Abstract
Objectives:
To identify factors that increase risk of gastrointestinal-related (GI-related) hospitalization of infants with cystic fibrosis (CF) during the first year of life.
Methods:
The Baby Observational and Nutrition Study was a longitudinal, observational cohort of 231 infants diagnosed with CF by newborn screening. We performed a post-hoc assessment of the frequency and indications for GI-related admissions during the first year of life.
Results:
Sixty-five participants had at least one admission in the first 12 months of life. High pancreatic enzyme replacement therapy (PERT) dosing (>2000 LU/kg/meal) (hazard ratio, HR=14.75, p=0.0005) and use of acid suppressive medications (HR=4.94, p=0.01) during the study period were positively associated with subsequent GI-related admissions. High levels of fecal calprotectin (fCP) (>200 μg/g) and higher relative abundance of fecal Klebsiella pneumoniae were also positively associated with subsequent GI-related admissions (HR=2.64, p=0.033 and HR=4.49, p=0.002, respectively). During the first 12 months of life, participants with any admission had lower weight-for-length z-scores (WLZ) (p=0.01). The impact of admission on WLZ was particularly evident in participants with a GI-related admission (p<0.0001).
Conclusions:
Factors associated with higher risk for GI-related admission during the first 12 months include high PERT dosing, exposure to acid suppressive medications, higher fCP levels, and/or relative abundance of fecal Klebsiella pneumoniae early in life. Infants with CF requiring GI-related hospitalization had lower WLZ at 12 months of age than those not admitted as well as those admitted for non GI-related indications.
Keywords: pancreatic enzyme replacement therapy, fecal calprotectin, Klebsiella pneumoniae, acid suppressive medications, malnutrition
Introduction
Cystic fibrosis (CF) is a multisystem disease that includes gastrointestinal (GI) manifestations such as meconium ileus (MI), pancreatic insufficiency (PI), fat malabsorption, rectal prolapse, gastroesophageal reflux, and alterations in the GI microbiome1. Many of these factors contribute to malnutrition and may be severe enough to warrant hospitalization. Although the implementation of universal newborn screening (NBS) for CF in the United States has improved outcomes2,3, infants with CF remain at risk for hospitalization during the first 12 months of life3–5. Agrawal et al. reported 0.67 per 10,000 admission for patients with CF <1 year of age in a post-NBS era in 2013. While this rate of admission is lower than for patients with CF >1 year of age, admission during the first 12 months of life contributes to the emotional and financial burden a diagnosis of chronic illness bestows on new parents5–6.
Nutritional management is prioritized in CF infants due to the impact of malnutrition on long-term pulmonary outcomes, cognitive function and mortality6–11. Nutritional challenges are often directly associated with early GI manifestations12. While respiratory findings that correlate with hospitalization during the first year of life have been reported previously3,4, factors that are associated with an increased risk of GI-related hospitalization during the first year of life, excluding meconium ileus (MI), remain incompletely understood. This study aims to identify potential factors that may increase risk of GI-related hospitalizations in infants with CF. By recognizing these factors, strategies may be developed to decrease the cost and burden associated with early hospitalization, improve overall GI symptomatology, and most importantly, optimize growth in the first year of life.
Methods
For our study, we performed a post-hoc analysis of data collected from the Baby Observational and Nutrition Study (BONUS). BONUS was a multicenter, longitudinal, prospective, observational cohort of infants diagnosed with CF by newborn screening (NBS). BONUS previously shed light on the impact of NBS on weight and length, PERT usage, and respiratory findings in the first year of life.3–4,13. Data was collected from each participant’s outpatient history, inpatient admission records, and laboratory values including oropharyngeal and fecal metagenomes during the first 12 months of life.
Participants and Study Design
The complete Methods and primary data from BONUS have previously been reported in detail3. In brief, BONUS was a prospective cohort study that enrolled infants with CF diagnosed by NBS from birth to 3.5 months of age at 28 CF Centers in the United States3 between January 7, 2012 through May 31, 2015. Infants were required to have a gestational age of 35 weeks or greater, birth weight greater than or equal to 2.5 kg and the ability to tolerate full oral feeds at enrollment. Infants were followed until 12 months of age with BONUS visits occurring simultaneously with routine standard-of-care visits in accordance with Cystic Fibrosis Foundation (CFF) guidelines6. Infants in whom a gastrostomy tube (Gtube)/percutaneous gastrostomy tube (PEG) was placed after enrollment remained in the study to observe natural history. Approval for BONUS was granted through the local institutional review board at each participating site (ClinicalTrials.gov NCT01424696) and informed consent was obtained from a parent or legal guardian of each participant.
The following parameters were collected:
- At enrollment:
- Birth and postnatal history were screened for a diagnosis of MI
- At each visit (monthly from birth-6 months of age and then every 2 months from 6 to 12 months of age):
- Anthropometrics
- Weight, length, and occipital-frontal circumference (OFC)14
- Medications
- Including pancreatic enzyme therapy (PERT), acid suppressive medications (proton pump inhibitors and H2-receptor antagonists) and antibiotics
- Closest to enrollment, 6 and 12 months of age (if a stool-containing diaper (S-CD) was available)
- Fecal calprotectin (fCP)
- Hospitalizations
- Type of hospitalization (respiratory, GI-related, or other based on primary indication for admission)
- Length of stay (LOS)
Definitions:
World Health Organization (WHO) standard growth curves were used to calculate weight, length, and OFC z-scores17–18. CFF guidelines recommend the following PERT dosing based on lipase units (LU): 450–900 LU/g of fat or 2,000–4,000 LU/120 ml of formula or when breastfeeding. PERT dosing for BONUS was stratified into 3 categories: <1000, 1000 to 2000, and >2000 LU/kg/meal13. Given that the mean PERT dose in our cohort was 1182 LU/kg/meal (range 492–3727 LU/kg/meal), we defined high PERT dosing as >2000 LU/kg/meal. Fecal calprotectin, a marker of intestinal inflammation was measured in μg/g, and a value of >/=200 μg/g was considered high based on ARUP laboratory cutoffs19.
Statistical Methods:
Descriptive analyses of categorical and continuous variables were performed using proportions, frequencies, medians and ranges. Differences and comparisons were performed with Chi-square test, Fisher’s exact test, Wilcoxon Rank Sum test and t-test, as appropriate to the variable’s level of measurement and distribution. Mixed model with first-order autoregressive covariance structure was used to investigate the association between hospital admission and various z-scores (weight z-score (WAZ), length z-score (LAZ), weight-for-length z-score (WLZ)) repeatedly measured during the first year of life. Kaplan Meier Curves were produced to illustrate the relationship between GI admission and time-static characteristics, such as at or closest to enrollment measurement of fCP, relative abundance of fecal K. pneumoniae and PERT dosing. Both Log Rank test and Cox proportional hazard model were used to investigate the effect on hospitalization and other outcomes of time-static covariates, such as at or closest to enrollment measurements of fCP, relative abundance of fecal K. pneumoniae, PERT dosing, mutation, genotype, and MI. PERT dosing, acid suppressive medication usage, antibiotics usage and feeding type were measured multiple times during the 12-month study period to incorporate the information from those multiple measured characteristics and their temporal order relative to the event, the Cox proportional hazards model with time-dependent explanatory variables was utilized20–21. The missing data for this study was acceptable for typical longitudinal observational studies, with missing values ranging from 0% for hospitalization, to 5% for fCP closest to enrollment, to 17% for fecal K. pneumoniae closest to enrollment. The typical pairwise deletion was used to handle missing values for various statistical testing. All statistical analyses were performed with SAS 9.4 (Cary, NC).
Results
The demographics of BONUS have been well described in greater detail in the original paper by Leung et al3. A total of 65 out of 231 infants enrolled in BONUS required admission during their first 12 months of life. Of the 231 infants in the study, 49 (21.2%) were hospitalized once and 16 (6.9%) were hospitalized at least twice. Four participants (1.7%) had three or more admissions which included separate admissions for respiratory and GI indications. Twenty-one participants (9.1%) had at least one admission for a GI-related cause, 40 (17.3%) had at least one admission for a respiratory indication, and 9 (3.9%) were admitted at least once for other causes. For infants with more than one GI admission, the second admission always occurred within two months of discharge from the first hospitalization.
There were no significant differences in median LOS among GI, respiratory and other indications for admission (p=0.27). A majority of the GI-related admissions (n=15, 57.7%) were for the following clinical diagnosis: failure to thrive, poor weight gain, feeding difficulty, or poor intake (Table 1). The median LOS for GI-related admissions was 5.0 days (range 1.0–44.0 days). Constipation accounted for 1 (3.9%) admission with a LOS of 1.0 days. Reflux/emesis accounted for 2 (7.7%) admissions with a median LOS of 9.0 days. Electrolyte disturbances accounted for 2 (7.7%) admissions with a median LOS of 6.5 days (range 5.0–8.0 days). There was only one admission for rectal prolapse with a LOS of 1.0 days. Gtube/PEG placement accounted for 4 (15.4%) admissions with a median LOS of 4.0 days (range 2.0–9.0 days).
Table 1:
Clinical Diagnosis for GI-related Hospitalizations
| Indication | Number of GI-related Hospitalizations | % of Total GI -related Hospitalizations | Average LOS days median (range) |
|---|---|---|---|
| FTT/Poor weight gain/Feeding difficulty/Poor intake | 15 | 57.7 | 4.0 (1.0 – 44.0) |
| Constipation | 1 | 3.9 | 1.0 |
| Reflux/Emesis | 2 | 7.7 | 9.0 (9.0 – 9.0) |
| Electrolyte disturbances | 2 | 7.7 | 6.5 (5.0 – 8.0) |
| Diarrhea | 1 | 3.9 | 5.0 |
| Rectal prolapse | 1 | 3.9 | 1.0 |
| Gtube/PEG placement | 4 | 15.4 | 4.0 (2.0 – 9.0) |
FTT: failure to thrive; Gtube: gastrostomy tube; PEG: percutaneous endoscopic gastrostomy tube
Clinical diagnosis for GI-related Hospitalizations in 21 infants with a total of 26 unique admissions (5 participants had >1 admission).
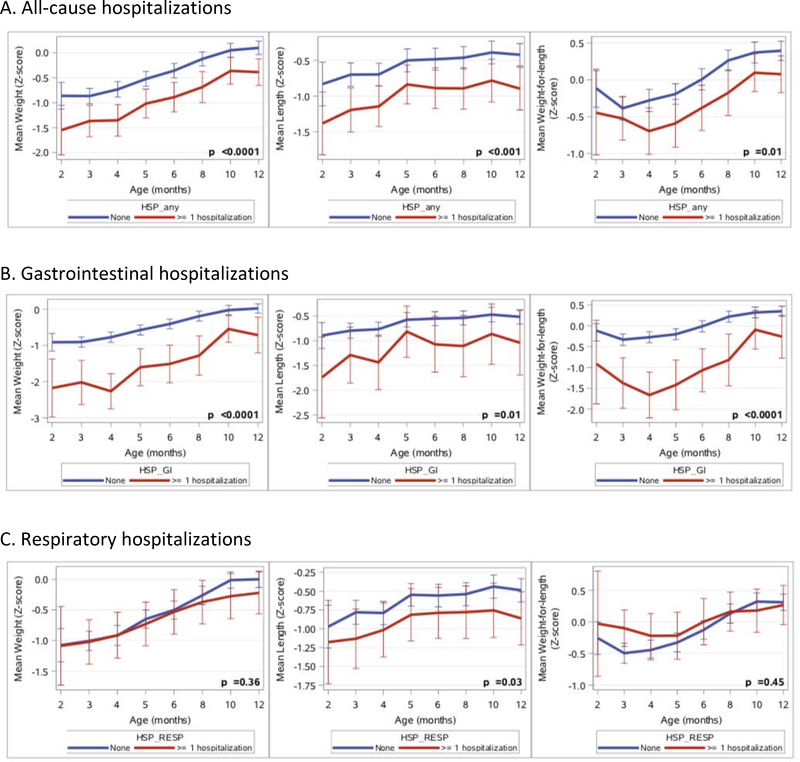
Mixed model analysis showed that all-cause admission was associated with lower WAZ (p<0.0001), lower LAZ (p<0.001) and lower WLZ (p=0.01), with GI-related admission having the greatest negative effect on anthropometric measures and respiratory admissions having a negligible effect (Figure 1). Among those participants who were not hospitalized, mean WLZ at 12 months of age were 0.39 (SD 0.81) compared to 0.08 (SD 0.97) for those who required any admission (p<0.05).
Figure 1:
Impact of hospitalization on growth of infants in the first year of life.
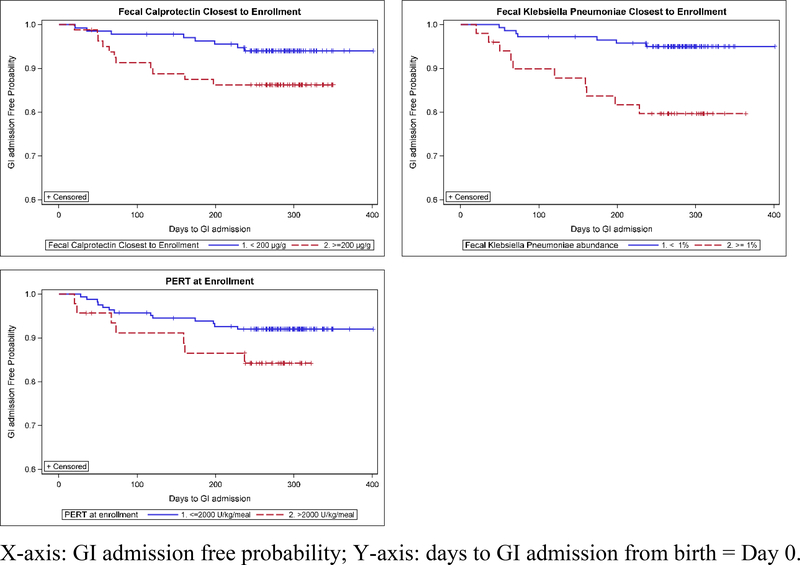
Factors and their relationship to GI-related admission are summarized in Table 2. Genotype, history of MI and feeding type were not associated with an increased risk of GI-related admissions in the first 12 months. We assessed the effect of medications on GI-related admissions. Log Rank test showed that high PERT dosing per meal (>2,000 LU/kg/meal) at enrollment, which was seen in 46 of 210 (21.9%) infants with PI, was associated with increased risk for a subsequent GI-related admission (p=0.031, Figure 2). When multiple measurements of PERT were modeled as time-dependent explanatory variables in the Cox proportional hazard model, the positive association between high PERT dosing per meal and GI-related admission was more significant (hazard ratio=14.75, p=0.0005). Acid suppressive medications were taken at some point in the first year of life (“ever taken”) in 153 (66.2%) of infants and were significantly associated with GI-related admission (p<0.001), with 116 of these (75.8%) ever using histamine (H2 blocker) and 85 (55.5%) ever using a proton pump inhibitor (PPI). Some infants took >1 acid suppressant. All 21 participants that had a GI-related admission were on acid suppressive treatment at some time during the first year of life with 18 (85.7%) participants on acid suppressive medications prior to the GI-related admission. Cox proportional hazard model showed that acid suppressive medications (either H2 blocker or PPI) were positively associated with subsequent GI-related admission (HR=4.94, p=0.003) but not respiratory admission (p=0.731). Antibiotics were “ever taken” at some point in the first year of life in 176 (76.2%) infants. Of the 21 participants admitted for GI indications, 12 (57.1%) had antibiotic treatment before admission. Cox proportional hazard model did not show significant association between antibiotic treatment and subsequent GI-related admission (HR=1.73, p=0.24).
Table 2:
Association of Risk Factors with GI-related Admissions
| Potential Associated Risk Factor | Total number n (%) n=231 |
Participants with GI-related Hospitalization n (%) n=21 |
Participants with no GI-related Hospitalization n (%) n=210 |
p value | |
|---|---|---|---|---|---|
| Phenotypic characteristics | |||||
| Genotype | F508del/F508del | 132 (57.1) | 12 (57.1) | 120 (57.1) | 0.94 a |
| F508del/other | 84 (36.4) | 8 (38.1) | 76 (36.2) | ||
| Other/other | 15 (6.5) | 1 (4.8) | 14 (6.7) | ||
| Meconium ileus | Yes | 27 (11.7) | 3 (14.3) | 24 (11.4) | 0.72 b |
| No | 204 (88.3) | 18 (85.7) | 186 (88.6) | ||
| Choice of feeding | |||||
| Feeding Type closest to enrollment* (n=229) | Breastfeeding ONLY | 66 (28.7) | 7 (33.3) | 59 (28.2) | 0.17 a |
| Formula ONLY | 117 (50.9) | 13 (61.9) | 104 (49.8) | ||
| Both | 47 (20.4) | 1 (4.8) | 46 (22.0) | ||
| Medications | |||||
| PERT dose at/near enrollment (PI infants only, n=210) | Normal (</=2000 LU/kg/meal) | 164 (78.1) | 13 (65.0) | 151 (79.5) | 0.16 b |
| High (>2000 LU/kg/meal) | 46 (21.9) | 7 (35.0) | 39 (20.5) | ||
| Acid suppressant (some infants took >1 during study period) | Never Taken | 78 (33.8) | 0 (0.0) | 78 (37.1) | <0.001 a |
| Ever Taken | 153 (66.2) | 21 (100) | 132 (62.9) | ||
| H2 receptor antagonist | 116 (75.8) | ||||
| Proton pump inhibitor | 85 (55.5) | ||||
| Antibiotic use | Never Taken | 55 (23.8) | 5 (23.8) | 50 (23.8) | 1.00 a |
| Ever Taken | 176 (76.2) | 16 (76.2) | 160 (76.2) | ||
| Stool markers | |||||
| Calprotectin level at enrollment* (n=216) | Normal (<200 μg/g) | 135 (62.2) | 8 (40.0) | 127 (64.5) | 0.03 a |
| High (>=200 μg/g) | 82 (37.8) | 12 (60.0) | 70 (35.5) | ||
| Relative abundance of K. pneumoniae in the fecal microbiota at enrollment* (n=193) | Normal (<1%) | 143 (74.1) | 8 (44.4) | 135 (77.1) | < 0.01 b |
| High (>=1%) | 50 (25.9) | 10 (55.6) | 40 (22.9) | ||
Chi square test
Fisher’s Exact test
Data not available for all subjects (see text)
Figure 2:
The relationship of GI admission and baseline measurements of fecal calprotectin, relative abundance of fecal K. pneumoniae and PERT dose
Fecal calprotectin levels at enrollment were available for 94% of infants and were found to be elevated in 37.8% of participants. Elevated levels of fCP at enrollment were positively associated with subsequent GI-related (p= 0.048) but not respiratory (p=0.2274) admissions. Higher relative abundance of K. pneumoniae in the fecal microbiota at enrollment was also associated with subsequent GI-related admissions (p=0.002) (Figure 2).
Discussion
This study fills a critical gap in the literature pertaining to factors that may be associated with increased risk of GI-related hospitalizations during the first year of life in the CF population. A noteworthy 1 in every 4 infants in the BONUS cohort required hospitalization in the first year of life, which was greater than Agarwal’s reported rate of 0.67 admissions per 10,000 for patients <1 year of age5. This discrepancy likely represents challenges in comparison of a relatively small prospective cohort with a large database driven population study that relies on ICD-9 codes for extrapolation of data. While GI-related admissions were less frequent than respiratory, 1 in 10 BONUS participants required at least one GI-related hospitalization, with a median of 6.87 days in the hospital. The rate of GI-related admissions in this study is much less that noted by Agrawal et al. for the total CF population of 3%5, although direct correlation of percentage of GI-related admissions for age was not available for comparison. This likely represents variation in specific GI manifestations of CF seen across different age groups.
The initial analysis of the BONUS study found that universal NBS led to significant improvements in both WAZ and LAZ compared to a similar cohort from 20 years ago3. It is encouraging that at 12 months of age, infants in the BONUS cohort who had never been hospitalized were at the population norm for weight and close to the population norm for length. While early recognition of disease has allowed for earlier and more aggressive intervention, malnutrition and stunting among infants with CF nevertheless persists3. Of the GI-related admissions in the BONUS study, the majority were for failure to thrive, poor weight gain, feeding difficulty, and poor intake. In total, nearly 60% of all GI-related admissions in the first year of life in the BONUS cohort were linked to malnutrition demonstrating that malnutrition continues to represent a significant risk to infants with CF. This illustrates the importance of adhering to the recommendations of the CFF to have a registered dietitian as part of the multidisciplinary team and close follow-up in the first 12 months of life6. Our analysis of the growth patterns of infants who were hospitalized in the first year of life indicates that those who were admitted for respiratory concerns had WAZ, LAZ and WLZ that were indistinguishable from infants who were never hospitalized; specifically, growth was at or close to the population norm at 12 months of age. On the contrary, those who were hospitalized because of GI symptoms had significantly lower anthropometric parameters relative to those who had never been hospitalized and the deficits were severe enough to skew these nutritional parameters for the group as a whole (“all-cause admission”). No catch-up growth was seen at 12 months of age in the infants hospitalized for GI concerns. The primary indications for GI-related admission was failure to thrive/poor feeding/poor intake, which directly impacts nutritional status. Unlike resolution from a pulmonary exacerbation or viral respiratory illness, catch-up growth requires persistent interventions over time22. Therefore, hospitalization for GI causes may serve to identify infants who require extra medical attention because adverse growth early in life is negatively associated with future pulmonary and other important clinical outcomes8,11.
Notably, the type of feeding (breastmilk, formula or combination) was not associated with increased risk of admission or in overall weight gain during the first 12 months of life in the initial analysis of the BONUS cohort3. These results support CFF guidelines recommending incorporation of breastfeeding/breastmilk into CF infants’ nutritional support, while providing as needed caloric additives6. However, due to our small sample size of CF infants with GI-related hospitalization, further research in this area in needed.
With regard to PERT dosing, there are conflicting results in the literature. Gelfond et al.8 found a mean PERT dose of 1882 LU/kg/meal and identified no association between PERT dose and weight gain or clinical benefit among the BONUS cohort; whereas Schechter et al. showed an improvement for WAZ and WLZ z-scores reached by 2 years of age in infants receiving >1500 LU/kg/meal23. The high PERT doses in both studies are below the cutoff point used in this analysis of 2,000 LU/kg/meal, which was associated with increased risk for GI-related admission. Using high PERT dosing has historically been thought to improve malabsorption and thus improve weight gain. Our results suggest that high PERT may be a result of, rather than a cause of, GI-related symptoms. Specifically, clinicians continue to utilize high PERT dosing when concerned about GI symptoms of malabsorption and poor weight gain. As an observational study, we cannot determine cause and effect. Further exploration is needed to determine the optimal PERT dosing to minimize GI symptoms and maximize growth.
Prescribing acid suppressive medications is common practice in CF. Approximately 65% of all persons with CF in the CFF Patient Registry are reported to be on acid suppressive medications24. There are many reasons for the use of these medications in this population including but not limited to gastroesophageal reflux, gastric hyperacidity, increasing the efficacy of PERT and concerns of micro-aspiration resulting in declining lung function and/or early lung disease25. PPI use among older persons with CF has been associated with higher risk for pulmonary exacerbations26, decline in lung function27 and increased rate of hospitalizations28. A previous analysis of the BONUS cohort found that use of acid suppressive medications with either H2-blocker or PPI medications was not associated with respiratory admissions4. The current analysis identified an almost 5-fold increased risk for GI-related admissions. Analytic limitation prevents us from understanding whether all acid suppressive medication strategies are equally associated with GI-related admission. As with PERT dosing, the finding of an increase in GI-related hospitalizations in infants with CF on either H2-blocker or PPI suggests that early initiation of acid suppressive medications may also be a surrogate marker of severity of GI symptoms and not a cause of increased admissions.
Garg et al. evaluated age-dependent variation of fCP, a marker of intestinal inflammation, in CF and found significantly lower levels than in healthy controls during the first year of life29. Ellemunter et al. evaluated longitudinal changes in fCP in CF patients 0 to 61 years old and found an elevation in two-thirds of patients in at least one sample over a period of 12 years28. In both studies however, elevated fCP was defined by >50 μg/g29–30. In our study we chose to use a higher cutoff value of 200 μg/g as used by ARUP and based on reports of elevated fCP in healthy infants in the first year of life31. In the BONUS cohort, elevated fCP at enrollment was predictive of GI admission. It is possible that this finding reflects impairment of intestinal absorption or increased caloric expenditure due to inflammation, resulting in nutritional failure requiring hospitalization. Further exploration is needed to confirm and delineate these relationships and whether this biomarker can be used clinically to identify infants who need closer follow-up or earlier intervention.
Previous work has identified an association between fCP and relative abundances of intestinal Proteobacteria32 in children with CF. Similarly, we identified an association between increased fecal abundance of the Proteobacteria, K. pneumoniae, from stool sample obtained closest to enrollment and during a subsequent GI-related admission. Klebsiella isolates have been associated with GI inflammation in premature infants33–34. Whether Klebsiella or other Proteobacteria cause or represent inflammation and increased risk of subsequent GI-related admission requires further investigation. Of note, a detailed description of alterations in the fecal microbiota in infants in the BONUS cohort published elsewhere noted no significant relationship between fecal taxonomy and calprotectin levels14.
The major limitation of this study is that it is a post hoc analysis and observational in nature, which can identify associations, but cannot establish causation. Of note, the core study design specifically excluded infants who were born prematurely, had very low birth weight, or had complex MI and were unable to tolerate full feedings at the time of enrollment; thus, our conclusions are generalizable only to “healthy” infants with CF. Furthermore, as only the primary diagnosis of admissions was captured, multi-system involvement was not elucidated. The small number of admissions overall during the BONUS study, particularly GI-related hospitalizations, also limited the power of these observations. In addition, the lack of assessment of social determinants of health in this study limits the generalizability of this data.
Despite our limitations, the results of this study highlight that even in a relatively healthy cohort of infants with CF, there is a significant risk of GI-related hospitalization. Anthropometrics measurements in infants with GI-related hospitalization did not show catch-up at 12 months of age. These infants must be identified early and followed closely to optimize growth, minimize negative long-term clinical outcomes, and improve survival. It is imperative to study a larger population of infants with GI-related hospitalization to better stratify risk factors for hospitalization in the first year of life including evaluation of social determinants of health and burden of disease to determine generalizable approaches to managing this vulnerable population.
Of course, risk factors will need to be reassessed once highly effective modulators (HEMs) are used in this very young, physiologically vulnerable age group. Restoration of CFTR function through HEMs may have a beneficial impact on both intestinal inflammation, malabsorption, and the gut microbiome35–37.
Conclusion
In conclusion, the most common reasons for GI-related hospitalization in infants with CF during the 12 months of life were failure to thrive and poor weight gain. Acid suppressive medications and high PERT dosage were associated with increased risk of GI-related admission. Theoretically, these factors may be indicative of malnutrition and its severity leading to clinician interventions. High fCP and Klebsiella pneumoniae abundance within the fecal microbiota early in life were associated with increased risk for subsequent GI-related admissions. Future research could be useful to note whether these objective measures can be used to identify those infants who may benefit from earlier or more aggressive interventions. Furthermore, these variables could be potentially modifiable risk factors. At present, given that the majority of infants with CF are not yet eligible for highly effective modulator therapy, the first year of life remains a critical time period. In light of the long-term implications of poor growth, further study is warranted to develop more effective outpatient nutritional and medical management strategies to minimize GI-related hospitalization as well as determine the impact of social determinants of health in families caring for an infant with CF.
What is known?
Infants with cystic fibrosis (CF) experience nutritional challenges and gastrointestinal symptoms early in life.
Risk of hospitalization during the first 12 months of life in infants with CF directly contributes to the emotional and financial burden of the disease.
Poor growth early in life negatively impacts long-term clinical outcomes.
What is new?
Infants with CF who require GI-related hospitalization in the first 12 months of life have potentially identifiable risk factors.
Risk factors include high PERT dosing, exposure to acid suppressive medications, lower birth weight-for length z-score, and higher fecal calprotectin (fCP) levels and/or K. pneumoniae relative abundance in the stool early in life.
Infants with CF requiring GI-related hospitalization had worse overall growth (WLZ) at 12 months of age than those not admitted and those admitted for non GI-related indications.
Acknowledgements:
We would like to thank the Cystic Fibrosis Foundation for their support in this ad hoc analysis as well as the Cystic Fibrosis Foundation Therapeutics Network Sites and the patients and families who participated in the BONUS study.
Conflicts of Interest and Source of Funding:
Dr. Sathe reports consulting for Alcresta Therapeutics and PBM BC Holdings, and research/grant support through the Cystic Fibrosis Foundation. Dr. Leung reports received research/grant support from the Cystic Fibrosis Foundation, BMS, Gilead and Abbvie and service on the medical advisory board for Gilead and Merck. Dr. Freeman reports research/grant support from the Cystic Fibrosis Foundation. Also of note, research reported in this publication was supported by Children’s HealthSM. The content is solely the responsibility of the authors and does not necessarily represent the official views of Children’s HealthSM.
BONUS and its lead investigators were supported by CFFT BONUS11KO, NIH R01DK095738, NIH P30DK089507, NIH R01DK095869, NIH K24HL141669, CFF SATHE18KO, CFFT SATHE18Y5, MISSION GRANT Children’s HealthSM
Footnotes
Trial Registration:
United States ClinicalTrials.Gov registry NCT01424696 (clinicaltrials.gov)
BONUS Study Investigators. This study was conducted with the participation of the study site principal investigators and coordinators listed below:
Drs. A. Stecenko, and M. Schechter, and E. Hunter (Emory University, Atlanta GA), Dr. W Hoover, H Hathorne, and K Brand (University of Alabama at Birmingham), Dr. A Filbrun and M Linn (University of Michigan, Ann Arbor MI), Dr. D. Borowitz, and N. Caci (Women and Children’s Hospital of Buffalo, Buffalo NY), Dr B. McWilliams and I. Brazil (Children’s Medical Center of Central Texas, Austin TX), Dr. J. Heubi, and M Bushman (Cincinnati Children’s Hospital Medical Center, OH), Dr. S. McColley and A. Bowen (Chicago Lurie Children’s Hospital), Dr. K McCoy and K Sakellaris (Nationwide Children’s Hospital, Columbus OH), Drs. P. Sharma and C. Cannon, and A. Hebert (University of Texas Southwestern Medical Center, Dallas TX), Dr. S. Sagel and M. Anthony (Children’s Hospital Colorado, Denver CO), Dr. M. Dyson and H. Urbanek (Cook Children’s Medical Center, Fort Worth TX), Dr. S. Millard and C. Gile (Henen DeVos Women and Children’s Center, Grand Rapids MI), Dr. G. Graff and L. Allwein (Hershey Medical Center, Hershey PA), Dr. P. Hiatt and N. Schaap (Baylor College of Medicine, Houston Texas), Dr. N. Krupp and L. Bendy (Riley Hospital for Children, Indianapolis IN), Dr. R. Ahrens and M. Teresi (University of Iowa, Iowa City IA), Dr. A. Berlinski and L.L. Ramsey (Arkansas Children’s Hospital, Little Rock AR), Dr. J. McNamara and M. Johnson (Minnesota Children’s Hospital and Clinics, Minneapolis MN), Dr. R. Brown and P. Berry (Vanderbilt Children’s Hospital, Nashville TN), Drs. N. Mehdi and J. Royall, and D. Thomas (Children’s Hospital of Oklahoma, Oklahoma City, OK), Dr. R. Rubenstein and E. Donnelly (Children’s Hospital of Philadelphia, PA), Dr. D. Weiner, S. Hurban, and E. Hartigan, (Children’s Hospital of Pittsburgh and University of Pittsburgh, PA), Dr. M. Powers, and K Simmons (Oregon Health & Sciences University, Portland OR), Dr. B. Chatfield, and H. Oldroyd (University of Utah, Salt Lake City, UT), Dr. L. Hoffman and S. McNamara (Seattle Children’s Hospital, Seattle WA), Dr. J. Wooldridge, and A. Cooper (Cardinal Glennon Children’s Hospital and St. Louis University, St. Louis MO), Dr. A. Faro, and D. Rodgers (St. Louis Children’s Hospital, St. Louis MO), Dr. C. Fortner and V. Suttmore (SUNY Upstate Medical University, Syracuse NY), J. Hocevar-Trnka, M. Kloster, M. Skalland, and A. Fowler (Cystic Fibrosis Foundation Therapeutics Development Network Coordinating Center, Seattle Children’s Research Institute, Seattle,WA).
References
- 1.Galante G and Freeman AJ. NeoReviews. 2019; 20(1):e12–e24. doi: 10.1542/neo.20-1-e12. [DOI] [PubMed] [Google Scholar]
- 2.Farrell PM, Kosorok MR, Rock MJ, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics. 2001;107:1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Leung DH, Heltshe SL, Borowitz D, et al. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. Baby observational and nutrition study (BONUS) investigators of the cystic fibrosis foundation therapeutics development network. JAMA Pediatr. 2017;171:546–554. doi: 10.1001/jamapediatrics.2017.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz D, Kopp BT, Salvator A, et al. Pulmonary findings in infants with cystic fibrosis during the first year of life: Results from the Baby Observational and Nutrition Study (BONUS) cohort study. Pediatr Pulm. 2019;54(5):581–6. doi: 10.1002/ppul.24261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Agarwal A, Mehta D, et al. Nationwide trends of hospitalizations for cystic fibrosis in the United States from 2003–2013. Intractable & Rare Dis Res. 2017;6(3):191–8. doi: 10.5582/irdr.2017.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl): S73–S93. doi. 10.106/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilschanski M, Braegger CP, Colombo C, et al. Highlights of the ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants and children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2016;63(6):671–675. doi: 10.1016/j.clnu.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162:530–535.e1. doi: 10.106/j.jpeds.2012.08.040 [DOI] [PubMed] [Google Scholar]
- 9.Stephenson AL, Mannik LA, Walsh S, et al. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: a population-based cohort study. Am J Clin Nutr. 2013;97(4):872–7. doi: 10.3945/ajcn.112.051409. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi M, Nathan N, Sarouk I, et al. Nutritional status in childhood as a prognostic factor in patients with cystic fibrosis. Lung. 2019;197(3):371–376. doi: 10.1007/s00408-019-00218-3. [DOI] [PubMed] [Google Scholar]
- 11.Sanders DB, Fink A, Mayer-Hamblett N, et al. Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr 2015;167:1081–1088.e1. doi: 10.1016/j.jpeds.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed A, Shores D. Gastrointestinal manifestation of cystic fibrosis: A primer for pediatricians. Contemp Pediatr. 2020;37(2). [Google Scholar]
- 13.Gelfond D, Heltshe SL, Skalland M, et al. Pancreatic Enzyme Replacement Therapy use in infants with cystic fibrosis by newborn screening. J Pediat Gastroenterol Nutr. 2018;66(4):657–663. doi: 10.1097/MPG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coburn-Miller C, Casey S, Loung Q, et al. Standardization of research-quality anthropometric measure of infants and implementation in a multicenter study. Clin Transl Sci. 2015;8:330–333. doi:1111/cts.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden HS, Eng A, Pope CE, et al. Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure. Nat Medicine. 2020;26:215–220. doi: 10.1038/s41591-019-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012;9:811–814 (2012). doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Child Growth Standards: length/height-for-age, weight-for age, weight-for height and body mass index-for-age: methods and development. WHO Multicenter Growth Reference Study Group. Geneva: World Health Organization. 2006. [Google Scholar]
- 18.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- 19.ARUP Laboratories, 500 Chipeta Way, SLC,UT 84108 800–522-2787. www.aruplab.com, Delgado Julio, MD, Lab. Director.
- 20.Allison PD. Survival Analysis Using SAS: A Practical Guide, second edition. SAS Press, Cary NC; 2010. ISBN 978–1-59994–640-5. [Google Scholar]
- 21.Hosmer DW, Lemeshow S, May S. Applied survival analysis: Regression modeling of time to event, second edition. John Wiley & Sons, Inc. Hoboken, NJ; 2008. ISBN: 978–0-75499–2. [Google Scholar]
- 22.Patterson KD, Kyraicou T, Desai M, et al. Factor affecting the growth of infants diagnosed with cystic fibrosis by newborn screening. BMC Pediatr. 2019;10:356. doi: 10.1186/s12887-019-1727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schecter MS, Michel S, Liu S, et al. Relationship of initial pancreatic enzyme replacement therapy dose with weight gain in infants with cystic fibrosis. JPGN 2018;76:520–6. doi: 10.1097/MPG.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annual Data Report 2018. Cystic Fibrosis Foundation Patient Registry. August 2019.
- 25.Houghton LA, Lee AS, Badri H, DeVault KR, Smith JA. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol. 2018;13(8):445–60. doi: 10.1038/nrgastro.2016.91. [DOI] [PubMed] [Google Scholar]
- 26.McCrory BE, Harper HN, McPhail GL. Use and incidence of adverse effects of proton pump inhibitors in patients with cystic fibrosis. Pharmacotherapy. 2018. doi: 10.1002/phar.2125. [DOI] [PubMed] [Google Scholar]
- 27.van Horck M, van de Kant K, Winkens B, et al. Risk factors for lung disease progression in children with cystic fibrosis. ERJ. 2018;51:1702509. doi: 10.1183/13993003.02509-2017. [DOI] [PubMed] [Google Scholar]
- 28.Ayoub F, Lascano J, Morelli G. Proton pump inhibitor use is associated with an increased frequency of hospitalization in patients with cystic fibrosis. Gastroenterology Res. 2017;10(5):288–293. doi: 10.14740/gr917w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg M, Leah ST, Coffey MJ, et al. Age-dependent variation of fecal calprotectin in cystic fibrosis and healthy children. JCF. 2017. 16:631–636. doi: 10.1016/j.jcf.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 30.Ellemunter H, Engelhardt A, Schüller K, Steinkamp G. Fecal Calprotectin in Cystic Fibrosis and Its Relation to Disease Parameters: A Longitudinal Analysis for 12 Years. J Pediatr Gastroenterol Nutr. 2017. Oct;65(4):438–442. doi: 10.1097/MPG.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 31.Roca M, Rodriguez VA, Donat E, et al. Fecal calprotectin and eosinophil-derived neurotoxin in healthy children between 0 and 12 years. JPGN 2017;65: 394–398. doi: 10.1097/MPG.0000000000001542. [DOI] [PubMed] [Google Scholar]
- 32.Manor O, Levy R, Pope CE, et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci Rep. 2016;4(6):22493. doi: 10.1038/srep22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho TTB, Groer MW, Kane B, et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr Res. 2019;85(3):361–368. doi: 10.1038/s41390-018-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope JL, Yang Y, Newsome RC, et al. Microbial colonization coordinates the pathogenesis of a Klebsiella pneumoniae infant isolate. Sci Rep. 2019;9(1):3380. 10.1038/s41598-019-39887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallings VA, Sainath N, Oberle M, et al. Energy balance and mechanism of weight gain with ivacaftor treatment of cystic fibrosis. J Pediatr. 2018;201:229–37. doi: 10.1016/j.jpeds.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Gelfond D, Heltshe S, Ma C, et al. Impact of CFTR modulation on intestinal pH, motility, and clinical outcomes in patients with cystic fibrosis and the G551D mutation. Clin Transl Gastroenterol. 2017;8(3):e81. doi: 10.1038/ctg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooi CY, Syed SA, Rossi L, et al. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci Rep. 2018;8(1):17834. doi: 10.1038/s41598-018-36364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]