Abstract
Purpose:
As part of the special issue on ‘Women in Science’, this review offers a perspective on past and ongoing work in the field of normal (non-cancer) tissue radiation biology, highlighting the work of many of the leading contributors to this field of research. We discuss some of the hypotheses that have guided investigations, with a focus on some of the critical organs considered dose-limiting with respect to radiation therapy, and speculate on where the field needs to go in the future.
Conclusions:
The scope of work that makes up normal tissue radiation biology has and continues to play a pivotal role in the radiation sciences, ensuring the most effective application of radiation in imaging and therapy, as well as contributing to radiation protection efforts. However, despite the proven historical value of preclinical findings, recent decades have seen clinical practice move ahead with altered fractionation scheduling based on empirical observations, with little to no (or even negative) supporting scientific data. Given our current appreciation of the complexity of normal tissue radiation responses and their temporal variability, with tissue- and/or organ-specific mechanisms that include intra-, inter- and extracellular messaging, as well as contributions from systemic compartments, such as the immune system, the need to maintain a positive therapeutic ratio has never been more urgent. Importantly, mitigation and treatment strategies, whether for the clinic, emergency use following accidental or deliberate releases, or reducing occupational risk, will likely require multi-targeted approaches that involve both local and systemic intervention. From our personal perspective as five ‘Women in Science’, we would like to acknowledge and applaud the role that many female scientists have played in this field. We stand on the shoulders of those who have gone before, some of whom are fellow contributors to this special issue.
Keywords: Normal tissue radiobiology, immune system, lung, cardiovascular system, brain
Introduction
There has been a trend for normal tissue biology to play second fiddle to other radiobiology fields, especially those more directly associated with cancer per se. As a result, participation in, and support for, this particular subfield has fluctuated over the decades, affected by scientific trends and economics (Groves and Williams 2019). This relative lack of engagement is unfortunate since it flies in the face of the fact that many of the major imperatives applied in radiation therapy were built on an understanding of the mechanisms of normal tissue radiation response (e.g. the use of fractionation, application of the therapeutic ratio, etc.); an understanding of these mechanisms also is a necessary tool in the fields of radiation epidemiology and protection.
In this review, and as part of the special issue on ‘Women in Science’, we discuss the past and current trends in normal tissue biology, tracing the evolution of our understanding of this complex field and speculating on future trends. Given our specific fields of expertise, we have chosen to focus on some of the critical organs considered dose-limiting in radiotherapy practice, namely the immune/inflammatory and cardiovascular systems, lung, and brain; we acknowledge (and apologize for the omission of) other equally important organs, such as the hematopoietic system, bone, etc. Finally, to all of those women whose work is recognized in this review, we salute and thank you.
Overview
The study of normal tissue reactions has been a subfield of radiation biology almost since the discovery of X-rays; the need to understand the impact of radiation exposure, whether beneficial or detrimental, on biological tissues was quickly recognized. Indeed, even as radiation was being developed as both a diagnostic tool and therapeutic application, its potential to induce injury in non-targeted tissues and organs, particularly in the skin, was quickly recognized (Johnston et al. 2010; Timins 2011). Interestingly, although ionizing radiation was identified as an environmental mutagen as early as the 1920s (Muller 1927), it was decades later before studies of workers, such as the radium dial painters (Sherk 2001) and uranium miners, (McLaughlin 2012) etc., and even some patient populations, such as those receiving Thorotrast®, etc. (Lipshutz et al. 2002) led to broad acknowledgement of its carcinogenic risk. Observations of leukemia in the survivors of the Japanese atomic bombs (Folley et al. 1952) and later reports of increased incidence of not only hematologic, but also solid tumors, following analyses of the Life Span Study (Hsu et al. 2013; Grant et al. 2017) left no further doubt. Ultimately, radiation-induced acute and late normal tissue effects, whether cancer or non-cancer, have been recognized as occurring in every tissue and organ, imposing limits on the use of radiation as a therapeutic modality.
Overall, it appears that enthusiasm for radiation’s clinical utilization took precedence over basic radiobiologic research for much of the first half of the 20th century. However, as the realization of its detrimental effects grew, and in conjunction with advancements in science and technology, came an evolution in our understanding of the mechanisms that drive the biological effects of radiation, including an appreciation of the complexity in response patterns, whether at the molecular, cellular or tissue/organ/systemic levels. For example, during the 1960s to 1980s, as radiation pathology became more sophisticated and precise (Fajardo 1982) and the interconnectivity between radiation physics and biology was better appreciated (Fowler et al. 1963; Williams and Newhauser 2019), competing hypotheses surfaced to explain the diverse range of observed normal tissue radiation injuries. One of the more simplistic explanations put forward, commonly referred to as the ‘target cell theory’, was that the majority of normal tissue deficits could be explained in terms of radiation sterilization of parenchymal clonogenic cells (Michalowski 1984). Alternatively, some investigators focused on the microcirculation together with the radiation sensitivity of endothelial cells, proposing that the critical event in normal tissue injury was loss of capillary function (Reinhold 1974). As part of a more integrated view, which took greater account of cellular diversity with respect to sensitivity and proliferation kinetics, Casarett and others suggested a more compartmentalized concept (Rubin and Casarett 1968), secondarily proposing that populations of injured cells communicate with each other. Every theory garnered its own supporters, generating competing philosophies and approaches across the radiation community. However, some scientists made stalwart efforts to rise above the fray and unite the various hypotheses under a more flexible umbrella. Notable among these was the pioneering radiobiologist, Tikvah Alper, who not only worked on ‘target theory’ in the 1950s (Alper 1956), but also proposed cell membranes as an alternative to DNA as a critical lesion (Gasinska 2016), as well as her coworker at the Gray Lab, Juliana Denekamp (Denekamp 1986), who adapted and modified her hypotheses in response to the emerging data, an approach that reflected not only her interest in a broad range of normal tissues, but also her conviction of letting the data guide the scientist and not the other way around (Emery et al. 1970; Stewart et al. 1978; Hirst et al. 1980; Stewart et al. 1980; Williams and Denekamp 1983, 1984; Douglas et al. 1986; Johansson et al. 2000, 2002).
Arguably, since the turn of the century, our understanding of the enormity and complexity of the biological radiation response in normal tissues has undergone its greatest evolution. (Figure 1). From the concept of tissue compartments communicating through unspecified channels (Rubin et al. 1998; Figure 1(A)), we have moved through an holistic overview of radiation disrupting homeostatic balance, resulting in autocrine and paracrine expression of chemokines and cytokines (McBride et al. 2004; Figure 1(B)), to our current, more granular, subcellular understanding of the mechanistic intra- and extracellular pathways involved at each phase of the response, developed through the integration of various -omics technologies and systems biology (Figure 1(C)) (Chua and Rothkamm 2013; Choudhary et al. 2020). In this review, we offer an overview of the current status of normal tissue radiobiology, providing a historical narrative of the progress that has been made in the field through a focus on some of the critical tissues and organs that are deemed dose-limiting in radiation therapy, namely the immune system, lung, cardiovascular system and brain.
Figure 1.
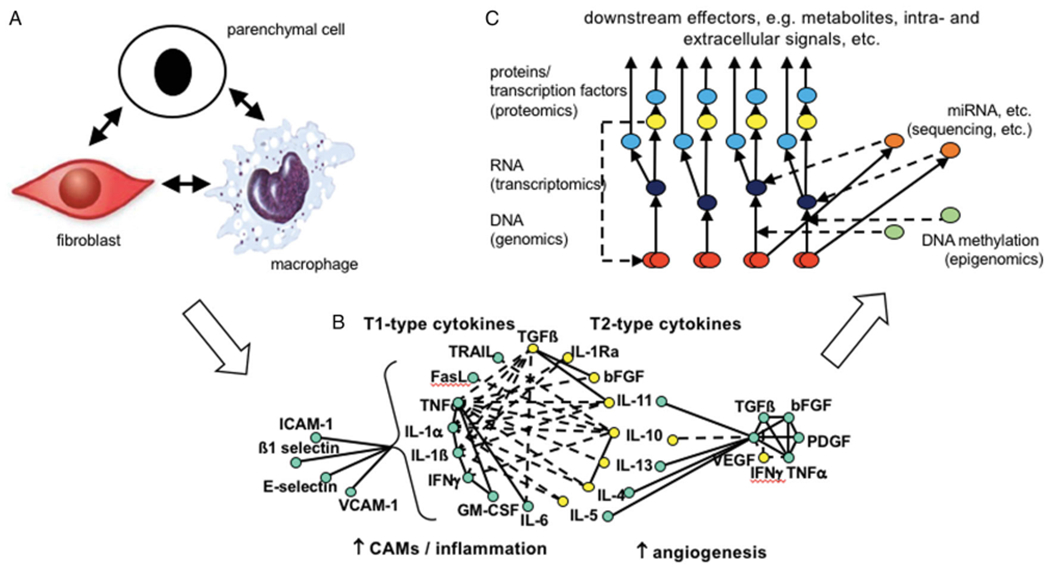
Examples of the evolution in hypotheses and approaches taken to decipher the mechanisms underlying normal tissue radiation responses. (A) A paradigm of tissue compartments communicating through unspecified channels (Rubin et al. 1998); (B) An holistic overview of radiation disrupting a tissue’s homeostatic balance, resulting in autocrine and paracrine expression of chemokines and cytokines (McBride et al. 2004); (C) A simplified overview of the potential integration of -omics data at the DNA (genes, red), RNA (transcripts, dark blue) and protein (red) levels, as well as the regulation (red broken lines) at the transcription (yellow) miRNA (orange) and epigenetic (green) levels (modified from Unger 2014.).
Immune system
Most of the radiobiological parameters that describe normal tissue effects, such as dose dependency, dose fractionation sensitivity, and latency, directly relate to tissue-specific, often highly differential, characteristics, such as intrinsic radiosensitivity, capacity for sublethal damage repair, turnover kinetics, and tissue organization (Hall and Giaccia 2019; Joiner MC & van der Kogel, 2019). However, despite significant cell type differentials within these parameters, the stages in progression of all radiation-damaged tissues appear to converge with respect to their interactions with the immune system, with the majority of observed responses progressing along common inflammatory and immune pathways before finally diverging into their tissue- or organ-specific pathologies.
Historically, skin was the first tissue in which the role of the immune system in the radiation response was recognized when, in 1895, many of the investigators using low power X-ray tubes in order to reproduce Röntgen’s findings subsequently developed dermatitis (Timins 2011). A unique aspect of radiation-induced skin injuries, their characteristic latency, led Marie and Pierre Curie and others to auto-experiment in attempts to elucidate relationships between latency, dose, and the persistence of radium and other radiation-induced lesions (Dutreix et al. 1998). Such studies resulted in early clinical dosimetrists using the skin inflammatory response to calibrate radiation tubes, with the minimal erythematous dose as a ‘unit’, i.e. the first bona fide biodosimeter; indeed, skin reactions have played a major role in the radiobiology modeling of many important response parameters (Willers H & Beck-Bornholdt 1996). Furthermore, and in parallel with these findings, was the early clinical realization that immune components themselves, and lymphocytes in particular, were directly and adversely affected by radiation (Heineke 1903; Heineke 1905). So, even though interest in the radiation response has broadened to most other tissues, each with its own pathophysiological format, inflammation and the immune system as a whole have long been considered to play critical roles in normal tissue effects.
The inflammatory kickoff
The immediate biochemical response to radiation damage is rapid and largely redox-regulated, leading to alterations in membrane permeability and purinergic signaling that affect the composition of intra- and extracellular milieus. Changes in redox-sensitive molecular switches can have many effects, including activation of multiple transcription pathways, with the release of damage-associated molecular patterns (DAMPs) that signal through members of the pattern recognition receptor (PRR) superfamily, including Toll-like receptors (Khodarev 2019); some DAMPs have been shown to translocate to the cytoplasm and may be released as paracrine factors. One of the proposed downstream outcomes is senescence, as elegantly shown by work from Judith Campisi’s group and others (Rodier et al. 2009); following irradiation, cells undergo stable cell cycle arrest and display persistent DNA segments with chromatin alterations, which reinforce senescence (DNA-SCARS) alongside a senescence-associated secretory phenotype (SASP), structures that have the potential to drive persistent immune infiltration (Le et al. 2010; Rodier et al. 2011). The possibility of persistent DNA damage driving immune involvement has been posited by some (Li et al. 2018; Ishida et al. 2019), and provides an explanation for the cyclical waves of inflammation that have long been seen as part of the late effects in different organs and tissues (Rubin et al. 1995; Fink et al. 2012; Gandhi and Chandna 2017). It also ties in with a general concept of danger-sensing by innate cells, as well as the inflammatory phenotype seen, for instance, in ATM patients (Härtlova et al. 2015; Beach et al. 2018).
Myeloid cells to the fore
Building on the ideas of Polly Matzinger regarding limitations in the self/non-self-immune recognition system (Matzinger 2002), radiation-damaged tissues may be considered as a hub for ‘danger’ signals, activating downstream pathways with a broad array of consequences (McBride et al. 2004). Subsequent expression of signaling cascades is dose-, volume- and tissue-dependent, involves multiple signaling families, and may be seen both intra- and extracellularly. For example, irradiated skin produces proinflammatory cytokines, such as the interleukins (IL)-1, IL-6, IL-17, IL-23, tumor necrosis factor (TNF)-α, as well as growth factors (e.g. transforming growth factor (TGF)-B) and chemokines (e.g. IL-8 and eotaxin), with many of these messengers being expressed in both tissue and plasma (Müller and Meineke 2007).
One effect of proinflammatory cytokine and chemokine expression is to orchestrate increased vascular permeability and mobilize circulating bone marrow-derived cells, in particular those of the myeloid lineage, causing them to undergo trans-endothelial migration into inflamed tissues (Figure 2). As a result, resident and infiltrating innate immune cells, in particular macrophages, are activated, promoting communication between endothelial cells, fibroblasts, and other cells, many of which contribute to further cytokine production and/or respond through the expression of an ever-changing array of PRR and cytokine receptors. The pluripotential nature of many of these signals increases the complexity of the response by further broadening the number of potential targets and outcomes. For example, danger signaling matures dendritic cells to present antigen to lymphocytes and break tolerance, whereas paracrine bystander effects can either increase or decrease cell survival and proliferation (Lotze et al. 2007; Tang et al. 2010). Furthermore, tissue irradiation – even localized exposures – frequently causes a persistent myeloid shift within the immune system, perhaps another illustration of the intimate link that ties peripheral inflammation to the bone marrow niche and adaptive hematopoiesis (Zhang et al. 1995; Zhao et al. 2014; Groves et al. 2015; Groves et al. 2018; Chavakis et al. 2019). The longevity of these responses is supported by data from the A-bomb survivor cohorts showing persistent residual injury, alongside hematopoietic myeloid bias and general immune-senescence (Yoshida et al. 2019).
Figure 2.
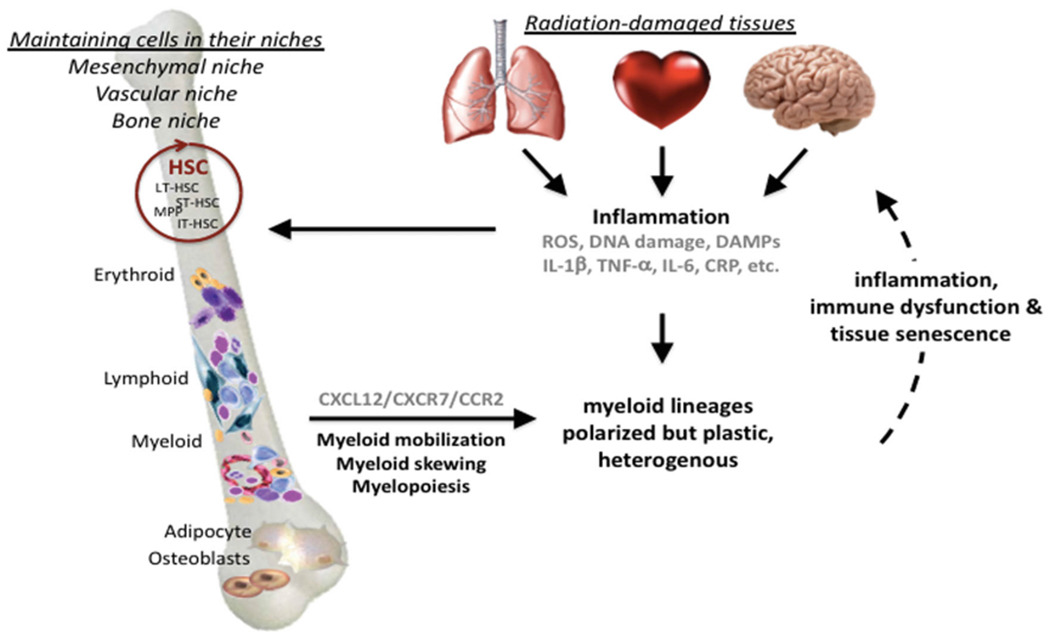
The feed-forward loop between radiation-induced inflammation, bone marrow myeloid skewing and persistent tissue damage. This illustrates the link between the initial tissue damage, which signals danger to the immune system, and the recurring waves of inflammatory responses, driven by persistent DNA damage and senescence-associated phenotypes. Positive feed-back to the bone marrow leads to hematopoiesis being adjusted toward emergency myelopoiesis, thereby maintaining a pool of inflammatory myeloid cells that prevents resolution and, instead, causing further inflammatory-related collateral tissue damage. Red circle = self-renewing hematopoietic stem cells.
The counter-response and impact on tissue-specific late effects
Localized irradiation can induce systemic consequences evolved to prepare the whole body for additional challenges. As a result, although the initial response is rapid, the subsequent contributions of the various cellular and molecular players change over time, possibly as part of homeostatic maintenance and restoration. For example, repeated adjustments and re-adjustments of the pro/anti-oxidant and pro/anti-inflammatory balances seen post-radiation within the cellular microenvironment vary the downstream net effects of redox-sensitive NF-κB, Nrf2 and pentose phosphate pathways. Homeostatic disruption following certain injuries, including high dose radiation, induces the entire cellular rheostat to react as if under threat, for example by exhibiting mitochondrial leakage and altered metabolic status (Yamamoto et al. 2018), with regulatory T cells, myeloid-derived suppressor cells and immune checkpoints operating to prevent excessive autoimmunity at the tissue level. While these networks are, to some extent, self-sustaining, an additional factor seen in irradiated tissues is programmed cell turnover and the release of more DAMPs; these events correspond with avalanches of cell loss resulting from the timed entry of cells into proliferation and subsequent cell death through mitotic catastrophe, phenomena that distinguish acute from late responding tissues. Critically, molecular ‘danger’ responses to radiation exposure occur in practically all tissues in the body; these responses differ qualitatively between tissues, although the relevance of such differences has yet to be determined.
The range of signal responses seen following radiation injury in tissues raises the question as to the role played by each specific signal or pathway within radiation-induced inflammatory lesions. For example, Angela Groves et al. highlighted a critical role for the IL-1ß/CCR2/CCL2 axis in the development of radiation lung fibrosis (Groves et al. 2018), secondarily noting that baseline cytokine expression differences exist between pneumonitis- versus fibrosis-prone mice, as seen by a number of investigators (Johnston et al. 1995; Chiang et al. 2005; Paun and Haston 2012). However, in similar studies focused on brain, late effects appeared more closely associated with waves of increased TNF-α expression, with the greatest increases seen following doses that caused radionecrosis (Chiang et al. 1997). This finding was supported by work from Jennifer Daigle et al., who showed that knocking out TNFR2, generally considered a negative control pathway, led to increased radiation-induced TNF-α levels, making mice more susceptible to brain injury (Daigle et al. 2001). Of course, the practice of using inbred murine models in this field likely adds to the confusion, given the known strain-dependent variations with respect to genetics (Franko et al. 1991; Haston 2012), which do not necessarily or consistently correlate with pathological outcomes (Sharplin & Franko, 1989b, 1989a). Other investigators have pointed to a more holistic, anti- versus proinflammatory mechanism as the signaling imperative, with the anti-inflammatory growth factor, TGF-B, being frequently identified as a critical player (Anscher et al. 1998a; Martin et al. 2000; Okunieff et al. 2002). Finally, taking a more cell-based approach, endothelial damage has been suggested as the primary cause of intestinal radiation damage (Paris et al. 2001), although this has been disputed (Brown 2008); indeed, even if true, it can be argued that any mechanistic involvement of these cells would likely require an immune component.
Interestingly, dose is a poorly defined variable in the context of the immune response to radiation. The production of a full spectrum of proinflammatory cytokines appears to require moderate doses in the hypofractionated range; for example, from our own work, as well as that of Claire Vanpouille-Box and Sandra Demaria, it appears that the optimal dose needed for radiation to act as an immune adjuvant in the clinical setting is in the range of 6-8 Gy (Demaria and Formenti 2012; Schaue et al. 2012; Vanpouille-Box et al. 2018). Although the resultant type I interferon response acts as a cross-over to the adaptive immune arm, inadvertently this may drive chronic inflammatory pathologies through relentless monocyte recruitment via CCL2/CCR2, preventing the development of tissue-healing phenotypes (Lee et al. 2009). However, not yet fully addressed and a question relevant to this review, is whether human tumors induce the same danger response(s) as their normal tissue counterparts, given the evident differential in benign versus oncogenic microenvironmental influences. Ironically, lower radiation doses have long been considered to be anti-inflammatory and have been used to treat patients with a variety of inflammatory diseases (Trott and Kamprad 1999), including for COVID-19 (Prasanna et al. 2020).
Lung
Clinical incidence and observations
The first reported observations of pulmonary damage following radiation therapy for breast cancer were reported in 1922 (Groover et al. 1922; Hines 1922), with numerous subsequent reports describing clinical, radiological, and histological manifestations of lung injury that appeared several weeks to months after treatment of the thoracic region with radiation therapy (Desjardins 1926; McIntosh and Spitz 1939; Warren and Spencer 1940; Leach et al. 1942). By 1940, clinical reports, with supporting data from animal studies, provided a well-defined clinical picture of the lung damage that results from radiation exposure, which manifests in two stages with respect to time and histological sequelae: an early onset, acute radiation pneumonitis or alveolitis followed by late onset, chronic fibrosis. Presciently, one of the early reports described two major issues regarding the observation of lung damage after radiation therapy that remain a challenge for physicians today: firstly, the contribution of pneumonitis/fibrosis to morbidity/mortality, and, secondly, an inability to differentiate radiation-induced changes from disease progression (McIntosh and Spitz 1939). Indeed, despite improvements in radiation delivery, the lung remains a major dose limiting organ in the treatment of thoracic tumors, as well as following the use of total body irradiation, e.g. as part of conditioning regimens for stem cell transplantation (Shinde et al. 2019; Jang et al. 2020).
In general, radiation oncologists consider the lung to be an acutely radiosensitive organs, with clinical manifestations taking weeks to months to appear. Early clinical symptoms range from mild dyspnea, nonproductive cough, chest discomfort, and low grade fever to respiratory insufficiency, cyanosis, and fulminant organ failure in the most extreme cases (Davis et al. 1992). In addition, focal to diffuse ground-glass opacification and increased lung density, observed by computed tomography (CT) and single photon-emission computed tomography (SPECT)-defined reductions in lung ventilation and perfusion, are common during the symptomatic phase (Marks et al. 2003). In contrast, the late phase of chronic lung fibrosis is a progressive disease that occurs months to years after treatment. The disease tends to stabilize approximately 2 years post-treatment, although lung volumes may continue to retract until ~5 years post-exposure; of note, fibrosis can develop in patients who have not presented with the acute clinical symptoms of radiation pneumonitis (Movsas et al. 1997). Corresponding characterization of lung changes in animal models have been made by multiple preclinical researchers, with the seminal work from Elizabeth Travis requiring specific mention (Travis 1980; Travis et al. 1980).
Bench to bedside and back
Throughout the latter half of the 20th century, translational radiation biologists and clinicians worked together to define the best treatment regimens for thoracic patients through a bi-directional flow of information from bench to beside. Although there was early recognition of normal tissue sparing through the use of split dose radiation (Regaud and Nogier 1911), attribution of the phenomenon to sublethal damage repair, reassortment of cells between fractions, and cell repopulation was not made until the 1960s, through the (mostly in vitro) work from such investigators as Mortimer Elkind and H. Rodney Withers (Elkind et al. 1965; Withers and Elkind 1969). Preceding and subsequent reports from small clinical trials, assessing responses in patients undergoing thoracic irradiation (Newton and Spittle 1969), also demonstrated a clear difference in survival outcomes between those patients receiving >3 Gy per fraction compared to those receiving treatment in <1.5 Gy per fraction, providing some of the earliest clinical evidence that the tolerance of the lung to radiation can be improved when treatment is limited to 1.5-2 Gy fractions.
In the early 1970s, collaborative work from Shirley Hornsey and Stan Field helped propel greater acceptance of the mouse lung as a surrogate for the human (Hornsey et al. 1975; Field et al. 1976). Subsequently, the majority of investigators used murine models to define the relationships among total dose, fraction number and size, and overall treatment time based on similarities in overall survival time (e.g. 40-180 days), pulmonary symptoms, and histopathologic sequelae (Travis 1980). The predictive value of data generated from such preclinical models, as well as their translation to the clinic, was further enhanced by the characterization of a noninvasive functional assay by Elizabeth Travis and colleagues, which enabled monitoring of the onset, severity, and duration of lung injury using whole body plethysmography (Travis et al. 1979; Travis et al. 1980). Interestingly, using this breathing rate assay in a CBA mouse model of whole lung irradiation, Travis and Down confirmed the dissociation of early pneumonitis from late fibrosis using treatment fractionation (Travis and Down 1981), an observation that was the basis for the hypothesis that the affected cells and molecular mechanisms underlying acute versus late stages of injury, not only in lung but also in other tissues, may be independent (Travis et al. 1984).
Key players in radiation-induced lung disease (RILD)
Despite its putative acute radiation sensitivity, following clinically relevant, or even relatively high (~10 Gy single dose), doses, histological features of lung damage do not become evident under light microscopy until several weeks after exposure, thus, the classic target cell theory appears insufficient to describe the early lung response. However, exhaustive characterization of the natural progression of RILD at the cellular level, using both transmission and scanning electron microscopy, has shown that, in reality, sub-structural changes appear in the lung of irradiated mice within hours of exposure (Maisin 1970; Oledzka-Slotwinska & Maisin 1970; Penney and Rubin 1977) (Figure 3). For example, a dose-dependent infiltration of inflammatory cells, including lymphocytes, macrophages, and neutrophils, into the lung tissue has been observed (Johnston et al. 2010; Johnston et al. 2011), although this is likely a component of the natural wound response of the organ. Release of surfactant into the alveolar space from the type II pneumocytes also is a rapid and early event (Rubin et al. 1980; Rubin et al. 1983); of note, although our group has shown that apoptosis of surfactant producing-type II pneumocytes appears to correlate with morbidity/mortality during the acute pneumonitic phase (Jackson et al., 2018), other groups have suggested that damage in alternative cell types may play an equally significant role (Figure 3), with candidates including club (Clara) cells (Kathiriya et al. 2020) and lung endothelial cells (Qiu et al. 2011). Interestingly, the potential for epithelial cells to play a critical role in both the initiation and progression of RILD has been a perennial hypothesis (Maisin et al. 1982), most recently in the context of combined injuries (Manning, Johnston, Hernady et al. 2013; Manning, Johnston, Reed et al. 2013).
Figure 3.
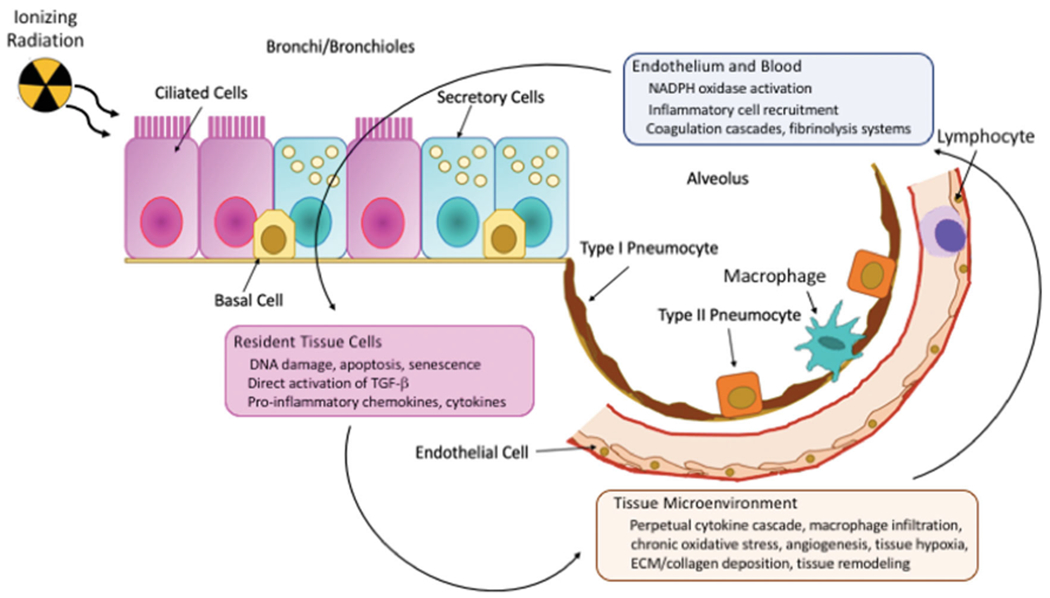
The development of radiation-induced lung injury is biologically complex. Of the 90+ cell types in the lung, there is no single target cell that initiates the response, although several are currently considered candidates. Progression to the early (pneumonitic) and late (fibrotic) phases involves multiple, parallel events, including initial and/or delayed hypoxia due to occlusion and permanent loss of blood vessels, respectively, waves of inflammatory cell recruitment and activation (including macrophages, lymphocytes and platelets), and chronic oxidative stress. Adapted from Bentzen 2006, Schaue et al. 2012 and Leiva-Juárez et al. 2018.
Identifying a single cell type as being singularly responsible for the early lung response is contentious given that, during the immediate and latent (i.e. asymptomatic) phases, ultrastructural changes have been seen in almost all of the 90+ cell types found within the lung (Maisin 1970). Pathologic alterations have included changes in the pulmonary vasculature (e.g. cytoplasmic swelling of endothelial cells with vessel occlusion and platelet aggregation, together with interstitial edema (Maisin 1970; Moosavi et al. 1977; Penney and Rubin 1977)) and the inflammatory environment (e.g. recruitment of bone-marrow derived inflammatory cells and shifts in macrophage polarization (Groves et al. 2016; Park et al. 2019)). In addition, coincident with the pulmonary cellular events, a broad spectrum of pro- and anti-inflammatory signals are expressed within milliseconds of the ionizing radiation event and subsequently span the symptomatic latent phase and beyond. Described by some as a ‘perpetual cascade’ (Rubin et al. 1995), elevated circulating and lung tissue levels of proinflammatory and profibrogenic cytokines, chemokines and growth factors have been demonstrated across the timeline of RILD progression, with levels and temporal expression patterns being dose, volume and strain-specific (Johnston et al. 1995; Chiang et al. 2005; Cappuccini et al. 2011; Jackson et al., 2018). Without spontaneous resolution or pharmacological intervention to subdue the resultant vascular dysfunction, cytokine storm, and inflammatory processes, ultimately, clinically-symptomatic lung damage ensues. Successful identification of specific interventions has proved elusive, despite the plethora of altered signaling pathways offering a large array of tantalizing targets, e.g. oxidative stress (Antonic et al. 2015; Murigi et al. 2015), angiogenesis (Mahmood et al. 2014; Ackermann et al. 2017; Jackson et al. 2017), inflammation (Williams et al. 2004; Tian et al. 2018), as well as specific molecules such as gastrin-releasing peptide (Zhou et al. 2013). Indeed, the observations of vascular injury and ischemia/reperfusion, chronic inflammation and, most especially, persistent DNA damage have led many investigators to describe RILD as an aberrant wound healing response (Fleckenstein et al. 2007; Ghita et al. 2019), with the breadth of homeostatic disruption suggesting that resolution will require a multi-targeted approach (Williams et al. 2010).
As mentioned earlier and in the preceding discussion of the immune system, species, sex and, in particular, strain affect expression of radiation-induced lung injury across animal models, and these factors have been closely studied in mouse lung (Dabjan et al. 2016). For example, Janet Sharplin and Allan Franko performed a comprehensive survival and histological analysis of acute and late lung injury seen among the most commonly used mouse strains (Sharplin & Franko, 1989b, 1989a). Interestingly, exploitation of such strain differences in the progression and manifestation of lung injury has enabled some researchers to identify genes and/or protein products that may influence susceptibility to pneumonitis and/or fibrosis (Haston and Travis 1997; Haston et al. 2002; Paun and Haston 2012; Jackson et al. 2016; Jackson et al., 2018). Importantly, using RNA sequencing and systems analysis of tissues from irradiated fibrosis-prone mice, progressive upregulation of pathways has been demonstrated in association with persistent DNA damage, acute and chronic inflammation, and cellular senescence (Beach et al. 2017), which, together with indications of myeloid phenotypic shifts during RILD progression (Groves et al. 2015; Groves et al. 2018), draws direct parallels with the mechanisms implicated as part of the immune response; potential interactions among the various cell types, together with direct pathways and feedback loops, are illustrated in Figure 3.
Cardiovascular system
Radiation-induced heart disease (RIHD) in the clinic
By the late 1950s, publications began reporting adverse cardiac effects following irradiation, seen not only in animal models (Kohn et al. 1957; Senderoff et al. 1959), but also in clinical subjects (Catterall 1960; Jones and Wedgwood 1960). By the early 1970s, the use of large thoracic fields, such as mantle or mediastinal irradiation, both of which involve high doses of radiation to the heart, was clearly seen as associated with late cardiac disease (Landberg et al. 1972; Martin et al. 1975; McReynolds et al. 1976). Indeed, since that time, systematic analyses of cardiac disease risk in survivors of Hodgkin’s disease have provided unequivocal proof of RIHD, including the influence of other cardiovascular risk factors (Hancock et al. 1993a; Darby et al. 2005; Hooning et al. 2007; Taylor et al. 2007; Henson et al. 2013; van Nimwegen et al. 2016). Futhermore, for several decades, radiation therapy for breast cancer, especially of the left breast, included all or part of the heart. Retrospective analyses of large cohorts of long-term breast cancer survivors, looking at cardiac morbidity and mortality, have provided insight into the radiation sensitivity of substructures in the heart, as well as determining the risk of delayed cardiac disease from even low radiation doses (Darby et al. 2005).
While the clinical approaches used to administer radiation therapy to thoracic tumors have undergone considerable improvements in recent decades, resulting in an increase in tumor control and a reduction in normal tissue radiation exposure, nonetheless sub-cohorts of patients with esophageal cancer, lung cancer, lymphomas and thymomas continue to receive significant radiation doses to the heart (Tomita et al. 2020; Garant et al. 2021). In these patient populations, radiation-induced heart disease (RIHD) has been shown to negatively affect long-term survival (Dess et al. 2017). Specific attention is drawn to late effects studies from clinicians in the field, such as Sarah Donaldson and her various collaborators, who through the auspices of national clinical trials groups, have tracked the cardiac outcomes of cancer patient populations (Hancock et al. 1993b), with a particular focus on survivors of pediatric disease (Mefferd et al. 1989; Mulrooney et al. 2009; Inskip et al. 2016), reconstructing the radiation dose to the heart in large cohorts of cancer patients and determining dose response (Taylor et al. 2007; Taylor et al. 2008; Taylor et al. 2009a; Taylor et al. 2009b; Ntentas et al. 2020). In a much cited article, Darby and colleagues concluded that radiotherapy for breast cancer increased the rate of major coronary events by 7.4% per Gy mean dose to the heart (Darby et al. 2013). Although not studied as extensively as the heart per se, large and small blood vessels in the radiation therapy field also show significant damage, as observed by Nicola Russell, Fiona Stewart and colleagues in tissue samples from cancer patients (Russell et al. 2009; Russell et al. 2015).
Taken together, these studies indicate that radiation-induced cardiovascular disease in cancer survivors can involve loss and deformation of the microvasculature, accelerated atherosclerosis and artery wall fibrosis, while in the heart, acute and chronic pericarditis, myocardial fibrosis, cardiac valve dysfunction, conduction defects, and ischemic heart disease can develop. Moreover, as seen in other critical organs and tissues, despite the development of FDA-approved mitigators for the acute radiation syndrome following situations of accidental or malicious radiation exposure, the development of delayed effects of acute radiation exposure (DEARE) in long-term survivors remains a concern; the heart is among the organs highlighted as being prone to developing clinically significant DEARE with symptoms similar to those seen in thoracic cancer survivors (Micewicz et al. 2019).
Evolving preclinical studies
Using animal models, early preclinical workers mapped out the pathology of RIHD, and determined α/β values for various biological endpoints of whole heart irradiation using dog (Gillette et al. 1992), rabbit (Fajardo and Stewart 1971) and rat models (Lauk et al. 1987). However, the majority of these studies employed whole thorax irradiation or exposure of the whole heart with some shielding of the lungs, limiting their translation to current clinical practice. Importantly, as seen in other complex normal tissues and organs, we have come to realize that the heart should not be considered as homogeneous; instead, the various cardiac substructures, such as the ventricles, atria, and coronary arteries, may exhibit differential radiation sensitivities, each of which needs to be taken into consideration when optimizing radiation therapy planning (Ghita et al. 2020) (Figure 4). Furthermore, it is important to keep in mind that in most, if not all, animal models of heart exposure using photons, a portion of the lungs will be exposed to radiation. This is relevant since models using precise exposures of lungs and heart with proton beams have shown that the two organ systems influence each other during the development of their respective radiation injuries, although the mechanisms by which this occurs are not yet fully understood (Ghobadi et al. 2012; van der Veen et al. 2015).
Figure 4.
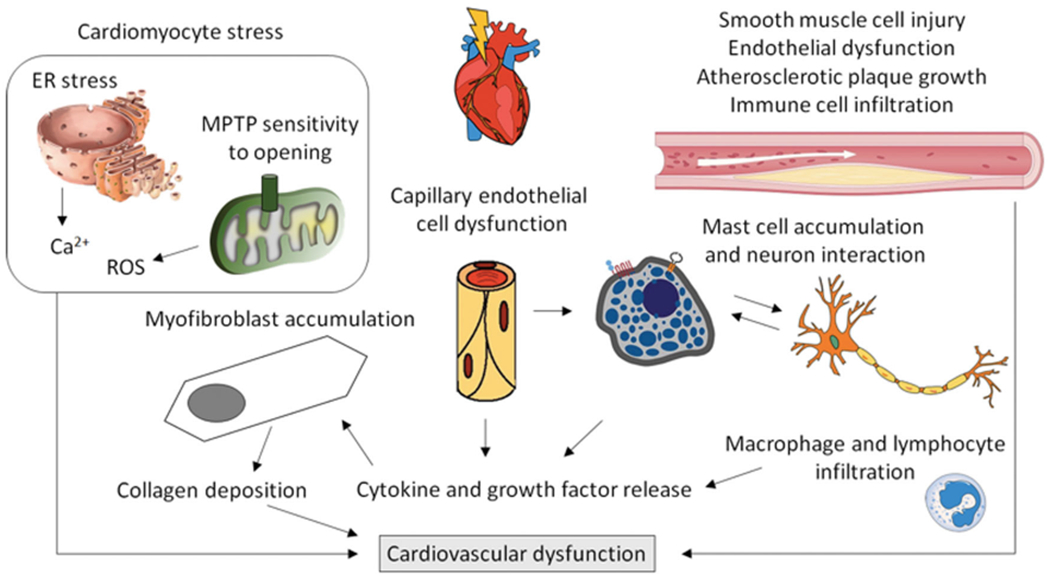
Research in preclinical models and analyses of patient samples have identified several cell types and cellular mechanisms that may contribute to the development of cardiovascular dysfunction after exposure to ionizing radiation. Additional insight into biological mechanisms needs to be obtained. ER: Endoplasmic Reticulum; MPTP: mitochondrial permeability transition pore.
Fortunately, the more recent developments of sophisticated imaging and irradiation technologies have enabled investigators in the field to administer partial heart irradiation to animal models, allowing researchers to more closely recapitulate current cardiac therapeutic exposures (Ghita et al. 2020; Dreyfuss et al. 2021; Lee et al. 2021). As a result, the data emerging from this work, using models with image-guided whole- or partial-heart irradiation, have provided insight into some of the biological mechanisms by which the heart responds to radiation injury. For instance, both genetic animal models and the combination of local heart irradiation with pharmacological modifiers confirm a role for TGF-B in the development of cardiac radiation fibrosis (Boerma et al. 2013; Seemann et al. 2013). This finding is not novel, since TGF-B has been consistently associated with radiation-induced fibrosis in other tissues over the years (Richter et al. 1997; Anscher et al. 1998b; Straub et al. 2015), but now provides a link between tissue remodeling and inflammatory processes (Richter et al. 1997; Yu et al. 2017). Interestingly, while radiation seems to induce an accumulation of proinflammatory macrophages in atherosclerotic vasculature, studies involving inhibition of macrophage function using thalidomide did not affect RIHD outcomes in a mouse model (Hoving et al. 2013; Gabriels et al. 2014). Furthermore, results from local heart irradiation in a genetic rat model suggest that mast cells may play a predominantly protective role in the development of RIHD (Boerma et al. 2005); other immune cells have not yet been studied extensively. Proteomic assessments of irradiated human and animal hearts have identified additional molecular pathways that may be involved, such as the peroxisome proliferator-activated receptor-α pathway that is central to the regulation of cardiac metabolism, supporting further mechanistic studies (Subramanian et al. 2017; Azimzadeh et al. 2020). In addition, local heart irradiation has been shown to cause persistent changes in cardiac mitochondrial morphology (Sridharan et al. 2014), supported by studies in a consomic rat strain (Schlaak et al. 2020), again expanding the potential for mitigation ‘targeting’ beyond the DNA.
Variations in RIHD: cardiovascular risk factors, lifestyle and sex
As may be expected, prior cardiovascular disease, as well as common cardiovascular risk factors, appear to increase the incidence of RIHD (Darby et al. 2013). This may explain why the rate of RIHD seems relatively high in cohorts of lung cancer patients since, in general, they are likely to have poor cardiovascular health (Dess et al. 2017). Lifestyle factors may also influence the risk of cardiovascular disease following low dose radiation exposures, as determined by data from the Japanese Life Span Study (Douple et al. 2011) and relevant occupational populations (Gillies et al. 2017). A recent review article from Tapio et al. provides an overview of our current understanding of the roles that lifestyle and genetic factors may play in predisposition to radiation-induced cardiovascular disease (Tapio et al. 2021), however additional research is still needed to determine the precise genetic factors involved.
Interestingly, there are well-known differences in the risk of developing cardiovascular disease between male and female individuals (Kouvari et al. 2020), and the disease itself presents differently in the two sexes (Cushman et al., 2021). However, little is known about the influence of sex on the development of RIHD. While both male and female patients are included in clinical studies of thoracic cancer therapy, very few reports make a direct comparison between the sexes (Christiansen et al. 2016). Moreover, until recently, the majority of experiments in animal models were performed with only one sex, and differences in size and anatomy between male and female animals may have led to radiation exposures of different portions of the lungs (Schlaak et al. 2019). Such factors will need to be taken into consideration in future studies when determining whether sex plays a role in the development of RIHD.
Brain
Evolving views of radiation-induced brain injury (RIBI)
Historically, since the majority of cells within the brain are not actively dividing, it was long considered to be a relatively radioresistant organ, with necrotic damage only seen following high doses (Greene-Schloesser et al. 2012). Even until recently, given the advancements made in conformal radiotherapy delivery and the use of fractionation, with single exposures being typically less than 2 Gy, the risk of RIBI induction has been deemed limited, particularly with respect to acute injury (Greene-Schloesser and Robbins 2012; Greene-Schloesser et al. 2012). However, countering this dogma has been the clinical observations, reported since the mid-1980s, of a biphasic delayed response: an early reversible injury, occurring at 1–6 months post-irradiation, involving transient demyelination with somnolence (Mandell et al. 1989); and a late irreversible, progressive injury, characterized histopathologically by vascular abnormalities, demyelination, and ultimately white matter necrosis (Schultheiss et al. 1995), and often associated with cognitive dysfunction (Hochberg and Slotnick 1980; Crossen et al. 1994). Importantly, a retrospective analysis of men, who had received relatively low doses of brain radiation (>250 mGy) as part of their treatment for cutaneous hemangioma during childhood, demonstrated a strong treatment influence on learning ability and logical reasoning into adulthood, suggesting sensitivity over a wider dose range and persistence not previously suspected (Hall et al. 2004). Significantly, a large proportion of patients receiving fractionated RT exhibit profound cognitive dysfunction following treatment (Greene-Schloesser and Robbins 2012; Makale et al. 2017), an apparent contradiction to the sparing effect of fractionation.
As interest in these effects increased, preclinical studies of RIBI primarily focused on effects in the subgranular zone of the dentate gyrus of the hippocampus (Makale et al. 2017), one of two distinct neurogenic niches within the vertebrate brain that, therefore, contain actively dividing cells. A range of doses, including low single fraction doses (e.g. ≤2 Gy), have been shown to induce apoptosis and alter differentiation of neural stem and progenitor cells (Monje et al. 2002; Mizumatsu et al. 2003; Monje et al. 2003; Monje 2008). As a result, novel RT-avoidance techniques were developed, where the hippocampal neurogenic niche is spared, dramatically reducing the dose delivered to this region while maintaining appropriate tumor coverage. Subsequently, both clinical and preclinical work with hippocampal avoidance have shown attenuation of RIBI and subsequent cognitive dysfunction (Tome et al. 2015; Redmond et al. 2017; Gui et al. 2019; Brown et al., 2020; Gui et al. 2020). However, while neurogenesis is undoubtedly important to the function of the brain and cognitive processes (Dietrich et al. 2008; Monje and Dietrich 2012), recent evidence demonstrates the exquisite radiation sensitivity of brain components previously considered radiation resistant, such as mature neuronal, glial, and brain endothelial structures (Tofilon and Fike 2000; Greene-Schloesser et al. 2013; Peiffer et al. 2014; Andrews et al. 2017; Begolly et al. 2018; Andrews et al. 2019). This evolution has paved the way for a more in-depth understanding of RIBI and opened new avenues for the development of novel treatment strategies.
The role of neuronal dysfunction in RIBI
Neuroinflammation and reactive gliosis (Figure 5) result from radiation-induced damage to neurons and glial cells, stimulating the release of cytokines, chemokines, and other molecules into the parenchyma (Lumniczky et al. 2017). Currently, chronic neuroinflammation is viewed as a key process in RIBI, including the development of long-term cognitive deficits, due to the resultant persistent disruption of the neuronal microenvironment and downstream deleterious effects on mature neurons (Andrews et al. 2017; Lumniczky et al. 2017; Andrews et al. 2019; Paladini et al. 2021). Interestingly, while radiation alone appears to induce chronic neuroinflammation, preclinical data show that tumor breakdown after RT further exacerbates inflammation, associated with greater and more persistent neurobehavioral dysfunction (Parihar et al. 2014; Chu et al. 2020). Microglial activation has been seen as a common feature following irradiation (Chiang et al. 1993; Kyrkanides et al. 1999; Yang et al. 2016), with an overlap in the expression of key proinflammatory signals among brain and other organs of interest, namely the interleukins (Hong et al. 1995; Kyrkanides et al. 1999), TNF-α (Kyrkanides et al. 1999; Daigle et al. 2001; Cho et al. 2017), chemokines (Olschowka et al. 1997; Moravan et al. 2016), etc., as well as anti-inflammatory signals, such as TGF-B (Kim et al. 2002; Pineda et al. 2013). Interestingly, recent evidence also has suggested a role for phenotypic polarization in brain injury resolution (Wang et al. 2021), drawing parallels with the lung and further supporting a role for the immune system.
Figure 5.
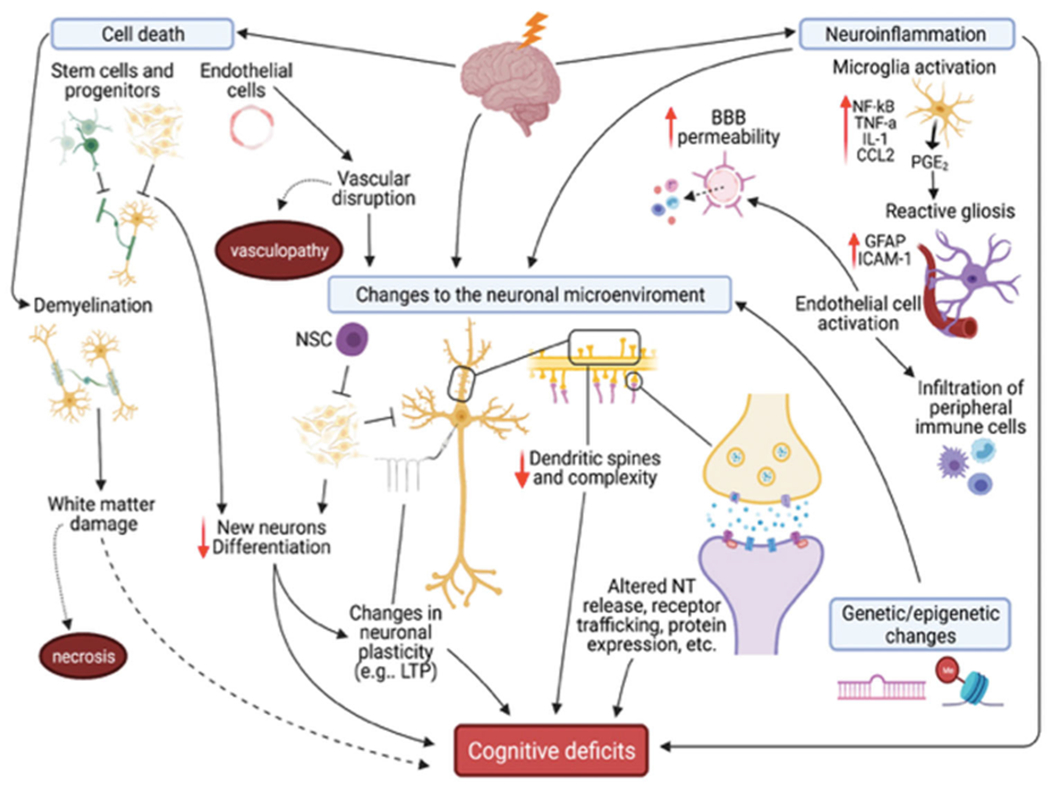
Clinical and preclinical research supports several mechanisms underlying RIBI and subsequent cognitive deficits, including apoptotic cell death, neuroinflammation, genetic/epigenetic changes, and changes to the neuronal microenvironment, which are prolonged by continued neuroinflammation and vascular damage. Damage depends on total dose delivered, fractionation, and dose-rate, and not all models display all changes. NSC: neural stem cell; LTP: long-term potentiation; NT: neurotransmitter.
Pharmacological inhibition of neuroinflammation, in addition to the use of novel RT delivery methods (e.g. FLASH), has been shown to attenuate neurobehavioral dysfunction in both tumor-bearing and non-tumor-bearing preclinical models, suggesting potential mechanisms for ameliorating RIBI (Monje et al. 2003; Acharya et al. 2016; Baulch et al. 2016; Feng et al. 2016; Feng et al. 2018; Montay-Gruel et al. 2018; Montay-Gruel et al. 2021). However, it is important to note that these effects are exacerbated by the early loss of endothelial cells and their subsequent activation, likely due to an increase in the permeability of the blood-brain barrier (BBB), leading to the infiltration of peripheral immune cells through chemotactic mechanisms (Belarbi et al. 2013; Morganti et al. 2014; Acharya et al. 2015a; Andrews et al. 2017). BBB perturbation also impacts the structure and function of mature neurons and the cerebrovasculature (Andrews et al. 2017) and has led many investigators to embrace a vascular hypothesis to explain the mechanisms of late brain injury (Greene-Schloesser et al. 2012).
A more recent, but growing, body of evidence has supported synaptic dysfunction, found within the hippocampus and prefrontal cortex (PFC), primarily at glutamatergic synapses, as another important mechanism underlying RIBI (Chakraborti et al. 2012; Parihar and Limoli 2013; Parihar et al. 2015; Kugelman et al. 2016; Duman et al. 2018; Zhang et al. 2018; Zhang et al. 2020). At early time points following exposure, an increase in hippocampal dendritic spines and excitatory synapses has been observed (Duman et al. 2018), in addition to increased excitability of PFC neurons (Zhang et al. 2020). These changes are followed by a reduction in density and complexity of spines, protein expression, and decreased neuronal excitability, seen days, weeks, and months after exposure (Chakraborti et al. 2012; Parihar and Limoli 2013; Shirai et al. 2013; Acharya et al. 2015b; Parihar et al. 2015; Feng et al. 2016; Duman et al. 2018; Zhang et al. 2018; Zhang et al. 2020). Functional connections between the hippocampus and PFC are also altered (Zhang et al. 2018; Zhang et al. 2020), likely mediated by radiation-induced presynaptic inhibition of hippocampal neurons that causes enhanced excitation within the PFC (Zhang et al. 2020) and altered expression of excitatory synaptic proteins (Parihar and Limoli 2013; Parihar et al. 2015). Treatment with memantine, an N-methyl-D-aspartate receptor antagonist, reduces these effects, suggesting these changes are a function of toxicity at glutamatergic synapses (Shi et al. 2006; Duman et al. 2018; Lynch 2019; Brown et al., 2020). The PFC is essential to cognition and is now being viewed by some as an important, but understudied region involved in RIBI (Makale et al. 2017). Taken together, cognitive dysfunction would arise from not only altered neuronal structure and function within the hippocampus and PFC, but also from changes in connectivity between these regions.
Individual variations in RIBI: sex and genetics
Sexual dimorphism affects numerous aspects of the CNS, including development and growth of the brain and its response to injury, and also has been seen with respect to RIBI and cognitive dysfunction. In the clinic, females often appear more sensitive to the development of RIBI (Ris and Noll 1994; Moore BD, 3rd, 2005; Panwala et al. 2019), although not all studies have confirmed a sex difference (Tonning Olsson et al. 2014). Sex differences are also seen in preclinical radiation studies, with males and females showing different mechanisms of injury and often a different time course of neurobehavioral dysfunction (Hinkle et al. 2019). For example, expression of microRNA (miR-29 family) is differentially altered in male and female brains, with the maintenance of DNA methylation in the frontal cortex of females hypothesized to be a protective mechanism by attenuating radiation-induced DNA hypomethylation in the brain (Kovalchuk and Baulch 2008; Acharya et al. 2017). Interestingly, in contrast to clinical findings, several preclinical studies have reported a lack of neurobehavioral deficits in female subjects exposed to low doses of high LET radiation, with, instead, males displaying greater microglia activation and decreased dendritic spines and synapses within the hippocampus (Krukowski et al. 2018; Hinkle et al. 2019; Parihar et al. 2020). Arguably, increased basal microglia activation within the female brain might provide a protective mechanism for radiation-induced deficits (Parihar et al. 2020). However, several earlier studies from the Kovalchuk laboratory (Kovalchuk et al. 2016a; Kovalchuk et al. 2016b) suggested that the female PFC is profoundly sensitive to low doses of photon radiation from both bystander effects and clinically-relevant scatter radiation, even though both sexes display decreased dendritic spines and behavioral deficits.
Cognitive deficits in cancer survivors also differ by individual, with not every patient receiving cranial RT developing cognitive dysfunction (Wefel et al. 2016). Genetic differences in oxidative stress, repair, and neuronal function exist within the CNS and impact cognition. For example, apolipoprotein (Apo), a glycoprotein critical for neural repair, has been associated with neurobehavioral outcomes in clinical and preclinical studies (Ahles et al. 2003; Correa et al. 2014; Koleck et al. 2014) and the ApoB/ApoA1 ratio has been shown to correlate with the development of radiation-induced cerebrovascular dysfunction (Li et al. 2020). ApoE status is modulated by sex in Alzheimer’s disease, with female ApoE4 carriers being at greater risk (Hohman et al., 2018). Supportive, albeit limited, preclinical work also has reported greater radiation-induced neurobehavioral deficits in ApoE4 female mice compared to males (Villasana et al. 2006), but exactly how this applies to clinical populations is not known. Other candidate genes important for neuronal functioning, including brain-derived neurotrophic factor, catechol-O-methyltransferase, monoamine oxidase, dopamine, and the folate pathway (Krull et al. 2013; Correa et al. 2019), also have been proposed as being involved in treatment-induced deficits in attention and executive function. Preclinical studies using normal tissue models support these pathways in individual variations in radiation-induced damage and the severity of subsequent deficits in attention and memory (Villasana et al. 2006; Davis et al. 2014).
Discussion
There is a fundamental conundrum that lies at the heart of normal tissue radiation biology: namely, what aspect(s) of the physicochemical injury and/or the biological response leads to the characteristic effects seen after radiation injury when compared with almost all other toxic insults? In other words, why do we get outcomes beyond wound repair and scarring if the basic outcomes from radiation damage are either cell death or survival? From a purely physical point of view, the sheer magnitude in number of ionizing events and their random distribution within each cell likely plays a role, with the list of potential targets expanding beyond nuclear DNA to include cellular structures, such as the mitochondria, cellular membranes, and even specific biomolecules, offering explanations for the observation of downstream genomic, epigenomic, transcriptomic, etc., changes. From the biological perspective, although cell death and associated loss of function are critical events in some of the more classic, acute radiation effects, e.g. the acute radiation syndromes and radiation dermatitis, the evolving and seemingly indiscriminate nature of progression to the delayed and late effects observed across different tissues has proved immensely complex, making identification of global (or even tissue-specific) mechanisms elusive. Nonetheless, as should be evident from this review, commonalities have begun to emerge, despite, or maybe because, of our growing appreciation of the multifaceted nature that these interactions display at the intra- and extracellular, microenvironmental, tissue and systemic levels.
In the broadest of terms, and irrespective of the tissue, as the timeline of radiation-induced effects unfolds, the acute expression of damage to cellular structures and tissue architecture transitions to the pathological changes associated with delayed radiation effects. When present, these latter events are characterized not only by an overt and dysregulated wound healing response, including the infiltration, and frequently chronic presence, of inflammatory cells (Zhao and Robbins 2009; Ratikan et al. 2015; Wang et al. 2019), altered vasculature with poor perfusion and ischemia (Chin et al. 2013; Wang et al. 2019; Zhuang et al. 2019), and aggravated connective tissue accumulation/scarring (Hwang et al. 2006; Curigliano et al. 2016; Veiga et al. 2020), but also by more subtle alterations that can affect the homeostatic status of a tissue’s microenvironment, such as shifts in macrophage polarization (Groves et al. 2016; Andrews et al. 2019), epithelial-mesenchymal cell/fibroblast-myofibroblast transition (Judge et al. 2015; Park et al. 2019) and the development of a senescence phenotype (Turnquist et al. 2019; Hansel et al. 2020). Although each of these events is not specific to radiation injury and have been seen under an array of disease conditions, it is their combined presence and involvement that characterizes the radiation response. Interestingly, and with potential relevance to the translation of data from the normal tissue radiobiology field to the clinic, many if not all of these pathological processes can precede radiotherapy as part of the general imbalance that comes with a growing tumor, whether treated or untreated (Zawaski et al. 2017; Oh et al. 2019), making understanding their role in a dysregulated microenvironment all the more necessary.
Not surprisingly, each transition in the radiation response is preceded or accompanied by changes in regulation, accomplished through a host of signaling pathways, likely reflecting attempts by the tissue to maintain or restore balance within the microenvironment (Williams et al. 2016; Davis et al. 2017). As highlighted previously, many of the altered regulatory pathways are associated with DNA damage, oxidative stress and inflammation, all seen both acutely and persistently. When signal transduction leads to paracrine signaling, this introduces the potential for abscopal or systemic involvement, even when the final overt effect remains localized. Indeed, as noted, there is communication between heart and lung following irradiation (Ghobadi et al. 2012; van der Veen et al. 2015), brain and gut (Jones et al. 2020), as well as between lung and brain, especially in the presence of tumor (Zhang et al. 2016). Clinically, the systemic phenomenon following irradiation has opened up new therapeutic avenues, most notably through the recent increased use of immunotherapy (Schaue 2017).
Whichever way one looks at this, it is hard to avoid the conclusion that the interplay between tissue-resident immune/non-immune cell populations and bone marrow-derived infiltrating immune cells, as well as the attempts to maintain tissue homeostasis, mediated in large part by cytokines and chemokines, are all clearly important to normal tissue radiobiology; nonetheless, a multitude of questions remain. Many of the so-called acute radiation responses, for example in lung, heart, CNS and skin, are obviously inflammatory in nature, rather than resulting from stem/progenitor cell death leading to functional cell depletion, throwing cold water on some of the old hypotheses, such as the target cell theory. The fact that dysregulated immune cell networks seem to play a prominent role in a wide range of radiation late effects, including life shortening and even cancer induction, as noted in Patricia Lindop’s work (Lindop and Rotblat 1961), only underscores this point. This is not to say that the classical responses with defined function endpoints seen in many normal tissues such as lung and kidney do not involve cell turnover and cell loss; nevertheless, it would be a mistake to underestimate the complexity of the interplay between immune and/or the non-immune elements within each tissue. Importantly, such considerations may prove critical with respect to the role of normal tissue radiobiology in radiation protection, especially when performing risk calculations in the low dose range where cell death is less evident. Indeed, although normal tissue effects are considered nonstochastic in nature, with a practical threshold of ≥0.5 Gy for most tissues (Stewart et al., 2012), the increasing concerns regarding radiation-induced effects associated with the growing use of medical imaging (Kuefner et al. 2015; Jaschke et al. 2020), air travel (Beck et al. 2008; Grajewski and Pinkerton 2013) and proposed space expeditions (Boerma et al. 2015; Furukawa et al. 2020), suggests that a more rigorous analysis of induction pathways, including the identification of contributing factors and critical events (Preston et al. 2020), needs to be undertaken to ensure future patient and worker safety.
In conclusion, it should be considered telling that, from the clinical radiotherapy perspective and despite the many advances in delivery systems, normal tissue toxicity remains a limiting factor for many treatment regimens (Pollom et al. 2017; Constine et al. 2019; De Ruysscher et al. 2019) and, therefore, needs to be an ongoing, if not expanding, area of research. In order to identify and develop appropriate countermeasures and treatment strategies, work must continue on defining key cells, their critical substructures and contributions to the various radiation-induced diseases. Such an understanding, in the context of individual susceptibilities, based on sex, genetic traits, lifestyle factors, preexisting diseases, etc., will be of enormous value toward individualizing radiation therapy, both for increased efficacy and safety. Indeed, many of the recent technological advances, such as FLASH (Vozenin et al. 2019), microbeam radiotherapy (Smyth et al. 2016), particle therapy (Mohan and Grosshans 2017), etc., all emphasize their ability to reduce normal tissue toxicity and subsequent improvement in the therapeutic ratio as support for their development and use. An appreciation of radiation sensitivities and temporal patterns enables a more science-based approach to prevention and mitigation; under best-case-scenarios, this would be combined with targeted (localized) and systemic interventions, with scheduling driven by easily accessible biomarkers. Importantly, given the coordinated multi-system nature of the biological response to radiation injury and the manifestation of normal tissue effects, translation from the bench to the clinic will require close collaborative efforts between physicians and basic scientists in equal measure. Following the examples set by earlier leaders in our field, such as Tikvah Alper, Juliana Denekamp, Elizabeth Travis, Fiona Stewart, and others too many to mention, we believe that through open minds, an appreciation for meticulous science, and collaborative efforts, we can look forward to finally solving the enigma of normal tissue radiation effects.
Funding
Dr. Boerma’s work was supported by National Institute of General Medical Sciences (NIGMS) P20 GM109005; National Institute of Allergy and Infectious Diseases (NIAID) U01 AI148308 and U01 AI133561. Dr. Davis’ work was supported by National Aeronautics and Space Administration (NASA) 80NSSC18K1080. Dr. Jackson’s work was supported by Biomedical Advanced Research and Development Authority (BARDA) HHSO10020150009I, HHSO1003300IT, HHSO10033003T, and HHSO10033004T; NIAID R44AI138865, and U19 AI150574. Dr. Schaue’s work was supported by the National Cancer Institute (NCI) R01 CA226875, R01 CA238998, and R21 CA228542; NIAID U01 AI148322. Dr. Williams’ work was supported by NCI R01 CA220467, R01 HL127001; NASA 80NSSC21K0542.
Abbreviations:
- Apo
apolipoprotein
- ATM
ataxia–telangiectasia mutated
- BBB
blood-brain barrier
- CCL2
chemokine ligand 2
- CCR2
chemokine receptor 2
- CT
computed tomography
- CNS
central nervous system
- DAMPs
damage-associated molecular patterns
- DEARE
delayed effects of acute radiation exposure
- DNA-SCARS
DNA segments with chromatin alterations reinforcing senescence
- FLASH
ultra-high dose rate
- IL-
interleukin-
- LET
linear energy transfer
- miR
microRNA
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- PRR
pattern recognition receptor
- PFC
prefrontal cortex
- RIBI
radiation-induced brain injury
- RIHD
radiation-induced heart disease
- RILD
radiation-induced lung disease
- RT
radiotherapy
- SASP
senescence-associated secretory phenotype
- SPECT
single photon-emission computed tomography
- TGF-B
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- TNFR2
tumor necrosis factor receptor 2
Biographies
Notes on contributors
Marjan Boerma is an Associate Professor in Pharmaceutical Sciences at the University of Arkansas for Medical Sciences (UAMS), and the director of the College of Pharmacy Division of Radiation Health and the Center for Studies of Host Response to Cancer Therapy. Her research focuses on cancer survivorship issues, particularly radiation-induced heart disease, risks of cardiovascular disease from exposure to radiation during space travel, and the development of medical countermeasures against nuclear accidents or terrorism
Catherine M. Davis is an Assistant Professor of Pharmacology and Molecular Therapeutics at the Uniformed Services University of the Health Sciences and a member of the Scientific Research Department at the Armed Forces Radiobiology Research Institute. Her research focuses on radiation-induced cognitive dysfunction, primarily addressing risks to the central nervous system from radiation exposure during space travel or military operations.
Isabel L. Jackson is an Associate Professor in Radiation Oncology and Director of the Division of Translational Radiation Sciences (DTRS) at the University of Maryland. Dr. Jackson is a subject matter expert in the field of tumor and normal tissue radiobiology, with specialized expertise in medical countermeasure (MCM) development for acute radiation sickness and delayed effects of acute radiation exposure.
Dörthe Schaue is an Associate Professor in the Department of Radiation Oncology at the University of California Los Angeles. Her research interests include tumor-specific immune responses in the context of radiation therapy, radiation-induced normal tissue responses, immune imbalances, inflammaging and countermeasure development.
Jacqueline P. Williams is a Professor in the Departments of Environmental Medicine and Radiation Oncology at the University of Rochester. Her research interests include investigation of the normal tissue effects that follow high dose (therapy- or accident-related) or low dose (space travel-related) irradiation, with a focus on lung, skin, bone marrow and CNS effects.
Footnotes
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
References
- Acharya MM, Baddour AA, Kawashita T, Allen BD, Syage AR, Nguyen TH, Yoon N, Giedzinski E, Yu L, Parihar VK, et al. 2017. Epigenetic determinants of space radiation-induced cognitive dysfunction. Sci Rep. 7:42885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, Le MT, Kawashita T, Giedzinski E, Parihar VK, et al. 2016. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Patel NH, Craver BM, Tran KK, Giedzinski E, Tseng BP, Parihar VK, Limoli CL. 2015a. Consequences of low dose ionizing radiation exposure on the hippocampal microenvironment. PLoS One. 10(6):e0128316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Rosi S, Jopson T, Limoli CL. 2015b. Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus. Cell Transplant. 24(4):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Kim YO, Wagner WL, Schuppan D, Valenzuela CD, Mentzer SJ, Kreuz S, Stiller D, Wollin L, Konerding MA. 2017. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis. 20(3):359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackson IL, Baye F, Goswami C, Katz BP, Zodda A, Pavlovic R, Gurung G, Winans D, Vujaskovic Z. 2018. Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease. Dis Model Mech. 10:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. 2003. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 12(6):612–619. [DOI] [PubMed] [Google Scholar]
- Alper T 1956. The modification of damage caused by primary ionization of biological targets. Radiat Res. 5(5):573–586. [PubMed] [Google Scholar]
- Andrews RN, Dugan GO, Peiffer AM, Hawkins GA, Hanbury DB, Bourland JD, Hampson RE, Deadwyler SA, Cline JM. 2019. White matter is the predilection site of late-delayed radiation-induced brain injury in non-human primates. Radiat Res. 191(3):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, Hampson RE, Deadwyler SA, Cline JM. 2017. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 187(5):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, Jirtle RL. 1998a. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 41(5):1029–1035. [DOI] [PubMed] [Google Scholar]
- Anscher MS, Kong FM, Jirtle RL. 1998b. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer. 19(2):109–120. [DOI] [PubMed] [Google Scholar]
- Antonic V, Rabbani ZN, Jackson IL, Vujaskovic Z. 2015. Subcutaneous administration of bovine superoxide dismutase protects lungs from radiation-induced lung injury. Free Radic Res. 49(10):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh O, Azizova T, Merl-Pham J, Blutke A, Moseeva M, Zubkova O, Anastasov N, Feuchtinger A, Hauck SM, Atkinson MJ, et al. 2020. Chronic occupational exposure to ionizing radiation induces alterations in the structure and metabolism of the heart: a proteomic analysis of human Formalin-Fixed Paraffin-Embedded (FFPE) cardiac tissue. IJMS. 21(18):6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, Giedzinski E, Syage A, Park AL, Benke SN, et al. 2016. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci USA. 113(17):4836–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TA, Groves AM, Johnston CJ, Williams JP, Finkelstein JN. 2018. Recurrent DNA damage is associated with persistent injury in progressive radiation-induced pulmonary fibrosis. Int J Radiat Biol. 94(12):1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TA, Johnston CJ, Groves AM, Williams JP, Finkelstein JN. 2017. Radiation induced pulmonary fibrosis as a model of progressive fibrosis: Contributions of DNA damage, inflammatory response and cellular senescence genes. Exp Lung Res. 43(3):134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P, Bartlett DT, Bilski P, Dyer C, Flückiger E, Fuller N, Lantos P, Reitz G, Rühm W, Spurny F, et al. 2008. Validation of modelling the radiation exposure due to solar particle events at aircraft altitudes. Radiat Prot Dosimetry. 131(1):51–58. [DOI] [PubMed] [Google Scholar]
- Begolly S, Olschowka JA, Love T, Williams JP, O’Banion MK. 2018. Fractionation enhances acute oligodendrocyte progenitor cell radiation sensitivity and leads to long term depletion. Glia. 66(4):846–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. 2013. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 73(3):1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen SM. 2006. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 6(9):702–713. [DOI] [PubMed] [Google Scholar]
- Boerma M, Nelson GA, Sridharan V, Mao XW, Koturbash I, Hauer-Jensen M. 2015. Space radiation and cardiovascular disease risk. World J Cardiol. 7(12):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. 2013. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 8(7):e70479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Wang J, Wondergem J, Joseph J, Qiu X, Kennedy RH, Hauer-Jensen M. 2005. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 65(8):3100–3107. [DOI] [PubMed] [Google Scholar]
- Brown M 2008. What causes the radiation gastrointestinal syndrome?: overview. Int J Radiat Oncol Biol Phys. 70(3):799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Bovi JA, Robinson C, Konski A, Khuntia D, et al. ; for NRG Oncology. 2020. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III trial NRG oncology CC001. J Clin Oncol. 38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccini F, Eldh T, Bruder D, Gereke M, Jastrow H, Schulze-Osthoff K, Fischer U, Köhler D, Stuschke M, Jendrossek V. 2011. New insights into the molecular pathology of radiation-induced pneumopathy. Radiother Oncol. 101(1):86–92. [DOI] [PubMed] [Google Scholar]
- Catterall M 1960. The effect of radiation upon the heart. Br J Radiol. 33:159–164. [DOI] [PubMed] [Google Scholar]
- Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. 2012. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 7(7):e40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Mitroulis I, Hajishengallis G. 2019. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 20(7):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. 1997. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 72(1):45–53. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. 2005. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 62(3):862–871. [DOI] [PubMed] [Google Scholar]
- Chiang CS, McBride WH, Withers HR. 1993. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother Oncol. 29(1):60–68. [DOI] [PubMed] [Google Scholar]
- Chin MS, Freniere BB, Bonney CF, Lancerotto L, Saleeby JH, Lo YC, Orgill DP, Fitzgerald TJ, Lalikos JF. 2013. Skin perfusion and oxygenation changes in radiation fibrosis. Plastic and Reconstructive Surgery. 131(4):707–716. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Lee WH, Hwang OMH, Sonntag WE, Lee YW. 2017. Role of NADPH oxidase in radiation-induced pro-oxidative and pro-inflammatory pathways in mouse brain. Int J Radiat Biol. 93(11):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Burns SC, Mirsafian H, Li W, Vo DT, Qiao M, Lei X, Smith AD, Penalva LO. 2020. Publisher correction: genomic analyses of early responses to radiation in glioblastoma reveal new alterations at transcription, splicing, and translation levels. Sci Rep. 10(1):13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JR, Massey R, Dalen H, Kanellopoulos A, Hamre H, Ruud E, Kiserud CE, Fosså SD, Aakhus S. 2016. Right ventricular function in long-term adult survivors of childhood lymphoma and acute lymphoblastic leukaemia. Eur Heart J Cardiovasc Imaging. 17(7):735–741. [DOI] [PubMed] [Google Scholar]
- Chu C, Davis CM, Lan X, Hienz RD, Jablonska A, Thomas AM, Velarde E, Li S, Janowski M, Kai M, et al. 2020. Neuroinflammation after stereotactic radiosurgery-induced brain tumor disintegration is linked to persistent cognitive decline in a mouse model of metastatic disease. Int J Radiat Oncol Biol Phys. 108(3):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua ML, Rothkamm K. 2013. Biomarkers of radiation exposure: can they predict normal tissue radiosensitivity? Clin Oncol (R Coll Radiol)). 25(10):610–616. [DOI] [PubMed] [Google Scholar]
- Constine LS, Ronckers CM, Hua CH, Olch A, Kremer LCM, Jackson A, Bentzen SM. 2019. Pediatric normal tissue effects in the Clinic (PENTEC): an international collaboration to analyse normal tissue radiation dose-volume response relationships for paediatric cancer patients. Clin Oncol. 31(3):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Satagopan J, Baser RE, Cheung K, Richards E, Lin M, Karimi S, Lyo J, DeAngelis LM, Orlow I. 2014. APOE polymorphisms and cognitive functions in patients with brain tumors. Neurology. 83(4):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Satagopan J, Martin A, Braun E, Kryza-Lacombe M, Cheung K, Sharma A, Dimitriadoy S, O’Connell K, Leong S, et al. 2019. Genetic variants and cognitive functions in patients with brain tumors. Neuro Oncol. 21(10):1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. 1994. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 12(3):627–642. [DOI] [PubMed] [Google Scholar]
- Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. 2016. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 66(4):309–325. [DOI] [PubMed] [Google Scholar]
- Cushman M, Shay CM, Howard VJ, Jiménez MC, Lewey J, McSweeney JC, Newby LK, Poudel R, Reynolds HR, Rexrode KM, et al. ; American Heart Association. 2021. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American heart association national survey: a special report from the American Heart Association. Circulation. 143(7):e239–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabjan MB, Buck CM, Jackson IL, Vujaskovic Z, Marples B, Down JD. 2016. A survey of changing trends in modelling radiation lung injury in mice: bringing out the good, the bad, and the uncertain. Lab Invest. 96(9):936–949. [DOI] [PubMed] [Google Scholar]
- Daigle JL, Hong JH, Chiang CS, McBride WH. 2001. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 61(24):8859–8865. [PubMed] [Google Scholar]
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. 2013. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 368(11):987–998. [DOI] [PubMed] [Google Scholar]
- Darby SC, McGale P, Taylor CW, Peto R. 2005. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 6(8):557–565. [DOI] [PubMed] [Google Scholar]
- Davis CM, Decicco-Skinner KL, Roma PG, Hienz RD. 2014. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat Res. 181(3):258–271. [DOI] [PubMed] [Google Scholar]
- Davis CM, Liu X, Alkayed NJ. 2017. Cytochrome P450 eicosanoids in cerebrovascular function and disease. Pharmacol Ther. 179:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SD, Yankelevitz DF, Henschke CI. 1992. Radiation effects on the lung: clinical features, pathology, and imaging findings. AJR Am J Roentgenol. 159(6):1157–1164. [DOI] [PubMed] [Google Scholar]
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. 2019. Radiotherapy toxicity. Nat Rev Dis Primers. 5(1):13. [DOI] [PubMed] [Google Scholar]
- Demaria S, Formenti SC. 2012. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J 1986. Cell kinetics and radiation biology. Int J Radiat Biol Relat Stud Phys Chem Med. 49(2):357–380. [DOI] [PubMed] [Google Scholar]
- Desjardins AU. 1926. The reaction of the pleura and lungs to roentgen rays. Am J Roentgenol Radium Ther Nucl Med. 16:444–453. [Google Scholar]
- Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L, Murthy VL, Hearn JWD, Kong FM, Kalemkerian GP, et al. 2017. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 35(13):1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. 2008. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 13(12):1285–1295. [DOI] [PubMed] [Google Scholar]
- Douglas BG, Grulkey WR, Chaplin DJ, Lam G, Skarsgard LD, Denekamp J. 1986. Pions and pig skin: preclinical evaluation of RBE for early and late damage. Int J Radiat Oncol Biol Phys. 12(2):221–229. [DOI] [PubMed] [Google Scholar]
- Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, Fujiwara S, Shore RE. 2011. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 5 Suppl 1(0 1):S122–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss AD, Goia D, Shoniyozov K, Shewale SV, Velalopoulou A, Mazzoni S, Avgousti H, Metzler SD, Bravo PE, Feigenberg SJ, et al. 2021. A novel mouse model of radiation-induced cardiac injury reveals biological and radiological biomarkers of cardiac dysfunction with potential clinical relevance. Clin Cancer Res. 27(8):2266–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman JG, Dinh J, Zhou W, Cham H, Mavratsas VC, Paveskovic M, Mulherkar S, McGovern SL, Tolias KF, Grosshans DR. 2018. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 20(5):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutreix J, Tubiana M, Pierquin B. 1998. The hazy dawn of brachytherapy. Radiother Oncol. 49(3):223–232. eng. [DOI] [PubMed] [Google Scholar]
- Elkind MM, Sutton-Gilbert H, Moses WB, Alescio T, Swain RW. 1965. Radiation response of mammalian cells grown in culture. V. Temperature dependence of the repair of X-ray damage in surviving cells (aerobic and hypoxic). Radiat Res. 25:359–376. [PubMed] [Google Scholar]
- Emery EW, Denekamp J, Ball MM, Field SB. 1970. Survival of mouse skin epithelial cells following single and divided doses of x-rays. Radiat Res. 41(3):450–466. [PubMed] [Google Scholar]
- Fajardo LF. 1982. Pathology of Radiation Injury. New York: Masson. [Google Scholar]
- Fajardo LF, Stewart JR. 1971. Capillary injury preceding radiation-induced myocardial fibrosis. Radiology. 101(2):429–433. [DOI] [PubMed] [Google Scholar]
- Feng X, Jopson TD, Paladini MS, Liu S, West BL, Gupta N, Rosi S. 2016. Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. J Neuroinflammation. 13(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Liu S, Chen D, Rosi S, Gupta N. 2018. Rescue of cognitive function following fractionated brain irradiation in a novel preclinical glioma model. Elife. 7:e38865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SB, Hornsey S, Kutsutani Y. 1976. Effects of fractionated irradiation on mouse lung and a phenomenon of slow repair. Br J Radiol. 49(584):700–707. [DOI] [PubMed] [Google Scholar]
- Fink J, Born D, Chamberlain MC. 2012. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 12(3):276–285. [DOI] [PubMed] [Google Scholar]
- Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. 2007. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 68(1):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley JH, Borges W, Yamawaki T. 1952. Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med. 13(3):311–321. [DOI] [PubMed] [Google Scholar]
- Fowler JF, Bewley DK, Morgan RL, Silvester JA, Alper T, Hornsey S. 1963. Dose-effect relationships for radiation damage to organized TISSUES. Nature. 199:253–255. [DOI] [PubMed] [Google Scholar]
- Franko AJ, Sharplin J, Ward WF, Hinz JM. 1991. The genetic basis of strain-dependent differences in the early phase of radiation injury in mouse lung. Radiat Res. 126(3):349–356. [PubMed] [Google Scholar]
- Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S, Katsube T, Wang B, Tsuruoka C, Shirai T, Nakamura AJ, et al. 2020. Space Radiation Biology for “Living in Space”. Biomed Res Int. 2020:4703286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels K, Hoving S, Gijbels MJ, Pol JF, Te Poele JA, Biessen EA, Daemen MJ, Stewart FA, Heeneman S. 2014. Irradiation of existing atherosclerotic lesions increased inflammation by favoring pro-inflammatory macrophages. Radiother Oncol. 110(3):455–460. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Chandna S. 2017. Radiation-induced inflammatory cascade and its reverberating crosstalks as potential cause of post-radiotherapy second malignancies. Cancer Metastasis Rev. 36(2):375–393. [DOI] [PubMed] [Google Scholar]
- Garant A, Spears G, Routman D, Whitaker T, Liao Z, Harmsen W, Liu A, Haddock M, Hallemeier C, Lin S, et al. 2021. A multi-institutional analysis of radiation dosimetric predictors of toxicity following trimodality therapy for esophageal cancer. Pract Radiat Oncol. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Gasinska A 2016. The contribution of women to radiobiology: Marie Curie and beyond. Rep Pract Oncol Radiother. 21(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghita M, Dunne V, Hanna GG, Prise KM, Williams JP, Butterworth KT. 2019. Preclinical models of radiation-induced lung damage: challenges and opportunities for small animal radiotherapy. Br J Radiol. 92(1095):20180473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghita M, Gill EK, Walls GM, Edgar KS, McMahon SJ, Osorio EV, Bergom C, Grieve DJ, Watson CJ, McWilliam A, et al. 2020. Cardiac sub-volume targeting demonstrates regional radiosensitivity in the mouse heart. Radiother Oncol. 152:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobadi G, van DV, Bartelds B, de Boer RA, Dickinson MG, de Jong JR, Faber H, Niemantsverdriet M, Brandenburg S, Berger RM, et al. 2012. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys. 84(5):e639–e646. [DOI] [PubMed] [Google Scholar]
- Gillette SM, Gillette EL, Shida T, Boon J, Miller CW, Powers BE. 1992. Late radiation response of canine mediastinal tissues. Radiother Oncol. 23(1):41–52. [DOI] [PubMed] [Google Scholar]
- Gillies M, Richardson DB, Cardis E, Daniels RD, O’Hagan JA, Haylock R, Laurier D, Leuraud K, Moissonnier M, Schubauer-Berigan MK, et al. 2017. Mortality from Circulatory Diseases and other Non-Cancer Outcomes among Nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat Res. 188(3):276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajewski B, Pinkerton LE. 2013. Exposure assessment at 30 000 feet: challenges and future directions. Ann Occup Hyg. 57(6):692–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, Cahoon EK, Milder CM, Soda M, Cullings HM, et al. 2017. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958-2009. Radiat Res. 187(5):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Moore E, Robbins ME. 2013. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 19(9):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Robbins ME. 2012. Radiation-induced cognitive impairment-from bench to bedside. Neuro Oncol. 14(Suppl 4): iv37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. 2012. Radiation-induced brain injury: A review. Front Oncol. 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover TA, Christie AC, Merritt EA. 1922. Observations on the use of copper filter in the roentgen treatment of deep-seated malignancies. South Med J. 15:440–444. [Google Scholar]
- Groves AM, Johnston CJ, Misra RS, Williams JP, Finkelstein JN. 2015. Whole-lung irradiation results in pulmonary macrophage alterations that are subpopulation and strain specific. Radiat Res. 184(6):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AM, Johnston CJ, Misra RS, Williams JP, Finkelstein JN. 2016. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int J Radiat Biol. 92(12):754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AM, Johnston CJ, Williams JP, Finkelstein JN. 2018. Role of infiltrating monocytes in the development of radiation-induced pulmonary fibrosis. Radiat Res. 189(3):300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AM, Williams JP. 2019. Saving normal tissues – a goal for the ages. Int J Radiat Biol. 95(7):920–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Chintalapati N, Hales RK, Voong KR, Sair HI, Grimm J, Duhon M, Kleinberg LR, Vannorsdall TD, Redmond KJ. 2019. A prospective evaluation of whole brain volume loss and neurocognitive decline following hippocampal-sparing prophylactic cranial irradiation for limited-stage small-cell lung cancer. J Neurooncol. 144(2):351–358. [DOI] [PubMed] [Google Scholar]
- Gui C, Vannorsdall TD, Kleinberg LR, Assadi R, Moore JA, Hu C, Quinones-Hinojosa A, Redmond KJ. 2020. A Prospective Cohort Study of Neural Progenitor Cell-Sparing Radiation Therapy Plus Temozolomide for Newly Diagnosed Patients With Glioblastoma. Neurosurgery. 87(1):E31–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P, Adami HO, Trichopoulos D, Pedersen NL, Lagiou P, Ekbom A, Ingvar M, Lundell M, Granath F. 2004. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ. 328(7430):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ, Giaccia A. 2019. Radiobiology for the radiologist. 8th ed. Philadelphia (PA): Wolters Kluwer. [Google Scholar]
- Hancock SL, Donaldson SS, Hoppe RT. 1993b. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 11(7):1208–1215. [DOI] [PubMed] [Google Scholar]
- Hancock SL, Tucker MA, Hoppe RT. 1993a. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 270(16):1949–1955. [PubMed] [Google Scholar]
- Hansel C, Jendrossek V, Klein D. 2020. Cellular senescence in the lung: the central role of senescent epithelial cells. Int J Mol Sci. 21(9):3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, Lienenklaus S, Nilsson LM, Kröger A, Nilsson JA, et al. 2015. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 42(2):332–343. [DOI] [PubMed] [Google Scholar]
- Haston CK. 2012. Mouse genetic approaches applied to the normal tissue radiation response. Front Oncol. 2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haston CK, Travis EL. 1997. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 57(23):5286–5291. [PubMed] [Google Scholar]
- Haston CK, Zhou X, Gumbiner-Russo L, Irani R, Dejournett R, Gu X, Weil M, Amos CI, Travis EL. 2002. Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Res. 62(13):3782–3788. [PubMed] [Google Scholar]
- Heineke H 1903. Über die Einwirkung der Röntgenstrahlen auf Tiere. Müunch Med Wochenschrift. 50:2090–2092. [Google Scholar]
- Heineke H 1905. Experimentelle Untersuchungen über die Einwirkung der Röntgenstrahlung auf das Knockenmark, nebst einigen Bemerkungen uüber die Röntgentherapie der Leukoämie und Pseudoleukoämie und des Sarcoms. Deutsche Zeitschrift f Chirurgie. 78(1-3):196–230. [Google Scholar]
- Henson KE, McGale P, Taylor C, Darby SC. 2013. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 108(1):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LE. 1922. Fibrosis of the lung following roentgen-ray treatments for tumor. JAMA. 79(9):720. [Google Scholar]
- Hinkle JJ, Olschowka JA, Love TM, Williams JP, O’Banion MK. 2019. Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Sci Rep. 9(1):18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst DG, Denekamp J, Hobson B. 1980. Proliferation studies of the endothelial and smooth muscle cells of the mouse mesentery after irradiation. Cell Tissue Kinet. 13(1):91–104. [DOI] [PubMed] [Google Scholar]
- Hochberg FH, Slotnick B. 1980. Neuropsychologic impairment in astrocytoma survivors. Neurology. 30(2):172–177. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, for the Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative, et al. 2018. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol. 75(8):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. 1995. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 33(3):619–626. [DOI] [PubMed] [Google Scholar]
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. 2007. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Nat Cancer Inst. 99(5):365–375. [DOI] [PubMed] [Google Scholar]
- Hornsey S, Kutsutanl Y, Field SB. 1975. Damage to mouse lung with fractionated neutrons and x rays. Radiology. 116(1):171–174. [DOI] [PubMed] [Google Scholar]
- Hoving S, Seemann I, Visser NL, Te Poele JA, Stewart FA. 2013. Thalidomide is not able to inhibit radiation-induced heart disease. Int J Radiat Biol. 89(9):685–691. [DOI] [PubMed] [Google Scholar]
- Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, et al. 2013. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 179(3):361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. 2006. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 21(3):457–467. [DOI] [PubMed] [Google Scholar]
- Inskip PD, Sigurdson AJ, Veiga L, Bhatti P, Ronckers C, Rajaraman P, Boukheris H, Stovall M, Smith S, Hammond S, et al. 2016. Radiation-Related New Primary Solid Cancers in the Childhood Cancer Survivor Study: Comparative Radiation Dose Response and Modification of Treatment Effects. Int J Radiat Oncol Biol Phys. 94(4):800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Ishida M, Tashiro S, Takeishi Y. 2019. DNA Damage and Senescence-Associated Inflammation in Cardiovascular Disease. Biol Pharm Bull. 42(4):531–537. [DOI] [PubMed] [Google Scholar]
- Jackson IL Zhang Y Bentzen SM Hu J Zhang A Vujaskovic Z 2016. Pathophysiological mechanisms underlying phenotypic differences in pulmonary radioresponse. Sci Rep. 6:36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IL, Zodda A, Gurung G, Pavlovic R, Kaytor MD, Kuskowski MA, Vujaskovic Z. 2017. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. Br J Pharmacol. 174(24):4738–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JK, Reilly M, Yaghmour G, Rashid F, Ballas LK. 2020. Acute Respiratory Events and Dosimetry of Total Body Irradiation Patients Using In Vivo Lung Dose Monitoring and Custom Lung Block Adaptation. Pract Radiat Oncol. 10(5):e397–e405. [DOI] [PubMed] [Google Scholar]
- Jaschke W, Bartal G, Martin CJ, Vano E. 2020. Unintended and Accidental Exposures, Significant Dose Events and Trigger Levels in Interventional Radiology. Cardiovasc Intervent Radiol. 43(8):1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Svensson H, Denekamp J. 2000. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiat Oncol Biol Phys. 48(3):745–750. [DOI] [PubMed] [Google Scholar]
- Johansson S, Svensson H, Denekamp J. 2002. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int J Radiat Oncol Biol Phys. 52(5):1207–1219. [DOI] [PubMed] [Google Scholar]
- Johnston J, Comello RJ, Vealé BL, Killion J. 2010. Radiation Exposure Dose Trends and Radiation Dose Reduction Strategies in Medical Imaging. J Med Imaging Radiat Sci. 41(3):137–144. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. 2010. Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiat Res. 173(4):522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CJ, Manning C, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. 2011. Effect of total body irradiation on late lung effects: hidden dangers. Int J Radiat Biol. 87(8):902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CJ, Piedboeuf B, Baggs R, Rubin P, Finkelstein JN. 1995. Differences in correlation of mRNA gene expression in mice sensitive and resistant to radiation-induced pulmonary fibrosis. Radiat Res. 142(2):197–203. [PubMed] [Google Scholar]
- Joiner MC. van der Kogel Aj 2019. Basic Clinical Radiobiology. 5th ed. Boca Raton (FL): CRC Press, Taylor & Francis Group. [Google Scholar]
- Jones CB, Davis CM, Sfanos KS. 2020. The potential effects of radiation on the gut-brain axis. Radiat Res. 193(3):209–222. [DOI] [PubMed] [Google Scholar]
- Jones A, Wedgwood J. 1960. Effects of radiations on the heart. Br J Radiol. 33:138–158. [DOI] [PubMed] [Google Scholar]
- Judge JL, Owens KM, Pollock SJ, Woeller CF, Thatcher TH, Williams JP, Phipps RP, Sime PJ, Kottmann RM. 2015. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol. 309(8):L879–L887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiriya JJ, Brumwell AN, Jackson JR, Tang X, Chapman HA. 2020. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell. 26(3): 346–358. e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarev NN. 2019. Intracellular RNA sensing in mammalian cells: role in stress response and cancer therapies. Int Rev Cell Mol Biol. 344:31–89. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lim DJ, Chung YG, Cho TH, Lim SJ, Kim WJ, Suh JK. 2002. Expression of TNF-alpha and TGF-beta 1 in the rat brain after a single high-dose irradiation. J Korean Med Sci. 17(2):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn HI, Kallman RF, Berdjis CC, De Ome KB. 1957. Late effects of whole-body x-irradiation in the mouse; some gross and histologic aspects of the development of morbidity prior to the terminal state, with special reference to the gonad, uterus, heart, liver, kidney, and submaxillary gland. Radiat Res. 7(4):407–435. [PubMed] [Google Scholar]
- Koleck TA, Bender CM, Sereika SM, Ahrendt G, Jankowitz RC, McGuire KP, Ryan CM, Conley YP. 2014. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum. 41(6):E313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvari M, Souliotis K, Yannakoulia M, Panagiotakos DB. 2020. Cardiovascular diseases in women: policies and practices around the globe to achieve gender equity in cardiac health. Risk Manag Healthc Policy. 13:2079–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Baulch JE. 2008. Epigenetic changes and nontargeted radiation effects-is there a link? Environ Mol Mutagen. 49(1):16–25. [DOI] [PubMed] [Google Scholar]
- Kovalchuk A, Mychasiuk R, Muhammad A, Hossain S, Ilnytskyy S, Ghose A, Kirkby C, Ghasroddashti E, Kovalchuk O, Kolb B. 2016a. Liver irradiation causes distal bystander effects in the rat brain and affects animal behaviour. Oncotarget. 7(4):4385–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk A, Mychasiuk R, Muhammad A, Hossain S, Ilnytskyy Y, Ghose A, Kirkby C, Ghasroddashti E, Kolb B, Kovalchuk O. 2016b. Profound and sexually dimorphic effects of clinically-relevant low dose scatter irradiation on the brain and behavior. Front Behav Neurosci. 10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Grue K, Frias ES, Pietrykowski J, Jones T, Nelson G, Rosi S. 2018. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav Immun. 74:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Bhojwani D, Conklin HM, Pei D, Cheng C, Reddick WE, Sandlund JT, Pui CH. 2013. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 31(17):2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuefner MA, Brand M, Engert C, Schwab SA, Uder M. 2015. Radiation induced DNA double-strand breaks in radiology. Rofo. 187(10):872–878. [DOI] [PubMed] [Google Scholar]
- Kugelman T, Zuloaga DG, Weber S, Raber J. 2016. Post-training gamma irradiation-enhanced contextual fear memory associated with reduced neuronal activation of the infralimbic cortex. Behav Brain Res. 298(Pt B):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O’Banion MK. 1999. TNFa and IL-1ß mediate ICAM-1 induction via microglia astrocyte interaction in CNS radiation injury. J Neuroimmunol. 95(1-2):95–106. [DOI] [PubMed] [Google Scholar]
- Landberg T, Baldetorp L, Lindberg LG, Svahn-Tapper G. 1972. Radiation sensitivity of tissues irradiated during mantle treatment of Hodgkin’s disease. Acta Radiol Ther Phys Biol. 11(6):521–535. [DOI] [PubMed] [Google Scholar]
- Lauk S, Ruth S, Trott KR. 1987. The effects of dose-fractionation on radiation-induced heart disease in rats. Radiother Oncol. 8(4):363–367. [DOI] [PubMed] [Google Scholar]
- Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, Laverdière C, Kokta V, Haddad E, Beauséjour CM. 2010. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 9(3):398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JE, Farrow JH, Foote FW, Wawro NW. 1942. Fibrosis of the lung following roentgen irradiation for cancer of the breast. Am J Roentgenol. 50:772. [Google Scholar]
- Lee CL, Lee JW, Daniel AR, Holbrook M, Hasapis S, Wright AO, Brownstein J, Da Silva Campos L, Ma Y, Mao L, et al. 2021. Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation. Radiother Oncol. 157:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, Nacionales DC, Butfiloski EJ, van Rooijen N, Akira S, et al. 2009. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 175(5):2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva-Juárez MM, Kolls JK, Evans SE. 2018. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 11(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, You L, Xue J, Lu Y. 2018. Ionizing Radiation-Induced Cellular Senescence in Normal, Non-transformed Cells and the Involved DNA Damage Response: A Mini Review. Front Pharmacol. 9:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zheng D, Xie F, Huang X, Zhuo X, Lin J, Li Y, Tang Y. 2020. The correlation between serum apolipoprotein B/apolipoprotein A1 ratio and brain necrosis in patients underwent radiotherapy for nasopharyngeal carcinoma. Brain Behav. 10(3):e01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindop PJ, Rotblat J. 1961. Shortening of Life and Causes of Death in Mice Exposed to a Single Whole-Body Dose of Radiation. Nature. 189(4765):645–648. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Brennan TV, Warren RS. 2002. Thorotrast-induced liver neoplasia: a collective review. J Am Coll Surg. 195(5):713–718. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tör M, Billiar T. 2007. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 220:60–81. [DOI] [PubMed] [Google Scholar]
- Lumniczky K, Szatmari T, Safrany G. 2017. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front Immunol. 8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M 2019. Preservation of cognitive function following whole brain radiotherapy in patients with brain metastases: Complications, treatments, and the emerging role of memantine. J Oncol Pharm Pract. 25(3):657–662. [DOI] [PubMed] [Google Scholar]
- Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Medhora M, Hill RP. 2014. Targeting the Renin-angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int J Radiat Oncol Biol Phys. 89(4):722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisin JR. 1970. The ultrastructure of the lung of mice exposed to a supra-lethal dose of ionizing radiation on the thorax. Radiat Res. 44(2):545–564. [PubMed] [Google Scholar]
- Maisin JR, Van Gorp U, de Saint-Georges L. 1982. The ultrastructure of the lung after exposure to ionizing radiation as seen by transmission and scanning electron microscopy. Scan Electron Microsc. (Part 1):403–412. [PubMed] [Google Scholar]
- Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. 2017. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell LR, Walker RW, Steinherz P, Fuks Z. 1989. Reduced incidence of the somnolence syndrome in leukemic children with steroid coverage during prophylactic cranial radiation therapy. Results of a pilot study. Cancer. 63(10):1975–1978. [DOI] [PubMed] [Google Scholar]
- Manning CM, Johnston CJ, Hernady E, Miller JN, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. 2013. Exacerbation of lung radiation injury by viral infection: the role of Clara cells and Clara cell secretory protein. Radiat Res. 179(6):617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CM, Johnston CJ, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. 2013. Lung irradiation increases mortality after influenza A virus challenge occurring late after exposure. Int J Radiat Oncol Biol Phys. 86(1):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LB, Yu X, Vujaskovic Z, Small W Jr., Folz R, Anscher MS. 2003. Radiation-induced lung injury. Semin Radiat Oncol. 13(3):333–345. [DOI] [PubMed] [Google Scholar]
- Martin M, LeFaix J-L, Delanian S. 2000. TGF-ß1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 47(2):277–290. [DOI] [PubMed] [Google Scholar]
- Martin RG, Ruckdeschel JC, Chang P, Byhardt R, Bouchard RJ, Wiernik PH. 1975. Radiation-related pericarditis. Am J Cardiol. 35(2):216–220. [DOI] [PubMed] [Google Scholar]
- Matzinger P 2002. The danger model: a renewed sense of self. Science. 296(5566):301–305. [DOI] [PubMed] [Google Scholar]
- McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. 2004. A sense of danger from radiation. Radiat Res. 162(1):1–19. [DOI] [PubMed] [Google Scholar]
- McIntosh HC, Spitz S. 1939. A study of radiation pneumonitis. AJR Am J Roentgenol. 41:605–615. [Google Scholar]
- McLaughlin J 2012. An historical overview of radon and its progeny: applications and health effects. Radiat Protect Dosimetry. 152(1-3):2–8. [DOI] [PubMed] [Google Scholar]
- McReynolds RA, Gold GL, Roberts WC. 1976. Coronary heart disease after mediastinal irradiation for Hodgkin’s disease. Am J Med. 60(1):39–45. [DOI] [PubMed] [Google Scholar]
- Mefferd JM, Donaldson SS, Link MP. 1989. Pediatric Hodgkin’s disease: pulmonary, cardiac, and thyroid function following combined modality therapy. Int J Radiat Oncol Biol Phys. 16(3):679–685. [DOI] [PubMed] [Google Scholar]
- Micewicz ED, Iwamoto KS, Ratikan JA, Nguyen C, Xie MW, Cheng G, Boxx GM, Deriu E, Damoiseaux RD, Whitelegge JP, et al. 2019. The Aftermath of Surviving Acute Radiation Hematopoietic Syndrome and its Mitigation. Radiat Res. 191(4):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowski A 1984. A critical appraisal of clonogenic survival assays in the evaluation of radiation damage to normal tissues. Radiother Oncol. 1(3):241–246. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. 2003. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 63(14):4021–4027. [PubMed] [Google Scholar]
- Mohan R, Grosshans D. 2017. Proton therapy - Present and future. Adv Drug Deliv Rev. 109:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M 2008. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 14(3):238–242. [DOI] [PubMed] [Google Scholar]
- Monje M, Dietrich J. 2012. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 227(2):376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. 2002. Irradiation induces neural precursor-cell dysfunction. Nat Med. 8(9):955–962. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. 2003. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 302(5651):1760–1765. [DOI] [PubMed] [Google Scholar]
- Montay-Gruel P, Acharya MM, Goncalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondre M, Ollivier J, et al. 2021. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin Cancer Res. 27(3):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, et al. 2018. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 129(3):582–588. [DOI] [PubMed] [Google Scholar]
- Moore BD. 2005. Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 30(1):51–63. [DOI] [PubMed] [Google Scholar]
- Moosavi H, McDonald S, Rubin P, Cooper R, Stuard ID, Penney D. 1977. Early radiation dose-response in lung: an ultrastructural study. Int J Radiat Oncol Biol Phys. 2(9-10):921–931. [DOI] [PubMed] [Google Scholar]
- Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. 2016. Brain radiation injury leads to a dose- and time-dependent recruitment of peripheral myeloid cells that depends on CCR2 signaling. J Neuroinflamm. 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Jopson TD, Liu S, Gupta N, Rosi S. 2014. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PloS One. 9(4):e93650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsas B, Raffin TA, Epstein AH, Link CJ Jr. 1997. Pulmonary radiation injury. Chest. 111(4):1061–1076. [DOI] [PubMed] [Google Scholar]
- Muller HJ. 1927. Artificial Transmutation of the Gene. Science. 66(1699):84–87. [DOI] [PubMed] [Google Scholar]
- Müller K, Meineke V. 2007. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 35(4 Suppl 1):96–104. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, et al. 2009. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 339(dec08 1):b4606–b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murigi FN, Mohindra P, Hung C, Salimi S, Goetz W, Pavlovic R, Jackson IL, Vujaskovic Z. 2015. Dose Optimization Study of AEOL 10150 as a Mitigator of Radiation-Induced Lung Injury in CBA/J Mice. Radiat Res. 184(4):422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton KA, Spittle MF. 1969. An analysis of 40 cases treated by total thoracic irradiation. Clin Radiol. 20(1):19–22. [DOI] [PubMed] [Google Scholar]
- Ntentas G, Darby SC, Aznar MC, Hodgson DC, Howell RM, Maraldo MV, Ahmed S, Ng A, Aleman BMP, Cutter DJ. 2020. Dose-response relationships for radiation-related heart disease: Impact of uncertainties in cardiac dose reconstruction. Radiother Oncol. 153:155–162. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Ahn EJ, Kim O, Kim D, Jung TY, Jung S, Lee JH, Kim KK, Kim H, Kim EH, et al. 2019. The Role Played by SLUG, an Epithelial-Mesenchymal Transition Factor, in Invasion and Therapeutic Resistance of Malignant Glioma. Cell Mol Neurobiol. 39(6):769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunieff P, Rubin P, Williams JP, Finkelstein JN, Li A, Fu D, Neta R, Whal S, Liu W, Ding I. 2002. TGFß1 involvement in the development of radiation-induced soft tissue fibrosis. Radiat Res. 54(4):1174–1179. [Google Scholar]
- Oledzka-Slotwinska H, Maisin JR. 1970. Electron microscopic and histochemical observations on the pulmonary alveolar surfactant in normal and irradiated mice. Lab Invest. 22(2):131–136. [PubMed] [Google Scholar]
- Olschowka JA, Kyrkanides S, Harvey BK, O’Banion MK, Williams JP, Rubin P, Hansen JT. 1997. ICAM-1 induction in the mouse CNS following irradiation. Brain Behav Immun. 11(4):273–285. [DOI] [PubMed] [Google Scholar]
- Paladini MS, Feng X, Krukowski K, Rosi S. 2021. Microglia depletion and cognitive functions after brain injury: from trauma to galactic cosmic ray. Neurosci Lett. 741:135462. [DOI] [PubMed] [Google Scholar]
- Panwala TF, Fox ME, Tucker TD, King TZ. 2019. The Effects of Radiation and Sex Differences on Adaptive Functioning in Adult Survivors of Pediatric Posterior Fossa Brain Tumors. J Int Neuropsychol Soc. 25(7):729–739. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Acharya MM, Roa DE, Bosch O, Christie LA, Limoli CL. 2014. Defining functional changes in the brain caused by targeted stereotaxic radiosurgery. Transl Cancer Res. 3(2):124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Angulo MC, Allen BD, Syage A, Usmani MT, Passerat de la Chapelle E, Amin AN, Flores L, Lin X, Giedzinski E, et al. 2020. Sex-Specific Cognitive Deficits Following Space Radiation Exposure. Front Behav Neurosci. 14:535885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Limoli CL. 2013. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 110(31):12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL. 2015. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct. 220(2):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. 2001. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 293(5528):293–297. [DOI] [PubMed] [Google Scholar]
- Park HR, Jo SK, Jung U. 2019. Ionizing Radiation Promotes Epithelial-to-Mesenchymal Transition in Lung Epithelial Cells by TGF-β-producing M2 Macrophages. In Vivo. 33(6):1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paun A, Haston CK. 2012. Genomic and genome-wide association of susceptibility to radiation-induced fibrotic lung disease in mice. Radiother Oncol. 105(3):350–357. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Creer RM, Linville C, Olson J, Kulkarni P, Brown JA, Riddle DR, Robbins ME, Brunso-Bechtold JE. 2014. Radiation-induced cognitive impairment and altered diffusion tensor imaging in a juvenile rat model of cranial radiotherapy. Int J Radiat Biol. 90(9):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney DP, Rubin P. 1977. Specific early fine structural changes in the lung irradiation. Int J Radiat Oncol Biol Phys. 2(11-12):1123–1132. [DOI] [PubMed] [Google Scholar]
- Pineda JR, Daynac M, Chicheportiche A, Cebrian-Silla A, Sii Felice K, Garcia-Verdugo JM, Boussin FD, Mouthon MA. 2013. Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med. 5(4):548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollom EL, Chin AL, Diehn M, Loo BW, Chang DT. 2017. Normal tissue constraints for abdominal and thoracic stereotactic body radiotherapy. Semin Radiat Oncol. 27(3):197–208. [DOI] [PubMed] [Google Scholar]
- Prasanna PG, Woloschak GE, DiCarlo AL, Buchsbaum JC, Schaue D, Chakravarti A, Cucinotta FA, Formenti SC, Guha C, Hu DJ, et al. 2020. Low-Dose Radiation Therapy (LDRT) for COVID-19: benefits or risks? Radiat Res. 194(5):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston RJ, Rühm W, Azzam EI, Boice JD, Bouffler S, Held KD, Little MP, Shore RE, Shuryak I, Weil MM. 2020. Adverse outcome pathways, key events, and radiation risk assessment. Int J Radiat Biol. 97(6):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Li J, He TC. 2011. Endothelial cell damage induces a blood-alveolus barrier breakdown in the development of radiation-induced lung injury. Asia Pac J Clin Oncol. 7(4):392–398. [DOI] [PubMed] [Google Scholar]
- Ratikan JA, Micewicz ED, Xie MW, Schaue D. 2015. Radiation takes its Toll. Cancer Lett. 368(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond KJ, Hales RK, Anderson-Keightly H, Zhou XC, Kummerlowe M, Sair HI, Duhon M, Kleinberg L, Rosner GL, Vannorsdall T. 2017. Prospective study of hippocampal-sparing prophylactic cranial irradiation in limited-stage small cell lung Cancer. Int J Radiat Oncol Biol Phys. 98(3):603–611. [DOI] [PubMed] [Google Scholar]
- Regaud C, Nogier C. 1911. Stérilisation reontgénienne, totale et définitive, sans radiodermite, des testicules du belier adulte. Comptes Rendus de la Societe de Biologie. 70:202–203. [Google Scholar]
- Reinhold HS. 1974. The influence of radiation on blood vessels and circulation. Chapter II. Cell viability of the vessel wall. Curr Top Radiat Res Q. 10(1):9–28. [PubMed] [Google Scholar]
- Richter KK, Langberg CW, Sung CC, Hauer-Jensen M. 1997. Increased transforming growth factor beta (TGF-ß) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int J Radiat Oncol Biol Phys. 39(1):187–195. [DOI] [PubMed] [Google Scholar]
- Ris MD, Noll RB. 1994. Long-term neurobehavioral outcome in pediatric brain-tumor patients: review and methodological critique. J Clin Exp Neuropsychol. 16(1):21–42. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 11(8):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, et al. 2011. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 124(Pt 1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin P, Casarett GW. 1968. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 22(4):767–778. [DOI] [PubMed] [Google Scholar]
- Rubin P, Finkelstein JN. Williams JP. 1998. Paradigm shifts in the radiation pathophysiology of late effects in normal tissues: molecular vs classic concepts. In: Tobias JS, Thomas PRM, editors. Current Radiation Oncology. 3rd ed. London: Arnold; p. 1–26. [Google Scholar]
- Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. 1995. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 33(1):99–109. [DOI] [PubMed] [Google Scholar]
- Rubin P, Shapiro DL, Finklestein JN, Penney DP. 1980. The early release of surfactant following lung irradiation of alveolar type II cells. Int J Radiat Oncol Biol Phys. 6(1):75–77. [DOI] [PubMed] [Google Scholar]
- Rubin P, Siemann DW, Shapiro DL, Finkelstein JN, Penney DP. 1983. Surfactant release as an early measure of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 9(11):1669–1673. [DOI] [PubMed] [Google Scholar]
- Russell NS, Floot B, van Werkhoven E, Schriemer M, de Jong-Korlaar R, Woerdeman LA, Stewart FA, Scharpfenecker M. 2015. Blood and lymphatic microvessel damage in irradiated human skin: The role of TGF-β, endoglin and macrophages. Radiother Oncol. 116(3):455–461. [DOI] [PubMed] [Google Scholar]
- Russell NS, Hoving S, Heeneman S, Hage JJ, Woerdeman LAE, de Bree R, Lohuis PJFM, Smeele L, Cleutjens J, Valenkamp A, et al. 2009. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol. 92(3):477–483. [DOI] [PubMed] [Google Scholar]
- Schaue D 2017. A century of radiation therapy and adaptive immunity. Front Immunol. 8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D, Kachikwu EL, McBride WH. 2012. Cytokines in radiobiological responses: a review. Radiat Res. 178(6):505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D, Ratikan JA, Iwamoto KS, McBride WH. 2012. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 83(4):1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, Frei A, Schottstaedt AM, Tsaih SW, Fish BL, Harmann L, Liu Q, Gasperetti T, Medhora M, North PE, et al. 2019. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am J Physiol Heart Circ Physiol. 316(6):H1267–H1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, Frei A, SenthilKumar G, Tsaih SW, Wells C, Mishra J, Flister MJ, Camara AKS, Bergom C. 2020. Differences in expression of mitochondrial complexes due to genetic variants may alter sensitivity to radiation-induced cardiac dysfunction. Front Cardiovasc Med. 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. 1995. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 31(5):1093–1112. [DOI] [PubMed] [Google Scholar]
- Seemann I, Te Poele JA, Luikinga SJ, Hoving S, Stewart FA. 2013. Endoglin haplo-insufficiency modifies the inflammatory response in irradiated mouse hearts without affecting structural and mircovascular changes. PLoS One. 8(7):e68922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderoff E, Kavee DJ, Johnson JR, Baronofsky ID. 1959. Survival following acute coronary artery ligation subsequent to irradiation of canine heart. Proc Soc Exp Biol Med. 100(1):1–3. [DOI] [PubMed] [Google Scholar]
- Sharplin J, Franko AJ. 1989a. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res. 119(1):1–14. [PubMed] [Google Scholar]
- Sharplin J, Franko AJ. 1989b. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 119(1):15–31. [PubMed] [Google Scholar]
- Sherk HH. 2001. Harrison S. Martland, MD. The Radium watch-dial workers and the evolution of industrial medicine. N J Med. 98(2):49–52. [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK. 2006. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 166(6):892–899. [DOI] [PubMed] [Google Scholar]
- Shinde A, Yang D, Frankel P, Liu A, Han C, Del Vecchio B, Schultheiss T, Cheng J, Li R, Kim D, et al. 2019. Radiation-related toxicities using organ sparing total marrow irradiation transplant conditioning regimens. Int J Radiat Oncol Biol Phys. 105(5):1025–1033. [DOI] [PubMed] [Google Scholar]
- Shirai K, Mizui T, Suzuki Y, Okamoto M, Hanamura K, Yoshida Y, Hino M, Noda SE, Al-Jahdari WS, Chakravarti A, et al. 2013. X irradiation changes dendritic spine morphology and density through reduction of cytoskeletal proteins in mature neurons. Radiat Res. 179(6):630–636. [DOI] [PubMed] [Google Scholar]
- Smyth LM, Senthi S, Crosbie JC, Rogers PA. 2016. The normal tissue effects of microbeam radiotherapy: what do we know, and what do we need to know to plan a human clinical trial? Int J Radiat Biol. 92(6):302–311. [DOI] [PubMed] [Google Scholar]
- Sridharan V, Aykin-Burns N, Tripathi P, Krager KJ, Sharma SK, Moros EG, Corry PM, Nowak G, Hauer-Jensen M, Boerma M. 2014. Radiation-induced alterations in mitochondria of the rat heart. Radiat Res. 181(3):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, Aleman BM, Edgar AB, Mabuchi K, Muirhead CR, Authors on behalf of ICRP, et al. 2012. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs-threshold doses for tissue reactions in a radiation protection context. Ann Icrp. 41(1-2):1–322. [DOI] [PubMed] [Google Scholar]
- Stewart FA, Denekamp J, Hirst DG. 1980. Proliferation kinetics of the mouse bladder after irradiation. Cell Tissue Kinet. 13(1):75–89. [DOI] [PubMed] [Google Scholar]
- Stewart FA, Michael BD, Denekamp J. 1978. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res. 75(3):649–659. [PubMed] [Google Scholar]
- Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. 2015. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 141(11):1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Seemann I, Merl-Pham J, Hauck SM, Stewart FA, Atkinson MJ, Tapio S, Azimzadeh O. 2017. Role of TGF Beta and PPAR Alpha Signaling Pathways in Radiation Response of Locally Exposed Heart: Integrated Global Transcriptomics and Proteomics Analysis. J Proteome Res. 16(1):307–318. [DOI] [PubMed] [Google Scholar]
- Tang D, Loze MT, Zeh HJ, Kang R. 2010. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 6(8):1181–1183. [DOI] [PubMed] [Google Scholar]
- Tapio S, Little MP, Kaiser JC, Impens N, Hamada N, Georgakilas AG, Simar D, Salomaa S. 2021. Ionizing radiation-induced circulatory and metabolic diseases. Environ Int. 146:106235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, McGale P, Povall JM, Thomas E, Kumar S, Dodwell D, Darby SC. 2009a. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys. 73(4):1061–1068. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Nisbet A, McGale P, Darby SC. 2007. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 69(5):1484–1495. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Nisbet A, McGale P, Goldman U, Darby SC, Hall P, Gagliardi G. 2009b. Cardiac doses from Swedish breast cancer radiotherapy since the 1950s. Radiother Oncol. 90(1):127–135. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, Darby SC. 2008. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 72(2):501–507. [DOI] [PubMed] [Google Scholar]
- Tian X, Wang F, Luo Y, Ma S, Zhang N, Sun Y, You C, Tang G, Li S, Gong Y, et al. 2018. Protective Role of Nuclear Factor-Erythroid 2-Related Factor 2 Against Radiation-Induced Lung Injury and Inflammation. Front Oncol. 8:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timins JK. 2011. Communication of benefits and risks of medical radiation: a historical perspective. Health Phys. 101(5):562–565. [DOI] [PubMed] [Google Scholar]
- Tofilon PJ, Fike JR. 2000. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 153(4):357–370.2.0.co;2] [DOI] [PubMed] [Google Scholar]
- Tome WA, Gokhan S, Brodin NP, Gulinello ME, Heard J, Mehler MF, Guha C. 2015. A mouse model replicating hippocampal sparing cranial irradiation in humans: A tool for identifying new strategies to limit neurocognitive decline. Sci Rep. 5:14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita N, Okuda K, Ogawa Y, Iida M, Eguchi Y, Kitagawa Y, Uchiyama K, Takaoka T, Nakanishi R, Shibamoto Y. 2020. Relationship between radiation doses to heart substructures and radiation pneumonitis in patients with thymic epithelial tumors. Sci Rep. 10(1):11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. 2014. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 51(4):515–521. [DOI] [PubMed] [Google Scholar]
- Travis EL. 1980. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 6(3):345–347. [DOI] [PubMed] [Google Scholar]
- Travis EL, Down JD. 1981. Repair in mouse lung after split doses of X rays. Radiat Res. 87(1):166–174. [PubMed] [Google Scholar]
- Travis EL, Down JD, Holmes SJ, Hobson B. 1980. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res. 84(1):133–143. [PubMed] [Google Scholar]
- Travis EL, Parkins CS, Holmes SJ, Down JD, Fowler JF. 1984. WR-2721 protection of pneumonitis and fibrosis in mouse lung after single doses of x rays. Int J Radiat Oncol Biol Phys. 10(2):243–251. [DOI] [PubMed] [Google Scholar]
- Travis EL, Vojnovic B, Davies EE, Hirst DG. 1979. A plethysmographic method for measuring function in locally irradiated mouse lung. Br J Radiol. 52(613):67–74. [DOI] [PubMed] [Google Scholar]
- Trott KR, Kamprad F. 1999. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother Oncol. 51(3):197–203. [DOI] [PubMed] [Google Scholar]
- Turnquist C, Beck JA, Horikawa I, Obiorah IE, Von Muhlinen N, Vojtesek B, Lane DP, Grunseich C, Chahine JJ, Ames HM, et al. 2019. Radiation-induced astrocyte senescence is rescued by A133p53. Neuro Oncol. 21(4):474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger K 2014. Integrative radiation systems biology. Radiother Oncol. 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen SJ, Ghobadi G, de Boer RA, Faber H, Cannon MV, Nagle PW, Brandenburg S, Langendijk JA, van Luijk P, Coppes RP. 2015. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 114(1):96–103. [DOI] [PubMed] [Google Scholar]
- van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, Hauptmann M, Kooijman K, Roesink J, van der Maazen R, Darby SC, et al. 2016. Radiation dose-response relationship for risk of coronary heart disease in survivors of hodgkin lymphoma. JCO. 34(3): 235–243. [DOI] [PubMed] [Google Scholar]
- Vanpouille-Box C, Formenti SC, Demaria S. 2018. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res. 24(2):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga C, Chandy E, Jacob J, Yip N, Szmul A, Landau D, McClelland JR. 2020. Investigation of the evolution of radiation-induced lung damage using serial CT imaging and pulmonary function tests. Radiother Oncol. 148:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. 2006. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 166(6):883–891. [DOI] [PubMed] [Google Scholar]
- Vozenin MC, Hendry JH, Limoli CL. 2019. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin Oncol (R Coll Radiol)). 31(7):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pan H, Lin Z, Xiong C, Wei C, Li H, Tong F, Dong X. 2021. Neuroprotective Effect of Fractalkine on Radiation-induced Brain Injury Through Promoting the M2 Polarization of Microglia. Mol Neurobiol. 58(3):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, Jiang X. 2019. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 15(10):2128–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Spencer J. 1940. Radiation reaction in the lung. Am J Roentgenol Radium Ther. 43:682–701. [Google Scholar]
- Wefel JS, Noll KR, Scheurer ME. 2016. Neurocognitive functioning and genetic variation in patients with primary brain tumours. Lancet Oncol. 17(3):e97–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willers H, Beck-Bornholdt HP. 1996. Origins of radiotherapy and radiobiology: separation of the influence of dose per fraction and overall treatment time on normal tissue damage by Reisner and Miescher in the 1930s. Radiother Oncol. 38(2):171–173. [DOI] [PubMed] [Google Scholar]
- Williams JP, Calvi L, Chakkalakal JV, Finkelstein JN, O’Banion MK, Puzas E. 2016. Addressing the Symptoms or Fixing the Problem? Developing Countermeasures against Normal Tissue Radiation Injury. Radiat Res. 186(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MV, Denekamp J. 1983. Modification of the radiation response of the mouse kidney by misonidazole and WR-2721. Int J Radiat Oncol Biol Phys. 9(11):1731–1736. [DOI] [PubMed] [Google Scholar]
- Williams MV, Denekamp J. 1984. Radiation induced renal damage in mice: influence of fraction size. Int J Radiat Oncol Biol Phys. 10(6):885–893. [DOI] [PubMed] [Google Scholar]
- Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, Finkelstein JN. 2004. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 161(5):560–567. [DOI] [PubMed] [Google Scholar]
- Williams JP, Johnston CJ, Finkelstein JN. 2010. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 11(11):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Newhauser W. 2019. Normal tissue damage: its importance, history and challenges for the future. Br J Radiol. 92(1093):20180048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers HR, Elkind MM. 1969. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiat Res. 38(3):598–613. [PubMed] [Google Scholar]
- Yamamoto K, Ikenaka Y, Ichise T, Bo T, Ishizuka M, Yasui H, Hiraoka W, Yamamori T, Inanami O. 2018. Evaluation of mitochondrial redox status and energy metabolism of X-irradiated HeLa cells by LC/UV, LC/MS/MS and ESR. Free Radic Res. 52(6):648–660. [DOI] [PubMed] [Google Scholar]
- Yang N, Gao X, Qu X, Zhang R, Tong F, Cai Q, Dong J, Hu Y, Wu G, Dong X. 2016. PIDD Mediates Radiation-Induced Microglia Activation. Radiat Res. 186(4):345–359. [DOI] [PubMed] [Google Scholar]
- Yoshida K, French B, Yoshida N, Hida A, Ohishi W, Kusunoki Y. 2019. Radiation exposure and longitudinal changes in peripheral monocytes over 50 years: the Adult Health Study of atomic-bomb survivors. Br J Haematol. 185(1):107–115. [DOI] [PubMed] [Google Scholar]
- Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, Greter M. 2017. The Cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 47(5):903–912. e904. [DOI] [PubMed] [Google Scholar]
- Zawaski JA, Sabek OM, Voicu H, Eastwood Leung HC, Gaber MW. 2017. Effect of brain tumor presence during radiation on tissue toxicity: transcriptomic and metabolic changes. Int J Radiat Oncol Biol Phys. 99(4):983–993. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Harada A, Bluethmann H, Wang JB, Nakao S, Mukaida N, Matsushima K. 1995. Tumor necrosis factor (TNF) is a physiologic regulator of hematopoietic progenitor cells: increase of early hematopoietic progenitor cells in TNF receptor p55-deficient mice in vivo and potent inhibition of progenitor cell proliferation by TNF alpha in vitro. Blood. 86(8):2930–2937. [PubMed] [Google Scholar]
- Zhang W, Ning N, Li X, Niu G, Bai L, Guo Y, Yang J. 2016. Changes of brain glucose metabolism in the pretreatment patients with non-small cell lung cancer: a retrospective PET/CT st udy. PloS One. 11(8):e0161325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhou W, Lam TT, Li Y, Duman JG, Dougherty PM, Grosshans DR. 2020. Cranial irradiation induces axon initial segment dysfunction and neuronal injury in the prefrontal cortex and impairs hippocampal coupling. Neurooncol Adv. 2(1):vdaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhou W, Lam TT, Weng C, Bronk L, Ma D, Wang Q, Duman JG, Dougherty PM, Grosshans DR. 2018. Radiation induces age-dependent deficits in cortical synaptic plasticity. Neuro Oncol. 20(9):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Ma C, O’Connell RM, Mehta A, DiLoreto R, Heath JR, Baltimore D. 2014. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 14(4):445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Robbins ME. 2009. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 16(2):130–143. [DOI] [PubMed] [Google Scholar]
- Zhou S, Nissao E, Jackson IL, Leong W, Dancy L, Cuttitta F, Vujaskovic Z, Sunday ME. 2013. Radiation-induced lung injury is mitigated by blockade of gastrin-releasing peptide. Am J Pathol. 182(4):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H, Shi S, Yuan Z, Chang JY. 2019. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]


