Abstract
The systemic pharmacotherapeutic efficacy of immunomodulatory drugs is heavily influenced by its route of administration. A few common routes for the systemic delivery of immunotherapeutics are intravenous, intraperitoneal, and intramuscular injections. However, the development of novel biomaterials, in adjunct to current progress in immunoengineering, is providing an exciting area of interest for oral drug delivery for systemic targeting. Oral immunotherapeutic delivery is a highly preferred route of administration due to its ease of administration, higher patient compliance, and increased ability to generate specialized immune responses. However, the harsh environment and slow systemic absorption, due to various biological barriers, reduces the immunotherapeutic bioavailability, and in turn prevents widespread use of oral delivery. Nonetheless, cutting edge biomaterials are being synthesized to combat these biological barriers within the gastrointestinal (GI) tract for the enhancement of drug bioavailability and targeting the immune system. For example, advancements in biomaterials and synthesized drug agents have provided distinctive methods to promote localized drug absorption for the modulation of local or systemic immune responses. Additionally, novel breakthroughs in the immunoengineering field show promise in the development of vaccine delivery systems for disease prevention as well as combating autoimmune diseases, inflammatory diseases, and cancer. This review will discuss current progress made within the field of biomaterials and drug delivery systems to enhance oral immunotherapeutic availability, and how these new delivery platforms can be utilized to deliver immunotherapeutics for resolution of immune‐related diseases.
Keywords: immunotherapy, oral bioavailability, oral immunotherapeutic delivery, oral vaccination
1. INTRODUCTION
Drug delivery is the process of administering a pharmaceutical compound in order to achieve a therapeutic effect. Oral administration is a preferred method for delivery because of its convenient and noninvasive delivery of drugs. However, a variety of obstacles limit the efficacy of oral drug administration, namely, the acidic and enzymatic degradation in the stomach, the range of pH throughout the gastrointestinal (GI) tract (pH ranging from 1 to 7), first‐pass metabolism, the steric barrier of the mucosal system and the physical barrier of the epithelial layers, to name a few. Each of these challenges contribute to the complex course an immunotherapeutic has to take through the body's intricate GI tract prior to reaching its target location within the intestine for absorption and systemic bioavailability. For the purpose of this review, bioavailability is defined as the potential of orally delivered drugs to reach systemic circulation. These issues are further escalated and complicate delivery of biologics such as vaccines and antibodies that need to be delivered systemically for modulating disease outcomes. Biomaterials and drug delivery systems (DDS) can play an important role in developing strategies to overcome these issues and allow for delivery of therapeutics to the immune system locally in mucosa or systemically. Notably for delivery of fragile immunotherapeutics, such as antibodies, mRNA, and DNA, specialized DDS are required, which can overcome the challenges associated with oral delivery. While there have been recent reviews that also suggest the importance of DDS for oral delivery, 1 , 2 , 3 , 4 this review will focus on discussion of immune engineering and immunotherapeutic delivery via oral route. In this review, we will first discuss the major immunotherapeutics that can potentially be delivered orally, and challenges associated with poor oral drug targeting. Next, we will discuss current oral to systemic delivery strategies, specific delivery mechanisms, and the promising future of oral drug administration for the systemic modulation of immune responses.
2. IMMUNOTHERAPEUTICS AND THEIR TARGETS
Human intestines house approximately 1012 lymphoid cells per meter and is known to have the highest density of immune cells in the body. 5 Thus, this tissue provides an attractive target for different therapeutics that can modulate immune‐related diseases including cancer, autoimmune diseases, and infection.
Since the GI tract is inherently tolerance‐inducing, it also provides the opportunity to generate tolerance toward molecules that the body has not been exposed to previously. For example, intravenously delivered therapeutics such as checkpoint inhibitors 6 , 7 or anti‐VEGF 8 can generate neutralizing antibodies, called antidrug antibodies, 9 which severely reduce their efficacy. Therefore, generating strategies for presentation of these therapeutics to the oral or mucosal immune system provides a unique opportunity to generate tolerance toward intravenously infused drugs, and thus improve efficacy by diminishing the generation of antidrug antibodies.
Although, GI tract is naturally tolerance inducing, it is also the site of entry for most of the pathogens, and developing immune responses that can eliminate these pathogens in the tolerance‐inducing environment is especially challenging. Therefore, generating DDS that can orally deliver therapeutics (e.g., vaccines) targeted toward immune cells such as dendritic cells (DCs) can be highly beneficial. This topic will be discussed in detail later in this review.
In addition to vaccines, another important class of immunotherapeutics are interleukins (ILs, e.g., IL‐10, IL‐4, IL‐2), which can have dramatic effect on immune responses in the gut. These ILs have their specific receptors on different immune cells that line the intestine, and thus provide druggable targets for generating immunotherapy, which can be either pro‐ or anti‐inflammatory. In addition to ILs, growth factors can also generate robust immune responses by proliferating specific type of immune cells. For example, granulocyte macrophage colony stimulating factor (GM‐CSF) can be utilized to proliferate innate cells (e.g., DCs), 10 , 11 and is utilized in clinic for treatment of cancer. 12 Targeting GM‐CSF specifically to colon tumors can be achieved by developing DDS that deliver this growth factor at the site of lesion, thereby making the therapy more effective. Moreover, small molecules such as rapamycin 13 , 14 that target the mTOR pathway 15 if targeted to specific sites of the immune system within the gut can provide site‐specific immune suppression. Lastly, delivery of specialized probiotics in the GI tract that can modulate the immune function is another major area where DDS can make a large impact. Some of the strategies that DDS utilizes to deliver these immunotherapeutics are discussed in this review.
Despite tremendous promise of DDS there still exists natural GI tract barriers that have to be overcome if oral delivery is to be considered for immune engineering. These natural barriers are briefly discussed below, and for further information on this topic readers are encouraged to read more specialized reviews. 1 , 16
3. GI TRACT BARRIERS THAT PREVENT ORAL IMMUNOTHERAPEUTIC DELIVERY
3.1. Physiochemical barrier
Orally delivered pharmaceuticals travel through the upper (mouth to the duodenum of the small intestine) and lower (most of the small intestine to the large intestine) segments of the GI tract, and the latter segment contains the most barriers for oral delivery yet houses most of the drug absorption. As the drug travels through the upper segment of the GI tract, it encounters the degradative environment of the stomach (pH from 1 to 3) and is also met by strong proteolytic gastric enzymes (i.e., lipase, pepsin, amylase). 17 This acidic environment and increased proteolytic activity within the upper GI tract can lead to the degradation of drugs before they reach the small intestine for absorption, therefore, limiting the efficacy of the drug. 18 Furthermore, these pharmaceuticals must also be able to overcome mechanical stress (gastric flow) that resist the progression of the drug. 18 Notably, proteins and other large biologics, like immunoglobulins, undergo stability and absorption challenges due to rapid degradation in the gut. 18 In one study, bovine milk immunoglobulin exhibited a 96% reduction in its rotavirus‐neutralizing activity in vitro when incubated with pepsin at a pH of 2, thus demonstrating the consequential effects of the GI environment on large biologics. 19 Therefore, it is important that biologics must be specially modified to endure the natural characteristics of the gut.
Additionally, orally delivered drugs also have reduced systemic availability as compared to drugs that are delivered intravenously or intranasally due to the phenomena known as first‐pass metabolism. 20 The first‐pass effect describes how the concentration of an orally administered drug is reduced prior to meeting systemic circulation due to decreased gastric residence time and enzymatic degradation. 20 Kolars et al. demonstrated this effect in their study, where cyclosporin was delivered to the small bowel of two patients following liver transplantation. 21 Approximately 25% and 51% of total cyclosporin‐derived metabolites were observed in portal blood for the patients after 60 min of delivery, thus indicating heightened metabolic degradation of cyclosporin. 21 This reduced availability of the drug in the systemic circulation directly decreases the sustained response that the oral therapeutics were initially intended to produce. The reduction of drug available in the systemic circulation is likely attributed to the drastic physiochemical conditions existent in the GI tract, such as the steric barriers of the mucosal immune system and the physical barrier of the intestinal epithelial layer. 18 , 20 In order to overcome the first‐pass effect, oral drugs are typically administered at a larger concentration; however, this then affects the toxicity and efficacy of various pharmaceuticals. 20
3.2. Epithelial barrier as immune defense
Immunotherapeutics must also overcome the challenge of limited traffic time in the GI tract and limited surface available for absorption (Figure 1, all schematic figures generated using Biorender.com, unless otherwise stated).
FIGURE 1.
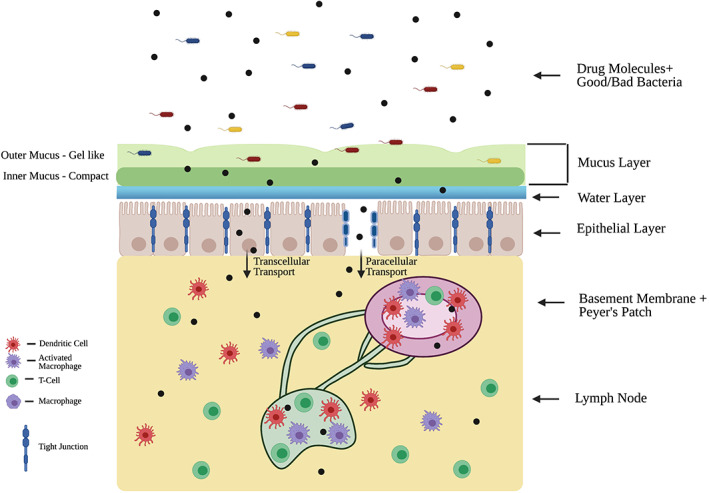
Drug molecules must bypass various barriers in the intestinal tract to reach systemic circulation. Some of these barriers include microbiota, mucosa, epithelial cells, and the immune system. Microbiota maintain immune homeostasis in the gut. A double‐layered mucosa coats the epithelium, which is bound by tight junctions. The basement membrane forms a dense layer under the epithelial layer. The gut barrier also houses several key immunological components (Peyer's patches, lymph nodes, dendritic cells, macrophages, T cells), which play an important role in preventing foreign pathogens/materials from invading systemic circulation
The epithelial layer of the GI tract contains tight junctions that further regulate the movement of substances within and through this surface, forming the first line of defense of the immune system. 22 These tight junctions create a barrier that affects both the paracellular and transcellular transportation of molecules through epithelial tissue, thus molecules attempting to reach systemic circulation must cater to the underlying mechanisms of active/passive transport through this layer. 23 Encountering these challenges therefore reduces gastric residence time of a pharmaceutical and adds to the overlying challenge of delivering a sustained effect of orally administered drugs.
Notably, epithelium that act as a defense mechanism can also be utilized to deliver drugs to the immune system as well. For example, Pridgen et al. demonstrated that polyclonal IgG Fc conjugated with poly(lactic acid)‐polyethylene glycol nanoparticles could be utilized to target the Fc receptor (FcRn) presented by the epithelial cells. Moreover, this study showed that these nanoparticles conjugated to IgG Fc were able to transcytose through the epithelium in vitro. 24 Lastly, this study also showed that orally administered FcRn‐targeted nanoparticles increased he mean absorption efficiency of the nanoparticles ~10‐fold as compared to the control of nanoparticles that were not targeted to FcRn (Figure 2). 24 This strategy of utilizing FcRn to target the epithelium can be utilized to generate immunity against infectious pathogens (e.g., M72 antigen against Tuberculosis), but also toward generating tolerance in autoimmune diseases (e.g., collagen for treatment of rheumatoid arthritis).
FIGURE 2.
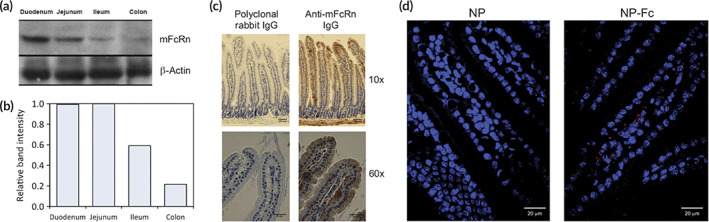
Nanoparticles conjugated with IgG Fc can be targeted to the intestinal epithelium. (a–c) FcRn is expressed in different parts of the intestines. (d) Nanoparticle intestinal uptake in mice shown here in red Source: Reprinted/adapted from Pridgen et al. Sci Transl Med. 2013;5(213):213ra167, © The Authors, some rights reserved, exclusive licensee AAAS. Distributed under a CC BY‐NC 4.0 license http://creativecommons.org/licenses/by‐nc/4.0/
3.3. Intestinal microbiota
Microbiota consists of microorganisms that reside in the gut of mammals and are necessary for the maintenance of immune homeostasis in the gut. 25 Gut microbiota act in a mutually beneficial relationship with the host to both strengthen the immune system through a series of microbiota‐dependent cascades within the epithelium as well as allow microbiota to thrive in the mucus. 26 However, such microbiota can still serve as a threat if the immune system is weakened. 26 Furthermore, it is important to note that while the GI tract serves an essential function in the digestion of foods through the existence of specific characteristics within its dynamic environment and complex regulative mechanisms, it can also impair the efficacy of orally administered pharmaceuticals. 25 , 26
Interestingly, microbiota which prevent the drug permeation through the gut, also can be used as a therapeutic themselves for immunotherapies. For example, Lin et al. demonstrated that a probiotic, Escherichia coli Nissle 1917 (EcN) can be delivered to the Peyer's patches that can then induce anti‐inflammatory responses in the intestine (Figure 3). Interestingly, this study took advantage of β‐glucan embedded on yeast membrane to target the M cells, by coating EcN with yeast membrane. 27 Importantly, this study demonstrated that delivery of yeast membrane coated EcN (EcN@YM) when delivered orally, could localize to the Peyer's patches (immune organ), where they can generate an immune response to prevent degradation of the intestinal barrier. 27
FIGURE 3.
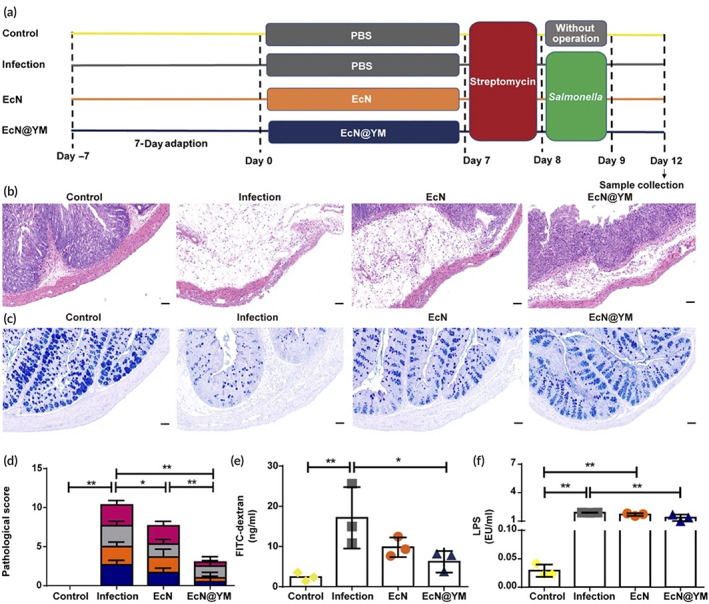
Yeast membrane coated Escherichia coli Nissle 1917 (EcN@YM), prevents intestinal barrier impairment from Salmonella infection. Using the experimental design (a) this study shows that EcN@YM prevented Salmonella mediated submucosal edema (b), depletion of goblet cells (c), pathological score (d), and an increase in intestinal permeability (e and f) Source: Reprinted/adapted from Lin et al. Sci Adv. 2021; 7(20):eabf0677. © The Authors, some rights reserved, exclusive licensee AAAS. Distributed under a CC BY‐NC 4.0 license http://creativecommons.org/licenses/by‐nc/4.0/
3.4. Mucosal immune system of the gut
Among the first lines of immunological protection in the GI tract is the specialized mucosal layer that lines the surface of the epithelium. The mucosal layer is a gel‐like structure composed of glycoproteins called mucins which are secreted by goblet cells that line the intestine. 28 Unfortunately, the constant production of mucus in the GI tract also largely reduces the availability of orally administered therapeutics to their targets. The mucosal system functions as a specialized immune defense system of the GI tract by detecting luminal foreign entities and either removing or neutralizing them while protecting the body's natural microbial flora. 29 , 30 Interestingly, mucosal surfaces vary in thicknesses along the GI tract due to the structure of their charged glycoproteins. This forms a steric barrier that restricts movement throughout its layer and drugs must permeate the mucosal barrier before entering systemic circulation. 31 Mucus is constantly being secreted and cleared quickly, thus trapping and removing foreign structures jointly and decreasing residence time of delivered drugs. 31 The gut mucosa poses a particularly significant challenge for immunotherapeutics, such as antibodies and other large proteins, due to its dynamic nature and steric barriers. Particularly, it was shown that the diffusion coefficient decreased with increasing molecular weight of proteins when tested in vitro in porcine intestinal mucus, thus demonstrating the size‐dependent barricade of the steric mucosal barrier. 32 Large proteins, especially antibodies, are also found to bind with mucins through electrostatic forces or strong hydrogen bonds thus essentially being trapped and unable to reach systemic circulation. 33 , 34 Therefore, for developing oral to systemic immunotherapeutics it is necessary to design them so that these are able to overcome the mucosal barriers.
Notably, Howe et al. demonstrated that not only the route of administration (systemic vs. oral) of therapeutics but also whether the therapeutic is associated with a nanoparticle is important for the development of an immune response. Specifically, this study demonstrated that oral delivery of ovalbumin (OVA) protein alone induce tolerance, whereas OVA conjugated to nanoparticle induced immunogenic response (Figure 4). 35 Moreover, subcutaneous boosting with OVA further increased the production of IgA titer, which is important for developing immunity against oral pathogens. 35 These data suggest that the mucosal immune systems barriers can be taken advantage of depending on the type of immunity desired (tolerogenic versus immunogenic). Therefore, stronger IgA‐based immunity against mucosal infections such as SARS‐COV2 may be generated by not only immunizing in the non‐mucosal tissue (e.g., intramuscular injections) but also orally with appropriate adjuvants.
FIGURE 4.
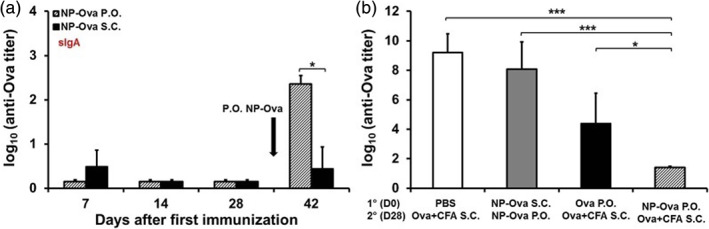
Induction of intestinal IgA and serum IgG2c antibody response depends on the immunization route that was used for priming Source: Reprinted/adapted from Howe et al. PLoS One. 2015;10(2):e0118067, with permission from Creative Commons Attribution (CC BY) license, PLOS One
The commensal bacteria reside primarily on the outer mucosal layer and actively interact with the mucin. For example, Bacteroides thetaiotaomicron, a type of commensal bacteria, has been shown to increase goblet cell differentiation, which are then responsible for generating mucin. 36 , 37 , 38 Different probiotic agents, which function to grow and restore intestinal flora, have also been found to stimulate mucin protein production and thus help to enhance pathogenic resistance. 39 , 40 Therefore, it is evident that commensal bacteria play a significant role in maintaining immunity at the mucosal layer. It is important to note, though, that the success behind this symbiotic relationship is still being widely investigated and conflicting evidence suggests that the penetration of commensal bacteria through the mucosal layer is associated with inflammatory diseases, such as inflammatory bowel disease or Crohn's disease. 41
Although the outer mucosal layer houses various microbiota that live in a symbiotic relationship with the host, the inner mucosal layer is nearly devoid of any bacteria. 42 Structurally, the inner mucosal layer is more compact than the outer layer, and thus serves more as a physical line of defense against pathogenic agents or foreign entities. 42 Drug delivery vehicles, therefore, must also seek new pathways to combat against the constantly recycled inner and outer layer of mucus. 43 This may involve coating the drug carrier with a specialized polymer that allows for increased mucosal penetration or adhesion. 31 , 44 , 45
Because the intestinal mucus provides a niche for various types of microbiota, it is important that the intestinal immune system be able to differentiate between commensal bacteria and pathogenic bacteria. The exact method in which a homeostatic environment is maintained is still unknown, though various studies have shown that the presence of antigen‐presenting cells, specifically DCs, along with assorted populations of B and T cells within the mucosa are likely related to the discrimination between commensal and pathogenic bacteria. 46 The subset of DCs found in the mucosa have been compared to similar DCs found in the respiratory tract where they play a notable role in tolerance, and thus building a specific tolerance toward commensal bacteria. 47 Aside from commensal bacteria, the specialized immune system in the mucosa can also recognize certain food antigens to help prevent immune responses against the food antigens. 46 Since the mucosa and the intestinal immune system are so tightly related, malfunctions at the mucosal layer, where immune responses can be triggered against non‐pathogenic bacteria, are the general basis for intestinal inflammatory diseases, such as Coeliac disease or Crohn's disease. 46 However, this relationship between the mucosa and the intestinal immune system demonstrates the opportunity to target the mucosal layer for oral delivery of drugs. Moreover, targeting DCs or mimicking the commensal bacteria can be an alternative approach to deliver immunotherapeutics orally to the mucosal immune system and potentially treat these inflammatory diseases.
An understanding of each of the above discussed challenges is necessary to advance the construction of novel biomaterials to tackle specific barriers in oral administration. Oral DDS takes into account these barriers in order to improve drug availability for oral to systemic delivery. Few of the strategies to achieve this include modulating the epithelial barrier for drug absorption, formulating therapeutics to better adhere to the mucosal layer, or targeting specific immune cells in the gut (Figure 1). These DDS strategies are further discussed in detail in the following sections with examples expanding the potential application of these DDS strategies on immune engineering and immunotherapeutic delivery via oral route.
4. MUCOADHESION TO IMPROVE DRUG DELIVERY
To optimize the amount of drug that is absorbed in the body, it is imperative that the carrier either releases a large amount of its contents in a short amount of time or releases a known amount throughout a set time. Currently, there is larger focus in the former, but recent developments of novel biomaterials have utilized mucoadhesion to increase the residence time of the drug in the GI tract. This method can enhance the therapeutic effect by increasing the absorption at the target site and can often be combined with enteric polymers (e.g., Eudragit) for gastric resistance and GI targeting. 48 This effect was demonstrated in an in vivo study with diabetic rats using orally administered insulin enterically coated with Eudragit as well as a polymeric mucoadhesive layer consisting of polycarbophil–cysteine that showed a sustained decrease in blood glucose over a time period of 80 h before steadily reaching its initial value again. Moreover, no significant effect was observed when insulin was orally administered without the polymeric coating. 49 Oral delivery techniques such as these, show promise in developing oral DDS that may allow for an increased gastric residence time with a controlled and sustained release of the orally delivered pharmaceutical (Figure 5). Mucoadhesion utilizes the formation of chemical bonds, most commonly hydrogen or ionic bonds, or even stronger covalent bonds between the mucosa and the mucoadhesive materials to prolong the residence time of absorption in the GI tract. 48 In addition to expanding the absorption window of an orally delivered drug, formation of these chemical/ionic bonds in combination with specialized polymers can enhance permeation and prevent degradation of delivered agents. Some of the polymers that can achieve this include chitosan; however, this bond is not strong enough on its own to sustain the mucoadhesion and thus only slightly increases the residence time. 50 However, when mucoadhesive technologies are paired with thiolated polymers, mucoadhesive properties can dramatically increase, as shown in an in vitro study demonstrating that thiolated polymers showed significant stability as opposed to non‐thiolated polymers and did not exhibit any disintegration behavior over the observation period of 48 h. 51 Thiolated polymers interact with the mucosal layer to form strong covalent disulfide bonds (Figure 2) that promote a more structurally stable carrier, in turn increasing the residence time in the GI tract at the target location. 32 In another study, thiolated chitosan micelles demonstrated up to a 56‐fold higher degree of attachment to intestinal mucosa compared to unmodified chitosan micelles, thus demonstrating the potential of thiolation for improved mucoadhesion and drug delivery. 52
FIGURE 5.
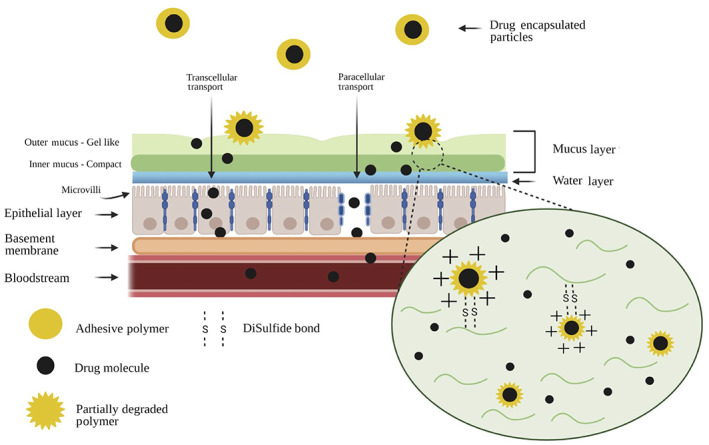
Mucoadhesive polymer coating on drugs can provide higher residence time in the gut. These mechanisms include disulfide bonds and ionic interactions with the mucosa
The strategies of mucoadhesion can be utilized to increase residence time of immunotherapeutics such as cytokines and growth factors. Interestingly, diseases such as Ulcerative colitis and Inflammatory Bowel Diseases may benefit from local delivery of anti‐inflammatory cytokines such as IL‐10 53 and IL‐27, 54 and antibodies such as anti‐IL‐22. 55 The extended residence of these therapeutics will ensure reduction of inflammatory cascade induced by pro‐inflammatory cells (e.g., T helper type 1 and T helper type 17) in the intestine, and provide immune homeostasis.
Interestingly, Chung et al. reported that orally delivered IL‐10 releasing poly(lactic acid) microparticles ameliorated local GI polyposis in mice. This study was able to demonstrate that these particles were taken up in the Peyer's patches, which could then polyp numbers and anemia in mice significantly as compared to the control. 56 Importantly, this study demonstrated that these particles could increase the survival of mice significantly as compared to controls. Therefore, delivery of anti‐inflammatory cytokines can be a powerful tool to locally modulate the immune response to address chronic diseases.
Another strategy for overcoming the mucosal barrier includes nanoparticle systems that use specialized mucolytic agents. These are conjugated on the surface of the particles and have the ability to cleave mucus substructures, which then allows for the drug carrier to bypass the mucosal layer. 52 De Sousa et al. examined papain (PAP) and bromelain (BRO) as mucolytic agents, both of which were shown to permeate through nine 2 mm intestinal mucus gel segments while the unmodified nanoparticles were found to only permeate through the first few segments. 57 The diffusion coefficient of intestinal mucin also exhibited a 2‐fold increase in the presence of PAP and BRO as opposed to unmodified nanoparticles. 57 These results demonstrate the ability of mucolytic agents to permeate the mucosal layer in the GI tract. Since both the PAP and BRO enzymes are digested in the gastric environment, utilizing enteric coatings would be one idea to consider for the purpose of ensuring the enzymes are not degraded by the harsh gastric acidity for the successful delivery of immunotherapeutics.
The combinatorial delivery of multiple targeting mechanisms allows for the strength of one agent to compliment the strength of the other for an effective therapeutic delivery. For example, self‐nanoemulsifying drug delivery systems (SNEDDS) as drug carriers were designed to increase drug dissolution and solubility due to their lipophilic nature. 58 Interestingly, SNEDDS cannot adhere to the mucosal layer within the GI tract due to the net‐negative charge of the mucus. An overall positive charge can be generated if SNEDDS are paired with mucoadhesives (e.g., chitosan derivatives) for binding to the negative mucosal layers, and thus increasing availability of drugs with poor solubility in these environments. The slightly negative charge of the mucosal layer explains the partial ionic binding of the positively charged acyl chitosan to the mucosal layer. This novel system has found success with drugs like saquinavir 59 in mice experiments as well as tipranavir 60 and cyclosporin A, 61 which were found to have enhanced effects with orally delivered drugs as compared to the control in clinical trials. 62 , 63 An interesting application of this combination strategy of SNEDDS can be delivery of anti‐inflammatory agents for chronic autoimmune diseases such as type 1 diabetes, multiple sclerosis, and rheumatoid arthritis. For example, rheumatoid arthritis patients are required to inject themselves with anti‐TNFα every few weeks to limit the damage by immune cells to the tissues. Therefore, a strategy that allows for antibodies to be taken orally will allow for delivery of anti‐inflammatory agents from oral to systemic route, thereby potentially increasing compliance as well. However, after achieving mucosal penetration, the drug then must overcome transportation through the epithelial barrier before these immunotherapeutics can be delivered systemically, and different methods to overcome this barrier are discussed below.
5. OVERCOMING THE PHYSICOCHEMICAL CHALLENGES OF THE EPITHELIAL BARRIER
Development for the enhancement of drug delivery through the epithelia is largely dependent on the mechanism of permeation across the epithelial barrier. These primarily consist of simple passive diffusion, carrier‐mediated diffusion, active transport, and transcytosis initiated by epithelial cell‐mediated endocytosis. 64 , 65 Several studies provide evidence for simple passive diffusion as the primary mechanism of drug permeation. 66 , 67 , 68 , 69 , 70 Simple passive diffusion involves the law of diffusion in which a molecule moves from an area of higher concentration to that of lower concentration and while this may be the most efficient mechanism since it does not require an energy input, many complications can arise due to varying properties of drug molecules, such as size and charge. 65 Carrier‐mediated diffusion is also another common mechanism of drug absorption and utilizes a transmembrane carrier to transport a drug molecule across the epithelia. 65 Active transport and transcytosis are not as common as passive diffusion and are significantly more energy‐expending processes. While active transport is not common for most therapeutics, some examples include levodopa 69 for Parkinson's disease or fluorouracil 70 , 71 for cancer.
Transport of these therapeutics is further supported by the microstructure of the epithelial barrier, namely villi and microvilli. Since the villi and microvilli are finger‐like projections that extend off the epithelial barrier along the length of the intestine on the apical side facing the intestinal lumen, it helps to increase the amount of surface area that is available for absorption (Figure 5). Moreover, polarized regions on the apical surface form a specialized network that allows for sorting and packaging of materials in and out of the cell, which is particularly important for transcytosis and delivery of drugs to systemic circulation. 72 Indeed, this process is the primary method for absorption of many immunoglobulins 73 and proteins. 74
In addition to villi and microvilli, other microstructures of epithelial cells are to be taken into consideration when designing DDS for therapeutic delivery. For example, tight junctions within the epithelial barrier make it difficult for large drug molecules (≥6 nanometers) to pass through the epithelial layer. 75 Two principal routes of absorption through the epithelial layer are the transcellular route and the paracellular route (Figures 1 and 5). The transcellular method involves transportation across the apical side of the epithelial cell membrane, transportation within the cell, and subsequent removal at the basolateral side of the epithelial cell. 76 On the other hand, paracellular route involves permeation of the drugs between the cells. 77 Two main characteristics of the drugs that determine permeation through paracellular route include the charge of the drugs and their size. 75 , 78
In order to utilize the mechanisms of paracellular drug delivery, strategies have been designed to enhance absorption and permeation between the cells. This process entails temporarily breaking down the epithelial cell membrane barrier or opening up intercellular tight junctions. 79 Surfactants have been extensively researched for their ability to open epithelial tight junctions for drug permeation. Because surfactants are amphiphilic, the hydrophilic and hydrophobic components can align themselves at the epithelial interface in order to decrease surface tension and thus facilitate the transportation of molecules across the epithelial layer. Although surfactants, such as sodium dodecyl sulfate or polysorbate‐80 were found to increase drug absorption, they were also found to produce irreversible membrane damage. 78 , 80 Various animal studies have shown that transient permeabilizing agents are less cytotoxic when compared to agents that generate irreversible membrane permeation. Examples of such agents include ethylenediaminetetraacetic acid (EDTA), which function as calcium chelators, or vehicles comprised of fatty acid chains such as caprate or laureate, which operate through modulation of filament interactions in the membrane. 81 Recently, negatively charged nanoparticles have also been found to enhance membrane permeability apparently with very little toxicity or permanence. 82
As opposed to surfactants, small and negatively charged nanoparticles work by enhancing transcellular permeation. Regardless, permeabilizing agents that disrupt the integrity of the cell membrane also allow for the opportunity of solutes, other than the targeted drug, to pass through the membrane, thus compromising clinical implementation of such agents. Nevertheless, clinical trials have utilized these agents and found success through the combination of both paracellular and transcellular enhancements of permeation. For example, GI permeation enhancement technology (GIPET) is a formulation that is being developed by Merrion Pharmaceuticals that has found extensive success in clinical trials and has been pre‐approved per United States Food and Drug Administration (U.S. FDA) standards. 83 The GIPET formulation utilizes fatty acid compounds to enhance membrane absorption and, in clinical trials, has been shown to increase the oral bioavailability by 12‐fold. 84 Other permeabilizing agents that have found clinical success include Chiasma's transient permeability enhancer (TPE) technology, which increases the GI absorption of large macromolecules, shown through clinical trials with Octreolin for acromegaly, 85 or Oramed Pharmaceutical's formulations of combining the delivery of enteric coatings and permeation enhancers (e.g., EDTA) to amplify absorption of orally delivered insulin. 83 The success of these technologies is likely attributed to the utilization of transient permeabilizing agents that both work to increase drug absorption while also working rapidly to reverse any structural effects and minimize any extensive damage to membrane function.
In addition to synthetic surfactants, Zonula occludens toxin (Zot) coatings have also been studied for manipulating tight junction openings in the intestine for paracellular delivery. In vitro studies in rabbit ileum with Zot shows that the effects of pharmaceuticals delivered orally peaked at around 80 min and with reversible effects in a time‐dependent manner, which demonstrates the reversibility potential for Zot coatings for oral drug delivery. 86 The mechanism behind tight junction manipulation by Zot involves a rearrangement of the epithelial cytoskeleton induced by a series of complex protein kinase C‐dependent signaling cascades. 87 , 88 Notably, Zot works similarly to zonulin, a protein abundant in the digestive tract that plays a significant role in tight junction regulation, both of which bind to the same receptor on intestinal epithelial cells, suggesting that this may be the reason for the effectiveness of Zot in epithelial permeation. 88 Importantly, these studies utilizing chitosan and Zot coatings reported no apparent toxicity. 50 , 86 However, further research needs to be done to further elucidate this mechanism and analyze long‐term organ‐level and organism‐level toxicity in larger animal models.
This enhancement of active transport of drugs systemically from oral route can be highly beneficial for treating acute immune system‐related diseases, such as infections. In fact, targeting the mucosal immune system can be highly beneficial in generating immune responses against pathogens. For example, Wei et al. demonstrated that molecular‐motors consisting of a magnesium‐based core, can be utilized to deliver vaccines in the gut, by actively transporting the vaccine components into the intestinal tissue (Figure 6). In fact, they demonstrated that the active transport due to the “motor” effect of the particles allows the payload to be distributed uniformly throughout the intestinal length. 89 These particles were also able to significantly increase IgA antibody titers in the feces as compared to the controls, which indicates that active transport of vaccines via motor‐based particles led to higher immune response. 89 Therefore, this strategy of active transport might be beneficial to study mucosa‐related infections, such as Listeria and Helicobacter Pylori, where local short‐term generation of immune responses can have a profound effect on disease outcomes.
FIGURE 6.
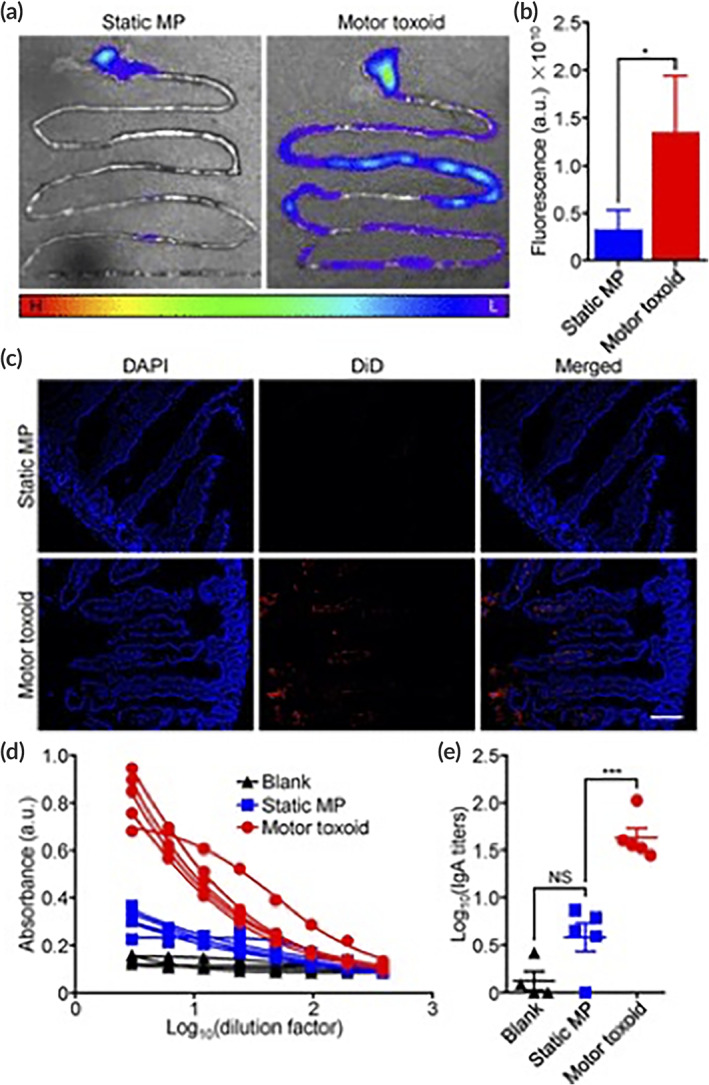
Delivery of Staphylococcal α‐toxin via “motor”‐based microparticles vaccines, enhanced distribution of the payload throughout the intestine (a–c), and lead to increase in IgA titers in mice (d and e) Source: Reprinted/Adapted from Wei et al. Nano Lett. 2019;19;1914–1921, with permission from Copyright 2019 American Chemical Society
In addition to temporarily opening tight junctions, it is also possible to increase passive transport using prodrugs strategies. Specifically, drugs are chemically modified to attribute them the properties of enhanced passive transport. Prodrugs are administered as inactive substances and converted to its pharmaceutically active form when metabolized with favorable physicochemical conditions in the body. 76 Prodrug design entails attaching of hydrophilic groups to enhance drug solubility and using lipophilic molecules to increase passive diffusion through the epithelial cell membrane. 90 Design of prodrugs must also be specific to particular properties of the pharmaceutically active drug, as some prodrugs, if prematurely activated before or during diffusion through the epithelial membrane, can cause trapping of the active drug within the cells of the membrane, and thus not be able to produce the full effect at the targeted site. For example, the L‐valyl prodrug of zanamivir (antiviral drug) was shown to improve epithelial cell permeability with around a 3‐fold increase in absorption as opposed to the acyloxy ester prodrugs that inhibited uptake. 91 Other mechanisms of prodrug design include targeting transporters located on both the apical and basolateral side of intestinal epithelial cells to allow for enhanced drug absorption. The human peptide transporter 1 (PEPT1) based prodrugs are an example of targeting specific uptake transporters that show promise for oral drug delivery. 90
In another study, it was found that oral delivery of insulin with the cell penetrating peptide (CPP) penetratin showed up to a 78.6‐fold increase in its hypoglycemic effects, lasting up to 18 h, as compared to the insulin control, giving a pharmacological availability of 18.2%. 92 Additionally, in situ experimentation found a dose‐dependent increase in insulin absorption in the ileum when administered with oligoarginine, another CPP. 93 While the mechanism behind CPPs are not fully understood, a form of endocytosis has been proposed as the method of cell penetration and targeted antigen delivery. 94 , 95 Since insulin is a peptide, other immunomodulatory peptides and proteins can be potentially delivered using CPP for oral to systemic delivery; however, this area needs to be researched further, and is discussed in the following section.
6. CURRENT ORAL TO SYSTEMIC IMMUNOTHERAPEUTIC DELIVERY STRATEGIES
Copious research has been done that demonstrates the potential for various therapeutics that can be used to modulate the immune system against a host of diseases such as autoimmune diseases and cancer. 96 , 97 Direct delivery to systemic circulation is usually obtained through intravenous administration; however, noninvasive routes of administration, particularly oral formulations, are especially advantageous in being cost‐effective and efficient methods of drug delivery for higher patient compliance. Immunotherapeutics can take many different forms; however, many promising agents, such as monoclonal antibodies and other relevant proteins, as well as genetically modifying agents, such as siRNA and mRNA, are easily degraded in the presence of gastric enzymes or impermeable to the complex layered GI immune system, and thus face decreased absorption and reduced drug effect with oral administration. In order to overcome the challenges attributed to oral administration, complex delivery systems and biomaterials have been, and continue to be, developed to further increase bioavailability and evolve this route into a more viable option. The focus of these delivery systems and biomaterial strategies specifically targets the physical and biological barriers en route for orally delivered drugs to reach systemic circulation.
Natural polymers as well as synthetically formulated biomaterials have been developed to increase the time spent during absorption in the GI tract. As previously discussed, chitosan is an example of a natural polymer that increases the time of absorption for orally delivered pharmaceuticals. For example, pre‐clinical trials using chitosan‐based nanoparticles loaded with 10‐Hydroxycamptothecin (HCPT) have found success in increased cell uptake and drug absorption for immunotherapy of melanoma 98 and has also been reported to enhance more efficient drug delivery of peptides, such as with eugenol‐loaded chitosan nanoparticles, which produced anti‐inflammatory effects in an aggressive model of rheumatoid arthritis. 99 The MucoJet is another example of a novel technology that has found success with in vivo animal studies to penetrate the mucosal layer via oral administration and elicit antibody production. 100 , 101 The MucoJet is a small immunotherapeutic delivery system consisting of a small plastic device that can be activated by the user by providing a pressure termed “click” in their work (Figure 7). 101 The polymeric membrane dissolves due to the “click,” causing the water reservoir to contact the chemical propellant (citric acid and sodium bicarbonate), generating carbon dioxide gas (Figure 7). 101 This gas production increases the pressure to around ~30 kPa, providing the device with sufficient force to penetrate the mucosal layer and deliver a vaccine solution. 101 Aran et al. utilized this technology to deliver ovalbumin to rabbits, which produced high titers of antigen‐specific immunoglobulins G and A.
FIGURE 7.
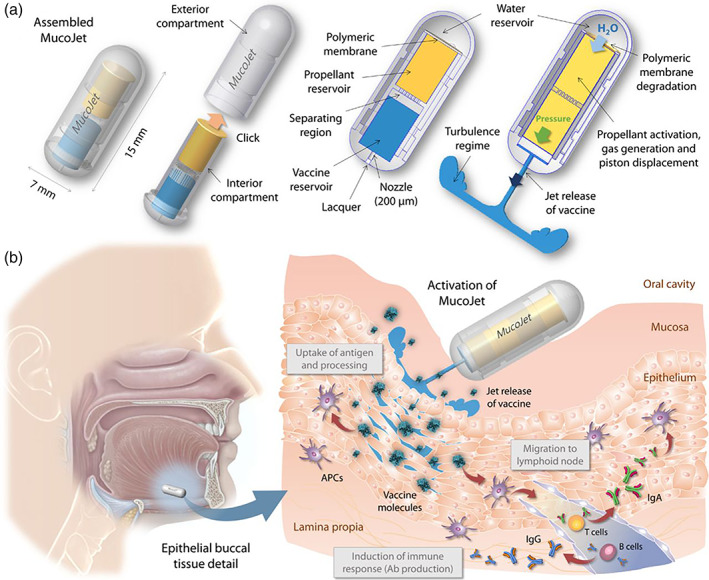
(a) The two components of the MucoJet are clicked together by the user prior to administration. The polymeric membrane is then dissolved. Water contact with the chemical propellant causes a chemical reaction that produces carbon dioxide gas, inducing a sufficient jet velocity and pressure for the vaccine solution to penetrate the buccal mucosa layer. (b) The MucoJet is delivered by mouth to buccal tissue (left). Upon penetration through the mucosal layer, the vaccine solution is delivered to antigen‐presenting cells (APCs) in the mucosa‐associated lymphoid tissue (MALT) to generate an immune response (right) Source: Reprinted/adapted from Aran et al. Sci Transl Med. 2017;9(380):eaaf6413. © The Authors, some rights reserved, exclusive licensee AAAS. Distributed under a CC BY‐NC 4.0 license http://creativecommons.org/licenses/by‐nc/4.0/
Each of these mentioned strategies provide advanced progress in increasing the bioavailability of orally delivered drugs, thus, making it a more attractive means of drug delivery. Although each of these strategies have made considerable progress, the safety, cost, and clinical translation of these technologies still remain unclear. Therefore, it is particularly important to address specific challenges of oral administration with novel biomaterials to make this route a viable candidate for immunotherapeutic delivery.
In addition to oral to systemic delivery of immunotherapeutics, delivery of immunotherapeutics to the immune system of the mucosa has gained a lot of interest in recent years. Interestingly, mucosa is the site where most of the pathogens enter the body (>90%), 102 and hence it can be advantageous to utilize immunoengineering technologies to deliver immunotherapeutics to the mucosal immune system.
7. TARGETING M‐CELLS FOR IMMUNOTHERAPEUTIC DELIVERY TO THE MUCOSAL IMMUNE SYSTEM
Microfold (M) cells are specialized intestinal epithelial cells that are commonly found in mucosa‐associated lymphoid tissue (MALT) and in gut‐associated lymphoid tissue (GALT). In the case of GALT, these cells specialize in transporting antigens from the lumen of the small intestine toward lymphoid tissue. 103 More specifically, at the inductive site, M cells transcytose antigens to be processed in the Peyer's patches, which is compacted with various immune cells, such as DCs, B cells, and T cells (Figure 8). 103 , 104 Upon activation, immune cells produce cytokines and altogether are involved in the release of antibodies for an antigen‐specific immune response (Figure 8). 103 , 105 Since M cells interact with these specialized cells responsible for generating a vaccine response, they are an ideal target for generating a vaccine response. 105 Current potent examples of oral vaccines include rotaviruses, polioviruses, and cholera vaccines. 106
FIGURE 8.
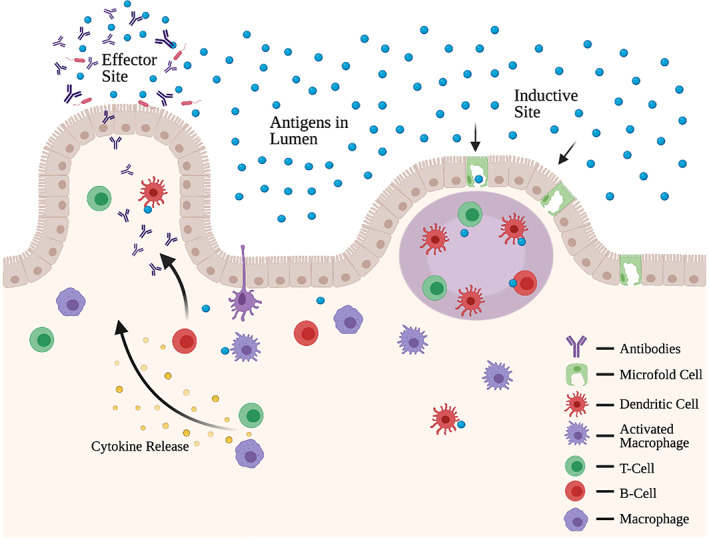
Inductive sites (right) made up of T and B cells within the Peyer's patches and effector sites (left) within the lamina propria comprise of intestinal immunity within gut‐associated lymphoid tissue. M‐cells along the epithelia allows for antigen uptake
Effective targeting of M cells for oral vaccine delivery can be challenging due to the structure of the cell and the environment in which they reside. M cells have the ability to uptake foreign entities and bypass the apical epithelial layer to directly deliver these foreign particles to the basolateral layer. 107 Although M cells may be an ideal target for immunotherapeutic delivery, only 1 in 10 million epithelial cells in the GI tract are M‐cells. 108 While it is possible to amplify the frequency of M cells, it might be advantageous to target existing M cells for efficient drug delivery.
Various animal studies have taken advantage of this efficacy for M cell targeting in inducing immunological responses for oral vaccination at the mucosal surface. For example, M cell targeting using a ligand reovirus protein sigma1 has been shown to facilitate oral tolerance in mice and thus exhibit the key role of antigen uptake and M cell targeting in mucosal immunity. 109 Studies have also looked at the specific relationship between M cells and secretory immunoglobulin A (sIgA), which is one of the main defense mechanisms of the MALT. 107 sIgA plays a significant role in regulating immunity in the mucosal epithelial by binding and removing foreign antigens and pathogens that are found within the mucosal surfaces. Notably, sIgA is also associated with helping immune tolerance by binding to dietary antigens and organisms in the microbiota. 110 Importantly, sIgA that is complexed with antigens, can also be endocytosed by M‐cells via reverse transcytosis. 111 , 112 This reverse transcytosis then provides the antigens directly to immune cells, such as DCs, which are present in the MALT. 111 In addition to sIgA, studies have also exhibited success with M cell targeting by utilizing Claudin 4 targeting peptide (CPE) which was found to enhance mucosal IgA responses. 113 Additionally, studies have also utilized p24 gag antigens linked to sIgA to elicit HIV‐specific immune responses. 114
Plant lectins are another notable method used to target M‐cells. Ulex europaeus agglutinin 1 (UEA‐1) lectin binds specifically to the alpha1,2 fucose residue that is expressed on the apical surface of the mouse M‐cells. 115 This selective binding promotes rapid uptake of antigens by M cells. Notably, the apical surface of the M cell faces the lumen, thus, making M cells an optimal target for oral vaccinations since the lumen is where oral vaccinations would be found. It is important to note, however, that a major drawback to utilizing plant lectins are their potential to produce antinutritional/toxic effects, such as those observed in studies showing significant weight loss in pigs after being fed Phaseolus vulgaris agglutinin (PHA), a kidney bean lectin. 116
Plant lectins can also have an affinity for specific glycoproteins and, therefore, these can be utilized to target glycosylated proteins on M cells. Glycoproteins are located on cell surfaces to aid in immune defense and, due to their unique patterns and structures, can also serve as an identity marker. However, little is actually known about the structure and function of glycoproteins on M‐cells. Interestingly, M‐cells have a distinct glycosylation protein profile as compared to other localized cells which, in turn, provides a mechanism to differentiate M cells from its surrounding cells. 103 Specifically, the glycocalyx, which is a form of glycolipid/glycoprotein coating that serves as a barrier between a cell and its surroundings, is thinner on M cells than the glycocalyx of its neighboring cells. 103 The reduced glycocalyx on M cells adds to its overall unique structure and allows easier access to the intestinal lumen for more efficient uptake of antigens, thus making it a targeted region of interest for the improvement of immunotherapeutic delivery. Additionally, glycosylation proteins vary in different locations of the intestine and also differ between species. This can potentially be used to target specific locations in the small intestine, for a targeted delivery and localized drug activation. To date, only little is known about the types of receptors that exist on the surface of M‐cells for recognition and subsequent endocytosis, therefore, it is important to further explore how M‐cells can be targeted to uptake specific antigens for immune targeting while avoiding the absorption of toxic and invasive pathogens.
8. ORAL VACCINATION—STATE OF THE ART AND ROLE OF IMMUNOENGINEERING
Although there are more than 20 actively administered vaccines in the United States, only rotavirus, adenovirus, cholera vaccine, and oral typhoid vaccines are administered orally. 117 Currently, most vaccines are delivered by intradermal or intramuscular injections, which are associated with problems such as safety and high cost of mass immunization. 118 , 119 Unfortunately, vaccines administered either intradermally or intramuscularly, provide only partial, or in some cases, no protection at the mucosal site, where most (>90%) of the pathogens access the body. 102 Therefore, targeting and generating mucosal immune responses against pathogenic proteins or self‐proteins for tolerance can be highly beneficial. Notably, the mucosal immune system tends to be immunosuppressive and, therefore, provides an attractive target for generating tolerance inducing vaccines as well. However, there are very few oral or intranasal vaccines available, and this can be directly linked to the lack of delivery systems capable of delivering proteins (antigen) and adjuvants (provides context for vaccines—immunogenic/tolerogenic) to the mucosal immune system.
Biomaterials, such as microparticle‐based systems, can target the GALT typically by introducing antigens to the inductive sites on the surfaces of tissues to streamline an immune response to the effector sites (Figure 6). As briefly discussed in the previous section, antigens that are transcytosed by specialized M‐cells are presented to antigen‐presenting cells (e.g., DCs, B lymphocytes, and macrophages) for the induction of immune responses. 120 Producing a sustained immune response with mucosal vaccination by targeting DCs can be challenging, but have found success in mice studies through the manifestation of immunologic memory via directly inducing cytotoxic T cell activation. 121
Another area where mucosa‐targeted vaccines can have a major impact is with autoimmune diseases, where tolerance against antigens of interest is desired. For example, in autoimmune diseases, such as rheumatoid arthritis (RA), the body mistakes self‐antigens (i.e., collagen in the case of RA) as foreign, which leads to immune responses being mounted against the self‐antigen. Specifically, delivery of antigens orally has been tested in clinical settings with mixed results, and no treatment has yet been approved. 122 , 123 , 124 One potential avenue to generate a robust tolerance‐inducing response is by directly delivering antigens of interest to the cells of the mucosal immune system. Moreover, a formulation that can deliver tolerance‐inducing molecules, to provide context, along with an antigen can also greatly improve immune responses.
Considerable progress has been made for the development of oral vaccine delivery systems and has also been tested in pre‐clinical models. For example, chitosan and alginate microparticles can be taken up by M cells in the Peyer's patches which can directly be absorbed by the MALT to induce subsequent immune responses. 125 Polymeric nanoparticles such as poly(lactide‐co‐glycolide) (PLGA) 126 , 127 have also found success in inducing immunoglobulin G (IgG) immune response to promote the linked systemic and mucosal responses necessary for sustained immunity. 128 Other potential candidates include the encapsulation of antigens or immunomodulatory agents using liposomes, 129 bilosomes, 130 bacterial outer membrane vesicles (OMVs), 131 virus‐like particles (VLPs), 132 and chemically processed pollen grains, 133 which have found pre‐clinical success against viral respiratory diseases or bilosome‐entrapped antibiotics with success against the bacterium Burkholderia pseudomallei. 130 The pre‐clinical success of these biomaterials demonstrates how important it is to further our understanding for the enhancement of oral vaccine delivery systems (Table 1).
TABLE 1.
A representative list of biomaterials as oral drug delivery systems
| Agent type | Examples | Functionality | |
|---|---|---|---|
| Mucosa targeting | Polymers |
• Thiolated polymers (polycarbophil–cysteine) 51 • Chitosan‐stearic acid‐thioglycolic acid 52 |
• Formation of non‐covalent bonds or stronger covalent bonds to increase residence time • Enhance permeability |
| Mucolytic enzymes |
• Papain 57 • Bromelain 57 |
• Conjugated on particle surface to cleave mucus substructures • Degraded in gastric environment |
|
| Self‐nanoemulsifying drug delivery systems (SNEDDS) | • Captex 300‐Kollipor EL‐propylenglycol 58 |
• Homogenous mixtures of oil, surfactant, and co‐solvent to self‐emulsify in aqueous medium • Ideal for poorly water‐soluble drugs |
|
| M‐cell targeting | Plant lectins | • Ulex europaeus agglutinin 1 (UEA‐1) lectin 115 |
• Possible antinutritional and toxic effects • High affinity to M‐cells as well as glycoproteins |
| Proteins |
• Protein sigma1 109 • Claudin 4 targeting peptide (CPE) 113 |
• Facilitates oral tolerance in pre‐clinical studies | |
| Epithelia targeting | Transient permeabilizing agents |
• Ethylenediaminetetraacetic acid (EDTA) 81 • Gastrointestinal permeation enhancement technology (GIPET) 83 • Chiasma's transient permeability enhancer (TPE) 85 |
• Enhances transcellular permeation • Less toxicity associated with reversibility |
| Surfactants |
• Sodium dodecyl sulfate (SDS) 78 • Polysorbate 80 (PS‐80) 80 |
• Amphiphilic structure decreases surface tension • Facilitates epithelial tight junction opening • Irreversible membrane damage |
|
| Bacterial surface protein | • Zonula occludens toxin (Zot) 50 , 86 |
• Rearrangement of epithelial cytoskeleton for tight junction opening • Reversible with no significant toxicity in pre‐clinical studies |
9. CONCLUSION
Oral routes of administration play a significant role in drug delivery. They prove to be an effective alternative to injected routes of administration due to their high patient compliance and convenience for achieving a specialized immune response. However, even with their copious advantages, numerous orally delivered drugs are associated with low bioavailability. This is generally attributed to degradative conditions and biological barriers, such as the mucosa or epithelia. 134 Nevertheless, particulate systems that utilize various mucoadhesive and permeabilizing technologies have found success in the clinical translation of these formulated carriers. However, further research is needed for these methods to be utilized for immune engineering. These novel technologies must also be careful to not disrupt or destroy the natural immune function of the GI tract when drugs are delivered orally and therefore should be both transient and effective in its design. Sublingual and buccal routes of administration are also effective methods of immunotherapeutic delivery that differ from the traditional method of oral drug administration. These routes bypass the first‐pass metabolic effect and allow for rapid onset of effects. However, only few developments using these delivery methods have been U.S. FDA approved, due to the uniqueness of its formulations and need for proof of safety and efficacy. Nevertheless, it is important to consider them as viable options for immunotherapeutic delivery. Convenience of sustained administration and high patient compliance make oral routes of administration more attractive methods of immunotherapeutic delivery, as opposed to injectable deliveries. It is expected that the future research in these systems will revolve around immunoengineering concepts for constructing biomaterials that target various cells and organs of the immune system while upholding the integrity of the GI tract as a whole.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHOR CONTRIBUTIONS
Tien Le: Conceptualization; writing ‐ original draft; writing‐review & editing. Brian Aguilar: Writing ‐ original draft; writing‐review & editing. Joslyn Mangal: Conceptualization; writing ‐ original draft; writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10243.
ACKNOWLEDGMENT
The authors would like to acknowledge funding sources to Abhinav P. Acharya that supported this work—NIH R01AR078343 and NIH R01AI155907.
Le T, Aguilar B, Mangal JL, Acharya AP. Oral drug delivery for immunoengineering. Bioeng Transl Med. 2022;7(1):e10243. 10.1002/btm2.10243
Funding information National Institute of Health, Grant/Award Numbers: R01AI155907, R01AR078343
DATA AVAILABILITY STATEMENT
Any information pertaining to this manuscript will be provided by the authors upon request.
REFERENCES
- 1. Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2020;5:127‐148. [Google Scholar]
- 2. Gleeson JP, Fein KC, Whitehead KA. Oral delivery of peptide therapeutics in infants: challenges and opportunities. Adv Drug Deliv Rev. 2021;173:112‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakhru SH, Furtado S, Morello AP, Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev. 2013;65(6):811‐821. [DOI] [PubMed] [Google Scholar]
- 4. Leong KW, Sung H. Nanoparticle‐ and biomaterials‐mediated oral delivery for drug, gene, and immunotherapy. Adv Drug Deliv Rev. 2013;65(6):757‐758. [DOI] [PubMed] [Google Scholar]
- 5. Mestecky J, McGhee JR. Immunoglobulin a (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153‐245. [DOI] [PubMed] [Google Scholar]
- 6. Acharya AP, Sinha M, Ratay ML, et al. Localized multi‐component delivery platform generates local and systemic anti‐tumor immunity. Adv Funct Mater. 2016;27(5):1604366. [Google Scholar]
- 7. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al. Five‐year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535‐1546. [DOI] [PubMed] [Google Scholar]
- 8. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of Bevacizumab for newly diagnosed Glioblastoma. N Engl J Med. 2014;370(8):699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enrico D, Paci A, Chaput N, Karamouza E, Besse B. Antidrug antibodies against immune checkpoint blockers: impairment of drug efficacy or indication of immune activation? Clin Cancer Res. 2020;26(4):787‐792. [DOI] [PubMed] [Google Scholar]
- 10. Acharya AP, Dolgova NV, Clare‐Salzler MJ, Keselowsky BG. Adhesive substrate‐modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736‐4750. [DOI] [PubMed] [Google Scholar]
- 11. Mangal JL, Inamdar S, Yang Y, et al. Metabolite releasing polymers control dendritic cell function by modulating their energy metabolism. J Mater Chem B. 2020;8:5195‐5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu AL, Gilman AL, Ozkaynak F, et al. Anti‐GD2 antibody with GM‐CSF, interleukin‐2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisher JD, Balmert SC, Zhang W, et al. Treg‐inducing microparticles promote donor‐specific tolerance in experimental vascularized composite allotransplantation. PNAS. 2019;116(51):25784‐25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratay ML, Balmert SC, Acharya AP, Greene AC, Meyyappan T, Little SR. TRI microspheres prevent key signs of dry eye disease in a murine, inflammatory model. Sci Rep. 2017;7:17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gamboa JM, Leong KW. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv Drug Deliv Rev. 2013;65(6):800‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El‐Kattan A, Varma M. Oral absorption, intestinal metabolism and human oral bioavailability. In: Paxton J, ed. Topics on Drug Metabolism; United Kingdom: IntechOpen Limited; 2012. 10.5772/31087 [DOI] [Google Scholar]
- 18. Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J. 2012;20(4):331‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petschow BW, Talbott RD. Reduction in virus‐neutralizing activity of a bovine colostrum immunoglobulin concentrate by gastric acid and digestive enzymes. J Pediatr Gastroenterol Nutr. 1994;19(2):228‐235. [DOI] [PubMed] [Google Scholar]
- 20. Pond SM, Tozer TN. First‐pass elimination basic concepts and clinical consequences. Clin Pharmacokin. 2012;9:1‐25. [DOI] [PubMed] [Google Scholar]
- 21. Kolars JC, Awni WM, Merion RM, Watkins PB. First‐pass metabolism of cyclosporin by the gut. Lancet. 1991;338(8781):1488‐1490. [DOI] [PubMed] [Google Scholar]
- 22. Keselowsky BG, Acharya A, Lewis JS. Innate and adaptive immunity: the immune response to foreign materials. In: Wagner WR, Sakiyama‐Elbert SE, Zhang G, Yaszemski MJ, eds. Biomaterials Science. 4th ed. Amsterdam: Elsevier Science; 2020:747‐775. [Google Scholar]
- 23. Tscheik C, Blasig IE, Winkler L. Trends in drug delivery through tissue barriers containing tight junctions. Tissue Barriers. 2013;1(2):e24565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pridgen EM, Alexis F, Kuo TT, et al. Transepithelial transport of fc‐targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5(213):213ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cerf‐Bensussan N, Gaboriau‐Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735‐744. [DOI] [PubMed] [Google Scholar]
- 27. Lin S, Mukherjee S, Li J, Hou W, Pan C, Liu J. Mucosal immunity‐mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer's patches. Sci Adv. 2021;7(20):eabf0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2013;15(1):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James SP. The gastrointestinal mucosal immune system. Dig Dis. 1993;11(3):146‐156. [DOI] [PubMed] [Google Scholar]
- 30. Johansson MEV, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernkop‐Schnürch A, Fragner R. Investigations into the diffusion behaviour of polypeptides in native intestinal mucus with regard to their peroral administration. Pharm Pharmacol Commun. 1996;2:361‐363. [Google Scholar]
- 33. Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus‐like particles in human cervical mucus. Biophys J. 2011;81(4):1930‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sigurdsson HH, Kirch J, Lehr C. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 35. Howe SE, Konjufca VH. Per‐oral immunization with antigen‐conjugated nanoparticles followed by sub‐cutaneous boosting immunization induces Long‐lasting mucosal and systemic antibody responses in mice. PLoS One. 2015;10(2):e0118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sicard J, Bihan G, Vogeleer P, Jacques M, Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Cell Infect Microbiol. 2017;7:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wrzosek L, Miquel S, Noordine M, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up‐regulate MUC‐2 mucin gene expression in a Caco‐2 cell‐culture model. Pediatr Surg Int. 2002;18(7):586‐590. [DOI] [PubMed] [Google Scholar]
- 40. Fata G, Weber P, Mohajeri MH. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins. 2018;10(1):11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012;3(6):544‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johansson MEV, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. PNAS. 2011;108:4659‐4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corfield AP. The interaction of the gut microbiota with the mucus barrier in health and disease in human. Microorganisms. 2018;6(3):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai SK, Wang Y, Hanes J. Mucus‐penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Banerjee A, Mitragotri S. Intestinal patch systems for oral drug delivery. Curr Opin Pharmacol. 2017;36:58‐65. [DOI] [PubMed] [Google Scholar]
- 46. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331‐341. [DOI] [PubMed] [Google Scholar]
- 47. Stumbles PA, Thomas JA, Pimm CL, et al. Resting respiratory tract dendritic cells preferentially stimulate helper cell type 2 (TH2) responses and require obligatory cytokine signals for induction of TH1 immunity. J Exp Med. 1998;188:2019‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernkop‐Schnürch A. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol. 2005;2(1):83‐87. [DOI] [PubMed] [Google Scholar]
- 49. Marschutz MK, Caliceti P, Bernkop‐Schnurch A. Design and in vivo evaluation of an oral delivery system for insulin. Pharm Res. 2000;17:12. [DOI] [PubMed] [Google Scholar]
- 50. Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non‐parenteral drug delivery. Pharmaceutics. 2017;9(4):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leitner VM, Marschütz MA, Bernkop‐Schnürch A. Mucoadhesive and cohesive properties of poly(acrylic acid)‐cysteine conjugates with regard to their molecular mass. Eur J Pharm. 2003;18(1):89‐96. [DOI] [PubMed] [Google Scholar]
- 52. Mahmood A, Lanthaler M, Laffleur F, Huck CW, Bernkop‐Schnürch A. Thiolated chitosan micelles: highly mucoadhesive drug carriers. Carbohydr Polym. 2017;167:250‐258. [DOI] [PubMed] [Google Scholar]
- 53. Loos M, Remaut E, Rottiers P, De Creus A. Genetically engineered Lactococcus lactis secreting murine IL‐10 modulates the functions of bone marrow‐derived dendritic cells in the presence of LPS. Scand J Immunol. 2009;69(2):130‐139. [DOI] [PubMed] [Google Scholar]
- 54. Hanson ML, Hixon JA, Li W, et al. Oral delivery of IL‐27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146(1):210‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL‐22 cascade in IBD. J Gastroenterol. 2018;53(4):465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chung AY, Li Q, Blair SJ, et al. Oral interleukin‐10 alleviates polyposis via neutralization of pathogenic T‐regulatory cells. Cancer Res. 2014;74(19):5377‐5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Sousa IP, Cattoz B, Wilcox MD, et al. Nanoparticles decorated with proteolytic enzymes, a promising strategy to overcome the mucus barrier. Eur J Pharm Biopharm. 2015;97:257‐264. [DOI] [PubMed] [Google Scholar]
- 58. Efiana NA, Mahmood A, Lam H, Zupančič O, Leonaviciute G, Bernkop‐Schnürch A. Improved mucoadhesive properties of self‐nanoemulsifying drug delivery systems (SNEDDS) by introducing acyl chitosan. Int J Pharm. 2017;519(1–2):206‐212. [DOI] [PubMed] [Google Scholar]
- 59. Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347(1–2):93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yeni P. Tipranavir: a protease inhibitor from a new class with distinct antiviral activity. J Acquir Immune Defic Syndr. 2003;34:S92‐S94. [DOI] [PubMed] [Google Scholar]
- 61. Gursoy RN, Benita S. Self‐emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173‐182. [DOI] [PubMed] [Google Scholar]
- 62. Markowitz M, Slater LN, Schwartz R, et al. Long‐term efficacy and safety of tipranavir boosted with ritonavir in HIV‐1‐infected patients failing multiple protease inhibitor regimens: 80‐week data from a phase 2 study. J Acquir Immune Defic Syndr. 2007;45(4):401‐410. [DOI] [PubMed] [Google Scholar]
- 63. Yocum DE, Allard S, Cohen SB, et al. Microemulsion formulation of cyclosporin (Sandimmun Neoral®) vs Sandimmun®: comparative safety, tolerability and efficacy in severe active rheumatoid arthritis. Rheumatology. 2000;39(2):156‐164. [DOI] [PubMed] [Google Scholar]
- 64. Salamat‐Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666‐1691. [DOI] [PubMed] [Google Scholar]
- 65. Alagga AA, Gupta V, Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. J Clin Pharmacol. 2002;42(6):620‐643. 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 66. Chetty DJ, Chen LH, Chien YW. Characterization of captopril sublingual permeation: determination of preferred routes and mechanisms. J Pharm Sci. 2001;90(11):1868‐1877. [DOI] [PubMed] [Google Scholar]
- 67. Chen LL, Chetty DJ, Chien YW. A mechanistic analysis to characterize oramucosal permeation properties. Int J Pharm. 1999;184(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 68. Squier CA, Kremer MJ, Bruskin A, Rose A, Haley JD. Oral mucosal permeability and stability of transforming growth factor beta‐3 in vitro. Pharm Res. 1999;16(1):1557‐1563. [DOI] [PubMed] [Google Scholar]
- 69. Wade DN, Mearrick PT, Morris JL. Active transport of L‐dopa in the intestine. Nature. 1973;242:463‐465. [DOI] [PubMed] [Google Scholar]
- 70. Yuasa H, Matsuhisa E, Watanabe J. Intestinal brush border transport mechanism of 5‐fluorouracil in rats. Biol Pharm Bull. 1996;19(1):94‐99. [DOI] [PubMed] [Google Scholar]
- 71. Yamamoto S, Kawasaki T. Active transport of 5‐fluorouracil and its energy coupling in Ehrlich ascites tumor cells. J Biochem. 1981;90(3):635‐642. [DOI] [PubMed] [Google Scholar]
- 72. Garcia‐Castillo MD, Chinnapen DJ, Lencer WI. Membrane transport across polarized epithelia. Cold Spring Harb Perspect Biol. 2017;9(9):a027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944‐956. [DOI] [PubMed] [Google Scholar]
- 74. Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575‐625. [DOI] [PubMed] [Google Scholar]
- 75. Lingaraju A, Long TM, Wang Y, Austin JR, Turner JR. Conceptual barriers to understanding physical barriers. Semin Cell Dev Biol. 2016;42:13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Laksitorini M, Prasasty VD, Kiptoo PK, Siahaan TJ. Pathways and progress in improving drug delivery through the intestinal mucosa and blood‐brain barriers. Ther Deliv. 2014;5(1):1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564‐580. [DOI] [PubMed] [Google Scholar]
- 78. Anderberg EK, Artursson P. Epithelial transport of drugs in cell culture. VIII: effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco‐2) cells. J Pharm Sci. 1993;82(4):392‐398. [DOI] [PubMed] [Google Scholar]
- 79. Cao S, Xu S, Wang H, et al. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech. 2019;20(190):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res. 1994;11:1132‐1142. [DOI] [PubMed] [Google Scholar]
- 81. Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1991;56(3):395‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lamson NG, Berger A, Fein KC, Whitehead KA. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020;4(1):84‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14(1):10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maher S, Leonard TW, Jacobsen J, Brayden DJ. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv Drug Deliv Rev. 2009;61(15):1427‐1449. [DOI] [PubMed] [Google Scholar]
- 85. Melmed S. Efficacy and Safety of Octreotide (MYCAPSSA™ [Formerly Octreolin™]) for Acromegaly. Identifier NCT01412424; 2012, March‐2014, May. Accessed July 20th, 2021. https://clinicaltrials.gov/ct2/show/study/NCT01412424
- 86. Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99(6):1158‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C‐dependent actin reorganization, in vitro. J Clin Invest. 1995;96(2):710‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marinaro M, Fasano A, Magistris MT. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71:1897‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wei X, Beltrán‐Gastélum M, Karshalev E, et al. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 2019;19(3):1914‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dahan A, Zimmermann EM, Ben‐Shabat S. Modern prodrug design for targeted oral drug delivery. Molecules. 2014;19(1):16489‐16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gupta SV, Gupta D, Sun J, et al. Enhancing the intestinal membrane permeability of zanamivir: a carrier mediated prodrug approach. Mol Pharm. 2011;8(6):2358‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nielsen EJ, Yoshida S, Kamei N, et al. In vivo proof of concept of oral insulin delivery based on a co‐administration strategy with the cell‐penetrating peptide penetratin. J Control Release. 2014;189:19‐24. [DOI] [PubMed] [Google Scholar]
- 93. Morishita M, Kamei N, Ehara J, Isowa K, Takayama K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J Control Release. 2007;118(2):177‐184. [DOI] [PubMed] [Google Scholar]
- 94. Madani F, Lindberg S, Langel U, Futaki S, Graslund A. Mechanisms of cellular uptake of cell‐penetrating peptides. J Biophys. 2011;2011:414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang J, Luo Y, Shibu MA, Toth I, Skwarczynskia M. Cell‐penetrating peptides: efficient vectors for vaccine delivery. Curr Drug Deliv. 2019;16(5):430‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chivere VT, Kondiah PPD, Choonara YE, Pillay V. Nanotechnology‐based biopolymeric oral delivery platforms for advanced cancer treatment. Cancers (Basel). 2020;12(2):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prosperi D, Colombo M, Zanoni I, Granucci F. Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Semin Immunol. 2017;34:61‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guo H, Li F, Qiu H, et al. Preparation and characterization of chitosan nanoparticles for chemotherapy of melanoma through enhancing tumor penetration. Front Pharamcol. 2020;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jabbari N, Eftekhari Z, Roodbari NH, Parivar K. Evaluation of encapsulated eugenol by chitosan nanoparticles on the aggressive model of rheumatoid arthritis. Int Immunopharmacol. 2020;85:106554. [DOI] [PubMed] [Google Scholar]
- 100. Aran K, Choolijian M, Paredes J, et al. An oral microjet vaccination system elicits antibody production in rabbits. Sci Transl Med. 2017;9(380):eaaf6413. [DOI] [PubMed] [Google Scholar]
- 101. Miller CS, Greenberg RN. MucoJet: a novel oral microjet vaccination system. Oral Dis. 2018;24:1145‐1147. [DOI] [PubMed] [Google Scholar]
- 102. Miquel‐Clopés A, Bently EG, Stewart JP, Carding SR. Mucosal vaccines and technology. Clin Exp Immunol. 2019;196(2):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Corr SC, Gahan CCGM, Hill C. M‐cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 104. Gardner AB, Lee SKC, Woods EC, Acharya AP. Biomaterials‐based modulation of the immune system. BioMed Res Int. 2013;2013:732182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Azizi A, Kumar A, Diaz‐Mitomas F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6(1):e1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592‐605. [DOI] [PubMed] [Google Scholar]
- 107. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301‐332. [DOI] [PubMed] [Google Scholar]
- 108. Kuolee R, Chen W. M cell‐targeted delivery of vaccines and therapeutics. Expert Opin Drug Deliv. 2008;5:693‐702. [DOI] [PubMed] [Google Scholar]
- 109. Suzuki H, Sekine S, Kataoka K, et al. Ovalbumin‐protein sigma 1 M‐cell targeting facilitates oral tolerance with reduction of antigen‐specific CD4+ T cells. Gastroenterology. 2008;135(3):917‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Brandtzaeg P. The gut as communicator between environment and host: immunological consequences. Eur J Pharmacol. 2011;668:S16‐S32. [DOI] [PubMed] [Google Scholar]
- 111. Rochereau N, Drocourt D, Perouzel E, et al. Dectin‐1 is essential for reverse transcytosis of glycosylated SIgA‐antigen complexes by intestinal M cells. PLoS Biol. 2013;11(9):e1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Corthésy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 113. Lo D, Ling J, Eckelhoefer AH. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses. BMC Biotechnol. 2012;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rochereau N, Pavot V, Verrier B, et al. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur J Immunol. 2015;45:773‐779. [DOI] [PubMed] [Google Scholar]
- 115. Kim S, Lee K, Jang Y. Mucosal immune system and M cell‐targeting strategies for oral mucosal vaccination. Immune Netw. 2012;12(5):165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vasconcelos IM, Oliveira JT. Antinutritional properties of plant lectin. Toxicon. 2004;44(4):385‐403. [DOI] [PubMed] [Google Scholar]
- 117. List of Vaccines Used in United States . Centers for Disease Control and Prevention website. Updated April 13, 2018. Accessed May 11, 2021. https://www.cdc.gov/vaccines/vpd/vaccines-list.html
- 118. Rouphael NG, Pain M, Mosley R, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV‐MNP 2015): a randomised, partly blinded, placebo‐controlled, phase 1 trial. Lancet. 2017;390(10095):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ozawa S, Clark S, Portnoy A, et al. Estimated economic impact of vaccinations in 73 low‐ and middle‐income countries, 2001–2020. Bull World Health Organ. 2017;95(9):629‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45‐S53. [DOI] [PubMed] [Google Scholar]
- 121. Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. PNAS. 2005;102(8):2880‐2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Toussirot EA. Oral tolerance in the treatment of rheumatoid arthritis. Curr Drug Targets Inflamm Allergy. 2002;1(1):45‐52. [DOI] [PubMed] [Google Scholar]
- 123. Park K, Park M, Cho M, et al. Type II collagen oral tolerance; mechanism and role in collagen‐induced arthritis and rheumatoid arthritis. Mod Rheumatol. 2009;19(6):581‐589. [DOI] [PubMed] [Google Scholar]
- 124. Trentham DE, Dynesius‐Trentham RA, Orav EJ, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261(5129):1727‐1730. [DOI] [PubMed] [Google Scholar]
- 125. van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials. 2001;22(7):687‐694. [DOI] [PubMed] [Google Scholar]
- 126. Choe S, Acharya AP, Keselowsky BG, Sorg BS. Intravital microscopy imaging of macrophage localization to immunogenic particles and co‐localized tissue oxygen saturation. Acta Biomater. 2010;6(9):3491‐3498. [DOI] [PubMed] [Google Scholar]
- 127. Acharya AP, Carstens MR, Lewis JS, et al. A cell‐based microarray to investigate combinatorial effects of microparticle‐encapsulated adjuvants on dendritic cell activation. J Mater Chem B. 2016;4(9):1672‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sarti F, Perera G, Hintzen F, et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials. 2011;32(16):4052‐4057. [DOI] [PubMed] [Google Scholar]
- 129. Liu J, Wu J, Wang B, et al. Oral vaccination with a liposome‐encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J Med Virol. 2013;86(5):886‐894. [DOI] [PubMed] [Google Scholar]
- 130. D'Elia RV, Woods S, Butcher W, et al. Exploitation of the bilosome platform technology to formulate antibiotics and enhance efficacy of melioidosis treatments. J Control Release. 2019;298:202‐212. [DOI] [PubMed] [Google Scholar]
- 131. Acevedo R, Fernandez S, Zayas C, et al. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Akahata W, Yang Z, Andersen H, et al. A VLP vaccine for epidemic Chikungunya virus protects non‐human primates against infection. Nat Med. 2010;16(3):334‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Atwe SU, Ma Y, Gill HS. Pollen grains for oral vaccination. J Control Release. 2014;194:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shen Z, Mitragotri S. Intestinal patches for oral drug delivery. Pharm Res. 2002;19(4):391‐395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any information pertaining to this manuscript will be provided by the authors upon request.


