Abstract
Objectives:
Treatment with trastuzumab and chemotherapy significantly improves the outcome in patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (AGC). CT-P6 (trastuzumab-pkrb; Herzuma) is a trastuzumab biosimilar approved for the treatment of HER2-positive gastric cancer. In this study, we aimed to compare the efficacy and safety of CT-P6 and reference trastuzumab as first-line treatment for HER2-positive AGC.
Materials and Methods:
The medical records of 102 patients with HER2-positive AGC treated with first-line trastuzumab-based chemotherapy were retrospectively reviewed. These patients were treated with either reference trastuzumab (n=72) or a biosimilar (n=30). Treatment outcomes, such as objective response rate, progression-free survival (PFS), and overall survival (OS), were compared between the reference and biosimilar groups.
Results:
The objective response rate of both groups (52.8% and 56.8% in the reference and biosimilar groups, respectively) were comparable (P=0.72). No statistically significant difference was observed with the reference versus biosimilar trastuzumab for PFS (median PFS, 6.9 vs. 5.4 mo; P=0.98) or OS (median OS, 12.3 mo vs. not reached; P=0.42). Safety profiles were similar between the 2 groups.
Conclusions:
Biosimilar trastuzumab showed equivalent outcome to reference trastuzumab, with similar adverse events. Biosimilar trastuzumab can suitably and safely replace trastuzumab as a reference for the treatment of HER2-positive AGC.
Key Words: gastric cancer, trastuzumab, biosimilar, CT-P6
Although the incidence of gastric cancer (GC) is steadily declining, GC remains an important cancer worldwide that accounted for over 1 million new cases in 2020, ranking fifth for incidence and fourth for mortality globally.1 Since complete surgical resection is the only potentially curative treatment for GC, early diagnosis is essential in GC to ensure a good prognosis. However, GC is often at an advanced stage at the time of diagnosis. At advanced stages, GC can be treated with palliative chemotherapy but can rarely be cured.2 Cytotoxic chemotherapy remains the backbone of palliative treatment, with a median overall survival (OS) of less than a year. Platinum-based and fluoropyrimidine-based combination therapy is the preferred first-line treatment for advanced gastric cancer (AGC).3
Human epidermal growth factor receptor 2 (HER2) plays a central role in the pathogenesis of several human cancers.4 HER2 is also overexpressed or amplified in 20% to 25% of gastric and gastroesophageal junction cancer.5 In the Trastuzumab for Gastric Cancer (ToGA) trial, the addition of the HER2-targeting monoclonal antibody trastuzumab to platinum-fluoropyrimidine chemotherapy in HER2-positive AGC improved response rates, progression-free survival (PFS), and OS compared with chemotherapy alone.6 Median OS was 13.8 months for patients assigned to trastuzumab plus chemotherapy arm compared with 11.1 months in the chemotherapy group (hazard ratio=0.74; 95% confidence interval [CI]: 0.60-0.91; P=0.0046). Trastuzumab, in combination with platinum-based chemotherapy, is the standard of care as frontline therapy for HER2-positive AGC.
A biosimilar is a biological product that is highly similar to the reference product and has no clinically meaningful differences in terms of the safety, purity, and potency of the product.7–9 CT-P6 (Herzuma; Celltrion Inc., Incheon, South Korea), a trastuzumab biosimilar, has equivalent pharmacokinetics to the reference trastuzumab in healthy subjects.10 CT-P6 was approved for the treatment of patients with HER2-positive early-stage breast cancer based on the results of a phase 3 trial.11 The use of CT-P6 has also been extrapolated to the treatment of metastatic breast cancer and metastatic GC. The approval of trastuzumab biosimilars may provide more patients with access to trastuzumab in clinical practice.
Although trastuzumab biosimilars are of growing importance in AGC, no studies have directly compared the efficacy and safety between the trastuzumab biosimilar CT-P6 and reference trastuzumab in patients with AGC. Herein, we investigated whether the reference trastuzumab and its biosimilar are interchangeable and compared the efficacy and side effects of these molecules in treating patients with HER2-positive AGC.
MATERIALS AND METHODS
Patient Selection Criteria
One hundred two patients with HER2 overexpression or HER2-amplified AGC who received first-line trastuzumab-based chemotherapy between February 2011 and December 2020 at the Gachon University Gil Medical Center, Republic of Korea, were retrospectively reviewed. The selection criteria for this study were as follows: at least 18 years of age; histologically confirmed gastric adenocarcinoma with distant metastases or recurrent disease; documented HER2 overexpression by immunohistochemistry and/or HER2 gene amplification by silver in situ hybridization; no previous chemotherapy except adjuvant chemotherapy completed at least 6 months before enrollment; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2; adequate organ function; no serious clinical complications. This retrospective study was reviewed and approved by the Institutional Review Board of Gachon University Gil Medical Center (Incheon, Republic of Korea).
Treatment Methods
All patients received trastuzumab in combination with fluoropyrimidine/platinum chemotherapy. Trastuzumab was administered by intravenous infusion at a loading dose of 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg doses every 3 weeks. Since the biosimilar of trastuzumab (CT-P6; Celltrion Inc.) was approved for AGC, physicians decided whether patients receive a biosimilar or reference trastuzumab, called Herceptin (Genentech, San Francisco, CA). Dose reductions of chemotherapy, including the initial dose, and treatment delays, were decided at each physician’s discretion according to the patient’s general condition, organ function, and the severity of hematological and nonhematological toxicities. Treatment was repeated until disease progression, unacceptable toxicity, or patient refusal. When a maximal clinical benefit was obtained, the treatment was stopped after 8 cycles, according to the investigator’s decision.
Assessment of Efficacy and Toxicity
Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.12 Objective response rate (ORR) was defined as the proportion of confirmed complete response (CR) or partial response at the best response. Disease control rate was defined as the percentage of confirmed CR, partial response, or stable disease at the best response. Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. PFS was measured from the date of initial treatment to the date of disease progression, death from any cause, or the last follow-up visit. OS was defined as the time from the first day of treatment until either the time of death from any cause or the last follow-up visit.
Statistical Analysis
Clinical variables were compared between groups using an independent t test or Pearson χ2 test, as appropriate. Tumor response to chemotherapy was compared across treatments using the Pearson χ2 test. The PFS and OS were estimated using the Kaplan-Meier method, and the significant differences between these curves were determined using the log-rank test. All statistical analyses were conducted using the R software (version 3.6.3), an open-source statistical software program. Statistical significance was set at P-value <0.05.
RESULTS
Patient Characteristics
From February 2011 to December 2020, 30 patients received biosimilar trastuzumab (CT-P6) in combination with platinum-based chemotherapy (biosimilar group), whereas 72 patients received reference trastuzumab with platinum-based chemotherapy (reference group). The baseline characteristics of the patients are summarized in Table 1. The backgrounds of the patients were not significantly different, excluding ECOG PS. Patients in the reference group had better PS than those in the biosimilar group.
TABLE 1.
Patient Baseline Demographics and Disease Characteristics
| n (%) | |||
|---|---|---|---|
| Characteristics | Reference Trastuzumab (N=72) | Biosimilar Trastuzumab (N=30) | P |
| Age, median (range) (y) | 63 (28-86) | 66 (28-83) | 0.216 |
| Sex | |||
| Male | 59 (81.9) | 29 (96.7) | 0.060 |
| ECOG performance status | 0.043 | ||
| 0 | 21 (29.2) | 2 (6.7) | |
| 1 | 39 (54.2) | 22 (73.3) | |
| 2 | 12 (16.7) | 6 (20) | |
| Previous gastrectomy | 19 (26.4) | 10 (33.3) | 0.479 |
| Primary site | 0.147 | ||
| EG junction | 4 (5.6) | 2 (6.7) | |
| Cardia | 4 (5.6) | 0 (0) | |
| Body | 41 (56.9) | 12 (40) | |
| Antrum | 23 (31.9) | 16 (53.3) | |
| Disease status | 0.437 | ||
| Initially metastatic | 55 (76.4) | 25 (83.3) | |
| Recurrent | 17 (23.6) | 5 (16.7) | |
| No. metastatic sites | 0.639 | ||
| 1 | 30 (41.7) | 11 (36.7) | |
| ≥2 | 42 (58.3) | 19 (63.3) | |
| Histologic grade | 0.218 | ||
| Well-differentiated/moderately differentiated | 36 (50) | 19 (63.3) | |
| Poorly differentiated/signet ring cell | 36 (50) | 11 (36.7) | |
| HER2 status | 0.858 | ||
| IHC 3+ | 37 (51.4) | 16 (53.3) | |
| IHC 2+ and ISH-positive | 35 (48.6) | 14 (46.7) | |
| LVEF at baseline, median (range) | 66 (30-83) | 60 (50-74) | 0.070 |
| Chemotherapy backbone | 1.000 | ||
| XP | 69 (95.8) | 30 (100) | |
| FP | 2 (2.8) | 0 (0) | |
| XELOX | 1 (1.4) | 0 (0) | |
ECOG indicates Eastern Cooperative Oncology Group; EG, esophagogastric; FP, fluorouracil+cisplatin; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; LVEF, left ventricular ejection fraction; XELOX, capecitabine+oxaliplatin; XP, capecitabine+cisplatin.
Treatment Exposure and Tumor Response
The number of chemotherapy cycles was not significantly different between the 2 groups: median of 6 cycles (range: 1 to 19) in the reference group and 5 cycles (range: 1 to 26) in the biosimilar group. Tumor responses are shown in Table 2. Only 2 patients achieved a confirmed CR in the reference group, and no patients achieved CR in the biosimilar group. The ORR of the 2 groups was not significantly different (52.8% in the reference group vs. 56.7% in the biosimilar group, respectively; P=0.720). The disease control rates of the reference and biosimilar were 69.4% and 73.3%, respectively (P=0.695).
TABLE 2.
Treatment Delivery and Objective Responses
| n (%) | |||
|---|---|---|---|
| Reference Trastuzumab (N=72) | Biosimilar Trastuzumab (N=30) | P | |
| Treatment delivery, median (range) | |||
| Total cycles | 6 (1-19) | 5 (1-26) | 0.834 |
| No. chemotherapy cycles | 6 (1-13) | 5 (1-9) | 0.557 |
| Conversion surgery | 15 (16.3) | 3 (10.0) | 0.557 |
| Best overall response | |||
| CR | 2 (2.8) | 0 (0) | |
| PR | 36 (50) | 17 (56.7) | |
| SD | 12 (16.7) | 5 (16.7) | |
| PD | 5 (6.9) | 1 (3.3) | |
| NE | 17 (23.6) | 7 (23.3) | |
| ORR | 38 (52.8) | 17 (56.7) | 0.720 |
| DCR | 50 (69.4) | 22 (73.3) | 0.695 |
CR indicates complete response; DCR, disease control rate; NE, not evaluated; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Survival
The median follow-up time for PFS was 48.0 months in the reference group and 7.2 months in the biosimilar group. After the median follow-up period, 81 patients (64/70 patients [91.4%] in the reference group and 17/32 patients [53.1%] in the biosimilar group) had progressed or died. The survival curves are shown in Figure 1. The median PFS was 6.9 months (95% CI, 5.4-8.9) in the reference group and 5.4 months (95% CI, 4.6-could not be calculated) in the biosimilar group (P=0.98). The median OS was 12.3 months (95% CI, 9.3-21.7) in the reference group and was not reached (95% CI, 8.2-lower limit could not be calculated) in the biosimilar group (P=0.42). There was no difference in the outcomes between the 2 groups.
FIGURE 1.
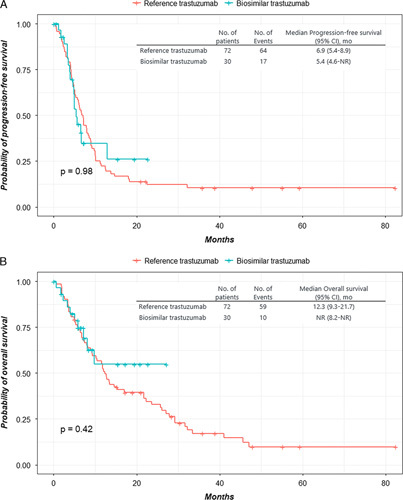
The Kaplan-Meier curves for progression-free survival (A) and overall survival (B) for patients taking reference trastuzumab versus trastuzumab biosimilar (CT-P6). CI indicates confidence interval; NR, not reported.
Safety
The hematological and nonhematological adverse events are listed in Table 3. The proportion of patients reporting at least 1 adverse event and grade 3 or worse treatment-related adverse events were similar between the 2 groups. The most frequent adverse events in both groups were hematological toxicities, such as anemia, leukopenia, neutropenia, and thrombocytopenia. Heart failure was reported in 9 patients in the reference group (12.5%) and 3 patients in the biosimilar group (10.0%). However, no grade 3 or 4 heart failure was reported. Of these patients, trastuzumab was discontinued in only 1 patient in the biosimilar group because of a confirmed decrease in left ventricular ejection fraction (LVEF). The LVEF measurements are presented in Table 4. Of the 102 patients who received trastuzumab-based chemotherapy, only 27 patients (37.5%) in the reference group and 12 patients (40.0%) in the biosimilar had both baseline and posttreatment LVEF measurements recorded using the echocardiogram. Six patients (22.2%) in the reference group and 2 patients (16.7%) in the biosimilar group showed a ≥10% decrease in LVEF. Among these patients, 1 patient (3.7%) in the reference group and 1 patient (8.3%) in the biosimilar group showed a ≥10% decrease in LVEF and final LVEF that decreased to or below 50%.
TABLE 3.
Adverse Events in Patients With Advanced Gastric Cancer After Treatment With Reference and Biosimilar Trastuzumab
| n (%) | ||||
|---|---|---|---|---|
| Reference Trastuzumab (N=72) | Biosimilar Trastuzumab (N=30) | |||
| Adverse Event | Any Grade | Grade 3-4 | Any Grade | Grade 3-4 |
| Hematological | ||||
| Anemia | 65 (90.3) | 13 (18.1) | 30 (100) | 12 (40) |
| Leukopenia | 37 (51.4) | 6 (8.3) | 21 (70) | 5 (16.7) |
| Neutropenia | 40 (55.6) | 15 (20.8) | 21 (70) | 11 (36.7) |
| Thrombocytopenia | 35 (48.6) | 4 (5.6) | 18 (60) | 3 (10) |
| Febrile neutropenia | 6 (8.3) | 6 (8.3) | 4 (13.3) | 4 (13.3) |
| Nonhematological | ||||
| Fatigue | 24 (33.3) | 0 (0) | 8 (26.7) | 0 (0) |
| Anorexia | 35 (48.6) | 0 (0) | 12 (40) | 0 (0) |
| Nausea | 25 (34.7) | 1 (1.4) | 9 (30) | 0 (0) |
| Vomiting | 17 (23.6) | 4 (5.6) | 6 (20) | 0 (0) |
| Diarrhea | 10 (13.9) | 0 (0) | 8 (26.7) | 0 (0) |
| Constipation | 4 (5.6) | 0 (0) | 2 (6.7) | 0 (0) |
| Stomatitis | 16 (22.2) | 3 (4.2) | 5 (16.7) | 1 (3.3) |
| Hand-foot syndrome | 12 (16.7) | 0 (0) | 0 (0) | 0 (0) |
| CIPN | 9 (12.5) | 1 (1.4) | 1 (3.3) | 0 (0) |
| Heart failure | 9 (12.5) | 0 (0) | 3 (10) | 0 (0) |
| Increased serum creatinine | 5 (6.9) | 1 (1.4) | 6 (20) | 0 (0) |
| Increased AST or ALT | 2 (2.8) | 0 (0) | 1 (3.3) | 0 (0) |
| Hyperbilirubinemia | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; CIPN, chemotherapy-induced peripheral neuropathy.
TABLE 4.
Change in Left Ventricular Ejection Fraction From Baseline During Trastuzumab-based Chemotherapy
| n (%) | ||
|---|---|---|
| Reference Trastuzumab (N=27) | Biosimilar Trastuzumab (N=12) | |
| No decrease | 7 (25.9) | 4 (33.3) |
| Decrease of <10 points from baseline | 14 (51.9) | 6 (50.0) |
| Decrease of ≥10 points from baseline | 6 (22.2) | 2 (16.7) |
| <50 points and decrease of ≥10 points from baseline | 1 (3.7) | 1 (8.3) |
DISCUSSION
The results of this study demonstrated similar clinical efficacy and safety between the biosimilar trastuzumab and its reference product in patients with HER2-positive AGC. To the best of our knowledge, this study is the first to directly compare the efficacy and safety of trastuzumab biosimilar to reference trastuzumab in patients with AGC. The ORRs of both groups were comparable (52.8% in the reference group and 56.7% in the biosimilar group). Furthermore, no statistically significant difference was observed in the survival outcomes between the 2 molecules. Although the patients in the biosimilar group had worse PS than those in the reference group, comparable efficacies were demonstrated between the 2 drugs in this study. Furthermore, biosimilar trastuzumab was well tolerated and demonstrated similar safety profiles compared with reference trastuzumab.
Compared with chemotherapy alone, treatment with trastuzumab in combination with chemotherapy significantly improved OS among patients with HER2-positive AGC.6 However, targeted cancer drugs such as trastuzumab are relatively more expensive compared with other chemotherapy drugs. Despite its clinical benefits, this high price can represent a substantial barrier to trastuzumab treatment, especially in a financially constrained environment.13 Since the price of biosimilars is generally lower than that of the reference products, the cost saved by switching patients to biosimilars could enable improved access to these clinically effective drugs.
Several trastuzumab biosimilar candidates have been developed in recent years. The US Food and Drug Administration (FDA) has approved 5 trastuzumab biosimilars: trastuzumab-anns, trastuzumab-dkst, trastuzumab-dttb, trastuzumab-pkrb, and trastuzumab-qyyp.14 CT-P6 (trastuzumab-pkrb), produced by the South Korean Biotechnology Company Celltrion, has demonstrated comparable pharmacodynamics and efficacy to trastuzumab in clinical trials.10,11,15,16 Trastuzumab biosimilars have been studied only in patients with breast cancer, and extrapolation has been granted from early breast cancer to metastatic breast cancer and metastatic GC. Extrapolation is an established regulatory principle that refers to the approval of a biosimilar for use in an indication held by the reference product, not directly studied in a comparative clinical trial with a biosimilar.17 Although some controversy exists regarding this topic, this concept is critical to the goals of an abbreviated pathway—improving access and options at a potentially lower cost. Thus, the use of biosimilars for the treatment of patients with HER2-positive AGC could be considered an effective way to provide budgetary savings and increase patient access to biological medicines.
Recently, a phase Ib/II study (PANTHERA trial) has shown that biosimilar trastuzumab (CT-P6) is effective in patients with HER2-positive AGC when used in combination with pembrolizumab and chemotherapy.18 These findings also have implications for reducing the costs of care by using biosimilars. The use of biosimilar trastuzumab is expected to increase in both clinical practice and clinical trials. However, no studies have directly compared the efficacy and safety of trastuzumab biosimilar to reference trastuzumab in patients with AGC. Therefore, the results of this study highlighting the efficacy and safety between the 2 drugs will guide future clinical trials.
There are several limitations to the current study. First, this was a retrospective analysis based on chart review. A retrospective study could provide important insights into the actual clinical use of biosimilar trastuzumab. However, due to reliance upon electronic medical records, if adverse events were not recorded, it was assumed that they did not occur. Second, this study included a small population (n=122) from a single institution. Third, since follow-up echocardiography was not routinely performed after trastuzumab treatment, it was difficult to accurately compare the cardiovascular safety between the 2 drugs. Echocardiography was performed to assess cardiac function when heart failure was clinically suspected. We might have missed patients who had no symptoms of heart failure but had decreased cardiac function. In our study, follow-up echocardiography was performed for only 12 patients (40%) in the biosimilar group and 27 patients (37.5%) in the reference group. Therefore, larger prospective studies are needed to confirm our findings.
The findings of this retrospective study demonstrated that biosimilar trastuzumab (CT-P6) is as efficacious as reference trastuzumab in terms of response rates, PFS and OS with comparable toxicity. in patients with HER2-positive AGC. The results of this study will help investigators in planning clinical studies in patients treated with biosimilar trastuzumab. Further prospective studies with large numbers of patients are, however, needed to validate these results.
Footnotes
W.K.L. and S.J.S. are co-correspondence author.
The authors declare no conflicts of interest.
Contributor Information
Joo-Hwan Park, Email: replica1874@hanmail.net.
Ja Hyun Yeo, Email: btceo@naver.com.
Young Saing Kim, Email: zoomboom@hanmail.net.
Inkeun Park, Email: ingni79@gilhospital.com.
Hee Kyung Ahn, Email: hkahn@gilhospital.com.
Eun Kyung Cho, Email: ekcho@gilhospital.com.
Dong Bok Shin, Email: dbs@gilhospital.com.
Jun-Young Yang, Email: yangjy@gilhospital.com.
Hyung-Sik Kim, Email: hskim@gilhospital.com.
Woon Kee Lee, Email: lwk@gilhospital.com.
Sun Jin Sym, Email: sympson@gilhospital.com.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Pyrhonen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. [DOI] [PubMed] [Google Scholar]
- 4. Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12(suppl 1):S9–S13. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration (FDA). Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry; 2015.
- 8. European Medicines Agency. Guidelines on biosimilar medicinal products. 2014. Available at: www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-biosimilar . Accessed January 22, 2019.
- 9. US Department of Health and Human Services; US Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. 2015. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf . Accessed April 29, 2016.
- 10. Esteva FJ, Lee SJ, Stebbing J, et al. Phase I clinical trial comparing PK and safety of trastuzumab and its biosimilar candidate CT-P6. Breast. 2017;32:S68–S69. [Google Scholar]
- 11. Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18:917–928. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13. Lammers P, Criscitiello C, Curigliano G, et al. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the United States and emerging markets. Pharmaceuticals (Basel). 2014;7:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration (FDA). Biosimilar product information. 2018. Available at: www.fda.gov/drugs/biosimilars/biosimilar-product-information . Accessed December 8, 2019.
- 15. Esteva FJ, Baranau YV, Baryash V, et al. Efficacy and safety of CT-P6 versus reference trastuzumab in HER2-positive early breast cancer: updated results of a randomised phase 3 trial. Cancer Chemother Pharmacol. 2019;84:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Im Y-H, Odarchenko P, Grecea D, et al. Double-blind, randomized, parallel group, phase III study to demonstrate equivalent efficacy and comparable safety of CT-P6 and trastuzumab, both in combination with paclitaxel, in patients with metastatic breast cancer (MBC) as first-line treatment. J Clin Oncol. 2013;31(suppl):629. [Google Scholar]
- 17. US Food and Drug Administration (FDA). Biosimilar development, review, and approval. 2017. Available at: www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval . Accessed April 13, 2021.
- 18. Rha SY, Lee C-K, Kim HS, et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: a multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol. 2020;38(suppl):3081. [Google Scholar]


