Abstract
More than five decades have been invested in understanding glucose biosensors. Yet, this immensely versatile field has continued to gain attention from the scientific world to better understand and diagnose diabetes. However, such extensive work done to improve glucose sensing devices has still not yielded desirable results. Drawbacks like the necessity of the invasive finger‐pricking step and the lack of optimization of diagnostic interventions still need to be considered to improve the testing process of diabetic patients. To upgrade the glucose‐sensing devices and reduce the number of intermediary steps during glucose measurement, fourth‐generation glucose sensors (FGGS) have been introduced. These sensors, made using robust electrocatalytic copper nanostructures, improve diagnostic efficiency and cost‐effectiveness. This review aims to present the essential scientific progress in copper nanostructure‐based FGGS in the past 10 years (2010 to present). After a short introduction, we presented the working principles of these sensors. We then highlighted the importance of copper nanostructures as advanced electrode materials to develop reliable real‐time FGGS. Finally, we cover the advantages, shortcomings, and prospects for developing highly sensitive, stable, and specific FGGS.
Keywords: diabetes management, early detection, electrode materials, hybrid copper nanostructures, nonenzymatic glucose sensors
1. INTRODUCTION
Glucose is the primary source of energy in living cells and plays a critical role in biology. Diabetes can result in elevated blood glucose levels that pose a severe hazard to human health. 1 , 2 Diabetes is an overgrowing global public disease and is characterized by insufficient insulin formation or distribution in the body, causing the death of 1.6 million people per year worldwide. 3 , 4 , 5 , 6 It is a chronic condition that requires daily monitoring of blood glucose levels, 7 and in severe cases, insufficient insulin levels can result in diabetic ketoacidosis, leading to seizures. 8 Diabetes complications can also include neuro and cardiovascular diseases in addition to kidney disorders 9 and new risks such as heart failure, kidney dysfunction, poor vision, nerve damage, and disability. 10 , 11 , 12 These complications often result due to poor blood glucose control. Regulated and routine blood glucose tests are necessary when coping with emergencies, including hypoglycemia (low blood sugar level). 13 , 14 , 15 Detecting glucose levels rapidly and reliably in clinical and biological samples remains a major challenge. 16 , 17 Limiting sugar consumption and continuously tracking blood glucose levels is critical to managing diabetes and can significantly reduce life‐threatening diabetes and provide sufferers with a healthy lifestyle. 18 , 19 , 20 , 21 , 22 Electrochemical glucose sensors 23 , 24 , 25 with high sensitivity, good selectivity, rapid test, low‐cost, reliable, and accurate in situ detection, 26 , 27 , 28 , 29 have attracted great attention when compared to other sensing technologies like chemiluminescence, 30 surface‐enhanced Raman scattering, 31 mass spectrometry, 32 calorimetry, 33 fluorescence spectroscopy, 34 , 35 and optical sensors. 36 In addition, substantial efforts have been made to investigate glucose sensing in various potential fields such as the pharmaceutical industry, pathology, physiology, food processing, and bio‐fermentation. 37 Glucose sensors account for approximately 85% of the biosensors industry because they represent the direct health consequences of diabetes, which affects over 400 million people worldwide. 38 , 39 , 40 Reliability and economic glucose sensors with good sensitivity and low detection limits are crucial to combat the prevailing situation. This current review targets the rapid development and recent advances of Cu‐based electrochemical biosensors for glucose detection. It summarizes all the generations of electrochemical glucose sensors, followed by the fundamentals of fourth‐generation glucose sensors (FGGS). In the end, future challenges and perspectives for further development of Cu‐based FGGS are proposed.
Electrochemical sensors are used to monitor blood glucose levels rapidly. 41 These devices allow for real‐time detection. Furthermore, continuous glucose monitors have been used to enable autonomous insulin delivery, where glucose measurements automatically adjust insulin delivery in closed‐loop systems. In this manner, insulin can be administered to the patient in cases of hyperglycemia. 41 Enzymatic glucose sensors (EGS) are based on glucose oxidase or glucose dehydrogenase enzymes and exhibit a very high and reliable sensitivity. 42 However, some limitation of such sensors, including chemical and thermal conditions, instability, and relatively high complexity of the test samples. 43 Fluctuations in external factors like pH, humidity level, and temperature, and so on, hinder further exploration in the field of enzyme‐based glucose biosensors. 44 , 45 , 46
Enzymes‐based glucose sensors are divided into three significant generations. 47 , 48 , 49 The first generation requires free oxygen to immobilize the enzyme (GOx) on the electrode. Oxygen dependency of these sensors has limited applications in oxygen‐deficient blood samples. 15 , 50 The second generation of enzyme‐based glucose sensors included an artificial mediator, which directly reacts with the enzyme glucose oxidase leading to less sensitivity and accuracy. Artificial mediators involved one‐electron reversible redox ferrocene derivatives and ferrocyanide. 51 The third generation was investigated to compensate for the shortcomings of the previous generations. However, minor changes in pH, temperature, and humidity were still susceptible to enzymatic denaturation. 3 , 12 The immobilization of enzymes on the conducting electrode's surface is complex, and its quantity cannot be precisely controlled. The high cost, complicated fabrication procedure, short shelf life, and poor reproducibility of enzyme‐based glucose sensors have always been challenging for researchers. 52 , 53 A description of enzymatic glucose oxidation mechanisms, viewed as first‐, second‐, and third‐generation sensors, is depicted in Figure 1. 3 The aforementioned disadvantages of EGS attracted researchers to develop fourth‐generation metal‐based enzyme‐free glucose sensors (FGGS) 54 , 55 , 56 that oxidize glucose directly on the electrode surface. 57 , 58 , 59 FGGS that do not rely on enzymes have gained widespread attention 60 and are considered ideal for glucose analysis because of their low cost, efficient sensitivity, high selectivity, and good stability.
FIGURE 1.
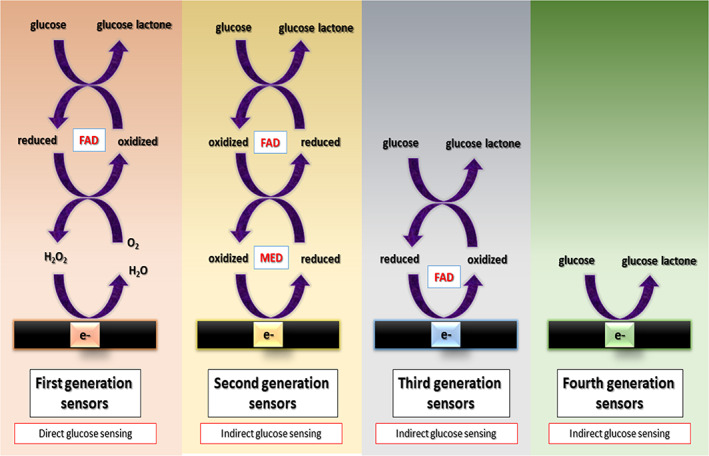
A description of the mechanisms of enzymatic glucose oxidation in first‐, second‐, third‐, and fourth‐generation glucose sensors 3
2. WORKING PRINCIPLE OF FGGS
Among the electrochemical detection techniques, two basic methods, amperometry and potentiometry, have been widely used. 15 , 50 The potential difference between a reference electrode and a working electrode is determined in potentiometric sensors at zero applied currents. The potential of the working electrode varies with the concentration of glucose. It has been shown that these sensors can evaluate glucose concentrations of 10 M or higher (an average human's blood glucose level is in the range of 4–7 mM). 12 Nonenzymatic electrodes have recently been developed by combining various metals and metal nanoparticles (NPs), including metal/metal oxide and alloy composites, for high sensitivity and low detection limit of the FGGS. 64 Bimetallic NPs can also be used in FGGS due to their superior electronic properties and increased catalytic activity. Similarly, alloys and metal oxides can be employed because they improve glucose oxidation and reduce the poisoning in the sensing electrodes of the sensor. 43
The previous decade has seen extensive advancements in the working mechanisms and principles of FGGS. 61 , 62 , 63 Like metal oxide‐based non‐EGS (NEGS), the copper‐based FGGS functions at varying pH. The functioning of the sensor depends on the stimulation of the metal oxide surface. This occurs in the vicinity of highly reactive hydroxide ions, which also serve the catalytic purpose during the oxidation of glucose molecules. Tian et al. developed the following mechanism for glucose sensing using copper oxide‐based NEGS. 64
The mechanism of this reaction is based on the electrochemical function of copper oxide that changes its oxidation states during the reaction. 65 , 66 This is evident in the above‐mentioned chemical reactions that occur in the FGGS. During the sensing process, as the voltage shifts, Cu2+ cations present in CuO get oxidized to Cu3+, and CuOOH is formed (Figure 2). This then allows the oxidation of glucose to develop gluconolactone in the next step of the reaction. During the same stage, Cu3+ gets reduced to Cu2+, leading to the formation of CuO or Cu(OH)2. 64 , 67 These step‐by‐step reactions cause a shift in the transfer rate of electrons on the electrode surface, thereby causing an increase in the overall electrical current generated. This is then recorded by the sensing detector, which detects glucose molecules in the given sample.
FIGURE 2.
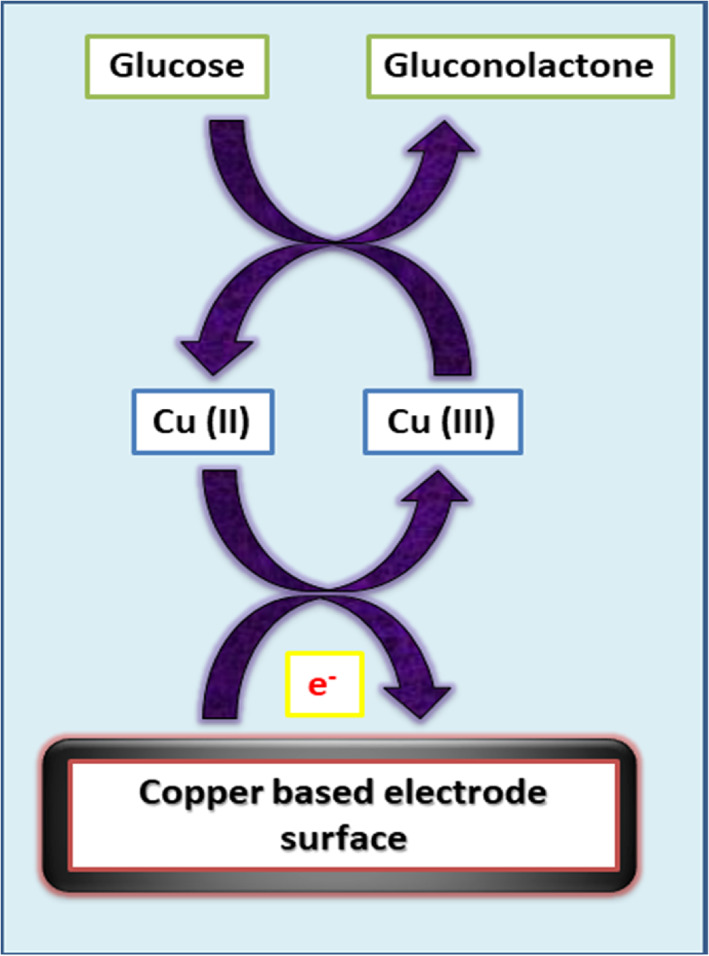
Reactions that occur in a copper‐based fourth‐generation glucose sensors (FGGS)
Metal oxide‐based NEGS like Co3O4, MnO2, CuO, Cu2O have almost similar glucose‐sensing mechanisms (Figure 3). This could occur by any or all of the following three methods 62 : (1) the copper oxide gets activated under strongly alkaline conditions, (2) formation of intermediary by‐products that function as a catalyst to oxidize glucose molecules, and (3) the intermediary by‐products then undergo reduction to give the original copper oxide.
FIGURE 3.
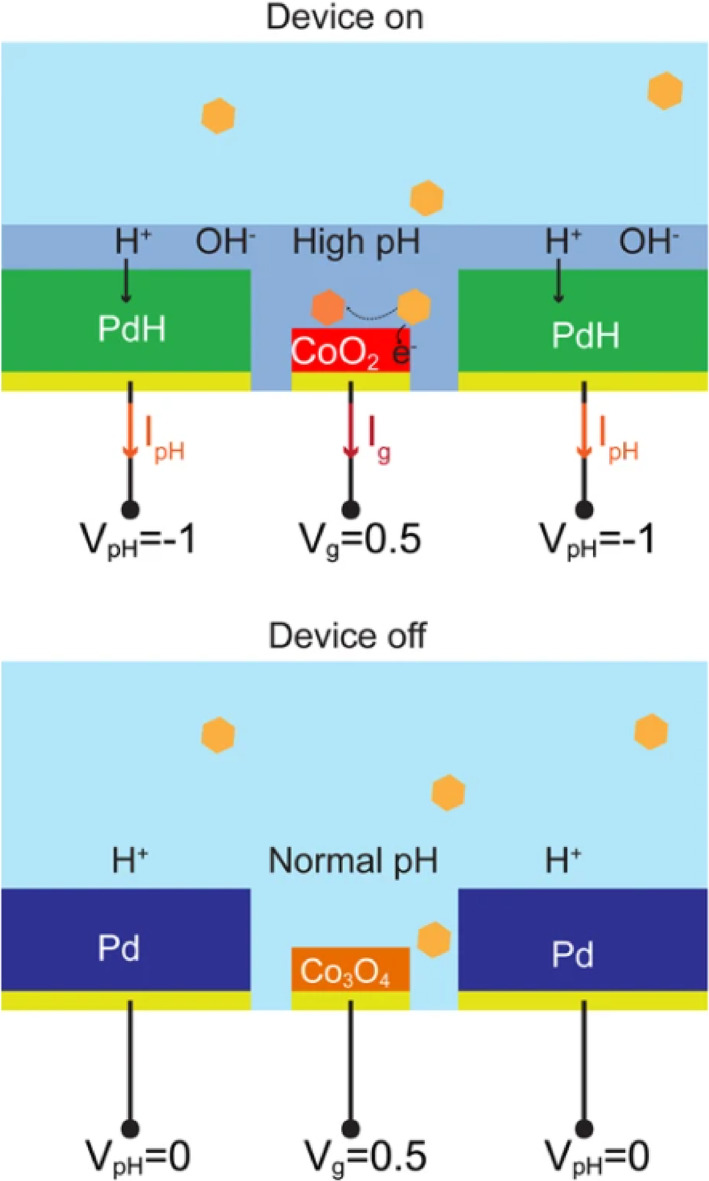
Operating principle for glucose sensing. When the device is on (top), V pH = −1 V, the Pd contact absorbs H+ from the solution and increases its pH. At high pH, the Au/Co3O4 contact is in its more reactive CoO2 oxidized state. With V g = 0.5 V, the CoO2 contact oxidizes glucose and the resulting I g is collected, which increases with increased glucose concentration. When the device is off (bottom), V pH = 0 V, the pH is at physiological values, typically pH 7, no sensing occurs from the Au/Co3O4 and I g = 0 A. Reprinted with permission from Reference 134, Copyright @ 2019 (Nature)
2.1. Mechanism of FGGS
The CuO‐based glucose sensors combined with gold NPs (AuNPs) and modified with CuO nanowires electrode (CuO NWs) gave a linear range of 0.5 μM to 5.9 mM and sensitivity of 4398.8 μA mM−1 cm−2 and a rapid response rate of 5 s. 68 The synergistic mechanism proposed for CuO in alkaline media requires oxides, hydroxides, and oxyhydroxides for the electrochemical oxidation of glucose. 69 The strong catalytic properties of Cu and its derivatives have been reported to accelerate glucose oxidation. 110 Cu in CuO is electrochemically oxidized to strong oxidizing species such as Cu(OH)−4 or CuOOH−. Thus, the +2 oxidation state changes to +3 111 :
Cu(III) catalyzes glucose's oxidation into gluconolactone and hydrolyzed into gluconic acid, as shown in Figure 4. 112 The reduction of Cu(III) to Cu(II) can be demonstrated by oxidation and reduction peaks. Cu(III) is the most responsible medium for electron transfer compared to other valence Cu ions. The stability of the AuNPs modified CuO NWs electrode was also investigated for more than 10 days with an interval of 2 days, which showed comparatively better stability than a bare CuO NWs electrode. The high catalytic capability of CuO NWs/AuNPs compared to bare CuO NWs could be attributed to incorporating AuNPs on the surface of CuO NWs, which significantly enhances the surface volume ratio of the designed electrode. The reported glucose sensor's properties were highly effective and reliable in testing human blood. Because of its high sensitivity and low limit of detection (LOD), it is suitable for noninvasive glucose detection in saliva and urine.
FIGURE 4.
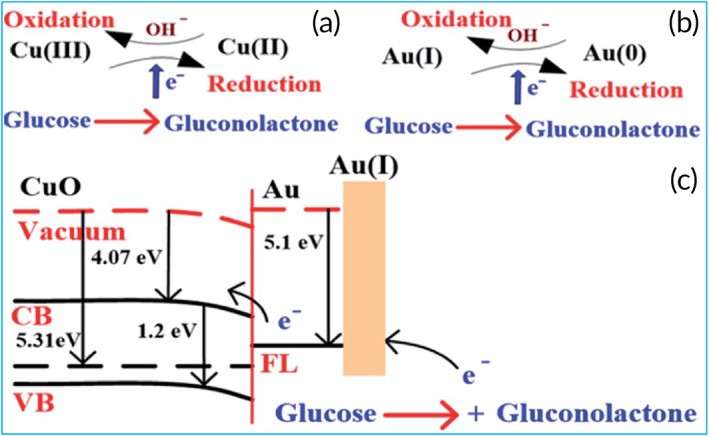
(a) Glucose detection mechanism in CuO, (b) glucose detection mechanism in Au, and (c) glucose detection mechanism in CuO nanowires (CuO NWs)/Au nanoparticle (AuNP) structure under applied potential. Reproduced with permission from Reference 68, Copyright @ 2019 (The Royal Society of Chemistry)
The sensitivity, detection range, detection limit, and response time of FGGS composed of copper and copper oxide nanostructures are given in Table 1.
TABLE 1.
Comparison of the performance of different Cu‐based nonenzymatic glucose sensors
| Electrodes/samples/electrocatalysts | Sensitivity (μA mM−1 cm−2) | Applied potential (V) | Limit of detection (LOD) (μM) | Linear range (mM) | Response time (s) | References |
|---|---|---|---|---|---|---|
| Green synthesis of Cu spherical NPs | 1065.21 | — | 0.046 | 1–7.2 | <3 | 119 |
| CuO PN | 3072 | 0.6 V Ag/AgCl | 0.41 | 0.005–0.225 and 0.225–0.825 | ∼0.8 | 120 |
| CuO‐flower | 2062 | 0.5 V Ag/AgCl | 0.25 | 0.001–1 | 1.6 | 121 |
| Cu2O/Cu/CC | 6952 | 0.60 V Hg/HgO | 0.06 | 0.001–1.555 | <2 | 124 |
| Cu‐MOF/MWNTs/GCE) | 3878 | — | 0.4 | 0.0005–11.84 | 0.3 | 126 |
| Au NPs‐modified CuO NWs | 4398.8 | 0.6 V Ag/AgCl | 0.5 | 0.0005–5.9 | Approx. 5 | 68 |
| CuO‐ZnO NRs/FTO | 2961.8 | — | 0.40 | 0.001–8.45 | <2 | 122 |
| Cu x O nanosheets/Cu | 1541 | 0.60 V Ag/Agl | 0.57 | 4 | ∼3 | 152 |
| Cu3(BTC)2‐derived CuO nanorod | 1523.5 | 0.6 V Ag/AgCl | 1 | Up to 1.25 | 5 | 153 |
| Cu/Ni/Au nanoporous film |
4135 2972 |
— | 0.1 |
0.0005–3 3–7 |
— | 154 |
| Cu+2/PANI/rGO/FR4 nanocomposite |
4168.37 525.4 |
0.66 V Ag/AgCl | 4.93 |
0.0028–0.0222 0–4 |
<5 | 155 |
| CuS nanosheets/Cu2O/CuO NWAs/Cu foil | 4262 | 0.60 V Ag/AgCl | 0.89 | 0.002–4.1 | 350–800 | 156 |
| Copper oxide/CPE | 1183.59 | — | 672.8 | 1.6–62.5 | 120 | 157 |
| MWCNT‐CuBTC | 14,949 | 0.6 | 10 | 0.2–1 | — | 158 |
| MOF‐derived CuO architectures | 10–120 | — | 0.1 | 0.01–0.12 | ~6 | 159 |
| CuO/CuBi2O4 | 330 | — | 0.7 | 0.000001–100 | — | 160 |
| CuS microflowers | 1007 | 0.5 | — | 0.2–5.4 | ~4 | 161 |
| Cu2O‐c/SPCE | 2376.7 | −1.0 to 1.2 V. Ag/AgCl | 0.003 | 0.000031–1.42 | — | 162 |
| Cu2+/MWCNT‐COOH | 1732 | — | 0.02 | 0.00002–8.0 | ~2 | 163 |
| CuO NPs/PEDOT:PSS/PGE | 663.2 | +0.70 V | — | 10 | — | 164 |
| CuO hollow sphere | 25.0 ± 0.8 | — | — | 0.001–3 | — | 165 |
| CuO hollow sphere | 13.6 ± 0.3 | — | — | 3–11.5 | — | 165 |
| CuO microspheres | 26.59 | — | 20.6 | 2–9 | — | 166 |
| Cu‐GNE | — | 0.5 | 0.12 | 1 | ~2 | 167 |
| Cu NWs/PANI/rGO | 843.06 | 0.64 | 1600 | 0–4 | — | 168 |
| CuO‐C‐dots | 110 and 63.3 | +0.50 | 200 | 0.5–2 and 2–5 | — | 169 |
| Cu x O/Cu | 1210 ± 124 | −0.2 and +0.6 | 10 | 0.01–7 | ~1 | 133 |
| CuO nanoleaves | 1467.32 | +0.6 | 0.012 | 0.005–5.89 | ~3.5 | 104 |
| CuNPs/PoPD/GCE | — | 0.5 | 0.25 | 0.005–1.6 | ~1 | 170 |
| CoNiCu alloy nanotubes | 791 | — | 0.5 | 0.05–1.551 | — | 106 |
Abbreviations: BTC, benzene tricarboxylate; CC, carbon cloth; CuBTC, copper‐1,3,5‐benzenetricarboxylic acid; Cu‐GNE, Cu nanoparticles on a linear graphene edge nanoelectrode; Cu‐MOF, Cu‐metal–organic frameworks; CPE, carbon paste electrode; FTO, fluorine doped tin oxide; GCE, glassy carbon electrode; MWCNT, multiwall carbon nanotubes; MWNTs, multiwalled carbon nanotubes; NPs, nanoparticles; NRs, nanorods; NWAs, nanowire arrays; PANI, polyaniline; PN, porous nanostructure; rGO, reduced graphene oxide.
3. COPPER NANOSTRUCTURES AS ADVANCED ELECTRODE MATERIALS FOR FGGS
A variety of nanostructured electrocatalysts are being investigated to develop advanced FGGS, as simple electrodes cannot compete with their level of glucose detection. 65 , 66 , 67 FGGS, which are based on the electrochemical oxidation of glucose that operates through a variety of inorganic catalysts including noble metals (Ag, Au, Pt, and Pd) and their alloys (Pt–Pd, Pt–Au, and Au–Pd), metal oxides (Co3O4, NiO, CuO, Cu2O, ZnO, and so on) or bimetallic electrodes and carbon‐based nanomaterials have been extensively explored for their excellent 68 , 69 glucose detecting capabilities. 70 , 71 , 72 Pt‐based nanosensors have shown high catalytic efficiency owing to their large surface area and their ability to control the kinetics of the reaction. 76 However, Pt and its derivatives are costly, which limits its practical application. 77 The same has been observed with Au‐ and Pd‐based NEGS. Therefore, widespread applications of noble metals have been hampered by disadvantages like low selectivity, high cost, toxicity, and metal scarcity, making their use impractical on a larger scale production. 73 , 74
In addition, metallic, 75 , 78 , 79 , 80 , 81 metal‐alloy based, 82 , 83 , 84 metal hydrate, 85 metal sulfide, 86 and metal oxides 87 , 88 , 89 , 90 have been successfully used to study nonenzymatic glucose sensing. These metallic‐based glucose sensors make use of a variety of sensing techniques like the fluorescent carbon dots‐based fluorescent method, 91 optical methods, 92 Raman spectroscopy, 93 and others. To achieve efficient reproducibility of glucose sensors, the continuous development of nanomaterials from other transition metals and their oxides is widely researched. 94 Subsequently, researchers are excited about FGGS since they can compare different transition metals with excellent redox activity and select those with superior stability and selectivity. 95 , 96 Transition metal oxides and their alloys, such as ZnO, CuO, NiO, and CO3O4, are widely used for glucose biosensors due to their high electrochemical activity, low cost, and the low potential requirement for electron transfer reactions. 97 , 98 , 99 , 100
Copper oxides (CuO and Cu2O) are particularly significant among transition metal oxides because of their excellent thermal, mechanical, and chemical stability. Much attention has been paid to developing copper oxide electrode materials for FGGS with high electrocatalytic activities. 101 , 102 X‐ray diffraction (XRD) characterization of copper particles, their oxides, carbon quantum dots loaded with copper oxide NPs (CQDs/Cu2O NPs), or CoNiCu alloy nanotubes (NTs) arrays transferred on indium tin oxide, helps to determine their crystal structure, orientation, shape, and size as well as other structural parameters such as average grain size, strain and crystal defects, characteristics that are important for FGGS applications 103 , 104 , 105 , 106 (Figure 5).
FIGURE 5.
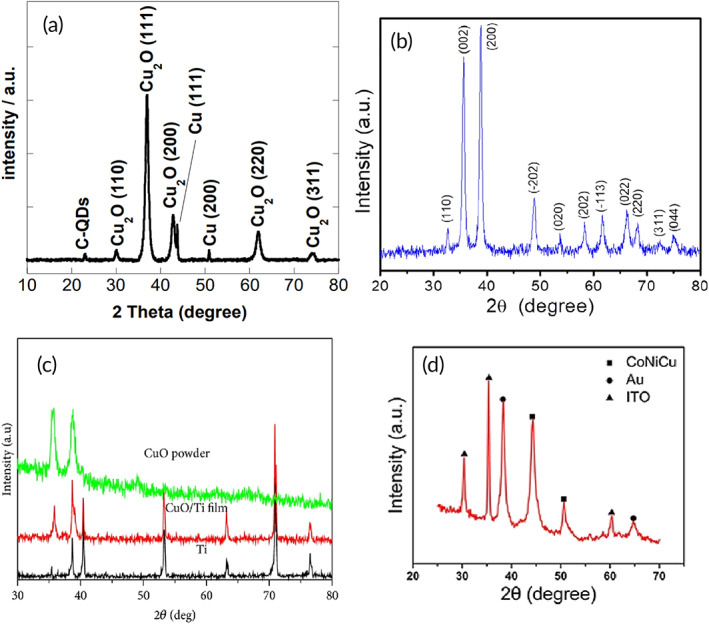
X‐ray diffraction (XRD) characterization of copper particles used in nonenzymatic glucose (NEGS). (a) XRD pattern of carbon quantum dots loaded with copper oxide nanoparticles (CQDs/Cu2O NPs). Adapted with permission from Reference 103, Copyright @ 2016 (MDPI). (b) XRD pattern of CuO nanoleaves. Adapted with permission from Reference 104, Copyright @ 2021 (IOP Science). (c) XRD patterns of Ti substrate, CuO film on Ti substrate, and CuO powder. Adapted with permission from Reference 105, Copyright @ 2014 (Hindawi). (d) XRD patterns of prepared CoNiCu alloy nanotubes arrays transferred on indium tin oxide. Adapted with permission from Reference 106, Copyright @ 2019 (Frontiers)
3.1. Electrochemical detection of glucose using copper‐based FGGS
Because of their outstanding chemical and thermal stability, various nanomaterials exhibit remarkable sensitivity and selectivity in glucose sensing. 82 Their electrochemical properties, high electrode catalytic activity, low cost, strength, natural abundance, nontoxicity, and environmentally friendly nature 107 , 108 , 109 , 110 have made Cu and its oxides a potential candidate for various applications such as photoelectric devices, gas sensing devices, lithium‐ion batteries, and especially as electrochemical sensors due to their optical nature and electrical characteristics. 82 , 83 , 84 CuNPs are an effective electrode material for glucose detection and are extremely sensitive to glucose oxidation due to their excellent electrical conductivity. 85 CuNPs have a high specific surface area, which improves FGGS activity significantly, and their synthesis techniques have evolved to include hydrothermal, pyrolysis, and electrodeposition. 113 , 114 , 115 , 116 , 117 CuO is a p‐type semiconductor with a narrow bandgap of 1.2 eV, which is more stable than simple Cu for glucose analysis. CuO nanomaterials show excellent electrocatalytic activity, proper redox potential, and low overpotential during electron transfer experiments. 91 , 92 , 93 CuO and CuS act as excellent electronic mediators in glucose oxidation due to the redox pairs of Cu2+ and Cu3+. 94
Various CuO nanostructures have been extensively researched and synthesized into multiple shapes with individual properties and performance through the development of nanotechnology, such as NPs, nanorods, nanofibers, nanospheres, flower‐like structures, and so on. 95 , 96 In a study by Ding et al., they developed an FGGS based on CuCo2O4 using electrospinning technology and carbonization treatment to prepare CuCo2O4–carbon nanofibers (CNFs) 118 (Figure 6). This sensor exhibited an enhanced activity with two linear ranges of 0.01–0.5 and 0.5–1.5 mM and a high sensitivity of 2932 and 708 μA mM−1 cm−2.
FIGURE 6.
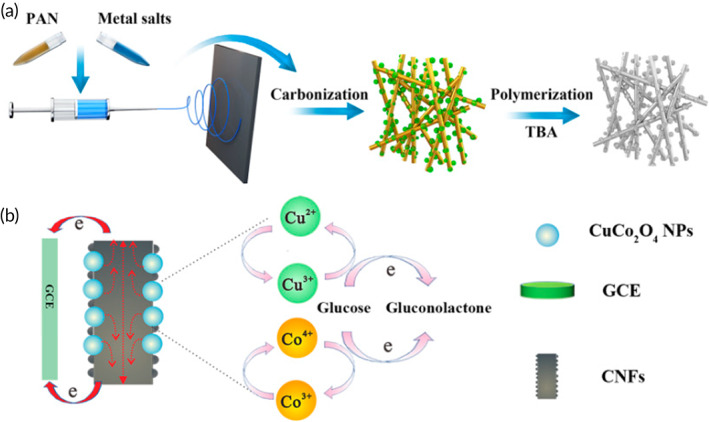
(a) Schematic diagram for the preparation of CuCo2O4–carbon nanofibers (CNFs) and (b) the representation of the proposed mechanism for electrocatalytic oxidation of glucose based on poly(thiophene‐3‐boronic acid) (PTBA)/CuCo2O4–CNFs/glassy carbon electrode (GCE). Reproduced with permission from Reference 118, Copyright @ 2019 (MDPI)
A simple, cost‐effective microwave‐based method for synthesizing a sensitive FGGS with CuO nanodisks was investigated, which maintained its remarkable selectivity, a high sensitivity of 627.3 μA·mM−1·cm−2, and broad linear range from 2.0 M to 2.5 mM. 98 Furthermore, glucose sensors showed high reproducibility and longstanding stability with only 9% sensitivity damage in 14 days with an interval of 2 days in the open air. The sensing ability of the proposed electrode was evaluated in human urine samples and can be attributed to the development of noninvasive biosensors in ambient conditions. The shape and size of CuO nanodisks composed of tiny nanorods are confirmed by transmission electron microscopy (TEM) while their high crystalline nature is determined from selected area electron diffraction patterns.
Dayakar et al. fabricated FGGS with pristine CuNPs on glassy carbon electrode (GCE). They prepared this sensor by the simple green method using leaf extract of Ocimum tenuiflorum. 99 , 119 Less toxic, smooth surface, and small‐sized NPs synthesized via this green method outperformed the catalytic activity toward glucose oxidation than other synthesized nanostructures. Furthermore, the modified Cu/GCE electrode exhibited the current response, which remained at 93.2% of its original value when stored for 10 days at room temperature, reflecting its long‐term stability toward glucose oxidation. The proposed electrode presented outstanding analytical sensing properties such as reproducibility, limited interference, and a sensitivity of 1065.21 μA mM−1 cm−2, with a detection limit of 0.038 μM (S/N = 3), and linear response ranges from 1 to 7.2 mM with a fast response of 3 s.
CuO porous nanostructure (CuO PN) electrodes have been shown to enhance glucose detection capabilities, showing a linear range of 0.005–0.225 mM, a high sensitivity of about 3072 μA mM−1 cm−2, and a low detection limit of about 0.41 μM. 100 Furthermore, the proposed sensor maintained its high stability over a month of monitoring, recording a 17% loss in current density under regular measurements. Thus, this cost‐effective and highly stable porous CuO FGGS was used for detecting glucose in human saliva with a high sensitivity of ∼2299 μA mM−1 cm−2.
Ashok and colleagues synthesized CuNPs using three methods, the colloidal method with NaBH4 as a reducing agent producing the best homogeneous phase of CuO NPs (Cu‐colloids). 121 Simultaneously, combustion‐based techniques, which were exploring glycine (Cu‐gly) and hydrazine (Cu‐hyd), did not yield any satisfactory results. 101 , 102 Flower‐shaped Cu‐colloidal particles showed a maximum electro‐oxidation current of glucose, with a low detection limit of 0.25 μM, a high sensitivity of 2062 μAmM−1 cm−2 for glucose, in a wide linear range of 1–850 μM. The Cu‐colloid particles' excellent electrocatalytic activity is related to their unique blossomed flower‐shaped morphology and the pointed tips of opened mesoporous or microporous petals. 121 This structure provided more active sites with a large surface area, promoting chemisorption of oxygen and charge transportation. The scanning electron microscopy (SEM) analysis demonstrates the morphology and composition of the Cu particles (Figure 7).
FIGURE 7.
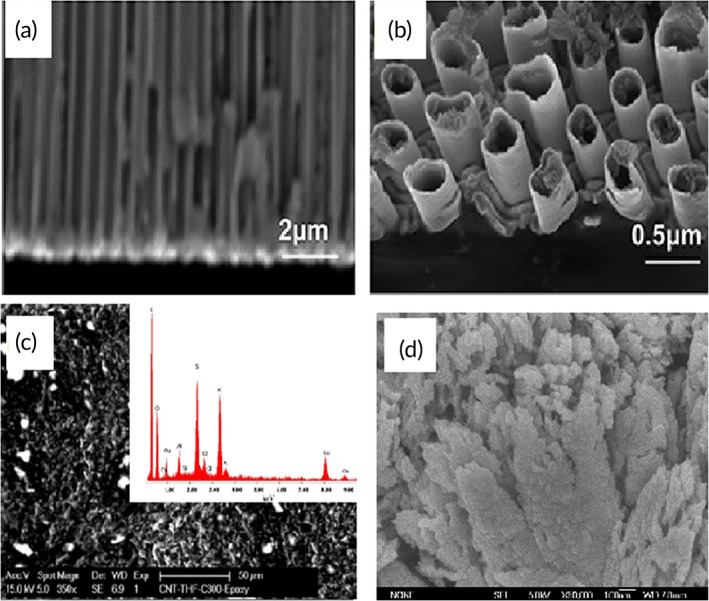
Scanning electron microscopy (SEM) characterization of copper particles used in NEGS. (a,b) Products deposited on CoNiCu alloy nanotubes with anodic aluminum oxide template. Adapted with permission from Reference 106, Copyright @ 2019 (Frontiers). (c) MWCNT‐copper‐1,3,5‐benzentricarboxylic acid (CuBTC) composite electrode. Adapted with permission from Reference 158, Copyright @ 2020 (MDPI). (d) CuO film on Ti substrate. Adapted with permission from Reference 105, Copyright 2014 (Hindawi)
Most Cu or Ni‐based NEGS are synthesized by modifying the substrate with NPs, scattered structures, or metal‐carbon hybrids. 103 In addition to CuO, nanostructures of cuprous oxide (Cu2O) have also been investigated to study their electrocatalytic properties to fabricate FGGS. Zhang et al. synthesized a self‐supported Cu2O/Cu/CC (carbon cloth) using a single step, simple potentiostatic electrochemical deposition on CC. 104 , 105 The flexible glucose sensor (Cu2O/Cu/CC) demonstrated a superior sensitivity of 6952 μA mM−1 cm−2, reproducibility (relative standard difference [RSD] = 2.74%) with an extremely low detection limit of 0.6 μM with a fast response time of less than 2 s. Moreover, 90.2% of the original sensitivity of the electrode was maintained during a 1‐month stability test.
The sensitivity and conductivity of the purest Cu electrode can be easily contaminated by oxidation; thus, researchers are focused on improving their efficiency by incorporating other components. 125 Wu et al. manufactured a high‐performance multilayer composite film‐based FGGS through a layer‐by‐layer method, employing Cu‐metal organic frameworks (Cu‐MOF), multiwalled carbon NTs (MWNTs) modified GCE, given in Scheme 1. 107 , 126 The glucose sensor showed an excellent sensitivity of 3878 μA mM−1 cm−2, a more comprehensive linear range of 0.5 μM–11.84 mM, with a low LOD of 0.4 μM and was free of interference. Researchers found that the hybrid composite's enhanced catalytic properties were due to its large surface area, multiple active sites, accounting for its excellent electrical conductivity of MWNTs and good selectivity of Cu‐MOF. The glucose detection efficiency of the developed sensor was tested in actual blood samples, yielding satisfactory and feasible results. However, due to the high alkaline conditions, its practical application was hindered.
SCHEME 1.
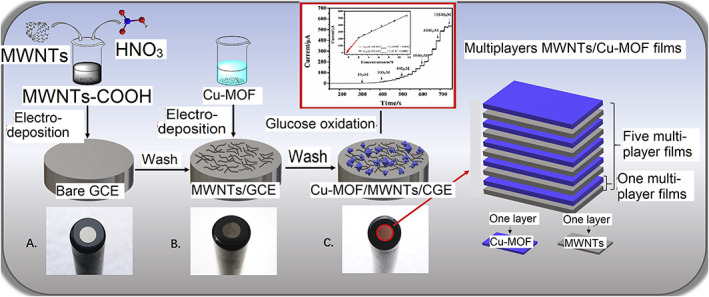
Schematic illustration of the Cu‐metal organic frameworks (Cu‐MOF) and multilayer films of Cu‐MOF/multiwalled carbon nanotubes (MWNTs)/glassy carbon electrode (GCE). Reproduced from Reference 126, Copyright 2019 (Elsevier)
3.2. Advantages and challenges of copper‐based FGGS
FGGS have received widespread attention in recent times because of their ability to deliver reproducible results with higher stability than traditional EGS. This, therefore, increases the practicability of FGGS in clinical applications and several recent studies support this advantage. 57 , 123 , 127 , 128 , 129 Furthermore, these NEGS remain functional even after 1 month and monitor glucose levels in undiluted whole blood after sterilization and thus exhibit long‐term stability. 24 , 130 , 131 This was previously not feasible with traditional glucose monitors that are active only for 7–14 days and get inactivated due to biofouling. In addition, NEGS show low detection time and rapid response rate, which adds to the advantage of these sensors over EGS. This was seen in the work by Yan et al., who demonstrated the practical clinical application of an FGGS based on copper sulfide nanoflakes‐reduced graphene oxide that showed a rapid response rate as low as 6 s and a low detection limit of 0.19 μM in human blood and urine samples. 132
Hence, recently the scientific focus has shifted toward developing nanomaterials‐based NEGS that provide better linear range and ease in operation. Nanomaterials also possess sizes equivalent to enzyme molecules that aid in their functionalization. Highly conductive carbon‐based nanomaterials are the best choice for electro‐oxidation of glucose; however, their stability is a significant concern. As a result, researchers have concluded that copper and its bimetallic nanomaterials have a promising potential for fostering and promoting FGGS in mass production. Because of its unusual electrocatalytic activity and use in many electrochemical devices, copper‐based FGGS have gained widespread popularity in recent decades. Copper is abundantly available in nature, low cost, and environmentally friendly and shows high catalytic activity. 113 , 114 Moreover, copper‐based FGGS with other metals in combination have displayed excellent sensing properties. In research carried out by Suneesh and colleagues, an FGGS based on Co–Cu alloy NPs was developed, which served as an excellent sensing device for quantifying glucose levels. 113
Despite the innumerable research done on FGGS and the use of nanomaterials in their construction, there are still a few challenges that need to be tackled before these sensors can be availed. The major obstacles that need to be addressed include miniaturization of the sensor, reduction in the sample required during sensing, and quick delivery of results. Furthermore, more work is necessary to improve the shelf life and reduce the cost of such test strips based on NEGS. The application of nanomaterials in the development of FGGS has also gained widespread attention, especially to create sweat based platforms and in vivo implantable glucose sensors for glucose measurements. However, such sensors to detect glucose concentrations in these fluids would require greater sensitivity of copper‐based FGGS. Also, these sensors show a highly accurate correlation between glucose levels measured in interstitial fluids and blood when measured using the commercially available blood‐glucose meter. This correlation has been observed in several recent studies, 134 , 135 , 136 , 137 , 138 and thus, such sensors can be potentially used in clinical applications for glucose measurements.
In addition, the biocompatibility and shelf life of copper‐based FGGS depend on the morphology of the copper architectures and the attached functional groups. 138 This is especially true in the case of implantable sensors made using NPs. Immune reaction against NPs is inevitable; hence, such reactions can be controlled by monitoring the size and shape of the NPs that indirectly influence the attachment of neutrophils or macrophages to them. 139 , 140 , 141 For example, particles that are not spherical and are over 6 μm in diameter may exhibit lowered macrophage adhesions and, therefore, will have better functional viability within the system. 138 Hence, the physical features of the NPs, their chemical nature, like their surface chemistry, influence their biocompatibility and enhance the sensor's overall life. 139
Furthermore, there is no sophisticated control over the protective sheath, thickness, and pore size of the nanoporous layer that would allow FGGS to work on plasma, human serum, and blood when undiluted. Moreover, disturbances caused by various electro‐active and electro‐inactive chemical species must still be adjusted. 61 Therefore, although these sensors offer promising alternatives to traditional, invasive blood glucose monitoring; further works need to be done produce better electrode protective films in FGGS, before these sensors are made available commercially on a large scale.
3.3. Comparison of FGGS composed of Cu nanostructures with EGS
The current glucose‐sensing devices available are based on EGS. A few studies have also demonstrated the catalytic effect of copper oxide in enzymatic glucose oxidation and hydrogen peroxide detection with good stability and ultra‐sensitive response. Umar et al., for example, established a reproducible glucose biosensor based on well‐crystallized flower‐shaped CuO nanostructures formed of thin nanosheets. 142 The designed biosensor showed a response time of less than 5 s, a high sensitivity of 47.19 μA mM−1 cm−2, and a LOD of 1.37 M. Several studies have shown that combining graphene with CuO NPs will produce more synergistic results and hence improve glucose detection. For instance, Qian et al. suggested a simple and straightforward method for depositing Cu2O NPs on graphene sheets (Cu2O@CRG) using sodium citrate as a reluctant agent, 143 demonstrating better sensitivity and selectivity in alkaline media than Cu2O or CRG. However, these sensors possess several drawbacks related to the inherent nature of the enzymes, like their minimal reproducibility and decreased stability when used for long durations. Also, the catalytic function of enzymes is easily affected by the external pH, temperature, absence or presence of humid conditions, and other chemicals in the vicinity. 144
To reduce the drawbacks incurred by these EGS and the volatile nature of enzymes, the NEGS were introduced (Figure 8). As discussed earlier, these sensors involve direct electrocatalytic oxidation of glucose molecules on their electrodes' surface. Because of their high electrocatalytic activity and advantages like inexpensive availability, nontoxic nature, ability to be quickly processed, and readily stored, copper, copper oxides‐based nanomaterials, and their hybrids have sparked significant interest for FGGS, too. 145 For example, Zhang et al. developed a nonenzymatic glucose‐sensing platform based on one‐dimensional Cu NWs, both sensitive and selective. 146 Wang et al. created a sensitive FGGS using CuO flowers and nanorods as the sensing material. 147 Moreover, significant efforts have been made to combine copper or copper oxides with carbon‐based nanomaterials to enhance their catalytic activity. 148 , 149 Luo et al. designed an FGGS based on Cu–graphene nanocomposites, which demonstrated a significantly higher current and a lower negative onset potential for glucose oxidation than Cu NPs. 148 Field emission SEM (FESEM) analysis of copper particles helps analyze the arrangement of the physical features of the crystalline particles (Figure 9).
FIGURE 8.
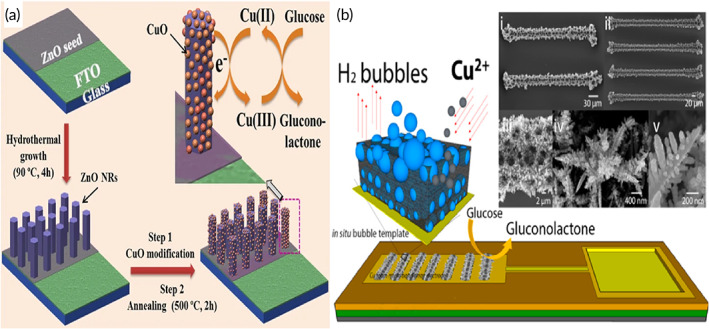
Nonenzymatic glucose (NEGS) with copper‐based electrodes. (a) Fabrication and application of NEGS for glucose detection. Adapted with permission from Reference 122, Copyright @ 2017 (Nature). (b) Hydrogen bubble template‐based electrodeposition process of the Cu foam and the SEM images of the resultant Cu Foam electrodeposits. Adapted with permission from Reference 171, Copyright @ 2019 (American Chemical Society)
FIGURE 9.
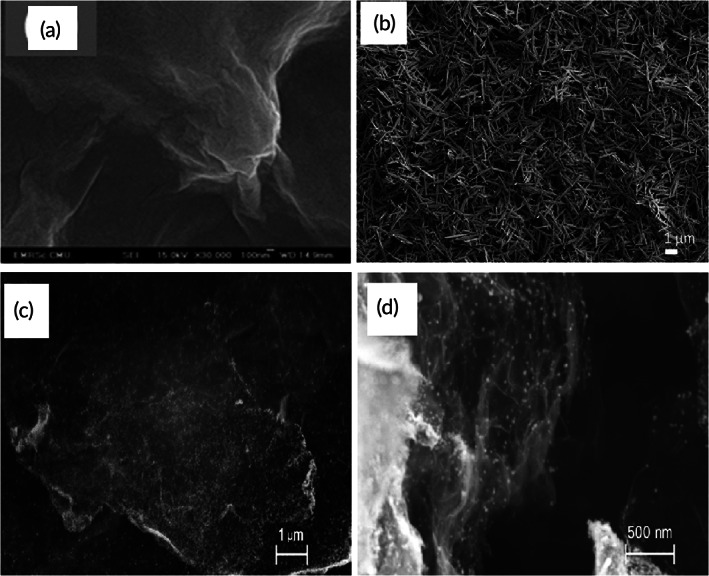
Field emission scanning electron microscopy (FESEM) characterization of copper particles used in nonenzymatic glucose (NEGS). (a) Bare Cu(II)/GO modified SPCE. Adapted with permission from Reference 172, Copyright @ 2021 (Nature). (b) Engineered hierarchical CuO nanoleaves. Adapted with permission from Reference 104, Copyright @ 2021 (IOP Science). (c,d) Copper nanoparticles electrochemically deposited on the PRG sheets. Adapted with permission from Reference 173, Copyright @ 2015 (PLos One)
Nanozymes have also gained popularity in recent decades. These nanozymes are nanomaterials that possess properties akin to enzymes and have been extensively studied for sensing purposes. 150 Wei and Wang were the first to equate catalytic NPs with artificial enzymes. 151 However, like EGS, NEGS face a few setbacks that hinder their practical application at a clinical level. This includes their lowered selectivity because of the lack of a prominent recognition element in the device, and their ability to function correctly under highly alkaline conditions, which means that these sensors will not show their best function within physiological pH. Also, most recent studies have focused on improving the material structure of nanomaterials used in NEGS and less work has been done to enhance targeted and highly sensitive glucose detection. EGS, on the contrary, have shown to give better glucose detection results. Therefore, the scientific focus must steer toward a better understanding of the mechanisms involved in the catalytic processes. Also, further works need to be done to explore other ways to develop nanomaterials that mimic enzymes like in EGS, and possess versatile 3D structures and have better application in the sensing process.
4. CONCLUSION AND FUTURE PERSPECTIVE
Recent advances in the fabrication of FGGS have significantly improved. However, the practical application of these devices continues to face significant challenges and hurdles. Efforts are being made to investigate Cu as a competing electrode for FGGS by improving the surface area, shape, and size to volume ratio, enhancing catalytic properties and stability, and detection capability. Reduced stability, shorter shelf life, and enzyme denaturation have limited the application of EGS, focusing researchers toward the tremendously growing field of FGGS. Even though numerous articles have been published demonstrating the efficacy of transition elements as electrocatalytic nanostructures for FGGS advancement, enzyme‐based glucose sensors still outperform the former category for their sensitivity and biocompatibility. To commercially enlist the FGGS, copper‐based characteristics such as low cost, stability, simplicity, and natural abundance can be leveraged to achieve the central goal. When exposed to air, Cu‐based biosensors are easily oxidized, reducing their stability. This can be improved by incorporating other nanomaterials within them, but this complicates FGGS fabrication.
Cu‐based electrodes perform best in alkaline media that operate in a synergistic environment, though the exact mechanism remains unknown. The researchers hope to develop FGGS to detect low glucose levels in blood samples and other bodily fluids. These significant challenges in making Cu‐based glucose sensors stable, reproducible, competitive, and commercially available with EGS are within reach, but the field remains exciting. Cu and its oxides are among the best electrocatalytic nanostructures for manufacturing glucose biosensors, but complete dedication is required to eliminate the shortcomings mentioned above. These efforts should be taken seriously to overcome the difficulties associated with maintaining optimal glucose levels in the blood. These findings contribute to the investigation of future research for the development of advanced versions of Cu‐based FGGS.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Tasbiha Awan: Data curation (equal); methodology (equal). Gowhar Naikoo: Conceptualization (equal); investigation (equal); writing–original draft (equal). Hiba Salim: Formal analysis (equal); methodology (equal). Fareeha Arshed: Formal analysis (equal); methodology (equal). Israr Hassan: Data curation (equal); formal analysis (equal); methodology (equal). Mona Pedram: Formal analysis (equal); methodology (equal). Waqar Ahmed: Formal analysis (equal); methodology (equal). Hakkim Faruck: Data curation (equal); investigation (equal); methodology (equal). Alaa Aljabali: Investigation (equal); writing–original draft (equal); writing–review and editing (equal). Vijay Mishra: Investigation (equal). Ángel Serrano‐Aroca: Formal analysis (equal); investigation (equal); methodology (equal). Rohit Goyal: Formal analysis (equal); investigation (equal); methodology (equal). Poonam Negi: Investigation (equal). Martin Birkett: Investigation (equal); writing–review and editing (equal). Mohamed Nasef: Writing–review and editing (equal). Nitin Charbe: Writing–review and editing (equal). Hamid A. Bakshi: Conceptualization (equal); investigation (equal); project administration (equal); supervision (equal); writing–original draft (equal); writing–review and editing (equal). Murtaza Tambuwala: Conceptualization (equal); investigation (equal); project administration (equal); supervision (equal); writing–original draft (equal); writing–review and editing (equal). [Correction added on September 24, 2021 after first online publication: Contribution details of Hamid A. Bakshi has been added.]
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10248.
ACKNOWLEDGMENT
Gowhar A. Naikoo, Tasbiha Awan, Hiba Salim, Israr Ul Hassan acknowledge the support received from The Research Council (TRC), Oman under the grant (Ref: BFP/RGP/HSS/18/122) to accomplish this work successfully.
Naikoo GA, Awan T, Salim H, et al. Fourth‐generation glucose sensors composed of copper nanostructures for diabetes management: A critical review. Bioeng Transl Med. 2022;7(1):e10248. doi: 10.1002/btm2.10248
Funding information The Research Council (TRC), Oman, Grant/Award Number: BFP/RGP/HSS/18/122
Contributor Information
Gowhar A. Naikoo, Email: gahmed@duedu.om.
Hamid A. Bakshi, Email: bakshi-h@ulster.ac.uk.
Murtaza M. Tambuwala, Email: m.tambuwala@ulster.ac.uk.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814‐825. 10.1021/cr068123a [DOI] [PubMed] [Google Scholar]
- 2. Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10:4558‐4576. 10.3390/s100504558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toghill KE, Compton RG. Electrochemical nonenzymatic glucose sensors: a perspective and an evaluation. Int J Electrochem Sci. 2010;5:1246‐1301. [Google Scholar]
- 4. Li Z, Chen Y, Xin Y, Zhang Z. Sensitive electrochemical nonenzymatic glucose sensing based on anodized CuO nanowires on three‐dimensional porous copper foam. Sci Rep. 2015;5:16115. 10.1038/srep16115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archana V, Xia Y, Fang R, Gnana Kumar G. Hierarchical CuO/NiO‐carbon nanocomposite derived from metal organic framework on cello tape for the flexible and high performance nonenzymatic electrochemical glucose sensors. ACS Sustain Chem Eng. 2019;7:6707‐6719. 10.1021/acssuschemeng.8b05980 [DOI] [Google Scholar]
- 6. Wu L, Lu ZW, Ma Y, et al. Cu(II) metal‐organic framework encapsulated in carbon paste electrode for high‐performance non‐enzymatic glucose sensing. Chinese J Anal Chem. 2020;48:e20038‐e20046. 10.1016/S1872-2040(20)60006-8 [DOI] [Google Scholar]
- 7. Zhu X, Ju Y, Chen J, Liu D, Liu H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018;3:1135‐1141. 10.1021/acssensors.8b00168 [DOI] [PubMed] [Google Scholar]
- 8. Hadgu FB, Sibhat GG, Gebretsadik LG. Diabetic ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes in Tigray, Ethiopia: retrospective observational study. Pediatr Heal Med Ther. 2019;10:49‐55. 10.2147/phmt.s207165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jean‐Marie E. Diagnosis and classification of diabetes mellitus. Encycl Endocr Dis. 2018;1:105‐109. 10.1016/B978-0-12-801238-3.65822-1 [DOI] [Google Scholar]
- 10. World Health Organization , Global Report on Diabetes. France: World Health Organization; 2016. ISBN 978 92 4 156525 7. [Google Scholar]
- 11. Bandodkar AJ, Imani S, Nuñez‐Flores R, et al. Re‐usable electrochemical glucose sensors integrated into a smartphone platform. Biosens Bioelectron. 2018;101:181‐187. 10.1016/j.bios.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahman MM, Ahammad AJS, Jin JH, Ahn SJ, Lee JJ. A comprehensive review of glucose biosensors based on nanostructured metal‐oxides. Sensors. 2010;10:4855‐4886. 10.3390/s100504855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795‐800. 10.2337/dc10-1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real‐time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43:2736‐2743. 10.2337/dc20-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juska VB, Pemble ME. A critical review of electrochemical glucose sensing: Evolution of biosensor platforms based on advanced nanosystems. Sensor. 2020;20:6013. 10.3390/s20216013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbas Q, Raza R, Shabbir I, Olabi AG. Heteroatom doped high porosity carbon nanomaterials as electrodes for energy storage in electrochemical capacitors: A review. J Sci Adv Mater Devices. 2019;4:341‐352. 10.1016/j.jsamd.2019.07.007 [DOI] [Google Scholar]
- 17. Rickard DT. Covellite formation in low temperature aqueous solutions. Miner Depos. 1972;7:180‐188. 10.1007/BF00207153 [DOI] [Google Scholar]
- 18. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self‐monitoring of diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2018;12:181‐187. 10.1016/j.dsx.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 19. Bailey TS, Grunberger G, Bode BW, et al. American association of clinical endocrinologists and American college of endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22:231‐262. 10.4158/EP151124.CS [DOI] [PubMed] [Google Scholar]
- 20. Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178‐192. 10.1016/j.diabres.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 21. Chung RJ, Wang AN, Liao QL, Chuang KY. Nonenzymatic glucose sensor composed of carbon‐coated nano‐zinc oxide. Nanomaterials. 2017;7:36. 10.3390/nano7020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Si P, Huang Y, Wang T, Ma J. Nanomaterials for electrochemical nonenzymatic glucose biosensors. RSC Adv. 2013;3:3487. 10.1039/c2ra22360k [DOI] [Google Scholar]
- 23. Cho SJ, Noh HB, Won MS, Cho CH, Kim KB, Shim YB. A selective glucose sensor based on direct oxidation on a bimetal catalyst with a molecular imprinted polymer. Biosens Bioelectron. 2018;99:471‐478. 10.1016/j.bios.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 24. Lee WC, Kim KB, Gurudatt NG, et al. Comparison of enzymatic and nonenzymatic glucose sensors based on hierarchical Au–Ni alloy with conductive polymer. Biosens Bioelectron. 2019;130:48‐54. 10.1016/j.bios.2019.01.028 [DOI] [PubMed] [Google Scholar]
- 25. Leonardi SG, Marini S, Espro C, Bonavita A, Galvagno S, Neri G. In‐situ grown flower‐like nanostructured CuO on screen printed carbon electrodes for nonenzymatic amperometric sensing of glucose. Microchim Acta. 2017;184:2375‐2385. 10.1007/s00604-017-2232-1 [DOI] [Google Scholar]
- 26. Wu H, Tian Q, Zheng W, et al. Nonenzymatic glucose sensor based on molecularly imprinted polymer: a theoretical, strategy fabrication and application. J. Solid State Electrochem. 2019;23:1379‐1388. 10.1007/s10008-019-04237-1 [DOI] [Google Scholar]
- 27. Wang S, Su P, Yang HEY. Polyamidoamine dendrimer as a spacer for the immobilization of glucose oxidase in capillary enzyme microreactor. Anal Biochem. 2010;405:230‐235. 10.1016/j.ab.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 28. Figiela M, Wysokowski M, Galinski M, Jesionowski T, Stepniak I. Synthesis and characterization of novel copper oxide‐chitosan nanocomposites for nonenzymatic glucose sensing. Sens Actuators B Chem. 2018;272:296‐307. 10.1016/j.snb.2018.05.173 [DOI] [Google Scholar]
- 29. Lopa NS, Rahman MM, Ahmed F, et al. Simple, low‐cost, sensitive and label‐free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens Bioelectron. 2019;126:381‐388. 10.1016/j.bios.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 30. Mao X, Lu Y, Zhang X, Huang Y. β‐Cyclodextrin functionalization of metal‐organic framework MOF‐235 with excellent chemiluminescence activity for sensitive glucose biosensing. Talanta. 2018;188:161‐167. 10.1016/j.talanta.2018.05.077 [DOI] [PubMed] [Google Scholar]
- 31. Fu C, Jin S, Oh J, Xu S, Jung YM. Facile detection of glucose in human serum employing silver‐ion‐guided surface‐enhanced Raman spectroscopy signal amplification. Analyst. 2017;142:2887‐2891. 10.1039/c7an00604g [DOI] [PubMed] [Google Scholar]
- 32. Tsao CW, Yang ZJ. High sensitivity and high detection specificity of gold‐nanoparticle‐grafted nanostructured silicon mass spectrometry for glucose analysis. ACS Appl Mater Interfaces. 2015;7:22630‐22637. 10.1021/acsami.5b07395 [DOI] [PubMed] [Google Scholar]
- 33. Shen W, Sun J, Seah JYH, Shi L, Tang S, Lee HK. Needle‐based sampling coupled with colorimetric reaction catalyzed by layered double hydroxide peroxidase mimic for rapid detection of the change of D‐glucose levels with time in bananas. Anal Chim Acta. 2018;1001:32‐39. 10.1016/j.aca.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 34. Chen X, Wang J, Yang C, Ge Z, Yang H. Fluorescence resonance energy transfer from NaYF4:Yb,Er to nano gold and its application for glucose determination. Sensors Actuators, B Chem. 2018;255:1316‐1324. 10.1016/j.snb.2017.08.124 [DOI] [Google Scholar]
- 35. Naaz S, Poddar S, Bayen SP, et al. Tenfold enhancement of fluorescence quantum yield of water soluble silver nanoclusters for nano‐molar level glucose sensing and precise determination of blood glucose level. Sens Actuators B Chem. 2018;255:332‐340. 10.1016/j.snb.2017.07.143 [DOI] [Google Scholar]
- 36. Lau OW, Shao B. Determination of glucose using a piezoelectric quartz crystal and the silver mirror reaction. Anal Chim Acta. 2000;407:17‐21. 10.1016/S0003-2670(99)00783-7 [DOI] [Google Scholar]
- 37. Dhara K, Mahapatra DR. Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim Acta. 2018;185:49. 10.1007/s00604-017-2609-1 [DOI] [PubMed] [Google Scholar]
- 38. Galindo R, Gutiérrez S, Menéndez N, Herrasti P. Catalytic properties of nickel ferrites for oxidation of glucose, β‐nicotiamide adenine dinucleotide (NADH) and methanol. J Alloys Compd. 2014;586:S511‐S515. 10.1016/j.jallcom.2013.01.049 [DOI] [Google Scholar]
- 39. Rane AV, Kanny K, Abitha VK, Thomas S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. Synth Inorg Nanomater. 2018:121‐139. 10.1016/b978-0-08-101975-7.00005-1 [DOI] [Google Scholar]
- 40. Ramachandran A, Rupanagudi KV, Khanna P, et al. Hepatic lipoprotein receptor related protein modulators as potential therapeutics for Alzheimer's disease. Alzheimer's Dement. 2019;15:P271. [Google Scholar]
- 41. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:S‐25‐S‐37. 10.1089/dia.2017.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson R, Elizabeth Q. Glucose oxidase: an ideal enzyme review article glucose oxidase. Biosens Biwlecrronia. 2016;7:165‐185. [Google Scholar]
- 43. Chandrasekaran NI, Matheswaran M. Unique nonenzymatic glucose sensor using a hollow‐shelled triple oxide Mn‐Cu‐Al nanocomposite. ACS Omega. 2020;5:23502‐23509. 10.1021/acsomega.0c00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gnana Kumar G, Amala G, Gowtham SM. Recent advancements, key challenges and solutions in nonenzymatic electrochemical glucose sensors based on graphene platforms. RSC Adv. 2017;7:36949‐36976. 10.1039/c7ra02845h [DOI] [Google Scholar]
- 45. Niu X, Li X, Pan J, He Y, Qiu F, Yan Y. Recent advances in nonenzymatic electrochemical glucose sensors based on non‐precious transition metal materials: opportunities and challenges. RSC Adv. 2016;6:84893‐84905. 10.1039/c6ra12506a [DOI] [Google Scholar]
- 46. Adeel M, Rahman MM, Caligiuri I, Canzonieri V, Rizzolio F, Daniele S. Recent advances of electrochemical and optical enzyme‐free glucose sensors operating at physiological conditions. Biosens Bioelectron. 2020;165:112331. 10.1016/j.bios.2020.112331 [DOI] [PubMed] [Google Scholar]
- 47. Wei M, Qiao Y, Zhao H, et al. Electrochemical nonenzymatic glucose sensors: recent progress and perspectives. Chem Commun. 2020;56:14553‐14569. 10.1039/d0cc05650b [DOI] [PubMed] [Google Scholar]
- 48. Thatikayala D, Ponnamma D, Sadasivuni KK, et al. Progress of advanced nanomaterials in the nonenzymatic electrochemical sensing of glucose and H2O2 . Biosensors. 2020;10:151. 10.3390/bios10110151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun A, Zheng J, Sheng Q. A highly sensitive nonenzymatic glucose sensor based on nickel and multi‐walled carbon nanotubes nanohybrid films fabricated by one‐step co‐electrodeposition in ionic liquids. Electrochim Acta. 2012;65:64‐69. 10.1016/j.electacta.2012.01.007 [DOI] [Google Scholar]
- 50. Zhu Z, Garcia‐Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI. A critical review of Glucose biosensors based on Carbon nanomaterials: Carbon nanotubes and graphene. Sensors (Switzerland). 2012;12:5996‐6022. 10.3390/s120505996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tian K, Prestgard M, Tiwari A. A review of recent advances in nonenzymatic glucose sensors. Mater Sci Eng C. 2014;41:100‐118. 10.1016/j.msec.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 52. Mohapatra J, Ananthoju B, Nair V, et al. Enzymatic and nonenzymatic electrochemical glucose sensor based on carbon nano‐onions. Appl Surf Sci. 2018;442:332‐341. 10.1016/j.apsusc.2018.02.124 [DOI] [Google Scholar]
- 53. Arif D, Hussain Z, Sohail M, Liaqat MA, Khan MA, Noor T. A nonenzymatic electrochemical sensor for glucose detection based on Ag@TiO2@ metal‐organic framework (ZIF‐67) nanocomposite. Front Chem. 2020;8:573510. 10.3389/fchem.2020.573510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kang L, He D, Bie L, Jiang P. Nanoporous cobalt oxide nanowires for nonenzymatic electrochemical glucose detection. Sens Actuators B Chem. 2015;220:888‐894. 10.1016/j.snb.2015.06.015 [DOI] [Google Scholar]
- 55. Wang S, Li S, Wang W, et al. A nonenzymatic photoelectrochemical glucose sensor based on BiVO4 electrode under visible light. Sens Actuators B Chem. 2019;291:34‐41. 10.1016/j.snb.2019.04.057 [DOI] [Google Scholar]
- 56. Parlak O, İncel A, Uzun L, Turner APF, Tiwari A. Structuring au nanoparticles on two‐dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens Bioelectron. 2017;89:545‐550. 10.1016/j.bios.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 57. Niu X, Li Y, Tang J, Hu Y, Zhao H, Lan M. Electrochemical sensing interfaces with tunable porosity for nonenzymatic glucose detection: a Cu foam case. Biosens Bioelectron. 2014;51:22‐28. 10.1016/j.bios.2013.07.032 [DOI] [PubMed] [Google Scholar]
- 58. Tobaldi DM, Espro C, Leonardi SG, et al. Photo‐electrochemical properties of CuO‐TiO2 heterojunctions for glucose sensing. J Mater Chem C. 2020;8:9529‐9539. 10.1039/d0tc01975e [DOI] [Google Scholar]
- 59. Liu X, Yang W, Chen L, Jia J. Three‐dimensional copper foam supported CuO nanowire arrays: an efficient nonenzymatic glucose sensor. Electrochim Acta. 2017;235:519‐526. 10.1016/j.electacta.2017.03.150 [DOI] [Google Scholar]
- 60. Basu D, Basu S. Performance studies of Pd–Pt and Pt–Pd–Au catalyst for electro‐oxidation of glucose in direct glucose fuel cell. Int J Hydrogen Energ. 2012;37:4678‐4684. 10.1016/j.ijhydene.2011.04.158 [DOI] [Google Scholar]
- 61. Hwang DW, Lee S, Seo M, Chung TD. Recent advances in electrochemical nonenzymatic glucose sensors—A review. Anal Chim Acta. 2018;1033:1‐34. 10.1016/j.aca.2018.05.051 [DOI] [PubMed] [Google Scholar]
- 62. Dong Q, Ryu H, Lei Y. Metal oxide based nonenzymatic electrochemical sensors for glucose detection. Electrochim Acta. 2021;370:137744. 10.1016/j.electacta.2021.137744 [DOI] [Google Scholar]
- 63. Li C, Su Y, Zhang S, Lv X, Xia H, Wang Y. An improved sensitivity nonenzymatic glucose biosensor based on a CuxO modified electrode. Biosens Bioelectron. 2010;26:903‐907. 10.1016/j.bios.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 64. Tian K, Baskaran K, Tiwari A. Nonenzymatic glucose sensing using metal oxides—Comparison of CuO, Co3O4, and NiO. Vacuum. 2018;155:696‐701. 10.1016/j.vacuum.2018.06.060 [DOI] [Google Scholar]
- 65. Zhang X, Gu A, Wang G, et al. Fabrication of CuO nanowalls on Cu substrate for a high performance enzyme‐free glucose sensor. CrystEngComm. 2010;12:1120‐1126. 10.1039/b919749d [DOI] [Google Scholar]
- 66. Reitz E, Jia W, Gentile M, Wang Y, Lei Y. CuO nanospheres based nonenzymatic glucose sensor. Electroanalysis. 2008;20:2482‐2486. 10.1002/elan.200804327 [DOI] [Google Scholar]
- 67. Cao F, Gong J. Nonenzymatic glucose sensor based on CuO microfibers composed of CuO nanoparticles. Anal Chim Acta. 2012;723:39‐44. 10.1016/j.aca.2012.02.036 [DOI] [PubMed] [Google Scholar]
- 68. Mishra AK, Mukherjee B, Kumar A, et al. Superficial fabrication of gold nanoparticles modified CuO nanowires electrode for nonenzymatic glucose detection. RSC Adv. 2019;9:1772‐1781. 10.1039/C8RA07516F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Parashuram L, Sreenivasa S, Akshatha S, Udayakumar V, Sandeep Kumar S. A nonenzymatic electrochemical sensor based on ZrO2: Cu(I) nanosphere modified carbon paste electrode for electrocatalytic oxidative detection of glucose in raw Citrus aurantium var. sinensis . Food Chem. 2019;300:125178. 10.1016/j.foodchem.2019.125178 [DOI] [PubMed] [Google Scholar]
- 70. Wang G, Wei Y, Zhang W, Zhang X, Fang B, Wang L. Enzyme‐free amperometric sensing of glucose using Cu‐CuO nanowire composites. Microchim Acta. 2010;168:87‐92. 10.1007/s00604-009-0260-1 [DOI] [Google Scholar]
- 71. Wang J, De Zhang W. Fabrication of CuO nanoplatelets for highly sensitive enzyme‐free determination of glucose. Electrochim Acta. 2011;56:7510‐7516. 10.1016/j.electacta.2011.06.102 [DOI] [Google Scholar]
- 72. Wei H, Sun JJ, Guo L, Li X, Chen GN. Highly enhanced electrocatalytic oxidation of glucose and shikimic acid at a disposable electrically heated oxide covered copper electrode. Chem Commun. 2009;(20):2842‐2844. 10.1039/b904673a [DOI] [PubMed] [Google Scholar]
- 73. Wang L, Zhang Q, Chen S, et al. Electrochemical sensing and biosensing platform based on biomass‐derived macroporous carbon materials. Anal Chem. 2014;86:1414‐1421. 10.1021/ac401563m [DOI] [PubMed] [Google Scholar]
- 74. Unmüssig T, Weltin A, Urban S, Daubinger P, Urban GA, Kieninger J. Nonenzymatic glucose sensing based on hierarchical platinum micro−/nanostructures. J Electroanal Chem. 2018;816:215‐222. 10.1016/j.jelechem.2018.03.061 [DOI] [Google Scholar]
- 75. Jia H, Chang G, Lei M, et al. Platinum nanoparticles decorated dendrite‐like gold nanostructure on glassy carbon electrodes for enhancing electrocatalysis performance to glucose oxidation. Appl Surf Sci. 2016;384:58‐64. 10.1016/j.apsusc.2016.05.020 [DOI] [Google Scholar]
- 76. Taurino I, Sanzó G, Mazzei F, Favero G, De Micheli G, Carrara S. Fast synthesis of platinum nanopetals and nanospheres for highly‐sensitive nonenzymatic detection of glucose and selective sensing of ions. Sci Rep. 2015;5:15277. 10.1038/srep15277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang H, Jiang F, Zhou R, et al. Effect of deposition potential on the structure and electrocatalytic behavior of Pt micro/nanoparticles. Int J Hydrogen Energy. 2011;36:15052‐15059. 10.1016/j.ijhydene.2011.08.072 [DOI] [Google Scholar]
- 78. Gu Y, Yuan R, Yan X, et al. Catalytic amplification based on hole‐transporting materials as efficient metal‐free electrocatalysts for nonenzymatic glucose sensing. Anal Chim Acta. 2015;889:113‐122. 10.1016/j.aca.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 79. Heli H, Amirizadeh O. Nonenzymatic glucose biosensor based on hyperbranched pine‐like gold nanostructure. Mater Sci Eng C. 2016;63:150‐154. 10.1016/j.msec.2016.02.068 [DOI] [PubMed] [Google Scholar]
- 80. Li C, Su Y, Lv X, et al. Controllable anchoring of gold nanoparticles to polypyrrole nanofibers by hydrogen bonding and their application in nonenzymatic glucose sensors. Biosens Bioelectron. 2012;38:402‐406. 10.1016/j.bios.2012.04.049 [DOI] [PubMed] [Google Scholar]
- 81. Su Y, Luo B, Zhang JZ. Controllable cobalt oxide/Au hierarchically nanostructured electrode for nonenzymatic glucose sensing. Anal Chem. 2016;88:1617‐1624. 10.1021/acs.analchem.5b03396 [DOI] [PubMed] [Google Scholar]
- 82. Wang C, Sun Y, Yu X, et al. Ag–Pt hollow nanoparticles anchored reduced graphene oxide composites for nonenzymatic glucose biosensor. J Mater Sci Mater Electron. 2016;27:9370‐9378. 10.1007/s10854-016-4979-2 [DOI] [Google Scholar]
- 83. Liu S, Zhang C, Yuan L, et al. Component‐controlled synthesis of small‐sized Pd–Ag bimetallic alloy nanocrystals and their application in a nonenzymatic glucose biosensor. Part Part Syst Charact. 2013;30:549‐556. 10.1002/ppsc.201200150 [DOI] [Google Scholar]
- 84. Liu P, Zhang M, Xie S, Wang S, Cheng W, Cheng F. Nonenzymatic glucose biosensor based on palladium‐copper oxide nanocomposites synthesized via galvanic replacement reaction. Sens Actuators B Chem. 2017;253:552‐558. 10.1016/j.snb.2017.07.010 [DOI] [Google Scholar]
- 85. Xia C, Ning W. A novel nonenzymatic electrochemical glucose sensor modified with FeOOH nanowire. Electrochem Commun. 2010;12:1581‐1584. 10.1016/j.elecom.2010.09.002 [DOI] [Google Scholar]
- 86. Sridhar V, Park H. Carbon encapsulated cobalt sulfide nano‐particles anchored on reduced graphene oxide as high capacity anodes for sodium‐ion batteries and glucose sensor. J Alloys Compd. 2018;764:490‐497. 10.1016/j.jallcom.2018.06.098 [DOI] [Google Scholar]
- 87. Li Y, Guan P, Yu F, Li W, Xie X. CeO2 nanorods embedded in Ni(OH2) matrix for the nonenzymatic detection of glucose. Nanomaterials. 2017;7:205. 10.3390/nano7080205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sun CL, Cheng WL, Hsu TK, Chang CW, Chang JL, Zen JM. Ultrasensitive and highly stable nonenzymatic glucose sensor by a CuO/graphene‐modified screen‐printed carbon electrode integrated with flow‐injection analysis. Electrochem Commun. 2013;30:91‐94. 10.1016/j.elecom.2013.02.015 [DOI] [Google Scholar]
- 89. Dong Q, Huang Y, Song D, Wu H, Cao F, Lei Y. Dual functional rhodium oxide nanocorals enabled sensor for both nonenzymatic glucose and solid‐state pH sensing. Biosens Bioelectron. 2018;112:136‐142. 10.1016/j.bios.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 90. Dong Q, Song D, Huang Y, et al. High‐temperature annealing enabled iridium oxide nanofibers for both nonenzymatic glucose and solid‐state pH sensing. Electrochim Acta. 2018;281:117‐126. 10.1016/j.electacta.2018.04.205 [DOI] [Google Scholar]
- 91. Sun X, Lei Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal Chem. 2017;89:163‐180. 10.1016/j.trac.2017.02.001 [DOI] [Google Scholar]
- 92. Ramon‐Marquez T, Sesay AM, Panjan P, Medina‐Castillo AL, Fernandez‐Gutierrez A, Fernandez‐Sanchez JF. A microfluidic device with integrated coaxial nanofibre membranes for optical determination of glucose. Sens Actuators B Chem. 2017;250:156‐161. 10.1016/j.snb.2017.04.140 [DOI] [Google Scholar]
- 93. Hu Y, Cheng H, Zhao X, et al. Surface‐enhanced Raman scattering active gold nanoparticles with enzyme‐mimicking activities for measuring glucose and lactate in living tissues. ACS Nano. 2017;11:5558‐5566. 10.1021/acsnano.7b00905 [DOI] [PubMed] [Google Scholar]
- 94. Zaidi SA, Shin JH. Recent developments in nanostructure based electrochemical glucose sensors. Talanta. 2016;149:30‐42. 10.1016/j.talanta.2015.11.033 [DOI] [PubMed] [Google Scholar]
- 95. Wang W, Li Z, Zheng W, Yang J, Zhang H, Wang C. Electrospun palladium (IV)‐doped copper oxide composite nanofibers for nonenzymatic glucose sensors. Electrochem Commun. 2009;11:1811‐1814. 10.1016/j.elecom.2009.07.025 [DOI] [Google Scholar]
- 96. Akbari A, Amini M, Tarassoli A, Eftekhari‐Sis B, Ghasemian N, Jabbari E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano‐Struct Nano‐Objects. 2018;14:19‐48. 10.1016/j.nanoso.2018.01.006 [DOI] [Google Scholar]
- 97. Guo MM, Xia Y, Huang W, Li Z. Electrochemical fabrication of stalactite‐like copper micropillar arrays via surface rebuilding for ultrasensitive nonenzymatic sensing of glucose. Electrochim Acta. 2015;151:340‐346. 10.1016/j.electacta.2014.11.041 [DOI] [Google Scholar]
- 98. Hosseini H, Rezaei SJT, Rahmani P, Sharifi R, Nabid MR, Bagheri A. Nonenzymatic glucose and hydrogen peroxide sensors based on catalytic properties of palladium nanoparticles/poly(3,4‐ethylenedioxythiophene) nanofibers. Sens Actuators B Chem. 2014;195:85‐91. 10.1016/j.snb.2014.01.015 [DOI] [Google Scholar]
- 99. Chu Z, Shi L, Liu L, Liu Y, Jin W. Highly enhanced performance of glucose biosensor via in situ growth of oriented Au micro‐cypress. J Mater Chem. 2012;22:21917. 10.1039/c2jm35554j [DOI] [Google Scholar]
- 100. Liu S, Yu B, Zhang T. A novel nonenzymatic glucose sensor based on NiO hollow spheres. Electrochim Acta. 2013;102:104‐107. 10.1016/j.electacta.2013.03.191 [DOI] [Google Scholar]
- 101. Li Y, Wei Y, Shi G, Xian Y, Jin L. Facile synthesis of leaf‐like CuO nanoparticles and their application on glucose biosensor. Electroanalysis. 2011;23:497‐502. 10.1002/elan.201000343 [DOI] [Google Scholar]
- 102. Liu M, Liu R, Chen W. Graphene wrapped Cu2O nanocubes: nonenzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron. 2013;45:206‐212. 10.1016/j.bios.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 103. Maaoui H, Teodoresu F, Wang Q, et al. Nonenzymatic glucose sensing using carbon quantum dots decorated with copper oxide nanoparticles. Sensor. 2016;16:1720. 10.3390/s16101720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahmad R, Khan M, Mishra P, et al. Engineered hierarchical CuO nanoleaves based electrochemical nonenzymatic biosensor for glucose detection. J Electrochem Soc. 2021;168:017501. 10.1149/1945-7111/abd515 [DOI] [Google Scholar]
- 105. Ji X, Wang A, Zhao Q. Direct growth of copper oxide films on Ti substrate for nonenzymatic glucose sensors. J Nanomater. 2014;2014:1‐5. 10.1155/2014/287303 [DOI] [Google Scholar]
- 106. Gong X, Gu Y, Zhang F, et al. High‐performance nonenzymatic glucose sensors based on CoNiCu alloy nanotubes arrays prepared by electrodeposition. Front Mater. 2019;6:1‐9. 10.3389/fmats.2019.00003 [DOI] [Google Scholar]
- 107. Zhang Y, Liu Y, Su L, et al. CuO nanowires based sensitive and selective nonenzymatic glucose detection. Sens Actuators B Chem. 2014;191:86‐93. 10.1016/j.snb.2013.08.096 [DOI] [Google Scholar]
- 108. Li J, Jin X, Li R, et al. Copper oxide nanowires for efficient photoelectrochemical water splitting. Appl Catal Environ. 2019;240:1‐8. 10.1016/j.apcatb.2018.08.070 [DOI] [Google Scholar]
- 109. Zhang Q, Wang J, Xu D, Wang Z, Li X, Zhang K. Facile large‐scale synthesis of vertically aligned CuO nanowires on nickel foam: growth mechanism and remarkable electrochemical performance. J Mater Chem A. 2014;2:3865. 10.1039/c3ta14767c [DOI] [Google Scholar]
- 110. Paulose R, Mohan R, Parihar V. Nanostructured nickel oxide and its electrochemical behaviour—A brief review. Nano‐Struct Nano‐Objects. 2017;11:102‐111. 10.1016/j.nanoso.2017.07.003 [DOI] [Google Scholar]
- 111. Zhu G, Xu H, Xiao Y, Liu Y, Yuan A, Shen X. Facile fabrication and enhanced sensing properties of hierarchically porous CuO architectures. ACS Appl Mater Interfaces. 2012;4:744‐751. 10.1021/am2013882 [DOI] [PubMed] [Google Scholar]
- 112. Gao Y, Kong Q, Zhang J, Xi G. General fabrication and enhanced VOC gas‐sensing properties of hierarchically porous metal oxides. RSC Adv. 2017;7:35897‐35904. 10.1039/c7ra06808e [DOI] [Google Scholar]
- 113. Rahmatolahzadeh R, Aliabadi M, Motevalli K. Cu and CuO nanostructures: facile hydrothermal synthesis, characterization and photocatalytic activity using new starting reagents. J Mater Sci Mater Electron. 2017;28:148‐156. 10.1007/s10854-016-5504-3 [DOI] [Google Scholar]
- 114. Zhao S, Wang T, Wang L, Xu S. A nonenzymatic glucose amperometric biosensor based on a simple one‐step electrodeposition of Cu microdendrites onto single‐walled carbon nanohorn‐modified electrode. J Solid State Electrochem. 2015;19:831‐839. 10.1007/s10008-014-2688-4 [DOI] [Google Scholar]
- 115. Effenberger FB, Sulca MA, Machini MT, et al. Copper nanoparticles synthesized by thermal decomposition in liquid phase: the influence of capping ligands on the synthesis and bactericidal activity. J Nanopart Res. 2014;16:2588. 10.1007/s11051-014-2588-7 [DOI] [Google Scholar]
- 116. Yadav S, Bajpai PK. Synthesis of copper sulfide nanoparticles: pH dependent phase stabilization. Nano‐Struct Nano‐Objects. 2017;10:151‐158. 10.1016/j.nanoso.2017.03.009 [DOI] [Google Scholar]
- 117. Tsolekile N, Parani S, Matoetoe MC, Songca SP, Oluwafemi OS. Evolution of ternary I–III–VI QDs: synthesis, characterization and application. Nano‐Struct Nano‐Objects. 2017;12:46‐56. 10.1016/j.nanoso.2017.08.012 [DOI] [Google Scholar]
- 118. Ding Y, Sun H, Ren C, Zhang M, Sun K. A nonenzymatic glucose sensor platform based on specific recognition and conductive polymer‐decorated CuCO2O4 carbon nanofibers. Materials (Basel). 2020;13:2874. 10.3390/ma13122874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dayakar T, Rao KV, Bikshalu K, Rajendar V, Park SH. Novel synthesis and characterization of pristine Cu nanoparticles for the nonenzymatic glucose biosensor. J Mater Sci Mater Med. 2017;28:109. 10.1007/s10856-017-5907-6 [DOI] [PubMed] [Google Scholar]
- 120. Chakraborty P, Dhar S, Deka N, Debnath K, Mondal SP. Nonenzymatic salivary glucose detection using porous CuO nanostructures. Sens Actuators B Chem. 2020;302:127134. 10.1016/j.snb.2019.127134 [DOI] [Google Scholar]
- 121. Ashok A, Kumar A, Tarlochan F. Highly efficient nonenzymatic glucose sensors based on CuO nanoparticles. Appl Surf Sci. 2019;481:712‐722. 10.1016/j.apsusc.2019.03.157 [DOI] [Google Scholar]
- 122. Ahmad R, Tripathy N, Ahn MS, et al. Highly efficient nonenzymatic glucose sensor based on CuO modified vertically‐grown ZnO Nanorods on electrode. Sci Rep. 2017;7:5715. 10.1038/s41598-017-06064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Niu X, Lan M, Zhao H, Chen C. Highly sensitive and selective nonenzymatic detection of glucose using three‐dimensional porous nickel nanostructures. Anal Chem. 2013;85:3561‐3569. 10.1021/ac3030976 [DOI] [PubMed] [Google Scholar]
- 124. Zhang H, Yu Y, Shen X, Hu X. A Cu2O/Cu/carbon cloth as a binder‐free electrode for nonenzymatic glucose sensors with high performance. New J Chem. 2020;44:1993‐2000. 10.1039/c9nj05256a [DOI] [Google Scholar]
- 125. Jiang D, Liu Q, Wang K, et al. Enhanced nonenzymatic glucose sensing based on copper nanoparticles decorated nitrogen‐doped graphene. Biosens Bioelectron. 2014;54:273‐278. 10.1016/j.bios.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 126. Wu L, Lu Z, Ye J. Enzyme‐free glucose sensor based on layer‐by‐layer electrodeposition of multilayer films of multi‐walled carbon nanotubes and Cu‐based metal framework modified glassy carbon electrode. Biosens Bioelectron. 2019;135:45‐49. 10.1016/j.bios.2019.03.064 [DOI] [PubMed] [Google Scholar]
- 127. Zhai D, Liu B, Shi Y, et al. Highly sensitive glucose sensor based on pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano. 2013;7:3540‐3546. 10.1021/nn400482d [DOI] [PubMed] [Google Scholar]
- 128. Ensafi AA, Abarghoui MM, Rezaei B. A new nonenzymatic glucose sensor based on copper/porous silicon nanocomposite. Electrochim Acta. 2014;123:219‐226. 10.1016/j.electacta.2014.01.031 [DOI] [Google Scholar]
- 129. Xue Y, Huang Y, Zhou Z, Li G. Nonenzymatic glucose biosensor based on reduction graphene oxide‐persimmon tannin‐Pt‐Pd nanocomposite. IOP Conf Ser Mater Sci Eng. 2018;382:022016. 10.1088/1757-899X/382/2/022016 [DOI] [PubMed] [Google Scholar]
- 130. Park S, Park S, Jeong RA, et al. Nonenzymatic continuous glucose monitoring in human whole blood using electrified nanoporous Pt. Biosens Bioelectron. 2012;31:284‐291. 10.1016/j.bios.2011.10.033 [DOI] [PubMed] [Google Scholar]
- 131. Mu Y, Jia D, He Y, Miao Y, Wu HL. Nano nickel oxide modified nonenzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens Bioelectron. 2011;26:2948‐2952. 10.1016/j.bios.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 132. Yan X, Gu Y, Li C, et al. A nonenzymatic glucose sensor based on the CuS nanoflakes‐reduced graphene oxide nanocomposite. Anal Methods. 2018;10:381‐388. 10.1039/c7ay02290e [DOI] [Google Scholar]
- 133. Fan H‐H, Weng W‐L, Lee C‐Y, Liao C‐N. Electrochemical cycling‐induced spiky CuxO/Cu nanowire array for glucose sensing. ACS Omega. 2019;4:12222‐12229. 10.1021/acsomega.9b01730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Strakosas X, Selberg J, Pansodtee P, et al. A nonenzymatic glucose sensor enabled by bioelectronic pH control. Sci Rep. 2019;9:10844. 10.1038/s41598-019-46302-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bruen D, Delaney C, Florea L, Diamond D. Glucose sensing for diabetes monitoring: recent developments. Sensor. 2017;17:1866. 10.3390/s17081866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang W, Du Y, Wang ML. Noninvasive glucose monitoring using saliva nano‐biosensor. Sens Bio‐Sensing Res. 2015;4:23‐29. 10.1016/j.sbsr.2015.02.002 [DOI] [Google Scholar]
- 137. Seshadri DR, Li RT, Voos JE, et al. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. Npj Digit Med. 2019;2:72. 10.1038/s41746-019-0150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ruckh TT, Clark HA. Implantable nanosensors: toward continuous physiologic monitoring. Anal Chem. 2014;86:1314‐1323. 10.1021/ac402688k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Boraschi D, Costantino L, Italiani P. Interaction of nanoparticles with immunocompetent cells: nanosafety considerations. Nanomedicine. 2012;7:121‐131. 10.2217/nnm.11.169 [DOI] [PubMed] [Google Scholar]
- 140. Lin SY, Hsu WH, Lo JM, Tsai HC, Hsiue GH. Novel geometry type of nanocarriers mitigated the phagocytosis for drug delivery. J Control Release. 2011;154:84‐92. 10.1016/j.jconrel.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 141. Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150‐3159. 10.4049/jimmunol.1103414 [DOI] [PubMed] [Google Scholar]
- 142. Umar A, Rahman MM, Al‐Hajry A, Hahn YB. Enzymatic glucose biosensor based on flower‐shaped copper oxide nanostructures composed of thin nanosheets. Electrochem Commun. 2009;11:278‐281. 10.1016/j.elecom.2008.11.027 [DOI] [Google Scholar]
- 143. Qian Y, Ye F, Xu J, Le ZG. Synthesis of cuprous oxide (Cu2O) nanoparticles/graphene composite with an excellent electrocatalytic activity towards glucose. Int J Electrochem Sci. 2012;7:10063‐10073. [Google Scholar]
- 144. Suginta W, Khunkaewla P, Schulte A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem Rev. 2013;113:5458‐5479. 10.1021/cr300325r [DOI] [PubMed] [Google Scholar]
- 145. Yang Z, Feng J, Qiao J, Yan Y, Yu Q, Sun K. Copper oxide nanoleaves decorated multi‐walled carbon nanotube as platform for glucose sensing. Anal Methods. 2012;4:1924. 10.1039/c2ay25283j [DOI] [Google Scholar]
- 146. Zhang Y, Su L, Manuzzi D, et al. Ultrasensitive and selective nonenzymatic glucose detection using copper nanowires. Biosens Bioelectron. 2012;31:426‐432. 10.1016/j.bios.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 147. Wang X, Hu C, Liu H, Du G, He X, Xi Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens Actuators B Chem. 2010;144:220‐225. 10.1016/j.snb.2009.09.067 [DOI] [Google Scholar]
- 148. Luo J, Jiang S, Zhang H, Jiang J, Liu X. A novel nonenzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal Chim Acta. 2012;709:47‐53. 10.1016/j.aca.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 149. Zhou X, Nie H, Yao Z, Dong Y, Yang Z, Huang S. Facile synthesis of nanospindle‐like Cu2O/straight multi‐walled carbon nanotube hybrid nanostructures and their application in enzyme‐free glucose sensing. Sens Actuators B Chem. 2012;168:1‐7. 10.1016/j.snb.2011.12.012 [DOI] [Google Scholar]
- 150. Hamman JH. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 2010;8:1305‐1322. 10.3390/md8041305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Morris GA, Kök SM, Harding SE, Adams GG. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev. 2010;27:257‐284. 10.1080/02648725.2010.10648153 [DOI] [PubMed] [Google Scholar]
- 152. Lv J, Kong C, Xu Y, et al. Facile synthesis of novel CuO/Cu2O nanosheets on copper foil for high sensitive nonenzymatic glucose biosensor. Sens Actuators B Chem. 2017;248:630‐638. 10.1016/j.snb.2017.04.052 [DOI] [Google Scholar]
- 153. Kim K, Kim S, Lee HN, Park YM, Bae YS, Kim HJ. Electrochemically derived CuO nanorod from copper‐based metal‐organic framework for nonenzymatic detection of glucose. Appl Surf Sci. 2019;479:720‐726. 10.1016/j.apsusc.2019.02.130 [DOI] [Google Scholar]
- 154. Liu G, Zhao J, Qin L, Liu S, Zhang Q, Li J. Synthesis of an ordered nanoporous Cu/Ni/Au film for sensitive nonenzymatic glucose sensing. RSC Adv. 2020;10:12883‐12890. 10.1039/d0ra01224f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Anand VK, Bukke A, Bhatt K, et al. Highly sensitive and reusable Cu+2/polyaniline/reduced graphene oxide nanocomposite ink‐based nonenzymatic glucose sensor. Appl Phys A Mater Sci Process. 2020;126:500. 10.1007/s00339-020-03620-4 [DOI] [Google Scholar]
- 156. Wei C, Zou X, Liu Q, Li S, Kang C, Xiang W. A highly sensitive nonenzymatic glucose sensor based on CuS nanosheets modified Cu2O/CuO nanowire arrays. Electrochim Acta. 2020;334:135630. 10.1016/j.electacta.2020.135630 [DOI] [Google Scholar]
- 157. Nurani DA, Wibowo R, El Fajri IF. Nonenzymatic glucose sensor based on electrodeposited copper on carbon paste electrode (Cu/CPE). AIP Conf Proc. 2016;1729(1). 10.1063/1.4946959 [DOI] [Google Scholar]
- 158. Remes A, Manea F, Motoc S, et al. Highly sensitive nonenzymatic detection of glucose at mwcnt‐cubtc composite electrode. Appl Sci. 2020;10:8419. 10.3390/app10238419 [DOI] [Google Scholar]
- 159. Vinoth V, Shergilin TD, Asiri AM, Wu JJ, Anandan S. Facile synthesis of copper oxide microflowers for nonenzymatic glucose sensor applications. Mater Sci Semicond Process. 2018;82:31‐38. 10.1016/j.mssp.2018.03.032 [DOI] [Google Scholar]
- 160. Na W, Lee J, Jun J, Kim W, Kim YK, Jang J. Highly sensitive copper nanowire conductive electrode for nonenzymatic glucose detection. J Ind Eng Chem. 2019;69:358‐363. 10.1016/j.jiec.2018.09.050 [DOI] [Google Scholar]
- 161. Radhakrishnan S, Kim H‐Y, Kim B‐S. A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection. Sens Actuators B. 2016;233:93‐99. 10.1016/j.snb.2016.04.056 [DOI] [Google Scholar]
- 162. Muthumariappan A, Sakthivel K, Chen SM, Chen TW, Mani G, Lou BS. Effects of annealing temperature on crystal structure and glucose sensing properties of cuprous oxide. Sens Actuators B Chem. 2018;266:655‐663. 10.1016/j.snb.2018.03.146 [DOI] [Google Scholar]
- 163. Zheng W, Li Y, Hu L, Lee LYS. Use of carbon supports with copper ion as a highly sensitive nonenzymatic glucose sensor. Sens Actuators B Chem. 2019;282:187‐196. 10.1016/j.snb.2018.10.164 [DOI] [Google Scholar]
- 164. Amirzadeh Z, Javadpour S, Shariat MH, Knibbe R. Nonenzymatic glucose sensor based on copper oxide and multi‐wall carbon nanotubes using PEDOT:PSS matrix. Synth Met. 2018;245:160‐166. 10.1016/j.synthmet.2018.08.021 [DOI] [Google Scholar]
- 165. Haghparas Z, Kordrostami Z, Sorouri M, Rajabzadeh M, Khalifeh R. Fabrication of nonenzymatic electrochemical glucose sensor based on nano‐copper oxide micro hollow‐spheres. Biotechnol Bioprocess Eng. 2020;25:528‐535. 10.1007/s12257-020-0058-x [DOI] [Google Scholar]
- 166. Saraf M, Natarajan K, Mobin SM. Nonenzymatic amperometric sensing of glucose by employing sucrose templated microspheres of copper oxide (CuO). Dalt Trans. 2016;45:5833‐5840. 10.1039/c6dt00670a [DOI] [PubMed] [Google Scholar]
- 167. Jiang J, Zhang P, Liu Y, Luo H. A novel nonenzymatic glucose sensor based on a Cu‐nanoparticle‐modified graphene edge nanoelectrode. Anal Methods. 2017;9:2205‐2210. 10.1039/c7ay00084g [DOI] [Google Scholar]
- 168. Anand VK, Bhatt K, Kumar S, et al. Sensitive and enzyme‐free glucose sensor based on copper nanowires/polyaniline/reduced graphene oxide nanocomposite ink. Int J Nanosci. 2021;20:2150020. 10.1142/S0219581X21500204 [DOI] [Google Scholar]
- 169. Sridara T, Upan J, Saianand G, Tuantranont A, Karuwan C, Jakmunee J. Nonenzymatic amperometric glucose sensor based on carbon nanodots and copper oxide nanocomposites electrode. Sensor. 2020;20:808. 10.3390/s20030808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu L, Chen Y, Lv H, Wang G, Hu X, Wang C. Construction of a nonenzymatic glucose sensor based on copper nanoparticles/poly(o‐phenylenediamine) nanocomposites. J Solid State Electrochem. 2015;19:731‐738. 10.1007/s10008-014-2659-9 [DOI] [Google Scholar]
- 171. Juska VB, Walcarius A, Pemble ME. Cu nanodendrite foams on integrated band array electrodes for the nonenzymatic detection of glucose. ACS Appl Nano Mater. 2019;2:5878‐5889. 10.1021/acsanm.9b01325 [DOI] [Google Scholar]
- 172. Phetsang S, Kidkhunthod P, Chanlek N, Jakmunee J, Mungkornasawakul P, Ounnunkad K. Copper/reduced graphene oxide film modified electrode for nonenzymatic glucose sensing application. Sci Rep. 2021;11:9302. 10.1038/s41598-021-88747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tehrani F, Reiner L, Bavarian B. Rapid prototyping of a high sensitivity graphene based glucose sensor strip. PLoS One. 2015;10:e0145036. 10.1371/journal.pone.0145036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


