To the Editor: During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, several new viral variants have emerged, leading to the virus becoming more contagious. However, efficient immune escape has not been observed, and vaccines have remained effective. Most recently, the B.1.1.529 (omicron) variant has been described, which the World Health Organization classified as a variant of concern on November 26, 2021.1
The omicron variant is characterized by a large number of mutations, with 26 to 32 changes in the spike (S) protein.2 Given that many of these mutations are in regions that are known to be involved in immune escape, we studied whether serum samples obtained from persons who had been vaccinated against SARS-CoV-2 or who had recovered from SARS-CoV-2 infection (i.e., convalescent) would be able to neutralize the omicron variant. The observation that the omicron variant is more likely than previous variants to cause reinfection suggests some level of immune escape.3
We obtained serum samples from persons who had been infected with the B.1.1.7 (alpha), B.1.351 (beta), or B.1.617.2 (delta) variant of SARS-CoV-2 and from persons who had received two doses of the mRNA-1273 vaccine (Spikevax, Moderna), the ChAdOx1-S vaccine (also known as ChAdOx1 nCoV-19; Vaxzevria, AstraZeneca), or the BNT162b2 vaccine (Comirnaty, Pfizer–BioNTech) or had received heterologous vaccination (i.e., one dose each) with the ChAdOx1-S and BNT162b2 vaccines. For all serum samples, we determined titers of neutralizing antibodies against the alpha, beta, delta, and omicron variants using a focus-forming assay with replication-competent SARS-CoV-2 viruses, as described previously.4 We also obtained serum samples from persons who had been infected and were subsequently vaccinated (convalescent–vaccinated) or had been vaccinated and had subsequent breakthrough infection (vaccinated–convalescent). We analyzed neutralizing antibody titers against the delta and omicron variants in these samples.
A total of 10 participants had been infected with the alpha variant, 8 with the beta variant, and 7 with the delta variant. Ten participants had received two doses of the mRNA-1273 vaccine, 10 the ChAdOx1-S vaccine, and 20 the BNT162b2 vaccine; 20 participants had received heterologous vaccination with the ChAdOx1-S and BNT162b2 vaccines. In addition, 5 participants had been infected and subsequently received one or two doses of the BNT162b2 vaccine, and 5 had been vaccinated with two doses of the mRNA-1273, ChAdOx1-S, or BNT162b2 vaccine and subsequently had breakthrough infection. The characteristics of the participants are shown in Tables S1 through S3 in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
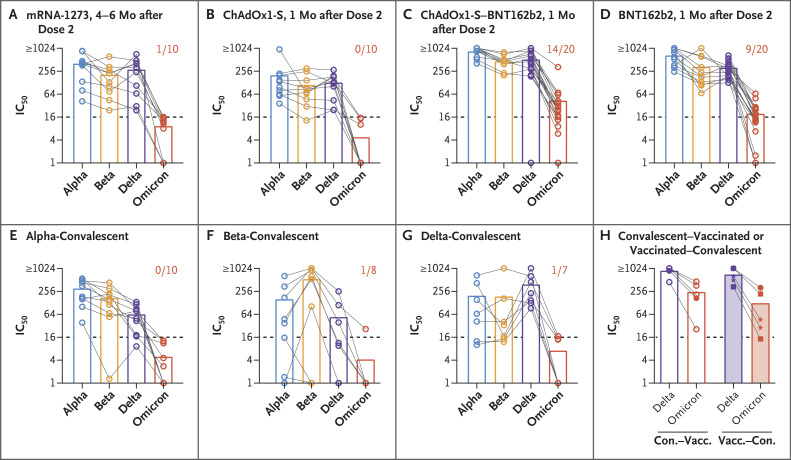
Serum samples from vaccinated persons neutralized the omicron variant to a much lesser extent than any other variant analyzed (alpha, beta, or delta) (Figure 1 and Table S4). We found some cross-neutralization of the omicron variant in samples obtained from persons who had received either homologous BNT162b2 vaccination or heterologous ChAdOx1-S–BNT162b2 vaccination but not in samples from persons who had received homologous ChAdOx1-S vaccination. We did not find neutralizing antibodies against the omicron variant in serum samples obtained 4 to 6 months after receipt of the second dose of the mRNA-1273 vaccine. However, in this group, the interval between receipt of the second dose and sampling was longer than for the other vaccination-regimen groups, for which serum samples were obtained only 1 month after receipt of the second dose. We did not analyze serum samples from persons who had received a third dose of vaccine. Serum samples that were obtained from convalescent participants largely did not neutralize the omicron variant, although cross-neutralization was observed against other variants. However, 9 of the 10 serum samples that were obtained from convalescent–vaccinated or vaccinated–convalescent participants were able to neutralize the omicron variant, although to a lesser degree than the delta variant.
Figure 1. Neutralization of the B.1.1.529 (Omicron) Variant as Compared with Other Variants of Concern.
Serum samples were obtained from participants who had received two doses of the mRNA-1273 vaccine (Panel A), two doses of the ChAdOx1-S vaccine (Panel B), heterologous ChAdOx1-S–BNT162b2 vaccination (Panel C), or two doses of the BNT162b2 vaccine (Panel D) or who had recovered from infection (i.e., convalescent) with the B.1.1.7 (alpha) variant (Panel E), the B.1.351 (beta) variant (Panel F), or the B.1.617.2 (delta) variant (Panel G). Samples were analyzed for 50% neutralization titers (IC50) against the alpha (blue), beta (orange), delta (purple), and omicron (red) variants. Bars indicate means, and symbols individual serum samples. Samples from the same participant are connected by lines. The dashed line in each panel indicates the limit of detection. The numbers in Panels A through G indicate the proportion of serum samples that were positive (>1:16) for the omicron variant. Serum samples from participants who had been infected and were subsequently vaccinated (convalescent–vaccinated; open bars) or who had been vaccinated and subsequently had breakthrough infection (vaccinated–convalescent; shaded bars) were analyzed for IC50 against the delta and omicron variants (Panel H). In the left part of Panel H (convalescent–vaccinated), open circles indicate participants who received a single dose of the BNT162b2 vaccine after infection, and closed circles those who received two doses of the BNT162b2 vaccine; in the right part (vaccinated–convalescent), closed circles indicate participants who had been vaccinated with two doses of the BNT162b2 vaccine before infection, stars those who had been vaccinated with two doses of the ChAdOx1-S vaccine, and squares those who had been vaccinated with two doses of the mRNA-1273 vaccine.
The omicron variant has already become the dominant variant in many countries and is causing considerable illness and death, although possibly to a somewhat lesser extent than previous variants. Although receipt of a third dose (booster) of the BNT162b2 vaccine may increase the level of cross-neutralizing antibodies to the omicron variant,5 on the basis of the data from the present study, the rapid development of new, variant-adapted vaccines is warranted.
Supplementary Appendix
Disclosure Forms
This letter was published on January 12, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern).
- 2.Network for Genomic Surveillance in South Africa (NGS-SA). SARS-CoV-2 sequencing update. November 26, 2021. (https://www.nicd.ac.za/wp-content/uploads/2021/11/Update-of-SA-sequencing-data-from-GISAID-26-Nov_Final.pdf).
- 3.Pulliamn JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the omicron variant in South Africa. December 2, 2021. (https://www.medrxiv.org/content/10.1101/2021.11.11.21266068v2). preprint. [DOI] [PMC free article] [PubMed]
- 4.Riepler L, Rössler A, Falch A, et al. Comparison of four SARS-CoV-2 neutralization assays. Vaccines (Basel) 2020;9:13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 omicron. Nature (in press) (https://www.nature.com/articles/d41586-021-03846-z). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.