Abstract
There have been several attempts to find promising biomaterials for skin regeneration, among which polylysine (a homopolypeptide) has shown benefits in the regeneration and treatment of skin disorders. This class of biomaterials has shown exceptional abilities due to their macromolecular structure. Polylysine‐based biomaterials can be used as tissue engineering scaffolds for skin regeneration, and as drug carriers or even gene delivery vectors for the treatment of skin diseases. In addition, polylysine can play a preservative role in extending the lifetime of skin tissue by minimizing the appearance of photodamaged skin. Research on polylysine is growing today, opening new scenarios that expand the potential of these biomaterials from traditional treatments to a new era of tissue regeneration. This review aims to address the basic concepts, recent trends, and prospects of polylysine‐based biomaterials for skin regeneration. Undoubtedly, this class of biomaterials needs further evaluations and explorations, and many critical questions have yet to be answered.
Keywords: cationic polymer, polyelectrolyte, poly‐l‐lysine, regenerative medicine, tissue engineering
Abbreviations
- Arg
arginine
- Asn
asparagine
- Asp
aspartic acid
- ECM
extracellular matrix
- FA
focal adhesion
- FAK
focal adhesion kinase
- Glu
glutamic acid
- Gly
glycine
- HA
hyaluronic acid
- hADSC
human adipose‐derived stem cell
- Hep
heptose
- Ile
isoleucine
- IM
internal membrane
- Kdo
ketodeoxyoctonate
- L‐b‐L
layer‐by‐layer assembly
- Leu
leucine
- LSGs
lineage‐specific genes
- Lys
lysine
- MEF
mouse embryo fibroblasts
- MSCs
mesenchymal stem cells
- NCAs
N‐carboxyanhydrides
- OM
intact outer membrane
- PAMAM
poly(amidoamine)
- PEC
polyelectrolyte complexes
- PECs
polyelectrolyte complexes
- PEI
polyethyleneimine
- PG
peptidoglycan layer
- PLL
poly‐l‐lysine
- PLGA
PLL‐coated poly(lactide‐co‐glycolide)
- PEM
polyelectrolyte multilayers
- Pro
proline
- PTK2
protein tyrosine kinase 2
- ROCK
Rho‐associated protein kinase
- PPP
pentose phosphate pathway
- PEP
phosphoenolpyruvate
- OAA
oxaloacetate
- Ser
serine
- Thr
threonine
- Trp
tryptophan
- Tyr
tyrosine
- Val
valine
- α‐KG
α‐ketoglutarate
- ε‐PL
ε‐poly‐l‐lysine
1. INTRODUCTION
The ultimate goal of tissue engineering is to develop materials that can mimic the natural properties of tissue, either as a substitute for tissue repair or to encourage regeneration. Tissues damage can occur due to various causes, such as accidents and disease. The materials utilized for tissue regeneration should mimic the natural behavior of tissue and possess suitable mechanical properties and architectural structure. In this regard, several sophisticated molecules and materials have been designed to meet these requirements. The design of scaffolds for tissue engineering is a tradeoff between the complexity of the molecules and the simplicity of the application, and requires profound knowledge about the relationship between material science and biology. 1 , 2 , 3 Polymers have attracted significant attention in the realm of regenerative medicine, because of their unique properties and chemical versatility. These macromolecules can be synthesized as various chemical and physical structures resulting in wide range of physicochemical properties. For example, polymers can be designed to meet the requirements for mimicking soft tissue (e.g., skin) and for hard tissue (e.g., bone). 4 , 5 In this regard, many natural and synthetic polymers, such as chitosan, 6 , 7 gelatin, 8 , 9 and agarose 10 , 11 have been investigated for tissue regeneration because of their beneficial properties.
These polymers can be categorized based on their charge, as neutral, cationic, or anionic polymers. For example, most of the polysaccharides are anionic polymers due to the presence of pendant anionic functional groups (e.g., hydroxyl and carboxyl) on their main chains. On the other hand, cationic polymers are positively charged polyelectrolytes, carrying positive charges either on their backbone or on the side chains, and have attracted much interest among the polymer families. Cationic polymers have been more often used in biomedical applications compared to anionic polymers, because of their interaction with negatively charged biomolecules, peptides, proteins, and nucleic acids. Polycationic polymers can interact with negatively charged cell membranes and enhance cellular activities. 12 , 13 , 14 , 15 , 16 Polycationic polymers such as poly‐l‐lysine (PLL), polyethyleneimine (PEI), and poly(amidoamine) (PAMAM) dendrimers have been widely utilized to carry cargos (e.g., drugs, genes, and so on) across the cell membrane. 17 , 18 , 19 They can be used to form polyelectrolyte complexes (PECs) by combining with anionic species such as polyanions, nucleic acid chains, and some drugs. 20 Besides, they can form layer‐by‐layer assemblies. 21 Moreover, some polycationic polymers exhibit intrinsic bioactivity, with antibacterial, antioxidant, and antitumor properties. 22 , 23
Polylysine is a cationic polymer, which has attracted significant attention in tissue engineering because of its biodegradability and biocompatibility. However, because of its poor mechanical properties, it is not often used alone, and it should be copolymerized or blended with other polymers to improve its mechanical properties. Controlled polymerization, grafting, and modification techniques have been used to prepare PLL‐based materials with the desired architecture, properties, and multi‐functionality as biomimicking scaffolds for tissue engineering applications. 22 Due to the unmet need for skin tissue regeneration and considering the adjustable and unique properties of the polylysine, it is necessary to review the polylysine performance in skin tissue engineering.
2. THE CHEMISTRY OF POLYLYSINE
Lysine (an essential amino acid for humans) is the primary building block of polylysine, and is available in two chiral forms: l‐lysine and d‐lysine. Polylysine can be synthesized through condensation polymerization or by fermentation, which results in α‐polylysine or ε‐polylysine, respectively. ε‐Polylysine allows the linking of ε‐amino and α‐carboxylic acid moieties and is utilized as a food preservative due to its nontoxicity and antibacterial properties. The conformation of poly‐l‐lysine (PLL) depends on pH, temperature, ionic strength, and solvent type. 24 , 25 PLL in aqueous solution exhibits the PII conformation at low temperature and neutral pH, while by raising the pH the conformation changes to an α‐helical conformation, and by raising the temperature it changes to a β‐sheet structure. It is thought that the steric interactions between the PLL side chains (uncharged), the hydrogen bonds of the solvent or PLL, and the proton donor and acceptor groups in the solvent, stabilize the PII conformation. 24 The different forms of lysine and their different polymerization structures are depicted in Figure 1.
FIGURE 1.
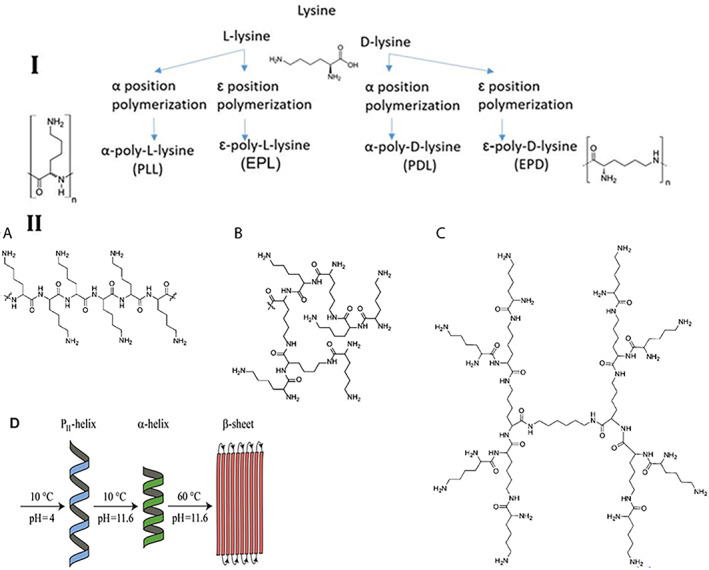
Polylysine structures. I. Lysine structure and polylysine formation. II. Polylysine structure (A) linear, (B) dendritic, (C) hyper‐branch, and (D) conformation of the polylysine at different temperatures and pH. Reproduced with permission from Reference [24]
Amino acid N‐carboxyanhydrides (NCAs), which are also known as leuchs anhydrides are well‐known reactive amino acid derivatives. 26 Ring‐opening polymerization of NCAs is a useful method for polypeptide synthesis because of its good yield. 27 , 28 PLL has been synthesized using the conversion of and ε‐primary amine‐protected l‐lysine derivative into the cyclic Nε‐(benzyloxycarbonyl)‐l‐lysine N‐carboxyanhydride monomer. NCA was polymerized via ring‐opening polymerization starting with a primary amine initiator which can operate in two ways: (1) attacking the C5 of NCA as a nucleophile (amine mechanism); and (2) deprotonating the N3 of NCA as a base (active monomer mechanism). Moreover, living polymerization using metal complexes has been used for NCA polymerization (Figure 2). 22 , 29
FIGURE 2.
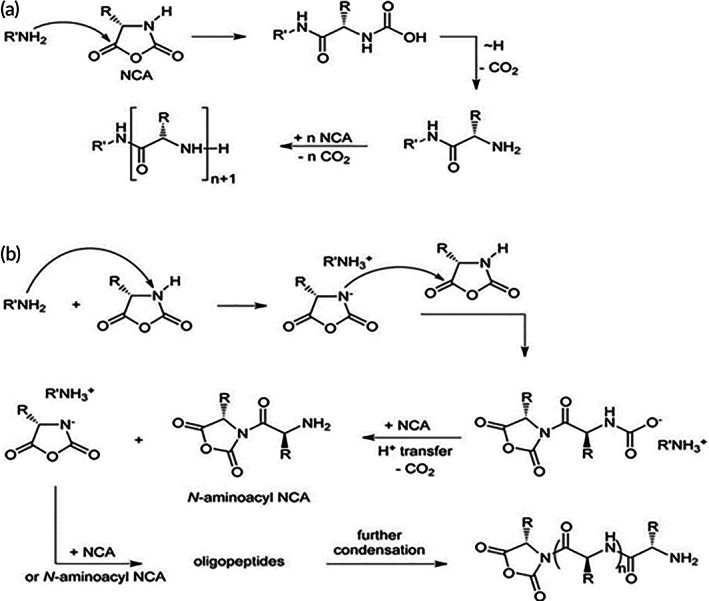
Synthesis of poly‐l‐lysine (PLL). PLL was synthesized using the conversion of the ε‐primary amine‐protected l‐lysine into the cyclic Nε‐(benzyloxycarbonyl)‐l‐lysine N‐carboxyanhydride monomer. NCA via ring‐opening polymerization was polymerized starting by primary amine initiator which can operate in two ways by (A) attacking the C5 of NCA as a nucleophile (amine mechanism) and (B) deprotonate the N3 of NCA as a base (active monomer mechanism)
3. INTERACTIONS BETWEEN CELLS AND POLYLYSINE SUBSTRATES
3.1. Simple polylysine substrates
The interactions between cells and their neighboring environment play a key role in governing cellular activity, such as during the processes of morphogenesis, embryogenesis, homeostasis, thrombosis, inflammation, and wound healing. 30 , 31 The extracellular matrix (ECM) binding‐site affects the shape of the cell and its motility. The first step in cell‐substrate interaction is the binding of integrin receptors to ECM molecules, followed by the aggregation of receptors at the contact point. 32 , 33 , 34 This is called the focal adhesion complex. Intracellular signaling cascades are triggered, resulting in the binding of the actin cytoskeleton to the ECM mediated by integrins. 35 , 36 Surface treatment of surfaces using polycationic polymers, such as polylysine, has been used to improve cell‐substrate interactions and facilitate cell adhesion. 37
The use of polyamine acids enhances cellular attachment to the substrate. Cellular functions are affected by this attachment and by ECM components. Some cells are able to produce ECM components, while other cells require an adjuvant to attach to the surface (especially in serum‐free media). Polylysine (because of its positively charged nature) can easily interact with the negatively charged surface on many types of cells. Therefore, cells can rapidly attach to polylysine substrates. Interestingly, some cells (e.g., sea urchin eggs) that normally have difficulty to attach to solid surfaces, can adhere to the polylysine surface, as well as mammalian cells. 37 Polylysine chains with higher molecular weights exhibit a higher tendency to bind to cell membranes. Moreover, flexible polycation chains exhibit higher cell‐binding compared to rigid chains. 38 It has been observed that culturing mesenchymal stem cells (MSCs) on PLL‐coated substrates could preserve stemness and prevent senescence of these cells. 39 PLL‐coated plates enhanced the growth of MSCs, and up‐regulated some genes that contribute to cell‐adhesion, cell differentiation, proliferation, and signaling. 39 Cell‐proliferation and osteogenic differentiation were improved by porous PLL‐coated poly(lactide‐co‐glycolide) (PLGA)/hydroxyapatite (HA) scaffolds. 40 Quirk et al. used coating with polylysine to attach the cells to the PLA surface. 41 The optimum concentration of polylysine for cellular attachment was reported to be approximately 0.05–0.5 μg/cm2 while higher concentrations may result in toxicity. 42 Sakai et al. used polylysine to improve the cellular attachment of BHK cells to a fibronectin surface. 43
3.2. Patterned polylysine substrates
Besides physical and chemical cues which affect the cell behavior, other geometrical cues or anisotropic physical features (e.g., parallel microgrooves and collagen fibers) also affect the cell orientation on both two‐ (2D and three‐dimensional (3D) substrates, which is known as the contact guidance phenomenon. 44 , 45 Contact guidance affects the shape, adhesion, and migration of cells. Indeed, cell migration, which is a key factor in many biological processes, is affected by both ECM guidance cues (e.g., a chemical species gradient or stiffness) and by contact guidance. Contact guidance results in cancer cell movement across the neighboring ECM and then to metastasis, by a process known as intravasation. 46 , 47 Furthermore, this phenomenon plays a key role in the wound healing process, where platelets and fibroblasts can induce a local contraction in the wound site, resulting in ECM fibers becoming radially oriented. This local fiber orientation guides the cell migration. 48
Appropriate signaling between cells, which is mediated via ECM proteins, significantly affects the redistribution of molecules that allow cell adhesion in dot or focal patterns. Cells are able to attach to the patterned polylysine substrate, while actin stress fibers are not created between neighboring dots. Irregular morphology was observed for cultured cells in which filopodia‐like extensions were abundant; besides no clustering was observed for focal adhesion molecules. 49 According to Lenhert et al., the cell spreading process includes three steps: (1) adhesion of the cells to the substrate, which depends on integrins; (2) integrin clustering followed by the creation of focal adhesion complexes. The focal adhesion complexes are assembled, the cells commence spreading, and Rac1 and Cdc42 are activated, resulting in the creation of lamellipodia and filopodia. The third step includes activation of RhoA, stress fiber formation, and creation of intracellular tension (Figure 3). 49
FIGURE 3.
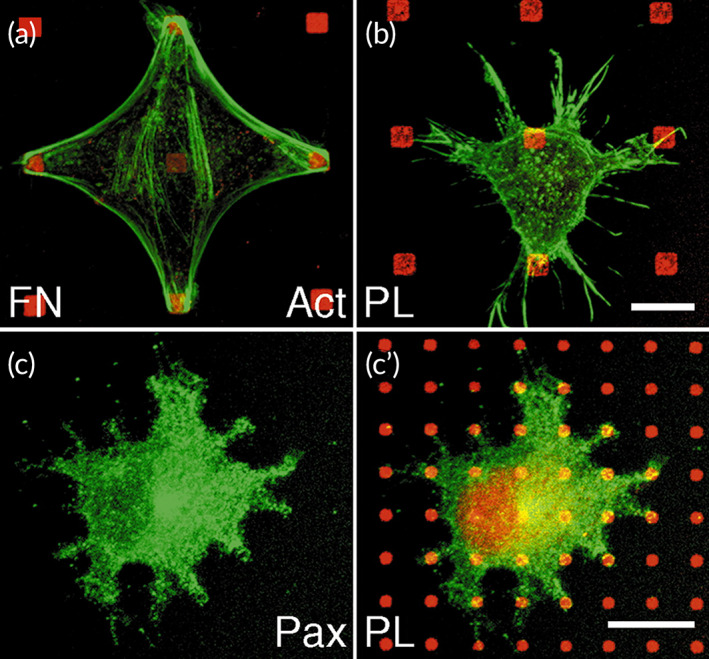
Cell spreading on polylysine. B16 cells were cultured on patterned substrata of fibronectin (red; A) or polylysine (red; B,C) and labeled for actin (green; A,B) and paxillin (green; C). Cells can adhere to polylysine dots, however, the actin cytoskeleton is not reorganized (B) and paxillin does not accumulate over the dots (C). Scale bars: 10 μm. Reprinted with permission from Reference [49]
Patterned co‐cultures can be created using three different techniques. Photolithography is a robust method to create patterned surfaces composed of different materials, that allow selective adhesion of various cells onto the predetermined area. For example, when co‐culturing hepatocytes and fibroblasts on micropatterned substrates, hepatocytes selectively adhered to the collagen islands which optimized the interaction between the different cells. 50 , 51 The second approach involves the delivery of cells to the specific areas on the substrate. For instance, patterning of various types of cells onto specific regions was achieved using a microfluidic device. 52 , 53 Furthermore, cell attachment to specific regions of the substrate can be excluded using elastomeric membranes. After removing the membrane, the remaining surface can be used to attach another different cell type. 54 However, these strategies suffer from some limitations. The construction of multicellular systems may be limited using photolithography, as each step requires the selective attachment of a single cell type. Moreover, geometrical constraints induced by laminar flow and separation between two different cell types because of channel interspacing are limitations associated with the microfluidic method. 55
Stimuli‐responsive surfaces can be used as a general strategy to overcome these limitations; cell repelling surfaces can be switched to cell adhesive surfaces via the application of a specific stimulus. 56 , 57 For example, the degree of hydrophilicity of electroactive or thermosensitive substrates can be tuned to enhance or diminish cell adhesion. However, high‐cost materials and sophisticated synthetic procedures are required to prepare such switchable surfaces, and this may limit their application. Accordingly, there is an urgent need to develop improved surface engineering strategies to fabricate patterned cell co‐cultures. Layer‐by‐layer assembly (L‐b‐L) of polyelectrolytes can be potentially used to fabricate such switchable surfaces, especially thin films. 58 Khademhosseini et al. co‐cultured either hepatocytes or embryonic stem cells with fibroblasts on a micropatterned hyaluronic acid (HA)/polylysine surface. This method was also used for patterned and controlled cell co‐culture. 59 HA is a biocompatible and biodegradable polysaccharide that has been widely studied for targeted drug delivery and for tissue engineering applications. Many polysaccharides, including HA, naturally repel cells and many other proteins. HA is an anionic polysaccharide that can form PEC or L‐b‐L assemblies by interacting with polycations such as PLL. Niepel et al. seeded human adipose‐derived stem cells (hADSCs) on a cross‐linked polyelectrolyte based on HA plus polylysine. It was found that a higher cross‐linking density of the substrate resulted in higher cell spreading and better osteogenic differentiation, while a substrate with a lower cross‐linking density better supported adipogenic differentiation. 60 Moreover, the hADSC fate was evaluated on the micropatterned polyelectrolyte of polylysine plus HA. When cell spreading was studied on a polyelectrolyte multilayer (PEM) with cross‐linking on a patterned substrate, there was enhanced rounding‐up of hADSCs. The patterned substrate affects both cell adhesion and differentiation, which are both important for designing novel tissue engineering scaffolds. For example, native multilayered microstructured large substrates improved chondrogenesis, while highly cross‐linked multilayered nanostructured small substrates enhanced osteogenesis (Figure 4). 21 Richert et al. fabricated a multi‐layered polyelectrolyte film based on polylysine/poly(l‐glutamic) acid and evaluated cellular attachment. It was observed that the pH used in the film fabrication process (i.e., film deposition) affected the cell adhesion, such that cells attached more firmly to films formed at pH 10.4, while they were repelled from films formed under acidic condition, that is, pH 4.4. These observations were related to the water swelling properties of these films which were high and low for films formed under basic and acidic conditions, respectively. Accordingly, the cell adhesion performance of PGA/PLL films could be adjusted depending on the pH of the film deposition process. This study confirmed the key role of multilayered films on tuning cell attachment/detachment properties. 61
FIGURE 4.
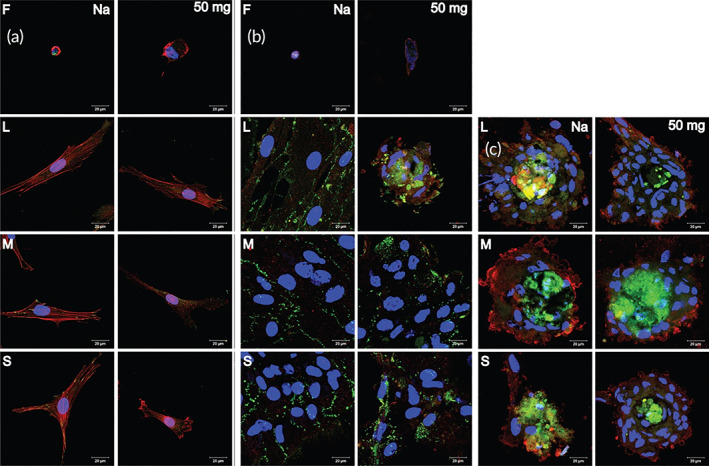
Polylysine and stem cell differentiation. (A) Confocal laser scanning microscopy images of adherent hADSC on flat (F) PEM. The platforms were cross‐linked using EDC (50, 10, and 2 mg mL−1). Afterward, the cells were cultured and incubated overnight (actin cytoskeleton (red), nucleus (blue), and vinculin in focal adhesions (green)). S, M, and L refer to small, medium, and large, respectively. PLL/HA and 50 mg mL−1 EDC cross‐linked nanostructured platforms were viewed. Nanostructured platforms were treated with PLL/HA and cross‐linked with 50 mg mL−1 EDC. (B) hADSC chondrogenic differentiation. (C) hADSC osteogenic differentiation. Reproduced with permission from Reference [21]
Focal adhesion (FA) complexes are the macromolecular assemblies responsible for the transduction of mechanical load via signaling molecules from the ECM to cell environment, that is, they serve as strain sensors or biosensors for various cells. These multiple protein structures contain integrins. Integrins are transmembrane heterodimeric proteins that connect the cells to ECM proteins (e.g., fibronectin, vitronectin, collagen, and laminin) via short amino acid sequences such as the arginylglycylaspartic acid motif. Meanwhile, they also connect the actin cytoskeleton to several proteins such as vinculin and filamin. Changes in the composition and morphology of focal adhesion complexes play a key role in cell migration. It has been proposed that the spreading of MSCs affects the cell differentiation process. The expression of lineage‐specific genes (LSGs) is influenced by phosphorylation of various gene products, such as protein tyrosine kinase 2 (PTK2) or focal adhesion kinase (FAK), and the Rho family of GTPases within the cytosol (Figure 5). MSC differentiation results in the formation of adipocytes when cell spreading is low, or osteocytes when cell spreading is high. Aspherical morphology and adipocyte differentiation have been observed in cells that were unable to undergo spreading. Furthermore, the cell morphology affects signal transduction and the shape influences signal transduction processes involving the Rho‐associated protein kinase (ROCK) pathway, resulting in adipocyte differentiation. 63 hADSC cultivated on a highly cross‐linked platform, were stained with alizarin red showing deposition of a mineralized matrix. There was only minimal staining in cells on a lower cross‐linked or native multilayer platform in conformity with the findings of McBeath et al. 64 This group studied stem cell distribution, RhoA activation, and cell differentiation. Cells growing on a glass substrate proliferated more than cells on a soft substrate. Consequently, the finding that during a long incubation period of 21 days, stem cells plated on a charged, rigid, glass substrate became differentiated, even with no chemical induction signals. 60
FIGURE 5.
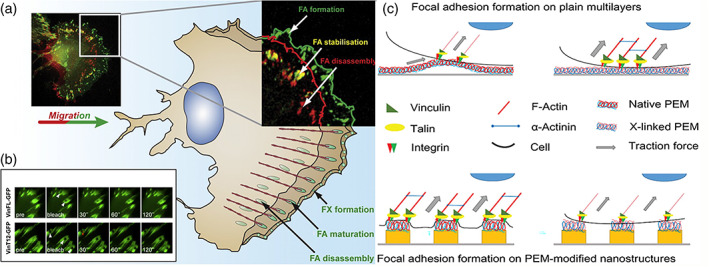
Polylysine and focal adhesions. (A) A schematic representation of the cell expressing β3‐integrin, B16 migrating melanoma, GFP, displaying, and disassembling sites of FA generation. Green mark was used to indicate fresh generated FA; structures that remained fixed within the 5‐min time intervals are labeled in yellow. (B) Fluorescence restoration of GFP vincular fusion complexes in mouse embryo fibroblasts (MEF). Reproduced with permission from Reference [62]. (C) Schematic description of the impact on the development of focal adhesion and actin polymerization of surface parameters. Reproduced with permission from Reference [21]
Better knowledge about the cell‐substrate interactions allows us to understand aspects of cellular activity, such as adhesion and migration and helps us to design a biomimetic surface for the best in‐vivo function and to achieve proper tissue regeneration.
4. POLYLYSINE ANTIBACTERIAL ACTIVITY
4.1. Gram‐negative bacteria such as Escherichia coli
ε‐Poly‐l‐lysine (ε‐PL) exhibits the most pronounced antibacterial activity within the polylysine family, and inhibits the proliferation of most microorganisms (Table 1 ). Generally, three mechanistic models including, barrel‐stave, toroidal, and the carpet mechanism have been proposed to explain the antibacterial activity of polycationic polymers like PLL. The barrel‐stave mechanism involves a rapid interaction with the hydrophobic core of the membrane lipid bilayer, while the other mechanisms rely on an interaction with the membrane surface and the headgroups. The residues with a hydrophobic nature not only can replace the headgroups of phospholipid units through a mechanism based on toroidal pore modeling, but can also induce pore lumen formation by altering the positive membrane curvature. The carpet‐like mechanism is based on a theory in which membranes are permeabilized in a nonspecific manner, involving only a contact between the positively charged peptide and the negatively charged head‐groups of the phospholipid. 71 , 72 The three models discussed above may not be sufficient to explain all the possible interactions within the membrane, so other models should be taken into consideration. 72 Scientists have also tried to explain the antimicrobial activity by other models, such as the grip‐and‐dip, as well as the disordered toroidal pore model. 73 , 74 Moreover, many believe that some interactions could be mediated by a process analogous to electroporation, called the sinking raft model. 75 It should be noted that in addition to the peptide structure and headgroup constitution of the membrane, the composition of existing acyl chains could also affect the peptide interaction with the membranes. 76
TABLE 1.
MICs of ε‐PL against parent strains and mutants of Escherichia coli and Listeria innocua detected by optical density measurements of growth at 620 nm
| Strain | Phenotype | MIC (mg/L) | Ref. |
|---|---|---|---|
| D21 | Parental wild‐type that express core portion of LPS | 87.5 | 65 |
| E. coli, JM109 | Express full core portion of the LPS, and has an extended O‐antigen polysaccharide chain. O‐rough:H48 | 87.5 | 66 |
| D21f2 | Truncated LPS after the Kdo carboxyl group. | 112.5 | 67 |
| JW4277‐1 | Lack type I fimbriae | 87.5 | 68 |
| D21f2 | Truncated LPS after the Kdo carboxyl group. | 112.5 | 69 |
| JW5917‐1 | Removed negative regulatory gene of CA synthesis. Uncontrolled | 175 | 68, 70 |
| L. innocua | Wild type | 750 | |
| E. coli K‐12 (LZB035) | Wild type | 74 |
Abbreviations: LPS, lipopolysaccharide; MIC, minimum inhibitory concentration.
Hyldgaard et al. clarified the mechanism of the antibacterial properties of ε‐polylysine against E. coli as Gram‐negative and Listeria innocua as Gram‐positive model bacteria. It was found that some components of the E. coli lipopolysaccharide layer, such as the divalent cations, along with heptose (Types I and II)‐phosphate could play a role in the binding process with polylysine. Polylysine destroyed the E. coli lipopolysaccharide layer and altered its morphology, while L. innocua showed only minimal morphological changes. The membranes of both the bacteria were attacked by polylysine, as shown by cytoplasmic membrane permeabilization. The carpet‐like model could also explain how polylysine could disrupt the membrane stability. This involves the polylysine binding to negatively charged phospholipids and releasing existing divalent cations, and consequently changing the membrane curvature into negative folding, thereby producing structures similar to vesicles or micelles (Figure 6). 69
FIGURE 6.
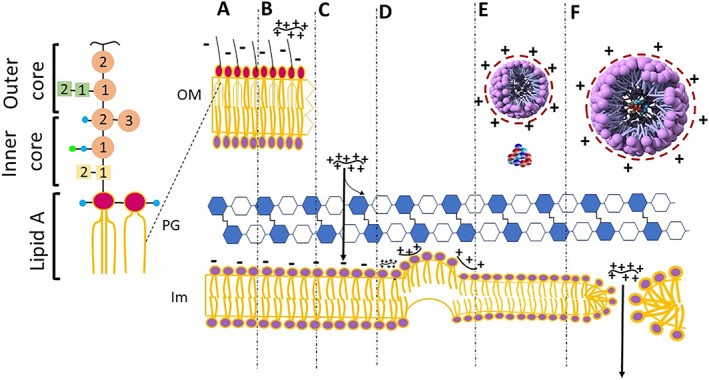
Description of the mechanisms of action suggested for ε‐PL on E. coli cells. (A) Intact outer membrane (OM), peptidoglycan layer (PG), and internal membrane (IM) unprotected cell wall. Lipopolysaccharide is the building blocks of the external leaflet of the external membrane. (B) The electronic interactions lead the collations of the red molecule (ε‐PL) with the LPS, which removes detergent‐like the LPS layer. (C) ε‐PL gets entry to a periplasmatic region, and there is still uncertainty about the possibility of ε‐PL interaction. (D) As ε‐PL exceeds a certain limiting value in the inner membrane, this confirms the negative curvature of one of the membrane leaflets (E) the phospholipids removed can shape hexagonal inverse micelles as well as the normal one, as indicated. (F) The phospholipids extracted from the inner membrane shape vesicles that creates holes behind where the surplus ε‐PL is released into the cytoplasm and can further damage the cell 69
The concentration of divalent cations in the ambient medium will competitively inhibit the binding of ε‐PL to the bacterial membranes. Moreover, an increase in the pH will decrease the positive charge on the ε‐PL, and subsequently, reduce the electrostatic interaction with the cells, and lessen the bactericidal activity. ε‐PL shows selective antimicrobial activity against a wide range of microorganisms, without any pronounced cytotoxicity towards eukaryotic cells, because the phospholipid head groups are different in eukaryotic and prokaryotic membranes.
In addition to membrane disruption, the antibacterial activity of ε‐PL could also involve other mechanisms. The formation of reactive oxygen species (ROS) and oxidative stress has been described as a result of cationic antibacterial compounds acting on cells. The findings of Del Carlo and Loeser in 2006 showed that ROS were produced by poly‐l‐lysine, and could cause substantial cell death in chondrocytes and bacteria that could be prevented by antioxidants. 77 Various genes associated with chemotaxis and oxidative/redox stress were shown to be induced by antibacterial compounds. The genes that were activated were concerned with the biosynthesis of adenosine triphosphate and the SOS response, and were associated with antibacterial activity. 71 , 72 RT‐qPCR results showed that the SOS response genes recA and lexA, were triggered by up‐regulation of the bacterial‐oxidative stress response as shown by the ROS‐related genes oxyR and sodA, and a decrease in the transcription of virulence genes eaeA and espA. The possible mechanisms of membrane disruption produced by ε‐PL are shown in Figure 6. The possible mechanisms of ROS and SOS responses produced by ε‐PL are shown in Figure 7. 78
FIGURE 7.
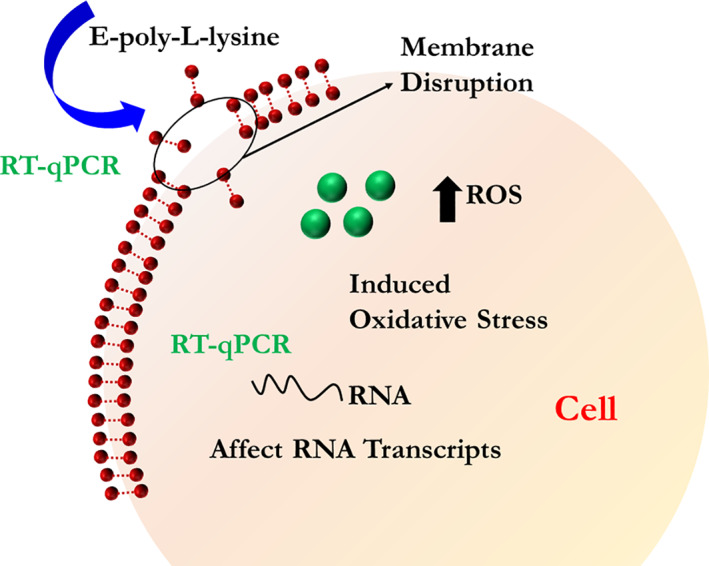
Bactericidal mechanism for ε‐PL against Escherichia coli O157: H7. ε‐PL bound to the membrane through the electrical forces resulting in both the destruction of the aimed membrane and formation changes and membrane fractures. The cytoplasm was entered through membrane pores by ε‐PL and two parallel reactions occurred afterward, that is, an increase in reactive oxygen species (ROS) levels and the DNA genome interactions, resulting in cell death. Reproduced with permission Reference [78]
4.2. Gram‐positive bacteria such as Staphylococcus aureus
The potential mechanism for the antimicrobial activity of ε‐PL against Gram‐positive bacteria such as S. aureus, is presented in Figure 8. In the first stage, the positively charged ε‐PL binds to negatively charged teichoic acid which is a component of the peptidoglycan layers within the cell wall of S. aureus. Next, ε‐PL can disrupt the cell wall peptidoglycan, causing fragility of the cell wall. The cell membrane is then disrupted by the ε‐PL, which further contributes to the disruption of the hydrophobic region and cell membrane curvature in both layers, resulting in increased permeability of the cell membrane. Lastly, ε‐PL gets inside the cells where it disrupts the main bacterial metabolism, thus, killing the cells. These mechanisms are shown in Figure 8. 79 Because polylysine destroys bacteria via membrane disruption (barrel stave and electroporation) and perturbation (charged lipid clustering) without affecting the cytoplasm, it is thought to be unlikely to produce any antimicrobial resistance even after repeated application. 80
FIGURE 8.
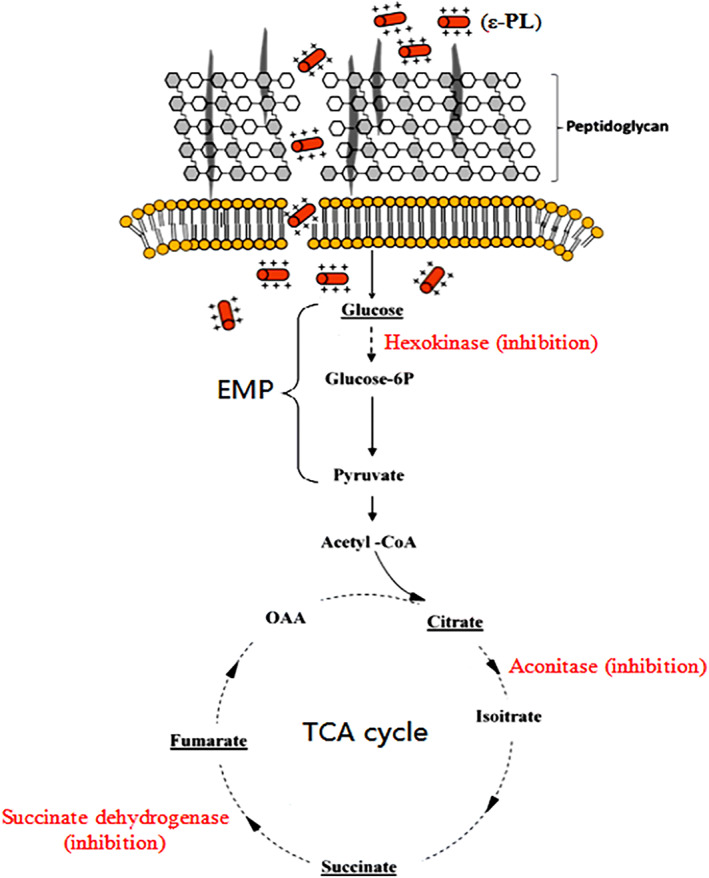
Proposed antibacterial mechanism of ε‐PL against Staphylococcus aureus. First, cationic ε‐PL attaches to negatively charged teichoic acid embedded in the peptidoglycan layers of the S. aureus cell wall. The peptidoglycan structure in the cell wall is destroyed by ε‐PL, leading to cell wall fragility. Next, the cell membrane is disturbed by ε‐PL, and this disturbance further induces the changes in hydrophobic region and bilayer curvature of cell membrane, causing increase of cell membrane permeability. Finally, ε‐PL enters into the cells and interrupts the primary metabolism of S. aureus; thus, killing the cells. Reproduced with kind permission from Reference [79]
4.3. Mycelial fungal cells such as Penicillium digitatum
Other have reports revealed that eukaryotic fungal cells (such as P. digitatum) could be inhibited by ε‐PL. 81 ε‐PL affected mycelial production, and the spore germination rate and germ tube duration were significantly inhibited. Moreover, ε‐PL could also affect the cell wall morphology in eukaryotic fungal cells, and disrupt their cell membrane. 82 These findings, therefore, showed that mycelial inhibition, spore development, and damage to the cell wall and membrane were all caused by ε‐PL and were linked to the death of eukaryotic fungal cells.
4.4. Yeast cells such as Saccharomyces cervisiae
It was observed that the fungicidal and fungistatic activities of ε‐PL were different against yeast species, as shown for S. cerevisiae cells. As shown in Figure 9, the potential mechanism has been suggested for the antifungal activity of ε‐PL, which binds to components of the cell membrane by electrostatic forces. If the concentration ε‐PL exceeds a threshold value, ε‐PL will rapidly become embedded in the S. cerevisiae cell membrane, following the mechanism of the carpet model, leading to the development of micelles, the folding of the phospholipid bilayer, and breaking open the cell membrane and eventually leading to cell death. However, a concentration of ε‐PL lower than the threshold level could still affect the structure, fluidity, and permeability of the cell membrane. The interference with central intracellular metabolism involving the carbon growth source could also contribute to the disruption of membrane function. Simultaneously, ε‐PL will also activate the intracellular generation of ROS and induce cell apoptosis. These cumulative steps caused by ε‐PL will contribute, in most cases, to inhibition of the growth of S. cerevisiae. 83
FIGURE 9.
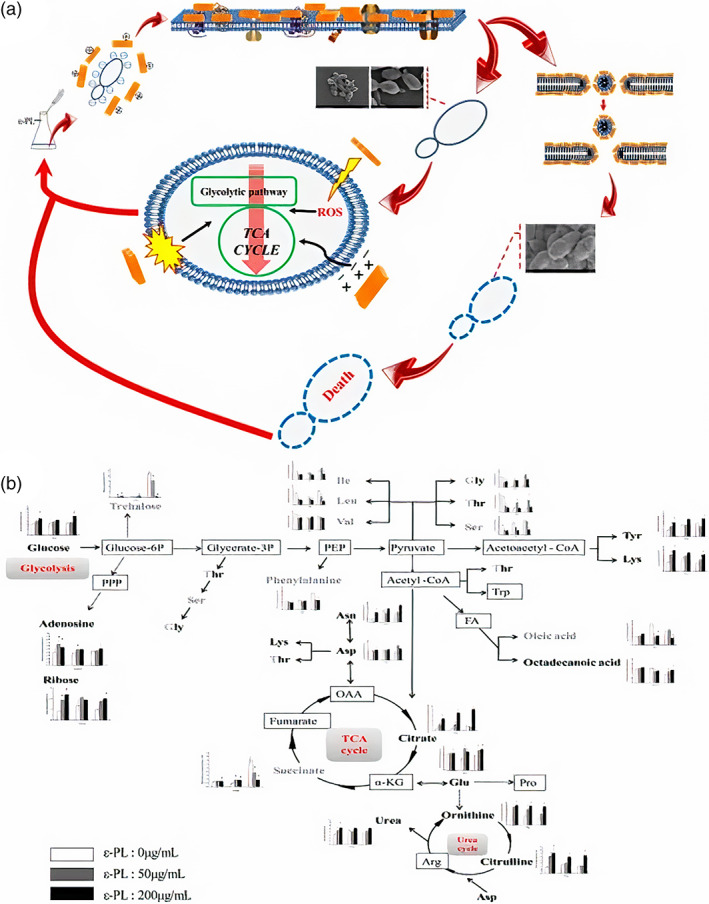
Antibacterial effects of ε‐PL versus Saccharomyces cerevisiae. (A) Mechanisms of action. (B) Variations in metabolite concentration at the sampling periods of 4, 8 and 12 h on the metabolic network. Reproduced with permission from Reference [83]
5. POLYLYSINE‐BASED SCAFFOLDS IN SKIN TISSUE ENGINEERING
Skin is the largest organ in the body, because it covers the entire body surface area (about 2 m2), and protects it against disease and infection. Various factors such as accidents or diseases can result in skin damage or dysfunction, resulting in vulnerability to further trauma, germs, toxins, and infectious parasites. 84 , 85 Wounds can be categorized into acute or chronic based on the length of time they have been present, and their likelihood to be repaired by normal skin regeneration. It is possible for acute injuries causing either superficial or deep wounds to repair completely within 21 days, and little to no scar tissue formation is usually observed. In contrast, chronic wounds usually develop when acute wounds fail to heal even after 3 months. Diabetic wounds, which are common in individuals suffering from diabetes, are considered to be chronic. A more complete classification of various wounds is represented in Figure 10. 86 The most important but challenging issue in wound treatment is to recover the tissue function with an acceptable aesthetic outcome in a short period of time. Large wounds especially in unhealthy condition such as diabetes, infectious disease and cancer can be very difficult to recover.
FIGURE 10.
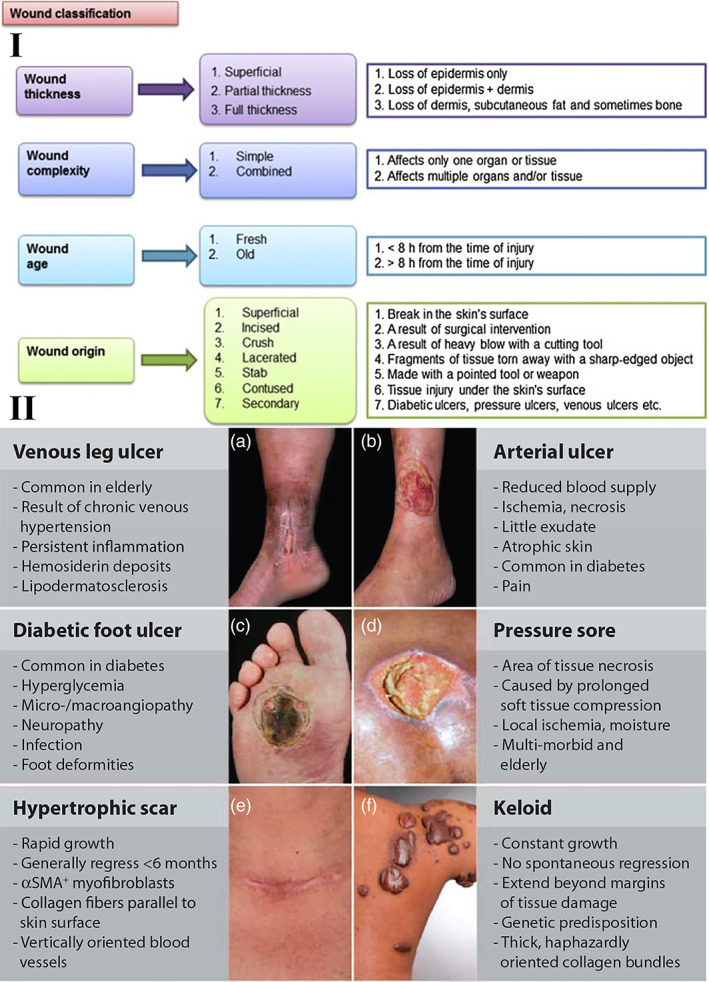
Wound classifications. I. Wound classification is based on thickness, complexity (simple or combined), age (fresh or old), and origin of occurrence. Reproduced with permission from Reference [86]. II. The most common wound healing pathologies are clinical features. A number of local and systematic factors leading to multiple wound‐healing pathologies may affect the repair process. (A) Medial aspect of the lower leg with venous leg ulcer (VLU). (B) Lateral aspect of the lower leg with an arterial ulcer. (C) Diabetic foot ulcer (DFU). (D) Pressure sore. (E) Hypertrophic scar after thyroid surgery. (F) Keloid. Reproduced with permission from Reference [87]
Inadequate wound healing or excessive scar formation can affect many people after surgery, major trauma, or disease. There is still an incomplete understanding of the cellular and molecular mechanisms underpinning tissue repair, the failure of wounds to heal, and current therapies are limited. Even in the present day, the poor rate of wound healing after surgery, trauma, chronic injuries, or severe diseases, impacts many people all around the world. Insufficient attention paid to the key factors involved in tissue regeneration, including, inflammatory effects, matrix deposition, adequate angiogenesis, and cell recruitment could explain some of the difficulties faced with adequate wound healing. Additional clinical conditions, such as advanced age, cardiovascular disease, or diabetes are often associated with poor wound healing, because of the disturbances they cause to normal cellular functions. A deep insight into the fundamental biology of tissue restoration and regeneration could be useful to identify new therapeutic approaches to enhance the body's natural wound healing ability. Figure 10 illustrates the intricacy of wound healing involving several kinds of cells. Normal healing of acute wounds takes place in several overlapping phases, including the initial inflammatory response, deposition of the matrix, cell proliferation, cell migration, and tissue remodeling (Figure 10). Nonhealing chronic wounds are the consequence of an interruption in or deregulation of one or more wound healing stages (Figure 11B). The first thing to happen is the rapid activation of the coagulation cascade to produce a fibrin clot, which the provides the essential matrix architecture for recruiting inflammatory and other cell types into the wound. Platelets immobilized within the fibrin clot secrete localized growth factors and chemokines into the wound vicinity in order to attract other cells. The importance of platelets for effective wound healing has been demonstrated by the therapeutic application of platelet‐rich plasma rich in preclinical studies of wound healing, 89 and the incidence of delayed healing in disorders related to platelets. 90 In addition to hemostasis, the early inflammatory responses (both local and systemic) activate wound healing responses (Figure 11A). Inflammation is more pronounce in deep and more severe injuries (Figure 11B), It is thought that the chronic inflammatory state of such injuries can fail to resolve, thus, producing chronic wounds. 91 More specifically, recent studies using molecular analysis of severely wounded tissue and wound fluid have shown a continuous competitive environment involving both pro‐inflammatory and anti‐inflammatory signals. 91 , 92 An increased number of neutrophils and macrophages are major components of the pro‐inflammatory cellular infiltrate that contributes to the delayed healing of chronic ulcers. 93 These cells secrete primary pro‐inflammatory cytokines, including IL‐1β and tumor necrosis factor‐α (TNFα), which have been shown to prolong the inflammatory process and thereby delay healing. 92 , 94 Increased IL‐1β and TNFα in chronic wounds have been shown to lead to an increase in metalloproteinase enzymes that cause excessive local degradation of the ECM, and therefore prevent the migration of other cells designed to heal the wound. 95 Recent studies have involved the inflammasome, a multiprotein complex produced by the innate immune system that continuously activates and secretes IL‐1β and IL‐18 inside chronic wounds. 96 , 97 Furthermore, the ongoing presence of a relatively high load of bacterial cells within chronic wounds (colonization) also continuously attracts pro‐inflammatory cells resulting in delayed wound healing (Figure 11). 87 A more complete insight into the mechanisms regulating the inflammatory response is required before better approaches to the healing of chronic wounds can be devised. 87
FIGURE 11.
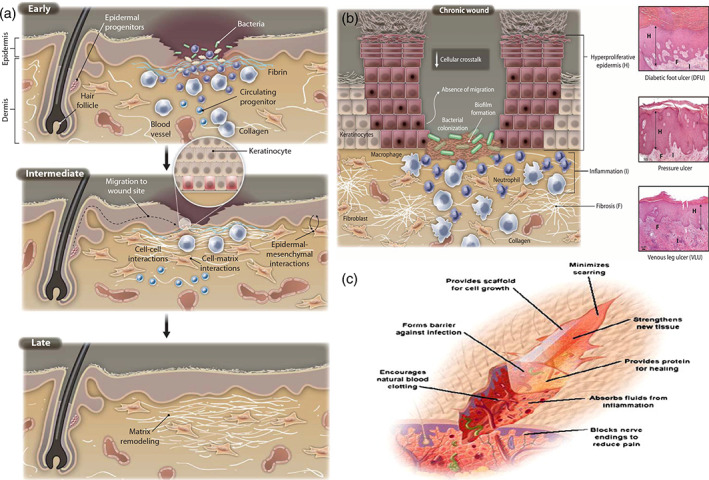
Molecular and cellular processes for the repair of normal skin. Molecular and cellular pathways for wound healing progression. The first step of wound healing comprises hemostasis and keratinocyte activation and inflammatory cells. The intermediate step includes keratinocytes, fibroblast proliferation, matrix deposition, along with angiogenesis. This spatiotemporal cycle is highly governed through several cell types that release various growth factors, cytokines, and chemokines to obtain wound closure and functional restoration. The chronic wound molecular pathology. Illustrations show the cellular and molecular mechanisms that are damaged in severe injuries. Although the increased inflammatory cells such as macrophages and neutrophils, they do not all work perfectly (validated using misshaped cells). Most fibroblasts mature into senescent ones. For chronic wounds, angiogenesis is decreased, the recruitment and activation of stem cells and the remodeling of ECM relative to the healing of injuries with the normal rate. Histology reflecting features of a diabetic foot ulcer (DFU), venous stasis ulcer (VLU), and bad sore. Such chronic wounds, though different etiology, have specific cellular characteristics depicted in (A): H, hyperproliferative epidermis; F, fibrosis, increased cellular inflammation. 87 (C) Schematic representation of the required properties of a wound dressing material. Reproduced with permission from Reference [88]
The ideal wound dressing should provide a suitable milieu for accelerating wound healing, minimizing the scar, increasing cellular activity (i.e., epithelial spreading, angiogenesis, and connective tissue synthesis). Wound dressings should also absorb wound exudate, maintain the proper moisture content and temperature within the wound (which also facilitates blood circulation and epithelial migration), exchange nutrients and oxygen, reduce pain, protect the wound against infection, and be nonadherent to the wound (i.e., easy to remove the wound dressing after healing). Moreover, dressings should encourage leucocyte migration, facilitate the activity of enzymes that can carry out debridement, and must also be sterile, nontoxic, and nonallergic. 84 Table 2 present the guidance for wound management and some synthetic commercial wound dressings available on the market are summarized in Table 3.
TABLE 2.
Wound management dressing guide
| Type of tissue in the wound | Goal | Dressing performance | Wound area preparation | Primary dressing | Secondary dressing | Ref. | |
|---|---|---|---|---|---|---|---|
| Necrotic, black, dry | Cutoff devitalized tissue, do not try debridement if vascular insufficiency suspected, remain dry and refer for vascular assessment | Hydration of wound bed, stimulate autolytic debridement | Surgical/mechanicaldebridement | Hydrogel | Film dressing | 98, 99 | |
| Sloughy, yellowish, brown, or black. Dry to low exudate | Eliminate slough, clean wound bed for granulation tissue | Rehydrate wound bed, adjust moisture balance, Stimulate autolytic debridement | Surgical/mechanical debridement | Hydrogel | Film dressing, low adherent (silicone) dressing | 100, 101 | |
| Sloughy, yellow, brown, black or gray Modest to high exudate | Eliminate slough, rinse wound bed for granulation tissue, controlling exudate | Absorb exudates, Protect peri‐wound skin to avoid maceration, Stimulate autolytic debridement | Surgical/mechanical debridement | Moisture absorbent dressing, For deep wounds, utilize cavity strips | film dressing | 102, 103 | |
| Granulating, clean, red Dry to low exudate | Boost granulation offer healthy wound bed for epithelialization | Maintain moisture balance Protect new tissue growth | Wound cleansing | Hydrogel, Low adherent (silicone) dressing, for deep wounds use cavity strips, rope or ribbon versions | Pad, avoid bandagesthat may cause occlusion and maceration. Tapes should be used with caution due to allergy potential and secondary complications | 104, 105, 106 | |
| Granulating, clean, red moderate to high exudate | Controlling the exudate, offer healthy wound bed for epithelialization | Maintain moisture balance, Protect new tissue growth | Wound cleansing consider barrier products | Absorbent dressing, low adherent (silicone) dressingFor deep wounds, utilize cavity strips | |||
| Epithelializing, red, pink, no to low exudate | Boost epithelialization and wound maturation | Protect new tissue growth | Hydrocolloid (thin), film dressing, low adherent (silicone) dressing | ||||
| Infected low to high exudate | Decrease bacterial load controlling the exudate controlling the odor | Antimicrobial performance moist wound healing odor absorption | Wound cleansing (consider antiseptic wound cleansing solution) consider barrier products | Antimicrobial dressing | |||
TABLE 3.
Synthetic commercially available wound dressings
| Wound dressing | Material | Function and Application | ‐Advantage | Disadvantage | Company/brand name | Ref. |
|---|---|---|---|---|---|---|
| Hydrocolloid | Bilayer structure inner layer: gelatin, pectin, carboxymethyl cellulose, elastomers outer layer: waterproof layer |
Maintains Moisture Low or medium exudative wound: pressure, diabetic wounds |
Could be removed without pain, maintains the wound moisture adheres to the dry and humid part | Not suitable for highexudation wounds. Useless for infection wound and necrotictissues | 3 M,Tegaderm, comfeel | 107, 108 |
| Hydrogel | Methacrylates, PVP, gelatin, pectin, chitosan | Donate moisture chronic dry wound (low humid), wound with necrotic tissue, mediumor deep wounds: low burning, pressure, and abrasion wounds | Provides a humid environment cools the wound and lowers pain. Useful for necrotic and infectious wounds | Dehydration, low absorbance | Aquaclear, purilon gel, hypergel sterigel | 109 |
| Alginate | Sodium/calcium alginate salts |
Absorb exudate High and medium exudative wound,deep wound: diabetic, pressure, abrasion, venues, burning type II |
Provides a humidenvironment, low allergic feature, useful for necrotic and infectious wounds | Dehydration, periodic control for deep wound | Algisite, algoderm, sorbsan | 110 |
| Foam | Silicone or PU foam | Absorb exudate high and medium exudative wound, diabetic, pressure, abrasion, venous, burning type II | Insulates heat and warms the wound, make pressure for venous ulcer | Allergic for delicate skin, useless for the dry and infectious wound | Allevyn, lyfoam, tegafoam | 111 |
| Semipermeable film | Transparent and semi‐permeable PU |
Maintains Moisture Used as a second layerfor hydrogels and foams, nonexudative wound: surgical wound |
Waterproof layer, permeable to oxygen, vapor. Barrier against infections | Useless for bloody/high exudative, necrotic, infectious wound | Tegaderm, hydrofim, polyskin | 112 |
| Antimicrobial dressings | Ionic silver, activated charcoal, Molecular iodine PHMB | Antibacterial | It can be used as different type of wound dressing such as Hydrofiber, Hydrocolloid and Gel or paste | It is not general dressing and based on wound the proper one should be selected | Smith & Nephew, Convatec, Coloplast Corp., | 113 |
The intrinsic properties of polylysine can play a role in the design of wound dressings. Polylysine has good biocompatibility together with pronounced antibacterial activity making it useful in wound dressings. Wang et al. synthesized a hydrogel derived from a combination of polyamino acids including ε‐polylysine and polyglutamic acid, and tested it as a wound dressing. This hydrogel had good biocompatibility, and showed antibacterial effects against both Gram‐positive and ‐negative bacteria, along with low inflammatory responses. 114 Zou et al. synthesized a hydrogel wound dressing based on ε‐polylysine and carboxymethyl chitosan, which exhibited inherent antibacterial activity along with tunable properties, such as the swelling ratio (varying from 800% to 2000%) and the Young's modulus (varying from 10 to 100 kPa). 80 Zhou et al. synthesized a photopolymerized hydrogel based on polylysine and methacrylamide, which exhibited good antibacterial and antifungal activity. Moreover, this hydrogel was also found to be non‐hemolytic and biocompatible. 115
The closure of surgical incisions and tissue flap reattachment using suturing or staples, can be limited by incomplete sealing, scar formation, and leaving the possibility of bacterial infection. Alternative methods of tissue closure, for example, fibrin glue or cyanoacrylate glue have been developed to seal and reattach tissue flaps. These adhesives benefit from several advantages over conventional methods, but there are also shortcomings, such as, inadequate adhesive properties, possibility of infection, and concerns about possible toxicity. A new medical adhesive was designed based on oxidized dextran combined with ε‐PL, which possessed low cytotoxicity and enhanced adhesive strength for sealing tissue. The adhesive is activated to become adherent by absorbing water from bodily fluids, and can seal surgical incisions completely. Experimental results revealed that the sealing performance of this adhesive was superior to fibrin glue. 116
Wang et al. synthesized an in‐situ gelling bioadhesive hydrogel based on ε‐polylysine for potentially infected wounds. Hydroxyphenylpropionic acid (HPA) was grafted onto an ε‐PL backbone and then cross‐linked using horseradish peroxidase (HRP) and hydrogen peroxide (H2O2). It was reported that the adhesive strength of the bioadhesive hydrogel (10–35 kPa) was higher than that of fibrin glue. Moreover, this platform showed good biocompatibility and antibacterial activity against both Gram‐positive and ‐negative bacteria. In‐vivo studies showed that the platform possessed an anti‐infective properties. 117 Self‐healing properties were introduced into the hydrogel by adding plasma amine oxidase to the ε‐PL, because of the formation of Schiff base reaction products. 118
As mentioned above, ε‐PL attaches to the bacterial surface destroying the cell membrane and causing cell lysis, resulting in cell death. SEM images were used to investigate the antimicrobial mechanism of ε‐PL, by visualizing morphological changes occurring during cell‐ ε‐PL interactions. As shown in Figure 12, rodlike (E. coli) and round (S. aureus) cellular morphology was preserved when the bacteria were cultured on a control hydrogel. By contrast, a disrupted and withered surface was observed for bacteria cultured on EHPA hydrogels. 117 S. aureus is a well‐known species that contributes to many wound infections. This bacterial species was embedded in EHPA hydrogels, which were then subcutaneously implanted in a mouse model to assess its potential as an antimicrobial hydrogel in clinical application. Sterile saline, noncontaminated EHPA hydrogels, S. aureus bacteria alone, and bacteria loaded‐EHPA hydrogels were all injected into groups of mice to evaluate the effects of the hydrogel on infection prevention and treatment. Three days after injection with bacteria alone, the positive control mice were close to death. The animals were divided into two groups; one group was sacrificed and dissected to observe the infected tissue. As shown in Figure 12, in the mice injected with bacteria alone, an abscess was observed. On the other hand, in the mice injected with bacteria loaded‐EHPA hydrogels, no infection was observed. In the sterile saline‐injected mice, the tissue morphology was similar to control mice. No pathological signs were observed for sterile hydrogel‐injected mice, showing the biocompatibility of the hydrogel after 7 days. Hematoxylin and eosin (H&E) staining showed widespread bacterial infection that could be prevented by the EHPA hydrogel (see Figure 12). Furthermore, an inflammatory response was also observed in tissue infected with bacteria alone, with numerous inflammatory cells migrating to the injection site. However, injection of the bacteria‐loaded EHPA hydrogel, sterile EHPA hydrogel, or sterile saline resulted in no inflammation (Figure 12). 117
FIGURE 12.
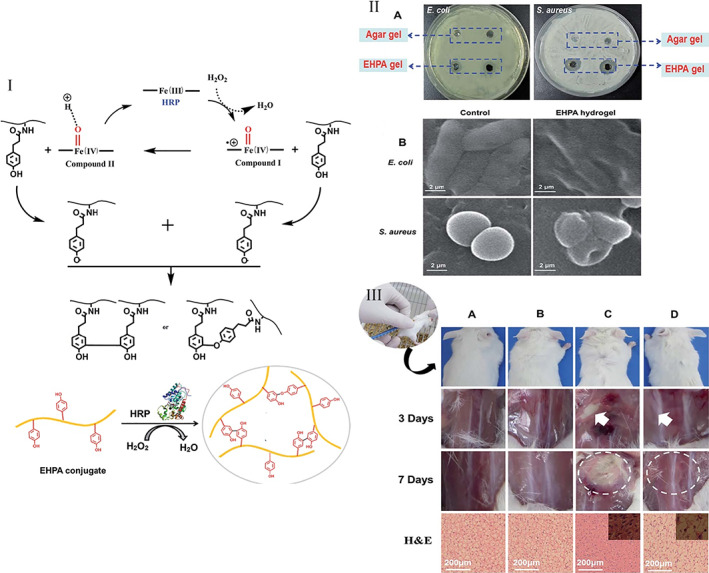
Antimicrobial effects of EHPA hydrogel. I. The proposed EHPA hydrogel‐forming mechanism through the oxidation reaction mediated by enzymes. II. Antibacterial activities against pathogenic Gram‐positive (Staphylococcus aureus) and ‐negative (Escherichia coli) bacteria by EHPA hydrogels (A) Antimicrobial ability of EHPA on agar through inhibition zones after 24 h. (B) SEM images of control sample of bacteria and after incubation in EHPA hydrogels. III. In vivo study. Images of the mice administered subcutaneously after 72 h and a week (n 1/4 3) with 10(8) CFU ml−1 S. aureus and histological analysis of inflammatory response to S. aureus, or S. aureus hydrogel, after a week (n 1/4 3). (A) Untreated (control), (B) treated with hydrogel, (C) treated with S. aureus and (D) hydrogel S. aureus‐treated groups. Hydrogel: 10 wt% EHPA crosslinked with HRP (0.05 mg ml−1) and H2O2 (0.06 wt%). Reproduced with permission from Reference [117]
6. POLYLYSINE FOR THERAPY OF SKIN DISEASE
The design and preparation of systems for the delivery of nucleic acids and therapeutic genes has gained attracted interest in recent years. Gene therapy strategies can be used for the treatment of many diseases, such as cancer, inherited disorders, viral infections, and wound management, especially in diabetic patients. Non‐viral gene delivery systems (nVGDS) can be used in gene therapy, where gene expression is promoted in fibroblasts to improve skin wound healing. 119
Various virus‐based vectors (e.g., retroviruses, adenoviruses, and adeno‐associated viruses) and also nonviral vectors (e.g., lipoplexes, polyplexes, and plasmid DNA) have been tested to deliver genetic material to target cells. 120 nVGDS have attracted much interest because they do not suffer from the safety issues related to viral vectors. nVGDS can enable the efficient transfer of genes to the intracellular compartment of cells. nVGDS based on polycations (e.g., polyethyleneimine [PEI]) which benefit from high transfection efficiency and low toxicity, are effective gene delivery platforms both in vitro and in vivo. However, ex vivo gene therapy (i.e., extraction of cells, genetic modification, and re‐transplantation of the modified cells) might benefit from the fact that the cell culture step gives better control of the whole process. Furthermore, exposure of the body to nVGDS is significantly reduced, resulting less risk of undesirable side effects. The transfer of foreign DNA into the cells, involves a multistep process essentially relying on endocytosis. 121 An endocytosis inhibitor brefeldin reduced DNA delivery mediated by many cargo‐vehicles, such as, PEI, adenovirus, and Lipofectamine‐2000. 92 All the non‐viral carriers are designed to package exogenous DNA (EDNA) or nucleic acids into a biocompatible complex which can easily be transported and adsorbed into the cells. After endocytosis into the cells, the EDNA will be contained inside endosomes and lysosomes, because these organelles are designed to break down foreign substances and microorganisms. The problem is then how to transfer these nucleic acids into the cytosol, and then into the nucleus without being degraded by hydrolytic enzymes. 122 Nuclear pores can allow certain nucleic acid sequences to penetrate into the nucleus. 123 However, the DNase II enzyme present in the extracellular space and in the cytoplasm is able to degrade DNA. The success of gene therapy using non‐viral vectors depends on their ability to protect the nucleic acids against degradation by nuclease enzymes, as well as to allow cell uptake. 124
Abbasi et al. conjugated palmitic acid (PA) to PLL to form a vehicle to transfer a plasmid DNA coding for enhanced green fluorescent protein (pEGFP) into skin fibroblasts. PLL‐PA was found to give the highest number of EGFP positive cells. EGFP is a frequently used reporter gene to measure the transfer of plasmids (pEGFP) for skin tissue regeneration. In addition, the relative efficiencies of various lipid‐substituted poly‐l‐lysines were studied. According to their results, the resistance to dissociation after heparin treatment was related to the degree of lipid substitution in the polymer composite. Lipid‐substituted PLLs exhibited the best complex formation with pEGFP. These polymers also showed good protection of pEGFP from in vitro digestion by DNase I and DNase II. Upon delivery by lipid substituted polymers, the intracellular pEGFP expression was stable for up to 7 days. Additional investigation using flow cytometry showed that modification of PLLs with stearic and myristic acid allowed successful protein expression, and myristic acid showed the lowest toxicity. They found that compared to pristine lipid alone, lipid conjugated PLL led to more efficient gene delivery. 125
Impaired angiogenesis is a significant clinical concern that might explain delayed wound healing, particularly in diabetes patients. One strategy to accelerate wound healing in diabetes patients, used an approach which could enhance angiogenesis. There were two peptides involved: (1) the TG peptide was covalently linked to the fibrin network for sustained release; (2) a polyR peptide was used to enhance the cellular uptake of these nanocondensates. To induce angiogenesis, both modified and unmodified polymers were condensed with plasmid‐DNA in vitro. HIF‐1α is an oxygen‐sensitive transcription factor that contains a domain for oxygen‐dependent degradation (ODD), and HIF‐1α is naturally degraded under normoxic conditions. The temporary‐induced expression of the angiogenesis‐linked genes Acta2, Pecam1, along with HIF‐1α and VEGF was observed after PLL‐g‐PEG polymer‐mediated DNA transfer of a HIF‐1α − ΔODD plasmid. In addition, the delivery of the modified HIF‐1α gene improved wound healing and increased the number of endothelial and smooth muscle cell precursors making more mature blood vessels. 126
Erlach et al. synthesized poly(2‐methyl‐2‐oxazoline)‐grafted poly(l‐lysine) particles as a nonviral delivery vehicle for therapeutic DNA (TDNA) for improved wound healing. The polyplexes were soluble in serum and after heating to 70°C, and they preserved the condensed DNA from digestion by DNase‐I. The efficacy of DNA‐PMOXA‐g‐PLL DNA transfection was strongly dependent upon the number of PMOXA molecules grafted onto the polymer chains; a low grafting density between 7% and 14% and a medium N/P ratio (3.125–6.25) was found to be the best. As an alternative to PLL‐g‐PEG‐DNA polyplexes formed from PLL20‐g7PMOXA4 may be promising for the transfection of TDNA. 127
7. TRANSLATIONAL SUCCESS AND CHALLENGES
Translational research tries to generate meaningful, applicable outcomes that advance human health and translate fundamental science findings more efficiently into practical usages. It is worth mentioning that healthcare is an important subject in translational research, particularly in using advanced materials. Different type of advanced biomaterials have been used as tissue replacements, regenerating scaffolds, drug carriers, and releasing vehicles known as a translational approach. Among these biomaterials, Polylysine can be engineered in different structures to be used in tissue engineering and as a drug/gene carrier. It is reported that polylysine/CAG‐peptide is used for the clinical treatment of cardiovascular diseases. 128 Moreover, polylysine/chitosan is proposed as a proper substrate for clinical usage as an anti‐hemorrhaging hydrogel. 129 However, it is reported that the polylysine show toxicity in some cases, and therefore it should be controlled for the desired application, which is known as a translation challenge. 130 It can be deduced that the successful translational of polylysine is a subtle balance between simplicity and complexity. Hence, the polylysine‐based products should be designed based on final application to achieve the proper results.
8. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Polylysine can be used as a biomaterial to improve wound healing and skin regeneration due to its beneficial features. Several promising results have been obtained based on the preclinical and clinical experiments made in the past years. Herein, we have summarized several instances of polylysine and its composites being used as antimicrobial materials to assist wound healing and act as a gene delivery vector to treat skin diseases. Polylysine could be applied in different formulations: scaffolds, gels, dressings/films, or nanoparticles because of its stimuli‐responsive performance, biocompatibility, and FDA approval (Figure 13).
FIGURE 13.
Future perspective and desired performance of the polylysine. Reprinted with permission from Reference [131]
Suppose everything goes well with polylysine usage in wound management; in that case, it is expected that the polylysine scaffold shows proper antibacterial effect along with proper cell adhesion and proliferation, which causes rapid wound healing. Moreover, as a gene delivery vehicle, polylysine is one of the most preferred carriers for gene delivery which can be tailored to play a suitable role in gene therapy. Despite some promising results with formulations based on polylysine obtained in skin regeneration and wound healing, several challenges remain. For example, preparation methods and characterization techniques should be optimized and standardized. Adjusting the suitable surface properties, design, and pore size required by cells to proliferate and principally to differentiate with the right phenotype is also another challenge. Drug delivery applications should be tailored to biological mechanisms involved in wound healing, and thus, considering the essential role of proper vascularization is another challenge in this realm. Moreover, gene transfer applications should be further investigated to better control the proton sponge effect for lysosomal escape.
CONFLICT OF INTEREST
Michael R. Hamblin declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; Hologenix Inc. Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc, Minneapolis‐St. Paul MN; USHIO Corp, Japan; Sanofi‐Aventis Deutschland GmbH, Frankfurt am Main, Germany.
AUTHOR CONTRIBUTIONS
Payam Zarrintaj: Conceptualization (lead); investigation (equal); project administration (equal); writing – original draft (lead); writing – review and editing (equal). Sadegh Ghorbani: Data curation (equal); investigation (supporting); writing – original draft (supporting). Mahmood Barani: Data curation (supporting); writing – original draft (supporting); writing – review and editing (supporting). Narendra Pal Singh Chauhan: Writing – original draft (supporting); writing – review and editing (supporting). Mohsen Khodadadi Yazdi: Writing – original draft (equal). Mohammad Reza Saeb: Conceptualization (equal); writing – review and editing (supporting). Joshua Ramsey: Data curation (supporting); writing – review and editing (supporting). Michael Hamblin: Conceptualization (equal); data curation (supporting); supervision (equal); writing ‐ review & editing (supporting). Masoud Mozafari: Conceptualization (equal); data curation (equal); supervision (equal); writing ‐ review & editing (equal). Ebrahim Mostafavi: Conceptualization (equal); funding acquisition (lead); supervision (equal); writing – review and editing (equal).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10261.
ACKNOWLEDGEMENTS
Michael R. Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700. Ebrahim Mostafavi would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035).
Zarrintaj P, Ghorbani S, Barani M, et al. Polylysine for skin regeneration: A review of recent advances and future perspectives. Bioeng Transl Med. 2022;7(1):e10261. doi: 10.1002/btm2.10261
Contributor Information
Michael R. Hamblin, Email: hamblin.lab@gmail.com.
Masoud Mozafari, Email: mozafari.masoud@gmail.com.
Ebrahim Mostafavi, Email: ebimsv@stanford.edu.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nature Protocols. 2016;11(10):1775. [DOI] [PubMed] [Google Scholar]
- 2. Seidi F, Yazdi MK, Jouyandeh M, et al. Crystalline polysaccharides: a review. 2021;275:118624. [DOI] [PubMed] [Google Scholar]
- 3. Zarrintaj P, Khodadadi Yazdi M, Youssefi Azarfam M, et al. Injectable cell‐laden hydrogels for tissue engineering: recent advances and future opportunities. 2021;27(11–12):821‐843. [DOI] [PubMed] [Google Scholar]
- 4. Vatankhah‐Varnosfaderani M, WFM D, Everhart MH, et al. Mimicking biological stress–strain behaviour with synthetic elastomers. Nature. 2017;549(7673):497. [DOI] [PubMed] [Google Scholar]
- 5. Farokhi M, Mottaghitalab F, Fatahi Y, et al. Silk fibroin scaffolds for common cartilage injuries: possibilities for future clinical applications. Eur Polym J. 2019;115:251‐267. [Google Scholar]
- 6. Mohebbi S, Nezhad MN, Zarrintaj P, et al. Chitosan in biomedical engineering: a critical review. Curr Stem Cell Res Ther. 2019;14(2):93‐116. [DOI] [PubMed] [Google Scholar]
- 7. Seidi F, Khodadadi Yazdi M, Jouyandeh M, et al. Chitosan‐based blends for biomedical applications. Int J Biol Macromol. 2021;183:1818‐1850. [DOI] [PubMed] [Google Scholar]
- 8. Bagher Z, Atoufi Z, Alizadeh R, et al. Conductive hydrogel based on chitosan‐aniline pentamer/gelatin/agarose significantly promoted motor neuron‐like cells differentiation of human olfactory ecto‐mesenchymal stem cells. Mater Sci Eng C. 2019;101:243‐253. [DOI] [PubMed] [Google Scholar]
- 9. Bagher Z, Alizadeh R, Farhadi M, et al. Conductive hydrogel based on chitosan‐aniline pentamer/gelatin/agarose significantly promoted motor neuron‐like cells differentiation of human olfactory ecto‐mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;101:243‐253. [DOI] [PubMed] [Google Scholar]
- 10. Zarrintaj P, Manouchehri S, Ahmadi Z, et al. Agarose‐based biomaterials for tissue engineering. Carbohydr Polym. 2018;187:66‐84. [DOI] [PubMed] [Google Scholar]
- 11. Yazdi MK, Taghizadeh A, Taghizadeh M, et al. Agarose‐based biomaterials for advanced drug delivery. J Control Release. 2020;326:523‐543. [DOI] [PubMed] [Google Scholar]
- 12. Calejo MT, Hasirci N, Bagherifam S, Lund R, Nyström B. Stimuli‐responsive structures from cationic polymers for biomedical applications. Cationic Polymers in Regenerative Medicine: Royal Society of Chemistry. 2014;13:149‐177. [Google Scholar]
- 13. Zarrintaj P, Ramseya JD, Samadib A, et al. Poloxamer: a versatile tri‐block copolymer for biomedical applications. 2020;110:37‐67. [DOI] [PubMed] [Google Scholar]
- 14. Bagheri B, Zarrintaj P, Samadi A, et al. Tissue engineering with electrospun electro‐responsive chitosan‐aniline oligomer/polyvinyl alcohol. 2020;147:160‐169. [DOI] [PubMed] [Google Scholar]
- 15. Youssefi Azarfam M, Nasirinezhad M, Naeim H, Zarrintaj P, Saeb MJJOCS. A green composite based on gelatin/agarose/zeolite as a potential scaffold for tissue engineering applications. 2021;5(5):125. [Google Scholar]
- 16. Chundawat NS, Pathan S, Singh GP, Deuri AS, Zarrintaj P, Chauhan NPSJCP. Synthesis and characterization of chitosan pyridyl imine palladium (CPIP) complex as green catalyst for organic transformations. 2021;75(6):2835‐2850. [Google Scholar]
- 17. Hajifathaliha F, Mahboubi A, Mohit E, Bolourchian N, Khalaj V, Nematollahi LJAPB. Comparison of linear poly ethylene imine (LPEI) and poly L‐lysine (PLL) in fabrication of CHOK1 cell‐loaded multilayer alginate microcapsules. 2020;10(2):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erfani A, Zarrintaj P, Seaberg J, Ramsey JD, Aichele CPJJOAPS. Zwitterionic poly (carboxybetaine) microgels for enzyme (chymotrypsin) covalent immobilization with extended stability and activity. 2021;138(22):50545. [Google Scholar]
- 19. Erfani A, Hanna A, Zarrintaj P, et al. Biodegradable zwitterionic poly (carboxybetaine) microgel for sustained delivery of antibodies with extended stability and preserved function. Soft Matter. 2021;17:5349‐5361. [DOI] [PubMed] [Google Scholar]
- 20. Antila HS, Härkönen M, Sammalkorpi M. Chemistry specificity of DNA–polycation complex salt response: a simulation study of DNA, polylysine and polyethyleneimine. Phys Chem Chem Phys. 2015;17(7):5279‐5289. [DOI] [PubMed] [Google Scholar]
- 21. Niepel MS, Ekambaram BK, Schmelzer CE, Groth T. Polyelectrolyte multilayers of poly (l‐lysine) and hyaluronic acid on nanostructured surfaces affect stem cell response. Nanoscale. 2019;11(6):2878‐2891. [DOI] [PubMed] [Google Scholar]
- 22. Samal SK, Dubruel P. Cationic Polymers in Regenerative Medicine. Royal Society of Chemistry; 2014. [Google Scholar]
- 23. Nourbakhsh M, Zarrintaj P, Jafari SH, et al. Fabricating an electroactive injectable hydrogel based on pluronic‐chitosan/aniline‐pentamer containing angiogenic factor for functional repair of the hippocampus ischemia rat model. Mater Sci Eng C. 2020;117:111328. [DOI] [PubMed] [Google Scholar]
- 24. Mirtič A, Grdadolnik J. The structure of poly‐L‐lysine in different solvents. Biophys Chem. 2013;175:47‐53. [DOI] [PubMed] [Google Scholar]
- 25. Adamczyk Z, Morga M, Kosior D, Batys P. Conformations of poly‐l‐lysine molecules in electrolyte solutions: modeling and experimental measurements. J Phys Chem C. 2018;122(40):23180‐23190. [Google Scholar]
- 26. Kricheldorf HR. Polypeptides and 100 years of chemistry of α‐amino acid N‐carboxyanhydrides. Angew Chem Int Ed. 2006;45(35):5752‐5784. [DOI] [PubMed] [Google Scholar]
- 27. Cheng J, Deming TJ. Synthesis of polypeptides by ring‐opening polymerization of α‐amino acid N‐carboxyanhydrides. Peptide‐Based Materials. Springer; 2011:1‐26. [DOI] [PubMed] [Google Scholar]
- 28. Song Z, Fu H, Wang J, et al. Synthesis of polypeptides via bioinspired polymerization of in situ purified N‐carboxyanhydrides. Proc Natl Acad Sci. 2019;116(22):10658‐10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Habraken GJ, Wilsens KH, Koning CE, Heise A. Optimization of N‐carboxyanhydride (NCA) polymerization by variation of reaction temperature and pressure. Polymer Chem. 2011;2(6):1322‐1330. [Google Scholar]
- 30. Bakhshandeh B, Zarrintaj P, Oftadeh MO, et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genetic Eng Rev. 2017;33(2):144‐172. [DOI] [PubMed] [Google Scholar]
- 31. Grinnell F. Cellular adhesiveness and extracellular substrata. International Review of Cytology. 1978;53:65‐144. [DOI] [PubMed] [Google Scholar]
- 32. Chen L, Yan C, Zheng Z. Functional polymer surfaces for controlling cell behaviors. Mater Today. 2018;21(1):38‐59. [Google Scholar]
- 33. Lih E, Oh SH, Joung YK, Lee JH, Han DK. Polymers for cell/tissue anti‐adhesion. Prog Polym Sci. 2015;44:28‐61. [Google Scholar]
- 34. Miyamoto S, Teramoto H, Coso OA, et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131(3):791‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park S, Kim J, Yoon S‐H. A review on quantitative measurement of cell adhesion strength. J Nanosci Nanotechnol. 2016;16(5):4256‐4273. [DOI] [PubMed] [Google Scholar]
- 36. Zhang H, Gu Q, Wallace GG, Higgins MJ. Effect of electrochemical oxidation and reduction on cell de‐adhesion at the conducting polymer–live cell interface as revealed by single cell force spectroscopy. Biointerphases. 2018;13(4):041004. [DOI] [PubMed] [Google Scholar]
- 37. Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975;66(1):198‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh A, Kasinath BS, Lewis E. Interaction of polycations with cell‐surface negative charges of epithelial cells. Biochimica et Biophysica Acta. 1992;1120(3):337‐342. [DOI] [PubMed] [Google Scholar]
- 39. Heo JS, Kim HO, Song SY, Lew DH, Choi Y, Kim S. Poly‐L‐lysine prevents senescence and augments growth in culturing mesenchymal stem cells ex vivo. BioMed Res Int. 2016;2016:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng S, Guan Y, Yu H, Huang G, Zheng C. Poly‐l‐lysine‐coated PLGA/poly (amino acid)‐modified hydroxyapatite porous scaffolds as efficient tissue engineering scaffolds for cell adhesion, proliferation, and differentiation. New J Chem. 2019;43(25):9989‐10002. [Google Scholar]
- 41. Quirk RA, Chan WC, Davies MC, Tendler SJ, Shakesheff KM. Poly (l‐lysine)–GRGDS as a biomimetic surface modifier for poly (lactic acid). Biomaterials. 2001;22(8):865‐872. [DOI] [PubMed] [Google Scholar]
- 42. Varani J, Inman DR, Fligiel SE, Hillegas WJ. Use of recombinant and synthetic peptides as attachment factors for cells on microcarriers. Cytotechnology. 1993;13(2):89‐98. [DOI] [PubMed] [Google Scholar]
- 43. Sakai Y, Sakoda A, Suzuki M. Attachment kinetics of animal cells immediately after contact onto specific and/or non‐specific surfaces. J Chem Eng Japan. 1995;28(5):590‐595. [Google Scholar]
- 44. Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. 2018;10(2):025003. [DOI] [PubMed] [Google Scholar]
- 45. Ohara PT, Buck RC. Contact guidance in vitro: a light, transmission, and scanning electron microscopic study. Exp Cell Res. 1979;121(2):235‐249. [DOI] [PubMed] [Google Scholar]
- 46. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor‐stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han W, Chen S, Yuan W, et al. Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci. 2016;113(40):11208‐11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil Cytoskeleton. 1999;42(4):331‐343. [DOI] [PubMed] [Google Scholar]
- 49. Lehnert D, Wehrle‐Haller B, David C, et al. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J Cell Sci. 2004;117(1):41‐52. [DOI] [PubMed] [Google Scholar]
- 50. Bhatia S, Balis U, Yarmush M, Toner M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883‐1900. [DOI] [PubMed] [Google Scholar]
- 51. Bhatia S, Toner M, Tompkins R, Yarmush M. Selective adhesion of hepatocytes on patterned surfaces a. Ann N Y Acad Sci. 1994;745(1):187‐209. [DOI] [PubMed] [Google Scholar]
- 52. Ostuni E, Kane R, Chen CS, Ingber DE, Whitesides GM. Patterning mammalian cells using elastomeric membranes. Langmuir. 2000;16(20):7811‐7819. [Google Scholar]
- 53. Takayama S, McDonald JC, Ostuni E, et al. Patterning cells and their environments using multiple laminar fluid flows in capillary networks. Proc Natl Acad Sci. 1999;96(10):5545‐5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rajan SAP, Hambright P, Burke RC, Hall AR. Microfluidics in cell and tissue studies. Tumor Organoids. Springer; 2018:149‐170. [Google Scholar]
- 55. Chiu DT, Jeon NL, Huang S, et al. Patterned deposition of cells and proteins onto surfaces by using three‐dimensional microfluidic systems. Proc Natl Acad Sci. 2000;97(6):2408‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zarrintaj P, Jouyandeh M, Ganjali MR, et al. Thermo‐sensitive polymers in medicine: a review. Eur Polymer J. 2019;117:402‐423. [Google Scholar]
- 57. Zarrintaj P, Bakhshandeh B, Saeb MR, et al. Oligoaniline‐based conductive biomaterials for tissue engineering. 2018;72:16‐34. [DOI] [PubMed] [Google Scholar]
- 58. Vázquez E, Dewitt DM, Hammond PT, Lynn DM. Construction of hydrolytically‐degradable thin films via layer‐by‐layer deposition of degradable polyelectrolytes. J Am Chem Soc. 2002;124(47):13992‐13993. [DOI] [PubMed] [Google Scholar]
- 59. Khademhosseini A, Suh KY, Yang JM, et al. Layer‐by‐layer deposition of hyaluronic acid and poly‐L‐lysine for patterned cell co‐cultures. Biomaterials. 2004;25(17):3583‐3592. [DOI] [PubMed] [Google Scholar]
- 60. Niepel MS, Almouhanna F, Ekambaram BK, Menzel M, Heilmann A, Groth T. Cross‐linking multilayers of poly‐l‐lysine and hyaluronic acid: effect on mesenchymal stem cell behavior. Int J Artif Organs. 2018;41(4):223‐235. [DOI] [PubMed] [Google Scholar]
- 61. Richert L, Arntz Y, Schaaf P, Voegel J‐C, Picart C. pH dependent growth of poly (L‐lysine)/poly (L‐glutamic) acid multilayer films and their cell adhesion properties. Surf Sci. 2004;570(1–2):13‐29. [Google Scholar]
- 62. Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol. 2011;90(2–3):157‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death & Differentiation. 2016;23(7):1128‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483‐495. [DOI] [PubMed] [Google Scholar]
- 65. Kolodziejek AM, Schnider DR, Rohde HN, et al. Outer membrane protein X (ail) contributes to Yersinia pestis virulence in pneumonic plague and its activity is dependent on the lipopolysaccharide core length. 2010;78(12):5233‐5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walker SL, Redman JA, Elimelech M. Role of cell surface lipopolysaccharides in escherichia c oli k12 adhesion and transport. Langmuir. 2004;20(18):7736‐7746. [DOI] [PubMed] [Google Scholar]
- 67. Burks GA, Velegol SB, Paramonova E, Lindenmuth BE, Feick JD, Logan BE. Macroscopic and nanoscale measurements of the adhesion of bacteria with varying outer layer surface composition. Langmuir. 2003;19(6):2366‐2371. [Google Scholar]
- 68. Chao Y, Zhang T. Probing roles of lipopolysaccharide, type 1 fimbria, and colanic acid in the attachment of Escherichia coli strains on inert surfaces. Langmuir. 2011;27(18):11545‐11553. [DOI] [PubMed] [Google Scholar]
- 69. Hyldgaard M, Mygind T, Vad BS, Stenvang M, Otzen DE, Meyer RL. The antimicrobial mechanism of action of epsilon‐poly‐l‐lysine. Appl Environ Microbiol. 2014;80(24):7758‐7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gottesman S, Trisler P, Torres‐Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K‐12: characterization of three regulatory genes. J Bacteriol. 1985;162(3):1111‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27‐55. [DOI] [PubMed] [Google Scholar]
- 72. Melo MN, Ferre R, Castanho MA. Antimicrobial peptides: linking partition, activity and high membrane‐bound concentrations. Nature Reviews Microbiology. 2009;7(3):245. [DOI] [PubMed] [Google Scholar]
- 73. Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem. 2005;280(40):33960‐33967. [DOI] [PubMed] [Google Scholar]
- 74. Sengupta D, Leontiadou H, Mark AE, Marrink S‐J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochimica et Biophysica Acta (BBA)‐Biomembranes. 2008;1778(10):2308‐2317. [DOI] [PubMed] [Google Scholar]
- 75. Teixeira V, Feio MJ, Bastos M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res. 2012;51(2):149‐177. [DOI] [PubMed] [Google Scholar]
- 76. Vad BS, Bertelsen K, Johansen CH, et al. Pardaxin permeabilizes vesicles more efficiently by pore formation than by disruption. Biophys J. 2010;98(4):576‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. DelCarlo M, Loeser RF. Chondrocyte cell death mediated by reactive oxygen species‐dependent activation of PKC‐βI. Am J Physiol‐Cell Physiol. 2006;290(3):C802‐C811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ye R, Xu H, Wan C, et al. Antibacterial activity and mechanism of action of ε‐poly‐L‐lysine. Biochem Biophys Res Commun. 2013;439(1):148‐153. [DOI] [PubMed] [Google Scholar]
- 79. Tan Z, Shi Y, Xing B, Hou Y, Cui J, Jia S. The antimicrobial effects and mechanism of ε‐poly‐lysine against Staphylococcus aureus. Bioresour Bioprocess. 2019;6(1):11. [Google Scholar]
- 80. Zou Y‐J, He S‐S, Du J‐ZJCJOPS. ε‐poly (L‐lysine)‐based hydrogels with fast‐acting and prolonged antibacterial activities. 2018;36(11):1239‐1250. [Google Scholar]
- 81. Liu K, Zhou X, Fu M. Inhibiting effects of epsilon‐poly‐lysine (ε‐PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol Technol. 2017;123:94‐101. [Google Scholar]
- 82. Wei M, Ge Y, Li C, et al. Antifungal activity of ε‐poly‐L‐lysine on Trichothecium roseum in vitro and its mechanisms. Physiol Molecular Plant Pathol. 2018;103:23‐27. [Google Scholar]
- 83. Bo T, Liu M, Zhong C, et al. Metabolomic analysis of antimicrobial mechanisms of ε‐poly‐l‐lysine on Saccharomyces cerevisiae. J Agric Food Chem. 2014;62(19):4454‐4465. [DOI] [PubMed] [Google Scholar]
- 84. Zarrintaj P, Moghaddam AS, Manouchehri S, et al. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine. 2017;12(19):2403‐2422. [DOI] [PubMed] [Google Scholar]
- 85. Hashemi S, Amirabad LM, Nazhvani FD, et al. Bilayer scaffolds for Interface tissue engineering and regenerative medicine: a systematic reviews. Adv Exp Med Biol. 2021;1‐31. [DOI] [PubMed] [Google Scholar]
- 86. Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm. 2015;97:417‐426. [DOI] [PubMed] [Google Scholar]
- 87. Eming SA, Martin P, Tomic‐Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine. 2014;6(265):265sr6‐265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jayakumar R, Prabaharan M, Kumar PS, Nair S, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322‐337. [DOI] [PubMed] [Google Scholar]
- 89. Brick N. Autologous platelet‐rich plasma for treating chronic wounds. Am J Nursing. 2013;113(8):54. [DOI] [PubMed] [Google Scholar]
- 90. Deppermann C, Cherpokova D, Nurden P, et al. Gray platelet syndrome and defective thrombo‐inflammation in Nbeal2‐deficient mice. J Clin Invest. 2013;123(8):3331‐3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20(5):517‐527. [DOI] [PubMed] [Google Scholar]
- 92. Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49(4):1013‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank SJJOID. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245‐253. [DOI] [PubMed] [Google Scholar]
- 94. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585‐601. [DOI] [PubMed] [Google Scholar]
- 95. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321‐325. [DOI] [PubMed] [Google Scholar]
- 96. Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer H‐D. The inflammasome mediates UVB‐induced activation and secretion of interleukin‐1β by keratinocytes. Curr Biol. 2007;17(13):1140‐1145. [DOI] [PubMed] [Google Scholar]
- 97. Stojadinovic O, Minkiewicz J, Sawaya A, et al. Deep tissue injury in development of pressure ulcers: a decrease of inflammasome activation and changes in human skin morphology in response to aging and mechanical load. PLoS One. 2013;8(8):p.e69223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar‐Jain P, Adivarekar RVJIJOBM. Hemostasis and anti‐necrotic activity of wound‐healing dressing containing chitosan nanoparticles. 2019;121:936‐946. [DOI] [PubMed] [Google Scholar]
- 99. Bennett‐Marsden M.. The role of the pharmacist.
- 100. Nather AA, ed., Surgery for diabetic foot: a practical operative manual. World Scientific Publishing Company, 2016, p 67.
- 101. Hampton S. A small study in healing rates and symptom control using a new sheet hydrogel dressing. J Wound Care. 2004;13(7):297‐300. [DOI] [PubMed] [Google Scholar]
- 102. Hasatsri S., Pitiratanaworanat A., Swangwit S., Boochakul C., C. J. D. R. Tragoonsupachai, and practice . Comparison of the morphological and physical properties of different absorbent wound dressings, Dermatol Res Pract vol. 2018, 2018, 9367034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lei J, Sun L, Li P, et al. The wound dressings and their applications in wound healing and management. Health Sci J. 2019;13(4):1‐8. [Google Scholar]
- 104. Vowden K, Vowden PJBJOCN. Understanding exudate management and the role of exudate in the healing process. 2003;8(Sup5):S4‐S13. [DOI] [PubMed] [Google Scholar]
- 105. Jones V, Grey JE, Harding KG. ABC of wound healing: wound dressings. BMJ: British Medical Journal . 2006;332(7544):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery (Oxford). 2011;29(10):491‐495. [Google Scholar]
- 107. Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna SJJOAPS. Wound dressings: current advances and future directions. Appl Polym. 2019;136(27):47738. [Google Scholar]
- 108. Williams CJBJON. Hydrocoll: a ‘new breed'of hydrocolloid wound dressing. 1998;7(21):1337‐1340. [DOI] [PubMed] [Google Scholar]
- 109. Zarrintaj P, Moghaddam AS, Manouchehri S, Atoufi Z, Amiri A, Amirkhani MA, Nilforoushzadeh MA, Saeb MR, Hamblin MR, Mozafari M . Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine. 2017;12(19):2403‐2422. [DOI] [PubMed] [Google Scholar]
- 110. Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ERJCP. Alginate‐based composite materials for wound dressing application: a mini review. 2020;236:116025. [DOI] [PubMed] [Google Scholar]
- 111. Chaganti P, Gordon I, Chao JH, Zehtabchi SJTAJOEM. A systematic review of foam dressings for partial thickness burns. 2019;37(6):1184‐1190. [DOI] [PubMed] [Google Scholar]
- 112. Stoica AE, Chircov C, Grumezescu AMJM. Nanomaterials for wound dressings: an up‐to‐date overview. 2020;25(11):2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Simões D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ. Recent advances on antimicrobial wound dressing: a review. Eur J Pharmaceut Biopharmaceutics. 2018;127:130‐141. [DOI] [PubMed] [Google Scholar]
- 114. Wang R, Zhou B, Xu DL, et al. Antimicrobial and biocompatible ε‐polylysine–γ‐poly (glutamic acid)–based hydrogel system for wound healing. J Bioactive Compatible Poly. 2016;31(3):242‐259. [Google Scholar]
- 115. Zhou C, Li P, Qi X, et al. A photopolymerized antimicrobial hydrogel coating derived from epsilon‐poly‐L‐lysine. Biomaterials. 2011;32(11):2704‐2712. [DOI] [PubMed] [Google Scholar]
- 116. Lee J‐H, Kim H‐L, Lee MH, Taguchi H, Hyon S‐H, Park J‐C. Antimicrobial effect of medical adhesive composed of aldehyded dextran and ε‐poly (L‐lysine). J Microbiol Biotechnol. 2011;21:1199‐1202. [DOI] [PubMed] [Google Scholar]
- 117. Wang R, Xu DL, Liang L, et al. Enzymatically crosslinked epsilon‐poly‐L‐lysine hydrogels with inherent antibacterial properties for wound infection prevention. RSC Adv. 2016;6(11):8620‐8627. [Google Scholar]
- 118. Wang R, Li Q, Chi B, et al. Enzyme‐induced dual‐network ε‐poly‐L‐lysine‐based hydrogels with robust self‐healing and antibacterial performance. Chem Commun. 2017;53(35):4803‐4806. [DOI] [PubMed] [Google Scholar]
- 119. Krueger GG. Fibroblasts and dermal gene therapy: a minireview. Hum Gene Ther. 2000;11(16):2289‐2296. [DOI] [PubMed] [Google Scholar]
- 120. Sung Y, Kim S. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pampinella F, Lechardeur D, Zanetti E, et al. Analysis of differential lipofection efficiency in primary and established myoblasts. Mol Ther. 2002;5(2):161‐169. [DOI] [PubMed] [Google Scholar]
- 122. Howell DP‐G, Krieser RJ, Eastman A, Barry MA. Deoxyribonuclease II is a lysosomal barrier to transfection. Mol Ther. 2003;8(6):957‐963. [DOI] [PubMed] [Google Scholar]
- 123. Barry ME, Pinto‐Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA. Role of endogenous endonucleases and tissue site in transfection and CpG‐mediated immune activation after naked DNA injection. Hum Gene Ther. 1999;10(15):2461‐2480. [DOI] [PubMed] [Google Scholar]
- 124. Lechardeur D, Sohn K‐J, Haardt M, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Therapy. 1999;6(4):482‐497. [DOI] [PubMed] [Google Scholar]
- 125. Abbasi M, Uludag H, Incani V, Hsu CYM, Jeffery A. Further investigation of lipid‐substituted poly (L‐lysine) polymers for transfection of human skin fibroblasts. Biomacromolecules. 2008;9(6):1618‐1630. [DOI] [PubMed] [Google Scholar]
- 126. Thiersch M, Rimann M, Panagiotopoulou V, et al. The angiogenic response to PLL‐g‐PEG‐mediated HIF‐1α plasmid DNA delivery in healthy and diabetic rats. Biomaterials. 2013;34(16):4173‐4182. [DOI] [PubMed] [Google Scholar]
- 127. von Erlach T, Zwicker S, Pidhatika B, et al. Formation and characterization of DNA‐polymer‐condensates based on poly (2‐methyl‐2‐oxazoline) grafted poly (l‐lysine) for non‐viral delivery of therapeutic DNA. Biomaterials. 2011;32(22):5291‐5303. [DOI] [PubMed] [Google Scholar]
- 128. Gao B, Zhang Q, Muhammad K, Ren X, Guo J, Xia S, Zhang W, Feng Y . A progressively targeted gene delivery system with a pH triggered surface charge‐switching ability to drive angiogenesis in vivo. Biomaterials science . 2019;7(5):2061‐2075. [DOI] [PubMed] [Google Scholar]
- 129. Nie W, Yuan X, Zhao J, Zhou Y, Bao HJCP. Rapidly in situ forming chitosan/ε‐polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydrate Polymers. 2013;96(1):342‐348. [DOI] [PubMed] [Google Scholar]
- 130. Isaksson K, Åkerberg D, Posaric‐Bauden M, Andersson R, Tingstedt BJJOMSMIM. In vivo toxicity and biodistribution of intraperitoneal and intravenous poly‐L‐lysine and poly‐L‐lysine/poly‐L‐glutamate in rats. Colloids and Surfaces B: Biointerfaces. 2014;25(5):1293‐1299. [DOI] [PubMed] [Google Scholar]
- 131. Patil NA, Kandasubramanian B. Functionalized polylysine biomaterials for advanced medical applications: a review. Eur Polymer Journal. 2021;110248. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


