Abstract
The Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, Calif.) utilizes capillary electrophoresis on a microchip device (LabChip 7500; Caliper Technologies, Mountain View, Calif.) that is capable of rapidly sizing small DNA fragments. To determine whether the system could replace conventional restriction fragment length polymorphism (RFLP) typing by agarose gel electrophoresis, we compared the analyzer with conventional flagellin RFLP for typing Campylobacter jejuni. Ninety-seven isolates representing 46 Fla types were initially analyzed. Correct Fla types were detected in 59% of the isolates. The major problem with the system was in resolving samples containing multiple DNA fragments differing from 8 to 20 bp. Overall, the bioanalyzer has the potential to replace conventional RFLP analysis by gel electrophoresis, but improvements in the chip separation are needed.
The Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, Calif.) (referred to here as the bioanalyzer) is the first commercially available system to utilize chip-based nucleic acid separation technology. The LabChip (developed by Caliper Technologies, Mountain View, Calif.) separates nucleic acid fragments by capillary electrophoresis in a chip with microfabricated channels and automates the detection as well as on-line data evaluation. We previously developed a typing system for Campylobacter jejuni based on restriction fragment length polymorphism (RFLP) analysis of the Campylobacter flaA flagellin gene (3, 4). While the system works well and has been utilized for several epidemiologic investigations (7; J. Hunt, L. Carroll, F. Leano, C. Hedberg, D. Boxrud, and I. Nachamkin, Abstr. 96th Gen. Meet. Am. Soc. Microbiol., abstr. C-279, 1996) the gel system has been somewhat difficult to standardize between laboratory investigators (I. Nachamkin, N. Stern, J. Hunt, M. A. Myszewski, L. Carroll, and H. Ung, Abstr. 96th Gen. Meet. Am. Soc. Microbiol., abstr. C-278, 1996), particularly in standardizing electrophoretic running conditions to allow for pattern recognition software to work between typing laboratories. To evaluate the bioanalyzer for RFLP analysis, we used Campylobacter flagellin gene typing as a model system.
C. jejuni isolates comprising a variety of flagellin gene types were obtained from our collection and were stored at −70°C prior to use. Flagellin gene typing was performed as previously described (4), except that we used a slightly modified set of primers to amplify flaA, based on a new consensus reverse sequence recommended by Wassenaar and Newell (8). The forward primer sequence 5′-ATGGGATTTCGTATTAACAC-3′ and reverse primer 5′-CAAAATGTTTTAAGATTACTACAG-3′ were used and improved typability (I. Nachamkin, unpublished data). Bacterial extracts were either prepared using the boiling method as previously described (4) or prepared using a commercially available bacterial DNA extract system (Instagene Matrix [catalog no. 7326030], Bio-Rad, Richmond, Calif.). Agarose gels were photographed and images were analyzed as previously described (4).
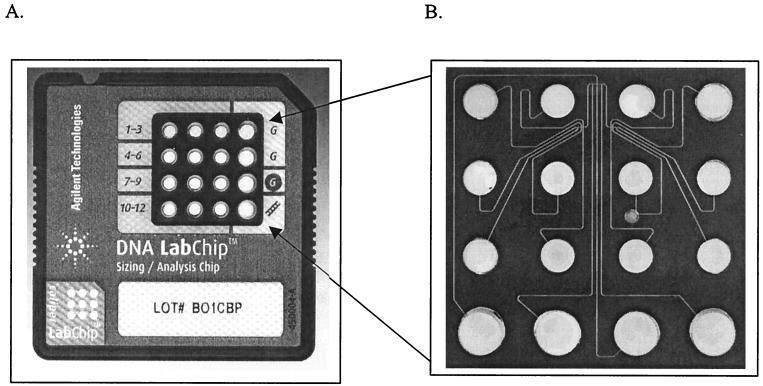
The bioanalyzer sizes and quantitates 12 samples on a disposable chip in approximately 30 min. Each glass chip possesses 16 wells interconnected via a network of microchannels which are filled with a gel-dye mix (Fig. 1). Twelve of the 16 wells are used for experimental samples, one is used for a kit-supplied molecular weight ladder, and three are used for loading the gel-dye mix. DNA 7500 LabChips were prepared and loaded with samples as recommended by the manufacturer with minor modifications. Briefly, microchannels were filled by pipetting 9 μl of gel-dye mix (consisting of a linear polymer and a fluorescent, intercalating dye of a proprietary nature) into the appropriate well and then forcing the mix into the microchannels by applying pressure to the well via a 1-ml syringe. Five microliters of marker mix was loaded into each sample well, followed by 1 μl of molecular weight ladder into the ladder well or samples into the sample well. The contents of each well on the chip were mixed in situ via gentle pipetting and then vortexed for 1 min at setting 4 using a Fisherbrand Vortex Genie-2 (Fisher Scientific, Pittsburgh, Pa.). After being vortexed, chips were immediately inserted into the bioanalyzer and processed. All experiments were performed using Agilent Biosizing software (version A.01.10). Previous studies showed that the bioanalyzer met analytical specifications with respect to sizing accuracy, sizing resolution, and quantitation accuracy (6).
FIG. 1.
LabChip 7500 used in the Agilent 2100 bioanalyzer. (A) Top side of the chip showing layout of marker and sample wells. (B) The chip performs capillary electrophoresis in a series of micro-fabricated channels.
In addition to the internal marker included on the LabChip, we included a 123-bp ladder (Gibco BRL) to be used as a marker for subsequent flagellin gene typing. An image of the chromatogram was produced by the bioanalyzer analysis software in TIFF format and imported into ProRFLP for typing analysis as previously described (4). Analyses of all samples on the bioanalyzer were performed in a blinded fashion.
Our initial study included 97 strains of C. jejuni representing 46 different flagellin gene types. The isolates were analyzed once in a series of nine different chip runs. RFLP patterns by conventional gel electrophoresis ranged from three to seven fragments for each strain. Of the 97 strains analyzed, 62 (59%) had the correct banding patterns detected by the bioanalyzer. There were 517 possible DNA fragments, of which the bioanalyzer showed concordant results for 90.3% (n = 467 bands). Thirty-one bands were not detected (6.0%), and/or there were 27 extra bands (5.2%) detected. We found that adjusting the bioanalyzer baseline resulted in correct results for 16 strain patterns. Of the other strains with discordant results, six were found to be due to technical errors (wrong sample loaded or well not loaded with gel properly), and the others were due to the inability of the chip to resolve two closely sized DNA fragments, generally within 8 to 20 bp (Fig. 2).
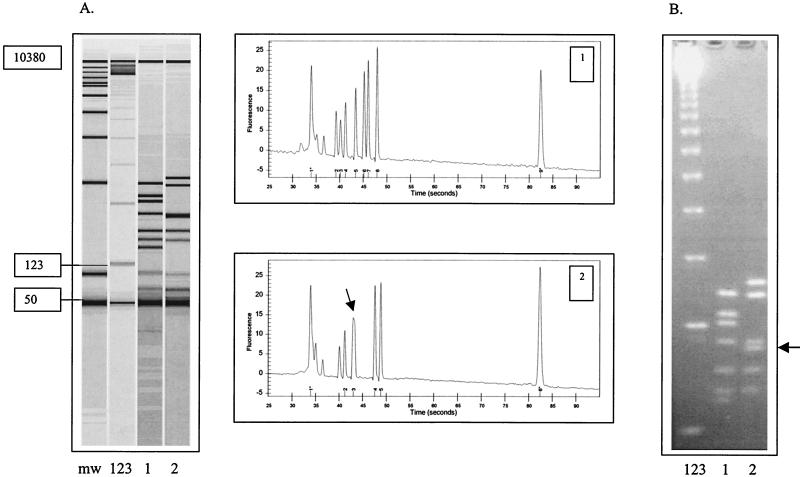
FIG. 2.
FlaA RFLP analysis of two strains of C. jejuni analyzed by the Agilent 2100 bioanalyzer (A) and conventional agarose gel electrophoresis (B). The 50- to 10,380-bp marker (lane mw) is used by the bioanalyzer to size the DNA fragments. A 123-bp ladder (lane 123) was included in both the LabChip and conventional agarose gel for flagellin gene typing. Lanes 1 and 2 show the flaA restriction fragment pattern of two C. jejuni isolates, 41-96-100 (lane 1) and 7-98-308 (lane 2), and the respective chromatograms generated by the Agilent bioanalyzer. Peak 1 (50 bp) and peak 9 (10,380bp) on each chromatogram represent internal markers. Note the wide unresolved peak (arrow) in sample 2 that is clearly resolved by agarose gel electrophoresis (arrows). For flagellin gene typing, only those bands above 123 bp are considered for analysis.
There was 100% concordance for 19 different Fla types; however, for certain Fla types, such as Fla-1, Fla-16, and Fla-80, none were analyzed correctly. There were five isolates representing Fla-1 patterns, and all five were typed incorrectly by the bioanalyzer. The Fla-1 profile contains six fragments, and the bioanalyzer could not differentiate two DNA fragments differing 9 to 12 bp in size. Similarly, for Fla-16, there were five DNA fragments, but the bioanalyzer could not differentiate two fragments differing 6 to 8 bp in size. For Fla-80, there were five fragments, and the bioanalyzer could not differentiate two fragments differing 10 to 12 bp in size.
After taking into account the problems observed in the initial evaluation, a second set of experiments was performed using 60 samples derived from the first set. These were selected to represent the more difficult strains, with most of the strains containing closely sized DNA fragments. Of these 60 strains, 38 strains (63%) showed results concordant with conventional RFLP analysis. We examined the correlation between band size and concordant-discordant results. The LabChip resolved closely sized bands of between 123 and 200 bp in size in 70.5% of samples where such banding patterns were present. In contrast, closely sized bands of between 200 and 300 bp in size were not resolved in 56% of samples where such banding patterns were present.
To look at interchip variability, 25 samples were run in duplicate on different chip sets and the patterns were compared. Nine of these samples initially exhibited results discordant with conventional RFLP analysis which were caused by the inability of the chip to resolve closely sized DNA fragments. All nine samples were discordant upon repeat analysis on two different chip sets. Sixteen samples initially showed concordant results with the expected RFLP patterns. Upon repeat testing on duplicate chip sets, all 16 samples showed concordant results with expected RFLP patterns.
Finally, we used the gel image generated by the bioanalyzer PC software to type the different RFLP patterns using the Campylobacter Fla typing database (freely available on request to I.N.) and ProRFLP software (4). Using images from the initial experiments that contained only the internal LabChip marker (50 to 7,500 bp), none of the RFLP patterns could be recognized in our database software analysis. In subsequent experiments, we also used a 123-bp ladder marker in the LabChip that we also include in our conventional gel electrophoresis. Using this as our standard for analysis in the ProRFLP software, the bioanalyzer RFLP patterns were now recognized in our database and gave Fla typing results concordant with conventional RFLP analysis.
RFLP analysis is widely used as a molecular epidemiologic tool for studying a variety of microbial pathogens (1) and has been extensively used by investigators studying Campylobacter spp. (5). Although restriction analysis of PCR products is not particularly technically complex, a laboratory must be suitably outfitted with the equipment necessary for performing gel electrophoresis and photography. If one wishes to develop a typing system that can be used in different laboratories, standardization of methods becomes a central issue as well.
The Agilent 2100 bioanalyzer is designed to rapidly analyze DNA fragments of various sizes and has the potential to allow any laboratory to perform molecular epidemiologic analysis, without the need for specialized electrophoresis equipment or the space associated with such equipment. Using the DNA 7500 LabChip, up to 12 samples can be analyzed in a single run, usually taking 1 to 2 h from start to finish, including postanalysis. Compared with conventional RFLP analysis, the costs for the bioanalyzer appear to be comparable, i.e., approximately $1.00 to $1.50 per sample plus the initial analyzer purchase (∼$18,000). For conventional analysis, one must acquire an electrophoresis chamber, power supply, and photographic equipment (digital) and include the cost of preparing the gels (∼$1.00/sample). Previous analysis of the system showed that the key to obtaining reproducible results was in chip preparation and proper assignment of 50- and 10,380-bp standards contained in the marker mix (6).
The bioanalyzer using the LabChip 7500 had difficulties in resolving some DNA fragments, generally within 8 to 20 bp in size. This resulted in the inability to correctly type a large proportion of strains in our analysis. This was not a consistent finding, since there were some strains with two fragments within this range that were resolved by the system. Resolution of closely sized bands appeared to be more problematic in the 200- to 300-bp region. Replicate analysis of problem samples using different chip sets showed highly reproducible results and suggests that adjustments to the separation matrix used in the chip or to the running conditions may be needed to better resolve these closely sized DNA fragments. Aside from this problem, however, the bioanalyzer correctly sized the majority of DNA fragments (∼90%) detected by conventional gel electrophoresis patterns.
While the performance characteristics of the bioanalyzer compared to conventional fla analysis was inadequate to replace our current system, the bioanalyzer might exhibit better performance for other RFLP applications, where resolution of closely sized DNA fragments is not an issue, but each individual application needs to be validated before using the system. Finally, if improvements can be made to the bioanalyzer, the system has the potential to ease the standardization of typing between laboratories. For example, in establishing regional or national monitoring typing for particular microbial pathogens, such as the PulseNet system developed by the Centers for Disease Control and Prevention (2), the bioanalyzer seems well suited for routine laboratory RFLP typing.
Acknowledgments
P.W. and L.J.K. were previously consultants for Caliper Technologies. This work was supported in part by a grant from the National Institutes of Health (CA78848-01) to P.W. and L.J.K.
REFERENCES
- 1.Arbeit R D. Laboratory procedures for the epidemiologic analysis of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 116–137. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Safer and healthier foods. Morb Mortal Wkly Rep. 1999;48:905–913. [PubMed] [Google Scholar]
- 3.Nachamkin I, Bohachick K, Patton C M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31:1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachamkin I, Ung H, Patton C M. Analysis of HL and O serotypes of Campylobacter strains by the flagellin gene typing system. J Clin Microbiol. 1996;34:277–281. doi: 10.1128/jcm.34.2.277-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newell D G, Frost J A, Duim B, Wagenaar J A, Madden R H, van der Plas J, On S L W. New developments in the subtyping of Campylobacter species. In: Nachamkin I, Blaser M J, editors. Campylobacter. Washington, D.C.: ASM Press; 2000. pp. 27–44. [Google Scholar]
- 6.Panaro, N. J., P. K. Yuen, T. Sakazume, P. Fortina, L. J. Kricka, and P. Wilding. Evaluation of the Agilent 2100 bioanalyzer. Clin. Chem., in press. [PubMed]
- 7.Smith K E, Besser J M, Hedberg C W, Ieano F T, Bender J B, Wicklund J H, Johnson B P, Moore K A, Osterholm M T. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. N Engl J Med. 1999;340:1525–1532. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 8.Wassenaar T, Newell D G. Genotyping of Campylobacter spp. Appl Environ Microbiol. 2000;66:1–9. doi: 10.1128/aem.66.1.1-9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]