Abstract
Brucella, a Gram-negative bacterium with a high infective capacity and a wide spectrum of hosts in the animal world, is found in terrestrial and marine mammals, as well as amphibians. This broad spectrum of hosts is closely related to the non-classical virulence factors that allow this pathogen to establish its replicative niche, colonizing epithelial and immune system cells, evading the host’s defenses and defensive response. While motility is the primary role of the flagellum in most bacteria, in Brucella, the flagellum is involved in virulence, infectivity, cell growth, and biofilm formation, all of which are very important facts in a bacterium that to date has been described as a non-motile organism. Evidence of the expression of these flagellar proteins that are present in Brucella makes it possible to hypothesize certain evolutionary aspects as to where a free-living bacterium eventually acquired genetic material from environmental microorganisms, including flagellar genes, conferring on it the ability to reach other hosts (mammals), and, under selective pressure from the environment, can express these genes, helping it to evade the immune response. This review summarizes relevant aspects of the presence of flagellar proteins and puts into context their relevance in certain functions associated with the infective process. The study of these flagellar genes gives the genus Brucella a very high infectious versatility, placing it among the main organisms in urgent need of study, as it is linked to human health by direct contact with farm animals and by eventual transmission to the general population, where flagellar genes and proteins are of great relevance.
Keywords: Brucella abortus, flagellum, flagellin, ORF, virulence factors
1. Introduction
Brucella abortus is a Gram-negative, non-motile, coccobacillary bacterium and a facultative intracellular pathogen, causing disease in both animals and humans [1]. Brucellosis, the name given to the disease caused by this pathogen, is a zoonosis described in cattle and other mammals. Being an opportunistic agent, it can infect humans, and diagnosis is difficult [2]. This disease can be characterized by its ability to become a chronic infection, which in bovines can cause abortions, stillbirths, the birth of weak calves, and sterility in males [3]. It is endemic in many areas of the world, and, in humans, the main sources of infection are through the consumption of contaminated meat and dairy products and through contact with secretions from infected animals [2,4,5]. Brucella enters the host organism through traumatized skin (wounds) or through the air to the mucosa in the form of an aerosol [6]. Once inside the host organism, Brucella spreads and multiplies in the lymph nodes, spleen, liver, bone marrow, mammary glands, and sexual organs via macrophages [7]. Brucella can compromise the gastrointestinal, hepatobiliary, genitourinary, skeletal muscle, cardiovascular system, and integumentary systems [8], which can become a chronic condition, crossing the blood-brain barrier through infected monocytes like a Trojan horse and achieving re-infection in the midbrain, causing neurobrucellosis [9]. If the bacteria are not killed, they can survive for long periods inside these phagocytic cells, in phagosomes, where they can multiply by inhibiting fusion with the lysosome through rapid acidification into the Brucella-containing vacuoles (BCVs) [4,7,10,11]. In non-phagocytic cells, Brucella tends to localize within the rough endoplasmic reticulum [2,12] where, like other intracellular bacteria, it survives in the host cell. The genus Brucella, identified in 1920 by Meyer and Shaw, currently comprises twelve species differentiated by their tropism, pathogenicity, and host phenotypic traits [13]. A decade ago, the genus contained six “classic” Brucella species (B. melitensis, B. abortus, B. suis, B. canis, B. ovis, and B. neotomae), which are also known as the “core” Brucella. Three of these species (B. melitensis, B. abortus and B. suis) are the pathogens that cause one of the most significant zoonoses worldwide [14]. The group of classic Brucella species was expanded in 2007 to include B. ceti and B. pinnipedialis, bacteria isolated from marine mammals [15]. Currently, new technological strategies like massive genetic sequencing are being used to group certain organisms with characteristics of the genus Brucella (wherein about 30 species and other serovars not previously described have been included) [16].
Due to its low infectious dose and easy transmission in the form of an aerosol, Brucella was one of the first microorganisms to enter consideration for use as a biological weapon, classified by the United States Army in the 1950s [17], and cataloged by the Centers for Disease Control and Prevention (CDC) and the National Institute of Allergy and Infectious Diseases (NIAID) as a Category-B bioterrorism agent [14]. Emphasizing the genes that command its infectious cycle, the study of Brucella offers a clearer view of its infective process and pathogenesis, and, in turn, a better understanding of how this genus establishes its replicative niche in host cells and can perpetuate its chronicity. Some years ago, an interesting finding was made regarding this bacterium, generally classified as non-motile, in terms of the expression of flagellar genes and a polar flagellum under certain conditions.
2. Bacterial Flagellum
The bacterial flagellum is a huge molecular complex made up of 20,000 to 30,000 protein subunits of approximately 30 different proteins [18]. The structural components of the flagellum can be divided into two parts: the basal body rings and the tubular axial structure [19]. The rings of the basal body form the rotary motor, together with the stator complex, composed of the cytoplasmic membrane proteins MotA and MotB [20,21]. The basal body rings consist of four main ring structures—the L ring, the P ring, the MS ring, and the C ring—where the latter two form the rotor–stator complex [22,23]. The latter complex is coupled to the proton flux, and all of this, in turn, surrounds the transporter gate formed by the FliE, FlgB, FlgC, and FlgF proteins [24]. The LP ring acts as bushing, supporting the distal rod in its rapid and stable rotation without much friction [25], which leads to flagellar movement [20,26]. Likewise, the axial structure consists of three main parts: the rod, the hook, and the filament [27,28]. The rod is a transmission shaft with an approximate length of 30 nm, which connects the rotor rings and the hook; this rod is a fairly complex helical cylinder composed of four flagellar proteins: FlgB, FlgC, FlgF, and FlgG [29,30]. The hook is a short, curved segment, approximately 55 nm long, which is made up of a helical set of subunits of the hook’s own flagellar protein (FlgE) and acts as a universal joint structure that transmits movement to the filament, regardless of its orientation. Between the filament and the hook, there is a short axial structural segment with a thinner and smoother appearance, called the hook and filament junction, formed by the adapter proteins FlgK and FlgL [24]. The filament is a thin helical structure of approximately 20 nm in diameter and typically grows to about 15 μm long, composed of approximately 20,000 flagellin protein (FliC) subunits [31,32,33]; it acts as a rigid helix to produce thrust for the cell to swim in aqueous environments [34,35,36]. This filament ends at its apex with the Cap protein (FliD), which forms a star-shaped homo-pentamer that covers the open end of the filament and helps the assembly of FliC [37,38,39,40]. FliD was originally found to be an inhibitor of flagellin polymerization in vitro [41] but it is required for the growth of flagellin in vivo [29]. The analysis of the flagellum structure in B. melitensis by transmission electron microscopy (TEM) has identified several characteristics of the sheathed flagella of other species, although little is known about flagellar sheaths in bacteria; indeed, Brucella is the only rhizobium that produces a sheathed flagellum [42,43]. Given the sheath surrounding the filament, the visible flagellum of B. melitensis has a diameter of 50 nm, which is larger than that of a sheathless flagellum. However, the diameter of the bacterial filament is usually about 20 nm but we show that the diameter of the inner filament in the sheath of B. melitensis is only 11 nm. Sheath production is not related to flagellar assembly in B. melitensis; this is because in bacterial mutants for structural flagellar proteins (ΔfliF, ΔflgE and ΔfliC), a filamentous appendage is still produced, despite the absence of FlgE or FliC proteins. However, the persistence of an empty sheath in flagellar mutants has often been described in bacteria that produce a sheathed flagellum, such as Vibrio species and H. pylori [44,45]. In this light, the flagellum sheath of B. melitensis is an extension of the outer membrane containing LPS, which is also observed in H. pylori, B. bacteriovorus, and some Vibrio species [46,47,48,49].
This process of conformation of the flagellar structure has been widely investigated in E. coli and Salmonella, being a regulated process where genes that are closely linked to the order of the formation of the flagella (class I, II, and III genes) are activated in tandem [50,51]. FlhDC is a master regulator, present in E. coli and Salmonella, that controls the expression of class II genes [52]; the products of these genes are structural proteins that comprise the basal body and hook as FliF and FlgE, respectively [53]. Likewise, the regulators of these class III proteins in E. coli are FliA (a sigma factor, also called σ28) and its anti-sigma, FlgM [32,50]. Once the structure of the hook is completed, the FlgM protein (anti-sigma) is secreted through its channel, which allows σ28 to activate the expression of class-III genes, including FliC, ending the formation of the filament [54].
3. Molecular Mechanism of Flagellar Expression in Brucella
Although the Brucella genome contains flagellar operons, there is no evidence of motility [55]. This information is quite interesting since it was previously reported that under specific growth conditions, B. melitensis, being non-motile, was able to form a flagellar appendage by a mechanism that has not yet been clarified [46], and recently, Brucella strains isolated from Ceratophyrus adornada frogs showed a phenotype with high motility [56]. In the genomic sequencing of isolated non-motile Brucella, the presence of non-functional flagellar genes has been found [57], while the analysis of genes encoding flagellar proteins in a recently described motile strain (B13-0095) revealed that all genes were fully functional [56], which could mean that these genes are expressed under environmentally selective pressure [55]. The action of certain bacterial enzymes participates indirectly to fulfill the purpose of achieving the replicative niche. An example of this is the nitrate reductase enzyme of Brucella, which is expressed basally in aerobic conditions but increases its expression in hypoxic conditions. This enzyme actively participates with other proteins, such as superoxide dismutase copper-zinc, (SOD Cu/Zn), performing adaptive functions against redox changes that may affect bacterial nidation during infection [58], a very important phenomenon in the survival of Brucella when it colonizes an organ, such as the uterus, in infected mammals [59]. This condition, wherein the presence of reactive oxygen species (ROS) increases, activates certain proteins such as MucR (a Ros-like regulator) in B. melitensis, B. suis, and B. abortus, which are also involved in the activation of flagellar genes in response to environmental stress [60,61]. The genes involved in the formation of the bacterial flagellum (E. coli; Salmonella spp.) differ in certain aspects from those described by Soler-Llorens, such as the location in the genome, or the size of genes and their regulation; however, they maintain the order of the assembly process of proteins of different classes and their regulatory proteins [56] (Figure 1).
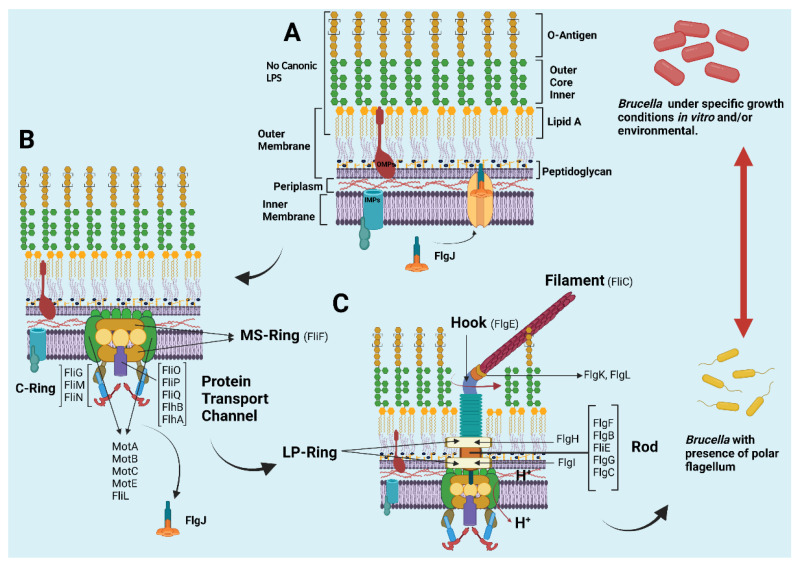
Figure 1.
Diagram of the bacterial flagellum of Brucella, according to the classical structure of the flagellum in Gram-negative bacteria and the most up-to-date literature. Brucella expresses its bacterial flagellum under specific and controlled in vitro growth conditions, and in the face of different environmental changes, the expression of the flagellum is involved in its survival in a hostile environment when it does not have a definitive host. (A) Under these specific conditions and after expression of the master regulators at gene level, FlgJ acts by drilling the membrane and the peptidoglycan (PG), a key protein in the construction of the flagellum base structure. (B) The correct functioning and conformation of this bacterial organelle depend on the perfect arrangement of the proteins that form the MS-ring, the transporter channel proteins, and the C-ring. Once the base of the flagellum has formed on the membrane, FlgJ is cleaved from the basal body. (C) This correct arrangement of previous proteins makes it possible to reach the end of the flagellum formation given by the cluster conglomerate of rod proteins, the LP-ring, followed by the hook, and finally, by the formation of the filament (FliC) that mediates the movement allowed by proton pumping from the periplasm. In Gram-negative bacteria, the final protein that “seals” the filament is called CapD (FliD); however, this has not yet been described in Brucella, even though Brucella presents the gene for its expression. LPS: lipopolysaccharide; OMP: outer membrane protein; IMP: inner membrane protein.
In Brucella, it has been suggested that certain proteins participate in this regulatory cascade of flagellar genes, with certain genes coding for the sigma factors RpoD, RpoN, RpoH1, RpoH2, RpoE1, and RpoE2, where the deletion of the rpoE1 gene increases the production of the flagellar protein FlgE [57]. In addition, this mutation also increases the gene expression of fliF, flgE, fliC, flaF, and flbT (Table 1), where the expression of these genes is also controlled by the master regulator ftcR, which is mediated by the rpoE1 gene product and other, as yet unknown, proteins [62].
Table 1.
The gene codes of Brucella abortus flagellar proteins are presented; in some cases, they are described as pseudogenes and have not been studied.
| Flagellar Structure | Proteins | Function | Gene Code | Location | Reference |
|---|---|---|---|---|---|
| Filament | CapD (FliD) | Term protein in the structure of the flagellum, adhesion to mucin. | BAB1_0534 | Distal end of filament | Database Kegg Genes |
| FliC | Filament subunits, immunity, coadjuvant in others species. | BAB2_1106 | Filament | [63] | |
| FlgL | Proteins that adhere the flagellum to the hook. | BAB2_1096 | Initial base of filament | [64] | |
| FlgK | BAB2_1097 | External OM | [64] | ||
| Hook | FlgE | Hook protein. | BAB2_1098 | External OM | [64] |
| Rod | FlgH | Structural proteins that form the L-ring. | BAB2_0156 | External OM | [64] |
| FlgI | Structural proteins that form the P-ring. | BAB2_0153, BAB2_0154 | PG | [64] | |
| FlgG | Structural proteins that form the channel. | BAB2_0151 | OM/PG | [64] | |
| FlgF | BAB2_0127 | [64] | |||
| FlgC | BAB2_0149 | [64] | |||
| FlgB | BAB2_0148 | [64] | |||
| FliE | BAB2_0122 | [65] | |||
| External position Export Gate | MotE | Rotor stabilizer proteins. | unknown | Intermembrane space | N.Y.I.B.* |
| MotC | BAB2_1102 | Intermembrane space | [64] | ||
| MotA | unknown | Intermembrane space | N.Y.I.B.* | ||
| MotB | BAB2_1103 | (PG, PS, IM) | [46] | ||
| MS-ring | FliL | Protein that binds Mot structures to the MS-ring. | pseudogene | (PG, PS, IM) | N.Y.I.B.* |
| FliF | Structural proteins that form the MS-ring. | BAB2_1105 | IM (MS-ring) | [66] | |
| C-ring | FliG | Structural proteins that form the C-ring. | pseudogene | Cytoplasm | N.Y.I.B.* |
| FliM | pseudogene | Cytoplasm | N.Y.I.B.* | ||
| FliN | BAB2_0122 | Cytoplasm | [67] | ||
| FliH/FliI/FliJ | Primary role: flagellar proteins of the export apparatus. Secondary role: Stabilizing proteins of the C-ring. |
pseudogene | Cytoplasm | N.Y.I.B.* | |
| Export Gate | FliO | Structural proteins that form the export gate. Type III secretion exporter | pseudogene | IM | N.Y.I.B.* |
| FliP | BAB2_0158 | [64] | |||
| FliQ | BAB2_1092 | [64] | |||
| FliR | BAB2_1088 | [64] | |||
| FlhA | BAB2_1089, BAB2_1091 | [46] | |||
| FlhB | BAB2:0120 | [46] | |||
| Regulator Genes | FtcR | Transcriptional regulators. | BAB2_1099 | Cytoplasm | [68] |
| FlaF | Transcriptional regulators. | BAB2_1095 | Cytoplasm | [62] | |
| FlbT | Transcriptional regulators. | BAB2_1094 | Cytoplasm | [55] | |
| FliK | Molecular ruler for hook length control. | pseudogene | Cytoplasm | N.Y.I.B.* | |
| FlgD | Cap foldases for hook. | BAB2_1093 | Cytoplasm | [64] | |
| FlgN | Chaperone for FlgK. | pseudogene | Cytoplasm | N.Y.I.B.* | |
| FlgA | Chaperone for FlgI. | BAB2_0152 | Cytoplasm | [64] | |
| fliR gene | Biosynthesis of FliR. | BAB2_1088 | Bacterial export protein | [64] | |
| FlgJ | Mannosyl-glycoprotein endo-beta-N-acetylglucosamidase in PG | BAB1_0260 | Cytoplasm/Inner membrane | [69] |
N.Y.I.B.*: not yet identified in Brucella.; OM: Outer membrane; PG: Peptidoglycan; IM: Inner membrane; PS: Periplasmic space.
Moreover, the mutation of rpoE1 increases the promoter activity of the flagellar master regulator ftcR, suggesting that the protein group with RpoE1 acts upstream of ftcR, repressing its expression. FtcR is a flagellar two-component regulator described in Brucella melitensis, which is involved in the expression of class II genes, flgE (hook proteins) and fliF (basal body proteins) [57,68], as well as certain proteins that regulate the expression of the class-III gene, such as FlaF, which represses FliC, and FlbT (Figure 2A). In addition to being a self-regulating protein, FlbT enables the production of the FliC protein, forming part of the expression of class III genes [70]. FliC production in Brucella melitensis is not subject to the basal body and hook termination, unlike E. coli and other bacteria [71,72]. In B. melitensis, the mutant for FlgE and FliF still produces flagellin, as previously indicated; therefore, the expression of FliC requires the FlbT regulator for its expression [71]. Genes have been shown to be involved in quorum sensing (QS) in Brucella; vjbR and blxR work as transcriptional regulators involved in the virulence of Brucella and participate in the formation of biofilms through the expression of flagellar genes [73]. VjbR is required by B. melitensis for transcription of the type-IV secretion system and the expression of several flagellar genes (fliF, flhA, motB, or flgE) that contribute to its virulence in mice, where ftcR is partially activated by VjbR [46,74] (Figure 2B). New studies have identified that FliC production is not controlled solely by the master regulator, FlbT. Along with the abovementioned components, we must mention YbeY endoribonuclease, one of the best-conserved enzymes in different organisms, as it is related to an important variety of metabolic activities including, for example, proper cell morphology, mRNA transcription levels, and virulence, as well as indirectly participating in the expression of certain flagellar proteins in B. abortus [75,76]. When the ybeY gene was mutated in Brucella abortus, it was found that the product of this gene participates indirectly in the gene expression of transcriptional regulatory proteins, since ftcR mRNA levels were elevated in the mutant strain. This is linked to the expression of FliC (flagellin), which is also significantly elevated in the ΔybeY strain [77], and, like FtcR, is required for FliC production. The increase observed in the expression of fliC mRNA in the ΔybeY strain could be due to the increase in FtcR levels (Figure 2C) [46,68].
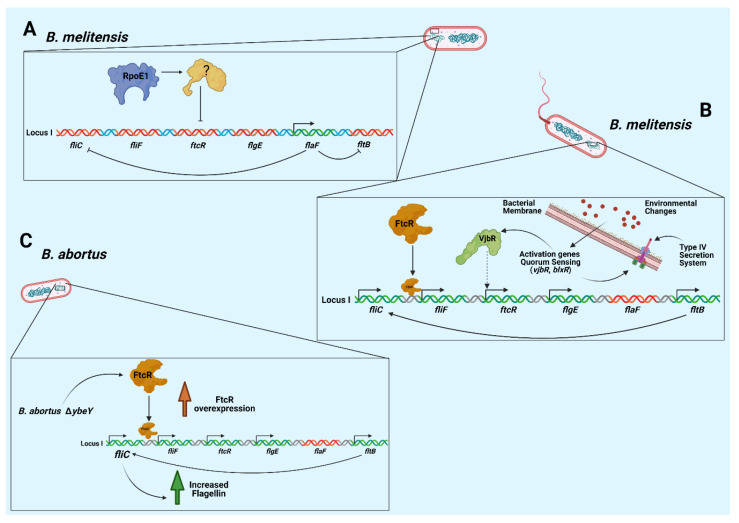
Figure 2.
Transcriptional regulation of some of the flagellar genes described in Brucella at locus I. (A) Repression of flagellar expression in B. melitensis is linked by the repressor RpoE1 (and other unknown proteins) to ftcR. This master regulator allows FlaF to be expressed and inhibits the expression of fliC and fltB. (B) The expression of a unipolar flagellum in B. melitensis is linked to FtcR, a direct regulator of flagellar protein expression; this is partially activated by the regulator VjbR, which is involved in quorum sensing and the expression of the type-IV secretion system. (C) In Brucella abortus, when the ybeY gene (BAB2_1156), an endoribonuclease, is deleted, there is an overexpression of the FtcR and this results in an increase in flagellin.
From the above information and added to the context of Brucella in the literature, it can be stated that the action of flagellar genes is not only subject to the expression of master regulators but is also related to the expression of other genes belonging to other bacterial metabolic cascades. This is clear from different studies conducted on mutant strains of different genes in Brucella (Table 2).
Table 2.
Summary of the effects related to the mutation of genes encoding flagellar proteins or genes, related to their expression in Brucella.
| Mutant |
Brucella Species |
Effect | References |
|---|---|---|---|
| ΔftcR | B. melitensis | Decreases flagellar gene expression. | [68] |
| ΔvjBR | B. melitensis | Decrease flagellar proteins expression. | [74,78,79] |
| ΔblxR | B. abortus | Regulates virulence factors (TSSIV and flagella). | [73] |
| ΔbvrR/bvrS | B. abortus | Decreases virulence. | [80] |
| ΔftcR, ΔfliF, ΔflgE, ΔfliC | B.melitensis | Empty sheath. | [62] |
| ΔflgJ | B. abortus | Decreases biofilms, virulence and infection capacity. | [69] |
| ΔybeY (BAB1_2156) | B. abortus | Multiple dysregulations of expression of bacterial proteins, including proteins and flagellar regulators. | [77] |
| ΔfliF, ΔflhA, ΔmotB, ΔflgE | B. melitensis | Dysfunction is the first step of infection or cycle of life. | [46] |
| ΔaibP (QS gen) | B. melitensis | Expression of fliC, fliF, flgE y flbT was significantly downregulated, with enhanced virB genes expression and VirB8 production. | [63] |
| ΔBM-LOV-HK (light-sensing histidine kinase) |
B. melitensis | Sigma factor rpoE1 downregulated, with flagellar, quorum sensing (QS). Type-IV secretion system genes were upregulated. | [81] |
| ΔbpdA | B. melitensis | Downregulation of flagellar promoter activities. Attenuation of virulence. Increase in biofilms. | [82,83] |
| ΔRpoE1 | B. melitensis | Overexpresses the flagellar protein FlgE. Increases promoter activity of the flagellar master regulator ftcR. | [62] |
4. Flagellar Proteins as Virulence Factors in Brucella spp.
Virulence factors are molecules produced by pathogens that favor their infective capacity, allowing them to colonize host tissue, evade the host immune response, enter and exit cells, or acquire nutrients (e.g., iron). These are key elements in the adaptation and survival of pathogens in the host organism [84]. The most important characteristic of Brucella is the ability to survive and multiply within both phagocytic and non-phagocytic cells [85]. There is also evidence that certain proteins that make up part of the flagellum participate in mechanisms that allow the bacterium to establish its replicative niche and its infective chronicity in the host [57,63,64,68,69]. These flagellar virulence factors have even been studied as immunogenic agents in murine models for the development of brucellosis vaccines [67]. Bacteria of the genus Brucella have been described for some time as non-motile organisms [1]; however, certain findings reveal the presence of operons that contain flagellar genes [86], and, in certain species of Brucella, under special conditions—either cultivational or environmental—functional bacterial flagella are present [46,87]. For the induction of the disease, as indicated previously, Brucella must adhere, invade, and survive within mammalian cells. As previously mentioned, the flagellar genes of B. abortus, present in its genome, are an interesting finding in relation to a bacterium described as non-motile [86]. This discovery may indicate that these proteins are involved in other vital functions for the establishment of bacterial infection, as in the case of the FlgJ protein, which, when encoded by the open reading frame BAB1_0260, participating in virulence and being mutated, limits the ability to form biofilms in vitro [69,88]. In E. coli, Salmonella, and other bacterial groups, this flagellar conformation and its subsequent formation begin with the action of the FlgJ protein. FlgJ is the rod cap protein most closely related to the early stages of flagellum formation and rod assembly; once the latter is formed, FlgJ is cleaved from the emerging flagellum and is essential for its genesis since it has dual canonical activity [89], hydrolysis on the PG and scaffold activity, enabling the assembly of the initial units of the flagellum in the periplasmic space [90]. It has been shown in S. enterica that the FlgJ muramidase plays an important role in flagellum synthesis, being secreted through the type-III flagellar export system and exhibiting its enzymatic activity in the C-terminal end [89,91]. This domain, of approximately 164 amino acids in length, presents two important catalytic residues for this muramidase activity. This is given by glutamic acid (E233) and aspartic acid (D248), the function of which is to make a hole in the PG, thus allowing the penetration of the shaft through the periplasmic space so that the hook and the filament can later assemble in the extracellular space [91]. It has been reported, however, that in S. enterica, mutants for flgJ maintain the ability to swim after long incubation times, which indicates that muramidase activity is not entirely necessary for flagellum formation, suggesting that flagellum formation is due to the assembly upon biogenesis of new membranes and new PG [92]. Likewise, in Rhodobacter sphaeroides, SltF is present, a muramidase associated with the flagellar system. Unlike the FlgJ of S. enterica, it is transported to the periplasm by the Sec translocase pathway, with the ability to produce a hole in the PG layer for the subsequent penetration of the flagellar structure [93,94]. In the case of Caulobacter crescentus, a bacterium that has a cell cycle very similar to Brucella [95], it possesses PleA, an enzyme homologous to lytic transglycosylase. This protein contains a region that is similar to a peptidoglycan-hydrolytic active site; a point mutation at this site in PleA results in the loss of the flagellum and pilus biogenesis. Furthermore, PleA is required for flagellar assembly, indicating its involvement during flagellum formation [96]. B. abortus has a flagellar protein with hydrolase activity on peptidoglycan, the FlgJ protein (BAB1_0260) located in genomic island three (GI-3) (https://www.ncbi.nlm.nih.gov/protein/82615263, accessed on 15 November 2021), which may be involved in the virulence of this species [69]. Although the muramidase activity has not yet been studied in Brucella, its activity in that part of the PG is doubtful; however, the bioinformatic bases suggest an important role for FlgJ in the initial stages of flagellar genesis, due to its glucosaminidase activity (https://www.kegg.jp/dbget-bin/www_bget?bmf:BAB1_0260, accessed on 15 November 2021). A comparative analysis of the amino acid sequence encoding FlgJ from Brucella with other bacteria reveals that the FlgJ from Brucella does not have the typical conserved sequences of FlgJ with muramidase activity found in E. coli and S. enterica (Figure 3). Even so, according to some databases, FlgJ from Brucella presents activity on the PG (acetylglucosaminidase activity). Like other FlgJ from other bacteria, it could be hypothesized that an extra element might be participating in this muramidase activity, to make a hole in the peptidoglycan before the assembly of proteins in the flagellum. This occurs in Rhodobacter sphaeroides or Caulobacter crescentus, as mentioned above, where other PG lytic enzymes would participate, together with the FlgJ from Brucella. This would, therefore, enable the correct assembly of the base of the flagella in the bacterial membrane. By obtaining mutants for the BAB1_0260 in B. abortus, it was possible to evaluate whether FlgJ was involved in pathogenicity as a virulence factor, showing that its deletion is not lethal to bacterial survival [69,97,98]. The FlgJ hydrolyzing activity on peptidoglycan (PG) plays an important role in the growth of this bacterium, specifically during the remodeling of PG during its cell division, which would explain why the elimination of the flgJ gene significantly reduces its growth. The bacterial flagellum is associated with functions that differ among bacteria. In addition to motility, it may be related to adherence and biofilm formation [99,100], processes closely related to the chronicity of the infections caused by different bacterial groups [101,102]. Biofilms are extracellular polymeric elements (EPS) produced by microorganisms, which include polysaccharides, proteins, nucleic acids, and lipids, which enable their adhesion to various surfaces and the interaction among bacteria (cell communication, competition, cooperation, or the horizontal movement of genes) [103]. The molecular mechanisms of how Brucella generates biofilms and the participating elements have scarcely been explored, since their replicative niche is cells in living tissue, which makes their study in this area difficult. This finding shows that FlgJ participates in the adherence or secretion of proteins involved in the process, making it clear that in the formation of biomass associated with B abortus 2308 biofilms, FlgJ is directly related to the levels of bacterial growth and division [69]. This suggests that reduced bacterial adherence decreases the colonization of host tissues, where several factors are involved, including the flagellum [104]. Likewise, microarray assay analyses have found that the effect of erythritol on the expression of B. melitensis genes (cultivated with or without erythritol) was shown to be the upregulation of two main virulence pathways, in response to erythritol exposure—the VirB type IV secretion system and flagellar proteins (the third-largest cluster of orthologous groups), suggesting a role for erythritol in virulence [82].
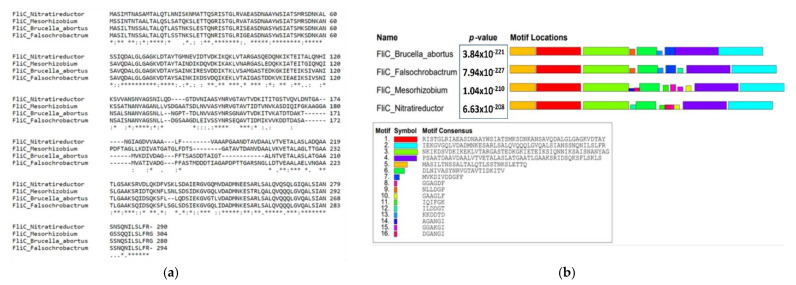
Figure 3.
p-value of conserved motifs related to flgJ gene of Brucella abortus 2308. Bioinformatics tool used: http://meme-suite.org (accessed on 15 November 2021).
5. Evolutionary Aspects of Brucella and Flagellar Genes
One of the orders that fall within the class of Alpha-proteobacteria is the order of the Rhizobiales; among the families of this order are the Brucellaceae, with genera such as Mycoplana, Ochrobactrum and Brucella, and the family Rhizobiaceae, which includes the genera Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium and Sinorhizobium. The genus Brucella developed as intracellular animal pathogens, while the genus Rhizobium is associated with soil and forms nitrogen-fixing nodules but, according to this evolutionary background, they have similar evolutionary characteristics [105]. Some evidence to hypothesize an evolutionary relationship between Brucellaceae and Rhizobiaceae is given by the presence of certain genes that they have in common; for example, some genes involved in symbiosis in Sinorhizobium meliloti are homologous to genes involved in Brucella pathogenesis [106]. The bacA gene that is involved in the symbiosis of Sinorhizobium meliloti has its homologous gene in Brucella abortus, which plays a role in the survival of Brucella in macrophages and in its pathogenesis in mice [106,107]. Along with the above findings and in relation to the bacterial flagellum, we should mention that Bradyrhizobium diazoefficiens, the nitrogen-fixing symbiont of soybean, possesses two flagellar systems that evolved independently [105,108,109]. This dual flagellar system, present in such bacteria, performs its function as a single flagellum, which is similar to the polar flagellum of Brucella spp. [109,110]. A comparative analysis of the genetic sequences encoding the FliC protein of Brucella abortus 2308 flagella revealed that bacteria of different genera in the order Rhizobiales show high homology of sequences and structural domains of the FliC protein (Figure 4). This is in conjunction with the consideration that a high percentage of bacteria of the Rhizobiales order are considered to be environmental microorganisms and present bacterial flagella [111,112,113].
Figure 4.
Bacterial FliC amino-acid sequence analysis. (a) Multiple alignments of sequences of the FliC protein of Brucella abortus (Q2YJF1) against the FliC protein of Falsochrobactrum sp. HN4 (A0A316J970), Mesorhizobium sp. UASWS1009 (A0A1C2EA77) and Nitratireductor pacificus pht-3B (K2M4Y7) (b). There are 16 conserved motifs in relation to the fliC gene present in the abovementioned species. Bioinformatics tool used: http://meme-suite.org (accessed on 15 November 2021).
Thus, we could speculate that Brucella diverged from common ancestors that are generally associated with soil, which are plant symbionts; that, like Brucella, they are intracellular and, therefore, do not require the expression of flagella. However, if the genes that encode the vast majority of the constituent proteins of a flagellum are subjected to selective pressure from their environment, the flagellum can be expressed [46,108]. Furthermore, all this is closely related to the proposition that Brucella’s ancestor was probably a free-living plan-related bacterium, possessing one chromosome and evolving into an animal parasite with two chromosomes [114] (Figure 5).
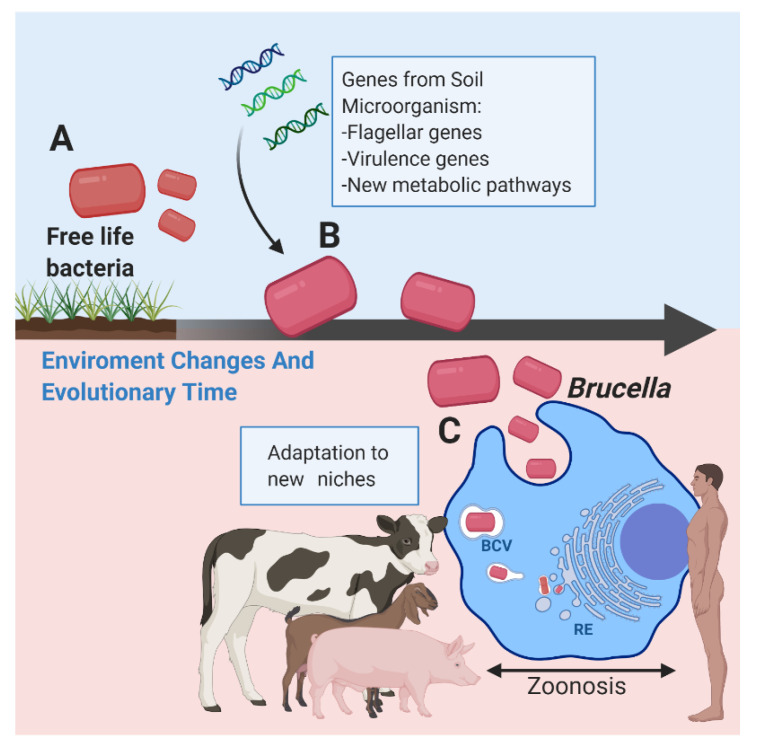
Figure 5.
(A) In terms of its evolutionary origin, it is postulated that Brucella may have been a free-living bacterium. (B) Over time, and according to its evolution, it remained in contact with other microorganisms, such as soil bacteria and fungi, managing to acquire certain genes that, therefore, improved its metabolic resources. (C) Environmental changes helped give it the ability to adapt to new hosts, such as eukaryotic cells. In Brucella, certain virulence factors that are different from other bacteria, like its LPS, its type-four secretion system (T4SS) and the BvrR/BvrS system, enable Brucella to interact with the host cell surface and form an early Brucella-containing vacuole (BCV) for its subsequent interaction with the endoplasmic reticulum (ER), where bacteria multiply and reach their replicative niche. Likewise, the presence of flagellar genes and the active participation of flagellum proteins in different functions, such as the translocation of proteins to the outside and the formation of adhered biofilm-associated biomass gave Brucella the ability to be a successful organism in achieving its infectious chronicity and evading the immune response of the host organism.
Within this evolutionary process, Brucella has acquired different foreign DNA fragments by horizontal transfer, which are distributed throughout the genome, and which encode several proteins, mainly of unknown function [115]. Yet, among these genes, there are some that encode virulence factors, where some flagellar proteins that are present in certain Brucella ORFs are worthy of note [69,86,116,117].
6. Flagellum and Immune Response against Brucella
The function of flagella as bacterial nanomachines is to mediate motility. The flagellar filament, composed of a single protein, flagellin, is assembled by the basal body via a pathway homologous to the type 3 secretion system (T3SS) [118]. In motile bacteria, such as Bartonella species (lineages 1–3, except Bartonella bovis), flagella are thought to mediate erythrocyte internalization as a mechanical force or as adhesion molecules [119]. The Bartonella species of lineage 4 that are non-motile, due to the loss of flagella, mediate interaction with erythrocytes through Trw T4SS, suggesting that Trw has functionally replaced the flagella in establishing interaction with erythrocytes [120]. Brucella are non-motile bacteria but flagella genes are essential for their virulence [121]. Given that Brucella is also found in erythrocytes [122], we could speculate that there is a mechanism similar to the one mentioned earlier, where flagellar genes play a fundamental role in the survival and stealth of Brucella, even, one could say, in the evasion of the immune response. Brucella spp. evade detection through TLR4 by producing a poorly recognized form of lipid A. Brucella lipid A contains a much longer fatty-acid residue (C28) than enterobacterial LPS (C12–C16), and this modification greatly decreases its endotoxic properties by reducing TLR4 agonist activity and, thus, the host immune response [123]. In addition to TLR4 evasion, Brucella evades detection by TLR5 by producing a flagellin that lacks the TLR5 agonist domain [124]. As a result, Brucella flagellin is a poor inducer of TLR5-mediated inflammatory responses [123]. In addition to manipulating T cell-mediated immune responses, Brucella can also control T cell-independent immune responses [125]. Therefore, it is likely that Brucella induces partial and transient immunosuppression to establish a chronic infection [126,127]. Conversely, certain flagellar proteins have been examined for their possible immunogenic value as vaccine candidates. Five flagellar genes (BAB1_0260 (FlgJ); BAB2_0122 (FliN); BAB2_0150; BAB2_1086; BAB2_1093) were taken into consideration for their ability to induce humoral and cellular responses and to protect mice against B. abortus. FlgJ and FliN proved to be protective antigens that produced humoral and cellular responses in mice [128]. In addition to the above findings, studies with the FliC protein have shown it to be a new potential antigen candidate for the development of a subunit vaccine against Brucella [129,130].
7. Conclusions and Outlook
In recent years, many new species of Brucella have been discovered, mainly pathogens of marine mammals and others capable of infecting terrestrial mammals, in addition to flagellated species present in amphibians [86]. This complicates the execution of control programs since the recently characterized Brucella species have high genetic flexibility and many of these isolates are motile, fast-growing, able to survive in the soil, more resistant to unfavorable conditions of environmental acidity, and more able to adapt to new non-mammalian hosts, such as amphibians, rapidly adapting to their environment to broaden their host range [87]. As Brucella is a pathogen with different routes of infection, it can survive in and out of mammalian hosts for a long time, even under unfavorable conditions. Brucella is characterized as being a stealthy microbe that tends to chronicity, instead of causing an acute fatal infection, standing out for being a successful microorganism by evading the immune response and keeping its hosts alive to maintain its own survival. Flagellar biogenesis shows interesting variations among different proteobacteria and even among members of Alphaproteobacteria. The flagellar genes of Brucella are needed to establish infection in vivo in mice; however, the molecular basis of the impact of the flagellum on virulence in Brucella is still to be determined. This is evidenced by certain species of Brucella, such as B. melitensis 16M, B. ovis, B. abortus 2308, and certain Brucella spp. in amphibians expressing flagellar proteins and/or flagella at the intracellular level [46,64,68,69,87]. This is an event of great importance, considering that today there is a strong relationship between humans and farm animals, which will eventually lead to an increase in this zoonosis caused by Brucella. If we pay attention to the routes of infection that Brucella has, in relation to its scope in the mechanisms of pathogenesis and transmission of the infection, we may hypothesize its relationship to the possible expression of the flagellum during invasion, and its persistence in red blood cells in the murine model [122]. This phenomenon is closely related to the role that the flagellum could have during its residence inside blood-sucking insects [131]. With all the above, we could hypothesize that the flagellum is playing an important role in bacterial survival at the extracellular, intracellular, or environmental level. The study of the bacterial flagellum in a non-motile bacterium is undoubtedly a challenge that deserves attention since it seems to be directly involved in its survival in different hosts and in a hostile environment.
Some methodologies have been proposed in the literature to explain the importance of flagellar genetic sequences involved in the abovementioned events. The creation of mutants, the study of biofilm formation, and the infective capacity both in vitro and in vivo [69] are part of the most successful methodology for preliminary assays. Likewise, the study of flagellar protein translocation by different types of transporters, where the role of these proteins in Brucella virulence could be evaluated [132], might point out certain functional evidence between different bacterial structures. Flow cytometry analysis in infected cells, to discern the appearance of markers involved in the immune response [130], as well as immunoprecipitation assays to see eventual protein interactions [130], and simple experiments such as staining of the flagella at different growth times under a variety of conditions [66] would provide further data to clarify certain unknowns about flagellar function and structure. Furthermore, in the field of immunology, the preparation of vaccines of different flagellar proteins in search of potential immunogenic agents [133], and the use of bioinformatics tools for comparative study with other bacteria [134,135], with differing degrees of similarity in the mechanisms of infection are appropriate methodologies to gain more background information. This will allow us to understand more fully the structure–function relationship of the flagellum in an organism described to date as a non-motile bacterium.
Acknowledgments
This work was supported by Grant 1180122 from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Santiago, Chile.
Author Contributions
Image making, literature search, hypothesis conjecture, original writing-drafting, writing-revising and editing; R.F.C.-R.; Imaging and research, M.F.-C.; research and writing, R.E.M.; research and writing, R.S.-S.; research and writing, Á.C.; fund acquisition, project administration, writing-revising and editing, Á.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
Grant 1180122 from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Santiago, Chile.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
https://www.ncbi.nlm.nih.gov/protein/82615263, visualized based on data from the website https://pubmed.ncbi.nlm.nih.gov/. https://www.kegg.jp/dbget-bin/www_bget?bmf:BAB1_0260, visualized based on data from the website https://www.genome.jp/ (all accessed on 15 November 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alton G.G., Jones L.M., Pietz D.E. Monograph Series. 2nd ed. World Health Organization, Laboratory Techniques in Brucellosis; Geneva, Switzerland: 1975. Geneva.55. [PubMed] [Google Scholar]
- 2.Corbel J.M. Brucellosis: An overview. Emerg. Infect. Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean A.S., Schelling E., Bonfoh B., Kulo A.E. Deletion in the Gene BruAb2_0168 of Brucella abortus Strains: Diagnostic Challenges. Clin. Microbiol. Infect. 2014;20:550–553. doi: 10.1111/1469-0691.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizarro-Cerdá J., Moreno E., Gorvel J.P. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2000;2:829–835. doi: 10.1016/S1286-4579(00)90368-X. [DOI] [PubMed] [Google Scholar]
- 5.Rivers R., Andrews E., Donoso G., Oñate A. Brucella abortus: Immunity, vaccines and prevention strategies based on nucleic acids. Arch. Med. Vet. 2006;38:7–18. doi: 10.4067/S0301-732X2006000100002. [DOI] [Google Scholar]
- 6.Zapata M.R., Santos J.S. Brucelosis. Med. Programa Form. Méd. Contin. Acreditado. 2014;11:3045–3053. doi: 10.1016/S0304-5412(14)70738-3. [DOI] [Google Scholar]
- 7.Ko J., Splitter G.A. Molecular Host-Pathogen Interaction in Brucellosis: Current Understanding and Future Approaches to Vaccine Development for Mice and Humans. Clin. Microbiol. Rev. 2003;16:65–78. doi: 10.1128/CMR.16.1.65-78.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn A.H., Probert W.S., Glaser C.A., Gupta N., Bollen A.W., Wong J.D., McDonald W.C. Human Neurobrucellosis with Intracerebral Granuloma Caused by a Marine Mammal Brucella spp. Emerg. Infect. Dis. 2003;9:485–488. doi: 10.3201/eid0904.020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miraglia M.C., Rodriguez A.M., Barrionuevo P., Rodriguez J., Kim K.S., Dennis V.A., Delpino M.V., Giambartolomei G.H. Brucella abortus Traverses Brain Microvascular Endothelial Cells Using Infected Monocytes as a Trojan Horse. Front. Cell Infect. Microbiol. 2018;8:200. doi: 10.3389/fcimb.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornand J., Gross A., Lafont V., Liautard J., Oliaro J., Liautard J.P. The innate immune response against Brucella in humans. Vet. Microbiol. 2002;90:383–394. doi: 10.1016/S0378-1135(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 11.Celli J., Chastellier C., de Franchini D.-M., Pizarro-Cerda J., Moreno E., Gorvel J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Głowacka P., Żakowska D., Naylor K., Niemcewicz M., Bielawska-Drózd A. Brucella—Virulence Factors, Pathogenesis and Treatment. Pol. J. Microbiol. 2018;67:151–161. doi: 10.21307/pjm-2018-029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz H.C., Vergnaud G. Molecular characterisation of Brucella species. Rev. Sci. Tech. 2013;32:149–162. doi: 10.20506/rst.32.1.2189. [DOI] [PubMed] [Google Scholar]
- 14.Pappas G., Panagopoulou P., Christou L., Akritidis N. Brucella as a biological weapon. Cell Mol. Life Sci. 2006;63:2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster J.T., Beckstrom-Sternberg S.M., Pearson T., Beckstrom-Sternberg J.S., Chain P.S.G., Roberto F.F., Hnath J., Brettin T., Keim P. Whole-genome-based phylogeny and divergence of the genus Brucella. J. Bacteriol. 2009;191:2864–2870. doi: 10.1128/JB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hordt A., Lopez M.G., Meier-Kolthoff J.P., Schleuning M., Weinhold L.M., Tindall B.J., Gronow S., Kyrpides N.C., Woyke T., Goker M. Analysis of 1000+ Type Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020;11:468. doi: 10.3389/fmicb.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen S.C., Boggiatto P., White D.M., McNunn T. Biosafety Concerns Related to Brucella and Its Potential Use as a Bioweapon. Appl. Biosaf. 2018;23:77–90. doi: 10.1177/1535676018771983. [DOI] [Google Scholar]
- 18.Chaban B., Hughes H.V., Semin M.B. The flagellum in bacterial pathogens: For motility and a whole lot more. Cell Dev. Biol. 2015;46:91–103. doi: 10.1016/j.semcdb.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Francis N.R., Sosinsky G.E., Thomas D., De Rosier D.J. Isolation, characterization, and structure of bacterial flagellar motors containing the switch complex. J. Mol. Biol. 1994;235:1261–1270. doi: 10.1006/jmbi.1994.1079. [DOI] [PubMed] [Google Scholar]
- 20.Blair D.F., Berg H.C. Restoration of torque in defective flagellar motors. Science. 1988;242:1678–1681. doi: 10.1126/science.2849208. [DOI] [PubMed] [Google Scholar]
- 21.Blair D.F., Berg H.C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990;60:439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H., Yonekura K., Namba K. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J. Mol. Biol. 2004;337:105–113. doi: 10.1016/j.jmb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Thomas D.R., Francis N.R., Xu C., DeRosier D.J. The three dimensional structure of the flagellar rotor from a clockwiselocked mutant of Salmonella enterica serovar typhimurium. J. Bacteriol. 2006;188:7039–7048. doi: 10.1128/JB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imada K. Bacterial flagellar axial structure and its construction. Biophys. Rev. 2017;10:559–570. doi: 10.1007/s12551-017-0378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Makino F., Miyata T., Minamino T., Kato T., Namba K. Structure of the molecular bushing of the bacterial flagellar motor. Nat. Commun. 2021;12:4469. doi: 10.1038/s41467-021-24715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson M.D., Tedesco P., Berg H.C., Harold F.M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc. Natl. Acad. Sci. USA. 1977;4:3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Pamphilis M.L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J. Bacteriol. 1971;105:376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Pamphilis M.L., Adler J. Fine structure and isolation of the hook–basal body complex of flagella from Escherichia coli and Bacillus subtilis. J. Bacteriol. 1971;105:384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homma M., De Rosier D.J., Macnab R.M. Flagellar hook and hook-associated proteins of Salmonella typhimurium and their relationship to other axial components of the flagellum. J. Mol. Biol. 1990;213:819–832. doi: 10.1016/S0022-2836(05)80266-9. [DOI] [PubMed] [Google Scholar]
- 30.Kubori T., Shimamoto N., Yamaguchi S., Namba K., Aizawa S.-I. Morphological pathway of flagellar assembly in Salmonella typhimurium. J. Mol. Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-M. [DOI] [PubMed] [Google Scholar]
- 31.Macnab R.M. Flagella and motility. In: Neidhart F.C., editor. Escherichia coli and Salmonella typhimurium. 2nd ed. American Society of Microbiology; Washington, DC, USA: 1996. pp. 123–145. [Google Scholar]
- 32.McCarter L.L. Regulation of flagella. Curr. Opin. Microbiol. 2006;9:180–186. doi: 10.1016/j.mib.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Paradis G., Chevance F.F.V., Liou W., Renault T.T., Hughes K.T., Rainville S., Erhardt M. Variability in bacterial flagella re-growth patterns after breakage. Sci. Rep. 2017;7:1282. doi: 10.1038/s41598-017-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macnab R.M. Type III flagellar protein export and flagellar assembly. Biochim. Biophys. Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Macnab R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 36.Minamino T., Imada K., Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol. BioSyst. 2008;4:1105–1115. doi: 10.1039/b808065h. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda T., Asakura S., Kamiya R. “Cap” on the tip of Salmonella flagella. J. Mol. Biol. 1985;184:735–737. doi: 10.1016/0022-2836(85)90317-1. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda T., Yamaguchi S., Hotani H. Flagellar growth in a filament less Salmonella fliD mutant supplemented with purfied hook associated protein 2. J. Biochem. 1993;114:39–44. doi: 10.1093/oxfordjournals.jbchem.a124136. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda T., Oosawa K., Hotani H. Self-assembly of the filament capping protein, FliD, of bacterial flagella into an annular structure. J. Mol. Biol. 1996;259:679–686. doi: 10.1006/jmbi.1996.0349. [DOI] [PubMed] [Google Scholar]
- 40.Maki S., Vonderviszt F., Furukawa Y., Imada K., Namba K. Plugging interactions of HAP2 pentamer into the distal end of flagellar filament revealed by electron microscopy. J. Mol. Biol. 1998;277:771–777. doi: 10.1006/jmbi.1998.1663. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda T., Kamiya R., Yamaguchi S. In vitro polymerization of flagellin excreted by a short-flagellum Salmonella typhimurium mutant. J. Bacteriol. 1984;159:787–789. doi: 10.1128/jb.159.2.787-789.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarter L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001;65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjoblad R.D., Emala C.W., Doetsch R.N. Invited review: Bacterial flagellar sheaths: Structures in search of a function. Cell Motil. 1983;3:93–103. doi: 10.1002/cm.970030108. [DOI] [PubMed] [Google Scholar]
- 44.Richardson K. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: Analysis of motility mutants in three animal models. Infect. Immun. 1991;59:2727–2736. doi: 10.1128/iai.59.8.2727-2736.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schirm M., Soo E.C., Aubry A.J., Austin J., Thibault P., Logan S.M. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 2003;48:1579–1592. doi: 10.1046/j.1365-2958.2003.03527.x. [DOI] [PubMed] [Google Scholar]
- 46.Fretin D., Fauconnier A., Kohler S., Halling S., Leonard S., Nijskens C., Ferooz J., Lestrate P., Delrue R.M., Danese I. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol. 2005;7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 47.Allen R.D., Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J. Bacteriol. 1971;107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geis G., Suerbaum S., Forsthoff B., Leying H., Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 1993;38:371–377. doi: 10.1099/00222615-38-5-371. [DOI] [PubMed] [Google Scholar]
- 49.Nedeljkovi’c M., Sastre D.E., Sundberg E.J. Bacterial Flagellar Filament: A Supramolecular Multifunctional Nanostructure. Int. J. Mol. Sci. 2021;22:7521. doi: 10.3390/ijms22147521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldridge P., Hughes K.T. Regulation of flagellar assembly. Curr. Opin. Microbiol. 2002;5:160–165. doi: 10.1016/S1369-5274(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 51.Soutourina O.A., Bertin P.N. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 2003;27:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 52.Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q., Mariconda S., Suzuki A., McClelland M., Harshey R.M. Uncovering a large set of genes that affect surface motility in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2006;188:7981–7984. doi: 10.1128/JB.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chevance F.F.V., Hughes K.T. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wareth G., Melzer F., Neubauer H. In Brucella: Selective pressure may turn some genes on instead of default off position. Med. Hypotheses. 2017;103:29–31. doi: 10.1016/j.mehy.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Soler-Llorens P.F., Quance C.R., Lawhon S.D., Stuber T.P., Edwards J.F., Ficht T.A., Robbe-Austerman S., O’Callaghan D., Keriel A.A. Brucella spp. Isolate from a Pac-Man Frog (Ceratophrys ornata) Reveals Characteristics Departing from Classical Brucellae. Front. Cell. Infect. Microbiol. 2016;6:116. doi: 10.3389/fcimb.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferooz J., Lemaire J., Delory M., De Bolle X., Letesson J. RpoE1, an extracytoplasmic function sigma factor, is a repressor of the flagellar system in Brucella melitensis. Microbiology. 2011;157:1263–1268. doi: 10.1099/mic.0.044875-0. [DOI] [PubMed] [Google Scholar]
- 58.Del Vecchio V.G., Kapatral V., Redkar R.J., Patra G., Mujer C., Los T., Ivanova N., Anderson I., Bhattacharyya A., Lykidis A., et al. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA. 2002;99:443–448. doi: 10.1073/pnas.221575398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirabella A., Terwagne M., Zygmunt M.S., Cloeckaert A., De Bolle X., Letesson J.J. Brucella melitensis MucR, an orthologue of Sinorhizobium meliloti MucR, is involved in resistance to oxidative, detergent, and saline stresses and cell envelope modifications. J. Bacteriol. 2013;195:453–465. doi: 10.1128/JB.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adetunji S.A., Faustman D.L., Adams L.G., Garcia-Gonzalez D.G., Hensel M.E., Khalaf O.H., Arenas-Gamboa A.M. Brucella abortus and Pregnancy in Mice: Impact of Chronic Infection on Fertility and the Role of Regulatory T Cells in Tissue Colonization. Infect. Immun. 2020;88:e00257-20. doi: 10.1128/IAI.00257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanna N., Ouahrani-Bettache S., Drake K.L., Adams L.G., Köhler S., Occhialini A. Global Rsh-dependent transcription profile of Brucella suis during stringent response unravels adaptation to nutrient starvation and cross-talk with other stress responses. BMC Genom. 2013;14:459. doi: 10.1186/1471-2164-14-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferooz J., Lemaire J., Letesson J. Role of FlbT in flagellin production in Brucella melitensis. Microbiology. 2011;157:1253–1262. doi: 10.1099/mic.0.044867-0. [DOI] [PubMed] [Google Scholar]
- 63.Terwagne M., Ferooz J., Rolán H.G., Sun Y.H., Atluri V., Xavier M.N., Franchi L., Núñez G., Legrand T., Flavell R.A., et al. Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell. Microbiol. 2013;15:942–960. doi: 10.1111/cmi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sidhu-Muñoz R.S., Tejedor C., Vizcaíno N. The Three Flagellar Loci of Brucella ovis PA Are Dispensable for Virulence in Cellular Models and Mice. Front. Vet. Sci. 2020;7:441. doi: 10.3389/fvets.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen E., Chaudhuri P., Gourley C., Harms J., Splitter G. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J. Bacteriol. 2011;193:5683–5691. doi: 10.1128/JB.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferooz J., Letesson J.-J. Morphological analysis of the sheathed flagellum of Brucella melitensis. BMC Res. Notes. 2010;9:333. doi: 10.1186/1756-0500-3-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Léonard S., Ferooz J., Haine V., Danese I., Fretin D., Tibor A., de Walque S., De Bolle X., Letesson J.J. FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae. J. Bacteriol. 2007;189:131–141. doi: 10.1128/JB.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson P.E., Gober J.W. FlbT, the post-transcriptional regulator of flagellin synthesis in Caulobacter crescentus, interacts with the 5′ untranslated region of flagellin mRNA. Mol. Microbiol. 2002;38:41–52. doi: 10.1046/j.1365-2958.2000.02108.x. [DOI] [PubMed] [Google Scholar]
- 69.Coloma-Rivero R.F., Gómez L., Alvarez F., Saitz W., Del Canto F., Céspedes S., Vidal R., Oñate A.A. The Role of the Flagellar Protein FlgJ in the Virulence of Brucella abortus. Front. Cell. Infect. Microbiol. 2020;10:178. doi: 10.3389/fcimb.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardissone S., Kint N., Viollier P.H. Specificity in glycosylation of multiple flagellins by the modular and cell cycle regulated glycosyltransferase FlmG. Elife. 2020;9:e60488. doi: 10.7554/eLife.60488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delory M., Hallez R., Letesson J.J., De Bolle X. An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M. J. Bacteriol. 2006;188:7707–7710. doi: 10.1128/JB.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwan W.R., Flohr N.L., Multerer A.R., Starkey J.C. GadE regulates fliC gene transcription and motility in Escherichia coli. World J. Clin. Infect. Dis. 2020;10:14–23. doi: 10.5495/wjcid.v10.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rambow-Larsen A.A., Rajashekara G., Petersen E., Splitter G. Putative quorum-sensing regulator BlxR of Brucella melitensis regulates virulence factors including the type IV secretion system and flagella. J. Bacteriol. 2008;190:3274–3282. doi: 10.1128/JB.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delrue R.M., Deschamps C., Léonard S., Nijskens C., Danese I. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell. Microbiol. 2005;7:1151–1161. doi: 10.1111/j.1462-5822.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 75.Pandey S.P., Winkler J.A., Li H., Camacho D.M., Collins J.J., Walker G.C. Central role for RNase YbeY in Hfq-dependent and Hfq-independent small-RNA regulation in bacteria. BMC Genom. 2014;15:121. doi: 10.1186/1471-2164-15-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandey S.P., Minesinger B.K., Kumar J., Walker G.C. A highly conserved protein of unknown function in Sinorhizobium meliloti affects sRNA regulation similar to Hfq. Nucleic Acids Res. 2011;39:4691–4708. doi: 10.1093/nar/gkr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Budnick J.A., Sheehan L.M., Colquhoun J.M., Dunman P.M., Walker G.C., Roop R.M., Caswell C.C. Endoribonuclease YbeY Is Linked to Proper Cellular Morphology and Virulence in Brucella Abortus. J. Bacteriol. 2018;200:105–118. doi: 10.1128/JB.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ke Y., Wang Y., Yuan X., Zhong Z., Qu Q., Zhou D., Zeng X., Xu J., Wang Z., Du X., et al. Altered Transcriptome of the B. melitensis Vaccine Candidate 16MΔvjbR, Implications for Development of Genetically Marked Live Vaccine. Indian J. Microbiol. 2012;52:575–580. doi: 10.1007/s12088-012-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uzureau S., Godefroid M., Deschamps C., Lemaire J., De Bolle X., Jean-Jacques Letesson J.-J. Mutations of the quorum sensing-dependent regulator VjbR lead to drastic surface modifications in Brucella melitensis. J. Bacteriol. 2007;189:6035–6047. doi: 10.1128/JB.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viadas C., Rodríguez M.C., Sangari F.J., Jean-Pierre Gorvel J.-P., García-Lobo J.M., López-Goñi I. Transcriptome analysis of the Brucella abortus BvrR/BvrS two-component regulatory system. PLoS ONE. 2010;21:e10216. doi: 10.1371/journal.pone.0010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gourley C.R., Petersen E., Harms J., Splitter G. Decreased in vivo virulence and altered gene expression by a Brucella melitensis light-sensing histidine kinase mutant. Pathog. Dis. 2015;73:1–14. doi: 10.1111/2049-632X.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen E., Rajashekara G., Sanakkayala N., Eskra L., Harms J., Splitter G. Erythritol triggers expression of virulence traits in Brucella melitensis. Microbes Infect. 2013;15:440–449. doi: 10.1016/j.micinf.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan M., Harms J.S., Marim F.M., Armon L., Hall C.L., Liu Y.P., Banai M., Oliveira S.C., Splitter G.A., Smith J.A. The Bacterial Second Messenger Cyclic di-GMP Regulates Brucella Pathogenesis and Leads to Altered Host Immune Response. Infect. Immun. 2016;84:3458–3470. doi: 10.1128/IAI.00531-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lood R., Frick I.-M. Protein-Based Strategies to Identify and Isolate Bacterial Virulence Factors. Methods Mol. Biol. 2017;1535:3–15. doi: 10.1007/978-1-4939-6673-8_1. [DOI] [PubMed] [Google Scholar]
- 85.De Figueiredo P., Ficht T., Rice-Ficht A., Rossetti C., Adams G.L. Pathogenesis and immunobiology of brucellosis review of Brucella host interactions. Am. J. Pathol. 2015;185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halling S.M. On the presence and organization of open reading frames of the nonmotile pathogen Brucella abortus similar to class II, III, and IV flagellar genes and to LcrD virulence superfamily. Microb. Comp. Genom. 1998;3:21–29. doi: 10.1089/omi.1.1998.3.21. [DOI] [PubMed] [Google Scholar]
- 87.Al Dahouk S., Köhler S., Occhialini A., de Bagüés M.P.J., Hammerl J.A., Eisenberg T., Vergnaud G., Cloeckaert A., Zygmunt M.S., Whatmore A.M., et al. Brucella spp. of amphibians comprise genomically diverse motile strains competent for replication in macrophages and survival in mammalian hosts. Sci. Rep. 2017;16:44420. doi: 10.1038/srep44420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Almirón M.A., Roset M.S., Sanjuan N. The aggregation of Brucella abortus occurs under microaerobic conditions and promotes desiccation tolerance and biofilm formation. Open Microbiol. J. 2013;22:87–91. doi: 10.2174/1874285801307010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nambu T., Inagaki Y., Kutsukake K. Plasticity of the domain structure in FlgJ, a bacterial protein involved in flagellar rod formation. Genes Genet. Syst. 2006;81:381–389. doi: 10.1266/ggs.81.381. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura S., Minamino T. Flagella-Driven Motility of Bacteria. Biomolecules. 2019;9:279. doi: 10.3390/biom9070279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nambu T., Minamino T., Macnab R.M., Kutsukake K. Peptidoglycan-hydrolyzing activity of the FlgJ protein, essential for flagellar rod formation in Salmonella typhimurium. J. Bacteriol. 1999;181:1555–1561. doi: 10.1128/JB.181.5.1555-1561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirano T., Minamino T., Macnab R.M. The role in flagellar rod assembly of the N-terminal domain of Salmonella FlgJ, a flagellum-specific muramidase. J. Mol. Biol. 2001;312:359–369. doi: 10.1006/jmbi.2001.4963. [DOI] [PubMed] [Google Scholar]
- 93.de la Mora J., Ballado T., González-Pedrajo B., Camarena L., Dreyfus G. The flagellar muramidase from the photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 2007;189:7998–8004. doi: 10.1128/JB.01073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.García-Ramos M., de la Mora J., Ballado T., Camarena L., Dreyfus G. Modulation of the enzymatic activity of the flagellar lytic transglycosylase SltF by rod components and the scaffolding protein FlgJ in Rhodobacter sphaeroides. J. Bacteriol. 2021;203:e00372-21. doi: 10.1128/JB.00372-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Bolle X., Crosson S., Matroule J.Y., Letesson J.J. Brucella abortus Cell Cycle and Infection Are Coordinated. Trends Microbiol. 2015;23:812–821. doi: 10.1016/j.tim.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Viollier P.H., Shapiro L. A lytic transglycosylase homologue, PleA, is required for the assembly of pili and the flagellum at the Caulobacter crescentus cell pole. Mol. Microbiol. 2003;49:331–345. doi: 10.1046/j.1365-2958.2003.03576.x. [DOI] [PubMed] [Google Scholar]
- 97.Ratushna V.G., Sturgill D.M., Ramamoorthy S., Reichow S.A., He Y., Lathigra R., Sriranganathan N., Halling S.M., Boyle S.M., Gibas C.J. Molecular targets for rapid identification of Brucella spp. BMC Microbiol. 2006;6:13–19. doi: 10.1186/1471-2180-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He Y., Xiang Z. Bioinformatics analysis of Brucella vaccines and vaccine targets using VIOLIN. Inmunome. 2010;27:S5. doi: 10.1186/1745-7580-6-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang K., Tong B.A., Liu J., Li C. A single-domain FlgJ contributes to flagellar hook and filament formation in the Lyme disease spirochete Borrelia burgdorferi. J. Bacteriol. 2012;194:866–874. doi: 10.1128/JB.06341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haiko J., Westerlund-Wikström B. The Role of the bacterial flagellum in adhesion and virulence. Biology. 2013;25:1242–1267. doi: 10.3390/biology2041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guttenplan S.B., Kearns D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013;37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall-Stoodley L., Stoodley P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 103.Burmølle M., Thomsen T.R., Fazli M., Dige I., Christensen L., Homøe P., Tvede M., Nyvad B., Tolker-Nielsen T., Givskov M., et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010;59:324–336. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 104.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;43:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 105.Klemm P., Vejborg R.M., Hancock V. Prevention of bacterial adhesion. Appl. Microbiol. Biotechnol. 2010;88:451–459. doi: 10.1007/s00253-010-2805-y. [DOI] [PubMed] [Google Scholar]
- 106.Mengucci F., Dardis C., Mongiardini E.J., Althabegoiti M.J., Partridge J.D., Kojima S., Homma M., Quelas J.I., Lodeiro A.R. Characterization of FliL Proteins in Bradyrhizobium diazoefficiens: Lateral FliL Supports Swimming Motility, and Subpolar FliL Modulates the Lateral Flagellar System. J. Bacteriol. 2020;202:e00708-19. doi: 10.1128/JB.00708-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gándara B., Merino A.L., Rogel M.A., Martínez-Romero E. Limited genetic diversity of Brucella spp. J. Clin. Microbiol. 2001;39:235–240. doi: 10.1128/JCM.39.1.235-240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.LeVier K., Phillips R.W., Grippe V.K., Roop R.M., 2nd, Walker G.C. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science. 2000;287:2492–2493. doi: 10.1126/science.287.5462.2492. [DOI] [PubMed] [Google Scholar]
- 109.Liu R., Ochman H. Origins of flagellar gene operons and secondary flagellar systems. J Bacteriol. 2007;189:7098–7104. doi: 10.1128/JB.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garrido-Sanz D., Redondo-Nieto M., Mongiardini E., Blanco-Romero E., Durán D., Quelas J.I., Martin M., Rivilla R., Lodeiro A.R., Althabegoiti M.J. Phylogenomic analyses of Bradyrhizobium reveal uneven distribution of the lateral and subpolar flagellar systems, which extends to Rhizobiales. Microorganisms. 2019;7:50. doi: 10.3390/microorganisms7020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mongiardini E.J., Quelas J.I., Dardis C., Althabegoiti M.J., Lodeiro A.R. Transcriptional Control of the Lateral-Flagellar Genes of Bradyrhizobium diazoefficiens. J. Bacteriol. 2017;199:e00253-17. doi: 10.1128/JB.00253-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun L., Yao L., Gao X., Huang K., Bai N., Lyu W., Chen W. Falsochrobactrum shanghaiense sp. nov., isolated from paddy soil and emended description of the genus Falsochrobactrum. Int. J. Syst. Evol. Microbiol. 2019;69:778–782. doi: 10.1099/ijsem.0.003236. [DOI] [PubMed] [Google Scholar]
- 113.Duarte C.M., Basile L.A., Zalguizuri A., Lepek V.C. The transcriptional factor TtsI is involved in a negative regulation of swimming motility in Mesorhizobium loti MAFF303099. FEMS Microbiol. Lett. 2016;363:222. doi: 10.1093/femsle/fnw222. [DOI] [PubMed] [Google Scholar]
- 114.Lai Q., Yu Z., Wang J., Zhong H., Sun F., Wang L., Wang B., Shao Z. Nitratireductor pacificus sp. nov., isolated from a pyrene-degrading consortium. Int. J. Syst. Evol. Microbiol. 2010;61:1386–1391. doi: 10.1099/ijs.0.024356-0. [DOI] [PubMed] [Google Scholar]
- 115.Scholz H.C., Hubalek Z., Sedlácek I., Vergnaud G., Tomaso H., Al Dahouk S., Melzer F., Kämpfer P., Neubauer H., Cloeckaert A., et al. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int. J. Syst. Evol. Microbiol. 2008;58:375–382. doi: 10.1099/ijs.0.65356-0. [DOI] [PubMed] [Google Scholar]
- 116.Wattam A., Foster J., Mane S., Beckstrom-Sternberg S., Beckstrom-Sternberg J., Dickerman A. Comparative Phylogenomics and Evolution of the Brucella Reveal a Path to Virulence. J. Bacteriol. 2014;196:920. doi: 10.1128/JB.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roop M., Gaines M., Anderson S., Caswell C., Martin W. Survival of the fittest: How Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 2009;198:4. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gómez L.A., Alvarez F.I., Fernández P.A., Flores M.R., Molina R.E., Coloma R.F., Oñate A.A. Immunogenicity and Protective Response Induced by Recombinant Plasmids Based on the BAB1_0267 and BAB1_0270 Open Reading Frames of Brucella abortus 2308 in BALB/c Mice. Front. Cell. Infect. Microbiol. 2016;6:117. doi: 10.3389/fcimb.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirov S.M. Bacteria that express lateral flagella enable dissection of the multifunctional roles of flagella in pathogenesis. FEMS Microbiol. Lett. 2003;224:151–159. doi: 10.1016/S0378-1097(03)00445-2. [DOI] [PubMed] [Google Scholar]
- 120.Walker T.S., Winkler H.H. Bartonella bacilliformis: Colonial types and erythrocyte adherence. Infect. Immun. 1998;31:480–486. doi: 10.1128/iai.31.1.480-486.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dehio C. Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell. Microbiol. 2008;10:1591–1598. doi: 10.1111/j.1462-5822.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lestrate P., Dricot A., Delrue R.M., Lambert C., Martinelli V., De Bolle X., Letesson J.J., Tibor A. Attenuated signature-tagged mutagenesis mutants of Brucella melitensis identified during the acute phase of infection in mice. Infect. Immun. 2003;71:7053–7060. doi: 10.1128/IAI.71.12.7053-7060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vitry M.A., Hanot Mambres D., Deghelt M., Hack K., Machelart A., Lhomme F., Vanderwinden J.M., Vermeersch M., De Trez C., Pérez-Morga D., et al. Brucella melitensis invades murine erythrocytes during infection. Infect. Immun. 2014;82:3927–3938. doi: 10.1128/IAI.01779-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lapaque N., Muller A., Alexopoulou L., Howard J.C., Gorvel J.P. Brucella abortus induces Irgm3 and Irga6 expression via type-I IFN by a MyD88-dependent pathway, without the requirement of TLR2, TLR4, TLR5 and TLR9. Microb. Pathog. 2009;47:299–304. doi: 10.1016/j.micpath.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Andersen-Nissen E., Smith K.D., Strobe K.L., Barrett S.L., Cookson B.T., Logan S.M., Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spera J.M., Ugalde J.E., Mucci J., Comerci D.J., Ugalde R.A. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc. Natl. Acad. Sci. USA. 2006;103:16514–16519. doi: 10.1073/pnas.0603362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Couper K.N., Blount D.G., Riley E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 128.Harms A., Dehio C. Intruders below the radar: Molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 2012;25:42–78. doi: 10.1128/CMR.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li X., Xu J., Xie Y., Qiu Y., Fu S., Yuan X., Ke Y., Yu S., Du X., Cui M., et al. Vaccination with recombinant flagellar proteins FlgJ and FliN induce protection against Brucella abortus 544 infection in BALB/c mice. Vet. Microbiol. 2012;161:137–144. doi: 10.1016/j.vetmic.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 130.Sadeghi Z., Fasihi-Ramandi M., Azizi M., Bouzari S. Mannosylated chitosan nanoparticles loaded with FliC antigen as a novel vaccine candidate against Brucella melitensis and Brucella abortus infection. J. Biotechnol. 2020;310:89–96. doi: 10.1016/j.jbiotec.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 131.Sadeghi Z., Fasihi-Ramandi M., Bouzari S. Brucella antigens (BhuA, 7α-HSDH, FliC) in poly I:C adjuvant as potential vaccine candidates against brucellosis. J. Immunol. Methods. 2021;113:172. doi: 10.1016/j.jim.2021.113172. [DOI] [PubMed] [Google Scholar]
- 132.Neglia G., Veneziano V., De Carlo E., Galiero G., Borriello G., Francillo M., Campanile G., Zicarelli L., Manna L. Detection of Brucella abortus DNA and RNA in different stages of development of the sucking louse Haematopinus tuberculatus. BMC Vet. Res. 2013;9:236. doi: 10.1186/1746-6148-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan X., Hu S., Yang Y., Xu D., Li H., Liu W., He X., Li G., Cai W., Bu Z. The Twin-Arginine Translocation System Is Important for Stress Resistance and Virulence of Brucella melitensis. Infect. Immun. 2020;88:e00389-20. doi: 10.1128/IAI.00389-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma Z., Li R., Hu R., Deng X., Xu Y., Zheng W., Yi J., Wang Y., Chen C. Brucella abortus BspJ Is a Nucleomodulin That Inhibits Macrophage Apoptosis and Promotes Intracellular Survival of Brucella. Front. Microbiol. 2020;11:599205. doi: 10.3389/fmicb.2020.599205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Z., Zhu Y., Sha T., Li Z., Li Y., Zhang F., Ding J. Design of a new multi-epitope vaccine against Brucella based on T and B cell epitopes using bioinformatics methods. Epidemiol. Infect. 2021;149:e136. doi: 10.1017/S0950268821001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
https://www.ncbi.nlm.nih.gov/protein/82615263, visualized based on data from the website https://pubmed.ncbi.nlm.nih.gov/. https://www.kegg.jp/dbget-bin/www_bget?bmf:BAB1_0260, visualized based on data from the website https://www.genome.jp/ (all accessed on 15 November 2021).