Abstract
Central venous catheter needleless connectors (NCs) have been shown to develop microbial contamination. A protocol was developed for the collection, processing, and examination of NCs to detect and measure biofilms on these devices. Sixty-three percent of 24 NCs collected from a bone marrow transplant center contained biofilms comprised primarily of coagulase-negative staphylococci.
Intravenous (i.v.) access lines (6, 7) and needleless connectors (NCs) (3, 4) have been demonstrated to be a risk factor for blood stream infection (BSI). Patients who require long-term i.v. access, such as bone marrow transplant patients, are at even greater risk for BSI. To deliver i.v. fluids (e.g., medication, blood products, or nutrients), tubing must be connected to i.v. catheters that enter the patient's bloodstream. Until recently, such connections have been made using beveled, hollow-bore needles that pierce an elastic membrane on a catheter end cap. Because of the potential for needle-stick injuries and health care worker exposure to bloodborne pathogens, many institutions have recently adopted the use of NCs. Though safer for health care workers, the potential for NCs to increase BSI risk to patients has been documented in outbreaks of nosocomial BSI (3, 4). In October 1998, the Centers for Disease Control and Prevention (CDC) was asked to investigate a BSI outbreak at a bone marrow transplant center in which NCs were involved. As part of this investigation, CDC assessed the ability of NCs to harbor biofilms that could act as a reservoir for BSI pathogens. It is well established that biofilms may develop on intravascular devices, including central venous catheters (CVCs) (1, 2, 8). Though contamination of NCs by various organisms has been observed (3, 4), the occurrence of biofilms on these devices has, to our knowledge, not been documented. The objectives of this study were (i) to develop a standardized protocol that could be used to collect, ship, and process NCs for biofilm contamination and (ii) to determine whether biofilms could develop on these devices and what organisms were the primary colonizers. Hickman NCs were collected from patients with long-term CVCs in a single bone marrow transplant center in which an outbreak of BSIs had occurred.
Collection and shipment of NCs.
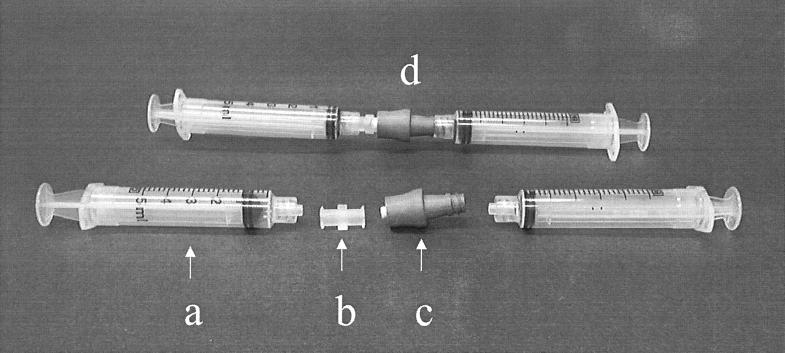
Female-female luer couplings (no. 06359-42; Cole Parmer, Niles, Ill.) were autoclaved and then used to connect two 5-ml syringes. One of the syringes contained 5-ml of phosphate-buffered saline (PBS; pH 7.2; Life Technologies, Grand Island, N.Y.). Syringe pairs were placed into zip-lock bags and shipped on ice packs to the bone marrow transplant center for the collection of NCs. By using an aseptic technique, the NCs were removed from the patient's CVC and placed into an unused sterile Petri dish and transported to the laboratory. After the two syringes were separated, the luer coupling remained on one syringe. The smaller end of the NC was then wiped with a sterile alcohol pledget and connected to the syringe without the luer coupling. The other end of the NC was connected to the luer coupling on the second syringe. Approximately half of the PBS was gently forced through the connector into the opposite syringe in order to fill the inner lumen of the connector. The NC, now attached to the twin syringes, was then placed inside a zip-lock bag and shipped on ice packs to the CDC laboratory for processing within 24 h of collection. The collection protocol is depicted in Fig. 1.
FIG. 1.
Depiction of the NC collection protocol, showing the syringe (a), the luer adapter (b), a NC (c), and a connector prepared for shipment (d).
Processing of NCs.
While still attached to syringes, the outer surfaces of connectors were disinfected prior to processing for biofilms by immersion in 0.525% sodium hypochlorite (10% solution of commercial bleach in filter sterile reverse osmosis water [FSROW]) for 10 min and then immersion in a 0.12 M Na2S2O3 solution for 1 min to inactivate the sodium hypochlorite, followed by air drying in a biological safety cabinet. The remaining volume of PBS was flushed into the syringe attached to the small end of the NC. The filled syringe was removed and emptied into a sterile 50-ml centrifuge tube containing 5 ml of sterile PBS. Because of the concern that organisms may have detached from the NC luminal biofilms during transit, this 5-ml volume of PBS was added to the tube containing the NC components. This additional 5 ml also provided a total volume of 10 ml, which was necessary to totally immerse the NC during processing. The NC was detached from the syringe and cut in half transversely at its joint using an alcohol-sterilized Handy Cut tool (Craftsman; Sears Roebuck and Co., Chicago, Ill.), and both halves were dropped into the tube. Connectors were then processed by three 30-s cycles of sonication at a frequency of 42 kHz (model 2510; Branson Co., Danbury, Conn.), followed by 30 s of vortexing (Vortex Genie 2; Scientific Products Co., Bohemia, N.Y.), and homogenization of the suspension in a tissue homogenizer for 60 s (Polyscience Tissue Homogenizer model K-120; Polysciences Co., Niles, Ill.) at approximately 16,000 rpm. This biofilm suspension was then either filtered through a 0.45-μm (pore-size) membrane filter using a sterile filtration apparatus in a biological safety cabinet, after which filters were placed onto trypticase soy agar (TSA) plates, or the suspension was serially diluted and plated onto TSA plates using the spread-plating technique. Plates were incubated for 48 h at 30°C, and the colonies were counted. Representative colonies (based on colony morphology) were subcultured and identified using conventional biochemical techniques.
Validation of the outer-surface disinfection method.
An experiment was performed to compare several disinfectants for their ability to inactivate and remove the bacteria, including bacterial endospores, from NC outer surfaces. A spore suspension of Bacillus stearothermophilus (ATCC 7953) in ethanol was diluted in sterile phosphate-buffered water, plated on TSA, and incubated at 56°C for 24 h to verify viability. To protect the inner lumen from disinfectants, 5-ml syringes were connected to each end of 20 sterile NCs, and the connector immersed in the B. stearothermophilus spore suspension (8 × 105 spores per ml of PBS) for 10 s and then air dried in a biological safety cabinet for 2 h. Four treatment methods were evaluated: (i) untreated control, rinsed in FSROW for 1 min; (ii) samples immersed in 70% ethanol for 30 s and FSROW for 1 min and then air dried; (iii) samples immersed in 0.525% sodium hypochlorite for 10 min and 0.12 M Na2S2O3 for 1 min and then air dried; and (iv) samples immersed in 2% glutaraldehyde (dilution of 25% glutaraldehyde solution [Bio-Rad, Cambridge, Mass.] in FSROW) for 10 min and 0.6 M NaHSO3 for 1 min and then air dried. To verify that there was no carryover of the disinfectant that might affect biofilm recovery from the inner lumen, connectors were cut in half following the above treatments, and placed into tubes containing 1.2 × 105 CFU of Enterobacter cloacae (CDC Dialysis and Medical Device Section Laboratory no. 95-12-25) ml−1 in 10 ml of PBS. E. cloacae had been grown on R2A medium (Difco Laboratories, Detroit, Mich.) to stationary phase (20 h), resuspended to a 0.5 McFarland equivalent (600-nm absorbance), and then diluted to the concentration used. The viable cell count of the inoculum was determined by plating on R2A medium. E. cloacae was chosen to simulate the organisms to potentially be present on the inner lumen of the connectors. Connectors were then sonicated and vortexed, as described above, after which the suspension was homogenized and plated on both R2A medium and TSA medium. The R2A medium plates were incubated at 30°C for 24 h and counted; total colony counts represented the number of E. cloacae. The TSA medium plates were incubated at 56°C for 24 h and counted; total colony counts represented the number of B. stearothermophilus. A carryover of disinfectant could be assumed if the colony counts of E. cloacae decreased from that of the control treatment. The efficiency of the disinfectant could be simultaneously determined by demonstrating a decrease in the number of B. stearothermophilus CFUs from that of the control.
Validation of biofilm recovery method.
To validate the reproducibility of the biofilm recovery method, two disk reactors, described elsewhere (5), containing 250 ml of sterile Ringer's lactate with 5% dextrose (D5RL; Baxter Healthcare Corp., Deerfield, Ill.) were each inoculated with 1 ml of a 2.5 × 105 ml−1 suspension of E. cloacae (CDC's Dialysis and Medical Device Section Laboratory no. 95-12-25). Sterile silicone tubing (1.65 mm, inner diameter; Cole Parmer, Niles, Ill.) from each reactor was connected to five sterile NCs in series by means of a peristaltic pump (model no. 7553-80, with an eight-cartridge pump head; Cole Parmer) at a flow rate of 1.5 ml min−1. The reactors were then placed on a stirring plate set at 100 rpm and mixed for 5 days at a temperature of 22 to 25°C. After 5 days, the flow rate through the connectors was stopped, and both lines containing the NC were removed from the reactors to a biological safety cabinet, with care taken to ensure that the solution stayed inside the connectors. The NCs were then disconnected from each other and processed for biofilm removal as described above.
Scanning electron microscopy.
NCs were cut apart as described above and fixed by placing them into 5% glutaraldehyde (Ted Pella, Redding, Calif.) in cacodylate buffer (0.067 M, pH 6.2) for fixation overnight at room temperature. The samples were then dehydrated in a graded series of ethanol (30, 50, 70, and 90%) for 10 min each at room temperature and immersed in hexamethyldisilazane (HMDS; Polysciences, Warrington, Pa.) for 4 h at room temperature. Finished specimens were mounted on aluminum stubs with silver paint, sputter coated with 25-nm gold particles, and examined with a Philips XL 20 SEM (FEI Company, a subsidiary of Philips, Hillsboro, Oreg.).
Since the goal of this study was to develop a sampling protocol and biofilm recovery technique that would measure only the organisms attached to the NC inner surfaces, it was important to choose a surface disinfectant that would inactivate even the most resistant organisms on the connector outer surfaces. Therefore, B. stearothermophilus was chosen as the indicator organism, since we assumed that if the spores of this organism were inactivated, all other non-endospore-forming organisms commonly found as contaminants on medical devices exposed outside the patient body (e.g., coagulase-negative staphylococci) would also be inactivated. Treatment of the NC outer surfaces with 0.525% sodium hypochlorite solution was more efficient at reducing viable endospores (3.3-log reduction) compared to either glutaraldehyde (2.2-log reduction) or 70% ethanol treatment (no reduction) (Table 1). It was also important to ensure that there was no carryover of the disinfectant that would affect the recovery of the biofilms on the inner surfaces. E. cloacae served as the indicator biofilm organism, since we had previously demonstrated its ability to grow and form biofilms on these devices (5). The results shown in Table 1 indicated that there was negligible carryover of each disinfectant tested (column 4).
TABLE 1.
Effect of different treatments on the inactivation of B. stearothermophilus endospores from NC outer surfaces and on the recovery of E. cloacae
| Treatment | Endospores recovered (Mean log CFU/NC [SD]) (n = 5) | Log reduction | E. cloacae recovered (Mean log CFU/NC [SD]) (n = 5) |
|---|---|---|---|
| Untreated | 3.79 (0.08) | 6.04 (0.05) | |
| 70% ethanol for 30 s | 3.85 (0.14) | −0.06 | 5.99 (0.09) |
| 0.525% sodium hyplochlorite for 10 min | 0.46 (1.03)a | 3.33 | 6.05 (0.04) |
| 2% glutaraldehyde for 10 min | 1.59 (1.45)a | 2.20 | 6.13 (0.12) |
Four of five plates recovered 0 CFU after sodium hypochlorite treatment, as did two of five plates for the glutaraldehyde treatment. Therefore, a “1” was added to all 0 (nonexisting) data points to make the data existent and to enable a calculation of the mean and standard deviation.
Our method for biofilm recovery was initially validated using E. cloacae biofilms grown in a model system. The mean recovery values of the biofilms on the NCs for two separate experiments were 4.29 (n = 5)- and 4.11 (n = 5)-log CFU per connector, using the sonication-vortexing-homogenization biofilm removal technique. The coefficients of variation for both experiments were 7.5 and 5.6%, which is considered reproducible as a biofilm recovery method. This method has been previously described as providing greater than 97% recovery of attached organisms from plastic surfaces (5).
The predominant organism isolated from NCs collected from the bone marrow transplant center was Staphylococcus epidermidis (6 of 24 samples), a common skin organism. S. epidermidis has also been commonly isolated from biofilms of indwelling medical devices (2). Several other coagulase-negative staphylococci, including S. hominis subsp. hominis (3 of 24), S. haemolyticus (3 of 24), and S. xylosus (1 of 24) were also isolated. Micrococcus luteus (2 of 24), other Micrococcus spp. (1 of 24), Kocuria kristinae (1 of 24), Curtobacterium spp. (1 of 24), and Klebsiella pneumoniae (2 of 24) were also found. Bacillus spp., a common soil organism not usually isolated from indwelling medical devices, was found in only 1 of 24 samples.
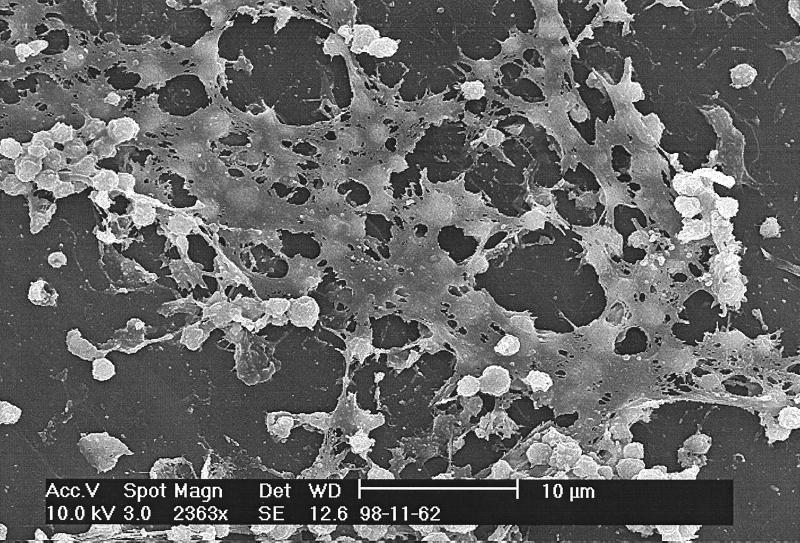
Of 24 NCs collected and examined, 15 (63%) contained detectable levels of attached organisms, and 3 of these contained more than 1,000 CFU per NC. Scanning electron microscopy provided the confirmatory evidence that organisms had developed biofilms. Figure 2 shows a staphylococcal biofilm on a connector surface, as evidenced by the presence of both bacterial cells and apparent extracellular polymeric substances. Anaissie et al. (1) showed that bacterial colonization of CVCs occurred rapidly and that biofilm was found on the CVCs of all patients in that study whose catheters had been in place for less than 3 days. These authors observed biofilms on all CVCs sampled, and they also showed that bacteria could adhere to medical devices within 24 h. Therefore, it would not be unreasonable for NCs in our study to contain significant levels of attached organisms and biofilms, since they were used for periods of up to 7 days (data not shown).
FIG. 2.
Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of an NC.
In conclusion, a protocol has been developed for the collection, shipment, and processing of NCs to determine the level of biofilm contamination on these devices. The attached bacteria were isolated from 63% of the 24 NCs sampled. The organisms isolated from these connectors were S. epidermidis (predominant isolate), S. hominis subsp. hominis, S. haemolyticus, S. xylosus, M. luteus, Micrococcus spp., K. pneumoniae, Curtobacterium spp., K. kristinae, and Bacillus spp. Several of the NCs examined contained more than 1,000 CFU per NC, and scanning electron microscopy revealed the presence of both bacterial cells and extracellular polymeric substances.
Acknowledgments
We acknowledge the generous technical support of Stacy Holt and Sigrid McAllister of the Hospital Infections Program of the CDC.
REFERENCES
- 1.Anaissie E, Samonis G, Kontoyiannis D, Costerton J, Sabharwal U, Bodey G, Raad I. Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. Eur J Clin Microbiol Infect Dis. 1995;14:135–137. doi: 10.1007/BF02111873. [DOI] [PubMed] [Google Scholar]
- 2.Christensen G D, Baldassari L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: American Society for Microbiology; 1994. pp. 45–78. [Google Scholar]
- 3.Danzig L E, Short L J, Collins K, Mahoney M, Sepe S, Bland L, Jarvis W R. Bloodstream infections associated with a needleless intravenous infusion system in patients receiving home infusion therapy. JAMA. 1995;273:1862–1864. [PubMed] [Google Scholar]
- 4.Do A N, Ray B J, Banerjee S N, Illian A F, Barnett B J, Pham M H, Hendricks K A, Jarvis W R. Bloodstream infection associated with needleless device use and the importance of infection-control practices in the home health care setting. J Infect Dis. 1999;179:442–448. doi: 10.1086/314592. [DOI] [PubMed] [Google Scholar]
- 5.Donlan R, Murga R, Carson L. Growing biofilms in intravenous fluids. In: Wimpenny J, Gilbert P, Walker J, Brading M, Bayston R, editors. Biofilms: the good, the bad, and the ugly. Contributions made at the Fourth Meeting of the Biofilm Club. United Kingdom: Powys; 1999. pp. 23–29. [Google Scholar]
- 6.Elliott T S J, Moss H A, Tebbs S E, Wilson I C, Bonser R S, Graham T R, Burke L P, Faroqui M H. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur J Clin Microbiol Infect Dis. 1997;16:210–213. doi: 10.1007/BF01709583. [DOI] [PubMed] [Google Scholar]
- 7.Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893–898. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- 8.Raad I I, Sabbagh M F, Rand K H, Sherertz R J. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn Microbiol Infect Dis. 1992;15:13–20. doi: 10.1016/0732-8893(92)90052-u. [DOI] [PubMed] [Google Scholar]