Method |
|---|---|---|
|
| ||
| Bowdoin College | Metrohm 905 Titrando | 1 |
| EPA Atlantic Coastal Environmental Sciences Division | Apollo SciTech Model AS-ALK2 | 1 |
| Northeastern University Marine Science Center | VINDTA 3C (Marine Analytics and Data) | 1 |
| Massachusetts Institute of Technology | Custom built by Andrew Dickson Laboratory UCSD | 1 |
| Woods Hole Oceanographic Institution | Metrohm 808 Titrando | 1 |
| University of Connecticut | Contros HydroFIA | 2 |
| University of New Hampshire | Contros HydroFIA | 2 |
2.3. Data analysis
Empirical relationships between physical variables (temperature, salinity, latitude) and TA were evaluated for both the entire dataset and groupings of stations by subregion (figure 1) using simple linear regression (salinity only) and multiple linear regression (MLR) using equations with similar form as Juranek et al [54] and Alin et al [3]:
| (1) |
| (2) |
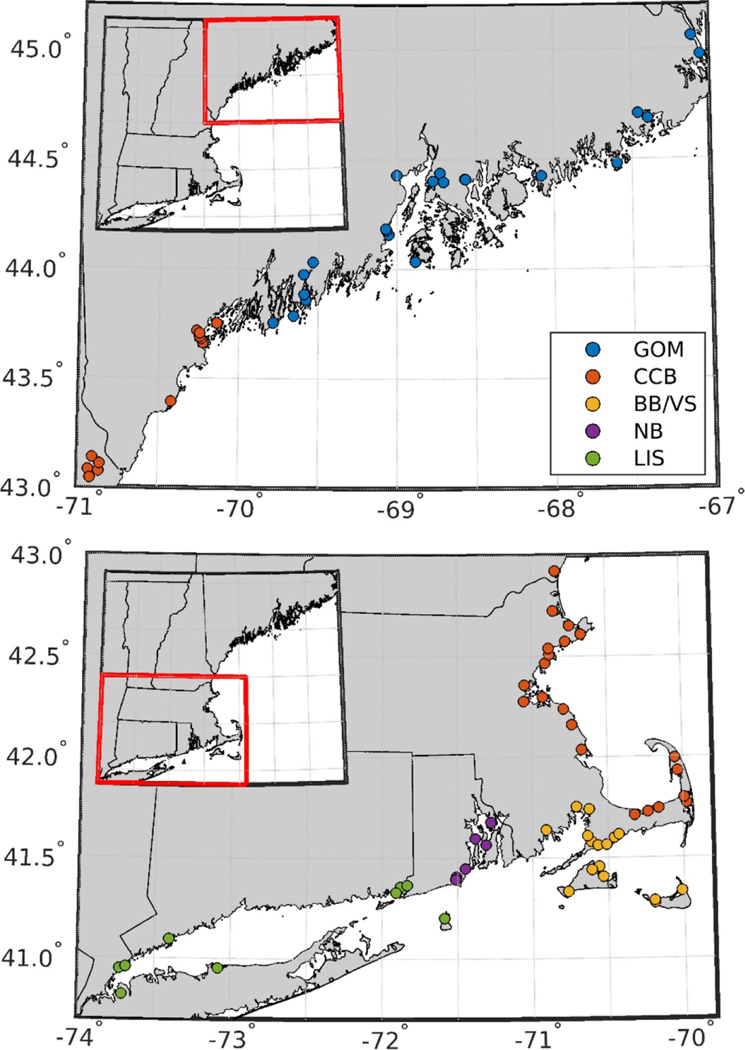
where TA is total alkalinity, S is salinity, T is temperature, Lat is latitude, the r-subscript indicates a reference temperature and salinity, defined as the mean temperature or salinity for the dataset analyzed and the subscript i indicates subregions. Subregion delineation was informed by Gledhill et al [18], with station groupings including LIS, Narragansett Bay (NB), Buzzards Bay/Vineyard Sound (BB/VS), Cape Cod Bay/Central Gulf of Maine (CCB), and northern Gulf of Maine (GOM) (see figure 1). Latitude was only included as a predictor variable when evaluating the entire dataset (equation (1)).
Figure 1.
Sampling locations. Sampling stations are colored by geographic groupings corresponding to regions in tables 2 and 3, and figure 3. Groupings are the northern Gulf of Maine (GOM, blue), central Gulf of Maine/Cape Cod Bay (orange, CCB), Buzzards Bay/Vineyard Sound (BB/VS, yellow), Narragansett Bay (NB, purple), and Long Island Sound (LIS, green).
3. Results
A total of 410 samples were collected. Field duplicates were collected at low and high tides at most stations, leading to 264 unique samples. Eight sets of field duplicates with a%-standard-deviation from the mean of more than 1% were excluded from this analysis. There was good agreement between the remaining pairs with a mean standard deviation between duplicates of 3.6 ± 0.3 μmol kg−1 (±SE, n = 145) or 0.20 ± 0.02%. The TA of 122 of 145 sets of duplicates (84.1%) differed by less than 10 μmol kg−1. Laboratory verification of a subset of salinity measurements (n = 11) showed average differences between field and lab salinity (±SD) of 1.9 ± 2.1, and salinometer measurements were used in place of field observations where available. Additionally, ten field salinity values were higher than typically observed in the coastal New England region (>34) and were excluded from the interpretation.
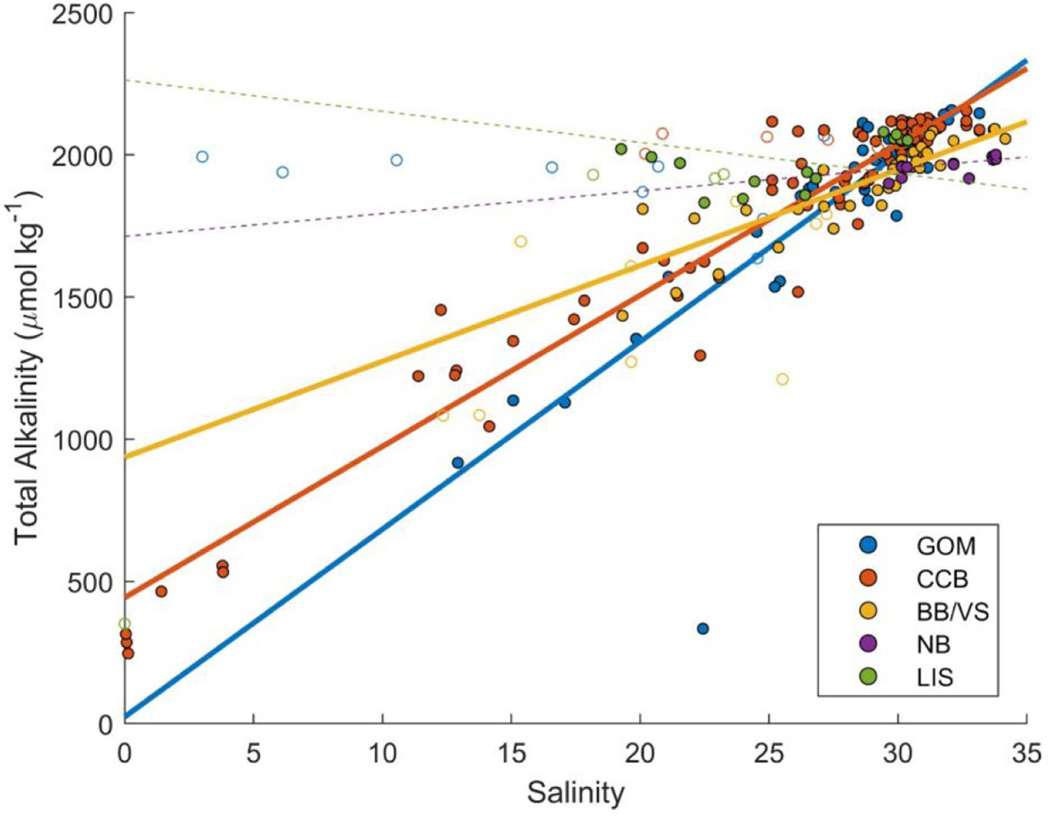
There was a high degree of geographic variation in TA (table 2), and also in station mean and distribution over a tidal cycle (figure 2). Simple regression analysis indicated a correlation between salinity and TA across the entire dataset (r2 = 0.668, p < 0.0001, not shown) that was improved (r2 = 0.820, p < 0.0001, table S1 and figure S1 (which are available online at stacks.iop.org/ERL/16/024009/mmedia)) by excluding data from stations where high tidal variability in either salinity or TA was not accompanied by variability in the other parameter (figure 3, open circles, see paragraph below). The best correlations, with both the highest r2 and lowest root mean squared error (RMSE), were achieved by incorporating temperature (for all data combined and by subregion) and latitude (for all data combined) as predictor variables via MLR (tables 3 and S2, figure 3 filled circles only). Despite a relatively high r2 for fits combining all data, RMSE was large (121.1 μmol kg−1). Analyzing the data in groupings by subregion improved the prediction for some regions and worsened the prediction for others (figure 3, tables 3, S1 and S2).
Table 2.
Number of sampling stations, mean total alkalinity (TA), and salinity by subregion. Values in parentheses are standard deviation. The number of observations may differ between TA and salinity due to quality control measures for each parameter.
| Region | Number of stations | TA (μmol kg−1) | Number of observations | Salinity | Number of observations |
|---|---|---|---|---|---|
|
| |||||
| GOM | 22 | 1899.9 (335.8) | 60 | 26.1 (7.1) | 60 |
| CCB | 33 | 1813.0 (457.7) | 95 | 25.1 (8.5) | 99 |
| BB/VS | 16 | 1824.8 (253.8) | 48 | 27.0 (5.5) | 43 |
| NB | 6 | 1968.3 (37.1) | 15 | 31.9 (1.7) | 14 |
| LIS | 9 | 1881.3 (378.7) | 9 | 20.4 (9.8) | 23 |
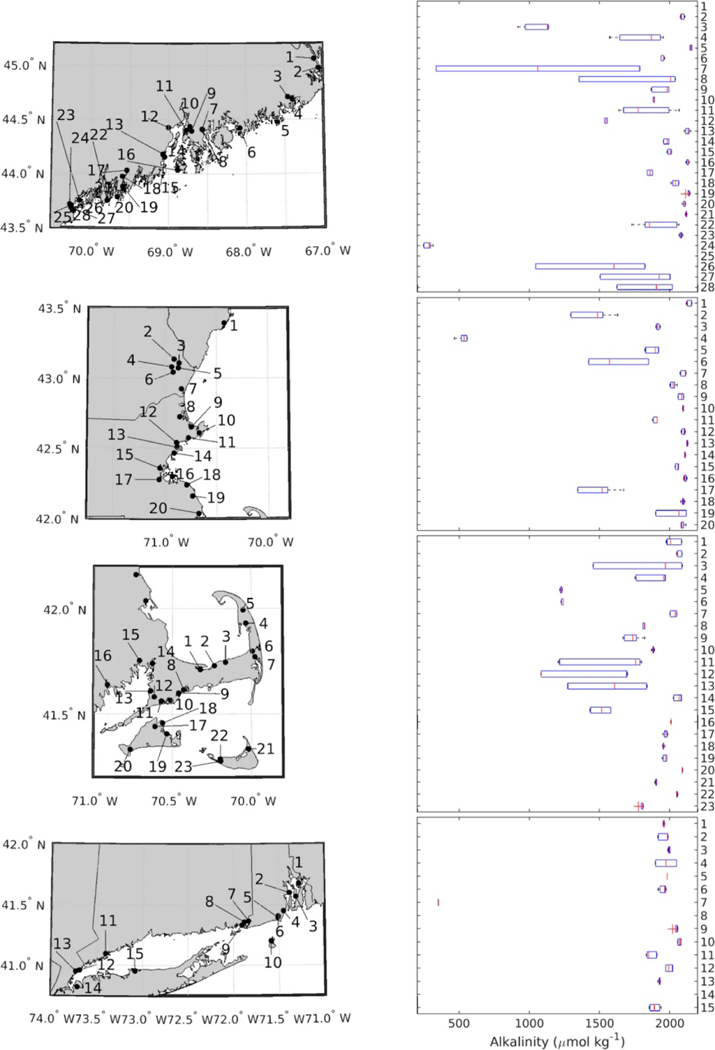
Figure 2.
Left panels show subsets of locations of sampling stations moving from north to south down the Northeast US coast: northeastern Maine (top), southwestern Maine, New Hampshire, northern Massachusetts (2nd), Cape Cod, Martha’s Vineyard, and Nantucket, Massachusetts (3rd), Rhode Island, Connecticut, and New York (bottom). Right panels show distribution of station alkalinity measurements. Boxplots are generated using all data from each station, representing up to six samples collected. Station numbers for each boxplot correspond to numbers in the map panels on the left. Red lines indicate the station median, the box the interquartile range, and whiskers correspond to ±2.7σ.
Figure 3.
Salinity-total alkalinity (TA) relationships. Each station is represented by at most three points, showing the TA and salinity values for low, mid, and high tide, as available. Open circles are from the ten stations where tidal variability in salinity and TA was unexpected. Data points are colored by regional grouping shown in figure 1. Lines are calculated from regression analyses using the mean temperature for each region. Dashed lines for LIS and NB are included for completeness, but the slopes with respect to salinity were not statistically significant (p > 0.05, table S1).
Table 3.
Summary fit statistics including number of samples (N), r2, p, and root mean square error (RMSE) for multiple linear regression analysis predicting total alkalinity from temperature and salinity for all data combined (All Data) and individual subregions of the northern Gulf of Maine (GOM), Cape Cod Bay/Central Gulf of Maine (CCB), Buzzards Bay/Vineyard Sound (BB/VS), Narragansett Bay (NB) and Long Island Sound (LIS). Fits for all data also include latitude as a predictor variable. Full fit statistics can be found in table S1.
| Region | N | r 2 | p | RMSE (μmol kg−1) |
|---|---|---|---|---|
|
| ||||
| All Data | 191 | 0.894 | <0.0001 | 121.1 |
| GOM | 45 | 0.773 | <0.0001 | 177.1 |
| CCB | 84 | 0.944 | <0.0001 | 110.9 |
| BB/VS | 34 | 0.814 | <0.0001 | 70.3 |
| NB | 14 | 0.176 | 0.566 | 33.5 |
| LIS | 14 | 0.674 | 0.0085 | 48.7 |
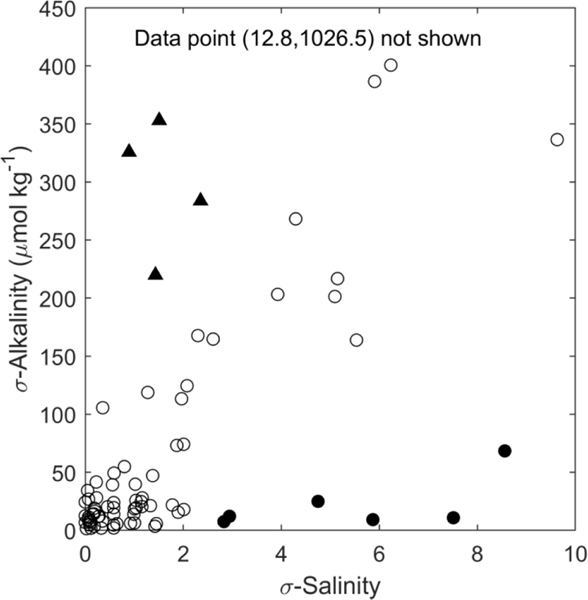
Station-level standard deviation was used to assess tidal variations in TA and salinity (figure 4).Three patterns of variation were identified: stations with (a) low or proportional variation in both TA and salinity; (b) low variation in salinity but high variation in TA (σTA > 200μmol kg−1, σsal < 2.5); (c) high variation in salinity but low variation in TA (σsal > 2.5, σTA < 100 μmol kg−1). The majority of sampling stations fell into the first (n = 71) category, four into the second category, and six into the third category. Five stations did not have enough samples to evaluate variability over a tidal cycle.
Figure 4.
Standard deviation in total alkalinity vs standard deviation in salinity over a tidal cycle from each sampling station. Open circles are stations with low or proportional variation in salinity and alkalinity (group 1), closed triangles have large variation in alkalinity but small variation in salinity (group 2), and closed circles have large variation in salinity but small variation in alkalinity (group 3).
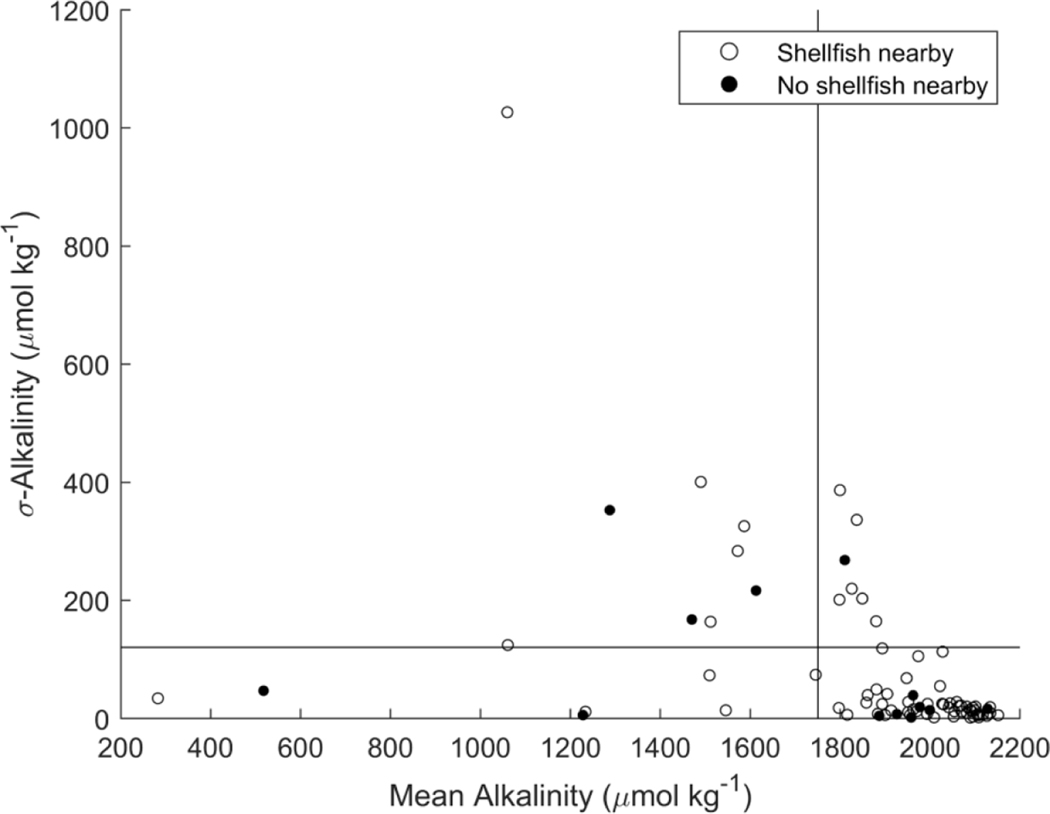
Participants identified 47 sampling locations that they believed were in close proximity to shellfish aquaculture or wild shellfish populations. Spatial data layers from the NODP identified 18 and 58 stations within 1 km of shellfish aquaculture operations or suitable shellfish habitat, respectively. Some stations overlapped these three data sources, and combining data sources yielded 72 stations monitored on Shell Day that were likely near shellfish populations. Five stations in close proximity to shellfish had mean TA that was lower than the 20th percentile in the dataset (TA < 1750.7 μmol kg−1), six stations had tidal variability in TA greater than the 80th percentile (σTA > 120.4 μmol kg−1), and six stations had both low mean and high tidal variability.
4. Discussion
4.1. Efficacy of sampling design
To the authors’ best knowledge, Shell Day was the most geographically extensive, single-day effort to sample and analyze carbonate system parameters of seawater in coastal New England (figure 1, table 2), and the first to evaluate a community science strategy for discrete carbonate system monitoring. Individual sampling programs have carried out more comprehensive monitoring of single embayments and estuaries (e.g. [11, 17, 55, 56]), but the single-day sampling over a tidal cycle provides a unique snapshot of variability in TA and salinity across both time and space (figure 2). Long term and high-precision observations may be required to discern location-specific drivers of OCA, but synoptic approaches such as Shell Day can help prioritize locations for more targeted assessments (see section 4.4).
The good agreement between field replicates indicates that the community-based sample collection and handling protocols generally yielded high-quality TA measurements (figure S1). The success of Shell Day suggests that community science organizations with capacity for additional water sample collection could collaborate with laboratories to add TA to sampling programs to improve understanding of OCA, while increased community science participation can serve to facilitate long-term observations. Such combined science and outreach efforts may also help communities and managers better understand the complex dynamics of OCA and increase public engagement in addressing this environmental challenge.
4.2. Empirical relationships between salinity, temperature and total alkalinity
The entire salinity-TA data set displays high variability (figure 3) and suggests that dilution with low TA freshwater exerts a strong control on coastal TA across the Northeast (figure 3, filled symbols). However, ten Shell Day sampling stations exhibited strong deviations from this overall trend (figure 3, open symbols), illustrating the potential importance of other localized processes. For instance, several stations exhibited TA of ca. 2000 μmol kg−1 and reported salinity that approached zero, implying differences in freshwater endmembers at the watershed scale (see section 4.3). Additionally, the geographic subsets of the data identified in figure 1 show distinct salinity-TA relationships, with differences in both the salinity-TA slope and the zero-salinity intercept. Many environmental and water quality factors may vary at the subregion and watershed scale, such as underlying gradients in both coastal and freshwater endmembers spanning this large region (e.g. figure S4, [18]) that could contribute to these differences in salinity-TA regressions (figure 3).
One project goal was to determine if a region-wide empirical relationship could be used to accurately estimate TA from salinity and temperature, two parameters typically monitored by community science programs. Although strong regional relationships between salinity, temperature, and TA have been observed in other studies (e.g. [3, 54, 57–59]), the Shell Day data suggest there is not likely to be a single Northeast-wide relationship that can accurately predict TA in coastal waters. For the entire region, the best empirical relationship still had a high RMSE (121.1 μmol kg−1), which contrasts with the tight salinity-TA relationships observed in the more open ocean environments of the Northwest Atlantic continental shelf [16, 60]. Even by subregion, RMSE was still high (>48 μmol kg−1) in all statistically significant empirical fits (table 3). This error is 10–50 times greater than the laboratory measurement uncertainty, which is typically on the order of 1–4 μmol kg−1.
Ultimately, the goal of empirical fits to predict TA should be to achieve a low RMSE so as to limit additional uncertainty in carbonate system calculations. For example, Alin et al [3] and Juranek et al [54] predicted TA with an overall RMSE of 6.4 and 4.8 μmol kg−1, in the Southern California Coastal Current and Northeast Pacific regions, respectively. In the nearshore and estuarine environments of Washington state, Fassbender et al [59] predicted TA from salinity with an error of 17 μmol kg−1, which they estimated was appropriate for ‘weather’ quality calculations of Ω and pH, but not ‘climate’ quality calculations [61], where a prediction error on TA needed to be <10 μmol kg−1. A similar error propagation analysis to determine maximum error acceptable for TA predictions is unfortunately not possible with this dataset owing to the lack of additional carbonate system measurements. Thus, given this high uncertainty, salinity and temperature alone, at least as measured in the Shell Day dataset, are not sufficient to estimate TA across the entire Northeast United States region. However, empirical relationships to predict TA may be possible using more localized datasets. This is supported by the reduced error when predicting TA from salinity and temperature for some of the subregions, reinforcing the need to understand drivers of the carbonate system at the watershed scale (tables 2 and S1).
Several factors could drive the high predictive error when estimating TA from proxies in the coastal environment of the Northeast. Processes that influence TA such as sulfate reduction, denitrification, calcification, or calcium carbonate dissolution could be responsible for some of the variation in the salinity-TA relationship, but we lacked the data to evaluate the contribution of these processes. Challenges with the collection of high-quality salinity data may also have led to increased variability in the salinity-TA relationship. For instance, imprecise calibration of handheld salinity meters or lower precision and accuracy of refractometers (used at 23 of the sampling locations) may have contributed to this poor correlation. Furthermore, if the water column was stratified at the time of sample collection, small differences between the depth of the salinity measurement and the depth that the water was sampled for TA analysis could lead to decoupling of salinity from TA. Empirical fits between temperature, salinity, and TA using only laboratory salinometer measurements on the water collected for TA analysis in the GOM region, rather than field observations, showed much smaller RMSE (30.1 vs 177.1 μmol kg−1, respectively) and higher r2 values (0.993 vs 0.772, respectively) (tables S1 and 3) than the overall GOM region, although this dataset was also much smaller. The reduced error implies that more accurate measurements of salinity may improve the predictive capacity of a subregional empirical relationship, although more data would be needed to fully evaluate this hypothesis.
4.3. Carbonate system variability
Station-level standard deviation values for salinity and TA provide insight into factors that influence tidal variability in TA during the time of sampling (figure 4). For instance, stations that displayed little or proportional variability in both TA and salinity (Group 1, figure 4) likely illustrate conservative mixing with low-TA freshwater as the dominant driver of TA variability over a tidal cycle. Most of the observations fall into this category, which reflects the strong impact of freshwater inputs on TA concentration (see also section 4.2). Stations with low variability in both TA and salinity may reflect either coastal or riverine endmembers, but given the limited nature of this dataset (three samples per station) and relatively high uncertainty in reported salinity (1.9 ± 2.1), it is not possible to distinguish natural variability in salinity from measurement uncertainty for observations where σsalinity was less than approximately 2 units.
Stations with large changes in TA but low salinity variation (Group 2, figure 4) could be influenced by alkalinity production from sediments [62–65]. For example, at a single sampling station, Wang et al [64] observed a nearly 200 μmol kg−1 increase in TA from high to low tide during summer, which was attributed to anoxic or suboxic processes occurring in marsh sediments such as sulfate reduction or denitrification that led to significant alkalinity export during ebb tide. Production of dissolved organic carbon can also lead to increased contributions of organic alkalinity that could cause tidal variations in TA without changes in freshwater inputs [66]. Organic acids have been estimated to modify coastal TA by up to 100 μmol kg−1 [64, 66, 67], potentially representing 20%–50% of the signal observed at these four sampling stations.
The six stations with large variation in salinity but little change in TA over a tidal cycle (Group 3) may reflect high alkalinity freshwater contributions. Compilations of river alkalinity measurements collected over the past several decades indicate that most observations of freshwater TA in the New England region are relatively low (200–1000 μmol kg−1, [17, 56, 68], figure S4) in comparison to expected seawater values (>2000 μmol kg−1), but a number of rivers that discharge into coastal Maine, New Hampshire, northern Massachusetts, and LIS have much higher TA (>1000 μmol kg−1, [55, 68], figure S4). However, more data along with repeat sampling over multiple tidal cycles would be needed to better understand these anomalous relationships between TA and salinity.
4.4. Using distributed monitoring for targeted assessments
Assessment of the vulnerability of communities, economies, and ecosystems to OCA requires detailed syntheses of social and biogeochemical conditions (e.g. [26, 69]). No single sampling program could provide those syntheses, but efforts like Shell Day may help identify locations for in-depth evaluation of vulnerability to OCA. Although explicit biological thresholds for mean or variability in TA for shellfish are not known, extreme values within the distribution of the Shell Day dataset may be used to suggest locations for further study. For example, stations in close proximity to shellfish that also had low mean TA, high tidal variability in TA, or both, are likely to experience higher levels of coastal acidification stress, or be at risk for future acidification due to low buffering capacity (figure 5, [18]). In addition, DIC tends to be higher than TA in rivers and groundwater in New England [17, 56, 70] and carbonate system buffering diminishes as DIC increases relative to TA [64, 71–73]. Thus, regions of low TA, especially if caused by mixing with high DIC, low TA freshwater, are likely to have a higher sensitivity to future increases in CO2 from either atmospheric or local biological sources. Highly variable environments have also been proposed as locations that promote adaptation and/or evolution of resilience to acidification stress ([74] and references therein), and the distributed, single-day monitoring approach may identify potentially resilient populations of shellfish.
Figure 5.
Station mean and standard deviation in total alkalinity. Open circles are locations identified as near shellfish aquaculture, wild populations, or suitable shellfish habitat. Closed circles indicate stations not adjacent to aquaculture or wild shellfish habitats. Vertical and horizontal lines indicate the extreme low and high values (20th and 80th percentiles for mean and standard deviation, respectively) in the distribution of the Shell Day data.
4.5. Recommendations for community-based sampling and measurements of seawater parameters
These results suggest two important practical considerations for future studies. First, the development of empirical relationships between salinity and TA relies on high-quality measurements of both salinity and TA, but during Shell Day, salinity was measured less accurately than anticipated. Refractometer-based salinity measurements often differed from laboratory measurements by several units, and even sensor-based salinity measurements sometimes yielded implausible values. These problems emphasize the importance of careful, well-documented calibration and verification procedures. In addition, in a strongly stratified water column, salinity measured in the water column using handheld instruments may differ from the actual salinity of the discrete water sample collected for TA analysis. At a minimum, salinity and TA should be measured at precisely the same water depth or, ideally, salinity should be measured on a subsample of the water sample used for TA measurements.
More broadly, Shell Day responds to the call to expand coastal monitoring, build partnerships that utilize existing monitoring efforts to observe coastal carbonate chemistry, and increase education and outreach on behalf of OCA as an indicator of climate change and water quality [46]. A natural expansion of this approach would be the addition of a second carbonate system parameter. The cost and calibration of equipment poses challenges for in situ measurements of pH and pCO2, while bottle sampling for dissolved inorganic carbon, pH, or pCO2 has significant challenges related to the collection, handling, and preservation of samples that are sensitive to gas exchange. Adjustments would need to be made to the sampling protocol, such as providing sampling devices designed to minimize gas exchange, using gas-impermeable borosilicate glass bottles, and more rapid preservation of samples immediately after collection. These approaches may not be appropriate for community science because sample preservation typically involves using a concentrated solution of mercuric chloride, a hazardous substance.
Community science driven seawater monitoring can serve many purposes. This project was designed to pilot a community science based approach to characterizing single-day variations in TA across a large geographic range. A targeted sampling design prioritizing specific ecosystems, communities of interest, and/or drivers of coastal carbonate chemistry could be developed in collaboration with academic researchers to address specific questions or enhance evaluation of regional differences in vulnerability to acidification. For example, the EPA’s National Coastal Condition Assessment added TA to its suite of standard measured parameters beginning in 2020. Future efforts could also target sites with shellfish aquaculture or large populations of wild shellfish, or be timed to address specific processes, such as the spring freshet, peak respiration, major storm events, or seasonal patterns of eutrophication.
5. Conclusions
The success of the Shell Day sampling effort illustrates the potential of community science to contribute to carbonate system monitoring. These results reveal ways to improve sampling methodology and show the value of TA as a potential tool for OCA studies. This project highlighted opportunities for laboratories and research facilities to collaborate with coastal monitoring programs and community science organizations, developed resources that could be used to support future events at other locations (e.g. Quality Assurance Project Plan, data sheets, sampling protocols, educational documents and videos), and identified areas of expansion such as procedures to collect samples for other carbonate system parameters. Community science efforts can provide a way for state and local governments to inventory monitoring assets, establish collaborations among laboratories to build capacity for seawater monitoring, and engage constituencies in education and outreach programs that increase public understanding of ocean and coastal acidification.
Supplementary Material
Acknowledgments
We are very grateful to the volunteers of the 59 water monitoring organizations who helped to collect samples on Shell Day, a list of whom can be found in the supplement to this manuscript. Support for this study was provided by the U.S. Integrated Ocean Observing System and the NOAA Ocean Acidification Program to the Northeastern Regional Association of Coastal Ocean Observing Systems (NERACOOS, NOAA Grant No. NA16NOS0120023), Woods Hole Sea Grant (NOAA Grant No. NA18OAR4170104) to JER and DCM; the North American Association for Environmental Education EE360 Fellowship program (Environmental Protection Agency Grant Number NT 83695801), Maine Sea Grant (NOAA Grant No. NA18OAR4170103), and a gift from The Ocean Foundation, via the World Ocean Initiative, supported by The Henry Foundation to PRG; MIT Sea Grant (NOAA Grant No. NA18OAR4170105) to JR and CB; AGU’s Centennial Grant to CB; the WHOI Investment in Science Program and the NSF Independent Research and Development Program to DCM; the WestWind Foundation to JER; AP, JG, ML, and PRG were supported by the U.S. Environmental Protection Agency (EPA). The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of EPA. The manuscript was submitted with EPA tracking number ORD-038339.
Footnotes
Data availability statement
The data that support the findings of this study are openly available at the following URL: https://doi.org/10.4211/hs.4364cffedc7e49d49255eef5f8e83148.
(Supplementary material for this article is available online)
References
- [1].Doney S C, Fabry V J, Feely R A and Kleypas J A 2009. Ocean acidification: the other CO2 problem Annu. Rev. Mar. Sci. 1 169–92 [DOI] [PubMed] [Google Scholar]
- [2].Ekstrom J A et al. 2015. Vulnerability and adaptation of US shellfisheries to ocean acidification Nat. Clim. Change 5 207–14 [Google Scholar]
- [3].Alin S R, Feely R A, Dickson A G, Hernández-Ayón J M, Juranek L W, Ohman M D and Goericke R 2012 Robust empirical relationships for estimating the carbonate system in the southern California current system and application to CalCOFI hydrographic cruise data (2005–2011) J. Geophys. Res. Oceans 117 C05033 [Google Scholar]
- [4].Bauer J E, Cai W-J, Raymond P A, Bianchi T S, Hopkinson C S and Regnier P A G 2013. The changing carbon cycle of the coastal ocean Nature 504 61–70 [DOI] [PubMed] [Google Scholar]
- [5].Cai W-J et al. 2011. Acidification of subsurface coastal waters enhanced by eutrophication Nat. Geosci. 4 766–70 [Google Scholar]
- [6].Doney S C 2010. The growing human footprint on coastal and open-ocean biogeochemistry Science 328 1512–6 [DOI] [PubMed] [Google Scholar]
- [7].Feely R A, Alin S R, Newton J, Sabine C L, Warner M, Devol A, Krembs C and Maloy C 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary Estuar. Coast Shelf Sci. 88 442–9 [Google Scholar]
- [8].Feely R A, Sabine C L, Hernandez-Ayon J M, Ianson D and Hales B 2008. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf Science 320 1490–2 [DOI] [PubMed] [Google Scholar]
- [9].Provoost P, van Heuven S, Soetaert K, Laane RWPM and Middelburg JJ 2010. Seasonal and long-term changes in pH in the Dutch coastal zone Biogeosciences 7 3869–78 [Google Scholar]
- [10].Takeshita Y, Frieder C A, Martz T R, Ballard J R, Feely R A, Kram S, Nam S, Navarro M O, Price N N and Smith J E 2015. Including high-frequency variability in coastal ocean acidification projections Biogeosciences 12 5853–70 [Google Scholar]
- [11].Wallace R B, Baumann H, Grear J S, Aller R C and Gobler C J 2014. Coastal ocean acidification: the other eutrophication problem Estuar. Coast Shelf Sci. 148 1–13 [Google Scholar]
- [12].Cai W-J, Wang Z A and Wang Y 2003. The role of marsh-dominated heterotrophic continental margins in transport of CO2 between the atmosphere, the land-sea interface and the ocean Geophys. Res. Lett. 30 1849 [Google Scholar]
- [13].Andersson A J, Mackenzie F T and Lerman A 2005. Coastal ocean and carbonate systems in the high CO2 world of the Anthropocene Am. J. Sci. 305 875–918 [Google Scholar]
- [14].Howarth R, Chan F, Conley D J, Garnier J, Doney S C, Marino R and Billen G 2011. Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems Front. Ecol. Environ. 9 18–26 [Google Scholar]
- [15].Wang Z A, Wanninkhof R, Cai W-J, Byrne R H, Hu X, Peng T-H and Huang W-J 2013. The marine inorganic carbon system along the Gulf of Mexico and Atlantic coasts of the United States: insights from a transregional coastal carbon study Limnol. Oceanogr. 58 325–42 [Google Scholar]
- [16].Wang Z A, Lawson G L, Pilskaln C H and Maas A E 2017. Seasonal controls of aragonite saturation states in the Gulf of Maine J. Geophys. Res. Oceans 122 372–89 [Google Scholar]
- [17].Rheuban J E, Doney S C, McCorkle D C and Jakuba R W 2019. Quantifying the effects of nutrient enrichment and freshwater mixing on coastal ocean acidification J. Geophys. Res. Oceans 124 9085–100 [Google Scholar]
- [18].Gledhill D K et al. 2015. Ocean and coastal acidification off New England and Nova Scotia Oceanography 28 182–97 [Google Scholar]
- [19].Goldsmith K A, Lau S, Poach M E, Sakowicz G P, Trice T M, Ono C R, Nye J, Shadwick E H, StLaurent K A and Saba G K 2019. Scientific considerations for acidification monitoring in the U.S. Mid-Atlantic Region Estuar. Coast Shelf Sci. 30 106189 [Google Scholar]
- [20].Saba G K et al. 2019. Recommended priorities for research on ecological impacts of ocean and coastal acidification in the U.S. Mid-Atlantic Estuar. Coast Shelf Sci. 30 106188 [Google Scholar]
- [21].Melzner F, Mark F C, Seibel B A and Tomanek L 2020. Ocean acidification and coastal marine invertebrates: tracking CO2 effects from seawater to the cell Annu. Rev. Mar. Sci. 12 499–523 [DOI] [PubMed] [Google Scholar]
- [22].Strong A L, Kroeker K J, Teneva L T, Mease L A and Kelly R P 2014. Ocean acidification 2.0: managing our changing coastal ocean chemistry BioScience 64 581–92 [Google Scholar]
- [23].Cooley S R, Jewett E B, Reichert J, Robbins L, Shrestha G, Wieczorek D and Weisberg S 2015. Getting ocean acidification on decision makers’ to-do lists: dissecting the process through case studies Oceanography 28 198–211 [Google Scholar]
- [24].Cooley S R, Ono C R, Melcer S and Roberson J 2016. Community-level actions that can address ocean acidification Front. Mar. Sci. 2 128 [Google Scholar]
- [25].Mathis J T, Cooley S R, Lucey N, Colt S, Ekstrom J, Hurst T, Hauri C, Evans W, Cross J N and Feely R A 2015. Ocean acidification risk assessment for Alaska’s fishery sector Prog. Oceanogr. 1 71–91 [Google Scholar]
- [26].Stewart-Sinclair P J, Last K S, Payne B L and Wilding T A 2020. A global assessment of the vulnerability of shellfish aquaculture to climate change and ocean acidification Ecol. Evol. 10 3518–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kroeker K J, Kordas R L, Crim R N and Singh G G 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms Ecol. Lett. 13 1419–34 [DOI] [PubMed] [Google Scholar]
- [28].Kroeker K J, Kordas R L, Crim R, Hendriks I E, Ramajo L, Singh G S, Duarte C M and Gattuso J-P 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming Glob. Change Biol. 19 1884–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Talmage S C and Gobler C J 2009. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica) Limnol. Oceanogr. 54 2072 [Google Scholar]
- [30].White M M, McCorkle D C, Mullineaux L S and Cohen A L 2013. Early exposure of bay scallops (Argopecten irradians) to high CO2 causes a decrease in larval shell growth PloS One 8 e61065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Waldbusser G G, Hales B, Langdon C J, Haley B A, Schrader P, Brunner E L, Gray M W, Miller C A and Gimenez I 2015. Saturation-state sensitivity of marine bivalve larvae to ocean acidification Nat. Clim. Change 5 273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ries J B, Cohen A L and McCorkle D C 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification Geology 37 1131–4 [Google Scholar]
- [33].Fitzer S C, Cusack M, Phoenix V R and Kamenos N A 2014. Ocean acidification reduces the crystallographic control in juvenile mussel shells J. Struct. Biol. 188 39–45 [DOI] [PubMed] [Google Scholar]
- [34].Schalkhausser B, Bock C, Stemmer K, Brey T, Pörtner H-O and Lannig G 2013 Impact of ocean acidification on escape performance of the king scallop, Pecten maximus, from Norway Mar. Biol. 160 1995–2006 [Google Scholar]
- [35].Schalkhausser B, Bock C, Pörtner H-O and Lannig G 2014. Escape performance of temperate king scallop, Pecten maximus under ocean warming and acidification Mar. Biol. 161 2819–29 [Google Scholar]
- [36].Lagos N A, Benítez S, Duarte C, Lardies M A, Broitman B R, Tapia C, Tapia P, Widdicombe S and Vargas C A 2016. Effects of temperature and ocean acidification on shell characteristics of Argopecten purpuratus: implications for scallop aquaculture in an upwelling-influenced area Aquac. Environ. Interact. 25 357–70 [Google Scholar]
- [37].Grear J S, O’Leary C A, Nye J A, Tettelbach S T and Gobler C J 2020. Effects of coastal acidification on North Atlantic bivalves: interpreting laboratory responses in the context of in situ populations Mar. Ecol. Prog. Ser. 9 89–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grear J, Pimenta A, Booth H, Horowitz D B, Mendoza W and Liebman M 2020. In situ recovery of bivalve shell characteristics after temporary exposure to elevated pCO2 Limnol. Oceanogr. 65 2337–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dickson A G, Sabine C L and Christian J R 2007. Guide to Best Practices for Ocean CO2 Measurements (Sydney, BC: North Pacific Marine Science Organization) (https://repository.oceanbestpractices.org/handle/11329/249)
- [40].Pimenta A R and Grear J S 2018. Guidelines for measuring changes in seawater pH and associated carbonate chemistry in coastal environments of the Eastern United States U.S. Environmental Protection Agency (https://repository.oceanbestpractices.org/handle/11329/878) [Google Scholar]
- [41].Shamberger K E F, Cohen A L, Golbuu Y, McCorkle D C, Lentz S J and Barkley H C 2014. Diverse coral communities in naturally acidified waters of a Western Pacific reef Geophys. Res. Lett. 41 499–504 [Google Scholar]
- [42].Loperfido J V, Beyer P, Just C L and Schnoor J L 2010. Uses and biases of volunteer water quality data Environ. Sci. Technol. 44 7193–9 [DOI] [PubMed] [Google Scholar]
- [43].Latimore J A and Steen P J 2014. Integrating freshwater science and local management through volunteer monitoring partnerships: the Michigan clean water corps Freshw Sci. 33 686–92 [Google Scholar]
- [44].US Environmental Protection Agency 2016. Environmental protection belongs to the public: a vision for citizen science at EPA Washington, DC (www.epa.gov/sites/production/files/2016-12/documents/nacept_cs_report_final_508_0.pdf)
- [45].Poisson A C, McCullough I M, Cheruvelil K S, Elliott K C, Latimore J A and Soranno P A 2020. Quantifying the contribution of citizen science to broad-scale ecological databases – Poisson – 2020 - Wiley online library Front. Ecol. Environ. 18 19–26 [Google Scholar]
- [46].Cross J N et al. 2019. Building the knowledge-to-action pipeline in North America: connecting ocean acidification research and actionable decision support Front. Mar. Sci. 6 356 [Google Scholar]
- [47].Northeast Coastal Acidification Network 2018. Ocean and coastal monitoring webinars for citizen scientists Home | NECAN (http://www.necan.org/ocean-and-coastal-monitoring-webinars-citizen-scientists)
- [48].Gassett P 2020. Building monitoring and response capacity for ocean and coastal acidification in the northeast United States Electronic Theses and Dissertations University of Maine 4377 (https://digitalcommons.library.umaine.edu/etd/4377)
- [49].Northeast Ocean Data Portal 2019. Mar Aquaculture Northeast United States (http://www.northeastoceandata.org/files/metadata/Themes/Aquaculture/Aquaculture.pdf)
- [50].Northeast Ocean Data Portal 2014. Feb Shellfish habitats for Maine, Massachusetts and New Hampshire Northeast United States (Boston, MA: The Nature Conservancy) (http://www.northeastoceandata.org/files/metadata/Themes/Habitat/ShellfishHabitat.pdf)
- [51].Gran G 1952. Determination of the equivalence point in potentiometric titrations. Part II Analyst 77 661–71 [Google Scholar]
- [52].Yao W and Byrne R H 1998. Simplified seawater alkalinity analysis: use of linear array spectrometers Deep Sea Res. Part Oceanogr. Res. Pap. 45 1383–92 [Google Scholar]
- [53].McDougall T J and Barker P M 2011. Getting started with TEOS-10 and the Gibbs Seater (GSW) Oceanographic Toolbox SCOR/IAPSO WG127 [Google Scholar]
- [54].Juranek L W, Feely R A, Gilbert D, Freeland H and Miller L A 2011. Real-time estimation of pH and aragonite saturation state from Argo profiling floats: prospects for an autonomous carbon observing strategy Geophys. Res. Lett. 38 L17603 [Google Scholar]
- [55].Hunt C W, Salisbury J E and Vandemark D 2011. Contribution of non-carbonate anions to total alkalinity and overestimation of CO2 in New England and New Brunswick rivers Biogeosciences 8 3069–76 [Google Scholar]
- [56].Hunt C W, Salisbury J E and Vandemark D 2014. CO2 input dynamics and air–sea exchange in a large New England estuary Estuar. Coasts 37 1078–91 [Google Scholar]
- [57].Lee K, Tong L T, Millero F J, Sabine C L, Dickson A G, Goyet C, Park G-H, Wanninkhof R, Feely R A and Key R M 2006. Global relationships of total alkalinity with salinity and temperature in surface waters of the world’s oceans Geophys. Res. Lett. 33 L19605 [Google Scholar]
- [58].Velo A, Pérez F F, Tanhua T, Gilcoto M, Ríos A F and Key R M 2013. Total alkalinity estimation using MLR and neural network techniques J. Mar. Syst. 111–112 11–18 [Google Scholar]
- [59].Fassbender A J, Alin S R, Feely R A, Sutton A J, Newton J A and Byrne R H 2017. Estimating total alkalinity in the Washington State coastal zone: complexities and surprising utility for ocean acidification research Estuar. Coasts 40 404–18 [Google Scholar]
- [60].Wanninkhof R, Barbero L, Byrne R, Cai W-J, Huang W-J, Zhang J-Z, Baringer M and Langdon C 2015. Ocean acidification along the Gulf coast and east coast of the USA Cont. Shelf Res. 15 54–71 [Google Scholar]
- [61].Newton J A, Feely R A, Jewett E B, Williamson P and Mathis J 2015. Global Ocean Acidification observing network: requirements and governance plan Second Edition, GOA-ON (http://www.goa-on.org/docs/GOA-ON_plan_print.pdf)
- [62].Wang Z A and Cai W-J 2004. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): a marsh CO2 pump Limnol. Oceanogr. 49 341–54 [Google Scholar]
- [63].Hu X and Cai W-J 2011. An assessment of ocean margin anaerobic processes on oceanic alkalinity budget Glob. Biogeochem. Cycles 25 GB3003 [Google Scholar]
- [64].Wang Z A, Kroeger K D, Ganju N K, Gonneea M E and Chu S N 2016 Intertidal salt marshes as an important source of inorganic carbon to the coastal ocean Limnol. Oceanogr. 61 1916. –31 [Google Scholar]
- [65].Thomas H et al. 2009. Enhanced ocean carbon storage from anaerobic alkalinity generation in coastal sediments (https://DalSpace.library.dal.ca//handle/10222/27463)
- [66].Song S, Wang Z A, Gonneea M E, Kroeger K D, Chu S N, Li D and Liang H 2020. An important biogeochemical link between organic and inorganic carbon cycling: effects of organic alkalinity on carbonate chemistry in coastal waters influenced by intertidal salt marshes Geochim. Cosmochim. Acta 275 123–39 [Google Scholar]
- [67].Waldbusser G G and Salisbury J E 2014. Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats Annu. Rev. Mar. Sci. 6 221–47 [DOI] [PubMed] [Google Scholar]
- [68].Lauerwald R, Hartmann J, Moosdorf N, Kempe S and Raymond P A 2013. What controls the spatial patterns of the riverine carbonate system?—A case study for North America Chem. Geol. 337–338 114–27 [Google Scholar]
- [69].United States 2019. May Congress.House.Committee on Science, Space, and Technology. Coastal communities ocean acidification act of 2019 Washington, D.C.: U.S. Government Publishing Office Report No.: 116–81 [Google Scholar]
- [70].Liu Q, Charette M A, Breier C F, Henderson P B, McCorkle D C, Martin W and Dai M 2017. Carbonate system biogeochemistry in a subterranean estuary—Waquoit Bay, USA Geochim. Cosmochim. Acta 15 422–39 [Google Scholar]
- [71].Frankignoulle M, Canon C and Gattuso J-P 1994. Marine calcification as a source of carbon dioxide: positive feedback of increasing atmospheric CO2 Limnol. Oceanogr. 39 458–62 [Google Scholar]
- [72].Egleston E S, Sabine C L and Morel F M M 2010. Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity Glob. Biogeochem. Cycles 24 GB1002 [Google Scholar]
- [73].Cai W-J et al. 2017. Redox reactions and weak buffering capacity lead to acidification in the Chesapeake Bay Nat. Commun. 8 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kapsenberg L and Cyronak T 2019. Ocean acidification refugia in variable environments Glob. Change Biol. 25 3201–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.[] Rheuban J E et al. 2020. Shell Day Data, HydroShare ( 10.4211/hs.4364cffedc7e49d49255eef5f8e83148) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.