Abstract
Fusarium oxysporum is an important plant pathogen and an emerging opportunistic human pathogen. Germination of conidial spores and their fusion via conidial anastomosis tubes (CATs) are significant events during colony establishment in culture and on host plants and, hence, very likely on human epithelia. CAT fusion exhibited by conidial germlings of Fusarium species has been postulated to facilitate mitotic recombination, leading to heterokaryon formation and strains with varied genotypes and potentially increased virulence. Ca2+ signalling is key to many of the important physiological processes in filamentous fungi. Here, we tested pharmacological agents with defined modes of action in modulation of the mammalian Ca2+ signalling machinery for their effect on germination and CAT-mediated cell fusion in F. oxysporum. We found various drug-specific and dose-dependent effects. Inhibition of calcineurin by FK506 or cyclosporin A, as well as chelation of extracellular Ca2+ by BAPTA, exclusively inhibit CAT induction but not germ-tube formation. On the other hand, inhibition of Ca2+ channels by verapamil, calmodulin inhibition by calmidazolium, and inhibition of mitochondrial calcium uniporters by RU360 inhibited both CAT induction and germ-tube formation. Thapsigargin, an inhibitor of mammalian sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), partially inhibited CAT induction but had no effect on germ-tube formation. These results provide initial evidence for morphologically defining roles of Ca2+-signalling components in the early developmental stages of F. oxysporum colony establishment—most notably, the indication that calcium ions act as self-signalling molecules in this process. Our findings contribute an important first step towards the identification of Ca2+ inhibitors with fungas-specific effects that could be exploited for the treatment of infected plants and humans.
Keywords: Ca2+ signalling, cell fusion, CAT, germination, Fusarium oxysporum
1. Introduction
Ca2+ signalling is known to play a major role in regulating important morphogenetic and physiological processes in filamentous fungi. Evidence has been obtained suggesting the involvement of Ca2+ in the regulation of morphological and pathogenic events in fungi, such as secretion [1,2], hyphal tip growth [1,3,4], hyphal branching [5,6], sporulation [7,8], dimorphism [9], cytoskeletal organization [1] and differentiation of infection structures [10]. As many drugs in humans target Ca2+-signalling machinery, deciphering the role of Ca2+ signalling in morphological and physiological changes in fungi can help to unravel their potential as antifungal drug targets.
Fungal spores serve as dissemination structures that can survive harsh environmental conditions outside the host. Spore germination, with cell-symmetry breaking through the emergence of a germ tube and/or CAT as the initiating process, is the first step in the life cycle of most filamentous fungi, including the economically important fungal pathogen Fusarium oxysporum. F. oxysporum produces three different types of vegetative, (i.e., asexual) spores: microconidia, macroconidia and chlamydospores [11]. Upon germination, microconidia of F. oxysporum undergo cell-cell fusion via conidial anastomosis tubes (CATs), which has been postulated to promote efficient colonization and horizontal gene transfer (HGT), leading to heterokaryon formation [12,13]. The process of HGT is important, as it can lead to strains with acquired or altered virulence and resistance [14]. This is particularly important in the context of acquiring resistance to antifungal drugs. The process of CAT-mediated cell fusion progresses in three stages: induction of CAT formation, CAT homing and CAT fusion [13]. Almost 60 mutants involved in different signalling pathways have been identified as defective at different stages of CAT fusion in Neurospora crassa [15]. In this model fungus, preliminary evidence was obtained for the role of Ca2+ signalling during CAT induction. The oscillatory recruitment of proteins MAK-2 and SO to CAT tips during CAT fusion was predicted from mathematical modelling to involve the pulsatile secretion of a self-signalling chemo-attractive ligand, which is not yet identified [16]. This pulsatile secretion and the merging of the plasma membranes of fusing CATs have been hypothesized to be regulated by Ca2+ signalling [17].
In fungi, there are two types of Ca2+ uptake systems involving Ca2+ channel transport across the plasma membrane: the low-affinity Ca2+ uptake system (LACS) and the high-affinity Ca2+ uptake system (HACS). The L-type Ca2+ channel Cch1 and regulatory proteins Mid1 and Ecm7 [18,19] belong to HACS. Cch1 has been as the mammalian orthologue of the pore-forming α1 subunit of voltage-gated calcium channels (VGCCs), although there is only 24% amino-acid sequence similarity between the two [20,21]. The HACS operates when the availability of external Ca2+ is low, whilst the LACS functions when there is a high availability of Ca2+ in the external medium. The only known member of LACS is Fig1 [22,23]. Upon stimulation, extracellular Ca2+ uptake takes place through either low-affinity or high-affinity Ca2+ channels in the plasma membrane. This increases the resting concentration of Ca2+ (50–200 nm in the case of most fungal cells) in the cytoplasm by around 1000-fold [2]. Opening of Ca2+ storage organelles, such as ER, Golgi, mitochondria and vacuoles, also contributes to the increase in intracellular Ca2+. This increase remains transient in cells due to sequestration of Ca2+ within organelles and/or as a result of its export by the action of Ca2+ antiporters and Ca2+ pumps. Meanwhile, the transient increase in intracellular [Ca2+]c forms a Ca2+ signal, which activates downstream signalling pathways through Ca2+-dependent proteins, such as calmodulin. Proteins such as calmodulin undergo conformational changes upon binding to four Ca2+ ions each [24]. Binding of the Ca2+-calmodulin complex in turn activates phosphatases, such as calcineurin, which regulates numerous downstream physiological processes and morphological changes through dephosphorylation of transcription factors, such as the well characterized Crz1 [2]. In mammalian cells, stimulus recognition through GPCRs at the plasma membrane leads to generation of inositol triphosphate (IP3). Binding of IP3 to its receptor, IP3R, in the ER releases Ca2+ into the cytoplasm. The ER membrane also contains ryanodine receptors (RyRs), which are opened by Ca2+ itself, leading to calcium-induced calcium release (CICR). The efflux of Ca2+ to the cytoplasm is followed by its sequestration to the ER through the Ca2+ ATPase pump [25].
Besides the use of mutants and the direct imaging/measurement of [Ca2+]c dynamics in living cells, pharmacological agents that modulate different components of the Ca2+-signalling machinery in animal cells have been extensively employed to study the role of Ca2+ signalling in filamentous fungi (e.g., [26,27]. The mode of action of most of these Ca2+ modulators have been well characterized in mammalian cells. 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) chelates extracellular Ca2+ and inhibits the availability of extracellular Ca2+ for influx to cells [28,29]. Verapamil inhibits Ca2+ channels in the plasma membrane and thus inhibits the influx of Ca2+ [30,31]. Calmidazolium blocks calmodulin and prevents binding of intracellular Ca2+ to calmodulin after a transient Ca2+ increase in the cytoplasm [2,24,32]. This prevents further downstream signalling upon stimulus recognition on the plasma membrane. FK506 (aka tacrolimus) [33] binds to FK506 binding proteins (FKBPs), which in turn bind to calcineurin and prevent its activation by the Ca2+-calmodulin complex [34,35,36]. An alternate inhibitory path exists for calcineurin. Cyclosporin A, a cyclic polypeptide, forms a complex with cyclophilin and inactivates calcineurin function [37]. Both these inhibitory pathways prevent the phosphatase activity of calcineurin, which otherwise dephosphorylates downstream transcription factors, affecting altered gene expression in response to Ca2+ signalling. In mammalian cells, thapsigargin binds irreversibly and specifically to F256 in the M3 helix transmembrane domain of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump on the ER membrane, leading to inhibition of Ca2+ influx to the ER lumen [27,38,39]. The mitochondrial calcium uniporter (MCU) in the mitochondrial inner membrane allows for sequestration of Ca2+ to the mitochondria following a Ca2+ signal in the cytoplasm [40,41,42]. Ruthenium red (RU360) has been shown to have an inhibitory effect on MCU [27].
Comparative genomic analyses and functional screening of components of the Ca2+-signalling machinery of animals and plants has revealed the conservation of numerous components in different filamentous fungi, including Fusarium oxysporum [8,43,44,45]. However, some important components have been shown to not be conserved [43,46]. Homologous sequence analysis of cation-signalling components to mammalian protein sequences revealed the absence of IP3R and RYR homologues in all fungi examined [47]. However, similar channels are present in the vacuole, which is a major Ca2+-storage organelle in fungi. The MCU also has homologues in filamentous fungi, including N. crassa, Aspergillus spp. and Fusarium spp. [27,47,48]. ER-localized SERCA-type Ca2+ -ATPases, Nca1/Eca1, Ca2+/Mn2+ -ATPase and Spf1 have been characterized in other filamentous fungi, although they have not yet been studied in Fusarium [8].
Although F. oxysporum is a major plant pathogen and an opportunistic human pathogen [49,50,51], there are very few studies on the role of Ca2+ signalling in this important species. Inhibition of calcineurin function, both through creation of respective deletion strains and by pharmacological inhibition using FK506 and Cyclosporin A, confirmed a role for calcineurin in germination of microconidia in F. oxysporum under rich media conditions [3,52]. Here, we used known Ca2+-signalling modulators to study the role of the Ca2+-signalling machinery in germ-tube formation and CAT-mediated cell fusion of F. oxysporum f. sp. Lycopersici (Fol) in order to report the first evidence concerning how inhibition of key Ca2+ signalling components affects the early stages of germling development in this fungal pathogen. New insights on this topic will hopefully contribute to the identification of Ca2+ inhibitors with fungus-specific effects that could be exploited for the treatment of infected plants and humans.
2. Materials and Methods
2.1. Culture Conditions of F. oxysporum
Microconidia of F. oxysporum f. sp. Lycopersici (isolate 4287) were harvested from liquid cultures grown at 25 °C and 250 rpm for 5 days in PDB [13]. Germination and CAT fusion assays were set up in 8-well borosilicate slide-culture chambers (Nalgene Nunc, Rochester, NY, USA), as previously described in detail [13]. Briefly, 300 µL of a 1 × 106 cells/mL spore suspension in media was added to each well and incubated for 12 h at 25 °C. Spore germination was analysed in both 1% PDB alone and in 1% PDB supplemented with 25 mM NaNO3. NaNO3 is required for CAT-mediated germling fusion to occur in F. oxysporum. Consequently, CAT induction and fusion were only analysed in the NaNO3-supplemented medium because the process is inhibited in the 1% PDB control condition. We have reported the absence of CAT fusion as an inhibition of the first stage in CAT-mediated cell fusion—which is CAT induction/CAT formation—because we did not find any condition involving the tested pharmacological agents in which CATs were formed but were unable to fuse.
2.2. Pharmacological Inhibition, Microscopy and Statistical Analysis
Pharmacological agents applied to assess the influence of Ca2+ inhibition on spore germination and CAT fusion are shown in Table 1. Stocks of the agents were prepared with either water or DMSO. An appropriate volume of each inhibitor stock solution was added to the growth medium (either 1% PDB or 1% PDB + 25 mM NaNO3) at the beginning of each experiment to provide the desired final concentration. Effects of these agents on germ-tube formation was analysed by cultivating microconidia in 1% PDB alone, in which only germ tubes were being formed, while the effect on CAT-mediated cell fusion was analysed by cultivation in fusion medium, i.e., 1% PDB supplemented with 25 mM NaNO3, in which both germ tubes and CATs were being formed. Unless otherwise stated, the final concentration of DMSO did not exceed 4%.
Table 1.
Pharmacological agents used to inhibit Ca2+ signalling in F. oxysporum.
| Name | Solvent | Source | Concentration |
|---|---|---|---|
| BAPTA | Water | Invitrogen Life Technologies | 0–15 mM |
| Calmidazolium | DMSO | Acros Organics | 0–20 µM |
| Cyclosporin A | DMSO | InvivoGen | 0–100 µM |
| FK506 | DMSO | InvivoGen | 0–20 µM |
| RU360 | Water | Calbiochem | 0–1 µM |
| Thapsigargin | DMSO | Sigma Aldrich | 0–100 µM |
| Verapamil | Water | Sigma Aldrich | 0–15 mM |
The formation of germ tubes and/or CATs was determined at 12 h post incubation (hpi) using simple DIC light microscopy and subsequent image quantification, as detailed by Kurian et al. (2018). A total of 360 imaging files, with each comprising a 4 × 4 image array, were collected for this study. From this collection of 5000 images, a minimum of 20 images were selected to morphologically evaluate at least 300 cells per test condition per experiment. Results were assembled from a minimum of three experimental repeats for each drug concentration tested, and the mean ± SEM was plotted. Plotting of graphs and performance of statistical analysis were performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com (accessed on 25 August 2021)). Non-parametric Mann-Whitney tests were conducted for the comparison of results from each drug concentration versus non-treated, and p-values ≤ 0.05 were reported as significant (*).
The minimum inhibitory concentration (MIC) is the concentration of a pharmacological agent resulting in ≥90% inhibition of its target molecule and process in comparison to the untreated control. MIC values were determined for germ-tube formation from the counts of germ tubes being formed, as well as non-germinated spores, upon treatment with a specified concentration of an inhibitor. Similarly, MIC values for CAT fusion were determined from counts of germinated spores undergoing CAT fusion upon treatment with specified concentrations of the same inhibitors.
3. Results
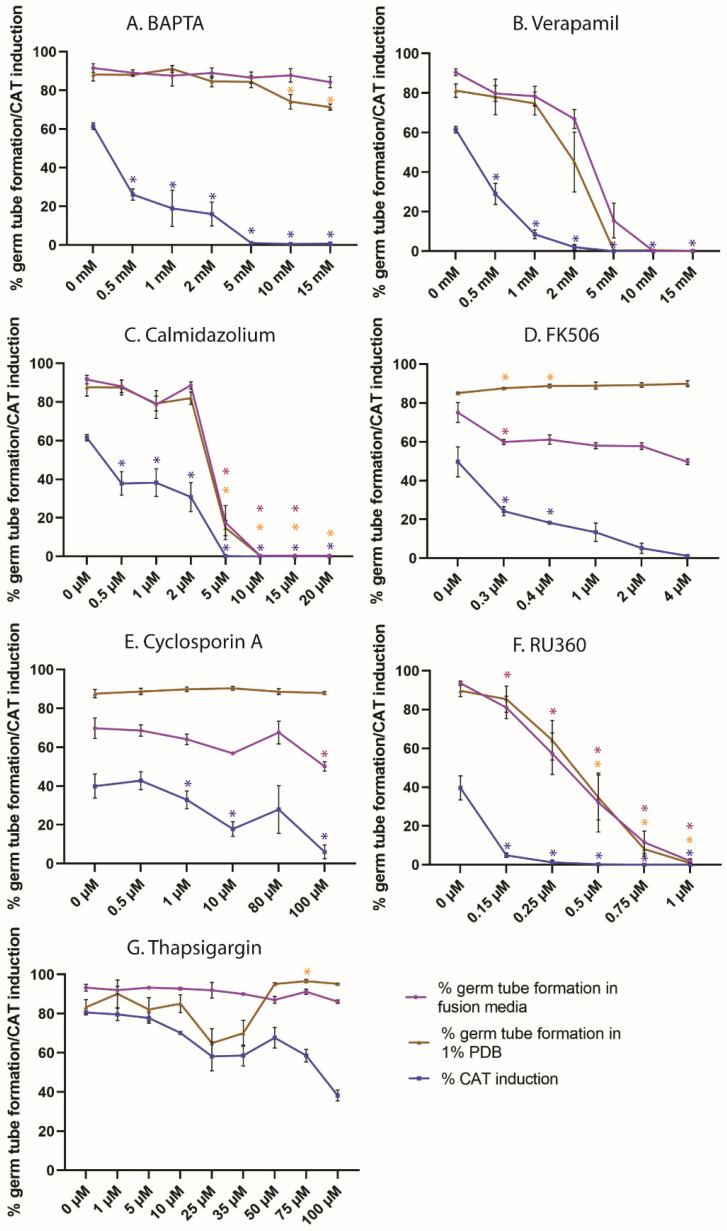
The dose-dependent inhibitory effects of the Ca2+ modulators BAPTA, verapamil, calmidazolium, thapsigargin, FK506 and RU360 on microconidial germ-tube formation and CAT-mediated germling fusion were quantitatively analysed (Figure 1). The tested concentration ranges of these inhibitors all fell within those previously reported to have inhibitory effects in fungi [34,48].
Figure 1.
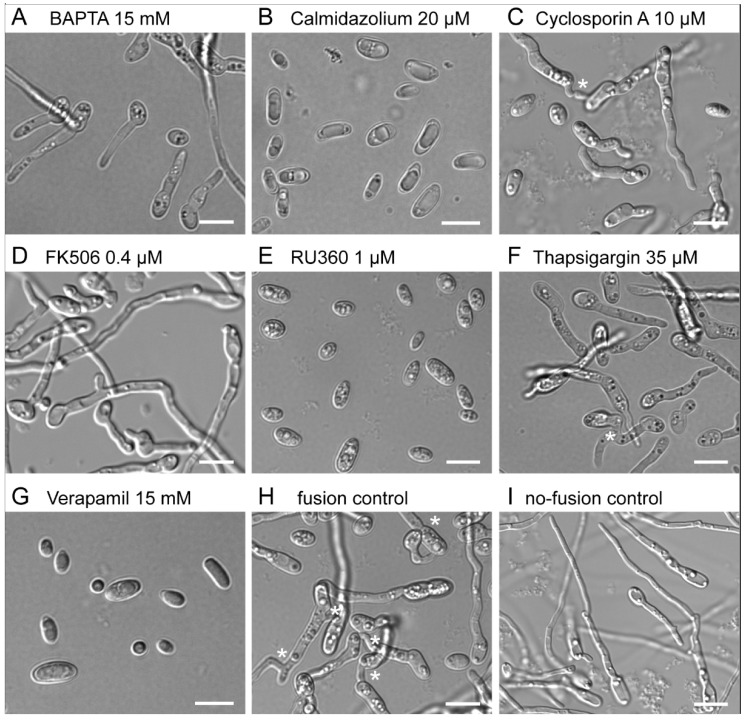
Effect of calcium-signalling inhibitors on germination and CAT-mediated cell fusion. (A) Chelation of extracellular Ca2+ with BAPTA inhibits the formation of CATs and thus blocks cell fusion very effectively. Germ-tube formation and elongation are unaffected. (B) Calmidazolium, which also blocks calmodulin/calcineurin signalling, already affects germination, germ-tube elongation and CAT-mediated cell fusion at low concentrations. Nevertheless, CAT formation is much more sensitive compared to germ-tube formation. (C) Cyclosporin A, on the other hand, which also acts on calcineurin signalling, has a stronger inhibitory effect on CAT-mediated cell fusion. (D) Inactivation of calcineurin signalling with FK506 has a strong inhibitory effect on CAT formation but not on germ-tube development. (E) Inhibition of mitochondrial calcium uniporters with RU360 results in a very similar phenotype compared to verapamil, though at much lower effective concentrations. (F) Inhibition of Ca2+ influx into the ER with thapsigargin has only a weak but specific effect on CAT formation. (G) Inactivation of Ca2+ plasma-membrane channels with verapamil supresses germination altogether. (H) In the positive control (1% PDB + NaNO3 fusion medium), germination, germ-tube elongation and germling fusion (*) occur with high frequencies, whereas (I) in the absence of NaNO3 (1% PDB only), CAT formation (and hence germling fusion) cannot take place, and only elongated germ tubes are produced. Scale bars, 10 µm.
3.1. Extracellular Ca2+ Is Required for CAT Induction but Not Germ-Tube Formation
We used BAPTA, which is cell-impermeable and hence chelates extracellular Ca2+, at concentrations between 0.5 and 15 mM to study the role of extracellular Ca2+ in conidial germ-tube formation and CAT fusion (Table 1 and Figure 2). Germ-tube formation in 1% PDB alone was unaffected by BAPTA concentrations of up to 5 mM but showed up to 10% reduction with 10–15 mM BAPTA. Germ-tube formation in fusion medium (1% PDB + NaNO3) was not inhibited by BAPTA at concentrations of up to 15 mM. However, CAT induction was very sensitive to BAPTA, and the MIC value of BAPTA for CAT fusion was determined as 5 mM. As BAPTA is a highly specific chelating agent compared to other less effective agents, such as ethylene glycol bis (2-Aminoethyl ether)-N,N,N′,N′ tetraacetic acid (EGTA), we assume complete removal of the available Ca2+ from the medium at 1 mM of BAPTA [53]. It is evident from these results that conidial germ-tube formation in Fol is still functional in the absence of extracellular Ca2+, either due to intracellular Ca2+ from storage organelles or via compensation by other extracellular ions. CAT induction, on the other hand, is strictly dependent on extracellular Ca2+, most probably because it is involved in the cell-cell communication between the fusing partners.
Figure 2.
Effect of calcium-signalling inhibitors on germination and CAT-mediated cell fusion. (A) BAPTA, a chelator of extracellular Ca2+, inhibits the formation of CATs. Germ-tube formation and elongation remain unaffected. (B) Verapamil, an inhibitor of Ca2+ channels on the plasma membrane, inhibits CAT formation and germ-tube formation. (C) Calmidazolium, blocking calmodulin/calcineurin signalling, inhibits germ-tube formation and CAT formation. (D) FK506, an inhibitor of calcineurin signalling, inhibits CAT formation but not germ-tube development. (E) Cyclosporin A, an inhibitor of calcineurin, inhibits CAT induction but not germ-tube formation. (F) RU360, an inhibitor of mitochondrial calcium uniporters, inhibits germ-tube formation and CAT formation. (G) Thapsigargin, an inhibitor of ER Ca2+ ATPases, has a strong inhibitory effect on CAT formation and cell fusion but not germ-tube formation. p-values ≤ 0.05, indicating significant differences between individual drug concentrations in comparison to corresponding non-treated controls, are marked with an asterisk (*).
3.2. Calcium Entry/Exit through Calcium Channels Is Required for Germ-Tube Formation and CAT Induction
Verapamil, an inhibitor of the CCH1 Ca2+ channel, was used at concentrations between 0.5 and 15 mM to determine the role of Ca2+ influx through the plasma membrane in spore germ-tube formation and CAT fusion (Table 1 and Figure 2). Cell-symmetry breaking by germ-tube formation alone in 1% PDB (w/o NaNO3) was inhibited by 5 mM verapamil (MIC = 5 mM). Cell-symmetry breaking by germ-tube formation in fusion medium (1% PDB + NaNO3) showed a similar dose-dependent inhibition but required 10 mM verapamil for inhibition (MIC = 10 mM). The presence of additional nutrient ions from NaNO3 are potentially responsible for this 5 mM concentration gap until complete inhibition between both media. CAT fusion responded more sensitively than germ-tube formation and was already fully inhibited by 2 mM verapamil in fusion medium (MIC = 2 mM). This shows that both CAT induction and germ-tube formation require an influx of extracellular Ca2+ through the CCH1 channel, albeit at different concentrations. As CCH1 is a component of HACS, which functions under low extracellular Ca2+ concentrations, our results indicate the requirement of a higher extracellular Ca2+ concentration for CAT induction. We suspect that a complete block of CCH1 by verapamil does not occur at the inhibitory concentration for CAT induction. A lower uptake of extracellular Ca2+ might still take place through the CCH1 channel, which might not be enough to induce CAT formation but could be sufficient for germ-tube formation. Complete inhibition of CCH1 might be caused by the inhibitory concentration of 10 mM verapamil for germ-tube formation.
3.3. Calmodulin Inhibition by Calmidazolium Inhibits CAT Induction and Germ-Tube Formation
The calmodulin inhibitor calmidazolium was used at concentrations between 0.5 and 20 µM to determine the function of intracellular Ca2+ in germ-tube formation and CAT fusion (Table 1 and Figure 2). The MIC value of calmidazolium in germ-tube formation was 10 µM in 1% PDB and fusion media. CAT induction was more sensitive than germ-tube formation to calmidazolium and had an MIC value of 5 µM. This shows that binding of intracellular Ca2+ to calmodulin is required for germ-tube formation and CAT induction, but as already observed with the other inhibitors, both developmental pathways display different requirements of intracellular Ca2+ levels.
3.4. Inhibition of Calcineurin Function Inhibits CAT Induction but Not Germ-Tube Formation
FK506 and cyclosporin A are indirect inhibitors of calcineurin function with different modes of action [36,54,55]. FK506 binds to FKBPs, while cyclosporin A binds to cyclophilins. Binding of either of these complexes to calcineurin blocks its function as phosphatase and prevents the dephosphorylation of downstream transcription factors. Here, we used concentration ranges of 0–4 µM of FK506 and 0–100 µM of cyclosporin A to determine the effect of calcineurin inhibition on germ-tube formation and CAT fusion. CAT fusion was inhibited by both agents; however, germ-tube formation was not (Table 1, Figure 2). The MIC value for the inhibition of CAT induction was 4 µM for FK506. Although less than 10% CATs were formed, we did not achieve complete inhibition of CAT induction, even at the highest tested concentrations of 100 µM cyclosporin A. Due to noticeable precipitation of the drug in the medium, it was not reasonable to raise the test concentration any further.
3.5. Calcium Sequestration through the Mitochondrial Calcium Uniporter Is Required for CAT Induction and Germination
In mammalian cells, RU360 inhibits the transfer of intracellular Ca2+ from the cytoplasm through the mitochondrial calcium uniporter onto the inner mitochondrial membrane. We tested the effects of 0.15–1 µM RU360 on germling morphogenesis (Table 1, Figure 2). Germ-tube formation in both 1% PDB and fusion medium exhibited a dose dependent inhibition, with MIC values of 0.75 µM and 1 µM in 1% PDB and fusion media, respectively. CAT fusion also exhibited dose-dependent inhibition, with an MIC value of 0.15 µM. Thus, our results indicate an inhibitory effect of RU360 on MCU in fungal cells. Germ-tube formation and CAT induction are hence dependent on intracellular Ca2+ sequestration to mitochondria, albeit at different concentrations of intracellular [Ca2+]. Influx of intracellular Ca2+ to mitochondria during CAT induction appears to occur at a higher concentration range of intracellular Ca2+ compared to germ-tube formation.
3.6. ER Calcium ATPase Inhibition by Thapsigargin Partially Inhibits CAT Induction but Not Germ-Tube Formation
Thapsigargin binds to the ER calcium ATPase and inhibits the sequestration of Ca2+ into the ER following a high concentration of [Ca2+] in the cytoplasm [39]. The test concentrations of 1–100 µM of thapsigargin resulted in less obvious dose-dependent effects in comparison to the other inhibitors (Table 1, Figure 2). Cell-symmetry breaking by germ-tube formation in 1% PDB alone was inhibited by more than 20 % at concentrations of 25 and 35 µM thapsigargin but not affected at higher concentrations. In fusion medium, 1–100 µM thapsigargin had only negligible inhibitory effects on germ-tube formation. CAT induction, on the other hand, was significantly inhibited by thapsigargin concentrations higher than 10 µM and decreasing to 40% at 100 µM. Notably, at (calculated) concentrations greater than 50 µM, the drug increasingly precipitated in the fusion medium, decreasing its actual concentration and bioavailability. Nevertheless, the data show that Ca2+ sequestration into the ER has a decisive role in cell-symmetry breaking by germ-tube and CAT formation, as well as CAT-mediated cell fusion.
4. Discussion
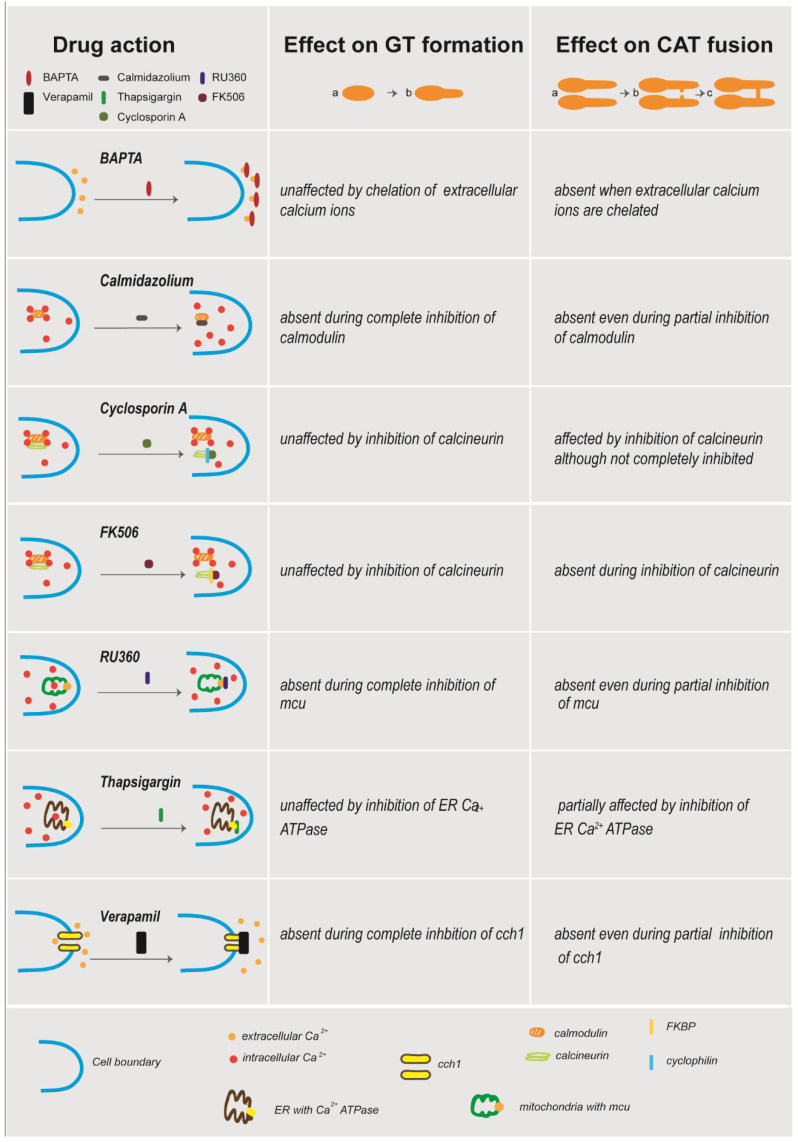
The goal of this study was to determine the role of Ca2+ signalling during germ-tube morphogenesis and CAT-mediated germling fusion in microconidia of F. oxysporum f. sp. lycopersici. Table 2 and Figure 3 summarize the results obtained with the different Ca2+-signalling inhibitors used in this investigation.
Table 2.
Pharmacological inhibition of germ-tube formation and CAT fusion. This table summarizes the effect of each pharmacological agent on germ-tube formation and CAT induction in F. oxysporum. The MIC values are derived from the corresponding graphs shown in Figure 2.
| Pharmacological Agent | Mode of Action in Mammalian Cells | MIC for Germ-Tube Formation | MIC for CAT Induction | |
|---|---|---|---|---|
| 1% PDB | Fusion Media | |||
| BAPTA | extracellular Ca2+ ion chelation | no inhibition | no inhibition | 5 mM |
| Calmidazolium | inhibits calmodulin | 10 µM | 10 µM | 5 µM |
| Cyclosporin A | inhibits calcineurin | no inhibition | no inhibition | >100 µM |
| FK506 | inhibits calcineurin | no inhibition | no inhibition | 4 µM |
| RU360 | inihibits mitochondrial Ca2+ uniporter | 0.75 µM | 1 µM | 0.15 µM |
| Thapsigargin | inhibits ER Ca2+ ion sequestration | no inhibition | no inhibition | no full inhibition |
| Verapamil | inhibits Cch1 | 5 mM | 10 mM | 2 mM |
Figure 3.
Schematic overview of the drug’s mode of action. The mode of action of each pharmacological agent in the fungal cell are correlated with the effect of each treatment on germ-tube (GT) formation and CAT formation.
Experiments using the Ca2+ chelator BAPTA showed that extracellular calcium is not required for germ-tube formation. A previous study of F. oxysporum showed the induction of CAT fusion (~40%) in 1% PDB upon addition of 25 mM CaCl2 [13]. In the same study, no significant improvement in germination was observed upon addition of CaCl2. Similarly, Ca2+ signalling was shown to be dispensable for cell-symmetry breaking by germ-tube formation for spores of Aspergillus nidulans, Magnaporthe oryzae (formerly M. grisea) and Curvularia trifolii and proposed to be complemented by other pathways, such as the MAP kinase pathway and the PKA pathway [56,57,58].
Our results reveal that extracellular Ca2+ is required for CAT induction in F. oxysporum. The same finding was previously reported for N. crassa [59]. In this model fungus, the Ca2+ sensor mutant Δcse-1 exhibited a defective fusion phenotype. Deletion mutants of PIK-1 and NFH-2, two possible interacting partners of CSE-1, also showed similar phenotypic defects in CAT-mediated cell fusion as the Δcse-1 mutant [59]. Ca2+ signalling has been proposed to regulate the interaction between these three proteins, leading to exocytosis of the chemoattractant and/or receptor during hyphal fusion [59,60]. In N. crassa, PEF1, a protein involved in membrane plugging during injury, was shown to be dependent on extracellular Ca2+ for its membrane recruitment [61]. Extracellular Ca2+ chelation with EGTA also impaired septal plugging in response to wounds, independent of PEF1. Cell lysis involving transient membrane rupture and fusion is the key final step during CAT-mediated cell fusion. However, CAT formation itself was impaired in our study, highlighting the importance of extracellular Ca2+ for cell-cell communication during CAT induction, homing and, ultimately, CAT fusion. An oscillatory high-Ca2+ gradient was observed during polarized tip extension in A. nidulans [1]. On the other hand, it has been reported that the hyphae of F. oxysporum can grow in the absence of a tip-focussed Ca2+ gradient [3]. Evidently, different Ca2+ signatures could be involved in different types of tip growth. Hence, our results suggest that even though germ-tube protrusion involves polarized growth, it differs from polarized growth exhibited by CATs in terms of Ca2+ requirement. We previously showed that the negatively chemotropic growth behaviour of germ tubes is mainly driven by the CDC42 GTPase, whereas positive chemotropic growth behaviour of CATs requires the activity of RAC1 [62]. Similarly, different Ca2+-signalling pathways feeding into different downstream processes may add another layer of regulation to achieve the generation of functionally distinct cell protrusions from the same cell. While CATs can employ extracellular calcium as a chemotropic cue for CAT homing, germ tubes remain unresponsive. Therefore, selective Ca2+ inhibition with BAPTA presents a useful tool for discrimination of the signalling mechanisms required for germ-tube protrusion from those involved in self-signalling during CAT-mediated cell fusion.
Using the L-type Ca2+ channel blocker verapamil, both-germ tube and CAT formation were inhibited. Nevertheless, CAT formation was again more sensitive to this inhibitor, as previously seen with other inhibitors of cellular key processes, such as GTPases or microtubules [62,63]. In S. cerevisiae, verapamil was shown to partially inhibit Cch1-Mid1 activity [30]. Inhibition of hyphal development, adhesion and gastrointestinal-tract colonization upon treatment with verapamil was reported in Candida albicans [31]. Inhibition of appressorium formation but not conidial germination with 500 µM verapamil was reported in M. grisea (now M. oryzae) [57]. Amiodarone, an antiarrhythmic drug with a proposed activity in opening Ca2+ channels in the plasma membrane, leading to loss of intracellular Ca2+, exhibited antifungal activity against F. oxysporum [64]. Ca2+ channels mediate the flow of free Ca2+ along a concentration gradient into the cytoplasm, either from the external medium or from intracellular Ca2+ stores. Homologues of Cch1 and Mid1, two components of HACS, are present in filamentous fungi [2,43] and yeast and have been shown to play similar roles in N. crassa and Giberella zeae (the teleomorph of F. graminearum) [23,65,66]. Other mechanisms complementing the absence of CCH1, such as LACS component Fig1 have been reported [23]. Several studies in N. crassa and S. cerevisea have also provided evidence for the presence of Ca2+ channels other than cch1-mid1 in the plasma membrane [27,67,68]. Additionally, localization of cch1 has not been performed in fungi; the associated protein Mid 1 has been shown to be localized in ER in B. cinerea [53]. Unless cch1 is localized in the fungal plasma membrane, the effects observed with verapamil cannot be validated. However, pulsatile increases in cytosolic-free Ca2+ ([Ca2+]c) were observed in growing hyphal tips of the wild type and FoCCH1 and FoMID1 mutants in the F. oxysporum strain O-685, which is different from the tomato pathogen F. oxysporum strain 4287 used in this study, although the pattern differed between each of the mutants and the wild type [3]. A similar study in F.graminearum reported variations in Ca2+ signatures influenced by external calcium and growth defects of CCH1, MID1 and FIG1 mutants [69]. Ca2+ accumulation through CCH1 and MID1 in the plasma membrane was found to be important for thigmotropic tip growth in hyphae of C. albicans, although it was later shown to act as a signal enhancer for directional growth, with the possibility of additional essential signalling components [70,71]. Our findings support the function of HACS involving the L-type Ca2+ channel in the induction of germ-tube formation and in CAT fusion in F. oxysporum, especially when the availability of Ca2+ in the growth medium is low, such as in 1% PDB with or without NaNO3.
Calmidazolium, an inhibitor of calmodulin, was found to inhibit both germ-tube formation and CAT fusion. CAT formation was again more sensitive to this agent. Deletion of the gene encoding the calmodulin-binding protein HAM-3 was shown to be required for cell fusion [72], and loss of the Ca2+-binding protein HAM-10 was found to lead to fusion defects [73,74]. We also found that inhibition of the mitochondrial Ca2+ uniporter with RU360 inhibited both germ-tube formation and CAT fusion. CAT fusion was also more sensitive to this agent. This shows that mitochondrial Ca2+ stores are important for germ-tube formation and CAT formation although at different levels of sensitivity. Homologues of MCU have been identified in pathogenic filamentous fungi, including Fusarium spp. [47]. RU360 was found to alter the Ca2+ signature of transient increases in [Ca2+]c during staurosporine-induced cell death in N. crassa [27,48]. On the other hand, inhibition of the ER Ca2+ ATPase by thapsigargin showed partial inhibitory effects on germination in 1% PDB or CAT induction in fusion medium. Germ-tube formation in fusion media was unaffected by inhibition of ER Ca2+ ATPase with thapsigargin. Our results with thapsigargin indicate that germ-tube formation could take place in the absence of Ca2+ uptake to the ER, albeit with reduced efficiency. A possible explanation is that the transient rise of cytoplasmic Ca2+ quickly falls back to resting levels due to alternative mechanisms involving other Ca2+-storage organelles, such as mitochondria or vacuoles, or by export to the extracellular medium. Germ-tube formation progresses since it is less sensitive to these changes, whereas CAT induction is inhibited, as it is dependent on Ca2+ uptake to the ER.
The HACS system involving CCH1 and MID1 has been shown to be sensitive to calcineurin and is therefore expected to have a functional role downstream of Ca2+ influx through these plasma membrane channels [75]. The calcineurin pathway is not essential for normal cell survival but is required for stress responses [76,77]. Our results indicate that inhibition of calcineurin by FK506-FKBP or cyclosporinA-cyclophilin inhibits CAT induction but not germ-tube formation in F. oxysporum. FK506 forms a complex with FKBPs, which, in turn, binds to the regulatory subunit B of calcineurin to inhibit its function [36]. Cyclosporin A forms a complex with cyclophilins and inhibits its action by binding to the same regulatory subunit of calcineurin [55]. Even though the inhibition of calcineurin by FK506 and cyclosporin A yielded the same result, where CAT induction alone was inhibited, FK506 is a more potent inhibitor of calcineurin, as previously reported [33,78]. In our study, CAT induction was found to be inhibited at lower concentrations of verapamil, calmidazolium and RU360 (Table 2) than germ-tube formation and therefore appeared to be much more sensitive to these agents. This further supports our notion that even though germ-tube formation and CAT induction both involve Ca2+ signalling, they are differentially regulated by Ca2+ channels, calmodulin and mitochondrial Ca2+ uniporters.
CATs were more sensitive to all pharmacological agents tested, which inhibited the process at much lower concentrations compared to germ-tube formation. Even though both cell protrusions do occur in the same medium (1% PDB + 25 mM NaNO3), germ-tube formation and CAT fusion occur at different time points during germling development [13]. Germ-tube formation is an early event in F. oxysporum, commencing ~2 h after incubation starts, whereas CAT fusion begins ~7 h after incubation starts. Our results also indicate the dependency of CAT fusion on a higher extracellular Ca2+ concentration, followed by its intracellular sequestration to mitochondria, as well as more Ca2+ binding to calmodulin when compared to germ-tube formation. Germ-tube formation, followed by continued germ-tube protrusion, increases the cell volume much more significantly than the protrusion of expansion-limited CATs. This might lead to further release of Ca2+ to the medium or alter the ratio of intracellular and extracellular Ca2+ levels. Thus, high extracellular Ca2+ availability following germ-tube formation might be a key prerequisite for CAT induction to occur in Fol. This is different from other filamentous fungi, such as N. crassa, in which CATs and germ tubes can be simultaneously formed by one and the same cell in Vogel’s media without other supplements.
5. Conclusions
Overall, our results provide clear evidence for a significant role of Ca2+ signalling in the initial morphogenesis of CATs and germ tubes. At the same time, our data strongly suggest a differential regulation of CAT induction and germ-tube formation by the Ca2+-signalling machinery. Our study provides further evidence of Ca2+ acting as a self-signal for CAT homing and fusion. Several features of cellular Ca2+ signals support this notion, including (1) their pulsatile nature in growing tips (fitting with the pulsatile ping-pong mechanism of SO and MAK-2 proteins to N. crassa tips [79]); (2) their ability to translate as elementary (localised and specific action) and global (waves and spikes) signals, which greatly facilitates signal translation across the intercellular space between sender and receiver [12]; and (3) their fast propagation dynamics, which is related to; (4) the fact that Ca2+ ions exist and function both intracellularly and extracellularly. The Ca2+ modulators used in this study were originally developed for use in mammals. As a result, their modes of action have been characterized in depth in mammalian cells. Hence, caution must be taken when interpreting the results as potential drug targets in F. oxysporum and other fungi until the specificity/selectivity for their fungal targets is confirmed. However, our studies highlight the utility of these pharmacological agents in studying calcium signalling in this important plant and human pathogen. This includes, for instance, the effect of verapamil, calmidazolium and RU360 in the inhibition of germ-tube formation, which presents important results for the development of novel antifungal drugs. Particularly with respect to increasing reports of acquired antifungal resistance of microbial pathogens, in future studies, it is crucial to identify fungus-specific targets in the universal Ca2+-signalling machinery of eukaryotes.
Author Contributions
Conceptualization, N.D.R., S.M.K. and A.L.; methodology, N.D.R., S.M.K. and A.L.; data acquisition and curation, S.M.K. and A.L.; analysis and data interpretation, S.M.K., N.D.R. and A.L.; manuscript drafting, S.M.K., A.L. and N.D.R.; manuscript revision and submission, S.M.K. and A.L.; funding acquisition, N.D.R. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the European Union grants PITN-GA-2013-607963 to N.D. Read and the Tyrolean Science Funding (TWF) grant 256524 to A.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the considerable number and large file size of the imaging data sets, as well as the requirement for specific image analysis software.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takeshita N., Evangelinos M., Zhou L., Serizawa T., Somera-Fajardo R.A., Lu L., Takaya N., Nienhaus G.U., Fischer R. Pulses of Ca2+ coordinate actin assembly and exocytosis for stepwise cell extension. Proc. Natl. Acad. Sci. USA. 2017;114:5701–5706. doi: 10.1073/pnas.1700204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S., Hou Y., Liu W., Lu C., Wang W., Sun S. Components of the Calcium-Calcineurin Signaling Pathway in Fungal Cells and Their Potential as Antifungal Targets. Eukaryot. Cell. 2015;14:324–334. doi: 10.1128/EC.00271-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.-S., Kim J.-E., Frailey D., Nohe A., Duncan R., Czymmek K.J., Kang S. Roles of three Fusarium oxysporum calcium ion (Ca2+) channels in generating Ca2+ signatures and controlling growth. Fungal Genet. Biol. 2015;82:145–157. doi: 10.1016/j.fgb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Riquelme M., Aguirre J., Bartnicki-García S., Braus G.H., Feldbrügge M., Fleig U., Hansberg W., Herrera-Estrella A., Kämper J., Kück U., et al. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018;82:82. doi: 10.1128/MMBR.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman-Gavrila L.B., Lew R.R. Calcium gradient dependence of Neurospora crassa hyphal growth. Microbiology. 2003;149:2475–2485. doi: 10.1099/mic.0.26302-0. [DOI] [PubMed] [Google Scholar]
- 6.Harris S.D. Hyphal branching in filamentous fungi. Dev. Biol. 2019;451:35–39. doi: 10.1016/j.ydbio.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen Q.B., Kadotani N., Kasahara S., Tosa Y., Mayama S., Nakayashiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 2008;68:1348–1365. doi: 10.1111/j.1365-2958.2008.06242.x. [DOI] [PubMed] [Google Scholar]
- 8.Lange M., Peiter E. Calcium Transport Proteins in Fungi: The Phylogenetic Diversity of Their Relevance for Growth, Virulence, and Stress Resistance. Front. Microbiol. 2020;10:3100. doi: 10.3389/fmicb.2019.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce K., Andrianopoulos A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015;39:797–811. doi: 10.1093/femsre/fuv035. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Gutjahr C., Bleckmann A., Dresselhaus T. Calcium Signaling during Reproduction and Biotrophic Fungal Interactions in Plants. Mol. Plant. 2015;8:595–611. doi: 10.1016/j.molp.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Okungbowa F.I., Shittu H.O. Fusarium wilts: An overview. Environ. Res. J. 2012;6:83–102. [Google Scholar]
- 12.Read N.D., Goryachev A.B., Lichius A. The mechanistic basis of self-fusion between conidial anastomosis tubes during fungal colony initiation. Fungal Biol. Rev. 2012;26:1–11. doi: 10.1016/j.fbr.2012.02.003. [DOI] [Google Scholar]
- 13.Kurian S.M., Di Pietro A., Read N.D. Live-cell imaging of conidial anastomosis tube fusion during colony initiation in Fusarium oxysporum. PLoS ONE. 2018;13:e0195634. doi: 10.1371/journal.pone.0195634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrabi R., Bahkali A.H., Abd-Elsalam K.A., Moslem M., Ben M’Barek S., Gohari A.M., Jashni M.K., Stergiopoulos I., Kema G.H., de Wit P.J. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiol. Rev. 2011;35:542–554. doi: 10.1111/j.1574-6976.2010.00263.x. [DOI] [PubMed] [Google Scholar]
- 15.Daskalov A., Heller J., Herzog S., Fleißner A., Glass N.L. Molecular Mechanisms Regulating Cell Fusion and Heterokaryon Formation in Filamentous Fungi. Microbiol. Spectr. 2017;5:215–229. doi: 10.1128/microbiolspec.FUNK-0015-2016. [DOI] [PubMed] [Google Scholar]
- 16.Goryachev A.B., Lichius A., Wright G.D., Read N.D. Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. BioEssays. 2012;34:259–266. doi: 10.1002/bies.201100135. [DOI] [PubMed] [Google Scholar]
- 17.Herzog S., Schumann M.R., Fleißner A. Cell fusion in Neurospora crassa. Curr. Opin. Microbiol. 2015;28:53–59. doi: 10.1016/j.mib.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Martin D.C., Kim H., Mackin N.A., Maldonado-Báez L., Evangelista C.C., Beaudry V.G., Dudgeon D.D., Naiman D.Q., Erdman S.E., Cunningham K.W. New Regulators of a High Affinity Ca2+ Influx System Revealed through a Genome-wide Screen in Yeast. J. Biol. Chem. 2011;286:10744–10754. doi: 10.1074/jbc.M110.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller E.M., Locke E.G., Cunningham K.W. Differential Regulation of Two Ca2+ Influx Systems by Pheromone Signaling in Saccharomyces cerevisiae. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer M., Schnell N., Chattaway J., Davies P., Dixon G., Sanders D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997;419:259–262. doi: 10.1016/S0014-5793(97)01466-X. [DOI] [PubMed] [Google Scholar]
- 21.Paidhungat M., Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 1997;17:6339–6347. doi: 10.1128/MCB.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller E.M., Mackin N.A., Erdman S.E., Cunningham K.W. Fig1p Facilitates Ca2+ Influx and Cell Fusion during Mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 23.Cavinder B., Trail F. Role of Fig1, a Component of the Low-Affinity Calcium Uptake System, in Growth and Sexual Development of Filamentous Fungi. Eukaryot. Cell. 2012;11:978–988. doi: 10.1128/EC.00007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clapham D.E. Calcium Signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Reddish F.N., Miller C.L., Gorkhali R., Yang J.J. Monitoring ER/SR Calcium Release with the Targeted Ca2+ Sensor Catch ER+ J. Vis. Exp. 2017;123:e55822. doi: 10.3791/55822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson G., Kozlova-Zwinderman O., Collis A.J., Knight M.R., Fincham J.R.S., Stanger C.P., Renwick A., Hessing J.G.M., Punt P.J., Van Den Hondel C.A.M.J.J., et al. Calcium measurement in living filamentous fungi expressing codon-optimized aequorin. Mol. Microbiol. 2004;52:1437–1450. doi: 10.1111/j.1365-2958.2004.04066.x. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves A.P., Cordeiro J.M., Monteiro J., Muñoz A., Correia-De-Sá P., Read N., Videira A. Activation of a TRP-like channel and intracellular calcium dynamics during phospholipase C-mediated cell death. J. Cell Sci. 2014;127:3817–3829. doi: 10.1242/jcs.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder U., Bencina M., Eigentler A., Meyer V., Marx F. The Aspergillus giganteus antifungal protein AFPNN5353activates the cell wall integrity pathway and perturbs calcium homeostasis. BMC Microbiol. 2011;11:209. doi: 10.1186/1471-2180-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popa C.-V., Dumitru I., Ruta L.L., Danet A.F., Farcasanu I.C. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 2010;277:4027–4038. doi: 10.1111/j.1742-4658.2010.07794.x. [DOI] [PubMed] [Google Scholar]
- 30.Teng J., Goto R., Iida K., Kojima I., Iida H. Ion-channel blocker sensitivity of voltage-gated calcium-channel homologue Cch1 in Saccharomyces cerevisiae. Microbiology. 2008;154:3775–3781. doi: 10.1099/mic.0.2008/021089-0. [DOI] [PubMed] [Google Scholar]
- 31.Yu Q., Ding X., Zhang B., Xu N., Jia C., Mao J., Zhang B., Xing L., Li M. Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res. 2014;14:633–641. doi: 10.1111/1567-1364.12150. [DOI] [PubMed] [Google Scholar]
- 32.Sunagawa M., Kosugi T., Nakamura M., Sperelakis N. Pharmacological Actions of Calmidazolium, a Calmodulin Antagonist, in Cardiovascular System. Cardiovasc. Drug Rev. 2006;18:211–221. doi: 10.1111/j.1527-3466.2000.tb00044.x. [DOI] [Google Scholar]
- 33.Hooks M.A. Tacrolimus, a New Immunosuppressant—A Review of the Literature. Ann. Pharmacother. 1994;28:501–511. doi: 10.1177/106002809402800414. [DOI] [PubMed] [Google Scholar]
- 34.Lamoth F., Alexander B.D., Juvvadi P.R., Steinbach W.J. Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species: Table 1. J. Antimicrob. Chemother. 2015;70:1408–1411. doi: 10.1093/jac/dku549. [DOI] [PubMed] [Google Scholar]
- 35.Juvvadi P.R., Lamoth F., Steinbach W.J. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014;28:56–69. doi: 10.1016/j.fbr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Handschumacher R. Specific interaction of the cyclophilin-cyclosporin complex with the B subunit of calcineurin. J. Biol. Chem. 1993;268:14040–14044. doi: 10.1016/S0021-9258(19)85206-7. [DOI] [PubMed] [Google Scholar]
- 37.Amor K.T., Ryan C., Menter A. The use of cyclosporine in dermatology: Part I. J. Am. Acad. Dermatol. 2010;63:925–946. doi: 10.1016/j.jaad.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 38.Hong M.-P., Vu K., Bautos J., Gelli A. Cch1 Restores Intracellular Ca2+ in Fungal Cells during Endoplasmic Reticulum Stress. J. Biol. Chem. 2010;285:10951–10958. doi: 10.1074/jbc.M109.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brini M., Carafoli E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 40.Garg V., Kirichok Y. Keeping a lid on calcium uptake. eLife. 2016;5:341. doi: 10.7554/eLife.17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zelter A., Bencina M., Bowman B.J., Yarden O., Read N.D. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 2004;41:827–841. doi: 10.1016/j.fgb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Benčina M., Bagar T., Lah L., Kraševec N. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet. Biol. 2009;46:S93–S104. doi: 10.1016/j.fgb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Rispail N., Soanes D.M., Ant C., Czajkowski R., Grünler A., Huguet R., Perez-Nadales E., Poli A., Sartorel E., Valiante V., et al. Comparative genomics of MAP kinase and calcium–calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Galagan J.E., Calvo S.E., Borkovich K.A., Selker E.U., Read N.D., Jaffe D., Fitzhugh W., Ma L.-J., Smirnov S., Purcell S., et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 47.Prole D.L., Taylor C.W. Identification and Analysis of Cation Channel Homologues in Human Pathogenic Fungi. PLoS ONE. 2012;7:e42404. doi: 10.1371/journal.pone.0042404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonçalves A.P., Cordeiro J.M., Monteiro J., Lucchi C., Correia-De-Sá P., Videira A. Involvement of mitochondrial proteins in calcium signaling and cell death induced by staurosporine in Neurospora crassa. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2015;1847:1064–1074. doi: 10.1016/j.bbabio.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Ploetz R.C. Fusarium Wilt of Banana. Phytopathology. 2015;105:1512–1521. doi: 10.1094/PHYTO-04-15-0101-RVW. [DOI] [PubMed] [Google Scholar]
- 50.Nucci F., Nouér S.A., Capone D., Anaissie E., Nucci M. Fusariosis. Semin. Respir. Crit. Care Med. 2015;36:706–714. doi: 10.1055/s-0035-1562897. [DOI] [PubMed] [Google Scholar]
- 51.Esnakula A.K., Summers I., Naab T.J. Fatal Disseminated Fusarium Infection in a Human Immunodeficiency Virus Positive Patient. Case Rep. Infect. Dis. 2013;2013:379320. doi: 10.1155/2013/379320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou Y.-H., Hsu L.-H., Wang H.-F., Lai Y.-H., Chen Y.-L. Calcineurin Regulates Conidiation, Chlamydospore Formation and Virulence in Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2020;11:539702. doi: 10.3389/fmicb.2020.539702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harren K., Tudzynski B. Cch1 and Mid1 Are Functionally Required for Vegetative Growth under Low-Calcium Conditions in the Phytopathogenic Ascomycete Botrytis cinerea. Eukaryot. Cell. 2013;12:712–724. doi: 10.1128/EC.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., Farmer J.D., Jr., Lane W.S., Friedman J., Weissman I., Schreiber S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-H. [DOI] [PubMed] [Google Scholar]
- 55.Jin L., Harrison S.C. Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin. Proc. Natl. Acad. Sci. USA. 2002;99:13522–13526. doi: 10.1073/pnas.212504399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osherov N., May G.S. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 2001;199:153–160. doi: 10.1111/j.1574-6968.2001.tb10667.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee S.C., Lee Y.H. Calcium/calmodulin-dependent signaling for appressorium formation in the plant pathogenic fungus Magnaporthe grisea. Mol. Cells. 1998;8:698–704. [PubMed] [Google Scholar]
- 58.Som T., Kolaparthi V.S. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 1994;14:5333–5348. doi: 10.1128/mcb.14.8.5333-5348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palma-Guerrero J., Hall C.R., Kowbel D., Welch J., Taylor J.W., Brem R.B., Glass N.L. Genome Wide Association Identifies Novel Loci Involved in Fungal Communication. PLoS Genet. 2013;9:e1003669. doi: 10.1371/journal.pgen.1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weichert M., Fleißner A. Anastomosis and Heterokaryon Formation. In: van den Berg M.A., Maruthachalam K., editors. Genetic Transformation Systems in Fungi. Volume 2. Springer International Publishing; Cham, Switzerland: 2015. pp. 3–21. [Google Scholar]
- 61.Schumann M.R., Brandt U., Adis C., Hartung L., Fleißner A. Plasma Membrane Integrity During Cell–Cell Fusion and in Response to Pore-Forming Drugs Is Promoted by the Penta-EF-Hand Protein PEF1 in Neurospora crassa. Genetics. 2019;213:195–211. doi: 10.1534/genetics.119.302363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lichius A., Goryachev A., Fricker M., Obara B., Castro-Longoria E., Read N.D. CDC-42 and RAC-1 regulate opposite chemotropisms in Neurospora crassa. J. Cell Sci. 2014;127:1953–1965. doi: 10.1242/jcs.141630. [DOI] [PubMed] [Google Scholar]
- 63.Lichius A., Roca G.M., Read N.D. How to Distinguish Conidial Anastomosis Tubes (CATs) from Germ Tubes, and to Discriminate between Cell Fusion Mutants Blocked in CAT Formation and Homing. The Neurospora Protocol Guide. 2010. [(accessed on 27 November 2021)]. Available online: http://www.fgsc.net/neurosporaprotocols/LICHIUS_ROCA%20_READ_2010_CAT_formation_protocol_final.pdf.
- 64.Courchesne W.E. Characterization of a Novel, Broad-Based Fungicidal Activity for the Antiarrhythmic Drug Amiodarone. J. Pharmacol. Exp. Ther. 2002;300:195–199. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- 65.Lew R.R., Abbas Z., Anderca M.I., Free S.J. Phenotype of a Mechanosensitive Channel Mutant, mid-1, in a Filamentous Fungus, Neurospora crassa. Eukaryot. Cell. 2008;7:647–655. doi: 10.1128/EC.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cavinder B., Hamam A., Lew R.R., Trail F. Mid1, a Mechanosensitive Calcium Ion Channel, Affects Growth, Development, and Ascospore Discharge in the Filamentous Fungus Gibberella zeae. Eukaryot. Cell. 2011;10:832–841. doi: 10.1128/EC.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Troppens D.M., Chu M., Holcombe L.J., Gleeson O., O’Gara F., Read N., Morrissey J.P. The bacterial secondary metabolite 2,4-diacetylphloroglucinol impairs mitochondrial function and affects calcium homeostasis in Neurospora crassa. Fungal Genet. Biol. 2013;56:135–146. doi: 10.1016/j.fgb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Bonilla M., Cunningham K.W. Calcium Release and Influx in Yeast: TRPC and VGCC Rule Another Kingdom. Sci. Signal. 2002;2002:pe17. doi: 10.1126/stke.2002.127.pe17. [DOI] [PubMed] [Google Scholar]
- 69.Kim H.-S., Kim J.-E., Son H., Frailey D., Cirino R., Lee Y.-W., Duncan R., Czymmek K., Kang S. Roles of three Fusarium graminearum membrane Ca2+ channels in the formation of Ca2+ signatures, growth, development, pathogenicity and mycotoxin production. Fungal Genet. Biol. 2018;111:30–46. doi: 10.1016/j.fgb.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Brand A.C., Morrison E., Milne S., Gonia S., Gale C.A., Gow N. Cdc42 GTPase dynamics control directional growth responses. Proc. Natl. Acad. Sci. USA. 2014;111:811–816. doi: 10.1073/pnas.1307264111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brand A., Shanks S., Duncan V.M., Yang M., Mackenzie K., Gow N.A. Hyphal Orientation of Candida albicans Is Regulated by a Calcium-Dependent Mechanism. Curr. Biol. 2007;17:347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simonin A.R., Rasmussen C.G., Yang M., Glass N.L. Genes encoding a striatin-like protein (ham-3) and a forkhead associated protein (ham-4) are required for hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 2010;47:855–868. doi: 10.1016/j.fgb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Fu C., Iyer P., Herkal A., Abdullah J., Stout A., Free S.J. Identification and Characterization of Genes Required for Cell-to-Cell Fusion in Neurospora crassa. Eukaryot. Cell. 2011;10:1100–1109. doi: 10.1128/EC.05003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu C., Ao J., Dettmann A., Seiler S., Free S.J. Characterization of the Neurospora crassa Cell Fusion Proteins, HAM-6, HAM-7, HAM-8, HAM-9, HAM-10, AMPH-1 and WHI-2. PLoS ONE. 2014;9:e107773. doi: 10.1371/journal.pone.0107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonilla M., Cunningham K.W. Mitogen-activated Protein Kinase Stimulation of Ca2+ Signaling Is Required for Survival of Endoplasmic Reticulum Stress in Yeast. Mol. Biol. Cell. 2003;14:4296–4305. doi: 10.1091/mbc.e03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Silao F.G.S., Bigol U.G., Bungay A.A.C., Nicolas M.G., Heitman J., Chen Y.-L. Calcineurin Is Required for Pseudohyphal Growth, Virulence, and Drug Resistance in Candida lusitaniae. PLoS ONE. 2012;7:e44192. doi: 10.1371/journal.pone.0044192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blankenship J., Heitman J. Calcineurin Is Required for Candida albicans To Survive Calcium Stress in Serum. Infect. Immun. 2005;73:5767–5774. doi: 10.1128/IAI.73.9.5767-5774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eckstein L.A., Van Quill K.R., Bui S.K., Uusitalo M.S., O’Brien J.M. Cyclosporin A Inhibits Calcineurin/Nuclear Factor of Activated T-Cells Signaling and Induces Apoptosis in Retinoblastoma Cells. Investig. Opthalmology Vis. Sci. 2005;46:782–790. doi: 10.1167/iovs.04-1022. [DOI] [PubMed] [Google Scholar]
- 79.Fleissner A., Leeder A.C., Roca M.G., Read N., Glass N.L. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Natl. Acad. Sci. USA. 2009;106:19387–19392. doi: 10.1073/pnas.0907039106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the considerable number and large file size of the imaging data sets, as well as the requirement for specific image analysis software.