Abstract
Bone grafts or prosthetic implant designing for clinical application is challenging due to the complexity of integrated physiological processes. The revolutionary advances of nanotechnology in the biomaterial field expedite and endorse the current unresolved complexity in functional bone graft and implant design. Rare earth (RE) materials are emerging biomaterials in tissue engineering due to their unique biocompatibility, fluorescence upconversion, antimicrobial, antioxidants, and anti‐inflammatory properties. Researchers have developed various RE smart nano‐biomaterials for bone tissue engineering and implantology applications in the past two decades. Furthermore, researchers have explored the molecular mechanisms of RE material‐mediated tissue regeneration. Recent advances in biomedical applications of micro or nano‐scale RE materials have provided a foundation for developing novel, cost‐effective bone tissue engineering strategies. This review attempted to provide an overview of RE nanomaterials' technological innovations in bone tissue engineering and implantology and summarized the osteogenic, angiogenic, immunomodulatory, antioxidant, in vivo bone tissue imaging, and antimicrobial properties of various RE nanomaterials, as well as the molecular mechanisms involved in these biological events. Further, we extend to discuss the challenges and prospects of RE smart nano‐biomaterials in the field of bone tissue engineering and implantology.
Keywords: bone grafts, bone tissue engineering, implantology, nanomaterials, RE materials
1. INTRODUCTION
Rare earth (RE) materials are found naturally in a thin layer of earth surfaces. 1 , 2 , 3 RE metals are found in the ores like basalts, granites, gneisses, shales, clays, and silicate rocks. Yttrium and lanthanides are the commonly known RE metals. The Finnish chemist Johan Gadolin isolated the first RE element yttrium in 1794 from gadolinite near Ytterby (Sweden). Seventeen lanthanides have been identified so far. 4 Among lanthanides, cerium is the most abundant element (60–68 ppm), followed by neodymium and lanthanum. 5 , 6 Praseodymium, samarium, gadolinium (Gd), and dysprosium have abundances in the range of 5–10 ppm, while other elements are less abundant, with lutetium being the least abundant (<0.5 ppm). 7 The electronic configuration of RE elements is ([Xe]4f n 5s25p6 [n = 0–14]) and usually exists as trivalent cations. The outer 5s substantially shield the 4f electrons and 5p electrons, and hence the electronic transitions from 4f to 4f or from 4f to 5d are barely affected by the surrounding environment. Therefore, the RE materials have sufficient energy levels and several unique spectroscopic characters such as extended lifetime emission and narrow bandwidth with sharp fluorescent emissions via photoluminescence. 8 , 9 Generally, photoluminescence obeys Stokes law that means the wavelength of the emitted fluorescence light is more extended than incident light, termed the “downconversion” luminescence. Downconversion luminescence converts higher‐energy photons into lower‐energy photons. For instance, ultraviolet (UV) radiation excites Eu3+, Tb3+, and Dy3 and emits in the visible region. UV excitation of Nd3+ emits in the near‐infrared (NIR) region. Excitation by long‐wavelength radiation (i.e., anti‐Stokes luminescence) of Er3+ or Tm3+ emits shorter‐wavelength light. The emitted fluorescence light is in a shorter wavelength and higher energy than the incident light; thereby, it is called anti‐Stokes luminescence or “upconversion” luminescence. Therefore, RE materials are gaining their significance in biomedical imaging owing to the reduction of autofluorescence and penetrating properties in the tissues of biological systems. 10 , 11 , 12
Various electronic configurations and variable valence states are crucial in enhancing the stability, broadening the absorption range endowed RE ions with flexible redox properties and unique luminous and electromagnetic characteristics. 13 , 14 , 15 These properties of RE elements attribute to the design of nanostructured materials either as major components or as dopants paving the way for new tissue engineering applications. The particle size ranging from 1 to 100 nm of nanoparticles and geometry has been reported to play an essential role in cell–material interactions, affecting cellular uptake, and cell functioning. 16 Most cell–nanoparticle interactions have been facilitated at nano biointerface by several factors such as nanoparticle's shape and surface morphology. 16 The shape/geometry of the nanoparticles directly influences their cellular uptake. It has been observed that rod‐shaped particles have the highest uptake, followed by spheres, cylinders, and cubes. 17 Similarly, the neodymium nanoparticle's shape influences the cellular activity in terms of altered mitochondrial membrane potential, reactive oxygen species (ROS), and eventually angiogenesis in endothelial cells. 18 The cellular uptake of nanomaterials such as liposomes, 19 iron oxide, 20 polymeric, 21 gold, 22 , 23 , 24 and silica nanoparticles 25 is size dependent. The particle size of the polystyrene spheres increased the binding and affected the immune response in human dendritic cells. 26 Similarly, the RE materials like ceria have the highest cellular uptake and reactive oxygen species production in human monocyte cell line U937, 27 size dependence cell viability in Hela and HEK cells, 28 and size dependence biodistribution of ceria was also observed in rat animal model. 28 Further, rare‐earth fluorides such as erbium showed good cell imaging features depends on their size. 29 Besides that, many factors, such as surface chemistry and oxidation states of RE metals like ceria, affected the physiological conditions. 30 Few studies reported that RE materials doped mesoporous silica nanoparticle and polymeric nanoparticles possess positively charged that could be facilitated the cell nanomaterial interactions. 21
Moreover, in vivo assay usually demands controlled particle size to use the enhanced permeation and retention effect, high colloidal stability, and low toxicity. 31 RE metal‐based nanoparticles are used in different imaging approaches other than luminescent imaging like magnetic resonance imaging (MRI) and computed tomography (CT). 32 RE materials hold a robust therapeutic potential owing to biocompatibility, optical, and physicochemical properties. Lanthanides are widely used in the electronic and painting industry due to their magnetic and adsorption properties. 33 , 34 The magnetic properties of some lanthanide cations such as Gd3+, Ho3+, and Dy3+ make RE‐based nanoparticles of these cations very useful in MRI because these cations can induce additional contrast between normal and abnormal regions. 35 , 36 In the biological field, various functions of RE elements have been reported. Recently, researchers have been trying to use the intrinsic optical properties of RE nanomaterials for in vivo imaging to monitor the physiologic processes. 37 , 38 , 39 Besides that, in compliance with unique features, these materials are used for in situ bio‐labeling of cellular organelles, photodynamic therapy in tumor targeting, site‐specific delivery of therapeutic molecules with a combination of fluorescence and the therapeutic effect as a theranostic tool. 40 , 41 , 42 , 43 , 44 Due to the high adsorbing affinity, RE has been widely used as a doping material with metal to produce alloy materials for bone and dental prostheses production. 45 , 46 RE nanoparticles can be incorporated into the connectivity centers or inside the metal–organic frameworks. 35 , 47 Highly porous and oriented structures allow RE nanoparticles to accommodate many different functional carrier cargoes like drugs, growth factors and make them attractive materials for biomedical applications. 33 The development of RE‐based smart nano‐biomaterials with osteogenic, angiogenic, and immunomodulatory potential and in vivo imaging has a massive scope in the field of bone tissue engineering and implantology. Significant advancements have been made with RE in bone grafts and prostheses design in the past two decades. Here, we have listed the advances and potential applications of these RE smart nano‐biomaterials in bone tissue engineering and implantology.
2. BONE CELL BIOLOGY
Bone is a metabolically growing vital organ that gives the body structural (mechanical stability) and functional properties. The bone progenitor cells carry out different functions such as bone formation, resorption, repair, and mineral homeostasis. The bone progenitor cells originate from two cell lineages, mesenchymal and hematopoietic. 48 Osteoblasts and osteocytes are differentiated from the mesenchymal stem cells (MSCs). Bone marrow mononuclear hematopoietic cells differentiate into osteoclasts. 49 , 50 , 51 Osteoclasts resorb old and defected bone matrix, and osteoblasts deposit new bone matrix in that place. This phenomenon is called bone remodeling. 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 Balanced osteoblast and osteoclast activity maintain healthy bone. 60 Certain pathological conditions disrupt the osteoblast and osteoclast function, causing bone loss or excessive bone mass. 61 Osteocytes are embedded in the bone matrix, comprise 95% of cells in bone, and have the most extended half‐life (25 years) among the bone cells. 62 A bone matrix consists of organic and inorganic components. The inorganic matrix, calcium, phosphorus, sodium, and magnesium are associated with bone mineral crystals. Bone mineral crystals have shown in the form of apatite, hydroxyapatite (HA), (CaO[PO4][OH2), and acid phosphate groups (HPO4)2 containing brushite (CaHPO2 42H2O). These minerals serve as an ion reservoir, which helps maintain their extracellular fluid concentrations for critical physiological functions and gives stiffness and strength to the bone. 63
Osteocytes sense biological and mechanical stimuli and produce a range of signaling molecules to control osteoblast 64 , 65 , 66 , 67 and osteoclast functions. 68 , 69 , 70 The balanced function of osteoblasts, osteoclasts, and osteocytes is vital for effective bone regeneration and implant success. 60 , 71 , 72 Researchers are currently developing bone‐biomaterials and implants that can modulate osteoblast, osteoclast, or osteocyte function. 73 , 74 , 75 RE materials have shown the potential to modulate osteoblast, osteoclast, or osteocyte function. 76 , 77 , 78 , 79 Therefore, RE‐based bone‐biomaterials and implants could be the next generation bone graft, implant, and prosthetics for effective bone tissue engineering.
Besides these, endothelial cells, immune cells, and neuronal cells regulate bone regeneration and homeostasis. These cells produce various signaling molecules affecting bone cells' functions in an autocrine or paracrine manner. Endothelial cells influence bone formation through neovessel formation and release of various growth factors needed for osteogenic differentiation of precursor cells. The endothelial cells lie nearby the bone cells and secrete growth factors like platelet‐derived growth factor (PDGF)‐BB and vascular endothelial growth factor (VEGF) to promote osteogenic differentiation of precursor cells. 80 , 81 Moreover, neovasculogenesis is crucial to supply oxygen and growth factors for the precursor cells migrated to the defect sites. Osteogenic cell‐secreted osteopontin induces early angiogenesis in developing bone. 82 , 83 The immune cells, including monocytes, neutrophils, dendritic cells, and B and T lymphocytes, play a vital role in osteoimmunomodulation. Biomaterial‐mediated M1 and M2 polarization of macrophages regulate different stages of bone defect healing. 84 , 85 The key molecules responsible for the signaling between osteoclasts and osteoblasts are regulated by immune cells. 86 , 87 , 88 The immune cell‐secreted tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐6, and IL‐1β enhance osteoclast differentiation and bone resorption via receptor activator for nuclear factor‐κB ligand (RANKL) secretion. 89 These pro‐inflammatory cytokines inhibit osteoblast differentiation. 89 Whereas anti‐inflammatory cytokines, including IL‐4 and IL‐10, increase bone formation by inducing osteoblast function and inhibiting osteoclastogenesis. 90 Chen et al. have summarized the biomaterial–immune cell interaction and its effect on bone defect healing and osseointegration. 84 , 85 Their review suggested the development of novel biomaterials with osteoimmunomodulatory properties for orthopedic and dental applications. Reports from literature had shown the immunomodulatory potential of RE materials, 91 , 92 which is thoroughly discussed in Section 5.1.2 of this review.
Neuronal cells also significantly contribute to maintaining skeletal homeostasis. The bone marrow consists of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). SNS closely associates with the blood vessels through the nutrient foramen and innervating different regions; some nerves reach bone marrow and connect with transcortical vessels in the bone. 93 Further, neuron regulates various hematopoietic cell functions via neurotransmitters' binding to beta‐adrenergic receptors. 94 The PNS may innervate the distal femoral metaphysis and uses acetylcholine as the primary neurotransmitter, which binds to muscarinic or nicotinic receptors. 95 Apart from the direct regulation of hematopoietic cells, PNS regulates bone remodeling. 96 , 97 , 98 , 99 Implant‐derived magnesium has been reported to promote bone healing via local neuronal production of calcitonin gene‐related polypeptide‐α (CGRP). 100 RE element Gd‐doped magnesium scaffold has been reported to enhance bone defect healing via neuronal CGRP‐mediated effect on osteogenesis and angiogenesis. 101 These findings further strengthen the scope of RE‐based biomaterials in orthopedics and implantology.
3. NANOMATERIALS AND CELLS INVOLVED IN BONE REGENERATION
The unprecedented pathological or congenital malfunctions affect bone metabolism by aberrant or restricted actions of the aforementioned bone cells. Thereby understanding the pathophysiology of these cells cues the novel therapeutic targets for bone‐related diseases. Many therapeutic strategies have been developed like small molecules, recombinant proteins, peptides, and plant‐based phytochemicals to eliminate bone therapy‐related complications. Recently, the role of nanoparticles has significantly compromised the need for bone therapeutics. The organic and inorganic components of the bone matrix directly facilitate bone regeneration and maintain bone homeostasis. RE nanomaterials can be designed in combination with organic and inorganic components of the bone matrix to improve bone regeneration. Various metal ions, including RE, had been reported to modulate the osteocyte, osteoblast, and osteoclast activity. Gold nanoparticles incorporated gelatin hydrogels promote proliferation and differentiation of human adipose‐derived stem cells toward osteoblast cells in a dose‐dependent manner. 102 Another study indicated that the gold nanoparticles suppress osteoclast formation in a dose‐dependent manner and increase bone density that can be useful in preventing and treating osteoporosis. 103 The gold nanoparticle‐labeled MSCs improve contrast for imaging, and gold nanoparticles preserve the migratory capacity of MSCs. 104 The gold nanoparticle‐functionalized mesoporous silica nanoparticles synergistically increase the immunomodulatory effects and direct osteogenic stimulation by increasing the osteogenic differentiation capability of MC3T3‐E1 cells and accelerate new bone formation in a critical‐sized cranial defect site in rats. 105 The therapeutic potential of Ag–Au–HA compositions would be excellent for bone regeneration and fracture healing. 106 Surface modification of bone grafts with silver nanoparticles, samarium, and TiO2 prevents the risk of contamination and infection in alveolar bone and dental implant surgery. 107 , 108 The iron oxide nanoparticles coated with dextrin and chitosan increase osteoblast proliferation and differentiation. 109 , 110 The inorganic nanoparticles like calcium phosphate nanoparticles increase the osteogenic differentiation of rat bone marrow stromal cells, 111 , 112 , 113 and magnesium‐containing biocomposites facilitate femur fracture repair. 100 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 In this pipeline, the RE nanoparticles have tremendous potential for bone graft development since it has versatile bio applications, including an antioxidant to antimicrobial effect. 122 , 123 , 124 Furthermore, RE metals can be doped in the abovementioned nanoparticles to redevelop the smart nano‐biomaterials with improved antimicrobial, immunomodulatory potential of ceria, 125 , 126 the osteo‐angiogenic effect of europium, 127 , 128 , 129 contrast imaging potential of Gd, 130 , 131 and laser irradiation property of neodymium. 132 , 133 This review exemplifies the role of various RE nanomaterials for the therapeutic modulation of these important bone cells.
4. BONE DEFECT HEALING
Critical size or large bone defects need medical interventions to restore. 134 , 135 , 136 A typical bone defect repair consists of four overlapping stages: the initial inflammatory response, soft callus formation, hard callus formation, and bone remodeling. 137 , 138 Bone defect healing starts with an initial anabolic phase, where local tissue volume increases through inflammation, and hematoma is formed at the defect site immediately. 139 , 140 It has been reported that mesoporous silica nanoparticles, silver and gold nanoparticles can induce inflammation, activating the inflammatory cascades to recruit endothelial cells and neutrophils. Ceria nanoparticles and europium‐doped mesoporous silica nanospheres (Eu‐MSNs) stimulated the pro‐inflammatory response in macrophages, osteogenic differentiation of BMSCs, and angiogenic activity of HUVECs. Bone precursor cells and endothelial cells contribute to form cartilaginous bony callus (soft callus), which bridge the gap between the bone fragments. 141 , 142 Chronic inflammation is deleterious to proceed to heal the wound trajectory. Therefore, immunomodulatory nanobiomaterials have been developed using RE nanoparticles such as ceria, which facilitate immunomodulatory action by switching M1 macrophage to M2. M2 macrophages recruit and activate precursor cells to form a cartilaginous soft callus. Soft callus, along with endothelial cells and osteoblasts, then progresses to hard callus formation, also known as primary bone formation; this stage represents the most active period of osteogenesis. 143 Following these processes, the bone remodeling phase begins with coordinated osteoblast and osteoclast activities. Reabsorption of callus tissues by osteoclast is followed by lamellar bone formation. The ROS‐producing ability of RE nanoparticles such as ceria activates the RANKL pathway to induce osteoclastogenesis. 78 , 79 Moreover, angiogenesis is a critical factor for bone remodeling because it provides the appropriate conditions for osteoblast and osteoclast activities. 143 , 144 , 145 The RE materials such as europium has the potent role of angiogenic activity via ROS production. 127 , 128
5. APPLICATION OF RE SMART NANO‐BIOMATERIALS IN BONE TISSUE ENGINEERING
Bio‐implants are orchestrated specialized materials that render the ability to replace or restore the specific functions of the damaged organs or tissues. 146 , 147 One of the recently identified such materials belongs to RE metal groups. The different RE nanomaterial synthesis methods and their physicochemical properties are listed in Table 1. In addition, RE nanomaterials have a lot of biological applications. Reports from literature had report antioxidants potential of ceria, 125 , 126 osteo‐angiogenic effects of europium, 127 , 128 , 129 laser irradiation property of neodymium, 132 , 133 and contrast imaging potential of Gd. 130 , 131 Various biological applications, especially concerning bone tissue engineering application of RE materials, are summarized in Table 2. The outcomes of bone fracture healing strategies are still not satisfactory due to the lack of osteoinduction, osteoconduction, immunomodulation, and osteointegration ability of biomaterials. The use of emerging RE nanomaterials has the potential to address these challenges. In the past two decades, significant advancements have been made using RE materials in bone implants and prostheses design. This review attempts to comprehensively exemplify the potential usage of RE elements in bone graft and implant development. We profoundly discuss the challenges in using RE nanomaterials in bone regenerative medicine, particularly in the osteogenic process.
TABLE 1.
RE nanoparticle synthesis and their properties
| S.no | Method | Nanoparticles | Reductant/modification | Properties | References |
|---|---|---|---|---|---|
| 1. | Hydrothermal | Cerium oxide (CeO2) | Sodium dodecyl sulfate | Weak agglomeration | 148 |
| 2. | Hydrothermal | Pr‐, Gd‐, and Sm‐doped ceria nanoparticles | 20% Pr and Sm 10% Gd | Weak agglomeration (13–25 nm) | 149 |
| 3. | Solution casting | Ce2O3 | PLGA | Sustained release of the ceria nanoparticles | 150 |
| 4. | Flame spray pyrolysis | Nanoceria | Heparin and 3‐amino propyl tri‐ethoxy silane | 12 nm | 151 |
| 5. | Sol–gel | Ce2O3, Ga2O3 doped ZnO | 0.2% Ce2O3 and 1.0% Ga2O3 | Mesoporous | 152 |
| 6. | Plasma spraying | CeO2 | Calcium silicate | Antioxidant | 153 |
| 7. | Plasma spraying | CeO2 | Titanium | Antioxidant | 154 |
| 8. | Sol–Gel | CeO2 nanoparticles | Oligochitosan alginate and gelatin | Injectable hydrogel | 155 |
| 9. | Microemulsion | Ceria nanoparticles | Alendronate‐PEG 600 | Endochondral ossification | 125 |
| 10. | Melt quench and Polymer foam replication | Ce2O3 and Ga2o3 | Borate (13‐93b3) | Bioactive glass powders | 156 |
| 11. | Plasma sprayed | CeO2 | Calcium silicate | Antimicrobial activity | 157 |
| 12. | Ultrasonication | EuF3‐ and TbF3‐coated multiwalled carbon nanotubes | Sodium dodecyl sulfate | 10 nm thickness of coating | 158 |
| 13. | Solution synthesis | Eu3+‐doped Y2O3 | Alumina nanoparticles | Ultrathin films | 159 |
| 14. | Microemulsion | Eu (DBM)3 dibenzoylmethanate phenanthroline nanoparticles | Triton X‐100, Octanol, and cyclohexane | 40 nm in size, spherical shape, and good dispersibility | 160 |
| 15. | Chemical etching | Re10Pb25F65 Re‐Er3+, Yb3+, Eu3+, Dy3+, Ho3+, Tm3+ | Oxyfluoride nano‐glass‐ceramics | 8 nm diameter | 10 |
| 16. | Solution Combustion‐fluoridation | RE‐doped Lu2O3 and Y2O3 powders | Eu3+‐doped and codoped with Yb3+/Ho3+ | 200–300 nm size | 161 |
| 17. | Co‐precipitation‐solvothermal | Eu‐doped Y2O3 | Aqueous and ethylene glycol | Y2O3:Eu wires and spherical, photoluminescence | 162 |
| 18. | Conjugation | Eu3+‐doped Gd3+ | Fe3O4 nanoparticles via a PEG‐NH2 linker | Water‐soluble cell fluorescence imaging | 40 |
| 19. | Microwave | Tb3+‐doped Eu3+ | Polyethyleneimine | 12 nm multicolor luminescent LaF3 | 163 |
| 20. | Sol–gel | Eu3+‐, Sm3+‐, and Tb3+‐doped TiO2 | Titanium (IV)‐isopropoxide, water, ethanol, and nitric acid in the molar ratio of 1:3:20:0.08 | Red emission in Eu3+, Sm3+ doped TiO2 | 164 |
| 21. | Sol–gel | Eu(III) | Europium(III)‐doped yttrium, lanthanum, and gadolinium oxides | Sub‐10 nm, luminescent properties | 165 |
| 22. | Emulsifier‐free emulsion polymerization | Eu nanoparticles | Oleic acid and sodium undecylenate modified Fe3O4 | 120 nm exhibit superparamagnetism | 166 |
| 23. | Conjugation | Gd‐FITC mesoporous silica nanoparticles | Diethylene triamine pentaacetic acid, phenyl thiourea, tetraethyl orthosilicate, and cetyltrimethylammonium bromide | Green fluorescence and paramagnetism | 167 |
| 24. | Thermolysis (>250°C) | Er3+/Yb3+ co‐doped NaGdF4 | Oleic acid, 1‐octadecene, sodium trifluoroacetate, polyacrylic acid, and chloroform RGD | 32 ± 9 nm in size, optical, and magnetic properties | 168 |
| 25. | Green chemistry | Gd nanoparticles | Dextran, ammonium hydroxide | Ultrafine sub‐10 nm | 169 |
| 26. | Molecular dynamics simulations | Metallofullerenol Gd@ C82(OH)22 | Fullerene C82 | Inhibition of MMP‐2 and MMP‐9 | 170 |
| 27. | Solvothermal | GdPO4•H2O nano‐bundles | NH4H2PO4, HA, and PLGA | ~1 μm in length, ~30 nm in width, paramagnetism | 171 |
| 28. | Polyol | Gadolinium (III) oxide | 3‐glycidyloxypropyl trimethoxysilane, Bisphosphonate | 70 nm, and long‐term follow‐up imaging studies | 131 |
| 29. | Lyophilization method | GdPO4/CTS | Chitosan | Porous scaffolds | 172 |
| 30. | Lyophilization method | Gd‐doped MCS/CTS (Gd‐MCS/CTS) scaffolds | CTAB, NH3·H2O, TEOS | Hierarchically porous structures | 173 |
| 31. | Thermolysis (>250°C) | Er3+/Yb3+ co‐doped NaGdF4 | Oleic acid, 1‐octadecene, sodium trifluoroacetate, polyacrylic acid, chloroform RGD | 32 ± 9 nm in size, optical, and magnetic properties | 168 |
| 32. | Green chemistry | Gd nanoparticles | Dextran, ammonium hydroxide | Ultrafine sub‐10 nm | 169 |
| 33. | Hydrothermal | Neodymium oxide | Acetic acid | Fibrous/rod‐like particle | 174 |
| 34. | Solvothermal | Neodymium oxide | Nitric acid/acetic acid | Fibrous/needle‐like particle | 175 |
| 35. | Chemical | Nd(OH)3 | Borohydride | 30–100 nm | 176 |
| 36. | Microemulsion | Nd(OH)3 | n‐butanol, n‐octane, CTAB | Cube, sphere, and oval like | 177 |
| 37. | Radiofrequency sputtering | Nd‐doped TiO2 | TiO2 and metallic Nd (RF:13.56 MHz) | Red luminescence | 178 |
| 38. | Wet co‐precipitation | NdPO4 | NH4H2PO4 | 92 nm, monoclinic, spherical | 179 |
| 39. | Inverse microemulsion and sol gel | Neodymium oxalate | Organically modified silane (Ormosil) | 10–40 nm, violet emission | 180 |
| 40. | Polyol | Neodymium oxide | Diethylene glycol, NaOH | 2–5 nm in size spherical shape | 181 |
| 41. | Sol–gel | CeO2, Pr2O3, and Nd2O3 | Citric acid | 10–30 nm, spherical shape | 182 |
| 42. | Chemical reduction | Nd | Sodium borohydride, hydrazine hydride, ammonia | Spherical, cube, and rod | 18 |
| 43. | Electrospinning | Nanofiber | Polyvinyl acetate | Crystalline 20 nm diameter | 183 |
| 44. | Sol–gel | Pr3+ | Citric acid, ammonia solution | Spinel cubic crystal and larger ionic radii | 184 |
| 45. | Polyol | Pr6O11 | Diethylene glycol and sodium hydroxide | 10 nm | 185 |
| 46. | Hydrothermal | Ce/Pr‐CQDS | EDTA, Glycine | Hydroxyl radical scavenging | 186 |
| 47. | Ball milling | SmCo5 and PrCo5 | Dry HEBM under argon, Wet HEBM–heptane, and oleic acid | 10 nm | 187 |
| 48. | Surface functionalization | Sm‐doped YVo4 | Citrate and polyvinyl pyrrolidine | 20–50 nm | 188 |
| 49. | Emulsion | Sm153 | EDTMP | 200–500 nm | 189 |
| 50. | Thermal decomposition | Y2O3 nanoparticles | Oleic acid | 30 nm, green fluorescence at room temperature | 190 |
| 51. | Microwave irradiation method | Terbium hydroxide nanorods | NH4OH | 340 nm length, 65 nm width | 191 |
| 52. | Solvothermal | YbFeO3(o‐YbFeO3) | Ytterbium acetate, Yb chlorides, and iron acetylacetonate | Hexagonal orthorhombic perovskite structure | 192 |
TABLE 2.
Applications of RE smart nano‐bio materials in bone tissue engineering
| S.No | RE materials | Biological property | Model | Mechanism/pathway | References |
|---|---|---|---|---|---|
| 1. | Nanoceria | Antioxidants | Homozygous tubby (tub/tub) mice | Neuroprotection genes | 193 |
| 2. | CeO2 nanoparticles | Antioxidant | Osteoblastic cell line (MC3T3‐E1) | ROS production | 153 |
| 3. | CeO2 nanoparticles | Antioxidant | MC3T3‐E1 | Wnt/β‐catenin | 194 |
| 4. | CeO2 nanoparticles | Antioxidant | MC3T3‐E1 | Osteoradionecrosis | 195 |
| 5. | Cerium (III) | Osteoclastogenesis | RAW264.7 | NADPH oxidase 1 | 79 |
| 6. | CeO2 nanoparticles | Pro‐angiogenic property | MSCs | Increased Ca2+ level, HIF‐1α, VEGF signaling | 196 |
| 7. | SmCeO2 | Pro‐angiogenic property | Endothelial cells | p38MAPK/HIF‐1α | 197 |
| 8. | CeO2 nanoparticles | Osteoinductive and anti‐inflammatory | BMSCs, RAW264.7 | BMP2 and TGF‐β1, CD206, IL‐1ra, and IL‐10 | 194 |
| 9. | Ce3+ | Osteoinductive and anti‐inflammatory | BMSCs | Smad/BMP | 198, 199 |
| 10. | Ceria nanoparticles | Endochondral ossification | Mice critical‐sized bone defects | DEAH (Asp‐Glu‐Ala‐His) box helicase 15 and p38 MAPK | 125 |
| 11. | Nanoceria | Anti‐angiogenic and pro‐inflammatory | Vldlr null mice | ERK 1/2, JNK, p38 MAP kinase, and Akt | 200 |
| 12. | Oligochitosan coated CeO2 nanoparticles | Anti‐angiogenic and pro‐inflammatory | Human retinal pigment epithelium‐19 and umbilical endothelium cell lines | Inhibition of VEGF and inflammatory‐related protein expression | 155 |
| 13. | Ce (III)‐based alginate/hyaluronate hydrogel | Osteoconductivity and antimicrobial ability | MG63 cells, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Candida albicans | MG63 cell viability | 201 |
| 14. | Ceria inclusion in the graphene hydroxyapatite (GR‐HA) matrix | Osteoconductivity and antimicrobial ability | MG63 cells, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa | Expression of the osteoblastic genes Runx2, Col 1, ALP, BMP‐2, OC and OPG | 202 |
| 15. | Ceria and silver‐reinforced HA composite | Antioxidant and antibacterial | E. coli and S. aureus | Mechanical integrity and cytocompatible | 126 |
| 16. | CeO2 incorporated calcium silicate | Dental implants and antimicrobial activity | E. faecalis | ALP, OCN, and BSP | 157 |
| 17. | Ceria nanoparticles on the poly‐l‐lactide scaffold | Cell‐material interactions | Human MSCs and osteoblast‐like cells (MG63) | Ce4+ enhances proliferation, migration, and adhesion behavior | 14 |
| 18. | Cerium | Osteogenic differentiation and mineralization | MC3T3‐E1 | Runx2, BMP2, ALP, BSP, Col I, and OCN | 77 |
| 19. | Nanoceria | Osteogenic differentiation | BMSCs | Dose‐dependent manner, 24–72 h | 203 |
| 20. | Ceria | Osteogenic differentiation | MSCs | TGF‐β/BMP | 204 |
| 21. | Ceria | Osteogenic differentiation | BMSCs | Smad/BMP | 205 |
| 22. | Ceria‐stabilized zirconia/alumina | Mandibular implant | Clinical report | Elasticity equivalent to that of a cobalt‐chromium | 206 |
| 23. | Cerium‐based zirconia/alumina composite | Osteogenic response | MC3T3‐E1 and male Sprague–Dawley rats | Osteogenic response in vitro and the osseointegration capability in vivo | 207 |
| 24. | Nano CeO2 | Bone regeneration | BMSCs and male Sprague–Dawley rats | Enhancing bone regeneration in a critical‐size defect rat model | 208 |
| 25. | CeO2 nanoparticles‐modified bioglass scaffolds | Osteogenic differentiation | Human BMSCs and in vivo rat, cranial defect models | ERK pathway, collagen deposition, osteoclast formation, and bone regeneration | 76 |
| 26. | Nanocrystalline CeO2 | Dentinogenesis | Chinchilla breed rabbits | Dentin and bone regeneration effectively | 209 |
| 27. | CeO2 nanoparticles | Chemotherapeutic action | Osteosarcoma cell line SAOS‐2 | pH‐sensitive manner | 210 |
| 28. | CeO2 nanoparticles | Osteoclastogenesis | Bone marrow‐derived macrophages | ROS‐mediated RANKL pathway | 78 |
| 29. | Eu(III) complex | Contrast agent | Bovine tibia specimens | Bone structure analysis | 211 |
| 30. | Gold nanoparticles conjugated with the europium | Luminescent probe | Human platelets | Targeted the platelets in low pH 6.5 | 212 |
| 31. | Europium hydroxide nanoparticles | Angiogenesis | Endothelial cells | PI3K/Akt | 213 |
| 32. | Europium (III) hydroxide | Pro‐angiogenic properties | Endothelial cells | MAPK pathway | 129 |
| 33. | Gd2O3:Eu3+ nanotubes | Bone mineral density | MC3T3‐E1 | High ALP activity, mineralization, BMP signaling pathway | 214 |
| 34. | Bioactive glass incorporated europium scaffolds | Luminescent property and new bone formation | Osteoporotic bone defects in OVX rats | Bone formation | 128 |
| 35. | Europium‐doped mesoporous silica nanospheres | Pro‐inflammatory and osteogenic differentiation | Macrophage and HUVECs | New bone formation at a critical‐sized cranial defect site | 215 |
| 36. | Europium‐doped bioactive glass nanoparticles | Osteogenic differentiation | Human MSCs | ALP activity, COL I secretion, ALP, Col I, OPN, Runx2 | 127 |
| 37. | Eu3+‐doped nanohydroxyapatite | Luminescent property and osteogenic differentiation | hASCs | GSK3β /β‐catenin | 216 |
| 38. | Gd doped FITC silica nanoparticles | Differentiation into adipocytes, osteocytes, and chondrocytes | Human MSCs | Green fluorescence and paramagnetism | 167 |
| 39. | RGD functionalized Er3+/Yb3+ co‐doped NaGdF4 | Tumor angiogenesis | U87MG tumor cells | Target the αvβ3 integrin–expressing tumor cells | 168 |
| 40. | Gd@C82(OH)22 | High antitumoral efficacy | Molecular dynamics simulations | Inhibit MMP‐2 activity | 170 |
| 41. | Gd‐based nanoparticles | Tumor angiogenesis | Balb/c tumor‐bearing mice | Determination of tumor boundary by MR imaging | 169 |
| 42. | GdPO4H2O nanobundles | MRI and X‐ray tracing and osteogenesis | MC3T3‐E1 and in vivo rabbit radius defects | OCN and mineralization | 171 |
| 43. | GdPO4/CTS scaffolds | Osteoconductivity | Rabbit BMSCs | ALP, Runx‐2, OCN, Col‐I, and Smad/Runx2 | 172 |
| 44. | Gd‐doped MCS/CTS | Osteogenic differentiation | Rabbit BMSCs | Wnt/β‐catenin | 173 |
| 45. | Gd‐BG scaffolds | Osteogenic differentiation | Human BMSCs | Akt/GSK3β | 217 |
| 46. | Ca−P‐coated Mg−Zn−Gd scaffolds | Orthotopic reconstruction of large‐sized orbital bone defect healing | Canines | CGRP‐mediated angiogenesis and osteogenesis | 101 |
| 47. | Gadolinium MRI enhancer | Assessment of perfusion in carpal bones | Kienbock's disease | Diagnose altered perfusion in patients with Kienbock's disease | 218 |
| 48. | Gadolinium (III) oxide nanoparticles | Monitor in vivo implantation | Condyle defect rat model | Long‐term follow‐up imaging studies | 131 |
| 49. | Gadolinium (III) nanocages | MRI imaging | KPC transgenic mouse models | Detect neuropilin‐1‐positive in pancreatic cancer | 219 |
| 50. | Gadolinium | Whole‐body magnetic resonance imaging | Breast cancer, prostate cancer, and lung cancer patients | Detection of bone metastasis | 130 |
| 51. | Yb3+/Ho3 Co‐doped apatite nanoparticles | Bone regeneration | MG63 cells and New Zealand white rabbits | Distinguish implanted material from bone tissue | 220 |
| 52. | Magnetic lanthanum‐doped HA/CS scaffolds | Macrophage polarization and bone regeneration | Rat bone marrow mesenchymal stem cells | Upregulation of Smad 1/5/9 pathway | 92 |
| 53. | Lanthanum phosphate chitosan scaffolds | Osteogenic differentiation | BMSCs and rat critical‐sized calvarial defect sites | Wnt/β‐catenin signaling pathway | 221 |
| 54. | La3+ ions calcium silicate chitosan bone scaffolds | Osteogenic differentiation | Rabbit BMSCs | TGF signal pathway | 222 |
| 55. | Nd: YVO4 | Laser oscillator for drill the cortical bone | Femoral bone of a pig | 160 mW for 0.75‐mm thick drilling | 223 |
| 56. | Nd: YAG silicon carbide on Ti6Al4V | Laser irradiation on bone healing | Osteoblast | Bone healing | 224 |
| 57. | High‐power, low‐level Nd: YAG laser | Laser irradiation on bone healing | MC3T3‐E1 osteoblasts | BMP‐2‐related signaling pathway | 132 |
| 58. | Nd:YAG laser with EMP | Healing intrabony defects | Periodontal disease | Probing depth decrease and increased clinical attachment level (CAL) | 225 |
| 59. | Nd: YAG laser with SRP | Periodontal inflammatory response | Periodontal inflammation | Plaque index (PI), gingival index (GI), probing pocket depth (PPD), and marginal bone loss | 226 |
| 60. | Nd2O3 | Inflammatory response | Human bronchial epithelial cells | STAT3 | 227 |
| 61. | Nd nanoparticles | Redox‐mediated angiogenic response | EA.hy926 endothelial cells | PKM2‐NOX4 signaling pathways | 18 |
| 62. | Nd:YAG | Laser irradiation | Male Wistar rat | Accelerate bone metabolism during tooth movement | 228 |
| 63. | Nd:YAG Q‐switch laser | Antimicrobial | Peri‐implantitis | Disinfected the contaminated implant | 229 |
| 64. | Nd‐Ca‐Si silicate glasses and alginate composite hydrogels | Anticancer and wound healing bioactivity | HUVEC cells, nude mice, and BALB/c mice | Thermal therapy for cancer treatment and burn wound healing | 230 |
| 65. | Samarium with EDTMP and Technetium‐99m | Targeted delivery for bone metastasis | Male Wistar rats | 150 min accumulation and release of EDTMP at bone tissue | 189 |
| 66. | Sm3+‐doped P2O5 glass‐reinforced hydroxyapatite | Osteogenesis and antimicrobial | MG63 cells, Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa | F‐actin cytoskeleton organization and cell proliferation in MG63 and potent antimicrobial activity | 231 |
| 67. | Y2O3 nanoparticles incorporated polycaprolactone scaffolds | Cell proliferation and angiogenesis | Fibroblasts (L‐929) and osteoblast‐like cells (UMR‐106) | VEGF and EGFR | 232 |
| 68. | Er:YAG laser irradiation | Evaluate the moisture content, roughness, and thickness | Cortical bone | Optical coherence tomography (OCT) | 233 |
Abbreviations: ALP, alkaline phosphatase; BMSCs; bone marrow mesenchymal stem cells; BSP, bone sialoprotein; CGRP, calcitonin gene‐related polypeptide‐α; EDTMP, ethylenediamine tetramethylene phosphonic acid; MSCs, mesenchymal stem cells; OCN, osteocalcin; VEGF, vascular endothelial growth factor.
5.1. Cerium
Cerium is the most abundant RE element, approximately 50–60 ppm found on the earth's surface. Cerium exhibits unique redox behavior due to its electron configuration, filling the 4f orbital in the ground state and standard oxidation numbers of +3 or + 4. Oxide forms of cerium include cerium oxide or ceria (CeO2), and dicerium trioxide or sesquioxide (Ce2O3) has been broadly utilized for various applications, such as electrolytes in fuel and solar cells, detection systems, surface polishing, and catalysis. The redox equilibrium between two oxidation states results in the ROS and reactive nitrogen species (RNS) regulation. At the nanoscale level, the reactivity of CeO2 is more effective as the high surface‐to‐volume ratio results in elevated surface oxygen vacancies, which is responsible for the enhanced biological activities such as antimicrobial, antioxidants, and angiogenic responses. 91 The applications of CeO2, especially in bone formation, are discussed in the following sections.
5.1.1. Redox modulator
Redox signaling is essential for physiological and pathological conditions. Under physiological conditions, there will be a balance between oxidants and antioxidants, which maintains the redox state at the threshold level. The redox states altered beyond the tolerable threshold level lead to apoptosis. Oxidative stress caused by generating abundant ROS in the living system is obnoxious. The body itself has a defense mechanism to modulate such redox states, whereas, in some pathological conditions like bone fracture microenvironment, the levels of ROS are abundantly high and affect bone reconstruction. Excessive ROS production can induce osteoclastogenesis and suppresses the osteoblastic differentiation process. Therefore, it is essential to balance the equilibrium by using antioxidants to modulate the redox states. Nanoceria acts as an antioxidant therapeutic. The different sizes (5, 15, 30, or 55 nm) of ceria particles biodistribution had been analyzed by intravenous injection in rats. 28 The nanoceria was detected in blood, brain, liver, and spleen. The liver and spleen contain a large percentage of the injected dose, with no significant clearance over 720 h and very little nanoceria entered brain parenchyma. Superoxide dismutase mimetic activity retains in PLGA encapsulated ceria nanoparticles for 90 days under different pH. 150 Plasma‐sprayed CeO2 coating enhances superoxide dismutase activity and reduces ROS in hydrogen peroxide (H2O2)‐treated osteoblasts. 153 The heparin‐functionalized nanoceria enhances cellular uptake and ROS scavenging. 151
Radiation causes bone damage, including a decrease in osteocyte number and osteoblastic activity. CeO2 nanoparticles exhibit protective effects on irradiation‐induced osteoradionecrosis in MC3T3‐E1 cells by reducing oxidative stress. 195 Further, increasing the content of CeO2 in HA coatings diminishes the H2O2‐induced inhibition of osteogenic differentiation and increases alkaline phosphatase (ALP) activity, calcium deposition activity, and mRNA expression levels of osteogenesis markers runt‐related transcription factor‐2 (RUNX2), ALP, and osteocalcin (OCN) in bone marrow mesenchymal stem cells (BMSCs). Furthermore, CeO2 induces the gene and protein expressions of β‐catenin and cyclin D1. 194 Similarly, Varini et al. found that mesoporous glasses with 1.2% and 3.6% CeO2 prevent oxidative stress improves MC3T3‐E1 cell proliferation. 234 The schematic representation of the preparation of the alginate/glass beads with ceria is given in Figure 1I. The topical application of water‐soluble CeO2 nanoparticles (nanoceria) accelerates the healing of full‐thickness dermal wounds in mice by reducing oxidative damage to cellular membranes. Furthermore, nanoceria enhances the proliferation and migration of fibroblasts, keratinocytes, and vascular endothelial cells. 235
FIGURE 1.
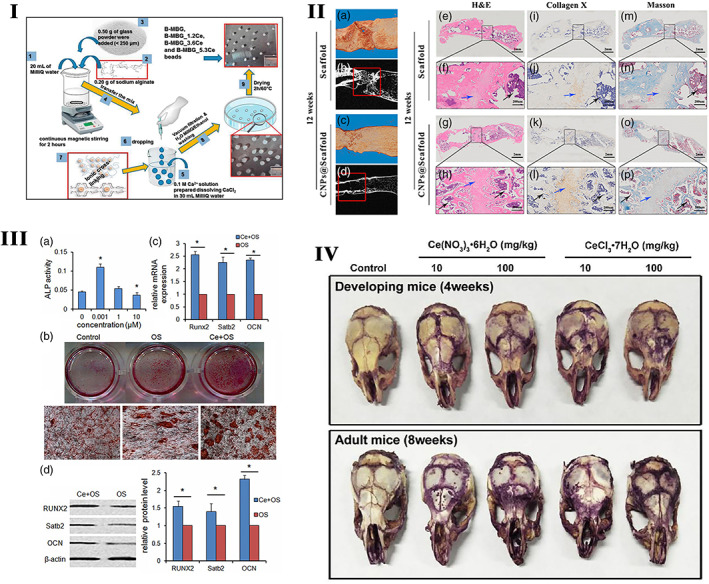
I. Schematic representation of the preparation of the alginate/glass beads with ceria to prevent oxidative stress in MC3T3‐E1. Source: Reprinted with permission from ref. 234. Copyright 2019, Elsevier. II. The effect of cerium‐doped nanoparticles on osteogenesis (a–d). Representative micro‐CT (b, d) and 3D reconstruction (a, c) images of femurs 12 weeks after ceria‐based scaffold implantation. The red solid line frame outlines the bone defect area. (e–h) H&E staining at 12 weeks post‐surgery. (i–l) Collagen X IHC staining at 12 weeks post‐surgery. (m–p) Masson's trichrome staining at 12 weeks post‐surgery. The solid black box represents the enlarged defect area. Blue arrowheads indicate hypertrophic chondrocytes, and black arrowheads represent new trabecular bone formed by endochondral ossification (n = 3/group). Source: Reprinted with permission from ref. 125. Copyright 2019, John Wiley & Sons, Inc. III Ce promotes bone marrow mesenchymal stem cells (BMSCs) osteogenic differentiation ex vivo. (a) BMSCs were treated with various concentrations of Ce (0, 0.001, 1, 10 μM) for 7 days and assessed by measuring the alkaline phosphatase (ALP) activity. (b) BMSCs were treated with standard, OS, and OS + Ce medium for 21 days and assessed by alizarin red S staining. (c) Quantitative real time PCR analysis indicated that the mRNA expressions of Runx2, Satb2, and OCN were significantly up‐regulated in the BMSCs treated with Ce (0.001 μM) for 7 days compared to the control group. (d) Western bolt analysis showed the expressions of RUNX2, Satb2, and OCN proteins were up‐regulated after treatment with Ce (0.001 μM) for 7 days. Data are presented as mean ± SD from a representative of three separate experiments. *p < 0.05. Source: Reprinted with permission from ref. 205. IJCEP Copyright 2014. (IV) The TRAP staining of mice skull treated with cerium for 9 days. Source: Reprinted with permission from ref. 79. Copyright 2019, Elsevier. TRAP, tartare resistant acid phosphatase
The imbalance in the microenvironmental conditions such as changes in pH, necrotic cells, and invasion of microorganisms elevates the ROS levels in bone fracture environments and osteoporotic conditions. 236 , 237 , 238 Elevated ROS levels hinder the recruitment of osteoblast precursors and delay the healing process. The H2O2 level above 0.3 mmol modulates oxidative stress and inhibits the osteogenic differentiation of odontoblastic cells and preosteoblastic MC3T3‐E1 cells via ERK and NFkB pathways. 239 In contrast, the odontoblasts cells treated with H2O2 at concentrations below 0.3 mmol/L display a significant increase in ALP activity and matrix mineralization. Another study demonstrated that H2O2‐induced oxidative stress enhances differentiation of calcifying vascular cells and inhibits differentiation of bone cells, which causes either atherosclerosis by the accumulation of lipids in the vessel wall or osteoporosis by lack of osteoblast mineralization 236 Even though nanoceria acts as an antioxidant, nanoceria also mimics the activity of superoxide dismutase, 240 , 241 catalase and nitric oxide synthase 242 maintaining some basal level ROS and redox states, which are mainly dependent on catalytic activity and oxidation potential such Ce3+ and Ce4+. 14 , 199 The catalytic properties and biomedical applications of cerium oxide nanoparticles were critically reviewed by Walkey et al., the interested readers can be read it for further information. 243 The microenvironmental conditions played a significant role in the production of ROS. Acidic environments like cancer, ceria nanoparticles favor the scavenging of superoxide radical over the hydroxyl peroxide resulting in accumulation of the ROS, which can be used for sensitization of cancer cells. 244 Zhou et al. claimed that elevation of intracellular ROS level by cerium (III) enhances the expression and activity of NADPH oxidase 1, which further activates the RANKL‐dependent osteoclasts differentiation, and the cerium (III) activated osteoclasts exhibit higher bone resorption activity. 79 The Figure 1IV depicted the osteoclastogenic effect of cerium by tartare resistant acid phosphatase (TRAP) staining. Another study reported that CeO2 nanoparticles facilitated osteoclast formation at lower concentrations via the RANKL pathway. A higher concentration of CeO2 inhibited osteoclastogenesis by inducing apoptosis in bone marrow‐derived macrophages by modulating cellular ROS levels. 78 Recent research attempts with poly(1,8 octanediol‐co‐citrate), beta‐tricalcium phosphate, and CeO2 nanoparticles had developed the porous, biocompatible, bioactive, and free‐radical scavenging RE nanomaterials. 245 The Ce6 upconversion nanoparticles act as photosensitizers that excite at 808 nm and convert NIR to visible photon energy. This event generates toxic ROS in cancer cells through the Fenton‐like reaction by Fe(OH)3 compound and enhances the tumor treatment efficacy. 246
5.1.2. Angiogenesis and immunomodulation
Insufficient blood vessel formation is a critical problem that hampers the clinical application of bone grafts. The scaffolds modified with CeO2 nanoparticles improve the proliferation and inhibit the apoptosis of MSCs. Meanwhile, it activates the calcium channel enhancing intracellular free Ca2+ level in MSCs, which subsequently augments the stability of hypoxia‐inducible factor‐1 alpha (HIF‐1α) and VEGF expression. The improved paracrine signaling of VEGF promotes the proliferation, differentiation, and tube formation ability of endothelial progenitor cells and significantly improves the blood vessel distribution inside of bone scaffolds. 196 Physicochemical properties like Ce3+/Ce4+ ratio, surface charge, size, and shape of cerium nanoparticles influence the angiogenesis process. The Ce3+/Ce4 ratio modulates the intracellular oxygen environment by stabilizing HIF‐1α endogenously and promotes angiogenesis. 247 Mesoporous sol–gel glasses substituted with Ce2O3, Ga2O3 (both 0.2% and 1.0%), and ZnO (0.4% or 2.0%), contain well‐interconnected ultra‐large pores (pores >400 μm) ideal for vascular ingrowth and proliferation of endothelial cells. 152 The functional nanoconjugates of SmCeO2 trigger endothelial cell proliferation and induce the growth of blood vessels in the chick embryo. The enhanced expression of pro‐angiogenic markers (p38MAPK/HIF‐1α) by these functional nanoconjugates might be the plausible signaling mechanism of the pro‐angiogenic property. 197 Endochondral bone regeneration is similar to long bone defect healing, which needs angiogenesis and osteogenesis. The micro emulsion‐based alendronate‐anchored polyethylene glycol‐modified ceria nanoparticles (CNPs) accelerated vascular invasion. They enhanced endochondral ossification‐based bone regeneration by activating RNA helicase, DEAH (Asp‐Glu‐Ala‐His) box helicase 15 (DHX15). CNPs enhance the proliferation and hypertrophic differentiation of BMSCs by stimulating the DHX15–p38 MAPK axis. Further inhibition of DHX15 by shRNA affected the expression of hypertrophic genes Runx2, MMP13, and Col10α1, which confirmed the importance of DHX15 in hypertrophic differentiation of BMSCs. 125 The effect of cerium‐doped nanoparticles on osteogenesis is shown in Figure 1II,III.
The aberrant angiogenesis causes lethal effects in some neurodegenerative conditions and cancer metastasis. Nanoceria inhibits the expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice representing a novel therapeutic strategy to treat age‐related macular degeneration (AMD) and other neurodegenerative diseases. Nanoceria causes inhibition of pro‐inflammatory cytokines and pro‐angiogenic growth factors and upregulation of several cytokines and anti‐angiogenic genes in the Vldlr_/_ retina. Nanoceria inhibits the activation of ERK1/2, JNK, p38 MAP kinase, and Akt. 200 Similarly, the water‐soluble oligochitosan‐coated CeO2 nanoparticle‐loaded injectable hydrogel shows biocompatibility and radical‐scavenging effect. 155 Furthermore, it downregulates the expression of angiogenic proteins and pro‐inflammatory cytokines in AMD cellular models like human retinal pigment epithelium‐19 and umbilical endothelium cell lines. 155 It also has been documented that nanoceria alleviates the endometrial lesions induced in the mice model by decreasing oxidative stress and inhibiting angiogenesis.
Moreover, nanoceria was also observed to protect endometriosis‐related adverse effects on the oocytes, which is critical for a successful pregnancy. 248 The genotoxicity studies in liver cells revealed that the high dose (1000 mg/kg body weight) of ceria nanoparticles induces DNA damage in peripheral blood leukocytes, micronucleus formation in blood cells, and total cytogenetic changes in the bone marrow. Ceria nanoparticles exhibit higher tissue distribution and greater clearance in large fractions through urine and feces than CeO2 bulk, whereas the maximum amount of micro‐sized CeO2 excretes in feces. 249 Nanoceria significantly inhibits the production of ROS in A2780 ovarian cancer cells. Nanoceria treatment also inhibits VEGF165‐induced proliferation, capillary tube formation, activation of VEGFR2 and MMP2 in HUVECs. Thus, nanoceria can be used as an anti‐angiogenic therapeutic agent during cancer treatment. 250 This pro‐angiogenic and anti‐angiogenic potential of ceria‐based nanoparticles might be related to the dose of ceria content in the nanoparticles, the cell type, and disease condition. Optimizing the proper dose of cerium in the ceria nanoparticles is crucial for pro‐angiogenic effect‐mediated bone defect healing.
Plasma spraying technique‐based CeO2‐coated (CS‐10Ce and CS‐30Ce) calcium silicate materials have shown good osteogenic responses in bone marrow‐derived MSCs (BMSCs) by increasing the expression of osteoinductive molecules BMP2 and TGF‐β1. This effect limits inflammatory reactions by up‐regulating the expressions of anti‐inflammatory M2 macrophage markers (CD206, IL‐1ra, and IL‐10) in RAW264.7 macrophages. 194 Ce4+/Ce3+ (i.e., 0.46, 1.23, and 3.23) ratios of CeO2 nanoparticles applied to titanium substrate surfaces by magnetron sputtering elevate the M2 macrophage polarization and anti‐inflammatory cytokine secretion resulting in new bone formation and osseointegration. 199 Since immunomodulation plays a vital role in bone defect healing and implant success, the immunomodulatory potential of nanoceria could be applied in bone tissue engineering and implantology. Similarly, the T cells, B cells, neutrophils, and other immune cells participate in the bone regeneration cascade. 251 , 252 The effect of RE metal, including cerium‐based nanomaterials, on the activation and expansion of the T cells, B cells, neutrophils, and other immune cells during bone defect healing is still unknown. 253
5.1.3. Antimicrobial activity
Due to the antioxidant property of ceria, it has been widely used as an antimicrobial agent. Alginate/hyaluronate and Ce (III) ions based hydrogel shows bioactive and antimicrobial ability against Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Candida albicans without compromising the osteoconductivity. The antimicrobial ability of Ce(III) is observed in Ce3+ ion incorporated hydrogel. A higher Ce(III) concentration in the hydrogel leads to an even stronger antimicrobial activity. The Ce3+ in cerium oxide is the key component of antioxidant activity to overcome free‐radical formation during the cellular growth process. Further, nanoceria decreases NO production in macrophages and in tissues of C57BLK6 mice for alleviating the pro‐inflammatory response caused by the infectious agents, which could be the mechanism of ROS scavenging ability of nanoceria‐mediated anti‐inflammation that serves as a treatment for a broad spectrum of inflammatory diseases. 201 , 254 The same research group also reported that ceria inclusion in the graphene hydroxyapatite (GR‐HA) matrix induces antimicrobial resistance against S. aureus, S. epidermidis, and P. aeruginosa of the composite. 202 Antioxidant ceria and antibacterial silver reinforce HA composite with enhanced mechanical and cytocompatible properties and show antibacterial efficacy of ~61% for Escherichia coliand ~53% for S. aureus. 126 Plasma‐sprayed CeO2‐incorporated calcium silicate coating in dental implants shows better biocompatibility, upregulates mRNA expression levels of ALP, OCN, and bone sialoprotein (BSP), and intensifies antimicrobial activity against Enterococcus faecalis 157
5.1.4. Osteogenesis
Unique biological properties of ceria nanoparticles such as antioxidants, anti‐inflammatory, pro‐angiogenic, and antimicrobial nature suggest ceria as an appropriate biomaterial for bone tissue engineering applications. The ceria nanoparticles on the poly‐l‐lactide scaffold surface promote hMSCs and osteoblast proliferation, migration, and adhesion. 14 The antioxidant properties of the CeO2‐incorporated HA coatings maintained intracellular SOD activity, reduced oxidative injure, and enhanced the osteogenic differentiation of BMSCs, probably through Wnt/β‐catenin signaling. 255 The cerium ions influence the formation and structure of HA, as indicated by the apatite structure maintained by Ce3+ ions. 256 The cerium has shown dose‐dependent osteogenic effects on MC3T3‐E1 cells. Cerium at concentrations of 0.0001, 0.001, 0.01, 0.1, or 1 μM promotes the proliferation and osteogenic differentiation of MC3T3‐E1 cells, as displayed by the upregulation of RUNX2 and BMP2 ALP, BSP, collagen I (COLI), and OCN. Whereas 1000 μM ceria inhibits osteogenic differentiation. 77 Similarly, exposure to 1% ceria reduces ALP activity in MC3T3‐E1 cells, and cerium trichloride (CeCl3) stimulates MC3T3‐E1 cell proliferation. 203 These results from the literature indicate that loading the proper dose of ceria in biomaterials is crucial for effective bone regeneration. The plasma‐sprayed CeO2 − coating with higher Ce4+ concentration elicits more significant effects than the CeO2 coating with Ce3+ concentration. The osteogenic differentiation is activated by RUNX2 expression and enhanced through increased ALP and OCN expression in BMSCs through the Smad‐dependent BMP signaling pathway. 198 The nanoceria‐mediated osteogenic differentiation of BMSCs is dose‐dependent between 24 and 72 h. Prolonged incubation with nanoceria, that is, 14 days, inhibits the osteogenic differentiation. In contrast, nanoceria inhibits the adipogenic differentiation of BMSCs on Day 17, which conferred that the biomaterial doped with ceria should not give prolonged release and should be optimized for a better bone regenerative effect. 257 Melt quench technique‐based bioactive borate (13‐93B3) glass powders containing up to 5 wt% Ce2O3 and Ga2O3 increases chemical durability, exhibits a good in vitro bioactive response, and has high in vitro HA forming ability making them promising candidates for bone tissue engineering applications. 156 Ceria promotes osteogenic differentiation in MSCs by interacting with BMP receptors and activates TGF‐β/BMP signaling pathway by upregulation of RUNX2, which further up‐regulates osteoblast marker genes COLI and BMP2 at early stages, ALP, and OCN at later stages of differentiation further inhibits the adipogenic differentiation of MSCs by downregulation of an adipocyte marker PPARγ2. 204
Smad‐dependent BMP signaling plays a vital role in the migration and osteogenic differentiation of BMSCs. Ceria promotes the phosphorylation of Smad1/5/8 and translocating to the nucleus via increased BMP2 expression. The activity of p‐Smad1/5/8 increases stromal cell‐derived factor‐1 (SDF‐1) and RUNX2 expression levels in BMSCs. 205 The foamed ceria made up of CeO2, and bovine hydroxyapatite (BHA) composites show potential free‐radical scavenging ability for developing orthopedic biomaterial. 258 , 259 Ceria‐stabilized zirconia/alumina nanocomposite exhibits an elastic and flexible property equivalent to a cobalt‐chromium alloy used as a mandibular implant. 206 Intramuscular injections of CeO2 enhance muscle mass, glycogen, ATP content, and type I fiber ratio, resulting in higher muscle endurance. 260 The cerium/zirconia/alumina composite enhances the osteogenic response in vitro and in vivo. 207 Nano CeO2‐containing calcium sulfate hemihydrate composite with 5% w/w shows a higher bone regenerative potential. 208 Freeze‐dried CeO2 nanoparticles‐modified bioglass scaffolds rapidly promote the proliferation and osteogenic differentiation of human BMSCs. The enhanced osteoinductivity of ceria‐bioglass scaffolds is mainly related to the activated ERK pathway. Rat cranial defect model revealed that ceria‐bioglass scaffolds accelerate collagen deposition, osteoclast formation, and bone regeneration compared to bioglass scaffolds. 76 Nanocrystalline CeO2 promotes dentinogenesis in the damaged teeth root. 209 All aforementioned osteogenic properties of cerium‐doped innovative nanomaterials indicate the potential applications of cerium in bone tissue engineering and implantology.
5.2. Europium
Europium is the least dense, the softest, and the most volatile member of the lanthanide series. The europium element was discovered in 1901 by French chemist Eugène‐Anatole Demarçay and was named for Europe. Europium occurs in minute amounts in many RE minerals such as monazite and bastnasite. The primary use of europium is in optical displays, TV screens, and fluorescent lamps. Europium is also used in scintillators for X‐ray tomography and as a source of blue color in light‐emitting diodes. 261 The bio labeling property of europium ions has been used to synthesize the cyclen‐based europium (III) complex as a lanthanide luminescent contrast agent for bone structure analysis by incorporating the iminodiacetate functionalities as selective Ca(II) binding motifs. This contrast agent selectively visualizes the damaged bone structure (microcracks). 211
The gold nanoparticles conjugated with the europium luminescent probe and the peptide (pHLIP•EuL•Au) target the platelets in low pH 6.5 and translocate the pHLIP across the membrane. 212 H2O2, a redox signaling molecule generated by europium hydroxide nanoparticles, activates the endothelial nitric oxide synthase that promotes nitric oxide production in a PI3K (phosphoinositide 3‐ kinase)/Akt‐dependent manner, eventually triggering angiogenesis. 213 The molecular mechanisms underlying the europium hydroxide nanorods (EHNs) induced angiogenesis are given in Figure 2IV. It has been further evidenced that microwave‐assisted synthesized europium (III) hydroxide nanorods exert pro‐angiogenic properties through ROS generation and activation of the MAPK pathway. 129 On the other hand, Gd2O3:Eu3+ nanotubes generate excessive ROS injury to the mitochondria and DNA in BMSCs, and the release of cathepsin B by lysosomal rupture triggered cell death necrosis. 262 The nanotubes of Gd2O3:Eu3+ remarkably enhance the bone mineral density and bone biomechanics as indicated by high ALP activity, mineralization and promoted the expression of osteogenesis genes in MC3T3‐E1 cells through activation of the BMP signaling pathway. 214 Mesoporous bioactive glass (MBG) incorporated europium scaffolds by an in situ co‐template methods have highly interconnective large pores (300–500 μm), high specific surface area (140–290 m2/g), and well‐ordered mesopores (5 nm) as well as uniformly distributed europium elements. Incorporating 2–5 mol% europium toward MBG scaffolds with luminescent property stimulates new bone formation (Figure 2III) in osteoporotic bone defects in OVX rats. 128
FIGURE 2.
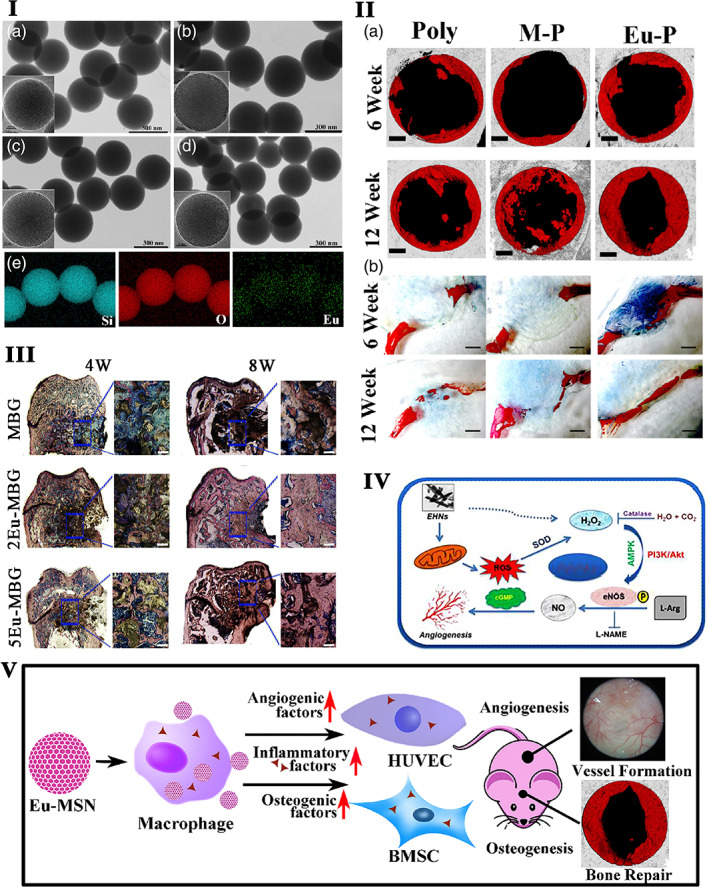
I. SEM images of pure MSNs (a), europium‐doped mesoporous silica nanospheres (1Eu‐MSNs) (b), 2Eu‐MSNs (c), and 3Eu‐MSNs (d) show uniformly spherical morphology with a size of 280–300 nm, and the inserted TEM images show the abundant mesoporous structure of nanoparticles. EDS mapping analysis (e) shows homogeneous element distribution of Si, O, and Eu in 2Eu‐MSNs typically. Source: Reprinted with permission from ref. 215. Copyright 2017, Elsevier. II. The effect of Eu‐MSNs on the in vivo osteogenesis. Representative micro‐CT images of new bone formation (the gray background represents normal skull, the black holes represent the cranial defect created by surgical operation with a diameter of 5 mm, and the red part represents the newly formed bone at the defect site, analyzed by CTAn software of micro‐CT) (a) in cranial defect at 6 weeks and 12‐week show larger new bone area in Eu‐MSNs‐polymer film (indicated as Eu‐P in figure) group. Immunofluorescent staining images (b) by VG stain in the cranial defects show that more new bone (red) was formed at the cross section of the defect in Eu‐P groups at 6 weeks and 12 weeks, indicating similar results as micro‐CT analysis (pure polymer film as Poly, MSNs‐polymer composite films as M‐P, and Eu‐MSNs‐polymer composite films with as Eu‐P), scale bar = 1 mm. Source: Reprinted with permission from ref. 215. Copyright 2017, Elsevier. II.) Osteogenic effect of europium. (a) Histological analysis and histomorphometric measurements of in vivo bone formation ability for MBG, 2Eu‐MBG, and 5Eu‐MBG scaffolds after implanted in the osteoporotic femur defects of OVX rats at 4 and 8 weeks. The scale bar is 100 μm. Source: Reprinted with permission from ref. 128. Copyright 2016, American Chemical Society. IV. Graphical representation of the hypothesized molecular mechanisms underlying the EHNs induced angiogenesis mediated through ROS‐NO‐cGMP signaling axis. 213 Source: Republished with permission of Royal Society of Chemistry, 2015, permission conveyed through Copyright Clearance Center, Inc. V. The prepared Eu‐MSNs showed an inflammatory stimulation on macrophages, which further induced the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) via upregulating the gene expression of COL‐I, OCN, ALP, and RUNX2 as well as the angiogenic differentiation of HUVECs via upregulating the gene expression of CD31, MMP9, VEGFR, and PDGFR. The particles were then applied for in vivo experiments and showed a satisfactory effect on the bone repair of cranial defect and neovascularization. Source: Reprinted with permission from ref. 215. Copyright 2017, Elsevier. EHNs, europium hydroxide nanorods; HUVECs, human umblical vein endothelial cells; PDGFR, platelet‐derived growth factor receptor; SEM, scanning electron microscope; TEM, transmission electron microspcope
Figure 2I showed that the morphology of europium‐doped mesoporous silica nanospheres (Eu‐MSNs) stimulated the pro‐inflammatory response in macrophages, osteogenic differentiation of BMSCs, and angiogenic activity human umblical vein endothelial cells (HUVECs). Further, the Eu‐MSNs accelerate the new bone formation in the critical‐sized cranial defect site via immunomodulatory effect. The overall mechanism is provided in Figure 2II,V. 215 Europium‐doped bioactive glass nanoparticles (BGNEu) significantly enhance human MSCs (hMSCs) osteogenic differentiation (ALP activity and COLI secretion) by activating osteogenic marker ALP, COLI, OPN, and RUNX2. 127 Nanohydroxyapatite (nHAp) doped with Li+ ions (5 mol% Li+:nHAp) and co‐doped with lanthanide ions like samarium (III) (Sm3+) and europium (III) (Eu3+) ions enhance the luminescent property. Further, these composite improve osteogenic differentiation of human adipose‐tissue‐derived stem cells (hASCs) by a decrease in the expression of glycogen synthase kinase 3β (GSK3β) and an increase in β‐catenin mRNA level. 216
5.3. Gadolinium
Gd occurs in many minerals and other RE materials, but it is obtained primarily from bastnasite. It was discovered by a Finnish chemist Johan Gadolin. 263 Gd is known for its high potential in MRI. Nevertheless, its MRI applications are overshadowed by their large sizes resulting in poor organ/tumor targeting. Hsiao et al. used Gd as a dopant in fluorescein isothiocyanate mesoporous silica nanoparticles that possess green fluorescence and paramagnetism for labeling hMSCs via endocytosis. These labeled hMSCs can proliferate and differentiate into adipocytes, osteocytes, and chondrocytes. 167 Further radiolabeled arginine‐glycine‐aspartic acid (RGD)‐functionalized Er3+/Yb3+ co‐doped NaGdF4 upconversion nanophosphors (UCNPs) had been developed to specifically target the αvβ3 integrin‐expressing U87MG tumor cells and xenografted tumor models for tumor angiogenesis. 168 Metallofullerenol Gd@C82(OH)22 effectively inhibits MMP‐2 activity by blocking the Zn21‐catalytic site directly or the S19 loop indirectly and inhibits the proteolysis of MMP‐9 via allosteric modulation with high antitumoral efficacy. 170 The biocompatible dextran‐coated ultrafine sub‐10 nm Gd‐based nanoparticles are found particularly capable of determining the tumor boundary with clearly enhanced tumor angiogenesis. 169
Solvothermal synthesized GdPO4H2O nanobundles incorporated HA and PLGA serve as a biodegradable and traceable bone implant for MRI and X‐ray tracing; this unique biomaterial promotes OCN expression in MC3T3‐E1 cells and bone mineralization in vivo rabbit radius defects (Figure 3I). 171 GdPO4/chitosan scaffolds prepared by the lyophilization method improve the osteoconductivity, resulting in admired cell spreading and in vivo bone tissue in‐growth. GdPO4 nanoparticles in the GdPO4/CTS scaffolds robustly promote osteogenic differentiation by upregulating the levels of ALP, RUNX2, OCN, and COLI expression in rabbit BMSCs via activation of the Smad/RUNX2 signaling pathway (Figure 3VI). 172 Gd‐doped MCS/CTS (Gd‐MCS/CTS) scaffolds show anabolic effects on rabbit BMSCs cell proliferation and osteogenic differentiation through the activation of the Wnt/β‐catenin signaling pathway (Figure 3IV,V). 173 Gd‐BG scaffolds promote the proliferation and osteogenic differentiation of human BMSCs via the Akt/GSK3β signaling pathway (Figure 3II,III). 217 Gd is a widely accepted contrast agent in MRI, cardiac applications such as effective MR angiography. 264 Gd ethoxybenzyl diethylenetriamine pentaacetic acid (Gd‐EOB‐DTPA) is the liver‐specific contrast enhancement agent presently used for diagnosing HCC. MRI with Gd‐EOB‐DTPA enhancement is superior to enhanced CT and conventional contrast‐enhanced MRI in diagnosing small liver lesions and differentiating benign and malignant nodules. Gd‐EOB‐DTPA excretes into the biliary tract through multidrug resistance‐associated protein 2 (MRP2) on the biliary tract. The period of this phase is called the hepatobiliary specific period or hepatobiliary phase. The remaining contrast agent, similar to Gd‐DTPA, can be excreted through the kidney. This dual clearance pathway can compensate for each other when the liver or kidney function is damaged, thereby ensuring higher safety. 265 Compared with conventional hepatobiliary MRI, enhanced MRI by Gd‐BOPTA combined with ultrasound has good diagnostic value in determining HCC. 266 Gd(III) complexes containing a polydentate carboxylate ligand exhibit good MRI contrast properties. 267 PEGGd2O3 NPs presented longer half‐life, similar acute toxicity and histological influence, more negligible effect on hepatic and renal functions, and stronger contrast enhancement in the tumor. 268 Gd2O3‐assembled mesoporous silica MCM‐41 nanocomposite has been identified both in vitro and in vivo as a safe MRI contrast medium with better efficacy than its commercially available counterpart Gd‐DTPA. 269 An ultrasmall, theranostic (3.0 ± 1.0 nm size) Gd‐based nanoparticle (AGuIX NPs) are used to improve radiographic delineation and increase the intratumoral dose‐effect delivered by the particles. 270
FIGURE 3.
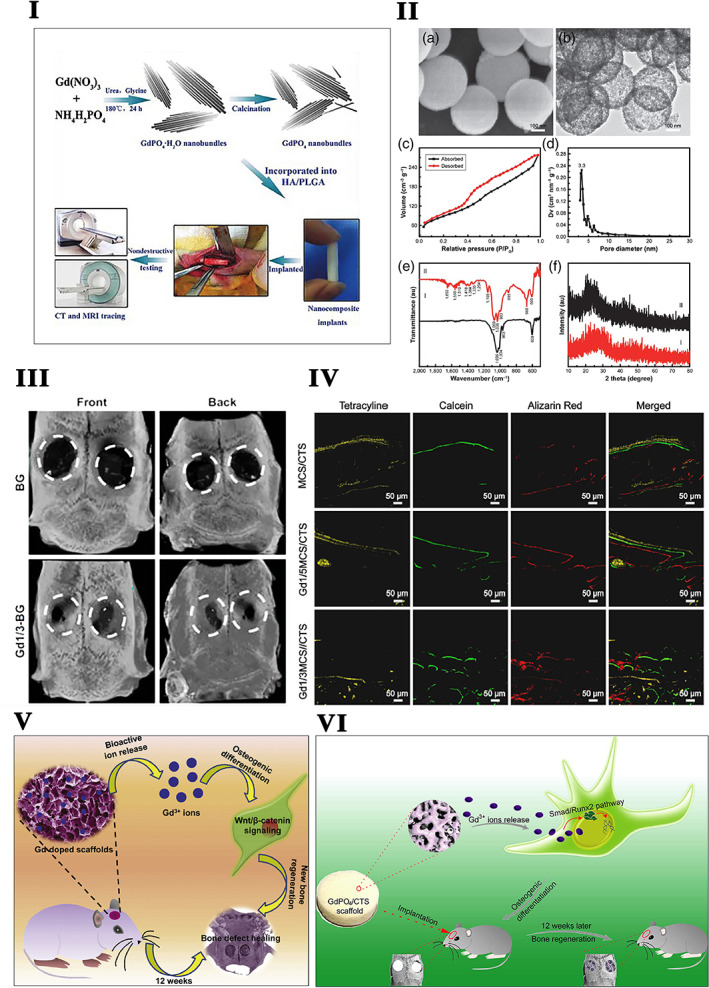
I. Schematic illustration of GdPO4·H2O and GdPO4 nanobundles synthesis and their application in biodegradable bone implants for MR and CT tracing. Source: Reprinted with permission from ref. 171. Copyright 2016, John Wiley & Sons, Inc. II. The structural property of the Gd‐BG scaffold. (a) SEM image and (b) TEM image of Gd‐BGS microspheres. (c) Nitrogen adsorption–desorption isotherm, (d) Barrett–Joyner–Halenda (BJH) pore‐size distribution curve of mesoporous Gd‐BGS microspheres. (e) The X‐ray diffraction patterns of samples: (I) Gd‐Bg microspheres and (II) Gd‐BG scaffolds. (f) The Fourier transform infrared spectra of samples: (I) Gd‐BG microspheres and (II) Gd‐BG scaffolds. Source: Reprinted with permission from ref. 217. Copyright 2019, Elsevier. III. Micro‐CT of rat cranial defects implanted with BG and Gd1/3‐BG scaffolds at 8 weeks after implantation. The images of reconstruction of micro‐CT for the bone regeneration of the defect area at Week 8. Source: Reprinted with permission from ref. 217. Copyright 2019, Elsevier. IV. Gd nanoparticle‐mediated bone tissue regeneration. Fluorochrome‐labeling analysis characterizing the new bone formation within MCS/CTS, Gd1/5MCS/CTS, and Gd1/3MCS/CTS scaffolds. Tetracycline (yellow), calcein (green), and alizarin red (red) were injected in rats at Weeks 3, 6, and 9. Source: Reprinted with permission from ref. 173. Copyright 2019, Elsevier. V. Schematic illustration of Gadolinium‐doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Source: Reprinted with permission from ref. 173. Copyright 2019, Elsevier. VI. Gadolinium phosphate/chitosan scaffolds promote new bone regeneration via Smad/Runx2 pathway. Source: Reprinted with permission from ref. 172. Copyright 2019, Elsevier. TEM, transmission electron microspcope
Further, it has been used as an MRI or X‐ray contrast agent of the osteoblasts applied in biodegradable HA/PLGA bone implants in vivo, providing a practical approach for recognizing the implants or the newly formed bone tissues. 171 GD MRI enhancer‐based dynamic contrast‐enhanced (DCE) MR examinations at 3 T assess perfusion in healthy carpal bones in a patient with osteonecrosis and Kienbock's disease. 218 The results suggested that areas of healthy bone show low perfusion. DCE‐MRI at 3 T diagnoses altered perfusion in patients with Kienbock's disease. RE element Gd‐doped magnesium scaffold (CaP‐coated Mg‐Zn‐Gd) enhances orthotopic reconstruction of large‐sized orbital bone defect healing in canines. The scaffolds triggered trigeminal neurons via CGRP promote endomucin expression in endothelial cells, facilitating angiogenesis and osteogenesis. 101 Gd (III) oxide nanoparticles (70 nm size) synthesized via the polyol method and surface functionalized with a bisphosphonate (BP) derivative (GBCAs)‐BP) show a strong affinity towards calcium phosphate. The CPC‐GBCAs‐BP functional material is longitudinally monitored after in vivo implantation in a condyle defect rat model. The BP functionalization prolongs the residence of the contrast agent within the CPC to allow long‐term follow‐up imaging studies. 131 Heat shock protein 16.5 (Hsp16.5) and peptide conjugated Gd (III) nanocages detect neuropilin‐1‐positive cells in genetically engineered mouse models. 219 Papageorgiou et al. used Gd for whole‐body magnetic resonance imaging, a radiation‐free alternative to the 99mTc‐HDP bone scan (BS) to detect metastasis of cancer bone. 130 Since Gd‐based contrast agents (GBCAs) are used for MRI enhancers in the bone; it has some adverse effects on the body. For instance, Gd concentration in bone is significantly higher in exposed subjects than in control subjects. Gd can be retained in bone up to 5 years after one GBCA administration. 271 The Gd‐exposed tibia shows a higher Gd concentration compared to the control group. 272 Based on the reports mentioned above from the literature, Gd can be used not only for the bone regeneration application but also to visualize the damaged bone and newly formed bone in vivo.
5.4. Neodymium
Neodymium is a ductile and malleable silvery‐white metal. Austrian chemist Carl Auer von Welsbach discovered neodymium in 1885. Neodymium occurs in the least amount in the rocks of Earth's crust. The major application of neodymium is in high‐strength permanent magnets used in high‐performance electric motors and generators, the electronics industry, and the ceramics industry for glazes and color glass in various shades from pink to purple. Neodymium‐stabilized yttrium aluminum garnet (YAG) is a component of many modern lasers, and neodymium glasses are used in fiber optics. 273 Neodymium is used in a laser oscillator to irradiate the specimen. Nd:YVO4 laser oscillator has a threshold average laser power of 160 mW required to drill through a 0.75‐mm thick cortical bone with a peak intensity of 1.3 GW/cm2. 223 Nd‐YAG laser irradiation in the near‐infrared ray (NIR) area has been reported to promote bone healing via the expression of ALP, RANKL, and OPG. It indicated that osteoblast‐like cells activate genes related to bone metabolism by combining mechanical stimulation and laser irradiation. 274 Nd:YAG laser irradiation stimulates cell growth in the nonsensitized osteoblasts and induces the expression of osteopontin, ALP, and RUNX2 in osteoblasts, type I COLI in fibroblasts, and vinculin in endothelial cells in low pulse energy levels. 275 Nd:YAG laser treatment improves zirconia bioactivity by increasing human osteoblast's cell viability, proliferation, and expression of COL1 and ALP activity. 276 Nd:YAG is frequently used as an alternate nonsurgical mechanical debridement of peri‐implant diseases. Single time Nd:YAG laser treatment effectively decreases the peri‐implant inflammatory parameters plaque index, bleeding on probing, and probing depth indicated that Nd:YAG laser‐assisted nonsurgical MD is more effective in reducing peri‐implant soft tissue inflammatory parameters than MD alone in the short term but not in long term. 277 The major challenge for orthodontic treatments lies in moving the tooth and shortening the time. Nd:YAG laser irradiation on orthodontic tooth movement with 1064 nm stimulates osteoblasts via producing ROS and nitric oxide. A higher RANKL/OPG ratio leads to the activation of osteoclasts. Higher RANKL expression was observed in the prolonged laser irradiation side, while no change was noticed in the expression of OPG. 228 It has been found that the Nd:YAG laser irradiation of bone for the long term severely delays bone healing as compared to positive control bur osteotomy sites and in patients with osteopenia or osteoporosis. 133 , 278 So the slight modification of Nd:YAG laser with silicon carbide on titanium‐6 aluminum‐4 vanadium (Ti6Al4V) alloys had been prepared to promote the osteoblast cell growth effectively. 224 To exterminate the delayed bone healing induced by Nd:YAG, Kim et al. use high‐power, low‐level Nd:YAG laser, which increases osteoblast activity very efficiently, accelerating the mineral deposition via activation of the BMP‐2‐related signaling pathway in MC3T3‐E1 osteoblasts. 132 A pulsed Nd:YAG laser is an effective physiotherapy modality used as a Class IV high‐intensity laser therapy combined with exercise, which effectively increases lumbar and total hip BMD after 24 weeks of treatment, with effects lasting up to 1 year. High‐intensity, pulsed, and high‐power laser irradiation applied once every 2 days for 2 weeks effectively enhanced bone regeneration in an osseous defect in rats. The power magnitude did not affect the osseous regeneration process but was presumed to be more efficient at the dose of 0.75 W, lower than 3 W. These data indicated that the Nd:YAG laser light could heal local bone loss after surgical treatment. 278
Enamel matrix proteins (EMPs) are widely used in periodontal surgery for the regeneration of periodontal tissues. The use of Nd:YAG laser with EMP heals the intrabony defects of periodontal disease. This treatment approach decreases the probing depth and increases the clinical attachment level compared to baseline values. 225 Similarly, Nd:YAG laser in combination with scaling and root planning (SRP) alleviates periodontal inflammatory parameters plaque index, gingival index, and probing pocket depth, as well as reduces marginal bone loss compared to treatment by SRP alone. 226 The nanophosphors of GdF3:Nd3+ coated with poly(maleic anhydride‐alt‐1‐octadicene) (PMAO) have no significant cellular toxicity for concentrations up to 200 mg ml−1. Furthermore, the incorporation of Gd into the nanocrystalline structure makes an ideal structure for use as MRI contrast agents (Figure 4IV). 280 Rocha et al. found that neodymium‐doped LaF3 core/shell nanoparticles emerge as relevant sub‐tissue optical probes for bioimaging. 281 Further experiments from their team reported that Nd3+‐doped LaF3 (Nd3+:LaF3) nanoparticles exhibit fluorescence in three main emission channels of Nd3+ ions like 910, 1050, and 1330 nm, respectively. The optimal fluorescence of Nd3+‐doped LaF3 nanoparticles in terms of relative emission intensities, penetration depths, and sub tissue optical dispersion is higher in 4F3/2→4I11/2 (1050 nm in the second biological window) than the 4F3/2→4I9/2 (910 nm, in the first biological window). 282
FIGURE 4.
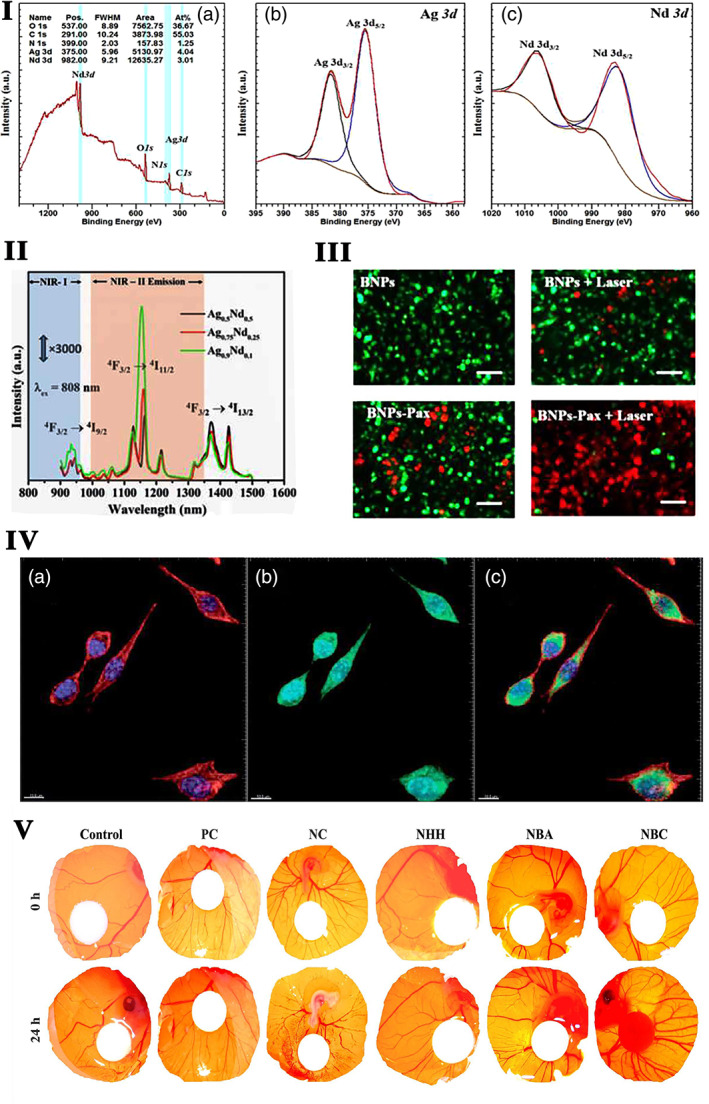
I. Photoemission spectra of BNPs: (a) survey spectra and high‐resolution spectra of (b) Ag 3d (c) Nd 3d. II. Emission spectra of Ag−Nd BNPs on excitation with 808 nm reveals mission ability in the NIR (750–1600 nm) region, with strong emission in the region of the second biological window, which is more transparent for deep tissue penetration. III. Fluorescence images of treated cells (scale bar = 100 μm). Source: Reprinted with permission from ref. 279. Copyright 2017, Elsevier. IV. Bimodal imaging by rare‐earth nanoparticles. Multiphoton microscopy images of fibroblast cells with PMAO coated GdF3:Nd3+ nanoparticles. (a) Image of the DAPI‐stained nuclei (blue channel) and phalloidin‐stained cytoplasm (red channel). (b) Observed emission of the nanoparticles under 488 nm excitation. The green color denotes emission correlated with the cytoplasm, and the light blue color denotes emission correlated with the nuclei. (c) Images of the DAPI, phalloidin, and fluorescent channels together. Source: Reprinted with permission from ref. 280. Republished with permission of Royal Society of Chemistry, 2013, permission conveyed through Copyright Clearance Center, Inc. V. The angiogenic property of Nd nanopolymorphs assessed using the chorioallantoic membrane (CAM) chick egg model. PC, positive control (20 ng VEGF‐treated CAM), NC, negative control (200 μM thalidomide‐treated CAM), NHH, Nd nanoparticles, NBA, Nd nanocubes, NBC, Nd nanorods. Source: Reprinted with permission from ref. 18. Copyright 2019, Elsevier
Nano‐sized neodymium oxide (Nd2O3) arrests the S‐phase of the cell cycle, disrupts mitochondrial membrane potential, and inhibits proteasome activity, leading to autophagy in non‐small cell lung cancer NCI‐H460 cell. 283 Microwave‐assisted polyol‐based chitosan‐functionalized silver‐neodymium bimetallic nanoparticles (Ag‐Nd BNPs, 10 nm) exhibit fluorescence in the NIR region and magnetic properties (Figure 4I,II). Ag‐Nd BNPs had excellent biocompatibility and also promoted the loading of the anticancer drug paclitaxel. The synergistic effect of paclitaxel and the photothermal property enables Ag‐Nd BNPs to destroy cancer cells in vitro at a low dose compared to single therapy (Figure 4III). 279 Nd‐diethylene triamine penta acetate acid (Nd‐DTPA) complex shows bright narrow‐band emission at 1330 nm for in vivo NIR‐II bioimaging with rapid renal excretion and high biocompatibility and optical‐guided small tumor (down to ~3 mm) detection. 284 Polyacrylic acid (PAA)‐modified NaLuF4:Gd/Nd nanorods are used in tiny tumor detection. The NIR‐II emission at 1056 nm and 1328 nm with high photostability of Nd can utilize for NIR‐II optical imaging of small tumor (5 mm) diagnosis and small blood vessel with a high resolution (~105 μm). 285 Recently, Ma et al. prepared implantable multifunctional material of Nd‐Ca‐Si silicate glasses and glass/alginate composite hydrogels, which have photothermal properties with unique temperature monitoring, photothermal function, and wound healing bioactivity that can be used for localized thermal therapy for cancer treatment. Besides, the composite hydrogel has bioactivity to repair heat damage‐caused wounds by PTT due to the bioactive silicate components. 230 These findings demonstrate that the explored lanthanide‐based probes are promising NIR contrast agents for future biomedical applications, such as early diagnosis of a small tumor, vascular‐related disease imaging, angiogenesis, and diagnosis. Recently Ansari et al. reported that surface‐modified mesoporous silica micro‐cocoon with neodymium hydroxide (Nd(OH)3) shows good cell viability even at high concentrations and hydrophilic conditions. These nontoxic cocoon‐shaped microstructures could be potentially suitable candidates for optical bio‐probes and drug delivery applications. 286 Nd2O3 exposure on human bronchial epithelial cells (16HBE) initiates an inflammatory response via the p‐STAT3 pathway. 227 Nd2O3‐treated 16HBE cells release the pro‐inflammatory cytokines IL‐6 and IL‐8 and upregulate circRNA 0039411 (circ_0039411) by sponging miR‐93‐5p. 227 These anticancer applications of RE smart nano‐biomaterials might be helpful to combine with the osteogenic treatment during cancer metastasis‐induced bone loss.
Our previous research revealed that neodymium nanoparticles exhibit a redox‐mediated angiogenic response in a shape‐dependent manner (Figure 4V). The redox signaling perceived via PKM2‐NOX4 signaling pathways activates the pro‐angiogenic factors, namely, VE‐cadherin, HIF1α, VEGF, and VEGFR2, to facilitate the angiogenic process in EA. Hy 926 cells. 18 The static magnetic field of neodymium is helpful to promote the bone formation faster after the bone is wounded. The implant stability quotient values and tissue response after implant placement under the influence of the magnetic field are significantly higher than on the nonmagnetic side. A positive correlation has existed between the magnetic field and osseointegration. 287 Nd:YAG laser irradiation significantly enhances the amount of orthodontic tooth movement, the expressions of ALP and RANKL at the pressure site, and no difference in OPG expression. 228 These effects stimulate osteoclast and osteoblast activation and accelerate bone metabolism during tooth movement. The laser melting method alloyed neodymium with Mg‐5.6, Zn‐0.5, and zirconia enhances corrosion resistance and exhibits excellent biocompatibility. 288 Shreds of evidence revealed that microorganisms play the chief role in causing peri‐implantitis. Short pulse laser‐induced by Nd:YAG Q‐switch laser in nanoseconds cleans contaminated implant surfaces to treat peri‐implantitis significantly. 229
Besides the application in laser irradiation, bone healing, and bioimaging, the nanoparticles of neodymium (III) hexacyanoferrate (II) (NdHCF) coated on the surface of carbon paste electrode are used for sensing the glucose by enzymatic reaction of the glucose oxidase (GOx) with NdHCF. 289 Pourjavid et al. developed the highly selective Nd(III) PVC‐based membrane sensor with sodium tetraphenylborate (NaTPB) and oleic acid (OA) as anionic additives and benzyl acetate (BA), dibutyl phthalate (DBP), o‐nitrophenyloctyl ether (NPOE), and acetophenone (AP) as plasticizing solvent mediators to trace Nd (III) ions in some binary mixtures such as mouth washing solutions, soil, and sediment samples. 290 Further, neodymium and fluorine‐doped TiO2 act as a photocatalyst, which increases the rate of methylene blue degradation to about 1.76 and 1.45 times higher than undoped TiO2 in ultraviolet light and visible light, respectively. 291
5.5. Lanthanum and other RE metal‐doped nano‐biomaterials
Lanthanum is the second most reactive and malleable silvery‐white rare‐earth metal. Lanthanum was discovered in 1839 by Carl Gustaf Mosander. Lanthanum occurs in the rare‐earth minerals monazite and bastnasite. Lanthanum compounds are used as hosts for phosphors in fluorescent lighting and X‐ray detectors. 292 Lanthanum oxide nanoparticles (LONPs) exert their action via the release of ROS. LONP extracts do not exert any acute systemic toxicity effects in mice. On the other hand, LONP exerts toxicity to the liver following oral administration, suggesting that these particles are absorbed from the gastrointestinal tract and deposited in the hepatobiliary system. LONP did not show any mutation in the Ames test, both in the presence or absence of S‐9. 293 The accumulation of lanthanides in hepatocytes gradually increases dose dependent with exposure to the elements like La and Ce. These lanthanides enter hepatocytes and accumulated in the nuclei, and induce oxidative damage in hepatic nuclei and mitochondria, as indicated by decreased levels of SOD, CAT, and GSH. 294 Hydrothermally prepared Yb3+, Ho3+ co‐doped fluorapatite (FA:Yb3+/Ho3+), and hydroxyapatite (HA:Yb3+/Ho3+) particles exhibited green (FA:Yb3+/Ho3+) and red (HA:Yb3+/Ho3+) upconversion emissions under 980 nm near‐infrared excitation due to its lattice structure and composition. The upconversion apatite particles are used to distinguish implanted material from bone tissue. An image superposition method provides a novel strategy for long‐term fluorescence tracking of implanted material or scaffold during bone regeneration (Figure 5II). 220 Magnetic lanthanum‐doped HA/CS scaffolds recruit rat BMSCs and modulate host‐to‐scaffold immune responses by promoting M2 macrophage polarization in vitro by upregulating the phosphorylation of the Smad 1/5/9 pathway that eventually promote bone regeneration. 92 Furthermore, lanthanum‐doped scaffolds promote osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) through the Wnt/β‐catenin signaling pathway and induce high expression of the osteogenic markers and enhance bone regeneration in rat critical‐sized calvarial defect sites. 221 Another study reported that La3+ ions in the bone scaffolds remarkably induce the osteogenic differentiation of rabbit BMSCs via the activation of the TGFβ signaling pathway. 222
FIGURE 5.
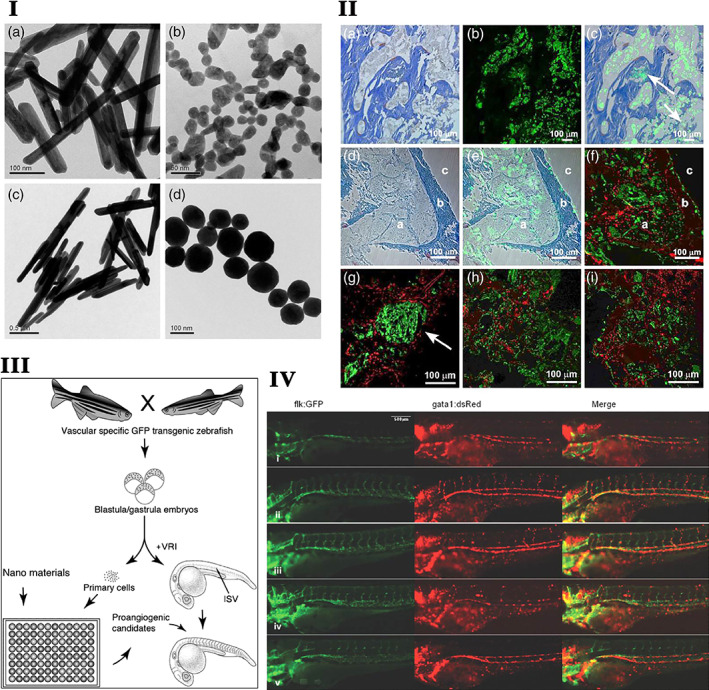
I. Representative TEM images of nanoparticles with proangiogenesis activity. (a) Eu rods, (b) Eu spheres, (c) Tb rods, and (d) Tb spheres. Source: Republished with permission of Royal Society of Chemistry. Reprinted with permission from ref. 295. Copyright 2016, Copyright Clearance Center, Inc. II. Yb(3+)/Ho(3+) co‐doped apatite upconversion nanoparticles to distinguish implanted material from bone tissue. (a) The light image of the Masson's stained histological section of new bone tissue (matured: blue, growing: red). (b) The upconversion green fluorescent image of the implanted FA:10Yb3+/0.5Ho3+ particles. (c) Their overlap image after 4 months. (d) The light image of the stained new bone tissue after 6 months. (e) Overlapping image of the light image and the upconversion green fluorescent image of the implanted FA:10Yb3+/0.5Ho3+ particles. (f) The superposition of the red fluorescent image of the new bone tissue under 561 nm laser excitation and the green fluorescent image of the FA:10Yb3+/0.5Ho3+ particles under 980 nm NIR excitation. The confocal superposition images of FA:10Yb3+/0.5Ho3+ particles (green) and new bone tissue (red) at 2 (g), 4 (h), and 6 (i) months after implantation. Source: Reprinted with permission from ref. 220. Copyright 2016, American Chemical Society. III. Schematic diagram showing the overall strategy and methodology of our experiments illustrating Tg(flk: EGFP) transgenic primary cell and whole embryo‐based high‐throughput screening for nanomaterials with proangiogenesis activity. Source: Reprinted with permission from ref. 295. Copyright 2016, Royal Society of Chemistry. IV. Lanthanide nanoparticles could recover circulation in VRI pretreated zebrafish embryos. Zebrafish embryos at 72 hpf. (i) Blank control, (ii) 100 μg ml−1 Eu rods, (iii) 100 μg ml−1 Eu spheres, (iv) 100 μg ml−1 Tb rods, and (v) 100 μg ml−1 Tb spheres. The green channel represents the blood vessels, while the red channel represents the mature blood cells. The merged pictures indicate that the embryonic circulation in the ISV region has recovered after the treatment of nanoparticles in this method. Source: Reprinted with permission from ref. 295. Republished with permission of Royal Society of Chemistry, 2016, Copyright Clearance Center, Inc. SEM, scanning electron microscope; TEM, transmission electron microspcope
Among 17 RE elements, the osteogenic and bone defect healing potential of only a few RE elements had been extensively explored. Bone regeneration related‐biological functions of other RE metal‐based nanomaterials are reported sporadically. Radiolabeled arginine‐glycine‐aspartic acid (RGD)‐functionalized Er3+/Yb3+ co‐doped NaGdF4 upconversion nanophosphors (UCNPs) had been developed to specifically target the αvβ3 integrin‐expressing U87MG tumor cells and xenografted tumor models for tumor angiogenesis. 168 It has been reported that Eu III(OH)3 and TbIII(OH)3 promote angiogenesis in the transgenic zebrafish model. (Figure 5I,III,IV) 295 Zou et al. reported that the one‐pot hydrothermal carbonization method synthesized praseodymium co‐doped carbon quantum dots (Ce/Pr‐C GR‐HA) enhance hydroxyl radical scavenging property with favorable biocompatibility and negligible cytotoxicity. These carbon dots are readily internalized into the cytoplasm and decrease ROS level. 186
Further radiolabeled arginine‐glycine‐aspartic acid (RGD)‐functionalized Er3+/Yb3+ co‐doped NaGdF4 UCNPs had been developed to specifically target the αvβ3 integrin‐expressing U87MG tumor cells and xenografted tumor models for tumor angiogenesis. 168 Samarium‐doped YVO4 nanoparticles (20–50 nm) show significant toxicity in RAW 264.7 macrophages at concentrations of 25 mg/ml than erbium‐doped YVO4. 188 Ethylenediamine tetramethylene phosphonic acid (EDTMP), and technetium‐99m‐labeled samarium nanoparticles accumulate in the bone tissue for extended periods (150 min), resulting in the prolonged release of EDTMP at the target site. This prolonged release may be a more optimal treatment for the management of cancer bone metastasis‐related pain. 189 Morais et al. fabricated samarium (Sm3+)‐doped P2O5 glass‐reinforced HA‐based bone composites, which enhance the F‐actin cytoskeleton organization and cell proliferation and expression of relevant osteoblastic genes. Also, Sm3+ doping reduces the adhesion of S. aureus and S. epidermidis on bone substitutes. The improved osteoblastic behavior and the antibacterial effects are dependent on the amount of samarium in the composite. 231 Augustine et al. reported that Y2O3 nanoparticles incorporated polycaprolactone scaffolds promote the expression of cell proliferation and angiogenesis‐related markers such as VEGF and endothelial growth factor receptor (EGFR) in fibroblasts (L‐929) and osteoblast‐like cells UMR‐106. 232
Erbium:YAG (Er:YAG) laser‐assisted bone irradiation promotes inflammatory cell infiltration, fibroblastic reaction, and revascularization adjacent to the irradiated bone surface. 296 Even though Er:YAG is being used in clinical practice, the water content of bone usually changes with the position. At the same time, the amount of water spray in the process of laser irradiation is also uncertain. In order to avoid this problem, Huang et al. used optical coherence tomography (OCT) to characterize the roughness and thickness of the heterogeneous layer on the cortical bone surface with different moisture contents that led to different ablation effects. The results from their study showed that OCT could quickly and accurately evaluate the differences between the moisture content, as compared to histology and scanning electron microscope (SEM). 233 NaYF4:Yb, Er@CaF2 nanoparticles with a small size (10–13 nm) robustly enhance (ca. 300 times) upconversion emission compared with the pristine nanoparticles. The CaF2 shell protects the rare‐earth ions from leaking when the nanoparticles are exposed to the buffer solution and ensure biological safety for the potential bio probe applications. 13 Nanoparticle‐based in vivo imaging is hindered by the autofluorescence of the host cells and tissues. This issue could be addressed by the use of HA:Yb/Ho as an upconversion material. Ytterbium (Yb) and holmium (Ho) co‐doped HA matrix favors by its bright fluorescence under NIR irradiation and enhances bone formation. 297 Another study conducted by Nethi et al. extensively studied the pro‐angiogenic properties of terbium hydroxide nanorods. They reported that the pro‐angiogenic property of Tb enhances wound healing in mouse models. 191
6. CHALLENGES OF RE ELEMENTS AND THEIR USE IN BONE REGENERATION
RE elements hold unique biological properties required for effective bone regeneration, such as pro‐angiogenic, immunomodulatory, antimicrobial, and osteogenic. 18 , 129 , 197 , 280 The various biological processes and signaling molecules involved in RE material‐mediated bone defects healing are depicted in Figure 6. The advances in RE nano‐biomaterials for bone tissue engineering and implantology are aforementioned in this review. Overall, potential applications of RE materials in bone tissue engineering and implantology are depicted in Figure 7. RE materials in bone tissue engineering and bone defect healing in the clinic are still a long way to go. One of the major challenges in RE material‐based bone regeneration is progenitor cells' recruitment and biological activity. Some RE nanomaterials are engaged in recruiting immature progenitor cells like MSCs and stimulating them to develop osteoblasts, mediated by a cascade of signals and the activations of several extra and intracellular receptors. The recruitment of progenitor cells is mainly regulated via epigenetic, cellular reprogramming, cell metabolism, and autophagy. 298 It has been reported that decreased level of autophagy in human MSCs reduces osteoblast differentiation. 299 The molecular mechanisms involved in RE material‐induced autophagy in bone cells are not yet fully elucidated. The recruitment and activation of immune cells are essential for effective and accelerated bone fracture healing. RE nanomaterials had been reported to modulate macrophage polarization during bone defect healing. However, the effect of RE nanomaterials on the expansion and activation of various immune cells regulating bone homeostasis, including T cells, B cells, and neutrophils, has not been investigated yet.
FIGURE 6.
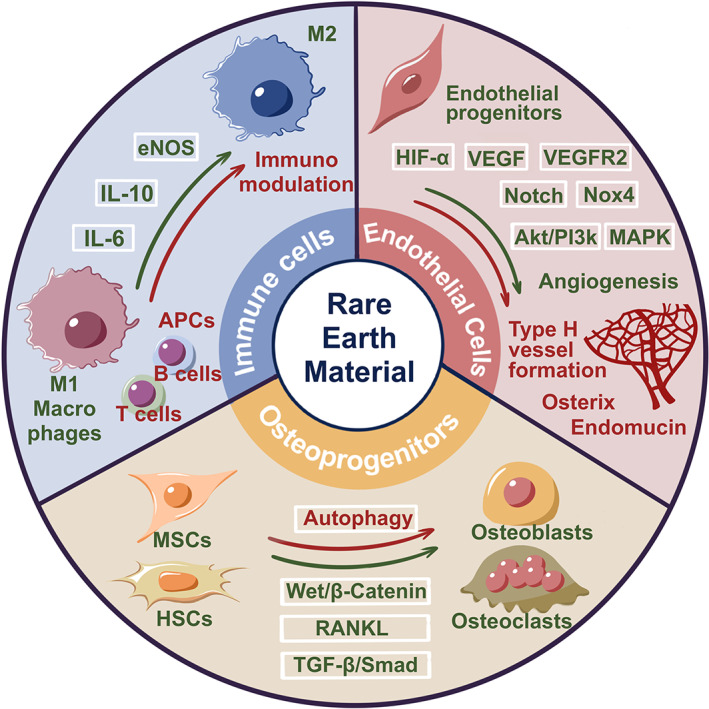
Advances and prospects of molecular mechanisms involved in RE smart nano‐biomaterial‐based bone tissue engineering and implant osseointegration. Green color text and arrows indicate the already explored mechanisms, and the red color text and arrows indicate the possible mechanisms that need to be explored
FIGURE 7.
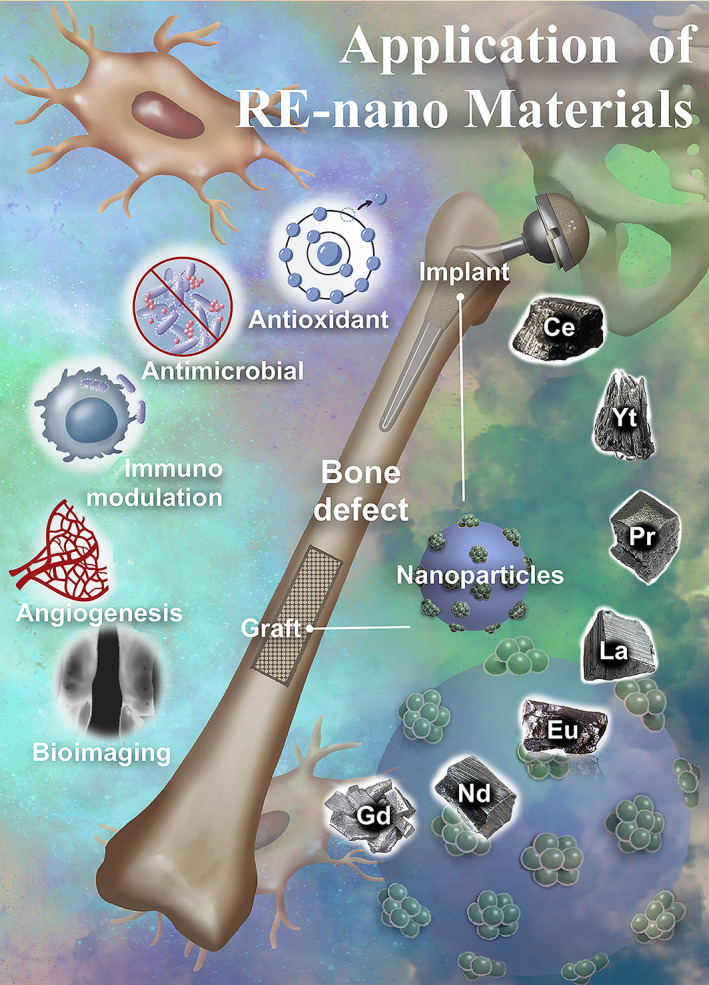
Potential applications of RE biomaterials in bone tissue engineering and implantology
Furthermore, in the bone fracture microenvironment, the ROS levels are abundantly high and affect bone reconstruction. 300 Excessive ROS production can induce osteoclastogenesis, 57 whereas hydrogen peroxide suppresses the osteoblastic differentiation process in primary mouse BMSCs. 301 There is an opposing role of RE materials in producing reactive oxygen species and altering the redox states in the bone defect site. Thereby, it is inevitable to tune or modulate the redox signaling intersecting the current problem. Most of these studies lack the in‐depth investigation on local and systemic adverse effects of in vivo applied RE nano‐biomaterials in long‐term use. Therefore, designing suitable graft materials and optimizing the proper dose of RE material to stimulate biological functions required for bone regeneration is the most challenging. The clinical usage of rare‐earth‐based materials in the tissue engineering field is restricted by a lack of site specificity and sustained delivery of RE elements. Direct injection of nanomaterials in the minor defects and fracture sites and systemic injection targeting osteoporotic bone/defect sites are under investigation. 302 , 303 , 304 Whereas nonunion fractures and critical‐sized bone defects need specialized treatment modalities. Mainstream reports from the literature had indicated the in vitro and in vivo osteogenic properties of RE nanoparticles. However, literature has indicated the inhibitory effect of RE nanoparticles on cell viability and osteogenic potential. 77 , 203 , 257 This inhibitory effect was mainly related to the dose of RE metals and the duration of the incubation period in vitro.
7. PROSPECTS OF RE METALS IN BONE TISSUE ENGINEERING AND IMPLANTOLOGY
Bone regeneration is a complex process involving numerous factors, including the recruitment of progenitor cells, inflammation, early angiogenesis, and osteogenesis. RE nanomaterials have autophagy augmenting potential, 305 whereas RE materials can induce autophagy, but there is no adequate evidence to prove this phenomenon. In order to intersect the role of RE materials induced autophagy would open up the new vistas in bone tissue engineering that can be applied to induce bone regenerative potential. RE nanomaterials contribute to sustaining mild ROS levels, which could modulate the redox state via autophagy in the MSCs to regulate the osteogenic processes effectively and eventually heal the bone defect.
Effective bone regeneration requires a continuous blood supply. Coordination between osteogenesis and angiogenesis is crucial for proper bone regeneration. 306 , 307 , 308 Osteogenesis, angiogenesis, and osseointegration are essential for the successful restoration of bone mass. Tuning of such factors by designing with RE nano biomaterials is critical for bone tissue engineering. 46 , 309 In recent years, great attention has been drawn to coupling angiogenesis and osteogenesis to promote type H vessel formation. Type H vessels are a subtype of the capillary with high expression of CD31 and endomucin and promote osteogenesis. Type H vessels can actively direct bone formation by producing factors that stimulate the proliferation and differentiation of osteoprogenitors in the bone marrow. 81 , 310 , 311 , 312 Type H vessel‐inducing potential of rare‐earth‐based nanomaterials is not adequately studied. Understanding the role of RE‐based materials on type H vessel formation may open up new vistas in the bone tissue engineering field.
Osteocytes play a vital role in bone modeling, remodeling, and homeostasis. The primary function of osteocytes is to convert mechanical stimuli to biological signalings that regulate the functions of osteoblasts, osteoclasts, and immune cells. The effect of RE nanomaterials on osteocytes function has not been reported yet. Future research should focus on designing RE smart nano‐biomaterials that can modulate osteocyte function and promote bone regeneration. Similarly, immunomodulation regulation‐based bone tissue engineering is currently a hot research topic. Investigating the use of RE nanomaterials on spatio temporal control of macrophage polarization and infiltration of various immune cells, including T cells, B cells, and neutrophils, would lead to the applicability of nano immunoenginneering approaches in bone tissue.
The majority of cancer easily metastasized in the bone. The cancer metastasized to the bone is very difficult to treat and causes excessive osteolysis. Scientists are desperately trying to develop therapeutic approaches to treat cancer metastasized in bone and simultaneously rescue bone loss. RE nano‐biomaterials have bone regenerative and anti‐cancer properties. RE nano‐biomaterials has the potential to be used for in vivo imaging of cancer during diagnosis and treatment. Similarly, RE nano‐biomaterials have shown the potential for imaging the newly formed and osteoporotic bone. Therefore, the prospect should be focused on designing RE innovative nano‐biomaterials‐based targeted therapy that can treat cancer metastasized in bone, rescue metastasis‐induced bone loss, and simultaneously visualize the remaining cancer mass and newly formed bone.
In‐depth analysis of local and systemic adverse effects of RE‐nanobiomaterials in large animal models close to humans is another prospect that streamlines the clinical application of RE nano‐bio materials. The clinical complication can be minimized by using rare‐earth nanomaterials as a co dopant in new scaffold‐based mechanics like 3D printing or electrospinning. 313 , 314 , 315 Electrospinning is the most practical and widely explored technique for synthetic membranous grafts. Biopolymers like collagen, silk, and synthetic polymers like polyethylene glycol (PEG) and poly(lactic acid) (PLLA) have been designed for tissue regeneration purposes. 316 , 317 Using the RE‐based nanomaterials with these techniques may yield a remarkable outcome in accelerating bone defect healing with structural and mechanical stability. RE materials doped electrospun or 3D‐printed scaffolds may aid to warrant the sustained release and site‐specific delivery of RE elements based on their physicochemical properties.
8. CONCLUSIONS
In summary, this review portrayed the technological innovations of RE‐based materials in bone tissue engineering. The intriguing features of RE materials such as biocompatibility, narrow band upconversion fluorescence property for deep tissue penetration, and excellent biological properties imply the promising potential of RE materials in biomedical applications. RE materials' antioxidant, immunomodulatory, angiogenic, and osteogenic properties could be utilized to fabricate cost‐effective bone grafts and implants. The mechanism of RE‐material‐based recruitment of progenitor cells, induction of early angiogenesis, and osteogenesis should be studied thoroughly. The role of RE materials on immunomodulation, autophagy machinery in osteoblasts and MSCs, type H vessel formation, osteocytes function, and endothelial regulations need to be thoroughly investigated. Nevertheless, dose optimization, mode of delivery, and local/systemic adverse effects should be thoroughly investigated in large animal models to guarantee the bench‐to‐bed translation. Overall, RE smart nano‐bio materials hold promising potential to substantiate the global demand for cost‐effective biomaterials for bone tissue engineering and implantology in the future.
CONFLICT OF INTERESTS
All authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Duraipandy Natarajan: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); writing – original draft (lead); writing – review and editing (lead). Zhitong Ye: Software (equal); visualization (equal). Liping Wang: Funding acquisition (equal); project administration (equal); resources (equal); supervision (equal). Linhu Ge: Funding acquisition (equal); project administration (equal); resources (equal); supervision (equal). Janak Lal Pathak: Conceptualization (lead); funding acquisition (lead); methodology (lead); project administration (equal); resources (equal); supervision (lead); validation (lead); writing – review and editing (lead).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10262.
ACKNOWLEDGMENTS
The first author (Duraipandy Natarajan) affectionately dedicates this work to the memory of his deceased honored Mother, Mrs. N. Rajalakshmi. This study was supported by the project of Guangzhou Science and Technology Bureau (202002030301), Department of Education of Guangdong Province (2018KTSCX186), and high‐level university construction funding of Guangzhou Medical University (B185006003014, B195002003017, 02‐410‐B205001293, 02‐412‐B205002‐1003017, and 06‐410‐2106035).
Natarajan D, Ye Z, Wang L, Ge L, Pathak JL. Rare earth smart nanomaterials for bone tissue engineering and implantology: Advances, challenges, and prospects. Bioeng Transl Med. 2022;7(1):e10262. doi: 10.1002/btm2.10262
Funding information Department of Education of Guangdong Province, Grant/Award Number: 2018KTSCX186; Guangzhou Science and Technology Bureau, Grant/Award Number: 202002030301; High‐Level University Construction Funding of Guangzhou Medical University, Grant/Award Numbers: 02‐410‐B205001293, 02‐412‐ B205002‐1003017, 06‐410‐2106035, B185006003014, B195002003017
Contributor Information
Liping Wang, Email: wangliplj@126.com.
Linhu Ge, Email: gelinhu@yeah.net.
Janak Lal Pathak, Email: j.pathak@gzhmu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study
REFERENCES
- 1. Escudero A, Becerro AI, Carrillo‐Carrión C, et al. Rare earth‐based nanostructured materials: synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics. 2017;6(5):881‐921. doi: 10.1515/nanoph-2017-0007 [DOI] [Google Scholar]
- 2. Du X, Graedel TE. Global in‐use stocks of the rare earth elements: a first estimate. Environ Sci Technol. 2011;45(9):4096‐4101. doi: 10.1021/es102836s [DOI] [PubMed] [Google Scholar]
- 3. Chakhmouradian AR, Wall F. Rare earth elements: minerals, mines, magnets (and more). Elements. 2012;8(5):333‐340. doi: 10.2113/gselements.8.5.333 [DOI] [Google Scholar]
- 4. Klinger JM. A historical geography of rare earth elements: from discovery to the atomic age. Extr Ind Soc. 2015;2(3):572‐580. doi: 10.1016/j.exis.2015.05.006 [DOI] [Google Scholar]
- 5. Fernandez V. Rare‐earth elements market: a historical and financial perspective. Resour Policy. 2017;53:26‐45. doi: 10.1016/j.resourpol.2017.05.010 [DOI] [Google Scholar]
- 6. Gursoy O, Timelli G. Lanthanides: a focused review of eutectic modification in hypoeutectic Al‐Si alloys. J Mater Res Technol. 2020;9(4):8652‐8666. doi: 10.1016/j.jmrt.2020.05.105 [DOI] [Google Scholar]
- 7. Bünzli J‐C. Lanthanides. Kirk‐Othmer Encyclopedia of Chemical Technology, Hoboken, New Jersey: John Wiley & Sons; 2013:1‐43. [Google Scholar]
- 8. Sun LD, Dong H, Zhang PZ, Yan CH. Upconversion of rare earth nanomaterials. Annu Rev Phys Chem. 2015;66:619‐642. doi: 10.1146/annurev-physchem-040214-121344 [DOI] [PubMed] [Google Scholar]
- 9. Jinsheng L, Lijuan W, Gangke X, Junping M, Yan D. Far infrared radiation property of rare earth mineral composite materials. J Rare Earths. 2006;24(1):281‐283. doi: 10.1016/s1002-0721(07)60381-0 [DOI] [Google Scholar]
- 10. Tikhomirov VK, Mortier M, Gredin P, Patriarche G, Gorller‐Walrand C, Moshchalkov VV. Preparation and up‐conversion luminescence of 8 nm rare‐earth‐doped fluoride nanoparticles. Opt Express. 2008;16(19):14544‐14549. doi: 10.1364/oe.16.014544 [DOI] [PubMed] [Google Scholar]
- 11. Wang HQ, Batentschuk M, Osvet A, Pinna L, Brabec CJ. Rare‐earth ion doped up‐conversion materials for photovoltaic applications. Adv Mater. 2011;23(22–23):2675‐2680. doi: 10.1002/adma.201100511 [DOI] [PubMed] [Google Scholar]
- 12. Lyu L, Cheong H, Ai X, et al. Near‐infrared light‐mediated rare‐earth nanocrystals: recent advances in improving photon conversion and alleviating the thermal effect. NPG Asia Mater. 2018;10(8):685‐702. doi: 10.1038/s41427-018-0065-y [DOI] [Google Scholar]
- 13. Wang YF, Sun LD, Xiao JW, et al. Rare‐earth nanoparticles with enhanced upconversion emission and suppressed rare‐earth‐ion leakage. Chemistry. 2012;18(18):5558‐5564. doi: 10.1002/chem.201103485 [DOI] [PubMed] [Google Scholar]
- 14. Naganuma T, Traversa E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials. 2014;35(15):4441‐4453. doi: 10.1016/j.biomaterials.2014.01.074 [DOI] [PubMed] [Google Scholar]
- 15. Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Redox‐active radical scavenging nanomaterials. Chem Soc Rev. 2010;39(11):4422‐4432. doi: 10.1039/b919677n [DOI] [PubMed] [Google Scholar]
- 16. Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol. 2014;12:5. doi: 10.1186/1477-3155-12-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613‐11618. doi: 10.1073/pnas.0801763105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duraipandy N, Kiran MS. Effects of structural distinction in neodymium nanoparticle for therapeutic application in aberrant angiogenesis. Colloids Surf B Biointerfaces. 2019;181:450‐460. doi: 10.1016/j.colsurfb.2019.05.073 [DOI] [PubMed] [Google Scholar]
- 19. Chono S, Tanino T, Seki T, Morimoto K. Uptake characteristics of liposomes by rat alveolar macrophages: influence of particle size and surface mannose modification. J Pharm Pharmacol. 2007;59(1):75‐80. doi: 10.1211/jpp.59.1.0010 [DOI] [PubMed] [Google Scholar]
- 20. Gal N, Lassenberger A, Herrero‐Nogareda L, et al. Interaction of size‐tailored PEGylated iron oxide nanoparticles with lipid membranes and cells. ACS Biomater Sci Eng. 2017;3(3):249‐259. doi: 10.1021/acsbiomaterials.6b00311 [DOI] [PubMed] [Google Scholar]
- 21. He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657‐3666. doi: 10.1016/j.biomaterials.2010.01.065 [DOI] [PubMed] [Google Scholar]
- 22. Mironava T, Hadjiargyrou M, Simon M, Jurukovski V, Rafailovich MH. Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology. 2010;4(1):120‐137. doi: 10.3109/17435390903471463 [DOI] [PubMed] [Google Scholar]
- 23. Vedantam P, Huang G, Tzeng TR. Size‐dependent cellular toxicity and uptake of commercial colloidal gold nanoparticles in DU‐145 cells. Cancer Nanotechnol. 2013;4(1–3):13‐20. doi: 10.1007/s12645-013-0033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662‐668. doi: 10.1021/nl052396o [DOI] [PubMed] [Google Scholar]
- 25. Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well‐suspended, uniform mesoporous silica nanoparticles. Small. 2009;5(12):1408‐1413. doi: 10.1002/smll.200900005 [DOI] [PubMed] [Google Scholar]
- 26. Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315‐322. doi: 10.1016/j.ijpharm.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 27. Lord MS, Jung M, Teoh WY, et al. Cellular uptake and reactive oxygen species modulation of cerium oxide nanoparticles in human monocyte cell line U937. Biomaterials. 2012;33(31):7915‐7924. doi: 10.1016/j.biomaterials.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 28. Yokel RA, Tseng MT, Dan M, et al. Biodistribution and biopersistence of ceria engineered nanomaterials: size dependence. Nanomedicine. 2013;9(3):398‐407. doi: 10.1016/j.nano.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 29. Semashko VV, Pudovkin MS, Cefalas AC, et al. Tiny rare‐earth fluoride nanoparticles activate tumour cell growth via electrical polar interactions. Nanoscale Res Lett. 2018;13(1):370. doi: 10.1186/s11671-018-2775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romer I, Briffa SM, Arroyo Rojas Dasilva Y, et al. Impact of particle size, oxidation state and capping agent of different cerium dioxide nanoparticles on the phosphate‐induced transformations at different pH and concentration. PLoS One. 2019;14(6):e0217483. doi: 10.1371/journal.pone.0217483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahman P, Green M. The synthesis of rare earth fluoride based nanoparticles. Nanoscale. 2009;1(2):214‐224. doi: 10.1039/b9nr00089e [DOI] [PubMed] [Google Scholar]
- 32. Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare‐earth based nanoparticles. ACS Nano. 2011;5(11):8488‐8505. doi: 10.1021/nn202378b [DOI] [PubMed] [Google Scholar]
- 33. Yada M, Kitamura H, Ichinose A, Machida M, Kijima T. Mesoporous magnetic materials based on rare earth oxides. Angew Chem Int Ed. 1999;38(23):3506‐3510. doi:10.1002/(Sici)1521‐3773(19991203)38:23<3506::Aid‐Anie3506>3.0.Co;2‐2 [DOI] [PubMed] [Google Scholar]
- 34. Zeng Z, Xu Y, Zhang Z, et al. Rare‐earth‐containing perovskite nanomaterials: design, synthesis, properties and applications. Chem Soc Rev. 2020;49(4):1109‐1143. doi: 10.1039/c9cs00330d [DOI] [PubMed] [Google Scholar]
- 35. Meiser F, Cortez C, Caruso F. Biofunctionalization of fluorescent rare‐earth‐doped lanthanum phosphate colloidal nanoparticles. Angew Chem Int Ed Engl. 2004;43(44):5954‐5957. doi: 10.1002/anie.200460856 [DOI] [PubMed] [Google Scholar]
- 36. Liu Q, Chen M, Sun Y, et al. Multifunctional rare‐earth self‐assembled nanosystem for tri‐modal upconversion luminescence/fluorescence/positron emission tomography imaging. Biomaterials. 2011;32(32):8243‐8253. doi: 10.1016/j.biomaterials.2011.07.053 [DOI] [PubMed] [Google Scholar]
- 37. Gai S, Li C, Yang P, Lin J. Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem Rev. 2014;114(4):2343‐2389. doi: 10.1021/cr4001594 [DOI] [PubMed] [Google Scholar]
- 38. Sun Y, Zhu X, Peng J, Li F. Core‐shell lanthanide upconversion nanophosphors as four‐modal probes for tumor angiogenesis imaging. ACS Nano. 2013;7(12):11290‐11300. doi: 10.1021/nn405082y [DOI] [PubMed] [Google Scholar]
- 39. Shen J, Sun LD, Yan CH. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Trans. 2008;42:5687‐5697. doi: 10.1039/b805306e [DOI] [PubMed] [Google Scholar]
- 40. Liu Z, Li B, Wang B, et al. Magnetic nanoparticles modified with DTPA‐AMC‐rare earth for fluorescent and magnetic resonance dual mode imaging. Dalton Trans. 2012;41(28):8723‐8728. doi: 10.1039/c2dt30125c [DOI] [PubMed] [Google Scholar]
- 41. Shen J, Zhao L, Han G. Lanthanide‐doped upconverting luminescent nanoparticle platforms for optical imaging‐guided drug delivery and therapy. Adv Drug Deliv Rev. 2013;65(5):744‐755. doi: 10.1016/j.addr.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 42. Dutta R, Pandey AC. The applicability of rare earth gadolinium oxide nanoparticles for biomedical applications. Nanosci Technol. 2015;2(2):1‐6. doi: 10.15226/2374-8141/2/2/00130 [DOI] [Google Scholar]
- 43. Singh S. Cerium oxide‐based nanozymes: redox phenomenon at biointerfaces. Biointerphases. 2016;11(4):04B202. doi: 10.1116/1.4966535 [DOI] [PubMed] [Google Scholar]
- 44. Zhu X, Zhang J, Liu J, Zhang Y. Recent progress of rare‐earth doped upconversion nanoparticles: synthesis, optimization, and applications. Adv Sci (Weinh). 2019;6(22):1901358. doi: 10.1002/advs.201901358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie Y, Li K, Zheng X. Biological coatings for implant surface modification. In The World Scientific Encyclopedia of Nanomedicine and Bioengineering II; Frontiers in Nanobiomedical Research (Vol. 3). Singapore: World Scientific Publishing Company; 2017;183‐228. doi: 10.1142/9789813202573_0005 [DOI] [Google Scholar]
- 46. Zhang K, Wang S, Zhou C, et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neouze M‐A, Schubert U. Surface modification and functionalization of metal and metal oxide nanoparticles by organic ligands. Monatsh Chem. 2008;139(3):183‐195. doi: 10.1007/s00706-007-0775-2 [DOI] [Google Scholar]
- 48. Yada M, Kitamura H, Ichinose A, Machida M, Kijima T. Mesoporous magnetic materials based on rare earth oxides. Angewandte Chemie International Edition. 1999;38(23):3506‐3510. doi:10.1002/(sici)1521‐3773(19991203)38:23<3506::aid‐anie3506>3.0.co;2‐2 [DOI] [PubMed] [Google Scholar]
- 49. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17(2):S77‐S83. doi: 10.1016/8756-3282(95)00183-e [DOI] [PubMed] [Google Scholar]
- 50. Horowitz M. Matrix proteins versus cytokines in the regulation of osteoblast function and bone formation. Calcif Tissue Int. 2003;72(1):5‐7. doi: 10.1007/s00223-002-1048-z [DOI] [PubMed] [Google Scholar]
- 51. Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2(1):81‐94. doi: 10.1023/a:1010011209064 [DOI] [PubMed] [Google Scholar]
- 52. Schneider GB, Relfson M, Nicolas J. Pluripotent hemopoietic stem cells give rise to osteoclasts. Am J Anat. 1986;177(4):505‐511. doi: 10.1002/aja.1001770408 [DOI] [PubMed] [Google Scholar]
- 53. Rimondi E, Zweyer M, Ricci E, Fadda R, Secchiero P. Receptor activator of nuclear factor kappa B ligand (RANKL) modulates the expression of genes involved in apoptosis and cell cycle in human osteoclasts. Anat Rec (Hoboken). 2007;290(7):838‐845. doi: 10.1002/ar.20550 [DOI] [PubMed] [Google Scholar]
- 54. Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008;138(2):415S‐418S. doi: 10.1093/jn/138.2.415S [DOI] [PubMed] [Google Scholar]
- 55. Bull H, Murray PG, Thomas D, Fraser AM, Nelson PN. Acid phosphatases. J Clin Pathol: Mol Pathol. 2002;55(2):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murrills RJ, Stein LS, Dempster DW. Stimulation of bone resorption and osteoclast clear zone formation by low pH: a time‐course study. J Cell Physiol. 1993;154(3):511‐518. doi: 10.1002/jcp.1041540309 [DOI] [PubMed] [Google Scholar]
- 57. Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen‐derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85(3):632‐639. doi: 10.1172/JCI114485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Osdoby P, Krukowski M, Oursler MJ, Salino‐Hugg T. The origin, development and regulation of osteoclasts. Bioessays. 1987;7(1):30‐34. doi: 10.1002/bies.950070107 [DOI] [PubMed] [Google Scholar]
- 59. Arnett TR, Dempster DW. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 1986;119(1):119‐124. doi: 10.1210/endo-119-1-119 [DOI] [PubMed] [Google Scholar]
- 60. Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone‐the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121‐145. doi: 10.1146/annurev-pathol-011110-130203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55(3):287‐299. doi: 10.1002/jcb.240550304 [DOI] [PubMed] [Google Scholar]
- 63. Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: structure, blood supply, cells, matrix, and mineralization. J Bone Joint Surg Am. 1996;45:371‐386. [PubMed] [Google Scholar]
- 64. Bukka P, McKee MD, Karaplis AC. Molecular regulation of osteoblast differentiation. In Bone Formation; Topics in Bone Biology (Vol. 1) London: Springer Nature; 2004;1‐17. doi: 10.1007/978-1-4471-3777-1_1 [DOI] [Google Scholar]
- 65. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233‐1239. doi: 10.1002/jcb.20958 [DOI] [PubMed] [Google Scholar]
- 66. Chambers TJ. Regulation of the differentiation and function of osteoclasts. J Pathol. 2000;192(1):4‐13. doi:10.1002/1096‐9896(2000)9999:9999<::AID‐PATH645>3.0.CO;2‐Q [DOI] [PubMed] [Google Scholar]
- 67. Franz‐Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176‐190. doi: 10.1002/dvdy.20603 [DOI] [PubMed] [Google Scholar]
- 68. Yang DQ, Feng S, Chen W, Zhao H, Paulson C, Li YP. V‐ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast‐mediated bone resorption. J Bone Miner Res. 2012;27(8):1695‐1707. doi: 10.1002/jbmr.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arey LB. Phagocytosis by osteoclasts. Anat Rec. 1917;13(5):269‐272. doi: 10.1002/ar.1090130504 [DOI] [Google Scholar]
- 70. Lacombe J, Karsenty G, Ferron M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle. 2013;12(17):2744‐2752. doi: 10.4161/cc.25825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119‐130. doi: 10.1016/j.bone.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cao W, Helder MN, Bravenboer N, et al. Is there a governing role of osteocytes in bone tissue regeneration? Curr Osteoporos Rep. 2020;18(5):541‐550. doi: 10.1007/s11914-020-00610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Steffi C, Shi Z, Kong CH, Wang W. Modulation of osteoclast interactions with orthopaedic biomaterials. J Funct Biomater. 2018;9(1):18. doi: 10.3390/jfb9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Borciani G, Montalbano G, Baldini N, Cerqueni G, Vitale‐Brovarone C, Ciapetti G. Co‐culture systems of osteoblasts and osteoclasts: simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020;108:22‐45. doi: 10.1016/j.actbio.2020.03.043 [DOI] [PubMed] [Google Scholar]
- 75. Liao X, Lu S, Zhuo Y, et al. Bone physiology, biomaterial and the effect of mechanical/physical microenvironment on MSC osteogenesis: a tribute to Shu Chien's 80th birthday. Cell Mol Bioeng. 2011;4(4):579‐590. doi: 10.1007/s12195-011-0204-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu B, Zhu DY, Yin JH, et al. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication. 2019;11(2):025012. doi: 10.1088/1758-5090/ab0676 [DOI] [PubMed] [Google Scholar]
- 77. Liu D, Zhang J, Li Y, Wang S, Yang M. The effects of Ce on the proliferation, osteogenic differentiation and mineralization function of MC3T3‐E1 cells in vitro. Biol Trace Elem Res. 2012;149(2):291‐297. doi: 10.1007/s12011-012-9423-8 [DOI] [PubMed] [Google Scholar]
- 78. Yuan K, Mei J, Shao D, et al. Cerium oxide nanoparticles regulate osteoclast differentiation bidirectionally by modulating the cellular production of reactive oxygen species. Int J Nanomedicine. 2020;15:6355‐6372. doi: 10.2147/ijn.S257741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou L, Tang S, Yang L, et al. Cerium ion promotes the osteoclastogenesis through the induction of reactive oxygen species. J Trace Elem Med Biol. 2019;52:126‐135. doi: 10.1016/j.jtemb.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 80. Towler DA. Vascular biology and bone formation: hints from HIF. J Clin Invest. 2007;117(6):1477‐1480. doi: 10.1172/JCI32518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376‐380. doi: 10.1038/nature13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin‐deficient mice. J Bone Miner Res. 2007;22(2):286‐297. doi: 10.1359/jbmr.061103 [DOI] [PubMed] [Google Scholar]
- 83. Lee E, Ko JY, Kim J, Park JW, Lee S, Im GI. Osteogenesis and angiogenesis are simultaneously enhanced in BMP2‐/VEGF‐transfected adipose stem cells through activation of the YAP/TAZ signaling pathway. Biomater Sci. 2019;7(11):4588‐4602. doi: 10.1039/c9bm01037h [DOI] [PubMed] [Google Scholar]
- 84. Chen Z, Klein T, Murray RZ, et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19(6):304‐321. doi: 10.1016/j.mattod.2015.11.004 [DOI] [Google Scholar]
- 85. Chen Z, Han S, Shi M, et al. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: from nanoimmunotoxicity to nanoimmunotherapy. Appl Mater Today. 2018;10:184‐193. doi: 10.1016/j.apmt.2017.12.003 [DOI] [Google Scholar]
- 86. Caetano‐Lopes J, Canhao H, Fonseca JE. Osteoimmunology‐ ‐the hidden immune regulation of bone. Autoimmun Rev. 2009;8(3):250‐255. doi: 10.1016/j.autrev.2008.07.038 [DOI] [PubMed] [Google Scholar]
- 87. Longoni A, Knezevic L, Schepers K, Weinans H, Rosenberg A, Gawlitta D. The impact of immune response on endochondral bone regeneration. NPJ Regen Med. 2018;3:22. doi: 10.1038/s41536-018-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Silver I. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts*1. Exp Cell Res. 1988;175(2):266‐276. doi: 10.1016/0014-4827(88)90191-7 [DOI] [PubMed] [Google Scholar]
- 89. Kukita A, Kukita T, Nagata K, et al. The transcription factor FBI‐1/OCZF/LRF is expressed in osteoclasts and regulates RANKL‐induced osteoclast formation in vitro and in vivo. Arthritis Rheum. 2011;63(9):2744‐2754. doi: 10.1002/art.30455 [DOI] [PubMed] [Google Scholar]
- 90. Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell‐macrophage crosstalk and bone healing. Biomaterials. 2019;196:80‐89. doi: 10.1016/j.biomaterials.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Abuid NJ, Gattás‐Asfura KM, LaShoto DJ, Poulos AM, Stabler CL. Biomedical applications of cerium oxide nanoparticles: a potent redox modulator and drug delivery agent. In Nanoparticles for Biomedical Applications. Chapter 17. Amsterdam, Netherlands: Elsevier; 2020;283‐301. doi: 10.1016/B978-0-12-816662-8.00017-5 [DOI] [Google Scholar]
- 92. Wang Q, Tang Y, Ke Q, et al. Magnetic lanthanum‐doped hydroxyapatite/chitosan scaffolds with endogenous stem cell‐recruiting and immunomodulatory properties for bone regeneration. J Mater Chem B. 2020;8(24):5280‐5292. doi: 10.1039/d0tb00342e [DOI] [PubMed] [Google Scholar]
- 93. Boeckx C, Benitez‐Burraco A. Osteogenesis and neurogenesis: a robust link also for language evolution. Front Cell Neurosci. 2015;9:291. doi: 10.3389/fncel.2015.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol Rev. 2018;98(3):1083‐1112. doi: 10.1152/physrev.00014.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Artico M, Bosco S, Cavallotti C, et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med. 2002;10(1):77‐80. [PubMed] [Google Scholar]
- 96. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305‐317. doi: 10.1016/s0092-8674(02)01049-8 [DOI] [PubMed] [Google Scholar]
- 97. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514‐520. doi: 10.1038/nature03398 [DOI] [PubMed] [Google Scholar]
- 98. Fielding C, Mendez‐Ferrer S. Neuronal regulation of bone marrow stem cell niches. F1000 Faculty Rev. 2020;9:614. doi: 10.12688/f1000research.22554.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Terzi A, Suter DM. The role of NADPH oxidases in neuronal development. Free Radic Biol Med. 2020;154:33‐47. doi: 10.1016/j.freeradbiomed.2020.04.027 [DOI] [PubMed] [Google Scholar]
- 100. Zhang Y, Xu J, Ruan YC, et al. Implant‐derived magnesium induces local neuronal production of CGRP to improve bone‐fracture healing in rats. Nat Med. 2016;22(10):1160‐1169. doi: 10.1038/nm.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang D, Ni N, Su Y, et al. Targeting local osteogenic and ancillary cells by mechanobiologically optimized magnesium scaffolds for orbital bone reconstruction in canines. ACS Appl Mater Interfaces. 2020;12(25):27889‐27904. doi: 10.1021/acsami.0c00553 [DOI] [PubMed] [Google Scholar]
- 102. Heo DN, Ko WK, Bae MS, et al. Enhanced bone regeneration with a gold nanoparticle‐hydrogel complex. J Mater Chem B. 2014;2(11):1584‐1593. doi: 10.1039/c3tb21246g [DOI] [PubMed] [Google Scholar]
- 103. Lee D, Heo DN, Kim HJ, et al. Inhibition of osteoclast differentiation and bone resorption by bisphosphonate‐conjugated gold nanoparticles. Sci Rep. 2016;6:27336. doi: 10.1038/srep27336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nold P, Hartmann R, Feliu N, et al. Optimizing conditions for labeling of mesenchymal stromal cells (MSCs) with gold nanoparticles: a prerequisite for in vivo tracking of MSCs. J Nanobiotechnology. 2017;15(1):24. doi: 10.1186/s12951-017-0258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liang H, Jin C, Ma L, et al. Accelerated bone regeneration by gold‐nanoparticle‐loaded mesoporous silica through stimulating immunomodulation. ACS Appl Mater Interfaces. 2019;11(44):41758‐41769. doi: 10.1021/acsami.9b16848 [DOI] [PubMed] [Google Scholar]
- 106. Kumar VB, Khajuria DK, Karasik D, Gedanken A. Silver and gold doped hydroxyapatite nanocomposites for enhanced bone regeneration. Biomed Mater. 2019;14(5):055002. doi: 10.1088/1748-605X/ab28e4 [DOI] [PubMed] [Google Scholar]
- 107. Sivolella S, Stellini E, Brunello G, et al. Silver nanoparticles in alveolar bone surgery devices. J Nanomater. 2012;2012:1‐12. doi: 10.1155/2012/975842 [DOI] [Google Scholar]
- 108. Wu J, Zhang G, Liu J, et al. Synthesis, characteristics, and antibacterial activity of a rare‐earth samarium/silver/titanium dioxide inorganic nanomaterials. J Rare Earths. 2014;32(8):727‐732. doi: 10.1016/s1002-0721(14)60133-2 [DOI] [Google Scholar]
- 109. Predoi D, Balcan RAV. Osteoblast interaction with iron oxide nanoparticles coated with dextrin in cell culture. J Optoelectron Adv Mater. 2008;10(1):152‐157. [Google Scholar]
- 110. Shi SF, Jia JF, Guo XK, et al. Biocompatibility of chitosan‐coated iron oxide nanoparticles with osteoblast cells. Int J Nanomedicine. 2012;7:5593‐5602. doi: 10.2147/IJN.S34348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yuan H, Barbieri D, Luo X, Van Blitterswijk CA, De Bruijn JD. 1.14 calcium phosphates and bone induction. In Comprehensive Biomaterials II. Amsterdam, Netherlands: Elsevier; 2017;333‐349. http://linkinghub.elsevier.com/retrieve/pii/B9780128035818102413 [Google Scholar]
- 112. Chen XR, Bai J, Yuan SJ, et al. Calcium phosphate nanoparticles are associated with inorganic phosphate‐induced osteogenic differentiation of rat bone marrow stromal cells. Chem Biol Interact. 2015;238:111‐117. doi: 10.1016/j.cbi.2015.06.027 [DOI] [PubMed] [Google Scholar]
- 113. Wang L, Fan H, Zhang ZY, et al. Osteogenesis and angiogenesis of tissue‐engineered bone constructed by prevascularized beta‐tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials. 2010;31(36):9452‐9461. doi: 10.1016/j.biomaterials.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 114. Munir K, Lin J, Wen C, Wright PFA, Li Y. Mechanical, corrosion, and biocompatibility properties of Mg‐Zr‐Sr‐Sc alloys for biodegradable implant applications. Acta Biomater. 2020;102:493‐507. doi: 10.1016/j.actbio.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 115. Chen Z, Mao X, Tan L, et al. Osteoimmunomodulatory properties of magnesium scaffolds coated with beta‐tricalcium phosphate. Biomaterials. 2014;35(30):8553‐8565. doi: 10.1016/j.biomaterials.2014.06.038 [DOI] [PubMed] [Google Scholar]
- 116. Ye G, Bao F, Zhang X, et al. Nanomaterial‐based scaffolds for bone tissue engineering and regeneration. Nanomedicine (Lond). 2020;15(20):1995‐2017. doi: 10.2217/nnm-2020-0112 [DOI] [PubMed] [Google Scholar]
- 117. Wang Q, Yan J, Yang J, Li B. Nanomaterials promise better bone repair. Mater Today. 2016;19(8):451‐463. doi: 10.1016/j.mattod.2015.12.003 [DOI] [Google Scholar]
- 118. Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029. doi: 10.1038/boneres.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chen H, Zeng Y, Liu W, Zhao S, Wu J, Du Y. Multifaceted applications of nanomaterials in cell engineering and therapy. Biotechnol Adv. 2013;31(5):638‐653. doi: 10.1016/j.biotechadv.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 120. Zhang ZG, Li ZH, Mao XZ, Wang WC. Advances in bone repair with nanobiomaterials: mini‐review. Cytotechnology. 2011;63(5):437‐443. doi: 10.1007/s10616-011-9367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Balasundaram G, Webster TJ. Nanotechnology and biomaterials for orthopedic medical applications. Nanomedicine (Lond). 2006;1(2):169‐176. doi: 10.2217/17435889.1.2.169 [DOI] [PubMed] [Google Scholar]
- 122. Samhadaneh DM, Mandl GA, Han Z, et al. Evaluation of lanthanide‐doped upconverting nanoparticles for in vitro and in vivo applications. ACS Appl Bio Mater. 2020;3(7):4358‐4369. doi: 10.1021/acsabm.0c00381 [DOI] [PubMed] [Google Scholar]
- 123. Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D. Advances in highly doped upconversion nanoparticles. Nat Commun. 2018;9(1):2415. doi: 10.1038/s41467-018-04813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Portioli C, Pedroni M, Benati D, et al. Citrate‐stabilized lanthanide‐doped nanoparticles: brain penetration and interaction with immune cells and neurons. Nanomedicine (Lond). 2016;11(23):3039‐3051. doi: 10.2217/nnm-2016-0297 [DOI] [PubMed] [Google Scholar]
- 125. Li J, Kang F, Gong X, et al. Ceria nanoparticles enhance endochondral ossification‐based critical‐sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. FASEB J. 2019;33(5):6378‐6389. doi: 10.1096/fj.201802187R [DOI] [PubMed] [Google Scholar]
- 126. Pandey A, Midha S, Sharma RK, et al. Antioxidant and antibacterial hydroxyapatite‐based biocomposite for orthopedic applications. Korean J Couns Psychother. 2018;88:13‐24. doi: 10.1016/j.msec.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 127. Li F, Wang M, Pi G, Lei B. Europium doped monodispersed bioactive glass nanoparticles regulate the osteogenic differentiation of human marrow mesenchymal stem cells. J Biomed Nanotechnol. 2018;14(4):756‐764. doi: 10.1166/jbn.2018.2504 [DOI] [PubMed] [Google Scholar]
- 128. Wu C, Xia L, Han P, et al. Europium‐containing mesoporous bioactive glass scaffolds for stimulating in vitro and in vivo osteogenesis. ACS Appl Mater Interfaces. 2016;8(18):11342‐11354. doi: 10.1021/acsami.6b03100 [DOI] [PubMed] [Google Scholar]
- 129. Patra CR, Bhattacharya R, Patra S, et al. Pro‐angiogenic properties of europium(III) hydroxide Nanorods. Adv Mater. 2008;20(4):753‐756. doi: 10.1002/adma.200701611 [DOI] [Google Scholar]
- 130. Papageorgiou I, Dvorak J, Cosma I, Pfeil A, Teichgraeber U, Malich A. Whole‐body MRI: a powerful alternative to bone scan for bone marrow staging without radiation and gadolinium enhancer. Clin Transl Oncol. 2020;22(8):1321‐1328. doi: 10.1007/s12094-019-02257-x [DOI] [PubMed] [Google Scholar]
- 131. Mastrogiacomo S, Kownacka AE, Dou W, et al. Bisphosphonate functionalized gadolinium oxide nanoparticles allow long‐term MRI/CT multimodal imaging of calcium phosphate bone cement. Adv Healthc Mater. 2018;7(19):e1800202. doi: 10.1002/adhm.201800202 [DOI] [PubMed] [Google Scholar]
- 132. Kim IS, Cho TH, Kim K, Weber FE, Hwang SJ. High power‐pulsed Nd:YAG laser as a new stimulus to induce BMP‐2 expression in MC3T3‐E1 osteoblasts. Lasers Surg Med. 2010;42(6):510‐518. doi: 10.1002/lsm.20870 [DOI] [PubMed] [Google Scholar]
- 133. McDavid VG, Cobb CM, Rapley JW, Glaros AG, Spencer P. Laser irradiation of bone: III. Long‐term healing following treatment by CO2 and Nd:YAG lasers. J Periodontol. 2001;72(2):174‐182. doi: 10.1902/jop.2001.72.2.174 [DOI] [PubMed] [Google Scholar]
- 134. Lasanianos NG, Kanakaris NK, Giannoudis PV. Current management of long bone large segmental defects. Orthop Trauma. 2010;24(2):149‐163. doi: 10.1016/j.mporth.2009.10.003 [DOI] [Google Scholar]
- 135. Berner A, Reichert JC, Muller MB, et al. Treatment of long bone defects and non‐unions: from research to clinical practice. Cell Tissue Res. 2012;347(3):501‐519. doi: 10.1007/s00441-011-1184-8 [DOI] [PubMed] [Google Scholar]
- 136. Vajgel A, Mardas N, Farias BC, Petrie A, Cimoes R, Donos N. A systematic review on the critical size defect model. Clin Oral Implants Res. 2014;25(8):879‐893. doi: 10.1111/clr.12194 [DOI] [PubMed] [Google Scholar]
- 137. Marsh DR, Li G. The biology of fracture healing: optimising outcome. Br Med Bull. 1999;55(4):856‐869. doi: 10.1258/0007142991902673 [DOI] [PubMed] [Google Scholar]
- 138. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551‐555. doi: 10.1016/j.injury.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kolar P, Schmidt‐Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16(4):427‐434. doi: 10.1089/ten.TEB.2009.0687 [DOI] [PubMed] [Google Scholar]
- 140. Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469(11):3118‐3126. doi: 10.1007/s11999-011-1865-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45‐54. doi: 10.1038/nrrheum.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Walters G, Pountos I, Giannoudis PV. The cytokines and micro‐environment of fracture haematoma: current evidence. J Tissue Eng Regen Med. 2018;12(3):e1662‐e1677. doi: 10.1002/term.2593 [DOI] [PubMed] [Google Scholar]
- 143. Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19(5):459‐466. doi: 10.1016/j.semcdb.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 144. Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br. 2007;89(4):425‐433. doi: 10.1302/0301-620X.89B4.18301 [DOI] [PubMed] [Google Scholar]
- 145. Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109‐117. doi: 10.1016/j.abb.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 146. Kang CW, Fang FZ. State of the art of bioimplants manufacturing: part I. Adv Manuf. 2018;6(1):20‐40. doi: 10.1007/s40436-017-0207-4 [DOI] [Google Scholar]
- 147. Kang CW, Fang FZ. State of the art of bioimplants manufacturing: part II. Adv Manuf. 2018;6(2):137‐154. doi: 10.1007/s40436-018-0218-9 [DOI] [Google Scholar]
- 148. Mei L, Zhenxue S, Zhaogang L, Yanhong H, Mitang W, Hangquan L. Effect of surface modification on behaviors of cerium oxide Nanopowders. J Rare Earths. 2007;25(3):368‐372. doi: 10.1016/s1002-0721(07)60438-4 [DOI] [Google Scholar]
- 149. Tok AIY, Du SW, Boey FYC, Chong WK. Hydrothermal synthesis and characterization of rare earth doped ceria nanoparticles. Mat Sci Eng A. 2007;466(1–2):223‐229. doi: 10.1016/j.msea.2007.02.083 [DOI] [Google Scholar]
- 150. Singh V, Singh S, Das S, Kumar A, Self WT, Seal S. A facile synthesis of PLGA encapsulated cerium oxide nanoparticles: release kinetics and biological activity. Nanoscale. 2012;4(8):2597‐2605. doi: 10.1039/c2nr12131j [DOI] [PubMed] [Google Scholar]
- 151. Ting SR, Whitelock JM, Tomic R, et al. Cellular uptake and activity of heparin functionalised cerium oxide nanoparticles in monocytes. Biomaterials. 2013;34(17):4377‐4386. doi: 10.1016/j.biomaterials.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 152. Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L, Vallet‐Regi M. Mesoporous bioactive scaffolds prepared with cerium‐, gallium‐ and zinc‐containing glasses. Acta Biomater. 2013;9(1):4836‐4844. doi: 10.1016/j.actbio.2012.09.024 [DOI] [PubMed] [Google Scholar]
- 153. Li K, Xie Y, You M, Huang L, Zheng X. Cerium oxide‐incorporated calcium silicate coating protects MC3T3‐E1 osteoblastic cells from H2O2‐induced oxidative stress. Biol Trace Elem Res. 2016;174(1):198‐207. doi: 10.1007/s12011-016-0680-9 [DOI] [PubMed] [Google Scholar]
- 154. Li K, Xie Y, You M, Huang L, Zheng X. Plasma sprayed cerium oxide coating inhibits H2O2‐induced oxidative stress and supports cell viability. J Mater Sci Mater Med. 2016;27(6):100. doi: 10.1007/s10856-016-5710-9 [DOI] [PubMed] [Google Scholar]
- 155. Wang K, Mitra RN, Zheng M, Han Z. Nanoceria‐loaded injectable hydrogels for potential age‐related macular degeneration treatment. J Biomed Mater Res A. 2018;106(11):2795‐2804. doi: 10.1002/jbm.a.36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Deliormanli AM. Synthesis and characterization of cerium‐ and gallium‐containing borate bioactive glass scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2015;26(2):67. doi: 10.1007/s10856-014-5368-0 [DOI] [PubMed] [Google Scholar]
- 157. Qi S, Wu J, Xu Y, et al. Chemical stability and antimicrobial activity of plasma‐sprayed cerium oxide‐incorporated calcium silicate coating in dental implants. Implant Dent. 2019;28(6):564‐570. doi: 10.1097/ID.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 158. Wei X‐W, Xu J, Song X‐J, et al. Multi‐walled carbon nanotubes coated with rare earth fluoride EuF3 and TbF3 nanoparticles. Mater Res Bull. 2006;41(1):92‐98. doi: 10.1016/j.materresbull.2005.07.029 [DOI] [Google Scholar]
- 159. Lian J, Yang L, Chen XY, et al. Deposition of ultrathin rare‐earth doped Y2O3phosphor films on alumina nanoparticles. Nanotechnology. 2006;17(5):1351‐1354. doi: 10.1088/0957-4484/17/5/030 [DOI] [Google Scholar]
- 160. Wang Y, Qin W, Zhang J, et al. Synthesis, photoluminescence and bioconjugation of rare‐earth (Eu) complexes‐embedded silica nanoparticles. Solid State Commun. 2007;142(12):689‐693. doi: 10.1016/j.ssc.2007.04.038 [DOI] [Google Scholar]
- 161. Yang X, Xiao S, Ding JW, Yan XH. Luminescence properties of rare earth doped YF3 and LuF3 nanoparticles. J Appl Phys. 2008;103(9):093101. doi: 10.1063/1.2903582 [DOI] [Google Scholar]
- 162. Yin S, Akita S, Shinozaki M, Li R, Sato T. Synthesis and morphological control of rare earth oxide nanoparticles by solvothermal reaction. J Mat Sci. 2007;43(7):2234‐2239. doi: 10.1007/s10853-007-2070-3 [DOI] [Google Scholar]
- 163. Mi CC, Tian ZH, Han BF, Mao CB, Xu SK. Microwave‐assisted one‐pot synthesis of water‐soluble rare‐earth doped fluoride luminescent nanoparticles with tunable colors. J Alloys Compd. 2012;525:154‐158. doi: 10.1016/j.jallcom.2012.02.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Antić Ž, Krsmanović RM, Nikolić MG, et al. Multisite luminescence of rare earth doped TiO2 anatase nanoparticles. Mater Chem Phys. 2012;135(2–3):1064‐1069. doi: 10.1016/j.matchemphys.2012.06.016 [DOI] [Google Scholar]
- 165. Gaspar RDL, Rodrigues EM, Mazali IO, Sigoli FA. Luminescent properties of passivated europium(iii)‐doped rare earth oxide sub‐10 nm nanoparticles. RSC Adv. 2013;3(8):2794–2801. doi: 10.1039/c2ra22532h [DOI] [Google Scholar]
- 166. Zhu H, Shang Y, Wang W, et al. Fluorescent magnetic Fe3O4/rare earth colloidal nanoparticles for dual‐modality imaging. Small. 2013;9(17):2991‐3000. doi: 10.1002/smll.201300126 [DOI] [PubMed] [Google Scholar]
- 167. Hsiao JK, Tsai CP, Chung TH, et al. Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small. 2008;4(9):1445‐1452. doi: 10.1002/smll.200701316 [DOI] [PubMed] [Google Scholar]
- 168. Lee J, Lee TS, Ryu J, et al. RGD peptide‐conjugated multimodal NaGdF4:Yb3+/Er3+ nanophosphors for upconversion luminescence, MR, and PET imaging of tumor angiogenesis. J Nucl Med. 2013;54(1):96‐103. doi: 10.2967/jnumed.112.108043 [DOI] [PubMed] [Google Scholar]
- 169. Zhang B, Yang W, Yu J, et al. Green synthesis of sub‐10 nm gadolinium‐based nanoparticles for sparkling kidneys, tumor, and angiogenesis of tumor‐bearing mice in magnetic resonance imaging. Adv Healthc Mater. 2017;6(4):1600865. doi: 10.1002/adhm.201600865 [DOI] [PubMed] [Google Scholar]
- 170. Kang SG, Araya‐Secchi R, Wang D, Wang B, Huynh T, Zhou R. Dual inhibitory pathways of metallofullerenol Gd@C(8)(2)(OH)(2)(2) on matrix metalloproteinase‐2: molecular insight into drug‐like nanomedicine. Sci Rep. 2014;4:4775. doi: 10.1038/srep04775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Huang J, Lv Z, Wang Y, et al. In vivo MRI and X‐ray bifunctional imaging of polymeric composite supplemented with GdPO4.H2 O Nanobundles for tracing bone implant and bone regeneration. Adv Healthc Mater. 2016;5(17):2182‐2190. doi: 10.1002/adhm.201600249 [DOI] [PubMed] [Google Scholar]
- 172. Zhao P‐P, Hu H‐R, Liu J‐Y, et al. Gadolinium phosphate/chitosan scaffolds promote new bone regeneration via Smad/Runx2 pathway. Chem Eng J. 2019;359:1120‐1129. doi: 10.1016/j.cej.2018.11.071 [DOI] [Google Scholar]
- 173. Liao F, Peng XY, Yang F, Ke QF, Zhu ZH, Guo YP. Gadolinium‐doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Mater Sci Eng C Mater Biol Appl. 2019;104:109999. doi: 10.1016/j.msec.2019.109999 [DOI] [PubMed] [Google Scholar]
- 174. Kępiński L, Zawadzki M, Miśta W. Hydrothermal synthesis of precursors of neodymium oxide nanoparticles. Solid State Sci. 2004;6(12):1327‐1336. doi: 10.1016/j.solidstatesciences.2004.07.003 [DOI] [Google Scholar]
- 175. Zawadzki M, Kępiński L. Synthesis and characterization of neodymium oxide nanoparticles. J Alloys Compd. 2004;380(1–2):255‐259. doi: 10.1016/j.jallcom.2004.03.053 [DOI] [Google Scholar]
- 176. Haik Y, Chatterjee J, Jen CC. Synthesis and stabilization of Fe–Nd–B nanoparticles for biomedical applications. J Nanopart Res. 2005;7(6):675‐679. doi: 10.1007/s11051-005-5467-4 [DOI] [Google Scholar]
- 177. Zhu W, Ma J, Xu L, Zhang W, Chen Y. Controlled synthesis of Nd(OH)3 and Nd2O3 nanoparticles by microemulsion method. Mater Chem Phys. 2010;122(2–3):362‐367. doi: 10.1016/j.matchemphys.2010.03.004 [DOI] [Google Scholar]
- 178. Pandiyan R, Micheli V, Ristic D, et al. Structural and near‐infra red luminescence properties of Nd‐doped TiO2 films deposited by RF sputtering. J Mater Chem. 2012;22(42):22424–22432. doi: 10.1039/c2jm34708c [DOI] [Google Scholar]
- 179. Verma S, Bamzai KK. Preparation by chemical co‐precipitation, spectral and electrical characteristics of neodymium orthophosphate nanoparticles. J Rare Earths. 2015;33(5):535‐544. doi: 10.1016/s1002-0721(14)60453-1 [DOI] [Google Scholar]
- 180. Que W, Kam CH. Up‐conversion luminescence of neodymium oxalate nanoparticles/TiO2/organically modified silane composite thin films derived at low temperature. Opt Mater. 2002;19(2):307‐312. doi: 10.1016/s0925-3467(01)00227-0 [DOI] [Google Scholar]
- 181. Bazzi R, Brenier A, Perriat P, Tillement O. Optical properties of neodymium oxides at the nanometer scale. J Lumin. 2005;113(1–2):161‐167. doi: 10.1016/j.jlumin.2004.09.120 [DOI] [Google Scholar]
- 182. Singh S, Srivastava P, Kapoor IPS, Singh G. Preparation, characterization, and catalytic activity of rare earth metal oxide nanoparticles. J Therm Anal Calorim. 2012;111(2):1073‐1082. doi: 10.1007/s10973-012-2538-5 [DOI] [Google Scholar]
- 183. Hassan MS, Kang Y‐S, Kim B‐S, Kim I‐S, Kim H‐Y, Khil M‐S. Synthesis of praseodymium oxide nanofiber by electrospinning. Superlattices Microstruct. 2011;50(2):139‐144. doi: 10.1016/j.spmi.2011.05.010 [DOI] [Google Scholar]
- 184. Pachpinde AM, Langade MM, Lohar KS, Patange SM, Shirsath SE. Impact of larger rare earth Pr3+ ions on the physical properties of chemically derived PrxCoFe2−xO4 nanoparticles. Chem Phys. 2014;429:20‐26. doi: 10.1016/j.chemphys.2013.11.018 [DOI] [Google Scholar]
- 185. Quievryn C, Bernard S, Miele P. Polyol‐based synthesis of praseodymium oxide nanoparticles. Nanomater Nanotechnol. 2014;7:4. doi: 10.5772/58458 [DOI] [Google Scholar]
- 186. Zou S, Guo F, Wu L, et al. One‐pot synthesis of cerium and praseodymium co‐doped carbon quantum dots as enhanced antioxidant for hydroxyl radical scavenging. Nanotechnology. 2020;31(16):165101. doi: 10.1088/1361-6528/ab5b40 [DOI] [PubMed] [Google Scholar]
- 187. Gabay AM, Akdogan NG, Marinescu M, Liu JF, Hadjipanayis GC. Rare earth‐cobalt hard magnetic nanoparticles and nanoflakes by high‐energy milling. J Phys Condens Matter. 2010;22(16):164213. doi: 10.1088/0953-8984/22/16/164213 [DOI] [PubMed] [Google Scholar]
- 188. Petrochenko PE, Zhang Q, Wang H, et al. In vitro cytotoxicity of rare earth oxide nanoparticles for imaging applications. Int J Appl Ceram Technol. 2012;9(5):881‐892. doi: 10.1111/j.1744-7402.2012.02784.x [DOI] [Google Scholar]
- 189. Patricio BF, Albernaz Mde S, Sarcinelli MA, de Carvalho SM, Santos‐Oliveira R, Weissmuller G. Development of novel nanoparticle for bone cancer. J Biomed Nanotechnol. 2014;10(7):1242‐1248. doi: 10.1166/jbn.2014.1812 [DOI] [PubMed] [Google Scholar]
- 190. Takahashi N, Gubarevich A, Sakurai J, et al. Preparation and optical properties of rare earth doped Y2O3 nanoparticles synthesized by thermal decomposition with oleic acid. Adv Mat Res. 2011;332‐334:1974‐1978. [Google Scholar]
- 191. Nethi SK, Barui AK, Bollu VS, Rao BR, Patra CR. Pro‐angiogenic properties of terbium hydroxide nanorods: molecular mechanisms and therapeutic applications in wound healing. ACS Biomater Sci Eng. 2017;3(12):3635‐3645. doi: 10.1021/acsbiomaterials.7b00457 [DOI] [PubMed] [Google Scholar]
- 192. Hosokawa S, Jeon H‐J, Iwamoto S, Inoue M. Synthesis of rare earth iron‐mixed oxide nanoparticles by solvothermal methods. J Am Ceram Soc. 2009;92(12):2847‐2853. doi: 10.1111/j.1551-2916.2009.03295.x [DOI] [Google Scholar]
- 193. Kong L, Cai X, Zhou X, et al. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol Dis. 2011;42(3):514‐523. doi: 10.1016/j.nbd.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li K, Yu J, Xie Y, You M, Huang L, Zheng X. The effects of cerium oxide incorporation in calcium silicate coating on bone mesenchymal stem cell and macrophage responses. Biol Trace Elem Res. 2017;177(1):148‐158. doi: 10.1007/s12011-016-0859-0 [DOI] [PubMed] [Google Scholar]
- 195. Wang C, Blough E, Dai X, et al. Protective effects of cerium oxide nanoparticles on MC3T3‐E1 osteoblastic cells exposed to X‐ray irradiation. Cell Physiol Biochem. 2016;38(4):1510‐1519. doi: 10.1159/000443092 [DOI] [PubMed] [Google Scholar]
- 196. Xiang J, Li J, He J, et al. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8(7):4489‐4499. doi: 10.1021/acsami.6b00158 [DOI] [PubMed] [Google Scholar]
- 197. Nethi SK, Nanda HS, Steele TWJ, Patra CR. Functionalized nanoceria exhibit improved angiogenic properties. J Mater Chem B. 2017;5(47):9371‐9383. doi: 10.1039/c7tb01957b [DOI] [PubMed] [Google Scholar]
- 198. You M, Li K, Xie Y, Huang L, Zheng X. The effects of cerium valence states at cerium oxide coatings on the responses of bone mesenchymal stem cells and macrophages. Biol Trace Elem Res. 2017;179(2):259‐270. doi: 10.1007/s12011-017-0968-4 [DOI] [PubMed] [Google Scholar]
- 199. Li J, Wen J, Li B, et al. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv Sci (Weinh). 2018;5(2):1700678. doi: 10.1002/advs.201700678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kyosseva SV, Chen L, Seal S, McGinnis JF. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp Eye Res. 2013;116:63‐74. doi: 10.1016/j.exer.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Morais DS, Rodrigues MA, Lopes MA, et al. Biological evaluation of alginate‐based hydrogels, with antimicrobial features by Ce(III) incorporation, as vehicles for a bone substitute. J Mater Sci Mater Med. 2013;24(9):2145‐2155. doi: 10.1007/s10856-013-4971-9 [DOI] [PubMed] [Google Scholar]
- 202. Morais DS, Fernandes S, Gomes PS, et al. Novel cerium doped glass‐reinforced hydroxyapatite with antibacterial and osteoconductive properties for bone tissue regeneration. Biomed Mater. 2015;10(5):055008. doi: 10.1088/1748-6041/10/5/055008 [DOI] [PubMed] [Google Scholar]
- 203. Schmidlin PR, Tchouboukov A, Wegehaupt FJ, Weber FE. Effect of cerium chloride application on fibroblast and osteoblast proliferation and differentiation. Arch Oral Biol. 2012;57(7):892‐897. doi: 10.1016/j.archoralbio.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 204. Liu DD, Zhang JC, Zhang Q, Wang SX, Yang MS. TGF‐beta/BMP signaling pathway is involved in cerium‐promoted osteogenic differentiation of mesenchymal stem cells. J Cell Biochem. 2013;114(5):1105‐1114. doi: 10.1002/jcb.24451 [DOI] [PubMed] [Google Scholar]
- 205. Hu Y, Du Y, Jiang H, Jiang GS. Cerium promotes bone marrow stromal cells migration and osteogenic differentiation via Smad1/5/8 signaling pathway. Int J Clin Exp Pathol. 2014;7(8):5369‐5378. [PMC free article] [PubMed] [Google Scholar]
- 206. Hagiwara Y, Nakajima K. Use of ceria‐stabilized zirconia/alumina nanocomposite for fabricating the frameworks of removable dental prostheses: a clinical report. J Prosthet Dent. 2016;116(2):166‐171. doi: 10.1016/j.prosdent.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 207. Oshima Y, Iwasa F, Tachi K, Baba K. Effect of nanofeatured topography on ceria‐stabilized zirconia/alumina nanocomposite on osteogenesis and osseointegration. Int J Oral Maxillofac Implants. 2017;32(1):81‐91. doi: 10.11607/jomi.4366 [DOI] [PubMed] [Google Scholar]
- 208. Xiang H, Wang Y, Chang H, et al. Cerium‐containing alpha‐calcium sulfate hemihydrate bone substitute promotes osteogenesis. J Biomater Appl. 2019;34(2):250‐260. doi: 10.1177/0885328219849712 [DOI] [PubMed] [Google Scholar]
- 209. Lukin AV, Lukina GI, Volkov AV, Baranchikov AE, Ivanov VK, Prokopov AA. Morphometry results of formed osteodefects when using nanocrystalline CeO2 in the early stages of regeneration. Int J Dent. 2019;2019:9416381. doi: 10.1155/2019/9416381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tapeinos C, Battaglini M, Prato M, La Rosa G, Scarpellini A, Ciofani G. CeO2 nanoparticles‐loaded pH‐responsive microparticles with antitumoral properties as therapeutic modulators for osteosarcoma. ACS Omega. 2018;3(8):8952‐8962. doi: 10.1021/acsomega.8b01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. McMahon B, Mauer P, McCoy CP, Lee TC, Gunnlaugsson T. Selective imaging of damaged bone structure (microcracks) using a targeting supramolecular Eu(III) complex as a lanthanide luminescent contrast agent. J Am Chem Soc. 2009;131(48):17542‐17543. doi: 10.1021/ja908006r [DOI] [PubMed] [Google Scholar]
- 212. Davies A, Lewis DJ, Watson SP, Thomas SG, Pikramenou Z. pH‐controlled delivery of luminescent europium coated nanoparticles into platelets. Proc Natl Acad Sci U S A. 2012;109(6):1862‐1867. doi: 10.1073/pnas.1112132109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nethi SK, Veeriah V, Barui AK, et al. Investigation of molecular mechanisms and regulatory pathways of pro‐angiogenic nanorods. Nanoscale. 2015;7(21):9760‐9770. doi: 10.1039/c5nr01327e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Liu H, Jin Y, Ge K, et al. Europium‐doped Gd2O3 nanotubes increase bone mineral density in vivo and promote mineralization in vitro. ACS Appl Mater Interfaces. 2017;9(7):5784‐5792. doi: 10.1021/acsami.6b14682 [DOI] [PubMed] [Google Scholar]
- 215. Shi M, Xia L, Chen Z, et al. Europium‐doped mesoporous silica nanosphere as an immune‐modulating osteogenesis/angiogenesis agent. Biomaterials. 2017;144:176‐187. doi: 10.1016/j.biomaterials.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 216. Alicka M, Sobierajska P, Kornicka K, Wiglusz RJ, Marycz K. Lithium ions (Li[+]) and nanohydroxyapatite (nHAp) doped with Li(+) enhance expression of late osteogenic markers in adipose‐derived stem cells. Potential theranostic application of nHAp doped with Li(+) and co‐doped with europium (III) and samarium (III) ions. Korean J Couns Psychother. 2019;99:1257‐1273. doi: 10.1016/j.msec.2019.02.073 [DOI] [PubMed] [Google Scholar]
- 217. Zhu DY, Lu B, Yin JH, et al. Gadolinium‐doped bioglass scaffolds promote osteogenic differentiation of hBMSC via the Akt/GSK3beta pathway and facilitate bone repair in vivo. Int J Nanomedicine. 2019;14:1085‐1100. doi: 10.2147/IJN.S193576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Muller G, Mansson S, Muller MF, Johansson M, Bjorkman A. Increased perfusion in dynamic gadolinium‐enhanced MRI correlates with areas of bone repair and of bone necrosis in patients with Kienbock's disease. J Magn Reson Imaging. 2019;50(2):481‐489. doi: 10.1002/jmri.26573 [DOI] [PubMed] [Google Scholar]
- 219. Kawano T, Murata M, Kang JH, et al. Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage‐based targeted contrast agent. Biomaterials. 2018;152:37‐46. doi: 10.1016/j.biomaterials.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 220. Li X, Chen H. Yb(3+)/Ho(3+) co‐doped apatite upconversion nanoparticles to distinguish implanted material from bone tissue. ACS Appl Mater Interfaces. 2016;8(41):27458‐27464. doi: 10.1021/acsami.6b05514 [DOI] [PubMed] [Google Scholar]
- 221. Hu H, Zhao P, Liu J, et al. Lanthanum phosphate/chitosan scaffolds enhance cytocompatibility and osteogenic efficiency via the Wnt/beta‐catenin pathway. J Nanobiotechnol. 2018;16(1):98. doi: 10.1186/s12951-018-0411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Peng XY, Hu M, Liao F, et al. La‐doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Biomater Sci. 2019;7(4):1565‐1573. doi: 10.1039/c8bm01498a [DOI] [PubMed] [Google Scholar]
- 223. Lee YM, Tu RY, Chiang AC, Huang YC. Average‐power mediated ultrafast laser osteotomy using a mode‐locked Nd:YVO4 laser oscillator. J Biomed Opt. 2007;12(6):060505. doi: 10.1117/1.2821149 [DOI] [PubMed] [Google Scholar]
- 224. Khosroshahi ME, Mahmoodi M, Saeedinasab H. In vitro and in vivo studies of osteoblast cell response to a titanium‐6 aluminium‐4 vanadium surface modified by neodymium:yttrium‐aluminium‐garnet laser and silicon carbide paper. Lasers Med Sci. 2009;24(6):925‐939. doi: 10.1007/s10103-008-0628-1 [DOI] [PubMed] [Google Scholar]
- 225. Dilsiz A, Canakci V, Aydin T. The combined use of Nd:YAG laser and enamel matrix proteins in the treatment of periodontal infrabony defects. J Periodontol. 2010;81(10):1411‐1418. doi: 10.1902/jop.2010.100031 [DOI] [PubMed] [Google Scholar]
- 226. Qadri T, Javed F, Poddani P, Tuner J, Gustafsson A. Long‐term effects of a single application of a water‐cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation. Lasers Med Sci. 2011;26(6):763‐766. doi: 10.1007/s10103-010-0807-8 [DOI] [PubMed] [Google Scholar]
- 227. Hua Q, Chen Y, Liu Y, et al. Circular RNA 0039411 is involved in neodymium oxide‐induced inflammation and Antiproliferation in a human bronchial epithelial cell line via sponging miR‐93‐5p. Toxicol Sci. 2019;170(1):69‐81. doi: 10.1093/toxsci/kfz074 [DOI] [PubMed] [Google Scholar]
- 228. Tsuka Y, Fujita T, Shirakura M, et al. Effects of neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser irradiation on bone metabolism during tooth movement. J Lasers Med Sci. 2016;7(1):40‐44. doi: 10.15171/jlms.2016.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Namour M, El Mobadder M, Magnin D, et al. Q‐switch Nd:YAG laser‐assisted decontamination of implant surface. Dent J (Basel). 2019;7(4):99. doi: 10.3390/dj7040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ma L, Zhou Y, Zhang Z, et al. Multifunctional bioactive Nd‐Ca‐Si glasses for fluorescence thermometry, photothermal therapy, and burn tissue repair. Sci Adv. 2020;6(32):eabb1311. doi: 10.1126/sciadv.abb1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Morais DS, Coelho J, Ferraz MP, et al. Samarium doped glass‐reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J Mater Chem B. 2014;2(35):5872‐5881. doi: 10.1039/c4tb00484a [DOI] [PubMed] [Google Scholar]
- 232. Augustine R, Dalvi YB, Yadu Nath VK, et al. Yttrium oxide nanoparticle loaded scaffolds with enhanced cell adhesion and vascularization for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;103:109801. doi: 10.1016/j.msec.2019.109801 [DOI] [PubMed] [Google Scholar]
- 233. Huang W, Gao C, Lan Y, et al. Optical coherence tomography characterizes the roughness and thickness of the heterogeneous layer on cortical bone surface induced by Er:YAG laser ablation at different moisture contents. Quant Imaging Med Surg. 2020;10(3):713‐726. doi: 10.21037/qims.2020.02.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Varini E, Sánchez‐Salcedo S, Malavasi G, Lusvardi G, Vallet‐Regí M, Salinas AJ. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: bioactivity, biocompatibility and reactive oxygen species activity. Mater Sci Eng C. 2019;105:109971. doi: 10.1016/j.msec.2019.109971 [DOI] [PubMed] [Google Scholar]
- 235. Chigurupati S, Mughal MR, Okun E, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194‐2201. doi: 10.1016/j.biomaterials.2012.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509‐519. doi: 10.1016/s0891-5849(01)00610-4 [DOI] [PubMed] [Google Scholar]
- 237. Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288(1):275‐279. doi: 10.1006/bbrc.2001.5747 [DOI] [PubMed] [Google Scholar]
- 238. Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Therapy. 2005;2(5):787‐796. doi: 10.2217/14750708.2.5.787 [DOI] [Google Scholar]
- 239. Bai XC, Lu D, Bai J, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF‐kappaB. Biochem Biophys Res Commun. 2004;314(1):197‐207. doi: 10.1016/j.bbrc.2003.12.073 [DOI] [PubMed] [Google Scholar]
- 240. Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29(18):2705‐2709. doi: 10.1016/j.biomaterials.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Li Y, He X, Yin JJ, et al. Acquired superoxide‐scavenging ability of ceria nanoparticles. Angew Chem Int Ed Engl. 2015;54(6):1832‐1835. doi: 10.1002/anie.201410398 [DOI] [PubMed] [Google Scholar]
- 242. Dowding JM, Dosani T, Kumar A, Seal S, Self WT. Cerium oxide nanoparticles scavenge nitric oxide radical (NO). Chem Commun (Camb). 2012;48(40):4896‐4898. doi: 10.1039/c2cc30485f [DOI] [PubMed] [Google Scholar]
- 243. Walkey C, Das S, Seal S, et al. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano. 2015;2(1):33‐53. doi: 10.1039/C4EN00138A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wason MS, Colon J, Das S, et al. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle‐induced ROS production. Nanomedicine. 2013;9(4):558‐569. doi: 10.1016/j.nano.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Dulany K, Hepburn K, Goins A, Allen JB. In vitro and in vivo biocompatibility assessment of free radical scavenging nanocomposite scaffolds for bone tissue regeneration. J Biomed Mater Res A. 2020;108(2):301‐315. doi: 10.1002/jbm.a.36816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Wu X, Yan P, Ren Z, et al. Ferric hydroxide‐modified upconversion nanoparticles for 808 nm NIR‐triggered synergetic tumor therapy with hypoxia modulation. ACS Appl Mater Interfaces. 2019;11(1):385‐393. doi: 10.1021/acsami.8b18427 [DOI] [PubMed] [Google Scholar]
- 247. Das S, Singh S, Dowding JM, et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33(31):7746‐7755. doi: 10.1016/j.biomaterials.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Chaudhury K, Babu KN, Singh AK, Das S, Kumar A, Seal S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine. 2013;9(3):439‐448. doi: 10.1016/j.nano.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 249. Kumari M, Kumari SI, Kamal SS, Grover P. Genotoxicity assessment of cerium oxide nanoparticles in female Wistar rats after acute oral exposure. Mutat Res Genet Toxicol Environ Mutagen. 2014;775‐776:7‐19. doi: 10.1016/j.mrgentox.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 250. Giri S, Karakoti A, Graham RP, et al. Nanoceria: a rare‐earth nanoparticle as a novel anti‐angiogenic therapeutic agent in ovarian cancer. PLoS One. 2013;8(1):e54578. doi: 10.1371/journal.pone.0054578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Baht GS, Vi L, Alman BA. The role of the immune cells in fracture healing. Curr Osteoporos Rep. 2018;16(2):138‐145. doi: 10.1007/s11914-018-0423-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yang W, Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat. 2018;13:25‐32. doi: 10.1016/j.jot.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Li R, Ji Z, Qin H, et al. Interference in autophagosome fusion by rare earth nanoparticles disrupts autophagic flux and regulation of an interleukin‐1beta producing inflammasome. ACS Nano. 2014;8(10):10280‐10292. doi: 10.1021/nn505002w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti‐inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848‐2856. doi: 10.1002/smll.200901048 [DOI] [PubMed] [Google Scholar]
- 255. Li K, Shen Q, Xie Y, You M, Huang L, Zheng X. Incorporation of cerium oxide into hydroxyapatite coating protects bone marrow stromal cells against H2O2‐induced inhibition of osteogenic differentiation. Biol Trace Elem Res. 2018;182(1):91‐104. doi: 10.1007/s12011-017-1066-3 [DOI] [PubMed] [Google Scholar]
- 256. Feng Z, Liao Y, Ye M. Synthesis and structure of cerium‐substituted hydroxyapatite. J Mater Sci Mater Med. 2005;16(5):417‐421. doi: 10.1007/s10856-005-6981-8 [DOI] [PubMed] [Google Scholar]
- 257. Zhang Q, Ge K, Ren H, Zhang C, Zhang J. Effects of cerium oxide nanoparticles on the proliferation, osteogenic differentiation and adipogenic differentiation of primary mouse bone marrow stromal cells in vitro. J Nanosci Nanotechnol. 2015;15(9):6444‐6451. doi: 10.1166/jnn.2015.10709 [DOI] [PubMed] [Google Scholar]
- 258. Gunduz O, Gode C, Ahmad Z, et al. Preparation and evaluation of cerium oxide‐bovine hydroxyapatite composites for biomedical engineering applications. J Mech Behav Biomed Mater. 2014;35:70‐76. doi: 10.1016/j.jmbbm.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 259. Ball JP, Mound BA, Monsalve AG, Nino JC, Allen JB. Biocompatibility evaluation of porous ceria foams for orthopedic tissue engineering. J Biomed Mater Res A. 2015;103(1):8‐15. doi: 10.1002/jbm.a.35137 [DOI] [PubMed] [Google Scholar]
- 260. Arya A, Sethy NK, Gangwar A, et al. Cerium oxide nanozyme modulate the ‘exercise’ redox biology of skeletal muscle. Mater Res Express. 2017;4(5):055401. doi: 10.1088/2053-1591/aa6922 [DOI] [Google Scholar]
- 261. Britannica TEoE . Europium https://www.britannica.com/science/europium
- 262. Jin Y, Chen S, Duan J, Jia G, Zhang J. Europium‐doped Gd2O3 nanotubes cause the necrosis of primary mouse bone marrow stromal cells through lysosome and mitochondrion damage. J Inorg Biochem. 2015;146:28‐36. doi: 10.1016/j.jinorgbio.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 263. Britannica TEoE . Gadolinium. https://www.britannica.com/science/gadolinium
- 264. Gale EM, Caravan P, Rao AG, et al. Gadolinium‐based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol. 2017;47(5):507‐521. doi: 10.1007/s00247-017-3806-0 [DOI] [PubMed] [Google Scholar]
- 265. Li XQ, Wang X, Zhao DW, et al. Application of Gd‐EOB‐DTPA‐enhanced magnetic resonance imaging (MRI) in hepatocellular carcinoma. World J Surg Oncol. 2020;18(1):219. doi: 10.1186/s12957-020-01996-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Ji S, Wang Z, Xia S. Application of ultrasound combined with enhanced MRI by Gd‐BOPTA in diagnosing hepatocellular carcinoma. Am J Transl Res. 2021;13(6):7172‐7178. [PMC free article] [PubMed] [Google Scholar]
- 267. Li T, Duan E‐Y, Liu C‐J, Ma J‐G, Cheng P. Application of Gd(III) complexes for magnetic resonance imaging and the improvement of relaxivities via nanocrystallization. Inorg Chem Commun. 2018;98:111‐114. doi: 10.1016/j.inoche.2018.10.012 [DOI] [Google Scholar]
- 268. Dai Y, Wu C, Wang S, et al. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomedicine. 2018;14(2):547‐555. doi: 10.1016/j.nano.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 269. Shao Y, Tian X, Hu W, et al. The properties of Gd2O3‐assembled silica nanocomposite targeted nanoprobes and their application in MRI. Biomaterials. 2012;33(27):6438‐6446. doi: 10.1016/j.biomaterials.2012.05.065 [DOI] [PubMed] [Google Scholar]
- 270. Dufort S, Appelboom G, Verry C, et al. Ultrasmall theranostic gadolinium‐based nanoparticles improve high‐grade rat glioma survival. J Clin Neurosci. 2019;67:215‐219. doi: 10.1016/j.jocn.2019.05.065 [DOI] [PubMed] [Google Scholar]
- 271. Lord ML, Chettle DR, Grafe JL, Noseworthy MD, McNeill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: results of a small pilot feasibility study. Radiology. 2018;287(1):96‐103. doi: 10.1148/radiol.2017171161 [DOI] [PubMed] [Google Scholar]
- 272. Lord ML, McNeill FE, Grafe JL, Noseworthy MD, Chettle DR. Self‐identified gadolinium toxicity: comparison of gadolinium in bone and urine to healthy gadolinium‐based contrast agent exposed volunteers. Physiol Meas. 2018;39(11):115008. doi: 10.1088/1361-6579/aaedc6 [DOI] [PubMed] [Google Scholar]
- 273. Britannica TEoE . Neodymium. https://www.britannica.com/science/neodymium
- 274. Tsuka Y, Kunimatsu R, Gunji H, et al. Examination of the effect of the combined use of Nd: YAG laser irradiation and mechanical force loading on bone metabolism using cultured human osteoblasts. J Lasers Med Sci. 2020;11(2):138‐143. doi: 10.34172/jlms.2020.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Chellini F, Sassoli C, Nosi D, et al. Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: "an in vitro study". Lasers Surg Med. 2010;42(6):527‐539. doi: 10.1002/lsm.20861 [DOI] [PubMed] [Google Scholar]
- 276. Fernandes BF, da Cruz MB, Marques JF, et al. Laser Nd:YAG patterning enhance human osteoblast behavior on zirconia implants. Lasers Med Sci. 2020;35(9):2039‐2048. doi: 10.1007/s10103-020-03066-3 [DOI] [PubMed] [Google Scholar]
- 277. Abduljabbar T, Javed F, Kellesarian SV, Vohra F, Romanos GE. Effect of Nd:YAG laser‐assisted non‐surgical mechanical debridement on clinical and radiographic peri‐implant inflammatory parameters in patients with peri‐implant disease. J Photochem Photobiol B. 2017;168:16‐19. doi: 10.1016/j.jphotobiol.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 278. Alayat MSM, Abdel‐Kafy EM, Thabet AAM, Abdel‐Malek AS, Ali TH, Header EA. Long‐term effect of pulsed Nd‐YAG laser combined with exercise on bone mineral density in men with osteopenia or osteoporosis: 1 year of follow‐up. Photomed Laser Surg. 2018;36(2):105‐111. doi: 10.1089/pho.2017.4328 [DOI] [PubMed] [Google Scholar]
- 279. Mishra SK, Kannan S. A bimetallic silver‐neodymium theranostic nanoparticle with multimodal NIR/MRI/CT imaging and combined chemo‐photothermal therapy. Inorg Chem. 2017;56(19):12054‐12066. doi: 10.1021/acs.inorgchem.7b02103 [DOI] [PubMed] [Google Scholar]
- 280. Mimun LC, Ajithkumar G, Pokhrel M, et al. Bimodal imaging using neodymium doped gadolinium fluoride nanocrystals with near‐infrared to near‐infrared downconversion luminescence and magnetic resonance properties. J Mater Chem B. 2013;1(41):5702‐5710. doi: 10.1039/C3TB20905A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Rocha U, Jacinto da Silva C, Ferreira Silva W, et al. Subtissue thermal sensing based on neodymium‐doped LaF(3) nanoparticles. ACS Nano. 2013;7(2):1188‐1199. doi: 10.1021/nn304373q [DOI] [PubMed] [Google Scholar]
- 282. Rocha U, Kumar KU, Jacinto C, et al. Neodymium‐doped LaF(3) nanoparticles for fluorescence bioimaging in the second biological window. Small. 2014;10(6):1141‐1154. doi: 10.1002/smll.201301716 [DOI] [PubMed] [Google Scholar]
- 283. Chen Y, Yang L, Feng C, Wen LP. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non‐small cell lung cancer NCI‐H460 cells. Biochem Biophys Res Commun. 2005;337(1):52‐60. doi: 10.1016/j.bbrc.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 284. Li Y, Li X, Xue Z, Jiang M, Zeng S, Hao J. Second near‐infrared emissive lanthanide complex for fast renal‐clearable in vivo optical bioimaging and tiny tumor detection. Biomaterials. 2018;169:35‐44. doi: 10.1016/j.biomaterials.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 285. Li X, Jiang M, Li Y, Xue Z, Zeng S, Liu H. 808nm laser‐triggered NIR‐II emissive rare‐earth nanoprobes for small tumor detection and blood vessel imaging. Korean J Couns Psychother. 2019;100:260‐268. doi: 10.1016/j.msec.2019.02.106 [DOI] [PubMed] [Google Scholar]
- 286. Ansari AA, Khan A, Alam M, Siddiqui MA, Ahmad N, Alkhedhairy AA. Optically active neodymium hydroxide surface‐functionalized mesoporous silica micro‐cocoons for biomedical applications. Colloids Surf B Biointerfaces. 2020;189:110877. doi: 10.1016/j.colsurfb.2020.110877 [DOI] [PubMed] [Google Scholar]
- 287. Gujjalapudi M, Anam C, Mamidi P, Chiluka R, Kumar AG, Bibinagar R. Effect of magnetic field on bone healing around endosseous implants ‐ an in‐vivo study. J Clin Diagn Res. 2016;10(10):ZF01‐ZF04. doi: 10.7860/JCDR/2016/21509.8666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Shuai C, Yang Y, Peng S, et al. Nd‐induced honeycomb structure of intermetallic phase enhances the corrosion resistance of mg alloys for bone implants. J Mater Sci Mater Med. 2017;28(9):130. doi: 10.1007/s10856-017-5945-0 [DOI] [PubMed] [Google Scholar]
- 289. Sheng Q, Shen Y, Zhang H, Zheng J. Neodymium (III) hexacyanoferrate (II) nanoparticles induced by enzymatic reaction and their use in biosensing of glucose. Electrochim Acta. 2008;53(14):4687‐4692. doi: 10.1016/j.electacta.2008.01.087 [DOI] [Google Scholar]
- 290. Pourjavid MR, Yousefi SR, Hosseini MH, Rezaee M, Razavi T, Sehat AA. Determination of neodymium(III) in aqueous and soil samples with use of a high‐sensitive and selective membrane sensor. Int J Electrochem Sci. 2012;7(6):5147‐5162. [Google Scholar]
- 291. Yang L, Liu P, Li X, Li S. The photo‐catalytic activities of neodymium and fluorine doped TiO2 nanoparticles. Ceram Int. 2012;38(6):4791‐4796. doi: 10.1016/j.ceramint.2012.02.067 [DOI] [Google Scholar]
- 292. Britannica TEoE . Lanthanum. https://www.britannica.com/science/lanthanum
- 293. Brabu B, Haribabu S, Revathy M, et al. Biocompatibility studies on lanthanum oxide nanoparticles. Toxicol Res. 2015;4(4):1037‐1044. doi: 10.1039/c4tx00198b [DOI] [Google Scholar]
- 294. Huang P, Li J, Zhang S, et al. Effects of lanthanum, cerium, and neodymium on the nuclei and mitochondria of hepatocytes: accumulation and oxidative damage. Environ Toxicol Pharmacol. 2011;31(1):25‐32. doi: 10.1016/j.etap.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 295. Zhao H, Osborne OJ, Lin S, et al. Lanthanide hydroxide nanoparticles induce angiogenesis via ROS‐sensitive Signaling. Small. 2016;12(32):4404‐4411. doi: 10.1002/smll.201600291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Pourzarandian A, Watanabe H, Aoki A, et al. Histological and TEM examination of early stages of bone healing after Er:YAG laser irradiation. Photomed Laser Surg. 2004;22(4):342‐350. doi: 10.1089/pho.2004.22.342 [DOI] [PubMed] [Google Scholar]
- 297. Li X, Zou Q, Li W, Chen H. Investigation on anti‐autofluorescence, osteogenesis and long‐term tracking of HA‐based Upconversion material. Sci Rep. 2018;8(1):11267. doi: 10.1038/s41598-018-29539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU. Autophagy is required for self‐renewal and differentiation of adult human stem cells. Cell Res. 2012;22(2):432‐435. doi: 10.1038/cr.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Oliver L, Hue E, Priault M, Vallette FM. Basal autophagy decreased during the differentiation of human adult mesenchymal stem cells. Stem Cells Dev. 2012;21(15):2779‐2788. doi: 10.1089/scd.2012.0124 [DOI] [PubMed] [Google Scholar]
- 300. Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15(10):468‐477. doi: 10.1016/j.molmed.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 301. Liu AL, Zhang ZM, Zhu BF, Liao ZH, Liu Z. Metallothionein protects bone marrow stromal cells against hydrogen peroxide‐induced inhibition of osteoblastic differentiation. Cell Biol Int. 2004;28(12):905‐911. doi: 10.1016/j.cellbi.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 302. Chen J, Ashames A, Buabeid MA, Fahelelbom KM, Ijaz M, Murtaza G. Nanocomposites drug delivery systems for the healing of bone fractures. Int J Pharm. 2020;585:119477. doi: 10.1016/j.ijpharm.2020.119477 [DOI] [PubMed] [Google Scholar]
- 303. Winkler T, Sass FA, Duda GN, Schmidt‐Bleek K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: the unsolved challenge. Bone Joint Res. 2018;7(3):232‐243. doi: 10.1302/2046-3758.73.BJR-2017-0270.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Shi C, Wu T, He Y, Zhang Y, Fu D. Recent advances in bone‐targeted therapy. Pharmacol Ther. 2020;207:107473. doi: 10.1016/j.pharmthera.2020.107473 [DOI] [PubMed] [Google Scholar]
- 305. Nollet M, Santucci‐Darmanin S, Breuil V, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10(11):1965‐1977. doi: 10.4161/auto.36182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Martinez P, Esbrit P, Rodrigo A, Alvarez‐Arroyo MV, Martinez ME. Age‐related changes in parathyroid hormone‐related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporos Int. 2002;13(11):874‐881. doi: 10.1007/s001980200120 [DOI] [PubMed] [Google Scholar]
- 307. Stegen S, van Gastel N, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19‐27. doi: 10.1016/j.bone.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 308. Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N. It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front Bioeng Biotechnol. 2017;5:68. doi: 10.3389/fbioe.2017.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Yin S, Zhang W, Zhang Z, Jiang X. Recent advances in scaffold design and material for vascularized tissue‐engineered bone regeneration. Adv Healthc Mater. 2019;8(10):e1801433. doi: 10.1002/adhm.201801433 [DOI] [PubMed] [Google Scholar]
- 310. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323‐328. doi: 10.1038/nature13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016;143(15):2706‐2715. doi: 10.1242/dev.136861 [DOI] [PubMed] [Google Scholar]
- 312. Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10(1):426‐436. doi: 10.7150/thno.34126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Maroulakos M, Kamperos G, Tayebi L, Halazonetis D, Ren Y. Applications of 3D printing on craniofacial bone repair: a systematic review. J Dent. 2019;80:1‐14. doi: 10.1016/j.jdent.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 314. Zhang M, Lin R, Wang X, et al. 3D printing of Haversian bone‐mimicking scaffolds for multicellular delivery in bone regeneration. Sci Adv. 2020;6(12):eaaz6725. doi: 10.1126/sciadv.aaz6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Midha S, Dalela M, Sybil D, Patra P, Mohanty S. Advances in three‐dimensional bioprinting of bone: progress and challenges. J Tissue Eng Regen Med. 2019;13(6):925‐945. doi: 10.1002/term.2847 [DOI] [PubMed] [Google Scholar]
- 316. Wang Y, Cui W, Zhao X, et al. Bone remodeling‐inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale. 2018;11(1):60‐71. doi: 10.1039/c8nr07329e [DOI] [PubMed] [Google Scholar]
- 317. Xie X, Chen Y, Wang X, et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J Mater Sci Technol. 2020;59:243‐261. doi: 10.1016/j.jmst.2020.04.037 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study


